Reproductive Dynamics of the Large Yellow Croaker Larimichthys crocea (Sciaenidae), A Commercially Important Fishery Species in China
- 1State Key Laboratory of Marine Environmental Science, College of Ocean and Earth Sciences, Xiamen University, Xiamen City, China
- 2State Key Laboratory of Marine Pollution, City University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Fishery Resources Monitoring Center of Fujian Province, Fuzhou City, China
The large yellow croaker Larimichthys crocea (Richardson, 1846) (Sciaenidae) is distributed in southern Yellow Sea, East China Sea, and northern South China Sea of China and is a commercially important nearshore fishery species. L. crocea was listed on the IUCN Red List as “Critically Endangered” in 2020 mainly due to the over-exploration of its spawning and over-wintering aggregations in the 1950s–1980s throughout its distribution region. However, detailed studies on reproductive dynamics of L. crocea were limited in the past three decades. In this study, the reproductive dynamics of L. crocea was examined in the traditional Guanjingyang (GJY) spawning ground, one of the 15 well-known ones in its distribution region. Samples were collected using set nets from April 2019 to November 2021 to ensure at least 20 samples for all 12 months. A total of 1,006 individuals were caught, ranging from 46 to 391 mm standard length (SL) and 1.45 to 1,110.05 g body weight (BW). A growth dimorphism was found between sexes with females heavier than males when body sizes exceeded 61 mm SL (non-parametric ANCOVA, p < 0.01). Gonad histology of all 1,006 individuals revealed, for the first time, that L. crocea was able to spawn almost year-round for both females and males. Two spawning peaks, spring and autumn, were identified in March and May and in November for females and in April to June and in October to November for males. The minimum sizes at sexual maturity were 160 mm SL for females and 112 mm SL for male. The sizes at 50% sexual maturity were 187.2 mm SL for females and 150.2 mm SL for males. Results showed that the minimum SL for female maturity decreased about 20% in the past six decades. The spawning peaks were 2 months earlier in spring and 1 month extension in autumn in GJY. Clearly, the national fishing moratorium regulation in May to August, an important fishery management measure in China, can only protect the spring spawning peak partly. Further evaluation on the influence of climate change on reproductive strategies and stock recruitment of L. crocea is highly recommended.
Introduction
Croakers and drums or sciaenids (family Sciaenidae) have long been important species of coastal fisheries in warm temperate and tropical nations. Estimated annual global sciaenid catches increased from approximately 241,300 tonnes (t) in 1950 to over 1,000,000 t in 1995 for the first time and over 1,500,000 t in 2006–2019 (www.fao.org/fishery/statistics/global-capture-production/en). Many sciaenid stocks are facing declines and some are of conservation concern by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and the International Union for Conservation of Nature (IUCN) (FAO, 2018; Oliveira and Oliveira, 2018; FAO, 2020; Cisneros-Mata et al., 2021). The global assessments of sciaenids (N = 286) using the 2001 IUCN Red List Categories and Criteria (version 3.1) revealed that 5.6% of sciaenids are threatened, including vulnerable (VU), endangered (EN) or critically endangered (CR), and 1.4% near threatened (NT) (www.iucnredlist.org). The fishery operations of sciaenids are mainly associated with their biological features, including targeting their nearshore and river margin spawning and nursery aggregations (Chao et al., 2015).
China (mainland, excluding Hong Kong, Macao and Taiwan, unless otherwise specified) is the largest capture fisheries country in the world (Kang et al., 2018). Sciaenid capture fisheries have been of significance in domestic marine fisheries. Among the 26 marine fish species and species groups available for statistical catch volumes, six are from sciaenids, including the large yellow croaker Larimichthys crocea, the small yellow croaker Larimichthys polyactis, the Mi-iuy croaker Miichthys miiuy, Pennahia species, Nibea species, and Collichthys species (MARA, 2021). The estimated capture productions of sciaenids varied from 223,121 t in 1956 to 734,285 t in 2020, exceeding one million t in 2012, 2013, and 2015, constituting an average of 9.2% (between 36.1% in 1968 and 1.0% in 1989) of the annual total marine fish capture fishery production (MOA, 1950–2018; MARA, 2019–2021) (Figure 1). L. crocea and L. polyactis have the longest statistical datasets since 1956, indicating their commercially importance in Chinese domestic marine fisheries.
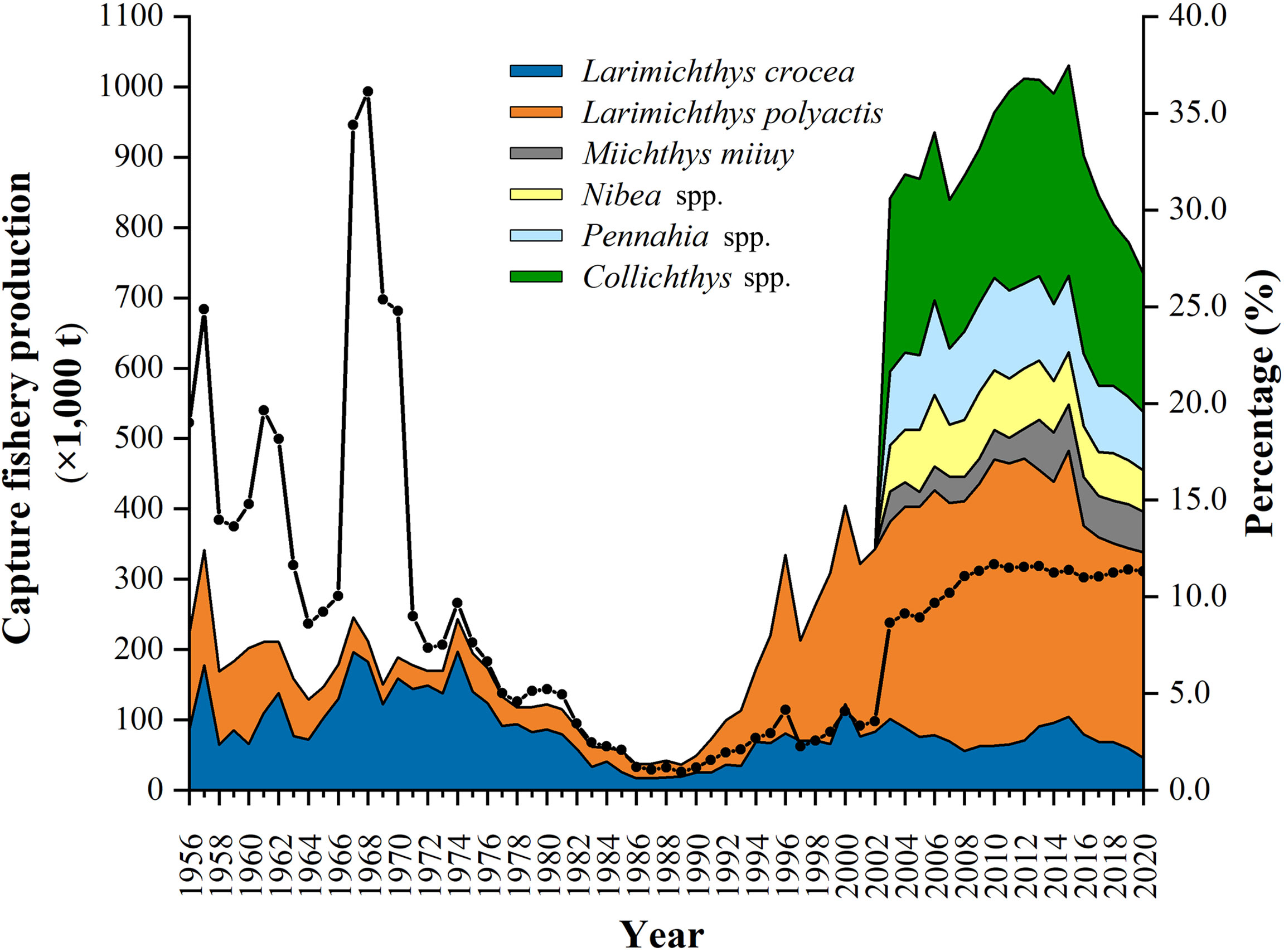
Figure 1 The estimated statistical annual sciaenid (three at species level and three at genus level) capture fishery productions (× 1,000 t) between 1956 and 2020 in China, together with their contributions (percentage, %) (solid line) to the annual total marine fish capture productions (MOA, 1950–2018; MARA, 2019–2021).
L. crocea is distributed in southern Yellow Sea, East China Sea, and northern South China Sea; therefore, it is largely endemic in Chinese waters (Liu et al., 2020a). In China, the annual catch volumes of L. crocea were over 50,000 t in the 1950s–early 1980s, exceeding 150,000 t in some years (Figure 1). The productions came mainly from the exploitation of the spawning and over-wintering aggregations in its entire distribution region (Liu and Sadovy de Mitcheson, 2008). The L. crocea fishery collapsed in the late 1980s, and the annual catches were less than 20,000 t; the aggregations of L. crocea were no longer significance and subsequently disappeared (Liu and Sadovy de Mitcheson, 2008). Various measures have been introduced for L. crocea management, including the prohibition of the drag seine nets since the mid-1950s, which particularly targeting the spawning aggregations, and the protection of nearshore spawning grounds by establishing protected areas, reducing fishing pressure, and controlling fishing gears since the 1980s (Liu and Sadovy de Mitcheson, 2008). Furthermore, L. crocea mariculture and juvenile restocking have been promoted, and the national fishing moratorium regulation in May to August in all Chinese seas has been enforced since the 1990s (MOA, 2000; Liu and Sadovy de Mitcheson, 2008; Liu et al., 2020b). Irrespective of the increase of L. crocea capture productions that has been reported in the past two decades (Figure 1), direct evidence for supporting stock recovery has not been reported since the 1990s, such as the reappearance of spawning aggregations, and the collection of fully mature females and males in the historical spawning grounds (Ye et al., 2020; Xu et al., 2021). Understanding the reproductive dynamics of commercially important fishes can provide valuable information for stock assessment and management measure evaluation (Sadovy and Domeier, 2005; Lowerre-Barbieri et al., 2011; Farley et al., 2013; Sadovy de Mitcheson et al., 2013).
Sansha Bay (26.40−27.00° N, 119.50−120.20° E), a typical semi-enclosed bay, is located in northern Fujian Province of China. Within Sansha Bay, there is a well-known L. crocea spawning ground, i.e., Guanjingyang (GJY) spawning ground, the only semi-enclosed bay type spawning ground among the 15 spawning grounds identified in its distribution region (Liu and Sadovy de Mitcheson, 2008; Zhang et al., 2011) (Figure 2). On the basis of the catch data, two spawning seasons were reported for L. crocea in GJY, i.e., in May to June and in September to October, referring to the spring and autumn spawning seasons, respectively; furthermore, the spring spawning season was considered as the main one (Chu and Wu, 1985; Zhang and Hong, 2015). The reductions of the minimum size [standard length (SL)] and body weight (BW) at female sexual maturity and the increase of growth rate in L. crocea were observed in GJY from the late 1950s to the late 1980s over three decades (Xu et al., 1980; Lin et al., 1992; Liu and Sadovy de Mitcheson, 2008). Although there were biological data available aforementioned, detailed studies through year-round sampling to determine the natural spawning season and the peak and the size at 50% maturity using the cost-effective gonad histology method have not been conducted for L. crocea in GJY, neither throughout the distribution region.
