Ecomorphological adaptation of Scorpaena porcus (Linnaeus, 1758): evidence from two different environments revealed by sagittae features and somatic growth rates
- 1Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Messina, Italy
- 2Department of Marine Studies, University of Split, Split, Croatia
- 3Department of Veterinary Sciences, University of Messina, Messina, Italy
- 4Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, Sea in Health and Life Srl, Capo Peloro, Messina, Italy
Improve the knowledge base on the ecomorphological adaptation of teleost species to different environments, trying to reconstruct how habitat can shape sagittae, is essential for conservational purposes, evolutionary evaluations, and population dynamics studies. Here is provided a comparative study between sagittae features, growth rates and age composition of two Scorpaena porcus populations inhabiting the Mediterranean waters, one from the Strait of Messina (Central Mediterranean Sea) and one from the Split area (Adriatic Sea). Additionally, the stomach contents of the Messina specimens have also been analysed to provide some preliminary information on the diet of S. porcus. A total of ninety individuals, half from each area, have been obtained from two extremely different environments. Results showed an overall different morphology, shape, and morphometry of sagittae among the size classes of the two investigated populations. Samples from Messina were characterized by a most elliptical and slender shape, with a more regular serration of margins than those from Split, which exhibited a wider sagitta, with a most enhanced anti-rostrum and longer rostrum. Concerning diet, specimens from Messina showed a preference for Crustacea (especially Brachyura and Amphipoda) and Teleost species, showing some differences with literature data from other geographical areas (Split one included). Results have confirmed the reliability of sagittae to detect the inter-population variability of S. porcus from different geographical areas, an essential tool for stock assessment, population studies and investigation on ecomorphological adaptation of teleost species to different habitats.
1 Introduction
The family Scorpaenidae counts more than 1400 species and represents one of the most diverse fish groups worldwide (Turan et al., 2009). It includes species adapted to live in many different environments, from the coastal shallow waters to the deep seas (Costello et al., 2001). Within the family, species belonging to Scorpaena genus are among the most abundant in all the temperate marine environments, and in several tropical seas, around the world, with 61 valid species included in the genus (Nelson et al., 2016; Fricke et al., 2018).
Among these, the black scorpionfish, Scorpaena porcus (Linnaeus, 1758), is one of the most common in the entire Mediterranean basin, Black Sea, and the Eastern Atlantic Ocean, from the British Islands to the Atlantic Moroccan coasts (Wheeler et al., 1986; Mahé et al., 2014). It dwells rocky, or mixed rocky-sandy, benthic habitats, from the shallow water to the bathyal plan, with a bathymetric distribution in the Mediterranean basin ranging between 20 and 200 m (up to 800 m) with a mean at 20 m (Fricke et al., 2018). It is very common also on seagrass beds, showing a sedentary lifestyle with nocturnal feeding habits (Poutiers, 1987; Pashkov et al., 1999). According to literature, this species is a small-body benthic predator, with a preference for small fishes and crustaceans (Compaire et al., 2018; Aydin and Mazlum, 2020; Ferri and Matić-Skoko, 2021). It is considered, together with the other scorpionfish species, as an essential predator for the well-functioning of the rocky-reefs ecosystems worldwide, both in temperate and tropical areas. Unlike other congeneric species, S. porcus is characterized by a slow-growth and a relatively short life, with 12 years as maximum recorded age (Kutsyn et al., 2019), and an enhanced geographical heterogeneity in life span and growth rates within the distribution areas (Jardas and Pallaoro, 1992; Pashkov et al., 1999; Kuzminova et al., 2011; Ferri et al., 2012; Kutsyn et al., 2019; Sahin et al., 2019). This high inter population variability has been related to the influence of the different fishing pressures and environmental factors experienced by the populations within the distribution range (Bradai and Bouain, 1988; Jardas and Pallaoro, 1992; Unsal and Oral, 1994; La Mesa et al., 2010; Kutsyn et al., 2019; Sahin et al., 2019; Ferri and Matić-Skoko, 2021). Many authors have also suggested S. porcus as indicator species for biomonitoring and environmental conditions’ assessments (Rudneva et al., 2005; Medinets and Medinets, 2010; Kuzminova et al., 2011; Ferri et al., 2012). In the Black Sea, this species showed a significant negative trend in body size related to several factors, as pollution, and temperature rise, being negatively affected by anthropogenetic pressure (Rudneva et al., 2005; Medinets and Medinets, 2010; Kuzminova et al., 2011). While, in the Adriatic Sea, variations in population dynamics, biological traits and biomass, related to fishing pressure, confirmed the sensitivity of the species to fisheries activities (Jardas et al., 1998; Jardas, 1999; Oven et al., 2000; Stagličić et al., 2011; Ferri et al., 2012; Ferri and Matić-Skoko, 2021). S. porcus represents one of the main target species of Mediterranean artisanal fisheries (especially in the eastern and western parts of the basin), representing a large portion of the total catch and, consequently, a large source of income (Jardas, 1999; García-Rodríguez et al., 2006; Forcada Almarcha et al., 2007; Goñi et al., 2008; Forcada et al., 2009; La Mesa et al., 2010; Ferri et al., 2012; Özgül et al., 2019) thanks to its relatively high commercial value in Croatia, Spain, and Italy. S. porcus annual total catch was 240 tons in the Eastern part (192 tons in Turkey) of the basin (104 tons from Black Sea, 36 from the Sea of Marmara, 88 from Aegean Sea and 12 from the Mediterranean Sea) in the first decade of 2000 (Bilgin and Çelik, 2009). In Italy the total landings of scorpionfish species in 2021 changed in relation to the geographic areas, from 41.2 tons in the Adriatic Sea, to 446.58 tons in the Ionia Sea (FAO, 2020). Concerning S. porcus conservation status, it was recorded a relevant decline in catches and average size during the last two decades in the Ionian coast of the Sicily, strictly related to fishing activities (Tiralongo, 2024). To improve the knowledge base on the intra-specific variability of S. porcus, analyzing the different growth and eco-morphological adaptations of populations inhabiting different geographical areas, is essential to enhance its conservation. In fact, in fishery biology, a proper assessment of fishes’ populations dynamics and stocks’ structure is a fundamental step to establish good management measures, monitoring species and communities’ response to management actions and different exploitation levels (Charles, 2001; Cochrane, 2002; Dimech et al., 2012; Perdichizzi et al., 2022). Moreover, being S. porcus a benthic species with a low home-range, a high spatial heterogeneity, and an enhanced ecological value, investigate how different habitats can influence its otoliths’ features (such as shape, morphology, and morphometry) and growth rates acquires great relevance and scientific interest, also in terms of taxonomical studies and evolution.
Sagittae, as the other otoliths, grow for the entire fishes’ life, with a daily metabolically inert deposition of calcium carbonate (Popper et al., 2005; Thomas and Swearer, 2019). Their peculiar physiology and growing mechanism have made otoliths (mainly sagittae) (Assis, 2003, 2005; Ladich and Schulz-Mirbach, 2016) an essential tool to study fish ages and growth (Newman and Dunk, 2002; Velasco et al., 2011). Thanks to their high variability in morphology and shape between and within species (Volpedo et al., 2008; Jaramilo et al., 2014; Zhuang et al., 2015a; D’Iglio et al., 2021a; D’iglio et al., 2022a; D’Iglio et al., 2023), they have been widely used also in several research field (e.g., taxonomy, paleontological studies, stomach content analyses, and stocks assessment in fisheries studies) (Benzinou et al., 2013; Higgins et al., 2013; Muniz et al., 2021; Neves et al., 2021; D’Iglio et al., 2021b; D’Iglio et al., 2022b). Concerning the intra-specific variations in shape and morphology, they can be related to many factors, ranging from those genetically driven (Vignon and Morat, 2010), to environmental conditions and diet (Schulz-Mirbach et al., 2010; Higgins et al., 2013). For this reason, sagittae are considered a good phenotypic marker, reliable to investigate the ecomorphological adaptation of teleost species to different environmental conditions and ecological dynamics (Abaad et al., 2016; Bose et al., 2020), as highlighted by the shape and morphological differences between stocks and populations (Benzinou et al., 2013; Higgins et al., 2013; Muniz et al., 2021; Neves et al., 2021). Diet composition and feeding habits were assessed as factors which influence on teleosts physiology, metabolism, and, consequently, on otoliths too (Mommsen, 1998; Sanchez-Jerez et al., 2002). Moreover, molecules incoming deriving from diet strongly influence otoliths’ growth process and the geographical differences in feeding habits, added to genetic and environmental variability, could allow their inter-population variability (Mille et al., 2016a, 2016b; Hüssy et al., 2021).
The present paper aims to investigate the intra-specific differences in age composition, growth, diet composition, and sagittae shape and morphology, between two populations of S. porcus inhabiting, respectively, the tidal ponds in the beach rock formations, along the Strait of Messina coast (Central Mediterranean Sea), and the coastal environment near the Split Area (Eastern Adriatic Sea). The stomach content analysis has been performed only on the specimens from Messina, using the obtained data to make a comparison with the data present in literature for the diet composition of the studied species in the Split area (Ferri and Matić-Skoko, 2021). The main purposes were: (i) to assess how sagittae contours, morphology and morphometry change among size classes in the two studied populations; (ii) to evaluate the reliability of shape and morphometrical analysis in the assessment of different S. porcus populations; (iii) to provide preliminary observations on growth and age composition between the studied areas; (iv) to analyse the stomach contents of the specimens from Messina, providing preliminary data on the diet composition and feeding habits of the samples of this area, useful to improve the knowledge base on their geographical variability and their influence on the growth and age composition of the specimens from the two different studied areas. These information are important to improve the knowledge base on the influences of the environment on inner ear morphology, otoliths and somatic growth.
2 Materials and methods
2.1 Sample collection and studied areas.
A total number of 90 S. porcus specimens were collected in December 2022 from two different localities of the Mediterranean Sea: 45 specimens from the tidal ponds occurring toward the Sicilian coast of the Messina’s Strait (Central Mediterranean Sea, 38°15’25”N, 15°36’51”E), and 45 specimens from the coastal environment near the Split area (Eastern Adriatic Sea, 43°26’0”N, 16°26’55”E) (Figure 1).
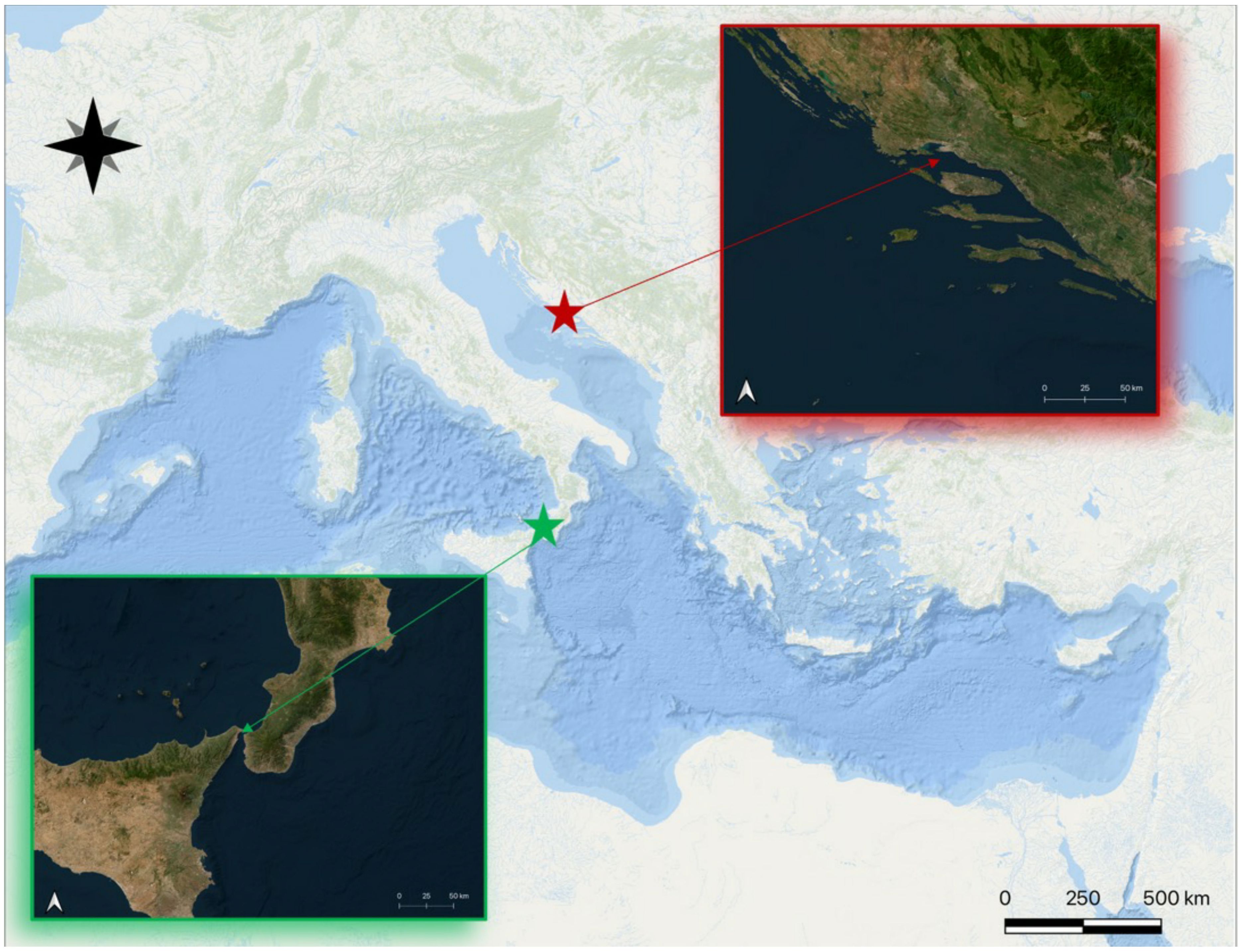
Figure 1 Map of the central Mediterranean Sea with the two studied areas highlighted in the boxes (Strait of Messina area in green and Split area in red).
Thanks to the limited depth (ranging from 0.2 to 1.2 m) of the tidal ponds occurring in the “beach rock” formations, a coastal biotope extending in the north-eastern Messina coast, between the two villages “Ganzirri” and “Torre Faro”, toward the Sicilian coast of the Strait of Messina, specimens were caught manually, using a sampling net from the rocky bench present in the beachfront, by local fishermen.
“Beach rock”. This sedimentary formation represents a rocky bench (2 kilometres long) that arrive from the beachfront, in the intertidal zone, to a depth of 2-3 meters, at the beginning of the infralittoral zone. It is considered an area of high interest, being part of the Oriented Natural Reserve of “Capo Peloro Lagoon” (Savoca et al., 2020), which, thanks to its irregular shape, is characterized by several tidal ponds, with different surface and connection dynamics to the sea, hosting very complex ecological communities. These are essential nursery areas for several teleost species and a shelter zone for numerous taxa (Gravem and Morgan, 2017; Capillo et al., 2018).
Concerning specimens from the Split area (Figure 1), they were collected using sets of trammel nets, 1.5 m high and 32 m long, with an inner layer mesh size of 28 mm and 150 mm mesh size of outer layers. Fish samples were captured in the nearshore, coastal waters in the Split area (43.5°N), in the eastern Adriatic, at depths ranging between 10 and 40 m (20 m in average). This biotope is characterized mainly by rocky substrata covered by photophilic algae alternating with patches of sand and Posidonia oceanica seagrass beds (Ferri et al., 2012; Ferri and Matić-Skoko, 2021).
2.2 Samples processing, otolith extraction and age reading
All sampled specimens were transported frozen to the laboratory, where they were measured (TL, cm) and weighed (BW, g). Sagittae were collected from each specimen, cleaned (15 min in 3% H2O2, followed by Milli-Q water) and, once dried, stored in Eppendorf microtubes. To assess the intra-specific variability in sagittae shape and morphometries in relation to fish size, all samples were divided into three size classes according to the following TL values: Class I included specimens smaller than 100 mm, Class II those with a TL ranging from 100 mm to 180 mm, and Class III larger than 180 mm. The left sagittae of both populations were photographed on the distal side using a stereomicroscope (Olympus SZX10) equipped with an Olympus DP-25 digital camera. Images were converted in binary format for shape analysis, using the ImageJ 1.48p free software, “available at http://rsb.info.nih.gov/ij/”. Otoliths morphologies were described following the terminology used by Tuset (Tuset et al., 2008), Nolf (Nolf, 1985) and Assis (Assis, 2000).
After images collection, left sagittae were used for age estimation. The reading process was carried out by two different operators, without data on fish length, one with experience in estimating the age of S. porcus, and one with experience in estimating the age of other teleost species. Both readers performed two different readings, with one month of distance one from the other. If readings were different for one or two years, it was performed a third reading, with the otolith discarding in the case of persisting differences. The relative precision between readings was tested by the index of average percentage error (APE) (Beamish and McFarlane, 1983) and the coefficient of variation (CV) (Chang, 1982). The annual age of the specimens was estimated with reflected light and on a black background using the stereomicroscope (Olympus SZX10) equipped with an Olympus DP-25 digital camera, at magnification 1.5-2.4x). The pattern of opaque and translucent zones was used for annuli counting. Indeed, according to literature (La Mesa et al., 2010; Carbonara and Follesa, 2019; Ferri and Matić-Skoko, 2021), an annulus is the combination of an opaque and a translucent (or hyaline) ring, appearing, under the reflecting light respectively light and dark. When this pattern was not clear, sagittae were grinded to facilitate the rings’ counting. It was not necessary the otoliths sectioning, being grinding enough to reveal the opaque and translucent zones’ pattern for those samples in which this was not clear from the whole otoliths viewing. Age was estimated by counting translucent zones (Landa and Hernández, 2020) and considering the 1st of July as the birthdate (La Mesa et al., 2010). In addition, during the age reading process, the edge of otoliths has been classified as opaque or translucent.
