Temporal Change and Fishing Down Food Webs in Small-Scale Fisheries in Morondava, Madagascar
- 1Blue Ventures Conservation, Level 2 Annex, Omnibus Business Centre, North Road, London, United Kingdom
- 2Centre for Ecology and Conservation, University of Exeter, Cornwall, United Kingdom
- 3BLOOM, Paris, France
- 4Louvain Coopération au Développement, Avenue du Grand Cortil, Louvain-la-Neuve, Belgium
Small-scale fisheries (SSFs) are often undervalued and unmanaged as a result of a lack of data. A study of SSFs in Menabe, western Madagascar in 1991 found diverse catches and a productive fishery with some evidence of declining catches. Here we compare data collected at the same landing site in 1991 and 2011. 2011 had seven times greater total monthly landings due to more people fishing and higher individual catches. Catch composition showed a lower mean trophic level in 2011 indicating overfishing, the true extent of which may be masked due to changes in technology and fishing behaviours. Limited management action since 2011 means these trends have likely continued and an urgent need for both greater understanding, and management of these fisheries remains if they are to continue providing food and income for fishing communities.
Introduction
Small-scale fisheries make significant contributions to nutrition, food security, and coastal economies, particularly in low-income countries (FAO, 2019). Yet there is often a lack of data documenting their contribution, and importantly their composition and change over time (Jackson et al., 2001; Andrew et al., 2007; Jacquet and Pauly, 2008). Fishing activities are increasing worldwide both as a result of a growing population and increased per capita consumption (Cai and Leung, 2017). This growing demand places additional pressure on the health of fish stocks. Although there is evidence of improving trends in the stock abundance of well managed fisheries, this is not the case for fisheries in much of the global south where effective governance, monitoring and management are often lacking (Costello et al., 2016; Hilborn and Hilborn, 2019).
In addition to the threats of overfishing, climate change is expected to impact fish and fisheries through a complex suite of linked processes (Brander, 2010). Larger declines are predicted particularly in tropical regions and therefore likely to disproportionately impact the communities that rely on the ocean for their livelihoods (Lam et al., 2016; Ding et al., 2017).
Madagascar is listed as the eleventh poorest country in the world (International Monetary Fund, 2019) and more than 77% of its people live on less than USD $1.90 per day (Alkire et al., 2019). Similar to many countries in the global south, the country lacks the institutional and governance capacity to monitor and manage the socially dynamic, multi-species and often geographically remote fisheries effectively. Therefore, policy and management often remain largely uninformed, ineffective and unable to identify - let alone respond to - changes in fisheries resources and sustainability (Le Manach et al., 2011; Le Manach et al., 2012).
Madagascar’s marine capture fisheries production was estimated to be around 125,000 tonnes in 2017 (FAO, 2019). A study published in 2011 estimated that Madagascar’s small-scale marine fisheries contributed as much as 65% of total fish production (Le Manach et al., 2011), and this same study highlighted that Madagascar’s national fisheries statistics were under-reported by up to 200% between the 1950s and 2000’s (Le Manach et al., 2011). In a country where coastal regions have historically had a higher incidence of poverty than the rest of the country as a whole (Horning, 2008; Moran et al., 2008), the contribution of small-scale fisheries to the national economy and food security is likely to be greatly undervalued.
In much of Madagascar, and particularly in the south-western regions of Atsimo Andrefana and Menabe, fishing and associated activities, including aquaculture and fish processing, are the principal, and often only, source of income for many people (Laroche et al., 1997; Lilette, 2006). This stretch of coastline is home to the Vezo, a semi-nomadic ethnic group of traditional fishers (Astuti, 1995; Sanders, 2005; Lilette, 2006; Cripps and Gardner, 2016). With up to 95% of households in these coastal communities being reliant on fishing as their primary source of income (Barnes-Mauthe et al., 2013), effective fisheries management is vital to ensure the livelihoods and food security of people most at risk from overfishing as well as other human-induced and external threats e.g. climate change.
In 1991, Laroche et al. (1997) surveyed the small-scale fisheries in coastal cities of Toliara, Morombe and Morondava. They found diverse catches, comprising of pelagic, reef and coastal species, with differing species composition across the three main coastal towns. Fisheries in Toliara and Morombe focused on reefs, while in Morondava it primarily focused on sandy coastal areas, with some hand line fishers targeting scattered coral banks. The town of Morondava, also the focus of the current study, had the highest diversity of catches, with a greater composition of high-value and higher trophic level species than the other towns. Despite lower catch rates, particularly noted for handlines, fishers were still able to generate a good income from targeting high-value species in the region in 1991.
Laroche et al. (1997) also highlighted that fishers consistently reported that they were already adapting their activities to mitigate impacts of declining catches on their lives. These adaptations included increasing effective fishing effort (e.g. more hooks, longer nets, and smaller mesh sizes), developing technical solutions (e.g. using longer lasting nylon nets instead of cotton), and seeking supplementary income sources (e.g. farming or dockwork (boutry) in the nearby port). The authors reflected on the reasons for fisheries declines, including changes in the market forces across all of the towns, while specifically focusing on the competition with the commercial shrimp-trawl fishery in Morondava. They concluded that the fisheries were overexploited and in need of improved management and called for the integration of traditional management with government policy, and attribution of exclusive access rights to traditional fisher groups. They also noted that the use of marine sanctuaries could be particularly useful for maintaining species diversity and abundance and suggested that management would be most effective if associated with strategies for supporting livelihood diversification and stabilising population growth.
Since Laroche et al. (1997), a handful of isolated studies have been conducted on Madagascar’s small-scale fisheries, notably those involving finfish (Davies et al., 2009; Doukakis et al., 2009; Brenier et al., 2011; Gough et al., 2020), marine invertebrates (McVean et al., 2005; Barnes and Rawlinson, 2009; Bemiasa, 2009), sharks (McVean et al., 2006; Cripps et al., 2015; Humber et al., 2017a) and marine turtles (Walker and Roberts, 2005; Humber et al., 2011; Humber et al., 2017b), as well as monitoring methods (Behivoke et al, 2021). However, the majority of these studies provide only temporal ‘snapshots’ of fishery status; and none have assessed medium term trends in small-scale finfish fisheries. The current deficiency of accurate fisheries information underlines the need to improve understanding of temporal trends in small-scale fisheries in order to develop recommendations for adaptive management.
Building upon the analysis presented in Gough et al. (2020) that provided evidence of overfishing in the region in 2011, the current study compares landings from 2011 with those from the same fishing communities in 1991 (Laroche et al., 1997). We look at what had changed over the two decades between the two studies and interpret findings with a view to guiding future research and management that will safeguard fisheries livelihoods and human wellbeing.
Materials and Methods
The Morondava Fishing Area
Morondava is a town of around 53,000 people situated on the Menabe coastal region of western Madagascar (INSTAT, 2020) (Figure 1A). It is characterized by a shallow underwater shelf, which is around 30 km in width and less than 20m deep. A scattered coral bank lies at the seaward periphery adjacent to the continental shelf edge. Numerous rivers and extensive mangrove forests result in high turbidity within the sandy-bottomed lagoon (Cooke et al, 2003).
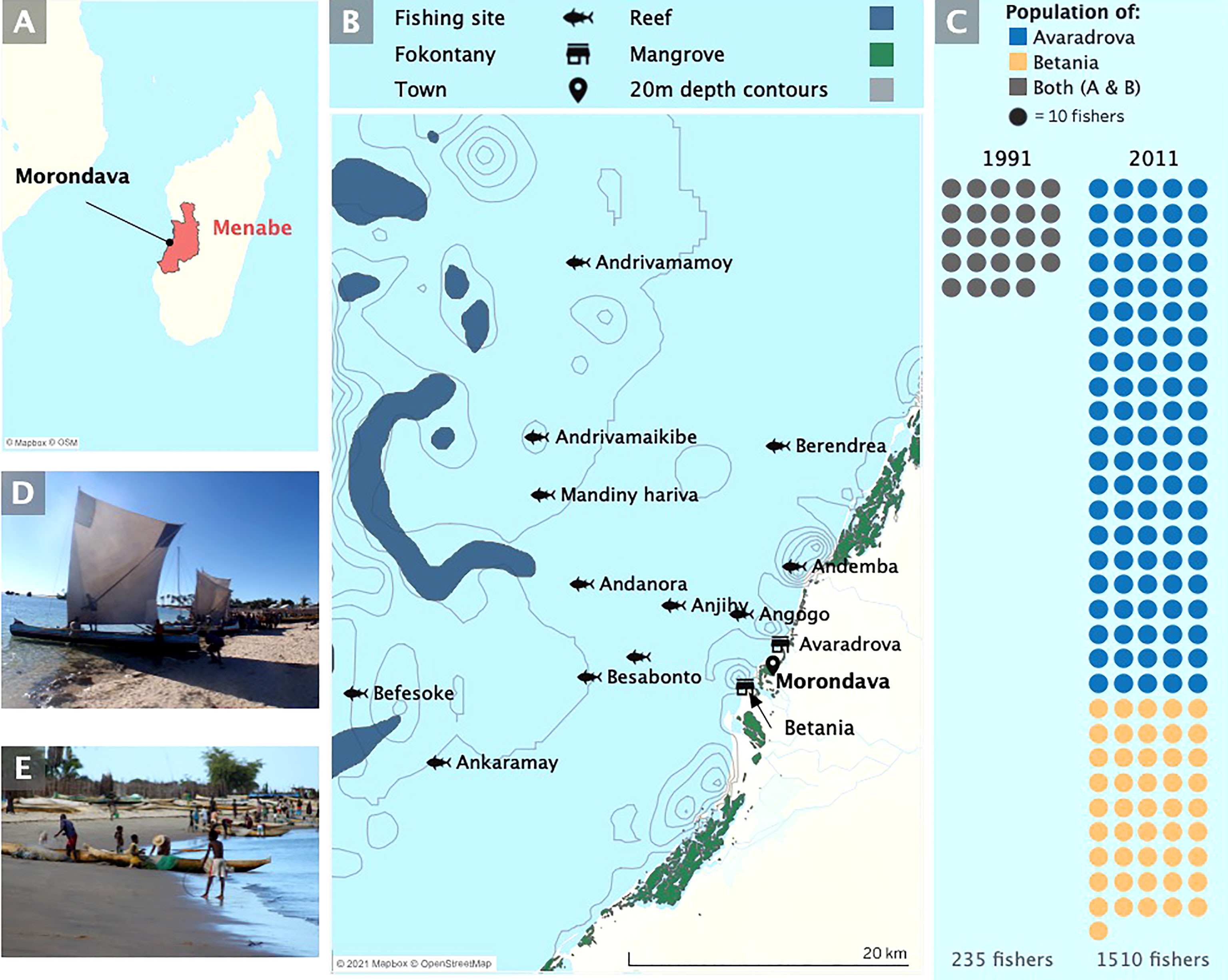
Figure 1 Map of study site (A) Location of Madagascar off the east coast of Africa in the Mozambique channel, and Morondava in the region of Menabe, west Madagascar. (B) The two villages (fokontany) of Betania and Avaradrova where landing surveys were carried out in both 1991 and 2011, and the commonly visited fishing sites recorded by fishers. (C) The number of fishers in each study as reported by village presidents (Note there was no village breakdown provided by Laroche et al., 1997). The two types of traditional boat used by the Vezo in the region’s small-scale traditional fishery (D) laka, and (E) molanga.
