Systematics of lionfishes (Scorpaenidae: Pteroini) using molecular and morphological data
- 1Department of Oceanography, National Sun Yat-sen University, Kaohsiung, Taiwan
- 2Biogeochemistry Lab, Taiwan Ocean Research Institute, Kaohsiung, Taiwan
Lionfishes (tribe Pteroini) are eye-catching due to their distinct elongated fins and warning coloration. The monophyly of the Pteroini is supported by recent phylogenetic analyses. However, the interrelationships between inter- and intra-genera of the Pteroini are contentious. In this study, 5,335 bps of two mitochondrial and five nuclear genes were sequenced to reconstruct the phylogeny of lionfishes. Our analyses showed Dendrochirus and Pterois were both not monophyletic and divided into Dendrochirus I, II, and III clades (D. I, II, and III) and Pterois I and II clades (Pt. I and II), respectively. Pt. I was sister to the Pt. II + D. I clades. D. II was the sister group of the Ebosia + Parapterois clade. The D. III clade was at the base of the Pteroini, followed by the genus Brachypterois. Morphologically, we provided combinations of characters to distinguish all clades. According to the molecular and morphological data, we propose a revised taxonomy of the Pteroini. D. I and Pt. I hold the generic names of Dendrochirus and Pterois, respectively. Neochirus gen. n. is proposed as a new genus for the D. II clade. The genera Pteropterus Swainson, 1839 and Nemapterois Fowler, 1938 are revalidated for Pt. II and D. III, respectively.
1 Introduction
Fishes of the tribe Pteroini (sensu Nelson et al., 2016), commonly known as lionfishes or turkeyfishes, are eye-catching with elongated fins and warning coloration (Randall, 2005a; Randall, 2005b). This fish group is well known for their venomous glands on the dorsal, anal, and pelvic fin spines (Allen and Eschmeyer, 1973), which are toxic and cause serious pain (Masuda et al., 1986; Randall, 2005a). Lionfishes are distributed throughout the tropical Indo-West Pacific region (Kochzius et al., 2003; Allen and Erdmann, 2008), and some species of Pterois were introduced to invaded waters through the aquarium trade, including Pterois volitans and Pterois miles in the Western Atlantic Ocean (Schofield, 2009; Schofield, 2010; Johnston and Purkis, 2011) and Pterois miles in the Mediterranean Sea (Bariche et al., 2013; Kletou et al., 2016).
The Pteroini are typified by the genus Pterois, established by Cuvier (1817), which currently comprises 12 nominal genera. However, Cuvier (1817) named this fish genus in French guise, Les Pterois, and it is therefore not available. Oken (1817) Latinized the genus Pterois, which is accepted as the first generic name by subsequently authors (Gill, 1903). Desmarest (1856) subsequent designated Scorpaena volitans as the type species of Pterois. Swainson (1839) established several genera comprising species separated from Pterois, including Brachyrus, Dendrochirus, Macrochirus, Pteroleptus, and Pteropterus. Bleeker (1863) established the genus Pseudomonopterus, typified by Pterois volitans. Bleeker (1876) revised Swainson’s (1839) genera and considered Brachyrus a synonym of Dendrochirus; meanwhile, he established the genus Parapterois. Swain (1882) synonymized four genera with Pterois, including Brachyrus, Macrochirus, Pteroleptus, and Pteropterus. Jordan and Starks (1904) propose the monotypic genus Ebosia based on Pterois bleekeri. Most authors subsequently considered the genus Dendrochirus valid in their works (Jordan and Seale, 1906; Herre, 1952; Smith, 1957; Matsunuma and Motomura, 2013; Matsunuma et al., 2017). Fowler (1938) erected two genera Brachypterois and Nemapterois, typified by Brachypterois serrulifer and Nemapterois biocellatus, respectively. The genus Parabrachirus was established by Matsubara (1943), and later this genus was considered an objective synonym of Parapterois by Smith (1957). Whitley (1951) proposed a new generic name, Ranipterois, to be a replacement name for the genus Brachypterois Fowler, 1938, since the latter was considered preoccupied by Brachypterois Jordan and Seale, 1906. However, the name Brachypterois (sensu Jordan and Seale, 1906) was actually a misspelling of Bathypterois Günther 1878. The replacement name, Ranipterois, is unneeded and becomes an objective synonym of Brachypterois Fowler, 1938 (Mandrytsa, 2001; Matsunuma et al., 2013). In Smith‘s (1957) work, three genera were considered junior synonyms of Pterois, including Macrochirus, Pseudomonopterus, and Pteroleptus. In addition, he treated Pteropterus as a valid genus distinguished from Pterois by counts of pectoral-fin rays, scale types, scale rows in the longitudinal series, and the state between orbit and suborbital ridge. Some authors generally consider Pteropterus a junior synonym of Pterois (Eschmeyer and Randall, 1975; Eschmeyer, 1986; Matsunuma and Motomura, 2015; Matsunuma and Motomura, 2016b). Eschmeyer and Randall (1975) considered Nemapterois a synonym of Dendrochirus. Mandrytsa (2001) synonymized Nemapterois with Dendrochirus; meanwhile, he listed several genera as junior synonyms of Pterois, including Macrochirus, Pseudomonopterus, and Pteroleptus. In total, five pterion genera are currently considered valid, including Brachypterois Fowler, 1938, Dendrochirus Swainson, 1839, Ebosia Jordan and Starks, 1904, Parapterois Bleeker, 1876, and Pterois Oken, 1817, comprising 30 species (Motomura, 2004a; Allen and Erdmann, 2008; Matsunuma et al., 2013; Matsunuma and Motomura, 2016a; Matsunuma and Motomura, 2016b; Nelson et al., 2016; Matsunuma et al., 2017; Matsunuma and Motomura, 2019; Matsunuma and Motomura, 2022).
Among the five valid genera, Dendrochirus and Pterois are the two major specious groups in the Pteroini (Smith, 1957; Eschmeyer and Randall, 1975; Masuda et al., 1986; Matsunuma and Motomura, 2013), comprising eight and 12 species, respectively (Matsunuma and Motomura, 2016b; Matsunuma and Motomura, 2019). In general, Dendrochirus can be distinguished from Pterois by two characteristics of the pectoral fin, viz., upper pectoral rays branched in Dendrochirus (versus all pectoral fin rays not branched throughout life in Pterois) (Swainson, 1839; Eschmeyer and Randall, 1975; Masuda et al., 1986; Poss, 1999; Matsunuma et al., 2017) and pectoral rays not free from membrane in Dendrochirus (versus upper pectoral rays free from membrane in Pterois) (Swainson, 1839; Smith, 1957).
The monophyly of the Pteroini has been tested by previous studies based on morphological and molecular evidence . Ishida (1994) reconstructed the phylogeny of the suborder Scorpaenoidei (Figure 1A) based on 95 osteological and myological characters and showed that Pterois is the basal group of the Pteroini, and the remaining four genera form an unresolved monophyletic group. Imamura (2004) analyzed the phylogeny of the Scorpaenoidea based on 111 osteological and myological characters, and his results supported the monophyly of the Pteroini despite only including species of Dendrochirus and Pterois. Smith et al. (2018) reconstructed the phylogeny of the order Scorpaeniformes based on 113 morphological and 5,280 molecular characters. In their result, the genera Dendrochirus and Pterois formed a monophyletic group but also only included one species for each genus. Taxon samplings in the above phylogenetic analyses are inadequate, with only one species included for each genus. The molecular phylogenies of Smith and Wheeler (2004) and Smith and Craig (2007) also showed the Pteroini as a monophyletic group, but their studies focused on resolving interrelationships of higher taxonomic ranks, and only two species of two pteroin genera were included. Kochzius et al. (2003) focused on Dendrochirus, including two species, and Pterois, including five species, based on short fragments of cyt b (421 bp) and 16S rRNA (543 bp). Their results (Figure 1B) did not support the monophyly of Dendrochirus and Pterois and showed D. zebra was clustered with the clade of Pterois, comprising P. antennata, P. mombasae, and P. radiata. Freshwater et al. (2009) reconstructed the phylogeny of Dendrochirus and Pterois using fragments of cyt b (891 bp) and one more species, D. biocellatus. In their phylogenetic tree (Figure 1C), the non-monophyly of Dendrochirus and Pterois was retrieved with similar topology to Kochzius et al. (2003), except that Dendrochirus was split into three clades.

Figure 1 Phylogenetic hypotheses of the tribe Pteroini in the previous studies. Morphological data: (A) Cladogram reconstructed based on 95 osteological and myological characters (Ishida, 1994). Molecular data: (B) Strict consensus tree reconstructed based on cyt b (421 bps) and 16S (543 bps) sequences by ML and MP analysis (Kochzius et al., 2003); and (C) NJ tree reconstructed based on 891 bps of cyt b (Freshwater et al., 2009).
