- 1Aquarium of the Pacific, Long Beach, CA, United States
- 2Research and Conservation, Georgia Aquarium, Atlanta, GA, United States
- 3Wildlife Genetics, Department of Comparative Medicine, Loyola University Medical School, Maywood, IL, United States
- 4Delaware Biotechnology Institute, Center for Bioinformatics & Computational Biology, University of Delaware, Newark, DE, United States
Maintaining self-sustaining populations of zoo and aquarium collections can be challenged when natural reproduction fails within mixed-sex populations; however, reproductive success can sometimes be restored with the application of reproductive technologies. Among a population of three female and one male Zebra Sharks (Stegostoma tigrinum), production of young failed despite constant male presence with two of the females. To determine if assisted techniques could be used to rescue sexual reproduction, artificial insemination was performed in a singleton female twice over a three-year period using freshly collected semen. Hatching success for eggs laid by all three females was monitored to compare natural and artificial insemination modes. After the first insemination (December 15th, 2011), 143 yolked eggs resulted in no sexually produced offspring and four genetically-confirmed, parthenogenetic offspring. After the second insemination (September 24th, 2013), 62 yolked eggs resulted in two sexually produced offspring, 18 and 33 days after insemination, and three parthenogenetic offspring > 213 days post-insemination. For the two females housed with the male, no sexual offspring resulted. All females produced at least one hatched parthenote. This study successfully employed artificial insemination to circumvent barriers to natural reproduction in Zebra Sharks. With further development, artificial insemination represents a powerful tool that could be used for maintaining genetic diversity for animals housed in aquaria and conservation-based breeding programs for elasmobranchs.
Introduction
Reproduction of animals in human care sometimes requires more than simple cohabitation of females and males as the physiological and behavioral processes involved can be complex (Ottinger and Mench, 1989). When natural reproduction fails, assisted techniques can be employed to overcome barriers. For example, artificial insemination (AI) is one of many reproductive technologies used to aid sexual reproduction in domestic and wildlife species (Malecki et al., 2008; Faigl et al., 2012). In its simplest form, AI is the process by which semen collected from a male donor is placed in the reproductive tract of a female. Thus, AI physically decouples the process of mating for species employing internal fertilization, which can offer alternatives when the barrier to reproduction is a lack of or inappropriate mating behavior (Huang et al., 2002; Zhang et al., 2004). AI also has implications for improved animal welfare as mating can present risks to one or both sexes (Ritter et al., 2019).
Many aspects of elasmobranch (sharks and rays) biology make them good candidates for implementation of artificial insemination for their care and management in zoos and aquariums. For example, copulation in elasmobranchs can be physically detrimental, especially to females (reviewed in Pratt et al., 2005). The risk of injury often leads to single-sexed populations being maintained in aquaria. While maintaining male-only or female-only collections minimizes risk of mating related injury and allows for population control, this challenges the ability to maintain genetically diverse populations across zoos and aquariums. In other wildlife species, AI has been used to overcome similar reproductive challenges, especially for species in peril (Blanco et al., 2009; Rodger et al., 2009; Howard et al., 2016), and may be an option for reproductive challenges for elasmobranchs in human care (Daly and Jones, 2017).
To date, AI has been attempted in only a handful of elasmobranch species with mixed success. A team of Japanese scientists reported successful AI of Cloudy Catsharks (Scyliorhinus torazame) in 1995 and Whitespotted Bamboo Sharks (Chiloscyllium plagiosum) in 1998, with fertility successes of ~77% and ~23%, respectively (Masuda et al., 2003). In 2005, Australian researchers attempted to synchronize AI with ovulation using sonography in Broadnose Sevengill Sharks (Notorynchus cepedianus), but failed to produce a pregnancy, despite documentation of ovulation (Daly and Jones, 2017). The cause of failure in this case was not identified but may have been due to errors in timing of insemination, semen quality, or method of insemination. More recently, Wyffels et al. (2021) reported success of AI in Whitespotted Bamboo Sharks by confirming sireship genetically rather than on fertility alone. However, in this study, a small number of offspring (1.1%) were produced by parthenogenesis, suggesting that presence of semen alone does not guarantee sexual reproduction. Parthenogenesis is the process whereby females produce viable offspring without genetic input from male conspecifics. Parthenogenesis has been documented across vertebrate species (see reviews: Booth and Schuett, 2016; Ramachandran and McDaniel, 2018), and has been observed in a number of elasmobranchs held in human care (Chapman et al., 2007; Feldheim et al., 2010; Harmon et al., 2016). In aquaria, parthenogenesis is documented when female-only populations (or individuals) give birth or lay fertile eggs; however, parthenogenesis has also been observed in the natural environment in endangered Smalltooth Sawfish (Pristis pectinata) (Fields et al., 2015). In both of these cases, parthenogenesis is hypothesized to occur due to a lack of male interaction, either by design in aquaria or due to population impacts leading to low encounter rates between males and females in the wild. Nevertheless, the co-occurrence of parthenogenesis and sexual reproduction for inseminated Whitespotted Bamboo Sharks as shown by Wyffels et al. (2021), represents a potential hurdle for implementation of AI that reliably results in sexually-produced offspring.
Development and use of AI in elasmobranchs also has conservation implications, given that many shark and ray species are imperiled (Dulvy et al., 2021), and aquariums and zoos are striving for sustainable methods of maintaining their collections. The option to breed animals in aquaria to produce genetically diverse offspring removes the need of institutions to source their collection from in situ populations and is an aim of some aquarium-driven conservation efforts (e.g. Wyffels et al., 2020a). Species like Zebra Sharks (Stegostoma tigrinum, formally Stegostoma fasciatum) that are both popular in collections and listed as endangered by the International Union for Conservation of Nature Red List of Threatened Species (Dudgeon et al., 2019) may serve as a good animal model for how AI can be used to achieve conservation goals as well as overcome reproductive hurdles as many institutions maintain individual animals or single-sex populations. Even when males and females are housed together, natural reproduction has had mixed success (Watson and Janse, 2017). In particular, successful natural reproduction of Zebra Sharks at Aquarium of the Pacific has failed to occur, despite cohabitation of a mature male with two mature females for 19 consecutive months. During this time, females laid yolked eggs, but none successfully hatched. Therefore, the goal of this study was to determine if sexual reproduction could be restored with implementation of assisted techniques. Two AI trials were conducted using freshly collected semen and the duration of fertility (i.e. length of time eggs with embryos were laid) was compared between females with physical access to the male and one isolated with access only to semen. Through these trials, we demonstrate the potential for AI to be used as an important tool for circumventing apparent barriers to natural reproduction in this species.
Materials and Methods
Husbandry
Zebra Sharks were housed in three different enclosures at Aquarium of the Pacific during the course of the study (Supplemental Table 1). All animals were obtained from Queensland, Australia, in 1992 (Fern – inseminated female), 2002 (Yin and Yang – females), and 2005 (Carlbe – male) and by the time of the study all were of adult length for females (> 169 cm total length) and males (> 147 cm total length) (Compagno, 2002) (Supplemental Table 2). The Zebra Shark male (Carlbe) was housed with two females (Yin and Yang) for the duration of the study in an approximately 1.4 million liter mixed tropical fish exhibit (Tropical Reef, “TR”), except for 5 months where he was isolated in an outdoor enclosure (0.25 million liters, “Holding 1”) between Nov 2012 and March 2013 and in 2014 from September to December (Supplemental Table 1). Tropical Reef is an indoor exhibit with artificial lighting (12 hr light:12 hr dark) and supplemented by natural light via skylights above the exhibit. He served as the semen donor for a third female (Fern) housed separately from the other Zebra Sharks in an outdoor ~0.41 million liter enclosure (Shark Lagoon, “SL”) seven months prior to the first insemination attempt and subsequently for duration of the study. All exhibits were filled with natural, filtered seawater kept at 23.8 – 25°C. Diet consisted of thawed seafood including shrimp, clam, squid and capelin fed at ~7% percent of their body weight weekly, along with a multivitamin supplement tablet (Mazuri Vita-Zu Shark/Ray; formula 5M24).