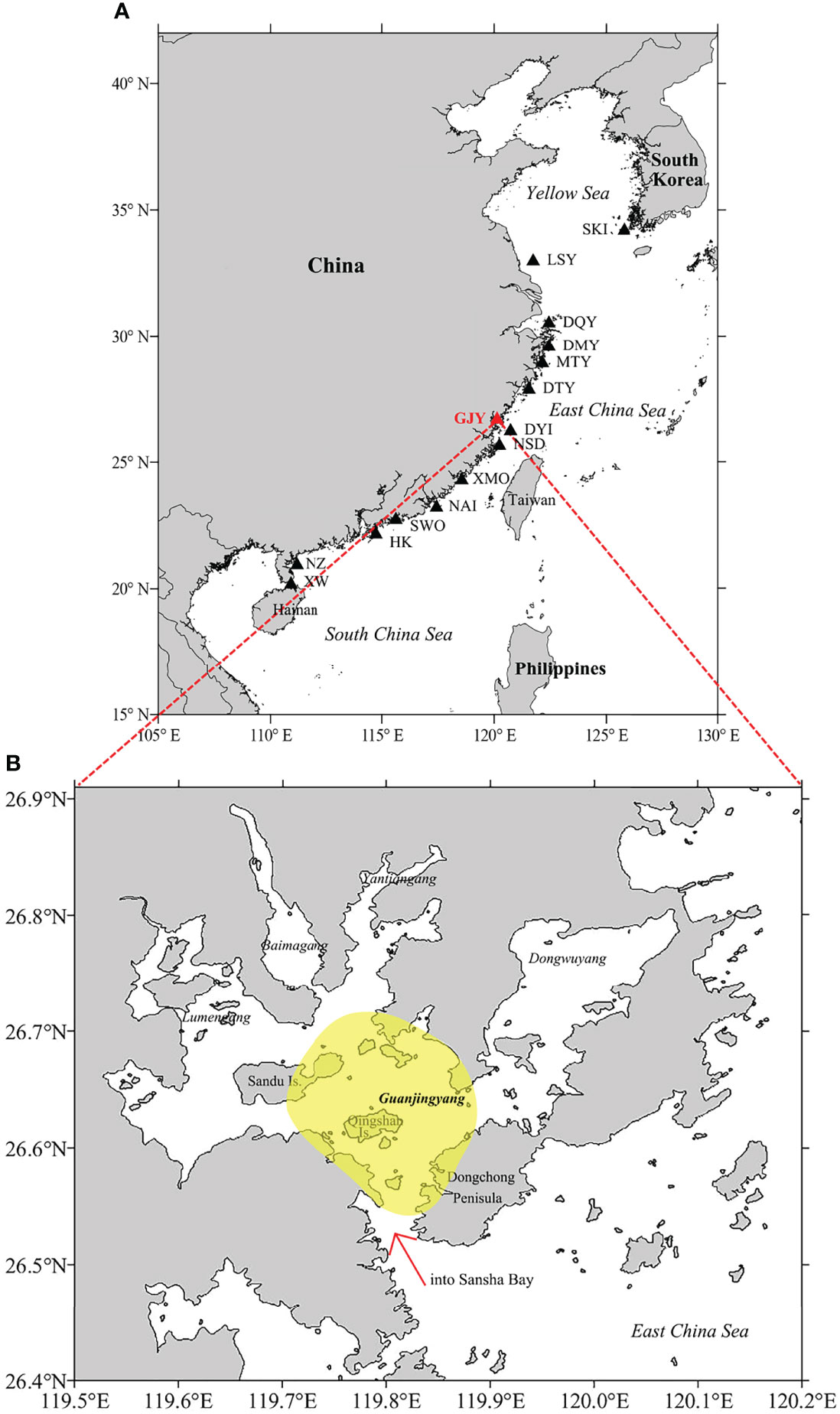
Figure 2 (A) Locations of the 15 spawning grounds for Larimichthys crocea in its distribution region (SKI, South Korea-inshore; LSY, Lusiyang; DQY, Daiquyang; DMY, Damuyang; MTY, Maotouyang; DTY, Dongtouyang; GJY, Guanjingyang; DYI, Dongying Island; NSD, Niushan Island; XMO, Xiamen offshore; NAI, Nanao-inshore; SWO, Shanwei offshore; HK, Hong Kong; NZ, Naozhou; XW, Xuwen). (B) Map of Sansha Bay indicating the sampling area (yellow), Guanjingyang (GJY) waters, Fujian Province, China (red arrow shows the only entrance of Sansha Bay).
This study was conducted in GJY L. crocea spawning ground, and the objectives were 1) to determine the current spawning season and its peak(s), 2) to examine the minimum sizes of sexual maturity and the sizes at 50% maturity for both females and males, and 3) to evaluate the changes of reproductive patterns over decades. The results will help us understand the current status of GJY spawning ground and discuss the national fishing moratorium regulation that applied since the 1990s.
Materials and Methods
Fish Sampling
Sample collection for L. crocea was conducted in GJY waters of Sansha Bay, Fujian Province (Figure 2). Sampling effort lasted from April 2019 to November 2021, with 23 months having L. crocea samples. Samples from the same month of different years were pooled together to gain enough number for all 12 months, i.e., at least 20 individuals per month. Every month, L. crocea samples were collected from four to six set nets that scattered in GJY waters. The sampling was completed in 2 days within the first 3 to 5 days of the full moon or new moon phases.
Fish Measurement and Calculation
All individuals collected were measured for SL (mm) and BW (g). The length–weight relationships for females and males were calculated as follows: BW = a × SLb, where a is the intercept and b is the slope (Froese, 2006).
The condition factor (K), that is the “a” aforementioned, was also calculated monthly as a = BW/SLb. The K is used to evaluate the degree of wellbeing and can provide information on the state of its sexual maturity and the environmental quality for reproduction (Le Cren, 1951; Azevedo et al., 2017). Generally, the higher K value indicates the better conditions for activities that require higher energy costs such as reproduction (Andrade et al., 2015). Intact paired gonad lobes were removed after dissection and weighed [gonad weight (GW), g]. The gonadosomatic index (GSI) was calculated as GSI (%) = GW/(BW − GW) × 100.
Gonad Histology
In the preliminary studies on sciaenids, the anterior, middle, and posterior proportions of each lobe showed no difference in developmental stages of germ cells (Lin et al., 1992; Yamaguchi et al., 2006; Tuuli et al., 2011; Zhang et al., 2019). Therefore, the middle portions of the lobes that provided the largest gonad section areas were cut and fixed in Dietrich’s fixative (Gray, 1975) for at least 1 week. The gonad tissues were then transferred to 70% ethanol for 48 h, dehydrated in at gradient of ethanol (from 95% to 100%), cleared in xylene and embedded in paraffin wax. Gonadal tissues were sectioned at 5–7 μm thickness using a rotary microtome. Slides were then counterstained with hematoxylin and eosin. For each gonad tissue, two to four slides were prepared.
Sexual Maturity Stages
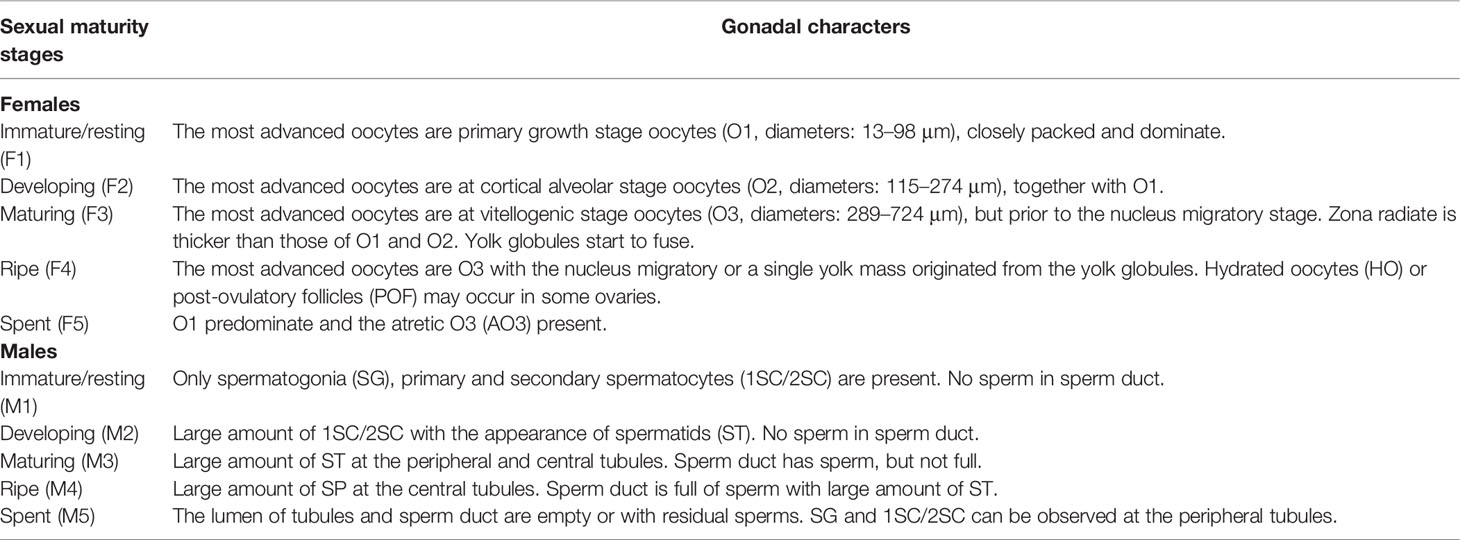
Gonad sections were examined under microscopy. For ovaries, oocytes were classified into six developmental stages, including primary-growth stage (O1), cortical-alveolus stage (O2), vitellogenic stage (O3), hydrated oocytes (HO), vitellogenic atretic oocyte (AO3), and post-ovulatory follicles (POF) (Grier, 1981). For testes, spermatogenic cysts were classified into five developmental stages: spermatogonia (SG), primary spermatocytes (1SC), secondary spermatocytes (2SC), spermatids (ST), and sperm (SP) (Wallace and Selman, 1981). The sexual maturity stages were determined by the presence of the most advanced oocyte stage and the occurrence of HO, POF, and AO3 in ovaries, and the relative proportions of 1SC, 2SC, and ST and the appearance of SP in the sperm duct (SD) in testes. Correspondingly, each ovary was allocated to one of the five maturity stages: immature F1, developing F2, mature F3, ripe F4, or spent F5; same to each testis: immature M1, developing M2, mature M3, ripe M4, or spent M5 (Yamaguchi et al., 2006; Brown-Peterson et al., 2011) (Table 1). The smallest SLs of F3 and M3 were considered as the minimum sizes for female and male maturity.