2.3 Stomach content analysis
Concerning stomachs, they were sampled only from Messina’s specimens, and stored in ethanol 70% + glycerin 5% for stomach content analysis. Each stomach content of specimens from Messina was analyzed under the stereomicroscope, to identify, at the lowest taxonomic level possible, each prey. Each preys’ items were counted and weighted, also detecting the digestion degree (1 = undamaged; 2 = almost digested; 3 = highly digested). The anatomical undigested preys’ parts (e.g., otoliths, telsons, carapaces, mouth parts, heads capsules, fish columns) were counted to assess the contribution of each prey taxon to the diet, grouping the unidentifiable preys’ items, due to the advanced state of digestion, into undetermined taxa. According to their presence in the stomachs, only one type of anatomical remains was counted for each prey group to avoid the double-counting.
Following indexes were calculated to evaluate the contribution of each preys’ taxa to the diet: the percentage of biomass composition (%W), the percentage of abundance composition (%N), and the frequency of occurrence (%F) (Hyslop, 1980). The Relative Importance Index was also calculated [IRI = %F (%N + %P)] expressing it also as percentage 9)(Cortés, 1997; D’Iglio et al., 2021b; D'Iglio et al., 2022b). Concerning the empty stomachs, it was calculated the Vacuity Index, VC = (Ne/N) × 100, as the percentage of empty stomachs (Ne) on the total stomach number (N).
2.4 Shape and morphometric analysis
Shape R (R software package, RStudio 2022.07.1 Build 554; R Gui 4.1.3 2022.03.10) was used to perform the Shape analysis from the otoliths’ outlines. This package has been developed for inter and inter specific analysis on shape variability in teleost’s otoliths (Libungan and Pálsson, 2015). Each binarized sagitta photo was analyzed for the outlines’ detection using a shape R specific function, with 0.05 as value for intensity threshold greyscale. A data file with studied specimens’ information (as body weight and fish length) was linked to extracted contours. These was used for each size classes to perform several otoliths’ measurements (maximum width, OW, mm, maximum length, OL, mm, perimeter, OP, mm, and surface, OS, mm2), through the getMeasurements function. Proper package functions were applied for the extraction, and subsequent adjusting, of Wavelet and Fourier coefficients, to assess the allometric relationships between fish lengths and otolith shapes. Wavelet coefficients were used to provide the comparison between the mean sagittae shape of the analyzed populations. The reconstruction’s quality was estimated analyzing the deviation of the coefficient’s reconstruction from the otolith outline (Supplementary Figure S1). Finally, a g-plots R package’s specific function was used to investigate how the position along the outline can influence the wavelet coefficients variation (Supplementary Figure S2).
Otoliths measurements performed with shape R were used to calculate several indexes. The sagittae length increase, related to the total fish length, was evaluated assessing otolith length to total fish length ratio (OL/TL); while, according to literature (Tuset et al., 2003a, 2003b, 2016a; Pavlov, 2016, 2021; Jawad et al., 2018), several shape indexes were used to evaluate intra and inter specific variability of sagittae shape: roundness (Ro = 4OS/πOL2), form factor (FF = 4πOS/OP2), aspect ratio (AR = OW/OL%), circularity (C = OP2/OS), rectangularity (Re = OS/[OL×OW]) and ellipticity [E = (OL–OW)/(OL+OW)].
2.5 Statistical analysis
Length frequency distributions of individuals from the two sampling areas were compared using the Kolmogorov–Smirnov two-sample test.
The von Bertalanffy growth model was fitted to the estimated age-length dataset using a non-linear least-square procedure of a Gauss–Newton algorithm, included in the statistical package STATISTICA (version 14.0.0.15). The von Bertalanffy growth parameters (L∞, K and t0) were calculated for both fish populations sampled in the Messina’s Strait and Split area and compared by the multivariate Hotelling’s T2-test. The growth performance index (Ф’ = 2 log L∞ + log K) was then calculated to compare the different populations of the black scorpionfish throughout its distribution range.
Univariate and multivariate statistical methods were applied to conduct investigations on sagittae features of specimens from Messina and Split using Prism V.8.2.1 (Graph- pad Software Ltd., La Jolla, CA 92037, USA), R vegan package V.2.5, and PAST V.4.
The intra-population variability of sagittae morphometries, between the different size classes investigated, was detected using a one-way analysis of variance (one-way ANOVA) or Kruskal–Wallis one-way ANOVA, followed by Tukey or Dunn’s post-hot test respectively. A Principal Component Analysis (PCA) was conducted to obtain an overview of the differences in otolith parameters between the size classes examined within each population. Additionally, the correlation between the measured parameters and fish body weight (BW) and total length (TL) was tested using the Spearman correlation analysis. To explore the intra-specific variability of otolith contours within each population, the shape indices were extrapolated and analysed through an ANOVA-like permutation test and a PCA, to obtain an overview of the differences in otolith shape between the size classes examined at the intra-population level. The significance level of p-value was set at < 0.05.
Concerning the inter-population variability, a one-way analysis of variance, or a corresponding non-parametric test in case of non-homogeneity of the data, was used to verify the existence of any differences in the sagittae extracted between Messina and Split specimens, using the origin site of the samples as the only independent factor, followed by a PCA. For a more detailed description of the two populations examined, a Mann-Whitney test was used to explore the variability of individual otolith morphometric parameters, within each size class, between the sampling sites identified in the current study. Additionally, to obtain an overview of the sagittae features of the two populations under examination, a Linear Discriminant Analysis (LDA) was applied to the dataset. A cross-validation of the LDA was carried out to provide a classification of the individuals for both the investigated areas, assessing the classification success rate as the specimens’ percentage and number correctly assigned to the original sample. Finally, to investigate the inter-population variability of otolith contours between specimens from Messina and Split, the shape indices were extrapolated and analysed through an ANOVA-like permutation test and a Principal Component Analysis (PCA), to obtain an overview of the differences in otolith shape between the two analyzed populations.
3 Results
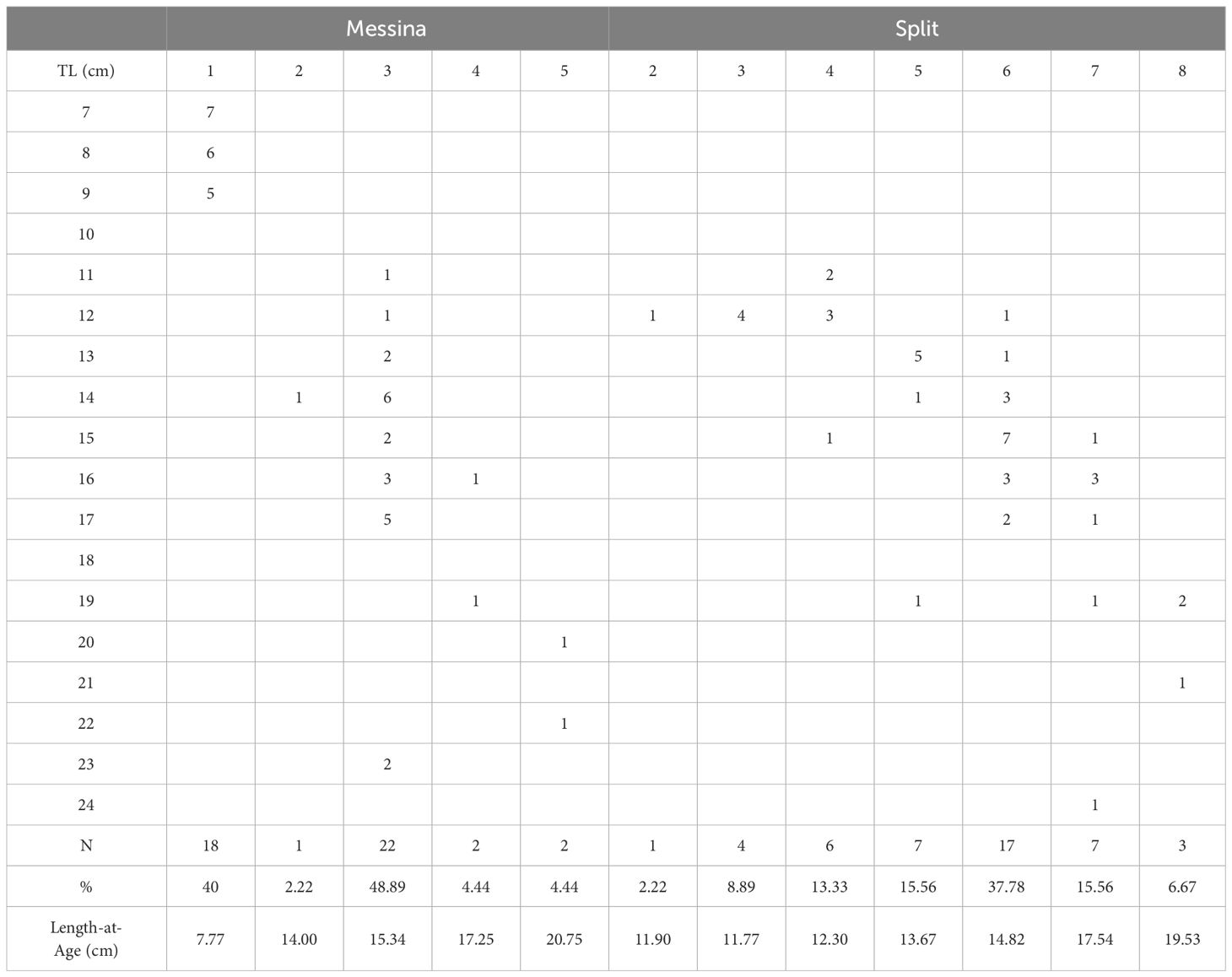
The 45 specimens from Messina showed a TL ranging from 6.5 to 23 cm, with a Mean TL ± SD of 12.61 ± 0.69 cm. Concerning Split area, specimens showed a TL ranging from 11.2 to 24.1 cm and a Mean TL ± SD of 14.71 ± 0.41 cm. Length-frequency distributions were significantly different between the two sampling areas (Kolmogorov–Smirnov test; P < 0.001).
3.1 Growth rates and age composition
Age counts were successfully provided for all the otoliths from Messina and Split, with no sample discarded, and a quite low variability of APE and CV indices (4.7% and 9.4%, respectively). The inner structure of sagittal otoliths consisted of a wide opaque nucleus, surrounded by an alternating pattern of translucent and opaque zones. Interpretation of otolith edge types revealed that translucent edge type was dominant in samples from Messina and Split (>90% in both areas). The age composition by area (Table 1) showed 3 to 5 years old as the main estimated ages (57.77%) for the Messina specimens, with a maximum age of 5 years. In Split area, the larger part of the specimens was between 5 and 8 years old (75.57%), with a maximum age of 8 years, showing that the specimens inhabiting the Messina’s tidal ponds was younger than that from Split.
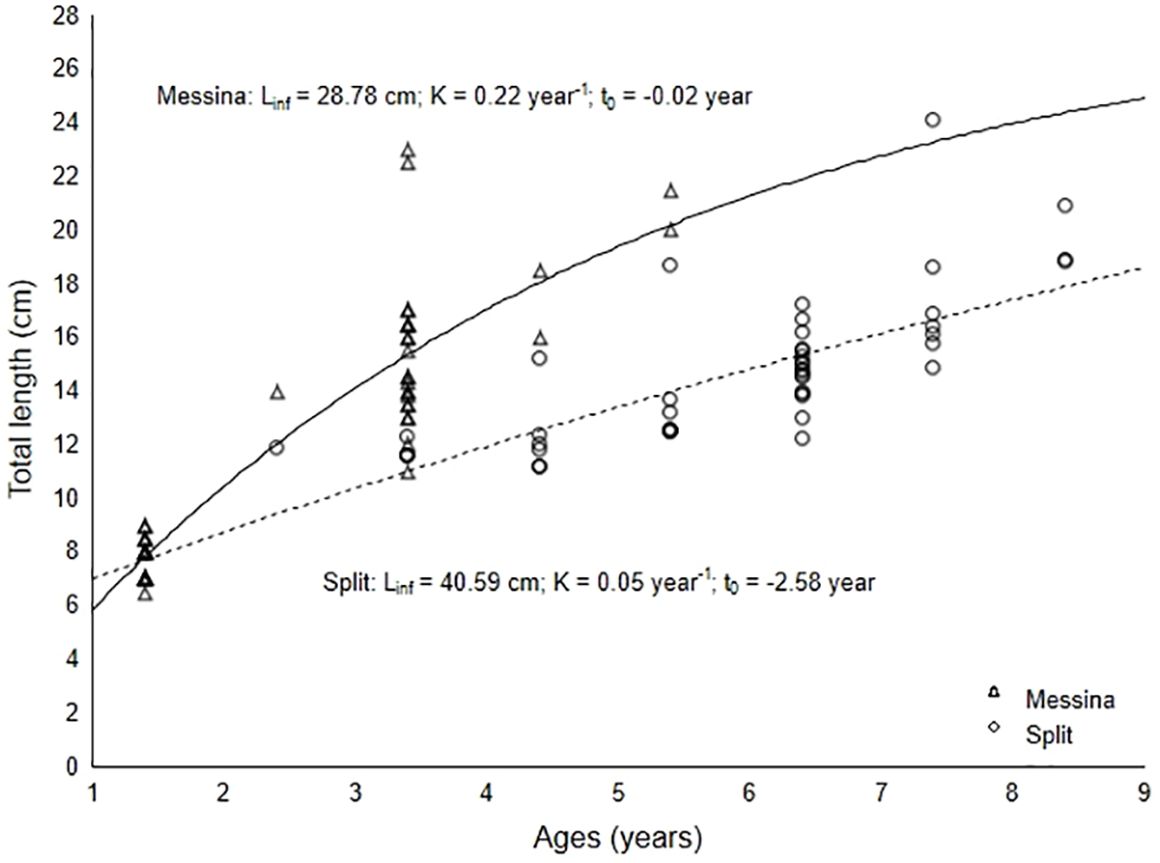
The von Bertalanffy growth curves were fitted to age-length dataset, estimated for individuals from the Messina Strait and Split area (Figure 2). Estimated parameters for S. porcus from the Messina Strait and Split area were L∞ = 28.78 cm, K = 0.22 year-1, t0 = -0.02 year (R2 = 0.89) and L∞ = 40.59 cm, K = 0.05 year-1, t0 = -2.58 year (R2 = 0.73), respectively. The growth performance index (Ф’) was 2.26 for the fish from the Messina Strait and 1.92 for the fish from the Split area. Specimens from Split Area showed a growth rate of slightly more than 1 cm per year between 4 and 7 years old.
The Hotelling’s T2-test indicated that the von Bertalanffy growth curves differed significantly between the two tested groups of individuals (T2 = 2885.92 > T20 (0.05, 3,86) = 12.20). The L∞ value was higher in fish from the Split area and the K value was higher in fish from the Messina Strait. Therefore, considering the growth coefficient and the growth performance index, fish from the Messina Strait grew faster than fish from the Split area. Statistical difference in length-at-age data (Table 1) was observed between the two investigated areas (t-test for paired comparison, P = 0.025).
3.2 Morphometric and shape analysis
Studied specimens showed an overall morphology of sagittae characterized by an oblong to lanceolate outline, with serrate to crenate margins. The anterior region was peaked, with rostrum, antirostrum and excisura ostii, that, together with the notch tilt angle, changed between the two populations in the analyzed size classes (Figures 3A–E). The posterior region was oblique to irregular, and the sulcus acusticus was heterosulcoid, ostial and median.
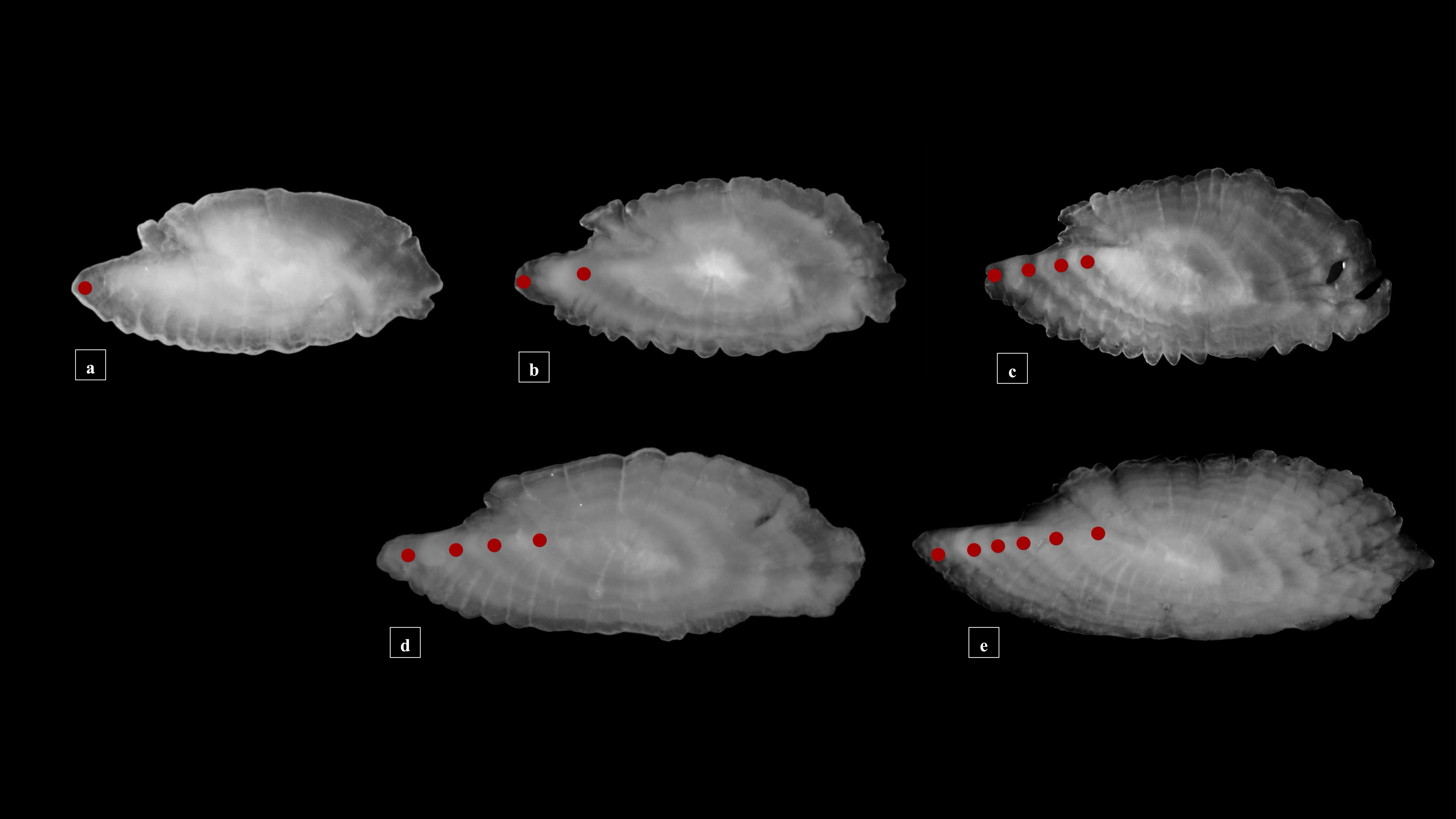
Figure 3 Stereoscope images of the medial view of the sagittae belonging to specimens from Messina (A-C) and Split (D, E) for the three investigated size classes [Class I: (A) Class II: (B–D) Class III: (C–E)]. Red dots indicate the end of each hyaline annulus in the reading area of each otoliths’ image.
