Potential role of climate change on the spread of salmonid skin condition: the biogeochemical hypothesis on ulcerative dermal necrosis on the Słupia River - Poland
- 1Department of Genetics and Marine Biotechnology, Institute of Oceanology of the Polish Academy of Sciences (IO PAN), Sopot, Poland
- 2Polish Angling Association (PZW), Słupsk, Poland
Ulcerative dermal necrosis (UDN) it is an idiopathic condition of fish skin that has been reported in Europe since 1820. UDN affects primarily an epidermal and dermal layer of the skin lesion, which in the early stages, occurs in the head area of migratory adult salmonids entering freshwater for upstream river migration. Studies show that acid-base water properties in estuaries are exceptionally dynamic, which results from the variability of the CO2 system. The carbonate system is shaped by the net effect of mineralization and primary production enhanced by: i) the constant inflow of nutrients and organic matter from the land and ii) the horizontal and vertical mixing of the two end-members of the total alkalinity, dissolved organic carbon and pCO2; both cause high acid-base gradients between the river and the ocean. Climate change affects the biogeochemical characteristics of estuaries. We show a strong positive correlation between local temperature anomalies along the Polish coast and the occurrence of UDN in Salmo trutta sp. spawners in the Słupia River, Poland. The results suggest that the biogeochemical processes associated with climate change may be at least one component of the UDN aetiology. They also highlight the need for systematic monitoring to understand these processes and their consequences. It is crucial for restoring and further preserving sustainability in the coastal system, which involves marine life and human well-being. Furthermore, salmonids are valuable commercial fish. Thus any health issues may have a profound effect on fisheries, local communities and the fish market in general.
1 Introduction
Ulcerative dermal necrosis (UDN) is a condition of the skin of wild and farmed salmonids (Eiras and Saraiva, 1988; Roberts, 1993; Rodgers, 1997). The disease can affect up to 75% of the wild spawners and has become a threat to the stock’s well-being and survival (Grudniewska et al., 2011). If ulceration covers 10% of the fish, the mortality is almost 50% (Noga, 2000). The ICES (2011) reports that adult fish affected by UDN often die before spawning, decreasing the population size.
The first outbreak of UDN occurred in 1820 (Grimble, 1899) and is continuing to emerge with an unrecognized pattern (Ciepliński et al., 2018). The outbreaks occur and gradually disappear (Roberts, 1993). The UDN has been reported in salmonids in Europe (Austria, Belgium, Canada, Finland, France, Germany, Ireland, Luxemburg, Poland, Sweden, Switzerland, UK), but there are also reports, though sparse, from North America (Munro, 1970; Johansson et al., 1982; Roberts, 1993; Grudniewska et al., 2012). Since 2014, there has been a noticeable increase in reports of severe UDN in salmonids in Swedish and Finnish rivers, where UDN had not been observed before (ICES, 2019). In addition, some reports of fish farms in Finland and Portugal affected by UDN are available (Lounatmaa and Janatuinen, 1978; Eiras and Saraiva, 1988), but they are rare, even for farms close to wild stocks (Roberts, 1993). The extent of the disease suggests that Baltic salmonids are not immunologically resistant to UDN, and the condition can spread in both the wild and on farms.
The aetiology of ulcerative dermal necrosis is currently unknown (Rodgers, 1997; Ciepliński et al., 2018). The condition starts with small grey lesions, usually in the head area of the fish (Roberts, 1993) (UDN images are available at https://www.facebook.com/UDNudnUDN). Ulceration may develop into skin necrosis that leads to secondary infection by Saprolegnia molds or bacteria Aeromonas spp. or Pseudomonas spp (Roberts, 1993; Ciepliński et al., 2018). There is no evidence that the cause of UDN is physical damage, such as abrasion of rocks and nets. Furthermore, despite thorough investigations on viruses (Roberts et al., 1972; Johansson et al., 1982; ICES, 2019), bacteria (Roberts, 1993; ICES, 2019), fungi (Carbery and Strickland, 1968; Willoughby, 1969; Roberts et al., 1972; Roberts, 1993) and autoimmune diseases (Roberts et al., 1972), no pathogen or other factor responsible for UDN has yet been identified. Quite evidently, UDN is a complex skin condition probably triggered by many factors, including environmental components. One of them can be the exposure of salmonids to increased ultraviolet radiation (UVR) linked with the destruction of stratospheric ozone and deforestation of river banks. A histological investigation of the effects of UVR on the skin of several fish species demonstrated the radiation lesion and the vulnerability of the damaged skin surface to the invasion of bacteria (Bullock, 1984). The harmful effects of exposure to UVR during all stages of a fish life cycle have been reviewed (Alves and Agustí, 2020). The potential involvement of UVR in the process that initiates UDN in salmonid fish has been discussed by Henard et al. (2022); it might be essential for salmonids residing at depths where exposure to UVR is significant (Holm et al., 2006; Rikardsen et al., 2007; Kristensen et al., 2018).
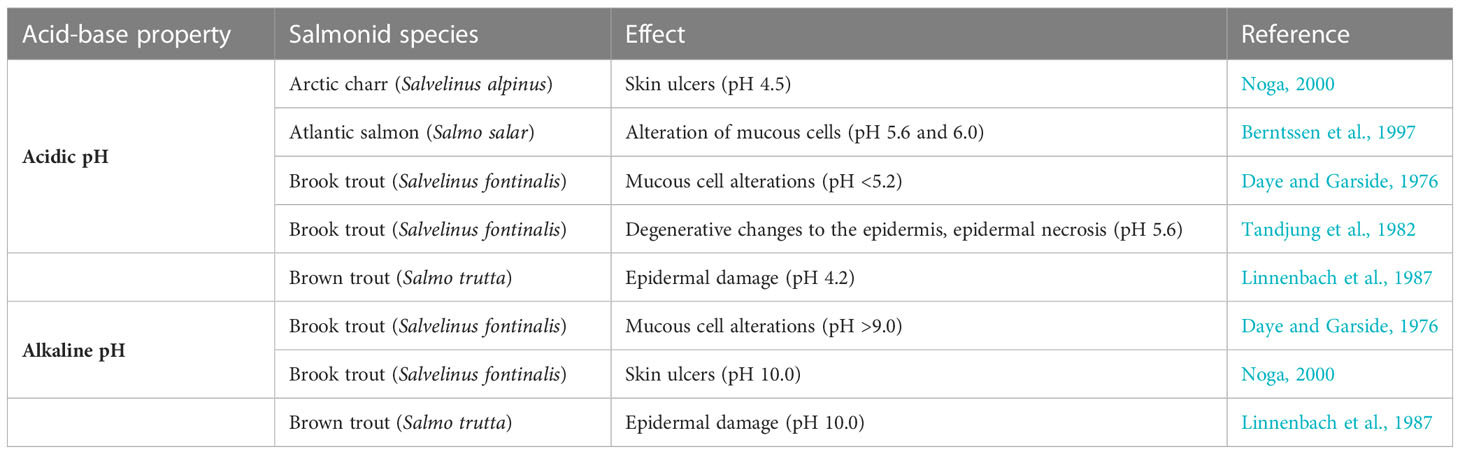
On the other hand, numerous studies show pH-related ulcer formation (Noga, 2000) and mucous cell alterations (Daye and Garside, 1976; Linnenbach et al., 1987; Segner et al., 1987). Sammut et al. (1995) reported the occurrence of ulcers with pH below 4 in the acidified estuarine regions of the Richmond River, Australia. The epithelial necrosis of the tissues of brook trout appears at low pH (below 5.2) and high alkaline conditions (more than 9.0). Tandjung et al. (1982) found degenerative changes in the epidermis during exposure of brook trout, Salvelinus fontinalis, from high to low pH, in extreme cases, leading to dermal necrosis. In fish, a slimy coat, the mucous that covers the epithelial surface, provides a physical and chemical protective layer. A thinned or damaged coating can result in a worsening of defense against harmful physical and chemical factors. The question arises if changing acid-base (AB) conditions in estuaries may impair mucous cells in fish skin and thereby reduce mucous production and/or secretion. Some studies link ulcers in salmonids with heavy metals such as aluminium or copper in water (Segner et al., 1988; Berntssen et al., 1997; Noga, 2000; Rajkowska-Myśliwiec et al., 2022), but the abundance and bioavailability of heavy metal ions are strictly correlated with acid-base conditions (Jin et al., 2021).
