Video surveys of sea snakes in the mesophotic zone shed light on trends in populations
- 1Australian Institute of Marine Science, Perth, WA, Australia
- 2Global FinPrint Project, Australian Institute of Marine Science (AIMS), University of Western Australia (UWA) (MO96), Crawley, WA, Australia
- 3Research & Collections, Western Australian Museum, Welshpool, WA, Australia
- 4School of Biological Sciences, The University of Western Australia, Perth, WA, Australia
- 5Stantec Australia, Perth, WA, Australia
- 6Australian Institute of Marine Science, Darwin, NT, Australia
Declines in abundance of sea snakes have been observed on reefs throughout the Indo-Pacific, although the reasons are unknown. To date, surveys have occurred on shallow reefs, despite sea snakes occurring over a large depth range. It is not known if populations of sea snakes in deep habitats have undergone similar declines. To address this, we analysed deep-water video data from a historical hotspot of sea snake diversity, Ashmore Reef, in 2004, 2016, and 2021. We collected 288 hours of video using baited remote underwater videos and a remotely operated vehicle at depths between 13 and 112 m. We observed 80 individuals of seven species with Aipysurus laevis (n = 30), Hydrophis peronii (n = 8), and H. ocellatus (n = 6) being the most abundant. Five of the species (A. duboisii, A. apraefrontalis, H. ocellatus, H. kingii, and Emydocephalus orarius) had not been reported in shallow waters for a decade prior to our study. We found no evidence of a decline in sea snakes across years in deep-water surveys, although abundances were lower than those in early shallow-water surveys. A comparison of BRUVS data from 2004 and 2016 was consistent with the hypothesis that predation by sharks may have contributed to the loss of sea snakes in shallow habitats. Our study highlights the use of underwater video to collect information on sea snakes in the mesophotic zone and also suggests that future monitoring should include these depths in order to capture a more complete representation of habitats occupied.
Introduction
Sea snakes are the most speciose group of marine reptiles (~70 known species) (Rasmussen et al., 2014). They play a key ecological role in the systems they inhabit both as mesopredators and prey (Shine et al., 2020). Sea snakes are economically important in several parts of the world where they are harvested for leather, meat, and traditional medicine (Zhou and Jiang, 2004). Although the taxonomy and phylogenetics of this group of species has received considerable attention in the recent past (e.g., Sanders et al., 2013; Rasmussen et al., 2014), our understanding of the distributions and ecology of sea snakes is based primarily on surveys in shallow waters (mostly <15 m) (See review by Lukoschek et al., 2013). Recent observations of these animals in localities (Park et al., 2017) and at depths (Crowe-Riddell et al., 2019) that were previously thought to be beyond habitable ranges have occurred due to expansion of sampling to isolated areas and the use of new, remotely-operated technologies to access relatively deep waters. These studies challenge traditional views of the biogeography, ecology and physiology of sea snakes, and highlight several key knowledge gaps in their biology (Bonnet et al., 2016). This point is further emphasised by the fact that more than one third of the species of sea snakes are currently categorised by the International Union for the Conservation of Nature (IUCN) Red List as Data Deficient (Elfes et al., 2013).
A number of recent studies suggest that sea snakes have undergone declines in some areas of the Indo-Pacific including the Great Barrier Reef (Lukoschek et al., 2007), New Caledonia (Goiran and Shine, 2013), and the Timor Sea (Lukoschek et al., 2013). Once recognised as a global hotspot for sea snake diversity and abundance, Ashmore Reef in north-western Australia has seen a dramatic decline in both the diversity and abundance of sea snakes within shallow waters over the last two decades, beginning in c. 2000 (Lukoschek et al., 2013; Somaweera et al., 2021). These observations are of particular concern, as some sea snakes have now disappeared from ranges that were largely restricted to the region of this reef. Species such as Aipysurus apraefrontalis and A. foliosquama that were once commonly observed at Ashmore are now categorised as Critically Endangered by the Environment Protection and Biodiversity Conservation (EPBC) Act. Similarly, A. fuscus which was also once abundant at this reef, is now categorised as Endangered by the IUCN (Udyawer et al., 2020). Although the exact reason for these declines is not known, Somaweera et al. (2021) outlined a number of possibilities including environmental disturbances, increased maritime activity, and a change in trophic structure of predators (sharks and large teleost mesopredators).
Surveys of sea snakes at Ashmore Reef and most other localities have typically occurred in shallow water and rely on diver-based or surface-based observations (but see Redfield et al., 1978; Udyawer et al., 2014). Given our improved understanding and developing knowledge of the depth range sea snakes occupy (0 – 250 m), it is important to determine if declines have occurred across the entire reef system or if deep habitats may still provide refuges for sea snakes. Sampling surveys that have used Baited Remote Underwater Video Stations (BRUVS) and a recent Remotely Operated Vehicle (ROV) survey of mesophotic habitats (> 50 m) offer an opportunity to examine patterns in the distribution and abundance of sea snakes across depth gradients at Ashmore Reef. BRUVS record abundances and behaviours of animals that are attracted to a bait bag. This technique is also used to sample reefs to depths beyond the range of SCUBA divers and collect a permanent video record of communities and the habitat where they were deployed (Ellis and Demartini, 1995; Langlois et al., 2020). Sea snakes are known to be attracted to the bait bag and are readily recorded by BRUVS (Udyawer et al., 2014). Surveys using ROV are becoming more common in the marine environment, although they are generally used to assess structural integrity of offshore structures used by oil and gas, with footage later repurposed to document fish and benthic life associated with the structures (e.g., Mclean et al., 2017). The artificial lighting, sound, and size of ROVs have been shown to influence fish behaviour (Stoner et al., 2008; Sward et al., 2019), although the effects on sea snake behaviour are unknown. Combined, BRUVS and ROVs could offer an effective means to determine species composition and abundance of sea snakes in deep-water habitats.
Here, we compare sightings of sea snakes on BRUVS surveys collected in 2004 and 2016 and a ROV survey in 2021 at Ashmore Reef. These video surveys encompass a period following a significant decline in abundance and diversity of sea snakes in shallow waters (Somaweera et al., 2021). Furthermore, our surveys span a time period over which there was a recovery of shark populations at the reef and subsequent changes in mesopredatory fish communities (Speed et al., 2018; Speed et al., 2019). We contrast observations of sighting of sea snakes in deep-water video surveys (mean depths > 70 m), with visual surveys in shallow water (mean depth < 15 m) (e.g., Lukoschek et al., 2013; Somaweera et al., 2021).