In Supplementary Tables S1, S2 are provided the morphometric mean values of the left sagittae for the two investigated populations, with the minimum and maximum range, divided in the three size classes.
Sagittae belonging to Messina specimens showed an oblong shape, with an enhanced margins serration. The rostrum and the antirostrum were short, with an arrow excisura ostii, a not acute notch, and an irregular posterior margin (Figures 4A–C). Concerning the intra specific differences among size classes, morphometrical analyses showed an enhanced variability, confirmed also by the mean otoliths’ shapes (Figure 4D). Sagittae of specimens belonging to Size Class I showed a most circular contour, then the other classes. Also, the posterior region showed a peculiar organization, with a most oblique margin than the other size classes. In the Size Class II, the contour was less circular than the Class I, with most irregular posterior margin and less acute notch of excisura. The Size Class III showed a very different shape, with an enhanced ellipticity (as highlighted by the highest E value among size classes: 0.41 ± 0.02 mm, see Supplementary Table S1) resulting in a less wide and longer sagitta than the other size classes.
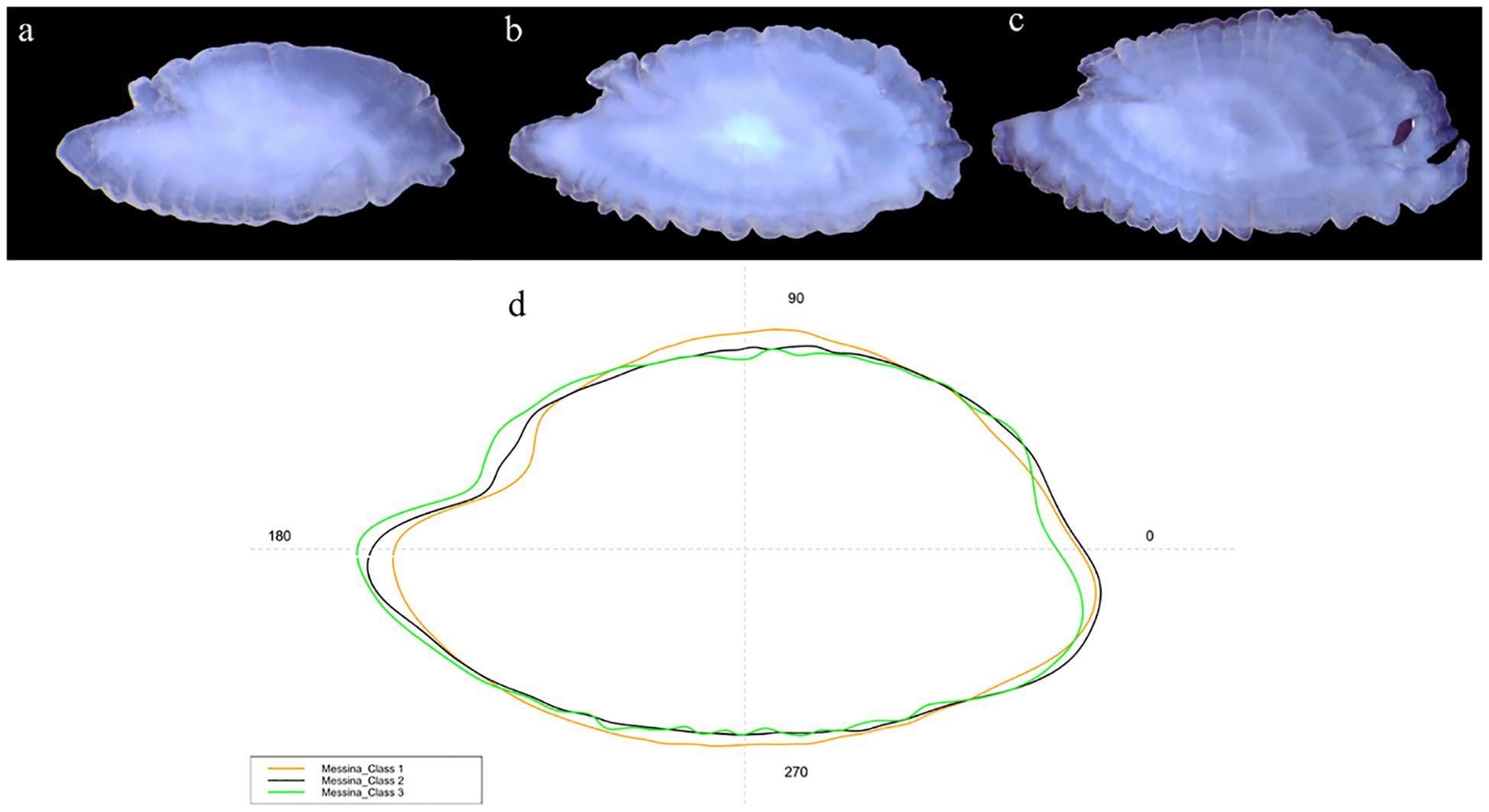
Figure 4 Stereoscope images of the medial view of the sagittae belonging to specimens from Messina, for the three investigated size classes [(A) Class I, (B) Class II, (C) Class III], with their mean otoliths shape (D).
Overall, the specimens examined showed significant variations in otoliths’ parameters between the size classes considered in the study.
The results of the differences are reported in Supplementary Table S3, showing the absence of significant differences for 90% of the parameters between Class II and Class III. Concerning the comparison between Size Class I and II, and between Size Class I and III, significant differences were detected for all the investigated morphometrical parameters, except for Re values.
PCA confirmed the results obtained by univariate analysis (Figure 5), explaining the main differences observed between the classes I vs II and I vs III.
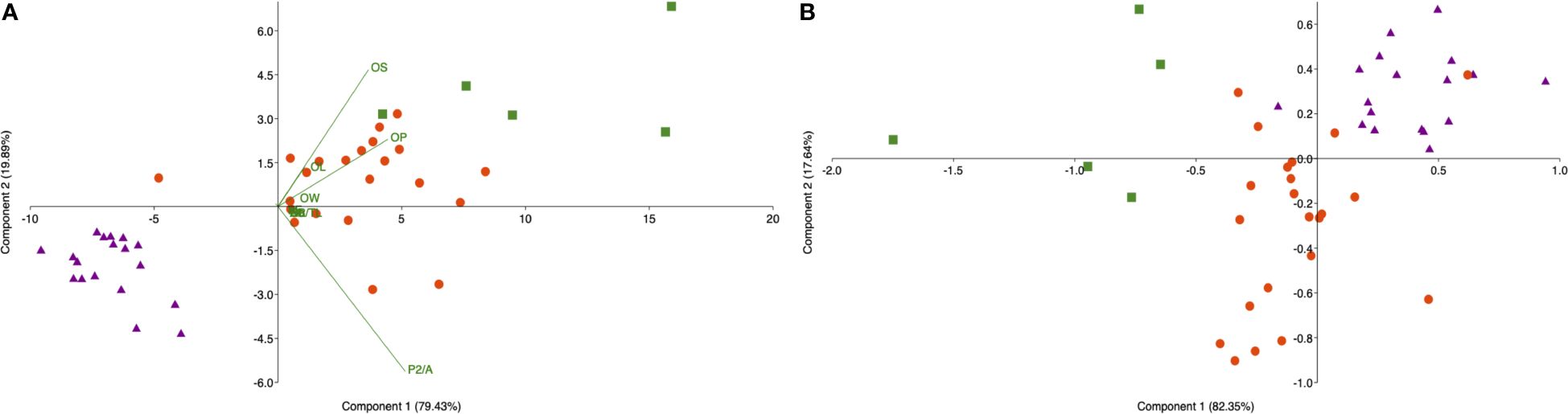
Figure 5 Principal Component Analysis (PCA) plot between the specimens belonging to size classes I (fill triangle dark magenta), II (dot orange red) and III (fill square forest green), calculated on sagittae parameters of Messina population (A); Principal Component Analysis (PCA) plot between the Messina’s specimens belonging to size classes I (fill triangle dark magenta), II (dot orange red) and III (fill square forest green), calculated on elliptic Fourier descriptors (B).
All sagittae measurements showed a significant correlation with specimens’ parameters TL and BW. Results of Spearman correlation analyses are reported in Supplementary Table S4.
The mean shape of otoliths differed significantly between the three size classes investigated (p< 0.001). Marked differences in otolith shape have also been confirmed by PCA, mainly between the classes I and III (Figure 5B).
Concerning sagittae belonging to Split specimens, they showed a lanceolate contour, with a marked irregularity of margins. The rostrum and antirostrum were long, with a wide excisura and a very acute notch (Figures 6A, B). Concerning the intra-specific differences among size classes, shape analysis showed a general uniform shape (Figure 6C). Class II was characterized by a more enhanced circular contour than the other class, with an oblique posterior margin and an acute notch. In Class III the sagittae were more elliptic than the Class II, as highlighted by the highest E value (0.41 ± 0.02 mm, see Supplementary Table S2), with an enhanced length and a reduced width.
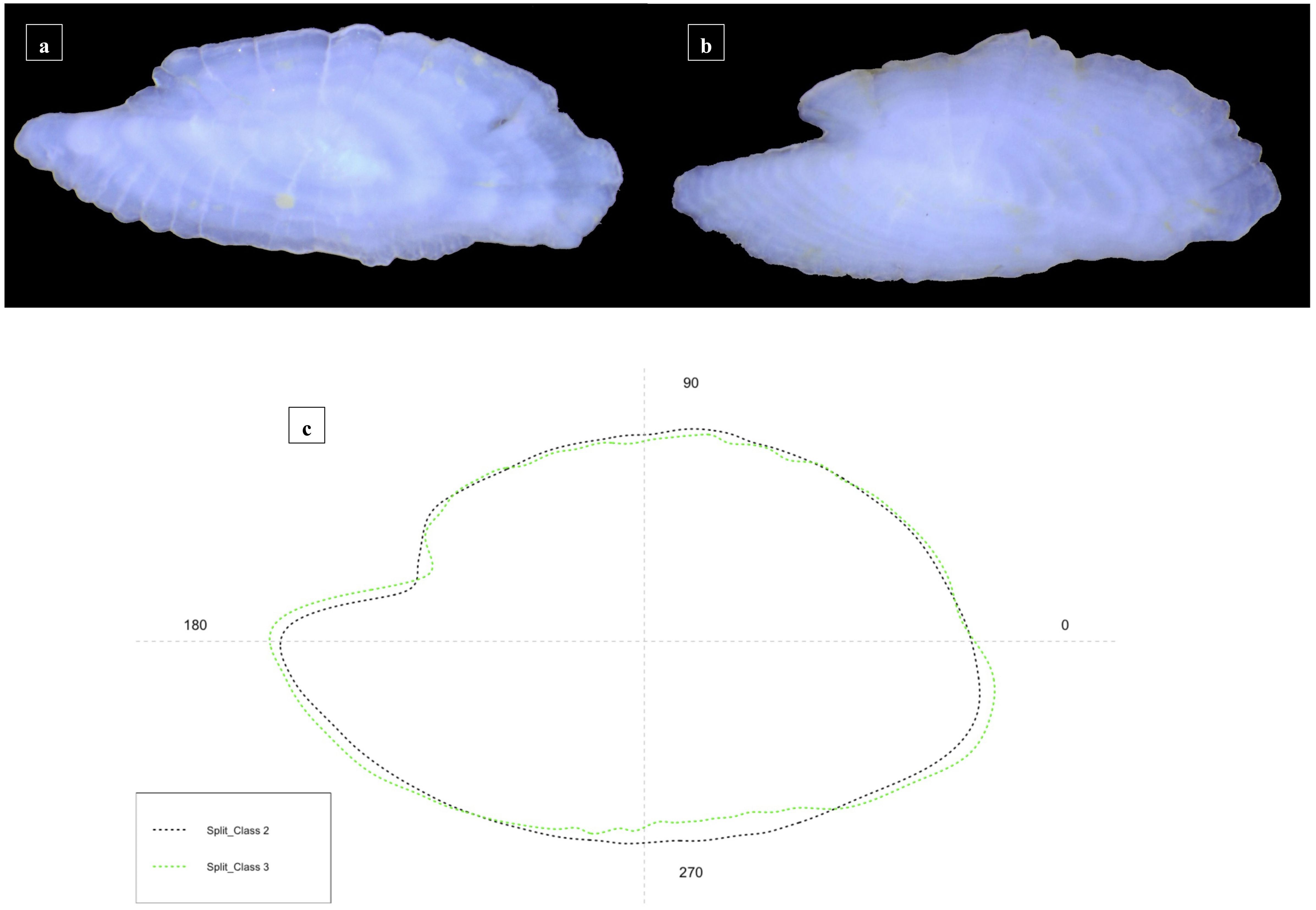
Figure 6 Stereoscope images of the medial view of the sagittae belonging to specimens from Split, for the two investigated size classes [(A) Class II, (B) Class III], with their mean otoliths shape (C).
The specimens examined showed overall a significant variation in sagittae parameters between the two size classes investigated in the study (p<0.05).
The results of the variations observed for the individual parameters of the otoliths extracted from each size class are reported in Supplementary Table S5. Significant differences between the two size classes (Size Classes II and III) of the Split population were detected for all the investigated morphometrical parameters, except for Re values.
PCA confirmed the results obtained by univariate analysis (Figure 7A), explaining the main differences observed between the size.
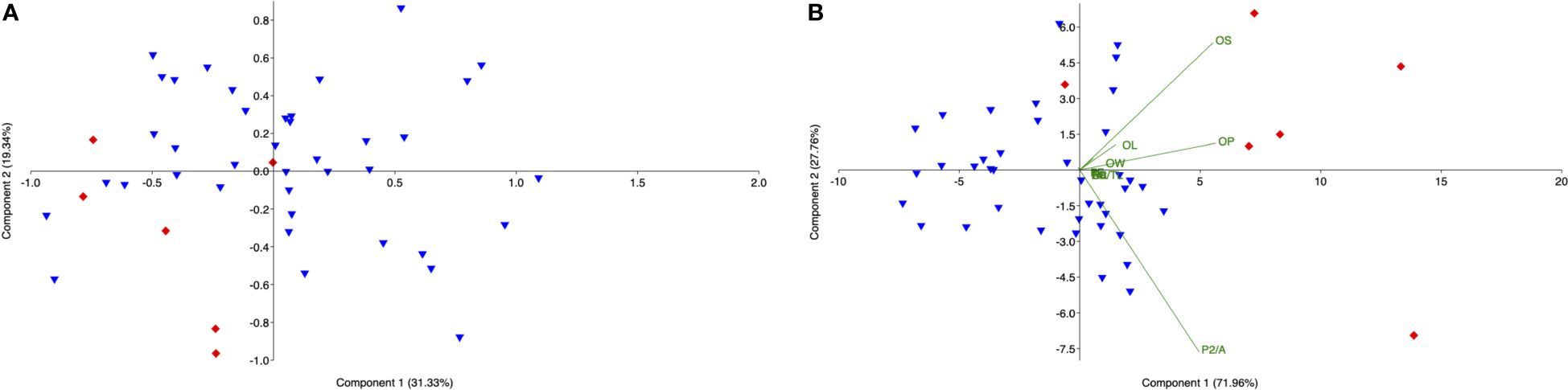
Figure 7 (A) Principal Component Analysis (PCA) plot between the specimens belonging to size classes II (Blue fill inverted triangle) and III (Red fill diamond), calculated on sagittae parameters from Split population; (B) Principal Component Analysis (PCA) plot between the specimens belonging to size classes II and III, from Split calculated on elliptic Fourier descriptors.
Almost all sagittae measurements showed significant correlation with specimen parameters TL and BW. Results of Spearman correlation analyses are reported in Supplementary Table S6.