Semen Collection and Artificial Insemination
Semen collection followed by AI was conducted twice during the study. In both cases, the male was guided by divers into a vinyl stretcher at the water surface. For the first collection, the male was restrained manually after inducing tonic immobility, and for the second collection, he was chemically sedated with MS-222 (75 mg/L). In preparation for semen collection, the male was rotated into dorsal recumbency and the cloaca elevated above the water. The pelvic fins and claspers were splayed laterally to expose the urogenital papilla. External pressure was applied bilaterally to the body wall overlying the ampullae and semen was expressed (1-2 mL in 2011 and 8 mL in 2013) into a sterile vial directly from the urogenital papilla. After collection, semen was stored at room temperature (22 – 25°C), which was similar to the animal’s exhibit temperature, for ~30 min prior to insemination. Ten minutes before the first AI procedure, 1-2 mL of semen was mixed with 10 mL of sterile seawater to increase volume available for delivery. For the second AI procedure, undiluted, raw semen (8 mL) was used.
Artificial insemination was conducted on December 15th, 2011, and again 649 days later (~22 months) on September 24th, 2013, in the same female (Fern). Fern had been actively laying eggs for at least one month prior to each insemination as evidenced by egg cases collected during husbandry cleaning of the habitat. Before the first insemination procedure, eggs were observed in both uteri and during the second insemination procedure, eggs were observed in the right cervix. In preparation for AI, divers guided the female into a vinyl stretcher at the water surface. She was rotated dorsoventrally in the stretcher to induce tonic immobility and her cloaca elevated out of the water. The inseminate was placed caudal to the nidamental gland in each of the paired oviducts using a syringe and catheter (10-French 45 cm polypropylene) inserted via the cloaca and advanced approximately 30 cm past the cervix of each oviduct as determined by digital palpation. There was moderate resistance to cloacal palpation during the 2011 procedure; however, for the 2013 procedure, the cervix was easily identified due to an obstructing egg case. Placement of one gloved finger through the cervix next to the egg case allowed positioning and advancement of the catheter into the oviducts. For the first insemination (2011), 5 mL of seawater-diluted semen was deposited in each oviduct, while 5 mL and 3 mL of undiluted semen was deposited in the right and left oviduct, respectively, for the second insemination (2013). No flushing of the catheter with additional seawater was performed after the insemination. After the AI procedure (5-15 min), Fern was rotated upright and released into the husbandry pool. She was allowed access to the exhibit after demonstrating normal swimming and navigating behavior and was then subsequently observed for 15 min. Fern was visually observed by the attending veterinarian daily for 1 week after the procedure as well as twice daily over the course of the study for any behavioral or appetite changes.
Egg Collection and Monitoring
Oviposition was monitored for both the artificially inseminated female (Fern) and the presumed naturally inseminated females (Yin and Yang) from January 2012, when egg laying activities began after the first insemination attempt, until Oct 16th, 2014, 388 days after the second insemination attempt. Females laid yolked and non-yolked (wind) egg cases; however, records of wind egg cases were not kept in this study. When a yolked egg case was discovered, the date of laying was recorded and the egg was externally tagged with a unique ID number. In some cases, the date of laying could be ascribed to a particular week rather than day; in this case, date of laying was attributed to four days before (i.e. approximate half-way point between weeks). Eggs were assigned maternity by exhibit to either Tropical Reef (Yin or Yang) or Shark Lagoon (Fern). If eggs could not be confirmed to either of these locations (i.e. laying exhibit was not recorded) or the yolk status of the egg was ambiguous (i.e. no records of whether yolk deterioration occurred or not), the egg data was removed from subsequent analyses.
After tagging, egg cases were incubated in floating baskets in a 4,000 liter, closed-recirculating system kept on artificial lighting (12 hr light:12 h dark) between 23.8 – 25°C. Eggs were examined weekly by candling using an underwater flashlight to determine the presence or absence of an embryo as well as status of the yolk (intact or deteriorating). If an embryo was detected, the date of its first observance and confirmation of movement during weekly checks was recorded. Eggs with broken, deteriorating yolks were removed from the tank and the discard date recorded. During the course of embryonic development, date of eclosion (i.e. point at which respiratory slits opened to flow-through seawater), embryo outcome (hatched or died before hatch), and date of hatching were noted. For embryos that died before hatching, a sample of embryonic tissue for genetic testing (see below) was collected except in cases of advanced autolysis. For hatchlings, a whole blood sample was collected and frozen at -80°C for paternity testing.
Paternity Testing
Whole blood or fin clip samples of hatchlings and adults as well as embryonic tissue samples were sent to the Loyola Wildlife Genetics Lab for paternity testing. Blood was sampled from the caudal vein from all animals (< 1 mL). Blood (5-10 μL) and fin-clip tissue samples were incubated at 37°C overnight with 5–10 U Proteinase K and DNA extracted following the protocol for the Puregene Core Kit A (Qiagen Sciences). Seven microsatellite loci developed for Zebra Sharks were amplified (Dudgeon et al., 2006). These loci were chosen because they showed high levels of polymorphism that would help identify the sire for each offspring. All loci were amplified in 12.5 μl volumes containing 1.2 μL 10X buffer, 2 mM MgCl2, 0.2 mM dNTPs, 5 pmol of each primer, 0.5 units of Taq polymerase (Promega, Corp) and 40-60 ng of template DNA. The program parameters were 95°C for 40 s, 60°C for 45 s and 72°C for 45 s for 35 cycles, followed by a seven min extension at 72°C for Sf41, Sf72, Sfa236, and Sfa248 in a Bio-rad iCycler (Bio-rad Laboratories, Hercules, CA). The annealing temperature was decreased to 57°C for Sfa382 and Sfa454 and to 52°C for Sfa205. Forward primers were fluorescently labeled with WELLRED™ dyes for analysis on a Beckman/Coulter CEQ 8000 capillary electrophoresis system (Beckman Coulter, Fullerton, California, USA) with system software version 8.0 (Beckman Coulter, Inc.). To verify correct assignment of microsatellite alleles, 10% of the total sample set was reamplified and run to confirm genotype consistency.
Data Analysis
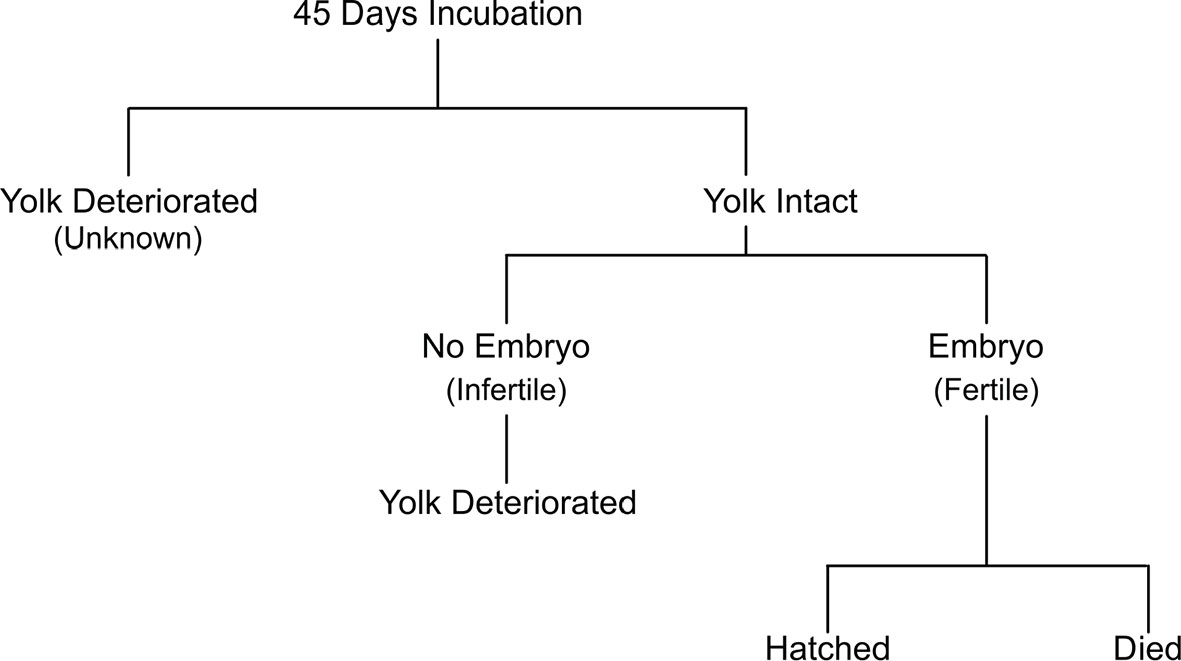
Where applicable, the number of days between oviposition and: yolk deterioration, first embryo detection, duration of development for expired embryos, eclosion, and/or hatching were calculated for each egg. Yolk deterioration is the loss of vitelline membrane integrity and recognizable as an irregular shaped yolk mass with non-homogeneous movement of the yolk inside of the egg case as it is rotated during candling. Since loss of yolk integrity complicated the ability to identify early-stage embryos, egg fertility was assigned using the following criteria: Fertile (visual confirmation of a moving embryo), Infertile (intact yolk and no embryo detection after 45 days post-laying), or Unknown (breakdown prior to 45 days) (Figure 1). The 45-day threshold for fertility assignments represents the average time at ~25°C to observe a developing embryo. Because fertility could not be confirmed or denied in Unknown eggs, a minimum fertility rate was calculated for each exhibit representing the percentage of eggs with embryos out of the number of yolked eggs. Hatchability represents the proportion of hatched embryos out of the number of yolked eggs. Embryo outcomes were designated as “hatched” or “died before hatch”. A clutch was defined as eggs laid on the same day and the difference between laying days was used to calculate mean and standard deviation of time between laying.
