Non-native fish species in the North Aegean Sea: a review of their distributions integrating unpublished fisheries data
- Fisheries Research Institute, Hellenic Agricultural Organization - DIMITRA (ELGO - DIMITRA), Nea Peramos, Greece
Fisheries-dependent data and information gathered through fisheries-related scientific surveys can significantly contribute to research on the patterns and processes underlying marine biological invasions and their interactions with fisheries. This study presents an overview of the presence of non-native fish species in the North Aegean Sea, offering insights into their distributions and impact on fisheries. The findings are based on a comprehensive compilation of commercial fisheries-dependent and scientific survey data collected in Greece from 2016 to 2023, along with a systematic review of the international scientific literature. The study resulted in the documentation of the presence of 37 non-native fish species, including several recorded for the first time in the area. The results suggest that non-native fish species invasions in the North Aegean Sea are dynamic. Although most species were classified as casual, and the frequencies of occurrence as well as the quantities in commercial fisheries catches of established species are smaller than in the South Aegean Sea, species introductions and the extent of occurrence and area of occupancy of several species have been increasing since the 2000s. The significance of non-native fish species is becoming increasingly relevant to commercial fisheries in the study area. This is particularly evident in the Northeastern Aegean Sea, where catches of non-native species are on the rise, and new species with potential commercial value have emerged in recent years.
Introduction
More than 1,000 marine non-native species have been recorded to date in the Mediterranean Sea (Galanidi et al., 2023). Hundreds of these species are Lessepsian, i.e., Red Sea endemics and species of Indo-Pacific biogeographical origin that have been entering via the Suez Canal since its opening in 1869 (Galil et al., 2017). Several Lessepsian species have expanded their invaded ranges northwards and westwards, leading to a compositional modification of the regional marine flora and fauna known as the “tropicalization” of the Mediterranean Sea (Bianchi and Morri, 2003). Fish, being a part of the food web and crucial to humans, are the most studied non-native species group in the Mediterranean (Azzurro et al., 2022a). More than a hundred have been introduced via the Suez Canal (Golani et al., 2021), and at least 51 have reached the Greek seas, mostly dispersing naturally along the Levantine coastline (Zenetos et al., 2020).
Scientific research on marine biological invasions has grown vigorously in the past few decades (Sagarin and Pauchard, 2010; Thomaz et al., 2015; Vaz et al., 2017). Species distribution modeling and ecosystem modeling are examples of modern approaches commonly applied to assess the current state or make future predictions regarding marine biological invasions (Corrales et al., 2020). These tools require comprehensive spatially explicit field observations across space and/or time but are often constrained by a lack of data (Corrales et al., 2017; Michailidis et al., 2019; Saygu et al., 2020), as most of the published information comprises first records of non-native species in newly invaded areas, with a few recent exceptions (e.g., Katsanevakis et al., 2020; Azzurro et al., 2022b; Ragkousis et al., 2023b). Data collection from commercial fisheries and research surveys at sea, through the implementation of multiannual programs according to the EU Data Collection Framework (DCF), can provide comprehensive, georeferenced information on non-native species occurrences, complementing data sourced from underwater biodiversity surveys, citizen science initiatives and other non-native species-targeted surveys. Additionally, the above-mentioned data are typically characterized by enhanced temporal and spatial scales and are often collected from offshore locations and/or large depths, where fieldwork may be difficult due to constraints of accessibility or cost. Therefore, the insights gained from analyzing those fisheries data can contribute significantly to advancing research in the field of marine biological invasions (Lefkaditou et al., 2010).
Although the largest species richness and most records of non-native fish species in the Aegean Sea occur in its southernmost part (Zenetos et al., 2020; Ragkousis et al., 2023a), several species have also been recorded in the colder and less haline North Aegean Sea. However, information on their distributions is fragmentary and largely restricted to first records. The North Aegean Sea has been identified as one of the Mediterranean hotspots characterized by a seawater warming trend over the last four decades (Pisano et al., 2020), and the area has recently experienced intense and long-duration marine heatwaves (Juza et al., 2022). Moreover, it has been predicted that the areas of the Mediterranean with the coldest waters, i.e., the Adriatic Sea, the Gulf of Lions and the Aegean Sea, will experience species loss in the coastal fish assemblages by the end of the 21st century (Ben Rais Lasram et al., 2010; Albouy et al., 2013; Libralato et al., 2015). Consequently, any niches left empty may open “invasion windows” for Lessepsian species (Johnstone, 1986; Oliverio and Taviani, 2003). This potential gradual restructuring of the biotic communities may affect ecosystem functioning and impact human activities in the area, similar to what is already happening in other parts of the Eastern Mediterranean (Edelist et al., 2013; Albano et al., 2021). The North Aegean is a productive sea within the generally oligotrophic Eastern Mediterranean, with high fisheries catches, particularly for small pelagics (Machias et al., 2007). Therefore, it is imperative that all available information on non-native species distributions in the area is compiled to accurately assess the current state of play and deliver a baseline study for the evaluation of future changes.
Publications regarding the importance of non-native species in commercial fisheries catches are scarce in Greece. Most published data were produced in experimental fishing surveys of diverse methodological approaches that were usually short-term and carried out in geographically restricted areas (e.g., Peristeraki et al., 2006; Corsini-Foka et al., 2015, 2017; Peristeraki et al., 2017). Most available information focuses on the South Aegean Sea, where the presence of non-native species is conspicuous (Corsini-Foka et al., 2010; Lefkaditou et al., 2010; Zenetos et al., 2020), whereas their presence in fisheries catches in the Northern Aegean Sea is, to our knowledge, largely undocumented. The only Greek fisheries data that are openly available are those kept by the Hellenic Statistical Authority (ELSTAT, 2023). However, these data do not include information on non-native species, as catches of such species are often reported by the fishers aggregated with the catches of related native species. Therefore, the DCF offers a unique opportunity to monitor non-native species spreading across the Greek seas and collect long-term data regarding their ranges and interactions with fisheries, following a systematic and comprehensive approach. To our knowledge, the only publication presenting a comprehensive review of fisheries-dependent data on the non-native species fished in the Greek seas is that by Lefkaditou et al. (2010).
This study is a review of the current state regarding the occurrences and importance for fisheries of non-native fish species in the North Aegean Sea, based on (a) an up-to-date systematic review of the international scientific literature, and (b) a compilation of data collected from the commercial fisheries and research surveys at sea from 2016 to 2023, implementing the Greek multiannual program according to DCF. This study aims to provide new information that may assist current and future research efforts regarding marine biological invasions and their effects in the area. The specific objectives of the study are (a) to reveal the current state of non-native fish species distributions, (b) to evaluate the present-day importance of non-native fish species to commercial fisheries, and (c) to assess trends in non-native fish species geographic range expansion and importance to commercial fisheries in the study area.