Ten samples from each of the F1, F2, and F3/F4 maturity stages determined aforementioned were randomly selected. For each sample of F1, F2, and F3/F4, the smallest and the largest O1, O2, and O3 in the gonad sections were measured, respectively. Briefly, for each oocyte measured, the longest and shortest diameters were measured, and the average size was used to present the size of the oocyte. Eventually, the size range was given for different developmental stages of oocytes.
Spawning Seasonality
The spawning season and spawning peak were determined by gonad histology. The criteria for the spawning season were the appearance of mature and/or ripe stages for females (F3 and/or F4) and males (M3 and/or M4); the months in which spent individuals (F5 or M5) occurred alone were not considered as spawning seasons (Sadovy, 1996). The spawning peak was defined as the months having at least 50% of females in F3 and/or F4 or of males in M3 and/or M4 (Sadovy, 1996).
GSI was also used to determine spawning peak. The spawning peak was assigned when the monthly average GSI% reached at least 50% of the average maximum GSI% recorded (Sadovy, 1996).
Size at 50% Sexual Maturity
Small juveniles can influence the determination of spawning seasonality (above) and size at 50% sexual maturity (SL50). To avoid this, only the individuals larger than the minimum sizes for female and male maturity (determined above) were used for analyses.
SL at which 50% of individuals attained sexual maturity (SL50) for females and males were determined by plotting the percentage of mature individuals (female: F3, F4, and F5; male: M3, M4, and M5) at 10-mm-SL-size class interval. Only the individuals during the spawning peak determined by gonad histology were used for analysis. A maturity curve was estimated by fitting a logistic equation as follows (Sparre and Venema, 1999; Crabtree et al., 1997):
where P is the percentage of mature individuals, a is a constant, and b represents the SL at the inflection point equivalent to the estimated SL50.
Data Analyses
The non-parametric Mann-Whitney U-test was performed to reveal the gender difference in K and GSI%. The non-parametric ANCOVA was conducted to reveal the difference between the b values of the length–weight relationships of females and males (Snedecor and Cochran, 1967), and log SL as covariate. The chi-square (χ2) was used to determine whether the sex ratio overall and monthly differed from the expected ratio of 1:1. All statistical analyses used a significance level of p ≤ 0.05. Analyses were conducted using Excel 2019, R version 3.6.3, MATLAB version R2020b, and IBM SPSS Statistics version 25.0.
Results
Biological Parameters
A total of 1,006 individuals were collected, ranging from 46 to 391 mm SL (180 ± 59 mm SL, mean ± SD) and 1.45 to 1,110.05 g BW (142.59 ± 157.00 g BW) (Table 2). Females (N = 523) ranged from 46 to 391 mm SL (177 ± 64 mm SL) and 1.45 to 1,110.05 g BW (148.32 ± 184.33 g BW), and males (N = 483) ranged from 100 to 337 mm SL (183 ± 52 mm SL) and 15.97 to 834.86 g (136.38 ± 120.52 g BW). Females were mainly in SL classes between 100 and 159 mm (44.55%) and males between 100 and 219 mm (77.43%), determined by the SL frequencies > 10% (Figure 3).
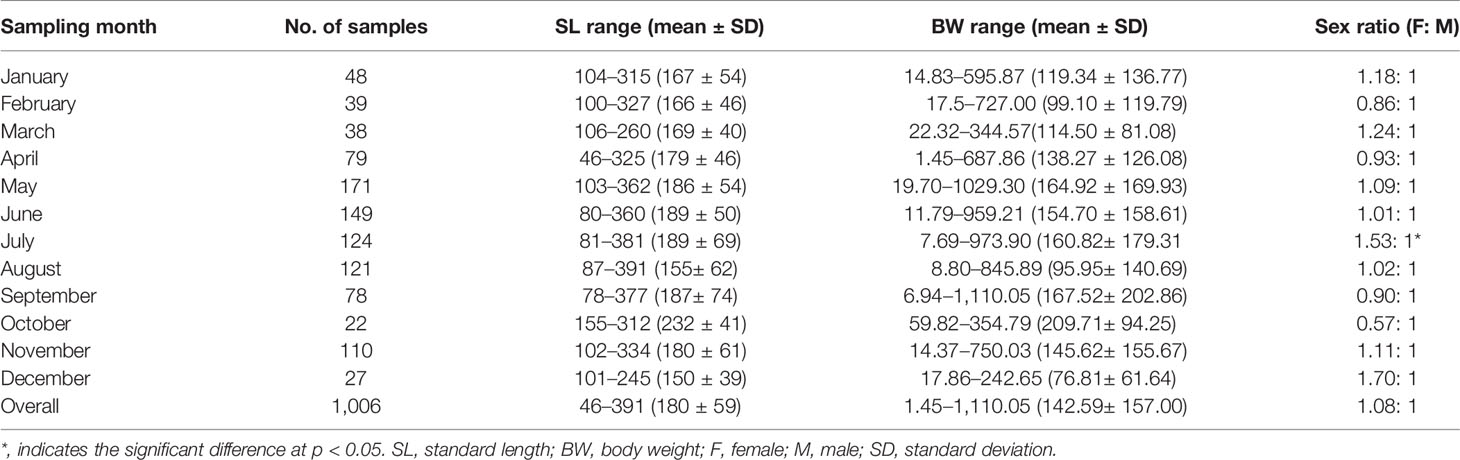
Table 2 The variation of sex ratio, body size (mm), and body weight (g) of Larimichthys crocea collected from April 2019 to November 2021.
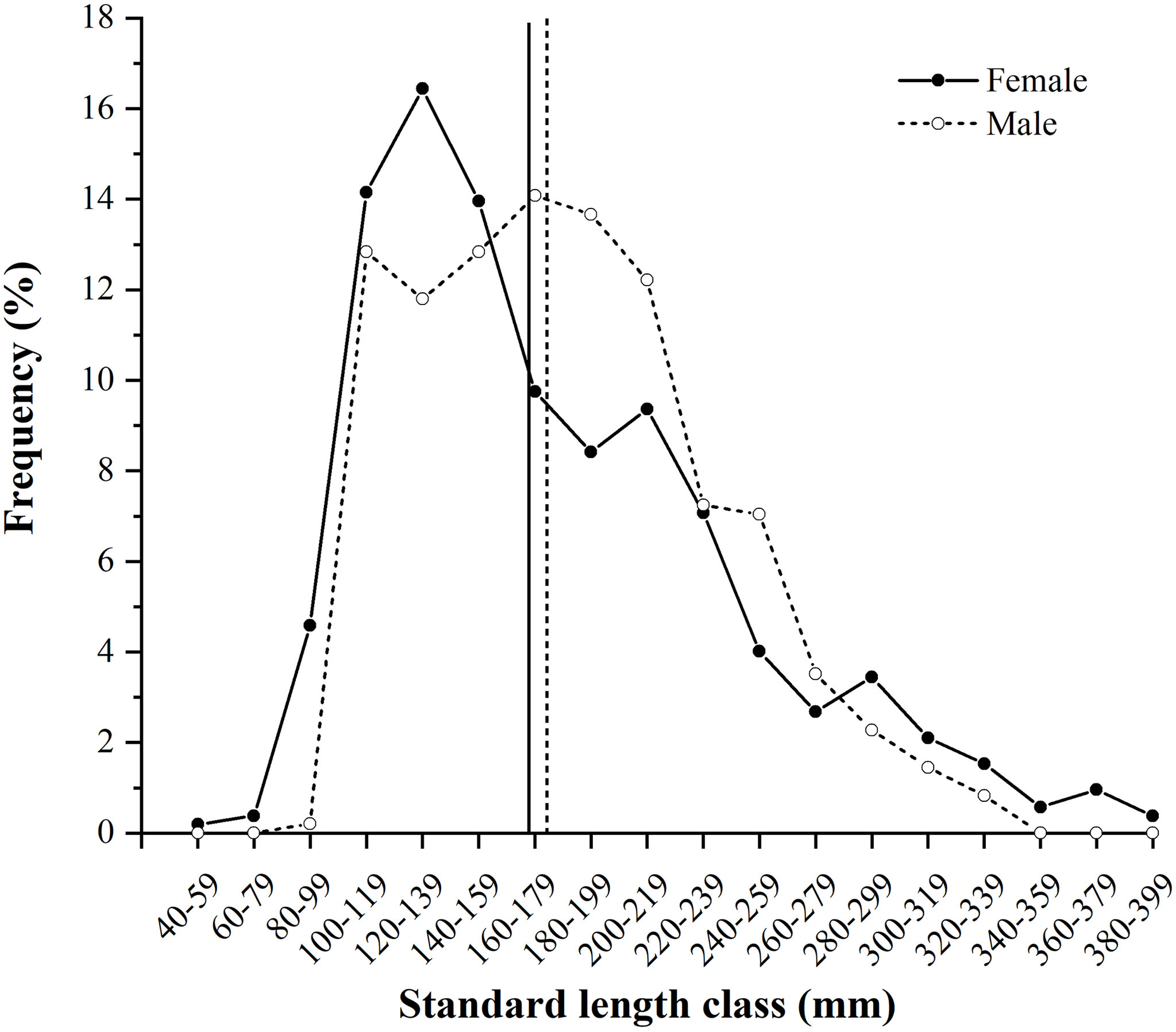
Figure 3 Size (standard length, mm) frequency (%) of Larimichthys crocea females (N = 523) and males (N = 483) collected from April 2019 to November 2021. Vertical solid and dashed lines indicate the average sizes of females and males, respectively.
The length–weight relationships were as follows: BW = 2.2089 × 10-5 × SL2.9709 (R² = 0.9431, N = 523) for females and BW = 2.6827×10-5 × SL2.9237 (R² = 0.9222, N = 483) for males. The significant difference was observed in length–weight relationships between sexes (non-parametric ANCOVA, p < 0.01), with a growth dimorphism showing females heavier than males when body sizes exceeded 61 mm SL. The overall sex ratio of female:male was 1.08:1, showing no significant difference between a 1:1 ratio (χ2 = 1.59, p > 0.05) (Table 2). Sex ratios showed monthly variation from 0.57: 1 in October to 1.70: 1 in December; the significance was only found in July (χ2 = 5.45, p < 0.05) (Table 2).
The K of males was significantly higher than that of females (Mann-Whitney U-test, U = 33735, p < 0.01) (Figure 4A). The K values were higher in March and May for females and in March to May for males.