Although the non-monophyly of Dendrochirus and Pterois is supported by molecular studies, the phylogenetic relationships of Brachypterois, Ebosia, and Parapterois in the Pteroini are still unclear. In addition, due to the limited sampling sizes of previous studies, the phylogenetic relationships at the intra-generic level are contentious. In addition, a close relationship between Dendrochirus and Pterois was proposed (Herre, 1952; Eschmeyer and Randall, 1975; Kochzius et al., 2003), but this phylogenetic hypothesis has never been well tested.
In the present study, we reconstruct the phylogeny of the Pteroini based on two mitochondrial and five nuclear genetic markers and include species of all five valid genera of Pteroini. This phylogenetic study is aimed at (a) examining the monophyly of pteroin genera, especially the specious Dendrochirus and Pterois; (b) investigating the interrelationships of genera within the Pteroini; and (c) examining the validity of the proposed diagnostic characters of each genus.
2 Materials and methods
2.1 Taxonomic sampling
In the present study, at least one species, including type species of all nominal genera except for Pteroleptus, was selected as representative of each pteroin genus. Two species of closely related genera, Hoplosebastes armatus and Scorpaenodes guamensis (Ishida, 1994; Smith et al., 2018), and four additional species of the Scorpaenoidei, including Neosebastes entaxis, Rhinopias eschmeyeri, Scorpaenopsis ramaraoi, and Thysanichthys crossotus, were selected as outgroups. Fish were purchased from fish landing sites or aquarium shops. The collection information is shown in Table S1. Additional seven COI sequences of various species, including five species for which we do not have access to sequencing and morphological examinations, were downloaded from GenBank and BOLD Systems (Table S3). In total, 19 out of 30 valid species of the Pteroini and six outgroups were included in the phylogenetic analyses (Table S3). Tissue samples were collected from muscle or fins and preserved in 95% ETOH and stored at −20°C. Voucher specimens were fixed in 10% neutral buffered formalin and transferred to 70% ETOH for permanent preservation. They were deposited in the Department of Oceanography, National Sun Yat-sen University, Kaohsiung (DOS), and Academia Sinica Institute of Zoology, Taiwan (ASIZP). Catalog numbers for voucher specimens are listed in Table S3.
2.2 Morphological analysis
Meristic counts were generally made on the left side and followed by Motomura (2004b) and Motomura et al. (2005). The standard length (SL) is measured as the direct distance from the tip of the upper lip to the middle of the posterior margin of the hypural plate. The last two dorsal and anal soft rays were counted as a single ray. Pectoral-fin rays are counted beginning with the uppermost ray. The longitudinal scale rows were taken from above the first pored lateral scale to the caudal-fin base; the number of near-vertical to oblique scale rows is above the lateral line. The terminology of head spines followed the diagram from Randall and Eschmeyer (2002). Terminologies of color patterns on the head were generally followed by Matsunuma and Motomura (2014); Matsunuma et al. (2017), and Matsunuma and Motomura (2019). Character mapping of selected characters was performed on the phylogeny using the stochastic mapping approach in Mesquite version 2.75 (Maddison and Maddison, 2011).
2.3 DNA extraction, amplification, and sequencing
Two mitochondrial (COI and cyt b) and five nuclear (gylt, plagl2, Ptr, rhodopsin, and zic1) genetic markers were selected for phylogenetic reconstruction. Genomic DNA was extracted from tissues using a GeneMark Easy Tissue and Cell Genomic DNA Purification Kit following the manufacturer’s protocol. Polymerase chain reactions (PCR) were performed in a 25 μl volume containing 3 μl of 10× Taq Buffer, 2 μl of dNTP mixture at 10 mM, 1 μl each of forward and reverse primers at 5 μM, 0.125 μl of Pro Taq Plus DNA polymerase (Protech Technology Enterprise, Taiwan), 1 μl of template DNA, and the remaining ultrapure water. In some cases when PCR failed, PCR reactions were performed in a 25 μl volume consisting of 12.5 μl of SuperRed PCR Master Mix (2x), 1 μl each of forward and reverse primers at 5 μM, 1 μl of template DNA, and the remaining ultrapure water. COI was amplified using combinations of universal COI primer pairs (Ward et al., 2005), FishF1, FishF2, FishR1, and FishR2. Cyt b was amplified using previously published primer pairs (Schmidt and Gold, 1993; Perdices et al., 2001), L14724 and H15915. Rhodopsin was amplified using combinations of previously published primer pairs (Chen et al., 2003; Chen et al., 2008), RH28F, RH1039R, and RH193F. The remaining nuclear genes (gylt, plagl2, Ptr, and zic1) were amplified using the primers reported by Li et al. (2007). The thermal cycle profiles of COI consisted of an initial denaturation step at 95°C for 4 min, followed by 35 cycles of 94°C for 30 s, 48°C for 30 s, and 72°C for 1 min; 92°C for 3 min, followed by 34 cycles of 92°C for 1 min, 53°C for 90 s, and 72°C for 3 min for cyt b; 94°C for 3 min, followed by 10 cycles of 94°C for 45 s, 58°C for 45 s, and 72°C for 75 s, and then additional 30 cycle of 94°C for 45 s, 55°C for 30 s, and 72°C for 75 s for five nuclear genes. All reactions ended in a final step at 72°C for 4–10 min. All PCR products were verified on 2% agarose gels and reliable enzymatically cleaned with the SAP-Exo Kit (Jena Bioscience, Jena, Germany). PCR products were sequenced in the forward and reverse directions by a biotechnology company (Genomics, Taiwan). Forward and reverse sequences were assembled and edited using BioEdit ver. 7.2.5 (Hall, 1999). Accession numbers for sequences generated in this study and downloaded from databases are listed in Table S3.
2.4 Alignment, model selection, and phylogenetic reconstruction
The alignments of multiple sequences were independently performed on each gene using clustalW (Thompson et al., 1994) in BioEdit version 7.2.5 (Hall, 1999). Substitution saturation of all genes was tested using DAMBE version 6.3.17 (Xia, 2013). Models of nucleotide substitution were determined for each genetic marker using PartitionFinder 2 (Lanfear et al., 2017). The optimal partitioning and model scheme identified the data as four partitions, including COI + cyt b (the best fit model as TRN + I + G), Ptr + rhodopsin + plagl2 (HKY + I + G), gylt (K80 + G), and zic1 (HKY + I). The sequences of seven genes were concatenated for phylogenetic analysis. For both datasets of COI and concatenated sequences, Maximum likelihood (ML) analysis was performed using the RAxML Blackbox web server (Kozlov et al., 2019). Bootstrap support was calculated with 100 reiterations. Maximum parsimony (MP) analysis was conducted using PAUP version 4.0 (Phylogenetic Analysis Using Parsimony) (Swofford, 2002) with 1,000 bootstrap reiterations. Bayesian inference (BI) of the combined molecular and selected seven morphological data was run using MrBayes version 3.2.2 (Ronquist et al., 2012). The parameter for the morphological coding was independently set to a gamma-shaped rate variation. The Markov chain Monte Carlo (MCMC) analysis was simultaneously run in four parallel chains for 3,000,000 generations with a sample frequency of every 1,000 generations, after checking that it was sufficient for convergence. A consensus tree was constructed after discarding the burn-in of the first 750,000 generations and evaluating statistical confidence in nodes by Bayesian posterior probabilities. A phylogenetic tree was displayed with FigTree version 1.4.3 (Rambaut, 2007).
3 Results
3.1 DNA sequences and phylogenetic analysis
Fourteen species of five pteroin genera and six outgroups were sequenced. A total of 245 sequences were generated in the present study. The concatenated sequences consist of two mitochondrial and five nuclear genetic markers, including COI (678 bp), cyt b (896 bp), gylt (732 bp), plagl2 (815 bp), Ptr (751 bp), rhodopsin (790 bp), and zic1 (673 bp), amounting to a total of 5,335 bp.
All genes were not found to have reached saturation. The individual gene trees of ML, BI, and MP are shown in Figure S1. Topologies among individual gene trees were not highly congruent, but ML, BI, and MP trees were reconstructed based on concatenated sequences as in previous studies (Schonhuth et al., 2015; Tavera et al., 2018; Costa et al., 2020; Sudasinghe et al., 2020; and many more). Only ML topology (Figure 2) was shown because the three phylogenetic results were consistent in most clades. The Pteroini were a monophyletic group with high support of 100 bootstrap values or posterior probabilities of 1 in all analyses (Figure 2). Eight major clades within the Pteroini were consistently recovered in the ML, BI, and MP trees. The two specious genera, Pterois and Dendrochirus, were both polyphyletic with Pterois divided into two groups (Pterois I and II) and Dendrochirus divided into three groups (Dendrochirus I, II, and III; Figure 2). Pterois I comprised the type species P. volitans, P. russelii, and P. lunulata. Pterois II included P. antennata, P. radiata, and P. paucispinula. Dendrochirus I was a monospecific clade comprising the type species Dendrochirus zebra. Dendrochirus II consisted of D. bellus and D. brachypterus, while Dendrochirus III was only composed of D. biocellatus.