The study was carried out in Betania and Avaradrova, two fokontany (a political and administrative subdivision equivalent to a village or group of villages) within the urban commune of Morondava (Figure 1B). Fishers in this region use two types of wooden canoe, locally known as pirogue. The larger canoe with outrigger (laka) is often used for offshore fishing trips and customarily uses a sail (Astuti, 1995) (Figure 1D). The smaller (molanga) is a simpler single hulled canoe usually powered by paddle and more frequently used for nearshore fishing excursions (Figure 1E).
Landings Data Collection
In 1991, Laroche et al. stationed surveyors at two landing sites in the commune of Morondava on the west coast of Madagascar; the first in the fokontany of Avaradrova, and the second in the fokontany of Betania (Figure 1B). The monitoring was carried out for 22 days a month over a 7-month period from April to October 1991. Each day the surveyors recorded the total fishing effort as the number of pirogues that went out to sea from the landing site and sampled ten of the returning pirogues for catch (although there is no record of sampling strategy for these ten pirogues). For each pirogue sampled, the surveyor recorded the number of individuals taking part in the fishing operation, the name of the fishing sites visited, the type of fishing gear employed and a visual estimation of the weight of each species caught, with surveyors being trained prior to sampling in estimating catch weights (Laroche et al., 1997). The number of fish in catches was not recorded. Note that only gillnet and handline fishing gears were recorded in 1991. The presence and recording of other gear types in the same study in other locations (Morombe and Toliara) suggests that if other gear types were being used in the Morondava fishery they would have been recorded.
In 2011, as part of a wider participatory project to understand fishing in the region (Gough et al., 2020), the present study stationed fisheries surveyors at the same landing sites as in Laroche et al. (1997). Two local surveyors were recruited from each village and following training and piloting of data collection in November-December 2010, sampling was undertaken for a minimum of two days every week at each landing site for the year (Jan-Dec) of 2011, with a break over the month of June due to local celebrations.
Fisheries surveyors recorded total fishing effort for the landing site as the number of boats (both laka and molanga) that went out to sea from the landing site each day of surveying (between 2 and 4 days per week). As the boats returned to the beach, the surveyors gathered information on each boat. The number of fishers, time spent fishing and location were recorded for each boat. Additionally, the weight of the total catch and the weight and number of each species were recorded as described in Gough et al. (2020). Surveyors received strong cooperation from fishers allowing them access to their catches during the survey period.
While fisheries monitoring is ongoing elsewhere in the region, monitoring in these two fokontany was not continued after 2011 following the cessation of project funding.
Raw data from the 1991 study were not available and so comparisons are made with results as presented in the paper published by Laroche et al. (1997).
Data Analysis
To ensure consistency and allow comparisons between the results from Laroche et al . and data obtained in 2011, we calculated the same five metrics for each fishing gear as Laroche et al. (1997) using the same methods and equations.
Catch per unit effort (CPUE) is a measure of how much biomass is caught by a single unit of fishing effort. Laroche et al. (1997) reported CPUE in kg fisher-1 hr-1, per the following formula.
Where Pik is the capture weight (kg) and ni is the number of fishing hours (# fishers x trip duration). Average CPUEi (expressed as kg trip-1) was also calculated for each gear type per calendar month.
Total fishing effort (fi) was also calculated as in Laroche et al. (1997) by gear type.
Where t is the number of fishing trips recorded per month and d is the number of fishing days per month and s is the number of days when surveys were conducted. In 2011, data collectors reported observing only 60% of fishing trips. Therefore, observation numbers were adjusted to provide an estimate of the total number of trips.
Monthly catch Estimation of the catch (Ci) for each month by fishing gear type was then calculated.
The following metrics as reported by Laroche et al. (1997) were also calculated for each gear;
FISHM: the number of people employed in the use of a particular fishing gear
LENG: the average duration of a fishing trip (minutes)
ACTI: the estimate of the daily proportion of pirogues fishing [ACTI = (number of trips during the whole survey period x 100)/total number of pirogues x number of days surveyed)].
Each of these results calculated for 2011 observations by gear type was then compared with the results reported by Laroche et al. (1997) for the data collected in 1991.
Raw data values and measures of variance were not presented in the 1997 publication; therefore, summary descriptive statistics are used to make comparisons between 1991 and 2011 metrics.
Laroche et al. (1997) provides a summary of the taxonomic composition of catches reporting the major family groups and their proportional contribution to catches. Laroche et al. (1997) grouped sharks and rays as well as grouping unidentified families and those with small contribution to the total catch as ‘other’. The proportional contribution of each family was calculated using 2011 data and then families grouped in the same way as presented by Laroche et al. to allow comparison. For each family/group the original (1991) proportional contribution was then subtracted from the present (2011) contribution to ascertain which families had increased and/or declined in catches between the two studies.
Laroche et al. (1997) provided a summary of the trophic group composition of reef and reef associated fish catches in 1991. To compare the present study with Laroche, the different fish species were classed according to their degree of reef association (Bellwood, 1988) as described by Laroche et al. (1997). Where new species were observed in 2011 but were not assigned in Bellwood (1988), the degree of reef association was taken from FishBase (Froese and Pauly, 2000). In order to allow direct comparison to Laroche et al. (1997), data on the species designated as reef species or reef-associated species were then retained and those on non-reef species not considered. Each of the remaining species was then allocated to one of five trophic groups: herbivore/detritivores, planktivores, macroinvertebrate feeders, piscivores, piscivore/macroinvertebrate feeder, or unassigned, using the same references as Laroche et al. to ensure comparability (Hiatt and Strasburg, 1960; Hobson, 1974). To be able to compare trophic group composition for the entire catch, and not just reef and reef associated species, trophic groups were assigned to the composition data reported by Laroche et al. (1997). This was then compared to the proportional composition for all species catches in 2011.
Finally, in addition to comparing the metrics presented in Laroche et al. (1997), trophic levels (TL) from FishBase (Froese and Pauly, 2000) were assigned to each fish family group (TLf) for both 2011 and 1991. The median trophic level was calculated for both 1991 and 2011 by taking the median value from the percentage frequency distribution, and the mean trophic level of the catch for both years was then calculated using the proportional composition of each family group (Pf).
Results
In 2011, surveys were conducted 2-4 days per week (overall 34% of total fishing days in Avaradrova and 40% of fishing days in Betania). Data collectors observed a total of 18,304 boats going fishing, with an average of 68 and 77 boats per day in Avaradrova and Betania respectively, which was estimated by the data collectors to represent 60% of the total number of boats out fishing on any given day. They collected samples from a total of 1,555 fishing trips (570 in Avaradrova and 985 in Betania) which indicates that samples were taken for between 6 and 8% of the active boats. The number of fishers on a single fishing trip ranged from 1 to 11 and catch surveys recorded 72,234 kg of fish landed, comprising a total of 447,585 individual fish from 32 families. A further 2,158 kg of crustaceans and 41 kg of holothurians was also landed but excluded from this analysis as the study by Laroche et al. focused solely on finfish. In the 1991 study, monitoring was carried out on 22 days per month, with 10 pirogues sampled for catch each day (Laroche et al., 1997). Although Laroche et al. reports recording the number of pirogues, these data are not published and so we do not know what proportion of the fleet, or consequently, the catch this sample represented in the paper.
Population of Fishers
Laroche et al. reported that, in 1991, of the estimated 40,000 inhabitants of Morondava, 235 reported fishing as their primary activity in the communities of Betania and Avaradrova. Official census data reports that the population of Morondava was 64,071 in 1993 (INSTAT, 1997) and has increased to an estimated 153,994 in the 2018 census (INSTAT, 2020). Interpolation of the INSTAT population data places the population of Morondava at an estimated 120,000 in 2011 with a growth rate slightly higher (3.5%) than the national average of 3%. The presidents of the two villages reported the population of fishers to total 1510 in 2011 (460/1300 inhabitants, in Betania and 1050/3250 inhabitants in Avaradrova) suggesting an estimated 9.8% annual growth rate (Figure 1C) and a 6.4 fold increase in the number of fishers over the 2 decades.
Fishing Gears and Fishing Grounds
Fishing in Morondava in 2011 was still undertaken using traditional fishing boats as described by Laroche et al. (1997) (Figures 1D, E). Laroche et al. reported that in 1991 fishers were using only handlines and fixed gillnets, with a distinct contrast between the fishing grounds used for each gear type. In 1991 handlines were commonly used in both inshore areas and offshore coral banks, which can be up to 30 km out to sea (Figure 1B), while gillnets were used close to the coast in the sandy lagoon area near the town of Morondava.
In 2011, handline fishers appeared to be almost exclusively fishing offshore. Gillnets and other nets, with the exception of shark nets, were still being used mainly in the inshore coastal waters, with a small increase in the use of gillnets in offshore areas (Figure 2). In addition to the handlines and gillnets reported by Laroche et al. however, the present study also recorded the use of beach seines, longlines, mosquito nets, shark nets, spears and spear guns in the Morondava fishery (Figure 2). Gough et al. (2009) provides a summary of the characteristics of each gear type used by Vezo fishers in southwest Madagascar.