2 Materials and methods
2.1 Study area
The study area comprised the North Aegean Sea (Figure 1) and was spatially divided into four sub-areas based on the Greek multiannual program: Northeastern Aegean Sea (CHIO-MIT), Thermaikos Gulf and adjacent seas (THERM), Thracian Sea (THR-LIM), and Thessaly and Sporades Islands (VOL-SPOR). The North Aegean Sea experiences the influence of a low-temperature and low-salinity (< 29 psu) plume of waters originating from the Black Sea (BSW) flowing through the Dardanelles Strait. In contrast, the Northeastern Aegean Sea is influenced by warm and highly saline (> 38.5 psu) waters originating from the Levantine Sea (LW) (Zervakis and Georgopoulos, 2002). Commercial fisheries in the North Aegean Sea are characterized by high catches of small pelagic fish predominantly captured by purse seiners. Bottom trawls are employed to target hake, mullets, shrimps, and cephalopods, while multi-gear small-scale coastal fisheries target a variety of pelagic and demersal species (Tsagarakis et al., 2010). In 2022, the total quantity of small- and medium-scale fisheries landings in the North and Northeastern Aegean Sea reached 35,636 tonnes, constituting 55% of the overall Greek fisheries landings. The highest landings were observed in the Thracian Sea (16,299 tonnes) and the area of Thermaikos Gulf and adjacent seas (14,121 tonnes), while landings in the Northeastern Aegean Sea amounted to 3,630 tonnes (ELSTAT, 2023).
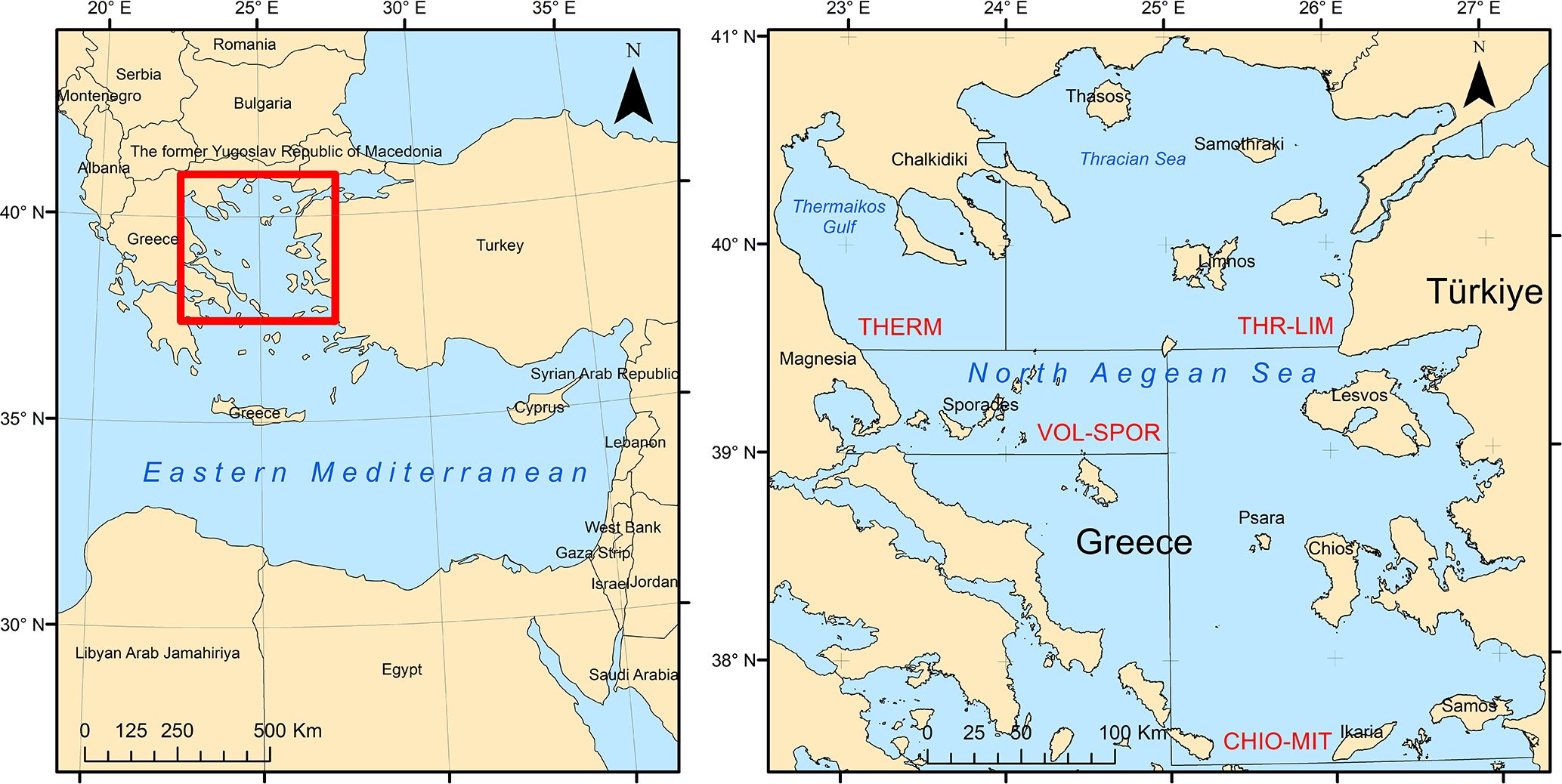
Figure 1 Map of the study area depicting the four subareas of the Greek multiannual program in the North Aegean Sea: CHIO-MIT = Northeastern Aegean Sea; THERM = Thermaikos Gulf and adjacent seas; THR-LIM = Thracian Sea – Limnos Island; and VOL-SPOR = Thessaly – Sporades Islands.
2.2 Systematic review of the scientific literature
A systematic review of the international scientific literature was performed using the extension of the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) approach developed for systematic reviews in ecology and evolutionary biology (Moher et al., 2009; O’Dea et al., 2021; Page et al., 2021). The aim of the review was to collect all scientific publications that contained spatial-temporal information on the distributions of non-native fish species in the study area. Records of range-expanding Atlantic species that were introduced in the Mediterranean via the Gibraltar strait (Evans et al., 2020) were also selected, as these species are often included in non-native species lists (e.g., Golani et al., 2021). The following information was retrieved: source of information, species, year of observation, subarea, country, location, geographic coordinates, depth, type of observation, substrate, and abundance. The implementation of the steps of PRISMA and the data cleaning performed are detailed in Supplementary File 1. The compiled information was tabulated, such as every row in the table contained the data retrieved for a particular species record (Supplementary File 1).
2.3 Preparation and compilation of field data
The compiled field data were primarily collected from: (a) the monitoring of commercial fisheries operations (2016–2023), and (b) the MEDITS bottom trawl scientific surveys (1996–2022). Records from commercial fisheries monitoring were included with the caveats that the 2016 records in the Fisheries Research Institute (FRI) database were incomplete and data collection in 2023 was still in progress. Additional species records based on samples provided by commercial fishers to FRI staff during 2007–2021 were also incorporated in the dataset. The commercial fisheries monitoring samples of 2016 and 2023 and the samples provided by commercial fishers were solely used in the compilation of the species inventory and the analysis of the spatial – temporal patterns in the distributions of the non-native fish species in the study area.
The compiled field data consisted of species abundance, biomass, and the associated spatial-temporal metadata, i.e., geographical coordinates, subarea, depth, and sampling year. For commercial fisheries samples, information regarding the observation type (on-board or onshore), the fishing gear used, and whether the species were landed or discarded, were also included. The sizes of samples are provided in Supplementary File 2. Non-native fish were recorded in samples caught with the following fishing gear: set gillnets (GNS), trammel nets (GTR), bottom otter trawls (OTB), beach and boat seines (SB-SV), purse seines (PS), set longlines (LLS), and pots/traps (FPO). Additionally, some non-native fish species individuals provided by professional fishers were caught with hand lines and pole lines (hand operated, LHP) and in stationary uncovered pound nets (FPN).