The mean shape of otoliths differed significantly between the two size classes investigated (p< 0.009). Slight differences in otolith shape have also been confirmed by PCA (Figure 7B).
3.3 Geographical differences between sagittae features
Concerning the number of individuals compared between the two zones, specimens belonging to Class III were much lesser than those belonging to Class II in both the studied areas. Overall, despite quite an overlap between the two areas, the two groups of individuals examined were different both for fish parameters and sagittae features (P<0.05), as well confirmed by PCA analysis (PC1 96.10%; PC2 3.51%) (Figure 8).
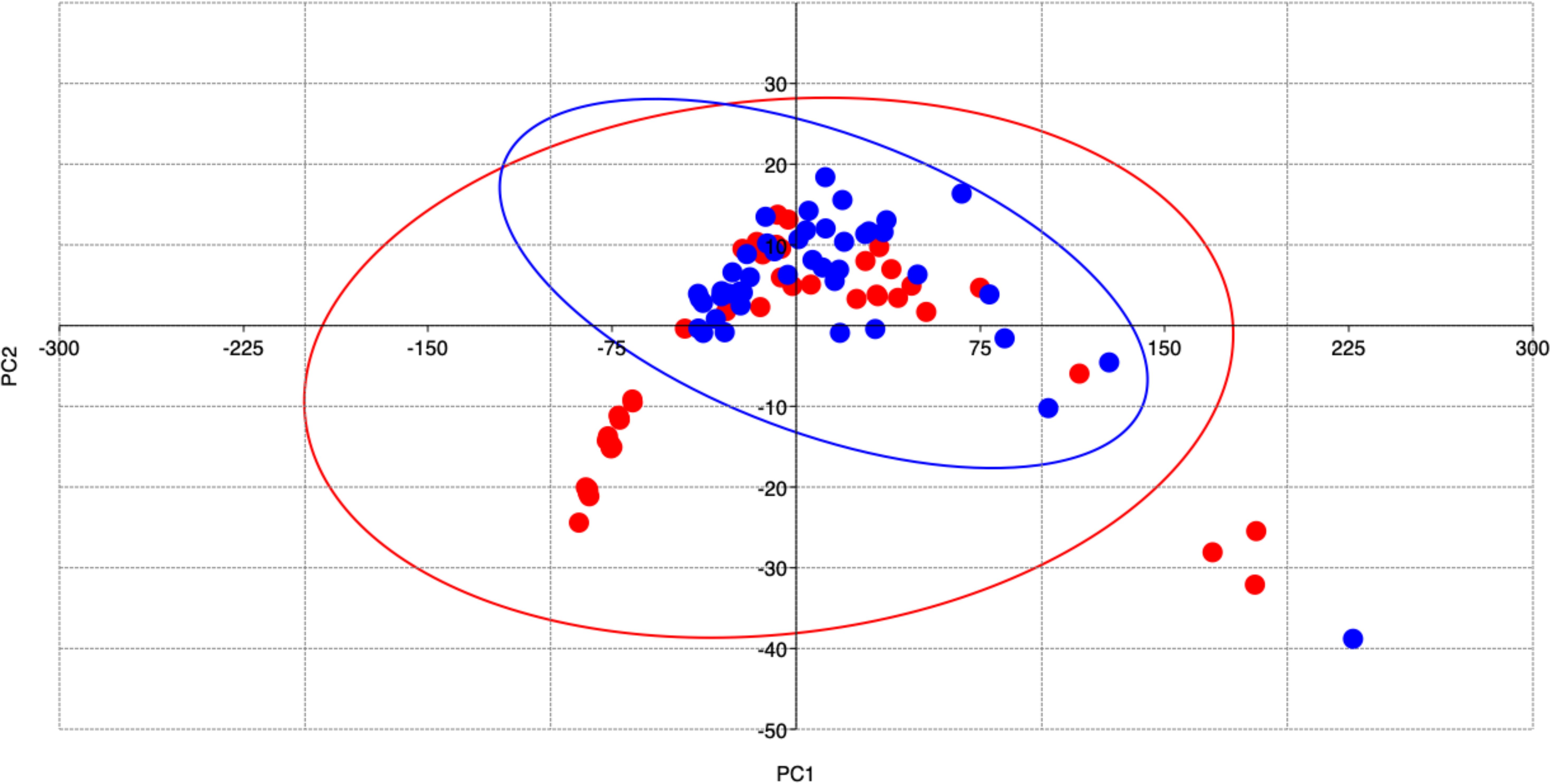
Figure 8 Principal Component Analysis showing fish and sagittae features differences between the sampling sites: Messina (red dots) and Split (blue dots).
Class I of the specimens from Messina was eliminated from subsequent analyses, as it was not comparable to a class equally represented in the other group from Split.
The results obtained through the Mann-Whitney test are reported in Table 2, showing, for the compared size Classes III, the presence of significant differences only for the Ro shape index.
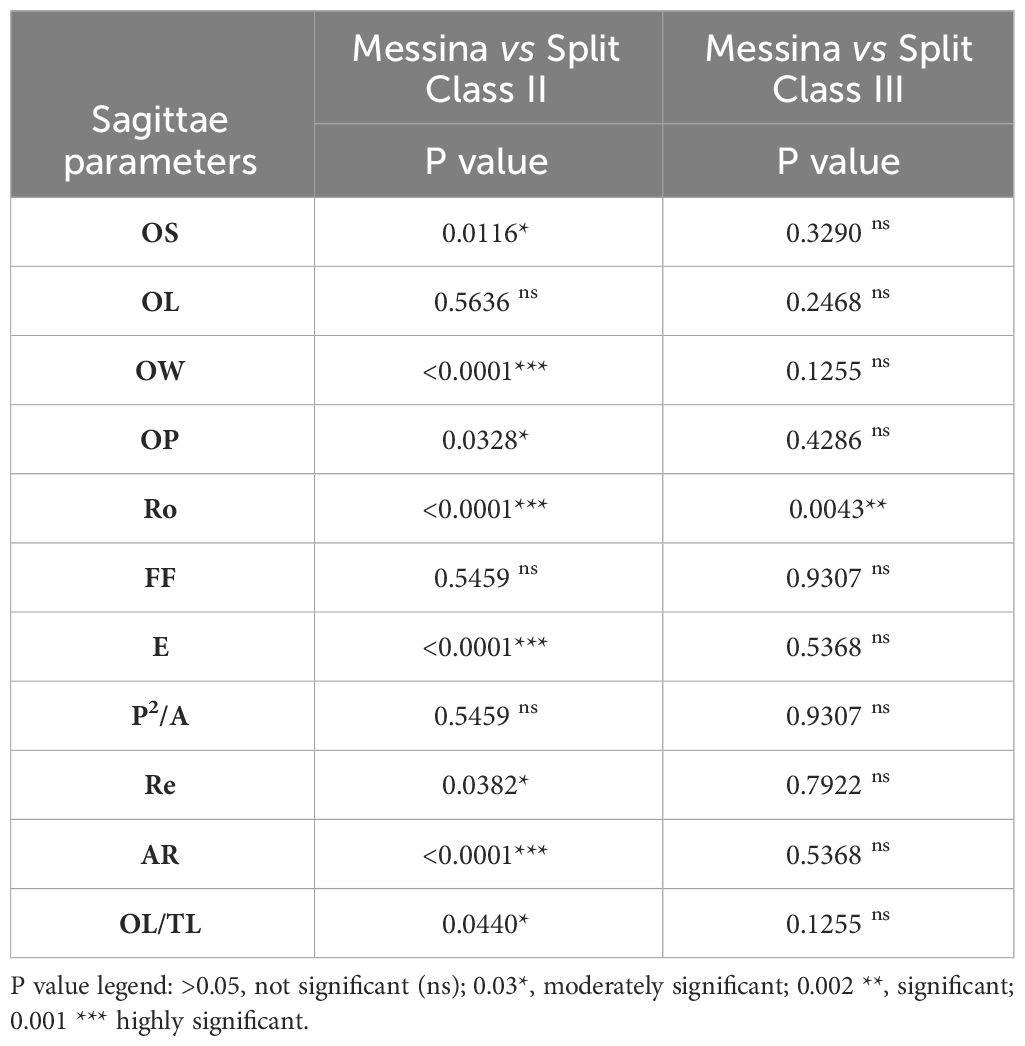
Table 2 Mann Whitney test results obtained for otoliths’ morphometric parameters comparison the same size classes from different sampling sites, Strait of Messina, and Split.
As highlighted by the LDA (Figure 9), the major differences concerned size classes II. An overall successful classification rate of 83.12% was achieved, concerning the LDA, for both the size classes combined (Supplementary Table S7). Specimens belonging to Class II showed the highest classifications, with values of 90.9% (from Messina) and 89.7% (from Split). Specimens belonging to Class III showed slightly lower values: 83.3% from Split and 80% from Messina.
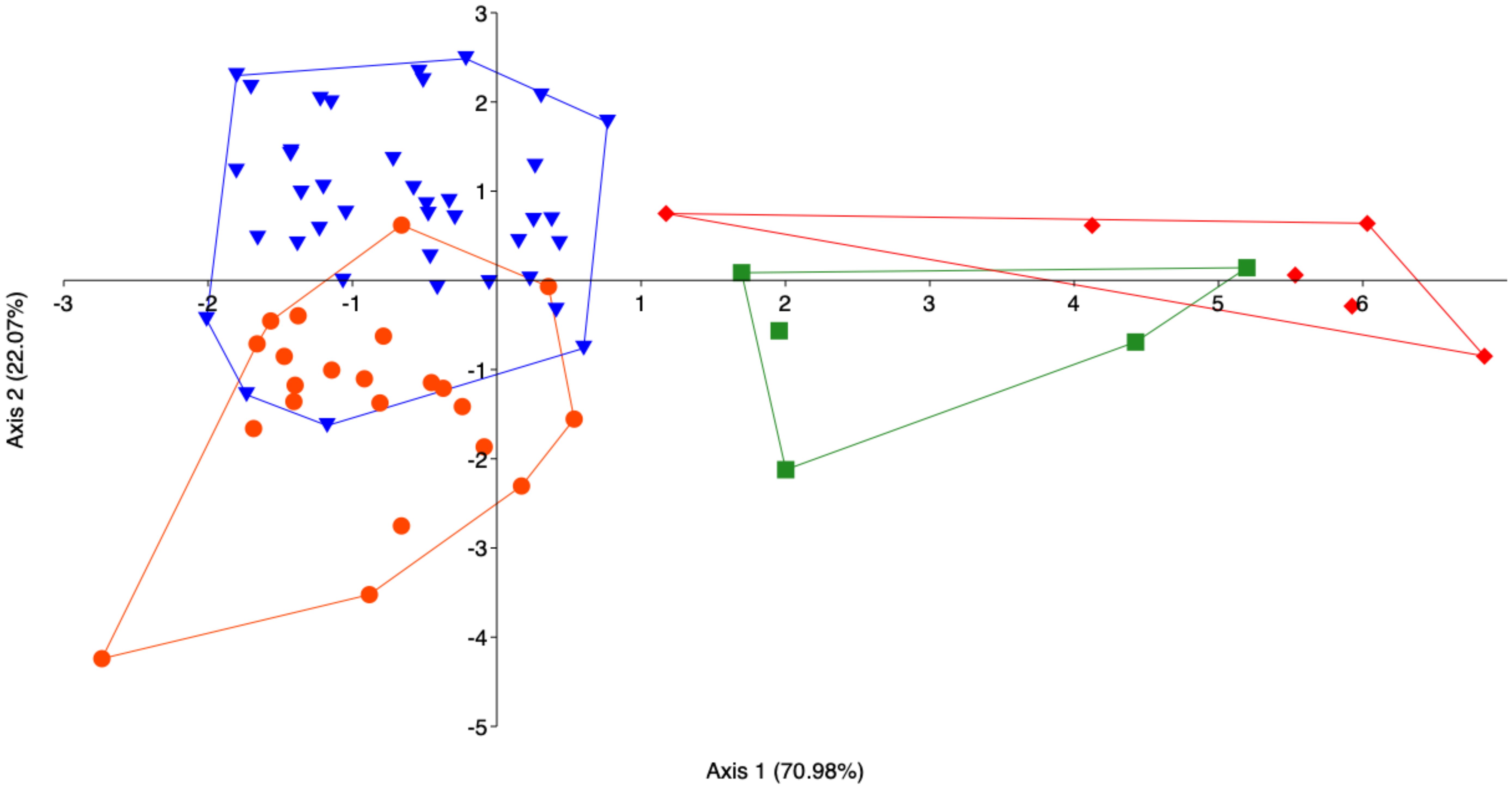
Figure 9 Linear Discriminant Analysis (LDA) between morphometric parameters (OL, OW, OP, OS, OL/TL, C, Re, E, AR, FF, Ro) of the investigated populations for the size classes II (orange-red dot Messina, Blue fill inverted triangle Split) and III (Forest green fill square Messina; Red diamond Split).
The analysis of the sagittae contours of the two populations examined showed an evident variability between the specimens of class II (p=0.001) and class III (p=0.017). Results of PCA and mean otoliths shapes confirmed the differences observed for each class between the sampling sites, showing a variance of 41.75% (PC1) and 17.16% (PC2) for Class II, and 53.67% (PC1) and 17.5% (PC2) for Class III (Figures 10A, B).
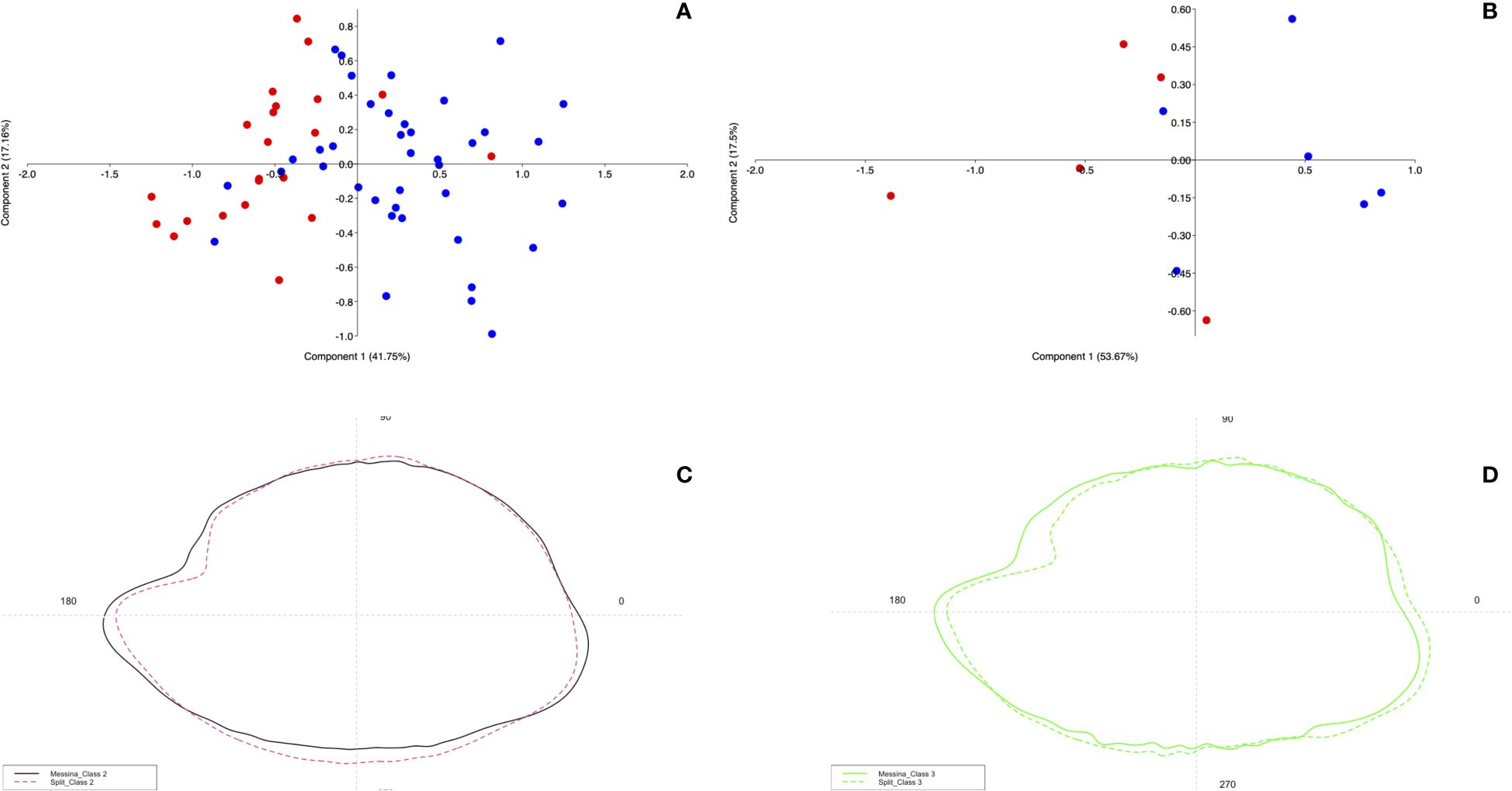
Figure 10 Results of PCA performed on Wavelet coefficient obtained by sagittae contours of specimens collected from Messina (red dots) and Split (blue dots). Results are reported for Class II and III, figures (A, B) respectively. Wavelet coefficient variance for each size classes investigated is expressed as percentage. Comparison of the mean otoliths shapes from the two populations is provided in figures (C, D) respectively for Class II and III.