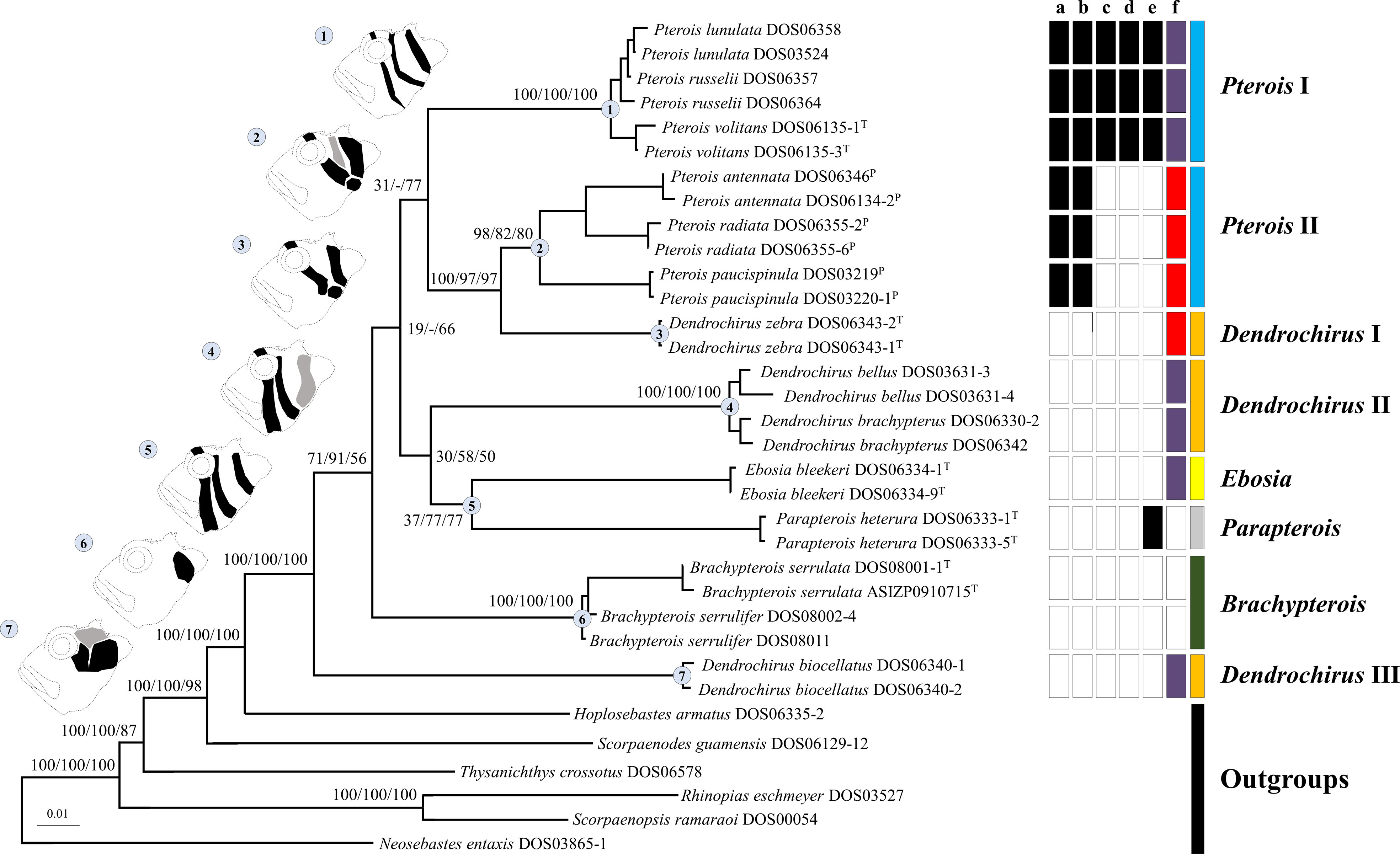
Figure 2 The ML topology of the Pteroini reconstructed based on concatenated sequences of the seven genetic markers. The numbers on nodes of major clades represented ML and MP bootstrap support, and BI posterior probabilities (Bs/Pp) values. Dash (–) denotes the unresolved clade in MP. Catalog numbers marked with T and P represent the type species of genera of the Pteroini and members of the genus Pteropterus, respectively. A representative diagram of the coloration pattern from cheek to postorbital region on the head and the corresponding clade are labeled with the same number. The normal pattern is denoted as black. The variable pattern is denoted as gray, which is absent in some individuals. The right of terminals represents the character state of each species based on morphological data. “a” shows branched rays of the pectoral fin: present (white) and absent (black). “b” shows upper pectoral rays free from membrane: absent (white) and present (black). “c” shows pectoral-fin rays: more than 15 (white) and less than 15 (black). “d” shows scale rows in the longitudinal series: less than 65 (white) and more than 65 (black). “e” shows the distance between the suborbital ridge and the orbit: close (white) and separated (black). “f” shows the number of short barbels on the snout tip: 0 (white), 2 (purple), and 3 (red). The last column represents the currently recognized pterion genera: Brachypterois (green), Dendrochirus (orange), Ebosia (yellow), Parapterois (gray), and Pterois (blue).
In the ML and BI trees, Dendrochirus III was at the basal node of the Pteroini, followed by the clade comprising two species of Brachypterois. The remaining taxa were divided into two clades. The first was composed of Dendrochirus II + Ebosia + Parapterois, in which Dendrochirus II was the sister group of the genera Ebosia and Parapterois. The second contained Dendrochirus I + Pterois I + Pterois II with Pterois I sister to the sub-clade of Dendrochirus I + Pterois II. The topology of the MP tree was nearly identical to the ML and BI trees except for that the interrelationships of Pterois I, Dendrochirus I + Pterois II, Dendrochirus II + Ebosia + Parapterois, and Brachypterois were unresolved.
To maximize taxon sampling, the ML tree of the COI gene was reconstructed based on fragments of 655 bps generated in this study and downloaded from databases (Table S3). The additional species from the online database completed the sampling of Brachypterois, and added one more species to Ebosia and Pterois, respectively. The eight major clades were also retrieved from the COI tree with high bootstrap supports (Figure 3), but their interrelationships were different from the concatenated tree (Figure 2). The Dendrochirus III clade was at the basal node, followed by Parapterois. The remaining taxa were divided into two clades. The first was composed of Dendrochirus II and Pterois I. The second comprised Brachypterois, Dendrochirus I, Ebosia, and Pterois II with Brachypterois at the basal node. In the COI tree, Brachypterois and Ebosia were monophyletic while the monophyly of Parapterois could not be determined since there is only one species included in the analysis.
