Corrigendum: Dugongs (Dugong dugon) along hyper-urbanized coastlines
- 1Department of Biological Sciences, National University of Singapore, Singapore, Singapore
- 2St. John’s Island National Marine Laboratory, Tropical Marine Science Institute, National University of Singapore, Singapore, Singapore
Coastal development and the increased anthropogenic use of sea spaces have rapidly degraded coastal habitats throughout Southeast Asia. We study how these activities impact dugong (Dugong dugon) population(s) along hyper-urbanized coastlines of the Johor and Singapore Straits through literature reviews and field surveys. Our review recovered sixty-nine live observations and carcass observations of dugongs between 1820 and 2021. The eastern Johor Strait is identified as a dugong hotspot. We observed peaks in observations coincident with the Northeast and Southwest monsoons. Distribution patterns of dugong observations were likely driven by a combination of natural and anthropogenic factors such as seasonality in seagrass abundance, tidal cycles, wind patterns and vessel traffic. Our field surveys ascertained active foraging sites along the anthropogenically disturbed Johor Strait and western Singapore Strait. Evident from our study is the importance of reef-associated seagrass meadows as refugia for foraging dugongs along areas of high anthropogenic use. This study provides an ecological baseline for dugong research along the Johor and Singapore Straits—within the data-poor western Malay Archipelago—, and aids in the design of sustainable management strategies and conservation programs for dugongs along areas where urbanization is commonplace.
Introduction
The dugong Dugong dugon (Müller, 1776) is the only extant species of the sirenian family Dugongidae. Global dugong populations have been assessed to be ‘Vulnerable to Extinction’ in IUCN’s Red List of Threatened Species (Marsh and Sobtzick, 2015). Female dugongs undergo relatively long calving intervals and gestation periods (two to seven years; 12 to 14 months, respectively), give birth to one calf at a time (albeit the occasional twin births), and practice prolonged maternal care (estimated suckling period of 18 months) (Marsh et al., 1984; Boyd et al., 1999; Kwan, 2002). The interactions between these reproductive traits and their vulnerability to anthropogenic threats—in particular, targeted or incidental catches, collisions with vessels, and habitat losses (Marsh et al., 2011)—exacerbate the extinction risks in this species.
Dugongs are especially impacted by the encroachment of anthropogenic activities into the marine environment, also known as ‘marine urbanization’. Given that an average coastal city (≤100 km from the sea) has a population approximately three times denser than the global average (Small and Nicholls, 2003), coastal spaces are significantly affected by this phenomenon (Firth et al., 2016). Marine urbanization is commonplace in Southeast Asia, a region which has experienced one of the highest rates of coastal population growth and resource exploitation globally (Jiang et al., 2001; Burke et al., 2011). The elevated demands for coastal resources and spaces, brought about by the sixfold increase in human populations between 1900 and 2000 (Jones, 2013), was met with the proliferation of port facilities, land reclamation and coastal protection via artificial structures (Jongman et al., 2012; Heery et al., 2018; Ma et al., 2019; Nelson Sella et al., 2019; Song et al., 2019; Veettil et al., 2020). The anthropogenic activities associated with these shoreline changes—dredging, reclamation, improper wastewater discharge and destructive fishing (Fortes, 1995; Duarte, 2002; Kirkman and Kirkman, 2002; Orth et al., 2006; Waycott et al., 2009; Yaakub et al., 2014; Unsworth et al., 2018), ensued losses of up to 50% of seagrass meadows in Southeast Asia (Macusi et al., 2011). As seagrass community specialists that rely heavily on these coastal habitats for foraging, birthing, and calving (Adulyanukosol et al., 2007; Tol et al., 2016), the trend for decreasing areas of seagrass meadows bodes poorly for dugongs in Southeast Asia (Hines, 2002; Marsh et al., 2002).
Within Southeast Asia, coastal habitat losses have been the most extreme in Singapore (Heery et al., 2018; Zhang and Hou, 2020). Between 1922 and 2011, the natural coastline length of Singapore decreased by 69% (Hilton and Manning, 1995; Lai et al., 2015), coinciding with areal losses of up to 85% in intertidal habitats including seagrass meadows. Seawalls, breakwaters, and other hard artificial structures were installed to maintain these shorelines against wave action (Lai et al., 2015). Coastal waterways in Singapore are also characterised by high volumes of vessel traffic, with 60% of territorial waters serving as shipping channels and port facilities (Chou, 2006). Currently the second busiest global port, an annual average of 593,297,000 tonnes of cargo, in addition to recreational and passenger vessels, ply along two waterways north and south of the main island of Singapore (AAPA, 2016). Shipping vessels using the Johor Strait, north to Singapore, are bound for three international ports — Johor Port and Tanjung Pelapas in Peninsular Malaysia, and Sembawang Wharves in Singapore. The Singapore Strait is the main passage for vessels bound for the Port of Singapore, a network of container terminals and port facilities at various locations along the main island of Singapore and offshore islands to her south.
Acute losses of foraging areas and high anthropogenic use of sea spaces along the Johor and Singapore Straits pose pressing constrains on available habitats for dugongs therein. Yet, the dugong populations that remain within these areas are some of the most poorly understood (Marsh et al., 2002). Little is known of the populations that utilize these highly urbanized coastal areas (Marsh et al., 2002; Perrin et al., 2002; Hines et al., 2012; Hines et al., 2015), barring sporadic sightings of individuals, carcass recoveries and observations of feeding trails (Tan et al., 2012; McKenzie et al., 2016). This paucity of scientific information impedes the conservation and management of dugongs that are locally classified as ‘Critically Endangered’ (Davison et al., 2008). We address this knowledge gap by evaluating the spatial and temporal distribution trends of extant dugong population(s) along these hyper-urbanized coastlines.
Methods
Study sites
This study focuses on two waterbodies north and south of the island nation of Singapore. The Johor Strait is a narrow water body (~1km at some points) bound by the southern tip of Peninsular Malaysia to the north and the island nation of Singapore to the south. Two bridges cross the Johor Strait: the Johor-Singapore Causeway and the Second Link. The former does not allow water exchanges, and thus effectively divides the Johor Strait into two (Ng et al., 2015). The Singapore Strait extends from the southern coast of the main island of Singapore to the Riau Islands in Indonesia and encompasses 43 islands. These areas, approximately 1° north of the equator, are characterized by hot and humid tropical weather patterns (28 - 30°C; 60 - 90% relative humidity on average) and experiences two monsoonal seasons: the Northeast monsoon from December to March, and the Southwest Monsoon from June to September. Higher mean monthly rainfall is observed during the Northeast (203 mm) as compared to the Southwest (138 mm) monsoons, while the average wind speed is approximately equal at ~2.2 m/s (Meteorological Service Singapore, 2021). The inter-monsoon periods between the Northeast and Southwest monsoons are characterized by more variable and lighter winds.
Literature review
Historical and current distribution trends of dugong populations within the Johor and Singapore Straits were first inferred by observation records retrieved from open-access Global Biodiversity Information Facility (GBIF) (GBIF.org, 2022) and Ocean Biodiversity Information System (OBIS) (OBIS, 2022) data repositories. A cleaning pipeline (adapted from Gomes et al., 2018) was implemented to remove records with imprecise coordinates from the GBIF and OBIS datasets. Geotagged records outside the natural range of dugongs (based on Marsh and Sobtzick, 2019) and records with missing, incomplete and/or duplicated coordinates were removed. A 1:10m physical vector of the ocean (Natural Earth, 2018) was used to remove records occurring on land. Using the List of Darwin Core Terms (Darwin Core, 2021) as a reference, ‘Human Observation’, ‘Machine Observation’ and ‘Observation’ records were classified as live observations while ‘Fossil Specimen’, ‘Material Sample’ and ‘Preserved Specimen’ records were classified as carcass observations.
We augment this dataset by including observation records obtained through literature searches of scientific publications (technical reports, natural history excerpts) and grey literature (newspaper and magazine articles), which included online sources (blog posts, webpages, and social media platforms). The search strings [(‘dugong’ OR ‘sea cow’) AND (‘Johor Strait’ OR ‘Singapore’ OR ‘Singapore Strait’)] and [(‘dugong feeding trails’ OR ‘dugong grazing trails’) AND (‘Johor Strait’ OR ‘Singapore’ Or ‘Singapore Strait’)] were used. All information with reference to dugongs, including sightings of live or dead dugongs, carcass recoveries, and feeding trails were collated and assessed. We also examined scientific specimens and records of dugongs deposited at the Lee Kong Chian Natural History Museum (LCKNHM), the national biodiversity repository of Singapore. A total of 69 records, comprising 25 live and 44 carcass observations from 1820 to 2021, detailed in Table S1, were obtained. Additional information attached to the records, which often detailed biometric information (sex, length and weight) and/or the circumstance of the observation (e.g., nylon rope around body or lacerations present), were used to identify patterns associated to dugong observations and key threats present in our study area.
We prepared a series of maps from the years 1954, 1993, 2002, 2012 and 2022 to plot long-term shoreline and coastal transformations for our study area. Photo editing software (Adobe Photoshop Creative Cloud 2018) was used to trace and superimpose the historic map of 1954 Singapore (1: 63,360; Survey Department, Federation of Malaya) and topographic maps of 1993, 2002 and 2012 Singapore (1:50,000; Singapore Armed Forces Mapping Unit). The resultant images were then superimposed onto 2012 and 2022 coastline shapefiles of our study area obtained from OpenStreetMaps. Live and carcass observations with specific location information (60 of 69 records) were overlaid onto these maps to visualize the changes in available habitat for the dugong over time. Taking the coordinates 1°22’00.0”N, 103°48’00.0”E as the center of Singapore, dugong observations were split into four locations: western Johor Strait [WJS], eastern Johor Strait [EJS], western Singapore Strait [WSS] and eastern Singapore Strait [ESS]. Euclidean-based hierarchical distance clustering and Z-score analysis of observation counts were conducted to identify dugong hotspots.