The interval (days) between AI and oviposition of Fertile eggs with either sexually or unisexually produced offspring was calculated for eggs laid by the inseminated female (Fern). Minimum and maximum fertility and hatchability was compared between females housed with the male and the female artificially inseminated as well as rate of sexually-produced and unisexually-produced (parthenogenetic) offspring. Minimum and maximum rates of parthenogenesis were calculated as a proportion of genetically confirmed or both genetically confirmed and not tested embryos to total yolked eggs, respectively. In addition, rate of yolk deterioration, embryo mortality, and hatching was determined for each female (Fern) or exhibit (Yin and Yang).
Results
Welfare
After two AI procedures, no behavioral problems were observed for either the male (Carlbe) or the female (Fern) post-handling as both exhibited normal swimming behaviors and resumed feeding at station within 24 hours. Fern was examined 60 days after the second procedure and no symptoms of infection nor abnormal findings were observed.
Sperm Collection and Sample Quality
Semen was expressed when pressure was applied both laterally and ventrally to the ampulla/seminal vesicle during both collection attempts; however, under sedation, the male was easier to handle without fin clamping to collect semen. Sperm motility was confirmed for both raw ejaculates prior to insemination, but not after the addition of seawater which activates motility of shark sperm (Wyffels et al., 2020b; Wyffels et al., 2021). Motility was visually observed using a phase contrast microscope at 400x magnification; however, detailed information quantifying percent motile sperm was not recorded in this retrospective study. Most sperm were individual and motile rather than aggregated in spermatozuegmata.
Genetics
Among the seven microsatellite loci used, the adults were highly polymorphic (heterozygous for 6 or all 7 loci; Supplemental Table 3). The number of alleles per locus seen in the four adults ranged from eight alleles for three markers, five, six and seven alleles for one marker each, and only two alleles for the seventh marker. The parthenogenetic offspring were homozygous at all loci (Table 2).
Artificial Insemination Efficacy
After the December 15th 2011 AI, Fern suspended egg laying for 22 days and laid only five yolked eggs between January 6th (first egg laid post-AI) and February 28th, 2012 (Figure 2). In the year following the 2011 AI, Fern laid 77 yolked eggs with a majority Infertile (n = 36, 47%), followed by Unknowns (n = 34, 44%) and Fertile (n = 7, 9%). Considering the seven Fertile eggs, three hatched and four embryos perished before hatching. Two of the three hatchlings were from the first two yolked eggs laid post-AI and were presumed to be parthenotes, while the third hatchling was genetically confirmed to be a parthenote (Table 1 and Supplemental Table 3). The four embryos that died before hatching were unable to be tested for sireship due to advanced post-mortem autolysis. The lack of genetic evidence of sexually produced embryos or hatchlings, the 2011 AI was deemed unsuccessful.
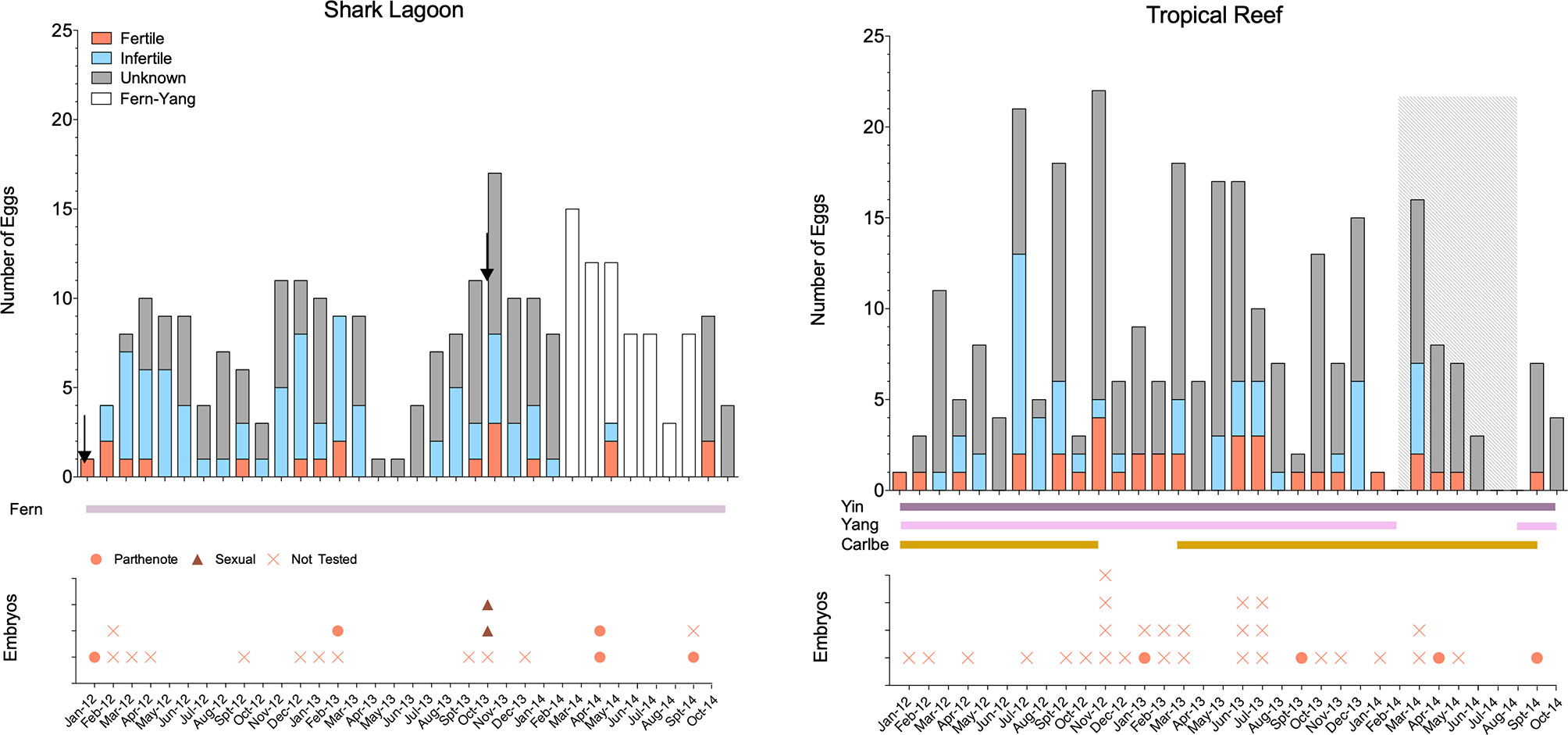
Figure 2 Yolked egg laying activity by month-year for Fern in Shark Lagoon (Left) and Yin and Yang in Tropical Reef (Right). Egg assignment into Infertile, Fertile and Unknown categories is based on the hierarchy shown in Figure 1. Shark residency for each exhibit is shown in solid, horizontal bars at the bottom of each plot. From February 2014 until August 2014 (gray rectangle) select eggs (Fern and Yang, open bars shown on left plot) from both exhibits were incubated together, precluding confirmation of dam, and were not included in analyses. Artificial inseminations performed in Fern (left) are show with arrows. Corresponding plots of hatched and unhatched embryos with genetic assignment are shown for each exhibit by month.
