Ramicrusta invasive alga causes mortality in Caribbean coral larvae
- 1Woods Hole Oceanographic Institution, Woods Hole, MA, United States
- 2Ursinus College, Collegeville, PA, United States
- 3Massachusetts Institute of Technology, Cambridge, MA, United States
- 4Marine Biological Laboratory, Woods Hole, MA, United States
The settlement of coral larvae is an important process which contributes to the success and longevity of coral reefs. Coral larvae often recruit to benthic structures covered with crustose coralline algae (CCA) which produce cues that promote settlement and metamorphosis. The Peysonneliaceae Ramicrusta spp. are red-brown encrusting alga that have recently become abundant on shallow Caribbean reefs, replacing CCA habitat, overgrowing corals and potentially threatening coral recruitment. In order to assess the threat of Ramicrusta to coral recruitment, we compared the survival and settlement of Porites astreoides and Favia fragum larvae to 0.5 – 2 mg ml-1 solutions of Ramicrusta sp. or CCA as well as sterile seawater (control). In all cases larval mortality was extremely high in the Ramicrusta treatments compared to the CCA and control treatments. We found 96% (± 8.9% standard deviation, SD) mortality of P. astreoides larvae when exposed to solutions of Ramicrusta and 0 - 4% (± 0 - 8.9% SD) mortality in the CCA treatments. We observed 100% F. fragum larval mortality when exposed to Ramicrusta and 5 – 10% (± 10 – 20% SD) mortality in the CCA treatments. Settlement or surface interaction of larvae in the CCA treatments was 40 - 68% (± 22 - 37% SD) for P. astreoides and 65 - 75% (± 10 - 19% SD) for F. fragum. Two P. astreoides larva that survived Ramicrusta exposure did settle/surface interact, suggesting that some larvae may be tolerant to Ramicrusta. These results suggest that Ramicrusta is a lethal threat to Caribbean coral recruitment.
Introduction
Coral reefs are biodiverse ecosystems that provide coastal protection, food and other economic benefits to coastal and island communities. Scleractinia corals form the structural framework of this habitat. Key requirements for the success and longevity of reefs is for larval corals to settle onto reefs, grow into larger colonies and regularly reproduce. Unfortunately, numbers of juvenile corals have recently decreased on many reefs (Edmunds et al., 2015; Hughes et al., 2019), suggesting that settlement and recruitment processes may be declining. Due to the small size of coral recruits and many possible stressors, specific factors attributing to this decline are challenging to document on reefs.
Ideal settlement substrates are one component contributing to successful coral recruitment. Some coral larvae preferentially settle on reef bedrock covered with crustose coralline algae (CCA; Rhodophyta family Corallinaceae). This relationship is related to chemical signals released by the CCA and/or associated microorganisms (Heyward and Negri, 1999; Negri et al., 2001; Sneed et al., 2014; Tebben et al., 2015). The presence of CCA is generally associated with high reef health or quality (Ritson-Williams et al., 2009). However, CCA has been replaced in some shallow reefs by encrusting red-brown colored algae of the Peyssonneliacean family, including Ramicrusta spp. (Pueschel and Saunders, 2009; Eckrich et al., 2011; Ballantine et al., 2016; Edmunds et al., 2019; Nieder et al., 2019). While Ramicrusta is known to have similar form and growth patterns as CCA, it behaves differently in that it frequently overgrows mature corals (Eckrich and Engel, 2013; Nieder et al., 2019). There is limited evidence of juvenile coral recruitment on Ramicrusta, with studies finding very limited (Edmunds et al., 2019) to no coral recruits on Ramicrusta coated surfaces (Eckrich and Engel, 2013). There are also differences in the microbial biofilms harbored by CCA and Ramicrusta which could additionally impact settlement (Wilson et al., 2020). Ramicrusta is considered invasive to shallow Caribbean reefs and studies suggest that the rapid spread of Ramicrusta may leave little suitable settlement substrate available for coral larvae (Eckrich and Engel, 2013; Edmunds et al., 2019).