Field surveys
We identified five seagrass meadows, based on our literature review, with consistent contemporary observations of dugong feeding trails (DFTs); four of these [Changi Beach Park Carpark 6, Changi Beach Park Carpark 7, Changi Civil Service Club, and Tanjung (=Cape in Malay) Chek Jawa] are within the Johor Strait and one [Terumbu (=Reef in Malay) Pandan] is within the Singapore Strait (see Table 1 for details). Field surveys to assess the distribution of DFTs were conducted at these five sites during the Southwest monsoon (June to September) and the second inter-monsoonal period (October to November) of 2018, 2019 and 2021.
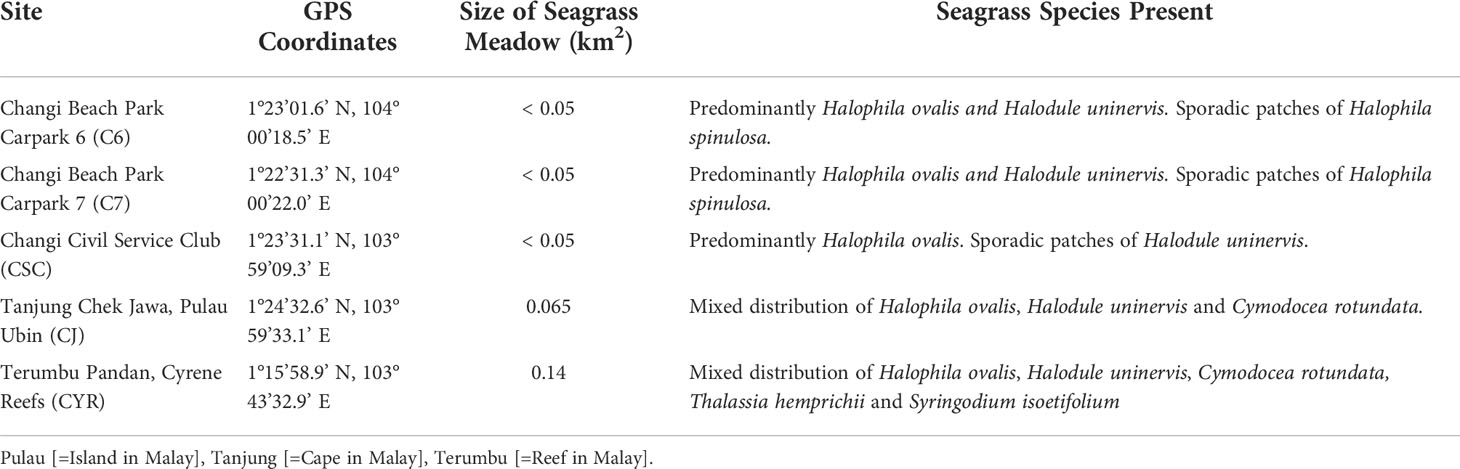
Table 1 Seagrass beds within territorial waters of Singapore surveyed for contemporary dugong feeding trails; area of seagrass meadow obtained from Yaakub et al. (2013).
Due to the turbid water conditions across all sites, strip transects parallel to the shoreline were conducted on foot during the low spring tides of each month. Three criteria were used to discriminate DFTs from other furrows present in the seagrass meadow. First, the difference in seagrass percentage cover between the seagrass bed directly adjacent to the furrow and within the furrow must be more than 70% (Preen, 1992; de Iongh et al., 1995; Preen, 1995; de Iongh et al., 1998; Nakaoka and Aioi, 1999; Lanyon and Sanson, 2006; Skilleter et al., 2007). Percentage cover was estimated with reference to Mellors (1991), and sample quadrats detailed in the Seagrass Watch program protocol (McKenzie et al., 2003). Based on the assumption that the dimensions of a DFT corresponds to the orofacial disk size of the dugong (Preen, 1992; Marshall et al., 2003), the second criterion for a furrow to be deemed as a DFT is that its width is approximately equal throughout. Thirdly, also based on the above assumption, a trail’s average width and depth should be between 10 - 28 cm and 2 - 6 cm respectively (Wake, 1975; Heinsohn et al., 1977; Anderson and Birtles, 1978; Preen, 1992; Nakaoka and Aioi, 1999; Petcharat and Lee, 2020). For each DFT, maximal length and GPS coordinates as well as averaged width and depth measurements were recorded over three random points. All measurements were recorded to the accuracy of ±0.5 cm. Seagrass species adjacent to each ascertained DFT were identified and recorded.
Results
A total of 7829 distinct dugong records throughout its entire range were obtained from the GBIF and OBIS datasets, which included observations between 1905 - 2021. Of these, 7722 records were of live observations. The majority of global dugong observations were along the northern coast of Australia (Figure S1). Records of live observations were common in eastern Africa (Kenya, Tanzania, Mozambique, Comoros and Madagascar) and the Pacific Islands east of Australia (New Caledonia and Vanuatu). In Asia, a majority of the observations, mostly recorded in Thailand, were of carcasses. Other than one live observation along the eastern Singapore Strait, there were no dugong records within the western Malay Archipelago (see boxed area in Figure S1).
When other information sources (scientific literature, grey literature, online sources and natural history museum records) were incorporated, a total of 70 distinct dugong records were recovered along the Johor and Singapore Straits (Table S1). This included the single live observation from the GBIF and OBIS datasets, which was subsequently removed from further analyses due to absence of information on the date of record. Of all records with spatial and temporal information (n = 69; 1820 - 2021), only 25 (36%) were of live dugongs while the remainder were of carcasses (Table 2). Most records were observations of single individuals (59 records). All seven records of dugong pairs were of mothers and their calves (Table S1). A herd of more than two dugongs were recorded only three times — these were along the Johor Strait, off the coast of Gelang Patah and Pulau Nanas, Malaysia (Table S1). Of the 19 records in which additional information was provided, nine (50%) involved fishing gear entanglement while six (33%) presented with multiple lacerations likely originating from fishing and boating activities (Table 2). Other than four records of live disentanglement, all records accompanied with circumstantial information were of carcasses.
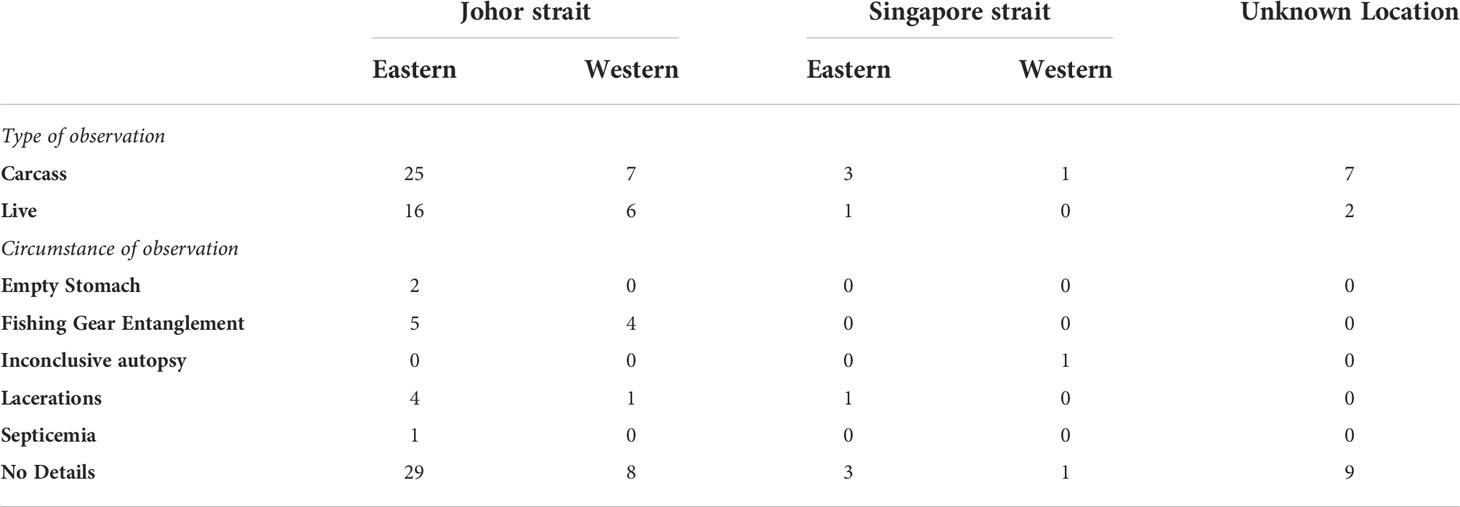
Table 2 Dugong observations along the coastlines of southern Johor and Singapore between 1820 and 2021. Refer to Table S1 for more details.
35 dugong observations (50.7% of all records) included information on sex, length, and/or weight (Table S1). Of these, there were approximately equal numbers of male and female dugongs (11 vs 8 respectively, n = 19), dugongs were of an average length of 2.0 m (n = 26) and an average weight of 81.1 kg (n = 9) (Figure S2). More observations were recorded from 1975 onwards (Figure 1A). Our cumulative data reveals a bimodal peak for carcass observations in March and July (Figure 1B), and a single peak in March for live observations.