Methods
Two video survey methods were used to collect information on sea snake abundance, which were Baited Remote Video Stations (BRUVS) and a Remotely Operated Vehicle (ROV). The original focus of the BRUVS surveys was to collect information on fish and shark communities, while the ROV survey focused on benthic mesophotic communities. BRUVS consist of digital cameras in underwater housings attached to a metal frame with a bait bag on an arm that are deployed for at least 60 minutes on the reef. The units did not contain any artificial lighting. BRUVS were deployed at Ashmore Reef in October 2004 (austral spring), primarily focussed on assessing shark stocks in the region (Meekan and Cappo, 2004). A second BRUVS survey in January 2016 (austral summer) also focused on reef sharks (Speed et al., 2018), as part of the Global FinPrint Project (https://globalfinprint.org/), although other large predatory teleost species were also targeted (Speed et al., 2019). Both surveys deployed BRUVS during daylight hours, with replicate units spaced > 400 m apart on the reef for > 60-minute periods. Bait bags were filled with 1 kg of pilchards (Sardinops spp). Depth of deployments in 2004 ranged between 13 – 58 m, and between 4 – 41 m depths in 2016 (For more details on deployments see Meekan and Cappo, 2004; Speed et al., 2018).
The second survey method using a ROV surveyed the mesophotic reefs around Ashmore (> 50 m depths) in April 2021 (austral autumn). The Schmidt Ocean Institute ROV SuBastian with the dimensions 2.7 x 2.2 x 1.8 m, and a top speed of 3 knots, was fitted with 4k UHD 2160 p video capability and auxiliary cameras on each side, as well as spot (28,000 lm), flood (8,500 lm), and strobe lights (100,000 lm) for illumination. ROV transect surveys spanned the 50-150m depth contours along the reef edge during daylight hours (6 am – 6 pm) over a 14-day period. Transects at 50 m, 100m, and 150m depth contours were done each day, with additional opportunistic surveys to collect invertebrates for a concurrent project being done over a larger depth range (30m – 150m).
We manually extracted screen captures and video clips of sea snake sightings on BRUVS and ROV footage that were reviewed to identify animals to species where possible by two of the authors (RS & VU) working independently. Where there was no consensus on species identification, or the image quality was not adequate to accurately identify an individual, it was listed as ‘Unidentified’.
To compare the spatial distribution of sightings of sea snakes in shallow-water at Ashmore Reef with the deep-water video records presented here, we collated previous records from the Atlas of Living Australia (https://www.ala.org.au/) (ALA, 2021). Shallow-water visual surveys at Ashmore Reef included: Scuba, manta tow, reef walking, boat surveys, and snorkelling (see Somaweera et al., 2021). We compared the differences in depth and horizontal spatial scales of shallow and deep-water surveys and distance of sightings from the reef was calculated using the ‘Generate Near Table’ function in ArcGIS, where distance measures were geodesic. We also compared temporal abundance records of sea snakes from shallow-water surveys at Ashmore Reef presented by Lukoschek et al. (2013) and Somaweera et al. (2021) with the deep water records collected using BRUVS and ROV in the present study.
We compared the results of BRUVS surveys in 2004 and 2016 to test the hypothesis that the recovery of populations of top order predators such as sharks led to the decline of sea snakes in shallow and deep-water habitats. We partition observations of sea snakes and sharks into ‘shallow’, which incorporates the depth range that previous shallow-water surveys have covered (0 – 30 m) and ‘deep’, which relates to observations between 30 – 60 m. Abundances of sea snakes and reef sharks were compared between time periods and depth bins (shallow and deep) using Poisson Generalised Linear Models (GLM). Model residuals were then plotted against fitted values to assess whether model assumptions were met (Zuur et al., 2007).
Results
Temporal sampling comparison
A total of 46 BRUVS were deployed around Ashmore Reef in 2004 (50.35 hours of video total) and 89 in 2016 (90.07 hours total). A ROV was deployed 14 times in 2021 (148 hours total) (Figure 1). The depth range of deep-water surveys reported here varied among survey type and year. In both the 2004 and 2016 surveys > 40% of deployments were below 30 m depth. Average maximum sampling depths differed between shallow-water visual surveys (14 ± 1.4 m SE) and deep-water video surveys (BRUVS & ROV surveys, 73 ± 19 m SE) (Supplementary Figure 1). ROV surveys in deep-water overlapped to a small extent with previous visual surveys in shallow water. The mean spatial separation from the reef edge of shallow and deep-water observations were significantly different at 0.29 km ± 0.04 SE and 7.4 km ± 0.49 SE respectively (Supplementary Figure 2).