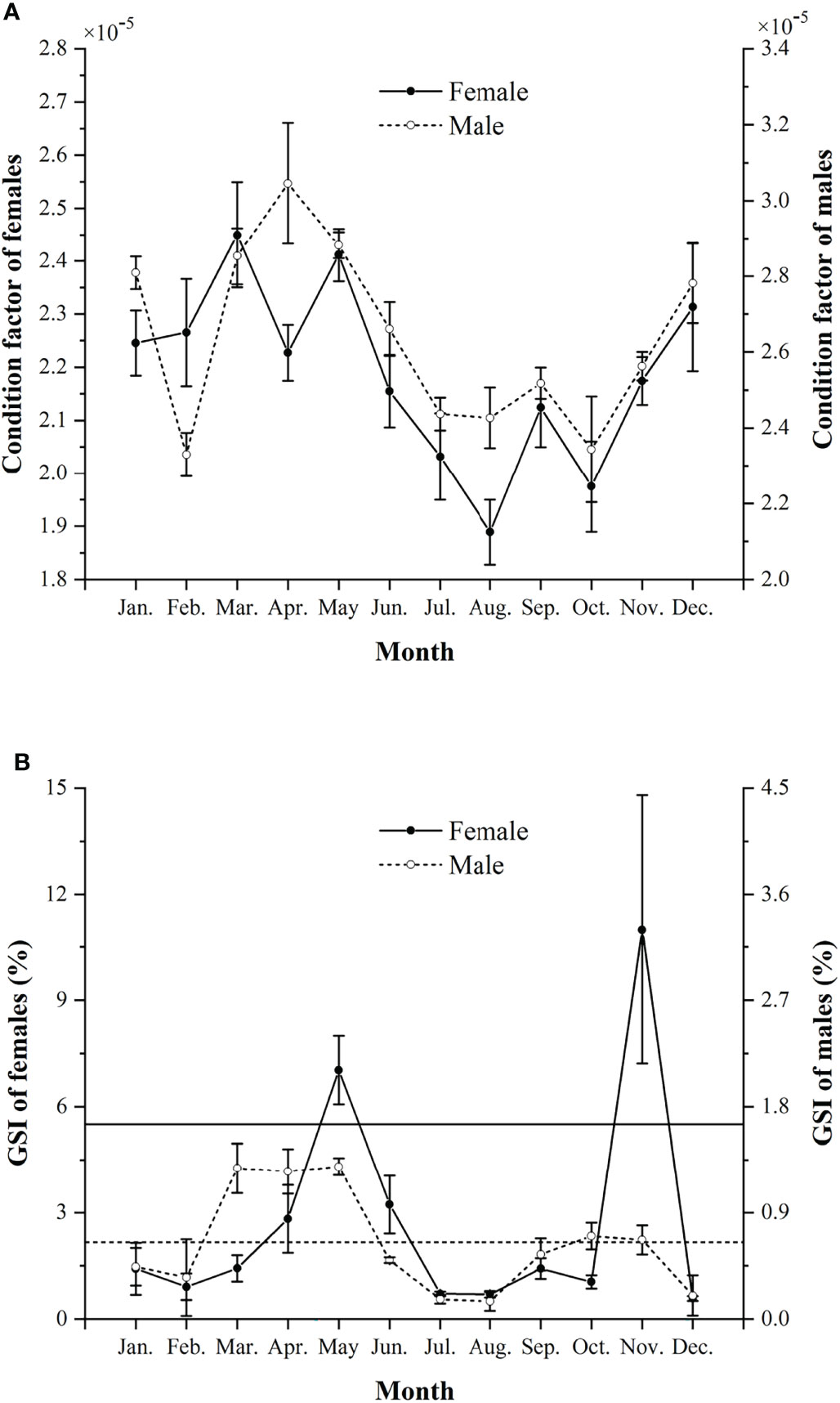
Figure 4 Monthly condition factors (mean ± SD) (A) and monthly gonadosomatic index (GSI %, mean ± SD) (B) in females and males of Larimichthys crocea from April 2019 to November 2021. Horizontal solid and dashed lines indicate the 50% of the maximum GSI% for females and males, respectively.
Spawning Seasonality
All five sexual maturity stages for females and males of L. crocea were observed (Table 1 and Figures 5 and 6). The oocyte sizes increased with developmental stages with large variation in O3 (Table 1).
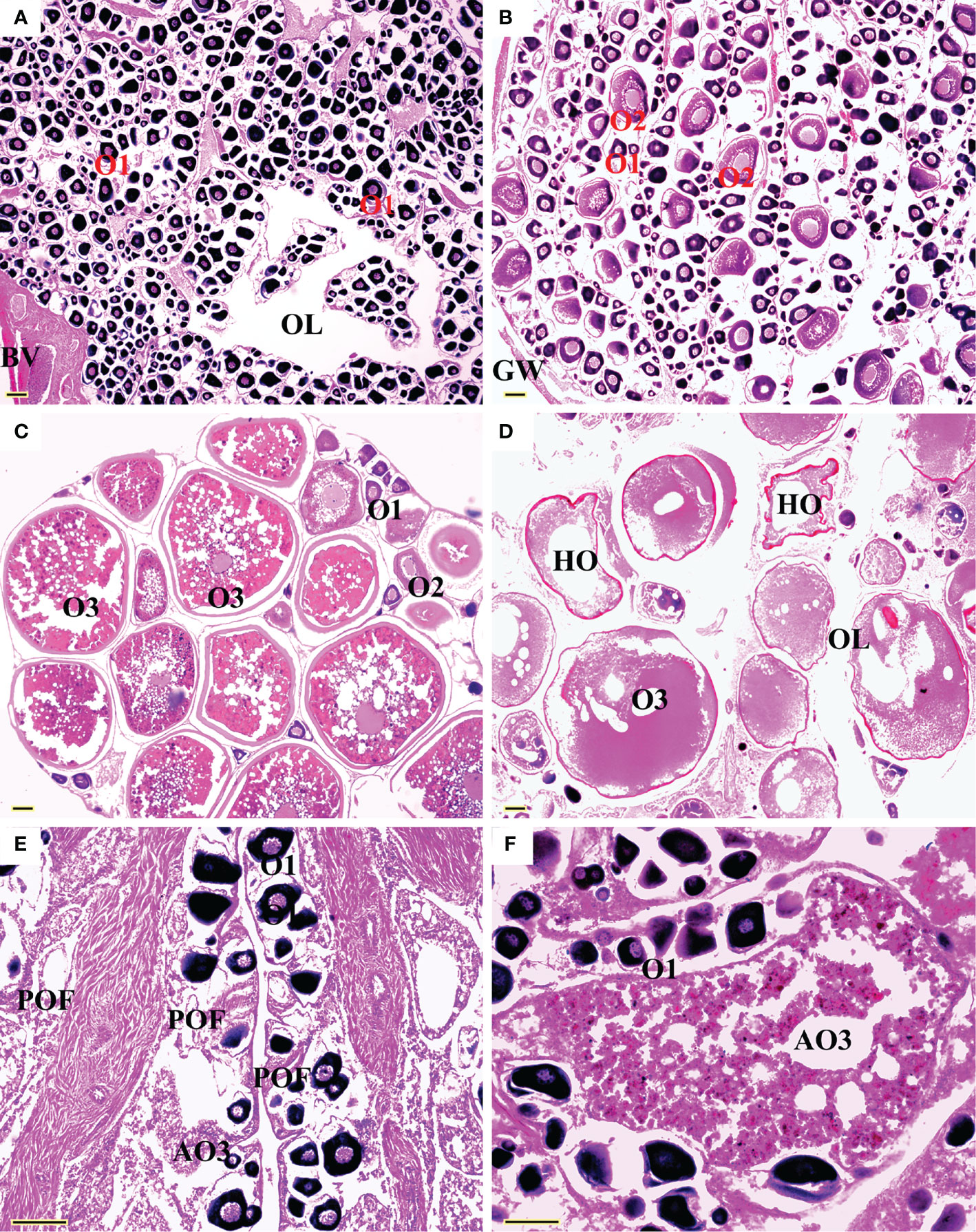
Figure 5 Sexual maturity stages in females of Larimichthys crocea. (A) F1: Immature/resting (175 mm SL, July 2021); (B) F2: Developing (212 mm SL, April 2021); (C) F3: Maturing (249 mm SL, June 2020); (D) F4: Ripe (198 mm SL, May 2019); (E) F5: Spent (212 mm SL, June 2021); (F) F5: Spent (360 mm SL, June 2021). AO3, atretic vitellogenic stage oocyte; BV, blood vessels; GW, gonadal wall; HO, hydrated oocyte; O1, primary growth stage oocyte; O2, cortical-alveolar stage oocyte; O3, vitellogenic stage oocyte; OL, ovarian lumen; POF, post-ovulatory follicles. Scale bars: 100 μm.
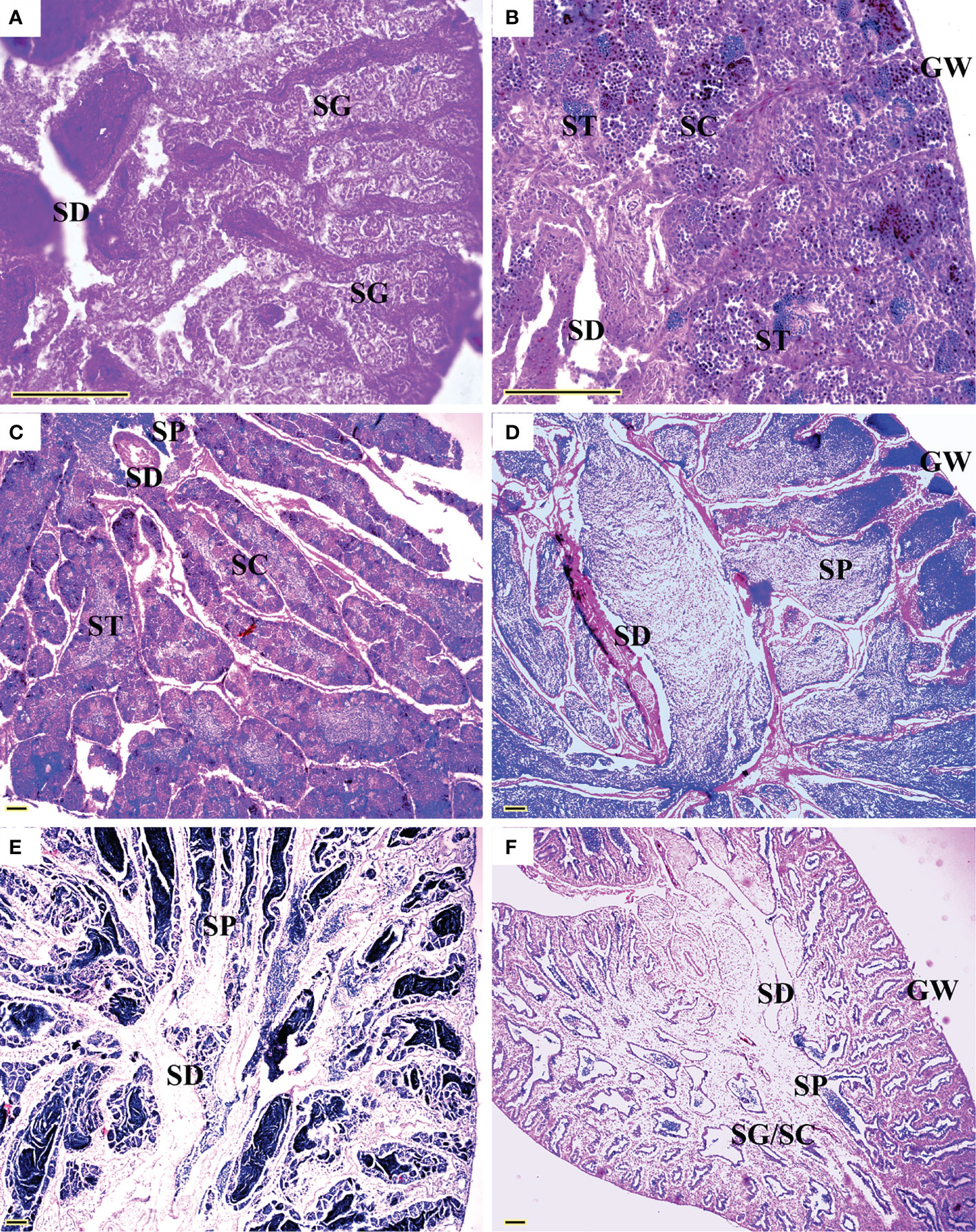
Figure 6 Sexual maturity stages in males of Larimichthys crocea. (A) M1: Immature/resting (144 mm SL, February 2021); (B) M2: Developing (167 mm SL, June 2020); (C) M3: Maturing (175 mm SL, May 2020); (D) M4: Ripe (248 mm SL, February 2021); (E) M4: Ripe (177 mm SL, March 2021); (F) M5: Spent (212 mm SL, July 2021). GW, gonadal wall; SC, spermatocytes; SD, sperm duct; SG, spermatogonia; SP, sperm; ST, spermatids. Scale bars: 100 μm.