WoRMS (WoRMS Editorial Board, 2023) was followed for the taxonomy and nomenclature of the species and FishBase (Froese and Pauly, 2023) for the characterization of their habitat. The biogeographic origin and the mode of introduction in the Mediterranean Sea of each species was determined based on Golani et al. (2021), and the scientific literature for species cases not treated therein. The years of first records and establishment status in Greece were obtained from Zenetos et al. (2020) and other scientific literature.
The establishment status of non-native fish species in the study area was assessed based on the operational criterion of at least three valid records well separated in space and/or time, or, alternatively, based on strong evidence of local, self-maintaining populations (Zenetos et al., 2010; Golani et al., 2021). In particular, to be classified as established in a subarea, a species should have records spread across a minimum distance of 100 km and/or from at least 3 consecutive years. These assessments were based on both the compiled field data and the results of the systematic review of the scientific literature. A species was classified as established in the study area if it was assessed as established in at least one subarea. The dispersal dynamics of the species were assessed without any reference to impacts, while the cases of rapid dispersal at multiple sites in the assessment area were classified as potentially invasive.
2.4 Data analysis
The cumulative occurrence records of all non-native fish species, derived from both field data and literature review results, were mapped across three temporal intervals: the 2000s, 2010s, and 2020s. This visualization aimed to elucidate the spatio-temporal dynamics of fish invasions within the study area (Azzurro et al., 2022a). For onshore records with no geographic coordinates available the maps depict the port location. Moreover, additional maps of cumulative occurrence records for the entire study period were produced for each species with at least ten records. Temporal dynamics was also illustrated by plotting cumulative numbers (%) of non-native fish species as a function of time for the period 1999 – 2023. The record of Lagocephalus spadiceus by Ananiadis (1952) was omitted from the plots. The cumulative numbers (%) of non-native fish species records and of the publications citing such records were also plotted to evaluate the effect of research effort. Additionally, the cumulative numbers of all non-native fish species, those assessed as established and those assessed as casual according to the operational criterion adopted, were plotted in separate graphs.
Lastly, the frequency of occurrence, the mean and maximum abundance and biomass, as well as the mean and maximum relative abundance and biomass were estimated for every non-native fish species. Estimates were performed for each combination of gear, subarea and year with non-native fish records. The MEDITS data were summarized for each combination of subarea and year.
All data analyses were carried out in R (R Core Team, 2023) and all maps were produced with the ArcGIS Pro software (ESRI, 2023).
3 Results
3.1 Non-native fish species distributions and establishment status in the study area
The systematic review of the scientific literature resulted in 185 published records of non-native fish species in the study area, in both Greece and Turkey (Supplementary File 1). After data cleaning, the records corresponded to 32 species (Table 1; Supplementary Files 1, 3). Eleven of these species were recorded solely in Turkish territorial waters. The compilation of the field data resulted in a dataset of 251 unpublished records, corresponding to 20 non-native fish species (Table 1; Supplementary File 3). Specifically, 224 field records were obtained from commercial fisheries, ten from the MEDITS surveys, and 17 records corresponded to specimens provided by commercial fishers and taxonomically verified by FRI staff (Supplementary File 3). The combination of all datasets resulted in an inventory of 37 non-native fish species with records in the study area, belonging to 20 orders and 26 families (Supplementary File 3). The most species-rich family was the Tetraodontidae, with six species.
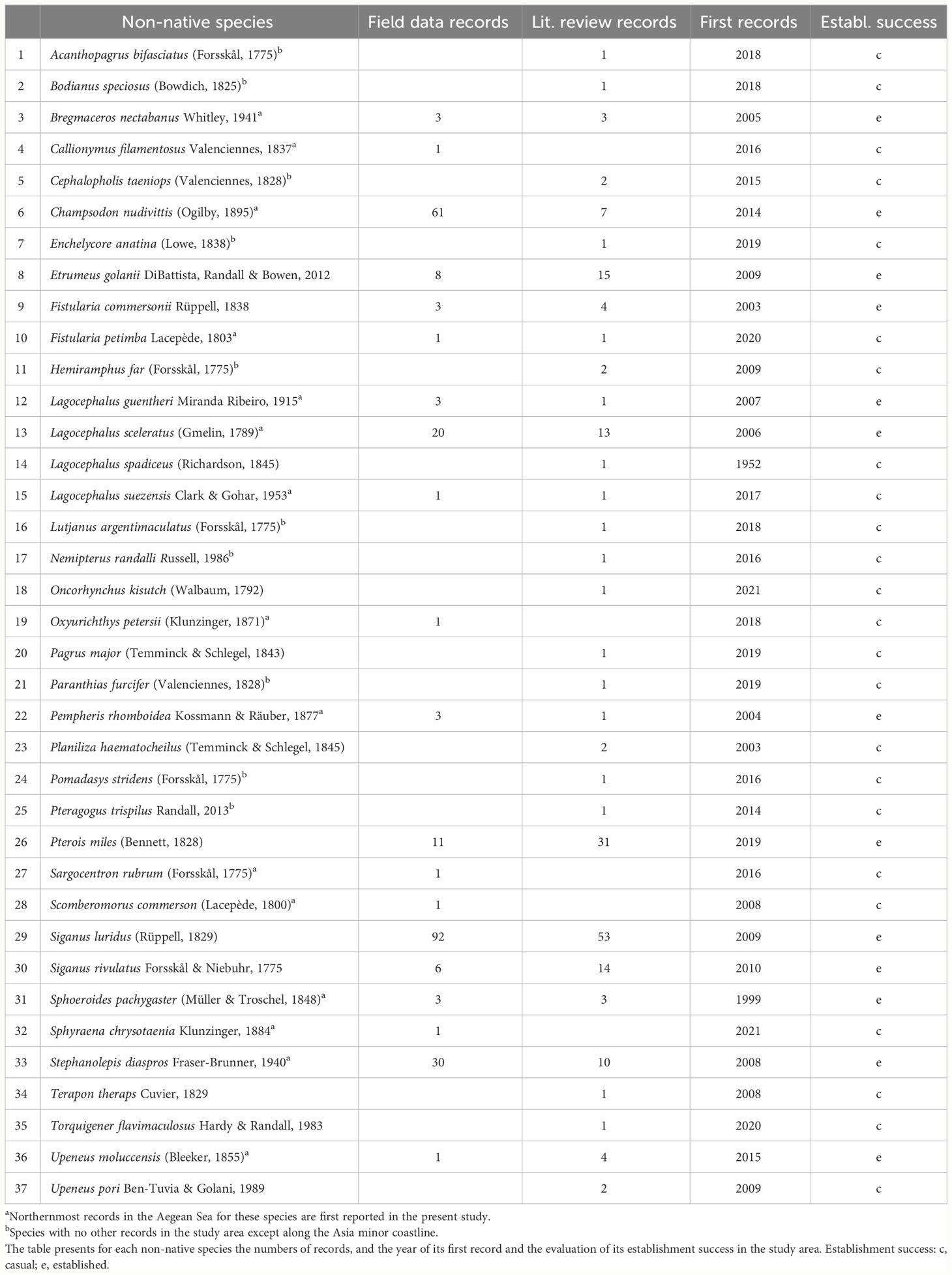
Table 1 Inventory of non-native fish species with records in the North Aegean Sea in the compiled field data (1996 – 2023) and/or found in the systematic review of the scientific literature.