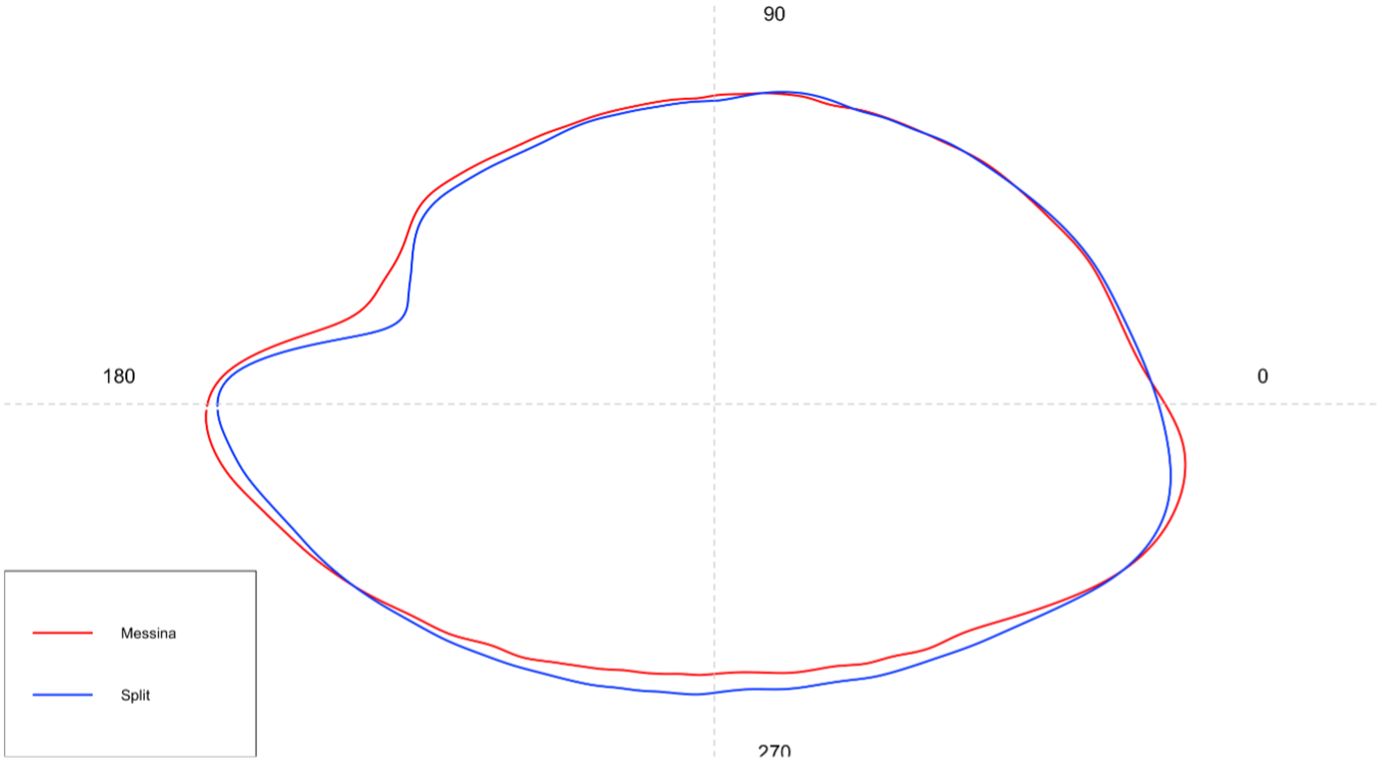
In Figures 10C, D is provided a comparison of the mean otoliths’ contours for the three investigated size classes. Specimens from Class II showed visible inter-population differences regarding rostrum and overall mean contour, oval in individuals from Split and elliptical in those from Messina. In the Class III, the differences detected for the second size class were confirmed, with more marked excisura ostii showed by Split specimens than Messina ones, which reported a less marked irregularity of the margins, a shorter rostrum and a less pointed antirostrum.
The shape analysis performed on the total interclass samples showed mean sagittae shapes clearly different between the two analyzed populations (Figure 11). The main differences were detected in the rostrum organization (longer and more pointed in Split population than in the Messina one), in the excisura ostii (deeper in Split population than in the Messina one), in the antirostrum organization (more prominent and pointed in Split population than in the Messina one), and in the overall sagittae shape, oval in Split specimens and elliptical in Messina ones.
3.4 Stomach content analysis
The analysis of the 49 sampled stomachs from Messina (9 empty stomachs, with a Vacuity Index of 4.9) has shown a total of 80 preys, belonging to 29 taxa. As reported in Table 3, Crustacea and Osteichthyes were the major taxa with the highest number of preys, with also the highest values of IRI %. The infraorder Brachyura was the taxon which showed the highest IRI % value (IRI % = 32.81), followed by the species, belonging to the infraorder Brachuyra, Pachygrapsus marmoratus (Fabricius, 1787) (IRI % = 27.59), that was the taxon with the highest relative abundance (N % = 18.75) among the preys. The order Amphipoda (IRI % = 10.74) and the species Xantho pilipes (A. Milne-Edwards, 1867) (IRI % = 9.86), belonging to the infraorder Brachuyra, were the other two most relevant taxa for relative importance. Concerning the Osteichthyes preys, those belonging to the species Chelon auratus (Risso, 1810) (IRI % = 3.24) and Thalassoma pavo (Linnaeus, 1758) (IRI % = 2.36) were the most relevant preys, with the genus Parablennius as taxon with the highest relative abundance value (N % = 2.50). Concerning the class Polychaeta, it was the major taxa with the less relative importance (IRI % = 0.15).
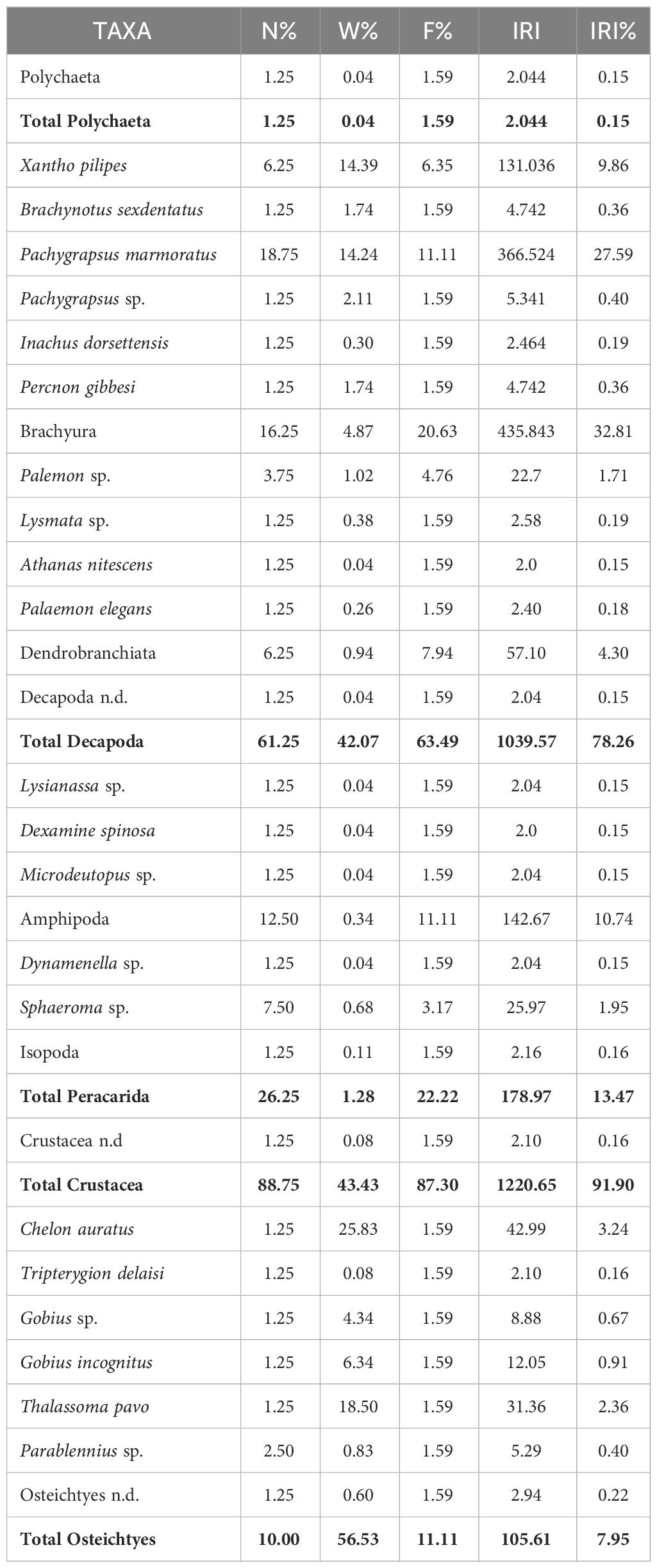
Table 3 Diet composition of S. porcus individuals with %N (relative abundance), %W (percentage in biomass), %F (frequency of occurrence), IRI (index of relative importance) and %IRI (index of relative importance expressed as percentage) expressed for each prey items.
4 Discussion
Data provided by the present work indicated a geographical variability for some biological features of S. porcus inhabiting two different biogeographic districts of the Mediterranean Sea: the Central Mediterranean Sea (Strait of Messina) and the Adriatic Sea (Split area) (Spanò and De Domenico, 2017). The relevant differences mainly involved the sagittae features of the black scorpionfish, also suggesting the reliability of otolith shape and morphometrics analyses as a useful tool for its populations’ discrimination.
Concerning growth and age composition, our preliminary observations indicated an estimated maximum age higher in Split (8) than in Messina (5), with the von Bertalanffy growth curves which have shown K parameters higher in Messina, and L∞ parameters higher in Split. The age composition was very different, with specimens from Messina that were mainly composed of individuals belonging to the third age class (3), with length-at-age values significantly higher than those shown by those from Split. All these data indicated a faster growth rate of the studied species in the Strait of Messina, as also highlighted by the growth performance index, which was higher in Messina specimens than Split. The high degree of population separation in S. porcus was widely reported, as highlighted by the differences in growth parameters and age composition detected between literature data from different geographical areas (Siblot-Boutéflika, 1976; Bradai and Bouain, 1988; Jardas and Pallaoro, 1992; Koca, 1997, 2002; Silvestri et al., 2002; Passalacqua et al., 2005; Bilgin and Çelik, 2009; La Mesa et al., 2010; Kendallha et al., 2018; Kutsyn et al., 2019; Sahin et al., 2019; Ferri and Matić-Skoko, 2021) (Table 4). Indeed, the overall comparison of the growth studies on this species from the Mediterranean basin highlighted a variability of Ф’ index between 1.92 and 2.3 (excluding the extreme value of 2.71 reported by Bilgin and Çelik, 2009, being based on an unrealistic L∞ of 140 cm). The Ф’ values shown by results were within the range of the previous studies, but with extreme values (1.92 and 2.26), reflecting the plasticity of S. porcus growth. The L∞ values reported by results were also in line with previous literature, in which they range from 19.8 and 60.94 cm. The only parameter not in line was the t0 from Messina specimens, lower than those reported by literature data from other Mediterranean geographical areas.
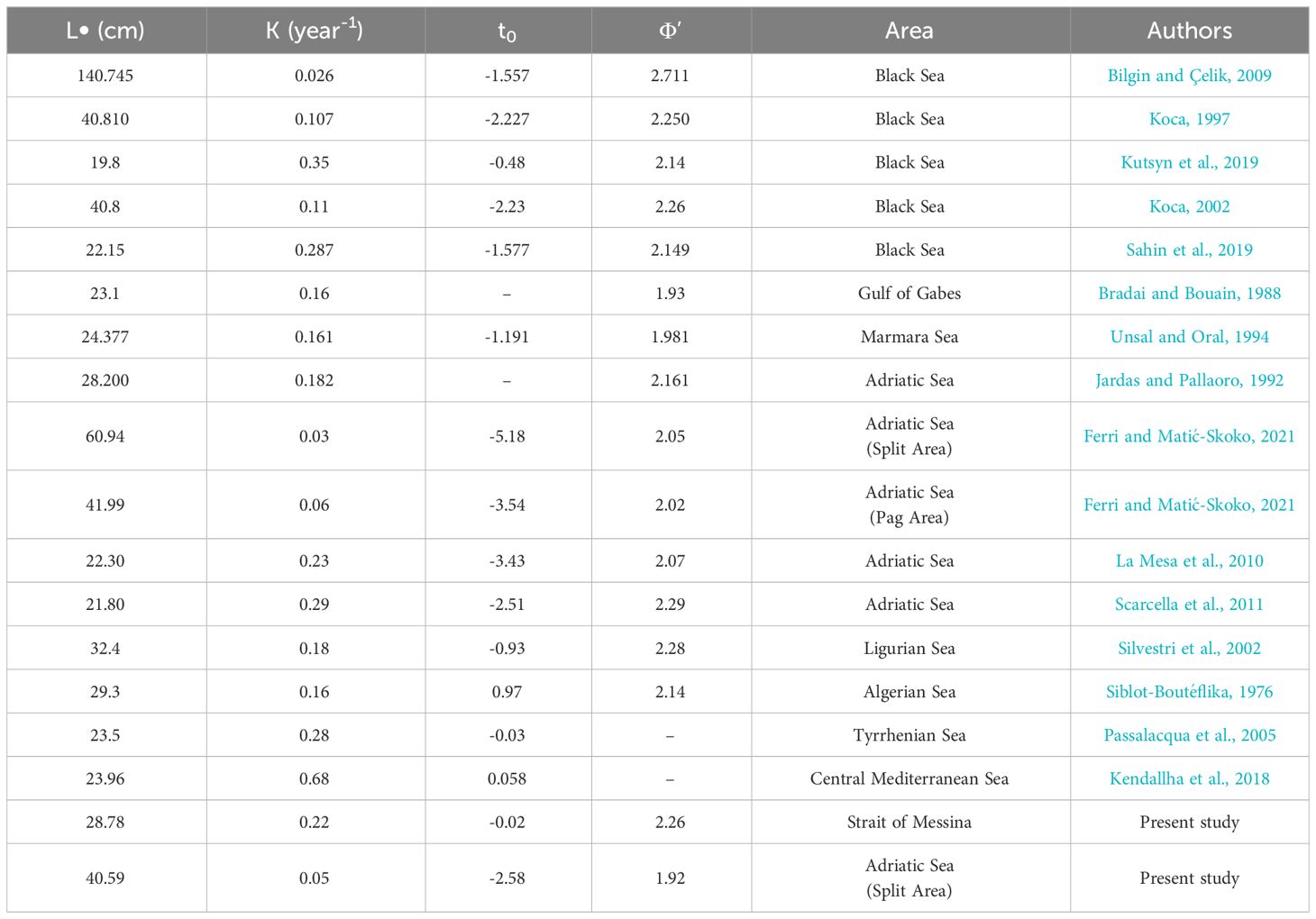
Table 4 Von Bertalanffy growth parameters and derived growth performance index (Ф’) of S. porcus from literature and the present study.
Despite this, it must be considered also the effect of the length composition of samples from the two studied areas, influencing the curve trajectory of the younger age classes, and, consequently, the reconstruction of the entire growth rate. Further analyses with wide length classes composition, especially regarding the Messina population, are required to confirm the differences reported by results for the growth rate in the two studied geographical areas. S. porcus is a low-range benthic species with a high site fidelity capable of exhibiting a high phenotypic heterogeneity in its biological traits, often responding to local environmental conditions (Özgül et al., 2019). The Strait of Messina represents a singularity inside the Mediterranean Sea, with a unique hydrographic regime and a peculiar seawater masses chemistry (Cucco et al., 2016; Savoca et al., 2020). It is located at the junction area between the Ionian and Tyrrhenian Sea. The meeting of these two water masses, different in physiochemical properties, occurs in an area with an enhanced morpho-bathymetrical irregularity of the bottom, resulting in a mixing process of the water masses, with strong currents regulated by tidal phases (Cucco et al., 2016; Longhitano, 2018). This intense hydro dynamism, with the massive presence of “upwelling” events, and the peculiar waters’ physio chemistry, have meant that this area has become an “Atlantic Island” inside the Mediterranean basin (as highlighted by the oxygen concentrations, nutrients, and temperature similar to those reported for the Atlantic Ocean) and a hot spot of biodiversity, with the presence of unique biological communities (De Domenico, 1987; Cortese and De Domenico, 1990; Spanò and De Domenico, 2017). Otherwise, the Adriatic Sea generally shows a pronounced seasonality and an enhanced longitudinal gradient in dissolved oxygen, temperature, nutrients, salinity, and chlorophyll-a (Lipizer et al., 2014), with urbane discharges, aeolian inputs, underground waters and surface runoff which represent the main nutrients sources. The eastern Adriatic Sea, to which Split area belongs, is outflowed by the East Adriatic current, bringing Levantine Intermediate Water from the Ionian Sea (Orlić et al., 2006; Zorica et al., 2019), with the water column characterized by a high homogeneity during winter, becoming more stratified during Summer (Vilibić and Orlić, 2001). According to literature, different oceanographic features can influence the growth of species, resulting in a faster growth in populations inhabiting cold and productive water masses, than those from less productive and warmer ones (Kikuchi et al., 2021). In addition to the oceanographic differences between the water masses, also the environmental conditions experienced by the studied groups in the two different habitats were completely different. Specimens from Messina were sampled in the tidal ponds present in the beach rock formations, with reduced depth and large variations, also daily, of both biotic and abiotic conditions, related to tidal cycles and storms (Capillo et al., 2018). This results in marked physiochemical differences between the two studied areas, with higher temperature and salinity values in tidal pools from Messina than those from Split, probably allowing for the higher growth rate and mean length at age found in specimens from Messina than those from Split. Indeed, tidal pools represent an “extreme” environment, and they are considered a perfect case of study to explore the behavioural, physiological and morphological adaptations of marine species to peculiar hydrographic and ecological conditions (Gomes et al., 1998; Capillo et al., 2018; Savoca et al., 2020). They are a shelter from the intense hydro-dynamism affecting the area, a nursery area and a perfect hunting ground for many predators, S. porcus included. Indeed, beach rock represents the only natural substrate for benthic species present at these depths (Spanò and De Domenico, 2017), and a shelter from the strong currents that make the coastal areas of the Strait of Messina a difficult environment for habitat exploitation (Gravem and Morgan, 2017). This biotope hosts very peculiar benthic communities, with substantially different species composition and richness to those found in other similar Mediterranean environments (Spanò and De Domenico, 2017). It also houses extended vermetid formations (protected by the European Community) with a unique arrangement, being on the substrate surface, and not in the typical trottoir formation, as in the rest of the Mediterranean Sea (Ingrosso et al., 2018). Conversely, the biotope of the coastal waters near Split is the classical environment inhabited by the studied species, and other Scorpaenids, in the Mediterranean Sea. It is characterized by depths ranging from 10 and 40 m, the presence of rocky bottoms covered by algae, sandy bottoms, and patches of P. oceanica, with a relatively high biodiversity (up to 27 different recorded fish species, belonging to Sparidae, Mullidae, Mugilidae, Centracanthidae, Labridae and Gobiidae families) (Institute of Oceanography and Fisheries, 2023). Specimens from Split here analyzed were sampled at an average depth of 20 m, very different from the 2-3 m depth of the tidal ponds of Messina’s beach rock formations. According to the literature, these differences in biotic and abiotic environmental conditions could drive the geographical variability in growth shown by results between the two studied areas (Bacha et al., 2010; Lek et al., 2012; Ferri and Matić-Skoko, 2021).