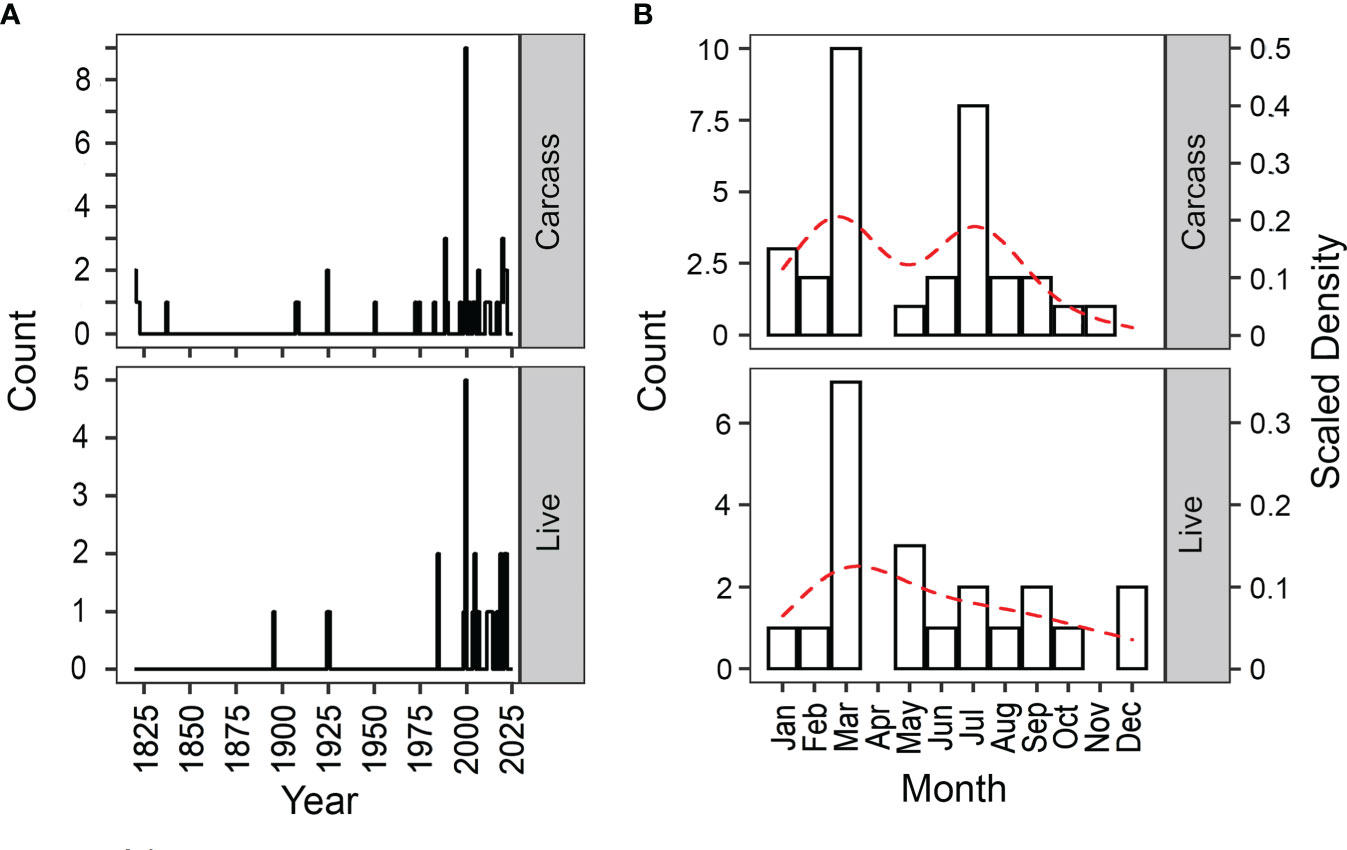
Figure 1 Temporal trends in dugong observations (1820 - 2021), by (A) year and (B) month, within the Johor and Singapore Straits. The dotted red line in (B) depicts the kernel density estimate (reflected on secondary axis) of live and carcass observations.
Prior to the establishment of the political border between Malaysia and Singapore in 1965, 64% (9 out of 14) of the records were vaguely reported to occur in ‘Singapore’ or ‘Johor Strait’. These observations with no specific location information (n = 9) were omitted and the remaining 60 dugong observations were used to evaluate spatial trends. The eastern Johor Strait is a historical and contemporary hotspot for dugongs, with 70% (42 out of 60 records) occurring in areas around Changi Beach, Pulau Tekong and Pulau Ubin (Insert A in Figure 2). 65% (31 out of 48) of dugong observations in the last 25 years (1997 - 2022) occurred in the eastern Johor Strait (Table S1), underpinning this area as most distinct out of the four broad areas. The three remaining broad areas are characterized by low dugong observations.
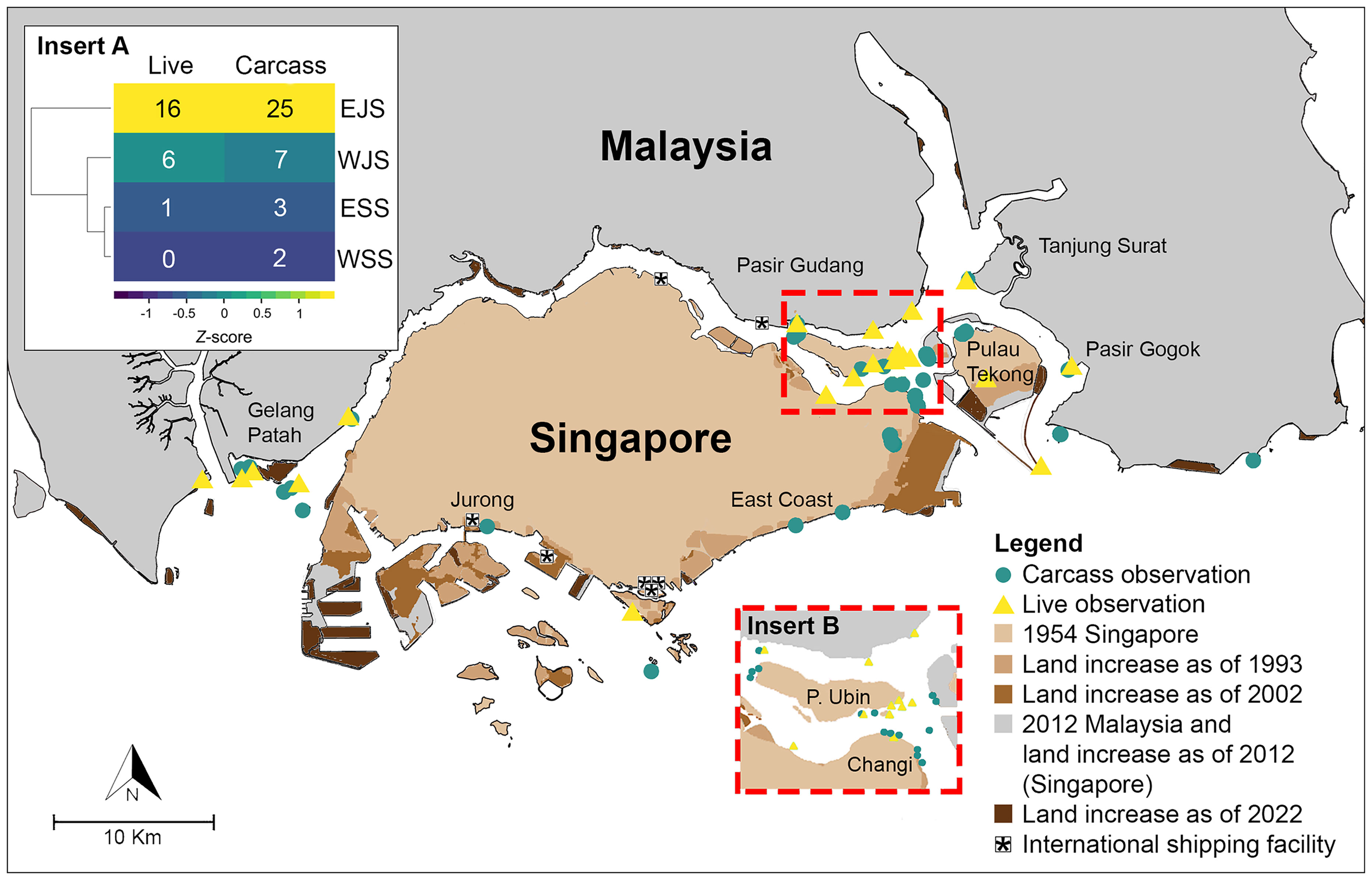
Figure 2 Distribution of live and carcass observations in Singapore between 1954 and 2020. Insert shows the section of the eastern Johor Strait where the most dugong observations were recorded. Records which were not accompanied with location information are not represented in this figure. Insert A: Hotspots of dugong observations revealed through Euclidean-based hierarchical distance clustering. EJS, WJS, ESS and WSS refers to the eastern and western Johor Strait and the eastern and western Singapore Strait respectively. Numbers depict the counts per observation in each broad locality.
Historical coastal land reclamation works along the Johor and Singapore Straits were extensive, and drastically altered habitats along the eastern and western coast of Singapore island, western coast of Johor and islands along the eastern Johor Strait and western Singapore Strait (Figure 5). Seven sites where carcasses were reported—previously coastal habitats—are present-day reclaimed land areas (Figure 2; Table S1). Specifically, between 1954 to 1993, major shoreline changes along the northeastern and southeastern coast of Singapore island and other islands in the western Singapore Strait were observed. Land reclamation works, between 1993 to 2022, expanded on already reclaimed areas along the eastern coast of Singapore island and Pulau Tekong. Recent large-scale coastal development is also observed in the islands within the western Singapore Strait and along the Pulai River Estuary in the western Johor Strait.
Six out of the 14 sites where DFTs are reported to occur, were solely identified from online sources (Table S2). Dugong feeding trails within the Johor Strait were recorded as early as 2005, these observations were concentrated along seagrass meadows at Changi Beach, Tanjung Chek Jawa and coastal sites along the Johor River (Figure 3A). Over the entire field survey period, 107 DFTs were observed and assessed at all five sites (Figure S3; Table 1). The mode intervals of maximal length, width, and depth of all DFTs were 125 - 175 cm, 17 - 19 cm, and 2.5 - 3.5 cm, respectively (Figure S3). 14% were narrower than 16 cm; 79% of all DFTs were 16 - 24 cm wide; and 7% were wider 24 cm. In the western Singapore Strait, DFTs were observed within the seagrass meadows adjacent to international shipping facilities (Figure 3B). Halophilia ovalis and Halodule uninervis were the most common species of seagrass along dugong feeding trails within our field survey sites (62.4% and 28.9% per feeding trail on average; Figure 3C).