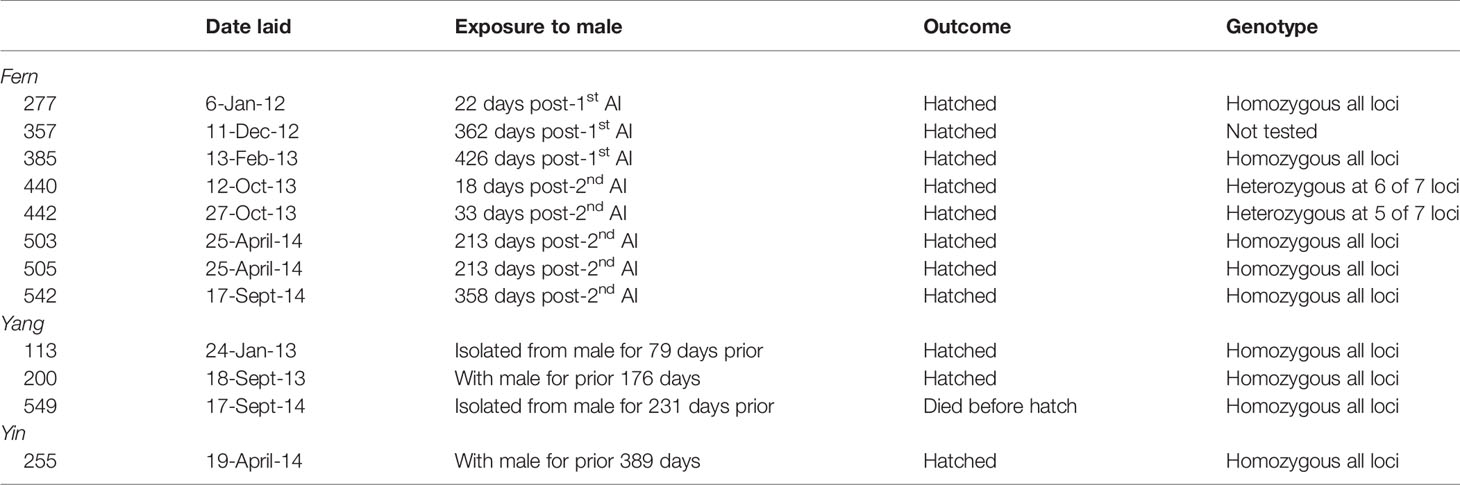
Table 1 Paternity of embryos and hatchlings by dam with days since possible semen exposure. Egg identification numbers are listed below each dam.
By contrast, the 2013 AI was successful and resulted in two sexually-produced offspring. Fern did not suspend oviposition after AI and laid a clutch 10 days post-procedure. During the latter part of the study (February – August 2014), Yang was moved to Holding 1 located near Shark Reef. Since eggs laid by either Fern or Yang at this time were incubated in the same basket without distinguishing eggs by female, these data were not included in subsequent analyses (n = 63) and represent a modest percentage (~15%) of total eggs. Fern laid 61 eggs during the year after the 2013 AI that could be confidently assigned to her. The majority were assigned an Unknown fertility status (n = 40, 66%), followed by Infertile (n = 13, 21%) and Fertile eggs (n = 8, 13%). Five of eight Fertile eggs hatched; two hatchlings were sexually reproduced and three hatchlings were parthenotes. The sexually-produced offspring were from the second and third clutches laid 18 and 33 days post-AI. Of the two Fertile eggs in the second clutch (10 eggs), one embryo died before hatching and the second (sexually-produced) hatched. Similarly, in the third clutch (4 eggs), the only Fertile egg was also sexually produced. In addition to the sexually produced hatchlings, three parthenotes hatched from eggs laid much later, 213 and 358 days post-AI.
Ovarian Activity and Fertility
Oviposition for the three females in two exhibits was monitored continuously for 1,036 days. During that time, 204 yolked eggs were laid in Shark Lagoon (Fern) and 277 were laid in Tropical Reef (Yin and Yang). In both exhibits, egg laying occurred year-round, with no distinct season or rest periods (Figure 2). In TR, the number of yolked eggs peaked during November in 2012 (n = 22) and March in 2013 and 2014 (n = 18 and 16). For Fern in SL, the number of yolked eggs peaked during November (n = 11) and December (n = 11) in 2012, October (n = 17) in 2013, and September (n = 16) in 2014. Defining clutches was difficult since wind eggs were not recorded and exact lay date was not known for every egg. Mean clutch for yolked eggs was 4 ± 3 and 3 ± 2 with eggs laid 15 ± 17 days and 12 ± 16 days for Tropical Reef and Shark Lagoon, respectively.
In both exhibits, the fertility status of a majority of eggs was categorized as Unknown (SL: n = 110, 54%; TR: n = 194, 70%), followed by Infertile (SL: n = 75, 37%; TR: n = 49, 18%), and finally Fertile (SL: n =19, 9%; TR: n = 34, 12%) (Table 2). Because of the high number of Unknown eggs, fecundity reported herein is potentially an underestimation. While the distribution of eggs by fertility status between exhibits was significantly different ( χ2 = 22.343, df = 2, p < 0.0001), minimum fertility (confirmed embryo presence) was not (p = 0.38). A substantial number of eggs experienced loss of yolk integrity during the first months of incubation (Table 2). In particular, for Fertile eggs that died before hatching, approximately 80% lost yolk integrity after the first three months of incubation. Yolk integrity loss for Unknown eggs (23 ± 11 days) was significantly faster than either Infertile (71 ± 19 days) or Fertile eggs (72 ± 20 days; KW2 = 318.12, p <0.0001), the latter of which were not different from each other (p = 0.57; Figure 3). Among Infertile eggs, the longest incubation time where the yolk remained intact was 149 days.
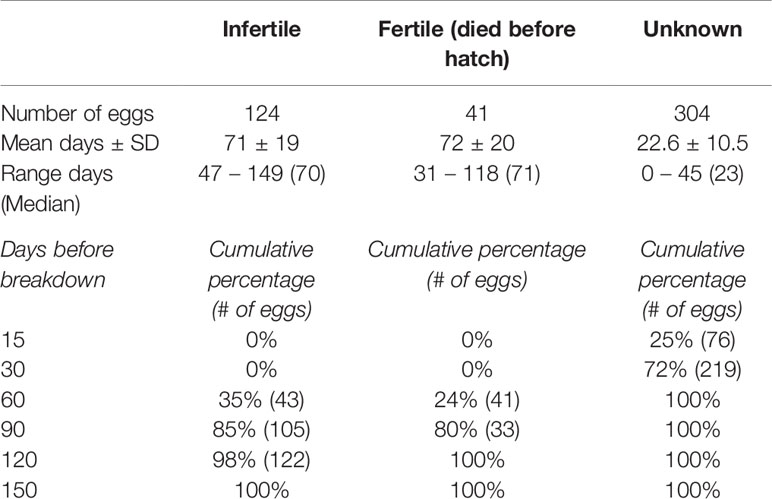
Table 2 Days until yolk deterioration for eggs categorized as Infertile, Fertile and Unknown fertility status.
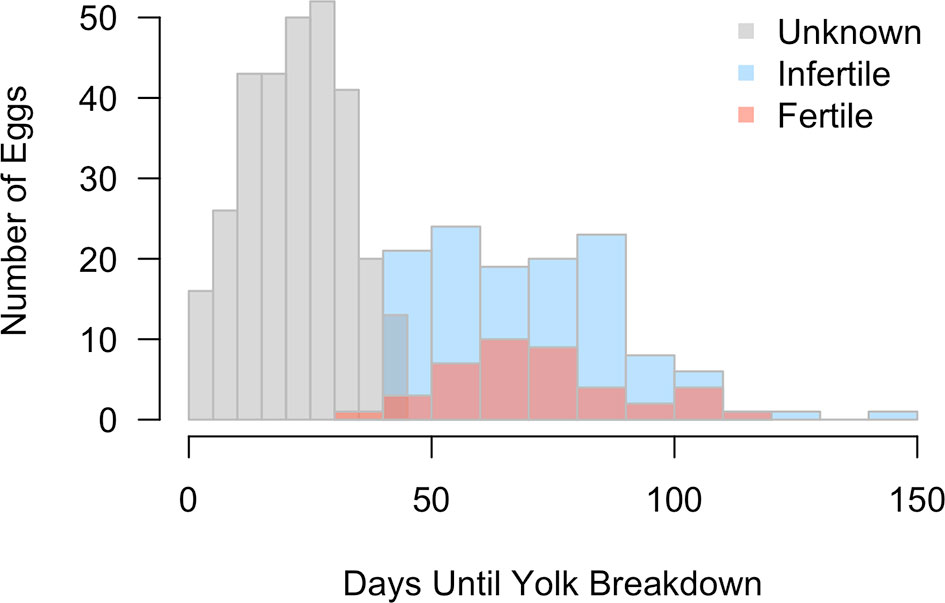
Figure 3 Distribution of days until yolk breakdown is shown for eggs of Fertile (salmon), Infertile (blue) and Unknown fertility status (gray). Yolk breakdown in Unknown eggs was significantly earlier than either confirmed Fertile or Infertile eggs.