Spawning seasons were almost year-round except July and August in females (Figure 7). The spawning peaks were March, May, and November for females and April to June and October to November for males (Figure 7). Females with HO and/or POF were collected in March, May, June, and November.
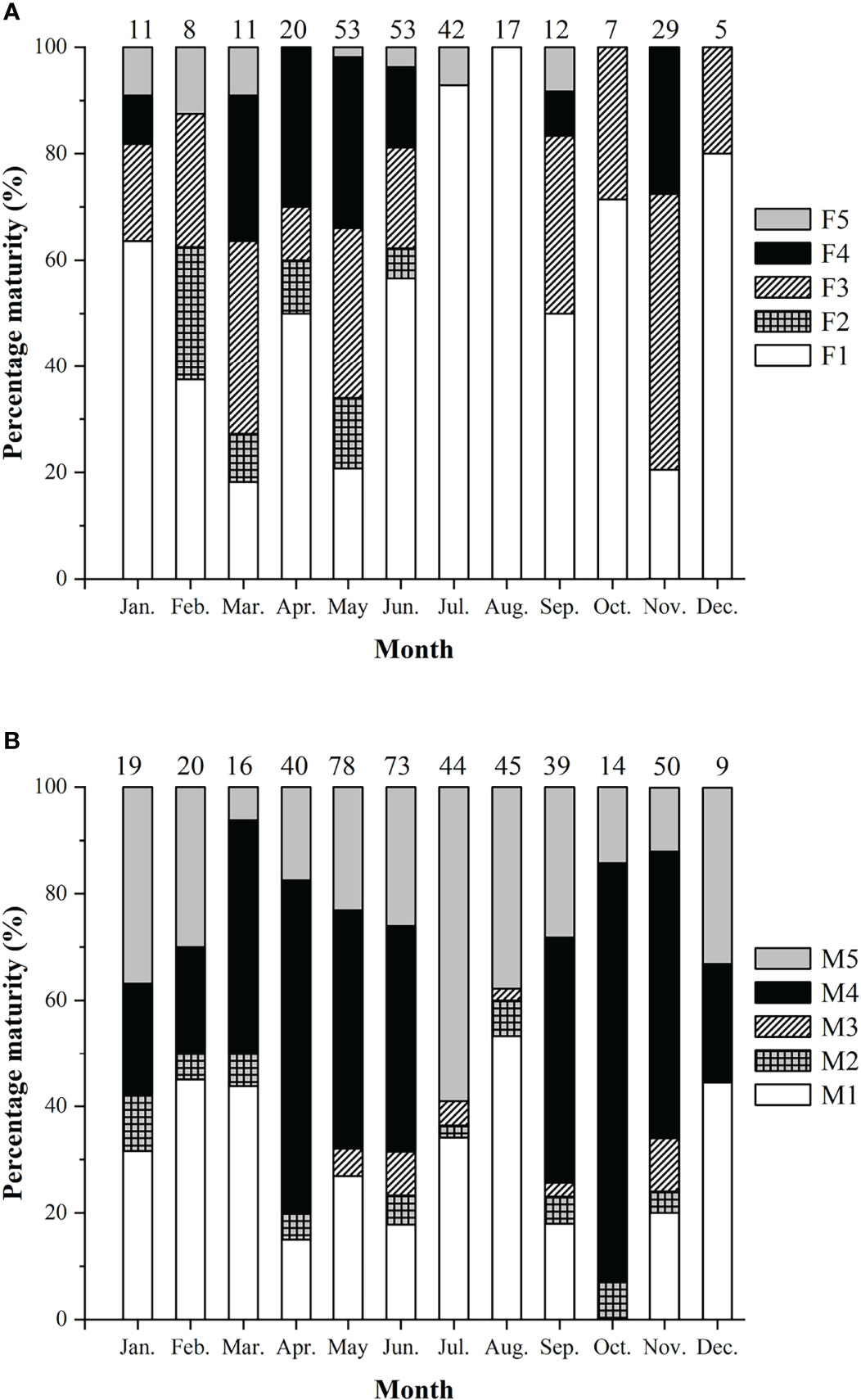
Figure 7 Percentage of sexual maturity stages of Larimichthys crocea. (A) Female. (B) Male. F1/M1: immature/resting; F2/M2: developing; F3/M3: maturing; F4/M4: ripe; F5/M5: spent. Numbers above the bars referred to the sample sizes.
Females and males showed monthly variations in GSI%, with females generally having higher GSI% than males (Mann-Whitney U-test, U = 75182, p < 0.01) (Figure 4B). Two spawning peaks were found in spring and autumn, i.e., in May and November for females, and in March to May and October to November for males. The significant difference of GSI% between the two spawning peaks was only found in males, with spring higher than autumn (Mann-Whitney U-test, U = 6435, p < 0.05).
Length at 50% Sexual Maturity
The minimum SLs for female and male maturity were 160 and 112 mm, respectively. The logistic equations were as follows: PSL = 100/{1 + exp[−0.0558 × (SL − 187.1963)]} (R2 = 0.9472, N = 168) for females and PSL = 100/{1 + exp[−0.0511 × (SL − 150.2256)]} (R2 = 0.9645, N = 263) for males (Figure 8). The estimated SL50 values of females and males were 187.2 and 150.2 mm, respectively.
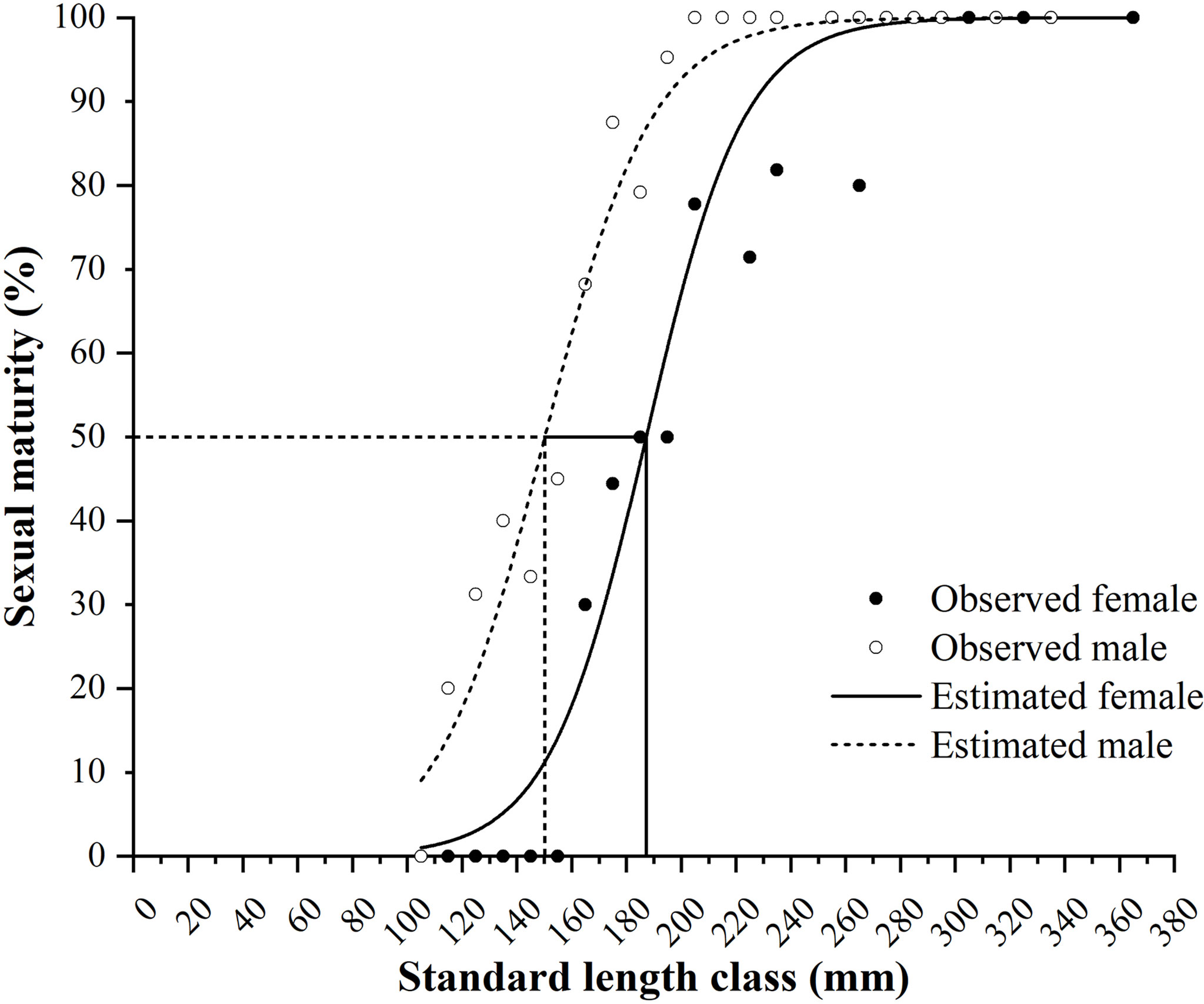
Figure 8 Female and male maturity of Larimichthys crocea in standard length class (mm) and the logistic curves. Vertical solid line and dash line indicate the estimated SL50 for females and males, respectively.
Discussion
Four biological changes on L. crocea were noticed over years in GJY spawning ground. First, the declines of the maximum size were observed over six decades. The maximum size in catches (N = 173) in May to June 1959 (spring spawning season) was 515 mm SL, with a high proportion (11%) larger than 400 mm SL (Xu et al., 1980). In 1986–1990, the maximum size in catches (N = 210) was 430 mm SL (Lin et al., 1992). In 2019–2021 (this study), the maximum sizes were 391 mm SL in total catches (N = 1,006) and 362 mm SL in May to June catches (N = 320) (Table 2). Unsustainable exploitation has confirmed to play a major role in the shift of size structure toward small individuals (Shin et al., 2005; Tu et al., 2018). This study showed that the maximum size declined nearly 30% in spring spawning season over six decades for L. crocea in GJY.