While there is evidence that Ramicrusta is a threat to adult corals, little is known about the effects of Ramicrusta on coral larvae and the settlement process. Here, we conducted a laboratory study using larvae from the Caribbean corals Porites astreoides and Favia fragum to investigate the impact of Ramicrusta and CCA solutions on larval mortality/survivorship and settlement. We show that Ramicrusta exposures cause high larval mortality compared to CCA.
Materials and methods
Sampling and coral spawning
Porites astreoides and Favia fragum adult colonies (12 -20 of each species) were collected from 2-12 m using hammer and chisel from the southern shore of St. John, U.S. Virgin Islands several days before spawning. Corals were held in shaded seawater tables consisting of flowing, non-filtered seawater. During their respective spawning periods, corals were placed overnight in 63 µm mesh baskets and the seawater level was dropped in the sea table to contain the larvae. The baskets were checked for larvae the following morning and the water level was subsequently raised to allow the corals to feed. P. astreoides coral larvae collection began several days before the new moon of June (Goodbody-Gringley et al., 2021) and F. fragum collection began 5-8 days after the new moon of July (Szmant-Froelich et al., 1985). Swimming larvae that were not exhibiting substratum searching behavior were collected and transferred into a container containing sterile seawater filtered using a 0.2 µm Supor filter (Millipore) under peristaltic pressure.
On the day of each experiment, igneous rocks containing pink coral crustose algae (CCA) or Ramicrusta, both identified based on morphology and coloration, were obtained from shallow 1-3 m southern shore St. John reefs. CCA was identified as a pink algal crust growing without the presence of Ramicrusta. Ramicrusta was consistently defined as a dark algal crust with mustard coloration (Ballantine et al., 2016).
Exposure experiments
To make the solutions, the Ramicrusta or CCA fragments were washed 5 times with filtered seawater and subsequently shaved off several rocks using a razor blade while carefully avoiding any substrate underneath. The shavings were weighed and ground into a powder using a mortar and pestle. Each powdered amount of Ramicrusta or CCA was added to a graduated cylinder with 60 ml of fresh 0.2 µm filtered sea water and stirred until a homogenous solution was formed. Each 60 ml solution was distributed into a 6-well plate, each well received 10 ml of a 0.5, 1, or 2 mg ml-1 solution of Ramicrusta or CCA. The concentrations were determined based on Pollock et al. (2017) who used a similar method to examine the optimal dosage of CCA for larvae settlement.
The P. astreoides and F. fragum experiments were conducted separately due to the differential spawning times. Each experiment used 6-well plates (16.8 ml well holding capacity, Costar, Corning Incorporated; Figure 1) and each plate was devoted to a separate treatment (5 wells per treatment, 10 ml sterile water or solution to each well).