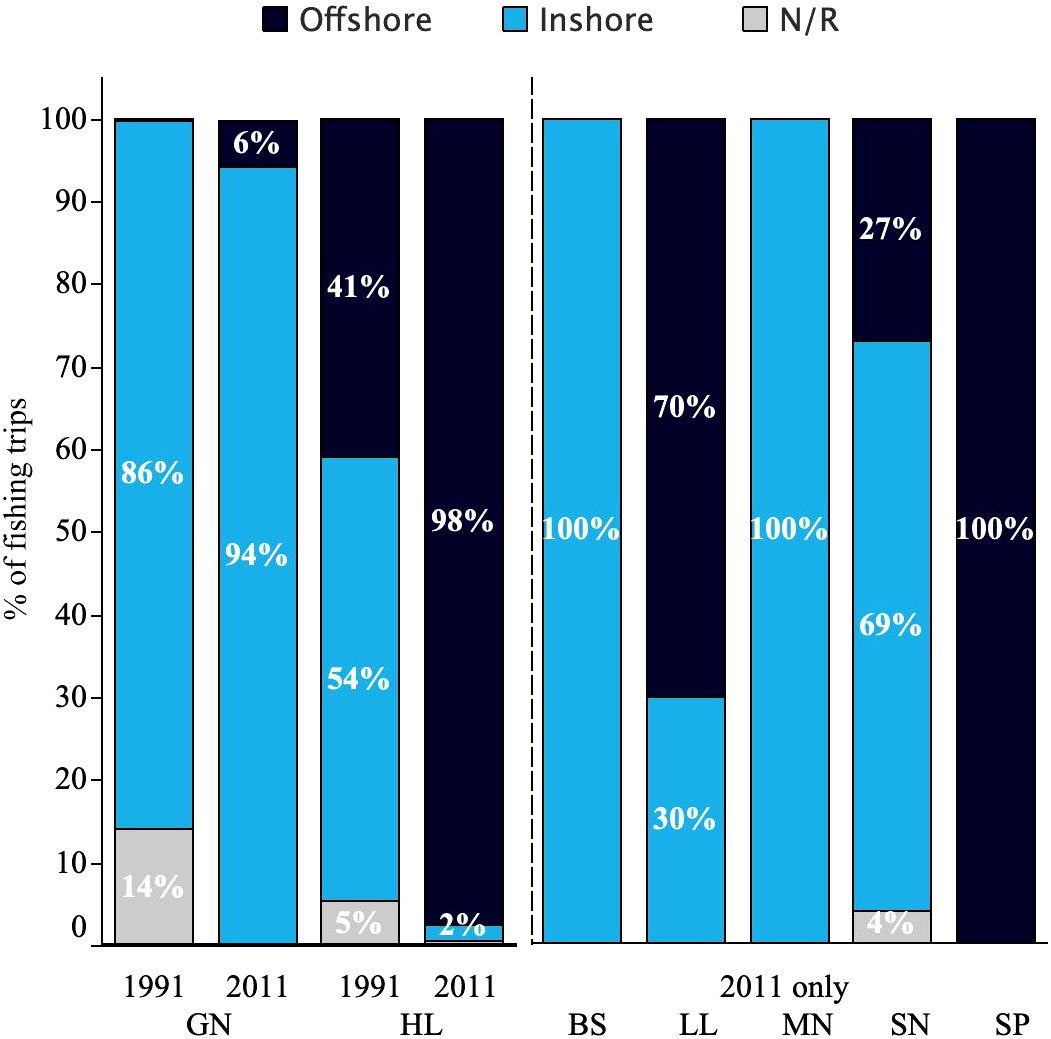
Figure 2 Proportional location of deployments of each gear in 1991 (Laroche et al., 1997) and 2011, this study (N/R, location not recorded; Gears, GN, gillnet; HL, handline; BS, beach seine; LL, longline; MN, mosquito net; SN, shark net; and SP, spear/speargun) by each location type. Temporal comparison (left) shown only for gillnet and handlines as other gears were not reported as being used in 1991.
Estimation of the Production Per Gear Type
The mean monthly catch in Morondava in 1991 (Laroche et al., 1997) was estimated at 16.8 tonnes per month and dominated by handline catches (80%) over gillnets (20%), whereas in 2011 mean monthly catch was estimated as almost sevenfold greater at 122.1 tonnes per month and dominated by gillnet catches (35.8%) over beach seine catches (27.0%) and handlines (26.5%; Table 1). Mean monthly landings from gillnets were estimated to be twelve times higher in 2011 than observed in 1991, while handline landings were estimated to have doubled since 1991. The mean monthly catch from only gillnet and handlines in 2011 was estimated to be 4.5 times larger than in 1991. Taken as a whole the overall mean monthly and daily catch per fisher increased from 71kg/fisher/month and 2.4kg/fisher/day in 1991 to 81kg/fisher/month and 2.7kg/fisher/day in 2011.
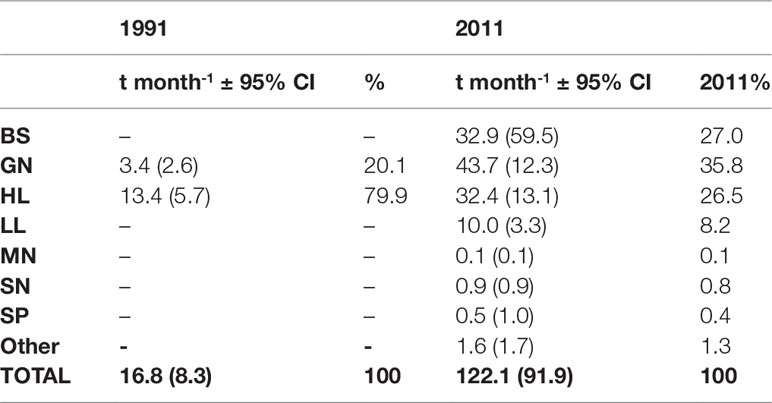
Table 1 Comparisons of estimated average monthly catch (± 95% confidence interval) by fishing gear between 1991 (Laroche et al., 1997) and 2011.
Species and Trophic Composition of Catches
In 1991, Laroche et al. reported 37 fish families amongst the catch, with seven families accounting for 80% of the catch. In 2011, 37 fish families contributed to catch from all gears with six families contributing more than 80% of the catch, with a similar result for handline and gillnet catches only (Table 2). In the 1991 data, the authors grouped sharks and rays which contributed to 11.1% of the catch. An additional 5.8% was grouped as ‘Other’, referring to unidentified finfish families and those with small contribution to the total catch with no further detail given on the families present. Only the Istiophoridae (billfish family) were known to be present in 1991 but absent in 2011, while only Hemiramphidae (halfbeaks) which were absent in 1991 were present in catches in 2011 although in small numbers (<0.1% of catch). Since further information was not available on which families comprised the “other” or “sharks” categories in the 1991 study, we are unable to know if there were further family groups lost or new ones appearing between the two studies (Table 2 and Figure 3).
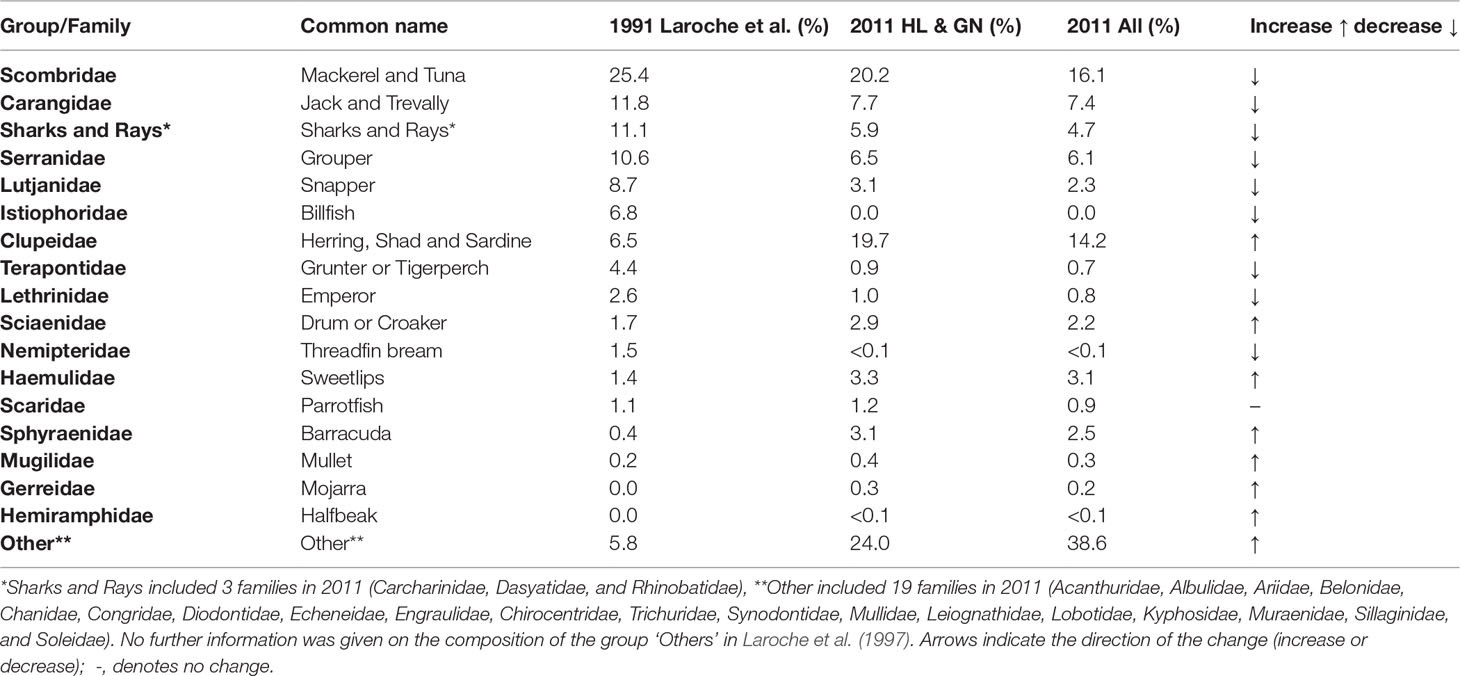
Table 2 The major fish families caught in Morondava (Avaradrova and Betania) and their percentage contribution to the total catch (by weight) in 1991 and 2011with arrows indicating the direction of the trend between the two studies.