Second, the reductions of the sizes at female and male sexual maturity were identified on L. crocea in GJY (Table 3). In nearly three decades from 1959 to 1986–1990, the minimum SL for female maturity declined 12.5%, with a further decline of 8.6% over the past three decades from 1986–1990 to 2019–2021. For males, the reduction of the minimum size at maturity was greater, nearly 32% over the past three decades from 1986–1990 to 2019–2021. Although the methods for determining SL50 (so called the majority proportion for maturity) were not standardized, the declines of SL50 were clear over the past three decades from 1986–1990 to 2019–2021: 16.5% and 19% for females and males, respectively. Furthermore, the estimated SL50 for female maturity on L. crocea was nearly 40 mm larger than that of males in 2019–2021; similar results were found in 1986–1990 (Table 3). For some sciaenids studied, the estimated SL50 of females were all larger than that of males (Tuuli et al., 2011; Militelli et al., 2012; Zhang et al., 2019). Larger mature females indicate higher fecundity, and this would have the most substantial impact on stock resistance and recovery (Farley et al., 2013; Bris et al., 2015; Sabrah et al., 2017). However, in other sciaenids, such as Plagioscion magdalenae and P. squamosissimus, SL50 in males was larger than for females (Castro, 1999; Marciano et al., 2005; Santos et al., 2010). This may be associated with specific characteristics or differential responses to fishery exploitation in different species (Santos et al., 2010). The over-exploitation of spawning and over-wintering aggregations and the loss of genetic diversity are likely to contribute to the size reduction at maturity which would have long-term impact on population structure and reproductive pattern (Liu and Sadovy de Mitcheson, 2008).

Table 3 Comparison of size at sexual maturity, and spawning season and peak of Larimichthys crocea over years in Guanjingyang spawning ground.
Third, a nearly year-round spawning pattern was observed for L. crocea females and males in GJY, the first time for the species. The same phenomenon of year-round spawning pattern has also been reported in other sciaenids, e.g., females of the tiger tooth croaker Otolithes ruber and the bigeye croaker Pennahia anea (Menon et al., 2015; Farkhondeh et al., 2018; Lanzuela et al., 2020). On the basis of the locations of these different studies, the year-round spawning pattern in sciaenids can be found in Indian and West Pacific Oceans, and from tropical to temperate.
Fourth, the shifts of spawning peaks were observed for the first time in L. crocea. The well-known two spawning peaks in GJY spawning ground were in May to June and in September to October with the spring was a major (Chu and Wu, 1985; Zhang and Hong, 2015) (Table 3). At least 2 months earlier in spring peak and 1 month later in autumn peak were noticed in this study based on cost-effective, gonad histology method; no any other reports mentioned the March and November spawning peaks in GJY.
The significant findings on year-round spawning activity and the shift of spawning peak in L. crocea merit further investigations. Temperature is likely to be the dominant factor influencing the variability of migration, spawning, and recruitment on animals (Gibson et al., 1993; Marshall and Elliott, 1998; Pankhurst and Munday, 2011; Golpour et al., 2021). The global warming is a non-negligible factor which can affect the reproductive dynamics of fishes. Elevated seawater temperature would stimulate the earlier spawning activity of spring and summer spawners while delaying the onset of sexual maturation of autumn spawners and extending the spawning duration of marine and freshwater fishes (Pankhurst and King, 2010; Yamamoto and Shiah, 2012; Rogers and Dougherty, 2019; Kawai et al., 2020). In GJY, the annual average temperature has increased by 1.65°C in the past four decades (Meteorology Bureau of Fujian Province, 1981–2010; Ningde Bureau of Statistics, 2011–2020) (Figure 9A). L. crocea can spawn in the wild at temperature of 18°C–24°C, and stop spawning at the temperature above 26°C (Liu, 2004; Yamada et al., 2007; Xu, 2018). The highest temperature in GJY was found in July, > 28°C (Xu, 2018). In this study, females did not spawn in July and August (Figure 7A) and were likely to be associated with high temperature, > 26°C, in summer (Figure 9B).
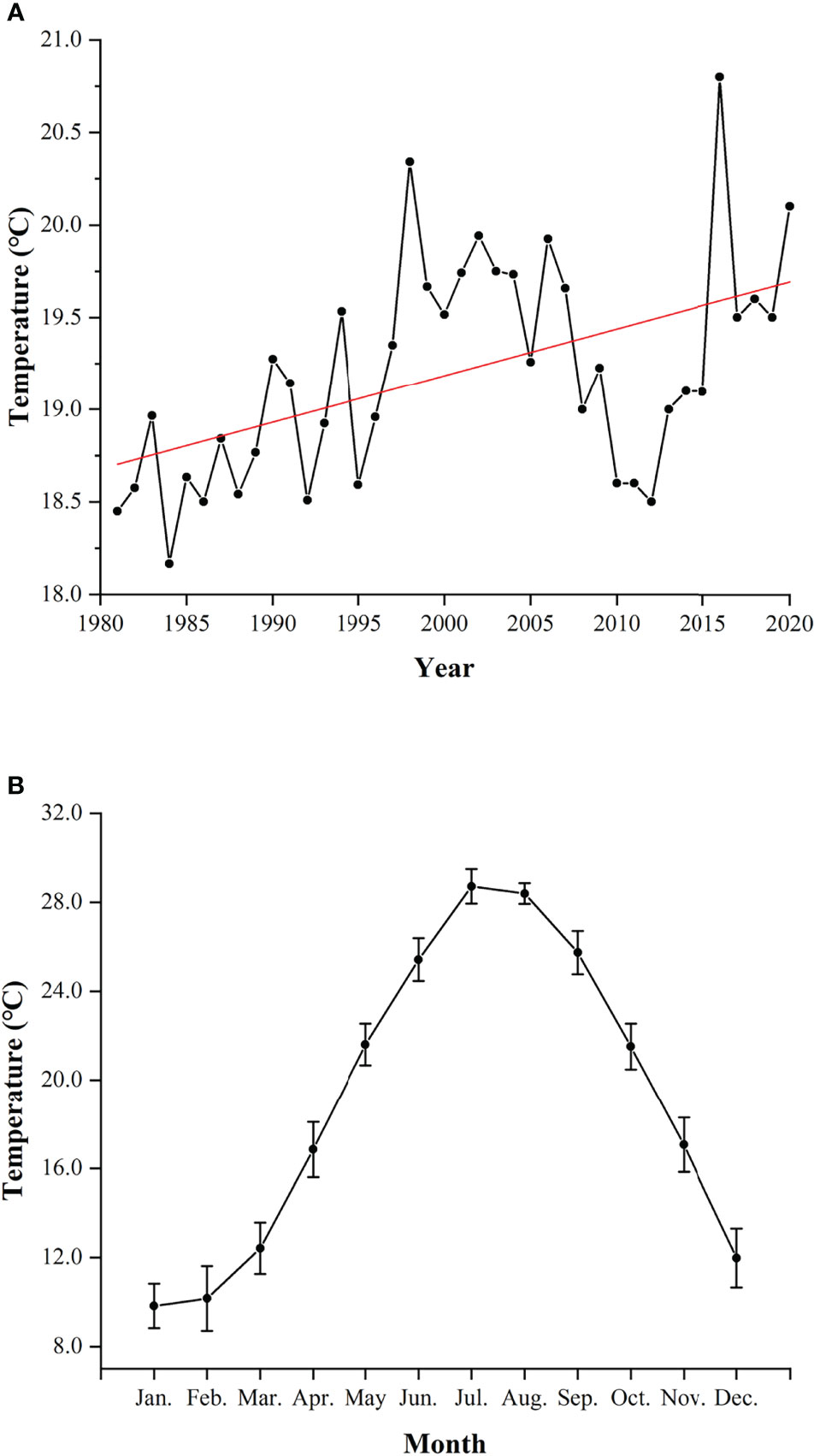
Figure 9 Annual average temperature (A) and monthly average temperature (mean ± SD) (B) of Guanjingyang from 1981 to 2020 (Meteorology Bureau of Fujian Province, 1981–2010; Ningde Bureau of Statistics, 2011–2020). The red line indicates the fitted linear equation: y = 0.025x - 31.51 (R2 = 0.2523).
This study provided two pieces of evidence that GJY still functions as the spawning ground for L. crocea. First, eggs of L. crocea were collected in April to June, August, October, and November (Dai, 2006; Shen, 2011; Xu, 2018; Jiang et al., 2021), matching largely to the two spawning peaks (spring and autumn) determined in this study except August. The L. crocea eggs mainly distributed in the eastern Sandu Island, GJY waters with extensions to the entry of Sansha Bay and Dongwuyang (Xu, 2018; Jiang et al., 2021) (Figure 10), indicating the spawning areas nearby in terms of the short embryonic development period (30–52 h under temperature of 18°C–23°C) (Sha, 1962; Liu, 1999). Second, females of L. crocea with HO and/or POF were collected in this study overlapped with the areas where eggs collected (Figure 10). Further studies are needed to investigate whether L. crocea forms spawning migration and enters Sansha Bay to reproduce, and the scale of the aggregations. At least from this study, part of the spawning stock is likely to remain in the Bay year-round and does not form spawning migration.