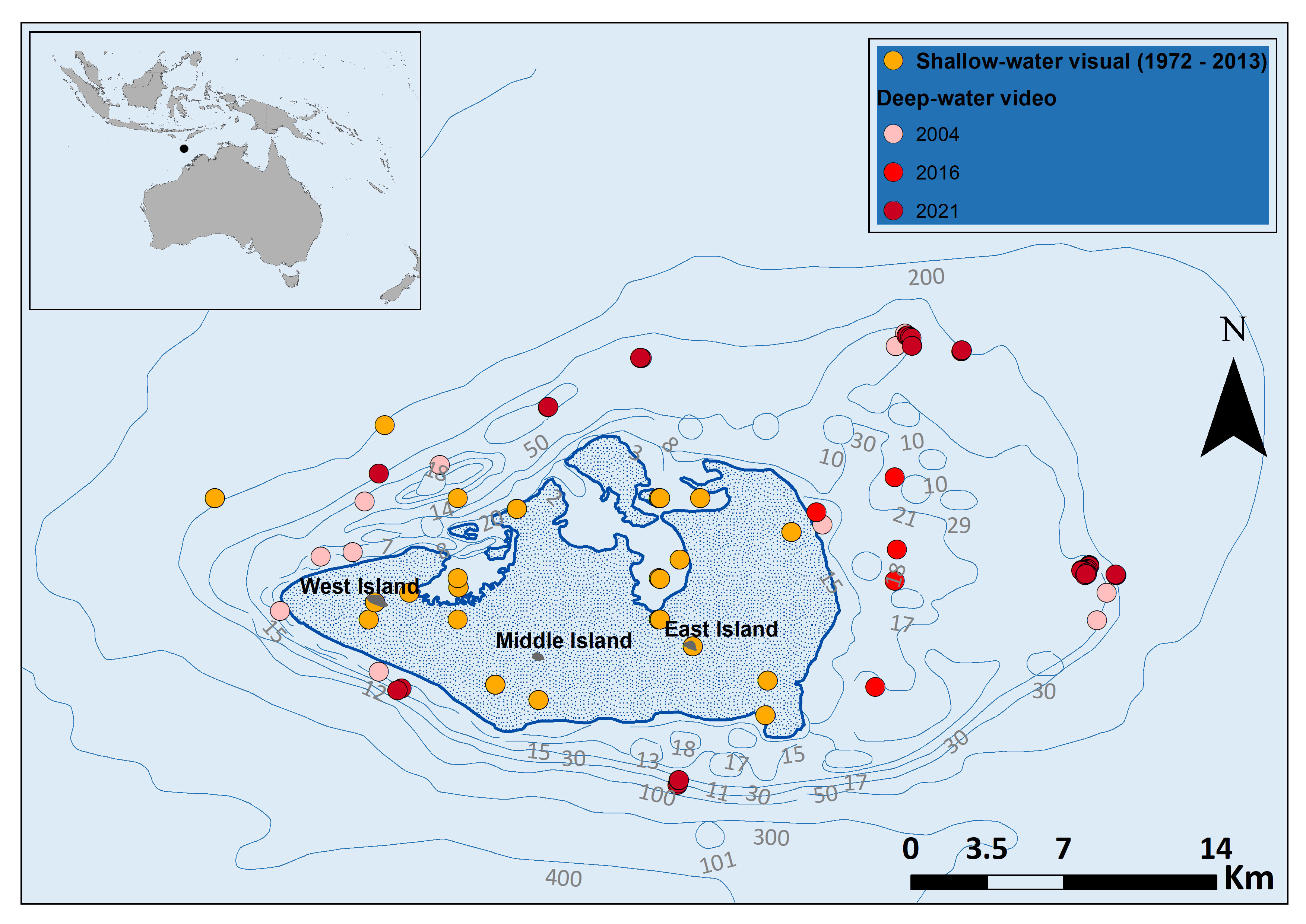
Figure 1 Sea snake sightings in and around Ashmore Reef. The deep-water video surveys in 2004 and 2016 were done using BRUVS, whereas the 2021 survey used an ROV. Shallow-water visual survey data downloaded from Atlas of Living Australia; occurrence download at https://doi.ala.org.au/doi/8b85f7f1-8ba7-40fe-a69c-6c48f5f45962;jsessionid=4A9BACF61A47968B6A7B1E2738C05DBF, Accessed September 2021. Thick blue line denotes the reef edge and shaded area includes shallow coral Reef habitats.
Snake observations
BRUVS deployments in 2004 identified sea snakes at depths between 13 – 58 m with a mean depth of 34 m (± 3.9 m SE). Snakes were observed across a more restricted depth range using BRUVS in 2016 (between 30-34 m), although had a comparable mean depth of 32 m (± 0.7 m). The ROV deployments in 2021 observed sea snakes at much greater depths ranging from 37 to 112 m with a mean of 65 m (± 2.9 m). The deepest observation was an individual of Hydrophis ocellatus at 112 m.
A total of 80 sightings of sea snakes were recorded in deep water, of which 70 were identified to species or genus level (Table 1), the remaining 10 observations were labelled as ‘Unidentified’ due to poor image quality. Of the total sightings, 60 were detected on the 2021 survey. When accounting for sampling effort, sightings of sea snakes across surveys varied from 0.3 hr-1 in 2004 to 0.06 hr-1 in 2016, to 0.4 hr-1 in 2021 (Figure 2A). All sightings in deep water surveys were lower than those in the historical peak of sea snake sightings in shallow water of 60 hr-1 in 1998 (Figure 2B).

Table 1 Sea snake taxa identified at Ashmore Reef using BRUVS surveys in 2004 and 2016, and a ROV survey in 2021.
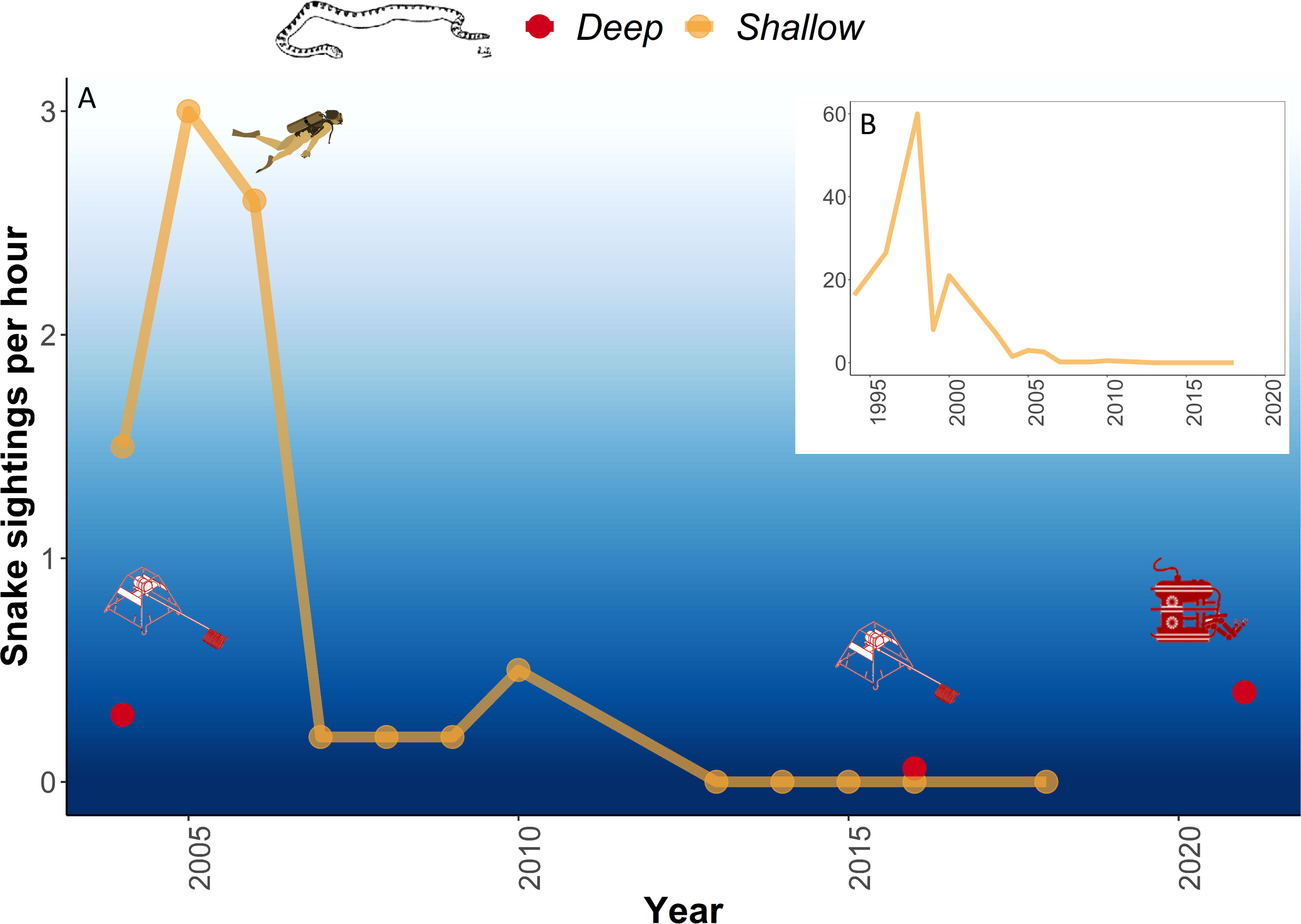
Figure 2 (A) Sea snake sightings per hour at Ashmore Reef between 2004 and 2021. Deep-water video observations were made by BRUVS in 2004 and 2016 and ROV in 2021. (B) Historical shallow-water observations of sea snakes recorded per hour at Ashmore Reef since the 1994 (redrawn from Somaweera et al., 2021). Diver image courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/).