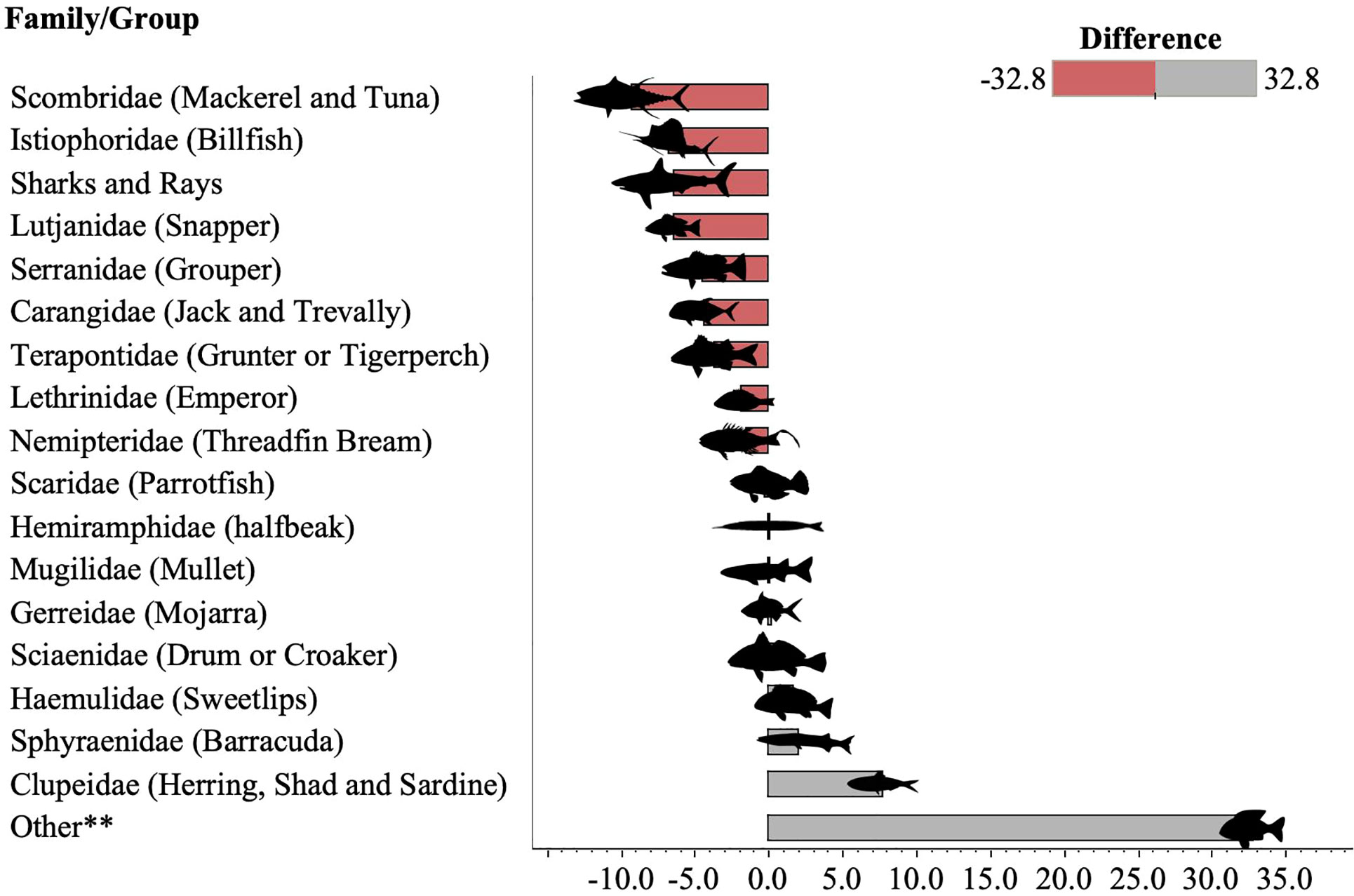
Figure 3 Change in fish family composition between 1991 (Laroche et al., 1997) and 2011 (this study) for all gear types. Red bars indicate fish families that reduced in proportional representation by weight in catches between 1991 and 2011 (e.g. sharks and rays dropped by 6.4% from 11.1% in 1991 to 4.7% in 2011, while Istiophoridae comprised 6.8% in 1991 but was absent from catches in 2011. Those in grey appeared more frequently in catches in 2011 (e.g. Sciaenidae increased by 0.5% from 1.7% in 1991 to 2.2% in 2011). **Other included 19 families in 2011 (Acanthuridae, Albulidae, Ariidae, Belonidae, Chanidae, Chirocentridae, Congridae, Diodontidae, Echeneidae, Engraulidae, Kyphosidae, Leiognathidae, Lobotidae, Mullidae, Muraenidae, Sillaginidae, and Soleidae, Synodontidae and Trichuridae). No further information was given on the composition of the group ‘Others’ in Laroche et al. (1997).
Laroche et al. (1997) highlighted the diversity of catches, with pelagic, reef, and coastal species represented. They further noted that Serranidae (groupers) were particularly targeted by fishers and comprised 10.6% of the catch. In the present study, this same family had declined in its contribution to catches comprising just 6.5% of the catch from HL and GN, and 6.1% of catch from all gear types (Table 2 and Figure 3). Indeed, of the top 10 fish groups reported by Laroche et al. (1997), only Clupeidae (herring, shad, sardine) and Sciaenidae (drums and croakers) had increased in prevalence in 2011, while all other families decreased (Table 2 and Figure 3).
Laroche et al. (1997) highlighted that piscivores and piscivore/macroinvertebrate feeders comprised a combined total of 91% of catches of reef and reef associated fish in 1991 (Figure 4A). In 2011, these trophic groups made a much smaller contribution of just 56% to the catches of reef and reef associated fish (Figure 4B) and 53% to handline and gillnet catches of reef and reef associated fish (Figure 4C). Assigning trophic groups to the non-reef families reported by Laroche et al. (1997) (Table 2), piscivores comprised an estimated 73% of the overall catch (including both reef and non-reef species) in 1991 (Figure 4D), while in 2011 this group comprised 62% of handline and gillnet catches (Figure 4E) and 52% to catches from all gear types (Figure 4F).
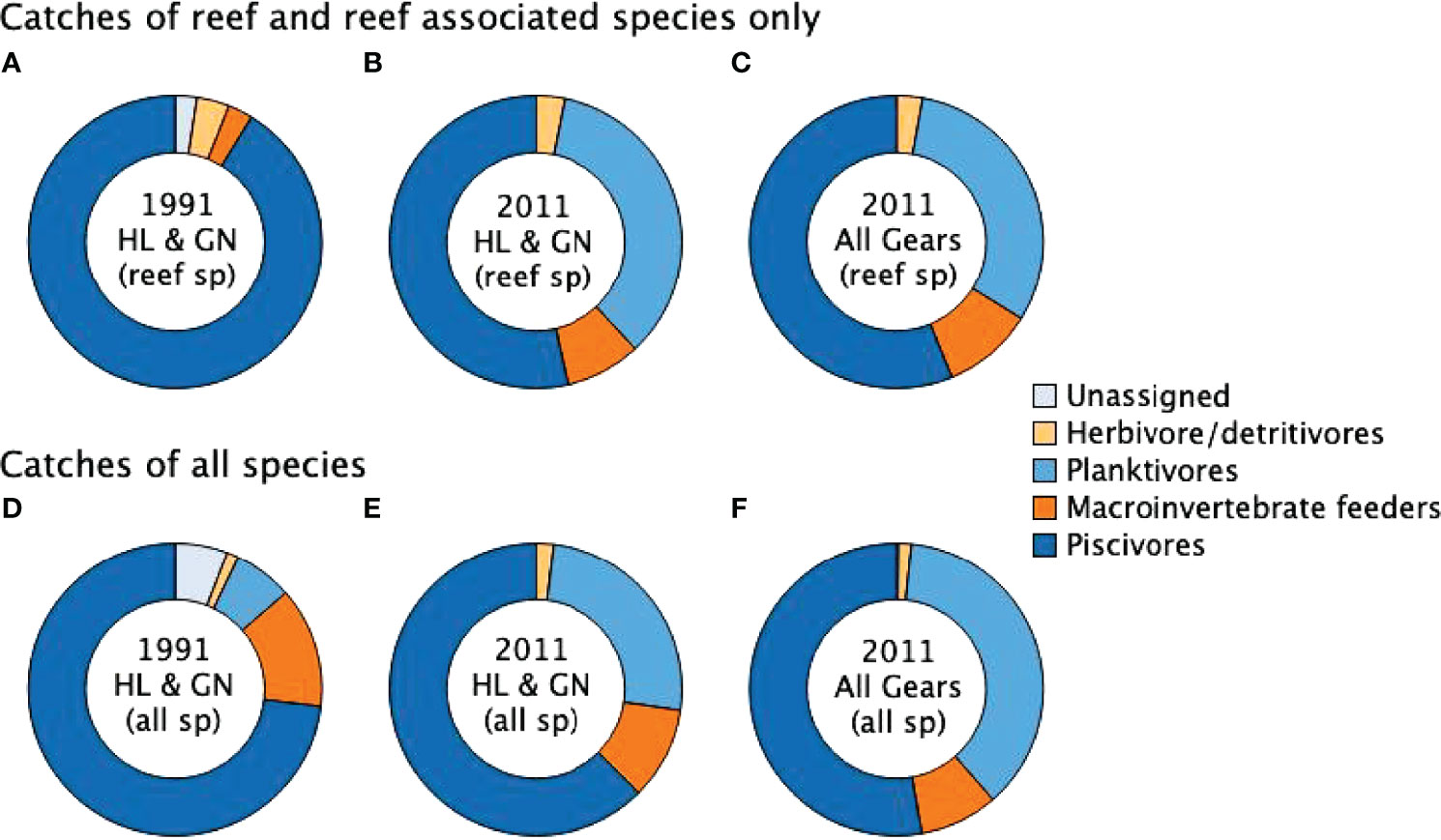
Figure 4 Change in the proportional contribution of fish from each trophic group between 1991 reported by Laroche et al. (1997) and 2011. (A) reef fish only reported by Laroche et al. and compares with (B) 2011 reef fish catches for handline and gillnets only, and (C) 2011 reef fish catches for all gear types. While (D) compares proportions of trophic groups from all species in 1991 (E) all species in 2011 from handline and gillnet catches only and (F) all species from all catches in 2011. Further breakdown of trophic group composition of catches by gear type are provided in Supplementary Data (S1).
The median trophic level (MTL) calculated from Laroche et al. (1997) was 4.0 in 1991 (mean 3.9) while in 2011 (across all gear types and for handline and gillnet catches only) the MTL was 3.65 (mean 3.7) a drop of 0.35 over the two decade period.
Main Characteristics of Fishing Techniques
Laroche et al. (1997) provided a summary of the following four variables for each of the landing sites; FISHM (the number of fishers employed in the use of a particular fishing gear), LENG [the average duration of a fishing trip (minutes)], CPUE (the amount of fish caught per fisher per hour), and ACTI (the estimate of the daily proportion of pirogues fishing). By comparing the 2011 data with that reported by Laroche et al. we found some notable change across the two decades.
