Submarine canyons as key habitats to preserve Risso’s dolphin (Grampus griseus) populations in the northwestern Mediterranean Sea
- Department of Projects, SUBMON - Conservation, Study and Awareness of the Marine Environment, Barcelona, Spain
This paper summarises the occurrence of Risso’s dolphin (Grampus griseus) over 12 years (2009 to 2021) in the northwestern Mediterranean. The study was conducted off the central coast of Catalonia (NE Spain) in an area covering 8,026 km2 and featuring a system of submarine canyons. The habitat is suitable for a wide diversity of species, including deep-diving cetaceans. In addition, a different dataset from other projects was included to compare distances to the coast from different periods. A visual effort of 8,756 km was carried out with the recording of 17 Risso’s dolphin sightings. The relative mean density of Risso’s dolphin was 0.0078 individuals/km2 (SD 0.1, n = 17), and the densities were higher in the southern canyons than in the northern canyons. Furthermore, the distance to the coast and the depth of any sighting were compared by gathering data from different periods and surveys (first period 1985–2014; second period 2016–2021) with 34 sightings analysed. Significant differences were found related to the distance to the coast, showing a displacement of the animals to more pelagic areas between periods. The spatial distribution of Risso’s dolphins was investigated by applying a generalized additive model based on sighting data collected during standardized vessel surveys. Four predictive variables were considered, taking into account the local physiographical features. The model showed that the spatial distribution of Risso’s dolphin in the area was related to the slope and slope variation. The species showed preferences for offshore areas as significant differences were obtained in terms of the distance of sightings to the coast. This paper provides new insights into the distribution of Risso’s dolphin in the central western Mediterranean Sea and identifies the submarine canyons of northern Catalonia as an essential habitat for the species.
1 Introduction
Risso’s dolphin, Grampus griseus (Cuvier, 1812), is a cosmopolitan cetacean species that occurs in temperate and tropical waters and that has a heterogeneous distribution in the Mediterranean Sea (Azzellino et al., 2008; Boisseau, 2010; Bearzi et al., 2011; Gaspari and Natoli, 2012; Azzellino et al., 2016). The species’ area of occurrence in the Mediterranean includes the Alboran Sea (Cañadas et al., 2002; Cañadas et al., 2005; Gannier, 2005), the Ligurian Sea (Di Sciara et al., 1993; Gannier, 2005; Azzellino et al., 2008; Moulins et al., 2008; Azzellino and Lanfredi, 2015; Azzellino et al., 2016), the Sardinian-Balearic Basin (Gómez de Segura et al., 2008; Arcangeli et al., 2018), Chicote et al., 2015 the Strait of Sicily (Corrias et al., 2021), the Tyrrhenian Sea (Raga and Pantoja et al., 2004; Arcangeli et al., 2012; Campana et al., 2015), the Adriatic Sea (UNEP MAP-RAC/SPA, 2014), and the Ionian (Frantzis and Herzing, 2002; Dimatteo et al., 2011; Carlucci et al., 2020; Menniti and Vella., 2022) and Aegean basins (Frantzis and Herzing, 2002). Very little is known about the waters of Levantine and North Africa (Kerem et al., 2012), although some efforts were done in 2018 in North Africa (ACCOBAMS, 2021).
The group size for the species has been described to vary between regions: six to 12 individuals around the British Isles (Evans et al., 2003; Evans, 2008); groups up to 20 individuals (modal six to 10), averaging 12.3 (1 to 55; N = 74) in the Azores; Pereira and Nuno (2008); 10–25 individuals in the Spanish Mediterranean (Cañadas et al., 2005; Gómez de Segura et al., 2008); 10–40 individuals in the Ligurian Sea (Airoldi et al., 2005; Azzellino et al., 2008); and the group size ranged between two and 42 Risso’s dolphin with a mean value of 19 ± 9 individuals in the Golf of Taranto (central-eastern Mediterranean Sea) (Cipriano et al., 2022). Association patterns occur in pairs and in numbers of three to 12 individuals and are defined as long-term, stable units of a stratified social organisation based on age and sex classes (Hartman et al., 2008).
Abundance estimates of Risso’s dolphin have been conducted at the local, regional, and basin scales. In the western Ligurian Sea (Northwestern Mediterranean), a long-term study of mark–recapture (1990–2014) resulted in a local population estimate of 100 individuals (95% CI of 60–220 individuals) (Azzellino et al., 2016). In the same study, the authors reported a significant decrease in the average population of Risso’s dolphin from 120 to 150 individuals (2000 to 2005) to 70 to 100 individuals (2010–2014). Also Airoldi et al. (2015) reported a decrease in the abundance of the population in the Ligurian Sea. Aerial seasonal surveys covering an area of 181,400 km2 of the northwestern Mediterranean Sea were conducted during the winter 2011–2012 and winter 2019 to provide estimates of abundance and distribution patterns for cetacean species, including Risso’s dolphin (Laran et al., 2021). The total estimated abundance of Risso’s dolphin was 2,000 individuals (95% CI: 700–5,900) in winter and 1,400 individuals (95% CI: 500–3,700) in summer. In the Spanish Mediterranean Sea, an abundance estimate based on line transect method was conducted in an area of 32,270 km², where aerial surveys in 2001–2003 yielded an estimate of 493 individuals (CV = 60.6%; Gómez de Segura et al., 2006). In the Alboran Sea, 864 individuals (CV = 15.65) were estimated between 2009 and 2012, based on modelling data from ship surveys, in an area covering approximately 45,000 km2 in the Alboran Sea (INDEMARES, 2013). In summer 2018, a large-scale Mediterranean Sea survey was conducted to estimate marine megafauna within the framework of the ACCOBAMS Survey Initiative (ASI project) framework. The abundance estimate for the Risso’s dolphins resulted in 24,106 individuals (95% CI = 13,986–41,548) (ACCOBAMS, 2021). The conservation status in the ACCOBAMS area for the Mediterranean population has recently been updated to endangered by the International Union for Conservation of Nature (IUCN) (Lanfredi et al., 2021).
The Mediterranean population of this species favours waters over steep slopes, submarine canyons, and seamounts (Cañadas et al., 2002; Azzellino et al., 2008; Bearzi et al., 2011; Azzellino et al., 2012; Azzellino et al., 2016) and prefers areas with depths of more than 500–2,500 m (Cañadas et al., 2002; Gómez de Segura et al., 2008). The range distance from the 200-m isobaths is around 5–30 km (Mangion and Gannier, 2002) and 14 km from the coast (Di Sciara et al., 1993). The depth range preferred by the species is explained by the trophic requirements of a mainly teuthophagus species occasionally foraging on fish and thaliaceans (Sekiguchi et al., 1992; Blanco et al., 2006; Luna et al., 2021). The adaptability of habitat use and evidence for genetic differentiation suggest the existence of various geographical units of the species within the Mediterranean (Gaspari, 2004; Jefferson et al., 2014).
A high degree of residency and site fidelity has been reported in different study areas from the Mediterranean Sea to the Atlantic Ocean based on the ecology and behaviour of the species and the availability of food resources (Hartman et al., 2008; De Boer et al., 2013; Remonato et al., 2013; Hartman et al., 2015; Maglietta et al., 2018). Studies based on photo-identification in West Provence (Golf of Lyon-Mediterranean Sea) for a part of the population called “resident” showed short movements; 63% of the individuals recaptured were within 50 km (Labach et al., 2015), sometimes recaptured after up to 18 years. However, the wide-range movements for “transient” animals, up to 493 km, were also recorded (Casacci and Gannier, 2000; Miragliuolo et al., 2004; Airoldi et al., 2005; Polo et al., 2009; Remonato et al., 2018). These long-distance movements, from offshore locations to the continental slope habitat, suggest that inter-regional movements are also possible (Delrocq and Gannier, 2016).
The range of species distribution in the Mediterranean extends from the eastern to the western end of the Mediterranean Sea, with a higher number of sightings in the western and northwest areas of the basin (Bearzi et al., 2011). Seasonal movements of the species have been monitored in the Ligurian Sea (northwestern Mediterranean Sea), where groups seem to follow a preferential route to the west, frequenting the same sites from year to year (Azzellino et al., 2008).
In this study, we analyse a long-term monitoring program on Risso’s dolphins from the central area of the northwestern Mediterranean to improve the knowledge on the ecology of the species by highlighting the relevance of the proximal areas of the submarine canyons as crucial habitats for the preservation of the species.