The most abundant species were Aipysurus laevis (n = 30), Hydrophis peronii (n = 8), and H. ocellatus (n = 6). Five of the species observed in 2021 were “re-discoveries” of species for Ashmore Reef, these included A. duboisii, A. apraefrontalis, H. ocellatus, H. kingii, and Emydocephalus orarius (Figure 3).

Figure 3 Photographs of sea snakes obtained during the ROV survey at Ashmore Reef in 2021. (A) Aipysurus apraefrontalis, (B) Aipysurus duboisii, (C) Emydocephalus orarius, (D) Hydrophis coggeri, (E) Aipysurus laevis (colour variations), (F) Hydrophis ocellatus, (G) Hydrophis kingii, and (H) Hydrophis peronii (with closeup of head).
The results from the Poisson GLM indicated that there were significant increases in the relative abundance of reef sharks and decreases in relative abundance of sea snakes between the 2004 and 2016 BRUVS surveys (Supplementary Table 1 & Figure 4). Depth bin (shallow vs deep) was not a significant term in either shark or snake models, nor was there an interaction between year and depth bin. The relative increase in shark numbers between surveys was an increase by a factor of 2.3 in 0-30m and 2.2 in the 30-60m. The relative decrease observed in sea snake numbers between surveys equated to a decrease by a factor of 15.7 in 0-30m and 3.3 in 30-60m.
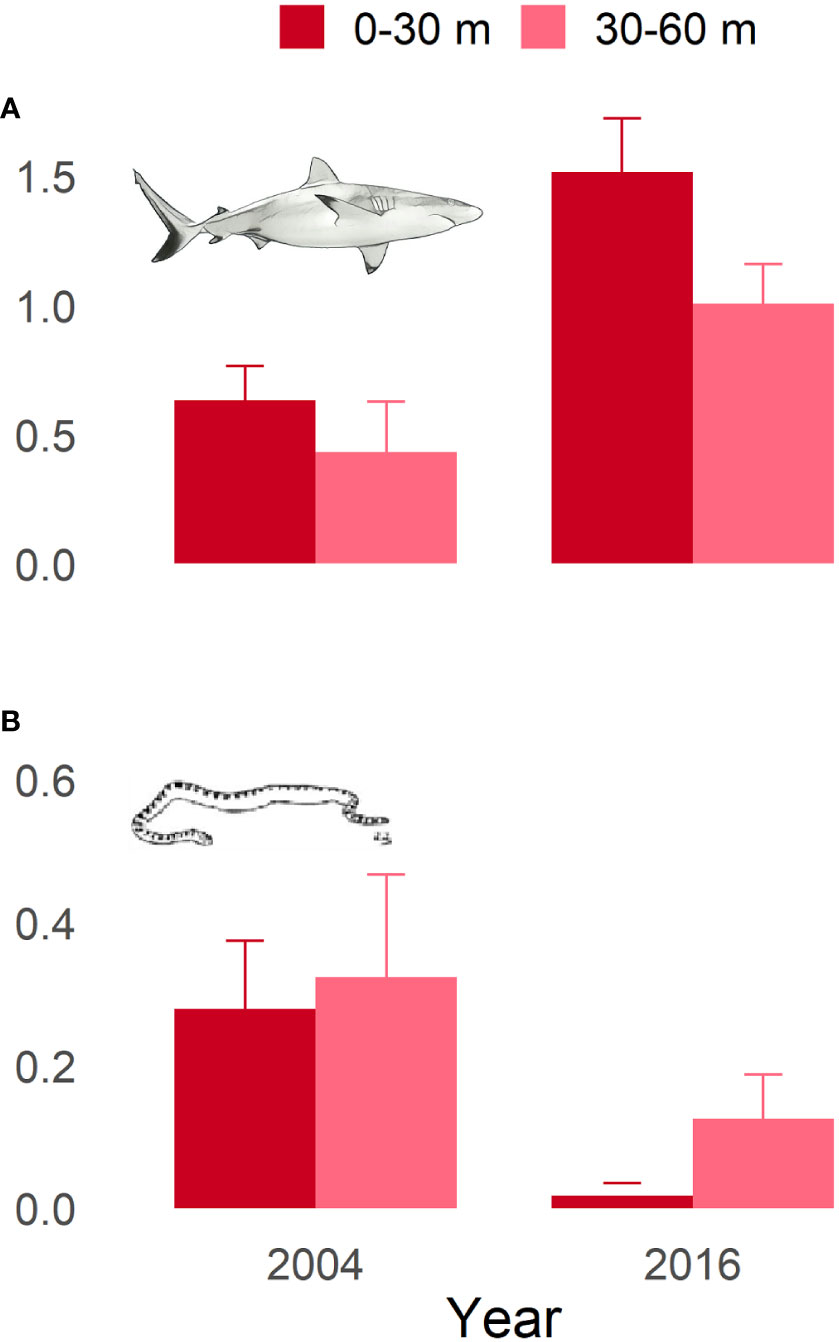
Figure 4 Mean hourly observations collected using BRUVS at Ashmore Reef by shallow (< 30 m) and deep (30 – 60 m) habitats for (A) reef sharks and (B) sea snakes.