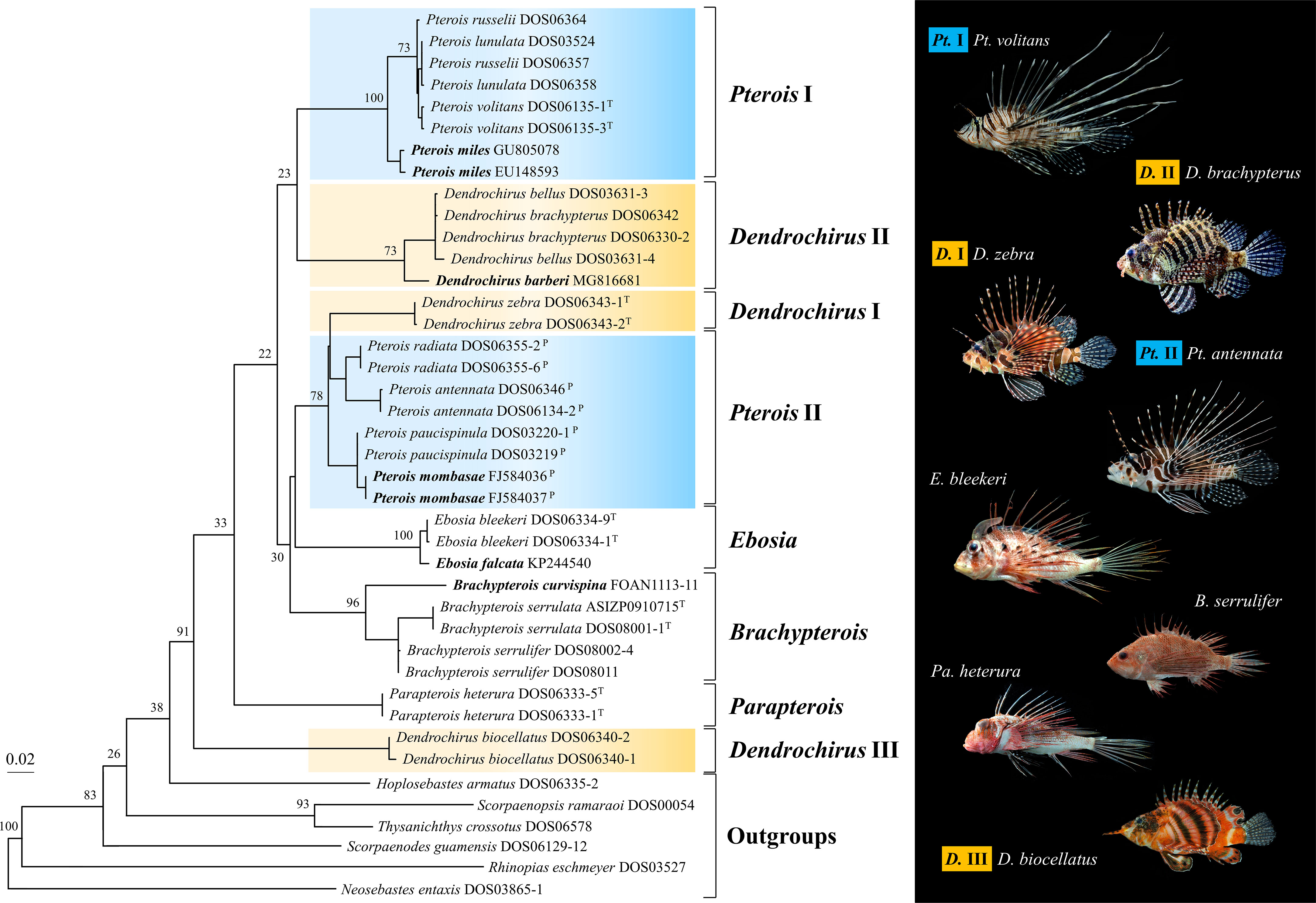
Figure 3 The maximum likelihood tree of the COI gene was reconstructed with sequences of the Pteroini from the present study, BOLD systems, and GenBank database. Bootstrap support values were shown on the major node. The bold species name represents the species of Pteroini lacking in Figure 2. Catalog numbers marked with T and P represent the type species of the genus Pteroini and members of the genus Pteropterus, respectively.
3.2 Morphological analysis
Seven coloration patterns (Figure 2) composed of several conspicuous reddish-brown to dark bands from cheek to postorbital region on head were recognized in seven clades (Ebosia and Parapterois shared the same coloration pattern in one clade). The first pattern was present in the Pt. I clade and was characterized by three narrow bands: the first band extending through the eye, from supraocular spine base to interopercle; the second band from the posteroventral margin of the orbit, reaching obliquely to subopercle; and the third band saddling the nape, reaching the central posterior of the opercle. The second pattern was found in Pt. II clade, characterized by three broad bands and a large black blotch: the first band obliquely crossing the eye, extending to the interopercle; the second band from the posteroventral margin of the orbit, reaching obliquely to the first band, but this band was absent in some individuals; and the third band saddling the nape, reaching the margin of the subopercle. The joining of the first and third bands is mostly connected with a large black blotch on the interopercle (rarely connected without a large black blotch, seen in Pterois cincta and P. radiata) (see Matsunuma and Motomura, 2016b, Figures 2-4, 6). The third pattern was present in the D. I clade and was characterized by two broad bands and a large black blotch: the first band crossing the eye to interopercle, with the lower tip forwardly curved; the second band saddling nape, reaching margin of subopercle, the lower tip backwardly curved. The large black blotch is also present on the interopercle but is not connected with the first and second bands. The fourth pattern was present in D. II clade characterized by three broad bands; the first band extending through eye, from supraocular spine base to the joining between interopercle and mandible; the second band from posterior of orbit and extending to interopercle; the third band saddling nape, reaching margin of the subopercle with the lower tip forwardly curved, but this band is absent in some individuals. The fifth pattern was present in the Ebosia + Parapterois clade and was characterized by three broad bands: the first band extending through the eye, from supraocular spine base to the joining between interopercle and mandible; the second band from posterior of the orbit and extending to the interopercle; the third band saddling the nape, reaching the central posterior of the opercle. The sixth pattern was found in the Brachypterois clade and was characterized by an irregular large black blotch present on the opercle. The seventh pattern was found in the D. III clade and was characterized by three irregular large blotches: the first blotch at the posteroventral margin of the orbit; the second at the interopercle; and the third at the nape. The third one is absent in some individuals, and the first and second blotches together look like a large blotch with a narrow notch on the upper side. In addition to color patterns from cheek to postorbital region on head, six characters were summarized to diagnose all clades except for D. II and D. III clades, including (a) absence of pectoral-fin branched rays (Figures 4A, B), (b) upper pectoral rays free from membrane (Figures 4A–C), (c) number of pectoral-fin rays, (d) number of scales in the longitudinal series, (e) distance between suborbital ridge to orbit (Figure 5), and (f) number of short barbels on the snout tip (Figure 2). The information on the remaining selected morphological characters is shown in Figure 2 and Table S2. In addition, the result of the ancestral state reconstruction for six diagnostic characters in the Pteroini using stochastic mapping is shown in Figure S2 and summarized in Figure 2.

Figure 4 Lateral view of the left pectoral-fin of five clades from Dendrochirus and Pterois. (A) Pterois I, P. volitans DOS06345, 55 mm SL. (B) Pterois II, P. antennata DOS06346, 68 mm SL. (C) Dendrochirus I, D. zebra DOS06343-1, 76 mm SL. (D) Dendrochirus II D. brachypterus DOS06342, 69 mm SL. (E) Dendrochirus III, D. biocellatus DOS06133, 64 mm SL.
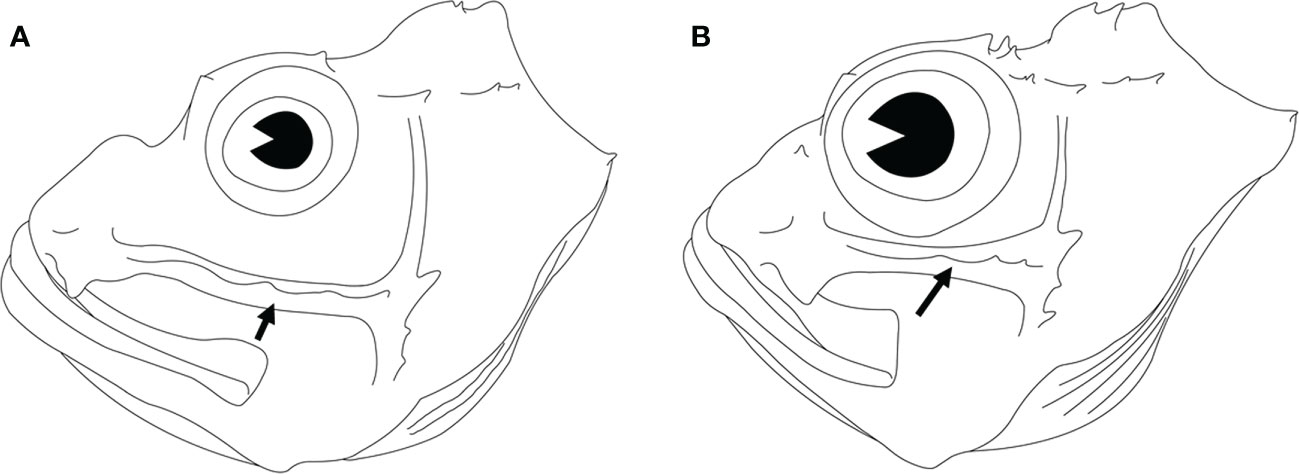
Figure 5 Lateral view of the heads of two lionfishes. The suborbital ridge is indicated by an arrowhead. (A) Pterois volitans DOS06135-1, 97 mm SL. (B) Pterois antennata DOS06346, 68 mm SL.
3.3 Taxonomy
Based on the color patterns on the head, six morphological characters, and phylogenetic analyses, the taxonomy of the two specious genera Dendrochirus and Pterois needed revisions. Pterois is divided into clades I and II. The type species of Pterois is in the Pterois I clade, where the generic name is attached, and the genus Pteropterus Swainson, 1839, is revalidated for the Pterois II clade. Dendrochirus is divided into clades I, II, and III. The type species of Dendrochirus is in the Dendrochirus I clade, where the generic name is attached. We propose a new genus, Neochirus gen. n., for the Dendrochirus II clade. Nemapterois Fowler, 1938, is revalidated for Dendrochirus III. Diagnoses of the new, two revalidated, and two revised genera are described, and keys to eight pteroin genera are provided. To avoid ambiguous readings of taxonomic status in the following sections, the genus name generally follows the taxonomic treatment herein unless otherwise noted.