Climate change may be a driver of long-term biogeochemical and AB properties in estuaries (Carstensen et al., 2018; Filho et al., 2022). Key climate processes that are likely to influence estuaries are sea-level rise, altered rain patterns, surface heat budget, wind conditions, ocean acidification, these altering the circulation and mixing, and nutrient reduction (HELCOM, 2007; Glamore et al., 2016; Carstensen et al., 2018). On the other hand, elevated atmospheric CO2 also increases the chemical weathering on land which changes the AB properties of freshwater systems, most often increasing the carbonates content (Raymond and Cole 2003). Also, higher temperatures influence the biological processes, i.e., primary production and remineralization of organic matter, driving pCO2 dynamics, and ultimately AB properties (Filho et al., 2022).
The UDN usually appears first in the coastal estuarine zone (Munro, 1970; Roberts et al., 1972; Johansson et al., 1982; Kurhalyuk et al., 2009; ICES, 2019), the region of high spatial variability of chemical characteristics and most dynamic of the whole migration route. Over a few kilometres, completely different water masses are present, oceanic and riverine. They differ in salinity (S), temperature in situ (Tis), oxygen saturation (O2%), partial pressure of carbon dioxide (pCO2), pH, total alkalinity (TA), dissolved inorganic carbon (DIC), organic matter content (OM), nutrients (Nu) and carbonate saturation (Ω). All of these cause completely different acid-base properties of water in the ocean and the river, mainly constituted by the CO2 system (Dickson et al., 2007). Thus, migratory fish pass not only the salinity (osmotic) gradient (Jonsson and Jonsson 2012) but also the less recognized acid-base gradient. Additionally, in the very mixing zone of the river and the sea, the AB conditions can vary significantly depending on the temperature, wind conditions, solar radiation driving the primary production (PP), and remineralization intensity (Stokowski et al., 2020). However, the influence of the variability of AB properties in estuaries on fish health and stress is not yet fully recognized, and studies focus mainly on the salinity gradient (Jonsson and Jonsson 2012).
The characteristics of the largest rivers in Poland have recently been investigated by Stokowski et al. (2020); Stokowski et al. (2021a); Stokowski et al. (2021b). Studies revealed a high dynamics in the carbonate system in the mixing zones of the Oder, Vistula, and Słupia rivers vicinities. Generally, the rivers were rich in total alkalinity and contain more CO2 than the Baltic Sea. In the case of the Vistula River, the annual pH in situ variability was 8.02 to 8.74 in the mixing zone (Stokowski et al., 2021b), which is much higher than in the open sea (+/- 0.3) (Omstedt et al., 2009). It shows that during migration, salmonids and any other anadromous fish pass through, in some cases, extreme AB gradients, which may cause significant stress and other not yet recognized effects. Although the AB properties of water are fundamental for the well-being of aquatic organisms (Powers, 1930), their role is underestimated and far from being clear.
Therefore, all the above matters plus the salmonids’ health are of major concern in terms of the sustainability of the coastal ecosystems, in a wide range of understanding. SDG 14 involves the following issues: “Careful management of this essential global resource is a key feature of a sustainable future. However, at the current time, there is a continuous deterioration of coastal waters owing to pollution, and ocean acidification is having an adversarial effect on the functioning of ecosystems and biodiversity. This is also negatively impacting small-scale fisheries.” (https://www.un.org/sustainabledevelopment/oceans/).
This study aims to investigate whether the UDN that occurs in salmonid spawners in the Słupia River, Poland, is associated with potentially suboptimal biogeochemical conditions related to climate change. We hypothesize that the dynamics of acid-base properties in an aquatic environment linked to climate changes may be at least one component of the UDN aetiology.
2 Study area
Słupia is a river in north-western Poland, a tributary of the Baltic Sea, with a water flow of 16 m3 s-1, a length of 138.6 km and a catchment area of 1620 km2. It is an important river for the migration of Salmo trutta and Salmo salar. The first major outbreak of UDN in the Słupia River was reported in 2007 (Grudniewska et al., 2011); since then salmonids in the river are reported to be affected by UDN (Ciepliński et al., 2018).
The biogenic substances (nitrogen and phosphorus), biological oxygen demand (BOD5) and heavy metals in the Słupia River show dynamics from 1988 to 2007. Generally, the Słupia River experienced a significant reduction in nutrient load related to the change in land use, now qualifying the Słupia River water as the first class of quality (Jarosiewicz, 2009). However, the characteristics of the carbonate system of the Słupia River are highly unknown. Based on Stokowski et al. (2021a) the total alkalinity in the river in May 2020 was ~2300 µmol kg-1. In other rivers draining the same catchment area, the TA is greater than 3000 µmol kg-1 (Vistula and Oder rivers). Therefore, the buffer capacity of the Słupia River is probably lower and more fragile to pH changes or may significantly vary and reach 3000 µmol kg-1 at some point. The Słupia River estuary is located in the temperate climatic zone in the southern Baltic Sea. The mean annual air temperature in Poland for 1991-2020 was 8.7°C, while for the seashore it is higher, amounting to 8.9°C (Miętus, 2021). The water temperature of the Słupia River increased by 0.26°C dec-1 between 1971 and 2015 (Ptak et al., 2016).
On the other hand, the Baltic Sea CO2 system is relatively well described (Thomas and Schneider, 1999; Beldowski et al., 2010; Kuliński et al., 2017). Furthermore, the vicinities of the most significant Polish rivers (Oder and Vistula rivers) have recently been described as showing significant acid-base gradients between rivers and the sea (Stokowski et al., 2020; Stokowski et al., 2021b).