Discussion
Our deep-water video surveys provide evidence of the persistence of species of sea snake that have not been seen in shallow water surveys for decades at Ashmore Reef, which was once a global hotspot of diversity and abundance for this marine predator guild. These “re-discoveries” of species in deep water suggest a possible refuge, although the relatively low abundances give cause for alarm for their long-term persistence. Of particular note from the ROV survey was the observation of the critically endangered species A. apraefrontalis. This species has undergone a decline in abundance of 90% or more of individuals since 2000 at Ashmore (Guinea, 2007) with the last confirmed sighting during a reef flat survey in 1998 (Guinea and Whiting, 2005). Due to the depth limitations of previous surveys, the habitat preference of this species was presumed to be within shallow waters (< 10 m) of the inner lagoon of Ashmore Reef (Mccosker, 1975; Minton and Heatwole, 1975). Similarly, other species such as A. duboisii, and H. ocellatus have not been observed at Ashmore Reef since the early 1970s (Lukoschek et al., 2013), whereas H. kingii and E. orarius (previously E. annulatus), the latter which have not been recorded since the mid-2000s. The re-sightings of these five species confirm that localised extinctions have not occurred over the past decades, although their absence on shallow-water surveys is concerning, as are the low abundances observed in deep water. The most common species of sea snake observed on the BRUVS and ROV surveys at Ashmore Reef was A. laevis, which is also the most common species sighted in shallow-water at Ashmore, and the most common species found in mesophotic habitats on other reefs and shoals in the region, including Scott Reef, Glomar Shoal, and Rankin Bank (AIMS unpublished data; Guinea, 2013; D’anastasi et al., 2016; Udyawer and Heupel, 2017).
Shallow-water visual surveys from 1972 to 2019 show a striking decline in sea snakes at Ashmore beginning around ca. 2000 (Lukoschek et al., 2013). Our limited BRUVS surveys support the idea that this also occurred in deep water between 2004 and 2016. However, it is likely that mesophotic waters act as a refuge for sea snakes, as has been suggested for a number of species of fishes (Thomas et al., 2015; Lindfield et al., 2016; Macdonald et al., 2016), particularly those of larger size classes (Williams et al., 2019). There are extensive deep-water habitats around Ashmore Reef (< 100 m in depth), which occur on the north and east sides of the reef (Berry, 1993). These seem to provide suitable habitat for sea snakes and were the locations where the majority of observations of sea snakes were made by video surveys. There is also the possibility that detectability of sea snakes varies with depth, which could be related to changes in foraging, resting, and predator avoidance in low light levels (e.g., Rickel and Genin, 2005), or changes due to habitat complexity, as have been observed in species of fish (Hixon, 2015). To test hypotheses of sea snake detectability, it would be necessary to sample across a wider range of shallow and mesophotic depths with consistent remote video techniques such as ROV or BRUVS.
BRUVS data collected in 2004 and 2016 provides support for the hypothesis that the decline in sea snake abundances at Ashmore Reef may have been influenced by a recovery of top-order predators. Over this time the numbers of reef sharks in shallow and deep habitats more than doubled, whereas numbers of sea snakes seen in the same videos declined by 15-fold in 0-30 m depths and more than double in 30-60 m depths. Sea snakes are known to be part of the diet of reef sharks and apex sharks (Lyle, 1987; Simpfendorfer et al., 2001) and such a decline is consistent with other studies that show an increase in the abundance of sharks can lead to changes in abundance and composition of mesopredatory fishes (Speed et al., 2018; Speed et al., 2019), rays (Bond et al., 2019), and moray eels (Clementi et al., 2021). Targeted shark fishing by Indonesian fishers to supply the trade in banquet foods (shark fin soup) has a centuries-old history at Ashmore Reef and shark populations have recovered since the 2000s due to better enforcement of marine park regulations that have established a no-take management zone at the reef (Speed et al., 2018; Meekan et al., 2020). If the decline in abundances of sea snakes was indeed driven by the recovery of top-order predators, it seems possible therefore that the large numbers of sea snakes recorded in this locality in early years of surveys may have been driven by the suppression of predation by targeted fishing of sharks. It must be acknowledged here that the numbers of sea snakes recorded in shallow-water surveys, prior to any BRUVS surveys being done, had considerable variation between samples, although indicated an overall decline. This variation may have been due to a number of potential biological drivers, environmental conditions, or other anthropogenic impacts that are not immediately evident (Although see Somaweera et al., 2021). Given that historical baseline data on sea snakes prior to the impacts of fishing in this location do not exist, it’s difficult to assess whether these high numbers of sea snakes were representative of natural baselines, or a potential artefact of reduced predation pressure through targeted fishing of known predators of sea snakes.
Why more sea snakes now occur in deep than shallow waters in the most recent surveys at Ashmore Reef remains unknown, although this is again consistent with the distribution of top-order predators, which are present in significantly greater numbers in shallow waters. BRUVS data suggested that there has been a decline in abundance of sea snakes between 2004 and 2016 in both shallow and deep water, but this pattern could not be confirmed with ROV data as we lacked any sampling using this technique prior to 2021. In any event, it may be difficult to compare abundances between these techniques given that BRUVS are stationary, have a fixed view once deployed and have baits that are likely to attract sea snakes. Future surveys may also consider the use of molecular techniques such as environmental DNA (eDNA), which may assist in confirming the presence of rare species that may otherwise go undetected by techniques such as BRUVS. In contrast to BRUVS, ROVs are mobile and have a changing field of view, and cover a larger area in transects, which arguably could increase the probability of detecting species. The lights and noise of the ROV may have also had an effect on the species and how many individuals were detected (Schramm et al., 2020). Additionally, the ROV sampled deeper water than BRUVS, with maximum depths of 112 and 58 m for the two techniques respectively. Nevertheless, the presence of sea snakes across surveys in deep water is a positive indication of species persistence, albeit in low numbers compared to sightings in shallow waters in early surveys.