There was little difference in the mean number of fishers operating a particular fishing gear on a single trip (FISHM) between 1991 and 2011. Although there was a drop in the mean number of fishers using handlines in Avaradrova, from 2 (error not reported) in 1991 to 1.4 fishers (± 0.5 SD) in 2011, suggesting handline fishers are increasingly operating alone in this village. The mean number of fishers on a trip employing gill nets in both villages remained at 1.0 (± 0.1 SD) in both villages, and handlines in Betania remained stable from 2 fishers in 1991 (error not reported) to 1.9 fishers (± 0.3 SD) in 2011 (Figure 5A). Fishers appeared, however, to be spending a markedly longer time at sea (LENG) in 2011 than in 1991 across all gear types (Figure 5B). The difference in the length of fishing trips using gillnets ranged from a mean of 36 minutes longer in Avaradrova, from 260 minutes in 1991 (error not reported) to 296 minutes (± 4.2 SD) in 2011 to a mean of over two hours longer for gillnet fishers in Betania from 200 minutes in 1991 (error not reported) to 333 minutes (± 87.4 SD). Handline fishers from both villages spent between 50 minutes to an hour longer fishing in 2011 than in 1991, on average from 500 minutes in 1991 (error not reported) to 550 (± 123.0 SD) in 2011 in Avaradrova, and from 550 minutes in 1991 (error not reported) to 608 (± 113.9 SD) in 2011 in Betania.
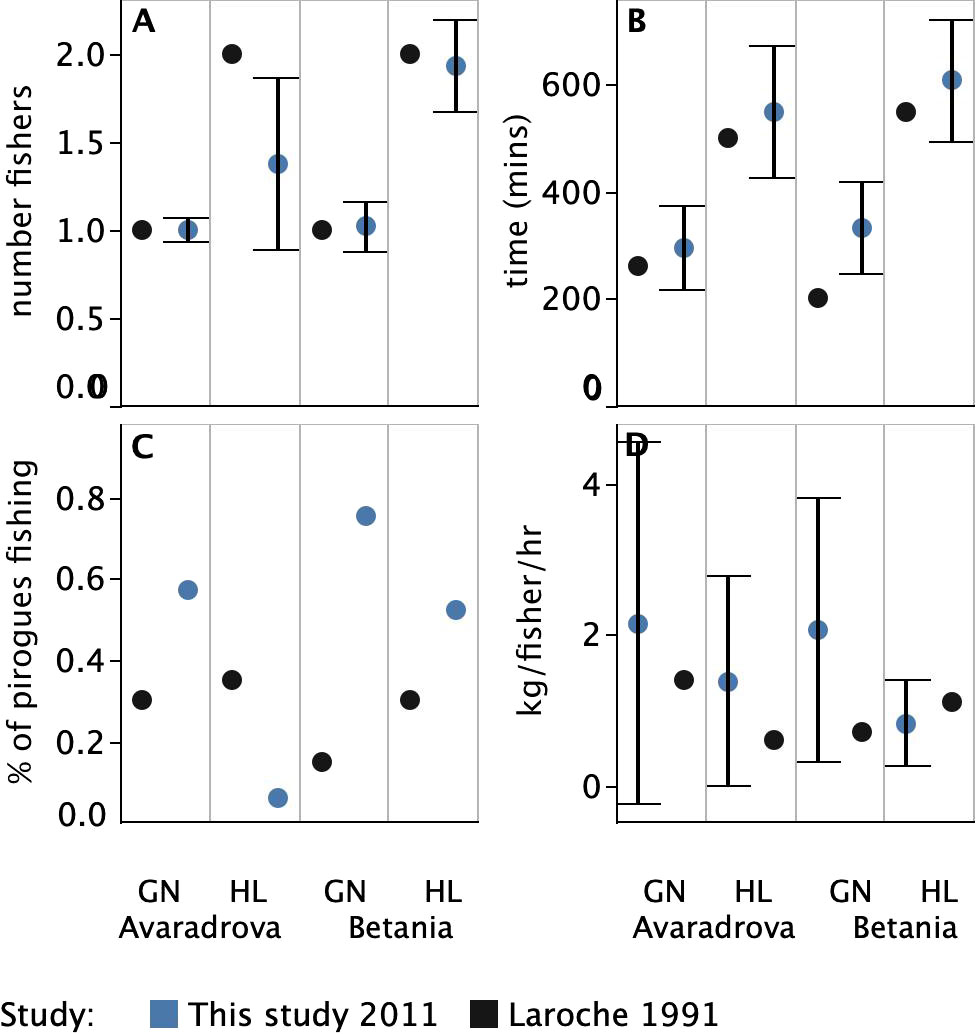
Figure 5 Comparisons between the 4 main fishing effort variables used by Laroche et al. (1997). (A) Average number fishing by gear type (FISHM). (B) Average duration (minutes) of a trip (LENG). (C) Average daily activity (ACTI - the estimate of the daily proportion of pirogues fishing), and (D) Average catch per unit effort (kg person-1 h-1) (CPUE). Error bars indicate standard deviation. Variation was not reported by Laroche et al. (1997) and thus error bars are not shown for 1991 values.
There was an increase in the total fishing effort (ACTI) for gillnets in both Avaradrova (from 0.3 in 1991 to 0.6 in 2011) and Betania (increasing from 0.2 in 1991 to 0.8 in 2011). Total effort exerted by handline fishers appeared to have declined in Avaradrova (0.4 in 1991 to 0.1 in 2011) but increased slightly in Betania (0.3 in 1991 to 0.5 in 2011) (Figure 5C).
Mean catch rate for an individual fisher on a fishing trip (CPUE) was higher for gillnets in both villages in 2011 when compared to 1991. CPUE was 2.2 kg/fisher/hr (± 2.4 SD) in 2011 for gillnets in Avaradrova and 2.1 kg/fisher/hr (± 1.8 SD) in Betania compared to 1.4 kg/fisher/hr and 0.7 kg/fisher/hr (error not reported) for each of these two sites respectively in 1991. Handline catches were also higher in 2011 in Avaradrova (1.4 kg/fisher/hr ± 1.4 SD) compared to 1991 (0.6 kg/fisher/hr error not reported). The only exception was that CPUE was slightly lower in 2011 in Betania (0.8 kg/fisher/hr ± 0.6 SD) compared to 1991 (1.1 kg/fisher/hr error not reported).
Discussion
This paper provides a useful snapshot of the temporal change in the small-scale fishery of Morondava focusing on the changes that occurred between 1991 and 2011. The main results of the comparison point towards overexploitation of the resources revealing that; 1) the catch composition had changed between the two studies, with fewer high-trophic level species in catches in 2011 than in 1991 (Figures 4A, B and Table 1) the estimated total monthly landings had dramatically increased between the two years. The seven-fold increase in catches appear to be explained by the substantial increase in the population of fishers between the two study periods (Figure 1C) and diversification of fishing gears, signified by the introduction of gears such as beach seines and long lines that were not reported in the 1991 study. We observed that gillnet and handline fishers were fishing further afield, spending more time fishing (Figure 5B) and returning with a slightly higher catch weight per fisher for a single fishing trip (CPUE) (Figure 5D). We examine each of these findings below and discuss what may be contributing to these results and what they may mean for the fishery and fishers of Morondava.
In 2011, Morondava fishers continued to catch a large diversity of demersal and pelagic species from offshore reef and sandy lagoon habitats, similar to that observed in 1991 by Laroche et al. (1997). While the number of fish families remained similar, some fish families that were present in 1991 became reduced or absent in the 2011 study. The reduction in the proportional contribution from families such as Istiophoridae (sailfish), and Serranidae (grouper) as well as sharks and rays was notable, as well as that from Lethrinidae (emperor) and Nemipteridae (sea bream), again larger and slow maturing species that show high vulnerability to fishing pressure (Cheung et al., 2005). Since many of these species are from high trophic levels, it explains the observed decline in median trophic level (MTL) of the catches. The 0.35 decline in trophic level (TL) observed in the Morondava fishery between 1991 and 2011 is higher than the reported global average of 0.1 TL per decade (Pauly et al., 1998). This provides evidence that the fishery may have been experiencing the phenomenon commonly referred to as ‘fishing down marine food webs’ and is considered indicative of a fishery under unsustainable exploitation (Pauly et al., 1998). A similar trend has been documented in the small-scale fishery of Toliara, southwest Madagascar (Brenier et al., 2011) as well as in a number of other small-scale tropical coastal fisheries (McClanahan and Mangi, 2004; Mangi and Roberts, 2006). Although change in trophic level could be explained by other factors besides resource overexploitation (Branch et al., 2010; Sethi et al., 2010) in this case the change is most likely associated with the change in catch composition (see below).
Essington et al. (2006) additionally defined ‘fishing through’ marine food webs describing how the MTL may decline due to the sequential addition of low TL catches rather than simply the decline of high TL species. The increased contribution of some low TL families, such as Clupeidae (sardine) in 2011 may reflect changes in markets and targeted fish species but is also likely a reflection of the introduction of new fishing gears, in particular the increased use of beach seines in the region over the 20 year period. The increased abundance of these species in catches has also been noted in a similar comparison conducted in the small-scale fishery of Toliara a region ca. 400km from the current site (Brenier et al., 2011).
Conversely, there may be some ‘fishing up’ the marine food web (Branch et al., 2010; Stergiou and Tsikliras, 2011) that is not captured by the overall change in TL as a number of higher TL families that were absent from the 1991 study were present in our 2011 surveys (although they may have been included in Laroche’s ‘other’ family group). Again, these are also likely to have appeared as a result of the introduction of new fishing gears and sites (Figure 3), for example Lobotidae (tripletails), being caught primarily by longline fishers at offshore sites, and Trichuridae (cutlassfish), and Chirocentridae (wolf herring) being caught by small mesh gillnets in inshore waters.
Fishers in Avaradrova, particularly those using handline, were fishing for longer periods (Figure 5B) and more frequently at offshore sites in 2011 than in 1991 (Figure 2). This may reflect the targeting of some of these larger bodied high trophic level fish families found predominantly in offshore areas, such as Scombridae (tuna and mackerel), or reef fishes such as those of the Serranidae (grouper), Lutjanidae (snapper) and Lethrinidae (emperor) families found on and around offshore reefs. Since these were some of the main families targeted in 1991 and their contribution to catches has declined (Figure 3), it is likely that this shift may be due to reduced catches of these species on nearshore reefs, driving fishers further afield. In addition, the diversification of fishing gears, such as the introduction of shark nets and longlines to target sharks and scombrids, as well as fishing further afield in deeper or previously less exploited waters may also be partially maintaining catches and masking the decline in higher trophic level value (Morato et al., 2006). The fish families caught by small-scale fishers are targeted for both local consumption and export markets. Increases in fishing effort across these families is therefore likely linked to both the requirements of feeding a growing local population and meeting the growing demands for export. A socio-economic survey carried out in the region in 2011 reported that 63% of fishers identified a large decrease in the abundance of fish in catches, and that they subsequently fished further away (26%) or more frequently (7%) to compensate for the changes (Dewar, 2012). These behavioural responses have been observed in numerous other small-scale fisheries around the tropics (Padilla et al., 2003; Eder, 2005; Sadovy, 2005; Fabinyi, 2010) and are often driven by declining catches and can sometimes mask trophic declines (Stergiou and Tsikliras, 2011).