Neochirus gen. n.
Type species. Dendrochirus brachypterus (Cuvier and Valenciennes, 1829)
Etymology. From the Greek nέας (neo, means new), and χϵίρ (cheír, means finger, hand), a reference to the split from Dendrochirus. Feminine.
Diagnosis. The new genus, Neochirus gen. n., can be distinguished from other pteroin genera by having head color pattern IV (Figure 2), a pair of short barbels on the tip of the snout, soft-rayed dorsal fin without ocellated spots, pectoral-fin rays 17–19, upper pectoral-fin rays not free from membrane (Figure 4D), anal-fin III, longitudinal scale rows less than 65, maxilla not covered by scales, mandible without spinous ridges, coronal and parietal spines separated, parietal spine not elongated throughout life.
Included species. Dendrochirus barberi (Steindachner, 1900), D. bellus (Jordan and Hubbs, 1925), D. brachypterus, D. hemprichi Matsunuma, Motomura, and Bogorodsky, 2017, and D. tuamotuensis Matsunuma and Motomura, 2013.
Dendrochirus Swainson, 1839
Type species. Dendrochirus zebra (Cuvier and Valenciennes, 1829)
Diagnosis. Distinguished from other pteroin genera by head color pattern III (Figure 2), three short barbels on the tip of the snout, a soft-rayed portion of the dorsal fin without ocellated spots, upper pectoral-fin rays free from membrane pectoral-fin rays more than 15, anal-fin III, longitudinal scale rows less than 65, maxilla not covered by scales, mandible without spinous ridges, coronal and parietal spines separated, and parietal spine not elongated throughout life.
Included species. Dendrochirus koyo Matsunuma and Motomura, 2019, D. zebra.
Nemapterois Fowler, 1938
Type species. Nemapterois biocellatus Fowler, 1938
Diagnosis. Distinguished from all other Pteroini by head color pattern VII (Figure 2), a pair of short barbels on the tip of the snout, a pair of preorbital barbel extraordinary long, soft-rays of dorsal fin with two large black ocelli, anal-fin III, maxilla not covered by scales, mandible without spinous ridges, coronal and parietal spines separated, parietal spine not elongated throughout life.
Included species. Dendrochirus biocellatus.
Pterois Oken, 1817
Type species. Pterois volitans Linnaeus, 1758
Diagnosis. distinguished from other pteroin genera by head color pattern I (Figure 2), a pair of short barbels on the tip of the snout, soft-rays of the dorsal fin without ocellated spots, pectoral-fin rays less than 15 and upper pectoral-fin rays free from membrane (Figure 4A) through life, anal-fin III; longitudinal scale rows more than 65, maxilla not covered by scales, mandible without spinous ridges, coronal and parietal spines separated, parietal spine not elongated throughout life.
Included species. Pterois andover Allen and Erdmann, 2008, P. longicauda Swainson, 1839, P. lunulata Temminck and Schlegel, 1843, P. miles (Bennett, 1828), P. russelii Bennett, 1831 and P. volitans.
Pteropterus Swainson, 1839
Type species. Pterois radiata Cuvier and Valenciennes, 1829
Diagnosis. Distinguished from other pteroin genera by head color pattern II (Figure 2), three short barbel on the tip of the snout, soft-rays of dorsal fin without ocellated spots, pectoral-fin rays more than 15 and upper pectoral-fin rays free from membrane (Figure 4B) through life, anal-fin III; longitudinal scale rows less than 65, maxilla not covered by scales, mandible without spinous ridges, coronal and parietal spines separated, parietal spine not elongated throughout life.
Included species. Pterois antennata (Bloch, 1787), P. brevipectoralis (Mandrytsa, 2002), P. cincta Rüppell, 1838, P. mombasae (Smith, 1957), P. paucispinula Matsunuma and Motomura, 2015, P. radiata, P. sphex Jordan and Evermann, 1903.
Keys to eight pteroin genera.
1a. No short barbels on snout tip....................................................2
1b. 2 or 3 short barbels on snout tip..............................................3
2a. Anal-fin III; maxilla covered by scales; mandible with spinous ridge; Head color pattern VI.................................... Brachypterois
2b. Anal-fin II; maxilla not covered by scales; mandible without spinous ridges; head color pattern V......................Parapterois
3a. Soft-rayed portion of terminal dorsal fin without two large black ocelli.......................................................................................4
3b. Soft-rayed portion of terminal dorsal fin with two large black ocelli; a pair of long preorbital barbels; head color pattern VII..................................................................Nemapterois
4a. Pectoral fin ray more than 15; longitudinal scales less than 65.............................................................................................5
4b. Pectoral fin ray less than 15; longitudinal scales more than 65; head color pattern I......................................................Pterois
5a. Coronal and parietal spines separated; parietal spine not elongated throughout life.............................................................6
5b. Coronal and parietal spines continuous; parietal spine elevated as a thin bony crest in adult males; head color pattern V................................................................................Ebosia
6a. Upper pectoral-fin rays not free from membrane.................7
6b. Upper pectoral-fin rays free from membrane throughout life, head color pattern II...........................................Pteropterus
7a. 3 short barbels on snout tip; head color pattern III............................................................................... Dendrochirus
7b. 2 short barbels on snout tip; Head color pattern IV.........................................................................Neochirus gen. n.
4 Discussion
In the present study, phylogenetic analyses of the Pteroini were conducted with the best sampling to date. Our results show the Pteroini are monophyletic with high branch supports, corroborating previous phylogenetic studies based on morphological or molecular data (Ishida, 1994; Imamura, 2004; Smith and Wheeler, 2004; Shinohara and Imamura, 2005; Smith and Craig, 2007; Lautredou et al., 2013; Betancur-R et al., 2017; Smith et al., 2018). In all phylogenetic analyses (Figures 2, 3), eight major clades within the Pteroini are consistently recovered, including the non-monophyletic Dendrochirus I–III and Pterois I–II (Kochzius et al., 2003; Freshwater et al., 2009) and three genera, Brachypterois, Ebosia, and Parapterois.
The genera Brachypterois, Ebosia, and Parapterois were not included in previous molecular studies (Kochzius et al., 2003; Freshwater et al., 2009). In the present study, we show that these genera are valid based on molecular and morphological data. Nemapterois was shown to be the most basal clade in the Pteroini, consistent with Freshwater et al. (2009). In some studies, the genus Brachypterois was considered the most basal within the Pteroini (Fowler, 1938; Kuiter and Tonozuka, 2001; Matsunuma et al., 2013). Fowler (1938) and Matsunuma et al. (2013) indicated that Brachypterois is characterized by a short dorsal-fin spine, unlike all other Pteroini with an elongated dorsal-fin spine. Kuiter and Tonozuka (2001) proposed that Brachypterois has a similar coloration pattern on its head to some members of the closely related outgroup Scorpaenodes. However, our phylogenetic results showed the Pteroini are sisters to the genus Hoplosebastes. Moreover, their comments were based on a few taxa without phylogenetic analysis.
In our analyses, the clade D. II + Ebosia + Parapterois is sister to Pt. I + Pt. II + D. I, which conflicts with the conclusions of Kochzius et al. (2003) and Freshwater et al. (2009). Ishida (1994)’s morphological phylogeny showed Brachypterois, Dendrochirus, Ebosia, and Parapterois formed a monophyletic group, but their interrelationships were unresolved. However, their samples of phylogenetic analyses based on morphological data within the Pteroini were restricted to genus-level, and intergeneric and intrageneric relationships were poorly determined due to the two non-monophyletic genera, Dendrochirus and Pterois (sensu Nelson et al., 2016), represented by only one species. Better sampling and more genetic markers do provide new insight into the phylogeny of the Pteroini.
Pterois volitans and Pt. miles were shown to be sister species in previous studies (Kochzius et al., 2003; Freshwater et al., 2009). However, our COI phylogeny shows Pt. lunulata, Pt. russelii, and Pt. volitans form a monophyletic group sister to Pt. miles (Figure 3). New insight into phylogeny is provided with better taxon sampling. However, phylogeny at the intra-genetic level was poorly studied in most pteroin genera except for a few studies such as Wilcox et al. (2017), and more studies are needed to provide interrelationships among species of the remaining genera.