2 Methodology
2.1 Study area
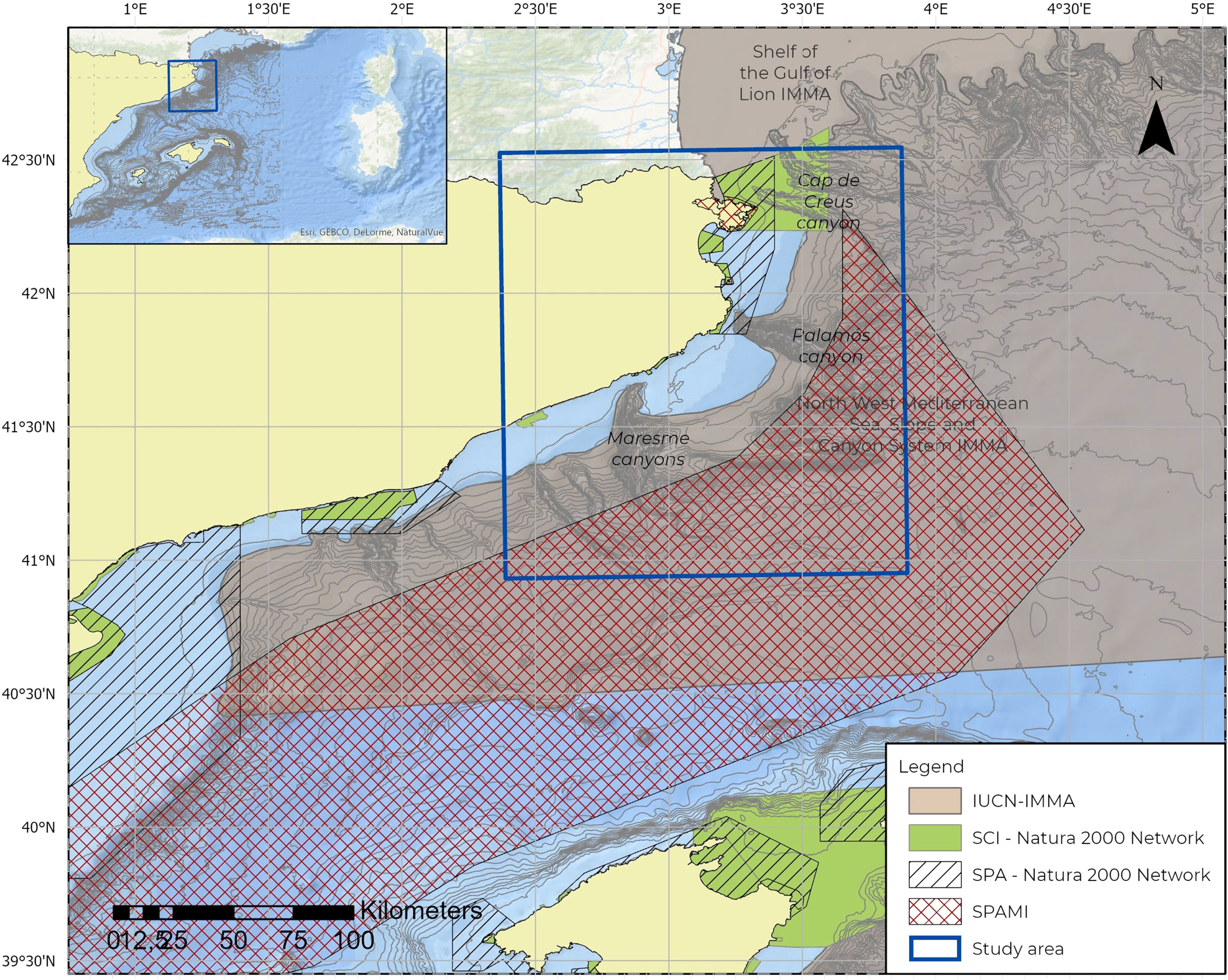
The study area (8,026 km2) is located in the Catalano-Balearic basin in the northwestern Mediterranean Sea, off the coast of Catalonia (NE Spain). The area extends from the continental shelf to 20 NM from the coast. It includes three different systems of submarine canyons: Creus, Palamós and Maresme, which reach depths of up to 2,000 m. The study area also encompassed several Spanish Marine Protected Areas (MPA) (Figure 1).
The Creus canyon is located at the western part of the Gulf of Lion continental margin and drags waters from the Rhône River. The head of the Creus canyon is located 5 km from the coast and reaches 6 km in width and almost 2,000 m in depth. The large amounts of organic material transported along the canyon play an essential role in maintaining biodiversity rates and its associated deep-sea ecosystems (Canals et al., 2009; Orejas et al., 2009).
The Palamós canyon is one of the most prominent topographical features of the Catalan Sea. This canyon is located 20 km south of Creus canyon and has a total length of 40 km and a maximum depth of 2,200 meters. The Palamós canyon is one of the most extensive and deep canyons in the northwestern Mediterranean and transports sediments from the coastal shelf to the open sea (Martín et al., 2006; Palanques et al., 2006; Palanques and Puig, 2007). This submarine canyon constitutes a notable “hot spot” for suspended and downward sediment flows in this margin (Martín, 2005; Martín et al., 2006).
The submarine canyon system of Maresme includes three different canyons, with one of them being the most relevant as it cuts deeply into the continental slope in a non-usual north–south direction (Díaz and Maldonado, 1990). The canyon’s width increases with depth, reaching up to 2,000 m with a width of 20 km (Canals et al., 2004).
2.2 Data collection
Data was collected from 2009 to 2021 in specific sighting surveys using sailing vessels 12–15 m in length. The research team included two observers at different heights, using a crow’s nest and an angle meter to calculate the perpendicular distance. Planned transects were designed as triangles to cross-depth contours as perpendicularly as possible (Figure 2A) and to cover as much of the area as possible (Figure 2B). The sighting effort was measured as the number of kilometres travelled with adequate sighting conditions (up to Beaufort Sea state 3) and observers at the lookout posts. The effort was recorded with a GPS navigation system, using Logger, the IFAW Data Logging Software (NMEA data automatically recorded every minute in a database), which continuously recorded the geographic position of the ship. Data on time, species, number of individuals, behaviour, presence of calves, movement to the vessel, and specific observations were also recorded. The group size was estimated by visual counts defined as the minimum number of individuals sighted at the same time (min), the maximum number of individuals sighted at the same time (max), and the most agreed number from the observers (best estimate). A group was defined as all the individuals that interacted socially and/or showed coordinated behaviour with a distance of less than five body lengths from the others (Whitehead, 2003).

Figure 2 (A) Map with on-effort track legs during the sampling period. (B) Effort distribution calculated on a 5 × 5-km grid shape file (EPSG:25831).
The authors had access to the Proyecto Mediterráneo dataset (Raga and Pantoja, 2004) and the ACCOBAMS Survey Initiative dataset (ASI 2018), which included 17 sightings of Risso’s dolphin in the study area since 1985. These datasets were added to the authors’ dataset, resulting in 34 Risso’s dolphin sightings that were used for the depth and distance-to-the-coast analysis.
2.3 Data analysis
2.3.1 Relative density
The study area was divided into a grid of 338 squares with a cell resolution of 5 × 5 km each, and the effort was calculated for each grid cell. The effort was evaluated in terms of kilometres of track lines (Figure 2A) per cell unit. Only the effort in “favourable conditions” (i.e., wind not exceeding 3 on the Beaufort scale) was considered. The tracks corresponding to the time spent with the same group of animals were also excluded. The relative density [density per unit of effort (animals/km)] was calculated as the number of individuals per kilometre of effort within each cell unit. The encounter rate (ER) was also calculated as sightings per kilometre for the different years. Geospatial analysis was performed with ArcMap 10.6.1. The 31N UTM Transverse Mercator projection was used for all GIS analyses (EPSG:25831).
2.3.2 General additive model
Presence–absence habitat models are suitable to relate species occurrence with information on the spatial characteristics of locations where the species was found (Elith and Leathwick, 2009). In this case, we used a generalized additive model (GAM) with binomial distribution and a logit link, using as a covariate of response the presence (1) and absence (0) of Risso’s dolphin in each grid cell. The benefit of additive modelling resides in its flexibility in capturing non-linear species–habitat relationships. When the data is related to certain variables but the relationships fail to be simply linear, GAM uses a link function to establish a relationship between the mean of the response variable and the smooth function of the explanatory variables. Consequently, the association between response and explanatory variables derives from the data itself and not from the model because no kind of parametric assumption is made (Hastie and Tibshirani, 1990; Yee and Mitchell, 1991). In this study, the GAM approach was applied to determine whether the selected variables affect the distribution of Grampus griseus in the study area. Zero-inflated models (Poisson regression and negative binomial) are used to model count data that has an excess of zero counts. Our data responds to binary data (1/0) and not to count data with an excess of zeros. Therefore, binary logistic regression (family = binomial), with a logit function, was applied.