3 Materials and methods
The percentage of Salmo trutta spawners affected by UDN (UDN%) was evaluated using data from the literature for 2007-2010 (Grudniewska et al., 2012), and the unpublished data provided by the Polish Angling Society for 2014-2022. There are no available data for 2011-2013. The UDN% is related to the spawners but not to the whole population of Salmo trutta in the Słupia River. Fish were caught using chamber traps during upstream migrations in autumn. Data from 2007 to 2010 based on reports of the Polish Angling Society branches, Slowinski National Park and “Troć” Fishing and Processing Cooperative. We consider them as preliminary because they were not subject to veterinary checks. From 2014 to 2022, the Polish Angling Society monitored UDN cases under the supervision of qualified ichthyologists experienced in the diagnostics of UDN. Therefore data are presented as two separate sets, 2014-2022 and 2007-2010 (Figures 1A, B). The number of fish caught each year and the percentage of Salmo trutta spawners affected by UDN (2014-2022), or UDN-LIKE (2007-2010) are presented in Table 1. The scarce data on UDN occurrence are available for other Polish rivers and Salmo salar (Grudniewska et al., 2012); in this study, we focus on Salmo trutta in the Słupia River.
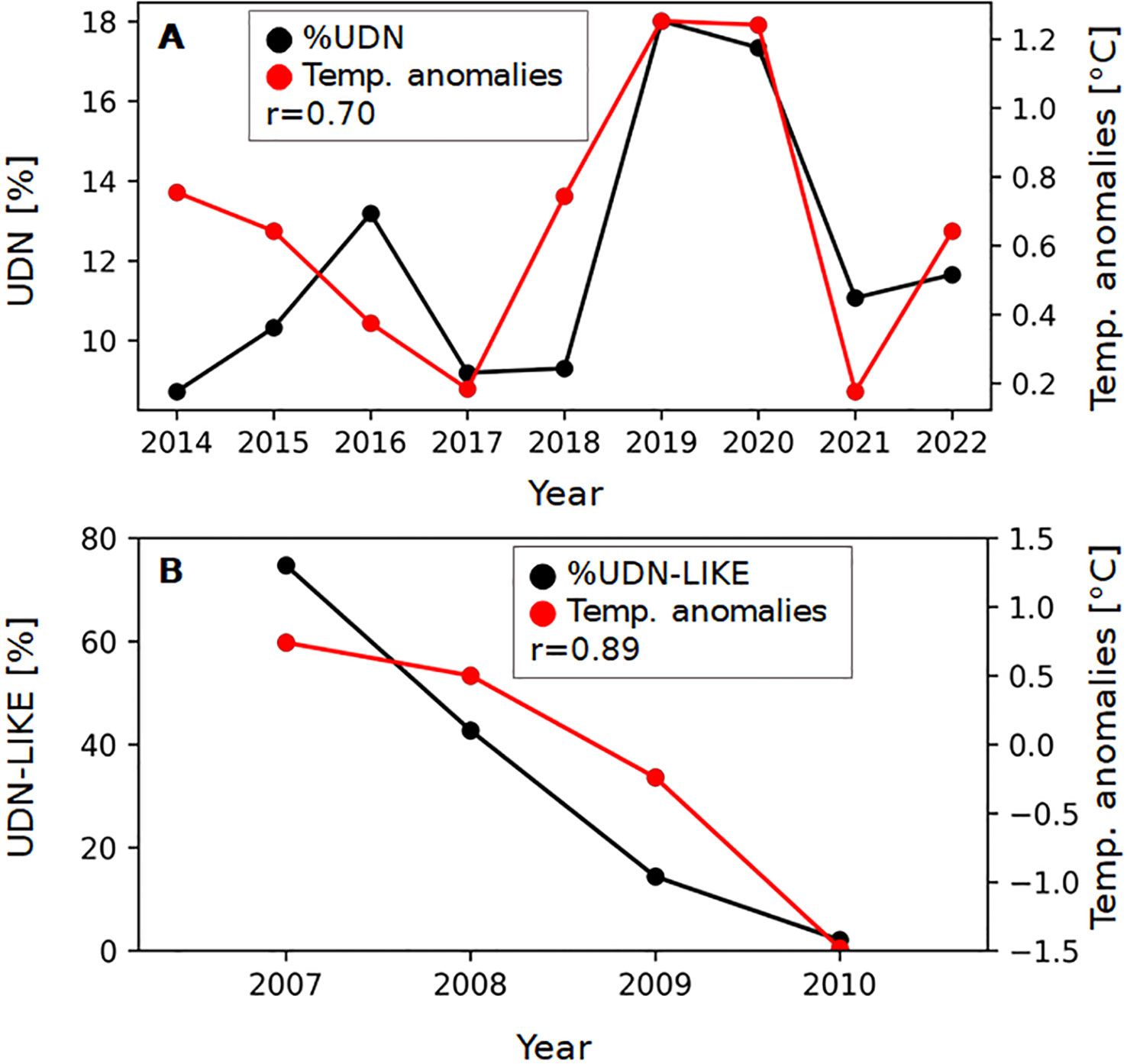
Figure 1 Relationship between the percentage of UDN cases (A) and UDN-LIKE cases (B) of Salmo trutta spawners in the Słupia River and the air temperature anomalies on the Polish coast. The Pearson correlation coefficient (r) describes the correlation between UDN% and air temperature anomalies on the Polish coast.
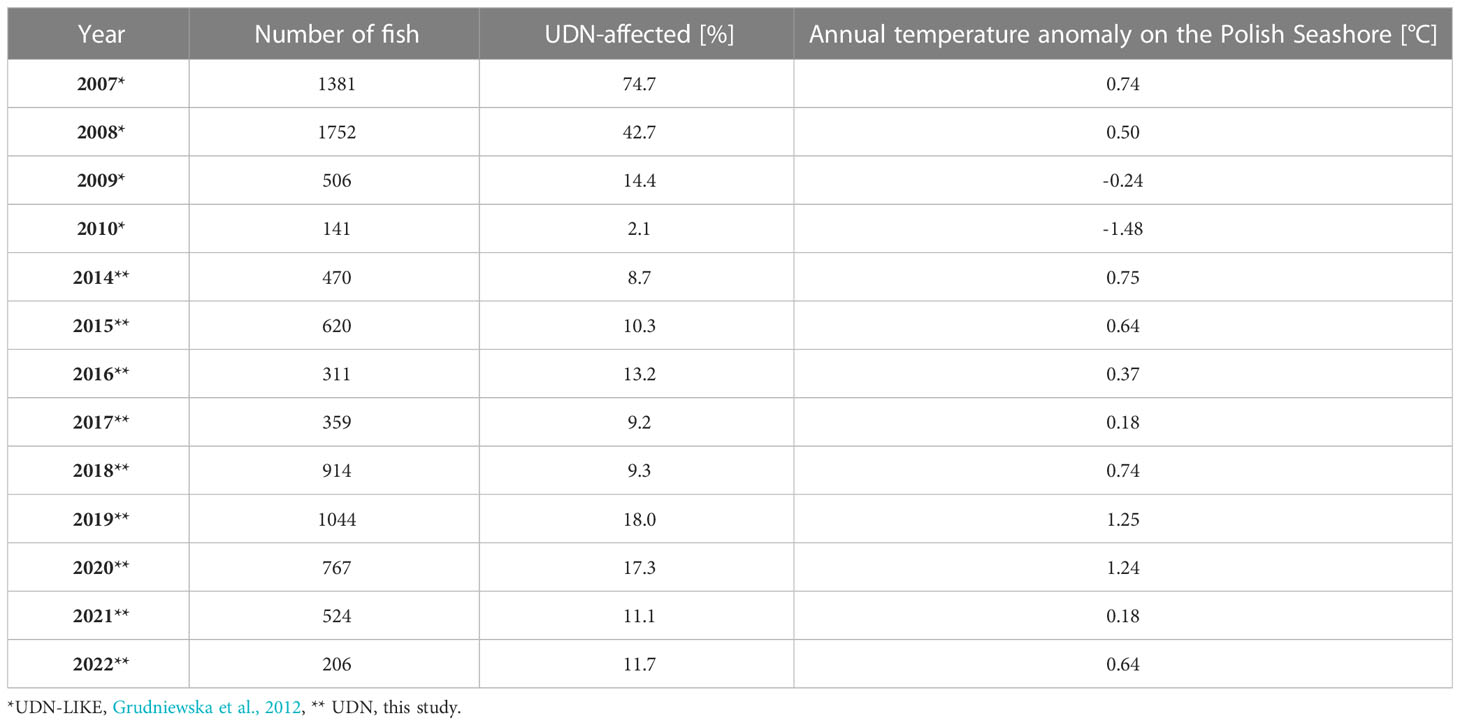
Table 1 The occurrence of UDN or UDN-LIKE in the Słupia River in 2014-2022 and 2007-2010, respectively.