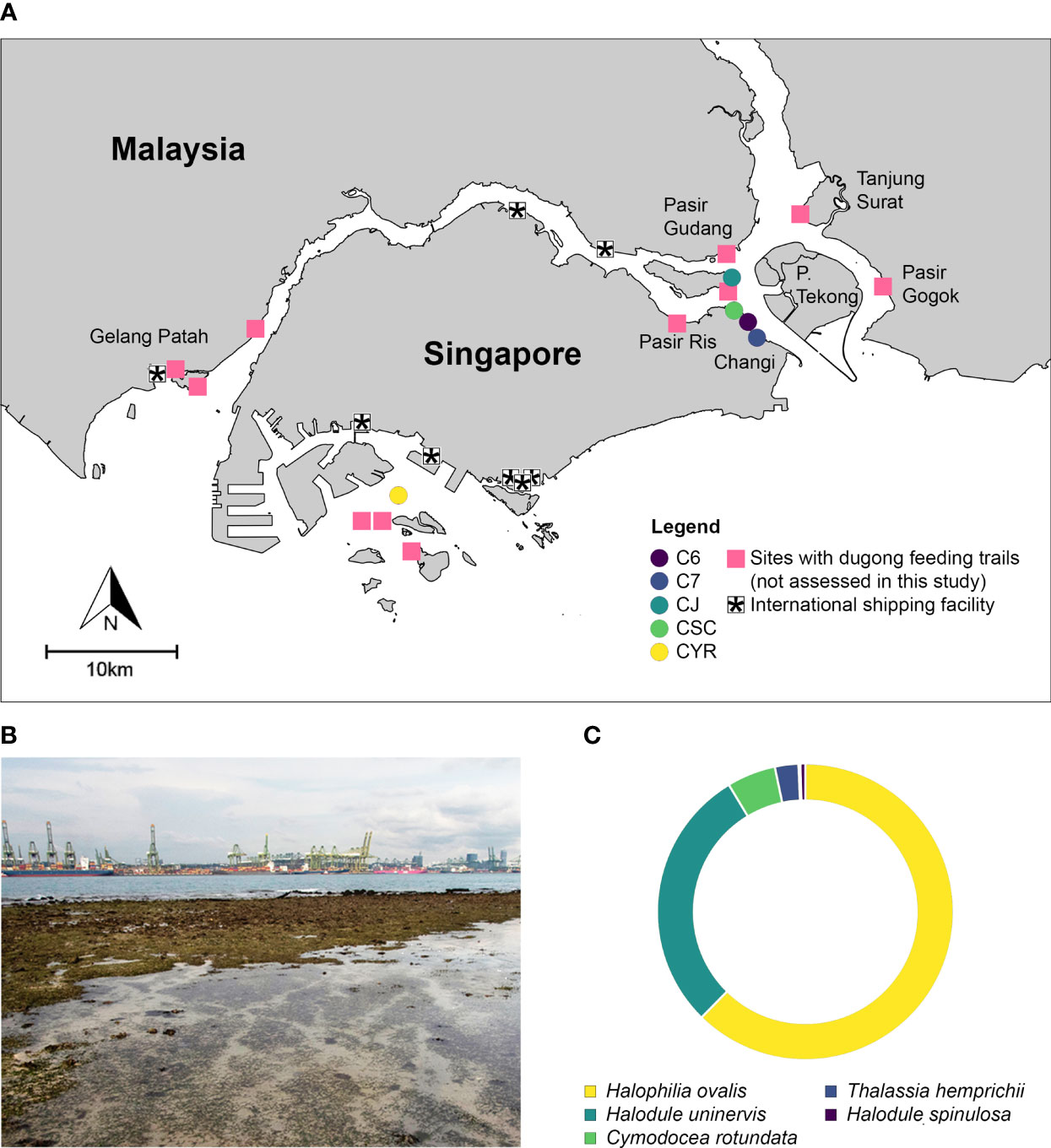
Figure 3 Dugong feeding trails along the coastlines of southern Johor and Singapore. (A) Location of sites where dugong feeding trails were reported and ascertained. (B) Dugong feeding trails along Terumbu Pandan, Cyrene Reefs, mere meters from international shipping lanes/facilities. (C) Proportion of seagrass species adjacent to an average dugong feeding trail within our field survey sites.
Discussion
Our study integrates historical and contemporary information, by coupling field surveys with extensive reviews of literature and publicly available data, to understand the distribution trends of dugongs along one of the busiest urban coastal areas. We identified the western Malay Archipelago as a region with scant information on dugong populations, with only one data point in the GBIF and OBIS combined dataset (Figure S1). Our review of published, grey, and online literature recovered an additional 69 live and carcass observations of dugongs in our study area (Table S1). Records of dugong observations steadily increased since 1975, in part due to concerted documentation and ease of record sharing over online and social media platforms. 74% these observations were supplemented with additional information that allude to the circumstances in which the individual was recovered; and about half (49%) included some biometric data. Although unstructured and opportunistic, the inclusion of such information enabled us to elucidate temporal trends and identify spatial hotspots for dugongs within this data-poor region.
Live and carcass observations increased between January and March during the Northeast monsoon, corroborating the trend observed in the 19th century when dugongs were actively hunted (Raffles, 1820). The peak in observations during these months were attributed, although not solely to, the increase in coastal anthropogenic activities resulting from fairer weather during the dry phase of the Northeast monsoon (Meteorological Service Singapore, 2021). We further hypothesize that this peak coincides with a period of improved foraging opportunity, when food availability increases and intertidal seagrass meadows become more accessible. The months between January and March have been proposed to be the growing season for seagrass species due to increased light availability, photosynthetically active radiation, and temperature (McKenzie et al., 2016). In addition to the increase in available seagrass biomass, new plant growth possesses higher nitrogenous content, suggested to be preferred by these nitrogen-limited hindgut fermenting mammals (Marsh et al., 2011). Further, over these months, changes to the mixed semidiurnal tidal cycle regime of the area renders reduced variability in tidal height (van Maren and Gerritsen, 2012). Consequently, intertidal seagrass meadows remain submerged for longer periods, and thus are more accessible for foraging dugongs. These factors, in tandem, suggest that dugongs increase their use of shallower habitats and, in turn, chances of human-dugong interactions between January and March.
Carcass observations peak again between May and July, during the Southwest monsoon. These months coincide with the proposed seasonal decline in seagrass biomass (McKenzie et al., 2016). Variations per tidal cycle and exposure times of intertidal seagrass meadow at night during this period are the highest in the year (van Maren and Gerritsen, 2012; McKenzie et al., 2016). This reduces the number of high tides after dusk — an optimal foraging period for dugongs, due to the increased accessibility to intertidal meadows and lowered chances of predation (Wirsing et al., 2007b; Sheppard et al., 2009; Sheppard et al., 2010; Derville et al., 2022). These sub-optimal elocal conditions, which limit the available window for foraging dugongs to access nearshore habitats, are accompanied by increases in mean wind speed (McKenzie et al., 2016) and vessel traffic (Department of Statistics Singapore, 2022a; Department of Statistics Singapore, 2022b). We suggest that the combination of wind or vessel-generated waves and vessel noise could have also negatively affected foraging activities along the nearshore habitats of the Johor and Singapore Straits (de Iongh et al., 2007; Sheppard et al., 2009; Budiarsa et al., 2021). Dugongs along these areas could have temporarily migrated to less anthropogenically disturbed seagrass meadows in the vicinity (de Iongh et al., 1998; Budiarsa et al., 2021), and account for the fewer records of live observations during this period.
Our results corroborate previous studies that identified the eastern Johor Strait as a critical area for dugongs (Raffles, 1820; Marsh et al., 2002; Hashim et al., 2017). Their presence can be due to the many shallow bays ideal for calving or nurturing offspring (Preen, 1989; Anderson, 1997; Adulyanukosol et al., 2007). Intertidal ecosystems in the eastern Johor Strait are strongly influenced by the high fluvial input from the Johor River (Loh et al., 2009; van Maren et al., 2014; Phang et al., 2015), and thus favor fast-growing seagrass species such Halophila ovalis and Halodule uninervis (Affendi et al., 2005; McKenzie et al., 2016). Adaptations in resource allocation (Benham et al., 2019) and settlement strategies (Lai et al., 2018) confer additional competitive advantages to H. ovalis — especially in sections with high vessel-mediated wave action within the narrow Johor Strait (Tan et al., 2016). Our field surveys identified H. ovalis and Hd. uninervis to be the most common seagrass species along DFTs across all sites. Digesta and observation studies revealed these species as important diet components for dugongs in the tropics (Marsh et al., 1982; Anderson, 1998; Nakaoka and Aioi, 1999; Masini et al., 2001; de Iongh et al., 2007; Tol et al., 2016). This is corroborated by the autopsy of a dugong carcass (Sivasothi, 2006), within our study site, which found approximately 30kg of H. ovalis at various stages of digestion within the digestive tract (Z. Jaafar, pers. obs.). Although these observations may simply reflect the availability and abundance of H. ovalis and Hd. uninervis (André et al., 2005), studies have proposed dugong foraging preferences towards seagrass species characterised by their digestibility, and high nitrogenous and fibre content (Johnstone and Hudson, 1981; Preen, 1992; Sheppard et al., 2006; Sheppard et al., 2008; Sheppard et al., 2010).
Urbanization of coastal areas and the resultant increase in anthropogenic uses of sea spaces have been identified as key drivers of dugong population decline in Southeast Asia (Hines, 2002; Mustika, 2006; Hines et al., 2012; Ponnampalam et al., 2015). Land reclamation activities along the Johor and Singapore Straits started in the mid-1800s (Richards et al., 1994; Corlett, 2000), and peaked in the years after 1965 (Hilton and Manning, 1995). Between 1960 and 2012 alone, land reclamation resulted in a 22% increase of the original land area of Singapore (Department of Statistics Singapore, 2021a). This consequently caused a tenfold reduction of intertidal habitats, seagrass meadows included, to approximately 3 km2 at present (Wee and Corlett, 1986; Hilton and Manning, 1995; Lai et al., 2015). Dredging and land reclamation works along the Johor Strait over the past decade were focused along the coastal areas of the Pulai and Johor Rivers (Choo and Ponnampalam, 2011; Rizzo and Glasson, 2012; Hashim et al., 2017). Ongoing and future reclamation plans in these areas, identified to be of high conservation importance (Bujang et al., 2006; Bujang et al., 2018; Rahman and Yaakub, 2020), will likely result in drastic losses of safe foraging, birthing, and calving grounds for dugong populations therein (Ponnampalam et al., 2022). In northeast Brazil, the anthropogenic-mediated loss of birthing grounds (Parente et al., 2004) was linked to the increased chances of mother-calf separation, and calf stranding rates of Antillean manatees (Balensiefer et al., 2017). This phenomenon is also reflected in our dataset; calf carcasses (≤ 2m in length; see Marsh, 1980; Cherdsukjai et al., 2020) accounted for more than 45% of the records with biometric information (Table S1). The autopsy of a 1.4m male recovered within our study site in 2021 concluded that pre-mature mother-calf separation was the likely cause of death as the suckling calf presented with no signs of physical trauma or disease, and its stomach was full of milk and seagrass (S. Z. H. Ng and Z. Jaafar, pers. obs.).