We start by fitting a linear model. Figure 3 represents the relationship between each possible pairing of environmental variables: depth, distance to coast, slope, and the variation of the slope. The relationship between variables (depth and distance to the coast) showed a covariance between them, tested through a Pearson correlation coefficient, so only one was included in the model. The selection of the model was based on the Akaike information criterion and the explanation of the model’s deviance. In all models, the significance of the deviance was tested with a χ2 test, and a visual inspection of the residuals was made, especially to look for trends.
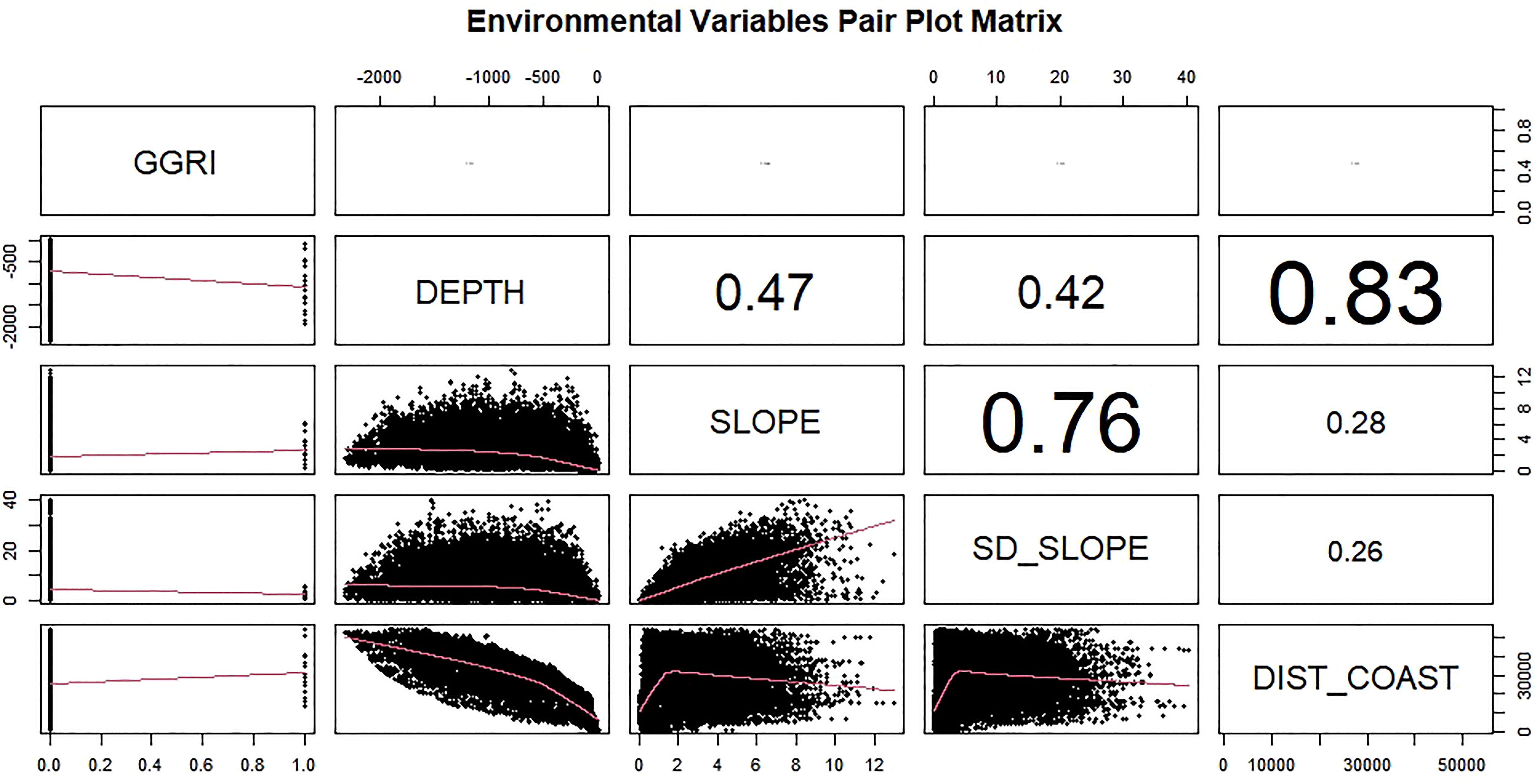
Figure 3 Linear correlation between environmental variables (DEPTH_MASK = depth, SLOPE = slope, SD_SLOPE = standard deviation slope, DIST COASt = distance coast).
The general structure of the selected model was as follows:
where p iis the proportion of positive observations in grid i, β0 is the intercept, fi is smooth functions of the predictor covariates, and zij is the value of the predictor covariate k in grid i. The models were fitted using the “mgcv” package version 1.7-26 for R version 3.0.2 (Wood and Wood, 2015), performing the manual selection.
A raster data layer of 1 km/1 km cell was created, combining effort and sighting in the study area. The cell value was indicated with 1 or 0 for the presence of Risso’s dolphin. The cell size was chosen since other studies in the Mediterranean indicate the size as appropriate for the spatial variation in environmental variables.
Due to data variation, only physiographical variables were measured for describing the presence/absence of Risso’s dolphin: depth, slope, slope variation, and distance to the coast. An environmental raster data layer of 1 km/1 km was created using a geographical information system (QGIS 3.14, QGIS Desktop 2.8.3). Physiographical variables were calculated for each cell, calculating the mean cell centroid coordinates. Depth was calculated from bathymetric data available at GEBCO (https://www.emodnet.eu/, 2020), using the GIS tool surf.contour, by calculating the difference between water isolines and the raster depth while avoiding the land parts of the raster. The slope was calculated with the Terrain analysis tool (Grass Package QGIS Desktop 2.8.4) and expressed in degrees. The variation in the slope was also calculated as the standard deviation of the slope. The distance to the coast was calculated with the r.neighbours tool (Grass Package 7.8.3 for QGIS).
2.4 Depth and distance to the coast
The depth and the distance to the coast of each sighting were compared to determine changes in Risso’s dolphin habitat distribution preference in the area. No data on Risso’s dolphin was registered in 2015. We established 2015 as the breaking point for the first and second study periods. Thus, data was clustered into two periods, according to the year they were recorded: first period 1985–2014 (n = 24) and second period 2016–2021 (n = 10) (Table 1).
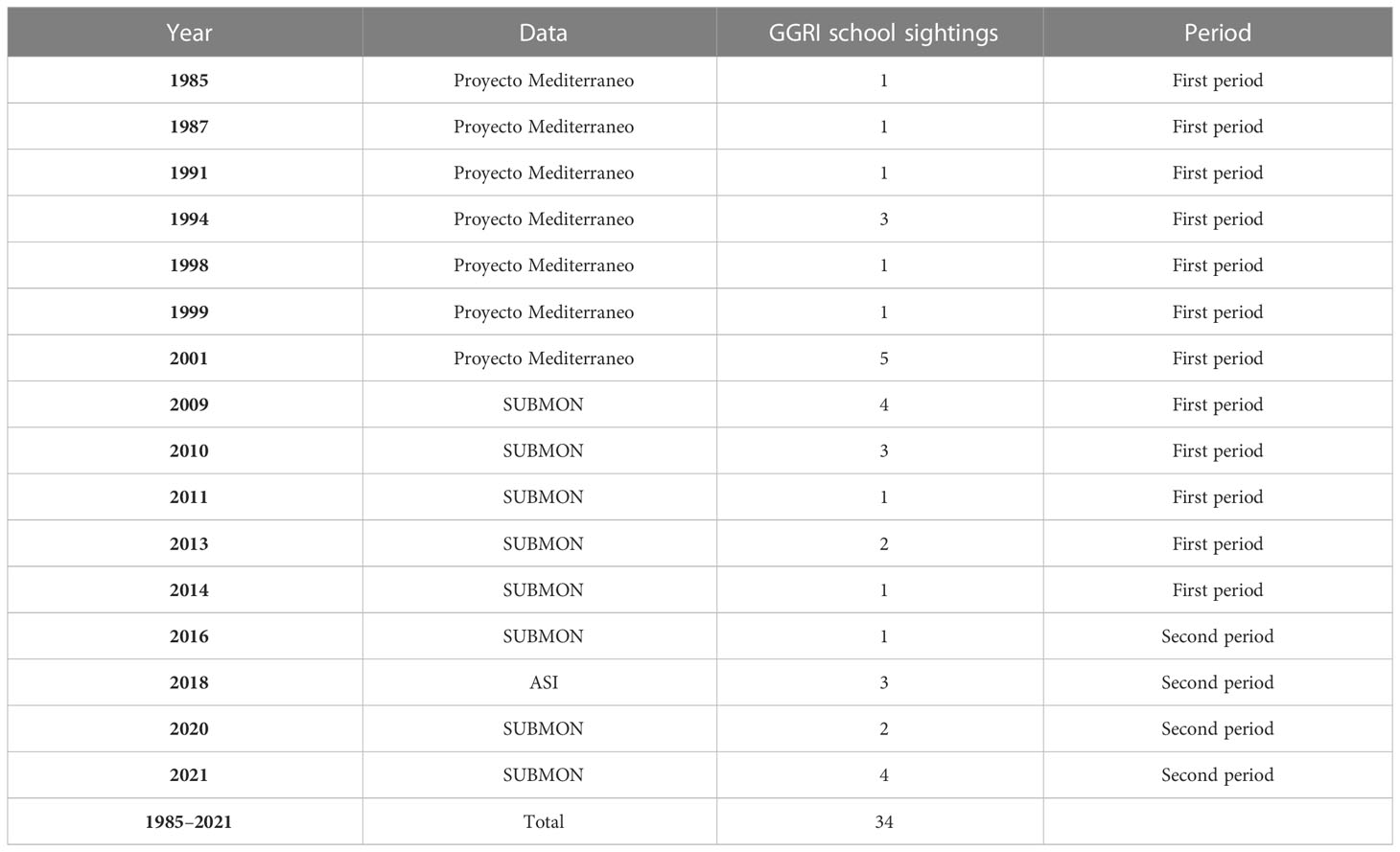
Table 1 Summary of the sightings for the different projects and years clustered into different periods.