It is well known that temperature can influence the metabolism, and consequently the growth rates, of several teleost species (Atkinson, 1994; Kozłowski et al., 2004; Jobling, 2011), but also food availability and quality can shape them (Mommsen, 1998; Bacha et al., 2010). Concerning diet composition, a comparison between the data provided by the stomach content analysis of the specimens from Messina, and the literature data regarding the studied species from Split, showed a similarity between the feeding habits of the black scorpion fish in the two areas, especially regarding the major taxa of the main preys. Results from the stomach content analysis on specimens sampled in Messina showed a high selectivity for decapods’ preys, as assessed also for other Scorpaenidae species from other geographical areas (Harmelin-Vivien et al., 1989; Başçïnar and Sağlam, 2009; Compaire et al., 2018; Aydin and Mazlum, 2020; Ferri and Matić-Skoko, 2021). The high relevance of brachyuran decapods (e.g., X. pilipes, P. marmoratus, Brachyura) reported by results was in line with literature data on the studied species, but some differences regarding the contribution of teleost fishes, peracarids crustaceans and molluscs were detected. The differences in feeding habits with S. porcus specimens from Split mainly consisted of the complete absence of Molluscs and Anomura from the diet of Messina’s specimens. According to the literature, these prey items represent an important source of food for Split population, together with teleost fishes and Caridea decapods, which showed a reduced occurrence in Messina specimens (Ferri and Matić-Skoko, 2021). Concerning peracarids crustaceans (e.g., Amphipoda and Isopoda), they were well represented in the stomach content from Messina, but they are very marginal in the diet composition reported in literature from Split. This variability in peracarids’ preys could be strictly related to the biocenosis present in the tidal pools of the beachrock formations of Messina, rich in Amphipods and Isopods (being the perfect habitats for them) (Dias, 2013). Indeed, the presence of these crustaceans in the diet of this Scorpaenidae species has been assessed also in other similar formations present in Spain (Gulf of Cadiz) (Compaire et al., 2018). The wide variability in feeding habits between groups of individuals inhabiting different geographical areas probably reflects the capability of this species to adapt its diet according to the prey’s availability, indicating a generalist and opportunistic feeding strategy (Harmelin-Vivien et al., 1989; Başçïnar and Sağlam, 2009; Rafrafi-Nouira et al., 2016; Compaire et al., 2018; Aydin and Mazlum, 2020; Ferri and Matić-Skoko, 2021). This is an essential feature which allowed this species to inhabit a wide range of Mediterranean areas and habitats. Further analyses on a wider temporal scale, with a larger samples’ number, representative of the different ontogenetic classes, are required to better reconstruct the feeding habits and diet composition of S. porcus specimens, during the entire year, in the tidal ponds of the Strait of Messina. This is essential to understand how much diet variations, added to the other genetic and environmental features, can allow for the geographical differences in growth rates between the two groups of individuals highlighted by results.
Fish growth and metabolism, such as biotic and abiotic habitat features, can also influence otoliths growth and, consequently, their morphometry and shape (Grønkjær, 2016). Lifestyle, diet composition, food quality and feeding fitness have a role in otoliths’ morphology, shape, growth patterns and physiology (Popper and Lu, 2000; Popper and Schilt, 2008; Schulz-Mirbach et al., 2019). According to the literature, otoliths’ shape and morphometry are influenced by water temperature, depth (e.g., the size of otoliths increases with greater depths and warmer water masses), genetics and lifestyle of the species (e.g., epipelagic species show smaller and more elongated sagittae than benthic ones) (Lombarte and Lleonart, 1993; Lombarte and Cruz, 2007; Volpedo et al., 2008; Lombarte et al., 2010; Tuset et al., 2015). Thanks to this high sensitivity to environmental conditions, otoliths (especially sagittae) have become an essential tool in fish stock assessment and population discrimination. This was confirmed by the results of sagittae analyses. The overall morphology and shape of both studied groups of individuals were in line with data from the literature, with several differences in morphometry. Tuset et al (Tuset et al., 2008). reported rectangularity and circularity values, for specimens from the western and central Mediterranean Sea, different from those of both Split and Messina specimens, with a most marked rectangular overall shape of the sagittae than the studied ones; while Yedier et al. (Yedi̇Er and Bostanci, 2021). reported, from the Black Sea, Aegean Sea and Sea of Marmora, different shape indices values, e.g., roundness, ellipticity and aspect ratio, with a most marked elliptical shape of the sagittae than the studied ones. This shape heterogeneity of sagittae was also evident when comparing Split and Messina specimens. As highlighted by the overall contours and the statistical analysis on the morphometrical parameters between the two groups of individuals, specimens from Split showed wider, double-picked (with an enhanced antirostrum) more circular sagittae, than those from Messina. Otherwise, these last showed more lanceolate, larger, with a most marked rostrum, sagittae, characterized by higher otolith surface values and a more elliptical than circular shape. Based on the obtained results, the morphology and morphometry of the sagittae from both the analyzed geographical areas showed peculiar features, different from those reported in the literature from other ones, highlighting the high geographical heterogeneity of the studied species’ otoliths.
Several factors could have induced these differences, being otoliths under a double control of genetic and environment, and sensitive to variation in physiological and metabolic individuals’ conditions. According to Vignon and Morat (Vignon and Morat, 2010), different environmental factors can reshape the overall sagittae outlines, while genetic variations at the intra-specific level, related to the long-time separation between the populations, only influence the shape of the otoliths locally (mainly at rostrum and antirostrum level). Concerning the environmental conditions experienced by the two groups of individuals, one of the most evident differences between the two sampling areas was the depth range, significantly higher in Split than in Messina. Indeed, it is widely reported how species and populations inhabiting deeper habitats show wider, more circular sagittae than those from shallower ones (Lombarte and Lleonart, 1993; Monteiro et al., 2005; Lombarte and Cruz, 2007). This finding is in line with results, which showed also a most lanceolate sagittae in specimens from Messina, inhabiting a very shallow environment, then those from Split. The soundscape can be another factor that can strongly influence the otolith morphology and development. Indeed, according to the sensory drive hypothesis postulated by Endler (Endler, 1992, 1993), there is a coevolution between detected signals and sensory systems, with the speciation that may be strongly influenced by the diversification of the organisms’ sensory interactions and environment. This is strongly evident in deep species and in species that use sound to communicate (such as rockfishes) (Tuset et al., 2016b). The soundscapes experienced by individuals drive a selection on the form-function of the fishes’ auditory system, with species or groups of individuals sharing a similar surrounding soundscape that could express an inner ears’ phenotypic similarity, expressed by otolith morphology and shape (Bose et al., 2020; Chapuis et al., 2023). This could be also the case of the two analyzed groups of individuals of S. porcus, with the differences related to the soundscapes of the two different studied areas that may have also influenced the detected sagittae variability in morphology, morphometry and shape. Also, metabolic rate and somatic growth can influence otolith features, such as morphometry, size and shape (Popper et al., 2005; Schulz-Mirbach et al., 2019). Messina specimens showed faster growth than the Split ones, which (if confirmed by the further analyses required to deeply investigate the growth rates of the studied species in the two studied areas) could be related to several environmental factors, such as the most enhanced availability of food in the tidal ponds habitats (Dias, 2013), probably resulting in a major food intake, and the “extreme” environmental conditions (such as high temperature and salinity, with large seasonal values’ fluctuation) experienced by specimens from Messina. Indeed, in addition to the enhanced availability of food in tidal ponds biocenosis, another factor strongly influencing growth and, consequently, sagittae features is the water temperature. It is widely reported how teleost species show enhanced growth rates in environments characterized by higher temperatures (Pepin, 1991; Jobling, 1997). The tidal ponds are characterized by large fluctuation of temperature, with a range (between 14 and 23° C) (Savoca et al., 2020) higher than that reported for the Split area (data reported in the literature for the first meter of the water column between 10 to 23°C) (Kraus et al., 2019). This different range of temperature which specimens from Messina probably experienced, in addition to other factors (such as food intake), could allowed for the faster growth shown by the results. It is necessary to enlarge the sample size also to obtain more solid data from the analyses on the intra and inter-population variability of sagittae, considering the differences in specimen numbers between size classes (especially between Size classes I, totally absent in Split, and III) in both the studied areas. Indeed, data from a larger number of specimens, with a similar sample size composition between the analyzed Size Classes, can be essential to obtain an even more complete picture of the inter and intra-population variability of the studied species. Bias related to sample size has great relevance, especially in inter-population studies involving otoliths’ shape and morphometrical data, with a non-representative number of specimens, or a not-comparable number of specimens from each population, representing one of the major concerns in this research field (Hilborn and Walters, 1992; Campana and Casselman, 1993; Lleonart and Maynou, 2003; Biolé et al., 2019). Future studies, involving more specimens, and combining otoliths’ shape analysis with biological or genetic analyses, are necessary to confirm and improve the data provided by results. This is essential to deepen the knowledge base regarding the inter-population differences in sagittae features and growth rates of the studied species for further stocks’ discrimination and population studies.
Concerning somatic growth, it is not clear its relation to metabolic rate, food intake and otoliths’ size and growth, also because faster fish growth and a most enhanced food intake do not always result in larger otoliths (Hoff and Fuiman, 1993; Grønkjær, 2016). Concerning the geographical differences among the size classes, the statistical analyses highlighted significant shape differences in intermediate specimens (11-18 cm), but no significant differences in morphometry between specimens from the two studied areas were found in those larger than 18 cm. This could be related to the life habits of the specimens from Messina, which could pass more time inside the tidal ponds of the beach rock formations during the first part of their life, searching for shelter and good hunting ground, as reported for other teleost species in the area (Capillo et al., 2018; Savoca et al., 2020). Indeed, tidal pools are recognized worldwide as an important nursery area for several teleost families, that in these environments can find shelter from predators and increased preys’ availability (Dias, 2013; Dias et al., 2016; Mendonça et al., 2019). This could explain the more significant morphometrical differences at the geographical level between specimens smaller than 18 cm than the larger ones. Future studies comparing the otoliths belonging to specimens smaller than 10 cm from both areas can confirm this thesis, being these absent from the Split samples here analyzed. Indeed, according to the literature, otolith shape and morphometry are under the control of both genetic and environmental influences (Cardinale et al., 2004). The environment can strongly shape the sensory organs, as also highlighted by the sensory drive hypothesis, tested by Tuset et al. (Tuset et al., 2015) on the sagittae of others Scorpaenidae species (Sebastes spp.). Specimens smaller than 18 cm, belonging to the first and second age groups, could be more influenced by the beach rocks’ tidal ponds than the third, spending more time in these habitats. Getting larger, S. porcus individuals could, conversely, spend more time in the nearshore coastal waters (re-entering in the tidal pounds searching for food, as reported for several transient species inhabiting these environments worldwide) (Dias, 2013), with biotopes and depths like those present in the Split area.
As shown by age composition results, tidal ponds in Messina represent a nursery area for S. porcus, characterized by a relevant abundance of small-age specimens. Considering the commercial and ecological value of this species, it is required an improved conservation of this sensitive and extreme biotope. It is necessary to enhance the management practice of commercial and recreational fishing, especially in the areas near this biotope. Moreover, being this area so close to the city, it is required to preserve it against increasing urbanization, creating buffer zones between the beach rock formation and the most anthropized zones. The conservation of these ecologically relevant habitats can improve not only the abundance and distribution of S. porcus, but also those of the several species inhabiting them, preserving the biodiversity of the entire marine coastal environment.
5 Conclusion
Assess and exploring the variations in ecomorphological features and population parameters at the geographical level is essential for both conservation purposes (being morphological features, such as those related to otoliths, and population dynamics widely used in stock assessment) and ecological studies (Aguirre and Lombarte, 1999; Lombarte et al., 2010; Zhuang et al., 2015b; Assis et al., 2020). The eco-morphological adaptation of species to different habitats and environmental features is the basis of phenotypic plasticity (i.e., the capability to express different phenotypes related to different environmental factors) (Via et al., 1995). This becomes an essential concept for the identification of marine teleost populations, being assumed that each population, living under peculiar environmental conditions, can display specific phenotypes under the regulation of genetic and/or environmental mechanisms (Swain and Foote, 1999; Grabowski et al., 2009; Berg et al., 2018). This process, being also the basis of the stock’s differentiation, assumes relevant importance in the conservation of fishery resources and the maintenance of marine ecosystems’ well-being.
In this regard, the present paper assessed both the reliability of S. porcus as a model species to explore the eco-morphological variability of otoliths and population dynamics plasticity, and the reliability of otoliths shape and morphometric analysis for the populations’ discrimination in the studied species. The low home-range distribution of this benthic species, its heterogeneity in growth somatic patterns and feeding habits related to different environments, added to the high geographical variability of sagittae in shape and morphometry reported by results, make S. porcus perfect to explore the influence of different environmental conditions on teleost species. Specimens inhabiting the tidal ponds of the beachrock formations could be a perfect case study to understand how teleost species can adapt to extreme environmental conditions and peculiar habitat features. Future analyses on population dynamics and seasonal distribution of the studied species in this peculiar environment are required, to understand the ecology of this species and its life habits in these habitats. Moreover, it will be also essential to provide valuable data about the species composition and ecological inter-specific dynamics existing in tidal ponds, recognized worldwide as fundamental nursery areas, and feeding grounds for several marine species, and, consequently, as important biocenosis for the well-being and the conservation of the marine biodiversity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving animals because ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because biological samples were obtained from landings of commercial fishing vessels and fisherman. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
CD: Writing – original draft, Visualization, Validation, Software, Investigation, Data curation, Conceptualization. SF: Writing – review & editing, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. JF: Writing – review & editing, Visualization, Validation, Supervision, Software, Methodology, Investigation, Conceptualization. MA: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation, Data curation, Conceptualization. NS: Writing – review & editing, Conceptualization, Validation, Supervision, Resources, Investigation, Funding acquisition. GC: Writing – review & editing, Visualization, Validation, Project administration, Methodology, Investigation, Data curation, Conceptualization. SS: Writing – review & editing, Visualization, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1347897/full#supplementary-material
References
Abaad M., Tuset V. M., Montero D., Lombarte A., Otero-Ferrer J. L., Haroun R. (2016). Phenotypic plasticity in wild marine fishes associated with fish-cage aquaculture. Hydrobiologia 765, 343–358. doi: 10.1007/s10750-015-2428-5
Aguirre H., Lombarte A. (1999). Ecomorphological comparisons of sagittae in Mullus barbatus and M. surmuletus. J. Fish Biol. 55, 105–114. doi: 10.1111/j.1095-8649.1999.tb00660.x
Assis C. A. (2000). Estudo morfológico dos otólitos sagitta, asteriscus e lapillus de Teleóstei (Actinopterygii, Teleostei) de Portugal continental. Sua aplicação em estudos de filogenia, sistematica e ecologia. Ecologia 1005, 1–1004.
Assis C. A. (2003). The lagenar otoliths of teleosts: Their morphology and its application in species identification, phylogeny and systematics. J. Fish Biol. 62, 1268–1295. doi: 10.1046/j.1095-8649.2003.00106.x
Assis C. A. (2005). The utricular otoliths, lapilli, of teleosts: Their morphology and relevance for species identification and systematics studies. Sci. Mar. 69, 259–273. doi: 10.3989/scimar.2005.69n2259
Assis I. O., da Silva V. E. L., Souto-Vieira D., Lozano A. P., Volpedo A. V., Fabré N. N. (2020). Ecomorphological patterns in otoliths of tropical fishes: assessing trophic groups and depth strata preference by shape. Environ. Biol. Fishes 103, 349–361. doi: 10.1007/s10641-020-00961-0
Atkinson D. (1994). Temperature and organism size—A biological law for ectotherms? Adv. Ecol. Res. 25, 1–58. doi: 10.1016/S0065-2504(08)60212-3
Aydin M., Mazlum R. E. (2020). Feeding ecology of black scorpion fish (Scorpaena porcus Linnaeus 1758) in SE black sea region, (Ordu) Turkey. J. Mar. Biol. Assoc. United Kingdom 100, 435–444. doi: 10.1017/S002531542000020X
Bacha M., Moali A., Benmansour N. E., Brylinski J. M., Mahé K., Amara R. (2010). Relationships between age, growth, diet and environmental parameters for anchovy (Engraulis encrasicolus L.) in the Bay of Bénisaf (SW Mediterranean, west Algerian coast). Cybium 34, 47–57. doi: 10.26028/cybium/2010-341-006
Başçïnar N. S., Sağlam H. (2009). Feeding habits of black scorpion fish scorpaena porcus, in the South-Eastern Black Sea. Turk J. Fish Aquat Sci. 9, 99–103.