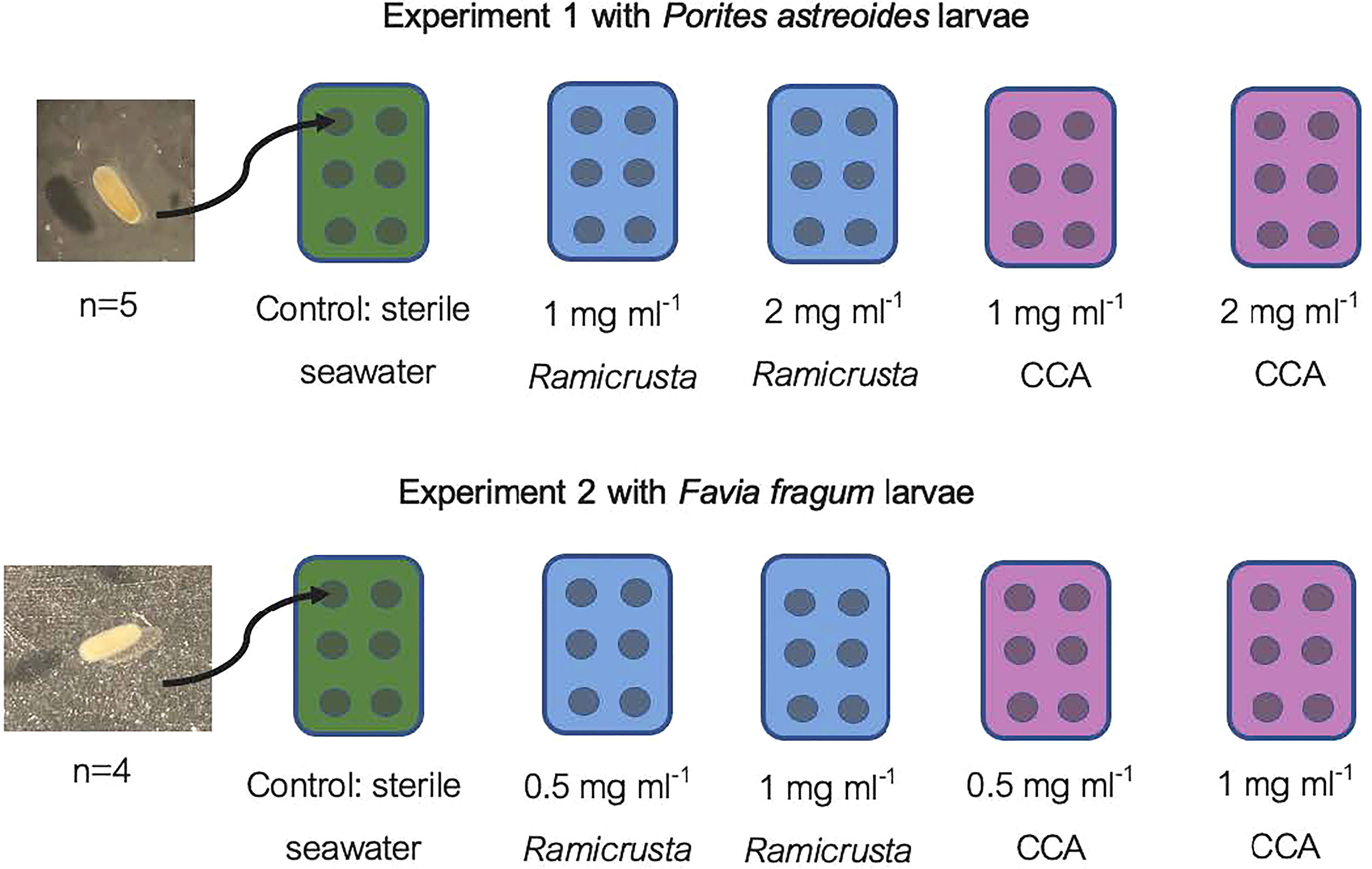
Figure 1 The experimental design for the larval exposure experiments. Each rectangle with six circles represents a 6-well plate and the circles represent 16.8 ml wells containing larvae and treatment. Green represents the sterile seawater, blue represents solutions containing Ramicrusta, and pink represents a solution containing CCA. Each well contained 10 ml of the solution or sterile seawater, and 5 wells each containing 4-5 larvae were examined per treatment.