All females were genetically confirmed to produce offspring; however, despite presence of the male in Tropical Reef, no sexually produced offspring resulted. Yin, Yang and Fern laid eggs that developed parthenogenetically over the entire course of the study (Table 1). The minimum (genetically confirmed) and maximum rate (includes untested embryos) of parthenogenesis for Fern in SL was 2.5% - 5.9% and 1.4% - 10.8% for Yin and Yang in TR. The pooled rate of parthenogenesis was 1.9% (n = 9) minimally and 8.7% (n = 42) maximally.
Incubation Characteristics
For embryos that hatched, mean time to visually detect developing embryos via candling was 45 ± 12 days. Time to eclosion did not differ between embryos that hatched (68 ± 8 days) and embryos that died before hatch (60 ± 21 days; t16.7 = 1.25, p = 0.2). Mean incubation time was 147 ± 6 days at ~25°C. Hatchability was higher for Fern (9 hatched, 4.4%) than for both females combined in TR (3 hatched, 1%).
Discussion
We demonstrate that AI can be successfully employed in the Zebra Shark and can be used as a tool to circumvent impediments to natural reproduction. At Aquarium of the Pacific, the male failed to sire sexually-produced offspring despite being housed with two reproductively active females. However, when AI was employed, his semen fertilized eggs and yielded hatchlings, suggesting the existence of other barriers to natural reproduction. Neither physical male access nor multiple AIs prevented development via parthenogenesis, indicating that sexual reproduction may be more difficult to guarantee than previously assumed in this species. The success of this work to produce sexual offspring via AI provides a baseline for future work to build upon to further refine this tool for conservation applications in this species.
Timing of Insemination
For many elasmobranch species, reproduction occurs in a distinct season (Wyffels et al., 2020b), such that semen of sufficient quantity and quality is introduced into the female reproductive tract occurs at purposeful times when she is at an appropriately receptive point to result in sexual reproduction. For oviparous species like Zebra Sharks, female ovulatory cycles can be easily tracked passively through the appearance of newly laid yolked egg cases in an animal’s enclosure, while male reproductive cycles are more difficult to monitor passively. Therefore, the challenge for successful reproduction in human care, both naturally and artificially, is to predict and promote the optimal timing and alignment of male and female cycles.
Ideally, reproductive information from natural settings can be leveraged to inform reproduction in aquariums, including appropriate timing of AI (Wyffels et al., 2020b). However, reproductive life history information is deficient for in situ Zebra Sharks (Dudgeon et al., 2019) as it is for many elasmobranch species (Simpfendorfer and Kyne, 2009; Dulvy et al., 2014). In Queensland, Australia, where the most well-studied wild population of Zebra Sharks exists, animals are known to form mixed-sex aggregations predictably and seasonally (Dudgeon et al., 2008; Dudgeon et al., 2009; Dudgeon et al., 2013). While copulation was not observed, some elasmobranch species form mixed-sex aggregations during their mating season and sexually segregate the remainder of the year (Pratt and Carrier, 2001). Thus, if one of the functions of this aggregation of Zebra Sharks is for mating, it suggests that insemination is season specific, and may not occur year-round. Data from aquarium settings corroborates this hypothesis. For example, at the Okinawa Churaumi Aquarium in Japan, Zebra Shark mating activity is observed only in the spring season with oviposition commencing shortly afterwards (Nozu et al., 2018). At Churaumi Aquarium the seawater temperature varies seasonally in this open system and likely contributes to resident female Zebra Sharks having clearly defined laying and resting periods.
In contrast, Zebra Sharks at Aquarium of the Pacific laid eggs nearly year-round with abbreviated and few “rest periods”, making determination of appropriate or optimal time to inseminate, from the female perspective, challenging. At Aquarium of the Pacific water temperature is held constant year-round for all exhibits; however, Fern was exposed to naturally varying day length (Shark Reef is located outside) while Ying, Yang and Carlbe in Tropical Reef have a more constant day:night cycle, although the exhibit has some natural lighting through large skylights. The lack of multiple different external stimuli, which may be important for keeping maintaining a cyclical reproduction (Mull et al., 2008; Wyffels et al. 2020a), may have contributed to a year-round laying cycle, making timing of insemination difficult to determine. Although Fern was actively laying prior to both inseminations, only the second attempt was successful. While it is difficult to speculate factors that led to success in one attempt but not the other, our trials combined with the limited data from in situ and aquarium populations, indicates future efforts should focus insemination trials during active laying periods.
Understanding timing of male reproductive cycling would help ensure the highest quality semen was being used for insemination and may increase the possibility of success. However, active observations of male reproductive status require physical examinations to track changes in testicular anatomy, steroid hormones and semen production (Nozu et al., 2017; Wyffels et al., 2020b). In the present study, the male was not undergoing routine physical examinations to quantify testicular activity and semen assessments were not performed regularly. While semen was assessed for sperm presence and motility prior to insemination, and both extraction attempts were successful, it is unknown whether the months sampled represented a peak in sperm productivity or if there were significant differences in semen quality between the insemination attempts that may have influenced why only the second attempt resulted in sexual reproduction. In other elasmobranch species that reproduce seasonally, semen production (and/or subsequent storage in ampullae) does not occur year-round (Maruska et al., 1996; Rossouw, 2014; Wyffels et al., 2020b). Insemination dose may also be a factor affecting AI success with the failed December 2011 AI delivering 1-2 mL of ejaculate and the successful September 2013 AI delivering 8 mL of ejaculate. Whether dose of semen (sperm number needed to achieve fertilization) or overall semen quality affected AI success is unknown for this study, but both have been shown to impact AI success (Wyffels et al., 2021). However, this study highlights the need to understand timing of both female and male reproductive cycles in order to ensure the best chance of success when employing assisted reproductive techniques.
The ability of females to store sperm could also influence AI success as well as inform the frequency by which these procedures need to be performed to ensure sexual reproduction occurs throughout a female’s laying cycle. In the current study, sexually produced offspring were produced 18 and 33 days post-insemination, suggesting that storage of sperm lasts for at least for one month in this species. In another oviparous elasmobranch species where AI has been successful, sperm storage was documented to occur up to 70 days in the Clearnose Skate Raja eglanteria with a single insemination (Luer et al., 2007) and 121 days post-insemination in the Whitespotted Bamboo Shark (Wyffels et al., 2021). Data are insufficient to evaluate how sperm concentration may have influenced results; however, this study does raise a number of interesting avenues for further research, considering that species where a lower volume of inseminate was introduced into the female reproductive tract resulted in successful hatches of sexually produced offspring. For instance, the small number of sexually produced offspring observed in this study could indicate that either Zebra Sharks have a more limited capacity of storing sperm or that volume or number of sperm inseminated was inadequate and that multiple inseminations might be needed in order for a female Zebra Shark to extend sexual reproduction beyond a month. Future AI studies should investigate timing of insemination, effect of inseminate volume and sperm concentration, and frequency of AI to determine what combination of factors yields the highest frequency of sexually produced young that can be sustained over longer periods post AI.