The annual air temperature anomaly on the Polish coast was obtained based on open-source data (https://danepubliczne.imgw.pl/) and the methodology proposed by Wójcik and Miętus, 2014. Air temperature anomalies were defined as a difference between the mean temperatures of 1951-2020 and the mean air temperature of a particular year in the seashore region (Wójcik and Miętus, 2014). The UDN% in Słupia River was correlated with the air temperature anomalies using the Pearson coefficient.
4 Results and discussion
We summarize all UDN reports available in the literature (Figure 2). The first UDN outbreak was reported in Scotland, dated 1820, thus in the heart of the Industrial Revolution period (1733 – 1913) (Grimble, 1899). The number of UDN cases is still growing in Europe (Figure 2) (Munro, 1970; Ljungberg and Johansson, 1977; Meier et al., 1977; Lounatmaa and Janatuinen, 1978; Johansson et al., 1982; Larsen and Jensen, 1982; Eiras and Saraiva, 1988; Roberts, 1993; Law, 2001; Kurhalyuk et al., 2009; Grudniewska et al., 2011; Kazuń et al., 2011; Kurhalyuk et al., 2011; Grudniewska et al., 2012; Grawiński and Kozińska, 2013; Tkachenko et al., 2014; Ciepliński et al., 2018). The first report of UDN for the Baltic region indicates the first occurrence in 1972 (Roberts, 1993), while the first significant outbreak of UDN in the Słupia River occurred in 2007 (Ciepliński et al., 2018). The inserts in Figure 2 show the general scientific interest in UDN based on the Scopus abstract and citation database. It shows that both the occurrence of UDN in European waters and the concern about the spread of the disease are lasting.
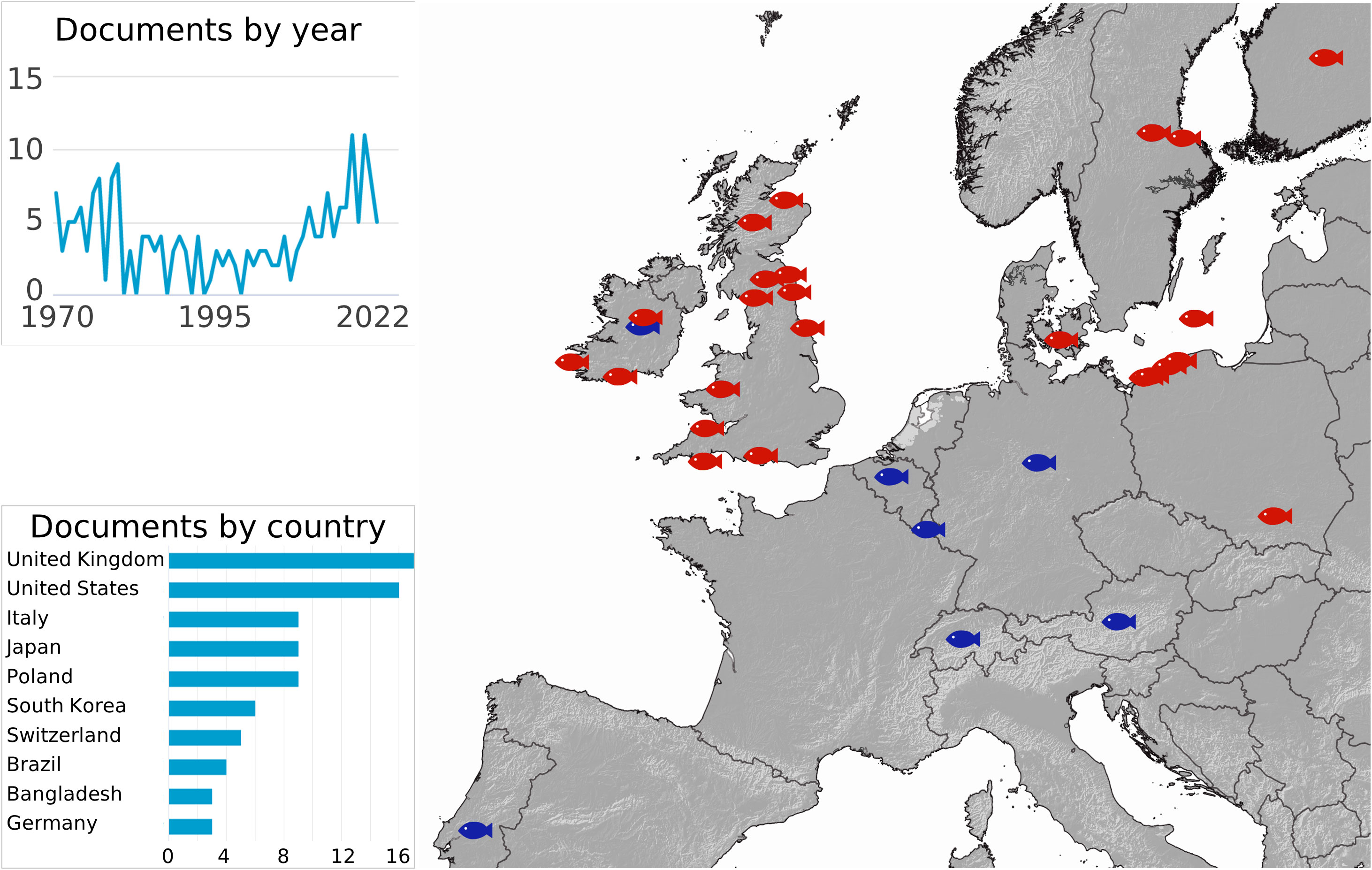
Figure 2 The map of the UDN occurrence (Grimble, 1899; Munro, 1970; Ljungberg and Johansson, 1977; Meier et al., 1977; Lounatmaa and Janatuinen, 1978; Johansson et al., 1982; Larsen and Jensen, 1982; Eiras and Saraiva, 1988; Roberts, 1993; Law, 2001; Kurhalyuk et al., 2009; Grudniewska et al., 2011; Kazuń et al., 2011; Kurhalyuk et al., 2011; Grudniewska et al., 2012; Grawiński and Kozińska, 2013; Tkachenko et al., 2014; Ciepliński et al., 2018). The inserts show the number of UDN-oriented publications by year and by country (Scopus). The red fish indicate the exact region, while the blue fish indicate the reported country of occurrence.