In the first experiment P. astreoides larvae (5 wells each containing 5 larvae) were added to the following treatments: sterile seawater (control), 1 mg ml-1 of CCA, 2 mg ml-1 of CCA, 1 mg ml-1 of Ramicrusta, and 2 mg ml-1 of Ramicrusta. Due to the high larval mortality associated with Ramicrusta in the first experiment, concentrations of solutions were reduced for the second experiment to examine the impact of a lower dose. Therefore, in the second experiment F. fragum larvae (5 wells each containing 4 larvae) were exposed to the following treatments: sterile seawater (control), 0.5 mg ml-1 of CCA, 1 mg ml-1 of CCA, 0.5 mg ml-1 of Ramicrusta, and 1 mg ml-1 of Ramicrusta. Both experiments were conducted at indoor ambient laboratory temperature (approximately 26-28° C) with regular 12 h light/12 h dark room lighting (no aquarium lighting) for 48 hours. When the experiment ended, the 5 wells of each treatment were examined under a dissecting microscope at 10.5x magnification. The larvae were first examined for mortality as “alive” or “dead/unaccounted” and then quantified by categorizing each as “interacting with substrate”, “swimming”, or “dead/unaccounted” (Birrell et al., 2008). Percentage of larval mortality (or settlement/surface interaction) in each well were further examined using statistical approaches.
The data were examined using SigmaPlot software (version 13, SYSTAT Software) using a Shapiro-Wilk test to determine if data were normally distributed. Based on the non-normality of our data distribution, we used a Kruskal-Wallis One-Way Analysis-of-Variance-on-Ranks (H test), and a Tukey post-hoc all pairwise multiple comparisons procedures with a significance level of p<0.05 to examine pairwise trends.
Results
For both species of coral, larval mortality was the highest in the Ramicrusta treatments and there was a significant effect of treatment on larval mortality (H test, p < 0.003, Supplementary Table 1). For P. astreoides, mean larval mortality was 96% (±8.9% standard deviation, SD) for both the 1 and 2 mg ml-1 Ramicrusta treatments (Figure 2A). Mortality in the 1 mg ml-1 CCA treatment was 4% (±8.9% SD) and there was no mortality in the 2 mg ml-1 CCA or sterile seawater treatments. Significantly more P. astreoides larvae survived in the control and 2 mg ml-1 CCA compared to the Ramicrusta treatments (p = 0.031, H test with Tukey