Molecular analyses (Kochzius et al., 2003; Freshwater et al., 2009), including the present study, consistently show Dendrochirus and Pterois (sensu Nelson et al., 2016) are not monophyletic. Dendrochirus (sensu Nelson et al., 2016) and the questionable genus Pteropterus were established by Swainson (1839) and distinguished from Pterois based on the upper pectoral ray not being free from membrane (vs. being free from membrane), and Dendrochirus (sensu Nelson et al., 2016) was further distinguished from Pterois by branched pectoral fin rays. The type designations of these two genera were not made by Swainson (1839), but Pterois radiata (=Pteropterus radiata) was the type species of Pteropterus by monotypy. Pterois radiata was described by Cuvier in Cuvier and Valenciennes (1829) based on a drawing of a fish with extremely short dorsal fin rays and pectoral fin rays clearly free from membrane. Swainson’s (1839) diagnosis of pectoral rays not free of membrane was probably a typo. Cuvier in Cuvier and Valenciennes (1829) conjectured the short rays being broken off, but Swainson (1839) considered the extremely short dorsal fin rays to be natural since the drawing was made by a zoological painter who would have been aware of the circumstance. Despite the debate, the designation of the neotype (Matsunuma and Motomura, 2016b) with a regular length of dorsal fin rays has solved the concern.
Subsequent authors distinguished Dendrochirus (sensu Nelson et al., 2016) from Pterois and Pteropterus, viz., Pt. II, by the presence of branched pectoral rays and upper pectoral rays not free from membrane (vs. all rays simple and upper rays free from membrane) (Smith, 1957; Eschmeyer and Randall, 1975; Masuda et al., 1986; Poss, 1999; Matsunuma et al., 2017). The character analyses show that pectoral fin rays that are unbranched and upper rays free from membrane are synapomorphies for the Pterois + (Dendrochirus + Pteropterus) clade rather than diagnostic characters to distinguish Dendrochirus from Pterois and Pteropterus (Figure 2). Character states of the pectoral ray in D. zebra may be secondary losses or two independent gains in Pterois and Pteropterus. Although D. zebra is characterized by pectoral rays that are not free from membrane, we noticed that this character may vary to a certain degree. Nearly half of the first five rays are free of membrane in our juvenile specimens (Figure 4C). Intraspecific variation is present, but we could not address more since we do not have access to specimens throughout their developmental stages. We keep this trait as a diagnostic character in the present study; however, further studies are needed to verify how it changes with development.
In addition, Smith (1957) proposed several diagnostic characters for Pteropterus, including longitudinal scales less than 65, pectoral fin rays more than 15, a suborbital ridge close to the orbit, and a dorsolateral body covered by ctenoid scales. However, Matsunuma and Motomura (2015) show that the dorsolateral bodies of some species are covered by ctenoid and cycloid scales, and this variation is supported by the present study. Therefore, the distribution of ctenoid scales on the dorsolateral body cannot be a diagnostic characteristic of Pteropterus. The remaining diagnostic characters are mapped to our phylogenetic tree, which showed that these meristic characters cannot distinguish Pteropterus from other genera within the Pteroini. Instead, pectoral-fin rays less than 15 and longitudinal scale rows more than 65 are diagnostic for Pterois within the Pteroini.
The D. II clade is represented by the D. brachypterus species group defined by Matsunuma et al. (2017). They indicated that the taxonomic status of the D. brachypterus species group still warrants investigation. According to the phylogenetic position in the Pteroini and several distinct diagnostic characters (see Results), we proposed the D. II clade as a new genus, Neochirus gen. n. The D. III clade was solely represented by D. biocellatus. The species D. biocellatus was originally described by Fowler (1938) as Nemapterois biocellatus based on a pair of extraordinary long preorbital barbels. Subsequently, Nemapterois was regarded as a junior synonym of Dendrochirus (sensu Nelson et al., 2016) (Eschmeyer and Randall, 1975; Mandrytsa, 2001). However, based on the phylogenetic position in the Pteroini and several distinct diagnostic characters, Nemapterois Fowler, 1938, is revalidated for the D. III clade.
The monotypic genus Pteroleptus Swainson, 1839 (=Pterois) was established based on the poorly known species, P. longicauda Swainson, 1839, described on the basis of Russell (1803)’s figure. No specimen of P. longicauda has been reported since Swainson’s (1839) original description, and the phylogenetic position of P. longicauda remains unclear within the Pteroini. However, Pteroleptus has been considered a synonym of Pterois by subsequent studies (Swain, 1882; Smith, 1957; Mandrytsa, 2001). Based on Russell’s (1803) plate of P. longicauda, it was like the members of the Pterois I clade by having several distinct characters, viz., the bands on the cheek were narrow, the pectoral-fin rays were less than 15, the scale rows in the longitudinal series more than 65, and the suborbital ridge was separated from the orbit. The only disagreement is that the pectoral rays of P. longicauda are not free from the membrane in Russell’s (1803) figure. Based on these characters, we consider P. longicauda a species of the Pterois I clade. The taxonomic status of P. longicauda needs further study.
Nomenclature
urn:lsid:zoobank.org:act:16414C1F-E381-4810-813C-AA4464558F98
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical review and approval was not required for the animal study because In the current study, all species were not protected species in Taiwan or listed in the CITES. No ethics declaration was required for this study because no experiment was conducted on live individuals. The samples were obtained from fish market and museum collection. Moreover, the fresh individuals from fish market were already dead.
Author contributions
T-KC and T-YL conceived the idea. T-KC, M-YL, and T-YL produced and analyzed the data. T-KC prepared the manuscript. The manuscript was revised by M-YL and T-YL. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the grant (110-2119-M-110-002) from the Ministry of Science and Technology, Taiwan.
Acknowledgments
We are grateful to S.-P. Huang (ASIZP) for his curatorial assistance, J.-F. Huang for collecting specimens, and J.-S. Lin for his assistance in the English editing of the draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1109655/full#supplementary-material
Supplementary Table 1 | Summary of voucher specimen catalog number, standard length (SL, mm) and sampling information for the selected taxa in the present study. N/A, not available.
Supplementary Table 2 | The selected characters for diagnosing genera of the tribe Pteroini based on the references. N/A, not available. 1 for Matsunuma et al. (2013); 2 for Motomura (2004a); 3 for Matsunuma and Motomura (2014); 4 for Smith (1957); 5 for Matsunuma and Motomura (2015); 6 for Matsunuma et al. (2017); 7 for Matsunuma and Motomura (2019). Refer to the reference list in the main text for their detailed information.
Supplementary Table 3 | Accession numbers of sequences analyzed in the present study. Members of the genus Pteropterus were marked with an asterisk (*). Accession numbers marked with a and b were downloaded from the BOLD system and GenBank, respectively. The rest of the sequences were generated in the present study.
References
Allen G. R., Erdmann M. V. (2008). Pterois andover, a new species of scorpionfish (Pisces: Scorpaenidae) from Indonesia and Papua new Guinea. Aqua Int. J. Ichthyol. 13 (3–4), 127–138.
Bariche M., Torres M., Azzurro E. (2013). The presence of the invasive lionfish Pterois miles in the Mediterranean Sea. Mediterr. Mar. Sci. 14, 292–294. doi: 10.12681/mms.428
Bennett E. T. (1831). Exhibition of the several species of Pterois contained in the Mauritius collection. Proc. Committee Sci. Correspondence Zoological Soc. London. 1830–31 (pt 1), 128.
Betancur-R R., Wiley E. O., Arratia G., Acero A., Bailly N., Miya M., et al. (2017). Phylogenetic classification of bony fishes. BMC Evol. Biol. 17, 162. doi: 10.1186/s12862-017-0958-3
Bleeker P. (1863). Dixième notice sur la faune ichthyologique de l'île de ternate. verslagen en mededeelingen der koninklijke akademie van wetenschappen. Afdeeling Natuurkunde. 15, 265–266.
Bleeker P. (1876). Genera familiae scorpaenoideorum conspectus analyticus. verslagen en mededeelingen der koninklijke akademie van wetenschappen. Afdeeling Natuurkunde. (Ser. 2) 9, 294–300.
Bloch M. E. (1787). Naturgeschichte der ausländischen fische. Berlin. 3, i–xii + 1–146, Pls. 181–216.