The possibility exists that the snakes observed in deep water around Ashmore Reef constitute separate populations to those that had declined in shallow-water habitats. For example, a previous study of the genetics of A. apraefrontalis populations across the North West Shelf of Australia identified genetic and morphological differences between specimens collected in shallow waters at Ashmore Reef and coastal populations in Exmouth Gulf (Sanders et al., 2015). Preliminary assessments of A. apraefrontalis from the deep-water ROV surveys in 2021 suggest the specimen sighted had morphological differences to individuals of the same species in shallow water, although genetic samples are required to confirm if these deep-water animals form a distinct population.
Our re-discoveries of species at Ashmore Reef also highlight the importance of spatial scale of sampling and design of surveys for marine fauna, which can be problematic when little information is known about depth and habitat use of target species. Observations of sea snakes in deep-water video surveys occurred at a greater distance from the edge of the reef slope than previous shallow-water surveys (Supplementary Figure 2), suggesting an importance or reliance on inter-reef habitat by some of these species. Indeed, a tracking study of A. laevis indicated frequent use of areas of non-continuous reef and foraging in areas of sand that intersect with coral or rock (Burns and Heatwole, 1998). Other studies of habitat use of Emydocephalus annulatus also showed a preference for coral rubble, when compared to coral, rock, or sand habitats (Shine et al., 2003; D’anastasi et al., 2016). Ideally, deep-water sampling should be incorporated into long-term surveys of sea snakes to ensure a more complete representation of distribution and status.
Whereas previous surveys using BRUVS have reported on sea snake abundance and distribution (e.g., Cappo et al., 2011; Heyward et al., 2012; Udyawer et al., 2014; Moore et al., 2017; Bouchet et al., 2020; Mclean et al., 2021), there have been comparatively few studies that have made use of ROV footage for this purpose (but see Crowe-Riddell et al., 2019). ROVs are used in the maintenance and operation of offshore oil and gas structures (Mclean et al., 2017) and provide a potentially effective method to collect data on the vertical habitat use of sea snakes where this industry occurs. Personal field observations by two of the authors (KM and NGW) suggest that the ROV seemed to have caused undetectable or very minimal disturbance to the behaviour of sea snakes. In combination with the high-definition camera system of the ROV, this enabled accurate identification of sea snakes, even at a distance of several meters from the camera. Such identifications were not always possible with footage obtained from BRUVS (e.g., Supplementary Figure 3). In summary, while there are limitations, both BRUVS and ROV survey methods have capabilities for observing and monitoring sea snake populations in habitats that are otherwise very difficult or impossible to access using other methods of visual survey.
Conclusion
Declines of sea snakes in the shallow waters at Ashmore Reef, once considered a global hotspot of sea snake diversity and abundance, have prompted concern for the persistence of some species. Our video surveys in the deep waters surrounding Ashmore Reef documented the presence of species that had not been recorded for over a decade during shallow-water sampling and show that these sea snakes still persist in these deep habitats, albeit at abundances that were well below those recorded in early surveys in shallow water. BRUVS surveys in 2004 and 2016 suggest that the recovery of populations of sharks and top-order teleosts may have been responsible for the decline in numbers of sea snakes in shallow habitats at Ashmore Reef, although additional sampling is required to confirm our hypothesis. Our observations show that deep-water and inter-reef habitats should be included in surveys to accurately assess population status of sea snakes. BRUVS and ROV techniques provide a practical means to achieve this goal.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
No ethics approvals were required for this project as all sampling was collected using non-invasive, underwater video techniques.
Author contributions
CS, NW, MM, CW, and KM collected data used in the manuscript. CS, NW, and KM devised the main conceptual idea for the manuscript. RS and VU provided identification of sea snakes based on video and still imagery from BRUVS and ROV surveys. CS, NW, MM, RS, VU, and KM made substantial contributions to drafting the manuscript, searching the literature, and interpreting results. All authors participated in drafting the final version of the manuscript and gave final approval of the version submitted and any revised versions.
Funding
Funding for this work was made possible through the Schmidt Ocean Institute, the Western Australian Museum (WAM), Australian Government Department of Agriculture, Water, and the Environment (DAWE), and the Australian Institute of Marine Science (AIMS).
Acknowledgments
We thank A. Carmignani (Curtin U/WAM), D. Stick (UWA), J. Kok (AIMS), C. Anderson (UWA/WAM), and I. Parnum (Curtin U) for their assistance with field work in 2021. This work also relied on BRUVS data collected during the Global FinPrint Project, funded by Paul G. Allen Philanthropies – grant number 11861. Data was collected in 2021 using ROV under permits issued by the Australian Government Director of National Parks (PA2019-00098-2) and the Australian Government Parks Australia (AU-COM2021-508).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.921542/full#supplementary-material
References
ALA. (2021). Atlas of living Australia. Available at: https://doi.ala.org.au/doi/8b85f7f1-8ba7-40fe-a69c-6c48f5f45962;jsessionid=4A9BACF61A47968B6A7B1E2738C05DBF (Accessed September 2021).
Berry P. (1993). Marine faunal surveys of ashmore reef and Cartier island north-western Australia. (Western Australian Museum).
Bond M., Valentin-Albanese J., Babcock E. A., Heithaus M., Grubbs R., Cerrato R., et al. (2019). Top predators induce habitat shifts in prey within marine protected areas. Oecologia. 190, 375–385. doi: 10.1007/s00442-019-04421-0
Bonnet X., Rasmussen A., Brischoux F. (2016). “Sea snakes,” in Reptile ecology and conservation: A handbook of techniques. Ed. Dodd C. K. (Great Britain: Oxford University Press).
Bouchet P. J., Letessier T. B., Caley M. J., Nichol S. L., Hemmi J. M., Meeuwig J. J. (2020). Submerged carbonate banks aggregate pelagic megafauna in offshore tropical Australia. Front. Mar. Sci. 7, 530. doi: 10.3389/fmars.2020.00530
Burns G., Heatwole H. (1998). Home range and habitat use of the olive sea snake, aipysurus laevis, on the great barrier reef, Australia. J. Herpetol., 350–358. doi: 10.2307/1565449
Cappo M., Stowar M., Syms C., Johansson C., Cooper T. (2011). Fish-habitat associations in the region offshore from James price point-a rapid assessment using baited remote underwater video stations (BRUVS). J. R. Soc. Western Aust. 94, 303–321.