Almost all of the reports are short-term observations in the UDN-affected regions, mainly focusing on demonstrating the occurrence or absence of the disease. There is one exception, the Słupia River, for which the long-term monitoring of UDN occurrence together with the percentage of affected spawners are available (Table 1). The observation of UDN% suggests the pattern of disease incidences in the Słupia River. We have presented it in this study by showing the UDN occurrence against the backdrop of climate change (Figures 1A, B). The assessment is based on the four-year observation by Grudniewska et al. (2012) and the nine-year targeted monitoring of UDN in the Słupia River performed by the Polish Angling Society.
However, there is a significant discrepancy (from 2.1% to 74.7%, Table 1; Figure 1) in the percentage of UDN cases in the Salmo trutta spawners in the Słupia in 2007-2010. As it has been mentioned before data for 2007-2010 can be treated only as a hint for later studies, because then UDN cases were not subjected to veterinary checks. It is probable that most of the UDN-like cases were not UDN but represented other skin lesions that can be observed in salmon skin during migration. However, in 2014-2022, all UDN cases were confirmed by qualified ichthyologists experienced in the diagnostics of UDN. In light of the multitude of data sources and the heterogeneous distribution of observed variables, caution is advised in interpreting our findings.
In this study, a significant, positive correlation between UDN% and local temperature anomalies has been shown (Figure 1A). The Pearson coefficient was 0.70 in 2014-2022. The relationship linking environmental factors and the appearance of UDN proves how important and necessary a long-term collection of UDN data is, especially from the point of view of the aetiology of the disease that remains unknown.
We hypothesize that the climate-change-driven dynamic of the acid-base properties in estuaries is one of the possible causes of UDN. We have considered that the correlation between UDN% and temperature anomalies (Figure 1A) may be a pure statistical coincidence, or other processes present in the environment may play a role. However, the link between climate change and the biogeochemistry of estuaries and aquatic organisms’ responses to pH changes (Table 2) makes the hypothesis reasonable.
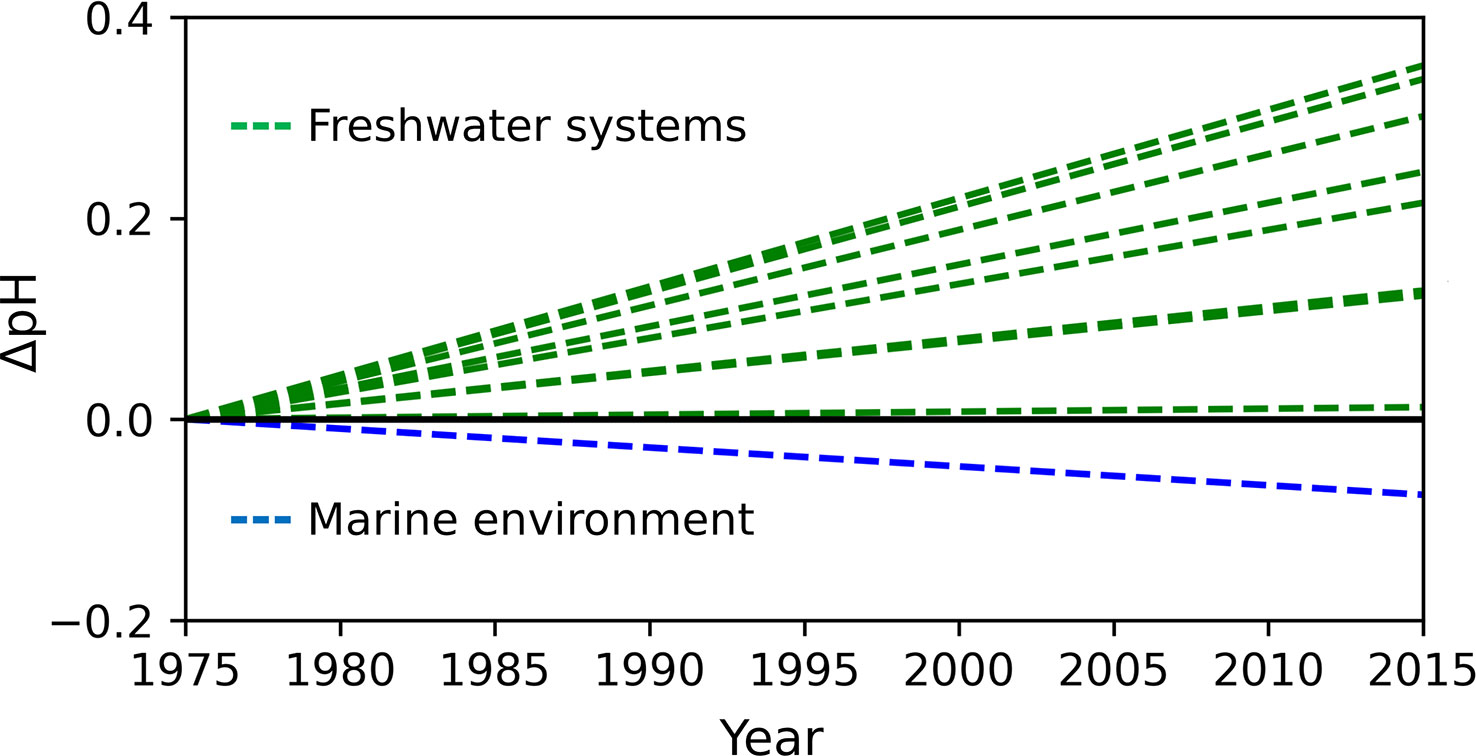
The change from saline to a freshwater environment is energetically demanding due to the change in osmoregulation, the stopping of eating, and the physical effort to overcome the river current. The salinity gradient is one of the chemical barriers that salmonids must overcome. However, seawater and river water also differ significantly in temperature, oxygen saturation and acid-base characteristics, i.e. pH, partial pressure of CO2 (pCO2), total alkalinity (TA), dissolved inorganic carbon (DIC), and calcium carbonate saturation (Ω). Furthermore, over the past few decades, we have observed an increasing pH mismatch between marine and freshwater systems (Doney et al., 2009; Kaushal et al., 2017; Carstensen et al., 2018) (Figure 3). The pH of the river’s end-members is increasing due to enhanced chemical weathering. Climate warming stimulates biological activity in soils and contributes to the release of organic acids and the accumulation of below-ground CO2 linked to microbial respiration, which affects the rate of chemical weathering (Beaulieu et al., 2012). The increased dissolution of carbonates and changes in precipitation patterns due to climate change lead to increased transport and concentration of total alkalinity in rivers (Beaulieu et al., 2012; Stets et al., 2014). On the other hand, oceanic pH is decreasing due to the absorption of anthropogenic CO2. All aforementioned mechanisms are related to climate change (Doney et al., 2009; Kaushal et al., 2017; Carstensen et al., 2018). Thus, the variability of temperature may be reflected in the dynamics of the pH difference between the river and the sea (Figure 3). The changing environmental conditions, including acid-base water properties, may cause detrimental effects and diseases in fish not acclimatized to new circumstances; one can be the UDN. As climate models project an increase in temperature, it is possible that in case of impaired adaptation of salmonids to new conditions, the UDN will continue to emerge.