Chen W. J., Bonillo C., Lecointre G. (2003). Repeatability of clades as a criterion of reliability: a case study for molecular phylogeny of acanthomorpha (Teleostei) with larger number of taxa. Mol. Phylogenet. Evol. 26, 262–288. doi: 10.1016/S1055-7903(02)00371-8
Chen W. J., Miya M., Saitoh K., Mayden R. L. (2008). Phylogenetic utility of two existing and four novel nuclear gene loci in reconstructing tree of life of ray-finned fishes: the order cypriniformes (Ostariophysi) as a case study. Gene. 423, 125–134. doi: 10.1016/j.gene.2008.07.016
Costa W. J. E. M., Henschel E., Katz A. M. (2020). Multigene phylogeny reveals convergent evolution in small interstitial catfishes from the Amazon and Atlantic forests (Siluriformes: Trichomycteridae). Zool. Scr. 49, 159–173. doi: 10.1111/zsc.12403
Cuvier G. (1817). “Le règne animal distribué d'après son organisation pour servir de base à l'histoire naturelle des animaux et d'introduction à l'anatomie comparée,” in Les Reptiles, les poissons, les mollusques et les annélides, 1, vol. 2. , i–xviii + 1–532.
Cuvier G., Valenciennes A. (1829). “Histoire naturelle des poissons,” in Tome Livre quatrième. Des acanthoptérygiens à joue cuirassée, vol. 4 . Ed. Levrault F. G. (Paris: Des acanthoptérygiens à joue cuirassée), i–xxvi + 2 pp. + 1–518, Pls. 72–99.
Desmarest E. (1856). “Reptiles et poissons,” in Encyclopédie d'histoire naturelle; ou, traité complet de cette science d'après les travaux des naturalistes les plus éminents de toutes les époques, etc.... par le dr. chenu, 1st edition, vol. 19. Ed. Chenu J. G. (E. Girard, Paris), 1–360. (Paris: Maresq)
Eschmeyer W. N. (1986). “Family no. 149: scorpaenidae. family no. 153: caracanthidae,” in Smiths’ sea fishes. Eds. Smith M. M., Heemstra P. C. (Berlin: Springer).
Eschmeyer W. N., Fricke R., van der Laan R. Catalog of fishes: genera, species, references. Available at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (Accessed 24/06/2020).
Eschmeyer W. N., Randall J. E. (1975). The scorpaenid fishes of the Hawaiian islands, including new species and new records (Pisces: Scorpaenidae). Proc. Calif. Acad. Sci. 40 (11), 265–334.
Fowler H. W. (1938). Descriptions of new fishes obtained by the united states bureau of fisheries steamer "Albatross", chiefly in Philippine seas and adjacent waters. U.S. Natl. Mus. 85 (3032), 31–135.
Freshwater D., Hamner R., Parham S., Wilbur A. (2009). Molecular evidence that the lionfishes Pterois miles and Pterois volitans are distinct species. J. N. C. Acad. Sci. 125, 39–46.
Gill T. N. (1903). On some fish genera of the first edition of cuvier's règne animal and oken's names. U.S. Natl. Mus. 26 (1346), 965–967.
Hall T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl. Acid S. 41, 95–98. doi: 10.14601/Phytopathol_Mediterr-14998u1.29
Herre A. W. (1952). A review of the scorpaenoid fishes of the Philippines and adjacent seas. Philipp J. Sci. 80 (4), 381–482.
Imamura H. (2004). Phylogenetic relationships and new classification of the superfamily scorpaenoidea (Actinopterygii: Perciformes). Species Divers. 9, 1–36. doi: 10.12782/specdiv.9.1
Ishida M. (1994). Phylogeny of the suborder scorpaenoidei (Pisces: Scorpaeniformes). Bull. Nansei Natl. Fish. Res. 27, 1–112.
Johnston M. W., Purkis S. J. (2011). Spatial analysis of the invasion of lionfish in the western Atlantic and Caribbean. Mar. pollut. Bull. 62, 1218–1226. doi: 10.1016/j.marpolbul.2011.03.028
Jordan D. S., Seale A. (1906). The fishes of samoa. description of the species found in the archipelago, with a provisional check-list of the fishes of Oceania. Fish. bull (Wash. D. C.). 25, 33–53. 173–455 + 457–488.
Jordan D. S., Hubbs C. L. (1925). Record of fishes obtained by David Starr Jordan in Japan. Memoirs Carnegie Museum. 10 (2), 93–346.
Jordan D. S., Starks E. C. (1904). A review of the scorpaenoid fishes of Japan. U.S. Natl. Mus. 27 (1351), 91–175.
Kletou D., Hall-Spencer J. M., Kleitou P. (2016). A lionfish (Pterois miles) invasion has begun in the Mediterranean Sea. Mar. Biodivers. Rec. 9, 46. doi: 10.1186/s41200-016-0065-y
Kochzius M., Soller R., Khalaf M. A., Blohm D. (2003). Molecular phylogeny of the lionfish genera Dendrochirus and Pterois (Scorpaenidae, Pteroinae) based on mitochondrial DNA sequences. Mol. Phylogenet. Evol. 28 (3), 396–403. doi: 10.1016/s1055-7903(02)00444-x
Kozlov A. M., Darriba D., Flouri T., Morel B., Stamatakis A. (2019). RAxML-NG: a fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinform. 35 (21), 4453–4455. doi: 10.1093/bioinformatics/btz305
Kuiter R. H., Tonozuka T. (2001). Pictorial guide to Indonesian reef fishes. part i. eels-snappers, muraenidae-lutjanidae (Australia: Zoonetics).
Lanfear R., Frandsen P. B., Wright A. M., Senfeld T., Calcott B. (2017). PartitionFinder 2: new methods for selecting partitioned models of evolution formolecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34 (3), 772–773. doi: 10.1093/molbev/msw260
Lautredou A. C., Motomura H., Gallut C., Ozouf-Costaz C., Cruaud C., Lecointre G., et al. (2013). New nuclear markers and exploration of the relationships among serraniformes (Acanthomorpha, Teleostei): the importance of working at multiple scales. Mol. Phylogenet. Evol. 67 (1), 140–155. doi: 10.1016/j.ympev.2012.12.020
Li C., Ortí G., Zhang G., Lu G. (2007). A practical approach to phylogenomics: the phylogeny of ray-finned fish (Actinopterygii) as a case study. BMC Evol. Biol. 7, 44. doi: 10.1186/1471-2148-7-44
Maddison W. P., Maddison D. R. (2011) Mesquite: a modular system for evolutionary analysis. Available at: http://mesquiteproject.org.
Mandrytsa S. A. (2001). Lateral line system and classification of scorpaenoid fishes (Scorpaeniformes: scorpaenoidei) (Perm: Perm University), 1–393.
Mandrytsa S. A. (2002). A new species of the genus Pteropterus (Scorpaenidae: Scorpaeniformes) from the Indian ocean. J. Ichthyol. 42, 129–130.
Masuda H., Amaoka K., Araga C., Uyeno T., Yoshino T. (1986). The fishes of the Japanese archipelago. vol. 1 (Tokyo, Japan: Tokai University Press).
Matsubara K. (1943). Studies on the scorpaenoid fishes of Japan (II). Trans. Sigenkagaku Kenkyusyo 2, 171–486.
Matsunuma M., Motomura H. (2013). Newly recognized diagnostic characters of the poorly known lionfish Pterois brevipectoralis (Scorpaenidae: Pteroinae), with notes on fresh coloration. Species Divers. 18, 163–173. doi: 10.12782/sd.18.2.163
Matsunuma M., Motomura H. (2014). A new species of scorpionfish, Ebosia saya (Scorpaenidae: Pteroinae), from the western Indian ocean and notes on fresh coloration of Ebosia falcata. Ichthyol. Res. 62, 293–312. doi: 10.1007/s10228-014-0445-4
Matsunuma M., Motomura H. (2015). Pterois paucispinula, a new species of lionfish (Scorpaenidae: Pteroinae) from the western pacific ocean. Ichthyol. Res. 62 (3), 327–346. doi: 10.1007/s10228-014-0451-6
Matsunuma M., Motomura H. (2016a). A new species of scorpionfish, Ebosia vespertina (Scorpaenidae: Pteroinae), from the southwestern Indian ocean. Ichthyol. Res. 63 (1), 110–120. doi: 10.1007/s10228-015-0479-2
Matsunuma M., Motomura H. (2016b). Redescriptions of Pterois radiata and Pterois cincta (Scorpaenidae: Pteroinae) with notes on geographic morphological variations in P. radiata. Ichthyol. Res. 63 (1), 145–172. doi: 10.1007/s10228-015-0483-6
Matsunuma M., Motomura H. (2019). Redescription of Dendrochirus zebra (Scorpaenidae: Pteroinae) with a new species of Dendrochirus from the ogasawara islands, Japan. Ichthyol. Res. 66, 353–384. doi: 10.1007/s10228-019-00681-1
Matsunuma M., Motomura H. (2022). Revision of the genus Parapterois (Scorpaenidae: Pteroinae) and resurrection of Parapterois nigripinnis (Gilchrist 1904). Ichthyol. Res. 69, 401–432. doi: 10.1007/s10228-021-00845-y
Matsunuma M., Motomura H., Bogorodsky S. V. (2017). Review of indo-pacific dwarf lionfishes (Scorpaenidae: Pteroinae) in the Dendrochirus brachypterus complex, with description of a new species from the western Indian ocean. Ichthyol. Res. 64 (4), 369–414. doi: 10.1007/s10228-017-0583-6
Matsunuma M., Sakurai M., Motomura H. (2013). Revision of the indo-West pacific genus Brachypterois (Scorpaenidae: Pteroinae), with description of a new species from northeastern Australia. Zootaxa. 3693 (4), 401–440. doi: 10.11646/zootaxa.3693.4.1
Motomura H. (2004a). Morphological comparison of a poorly known scorpionfish, Parapterois macrura, with a related species, P. heterura (Scorpaenidae: Pteroinae). Zool. Stud. 43 (1), 1–7.