Beyond the absence of some fish families in catches, intensive fishing has been demonstrated to lead to imbalances in ecosystem function, which has ramifications for community structure overall (Jennings and Kaiser, 1998; Jennings et al., 1999). Fishery removals may induce indirect trophic (food web) interactions, and thus fishing can have many effects on community structure (Botsford et al., 1997). These interactions become increasingly more complex in multispecies fisheries where multiple gear types are deployed (Jennings et al., 1999; Jennings, 2005) as observed in this study.
In addition to the changes in trophic composition, the overall average monthly landings for the Morondava landing sites in 2011 were estimated to be 700% higher than estimated by Laroche in 1991 (Table 1). It is likely that the increase in landings is due in part to increases in total fishing effort, particularly the number of fishers operating within the fishery. The population of Morondava has more than doubled from Laroche’s estimate of 40,000 to an estimated 120,000 in 2011. However, the population of fishers has increased more than 6-fold in this same period. This high growth is likely due to a combination of high fertility rates as well as the migration of fishers from communities to the south, who travel north as fisheries resources decline further south, attracted by the growth of export markets for shark fin and sea cucumbers and more plentiful resources further north (Cripps and Gardner, 2016). There is also a strong flow of agro-pastoralists and others from inland communities towards the coast as crop yields decline and communities face rising food and physical insecurity (Marikandia, 2001; Chaboud, 2006; Bruggemann et al., 2012). Migrants therefore have variable experience and expertise in fishing livelihoods depending on where they originated. Small-scale fisheries continue to make an important contribution to the livelihoods, income and food generation of the Vezo people living in the coastal town of Morondava and the wider south-west region of Madagascar. Yet with increasing numbers of people operating in the fishery, fishers often cite the number of fishers as being an important contributor to the changes they have seen in their catches over time, alongside the presence of an industrial shrimp-trawl fishery (Jones, 2011; Dewar, 2012).
Higher total monthly landings are, in part, explained by the diversification of fishing gears. Our results show the presence of shark nets, longlines, beach seines and mosquito nets in the Morondava fishery in 2011 (Figure 2 and Table 2), yet these gears were absent in the records from 1991. The absence of these gears in the 1991 study however, may not indicate absence in the fishery, but may be a result of the limited geographic and temporal scope of the original study. While these gears may not have been routinely employed in the fishing communities of Betania and Avaradrova, it is likely that these gears were being employed in the wider Menabe region in 1991 as they were reported to be common at the time in other Vezo fisheries, e.g. Toliara and Morombe (Laroche and Ramananarivo, 1995; Laroche et al., 1997). Other anthropological studies in the Menabe region also note the use of mosquito nets (Astuti, 1995) and shark nets (Sanders, 2005). However, it is likely that these gear types were not used as extensively in the study villages at the time of the original study or because they were used seasonally and outside of the Laroche et al. (1997) study period (Apr-Oct). Beach seines and Mosquito nets for example, were used between October and December, and mosquito nets were previously reported to be used only seasonally to target a small shrimp known locally as patsa (Acetes erythraeus) (Le Reste, 1970) rather than finfish. Shark nets, which were used in the present study between August and December, and Longlines (used all year round), may have previously only been more commonly used by migrant fishers operating on the nearby islands of Nosy Andriangory and Nosy Andramitaroky (Sanders, 2005).
Additionally, the slightly higher catches observed for gillnets and handlines may also suggest that they have improved in efficiency between the two studies. Observations were already reported in 1991 that fishers were using nets with smaller mesh sizes and increased numbers of hooks to compensate for declining catches (Laroche et al., 1997). However, since the 1997 paper does not report on net lengths or mesh sizes, it is not possible to quantify the change in more precise measures of fishing effort. Fisher interviews conducted in 2011 showed that they had been increasingly deploying nets with mesh sizes as small as 10 mm, and with numerous lengths of nets spliced together, forming barriers of up to 1 km in length in attempts to maintain catches (Dewar, 2012). This phenomenon of “technology creep” may also have allowed fishers to maintain or increase catch rates despite a declining ecological condition, through the use of increasingly effective and often destructive fishing gears (Rijnsdorp et al., 2006; Eigaard et al., 2014).
The authors of the 1991 study already noted that the fisheries of Morondava were showing signs of overexploitation and were in need of management intervention (Laroche et al., 1997). Yet it is notable that with reduced abundance of high trophic levels and a much larger monthly catch, overfishing has continued in these fisheries since the original study, with further evidence of overfishing highlighted by a wider analysis of fisheries catches in the region (Gough et al., 2020). The 1997 paper specifically noted the contribution that the commercial shrimp-trawl fishery made to declining catches through large bycatch rates and high discards, and this fishery continues to operate in the same area as the small-scale fishery and continues to be cited by local fishers to be contributing to fisheries declines in the region (Jones, 2011; Dewar, 2012). However, it is not possible to determine the impact of the trawl fishery on the small-scale fisher catches without investigating the trawl fishery itself.
We were unable to perform statistical comparisons due to the absence of raw data from 1991, but wherever possible we have noted where variation in 2011 overlaps with 1991. This highlights an issue around access to data needed in order to inform fisheries management. Data deficiency is often cited in respect to small scale fisheries, and this is often due to data not being collected. However, this study highlights that even where data exists it may not be accessible to fisheries managers in a form that enables further analysis. This is changing however with increasing innovation in fisheries data collection and management (Bradley et al., 2019) including new tools and methods that could be incorporated to allow more precision in understanding changes to effective fishing effort (Behivoke et al, 2021) and a growing trend in sharing of data for transparency, reproducibility and verification purposes (Pendleton et al., 2019).
Due to both studies providing a snapshot of the fishery across a single year we are unable to assess the contribution that inter-annual variation makes to the differences observed. While additional data have not been collected from the villages of Betania and Avaradrova since 2011, socio-economic assessments conducted in 2018 in 10 villages to the south of Morondava reported that 71% of fishers reported declining catches. Of those reporting declines, 31% attributed this to increasing numbers of fishers, and a further 21% to growing use of destructive fishing gears and methods (Blue Ventures, 2018, unpublished data). The villages in the 2018 study were located not more than 60 km south of Morondava and so it is likely that these results reflect the ongoing situation of overfishing and fisheries declines across the region, particularly given the management situation in the study villages has not changed dramatically since the 2011 study.
The original Laroche et al. (1997) paper highlighted the need for integrating traditional management with government policy and attributing exclusive access rights to traditional fisher groups and noted that the use of marine sanctuaries could be particularly useful for recovery of overexploited populations and for maintaining species diversity and abundance. While there isn’t yet any formal management in place in the Morondava fishery where this study was conducted, since 2003 a growing network of locally managed marine areas, which also include a number of permanent no-take-zones, have been established across the country. There is also growing evidence from the Toliara region that these no-take-zones are helping the recovery of fish biomass (Gilchrist et al., 2020).
A number of fishing villages to the south of the study location have been trialling periodic closures of their mangrove mud crab fisheries with strong social support from fishers (Rocliffe et al, in review), similar management could be supported in the communities engaged in this study. Additionally, the MIHARI (MItantana Harena Ranomasina avy eny Ifotony – translated as marine resource management at the local level) network, a civil society network established in 2012 by locally managed marine area leaders and supporting NGOs, put forward three position statements on three key issues, documented as motions during the 2017 national LMMA forum. These motions advocate for the formalisation of local management (MIHARI network, 2017b), the regulation of gears used in small-scale fisheries (MIHARI network, 2017c) and finally, to address the issue of spatial overlap with commercial shrimp-trawl operations and, the instalment of an exclusive fishing zone for small-scale traditional fishers (MIHARI network, 2017a). These motions are a significant first step towards LMMA leaders having a greater voice in management of their fisheries. These motions are under consideration by the Government, particularly as it aims to triple the area in marine protected areas (World Parks Congress, 2014), and would contribute to the management of the fisheries included in the study.
In conclusion this comparison between the fisheries data collected in 1991 and 2011 highlights a concerning trend in the small-scale fisheries of Morondava and given the lack of management in the region since the study it is likely that this trend has continued. A substantial increase in estimated total monthly landings for the fishery, as well as changes in family and trophic composition of catches supports other evidence of overfishing within the region’s fisheries (Brenier et al., 2011; Gough et al., 2020). It is likely that technological advancement, increases in effective fishing effort, and employment of less selective methods such as beach seining is contributing to larger catches in addition to masking some of the effects of overfishing by enabling fishers to continue to catch target species despite their reduced abundance. Failure to respond to the challenge of managing this and other comparable small-scale fisheries in western Madagascar may have enduring consequences for local food security in one of the poorest countries in the world, particularly as climate change is expected to cause further fisheries declines in the tropics. Regular monitoring, in addition to improvements in the precision of monitoring including the addition of length based measures (Gough et al., 2020) and improvements in monitoring effective fishing effort (Behivoke et al., 2021) would all support improvements in understanding the trends in the fishery and address some of the limitations of the current study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Data used in this study were collected by Blue Ventures which has an accord de siege with the Madagascar Government. Data were collected as part of an ongoing project to assess small-scale fisheries in the region of Menabe with a view to improving their management. All fish were sampled as part of the legal fishery with permission granted from each fisher. No animals were harmed for the purposes of this study (normal daily catches of a working fishery were observed), and participant’s names were not recorded. We obtained verbal consent from participants before conducting landings surveys due to low literacy levels in the region. During verbal consent, participants were informed about the survey, its purpose, and how the data would be used. Ethical approval for this research has been given by the Ethics committee at the University of Exeter.
Author Contributions
CG, EZ, MA and FM contributed to the conception and design of the study, as well as data collection. CG and FM conducted analyses and CG, AH, AB and BG developed the first drafts of the manuscript and all authors contributed to further manuscript revisions and read and approved the final version.
Funding
Funding for this study was providedby Louvain Development and the European Union’s Regional Coastal Management Programme of the Indian Ocean Countries (ReCoMaP/127769/M/ACT/RSA/005).