The field dataset includes the first records of four species in the study area: Callionymus filamentosus, Oxyurichthys petersii, Sargocentron rubrum, and Sphyraena chrysotaenia. This study also provides georeferenced metadata for the sole, so far, record of Scomberomorus commerson in the North Aegean Sea. Furthermore, the field data also include records that chronologically precede all previous published records of several species in Greek waters or the study area: the record of Fistularia petimba in the Thracian Sea (THR-LIM) in 2020, chronologically precedes the oldest published record of this species in Greece, off Samos Island in 2021 (Kondylatos and Nikolidakis, 2021). Furthermore, the records of Lagocephalus guentheri and Lagocephalus suezensis (Supplementary File 4) chronologically precede their previously published sightings in the study area. Finally, the field data include the until today northernmost records in the Aegean Sea of Bregmaceros nectabanus, Champsodon nudivittis, F. petimba, L. guentheri, Lagocephalus sceleratus, L. suezensis, Pempheris rhomboidea, Sphoeroides pachygaster, Stephanolepis diaspros, and Upeneus moluccensis (Table 1; Supplementary File 3).
The two specimens of L. suezensis that were recorded off Chalkidiki in 2017 were caught along with similarly sized juveniles of L. sceleratus (Supplementary File 3). The back of these specimens had dark spots of diverse sizes and irregular shape, whilst L. sceleratus juveniles had similarly sized dark spots of more regular shape. Both specimens were verified taxonomically with DNA barcoding and all barcodes resulted in top species matches for L. suezensis above 99.0% identity on GenBank (both samples provided partial barcodes, i.e., ~ 280 bp and 610 bp (Supplementary File 5).
Most of the recorded species are tropical species of Indo-Pacific origin or Red Sea endemics (28); only four have native geographic ranges in the Atlantic Ocean, three species are natively distributed in both the Indo-Pacific and the Atlantic Oceans, and only one has a cosmopolitan distribution (Supplementary File 3). Moreover, 28 of the species are considered Lessepsian, two species are of Atlantic origin that expanded their ranges in the Mediterranean by introduction via the Gibraltar strait, and seven were introduced via other human-mediated mechanisms (Supplementary File 3). Most species are reef-associated (18) or demersal (10), whilst the rest are pelagic (8) or benthopelagic species (1) (Supplementary File 3). Nineteen species were recorded only from depths shallower than 50 m, whereas nine species were collected from depths down to 100 m (Supplementary File 3). Eight species had records in deeper waters (< 200 m), however only C. nudivittis was collected over the continental slope (down to 315 m). The most eurybathic species (bathymetric range in the data > 100 m) were Siganus luridus, C. nudivittis, Etrumeus golanii, B. nectabanus, U. moluccensis, Pterois miles, S. diaspros, and S. pachygaster.
The establishment success in Greek waters for 27 of the recorded species (Supplementary File 3) was classified according to Zenetos et al. (2020): most of them (18) have established populations in Greek waters, four are considered invasive and the rest (4) are casual, while the establishment status of Pomadasys stridens was designated as “unknown”. The assessment of the establishment success in Greece of the remaining recorded species was based on the scientific literature and the authors’ judgement. Eight species were designated to be casual in Greece and two to be established. Establishment success in the study area was operationally assessed as follows: most species are casual (24) and the rest (13) are established in at least one subarea. The subarea with the most established species was the Northeastern Aegean Sea (CHIO-MIT) (11), whereas the other subareas had similarly low numbers of established species (3 - 4). Lagocephalus sceleratus was the only species with established populations in all subareas, followed by C. nudivittis and S. diaspros (three subareas each).
Interestingly, the field data provided strong evidence for the occurrence of self-sustaining, reproducing populations of L. sceleratus in the North Aegean Sea in the late 2010s to early 2020s: 42 juveniles (53 - 140 mm TL) were caught along the coasts of Northern Greece during the period 2017 – 2021 (Supplementary File 3). Most of them (36) were caught off Chalkidiki, in October and November 2017. The rest were caught at several locations in the Gulf of Kavala in 2020 and 2021. Local fishers also provided large-sized, adult specimens of L. sceleratus (500 - 700 mm TL) caught in the Gulf of Kavala (four specimens) in 2008 and 2011 and one specimen from Thermaikos Bay in 2021.
3.2 Spatio-temporal patterns in the distributions of non-native fish species
The exact numbers of non-native species shared between subareas and the species occurring in only one subarea are depicted in Figure 2. The number of species that were uniquely present in a single subarea was far larger in CHIO-MIT (16) than in THERM and THR-LIM (3 each) or in VOL-SPOR (1). Most of the species present in THERM, THR-LIM and VOL-SPOR were also present in CHI-MIT.
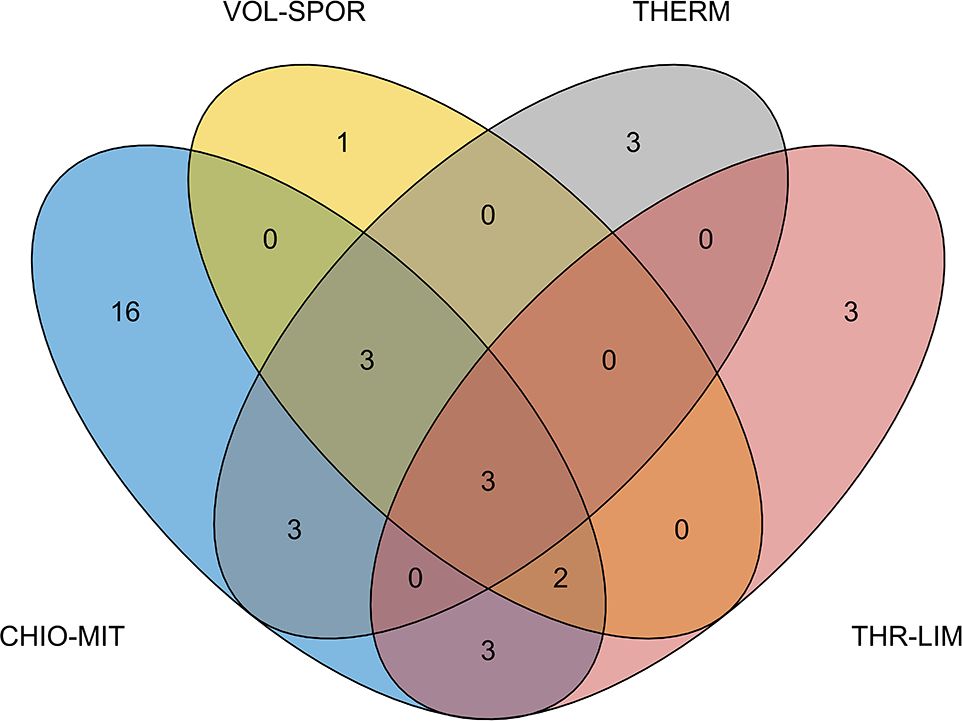
Figure 2 Venn diagram depicting non-native fish species turnover between subareas based on the results of the systematic review of the scientific literature and the field data compiled in this study. The plot indicates the numbers of non-native species shared between subareas or recorded in a single subarea. Subareas of the Greek multiannual program: CHIO-MIT = Northeastern Aegean Sea; THERM = Thermaikos Gulf and adjacent seas; THR-LIM = Thracian Sea – Limnos Island; and VOL-SPOR = Thessaly – Sporades Islands.