The difficulties in ascertaining their presence, on account of their elusive behaviour and lack of dorsal fins, result in elevated human-dugong interactions such as fishing gear entanglement and vessel collisions. This is exacerbated by the significant time dugongs spend near the water surface (Hodgson, 2004; Hagihara et al., 2014). Despite the low frequencies of such conflicts reflected in our dataset (vessel collisions, n = 6; fishing gear entanglement, n = 9), the probability of these observations in our study site is especially high, given the extent of coastal anthropogenic activities. Since its inception as a trading hub for the British in 1819, Singapore’s port capabilities have grown exponentially due to its strategic position as a primary shipping conduit linking the Indian and Pacific Oceans. Between the 1980s to the 2010s, the volume of cargo passing through Singapore increased almost twenty-fold, with a total of 626,521,200 tonnes reported in 2019 alone (Department of Statistics Singapore, 2021b). Similarly, major ports are in operation along the Malaysian coasts of the Johor Strait (Pasir [=Sand in Malay] Gudang and Tanjung Pelepas). Along this Strait, approximately 600m wide at the narrowest point, there are 108 sea-based mariculture farms registered with the Singapore Government. A total of seven Abandoned, Lost or Discarded Fishing Gears (ALDFGs), identified as mariculture-cage nets, have washed up on Singapore’s coastlines along the eastern Johor Strait between 2000 to 2019 (Gajanur and Jaafar, 2022). While fishing law enforcement has been shown to effectively reduce fishing related dugong mortality (Marsh, 2000), this issue remains in areas such as the Johor Strait, which abuts two countries with different sets of legislations and multiple stakeholders occupying the shorelines (Ponnampalam et al., 2015). Bans, both partial and entire, on trawl fishing in both countries (Fisheries act 1985, 2012; Fisheries act 1966, 2021) are in effect but their use is still prevalent (Hashim et al., 2017).
Dugongs have been documented to move in response to changes in environmental conditions and forage accessibility (Deutsch et al., 2022a; Deutsch et al., 2022b), and in some cases, display avoidance to areas of anthropogenic activity (Anderson and Birtles, 1978; Anderson, 1982; Preen, 1989;Adulyanukosol et al., 2007; Hodgson and Marsh, 2007; Wirsing et al., 2007a). Yet, our study ascertained dugong feeding activity along seagrass meadows immediately adjacent to areas of high anthropogenic use. The varying widths of DFTs within our small survey sites indicate that one, or a small herd of dugongs utilize these meadows on any given day. These sites are within, or mere meters away from, human activities such as recreation (swimming, SCUBA diving, picnics, boating, kayaking), fishing (both leisure and artisanal), industries (oil refineries) and shipping. The sustained use of high-risk areas by dugongs is not exclusive to our study area (see O’Shea et al., 2022). We suggest that key habitats within urbanized areas, especially those with lower chances of human-dugong interactions, remain as refugia. This is evident in our study where only two carcass observations were recovered from the highly disturbed western Singapore Strait, despite the active and sustained use of seagrass meadows within. These seagrass meadows are structurally different from those in the Johor Strait, as they occur offshore and are adjacent to fore reef zones with complex bathymetry (van Maren and Gerritsen, 2012); thus, potentially afford refuge from both natural and anthropogenic (Wirsing et al., 2007b; Wirsing et al., 2007c; Cleguer et al., 2020) threats. However, we are uncertain to their sustainability as viable long-term habitats, given plans for further coastal modifications.
Our study represents the first attempt in describing and evaluating dugong distribution in the hyper-urbanized coastal areas of Southeast Asia. We have identified spatial and temporal distribution trends, highlighted key threats, and ascertained the continued use of seagrass meadows in close proximity to areas of high anthropogenic disturbance. This information is especially useful in guiding conservation management strategies, with the latter highlighting the need to better understand the role of socially transmitted knowledge in sirenian use of space (O’Shea et al., 2022). Knowledge transfer via social learning is increasingly incorporated in the management strategies of threatened populations that display sociality (Brakes et al., 2019). We thus emphasize on the importance to identify, and stem the exploitation of, dugong foraging grounds along urbanized coastlines. We further recommend currently unprotected reef-associated seagrass meadows to receive increased scientific and conservation attention. These conservation strategies can benefit with increased transboundary collaboration. Researchers and conservation managers can leverage on the inherent fascination members of the public have for this flagship species to galvanize and rejuvenate protection efforts for dugongs and coastal ecosystems in peril (Long et al., 1999; Kurien, 2007; Dobbs et al., 2008; Petcharat and Lee, 2020). In areas where the future development of coastal space remains inevitable, whole losses of key seagrass habitats can be avoided through the wider adoption of clearly defined, multi-level stakeholder Environmental Impact Assessment frameworks (Yaakub et al., 2014). Coastal development projects can be coupled with ecologically sound mitigative measures (Chou and Tun, 2007; DHI Group, 2016) and designs that incorporate eco-engineering techniques (Loke et al., 2016; Strain et al., 2018; Tan et al., 2020) to alleviate anthropogenic stress on affected habitats. These steps are integral in the rapidly urbanizing Southeast Asian region, where ecological information—notably in the western Malay Archipelago—regarding dugong populations and seagrass ecosystems remain scarce (Marsh et al., 2002; Hines et al., 2005; Ooi et al., 2011; Fortes et al., 2018; Panyawai & Prathep, 2022).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
All authors contributed to the study’s conceptualization and design. SN collected the data, analyzed and interpreted the results. SN prepared the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by two EXPLORE Young Marine Scientist Research Awards from the Marine Science Research and Development Programme by the National Research Foundation, Singapore that were received by SN. Research funding from the Department of Biological Sciences, National University of Singapore, received by ZJ, was also used for the funding of this study.
Acknowledgments
We are grateful to Mr. Joseph Cham and Ms Celine Wong for field assistance. We thank the two reviewers whose comments improved this manuscript. This study was partially funded by the EXPLORE Young Marine Scientist Research Award by the Marine Science Research and Development Programme of the National Research Foundation, Singapore awarded to Sirius Ng. The authors would like to acknowledge the St. John’s Island National Marine Laboratory for providing the facility necessary for conducting the research. The Laboratory is a National Research Infrastructure under the National Research Foundation, Singapore. The study was conducted in accordance to permits NP/RP 18-057 and NP/RP 18-057a issued by National Parks Board Singapore.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.947700/full#supplementary-material
Supplementary Figure 1 | Global distribution of dugong observations (1905 - 2022), live (n = 7829) and carcass (n = 107), obtained from the Global Biodiversity Information Facility and Ocean Biodiversity Information System datasets. Only one dugong observation was recorded in the western Malay Archipelago (boxed area), specifically along the eastern Singapore Strait.
Supplementary Figure 2 | Biometric information, a) length (n = 26), b) weight (n = 9), and c) sex (n = 19), of dugong individuals as detailed in observation records between 1820 to 2021.
Supplementary Figure 3 | Dimensions of 107 dugong feeding trails found across five study sites in the Johor Strait and Singapore Strait.
References
AAPA (2016)World port rankings 2016. Available at: https://www.aapa-ports.org/unifying/content.aspx?ItemNumber=21048 (Accessed July 21, 2021).
Affendi Y. A., Ooi J. L. S., Phang S. M., Hussin A., Rosman F. R., Fauzi R. (2005). Dugongs in peril: the conservation and protection of dugongs in johor, Malaysia. Final report to Titan Chemicals Corp Bhd and United Nations Development Programme (UNDP). 110pp.
Anderson P. K. (1982). Studies of dugongs at shark bay, Western australia. ii.* surface and subsurface observations. Wildl. Res. 9, 85–99. doi: 10.1071/wr9820085
Anderson P. K. (1997). Shark bay dugongs in summer. i: lek mating. Behaviour 134, 433–462. doi: 10.1163/156853997X00629
Anderson P. K. (1998). Shark bay dugongs (Dugong dugon) in summer. ii: foragers in a halodule-dominated community. Mammalia 62, 409–426. doi: 10.1515/mamm.1998.62.3.409
Anderson P. K., Birtles A. (1978). Behaviour and ecology of the dugong, dugong dugon (Sirenia): observations in shoalwater and Cleveland bays, queensland. Wildl. Res. 5, 1–23. doi: 10.1071/wr9780001
André J., Gyuris E., Lawler I. R., André J., Gyuris E., Lawler I. R. (2005). Comparison of the diets of sympatric dugongs and green turtles on the orman reefs, Torres strait, Australia. Wildl. Res. 32, 53–62. doi: 10.1071/WR04015
Johor port berhad overview of johor port. Available at: https://www.johorport.com.my/about-us/overview (Accessed January 25, 2022).
Balensiefer D. C., Attademo F. L. N., Sousa G. P., Freire A. C. D. B., da Cunha F. A. G. C., Alencar A. E. B., et al. (2017). Three decades of antillean manatee (Trichechus manatus manatus) stranding along the Brazilian coast. Trop. Conserv. Sci. 10, 1–9. doi: 10.1177/1940082917728375
Benham C. F., Beavis S. G., Jackson E. L. (2019). Tolerance of tropical seagrasses zostera muelleri and halophila ovalis to burial: toward an understanding of threshold effects. Estuar. Coast. Shelf Sci. 218, 131–138. doi: 10.1016/j.ecss.2018.11.005
Boyd I. L., Lockyer C., Marsh H. D. (1999). “Reproduction in marine mammals,” in Biology of marine mammals (Washington D.C: Smithsonian Institution Press).