Conflict of Interest
Authors CG, AH, and MA are or have been employed by the NGO Blue Ventures, and EZ was employed by Louvain Coorporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the community-based fisheries surveyors involved in this research, a particular mention to Brian Jones, Thomas Beriziny, Breton (Toto) Vernes and Eloi Gustave who supported the authors in training surveyors, collecting and entering data and helping with translations. Our greatest thanks go to all the fishers who kindly allowed us to sample their catches during the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.884083/full#supplementary-material
References
Alkire S., Conceição P., Barham A., Calderón C., Conconi A., Dirksen J., et al. (2019). Global Multidimensional Poverty Index 2019 Illuminating Inequalities ( United Nations Development Programme and Oxford Poverty and Human Development Initiative) Oxford, University of Oxford. doi: 10.35648/20.500.12413/11781/ii022
Andrew N. L., Béné C., Hall S. J., Allison E. H., Heck S., Ratner B. D. (2007). Diagnosis and Management of Small-Scale Fisheries in Developing Countries. Fish. Fisheries. 8 (3), 227–240. doi: 10.1111/j.1467-2679.2007.00252.x
Astuti R. (1995). “The Vezo are Not a Kind of People”. Identity, Difference and “Ethnicity” Among a Fishing People of Western Madagascar. Am. Ethnologist. 22 (3), 464–482. doi: 10.1525/ae.1995.22.3.02a00010
Barnes-Mauthe M., Oleson K. L. L., Zafindrasilivonona B. (2013). The Total Economic Value of Small-Scale Fisheries With a Characterization of Post-Landing Trends: An Application in Madagascar With Global Relevance. Fisheries. Res. 147, 175–185. doi: 10.1016/j.fishres.2013.05.011
Barnes D. K. A. A., Rawlinson K. A. (2009). Traditional Coastal Invertebrate Fisheries in South-Western Madagascar. J. Mar. Biol. Assoc. United. Kingdom. 89 (8), 1589–1596. doi: 10.1017/S0025315409000113
Behivoke F., Etienne M. P., Guitton J., Randriatsara R. M., Ranaivoson E., Léopold M. (2021). Estimating Fishing Effort in Small-Scale Fisheries Using GPS Tracking Data and Random Forests. Ecol. Indic. 123, 1–7 doi: 10.1016/j.ecolind.2020.107321
Bellwood D. R. (1988). Seasonal Changes in the Size and Composition of the Fish Yield From Reefs Around Apo Island, Central Philippines, With Notes on Methods of Yield Estimation. J. Fish. Biol. 32 (6), 881–893. doi: 10.1111/j.1095-8649.1988.tb05431.x
Bemiasa J. (2009). Dynamique Des Pêcheries Traditionnelles Danchois, De Calmars Et De Poulpes Du Sud-Ouest De Madagascar : Utilisation Doutils Océanographiques Pour La Gestion Des Ressources ( University of Toliara). University of Toliara Institut Halieutique et des Sciences Marines, Madagascar Available at: https://archimer.ifremer.fr/doc/00000/6847/.
Botsford L. W., Castilla J. C., Peterson C. H. (1997). The Management of Fisheries and Marine Ecosystems, Science. Am. Assoc. Advancement. Sci. 277 (5325), 509–515. doi: 10.1126/science.277.5325.509
Bradley D., Merrifield M., Miller K. M., Lomonico S., Wilson J. R., Gleason M. G. (2019). Opportunities to Improve Fisheries Management Through Innovative Technology and Advanced Data Systems. Fish. Fisheries. 20 (3), 564–583. doi: 10.1111/faf.12361
Branch T. A., Watson R., Fulton E. A., Jennings S., McGilliard C. R., Pablico G. T., et al. (2010). The Trophic Fingerprint of Marine Fisheries. Nature. 468 (7322), 431–435. doi: 10.1038/nature09528
Brander K. (2010). Impacts of Climate Change on Fisheries. J. Mar. Syst. 79 (3–4), 389–402. doi: 10.1016/j.jmarsys.2008.12.015
Brenier A., Ferraris J., Mahafina J. (2011). Participatory Assessment of the Toliara Bay Reef Fishery, Southwest Madagascar. Madagascar. Conserv. Dev. 6 (2), 60–67. doi: 10.1579/0044
Bruggemann J. H., Rodier M., Guillaume M. M. M., Andréfouët S., Arfi R., Cinner J. E., et al. (2012). Wicked Social-Ecological Problems Forcing Unprecedented Change on the Latitudinal Margins of Coral Reefs: The Case of Southwest Madagascar. Ecol. Soc. 17 (4) 47. doi: 10.5751/ES-05300-170447
Cai J., Leung P. (2017). Short-Term Projection of Global Fish Demand and Supply Gaps. Fisheries and Aquaculture Technical Paper No 607. FAO, Rome
Chaboud C. (2006). Gérer Et Valoriser Les Ressources Marines Pour Lutter Contre La Pauvreté. Études Rurales. 178), 197–212. doi: 10.4000/etudesrurales.8388
Cheung W. W. L., Pitcher T. J., Pauly D. (2005). A Fuzzy Logic Expert System to Estimate Intrinsic Extinction Vulnerabilities of Marine Fishes to Fishing. Biol. Conserv. 124 (1), 97–111. doi: 10.1016/j.biocon.2005.01.017
Cooke A., Lutjeharms J. R. E., Vasseur P. (2003). “Marine and Coastal Ecosystems,” in The Natural History of Madagascar. Eds. Goodman S. M., Benstead J. P., 179–208. University of Chicago Press, Chicago
Costello C., Ovando D., Clavelle T., Kent Strauss C., Hilborn R., Melnychuk M. C., et al. (2016). Global Fishery Prospects Under Contrasting Management Regimes, Proceedings of the National Academy of Sciences of the United States of America. Natl. Acad. Sci. 113 (18), 5125–5129. doi: 10.1073/pnas.1520420113
Cripps G., Gardner C. J. (2016). Human Migration and Marine Protected Areas: Insights From Vezo Fishers in Madagascar, Geoforum journal Vol. 74 ( Elsevier Ltd), 49–62. doi: 10.1016/j.geoforum.2016.05.010
Cripps G., Harris A., Humber F., Harding S., Thomas T. (2015). A Preliminary Value Chain Analysis of Shark Fisheries in Madagascar,FAO, Rome and Indian Ocean Commission, Mauritius. doi: 10.1371/journal.pone.0065051
Davies T. E., Beanjara N., Tregenza T. (2009). A Socio-Economic Perspective on Gear-Based Management in an Artisanal Fishery in South-West Madagascar. Fisheries. Manage. Ecol. 16 (4), 279–289. doi: 10.1111/j.1365-2400.2009.00665.x
Dewar K. (2012). Gear Selectivity and the Influence of Socioeconomic Factors on Gear Choice in a Small-Scale Coastal Fishery in Western Madagascar ( Masters dissertation, University of Bangor). University of Bangor, Wales
Ding Q., Chen X., Hilborn R., Chen Y. (2017). Vulnerability to Impacts of Climate Change on Marine Fisheries and Food Security. Mar. Policy, 83, 55–61. doi: 10.1016/j.marpol.2017.05.011
Doukakis P., Jonahson M., Ramahery V., De Dieu Randriamanantsoa B., Harding S. (2009). Traditional Fisheries of Antongil Bay, Madagascar, Western Indian Ocean Journal of Marine Science. Western. Indian Ocean. Mar. Sci. Assoc. 6 (2) pp 175–181. doi: 10.4314/wiojms.v6i2.48237
Eder J. F. (2005). Coastal Resource Management and Social Differences in Philippine Fishing Communities. Hum. Ecol. 33 (2), 147–169. doi: 10.1007/s10745-005-2430-Z
Eigaard O. R., Marchal P., Gislason H., Rijnsdorp A. D. (2014). Technological Development and Fisheries Management,Reviews in Fisheries Science & Aquaculture Vol. 22 ( Taylor & Francis) , 156–174. doi: 10.1080/23308249.2014.899557
Essington T. E., Beaudreau A. H., Wiedenmann J. (2006). Fishing Through Marine Food Webs. Proc. Natl. Acad. Sci. 103 (9), 3171–3175. doi: 10.1073/pnas.0510964103
Fabinyi M. (2010). The Intensification of Fishing and the Rise of Tourism: Competing Coastal Livelihoods in the Calamianes Islands, Philippines. Hum. Ecol. 38 (3), 415–427. doi: 10.1007/s10745-010-9329-z
Froese R., Pauly D. (2000). FishBase 2000: Concepts, Designs and Data Source ( WorldFish) ICLARM, Los Banos, Laguna, Philippines. https://hdl.handle.net/20.500.12348/2428
Gilchrist H., Rocliffe S., Anderson L. G., Gough C. L. A. (2020). Reef Fish Biomass Recovery Within Community-Managed No Take Zones,Ocean and Coastal Management Vol. 192 ( Elsevier Ltd), 105210. doi: 10.1016/j.ocecoaman.2020.105210
Gough C., Beriziny T., Humber F., Harris A., Cripps G., Peabody S. (2009). Vezo Fishing : An Introduction to the Methods Used by Fishers in Andavadoaka Southwest Madagascar ( Blue Ventures) Blue Ventures, London, UK. 7:317
Gough C. L., Dewar K. M., Godley B. J., Zafindranosy E., Broderick C. (2020). Evidence of Overfishing in Small-Scale Fisheries in Madagascar. Front. Mar. Sci. Mar. Sci., 1–17. doi: 10.3389/fmars.2020.00317
Hiatt R. W., Strasburg D. W. (1960). Ecological Relationships of the Fish Fauna on Coral Reefs of the Marshall Islands, Ecological Society of America. Ecological Monographs 30, 1, 65–127. doi: 10.2307/1942181
Hilborn R., Hilborn U. (2019). Ocean Recovery: A Sustainable Future for Global Fisheries?, Oxford University Press. Ocean. Recovery. p181 doi: 10.1093/oso/9780198839767.001.0001
Hobson E. (1974). Feeding Relationships of Teleostean Fishes on Coral Reefs in Kona, Hawaii. Fishery. Bull. 72, 915–1031. link https://spo.nmfs.noaa.gov/sites/default/files/pdf-content/1974/724/hobson.pdf
Horning N. R. (2008). Strong Support for Weak Performance: Donor Competition in Madagascar. African Affairs, 107(428), 405–431. 10.1093/AFRAF/ADN036
Humber F., Andriamahaino E. T., Beriziny T., Botosoamananto R., Godley B. J., Gough C., et al. (2017a). Assessing the Small-Scale Shark Fishery of Madagascar Through Community-Based Monitoring and Knowledge. Oryx, Fisheries. Res. 186, 131–143. doi: 10.1016/j.fishres.2016.08.012
Humber F., Godley B. J., Nicolas T., Raynaud O., Pichon F., Broderick A. (2017b). Placing Madagascars Marine Turtle Populations in a Regional Context Using Community-Based Monitoring, Oryx Vol. 51 ( Cambridge University Press), 542–553. doi: 10.1017/S0030605315001398
Humber F., Godley B. J., Ramahery V., Broderick A. C. (2011). Using Community Members to Assess Artisanal Fisheries: The Marine Turtle Fishery in Madagascar. Anim. Conserv. 14 (2), 175–185. doi: 10.1111/j.1469-1795.2010.00413.x
INSTAT (1997). Recensement General De La Population Et De L’habitat - Etat De La Population (Antananarivo, Madagascar). Available at: https://www.instat.mg.