Furthermore, constituents of the media used to dilute or extend the ejaculate before insemination could be varied as male secretions or female uterine biochemical condition may influence sperm motility, and by extension, reproductive success (Luer et al., 2007). Artificial seawater is a common semen diluent for shark species (Minamikawa and Morisawa, 1996; Wyffels et al., 2021; this study), likely because seawater from male siphon sacs is supposed to help propel semen through the claspers during copulation (Gilbert and Heath, 1972). However, other media have also shown to be successful in AI trials such as elasmobranch modified semen extender (Luer et al., 2007). Luer and colleagues note the potential importance of male-derived fluids in extending the viability of sperm while in the female tract, some of which may be excluded from semen collected manually, depending on the method. Additionally, little is known about how the physiology of the female reproductive tract may influence sperm motility and viability, which could also play an influential role in success of AI. For instance, in the Banded Houndshark Triakis scyllium sperm motility was maintained over a longer time period in uterine fluid from conspecifics and blood plasma than in artificial seawater or artificial uterine fluid (Minamikawa and Morisawa, 1996), suggesting that the female body may play a role in sperm maintenance. The most appropriate semen diluent may be species-specific and reflect physiology and reproductive life history characteristics (García-Salinas et al., 2021; Wyffels et al., 2021). Future work should investigate if artificial seawater is the best diluent for Zebra Sharks or if other media may prove beneficial and lead to greater success in future AI trials.
Fecundity
A consistent and accurate definition of what constitutes a “fertile egg” varies depending on the user. For example, “fertile eggs” have been used to reference any egg with a yolk or eggs with confirmed embryos, despite the fact that these two definitions do not have the same, interchangeable meaning as not every yolked egg will result in embryo development. Fertility, when defined as presence of an embryo, is especially hard to determine if yolk breakdown obscures the ability to visually confirm embryo development, as was the case in this study. To circumvent this issue, the present study introduced a third category (Unknown) by which to classify egg fertility, due to the high incidence of deteriorated yolks observed within the first few weeks after laying when it was not yet possible to detect the presence of an embryo. For eggs that are truly Infertile (i.e. confirmation that no embryo development is taking place), yolks remained intact for up to 149 days (median 70 days). This is in sharp contrast to eggs categorized as Unknown, where by 30 days post-laying, 89% had deteriorated yolks. These two distinct peaks in yolk breakdown (i.e. earlier peak for Unknown eggs and later for Infertile eggs) suggest different biochemical processes may be at play. During development, embryos initiate a series of genetic programming changes as they divide and move through various stages of embryogenesis (Jukam et al., 2017), which can begin even at the one-cell stage in some species (Asami et al., 2022). As embryonic cells divide and epiboly of the yolk commences, this may put the egg on a path of “no return”, particularly once embryo-derived enzymes begin metabolizing egg nutrients (including the yolk) (Yadgary et al., 2011). If development proceeds successfully, embryos are macroscopic and identified via candling in approximately 45 days post-oviposition (this study). However, when embryo development was terminated, autolysis occurred rapidly rendering tissue unusable, and accounts for why tissue from deceased embryos was not useful for paternity testing. Therefore, we hypothesize that the rapid breakdown of yolks for eggs categorized as Unknown may be from failed embryo development. While we are unable to confirm presence of embryos for unknown eggs, future studies could test this hypothesis through thorough examinations of Unknown eggs before disposal. The yolks of Infertile eggs may able to remain relatively static for long periods of time since there are no embryo-derived signals inducing changes to the yolk (Réhault-Godbert et al., 2014; Zhu et al., 2020). Rather, natural breakdown likely is responsible and proceeds at a slower pace, which accounts for the significantly longer breakdown time than for eggs categorized as Unknown.
Females in both TR and SL were more fecund than Zebra Sharks from other aquariums (80-100 eggs per year versus to 20-50 eggs per year) (Robinson et al., 2011; Watson and Janse, 2017; Toledo and Alonso, 2021) but the increased number of eggs did not necessarily result in higher fertility. However, determining the effect this increased output may have on fertility is difficult as a consistent definition of fertility is not used across institutions. In the present study, fertility was assigned when an embryo could be visually confirmed, whereas in some reports, any egg with a yolk is defined as “fertile” (e.g. Kunze and Simmons, 2004; Watson and Janse, 2017). Considering that one-quarter of yolked eggs laid in this study did not result in embryo development, we recommend fertility to be assigned only when an embryo is confirmed (Wyffels et al. 2021). Minimum fertility in this study (12-19%) was comparable to sharks at Burj Al Arab Aquarium in Dubai (10-38% across four years; Robinson et al., 2011) and Loro Parque Aquarium in the Canary Islands (12-21% across five years; Toledo and Alonso, 2021) when this consistent definition of fertility was used. With regards to hatchability, our study fell on the lower end (1-4.4%) compared to Burj Al Arab Aquarium (3-22%) and Loro Parque Aquarium (0-13%). Notably, females from Burj Al Arab Aquarium and Loro Parque Aquarium have a defined and seasonal reproductive cycle and do not lay year-round as those at Aquarium of the Pacific. Egg quality may suffer from protracted oviposition, which may explain the lower hatchability rates if there is a cost to reproducing year round.
Parthenogenesis
In spite of female access to a male or sperm (either naturally or artificially), all females were confirmed to produce at least one parthenogenetic hatchling. In fact, the majority of hatchlings in this study were produced unisexually, indicating that parthenogenesis was the more evolutionarily-successful mode of reproduction. While parthenogenesis in Zebra Sharks has been documented previously (Robinson et al., 2011; Dudgeon et al., 2017), we demonstrate that parthenogenesis occurs despite male presence or when females are provided sperm via AI. Studies in other vertebrates hypothesize that species may “switch” to parthenogenesis during periods of isolation from male conspecifics as a last resort/last ditch effort to allow gene transfer to the next generation (Harmon et al., 2016; Dudgeon et al., 2017). Two females in our study were not isolated from a male and the third (Fern) was isolated for less than a year before AI. Nevertheless, they all continued to produce parthenotes. Fern readily switched between unisexual to sexual reproduction several times during the study. For example, the last hatched parthenogenetic egg of Fern’s before the second insemination was laid 256 days (~8.5 months) prior to the laying of the first sexually produced egg. Only 180 days (6 months) after a sexually produced egg was laid, Fern switched to begin laying parthenogenetic eggs once more. Switching from sexual to unisexual reproduction in less than one year’s time has been documented in Whitespotted Bamboo Sharks (Wyffels et al., 2021) and the Spotted Eagle Ray Aetobatus narinari (Harmon et al., 2016), the former demonstrated switching within 10, 80 and 83 days and the latter which continued to produce parthenogenically in subsequent pregnancies while isolated from mature males. Data from the present study suggests that a single insemination had only a temporary effect of halting unisexual reproduction, and suggests parthenogenesis may be the default mode of development for this species. This hypothesis raises questions about what factors or conditions are needed to override this method of reproduction.
Sireship was confirmed genetically for a majority of hatchlings (save for one deceased embryo), but a large number of developing embryos went untested due to advanced autolysis. Therefore, the maximum rate of parthenogenesis could be higher considering untested embryos from Fertile eggs and if embryo development is indeed occurring in some or all eggs categorized as Unknown fertility status (i.e. deteriorated yolks before 45 days). Parthenogenesis may be more common across species than presently assumed because it tends to be examined on a confirmatory basis when young are produced from females kept in single-sexed housing (Feldheim et al., 2010) rather than on an investigatory basis when young produced in a mixed-sex population where sexual reproduction is assumed to have occurred (Harmon et al., 2016). Compared with Whitespotted Bamboo Sharks, rate of parthenogenesis was slightly higher for the three Zebra Shark females in this study (2%) compared to the at least 6 females genetically confirmed parthenotes in Wyffels et al. (2021) (1.1%); however, if suspected parthenotes are included, the rate among these three Zebra Sharks is substantially higher (8.5%) and closer to presumed rates in parthenogenesis in this species (10-30%; Robinson et al., 2011). Further research should investigate how prevalent parthenogenesis is among elasmobranchs to determine if rates are species-specific or if the rate is more dependent on other environmental factors.