Cumulative occurrence maps (Figures 3, 4) indicate that records of non-native species were more numerous and widespread in the 2020s (252 records) than in the 2010s (152), while records from the 2000s were comparatively few (27). This trend was observed at the subarea level as well, except for THR-LIM, where records in the 2000s and 2010s were similar (8 and 7, respectively) but still fewer than in the 2020s (33). Most records were from CHIO-MIT (315), while the numbers of sightings in the other subareas were comparable (29 - 49). The apparent pronounced increase in non-native species records in the 2020s in THERM and THR-LIM is attributable to the rapid range expansion of C. nudivittis that has been occurring since 2020 (Figure 4).
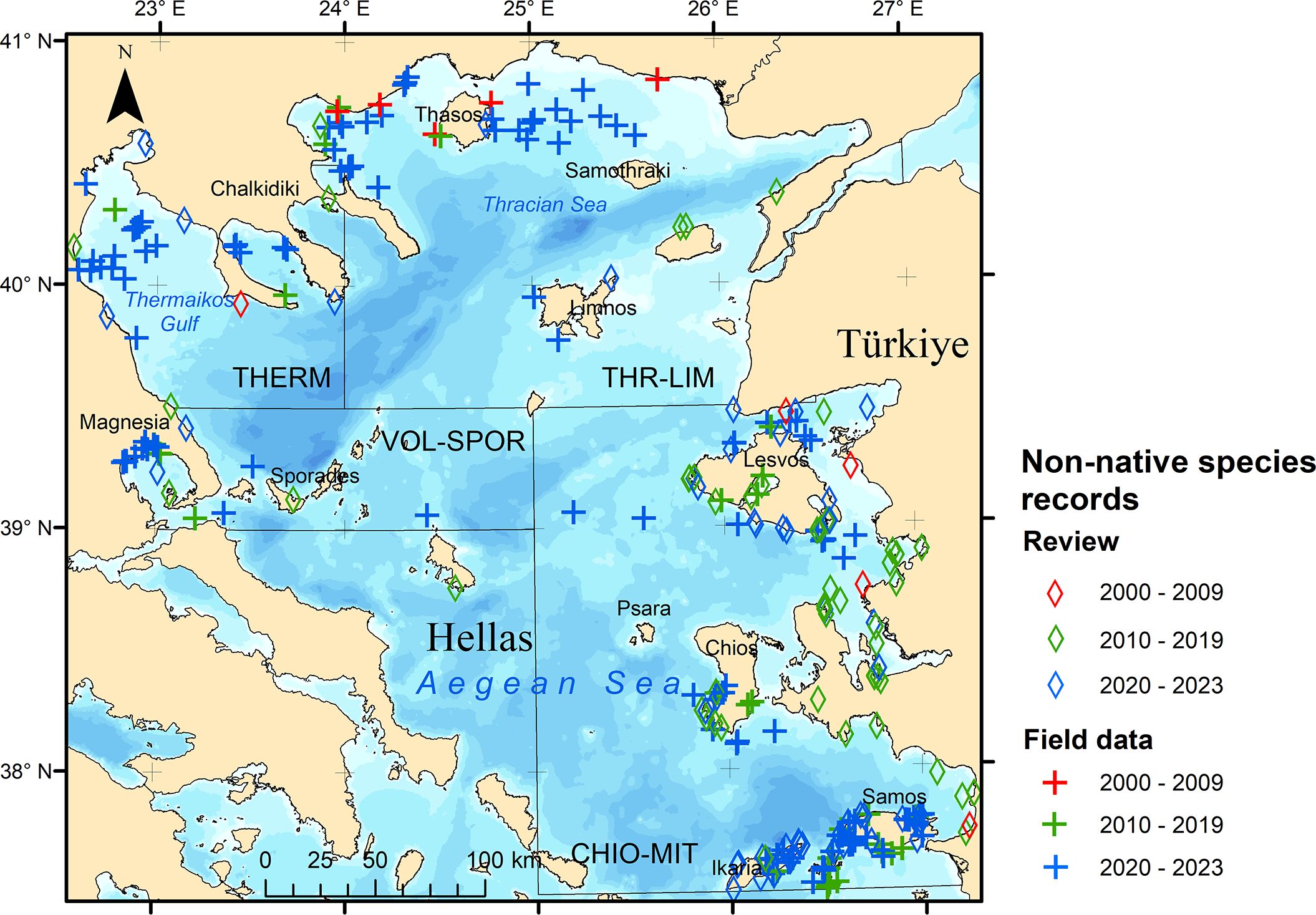
Figure 3 Cumulative occurrences of non-native fish species that have been recorded in the study area, based on the systematic review of the scientific literature (diamond symbols) and the field data compiled in this study (cross symbols). The records made in each decade are depicted in different colors: red (records made until 2009), green (records made during 2010 – 2019) and blue (records made since 2020). For the onshore records with no field geographic coordinates available, the map depicts the port locations.
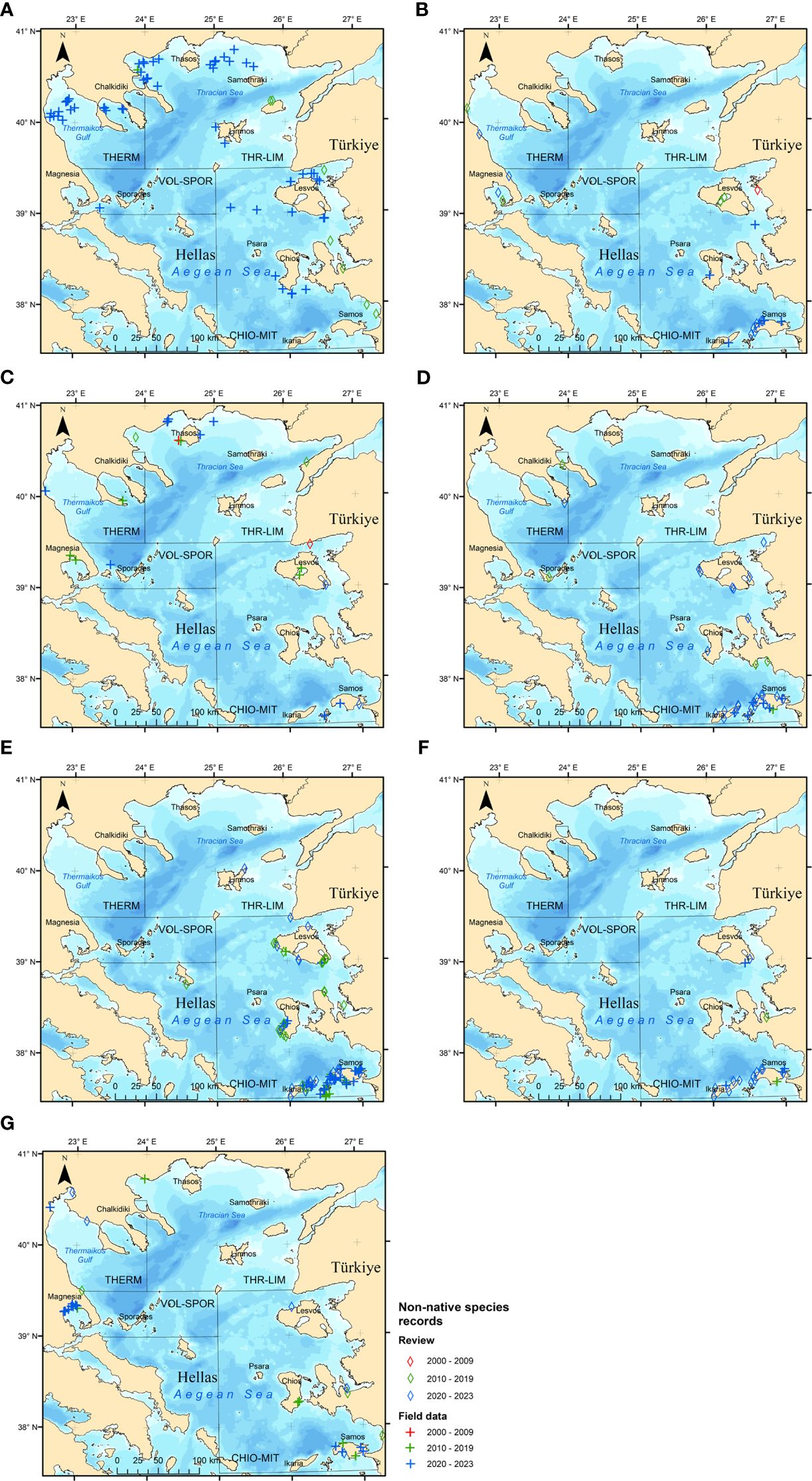
Figure 4 Cumulative occurrences of the non-native fish species with more than ten records in the study area, based on the systematic review of the scientific literature (diamond symbols) and the field data compiled in this study (cross symbols). The records are depicted in different colors depending on their dates: red (records made until 2009), green (records made during 2010 – 2019) and blue (records made since 2020). For the onshore records with no field geographic coordinates available, the maps depict the port locations. Species: (A) Champsodon nudivittis, (B) Etrumeus golanii, (C) Lagocephalus sceleratus, (D) Pterois miles, (E) Siganus luridus, (F) Siganus rivulatus, (G) Stephanolepis diaspros.