A parametric test (t-test) was applied to test the difference for central values with depth and distances to the coast during the two different periods since the data was found not to differ significantly from a normal distribution (depth: Shapiro–Wilk test, W = 0.96545, p-value = 0.3479, n = 34; distance to coast: Shapiro–Wilk test, W = 0.96545, p-value = 0.3479).
3 Results
From 2009 to 2021, a total of 8,756.68 km of effort was surveyed within the study area, and data from 17 sightings of Risso’s dolphins was registered. The overall ER was 0.0019 sightings/km, although this number changed between years. Table 2 show a summary of the total effort and ER for the different years. The average group size was 5.7 individuals ± 3.8 SD.
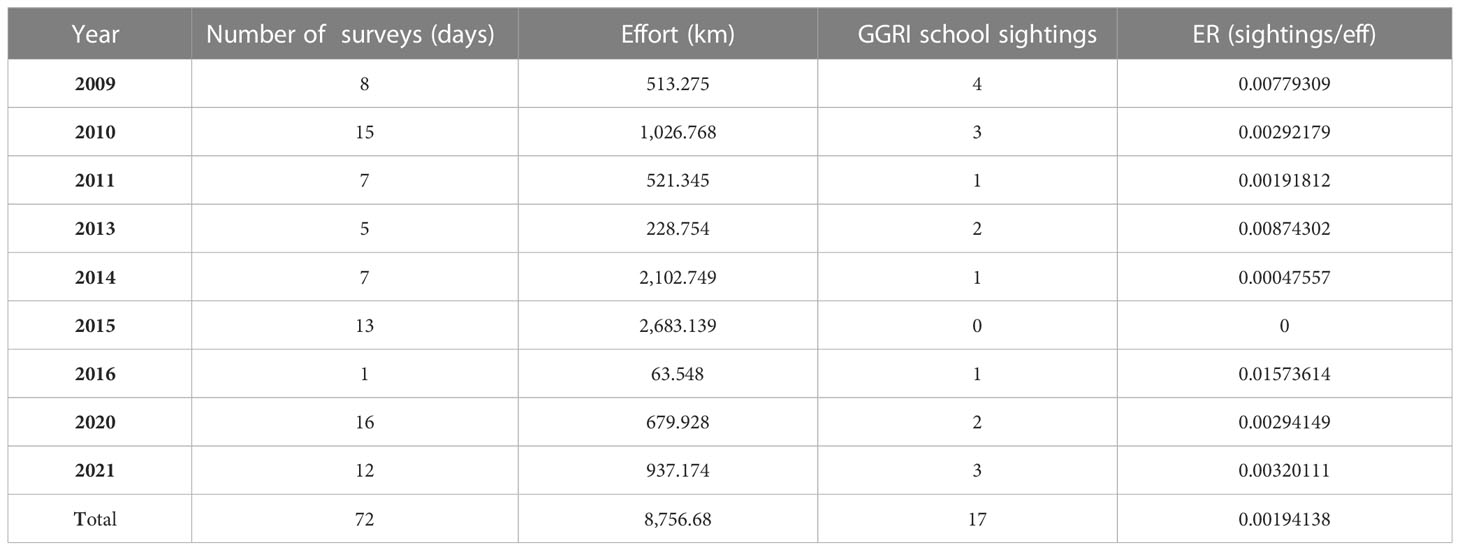
Table 2 Summary of the vessel surveys carried out in the study area showing the effort (km) and the number of schools of Risso’s dolphin (GGRI = Grampus griseus) observed and the encounter rate (ER = sightings/eff).
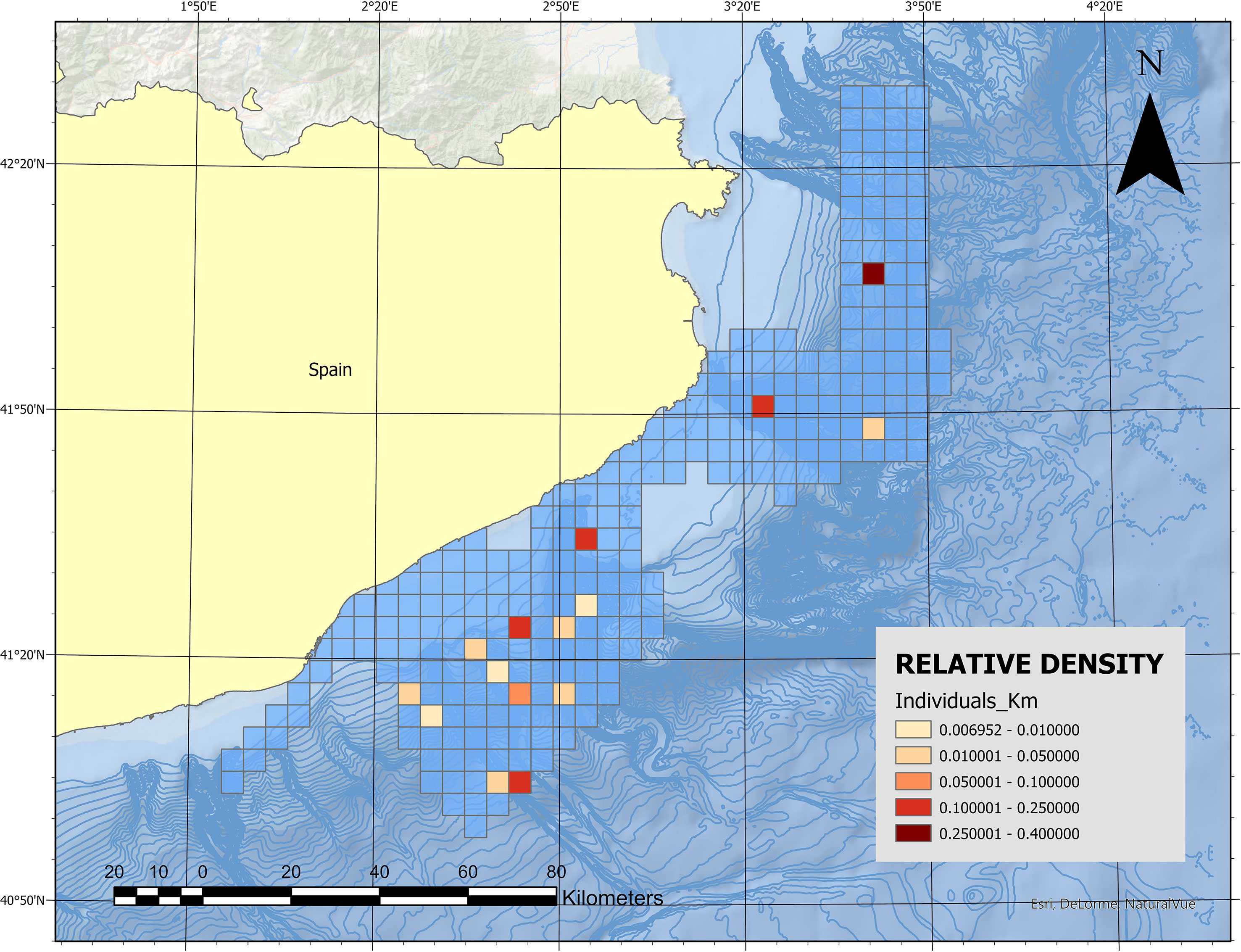
The mean relative density was 0.078 individuals/km2 (SD 0.1, n = 17) (Figure 4). The sightings were generally located in the southern and central submarine canyons, and no sightings were registered in the northern canyon.