Brakes P., Dall S. R., Aplin L. M., Bearhop S., Carroll E. L., Ciucci P., et al. (2019). Animal cultures matter for conservation. Science 363, 1032–1034. doi: 10.1126/science.aaw3557
Budiarsa A. A., de Iongh H. H., Kustiawan W., van Bodegom P. M. (2021). Dugong foraging behavior on tropical intertidal seagrass meadows: the influence of climatic drivers and anthropogenic disturbance. Hydrobiologia 848, 4153–4166. doi: 10.1007/s10750-021-04583-0
Bujang J. S., Zakaria M. H., Arshad A. (2006). Distribution and significance of seagrass ecosystems in Malaysia. Aquat. Ecosyst. Health Manage. 9, 203–214. doi: 10.1080/14634980600705576
Bujang J. S., Zakaria M. H., Short F. T. (2018). “Seagrass in Malaysia: Issues and challenges ahead,” in The wetland book II: Distribution, description, and conservation. Eds. Finlayson C. M., Milton G. R., Prentice R. C., Davidson N. C. (Dordrecht, The Netherlands:: Springer), 1875–1883.
Burke L., Reytar K., Spalding M., Perry A. (2011). Reefs at risk revisited (Washington D. C: World Resources Institute), 115.
Channel News Asia (2019)Need for third bridge between johor and Singapore to ease congestion: Malaysian home affairs minister. Available at: https://www.channelnewsAsia.com/Asia/johor-Singapore-third-bridge-home-minister-muhyiddin-852041 (Accessed January 21, 2022).
Cherdsukjai P., Buddhachat K., Brown J., Kaewkool M., Poommouang A., Kaewmong P., et al. (2020). Age relationships with telomere length, body weight and body length in wild dugong (Dugong dugon). PeerJ 8, 1–18. doi: 10.7717/peerj.10319
Choo C. K., Ponnampalam L. S. (2011). Empowering coastal communities for marine biodiversity management at the pulai river estuary. Project MAL/SGP/OP4/Y1/RAF/2008/FP-09(53) Final Report to the GEF-SGP. 41 pp.
Chou L. M. (2006). “Marine habitats in one of the world’s busiest harbours,” in The environment in Asia pacific harbours. Ed. Wolanski E. (Dordrecht: Springer Netherlands), 377–391. doi: 10.1007/1-4020-3655-8_22
Chou L. M., Tun K. P. P. (2007). Conserving reefs beside a marine landfill in Singapore. Coral Reefs 26, 719–719. doi: 10.1007/s00338-007-0220-3
Cleguer C., Garrigue C., Marsh H. D. (2020). Dugong (Dugong dugon) movements and habitat use in a coral reef lagoonal ecosystem. Endanger. Species Res. 43, 167–181. doi: 10.3354/esr01061
Corlett R. T. (2000). “Environmental heterogeneity and species survival in degraded tropical landscapes,” in The ecological consequences of environmental heterogeneity: 40th symposium of the British ecological society. (Oxford, UK: Cambridge University Press), 333–355.
Darwin Core (2021)Darwin Core quick reference guide. Available at: https://dwc.tdwg.org/terms/ (Accessed January 12, 2022).
Davison G. W., Ng P. K. L., Ho H. C. (2008). The Singapore red data book: threatened plants & animals of Singapore (Singapore: Nature Society of Singapore).
de Iongh H. H., Kiswara W., Kustiawan W., Loth P. E. (2007). A review of research on the interactions between dugongs (Dugong dugon müller 1776) and intertidal seagrass beds in Indonesia. Hydrobiologia 591, 73. doi: 10.1007/s10750-007-0785-4
de Iongh H. H., Langeveld P., van der Wal M. (1998). Movement and home ranges of dugongs around the lease islands, East Indonesia. Mar. Ecol. 19, 179–193. doi: 10.1111/j.1439-0485.1998.tb00461.x
de Iongh H. H., Wenno B. J., Meelis E. (1995). Seagrass distribution and seasonal biomass changes in relation to dugong grazing in the moluccas, East Indonesia. Aquat. Bot. 50, 1–19. doi: 10.1016/0304-3770(94)00438-R
Department of Statistics Singapore (2021a)Land area (as at June), annual. Available at: https://tablebuilder.singstat.gov.sg/table/TS/M890621 (Accessed December 17, 2021).
Department of Statistics Singapore (2021b)Sea Cargo and shipping statistics, annual. Available at: https://tablebuilder.singstat.gov.sg/table/TS/M650381 (Accessed December 17, 2021).
Department of Statistics Singapore (2022a)Vessel arrivals (>75 gt), monthly. Available at: https://data.gov.sg/dataset/vessel-arrivals-75-gt-monthly?view_id=e1ca2f7c-fe69-4662-b6c3-771738fa7316&resource_id=0db7afb5-bd73-4ae9-ba30-cc7037551cab (Accessed February 16, 2022).
Department of Statistics Singapore (2022b)Vessel calls (>75 gt), monthly. Available at: https://data.gov.sg/dataset/vessel-calls-75-gt-monthly?view_id=7ab93032-512d-487c-b509-5939f6016ac5&resource_id=876eedf5-4f78-4e61-aa0a-2a322e277922 (Accessed February 16, 2022).
Derville S., Cleguer C., Garrigue C. (2022). Ecoregional and temporal dynamics of dugong habitat use in a complex coral reef lagoon ecosystem. Sci. Rep. 12, 552. doi: 10.1038/s41598-021-04412-3
DESA U. N. (2019)World population prospects 2019, online edition. rev. 1. total population - both sexes. Available at: https://population.un.org/wpp/Download/Standard/Population/ (Accessed July 20, 2020).
Deutsch C. J., Castelblanco-Martínez D. N., Cleguer C., Groom R. (2022b). “Movement behavior of manatees and dugongs: II. small-scale movements reflect adaptations to dynamic aquatic environments,” in Ethology and behavioral ecology of sirenia. Ed. Marsh H. (Switzerland: Springer, Cham), 233–298.
Deutsch C. J., Castelblanco-Martínez D. N., Groom R., Cleguer C. (2022a). “Movement behavior of manatees and dugongs: I. environmental challenges drive diversity in migratory patterns and other large-scale movements,” in Ethology and behavioral ecology of sirenia. Ed. Marsh H. (Springer, Cham: Switzerland), 155–231.
DHI Group (2016)Reclaiming the land, protecting the environment: using EEMP to protect singapore’s natural heritage. Available at: http://www.dhigroup.com/upload/publications/scribd/228966594-Reclaiming-the-Land-Protecting-the-Environment-DHI-Case-Story-SG.pdf (Accessed January 10, 2020).
Dobbs K., Fernandes L., Slegers S., Jago B., Thompson L., Hall J., et al. (2008). Incorporating dugong habitats into the marine protected area design for the great barrier reef marine park, Queensland, Australia. Ocean Coast. Manage. 51, 368–375. doi: 10.1016/j.ocecoaman.2007.08.001
Duarte C. M. (2002). The future of seagrass meadows. Environ. Conserv. 29, 192–206. doi: 10.1017/S0376892902000127
Firth L. B., Knights A. M., Bridger D., Evans A. J., Mieszkowska N., Moore P. J., et al. (2016). “Ocean sprawl: challenges and opportunities for biodiversity management in a changing world,” in Oceanography and marine biology: An annual review, vol. 54 . Eds. Hughes R. N., Hughes D. J., Smith I. P., Dale A. C. (Boca Raton, Florida: CRC Press), 189–262.
Fortes M. D. (1995). Seagrasses of East Asia: environmental and management perspectives (Bangkok, Thailand: United Nations Environment Programme).
Fortes M. D., Ooi J. L. S., Tan Y. M., Prathep A., Bujang J. S., Yaakub S. M. (2018). Seagrass in southeast Asia: a review of status and knowledge gaps, and a road map for conservation. Bot. Mar. 61, 269–288. doi: 10.1515/bot-2018-0008
Gajanur A. R., Jaafar Z. (2022). Abandoned, lost, or discarded fishing gear at urban coastlines. Mar. pollut. Bull. 175, 113341. doi: 10.1016/j.marpolbul.2022.113341
GBIF.org (2022) GBIF occurrence download. https://doi.org/10.15468/dl.xazg6k Available at: https://github.com/ropensci/rgbif (Accessed April 05, 2022).
Gomes V. H., IJff S. D., Raes N., Amaral I. L., Salomão R. P., de Souza Coelho L., et al. (2018). Species distribution modelling: Contrasting presence-only models with plot abundance data. Sci. Rep. 8 (1), 1–12. doi: 10.1038/s41598-017-18927-1
Hagihara R., Jones R. E., Grech A., Lanyon J. M., Sheppard J. K., Marsh H. D. (2014). Improving population estimates by quantifying diving and surfacing patterns: A dugong example. Mar. Mammal Sci. 30, 348–366. doi: 10.1111/mms.12041
Hashim M., Ito S., Numata S., Hosaka T., Hossain M. S., Misbari S., et al. (2017). Using fisher knowledge, mapping population, habitat suitability and risk for the conservation of dugongs in johor straits of Malaysia. Mar. Policy 78, 18–25. doi: 10.1016/j.marpol.2017.01.002
Heery E. C., Hoeksema B. W., Browne N. K., Reimer J. D., Ang P. O., Huang D., et al. (2018). Urban coral reefs: degradation and resilience of hard coral assemblages in coastal cities of East and southeast Asia. Mar. pollut. Bull. 135, 654–681. doi: 10.1016/j.marpolbul.2018.07.041
Heinsohn G. E., Wake J., Marsh H. D., Spain A. V. (1977). The dugong (Dugong dugon (Müller)) in the seagrass system. Aquaculture 12, 235–248. doi: 10.1016/0044-8486(77)90064-3
Hilton M. J., Manning S. S. (1995). Conversion of coastal habitats* in Singapore: indications of unsustainable development. Environ. Conserv. 22, 307–322. doi: 10.1017/S0376892900034883
Hines E. M. (2002). Conservation of the dugong (Dugong dugon) along the Andaman coast of Thailand: an example of the integration of conservation and biology in endangered species research (British Columbia, Canada: University of Victoria).