Clementi G. M., Bakker J., Flowers K. I., Postaire B. D., Babcock E. A., Bond M. E., et al. (2021). Moray Eels are more common on coral Reefs subject to higher human pressure in the greater Caribbean. Iscience. 24, 102097. doi: 10.1016/j.isci.2021.102097
Crowe-Riddell J. M., D’anastasi B. R., Nankivell J. H., Rasmussen A. R., Sanders K. L. (2019). First records of sea snakes (Elapidae: Hydrophiinae) diving to the mesopelagic zone (> 200 m). Austral. Ecol. 44, 752–754. doi: 10.1111/aec.12717
D’anastasi B., Van Herwerden L., Hobbs J., Simpfendorfer C., Lukoschek V. (2016). Habitat and behavioural associations of aipysurus group sea snakes in Western Australia. Interim Rep. to Mar. Species Conserv. Depart. Environ. Aust. Govern.
Elfes C., Livingstone S., Lane A., Lukosche V., Sanders K., Courtney A., et al. (2013). Fascinating and forgotten: the conservation status of marine elapid snakes. Herpetol. Conserv. Biol. 8, 37–52.
Ellis D., Demartini E. E. (1995). Evaluation of video camera technique for indexing abundances of juvenile pink snapper pristipomoides filamentosus, and other Hawaiian insular shelf fishes. Fishery. Bull. 93, 67–77.
Goiran C., Shine R. (2013). Decline in sea snake abundance on a protected coral Reef system in the new caledonian lagoon. coral Reefs 32, 281–284. doi: 10.1007/s00338-012-0977-x
Guinea M. (2006). Sea Snakes of ashmore reef, Hibernia reef and Cartier island. DEWHA Final Rep. Survey. 2005, 1–37.
Guinea M. (2007). Sea Snakes of ashmore reef, Hibernia reef and Cartier island with comments on Scott reef. DEWHA Final Rep. Survey 2007, 1–20.
Guinea M. L. (2013). “Surveys of the sea snakes and sea turtles on reefs of the sahul shelf. draft final report 2012-2013”. (Darwin, Northern Territory).
Guinea M., Whiting S. (2005). Insights into the distribution and abundance of sea snakes at ashmore reef. Beagle, 199–206. (Darwin, Northern Territory).
Heyward A., Jones R., Travers M., Burns K., Suosaari G., Colquhoun J., et al. (2012). Montara: 2011 shallow reef surveys at ashmore, Cartier and seringapatam reefs. (Townsville, Qld: Australian Institute of Marine Science).
Hixon M. A. (2015). “Predation: piscivory and the ecology of coral-reef fishes,” in Ecology of fishes on coral Reefs. Ed. Mora C. (United Kingdom: Cambridge University Press), 41–53. University Printing House, Cambridge CB2 8BS.
Langlois T., Goetze J., Bond T., Monk J., Abesamis R. A., Asher J., et al. (2020). A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages. Methods. Ecol. Evol. 11, 1401–1409. doi: 10.1111/2041-210X.13470
Lindfield S. J., Harvey E. S., Halford A. R., Mcilwain J. L. (2016). Mesophotic depths as refuge areas for fishery-targeted species on coral Reefs. coral Reefs. 35, 125–137. doi: 10.1007/s00338-015-1386-8
Lukoschek V., Beger M., Ceccarelli D., Richards Z., Pratchett M. (2013). Enigmatic declines of australia’s sea snakes from a biodiversity hotspot. Biol. Conserv. 166, 191–202. doi: 10.1016/j.biocon.2013.07.004
Lukoschek V., Heatwole H., Grech A., Burns G., Marsh H. (2007). Distribution of two species of sea snakes, aipysurus laevis and emydocephalus annulatus, in the southern great barrier reef: metapopulation dynamics, marine protected areas and conservation. coral Reefs 26, 291–307. doi: 10.1007/s00338-006-0192-8
Lyle J. M. (1987). Observations on the biology of Carcharhinus cautus (Whitley), C. melanopterus (Quoy & gaimard) and C. fitzroyensis (Whitley) from northern Australia. Aust. J. Mar. Freshw. Res. 38, 701–710. doi: 10.1071/MF9870701
Macdonald C., Bridge T. C., Jones G. P. (2016). Depth, bay position and habitat structure as determinants of coral Reef fish distributions: Are deep reefs a potential refuge? Mar. Ecol. Prog. Ser. 561, 217–231. doi: 10.3354/meps11953
Mccosker J. (1975). Feeding behavior of indo-Australian hydrophiidae. Biol. Sea snakes, 217–232. (Baltimore).
Mclean D., Cure K., Wahab M. A., Galaiduk R., Birt M., Vaughan B., et al. (2021). A comparison of marine communities along a subsea pipeline with those in surrounding seabed areas. Continent. Shelf Res. 219, 104394. doi: 10.1016/j.csr.2021.104394
Mclean D., Partridge J., Bond T., Birt M., Bornt K., Langlois T. (2017). Using industry ROV videos to assess fish associations with subsea pipelines. Continental Shelf Res. 141, 76–97. doi: 10.1016/j.csr.2017.05.006
Meekan M., Cappo M. (2004). RE: Non-destructive techniques for rapid assessment of shark abundance in northern Australia. (Townsville, Australia).
Meekan M. G., Cappo M. M., Speed C. W. (2020). Response to comments on “Evidence for rapid recovery of shark populations within a coral Reef marine protected area”. Speed et al., 2018 220: 309–319. Biol. Conserv. 244, 108490. doi: 10.1016/j.biocon.2018.01.010
Minton S., Heatwole H. (1975). “Sea snakes from reefs of the sahul shelf,” in The biology of Sea snakes. Ed. Dunson W. A. (Baltimore: University Park Press), 141–144.