Motomura H. (2004b). New species of scorpionfish, Scorpaena cocosensis (Scorpaeniformes: Scorpaenidae) from the Cocos islands, Costa Rica, Eastern pacific ocean. Copeia. 4, 818–824. doi: 10.1643/CI-04-179R
Motomura H., Last P. R., Yearsley G. K. (2005). Scorpaena bulacephala, a new species of scorpionfish (Scorpaeniformes: Scorpaenidae) from the northern Tasman Sea. Zootaxa. 1043, 17–32. doi: 10.11646/zootaxa.1043.1.2
Nelson J. S., Grande T. C., Wilson M. V. H. (2016). Fishes of the world. 5th edn (Hoboken, New Jersey: John Wiley and Sons, Inc.).
Perdices A., Carmona J. A., Fernandez-Delgado C., Doadrio I. (2001). Nuclear and mitochondrial data reveal high genetic divergence among Atlantic and Mediterranean populations of the Iberian killifish Aphanius iberus (Teleostei: Cyprinodotidae). Hered. 87, 314–324. doi: 10.1046/j.1365-2540.2001.00888.x
Poss S. G. (1999). “Scorpaenidae. scorpionfishes (also, lionfishes, rockfishes, stingfishes, stonefishes, and waspfishes),” in FAO species identification guide for fishery purposes. the living marine resources of the Western central pacific. vol. 4. bony fishes part 2 (Mugilidae to carangidae). Eds. Carpenter K. E., Niem V. H. (Rome: FAO), 2291–2352.
Rambaut A. (2007). “FigTree,” in Program and documentation. Available at: http://tree.bio.ed.ac.uk/software/figtree/.
Randall J. E. (2005a). Reef and shore fishes of the south pacific (Hawaii: University of Hawaii press).
Randall J. E., Eschmeyer W. N. (2002). Revision of the indo-pacific scorpionfish genus Scorpaenopsis, with descriptions of eight new species. Indo-Pac. Fishes (34), 1–12.
Ronquist F., Teslenko M., Mark P., Ayres D. L., Darling A., Höhna S., et al. (2012). MRBAYES 3.2: efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 61 (3), 539–542. doi: 10.1093/sysbio/sys029
Rüppell W. P. E. S. (1838). “Neue wirbelthiere zu der fauna von abyssinien gehörig,” in Fische des rothen meeres, (Siegmund Schmerber: Frankfurt am Main) vol. i–ii + 1–148, 1–33.
Schmidt T. R., Gold J. R. (1993). Complete sequence of the mitochondrial cytochrome b gene in the cherryfin shiner, Lythrurus roseipinnis (Teleostei: Cyprinidae). Copeia. 1993, 880–883.
Schofield P. (2009). Geographic extent and chronology of the invasion of non-native lionfish (Pterois volitans [Linnaeus 1758] and P. miles [Bennett 1828]) in the Western north Atlantic and Caribbean Sea. Aquat. Invasions. 4, 473–479.
Schofield P. (2010). Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus 1758] and P. miles [Bennett 1828]) in the Western north Atlantic ocean, Caribbean Sea and gulf of Mexico. Aquat. Invasions. 5, S117–S122.
Schonhuth S., Lozano-Vilano L., Perdices A., Espinosa H., Mayden R. L. (2015). Phylogeny, genetic diversity and phylogeography of the genus Codoma (Teleostei: Cyprinidae). Zool. Scr. 44, 11–28. doi: 10.1111/zsc.12083
Shinohara G., Imamura H. (2005). Anatomical description and phylogenetic classification of the orbicular velvetfishes (Scorpaenoidea: Caracanthus). Ichthyol. Res. 52, 64–76. doi: 10.1007/s10228-004-0256-0
Smith J. L. B. (1957). The fishes of the family scorpaenidae in the western Indian ocean. part 2. the subfamilies pteroinae, apistinae, setarchinae and sebastinae. ichthyol. Ichthyol. Bull. J.L.B. Smith Inst. Ichthyol. 5, 75–87.
Smith W. L., Craig M. T. (2007). Casting the percomorph net widely: the importance of broad taxonomic sampling in the search for the placement of serranid and percid fishes. Copeia. 2007 (1), 35–55. doi: 10.1643/0045-8511(2007)7[35:CTPNWT]2.0.CO;2
Smith W. L., Evermann E., Richardson C. (2018). Phylogeny and taxonomy of flatheads, scorpionfishes, Sea robins, and stonefishes (Percomorpha: Scorpaeniformes) and the evolution of the lachrymal saber. Copeia. 106 (1), 94–119. doi: 10.1643/CG-17-669
Smith W. L., Wheeler W. C. (2004). Polyphylys of the mail-cheeked fishes (Teleostei: Scorpaeniformes): evidence from mitochondrial and nuclear sequence data. Mol. Phylogenet. Evol. 32, 627–646. doi: 10.1016/j.ympev.2004.02.006
Steindachner F. (1900). “Fische aus dem stillen ocean,” in Ergebnisse einer reise nach dem pacific (Schauinsland, 1896–1897). Anzeiger der Kaiserlichen Akademie der Wissenschaften, Wien, Mathematisch–Naturwissenschaftliche Classe. 37 (16), 174–178.
Sudasinghe H., Pethiyagoda R., Raghavan R., Dahanukar N., Rüber L., Meegaskumbura M. (2020). Diversity, phylogeny and biogeography of Systomus (Teleostei: Cyprinidae) in Sri Lanka. Zool. Scr. 49, 710–731. doi: 10.1111/zsc.12445
Swainson W. (1839). On the natural history and classification of fishes, amphibians, and reptiles, or monocardian animals (Longmans, London: Longman, Orne, Brown, Gree).
Swofford D. L. (2002). PAUP*, phylogenetic analysis using parsimony (and other methods) 4.0 beta 10 for 32-bit Microsoft windows (Sunderland, Massachusetts: Sinauer Associates).
Tavera J., Acero A., Wainwright P. C. (2018). Multilocus phylogeny, divergence times, and a major role for the benthic-to-pelagic axis in the diversification of grunts (Haemulidae). Mol. Phylogenet. Evol. 121, 212–223. doi: 10.1016/j.ympev.2017.12.032
Temminck C. J., Schlegel H. (1843). “Pisces,” in Ed. Siebold P.F. Fauna japonica, sive descriptio animalium. (quae in itinere per Japoniam suscepto annis 1823–1830 collegit, notis, observationibus et adumbrationibus illustravit Ph. Fr. de Siebold: Lugduni Batavorum) vol. 2–4, 21–72.
Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 (22), 4673–4680.
Ward R. D., Zemlak T. S., Innes B. H., Last P. R., Hebert D. N. (2005). DNA Barcoding australia’s fish species. Philos. T. R. Soc B. 360, 1847–1857. doi: 10.1098/rstb.2005.1716
Wilcox C. L., Motomura H., Matsunuma M., Bowen B. W. (2017). Phylogeography of lionfishes (Pterois) indicate taxonomic over splitting and hybrid origin of the invasive Pterois volitans. J. Hered. 2018, 162–175. doi: 10.1093/jhered/esx056
Keywords: lionfishes, morphology, phylogeny, Pteroini, taxonomy
Citation: Chou T-K, Liu M-Y and Liao T-Y (2023) Systematics of lionfishes (Scorpaenidae: Pteroini) using molecular and morphological data. Front. Mar. Sci. 10:1109655. doi: 10.3389/fmars.2023.1109655
Received: 28 November 2022; Accepted: 17 April 2023;
Published: 15 May 2023.
Edited by:
Anna Rita Rossi, Sapienza University of Rome, ItalyReviewed by:
Jose Julian Tavera, University of Valle, ColombiaGa Hun Boo, Sungkyunkwan University, Republic of Korea
Copyright © 2023 Chou, Liu and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Te-Yu Liao, swp0117@gmail.com
 Tak-Kei Chou1
Tak-Kei Chou1  Te-Yu Liao
Te-Yu Liao