Research effort regarding non-native fish species distributions in the study area, defined as the number of publications selected in the literature review, increased over time since the late 1990s (Figure 5A). The cumulative number of reported species also increased over time at a similar rate. The accumulation of publications and species numbers over time is best described by a linear function, with approximately half of the studies published and half of the species recorded by 2015. However, the accumulation of records over time is best described by an exponential function, with almost 90% of the sightings made after 2015.

Figure 5 Temporal progression of the dispersal of non-native fish species in the study area over the period 1999 – 2023, based on the systematic review of the scientific literature and the field data compiled in this study: (A) plot of the cumulative numbers (%) of non-native species, of non-native species records and of the publications citing non-native fish species records in the study area. Best fit lines of linear models fit to the non-native species numbers and publications numbers data and best fit curve of an exponential model fit to the non-native species records data are also depicted in the plot, as are the respective coefficients of determination (R2), (B–D) plots of the cumulative numbers of all non-native species, the non-native species assessed as established and the non-native species assessed as casual in the study area based on operational criteria. Subareas of the Greek multiannual program: CHIO-MIT = Northeastern Aegean Sea; THERM = Thermaikos Gulf and adjacent seas; THR-LIM = Thracian Sea – Limnos Island; and VOL-SPOR = Thessaly – Sporades Islands.
The rate of increase in the cumulative number of non-native species differs between subareas (Figures 5B–D), with a higher rate in CHIO-MIT than in other subareas. Non-native species numbers have continuously increased in CHIO-MIT since the late 1990s, whereas a significant increase in species numbers in the other subareas did not start before 2015. The temporal increase in the numbers of established species (Figure 5C) was much higher in CHIO-MIT than in the other subareas, where most species remained casual.
3.3 Non-native fish species effects on commercial fisheries
A total of 14 non-native fish species were recorded in the commercial fisheries monitoring samples during 2017–2022, which were caught with the following gear: GTR, GNS, OTB, PS, FPO, SB-SV, and LLS (Supplementary File 3). The species most frequently caught across gears, subareas and years was S. diaspros (18 combinations), followed by S. luridus (13), C. nudivittis (9), P. miles (7), and L. sceleratus (6). Stephanolepis diaspros and C. nudivittis were recorded in fisheries catches in all four subareas, whereas L. sceleratus was caught in three subareas. Several species were caught in only one subarea, mostly in CHIO-MIT, with the frequently caught S. luridus and P. miles included among them. Stephanolepis diaspros and S. luridus have been consistently present in commercial fisheries catches throughout the study period. On the other hand, P. miles has been constantly recorded since 2019, and C. nudivittis and E. golanii since 2020. Detailed results of the assessment of the importance of non-native fish species by fishing gear are presented in Supplementary File 2.
The total number of the caught non-native fish species differed between gear, subareas, and years (Supplementary File 3): more species were recorded in OTB (8), GTR (7) and GNS (6) than in PS (3), SB-SV (2), FPO (1), and LLS (1) catches. The highest number of species was recorded in CHIO-MIT (11), whereas six species were caught in THR-LIM, and four in both THERM and VOL-SPOR. Moreover, far more species were recorded in 2022 (8), 2021 (11) and 2020 (9) than in the three preceding years (3 in each year). In most samples with non-native species catches, a single species was detected, two species were less common, whereas three species were caught once.
Total non-native fish species quantity in the samples pooled across subareas and gear generally followed an increasing trend with time, rising from 38 individuals and 4 kg in 2017 to a maximum abundance of 521 individuals in 2022 and a maximum biomass of 30 kg in 2021. Pooled across years and gear, total non-native fish species quantity in samples was greater in CHIO-MIT (1,008 individuals and 85 kg) than in the other subareas (46 – 185 individuals and 2 – 3 kg). Moreover, the highest total catches were recorded in OTB and GTR samples (671 and 577 individuals and 13 kg and 67 kg, respectively).
In the hauls that contained non-native species, total non-native species abundance (individuals/haul) reached up to 9 in GNS, 180 in GTR, and 105 in OTB catches across subareas and years combinations (Figure 6). The respective ranges for biomass (g/haul) were 5 – 1700 in GNS, 20 – 11,225 in GTR and 8 – 7200 in OTB catches (Figure 6). Relative total non-native species abundance ranged from 0.25 – 24.14%, 0.44 – 24.49% and 0.02 – 8.01% in GNS, GTR and OTB catches, respectively, while relative total non-native species biomass ranged between 0.05 – 12.37% in GNS, 0.08 – 23.28% in GTR, and 0.01 – 7.11% in OTB catches (Figure 7). Higher quantities and proportions of non-native species were recorded in CHI-MIT than in the other subareas. Also, the quantities and proportions of non-native species in GNS and OTB hauls were higher in the early 2020s than in the late 2010s.