Moore C., Cappo M., Radford B., Heyward A. (2017). Submerged oceanic shoals of north Western Australia are a major reservoir of marine biodiversity. coral Reefs. 36, 719–734. doi: 10.1007/s00338-017-1564-y
Park J., Kim I.-H., Fong J. J., Koo K.-S., Choi W.-J., Tsai T.-S., et al. (2017). Northward dispersal of sea kraits (Laticauda semifasciata) beyond their typical range. PloS One. 12, e0179871. doi: 10.1371/journal.pone.0179871
Rasmussen A. R., Sanders K. L., Guinea M. L., Amey A. P. (2014). Sea Snakes in Australian waters (Serpentes: subfamilies hydrophiinae and laticaudinae)–a review with an updated identification key. Zootaxa 3869, 351–371. doi: 10.11646/zootaxa.3869.4.1
Redfield J., Holmes J., Holmes R. (1978). Sea Snakes of the eastern gulf of carpentaria. Mar. Freshw. Res. 29, 325–334. doi: 10.1071/MF9780325
Rickel S., Genin A. (2005). Twilight transitions in coral Reef fish: the input of light-induced changes in foraging behaviour. Anim. Behav. 70, 133–144. doi: 10.1016/j.anbehav.2004.10.014
Sanders K. L., Lee M. S., Bertozzi T., Rasmussen A. R. (2013). Multilocus phylogeny and recent rapid radiation of the viviparous sea snakes (Elapidae: Hydrophiinae). Mol. Phylogenet. Evol. 66, 575–591. doi: 10.1016/j.ympev.2012.09.021
Sanders K. L., Schroeder T., Guinea M. L., Rasmussen A. R. (2015). Molecules and morphology reveal overlooked populations of two presumed extinct Australian sea snakes (Aipysurus: Hydrophiinae). PloS One. 10, e0115679. doi: 10.1371/journal.pone.0115679
Schramm K. D., Marnane M. J., Elsdon T. S., Jones C., Saunders B. J., Goetze J. S., et al. (2020). A comparison of stereo-BRUVs and stereo-ROV techniques for sampling shallow water fish communities on and off pipelines. Mar. Environ. Res. 162, 105198. doi: 10.1016/j.marenvres.2020.105198
Shine R., Shine T. G., Brown G. P., Goiran C. (2020). Life history traits of the sea snake emydocephalus annulatus, based on a 17-yr study. coral Reefs. 39, 1407–1414. doi: 10.1007/s00338-020-01974-y
Shine R., Shine T., Shine B. (2003). Intraspecific habitat partitioning by the sea snake emydocephalus annulatus (Serpentes, hydrophiidae): the effects of sex, body size, and colour pattern. Biol. J. Linn. Soc. 80, 1–10. doi: 10.1046/j.1095-8312.2003.00213.x
Simpfendorfer C. A., Goodreid A. B., Mcauley R. B. (2001). Size, sex and geographic variation in the diet of the tiger shark, Galeocerdo cuvier, from Western Australian waters. Environ. Biol. Fishes. 61, 37–46. doi: 10.1023/A:1011021710183
Somaweera R., Udyawer V., Guinea M. L., Ceccarelli D. M., Clarke R. H., Glover M., et al. (2021). Pinpointing drivers of extirpation in sea snakes: a synthesis of evidence from ashmore reef. Front. Mar. Sci. 8, 559. doi: 10.3389/fmars.2021.658756
Speed C. W., Cappo M., Meekan M. G. (2018). Evidence for rapid recovery of shark populations within a coral Reef marine protected area. Biol. Conserv. 220, 308–319. doi: 10.1016/j.biocon.2018.01.010
Speed C. W., Rees M. J., Cure K., Vaughan B., Meekan M. G. (2019). Protection from illegal fishing and shark recovery restructures mesopredatory fish communities on a coral Reef. Ecol. Evol. 9, 10553–10566. doi: 10.1002/ece3.5575
Stoner A. W., Ryer C. H., Parker S. J., Auster P. J., Wakefield W. W. (2008). Evaluating the role of fish behavior in surveys conducted with underwater vehicles. Can. J. Fisheries Aquat. Sci. 65, 1230–1243. doi: 10.1139/F08-032
Sward D., Monk J., Barrett N. (2019). A systematic review of remotely operated vehicle surveys for visually assessing fish assemblages. Front. Mar. Sci. 6, 134. doi: 10.3389/fmars.2019.00134
Thomas C. J., Bridge T. C., Figueiredo J., Deleersnijder E., Hanert E. (2015). Connectivity between submerged and near-sea-surface coral Reefs: can submerged reef populations act as refuges? Divers. Distrib. 21, 1254–1266. doi: 10.1111/ddi.12360
Udyawer V., Cappo M., Simpfendorfer C. A., Heupel M. R., Lukoschek V. (2014). Distribution of sea snakes in the great barrier reef marine park: observations from 10 yrs of baited remote underwater video station (BRUVS) sampling. Coral Reefs. 33, 777–791. doi: 10.1007/s00338-014-1152-3
Udyawer V., Heupel M. (2017). Progress report: Spatial and temporal patterns in sea snake. (Townsville, Australia).
Udyawer V., Somaweera R., Nitschke C., D’anastasi B., Sanders K., Webber B. L., et al. (2020). Prioritising search effort to locate previously unknown populations of endangered marine reptiles. Global Ecol. Conserv. 22, e01013. doi: 10.1016/j.gecco.2020.e01013
Williams J., Jordan A., Harasti D., Davies P., Ingleton T. (2019). Taking a deeper look: Quantifying the differences in fish assemblages between shallow and mesophotic temperate rocky reefs. PloS One. 14, e0206778. doi: 10.1371/journal.pone.0206778
Zhou Z., Jiang Z. (2004). International trade status and crisis for snake species in China. Conserv. Biol. 18, 1386–1394. doi: 10.1111/j.1523-1739.2004.00251.x
Keywords: remotely operated vehicle, BRUVS, ROV, sharks, refugia, Hydrophis, Aipysurus
Citation: Speed CW, Wilson NG, Somaweera R, Udyawer V, Meekan MG, Whisson C and Miller K (2022) Video surveys of sea snakes in the mesophotic zone shed light on trends in populations. Front. Mar. Sci. 9:921542. doi: 10.3389/fmars.2022.921542
Received: 16 April 2022; Accepted: 07 September 2022;
Published: 26 September 2022.
Edited by:
Tracey T. Sutton, Nova Southeastern University, United StatesReviewed by:
Neville Scott Barrett, University of Tasmania, AustraliaJoel Williams, University of Tasmania, Australia
Copyright © 2022 Speed, Wilson, Somaweera, Udyawer, Meekan, Whisson and Miller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Conrad W. Speed, c.speed@aims.gov.au
 Conrad W. Speed
Conrad W. Speed Nerida G. Wilson
Nerida G. Wilson Ruchira Somaweera4,5
Ruchira Somaweera4,5  Vinay Udyawer
Vinay Udyawer Mark G. Meekan
Mark G. Meekan Karen Miller
Karen Miller