INSTAT (2020). Résultats Globaux Du Recensement Général De La Population Et De Lhabitation De 2018 De Madagascar - (RGPH-3) (Antananarivo, Madagascar). Available at: https://www.instat.mg/wp-content/uploads/Resultat-globaux-RGPH3-Tome-01.pdf.
International Monetary Fund (2019) World Economic Outlook Database. Available at: https://www.imf.org/external/pubs/ft/weo/2019/02/weodata/index.aspx.
Jackson J. B. C., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., et al. (2001). Historical Overfishing and the Recent Collapse of Coastal Ecosystems, Science. Am. Assoc. Advancement. Sci., 293629–637. doi: 10.1126/science.1059199
Jacquet J., Pauly D. (2008). Funding Priorities: Big Barriers to Small-Scale Fisheries. Conserv. Biol. 22 (4), 832–835. doi: 10.1111/j.1523-1739.2008.00978.x
Jennings S. (2005). Indicators to Support an Ecosystem Approach to Fisheries. Fish. Fisheries. 6 (3), 212–232. doi: 10.1111/j.1467-2979.2005.00189.x
Jennings S., Greenstreet S. P. R., Reynolds J. D. (1999). Structural Change in an Exploited Fish Community: A Consequence of Differential Fishing Effects on Species With Contrasting Life Histories. J. Anim. Ecol. 68 (3), 617–627. doi: 10.1046/j.1365-2656.1999.00312.x
Jennings S., Kaiser M. J. (1998). The Effects of Fishing on Marine Ecosystems, Advances in Marine Biology. Acad. Press. 34, 201–352. doi: 10.1016/S0065-2881(08)60212-6
Jones B. (2011). Socio-Economic Monitoring: A Baseline Assessment of the Fishing Villages of the Kirindy-Mite MPA ( Blue Ventures), Blue Ventures, London, UK.
Lam V. W. Y., Cheung W. W. L., Reygondeau G., Rashid Sumaila U. (2016). Projected Change in Global Fisheries Revenues Under Climate Change. Sci. Rep. 6, 6–13. doi: 10.1038/srep32607
Laroche J., Ramananarivo N. (1995). A Preliminary Survey of the Artisanal Fishery on Coral Reefs of the Tulear Region (Southwest Madagascar). Coral. Reefs. 14, 193–200. doi: 10.1007/BF00334341
Laroche J., Razanoelisoa J., Fauroux E., Rabenevanana M. W. (1997). The Reef Fisheries Surrounding the South-West Coastal Cities of Madagascar. Fisheries. Manage. Ecol. 4 (5023), 285–299. doi: 10.1046/j.1365-2400.1997.00051.x
Le Manach F., Gough C., Harris A., Humber F., Harper S., Zeller D. (2012). Unreported Fishing, Hungry People and Political Turmoil: The Recipe for a Food Security Crisis in Madagascar? Fisheries Centre Research Reports 2011, 19(4). University of British Columbia, Canada Mar. Policy. Pergamon. 36(1), 218–225. doi: 10.1016/J.MARPOL.2011.05.007
Le Manach F., Gough C., Humber F., Harper S., Zeller D. (2011). Reconstruction of Total Marine Fisheries Catches for Madagascar, (1950-2008) ( Sea Around Us). doi: 10.1017/CBO9781107415324.004
Le Reste L. (1970). Biologie de Acetes Erythraeus (Sergestidae) dans une baie du N.W de Madagascar (Baie d’Ambaro). ORSTOM, 8(2).
Lilette V. (2006). Mixed Results: Conservation of the Marine Turtle and the Red-Tailed Tropicbird by Vezo Semi-Nomadic Fishers. Conserv. Soc. 4 (2), 262–286. http://www.jstor.org/stable/26396661
Mangi S. C. C., Roberts C. M. M. (2006). Quantifying the Environmental Impacts of Artisanal Fishing Gear on Kenyas Coral Reef Ecosystems, Marine Pollution Bulletin, 52(12), 1646–1660. doi: 10.1016/j.marpolbul.2006.06.006
Marikandia M. (2001) The Vezo of the Fiherena Coast, Southwest Madagascar: Yesterday and Today, Ethnohistory, 48, 157–170. doi: 10.1215/00141801-48-1-2-157
McClanahan T. R., Mangi S. C. (2004) Gear-Based Management of a Tropical Artisanal Fishery Based on Species Selectivity and Capture Size, Fisheries Management and Ecology. 11(1), 51–60. doi: 10.1111/j.1365-2400.2004.00358.x
McVean A. R, Hemery G., Walker R. C. J., Ralisaona B. L. R., Fanning E. (2005) Traditional Sea Cucumber Fisheries in Southwest Madagascar: A Case-Study of Two Villages in 2002, Beche-De-Mer Information Bulletin, (21), 15–18. Available at: http://www.spc.int/DigitalLibrary/Doc/FAME/InfoBull/BDM/21/BDM21_15_McVean.pdf
McVean A. R., Walker R. C. J., Fanning E. (2006) The Traditional Shark Fisheries of Southwest Madgascar: A study in the Toliara region, Fisheries Research, 82(1–3), 280–289. doi: 10.1016/j.fishres.2006.06.016
MIHARI network (2017a) Motion- Creation of an Exclusive Fishing Zone for Small-Scale Fishers on the Coastal Strip. Available at: https://mihari-network.org/wp-content/uploads/2018/06/Motion-3-Creation-of-an-exclusive-fishing-zone-for-small-scale-fishers-on-the-coastal-strip.pdf
MIHARI network (2017b) Motion - Formalisation and Implementation of Dina in Natural Resource Management. Available at: https://mihari-network.org/wp-content/uploads/2018/06/Motion-1-Formalization-and-implementation-of-dina-in-natural-resource-management.pdf
MIHARI network (2017c) Motion - Regulation of Fishing Gear. Available at: https://mihari-network.org/wp-content/uploads/2018/06/Motion-2-–-Regulation-of-fishing-gear.pdf
Moran D. D., Wackernagel M., Kitzes J. A., Goldfinger S. H., Boutaud A. (2008) Measuring Sustainable Development - Nation by Nation. Ecological Economics Elsevier. 64(3), 470–474. Available at 10.1016/j.ecolecon.2007.08.017
Morato T., Watson R., Pitcher T. J., Pauly D. (2006) Fishing Down the Deep, Fish and Fisheries, 7(1), 24–34. doi: 10.1111/j.1467-2979.2006.00205.x
Padilla J.E., Mamauag S., Braganza G., Brucal N., Yu D, Morales A(2003). Sustainability Assessment of the Live Reef-Fish for Food Industry in Palawan, Philippines.Philippines: World Wildlife Fund.
Pauly D., Christensen V., Dalsgaard J., Froese R, Torres Jr F. (1998) Fishing Down Marine Food Webs, Science, 279(5352), 860–863. doi: 10.1126/science.279.5352.860
Pendleton L. H., Beyer H., Estradivari Grose S. O., Hoegh-Guldberg O., Karcher D. B., Kennedt E. (2019) Disrupting Data Sharing for a Healthier Ocean, ICES Journal of Marine Science, 76 (6), 1415–1423. doi: 10.1093/icesjms/fsz068
Rijnsdorp A. D., Daan N., Dekker W. (2006) Partial Fishing Mortality Per Fishing Trip: A Useful Indicator of Effective Fishing Effort in Mixed Demersal Fisheries, ICES Journal of Marine Science. Oxford University Press, 63(3), 556–566. doi: 10.1016/j.icesjms.2005.10.003
Sadovy Y. (2005) Trouble on the Reef: The Imperative for Managing Vulnerable and Valuable fisheries, Fish and Fisheries. John Wiley & Sons, Ltd (10.1111), 6(3), 167–185. doi: 10.1111/j.1467-2979.2005.00186.x
Sanders E. F. (2005) Fombandrazana Vezo: Ethnic Identity and Subsistence Strategies Among Coastal Fishers of Western Madagascar. ( Doctoral dissertation, University of Georgia)
Sethi S. A., Branch T. A., Watson R. (2010) Global Fishery Development Patterns Are Driven by Profit But not Trophic Level, Proceedings of the National Academy of Sciences of the United States of America, 107(27), 12163–12167. doi: 10.1073/pnas.1003236107
Stergiou K. I., Tsikliras A. C. (2011) Fishing Down, Fishing Through and Fishing Up: Fundamental Process Versus Technical Details, Marine Ecology Progress Series, 441, 295–301. doi:10.3354/meps09377
Keywords: trophic level, CPUE, fishing down marine food webs, overfishing, traditional fishers, participatory monitoring
Citation: Gough C, Ateweberhan M, Godley BJ, Harris A, Le Manach F, Zafindranosy E and Broderick AC (2022) Temporal Change and Fishing Down Food Webs in Small-Scale Fisheries in Morondava, Madagascar. Front. Mar. Sci. 9:884083. doi: 10.3389/fmars.2022.884083
Received: 25 February 2022; Accepted: 01 June 2022;
Published: 26 July 2022.
Edited by:
Pierre Failler, University of Portsmouth, United KingdomReviewed by:
Lida Teneva, Independent Researcher, Sacramento, CA, United StatesGildas Todinanahary, Université de Toliara, Madagascar
Copyright © 2022 Gough, Ateweberhan, Godley, Harris, Le Manach, Zafindranosy and Broderick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlotte Gough, charlie@blueventures.org
 Charlotte Gough
Charlotte Gough Mebrahtu Ateweberhan
Mebrahtu Ateweberhan Brendan J. Godley
Brendan J. Godley Alasdair Harris1
Alasdair Harris1  Frédéric Le Manach
Frédéric Le Manach Annette C. Broderick
Annette C. Broderick