The laboratory studies summarized in Table 2 show that the acid-base properties of the environment affect salmonids. Some dermal conditions, including epidermal damage (Linnenbach et al., 1987), ulcers, and necrosis in salmonids (Tandjung et al., 1982; Noga, 2000), are pH-related (Table 2). The alteration of mucous cells may decrease the resilience to environmental and biological factors (Daye and Garside, 1976; Berntssen et al., 1997). Thus, changes in the acid-base properties of water may affect salmonids migrating through estuaries (Table 2). Possibly, the fast, not gradual transition across the acid-base gradient during migration and the pH shock may multiply the detrimental effect. It may be the case in the Słupia River estuary characterized by a relatively short distance mixing zone (Kałas and Misiewicz, 2017). Fish skin is directly exposed to harmful factors in the surrounding water with a potential or confirmed detrimental effect on the organism. It is well established that skin is not only a passive biological barrier, but also a site of action of the system responding to stress – the cutaneous stress response system (CSRS) (Kulczykowska, 2019; Gozdowska et al., 2022), and in fish, the epithelial mucous layer constitutes the first line of defense. Therefore, in the nearest future, studies of the skin and mucous of sea trout spawners from regions of dynamic changes in acid-base water properties in the estuaries are required.
We consider that the acid-base gradient between seawater and river water may change into a harmful mismatch for migrating fish regarding climate change. When the adaptation of salmonids to new acid-base gradients in the estuaries becomes slower than the progress of environmental changes, it may cause adverse effects on fish.
5 Summary and perspectives
The UDN is one of the biggest threats to salmonids in Europe; however, the aetiology of this condition remains unknown. The biogeochemical changes related to acidity are probably one among many other processes in the environment which are associated with UDN aetiology. Furthermore, fish in reproductive migrations undergo stress which strongly affects their health.
Herein, we have presented the hypothesis that the acidity changes may be one of the environmental conditions favourable to UDN. In this study, we have chosen this factor as a starting point. We are conscious that more studies are required to verify the hypothesis - in the field and the laboratory. We will undertake this task soon because immediate action and adequate study are needed since climate change will continue, and the well-being of the wild fish population relies on the well-being of spawners. The susceptibility of salmonids to pH changes and the growing climate-driven pH mismatch between freshwater and marine systems may suggest that the spread of UDN is joined with a slow adaptation of fish to changing acid-base conditions. Therefore, the biogeochemical processes associated with climate change may be at least one component of the UDN aetiology. Our results highlight the need for systematic monitoring that could bring more data on UDN occurrence to resolve this problem.
Understanding these processes and their consequences is crucial for restoring and further preserving sustainability in the entire coastal system, which involves marine life as well as human well-being. Such a complex approach fits in with the foundations of the UN Decade of Ocean Science for Sustainable Development, in which one of the main objectives involves reversing the cycle of decline in ocean health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
The contribution of each author is listed below: • MS contributed substantially to data analysis, study conception and drafting of the manuscript. • WS contributed substantially to data acquisition. • EK contributed to the drafting of the manuscript. All authors have approved the manuscript and agree to its submission to Frontiers. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the IOPAN statutory task no. IV.2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alves R. N., Agustí S. (2020). Effect of ultraviolet radiation (UVR) on the life stages of fish. Rev. Fish Biol. Fisheries 30. doi: 10.1007/s11160-020-09603-1
Beldowski J., Löffler A., Schneider B., Joensuu L. (2010). Distribution and biogeochemical control of total CO2 and total alkalinity in the Baltic Sea. J. Mar. Syst. 81, 252–259. doi: 10.1016/j.jmarsys.2009.12.020
Berntssen M., Kroglund F., Rosseland B. O., Wendelaar Bonga S. E. (1997). Responses of skin mucous cells to aluminium exposure at low pH in Atlantic salmon (Salmo salar) smolts. Can. J. Fisheries Aquat. Sci. 54, 1039–1045. doi: 10.1139/f97-015
Beaulieu E., Goddëris Y., Donnadieu Y., Labat D., Roelandt C. (2012). High sensitivity of the continental-weathering carbon dioxide sink to future climate change. Nat. Climate Change 2, 346–349. doi: 10.1038/nclimate1419
Bullock M. A. (1984). The skin response of fish to ultraviolet radiation - a histological study. Scottish Mar. Biol. Assoc. Available at: https://core.ac.uk/download/pdf/9047783.pdf.
Carbery J. T., Strickland K. L. (1968). Resistance of rainbow trout to ulcerative dermal necrosis. Nature 217, 1158. doi: 10.1038/2171158a0
Carstensen J., Chierici M., Gustafsson B. G., Gustafsson E. (2018). Long-term and seasonal trends in estuarine and coastal carbonate systems. Global Biogeochem. Cycles 32 (3), 497–513. doi: 10.1002/2017GB005781
Ciepliński M., Kasprzak M., Grandtke M., Giertych M. J., Steliga A. (2018). Pattern of secondary infection with saprolegnia spp. in wild spawners of UDN-affected sea trout salmo trutta m. trutta (L.), the słupia river, n Poland. Oceanol. Hydrobiol. Stud. 47, 230–238. doi: 10.1515/ohs-2018-0022
Daye P. G., Garside E. T. (1976). Histopathologic changes in surficial tissues of brook trout, salvelinus fontinalis (Mitchill), exposed to acute and chronic levels of pH. Can. J. Zool. 54, 2140–2155. doi: 10.1139/z76-248
Dickson A. G., Sabine C. L., Christian J. R. (Eds.) (2007). Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication 3, 191 pp.
Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A. (2009). Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/annurev.marine.010908.163834
Eiras J., Saraiva A. (1988). First reported occurrence of ulcerative dermal necrosis (UDN) in Portugal (Aberdeen, Scotland, United Kingdom: European Association of Fish Pathologists) 8, 76–78. Available at: https://eafp.org/download/1988-Volume8/Issue%204/08_4%20p76.PDF.