comparison), but the other post-hoc comparisons were either not significant (p > 0.05) or non-testable due to the lack of significant difference between the rank sums (Supplemementary Table 1). There was 100% mortality in the F. fragum treatments with Ramicrusta and 10% (±20% SD) and 5% (±10% SD) mortality in the 0.5 and 1 mg ml-1 CCA treatments, respectively with complete survival of larvae in the sterile seawater (Figure 2B). Post-hoc statistical comparisons were either not significant (p > 0.05) or non-testable due to no significant difference between the rank sums (Supplemementary Table 1).
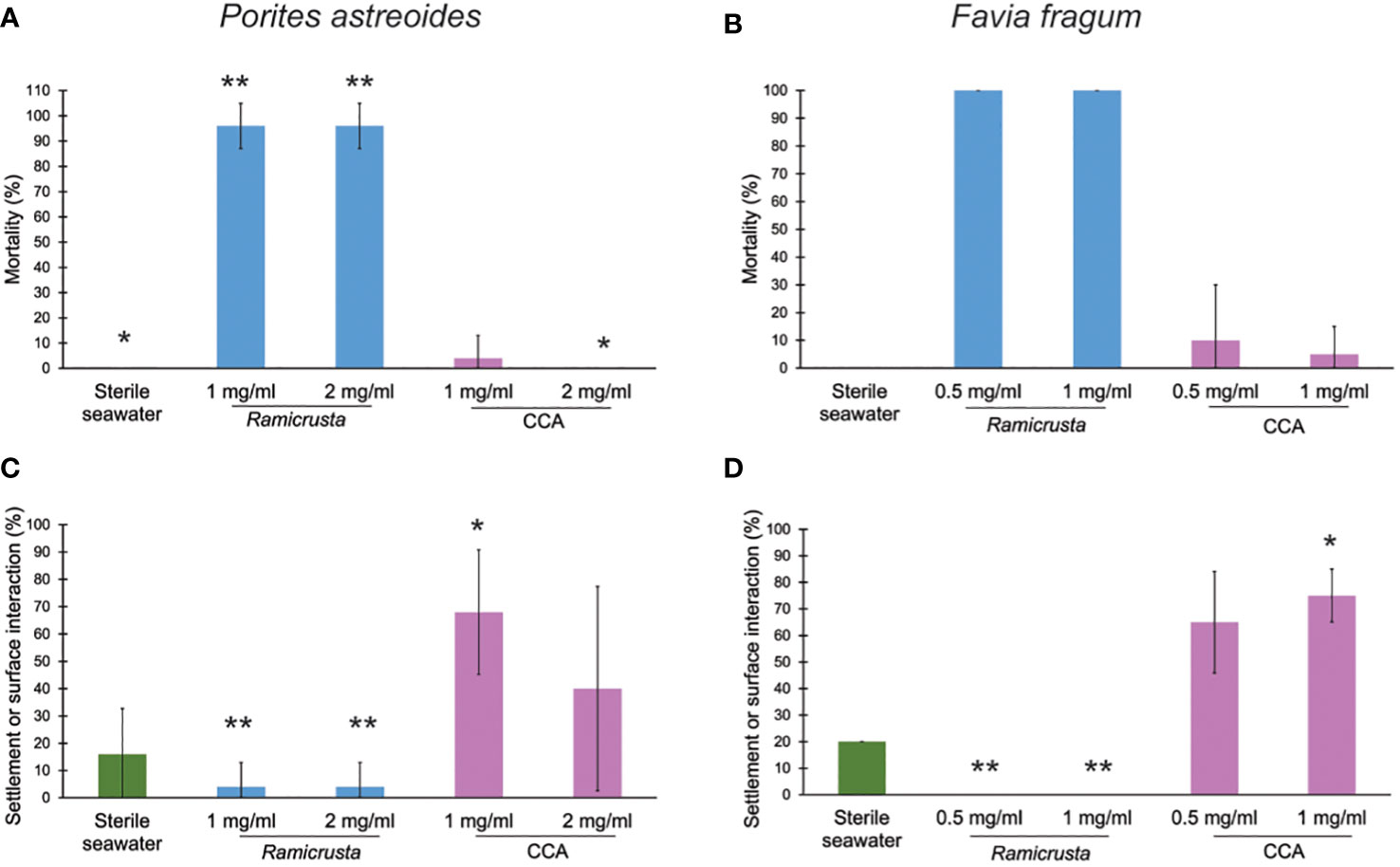
Figure 2 Ramicrusta exposure causes larval mortality and reduced settlement. The average percent (± SD) of P. astreoides (A) and F. fragum (B) larval mortality in the treatments (5 wells each containing 4-5 larvae) and percent of P. astreoides (C) and F. fragum (D) larvae that settled or interacted with the well surface (5 wells each containing 4-5 larvae). There was no mortality in the sterile seawater treatment of either experiment. Significantly different treatments according to Kruskal-Wallis H-test and Tukey Test post-hoc test are denoted by different asterisks groups (e.g., * and ** are significantly distinct) and are distinct for each figure panel (p < 0.05).
There was a significant effect of treatment on larval settlement and survivorship for both species of coral (H-test, p < 0.005, Supplementary Table 1) and the highest settlement and surface interaction occurred in the CCA treatments. Mean P. astreoides settlement/surface interaction was 68% (±23% SD) and 40% (±37% SD) for the 1 and 2 mg ml-1 CCA treatments, respectively (Figure 2C). One P. astreoides larva survived in each 1 mg ml-1 and 2 mg ml-1 Ramicrusta treatment. While both larvae showed evidence of settlement/surface interaction, the settlement/surface interactions in the Ramicrusta treatments were significantly lower than in the 1 mg ml-1 CCA treatment (Figure 2C, p = 0.019). Mean F. fragum settlement/surface interaction was 65% (±19.1% SD) and 75% (±10% SD) for the 0.5 and 1 mg ml-1 CCA treatments, respectively (Figure 2D). Significantly more larvae interacted with the surface in the CCA 1 mg ml-1 compared to each Ramicrusta treatments (p = 0.021), which is partially attributed to the complete mortality of F. fragum larvae exposed to Ramicrusta.