The sightings occurred in a depth ranging between 100 and 1,900 m, with a mean depth of 1,754 m (SD 567, n = 17). The mean distance from the coast was 31.7 km (Table 3).
Figure 5 represents the physiographical conditions in which the species was present (1) and absent (0), showing that Risso’s dolphins were present in deep zones, although they could be observed in shallower areas. The presence of Risso’s dolphin only occurred in offshore waters (>12 km perpendicular distance in the area), coincident with the presence of the submarine canyons in the area. The presence of Risso’s dolphin was also related to steep slopes.

Figure 5 Physiographical conditions in which the species Grampus griseus is present (1) and absent (0).
3.1 GAM results
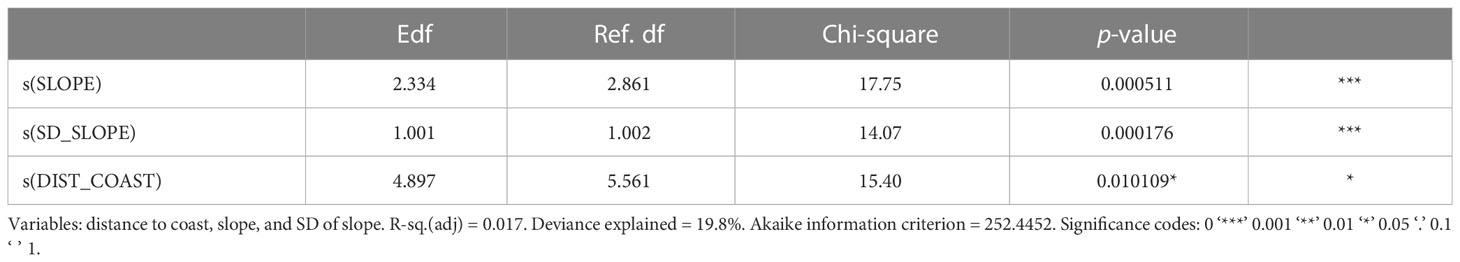
GAM developed for Risso’s dolphin reached 19.8% of explained deviance. Table 3 shows the results of the final model selected for the presence of Risso’s dolphin. The final occurrence probability model retained three covariates: distance to the coast, slope, and slope variation.
The spatial distribution of Risso’s dolphin was strongly related to the slope and to the slope variation (SD_SLOPE) (Table 3). These two variables are associated with the geomorphological features of submarine canyons within the study area, characterized by having steep slopes.
Figure 6 shows that the relationship with the distance to the coast was significant, indicating a direct relationship between the presence of Risso’s dolphin and the distance to the coast. The GAM identified that the habitat for Risso’s dolphin was an offshore one, with distances from the coast greater than 12 km and their presence increasing after 47 km from the coast. Medium slopes and slope variance, corresponding to the head and centre parts of the submarine canyons, also characterized it and were related to the presence of Risso’s dolphin in the model.

Figure 6 Predicted smooth splines of the response variable presence/absence of Grampus griseus as a function of the validated explanatory variables. The degrees of freedom for non-linear fits are in parentheses on the y-axis. Tick marks above the x-axis indicate the distribution of sightings. Dotted lines represent the 95% confidence intervals of the smooth spline functions.
3.2 Depth and distance to the coast
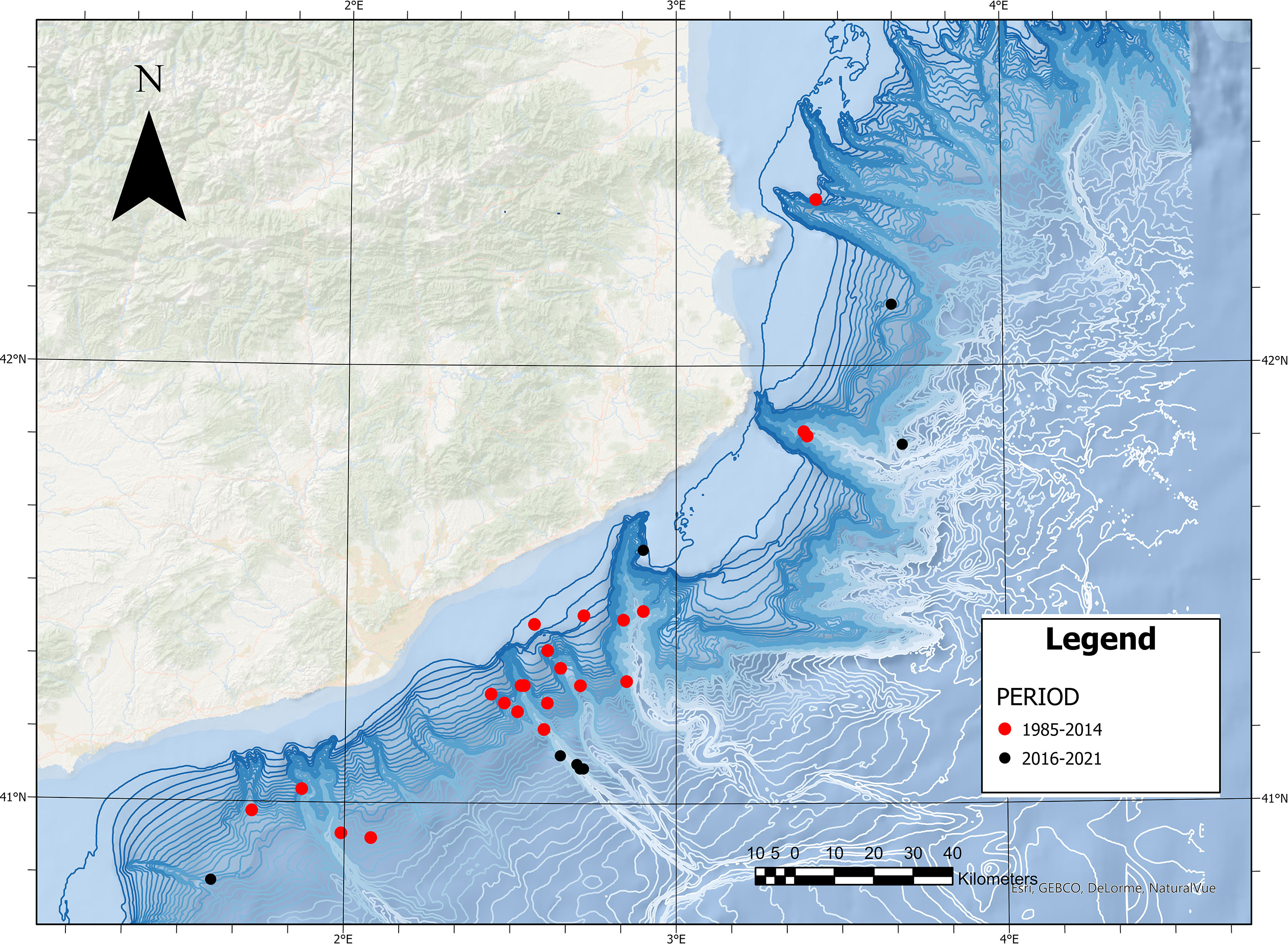
The distance to the coast of Risso’s dolphin sightings differed significantly between the two study periods (t-test: t = -2.9302, df = 14.14, p-value = 0.01087). While in the first period (1985–2014) the average distance to the coast was 30.77 km (SD =12.6 km), in the second period (2016–2021) the average distance was 47 km (SD = 10.7 km). The mean depth of Risso’s dolphin sightings showed no significant differences between the two study periods, although in the second period the sightings occurred in deeper waters [1,366.6 m (SD = 441 m)] than in the first study period [918 m (SD = 372 m)] (t-test: t = -2.1458, df = 12.298, p-value = 0.0525). Figure 7 shows the localization of the sighting during the two study periods. Table 4 shows the descriptive statistics of Risso's dolphin total sightings related to the physiographic variables.