Beamish R. J., McFarlane G. A. (1983). The forgotten requirement for age validation in fisheries biology. Trans. Am. Fish Soc. 112, 735–743. doi: 10.1577/1548-8659(1983)112<735:TFRFAV>2.0.CO;2
Benzinou A., Carbini S., Nasreddine K., Elleboode R., Mahé K. (2013). Discriminating stocks of striped red mullet (Mullus surmuletus) in the Northwest European seas using three automatic shape classification methods. Fish Res. 143, 153–160. doi: 10.1016/j.fishres.2013.01.015
Berg F., Almeland O. W., Skadal J., Slotte A., Andersson L., Folkvord A. (2018). Genetic factors have a major effect on growth, number of vertebrae and otolith shape in Atlantic herring (Clupea harengus). PloS One 13, e0190995. doi: 10.1371/journal.pone.0190995
Bilgin S., Çelik E.Ş. (2009). Age, growth and reproduction of the black scorpionfish, Scorpaena porcus (Pisces, Scorpaenidae), on the Black Sea coast of Turkey. J. Appl. Ichthyology 25, 55–60. doi: 10.1111/jai.2009.25.issue-1
Biolé F. G., Callicó Fortunato R., Thompson G. A., Volpedo A. V. (2019). Application of otolith morphometry for the study of ontogenetic variations of Odontesthes argentinensis. Environ. Biol. Fishes 102, 1301–1310. doi: 10.1007/s10641-019-00908-0
Bose A. P. H., Zimmermann H., Winkler G., Kaufmann A., Strohmeier T., Koblmüller S., et al. (2020). Congruent geographic variation in saccular otolith shape across multiple species of African cichlids. Sci. Rep. 10, 1–14. doi: 10.1038/s41598-020-69701-9
Bradai M. N., Bouain A. (1988). Age et croissance de Scorpaena porcus et Scorpaena scrofa du golfe de Gábes. Oebalia 15, 13–38.
Campana S. E., Casselman J. M. (1993). Stock discrimination using otolith shape analysis. Can. J. Fisheries Aquat. Sci. 50, 1062–1083. doi: 10.1139/f93-123
Capillo G., Panarello G., Savoca S., Sanfilippo M., Albano M., Volsi R. L., et al. (2018). Intertidal ponds of messina’s beachrock faunal assemblage, evaluation of ecosystem dynamics and communities’ interactions. AAPP Atti della Accademia Peloritana dei Pericolanti Classe di Sci. Fisiche Matematiche e Naturali 96, A41–A416. doi: 10.1478/AAPP.96S3A4
Carbonara P., Follesa M. C. (2019). Handbook on fish age determination: a Mediterranean experience (Rome: FAO).
Cardinale M., Doering-Arjes P., Kastowsky M., Mosegaard H. (2004). Effects of sex, stock, and environment on the shape of known-age Atlantic cod (Gadus morhua) otoliths. Can. J. Fisheries Aquat. Sci. 61, 158–167. doi: 10.1139/f03-151
Chang W. Y. B. (1982). A statistical method for evaluating the reproducibility of age determination. Can. J. Fisheries Aquat. Sci. 39, 1208–1210. doi: 10.1139/f82-158
Chapuis L., Yopak K. E., Radford C. A. (2023). From the morphospace to the soundscape: Exploring the diversity and functional morphology of the fish inner ear, with a focus on elasmobranchs. J. Acoust Soc. Am. 154, 1526–1538. doi: 10.1121/10.0020850
Cochrane K. (Ed.) (2002). A fishery manager’s guidebook – Management measures and their application (Rome: FAO Fisheries Technical Paper No. 424. Food & Agriculture Org).
Compaire J. C., Casademont P., Cabrera R., Gómez-Cama C., Soriguer M. C. (2018). Feeding of Scorpaena porcus (Scorpaenidae) in intertidal rock pools in the Gulf of Cadiz (NE Atlantic). J. Mar. Biol. Assoc. U. K. 98, 845–853. doi: 10.1017/S0025315417000030
Cortés E. (1997). A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fisheries Aquat. Sci (3), 726–738. doi: 10.1139/f96-316
Cortese G., De Domenico E. (1990). Some considerations on the levantine intermediate water distribution in the Straits of Messina. Boll. Ocean. Teor. Appl. 8, 197–207.
Costello M. J., Emblow C., White R. J. (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification Vol. 50 (Paris: Collection Patrimoines Naturels. Paris: Muséum national d’Histoire naturelle).
Cucco A., Quattrocchi G., Olita A., Fazioli L., Ribotti A., Sinerchia M., et al. (2016). Hydrodynamic modelling of coastal seas: The role of tidal dynamics in the Messina Strait, Western Mediterranean Sea. Natural Hazards Earth System Sci. 16, 1553–1569. doi: 10.5194/nhess-16-1553-2016
De Domenico E. (1987). Caratteristiche fisiche e chimiche delle acque nello Stretto di Messina. Doc. Trav. IGAL 11, 225–235.
Dias M. A. D. (2013). Tidal pools as nursery areas for marine fish larvae and juveniles – habitat use and trophic ecology. Mestrado em Ecologia Marinha, 1–92.
Dias M., Roma J., Fonseca C., Pinto M., Cabral H. N., Silva A., et al. (2016). Intertidal pools as alternative nursery habitats for coastal fishes. Mar. Biol. Res. 12, 331–344. doi: 10.1080/17451000.2016.1143106
D’Iglio C., Albano M., Famulari S., Savoca S., Panarello G., Di Paola D., et al. (2021a). Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 11, 16315. doi: 10.1038/s41598-021-95814-w
D’Iglio C., Albano M., Tiralongo F., Famulari S., Rinelli P., Savoca S., et al. (2021b). Biological and ecological aspects of the Blackmouth Catshark (Galeus melastomus rafinesque 1810) in the southern Tyrrhenian Sea. J. Mar. Sci. Eng. 9, 967. doi: 10.3390/jmse9090967
D’Iglio C., Famulari S., Albano M., Carnevale A., Di Fresco D., Costanzo M., et al. (2023). Intraspecific variability of the saccular and utricular otoliths of the hatchetfish Argyropelecus hemigymnus (Cocco 1829) from the strait of Messina (Central Mediterranean sea). PloS One 18, 1–31. doi: 10.1371/journal.pone.0281621
D’iglio C., Natale S., Albano M., Savoca S., Famulari S., Gervasi C., et al. (2022a). Otolith analyses highlight morpho-functional differences of three species of mullet (Mugilidae) from transitional water. Sustainability (Switzerland) 14, 1–25. doi: 10.3390/su14010398
D’Iglio C., Porcino N., Savoca S., Profeta A., Perdichizzi A., Armeli E., et al. (2022b). Ontogenetic shift and feeding habits of the European hake ( Merluccius merluccius L . 1758 ) in Central and Southern Tyrrhenian Sea ( Western Mediterranean Sea ): A comparison between past and present data. Ecol. Evol. 12, 1–12. doi: 10.1002/ece3.8634
Dimech M., Kaiser M. J., Ragonese S., Schembri P. J. (2012). Ecosystem effects of fishing on the continental slope in the Central Mediterranean Sea. Mar. Ecol. Prog. Ser. 449, 41–54. doi: 10.3354/meps09475
Endler J. A. (1992). Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. doi: 10.1086/285308
Endler J. A. (1993). Some general comments on the evolution and design of animal communication systems. Philos. Trans. - R. Soc. London B 340, 215–225. doi: 10.1098/rstb.1993.0060
FAO. (2020). FAO Fisheries and Aquaculture - FishStatJ - Software for Fishery and Aquaculture Statistical Time Series (FAO Fisheries and Aquaculture Division). Available online at: https://www.fao.org/fishery/en/topic/166235?lang=en (Accessed February 8, 2024).
Ferri J., Matić-Skoko S. (2021). The spatial heterogeneity of the black scorpionfish, scorpaena porcus (Scorpaenidae): Differences in length, dietary and age compositions. Appl. Sci. (Switzerland) 11, 11919. doi: 10.3390/app112411919
Ferri J., Stagličić N., Matić-Skoko S. (2012). The black scorpionfish, Scorpaena porcus (Scorpaenidae): Could it serve as reliable indicator of Mediterranean coastal communities’ health? Ecol. Indic 18, 25–30. doi: 10.1016/j.ecolind.2011.11.004
Forcada A., Valle C., Bonhomme P., Criquet G., Cadiou G., Lenfant P., et al. (2009). Effects of habitat on spillover from marine protected areas to artisanal fisheries. Mar. Ecol. Prog. Ser. 379, 197–211. doi: 10.3354/meps07892
Forcada Almarcha A., Bayle Sempere J. T., lizaso J. L. S. (2007). Evaluación de las áreas marinas protegidas y su efecto en pesquerías artesanales del Mediterráneo Occidental. Departamento Cienc. del Mar. y Biología Aplicada, 402. pp.
Fricke R., Golani D., Appelbaum-Golani B., Zajonz U. (2018). Scorpaena decemradiata new species (Teleostei: Scorpaenidae) from the gulf of aqaba, northern red sea, a species distinct from scorpaena porcus. Sci. Mar. 82, 169–184. doi: 10.3989/scimar.2018.82n3
García-Rodríguez M., Fernández Á.M., Esteban A. (2006). Characterisation, analysis and catch rates of the small-scale fisheries of the Alicante Gulf (SE Spain) over a 10 years time series. Fish Res. 77, 226–238. doi: 10.1016/j.fishres.2005.09.002
Gomes P. B., Belém M. J., da C., Schlenz E. (1998). Distribution, abundance and adaptations of three species of Actiniidae (Cnidaria, Actiniaria) on an intertidal beach rock in Cameiros beach, Pemambuco, Brazil. Miscellania Zoologica 21, 65–72.
Goñi R., Adlerstein S., Alvarez-Berastegui D., Forcada A., Reñones O., Criquet G., et al. (2008). Spillover from six western Mediterranean marine protected areas: Evidence from artisanal fisheries. Mar. Ecol. Prog. Ser. 366, 159–174. doi: 10.3354/meps07532
Grabowski T. B., Young S. P., Libungan L. A., Steinarsson A., Marteinsdóttir G. (2009). Evidence of phenotypic plasticity and local adaption in metabolic rates between components of the Icelandic cod (gadus morhua l.) stock. Environ. Biol. Fishes 86, 361–370. doi: 10.1007/s10641-009-9534-z
Gravem S. A., Morgan S. G. (2017). Shifts in intertidal zonation and refuge use by prey after mass mortalities of two predators. Ecology 98, 1006–1015. doi: 10.1002/ecy.1672
Grønkjær P. (2016). Otoliths as individual indicators: A reappraisal of the link between fish physiology and otolith characteristics. Mar. Freshw. Res. 67, 881–888. doi: 10.1071/MF15155
Harmelin-Vivien M. L., Kaim-Malka R. A., Ledoyer M., Jacob-Abraham S. S. (1989). Food partitioning among scorpaenid fishes in Mediterranean seagrass beds. J. Fish Biol. 34, 715–734. doi: 10.1111/j.1095-8649.1989.tb03352.x
Higgins R., Isidro E., Menezes G., Correia A. (2013). Otolith elemental signatures indicate population separation in deep-sea rockfish, Helicolenus dactylopterus and Pontinus kuhlii, from the Azores. J. Sea Res. 83, 202–208. doi: 10.1016/j.seares.2013.05.014
Hilborn C. R., Walters J. (1992). Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty (Chicago: Springer Science & Business Media). doi: 10.1086/417864
Hoff G. R., Fuiman L. A. (1993). Morphometry and composition of red drum otoliths: Changes associated with temperature, somatic growth rate, and age. Comp. Biochem. Physiol. A Physiol. 106, 209–219. doi: 10.1016/0300-9629(93)90502-U
Hüssy K., Limburg K. E., de Pontual H., Thomas O. R. B., Cook P. K., Heimbrand Y., et al. (2021). Trace element patterns in Otoliths: the role of biomineralization. Rev. Fisheries Sci. Aquaculture 29, 445–477. doi: 10.1080/23308249.2020.1760204
Hyslop E. J. (1980). Stomach contents analysis—a review of methods and their application. J. Fish Biol. 17, 411–429. doi: 10.1111/j.1095-8649.1980.tb02775.x
Ingrosso G., Abbiati M., Badalamenti F., Bavestrello G., Belmonte G., Cannas R., et al. (2018). Mediterranean bioconstructions along the Italian coast. Adv. Mar. Biol. 79, 61–136. doi: 10.1016/bs.amb.2018.05.001
Institute of Oceanography and Fisheries. (2023). Database and indicators of the state of the marine environment, mariculture and fisheries. Available online at: http://baltazar.izor.hr/azopub/bobazi.
Jaramilo A. M., Tombari A. D., Benedito Dura V., Eugeni Rodrigo M., Volpedo A. V. (2014). Otolith eco-morphological patterns of benthic fishes from the coast of Valencia (Spain). Thalassas 30, 57–66.
Jardas I. (1999). Review of long-term changes in trammel bottom set catches, crustacean, cephalopoda and fish communities along the eastern Adriatic (Croatian) coastal area. Acta Adriat 40, 67–78.
Jardas I., Pallaoro A. (1992). Age and growth of black scorpionfish, Scorpaena porcus L. 1758 in the Adriatic Sea. Rapport la Commission Internationale pour Mer Méditerranean 33, 296.
Jardas I., Pallaoro A., Kraljević M., Dulćić J., Cetinić P. (1998). Long-term changes in biodiversity of the coastal area of the eastern Adriatic: Fish, crustaceam and cephalopoda communities. Period Biol. 100, 19–28.
Jawad L. A., Sabatino G., Ibáñez A. L., Andaloro F., Battaglia P. (2018). Morphology and ontogenetic changes in otoliths of the mesopelagic fishes Ceratoscopelus maderensis (Myctophidae), Vinciguerria attenuata and V. poweriae (Phosichthyidae) from the Strait of Messina (Mediterranean Sea). Acta Zoologica 99 (2), 126–142. doi: 10.1111/azo.12197
Jobling M. (1997). “Temperature and growth: modulation of growth rate via temperature,” in Global warning: implication for freshwater and marine fish (Cambridge, Cambridge: Cambridge University Press, Cambridge), 225–253.
Jobling M. (2011). Temperature and growth: modulation of growth rate via temperature change. Global Warming, 225–254. doi: 10.1017/cbo9780511983375.010
Kendallha K., Shtewi H. H., Elsalini O. A., Shakman E. A. (2018). Biological aspects of black scorpionfish, Scorpaena porcus (Linnaeus 1758) in the western coast of Libya. Libyan J. Sci. 21, 13–23. doi: 10.55276/ljs.v21iA.138
Kikuchi E., Cardoso L. G., Canel D., Timi J. T., Haimovici M. (2021). Using growth rates and otolith shape to identify the population structure of Umbrina canosai (Sciaenidae) from the Southwestern Atlantic. Mar. Biol. Res. 17, 272–285. doi: 10.1080/17451000.2021.1938131
Koca H. U. (1997). A research on the determination of some parameters of Scorpion fish (Scorpaena porcus Linnaeus 1758) caught by bottom nets in the area of Sinop from the vievpoint of fishery biology (Sinop: Science and Engineering, Ondokuz Mayıs University).
Koca H. U. (2002). A Study on the determination of some parameters of the scorpion fish (Scorpaena porcus Linne. 1758) caught by bottom nets in the area of Sinop in terms of fishery biology. Turk J. Vet. Anim. Sci. 26, 65–69.