Hines E. M., Adulyanukosol K., Duffus D. A. (2005). Dugong (Dugong dugon) abundance along the Andaman coast of Thailand. Mar. Mammal Sci. 21, 536–549. doi: 10.1111/j.1748-7692.2005.tb01247.x
Hines E. M., Adulyanukosol K., Poochaviranon S., Somany P., Ath L. S., Cox N., et al. (2012). “Dugongs in Asia,” in Sirenian conservation (Florida, USA: University Press of Gainesville).
Hines E. M., Ponnampalam L. S., Izmal J. F., Whitty T. S., Jackson-Ricketts J., Kuit S. H., et al. (2015). Report of the third southeast Asian marine mammal symposium (SEAMAM III) (Bonn, Germany: UNEP/CMS Secretariat).
Hodgson A. J. (2004). Dugong behaviour and responses to human influences (Townsville, Australia: James Cook University).
Hodgson A. J., Marsh H. D. (2007). Response of dugongs to boat traffic: the risk of disturbance and displacement. J. Exp. Mar. Biol. Ecol. 340, 50–61. doi: 10.1016/j.jembe.2006.08.006
Jiang Y., Kirkman H., Hua A. (2001). Megacity development: managing impacts on marine environments. Ocean Coast. Manage. 44, 293–318. doi: 10.1016/S0964-5691(01)00052-7
Johnstone I. M., Hudson B. E. T. (1981). The dugong diet: mouth sample analysis. Bull. Mar. Sci. 31, 681–690.
Jones G. W. (2013). ‘The population of Southeast Asia” in Routledge Handbook of Southeast Asian Economics, ed. Coxhead I. (London: Routledge), 169–225.
Jongman B., Ward P. J., Aerts J. C. J. H. (2012). Global exposure to river and coastal flooding: long term trends and changes. Glob. Environ. Change 22, 823–835. doi: 10.1016/j.gloenvcha.2012.07.004
Kirkman H., Kirkman J. A. (2002). The management of seagrasses in southeast Asia. Bull. Mar. Sci. 71, 1379–1390.
Kurien J. (2007). “The blessing of the commons: small-scale fisheries, community property rights, and coastal natural assets,” in Reclaiming nature: Environmental justice and ecological restoration. Eds. Stanton E., Boyce J., Narain S. (London, UK: Anthem Press), 23–54. doi: 10.7135/UPO9781843313465.002
Kwan D. (2002). Towards a sustainable indigenous fishery for dugongs in Torres strait: a contribution of empirical data analysis and process (Townsville, Australia: James Cook University).
Lai S., Loke L. H. K., Hilton M. J., Bouma T. J., Todd P. A. (2015). The effects of urbanisation on coastal habitats and the potential for ecological engineering: a Singapore case study. Ocean Coast. Manage. 103, 78–85. doi: 10.1016/j.ocecoaman.2014.11.006
Lai S., Yaakub S. M., Poh T. S. M., Bouma T. J., Todd P. A. (2018). Unlikely nomads: settlement, establishment, and dislodgement processes of vegetative seagrass fragments. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00160
Lanyon J. M., Sanson G. D. (2006). Mechanical disruption of seagrass in the digestive tract of the dugong. J. Zool. 270, 277–289. doi: 10.1111/j.1469-7998.2006.00135.x
Loh K. S., Sivasothi N., Todd P. A. (2009). “Mass mortality and recruitment of intertidal marine invertebrates at chek jawa, Singapore,” in Proceedings of the 14th NUROP congress (Singapore: National University of Singapore).
Loke L. H., Liao L. M., Bouma T. J., Todd P. A. (2016). Succession of seawall algal communities on artificial substrates. Raffles Bull. Zool. 31, 1–10.
Long W. J. L., Coles R. G., McKenzie L. J. (1999). Issues for seagrass conservation management in Queensland. Pac. Conserv. Biol. 5, 321–328. doi: 10.1071/pc000321
Macusi E. D., Deepananda A. K., Conte A. R., Katikiro R. E., Fadli N., Jimenez L. A. (2011). Human induced degradation of coastal resources in Asia pacific and implications on management and food security. J. Nat. Stud. 9, 13–28.
Ma T., Li X., Bai J., Cui B. (2019). Habitat modification in relation to coastal reclamation and its impacts on waterbirds along china’s coast. Glob. Ecol. Conserv. 17, e00585. doi: 10.1016/j.gecco.2019.e00585
Marsh H. D. (1980). Age determination of the dugong (Dugong dugon (Müller)) in northern Australia and its biological implications. Rep. Int. Whal. Commn. Special Issue 3, 181–201.
Marsh H. (2000). Evaluating management initiatives aimed at reducing the mortality of dugongs in gill and mesh nets in the great barrier reef world heritage area. Mar. Mammal Sci. 16, 684–694. doi: 10.1111/j.1748-7692.2000.tb00965.x
Marshall C. D., Maeda H., Iwata M., Furuta M., Asano S., Rosas F., et al. (2003). Orofacial morphology and feeding behaviour of the dugong, amazonian, west african and antillean manatees (Mammalia: Sirenia): functional morphology of the muscular-vibrissal complex. J. Zool. 259, 245–260. doi: 10.1017/S0952836902003205
Marsh H. D., Channells P. W., Heinsohn G. E., Morrissey J. (1982). Analysis of stomach contents of dugongs from Queensland. Wildl. Res. 9, 55–67. doi: 10.1071/wr9820055
Marsh H., Heinsohn G. E., Marsh L. M. (1984). Breeding cycle, life history and population dynamics of the dugong, dugong dugon (Sirenia: Dugongidae). Aust. J. Zool. 32, 767–788. doi: 10.1071/zo9840767
Marsh H. D., O’Shea T. J., Reynolds J. E. (2011) Ecology and conservation of the sirenia: dugongs and manatees (Cambridge, UK: Cambridge University Press). Available at: http://www.cambridge.org/gb/knowledge/isbn/item6470427/?site_locale=en_GB (Accessed February 20, 2022).
Marsh H. D., Penrose H., Eros C., Hugues J. (2002). The dugong (Dugong dugon) status reports and action plans for countries and territories in its range (Gland, Switzerland: United Nations Environment Programme).
Marsh H. D., Sobtzick S. (2019). Dugong dugon. IUCN Red List Threat Species 2019: e.T6909A160756767. doi: 10.2305/IUCN.UK.2015-4.RLTS.T6909A160756767.en. (Accessed on September 04, 2022).
Masini R. J., Anderson P. K., McComb A. J. (2001). A halodule-dominated community in a subtropical embayment: physical environment, productivity, biomass, and impact of dugong grazing. Aquat. Bot. 71, 179–197. doi: 10.1016/S0304-3770(01)00181-4
McKenzie L. J., Campbell S. J., Roder C. A. (2003). Seagrass-watch: manual for mapping & monitoring seagrass resources by community (citizen) volunteers. 2nd (Cairns, Australia: Queensland Fisheries Service, Northern Fisheries Center).
McKenzie L. J., Yaakub S. M., Tan R., Seymour J., Yoshida R. L. (2016). Seagrass habitats of Singapore: environmental drivers and key processes. Raffles Bull. Zool. 34, 60–77.
Mellors J. E. (1991). An evaluation of a rapid visual technique for estimating seagrass biomass. Aquat. Bot. 42, 67–73. doi: 10.1016/0304-3770(91)90106-F
Meteorological Service Singapore (2021)Records of climate station means (climatological reference period: 1991-2020). Available at: http://www.weather.gov.sg/climate-climate-of-Singapore/ (Accessed September 2, 2021).
Mustika P. L. K. (2006). Marine mammals in the savu Sea (Indonesia): indigenous knowledge, threat analysis and management options (Townsville, Australia: James Cook University).
Nakaoka M., Aioi K. (1999). Growth of seagrass halophila ovalis at dugong trails compared to existing within-patch variation in a Thailand intertidal flat. Mar. Ecol. Prog. Ser. 184, 97–103. doi: 10.3354/meps184097
Natural Earth (2018)1:10m physical vectors. Available at: https://www.naturalearthdata.com/downloads/10m-physical-vectors/ (Accessed January 15, 2022).
Nelson Sella K. A., Sicius L., Fuentes M. M. P. B. (2019). Using expert elicitation to determine the relative impact of coastal modifications on marine turtle nesting grounds. Coast. Manage. 47, 492–506. doi: 10.1080/08920753.2019.1642176
Ng H. H., Tan H. H., Lim K. K. P., Ludt W. B., Chakrabarty P. (2015). Fishes of the eastern johor strait. Raffles Bull. Zool. 31, 303–337.
OBIS (2022) Distribution records of dugong dugon (Müller 1776) (Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO). Available at: www.obis.org (Accessed January 11, 2022).
Ooi J. L. S., Kendrick G. A., Van Niel K. P., Affendi Y. A. (2011). Knowledge gaps in tropical southeast Asian seagrass systems. Estuar. Coast. Shelf Sci. 92, 118–131. doi: 10.1016/j.ecss.2010.12.021
Orth R. J., Carruthers T. J. B., Dennison W. C., Duarte C. M., Fourqurean J. W., Heck K. L., et al. (2006). A global crisis for seagrass ecosystems. BioScience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
O’Shea T. J., Beck C. A., Hodgson A. J., Keith-Diagne L., Marmontel M. (2022). “Social and reproductive behaviors,” in Ethology and behavioral ecology of sirenia. Ed. Marsh H. (Switzerland: Springer, Cham), 101–154.