Discussion
Ramicrusta is a rapidly expanding encrusting algae that is especially prolific on shallow Caribbean reefs. Here we used exposure experiments to show that Ramicrusta causes mortality in larvae from two species of prominent brooding Caribbean corals, suggesting that Ramicrusta may be toxic to the larvae. We observed limited larval mortality (<10%) in our CCA exposure experiments, and 40–80% of these CCA-exposed larvae exhibited settlement or surface interactions, thus suggesting that the experimental conditions and solution concentrations were appropriate for the larvae. Notably, one P. astreoides in each Ramicrusta treatment (1 and 2 mg ml-1) survived and showed evidence of settlement/substrate interaction, signifying that select coral larva may tolerate Ramicrusta, which could signify metamorphosis under stress (Kitchen et al., 2022).
Our data suggest that Ramicrusta may contain or produce metabolites toxic to coral larvae. A recent study showed that Ramicrusta textilis produced caffeine, among other metabolites (Weber et al., 2022). Caffeine is a common deterrent of herbivores and pathogens by land plants (Kim et al., 2010), and it has adverse growth impacts on insect larvae (Srinivasan and Kesavan, 1977; Maguire et al., 2017). The impact of caffeine, as well as other Ramicrusta producing metabolites, on coral larvae is an area for further investigation.
Our study exposed coral larvae to Ramicrusta that was dissolved in solution at specific concentrations. While our CCA exposure experiments at similar doses resulting in settlement suggests that these concentrations may be in-line with natural exposure and other larval studies (Pollock et al., 2017), future studies could expose larva to surfaces with encrusted with Ramicrusta (instead of solutions) to verify the impact of typical surface-type exposures on larval survival and settlement. Here we used solutions to achieve homogeneity in all replicates, which may be more inconsistent with Ramicrusta surfaces due to differential biofilms on the surfaces. Additionally, we only evaluated two concentrations of Ramicrusta solutions and response could be evaluated with lesser dilutions of Ramicrusta. However, our results are in line with prior observations of no to limited coral settlement on Ramicrusta coated surfaces (Eckrich and Engel, 2013; Edmunds et al., 2019). Our experiments used replicate wells within a plate for each treatment which may introduce a plate-type effect in our study. Future experiments should randomize treatments within each plate and/or use separate plates for each replicate within a treatment to strengthen the experimental design and resulting conclusions. It should also be noted that only two species of coral larvae were used in this experiment, and expansion to additional species are warranted, as well as molecular genotyping to verify the species of Ramicrusta. Here we observed slightly elevated settlement in F. fragum compared to P. astreoides larvae which signifies species-related differences. Indeed, F. fragum typically settles within the first 24 hours (Goodbody-Gringley, 2010), and P. astreoides can maintain longer larval durations (Harrison and Wallace, 1990).
Overall, our results add to the growing knowledge that Ramicrusta is harmful to corals and is a potential threat to Caribbean reefs. Research that explores herbivory controls as well as other intervention mechanisms to remove or inhibit Ramicrusta spread on reefs is urgently needed to prevent further reef decline.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Author contributions include study conception, design and funding (AA, TM), experimentation (KC, NA, SF), data analysis (KC, AA), writing (KC, AA), and editing (all authors). All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by the WHOI Summer Student Fellowship and NSF REU award OCE-2150401 (to KC), NSF awards 1938147, 1923962 and 2109622 (to AA) and Oceankind, Vere Initiatives and NSF 2024077 (to TM).