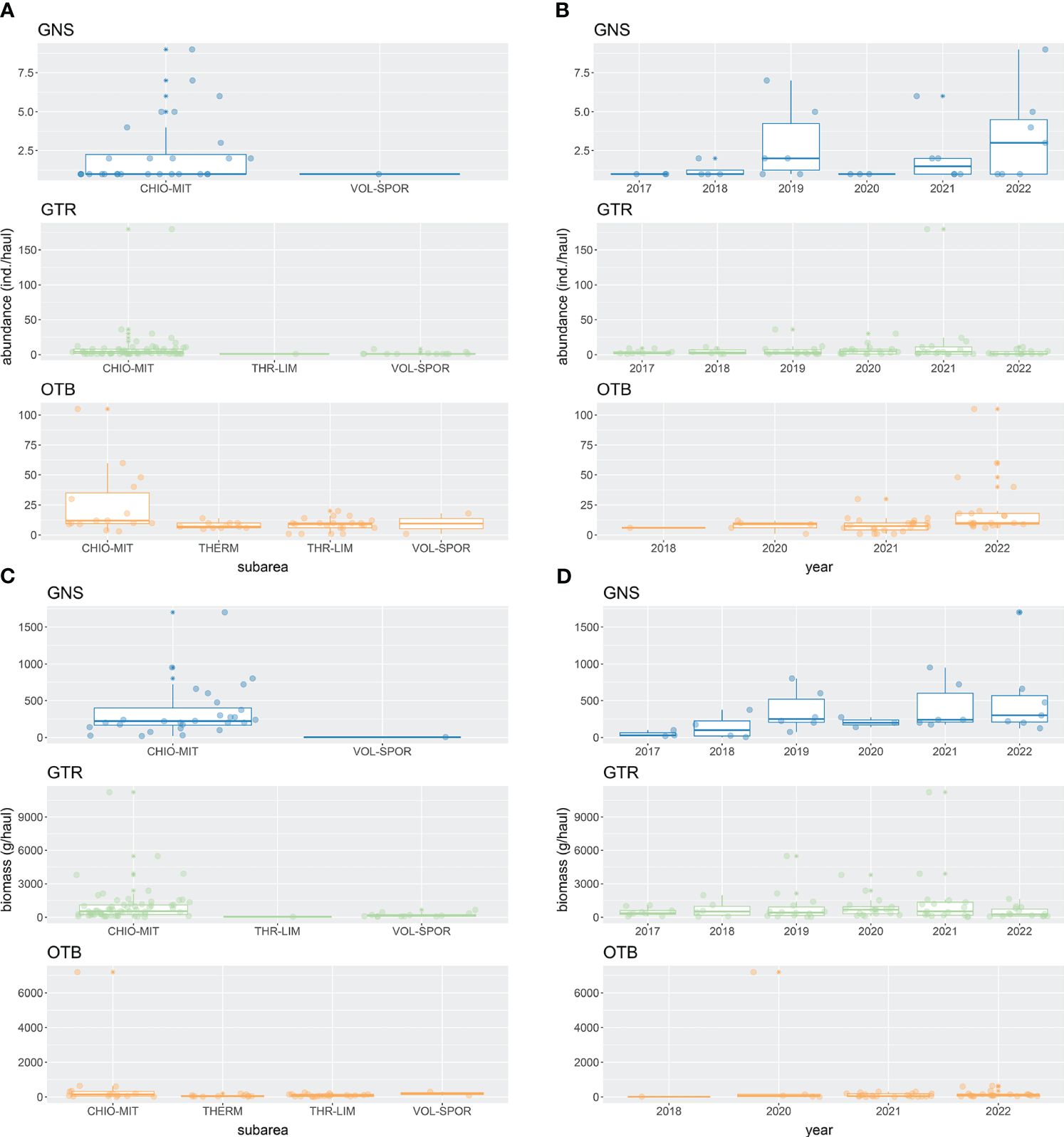
Figure 6 Boxplots summarizing the non-native fish species abundance (A, B) and biomass (C, D) per haul data across subareas and years. Only set gillnets (GNS), trammel nets (GTR) and bottom otter trawls (OTB) gear are considered. Only the hauls with at least one non-native species are represented in the plots. Data points are overlaid on the boxplots. Subareas of the Greek multiannual program: CHIO-MIT = Northeastern Aegean Sea; THERM = Thermaikos Gulf and adjacent seas; THR-LIM = Thracian Sea – Limnos Island; and VOL-SPOR = Thessaly – Sporades Islands.
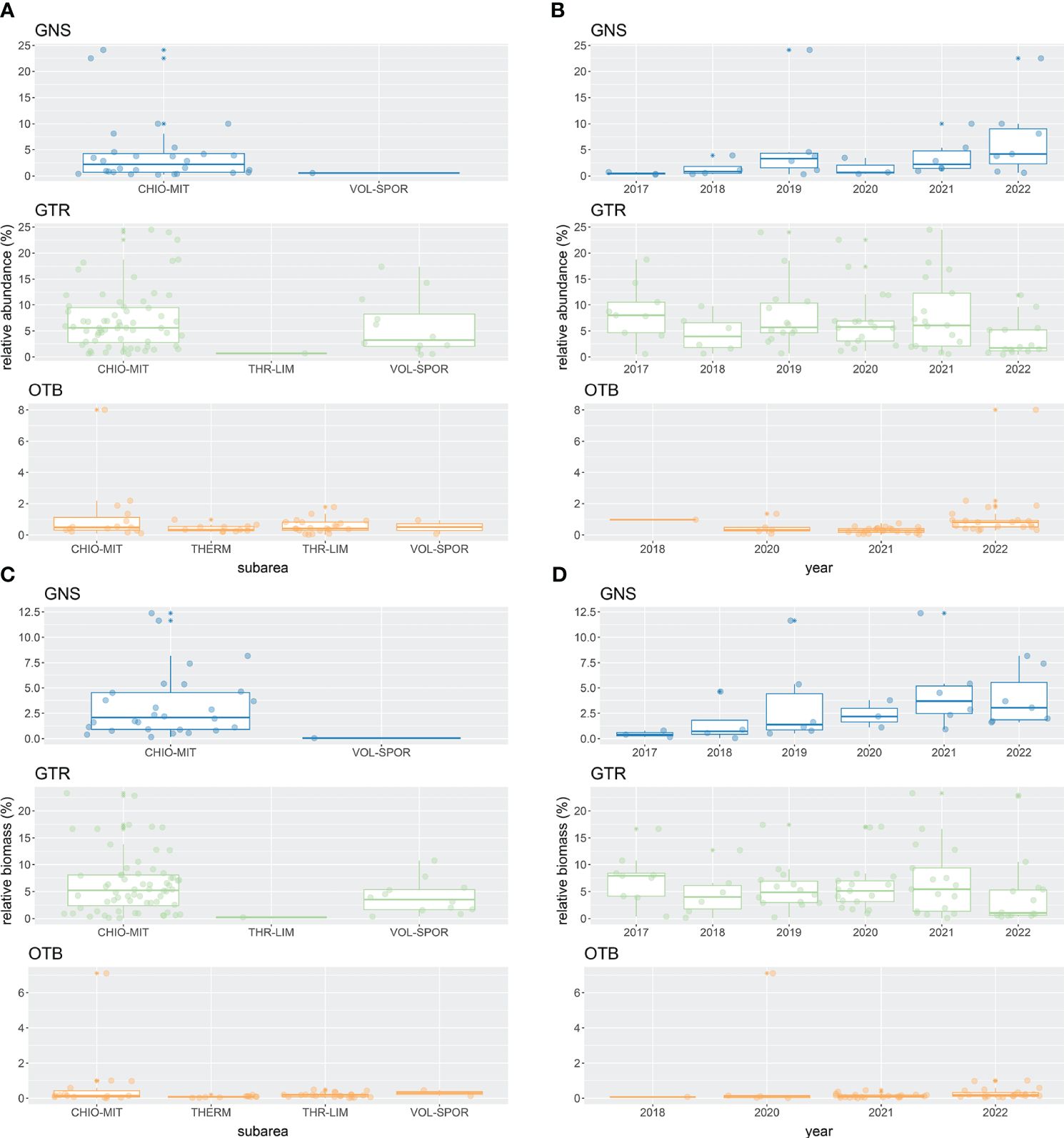
Figure 7 Boxplots summarizing the non-native fish species relative abundance (A, B) and biomass (C, D) per haul data across subareas and years. Only set gillnets (GNS), trammel nets (GTR) and bottom otter trawls (OTB) gear are considered. Only the hauls with at least one non-native species are represented in the plots. Data points are overlaid on the boxplots. Subareas of the Greek multiannual program: CHIO-MIT = Northeastern Aegean Sea; THERM = Thermaikos Gulf and adjacent seas; THR-LIM = Thracian Sea – Limnos Island; and VOL-SPOR = Thessaly – Sporades Islands.