Filho W. L., Nagy G. J., Martinho F., Saroar M., Erache M. G., Primo A. L., et al. (2022). Influences of climate change and variability on estuarine ecosystems: An impact study in selected European, south American and Asian countries. Int. J. Environ. Res. Public Health 19. doi: 10.3390/ijerph19010585
Glamore W. C., Rayner D. S., Rahman P. F. (2016). “Estuaries and climate change,” (UNSW: Technical Monograph prepared for the National Climate Change Adaptation Research Facility. Water Research Laboratory of the School of Civil and Environmental Engineering).
Gozdowska M., Sokołowska E., Pomianowski K., Kulczykowska E. (2022). Melatonin and cortisol as components of the cutaneous stress response system in fish: response to oxidative stress. Comp. Biochem. Physiol. -Part A : Mol. Integr. Physiol. 268. doi: 10.1016/j.cbpa.2022.111207
Grawiński E., Kozińska A. (2013). Badania nad patologią ryb południowego bałtyku – przegląd. Życie weterynaryjne 88, 851–860. XXXE. Paździor.
Grudniewska J., Bartel R., Bernaś R., Ciżmowski Ł., Jesiołowski M., Kacperska B., et al. (2011). Zmiany patologiczne w skórze u tarlaków łososia (Salmo salar) i troci (Salmo trutta m. trutta) z niektórych pomorskich rzek w 2009 roku. Komunikaty Rybackie 2, 7–12.
Grudniewska J., Bartel R., Terech-Majewska E., Kazuń B., Siwicki A. K. (2012). Ocena liczebności, kondycji oraz stanu zdrowotnego tarlaków troci (Salmo trutta trutta) i łososi (Salmo salar) zagrożonych chorobą UDN w niektórych polskich rzekach w 2010 roku. Komunikaty Rybackie 5, 1–7.
HELCOM. (2007). Climate change in the Baltic Sea area – HELCOM thematic assessment in 2007 balt. Sea Environ. Proc. No., 111. Available at: https://www.helcom.Ffi/wp-content/uploads/2019/08/BSEP111.pdf.
Henard C., Saraiva M. R., Ściślak M. E., Ruba T., McLaggan D., Noguera P., et al. (2022). Can ulcerative dermal necrosis (UDN) in Atlantic salmon be attributed to ultraviolet radiation and secondary saprolegnia parasitica infections? Fungal Biol. Rev. 40, 70–75. doi: 10.1016/j.fbr.2022.02.002
Holm M., Jacobsen J. A., Sturlaugsson J., Holst J. C. (2006). Behaviour of Atlantic salmon (Salmo salar l.) recorded by data storage tags in the NE Atlantic – implications for interception by pelagic trawls. ICES Document CM 2006/Q:12, 16.
ICES. (2011). Report of the Baltic salmon and trout assessment working group (WGBAST) (Riga, Latvia: ICES2011/ACOM:08). 297 pp.
ICES. (2019). Baltic Salmon and trout assessment working group (Wgbast). ICES Sci. Rep. 1, 312. doi: 10.17895/ices.pub.5974
Jarosiewicz A. (2009). Transformation of the water quality in the słupia river (Poland). Slupijas Upes (Polija) Ūdens Kvalitātes Izmaiņas 2, 64–70. doi: 10.17770/etr2009vol2.1024
Jin P., Zhang J., Wan J., Overmans S., Gao G., Ye M., et al. (2021). The combined effects of ocean acidification and heavy metals on marine organisms: a meta-analysis. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.801889
Johansson N., Svensson K. M., Fridberg G. (1982). Studies on the pathology of ulcerative dermal necrosis (UDN) in Swedish salmon, salmo salar l., and sea trout, salmo trutta l., populations. J. Fish Dis. 5, 293–308. doi: 10.1111/j.1365-2761.1982.tb00485.x
Jonsson B., Jonsson N. (2012). Ecology of Atlantic salmon and brown trout - habitat as a template for life histories. Fish Fish. 13. doi: 10.1111/j.1467-2979.2012.00472.x
Kałas M., Misiewicz E. (2017). Hydrophysical parameters of seawater in front of ustka during the summer season 2013. Biuletyn Instytutu Morskiego 32, 37–46. doi: 10.5604/12307424.1227776
Kaushal S. S., Duan S., Doody T. R., Haq S., Smith R. M., Newcomer Johnson T. A., et al. (2017). Human-accelerated weathering increases salinization, major ions, and alkalinization in fresh water across land use. Appl. Geochem. 83, 121–135. doi: 10.1016/j.apgeochem.2017.02.006
Kazuń B., Grudniewska J., Terech-Majewska E., Kazuń K., Głąbski E., Siwicki A. K. (2011). Ocena stanu zdrowotnego tarlaków troci (Salmo trutta trutta) z rzek pomorskich na podstawie badań mikrobiologicznych i immunologicznych, prowadzonych w 2010 roku. KOMUNIKATY RYBACKIE 5, 1–4.
Kristensen M., Righton D., del Villar-Guerra D., Baktoft H., Aarestrup K. (2018). Temperature and depth preferences of adult sea trout salmo trutta during the marine migration phase. Mar. Ecol. Prog. Ser. 599, 209–224. doi: 10.3354/meps12618
Kulczykowska E. (2019). Arginine vasotocin and isotocin: towards their role in fish osmoregulation. Fish Osmoregulation, 151–176. doi: 10.1201/b10994-6
Kuliński K., Schneider B., Szymczycha B., Stokowski M. (2017). Structure and functioning of the acid–base system in the Baltic Sea. Earth System Dynam. 8, 1107–1120. doi: 10.5194/esd-8-1107-2017
Kurhalyuk N., Tkachenko H., Pałczyńska K. (2009). Antioxidant enzymes profile in the brown trout (Salmo trutta trutta) with ulcerative dermal necrosis. Bull. Veterinary Institute Pulawy 53, 813–818.
Kurhalyuk N., Tkachenko H., Pałczyńska K. (2011). Resistance of erythrocytes from brown trout (Salmo trutta m. trutta l.) affected by ulcerative dermal necrosis syndrome. Pol. J. Vet. Sci. 14, 443–448. doi: 10.2478/v10181-011-0065-0
Larsen J. L., Jensen N. J. (1982). The ulcus syndrome in cod (Gadus morhua). prevalence in selected Danish marine recipiens and a control site in the period 1976-1979. Nord. Vet.-Med 34, 303–321.
Law M. (2001). Differential diagnosis of ulcerative lesions in fish. Environ. Health Perspect. 109, 681–686. doi: 10.1289/ehp.01109s5681
Linnenbach M., Marthaler R., Gebhardt H. (1987). Effects of acid water on gills and epidermis in brown trout (Salmo trutta l.) and in tadpoles of the common frog (Rana temporaria l.) [in low mountain lakes and brooks of West Germany]. Annales la Societe Royale Zoologique Belgique 117.
Ljungberg O., Johansson N. (1977). Ulceros dermal nekros hos laxfiskar. Svensk Veterindrtidning 4, 129–135.