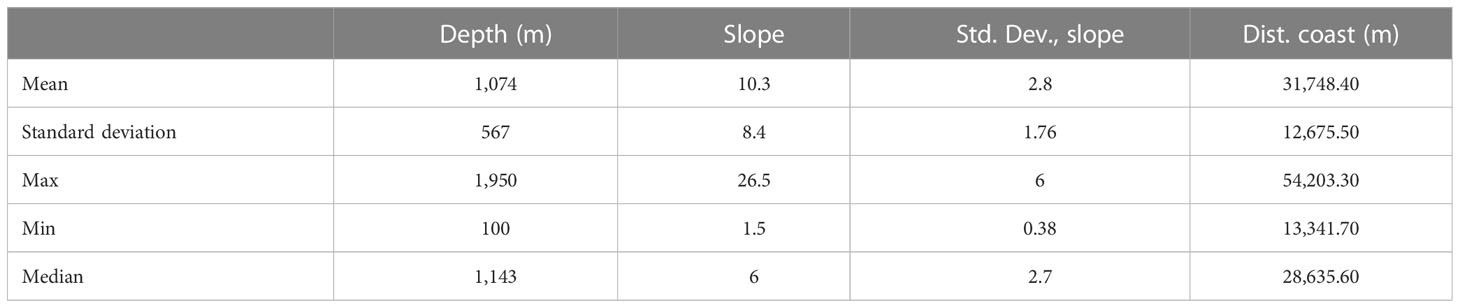
Table 4 Descriptive statistics of the distribution by depth and slope (degrees), standard deviation slope (Std. Dev., slope), and the distance to the coast (Dist. coast) for Risso’s dolphin sightings.
4 Discussion
The ER of Risso’s dolphin in the study area obtained in this study is consistent with the results obtained in the West Mediterranean Basin (Gómez de Segura et al., 2006; Laran et al., 2021) and Gulf of Taranto (Central-eastern Mediterranean Sea) (Carlucci et al., 2020) and higher than the ER from aerial surveys in a closer area (Gómez de Segura et al., 2008; ACCOBAMS, 2021). However, the ER value was lower than in the south-central Mediterranean Sea (Corrias et al., 2021).
On the contrary, the results from the ACCOBAMS report (ACCOBAMS, 2021) showed a density of 0.548 Risso’s dolphin/km2 for the Levantine Balearic area and 0.344 Risso’s dolphin/km2 for the Alboran Sea and northern Algeria. This data can be contrasted with the last estimate available for an area similar to these blocks, which was carried out by Gómez de Segura et al. (2006) with a result of 0.041 Risso’s dolphin/km2. These two values differ substantially, and given that the estimate using data from the ASI Project is based on information from only three sightings, this later density should be considered cautiously.
The relative density from our study shows that the highest values in the study area occurred in the southern and central submarine canyons (Maresme and Palamós canyons) (Figure 4), thus overlapping with the less protected area from the zone. These two submarine canyons have been included in the IMMAs proposal, but no specific binding protected figure has been established yet. Additionally, the SPAMI—Cetacean Migration Corridor, an MPA declared in 2018, is also farther away, only including a small area of the submarine canyon system.
The model explained a strong relationship between the presence of Risso’s dolphins with slope and slope variation, showing a preference for the steeper areas that, in the case of the study area, corresponds to the underwater canyons’ geomorphological features. Our findings are consistent with previous studies on Risso’s dolphin in the Mediterranean (Di Sciara et al., 1993; Cañadas et al., 2002; Gannier, 2005; Bearzi et al., 2011), which indicated a predilection for the continental slope with depths ranging from 500 to 1,500 m (Azzellino et al., 2012).
According to our model results, the distribution of Risso’s dolphins was also correlated with the distance to the coast as it was also found in the waters of Valencia and Murcia Regions (central Spanish Mediterranean) located southern to our study area (Gómez de Segura et al., 2008) but was not correlated with depth as it was in Gómez de Segura et al. (2006). This can be explained because distance from the coast is not related to depth in our area as it is more related into the southern area since the underwater canyons are quite close to the coast. Therefore, at the same depths, distances to the coast can vary substantially.
Our model explained 19.8% of the deviance; therefore, other factors could be related to the distribution of Risso’s dolphin in the area. Gómez de Segura et al. (2008) found that the second-best model for Risso’s dolphin incorporated the temporal variability of SST. Environmental variations could not be included in our model because of the big-scale temporal variability of the data. Moreover, data scarcity could explain the low adjustment of R2 and therefore the low deviance, thus producing a less accurate model.
Data analyzed from the different datasets (1985 to 2021) confirm that the species’ sightings in coastal areas and over the continental shelf have decreased while remaining stable in pelagic areas of the Western Mediterranean Sea ((Azzellino et al., 2016; ACCOBAMS, 2021). Data on the second period of the study (2016–2021) confirm that Risso’s dolphin sightings were made at a substantially greater distance from the coast (50 km, SD = 1.7 km) than those made in the first period of the study (1985–2014) (31.2 km, SD = 12.6 km), supporting the results from Azzellino et al. (2016) that suggest a displacement of the species to offshore areas.
The role of submarine canyon systems as a relevant habitat for the species is also supported by its feeding ecology. The species is considered mainly teutophagous: in the analyses of stomach content from individuals stranded in the northwestern Mediterranean conducted by Blanco et al. (2006), there was reported predation on cephalopod species from the middle slope, and more recently, Luna et al. (2021) identified cephalopod species associated to deeper waters of the continental shelf from stranded individuals along the study site. Considering trophic ecology, Borrell et al. (2021) conducted a study of stable isotopic niches of carbon, nitrogen, and sulphur for five species of cetaceans inhabiting the northwestern Mediterranean Sea, and the results placed the Risso’s dolphin in the highest trophic value, together with two deep-diver cetaceans—the long-finned pilot whale (Globicephala melas) and the Cuvier’s beaked whales (Ziphius cavirostris). Thus, similarities between the feeding strategies of the three species arise. The same study showed, for the species, a narrow range of isotopic sulphur values compared with the other deep-diver species, suggesting that Risso’s dolphin may occupy a relatively narrow range of the offshore habitat, primarily associated with submarine canyons, as has been suggested in distribution studies based on visual or acoustic data (Praca and Gannier, 2008; David and Di-Meglio, 2012).
Submarine canyon systems are marine ecosystems that support high levels of biodiversity (Fernandez et al., 2017; Santora et al., 2018). In the northwestern Mediterranean Sea, the submarine canyon systems and upwellings are two geomorphological and oceanographic features that encourage levels of productivity of outstanding biological and ecological relevance for the area. The interplay between the canyon topography and the oceanic currents has profound consequences for the high diversity that they support, affecting not only benthic communities but pelagic ones as well. In this sense, our results on the distribution of Risso’s dolphins show how relevant this deep habitat is for the species.
The results and data provided in this study will be incorporated into the conservation and management plans for the Risso’s dolphin in Spanish Mediterranean waters within the framework of the Marine Strategy Framework Directive. In a wider scope, our results will also contribute to establish the Important Marine Mammal Area of the North-West Mediterranean Sea, Slope, and Canyon System IMMA proposed by the International Committee on Marine Mammal Protected Areas, the IUCN, and World Commission on Protected Areas.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
Ethical review and approval were not required for the animal study because the study was carried out without any invasive technique.
Author contributions
CC, NA, and MG contributed to conception and design of the study and also to the data collection. CC and NA organized the database, and CC performed the statistical analysis. CC wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. NA is author of the picture from the cover. MG contributed to the final structure of the paper. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors gratefully acknowledge Fundación Biodiversidad—Ministry for Ecological Transition and Demographic Challenge from the Spanish Government for supporting this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
ACCOBAMS (2021). Absolute abundance of Risso's dolphin (Grampus griseus) in the north western part of the Pelagos Sanctuary. Pelagos Sanctuary Convention No. 03/2014, 59 pages. Istituto Tethys /EcoOcean Institut/GECEM/Fondazione CIMA/GIS3M.
Airoldi S., Azzellino A., David L., Dhermain F., Di-Meglio N., Jimenez V., et al. (2015). Final report “Absolute abundance of risso's dolphin (Grampus griseus) in the northwestern part of the pelagos sanctuary ” Pelagos sanctuary convention no. 59.
Airoldi S., Bendinoni F., Azzellini A., Fadda V., Profice A. (2005). “Abundance estimates of risso’s dolphins (Grampus griseus) in the western ligurian Sea through photographic mark-recapture,” in 19th Conference of the European Cetacean Society, La Rochelle, France.
Arcangeli A., Aissi M., Atzori F., Azzolin M., Buoninsegni J., Campana L., et al. (2018). “Improving knowledge and conservation of the Mediterranean population of risso's dolphins through effective partnerships,” in Preliminary report of the Mediterranean grampus project 2.0. Eds. Lanfredi C., Remonato E., Airoldi S.(La Spezia, Italy), 50.
Arcangeli A., Marini L., Crosti R. (2012). Changes in cetacean presence, relative abundance and distribution over 20 years along a trans-regional fixed line transect in the central tyrrhenian Sea. Mar. Ecol. 34. doi: 10.1111/maec.12006.
Azzellino A., Airoldi S., Gaspari S., Lanfredi C., Moulins A., Podestà M., et al. (2016). “Risso's dolphin, grampus griseus, in the Western ligurian Sea: trends in population size and habitat use,” in Advances in marine biology, vol. 75 . Eds. Sciara G. N. Di, Podestà M., Curry B. E. (Oxford: Academic Press), 205–232.