Future Directions
Artificial insemination represents an important tool for the conservation of endangered elasmobranch species. No negative effects were observed for males or females after semen extraction or insemination, although a female Zebra Shark at the Burgers’ Zoo in the Netherlands failed to lay yolked eggs for multiple years following AI (Watson and Janse, 2017). The use of this technique may enable better welfare for animals as male harassment and physical maiming is common and negatively impacts females when mixed-sex populations of Zebra Sharks are maintained (Adams, pers. observation). For animals that are relatively large, like Zebra Sharks, maintaining genetic diversity through physical movement of animals to enable natural reproduction is a logistical hurdle that AI may overcome when semen, instead of animals, is moved between institutions (Wyffels et al., 2021). While the technique requires refinement, AI paired with other reproductive technologies represents an important tool for maintaining genetic diversity of species in zoos and aquariums. In the present study, semen was administered within 30 min of extraction into the female reproductive tract. The development of cold storage protocols may extend the window by which semen is still viable for AI, allowing the opportunity for semen to be transferred between institutions nationally and internationally, thereby greatly expanding the genetic pool of founder individuals (Wyffels et al., 2021). This is especially significant for institutions that maintain female-only populations, which would enable them to genetically contribute to the Species Survival Plan for Zebra Sharks in managed care. Beyond development of cold storage protocols, cryopreservation paired with AI, represents another exciting avenue that would remove temporal barriers to reproduction if female and male reproductive cycles are not synchronous (García-Salinas et al., 2021). This has significant implications for Zebra Sharks as it represents a potential pathway where zoos and aquariums could become self-sustaining and maintain or increase the genetic diversity of their collections. For example, the possibility exists that these technologies could allow semen collected from in situ male Zebra Sharks to be cryopreserved and later used to inseminate ex situ females, obviating the need to remove animals from the wild all together. Artificial insemination as a tool also has implications for in situ conservation efforts. For example, for programs developed to reintroduce this species to areas where they have been locally extirpated, AI could promote genetic diversity in the released founder population from the ex situ brood stock. Considering that Zebra Sharks are endangered, further development of these tools has significant implications for the protection and recovery of this species.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Aquarium of the Pacific Research Advisory Committee.
Author Contributions
LA conceived the study, collected semen and inseminated females. NL, CP, JM, and EL provided husbandry care for sharks, maintained and monitored egg logs. JD conducted paternity testing on young and adults. KL and JW curated and analyzed data. LA, KL and JW interpreted the data, wrote and reviewed the manuscript. All authors approved the final manuscript.
Funding
This study was supported by Aquarium of the Pacific. Open access publication fees were supported by Georgia Aquarium.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to Shara Seals for veterinary technician assistance during artificial insemination procedures and AOP husbandry staff who assisted with handling during procedures and care of the sharks in this study. The authors also thank Allyson Stiles, Amiya Tucker and Mackenzie Lee for assistance in transcribing data into electronic form.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.886616/full#supplementary-material
References
Asami M., Lam B. Y. H., Ma M. K., Rainbow K., Braun S., VerMilyea M. D., et al. (2022). Human Embryonic Genome Activation Initiates at the One-Cell Stage. Cell Stem Cell 29, 209–216.e4. doi: 10.1016/j.stem.2021.11.012
Blanco J. M., Wildt D. E., Höfle U., Voelker W., Donoghue A. M. (2009). Implementing Artificial Insemination as an Effective Tool for Ex Situ Conservation of Endangered Avian Species. Theriogenology 71, 200–213. doi: 10.1016/j.theriogenology.2008.09.019
Booth W., Schuett G. W. (2016). The Emerging Phylogenetic Pattern of Parthenogenesis in Snakes. Biol. J. Linn. Soc 118, 172–186. doi: 10.1111/bij.12744
Chapman D. D., Shivji M. S., Louis E., Sommer J., Fletcher H., Prodöhl P. A. (2007). Virgin Birth in a Hammerhead Shark. Biol. Lett. 3, 425–427. doi: 10.1098/rsbl.2007.0189
Compagno L. J. V. (2002). “Sharks of the World,” in An Annotated and Illustrated Catalogue of Shark Species Known to Date (Rome: Food & Agriculture Org).
Daly J., Jones R. (2017). “The Use of Reproductive Technologies in Breeding Programs for Elasmobranchs in Aquaria (Chapter 36)” in The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and Their Relatives. Columbus, Ohio: Special Publication of the Ohio Biological Survey, vol. 363–374 (Columbus, Ohio: Ohio Biological Survey, Inc.)
Dudgeon C. L., Broderick D., Ovenden J. (2009). IUCN Classification Zones Concord With, But Underestimate, the Population Genetic Structure of the Zebra Shark Stegostoma Fasciatum in the Indo-West Pacific. Mol. Ecol. 18, 248–261. doi: 10.1111/j.1365-294X.2008.04025.x
Dudgeon C. L., Coulton L., Bone R., Ovenden J. R., Thomas S. (2017). Switch From Sexual to Parthenogenetic Reproduction in a Zebra Shark. Sci. Rep. 7, 40537. doi: 10.1038/srep40537
Dudgeon C. L., Feldheim K., Schick M., Overden J. R. (2006). Polymorphic Microsatellite Loci for the Zebra Shark Stegostoma Fasciatum. Mol. Ecol. Notes 6, 1086–1088. doi: 10.1111/j.1471-8286.2006.01442.x
Dudgeon C. L., Lanyon J. M., Semmens J. M. (2013). Seasonality and Site Fidelity of the Zebra Shark, Stegostoma Fasciatum, in Southeast Queensland, Australia. Anim. Behav. 85, 471–481. doi: 10.1016/j.anbehav.2012.12.013
Dudgeon C. L., Noad M. J., Lanyon J. M. (2008). Abundance and Demography of a Seasonal Aggregation of Zebra Sharks Stegostoma Fasciatum. Mar. Ecol. Prog. Ser. 368, 269–281. doi: 10.3354/meps07581
Dudgeon C. L., Simpfendorfer C. A., Pillans R. D. (2019). Stegostoma Fasciatum (Amended Version of 2016 Assessment). IUCN Red List Threat. Species, e.T41878A161303882.
Dulvy N. K., Fowler S. L., Musick J. A., Cavanagh R. D., Kyne P. M., Harrison L. R., et al. (2014). Extinction Risk and Conservation of the World’s Sharks and Rays. Elife 3, e00590. doi: 10.7554/eLife.00590
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing Drives Over One-Third of All Sharks and Rays Toward a Global Extinction Crisis. Curr. Biol. 31, 4773–4787.e8. doi: 10.1016/j.cub.2021.08.062
Faigl V., Vass N., Jávor A., Kulcsár M., Solti L., Amiridis G., et al. (2012). Artificial Insemination of Small Ruminants — A Review. Acta Vet. Hung. 60, 115–129. doi: 10.1556/avet.2012.010
Feldheim K. A., Chapman D. D., Sweet D., Fitzpatrick S., Prodöhl P. A., Shivji M. S., et al. (2010). Shark Virgin Birth Produces Multiple, Viable Offspring. J. Hered. 101, 374–377. doi: 10.1093/jhered/esp129
Fields A. T., Feldheim K. A., Poulakis G. R., Chapman D. D. (2015). Facultative Parthenogenesis in a Critically Endangered Wild Vertebrate. Curr. Biol. 25, R446–R447. doi: 10.1016/j.cub.2015.04.018
García-Salinas P., Gallego V., Asturiano J. F. (2021). Development of Sperm Cryopreservation Protocols for Sharks and Rays: New Tools for Elasmobranch Conservation. Front. Mar. Sci. 8, 689089. doi: 10.3389/fmars.2021.689089
Gilbert P. W., Heath G. W. (1972). The Clasper-Siphon Sac Mechanism in Squalus Acanthias and Mustelus Canis. Comp. Biochem. Physiol. Part A Physiol. 42, 97–119. doi: 10.1016/0300-9629(72)90371-4
Harmon T. S., Kamerman T. Y., Corwin A. L., Sellas A. B. (2016). Consecutive Parthenogenetic Births in a Spotted Eagle Ray Aetobatus Narinari. J. Fish Biol. 88, 741–745. doi: 10.1111/jfb.12819
Howard J. G., Lynch C., Santymire R. M., Marinari P. E., Wildt D. E. (2016). Recovery of Gene Diversity Using Long-Term Cryopreserved Spermatozoa and Artificial Insemination in the Endangered Black-Footed Ferret. Anim. Conserv. 19, 102–111. doi: 10.1111/acv.12229
Huang Y., Wang P., Zhang G., Zhang H., Li D., Du J., et al. (2002). Use of Artificial Insemination to Enhance Propagation of Giant Pandas at the Wolong Breeding Center. Proc. Int. Symposium Assisted Reprod. Technol. (ART) Conserv. Genet. Manage. Wildlife 8, 1–8. doi: 10.1023/A:1021259031267
Jukam D., Shariati S. A. M., Skotheim J. M. (2017). Zygotic Genome Activation in Vertebrates. Dev. Cell 42, 316–332. doi: 10.1016/j.devcel.2017.07.026
Kunze K., Simmons L. (2004). “Notes on Reproduction of the Zebra Shark, Stegostoma Fasciatum, in a Captive Environment” in Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays, and Their Relatives. Ed. Smith M. F. L. (Columbus, Ohio: Ohio Biological Survey), 493–497.