Lounatmaa K., Janatuinen J. (1978). Electron microscopy of an ulcerative dermal necrosis (UDN)-like salmon disease in Finland. J. Fish Dis. 1, 369–375. doi: 10.1111/j.1365-2761.1978.tb00041.x
Meier W., Klingler K., Müller R. (1977). Ulcerative dermal necrosis (UDN) of the river trout (Salmo trutta fario) in switzerland. II. predisposing and infection- precipitating factors. Schweiz Arch. Tierheilkd 119, 277–291.
Munro A. L. S. (1970). Ulcerative dermal necrosis, a disease of migratory salmonid fishes in the rivers of the British isles. Biol. Conserv. 2, 129–132. doi: 10.1016/0006-3207(70)90148-5
Noga E. J. (2000). Skin ulcers in fish: pfiesteria and other etiologies. Toxicologic Pathol. 28, 807–823. doi: 10.1177/019262330002800607
Omstedt A., Gustafsson E., Wesslander K. (2009). Modelling the uptake and release of carbon dioxide in the Baltic Sea surface water. Continental Shelf Res. 29, 870–885. doi: 10.1016/j.csr.2009.01.006
Powers E. P. (1930). The relation between pH and aquatic animals. Am. Nat. 64, 342–366. Available at: https://www.semanticscholar.org/paper/The-Relation-between-pH-and-Aquatic-Animals-Powers/5d749184ff0f5344c2b118f9c37ed39071ebae84.
Ptak M., Choiński A., Kirviel J. (2016). Long-term water temperature fluctuations in coastal rivers (southern Baltic) in Poland. Bull. Geogr. Phys. Geogr. Ser. 11, 35–42. doi: 10.1515/bgeo-2016-0013
Rajkowska-Myśliwiec M., Protasowicki M., Tański A., Watrak S. (2022). Heavy metals and the occurrence of ulcerative dermal necrosis (UDN) in Sea trout from the RIVER REGA, Poland–consumer health assessment. Int. J. Environ. Res. Public Health 19. doi: 10.3390/ijerph19042296
Raymond P. A., Cole J. J. (2003). Increase in the export of alkalinity from north america’s largest river. Science 301, 88–91. doi: 10.1126/science.1083788
Rikardsen A. H., Diserud O. H., Elliott J. M., Dempson J. B., Sturlaugsson J., Jensen A. J. (2007). The marine temperature and depth preferences of Arctic charr (Salvelinus alpinus) and sea trout (Salmo trutta), as recorded by data storage tags. Fisheries Oceanogr. 16, 436–447. doi: 10.1111/j.1365-2419.2007.00445.x
Roberts R. J. (1993). Ulcerative dermal necrosis (UDN) in wild salmonids. Fisheries Res. 17, 3–14. doi: 10.1016/0165-7836(93)90003-P
Roberts R. J., Shearer W. M., Munro A. L. S. (1972). Studies on ulcerative dermal necrosis of salmonids: IV. failure to detect epithelial auto-antibodies in sera from diseased fish. J. Fish Biol. 4, 21–25. doi: 10.1111/j.1095-8649.1972.tb05648.x
Rodgers C. J. (1997). A colour atlas of salmonid diseases. Aquaculture 152. doi: 10.1016/s0044-8486(97)89062-x
Sammut J., Melville M. D., Callinan R. B., Fraser G. C. (1995). Estuarine acidification: impacts on aquatic biota of draining acid sulphate soils. Aust. Geographical Stud. 33, 89–100. doi: 10.1111/j.1467-8470.1995.tb00687.x
Segner H., Linnenbach M., Marthaler R. (1987). Towards a use of epidermal mucous cells in the field assessment of acid stress in fish. J. Appl. Ichthyol. 3, 187–190. doi: 10.1111/j.1439-0426.1987.tb00540.x
Segner H., Marthaler R., Linnenbach M. (1988). Growth, aluminium uptake and mucous cell morphometrics of early life stages of brown trout, salmo trutta, in low pH water. Environ. Biol. Fishes 21, 153–159. doi: 10.1007/BF00004850
Stets E. G. G., Kelly V. J. J., Crawford C. G. G. (2014). Long-term trends in alkalinity in large rivers of the conterminous US in relation to acidification, agriculture, and hydrologic modification. Sci. Total Environ. 488–489, 280–289. doi: 10.1016/j.scitotenv.2014.04.054
Stokowski M., Makuch P., Rutkowski K., Wichorowski M., Kuliński K. (2021a). A system for the determination of surface water pCO2 in a highly variable environment, exemplified in the southern Baltic Sea. Oceanologia 63, 276–282. doi: 10.1016/j.oceano.2021.01.001
Stokowski M., Schneider B., Rehder G., Kuliński K. (2020). The characteristics of the CO2 system of the oder river estuary (Baltic Sea). J. Mar. Syst. 211, 103418. doi: 10.1016/j.jmarsys.2020.103418
Stokowski M., Winogradow A., Szymczycha B., Carstensen J., Kuliński K. (2021b). The CO2 system dynamics in the vicinity of the vistula river mouth (the southern Baltic sea): a baseline investigation. Estuarine Coast. Shelf Sci. 258, 107444. doi: 10.1016/j.ecss.2021.107444
Tandjung S. D., Rottiers D. V., Herman R. L., Godjah M. (1982). “Histopathological studies on the effects of acid stress to brook trout, salvelinus fontinalis (Mitchell) in the presence of sublethal concentration of aluminum,” in Proceedings of an International Symposium on Acidic Rain and Fishery Impacts on Northeastern North America. 357.
Thomas H., Schneider B. (1999). The seasonal cycle of carbon dioxide in Baltic Sea surface waters. J. Mar. Syst. 22, 53–67. doi: 10.1016/S0924-7963(99)00030-5
Tkachenko H., Kurhaluk N., Andriichuk A., Gasiuk E., Beschasniu S. (2014). Oxidative stress biomarkers in liver of sea trout (salmo trutta m. trutta l.) affected by ulcerative dermal necrosis syndrome. Turkish J. Fisheries Aquat. Sci. 14, 391–402. doi: 10.4194/1303-2712-v14_2_09
Willoughby L. G. (1969). Salmon disease in windermere and the river leven: the fungal aspect. Salmon Trout Magazine 186, 124–129.
Keywords: CO2 system, Salmo trutta, UDN, ocean acidification (OA), basification, coastal zone, estuary
Citation: Stokowski M, Sobiegraj W and Kulczykowska E (2023) Potential role of climate change on the spread of salmonid skin condition: the biogeochemical hypothesis on ulcerative dermal necrosis on the Słupia River - Poland. Front. Mar. Sci. 10:1104436. doi: 10.3389/fmars.2023.1104436
Received: 21 November 2022; Accepted: 28 April 2023;
Published: 12 May 2023.
Edited by:
Hongbo Jiang, Shenyang Agricultural University, ChinaReviewed by:
Agnieszka Paulina Kijewska, Gdański Uniwersytet Medyczn, PolandPatricia Noguera, University of Aberdeen, United Kingdom
Copyright © 2023 Stokowski, Sobiegraj and Kulczykowska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcin Stokowski, stokowski@iopan.pl
 Marcin Stokowski
Marcin Stokowski Wojciech Sobiegraj2
Wojciech Sobiegraj2  Ewa Kulczykowska
Ewa Kulczykowska