Azzellino A., Gaspari S., Airoldi S., Nani B. (2008). Habitat use and preferences of cetaceans along the continental slope and the adjacent pelagic waters in the western ligurian Sea. Deep Sea Res. Part I: Oceanographic Res. Papers. 55 (3), 296–323. doi: 10.1016/j.dsr.2007.11.006
Azzellino A., Lanfredi C. (2015). Analisi di distribuzione e abbondanza assoluta e relativa delle specie capodoglio (Physeter macrocephalus), grampo (Grampus griseus) e zifio (Ziphius cavirostris) nel santuario pelagos alla luce dei cambiamenti ambientali e dei fattori di pressione antropica. ACCOBAMS-SC14/2021/Doc16
Azzellino A., Panigada S., Lanfredi C., Zanardelli M., Airoldi S., Notarbartolo di Sciara G. (2012). Predictive habitat models for managing marine areas: spatial and temporal distribution of marine mammals within the pelagos sanctuary (Northwestern Mediterranean sea). Ocean Coast. Manage. 67, 63e74. doi: 10.1016/j.ocecoaman.2012.05.024
Bearzi G., Reeves R. R., Remonato E., Pierantonio N., Airoldi S. (2011). Risso’s dolphin grampus griseus in the Mediterranean Sea. Mamm. Biol. 76, 385–400. doi: 10.1016/j.mambio.2010.06.003
Blanco C., Raduan M. A., Raga J. A. (2006). Diet of risso’s dolphin (Grampus griseus) in the western Mediterranean Sea. Scientia Marina 70, 407–411. doi: 10.3989/scimar.2006.70n3407
Boisseau O., Lacey C., Lewis T., Moscrop A., Danbolt M., McLanaghan R. (2010). Encounter rates of cetaceans in the Mediterranean Sea and contiguous Atlantic area. J. Mar. Biol. Assoc. U. K. 90, 1589–1599. doi: 10.1017/S0025315410000342
Borrell A., Gazo M., Aguilar A., Raga J. A., Degollada E., Gozalbes P., et al. (2021). Niche partitioning amongst northwestern Mediterranean cetaceans using stable isotopes. Prog. Oceanography 193, 102559. doi: 10.1016/j.pocean.2021.102559
Campana I., Crosti R., Angeletti D., Carosso L., David L., Di-Méglio N., et al. (2015). Cetacean response to summer maritime traffic in the Western Mediterranean Sea. Mar. Environ. Res. 109, 1–8. doi: 10.1016/j.marenvres.2015.05.009
Cañadas A., Sagarminaga R., De Stephanis R., Urquiola E., Hammond P. S. (2005). Habitat selection models as a conservation tool: proposal of marine protected areas for cetaceans in southern Spain. Aquat. Conservation: Mar. Freshw. Ecosyst. 15, 495–521. doi: 10.1002/aqc.689
Cañadas A., Sagarminaga R., Garcıa-Tiscar S. (2002). Cetacean distribution related with depth and slope in the Mediterranean waters off southern Spain. Deep Sea Res. Part I: Oceanographic Res. Papers 49 (11), 2053–2073. doi: 10.1016/S0967-0637(02)00123-1
Canals M., Casamor J., Lastras G., Monaco A., Acosta J., Berné S., et al. (2004). The role of canyons in strata formation. Oceanography 17 (4), 80–91. doi: 10.5670/oceanog.2004.06
Canals M., Danovaro R., Heussner S., Lykousis V., Puig P., Trincardi P., et al. (2009). Cascades in Mediterranean submarine grand canyons. Oceanography 22, 26–43. doi: 10.5670/oceanog.2009.03
Carlucci R., Baş A. A., Liebig P., Renò V., Santacesaria F., Bellomo S., et al. (2020). Residency patterns and site fidelity of grampus griseus (Cuvier 1812) in the gulf of taranto (Northern Ionian Sea, central-Eastern Mediterranean Sea). Mamm Res. 65, 445–455. doi: 10.1007/s13364-020-00485-z
Casacci C., Gannier A. (2000). Habitat variability and site fidelity of the risso's dolphin in the northwestern Mediterranean: defining a home range for a nomad. Eur. Res. Cetacean. 14, 19–22.
Chicote C. A., Gazo M., Cañadas A., Pauner O., Saiz L., Pastor C., et al. (2015). Proyecto grampus: estudio y seguimiento de la población de calderón gris asociada a cañones submarinos en la costa catalana. Tech. report-Fundación Biodiversidad.
Cipriano G., Carlucci R., Bellomo S., Santacesaria F. C., Fanizza C., Ricci P., et al. (2022). Behavioral pattern of risso’s dolphin (Grampus griseus) in the gulf of taranto (Northern Ionian Sea, central-Eastern Mediterranean Sea). J. Mar. Sci. Eng. 10, 175. doi: 10.3390/jmse10020175
Corrias V., Filiciotto F., Giardina F. (2021). Sightings of risso’s dolphin (Grampus griseus) off the southern coast of linosa island (south-central Mediterranean Sea). Mediterr. Mar. Sci. 22 (2), 387–392. doi: 10.12681/mms.23648
David L., Di-Meglio N. (2012). “Role and importance of submarine canyons for cetaceansand seabirds in the north-westen Mediterranean Sea,” in Mediterranean Submarine canyons: ecology and governance. Ed. Würtz M. (Switzerland and M íalaga, Spain: IUCN Gland), 113–122.
De Boer M., Clark J., Leopold M., Simmonds M., Reijnders P. (2013). Photo-identification methods reveal seasonal and long-term site-fidelity of risso’s dolphins (Grampus griseus) in shallow waters (Cardigan bay, Wales). Open J. Mar. Science. 03, 66–75. doi: 10.4236/ojms.2013.32A007
Delrocq S., Gannier A. (2016). Contribution a l'etude du dauphin de risso en mediterranee occidentale: nouveaux resultats obtenus par photo-identification. Rev. d’Ecologie-la Terre la Vie 71 (2), 192–201. doi: 10.3406/revec.2016.1840
Díaz J., Maldonado A. (1990). Transgressive sand bodies on the maresme continental shelf, western Mediterranean Sea. Mar. Geology 91 (1-2), 53–72. doi: 10.1016/0025-3227(90)90132-4
Dimatteo S., Siniscalchi M., Esposito L., Prunella V., Bondanese P., Bearzi G., et al. (2011). Encounters with pelagic and continental slope cetacean species near the northern shore of the gulf of taranto, Italy. Ital. J. Zoology 78 (1), 130–132. doi: 10.1080/11250003.2010.532161
Di Sciara N., Venturino M. C., Zanardelli M., Bearzi G., Borsani J. B. (1993). Cetaceans in the central Mediterranean Sea: distribution and sighting frequencies. Bollettino di Zoologia 60, 131–138. doi: 10.1080/11250009309355800
Elith J., Leathwick J. R. (2009). Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. ecology evolution systematics 40, 677–697. doi: 10.1146/annurev.ecolsys.110308.120159
Evans P. G. H. (2008). “Risso’s dolphin grampus griseus,” in Mammals of the British islesHandbook, 4th Edition. Eds. Harris S., Yalden. D. W. (Southampton: The Mammal Society), 740–743.
Evans P. G. H., Anderwald P., Baines M. E. (2003). UK Cetacean Status Review. Report to English Nature and the Countryside Council for Wales. SeaWatch Foundation, Oxford. 160pp.
Fernandez U., Ramirez E., Aguzzi J., Allcock A. L., Davies J. S., Dissanayake A., et al. (2017). Ecological role of submarine canyons and need for canyon conservation: a review. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00005
Frantzis A., Herzing D. L. (2002). Mixed-species associations of striped dolphins (Stenella coeruleoalba), short-beaked common dolphins (Delphinus delphis), and risso’s dolphins (Grampus griseus) in the gulf of Corinth (Greece, Mediterranean Sea). Aquat. Mammals. 28, 188–197.
Gannier A. (2005). Summer distribution and relative abundance of delphinids in the Mediterranean Sea. Rev. D’Ecologie 60, 223–238. doi: 10.3406/revec.2005.1267
Gaspari S. (2004). Social and population structure of striped and risso’s dolphins in the Mediterranean Sea (Durham, UK: University of Durham).
Gómez de Segura A., Crespo E. A., Pedraza S. N., Hammond P. S., Raga J. A. (2006). Abundance of small cetaceans in the waters of the central Spanish Mediterranean. Mar. Biol. 150, 149–160. doi: 10.1007/s00227-006-0334-0
Gómez de Segura A., Hammond P. S., Raga J. A. (2008). Influence of environmental factors on small cetacean distribution in the Spanish Mediterranean. J. Mar. Biol. Assoc. U. K. 8, 1185–1192. doi: 10.1017/S0025315408000386.