Acknowledgments
Samples were collected under the USVI Territory Endangered Species Scientific Research Permit #DFW22067J and National Park Service Scientific Research and Collecting Permit #VIIS-2022-SCI-0005.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1158947/full#supplementary-material
References
Ballantine D. L., Ruiz H., Lozada-Troche C., Norris J. N. (2016). The genus Ramicrusta (Peyssonneliales, rhodophyta) in the Caribbean Sea, including ramicrusta bonairensis sp. nov. and Ramicrusta monensis sp. nov. Botanica Marina 59 (6), 417–431. doi: 10.1515/bot-2016-0086
Birrell C. L., McCook L. J., Willis B. L., Harrington L. (2008). Chemical effects of macroalgae on larval settlement of the broadcast spawning coral Acropora millepora. Mar. Ecol. Prog. Ser. 362, 129–137. doi: 10.3354/meps07524
Eckrich C. E., Engel M. S. (2013). Coral overgrowth by an encrusting red alga (Ramicrusta sp.): a threat to Caribbean reefs? Coral Reefs 32 (1), 81–84. doi: 10.1007/s00338-012-0961-5
Eckrich C., Engel M., Peachey R. (2011). Crustose, calcareous algal bloom (Ramicrusta sp.) overgrowing scleractinian corals, gorgonians, a hydrocoral, sponges, and other algae in lac bay, bonaire, Dutch Caribbean. Coral Reefs 30 (1), 131–131. doi: 10.1007/s00338-010-0683-5
Edmunds P., Steneck R., Albright R., Carpenter R., Chui A., Fan T.-Y., et al. (2015). Geographic variation in long-term trajectories of change in coral recruitment: a global-to-local perspective. Mar. Freshw. Res. 66 (7), 609–622. doi: 10.1071/MF14139
Edmunds P. J., Zimmermann S. A., Bramanti L. (2019). A spatially aggressive peyssonnelid algal crust (PAC) threatens shallow coral reefs in st. John, US virgin islands. Coral Reefs 38 (6), 1329–1341. doi: 10.1007/s00338-019-01846-0
Goodbody-Gringley G. (2010). Diel planulation by the brooding coral favia fragum (Esper 1797). J. Exp. Mar. Biol. Ecol. 389 (1-2), 70–74. doi: 10.1016/j.jembe.2010.03.016
Goodbody-Gringley G., Scucchia F., Ju R., Chequer A., Einbinder S., Martinez S., et al. (2021). Plasticity of Porites astreoides early life history stages suggests mesophotic coral ecosystems act as refugia in Bermuda. Front. Mar. Sci. 8, 702672. doi: 10.3389/fmars.2021.702672
Harrison P, Wallace C. (1990). Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z. (ed) Ecosystems of the world. (Amsterdam: Coral Reefs). Book 25.