Luer C. A., Walsh C. J., Bodine A. B., Wyffels J. T. (2007). Normal Embryonic Development in the Clearnose Skate, Raja Eglanteria, With Experimental Observations on Artificial Insemination. Environ. Biol. Fishes 80, 239–255. doi: 10.1007/s10641-007-9219-4
Malecki I. A., Rybnik P. K., Martin G. B. (2008). Artificial Insemination Technology for Ratites: A Review. Aust. J. Exp. Agric. 48, 1284–1292. doi: 10.1071/EA08141
Maruska K. P., Cowie E. G., Tricas T. C. (1996). Periodic Gonadal Activity and Protracted Mating in Elasmobranch Fishes. J. Exp. Zool. 276, 219–232. doi: 10.1002/(SICI)1097-010X(19961015)276:3<219::AID-JEZ6>3.0.CO;2-Q
Masuda M., Izawa Y., Kametuta S., Ikuta H., Isogai T. (2003). Artificial Insemination of the Cloudy Catshark. Japanese Assoc. Zoos Aquariums 44, 39–43.
Minamikawa S., Morisawa M. (1996). Acquisition, Initiation and Maintenance of Sperm Motility in the Shark, Triakis Scyllia. Comp. Biochem. Physiol. Part A Physiol. 113, 387–392. doi: 10.1016/0300-9629(95)02080-2
Mull C. G., Lowe C. G., Young K. A. (2008). Photoperiod and Water Temperature Regulation of Seasonal Reproduction in Male Round Stingrays (Urobatis Halleri). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 151, 717–725. doi: 10.1016/j.cbpa.2008.08.029
Nozu R., Murakumo K., Matsumoto R., Matsumoto Y., Yano N., Nakamura M., et al. (2017). High-Resolution Monitoring From Birth to Sexual Maturity of a Male Reef Manta Ray, Mobula Alfredi, Held in Captivity for 7 Years: Changes in External Morphology, Behavior, and Steroid Hormones Levels. BMC Zool. 2, 14. doi: 10.1186/s40850-017-0023-0
Nozu R., Murakumo K., Yano N., Furuyama R., Matsumoto R., Yanagisawa M., et al. (2018). Changes in Sex Steroid Hormone Levels Reflect the Reproductive Status of Captive Female Zebra Sharks (Stegostoma Fasciatum). Gen. Comp. Endocrinol. 265, 174–179. doi: 10.1016/j.ygcen.2018.03.006
Ottinger M. A., Mench J. A. (1989). Reproductive Behaviour in Poultry: Implications for Artificial Insemination Technology. Br. Poult. Sci. 30, 431–442. doi: 10.1080/00071668908417167
Pratt H. L., Carrier J. C. (2001). A Review of Elasmobranch Reproductive Behavior With a Case Study on the Nurse Shark, Ginglymostoma Cirratum. Environ. Biol. Fishes 60, 157–188. doi: 10.1023/A:1007656126281
Pratt H. L., Carrier J. C., Hamlett W. C. (2005). “Elasmobranch Courtship and Mating Behaviour,” in Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras. Ed. Hamlett W. C. (Boca Raton, FL: CRC Press), 129–169.
Ramachandran R., McDaniel C. D. (2018). Parthenogenesis in Birds: A Review. Reproduction 155, R245–R257. doi: 10.1530/REP-17-0728
Réhault-Godbert S., Mann K., Bourin M., Brionne A., Nys Y. (2014). Effect of Embryonic Development on the Chicken Egg Yolk Plasma Proteome After 12 Days of Incubation. J. Agric. Food Chem. 62, 2531–2540. doi: 10.1021/jf404512x
Ritter C., Beaver A., von Keyserlingk M. A. G. (2019). The Complex Relationship Between Welfare and Reproduction in Cattle. Reprod. Domest. Anim. 54, 29–37. doi: 10.1111/rda.13464
Robinson D. P., Baverstock W., Al-Jaru A., Hyland K., Khazanehdari K. A. (2011). Annually Recurring Parthenogenesis in a Zebra Shark Stegostoma Fasciatum. J. Fish Biol. 79, 1376–1382. doi: 10.1111/j.1095-8649.2011.03110.x
Rodger J. C., Paris D. B. B. P., Czarny N. A., Harris M. S., Molinia F. C., Taggart D. A., et al. (2009). Artificial Insemination in Marsupials. Theriogenology 71, 176–189. doi: 10.1016/j.theriogenology.2008.09.006
Rossouw G. J. (2014). Maturity, Spermatogenesis and Seasonal Reproductive Cycle of Male Rhinobatos Annulatus (Muller & Henl) From Algoa Bay, South Africa, and a Novel Description for Sperm Release From the Spermatocyst. Afr. Zool. 49, 128–136. doi: 10.10520/EJC152244
Simpfendorfer C. A., Kyne P. M. (2009). Limited Potential to Recover From Overfishing Raises Concerns for Deep-Sea Sharks, Rays and Chimaeras. Environ. Conserv. 36, 97–103. doi: 10.1017/S0376892909990191
Toledo H., Alonso E. (2021). Stegostoma Fasciatum Breeding Program at Loro Parque Aquarium. Drum Croak. 52, 84–90.
Watson L., Janse M. (2017). “Reproduction and Husbandry of Zebra Sharks, Stegostoma Fasciatum, in Aquaria” in The Elasmobranch Husbandry Manual Ii, vol. 421.
Wyffels J. T., Adams L. M., Bulman F., Fustukjian A., Hyatt M. W., Feldheim K. A., et al. (2021). Artificial Insemination and Parthenogenesis in the Whitespotted Bamboo Shark Chiloscyllium Plagiosum. Sci. Rep. 11, 9966. doi: 10.1038/s41598-021-88568-y
Wyffels J., Coco C., Schreiber C., Palmer D., Clauss T., Bulman F., et al. (2020a). Natural Environmental Conditions and Collaborative Efforts Provide the Secret to Success for Sand Tiger Shark Carcharias Taurus Reproduction in Aquaria. Zoo Biol. 39, 355–363. doi: 10.1002/zoo.21558
Wyffels J. T., George R., Adams L., Adams C., Clauss T., Newton A., et al. (2020b). Testosterone and Semen Seasonality for the Sand Tiger Shark Carcharias Taurus. Biol. Reprod. 102, 876–887. doi: 10.1093/biolre/ioz221
Yadgary L., Yair R., Uni Z. (2011). The Chick Embryo Yolk Sac Membrane Expresses Nutrient Transporter and Digestive Enzyme Genes. Poult. Sci. 90, 410–416. doi: 10.3382/ps.2010-01075
Zhang G., Swaisgood R. R., Zhang H. (2004). Evaluation of Behavioral Factors Influencing Reproductive Success and Failure in Captive Giant Pandas. Zoo Biol. 23, 15–31. doi: 10.1002/zoo.10118
Keywords: reproduction, sustainability, eclosion, paternity, fertility, oviparity
Citation: Adams L, Lyons K, Larkin E, Leier N, Monday J, Plante C, Dubach J and Wyffels J (2022) Artificial Insemination and Parthenogenesis in the Zebra Shark Stegostoma tigrinum. Front. Mar. Sci. 9:886616. doi: 10.3389/fmars.2022.886616
Received: 28 February 2022; Accepted: 04 May 2022;
Published: 02 June 2022.
Edited by:
Steven T Kessel, Shedd Aquarium, United StatesReviewed by:
Juan F. Asturiano, Universitat Politècnica de València, SpainRob Jones, The Aquarium Vet, Australia
Copyright © 2022 Adams, Lyons, Larkin, Leier, Monday, Plante, Dubach and Wyffels. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lance Adams, TEFkYW1zQGxiYW9wLm9yZw==
 Lance Adams
Lance Adams Kady Lyons
Kady Lyons Elizabeth Larkin1
Elizabeth Larkin1 Jennifer Wyffels
Jennifer Wyffels