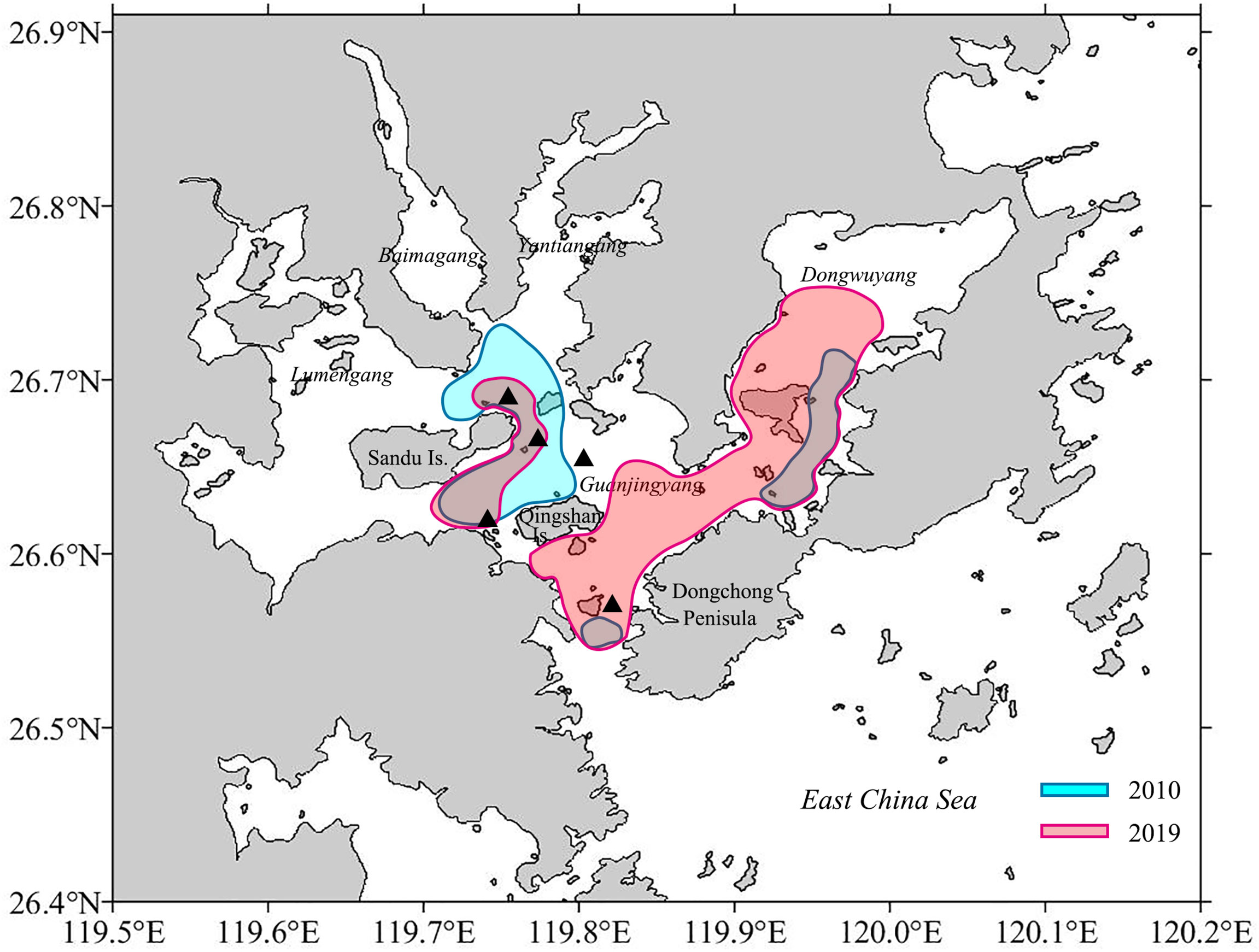
Figure 10 Possible spawning areas for Larimichthys crocea based on the egg collection (Xu, 2018; Jiang et al., 2021). The black triangle symbols indicate the area where females with HO and/or POF occurred in this study.
However, L. crocea larvae, as an important stage of life cycle, were rare and sporadic in plankton collections in GJY (Xu, 2018; Jiang et al., 2021). The changes in hydrological and ecological condition in Sansha Bay could have negative impact on survival rate of L. crocea larvae. For instance, the Noctiluca scintillans blooms and the red tides caused by the eutrophication would have detrimental effect on the survival of L. crocea larvae directly, such as hypoxia and toxins, or indirectly through the shortage of zooplankton as diets (Jiang et al., 2021). The environmental and ecological factors influence the survival of L. crocea larvae merit further investigation.
The management measures for L. crocea in Sansha Bay are diverse, with the prohibition of the drag seine nets in the 1950s, the establishment of the protected area for spawning aggregations in the 1980s, the conduction of long-term restocking programs since the 1990s, the introduction of national fishing moratorium regulation in May to August since the 1990s, and, to date, the regulation on the minimum catch size control (255 mm SL) (Liu and Sadovy de Mitcheson, 2008; http://hyyyj.fujian.gov.cn/). Evaluation after the implementation of the series measures is essential. On the basis of the fundamental information on the reproductive dynamics provided by this study, extra measures need to be considered, such as the protection of autumn spawning peak, the earlier regulation for protecting spring spawning peak, the control of fishing gears (e.g., set nets), and the increase of mesh size (1–5 mm are currently commonly used).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal collection and study was reviewed and approved by Fujian Province Ocean and Fisheries Bureau of China and Xiamen University of China.
Author Contributions
LY wrote the first draft and organized sampling trips. LY, YJ, QX, GD, and ML conducted the sample collection. LY, YJ, QX, and XC performed the histological analyses and data analyses. LY, YJ, and ML revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Fujian Province Ocean and Fisheries Bureau of China (contract nos. [3500]HTZB[GK]2019007-1-1 and 20200059) and the National Natural Science Foundation of China (grant no. 41976091). The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Bai-an Lin, Rui-hua Liu, Qing-qiang Ren, Jia-hao Song, Wei-di Yang, Lu-ping Fang and Guo-han Yang for sample collection and laboratory work, and the two reviewers and the handling editor for their helpful and constructive comments. We thank fishery authorities of Fujian Province and Ningde City for fish collection permit and Emily King for grammar corrections on the manuscript.
References
Andrade M. C., Jesus A. J. S., Giarrizzo T. (2015). Length-Weight Relationships and Condition Factor of the Eaglebeak Pacu Ossubtus xinguense Jégu 1992 (Characiformes, Serrasalmidae), and Endangered Species From Rio Xingu Rapids, Northern Brazil. Braz. J. Biol. 75 (3), 102–105. doi: 10.1590/1519-6984.01214BM
Azevedo J. W. J., Castro A. C. L., Silva M. H. L. (2017). Length-Weight Relation, Condition Factor and Gonadosomatic Index of the Whitemouth Croaker, Micropogonias furnieri (Desmarest 1823) (Actinopterygii: Sciaenidae), Caught in Le Leçóis Bay, State of Maranhão, Estern Amazon. Brazil. Braz. J. Oceanogr. 65 (1), 1–8. doi: 10.1590/s1679-87592017110506501
Bris A. L., Pershing A. J., Hernandez C. M., Mills K. E., Sherwood G. D. (2015). Modelling the Effects of Variation in Reproductive Traits on Fish Population Resilience. ICES J. Mar. Sci. 72 (9), 2590–2599. doi: 10.1093/icesjms/fsv154
Brown-Peterson N. J., Wyanski D. M., Saborido-Rey F., Macewicz B. J., Lowerre-Barbieri S. K. (2011). A Standardized Terminology for Describing Reproductive Development in Fishes. Mar. Coast. Fish.: Dyn Manage. Ecosyst. Sci. 3 (1), 52–70. doi: 10.1080/19425120.2011.555724
Castro A. (1999). Tamanho E Idade De Primeira Maturação Da Corvina, Plagioscion squamisissimus (Heckel 1940) (Teleostei, Sciaenidae), do Reservatório De Barra Bonita-SP. J. Boletim do Museu Paraense Emilio Goeldi Série Zoologia 15 (2), 119–132.
Chao N. L., Frédou F. L., Haimovici M., Peres M. B., Polidoro B., Raseira M., et al. (2015). A Popular and Potentially Sustainable Fishery Resource Under Pressure-Extinction Risk and Conservation of Brazilian Sciaenidae (Teleostei: Perciformes). Glob. Ecol. Conserv. 4, 117–126. doi: 10.1016/j.gecco.2015.06.002
Chu Y., Wu H. (1985). “Sciaenidae”, in The Fishes of Fujian Province (Part Ii). Ed. Chu Y. (Fujian, China: Fujian Science and Technology Press), 101–136.
Cisneros-Mata M.Á., True C., Enriquez-Paredes L. M., Sadovy Y., Liu M. (2021). Totoaba macdonaldi The IUCN Red List of Threatened Species 2021. e.T22003A2780880. doi: 10.2305/IUCN.UK.2021-2.RLTS.T22003A2780880.en
Crabtree R. E., Snodgrass D., Harnden C. W. (1997). Maturation and Reproductive Seasonality in Bonefish, Albula vulpes, From the Waters of the Florida Keys. Fish. Bull. 95, 456–465.
Dai Y. (2006). Distribution of Fish Eggs, Larva and Juveniles in Sansha Bay. Fujian J. Oceanogr. Taiwan 25, 256–261.
FAO (2018). The State of World Fisheries and Aquaculture-Meeting the Sustainable Development Goals (Rome: Food and Agriculture Organization of the United Nations).
FAO (2020). The State of World Fisheries and Aquaculture-Sustainability in Action (Rome: FAO). doi: 10.4060/ca9229en
Farkhondeh G., Safaie M., Kamrani E., Valinassab T. (2018). Population Parameters and Reproductive Biology of Otolithes ruber (Bloch & Schneider, 1801) (Teleostei: Sciaenidae) in the Northern Makran Sea. Iran. J. Ichthyol. 5 (3), 173–183. doi: 10.22034/iji.v5i3.297
Farley J. H., Williams A. J., Hoyle S. D., Davies C. R., Nicol S. J. (2013). Reproductive Dynamics and Potential Annual Fecundity of South Pacific Albacore Tuna (Thunnus alalunga). PloS One 8 (4), e60577. doi: 10.1371/journal.pone.0060577
Froese R. (2006). Cube Law, Condition Factor and Weight-Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 22, 241253. doi: 10.1111/j.1439-0426.2006.00805.x
Fujian Provincial Meteorological Bureau (1981-2010). Available at: http://fj.cma.gov.cn/ (Accessed February, 2022).
Gibson R. N., Ansell A. D., Robb L. (1993). Seasonal and Annual Variations in Abundance and Species Composition of Fish and Macrocrustacean Communities on a Scottish Sandy Beach. Mar. Ecol. Prog. Ser. 98, 89–105. doi: 10.3354/meps098089
Golpour A., Broquard C., Milla S., Dadras H., Baloch A. R., Saito T., et al. (2021). Determination of Annual Reproductive Cycle in Male Starlet, Acipenser ruthenus Using Histology and Ultrasound Imaging. Fish Physiol. Biochem. 47, 703–711. doi: 10.1007/s10695-020-00892-8
Gray P. (1975). The Microtomist’s Formulary and Guide (Huntington, NY: R. E. Krieger Publishing Co).
Grier H. J. (1981). Cellular Organization of the Testis and Spermatogenesis in Fishes. Am. Zool. 21 (2), 345–357. doi: 10.1093/icb/21.2.345
Jiang Y., Lin B., He H., Ding G., Yan L., Zhang G., et al. (2021). Species Composition and Assemblages of Ichthyoplankton in Sansha Bay, Fujian Province, China. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.758089
Kang B., Liu M., Huang X.-X., Li J., Yan Y.-R., Han C.-C., et al. (2018). Fisheries in Chinese Seas: What Can We Learn From Controversial Official Fisheries Statistics? Rev. Fish Biol. Fish. 28, 503–519. doi: 10.1007/s11160-018-9518-1
Kawai K., Fujita H., Sanchez G., Furusawa S., Umino T. (2020). Estimating the Spawning Season of Black Sea Bream Acanthopagrus schlegelii in Hiroshima Bay, Japan, From Temporal Variation in Egg Density. Fish. Sci. 86 (4), 645–653. doi: 10.1007/s12562-020-01433-1
Lanzuela N. S. B., Gallego E. M., Baltar J. E. P. (2020). Reproductive Biological Performance of Otolithes ruber (Bloch and Schneider 1801) in San Miguel Bay, Philippines. Ph. J. Fish. 27 (2), 127–136. doi: 10.31398/tpjf/27.2.2019C0006
Le Cren E. D. (1951). The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Eco. 20 (2), 201–219. doi: 10.2307/1540
Lin D., Zhang J., Luo J., Zheng Z., Shi X. (1992). Studies on the Gonad Development and the Annual Reproductive Cycle of the Cultured Large Yellow Croaker, Pseudosciaena crocea (Richardson). J. Fujian Norm. Univ. (Nat. Sci.) 8 (3), 81–87.