Heyward A., Negri A. (1999). Natural inducers for coral larval metamorphosis. Coral Reefs 18 (3), 273–279. doi: 10.1007/s003380050193
Hughes T. P., Kerry J. T., Baird A. H., Connolly S. R., Chase T. J., Dietzel A., et al. (2019). Global warming impairs stock–recruitment dynamics of corals. Nature 568 (7752), 387–390. doi: 10.1038/s41586-019-1081-y
Kim Y.-S., Choi Y.-E., Sano H. (2010). Plant vaccination: stimulation of defense system by caffeine production in planta. Plant Signaling Behav. 5 (5), 489–493. doi: 10.4161/psb.11087
Kitchen S. A., Jiang D., Harii S., Satoh N., Weis V. M., Shinzato C. (2022). Coral larvae suppress heat stress response during the onset of symbiosis decreasing their odds of survival. Mol. Ecol. 31 (22), 5813–5830. doi: 10.1111/mec.16708
Maguire R., Kunc M., Hyrsl P., Kavanagh K. (2017). Caffeine administration alters the behaviour and development of Galleria mellonella larvae. Neurotoxicol. Teratol. 64, 37–44. doi: 10.1016/j.ntt.2017.10.002
Negri A. P., Webster N. S., Hill R. T., Heyward A. J. (2001). Metamorphosis of broadcast spawning corals in response to bacteria isolated from crustose algae. Mar. Ecol. Prog. Ser. 223, 121–131. doi: 10.3354/meps223121
Nieder C., Chen P.-C., Allen Chen C., Liu S.-L. (2019). New record of the encrusting alga Ramicrusta textilis overgrowing corals in the lagoon of dongsha atoll, south China Sea. Bull. Mar. Sci. 95 (3), 459–462. doi: 10.5343/bms.2019.0010
Pollock F. J., Katz S. M., van de Water J. A., Davies S. W., Hein M., Torda G., et al. (2017). Coral larvae for restoration and research: a large-scale method for rearing acropora millepora larvae, inducing settlement, and establishing symbiosis. PeerJ 5, e3732. doi: 10.7717/peerj.3732
Pueschel C. M., Saunders G. W. (2009). Ramicrusta textilis sp. nov.(Peyssonneliaceae, rhodophyta), an anatomically complex Caribbean alga that overgrows corals. Phycologia 48, 480–491. doi: 10.2216/09-04.1
Ritson-Williams R., Arnold S. N., Fogarty N. D., Steneck R. S., Vermeij M. J., Paul V. J. (2009). New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithsonian Contributions to Mar. Sci. 38, 437. doi: 10.5479/si.01960768.38.437
Sneed J. M., Sharp K. H., Ritchie K. B., Paul V. J. (2014). The chemical cue tetrabromopyrrole from a biofilm bacterium induces settlement of multiple Caribbean corals. Proc. R. Soc. B: Biol. Sci. 281 (1786), 20133086. doi: 10.1098/rspb.2013.3086
Srinivasan A., Kesavan P. (1977). Toxic effects of caffeine on growth and metamorphosis of the larvae of Musca domestica. J. Toxicol. Environ. Health Part A Curr. Issues 2 (3), 569–576. doi: 10.1080/15287397709529457
Szmant-Froelich A., Reutter M., Riggs L. (1985). Sexual reproduction of Favia fragum (Esper): lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Bull. Mar. Sci. 37 (3), 880–892.
Tebben J., Motti C. A., Siboni N., Tapiolas D. M., Negri A. P., Schupp P. J., et al. (2015). Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 5, 10803. doi: 10.1038/srep10803
Weber L., Soule M. K., Longnecker K., Becker C. C., Huntley N., Kujawinski E. B., et al. (2022). Benthic exometabolites and their ecological significance on threatened Caribbean coral reefs. ISME Commun. 2 (1), 1–13. doi: 10.1038/s43705-022-00184-7
Keywords: coral reef, ecology, USVI, coral larvae, settlement, recruitment, Ramicrusta, Peysonneliaceae
Citation: Cayemitte K, Aoki N, Ferguson SR, Mooney TA and Apprill A (2023) Ramicrusta invasive alga causes mortality in Caribbean coral larvae. Front. Mar. Sci. 10:1158947. doi: 10.3389/fmars.2023.1158947
Received: 04 February 2023; Accepted: 30 March 2023;
Published: 18 April 2023.
Edited by:
Susana Carvalho, King Abdullah University of Science and Technology, Saudi ArabiaReviewed by:
Robert W. Thacker, Stony Brook University, United StatesMareen Moeller, University of Oldenburg, Germany
Copyright © 2023 Cayemitte, Aoki, Ferguson, Mooney and Apprill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy Apprill, aapprill@whoi.edu
 Kayla Cayemitte
Kayla Cayemitte Nadège Aoki
Nadège Aoki Sophie R. Ferguson
Sophie R. Ferguson T. Aran Mooney
T. Aran Mooney Amy Apprill
Amy Apprill