Panyawai J., Prathep A. (2022). A systematic review of the status, knowledge, and research gaps of dugong in southeast Asia. Aquat. Mamm. 48, 203–222. doi: 10.1578/AM.48.3.2022.203
Parente C. L., Vergara-Parente J. E., Lima R. P. (2004). Strandings of antillean manatees, Trichechus manatus manatus, in northeastern Brazil. Lat. Am. J. Aquat. Mamm. 3, 69–75. doi: 10.5597/lajam00050
Perrin W. F., Reeves R. R., Dolar M. L. L., Jefferson T. A., Marsh H. D., Wang J. Y., et al. (2002). Report of the second workshop on the biology and conservation of small cetaceans and dugongs of south-east Asia (Bonn, Germany: UNEP/CMS Secretariat).
Petcharat A., Lee Y. (2020). Measuring the nonuse value of the dugong (Dugong dugon) in Thailand. J. Asia-Pac. Biodivers. 13, 62–69. doi: 10.1016/j.japb.2019.12.002
Phang V. X. H., Chou L. M., Friess D. A. (2015). Ecosystem carbon stocks across a tropical intertidal habitat mosaic of mangrove forest, seagrass meadow, mudflat and sandbar. Earth Surf. Process. Landf. 40, 1387–1400. doi: 10.1002/esp.3745
Ponnampalam L. S., Izmal J. H. F., Adulyanukosol K., Ooi J. L. S., Reynolds J. E. (2015). Aligning conservation and research priorities for proactive species and habitat management: the case of dugongs dugong dugon in johor, Malaysia. Oryx 49, 743–749. doi: 10.1017/S0030605313001580
Ponnampalam L. S., Keith-Diagne L., Marmontel M., Marshall C. D., Reep R. L., Powell J., et al. (2022). “Historical and current interactions with humans,” in Ethology and behavioral ecology of sirenia. Ed. Marsh H. (Switzerland:Springer, Cham), 299–349.
Preen A. (1989). Observations of mating behavior in dugongs (Dugong dugon). Mar. Mammal Sci. 5, 382–387. doi: 10.1111/j.1748-7692.1989.tb00350.x
Preen A. (1992). Interactions between dugongs and seagrasses in a subtropical environment (Townsville, Australia: James Cook University).
Preen A. (1995). Impacts of dugong foraging on seagrass habitats: observational and experimental evidence for cultivation grazing. Mar. Ecol. Prog. Ser. 124, 201–213. doi: 10.3354/meps124201
Raffles T. S. B. (1820). XIII. some account of the dugong. by sir Thomas Stamford raffles, governor of sumatra; communicated in a letter to sir everard home, bart. v. p. r. s. Philos. Trans. R. Soc Lond. 110, 174–182. doi: 10.1098/rstl.1820.0014
Rahman S. A., Yaakub S. M. (2020). Socio-economic valuation of seagrass meadows in the pulai river estuary, peninsular Malaysia, through a wellbeing lens. Mar. Freshw. Res. 71, 877–891. doi: 10.1071/MF19208
Richards J. F., Flint E. P., Daniels R. C. (1994). Historic land use and carbon estimates for south and southeast Asia: 1880-1980 (Oak Ridge, TN, USA: Carbon Dioxide Information Analysis Center (CDIAC), Oak Ridge National Laboratory).
Rizzo A., Glasson J. (2012). Iskandar Malaysia. Cities 29 (6), 417–427. doi: 10.1016/j.cities.2011.03.003
Sheppard J. K., Carter A. B., McKenzie L. J., Pitcher C. R., Coles R. G. (2008). Spatial patterns of sub-tidal seagrasses and their tissue nutrients in the Torres strait, northern Australia: implications for management. Cont. Shelf Res. 28, 2282–2291. doi: 10.1016/j.csr.2008.03.033
Sheppard J. K., Jones R. E., Marsh H., Lawler I. R. (2009). Effects of tidal and diel cycles on dugong habitat use. J. Wildl. Manage. 73, 45–59. doi: 10.2193/2007-468
Sheppard J. K., Marsh H. D., Jones R. E., Lawler I. R. (2010). Dugong habitat use in relation to seagrass nutrients, tides, and diel cycles. Mar. Mammal Sci. 26, 855–879. doi: 10.1111/j.1748-7692.2010.00374.x
Sheppard J. K., Preen A. R., Marsh H. D., Lawler I. R., Whiting S. D., Jones R. E. (2006). Movement heterogeneity of dugongs, dugong dugon (Müller), over large spatial scales. J. Exp. Mar. Biol. Ecol. 334, 64–83. doi: 10.1016/j.jembe.2006.01.011
Sivasothi N. (2006)Dugong carcass washed ashore on southern tekong. Available at: http://rmbr.nus.edu.sg/news/index.php?entry=%2Fnews%2F20060608-dugong_tekong.txt (Accessed February 17, 2018).
Skilleter G. A., Wegscheidl C., Lanyon J. M. (2007). Effects of grazing by a marine mega-herbivore on benthic assemblages in a subtropical seagrass bed. Mar. Ecol. Prog. Ser. 351, 287–300. doi: 10.3354/meps07174
Small C., Nicholls R. J. (2003). A global analysis of human settlement in coastal zones. J. Coast. Res. 19, 584–599.
Song Y., Li D., Hou X. (2019). Characteristics of mainland coastline changes in southeast Asia during the 21st century. J. Coast. Res. 36, 261–275. doi: 10.2112/JCOASTRES-D-19-00018.1
Strain E. M. A., Olabarria C., Mayer-Pinto M., Cumbo V., Morris R. L., Bugnot A. B., et al. (2018). Eco-engineering urban infrastructure for marine and coastal biodiversity: which interventions have the greatest ecological benefit? J. Appl. Ecol. 55, 426–441. doi: 10.1111/1365-2664.12961
Tan K. S., Acerbi E., Lauro F. M. (2016). Marine habitats and biodiversity of singapore’s coastal waters: a review. Reg. Stud. Mar. Sci. 8, 340–352. doi: 10.1016/j.rsma.2016.01.008
Tan Y. M., Dalby O., Kendrick G. A., Statton J., Sinclair E. A., Fraser M. W., et al. (2020). Seagrass restoration is possible: insights and lessons from Australia and New Zealand. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00617/full
Tol S. J., Coles R. G., Congdon B. C. (2016). Dugong dugon feeding in tropical Australian seagrass meadows: implications for conservation planning. PeerJ 4, e2194. doi: 10.7717/peerj.2194
Unsworth R. K. F., Ambo-Rappe R., Jones B. L., La Nafie Y. A., Irawan A., Hernawan U. E., et al. (2018). Indonesia’s globally significant seagrass meadows are under widespread threat. Sci. Total Environ. 634, 279–286. doi: 10.1016/j.scitotenv.2018.03.315
URA (2019)URA master plan 2019. Available at: https://www.ura.gov.sg/maps/?service=MP (Accessed January 21, 2021).
van Maren D. S., Gerritsen H. (2012). Residual flow and tidal asymmetry in the Singapore strait, with implications for resuspension and residual transport of sediment. J. Geophys. Res. Oceans 117, 1–18. doi: 10.1029/2011JC007615
van Maren D. S., Liew S. C., Hasan G. M. J. (2014). The role of terrestrial sediment on turbidity near singapore’s coral reefs. Cont. Shelf Res. 76, 75–88. doi: 10.1016/j.csr.2013.12.001
Veettil B. K., Costi J., Marques W. C., Tran X. L., Quang N. X., Van D. D., et al. (2020). Coastal environmental changes in southeast Asia: a study from quang nam province, central Vietnam. Reg. Stud. Mar. Sci. 39, 101420. doi: 10.1016/j.rsma.2020.101420
Wake J. A. (1975). A study of the habitat requirements and feeding biology of the dugong dugong dugon (Müller) (Townsville, Australia: James Cook University).
Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Wee Y. C., Corlett R. (1986). The city and the forest: plant life in urban Singapore. (Singapore: Singapore University Press).
Wirsing A. J., Heithaus M. R., Dill L. M. (2007a). Can you dig it? use of excavation, a risky foraging tactic, by dugongs is sensitive to predation danger. Anim. Behav. 74, 1085–1091. doi: 10.1016/j.anbehav.2007.02.009
Wirsing A. J., Heithaus M. R., Dill L. M. (2007b). Fear factor: do dugongs (Dugong dugon) trade food for safety from tiger sharks (Galeocerdo cuvier)? Oecologia 153, 1031–1040. doi: 10.1007/s00442-007-0802-3
Wirsing A. J., Heithaus M. R., Dill L. M. (2007c). Living on the edge: dugongs prefer to forage in microhabitats that allow escape from rather than avoidance of predators. Anim. Behav. 74, 93–101. doi: 10.1016/j.anbehav.2006.11.016
Yaakub S. M., Chen E., Bouma T. J., Erftemeijer P. L. A., Todd P. A. (2014). Chronic light reduction reduces overall resilience to additional shading stress in the seagrass halophila ovalis. Mar. pollut. Bull. 83, 467–474. doi: 10.1016/j.marpolbul.2013.11.030
Yaakub S. M., Lim R. L., Lim W. L., Todd P. A. (2013). The diversity and distribution of seagrass in Singapore. Nat. Singap. 6, 105–111.
Keywords: distribution, coastal anthropogenic activities, feeding trails, Johor Strait, Singapore Strait, seagrass, threats, coastal development
Citation: Ng SZH, Ow YX and Jaafar Z (2022) Dugongs (Dugong dugon) along hyper-urbanized coastlines. Front. Mar. Sci. 9:947700. doi: 10.3389/fmars.2022.947700
Received: 19 May 2022; Accepted: 26 August 2022;
Published: 20 September 2022.
Edited by:
Michael Paul Jensen, Southwest Fisheries Science Center (NOAA), United StatesReviewed by:
Helene Denise Marsh, James Cook University, AustraliaNataly Castelblanco-Martínez, National Council of Science and Technology (CONACYT), Mexico
Copyright © 2022 Ng, Ow and Jaafar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sirius Z. H. Ng, c2lyaXVzLm5nQHUubnVzLmVkdQ==; Zeehan Jaafar, amFhZmFyekBudXMuZWR1LnNn
 Sirius Z. H. Ng
Sirius Z. H. Ng Yan Xiang Ow
Yan Xiang Ow Zeehan Jaafar
Zeehan Jaafar