Kozłowski J., Czarnołęski M., Dańko M. (2004). Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr. Comp. Biol. 44, 480–493. doi: 10.1093/icb/44.6.480
Kraus R., Grilli F., Supić N., Janeković I., Brailo M., Cara M., et al. (2019). Oceanographic characteristics of the Adriatic Sea–support to secondary HAOP spread through natural dispersal. Mar. pollut. Bull. 147, 59–85. doi: 10.1016/j.marpolbul.2018.10.062
Kutsyn D. N., Skuratovskaya E. N., Chesnokova I. I. (2019). Body size, age structure, growth, and maturation of black scorpionfish Scorpaena porcus (Scorpaenidae) from southwestern Crimea (Black Sea). J. Ichthyol 59, 864–869. doi: 10.1134/S0032945219060067
Kuzminova N., Rudneva I., Salekhova L., Shevchenko N., Oven L. (2011). State of black scorpionfish (Scorpaena porcus Linnaeus 1758) inhabited coastal area of Sevastopol region (Black sea) in 1998-2008. Turk J. Fish Aquat Sci. 11, 101–111. doi: 10.4194/trjfas.2011.0114
Ladich F., Schulz-Mirbach T. (2016). Diversity in fish auditory systems: One of the riddles of sensory biology. Front. Ecol. Evol. 4. doi: 10.3389/fevo.2016.00028
La Mesa M., Scarcella G., Grati F., Fabi G. (2010). Age and growth of the black scorpionfish, Scorpaena porcus (Pisces: Scorpaenidae) from artificial structures and natural reefs in the Adriatic Sea. Sci. Mar. 74, 677–685. doi: 10.3989/scimar.2010.74n4677
Landa J., Hernández C. (2020). Age estimation, corroboration and back-calculation of four-spot megrim (Lepidorhombus boscii) on the Porcupine Bank using otoliths. Estuar. Coast. Shelf Sci. 243, 106890. doi: 10.1016/j.ecss.2020.106890
Lek E., Fairclough D. V., Hall N. G., Hesp S. A., Potter I. C. (2012). Do the maximum sizes, ages and patterns of growth of three reef-dwelling labrid species at two latitudes differ in a manner conforming to the metabolic theory of ecology? J. Fish Biol. 81, 1936–1962. doi: 10.1111/j.1095-8649.2012.03446.x
Libungan L. A., Pálsson S. (2015). ShapeR: An R package to study otolith shape variation among fish populations. PloS One 10, 1–12. doi: 10.1371/journal.pone.0121102
Lipizer M., Partescano E., Rabitti A., Giorgetti A., Crise A. (2014). Qualified temperature, salinity and dissolved oxygen climatologies in a changing Adriatic Sea. Ocean Sci. 10, 771–797. doi: 10.5194/os-10-771-2014
Lleonart J., Maynou F. (2003). Fish stock assessments in the Mediterranean: State of the art. Sci. Mar 67 (S1), 37–49. doi: 10.3989/scimar.2003.67s137
Lombarte A., Cruz A. (2007). Otolith size trends in marine fish communities from different depth strata. J. Fish Biol. 71, 53–76. doi: 10.1111/j.1095-8649.2007.01465.x
Lombarte A., Lleonart J. (1993). Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fishes 37, 297–306. doi: 10.1007/BF00004637
Lombarte A., Palmer M., Matallanas J., Gómez-Zurita J., Morales-Nin B. (2010). Ecomorphological trends and phylogenetic inertia of otolith sagittae in Nototheniidae. Environ. Biol. Fishes 89, 607–618. doi: 10.1007/s10641-010-9673-2
Longhitano S. G. (2018). Between Scylla and Charybdis (part 2): The sedimentary dynamics of the ancient, Early Pleistocene Messina Strait (central Mediterranean) based on its modern analogue. Earth Sci. Rev. 179, 248–286. doi: 10.1016/j.earscirev.2018.01.017
Mahé K., Goascoz N., Dufour J. L., Iglesias S. P., Tetard A. (2014). Black scorpionfish Scorpaena porcus (Scorpaenidae): A first record in the eastern English Channel. Mar. Biodivers Rec 7, e6. doi: 10.1017/S1755267214000037
Medinets S., Medinets V. (2010). Results of investigations of atmospheric pollutants fluxes in zmeiny island in western part of the black sea in 2003-2007 years. J. Environ. Prot. Ecol. 11, 1030–1036. doi: 10.4194/1303-2712-v12_2_42
Mendonça V., Flores A. A. V., Silva A. C. F., Vinagre C. (2019). Do marine fish juveniles use intertidal tide pools as feeding grounds? Estuar. Coast. Shelf Sci. 225, 106255. doi: 10.1016/j.ecss.2019.106255
Mille T., Ernande B., Pontual H., Villanueva C., Mahé K. (2016a). Sources of otolith morphology variation at the intra-population level : directional asymmetry and diet marine fishes. SFI Days 2016.
Mille T., Mahé K., Cachera M., Villanueva M. C., De Pontual H., Ernande B. (2016b). Diet is correlated with otolith shape in marine fish. Mar. Ecol. Prog. Ser. 555, 167–184. doi: 10.3354/meps11784
Monteiro L. R., Di Beneditto A. P. M., Guillermo L. H., Rivera L. A. (2005). Allometric changes and shape differentiation of sagitta otoliths in sciaenid fishes. Fish Res. 74, 288–299. doi: 10.1016/j.fishres.2005.03.002
Muniz A. A., Moura A., Triay-Portella R., Moreira C., Santos P. T., Correia A. T. (2021). Population structure of the chub mackerel (Scomber colias) in the North-east Atlantic inferred from otolith shape and body morphometrics. Mar. Freshw. Res. 72, 341–352. doi: 10.1071/MF19389
Nelson J. S., Grande T. C., Wilson M. V. H. (2016). Fishes of the World, Fifth Edition. WILEY. doi: 10.1002/9781119174844
Neves J., Silva A. A., Moreno A., Veríssimo A., Santos A. M., Garrido S. (2021). Population structure of the European sardine Sardina pilchardus from Atlantic and Mediterranean waters based on otolith shape analysis. Fish Res. 243, 106050. doi: 10.1016/j.fishres.2021.106050
Newman S. J., Dunk I. J. (2002). Growth, age validation, mortality, and other population characteristics of the red emperor snapper, Lutjanus sebae (Cuvier 1828), off the Kimberley coast of north-western Australia. Estuar. Coast. Shelf Sci 55, 67–80. doi: 10.1006/ecss.2001.0887
Nolf D. (1985). “Otolithi piscium,” in Handbook of Paleoichthyology, vol. 10 . Ed. Fischer G.(Stuttgart, New York: Gustav Fischer Verlag ).
Orlić M., Dadić V., Grbec B., Leder N., Marki A., Matić F., et al. (2006). Wintertime buoyancy forcing, changing seawater properties, and two different circulation systems produced in the Adriatic. J. Geophys Res. Oceans 111, 1–21. doi: 10.1029/2005JC003271
Oven L. S., Rudneva I. I., Shevchenko N. F. (2000). Responses of Scorpaena porcus(Scorpaenidae) to anthropogenic impact. J. Ichthyol 40, 70–73.
Özgül A., Lök A., Tansel Tanrıkul T., Alós J. (2019). Home range and residency of Scorpaena porcus and Scorpaena scrofa in artificial reefs revealed by fine-scale acoustic tracking. Fish Res. 210, 22–30. doi: 10.1016/j.fishres.2018.10.008
Pashkov A. N., Shevchenko N. F., Oven L. S., Giragosov V. E., Kruglov M. V. (1999). Distribution, numbers and principal population indexes of Scorpaena porcus under anthropogenic pollution of the Black Sea. J. Ichthyol 39, 634–641.
Passalacqua M., Canta S., Bono G., Beltrano A. (2005). Scorpaena porcus Nell’area Marina Protetta (Amp) delle isole egadi. Biol. Mar. Medit 12, 418–421.
Pavlov D. A. (2016). Differentiation of three species of the genus Upeneus (Mullidae) based on otolith shape analysis. J. Ichthyol 56, 37–51. doi: 10.1134/S0032945216010094
Pavlov D. A. (2021). Otolith morphology and relationships of several fish species of the suborder Scorpaenoidei. J. Ichthyol 61, 33–47. doi: 10.1134/S0032945221010100
Pepin P. (1991). Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Can. J. Fisheries Aquat. Sci. 48, 503–518. doi: 10.1139/f91-065
Perdichizzi A., D’Iglio C., Giordano D., Profeta A., Ragonese S., Rinelli P. (2022). Comparing life-history traits in two contiguous stocks of the deep-water rose shrimp Parapenaeus longirostris (H. Lucas 1846) (Crustacea: Decapoda) in the southern Tyrrhenian Sea (Central Mediterranean sea). Fish Res. 248, 106206. doi: 10.1016/j.fishres.2021.106206
Popper A. N., Lu Z. (2000). Structure-function relationships in fish otolith organs. Fish Res. 46, 15–25. doi: 10.1016/S0165-7836(00)00129-6
Popper A. N., Ramcharitar J., Campana S. E. (2005). Why otoliths? Insights from inner ear physiology and fisheries biology. Mar. Freshw. Res. 56, 497–504. doi: 10.1071/MF04267
Popper A. N., Schilt C. R. (2008). Hearing and acoustic behavior: basic and applied considerations. Fish Bioacoustics 17–48, 17–48. doi: 10.1007/978-0-387-73029-5_2
Poutiers J. M. (1987). Fiches fao d’identification des especes pour les besoins de la peche. Mediterranee et mer noire zone de peche 37 révision 1 volume 1 vegetaux et invertebres. Vertebres 2, 1290–1300.
Rafrafi-Nouira S., El Kamel-Moutalibi O., Boumaïza M., Reynaud C., Capapé C. (2016). Food and feeding habits of black scorpionfish, Scorpaena porcus (Osteichthyes: Scorpaenidae) from the northern coast of Tunisia (Central Mediterranean). J. Ichthyol 56, 107–123. doi: 10.1134/S0032945216010112
Rudneva I. I., Shevchenko N. F., Zalevskaya I. N., Zherko N. V. (2005). Biomonitoring of the coastal waters of the Black Sea. Water Resour. 32, 215–222. doi: 10.1007/s11268-005-0028-x
Sahin C., Erbay M., Kalayci F., Ceylan Y., Yesilcicek T. (2019). Life-history traits of the Black Scorpionfish (Scorpaena porcus) in southeastern Black Sea. Turk J. Fish Aquat Sci. 19, 571–584. doi: 10.4194/1303-2712-v19_7_04
Sanchez-Jerez P., Gillanders B. M., Kingsford M. J. (2002). Spatial variability of trace elements in fish otoliths: Comparison with dietary items and habitat constituents in seagrass meadows. J. Fish Biol. 61, 801–821. doi: 10.1111/j.1095-8649.2002.tb00912.x
Savoca S., Grifó G., Panarello G., Albano M., Giacobbe S., Capillo G., et al. (2020). Modelling prey-predator interactions in Messina beachrock pools. Ecol. Modell 434, 1–17. doi: 10.1016/j.ecolmodel.2020.109206
Scarcella G., Grati F., Polidori P., Domenichetti F., Bolognini L., Fabi G. (2011). Comparison of growth rates estimated by otolith reading of Scorpaena porcus and Scorpaena notata caught on artificial and natural reefs of the northern Adriatic sea. Brazilian Journal of Oceanography 59, 33–42. doi: 10.1590/S1679-87592011000500006
Schulz-Mirbach T., Ladich F., Plath M., Heß M. (2019). Enigmatic ear stones: what we know about the functional role and evolution of fish otoliths. Biol. Rev. 94, 457–482. doi: 10.1111/brv.12463
Schulz-Mirbach T., Ladich F., Riesch R., Plath M. (2010). Otolith morphology and hearing abilities in cave- and surface-dwelling ecotypes of the Atlantic molly, Poecilia mexicana (Teleostei: Poeciliidae). Hear Res. 267, 137–148. doi: 10.1016/j.heares.2010.04.001
Siblot-Boutéflika D. (1976). Contribution à ľétude des Scorpaeni- dae de la région Alger (Marseille: Univ. Aix-Marseille II).
Silvestri R., Voliani A., Zucchi A. (2002). Nota sulla biologia di Scorpaena porcus Linneo 1758 nel Mar Ligure meridionale. Biol. Marina 9, 813–817.
Spanò N., De Domenico E. (2017). Biodiversity in central Mediterranean sea. Mediterr. Identities - Environment Society Culture 6, 129–148. doi: 10.5772/intechopen.68942
Stagličić N., Matić-Skoko S., Pallaoro A., Grgičević R., Kraljević M., Tutman P., et al. (2011). Long-term trends in the structure of eastern Adriatic littoral fish assemblages: Consequences for fisheries management. Estuar. Coast. Shelf Sci. 94, 263–271. doi: 10.1016/j.ecss.2011.07.005
Swain D. P., Foote C. J. (1999). Stocks and chameleons: The use of phenotypic variation in stock identification. Fish Res. 43, 113–128. doi: 10.1016/S0165-7836(99)00069-7
Thomas O. R. B., Swearer S. E. (2019). Otolith biochemistry—a review. Rev. Fisheries Sci. Aquaculture 27, 458–489. doi: 10.1080/23308249.2019.1627285
Tiralongo F. (2024). Unraveling the story of the black Scorpionfish (Scorpaena porcus linnaeus 1758): exploring local ecological knowledge and the exploitative history of a marine species. Fishes 9, 1–11. doi: 10.3390/fishes9010031
Turan C., Gunduz I., Gurlek M., Yaglioglu D., Erguden D. (2009). Systematics of Scorpaeniformes species in the Mediterranean Sea inferred from mitochondrial 16S rDNA sequence and morphological data. Folia biologica (Kraków) 57, 219–226. doi: 10.3409/fb57_1-2.219-226
Tuset V. M., Farré M., Otero-Ferrer J. L., Vilar A., Morales-Nin B., Lombarte A. (2016a). Testing otolith morphology for measuring marine fish biodiversity. Mar. Freshw. Res. 67, 1037–1048. doi: 10.1071/MF15052
Tuset V. M., Imondi R., Aguado G., Otero-Ferrer J. L., Santschi L., Lombarte A., et al. (2015). Otolith patterns of rockfishes from the northeastern pacific. J. Morphol 276, 458–469. doi: 10.1002/jmor.20353
Tuset V. M., Lombarte A., Assis C. A. (2008). Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 72, 7–198. doi: 10.3989/scimar.2008.72s1
Tuset V. M., Lombarte A., González J. A., Pertusa J. F., Lorente M. J. (2003a). Comparative morphology of the sagittal otolith in Serranus spp. J. Fish Biol. 63, 1491–1504. doi: 10.1111/j.1095-8649.2003.00262.x
Tuset V. M., Lozano I. J., Gonzĺez J. A., Pertusa J. F., García-Díaz M. M. (2003b). Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L. 1758). J. Appl. Ichthyology 19, 88–93. doi: 10.1046/j.1439-0426.2003.00344.x
Tuset V. M., Otero-Ferrer J. L., Gómez-Zurita J., Venerus L. A., Stransky C., Imondi R., et al. (2016b). Otolith shape lends support to the sensory drive hypothesis in rockfishes. J. Evol. Biol. 29, 2083–2097. doi: 10.1111/jeb.12932
Unsal N., Oral M. (1994). An investigation on the growth and reproduction characteristics of the black Scorpionfish in the Sea of Marmara. Turkish J. Zoology 20, 303–308.
Velasco E. M., Jiménez-Tenorio N., Del Arbol J., Bruzón M. A., Baro J., Sobrino I. (2011). Age, growth and reproduction of the axillary seabream, Pagellus acarne, in the Atlantic and Mediterranean waters off southern Spain. J. Mar. Biol. Assoc. United Kingdom 91, 1243–1253. doi: 10.1017/S0025315410000305
Via S., Gomulkiewicz R., De Jong G., Scheiner S. M., Schlichting C. D., Van Tienderen P. H. (1995). Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217. doi: 10.1016/S0169-5347(00)89061-8
Vignon M., Morat F. (2010). Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Mar. Ecol. Prog. Ser. 411, 231–241. doi: 10.3354/meps08651
Vilibić I., Orlić M. (2001). Least-squares tracer analysis of water masses in the South Adriatic, (1967-1990). Deep Sea Res. 1 Oceanogr Res. Pap 48, 2297–2330. doi: 10.1016/S0967-0637(01)00014-0
Volpedo A. V., Tombari A. D., Echeverría D. D. (2008). Eco-morphological patterns of the sagitta of Antarctic fish. Polar Biol. 31, 635–640. doi: 10.1007/s00300-007-0400-1
Wheeler A., Whitehead P. J. P., Bauchot M.-L., Hureau J.-C., Nielsen J., Tortonese E. (1986). Fishes of the north-eastern Atlantic and the Mediterranean. Copeia 1986 1, 266. doi: 10.2307/1444931
Yedi̇Er S., Bostanci D. (2021). Morphologic and morphometric comparisons of Sagittal otoliths of five Scorpaena species in the Sea of Marmara, Mediterranean Sea, Aegean Sea and Black Sea. Cah Biol. Mar. 62, 357–369. doi: 10.21411/CBM.A.6B8915B2
Zhuang L., Ye Z., Zhang C. (2015a). Application of otolith shape analysis to species separation in Sebastes spp. from the Bohai Sea and the Yellow Sea, northwest Pacific. Environ. Biol. Fishes 98, 547–558. doi: 10.1007/s10641-014-0286-z
Zhuang L., Ye Z., Zhang C., Ye Z., Li Z., Wan R., et al. (2015b). Stock discrimination of two insular populations of diplodus annularis (Actinopterygii: Perciformes: Sparidae) along the coast of Tunisia by analysis of otolith shape. J. Fish Biol. 46, 1–14. doi: 10.3750/AIP2015.45.4.04
Zorica B., Čikeš Keč V., Pešić A., Gvozdenović S., Kolitari J., Mandić M. (2019). Spatiotemporal distribution of anchovy early life stages in the eastern part of the Adriatic Sea in relation to some oceanographic features. J. Mar. Biol. Assoc. United Kingdom 99, 1205–1211. doi: 10.1017/S0025315418001145
Keywords: otolith, populations, growth, comparison, morphology, life-history
Citation: D’Iglio C, Famulari S, Ferri J, Albano M, Spanò N, Capillo G and Savoca S (2024) Ecomorphological adaptation of Scorpaena porcus (Linnaeus, 1758): evidence from two different environments revealed by sagittae features and somatic growth rates. Front. Mar. Sci. 11:1347897. doi: 10.3389/fmars.2024.1347897
Received: 01 December 2023; Accepted: 02 April 2024;
Published: 01 May 2024.
Edited by:
Cristina Porcu, University of Cagliari, ItalyReviewed by:
Andrea Bellodi, University of Cagliari, ItalyMarilena Donnaloia, COISPA Tecnologia & Ricerca, Italy
Jorge Landa, Spanish Institute of Oceanography (IEO), Spain
Copyright © 2024 D’Iglio, Famulari, Ferri, Albano, Spanò, Capillo and Savoca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josipa Ferri, jferri@unist.hr; Marco Albano, malbano@unime.it
†These authors have contributed equally to this work
 Claudio D’Iglio
Claudio D’Iglio Sergio Famulari
Sergio Famulari Josipa Ferri
Josipa Ferri Marco Albano
Marco Albano Nunziacarla Spanò
Nunziacarla Spanò Gioele Capillo
Gioele Capillo Serena Savoca
Serena Savoca