Liu J. (1999). Study on the Development of Pseudosciaena Crocea (Richarson) Embryo and Its Morphological Characteristics and the Ecology of Its Larval Juvenile Fish. Mar. Sci. 14 (7), 20–24.
Liu J. (2004). A Study on Twice Maturity Characteristic of Cultured Large Yellow Croaker in One Year. J. Jimei Univ. (Nat. Sci.) 9 (3), 200–204. doi: 10.19715/j.jmuzr.2004.03.002
Liu M., Cheng J.-H., Nguyen Van Q., Sasaki K., Hoshino K., Sakai T., et al. (2020a). Larimichthys Crocea The IUCN Red List of Threatened Species 2020, e.T49182559A49239394. doi: 10.2305/IUCN.UK.2020-1.RLTS.T49182559A49239394.en
Liu M., Jiang Y., Zheng L. M., Yang W. D., Chen D. (2020b). Large Yellow Croaker (Larimichthys crocea) Stock Assessment in Its Spawning Protection Area in Fujian Province, China. Xiamen University., 216.
Liu M., Sadovy de Mitcheson Y. (2008). Profile of a Fishery Collapse: Why Mariculture Failed to Save the Large Yellow Croaker. Fish Fish. 9 (3), 219–242. doi: 10.1111/j.1467-2979.2008.00278.x
Lowerre-Barbieri S. K., Ganias K., Soborido-Rey F., Murua H., Hunter J. R. (2011). Reproductive Timing in Marine Fishes: Variability, Temporal Scales, and Methods. Mar. Coast. Fish.: Dyn. Manage. Eco. Sci. 3, 71–91. doi: 10.1080/19425120.2011.556932
MARA (2019–2021). China Fishery Statistical Yearbooks 2019–2021 (Beijing: China Agriculture Press) Ministry of Agriculture and Rural Affairs.
Marciano F., Espíndola E., Rocha O., Moretto E. (2005). Aspectos Reprodutivos Da Corvina (Plagioscion squamosissimus) E do Tucunaré (Cichla Monoculus) No Reservatório Álvaro De Souza Lima (Bariri, SP), Em Quatro Épocas do Ano. J. Espécies invosoras em águas doces-estudos caso e propostas manejo. São Carlos: Editora da Universidade Federal São Carlos 417p, 181–194.
Marshall S., Elliott M. (1998). Environmental Influences on the Fish Assemblage of the Humber Estuary, U.K. Estuar. Coast. Shelf Sci. 46, 175–184. doi: 10.1006/ecss.1997.0268
Menon M., Maheswarudu G., Rohit P., Laxmilatha P., Das M., Rao K. N. (2015). Biology and Stock Assessment of the Bigeye Croaker Pennahia anea (Bloch, 1793) Landed Along Andhra Pradesh, North-East Coast of India. Indian J. Fish. 62 (1), 46–51.
Militelli M. I., Macchi G. J., Rodrigues K. A. (2012). Comparative Reproductive Biology of Sciaenidae Family Species in the Río De La Plata and Buenos Aires Coastal Zone, Argentina. J. Mar. Biol. Assoc. U. K. 93 (2), 413–423. doi: 10.1017/s0025315412001488
Ningde Bureau of Statistics (2011–2020). Available at: http://tjj.ningde.gov.cn/ (Accessed February, 2022).
Oliveira C. D. L., Oliveira C. Y. B. (2018). Production Evolution, Catch Estimate and Conservation Status of the Marine Sciaenidae (Pisces, Perciformes). Int. J. Fish. Aquat. Stud. 4 (3), 10–17. doi: 10.20431/2454-7670.0403002
Pankhurst N. W., King H. R. (2010). Temperature and Salmonid Reproduction: Implications for Aquaculture. J. Fish Biol. 76 (1), 69–85. doi: 10.1111/j.1095-8649.2009.02484.x
Pankhurst N. W., Munday P. L. (2011). Effects of Climate Change on Fish Reproduction and Early Life History Stages. Mar. Freshw. Res. 62 (9), 1015–1026. doi: 10.1071/mf10269
Rogers L. A., Dougherty A. B. (2019). Effects of Climate and Demography on Reproductive Phenology of a Harvested Marine Fish Population. Glob. Change Biol. 25 (2), 708–720. doi: 10.1111/gcb.14483
Sabrah M. M., Heneish R. A., Alwany M. E., Ahmad M. I. (2017). Sexual Maturity, Spawning Activity, Sex Ratio and Fecundity of Two Mullidae Species Dwelling the Gulf of Suez, Red Sea. Egypt. J. Aquat. Res. 43, 83–91. doi: 10.1016/j.ejar.2016.04.007
Sadovy Y. J. (1996). “Reproduction of Reef Fishery Species”, in Reef Fisheries. Eds. Polunin N. V. C., Robert C. M. (London: Chapman and Hall), 15–59.
Sadovy de Mitcheson Y., Craig M. T., Bertoncini A. A., Carpenter K. E., Cheung W. W. L., Choat J. H., et al. (2013). Fishing Groupers Towards Extinction: A Global Assessment of Threats and Extinction Risk in a Billion Dollar Fishery. Fish Fish. 14, 119–136. doi: 10.1111/j.1467-2979.2011.00455.x
Sadovy Y., Domeier M. (2005). Are Aggregation-Fisheries Sustainable? Reef Fish Fisheries as a Case Study. Coral Reefs 24, 254–262. doi: 10.1007/s00338-005-0474-6
Santos N. B., da Rocha R. M., Fredou F. L. (2010). Reproductive Biology of Plagioscion magdalenae (Teleostei: Sciaenidae) (Steindachner, 1878) in the Bay of Marajo, Amazon Estuary, Brazil. Neotrop. Ichthyol. 8 (2), 333–340. doi: 10.1590/s1679-62252010000200012
Sha X. (1962). A Description of Eggs and Larvae of the Large Yellow Croaker, Pseudosciaena crocea (Richardson). Studia Marina Sin. 2, 31–49.
Shen C. (2011). Species Composition and Abundance Temporal-Spatial Distribution of Egg, Fish, Larvae and Juveniles in Sansha Bay of Fujian. Mar. Fish. 33 (4), 7. doi: 10.13233/j.cnki.mar.fish.2011.04.004
Shin Y.-J., Rochet M.-J., Jennings S., Field J. G., Gislason H. (2005). Using Size-Based Indicators to Evaluate the Ecosystem Effects of Fishing. ICES J. Mar. Sci. 62, 384–396. doi: 10.1016/j.icesjms.2005.01.004
Snedecor G. W., Cochran W. G. (1967). Statistical Methods, 6th Edn (Ames: The Iowa State University Press).
Sparre P., Venema S. C. (1999). Introduction to Tropical Fish Stock Assessment. Part 2: Exercises Vol. 306 (Rome:Food and Agriculture Organization of the United Nations Fisheries Technical Paper), 1–106.
Tu C.-Y., Chen K.-T., Hsieh C.-h. (2018). Fishing and Temperature Effects on the Size Structure of Exploited Fish Stocks. Sci. Rep. 8(1), 1–10. doi: 10.1038/s41598-018-25403-x
Tuuli C. D., Sadovy de Mitcheson Y., Liu M. (2011). Reproductive Biology of the Greyfin Croaker Pennahia anea in the Northern South China Sea. Ichthyol. Res. 58 (4), 302–309. doi: 10.1007/s10228-011-0228-0
Wallace R. A., Selman K. (1981). Cellular and Dynamic Aspects of Oocyte Growth in Teleosts. Am. Zool. 21 (2), 325–343. doi: 10.1093/icb/21.2.325
Xu Z. (2018). Resources and Environmental Characteristics in Wild Large Yellow Croaker (Larimichthys crocea) Breeding Waters of Guanjingyang (Beijing: Ocean Press).
Xu P., Ke X., Su Y., Liu J., Zheng W. (2021). Protection and Utilization Status and Prospect of Large Yellow Croaker (Larimichthys crocea) Germplasm Resources. J. Fish. China. 46 (4), 676–684. doi: 10.11964/jfc.20210312688
Xu G., Zheng W., Wang Y. (1980). A Comparative Study of the Fecundity of Two Different Populations of the Large Yellow Croaker, Pseudosciaena crocea (Richardson). Studia Marina Sin. 16, 71–82.
Yamada U., Tokimura M., Horikawa H., Nakabo T. (2007). Fishes and Fisheries of the East China and Yellow Seas (Kanagawa: Tokai University Press), 808–820.
Yamaguchi A., Todoroki T., Kume G. (2006). Reproductive Cycle, Sexual Maturity and Diel-Reproductive Periodicity of White Croaker, Pennahia argentata (Sciaenidae), in Ariake Sound, Japan. Fish. Res. 82 (1–3), 95–100. doi: 10.1016/j.fishres.2006.08.012
Yamamoto Y., Shiah F. K. (2012). Spatial Variation in the Spawning Season of Bluegill Lepomis macrochirus in Lake Biwa, Japan. Zool. Stud. 51 (8), 1446–1453.
Ye G., Lin Y., Feng C., Chou L. M., Jiang Q., Ma P., et al. (2020). Could the Wild Population of Large Yellow Croaker Larimichthys crocea (Richardson) in China Be Restored? A Case Study in Guanjingyang, Fujian, China. Aquat. Living Resour. 33, 24. doi: 10.1051/alr/2020025
Zhang Q., Hong W., Yang S., Liu M. (2011). Discussion on the Division of Geographic Populations for the Large Yellow Croaker (Larimichthys crocea). Mod. Fish. Inf. 26 (2), 3–8. doi: 10.13233/j.cnki.mar.fish.2015.02.012 1572
Zhang Q., Hong W. (2015). Resource Status and Remediation Strategy for Large Yellow Croaker in Guanjingyang Bay. Mar. Fish. 37 (2), 179–186. doi: 10.13233/j.cnki.mar.fish.2015.02.012
Keywords: gonad histology, size at sexual maturation, spawning aggregation, spawning season, fishery management
Citation: Yan L-t, Jiang Y, Xu Q, Ding G-m, Chen X-y and Liu M (2022) Reproductive Dynamics of the Large Yellow Croaker Larimichthys crocea (Sciaenidae), A Commercially Important Fishery Species in China. Front. Mar. Sci. 9:868580. doi: 10.3389/fmars.2022.868580
Received: 02 February 2022; Accepted: 25 March 2022;
Published: 28 April 2022.
Edited by:
Amin Golpour Dehsari, Academy of Sciences of the Czech Republic (ASCR), CzechiaReviewed by:
Salvador Ruiz, University of Guadalajara, MexicoRoman Franěk, University of South Bohemia, Czechia
Copyright © 2022 Yan, Jiang, Xu, Ding, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Liu, minliuxm@xmu.edu.cn
 Li-ting Yan
Li-ting Yan Yan Jiang
Yan Jiang Qing Xu
Qing Xu Guang-mao Ding3
Guang-mao Ding3  Min Liu
Min Liu