Hartman K. L., Fernandez M., Wittich A., Azevedo J. M. N. (2015). Sex differences in residency patterns of risso’s dolphins (Grampus griseus) in the Azores: causes and management implications. Mar. Mammal Sci. 31, 1153–1167. doi: 10.1111/mms.12209
Hartman K., Visser F., Hendriks A. (2008). Social structure of risso's dolphins (Grampus griseus) at the Azores: a stratified community based on highly associated social units. Can. J. Zoology. 86, 294–306. doi: 10.1139/Z07-138
Hastie T. J., Tibshirani R. J. (1990). Generalized additive models (Boca Raton, Florida, USA: Chapman and Hall/CRC).
INDEMARES (2013). Informe final técnico. Available at: https://www.indemares.es/sites/default/files/informe_final_tecnico_alnitak.pdf.
Jefferson T., Weir C., Anderson R., Ballance L., Kenney R., Kiszka J. (2014). Global distribution of risso's dolphin (Grampus griseus): a review and critical evaluation. Mammal Rev. doi: 10.1111/mam.12008
Kerem D., Hadar N., Goffman O., Scheinin A., Kent R., Boisseau O., et al. (2012). Update on the cetacean fauna of the Mediterranean levantine basin. Open Mar. Biol. J. 6, 6–27. doi: 10.2174/1874450801206010006
Labach H., Dhermain F., Bompar J.-M., Dupraz F., Couvat J., David L., et al. (2015). Analysis of 23 years of risso’s dolphins photo-identification in the north-Western Mediterranean Sea, first results on movements and site fidelity sci. Rep. Port-Cros Natl. Park 29, 263–266.
Lanfredi C., Arcangeli A., David L., Holcer D., Rosso M., Natoli A. (2021). Grampus griseus (Mediterranean subpopulation) (The IUCN Red List of Threatened Species 2021: e.T16378423A190737150).
Laran S., Nivière M., Genu M., Dorémus G., Serre S., Spitz J., et al. (2021). Distribution et abondance de la mégafaune marine lors des campagnes SAMM cycle I et II en méditerranée (La Rochelle Université: Rapport final. Observatoire Pelagis), 76.
Luna A., Sánchez P., Chicote C., Gazo M. (2021). Cephalopods in the diet of risso's dolphin (Grampus griseus) from the Mediterranean Sea: a review. Mar. Mammal Sci. 38 (2). doi: 10.1111/mms.12869
Maglietta R., Renò V., Cipriano G., Fanizza C., Milella Stella E., Carlucci R., et al. (2018). DolFin: an innovative digital platform for studying. risso’s dolphins in the northern Ionian Sea (North-eastern central Mediterranean). Sci. Rep. 8 (1), 1–11.
Mangion P., Gannier A. (2002). Improving the comparative distribution picture for Risso’s dolphin and long-finned pilot whale in the Mediterranean Sea. Eur. Res. Cet. 16, 68–72.
Martín J. (2005). Sedimentary dynamics in the palamós submarine canyon (Universitat Politècnica de Catalunya), 198.
Martín J., Palanques A., Puig P. (2006). Composition and variability of downward particulate matter fluxes in the palamós submarine canyon (NW Mediterranean). J. Mar. Syst. 60 (1-2), 75–97. doi: 10.1016/j.jmarsys.2005.09.010
Menniti M. A., Vella A. (2022). Sighting of risso’s dolphin (Grampus griseus) during scientific research of the calabrian southern Ionian Sea (Central Eastern Mediterranean). Natural Eng. Sci. 7 (3), 248–259. doi: 10.28978/nesciences.1206056
Miragliuolo A., Mussi B., Bearzi G. (2004). Risso's dolphin harassment by pleasure boaters off the island of ischia, central Mediterranean Sea. Eur. Res. Cetaceans 15.
Moulins A., Rosso M., Ballardini M., Wurtz M. (2008). Partitioning of the pelagos sanctuary (northwestern Mediterranean Sea) into hotspots and coldspots of cetacean distributions. J. Mar. Biol. Assoc. U. K. 88, 1273–1281. doi: 10.1017/S0025315408000763
Orejas C., Gori A., Lo Iacono C., Puig P., Gili J. M., Dale M. R. T. (2009). Cold-water corals in the cap de creus canyon, northwestern Mediterranean: spatial distribution, density and anthropogenic impact. Mar. Ecol. Prog. Ser. 397, 37–51. doi: 10.3354/meps08314
Palanques A., Martín P., Puig J., Guillén J., Company, Sarda F. (2006). Evidence of sediment gravity flows induced by trawling in the palamo´s (Fonera) submarine canyon (northwestern Mediterranean). Deep-Sea Res. I 53, 201–214. doi: 10.1016/j.dsr.2005.10.003
Palanques A., Puig P. (2007). Near-bottom horizontal transfer of particulate matter in the palamós submarine canyon (NW Mediterranean). J. Mar. Res. 65 (2), 193–218.
Pereira J., Nuno D. S. G. (2008). Field notes on risso’s dolphin (Grampus griseus) distribution, social ecology, behaviour, and occurrence in the Azores. Aquat. Mammals 34 (4), 426–435. doi: 10.1578/AM.34.4.2008.426
Polo L., David L., Di-Meglio N., Rosso M. (2009). First analysis of long-term association of risso’s dolphin (Grampus griseus) population in the liguro – provençal basin and gulf of lion. Eur. Res. Cetacean 23.
Praca E., Gannier A. (2008). Ecological niches of three teuthophageous odontocetes in the northwestern Mediterranean Sea. Ocean Sci. 4 (1), 49–59. doi: 10.5194/os-4-49-2008
Raga J. A., Pantoja J. (2004). Proyecto mediterráneo: zonas de especial interés para la conservación de los cetáceos en el mediterráneo español (Organismo Autónomo Parques Nacionales).
Remonato E., Aimi A., Airoldi S. (2013). “Study of residency patterns, home ranges and movements of risso’s dolphins (Grampus griseus, cuvier 1812) in the Western ligurian Sea,” in Grampus griseus 200th anniversary: Risso’s dolphins in the contemporary world”. Report from the European Cetacean Society Conference Workshop, , 25 March 2012. 54–68.
Remonato E., Airoldi S., Rosso M., Lanfredi C., Bittau L., Renata M., et al. (2018). “Where are the risso’s dolphins (Grampus griseus) of the north Western Mediterranean Sea? study of movements within and outside the pelagos sanctuary,” in 32nd annual European Cetacean Society conference, La Spezia (Italy, 8-10 April, 2018.
Santora J. A., Zeno R., Dorman J. G., Syderman W. (2018). Submarine canyons represent an essential habitat network for krill hotspots in a Large marine ecosystem. Sci. Rep. 8, 7579. doi: 10.1038/s41598-018-25742-9
Sekiguchi K., Klages N. T. W., Best P. B. (1992). Comparative- analysis of the diets of smaller odontocete cetaceans along the coast of southern Africa. South Afr. J. Mar. Sci. 12, 843–861. doi: 10.2989/02577619209504746
UNEP MAP-RAC/SPA (2014). Status and Conservation of Cetaceans in the Adriatic Sea. By Holcer D., Fortuna C. M., Mackelworth P. C.. Draft internal report for the purposes of the Mediterranean Regional Workshop to Facilitate the Description of Ecologically or Biologically Significant Marine Areas, Malaga, Spain, 7–11 April 2014.
Whitehead H. (2003). Sperm whales: social evolution in the ocean (Chicago: University of Chicago Press).
Keywords: conservation, Risso’s dolphin, submarine canyons, marine protected area, distribution
Citation: Chicote CA, Amigó N and Gazo M (2023) Submarine canyons as key habitats to preserve Risso’s dolphin (Grampus griseus) populations in the northwestern Mediterranean Sea. Front. Mar. Sci. 10:1080386. doi: 10.3389/fmars.2023.1080386
Received: 26 October 2022; Accepted: 11 April 2023;
Published: 15 May 2023.
Edited by:
Antonella Arcangeli, Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), ItalyReviewed by:
Roberto Carlucci, University of Bari Aldo Moro, ItalyArianna Azzellino, Polytechnic University of Milan, Italy
Giulia Cipriano, University of Bari Aldo Moro, Italy
Copyright © 2023 Chicote, Amigó and Gazo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla A. Chicote, carlachicote@submon.org
†Present address: Manel Gazo, Department of Evolutionary Biology, Ecology and Environmental Science, University of Barcelona, Barcelona, Spain
 Carla A. Chicote
Carla A. Chicote Natalia Amigó
Natalia Amigó Manel Gazo
Manel Gazo