All non-native species were discarded except for E. golanii and S. chrysotaenia, which were landed, and S. luridus, which was either landed or discarded on a case-by-case basis.
4 Discussion
This study enhances our understanding of non-native fish distributions in the North Aegean Sea by synthesizing pertinent information from scientific literature and presenting unpublished occurrence records for several species in Greece, including several first-time records. The establishment success of most species was assessed as casual, with lower frequencies of occurrence and quantities in commercial fisheries catches compared to the South Aegean Sea. Nevertheless, non-native fish species invasions in the study area are dynamic, as evidenced by the overall increasing trend of species introductions, extent of occurrence, and area of occupancy observed for several species since the 2000s. The rapid dispersal of C. nudivittis and the substantial increase in the areas of occupancy of E. golanii, P. miles, and Siganus spp. in the Northeastern Aegean in the 2020s suggest their potential classification as invasive. Moreover, the significance of non-native fish species is growing in commercial fisheries, particularly in the Northeastern Aegean Sea, where non-native fish species catches are increasing, and new species of potential commercial interest have emerged in recent years.
4.1 Distributions of non-native fish species in the study area: current state
The compiled inventory encompasses records of 37 non-native fish species. The most recent published list (Zenetos et al., 2020) included 13 non-native fish species with records in the North Aegean Sea MSFD assessment area. All these species are included in this study, except Saurida lessepsianus, not found in the literature, and Tylosurus crocodilus, excluded due to questionable records. This work updates the list of Zenetos et al. (2020) with the first records in the study area of C. filamentosus, O. petersii, S. rubrum, and S. chrysotaenia. Additionally, a further seven species were not included in Zenetos et al. (2020) because their presence was reported after 2020, while 11 species with records solely in Turkish territorial waters were also incorporated into the inventory. The present study also provides evidence that potentially supports the upgrade of the O. petersii status from casual (Zenetos et al., 2020) to established; there are two previous records of O. petersii in the Aegean Sea, in Gokova Bay in 2005 (Akyol et al., 2006) and in Kastellorizo Island in 2010 (Apostolopoulos and Karachle, 2016). This study adds a third record of six individuals caught in Thermaikos Gulf in 2018.
Thirteen species were assessed using operational criteria as established in the study area. The large numbers of records (≥ 20) of C. nudivittis, E. golanii, L. sceleratus, P. miles, Siganus spp., and S. diaspros were deemed robust enough evidence for their establishment success in at least a part of the study area. In contrast, the actual establishment status of the species with few known occurrences that were operationally classified either as casual or as established was regarded as uncertain. Small numbers of records for a non-native species in an area, often at large distances apart, may suggest an ability for long-range dispersal and the existence of small satellite populations of the species at the periphery of its range (Lockwood et al., 2013). However, small populations of a non-native species may persist undetected due to insufficient research effort (false absences), while non-native species occurrence data may be sparse because collected information often remains unpublished. An example of the latter case is S. commerson, for which there are two published records in Greek waters, in the North Aegean Sea (this study) and off Rhodes Island (Corsini-Foka and Kalogirou, 2008), while it is known to be targeted by fisheries in the Dodecanese islands (Corsini-Foka et al., 2010). Another potential cause for false absences is the occasional taxonomic misidentifications: for example, F. petimba, L. suezensis, S. commerson, and S. chrysotaenia had only 1 - 2 published records in the study area, but these species closely resemble other taxonomically related species that are more familiar, so it is possible that they have occasionally been misidentified. Nevertheless, small non-native species populations may eventually lead to their local extinction instead of their establishment, due to factors such as environmental and demographic stochasticity, Allee effects, or biotic resistance (Lockwood et al., 2013). On the other hand, it is also possible that a small non-native species population may persist for years undetected, but eventually give rise to rapid growth and a large-scale invasion (Azzurro et al., 2016).
Champsodon nudivittis is the only non-native fish species that has dispersed widely in the study area and is not common in the South Aegean Sea. There are published records of C. nudivittis along the Turkish coasts of the North Aegean Sea since 2014. Nevertheless, field data have revealed a significant expansion in the extent of occurrence and area of occupancy of this species since 2020, suggesting the possibility that it has become invasive. The Champsodontidae are moderately deep-water species, and therefore their invasion of the Mediterranean Sea through the Suez Canal was likely initiated via a larval stage (Stern et al., 2019). The occurrence of C. nudivittis in the deep waters of the Levantine has been viewed as an indication that its thermal niche may be wider than previously thought or that it changed after its introduction in the Mediterranean Sea (Galil et al., 2019). Its wide thermal niche and the dispersal abilities of its larval stage were considered plausible drivers for its range expansion in the study area. Similarly, E. golanii, P. miles, and Siganus spp. dispersal dynamics may also be classified as potentially invasive: They rapidly dispersed at multiple sites and steadily increased their areas of occupancy in CHIO-MIT in the 2020s. Monitoring the progress of dispersal dynamics of their populations would provide valuable information for accurately assessing their status in the area. In comparison, the extent of occurrence and the area of occupancy for the commonly found L. sceleratus and S. diaspros did not exhibit significant changes during the study period.
The failure of non-native species to establish self-sustaining populations can result from various factors such as small propagule size, distant habitat, limited dispersal, unsuitable abiotic conditions, and biotic resistance (Lockwood et al., 2013). It has been shown that the dispersal rate of Lessepsian fishes is influenced by temperature (Ben Rais Lasram et al., 2008; Raitsos et al., 2010). Additionally, recent research has also underscored the fundamental role of salinity in determining the distribution of Lessepsian fish species in the Mediterranean Sea (D’Amen et al., 2023). The majority of non-native fish species recorded in the study area originate from the Indo-Pacific, suggesting that many unsuccessful introductions may be attributed to unsuitable temperature and salinity conditions. Indeed, Black Sea Waters (BSW) enter the North Aegean Sea through the Dardanelles Strait and form a low salinity (< 29 psu), buoyant, 20 – 50 m thick plume that flows to the southwest and also northwards into the Thracian Sea (Nittis and Perivoliotis, 2002; Zervakis and Georgopoulos, 2002). Additionally, the North Aegean Sea is under the influence of the several small rivers discharging in the area, of greater importance in Thermaikos Gulf (Kourafalou and Barbopoulos, 2003). Furthermore, satellite data have shown mean annual sea surface temperature (SST) in Thracian Sea and Northwestern Aegean Sea coastal waters to range from 18 to 20°C, with minimum values from 9 to 13°C (Androulidakis and Krestenitis, 2022). In comparison, the higher establishment success rates of non-native fish species in the Northeastern Aegean Sea and especially in the South Aegean Sea can be linked to a more suitable abiotic environment. The Northeastern Aegean Sea is influenced by highly saline (> 38.5 psu) waters from the Levantine Sea, and coastal waters SST has an annual average of 19 – 20°C and a minimum of 12 – 15°C (Androulidakis and Krestenitis, 2022). In the Southeastern Aegean Sea, mean and minimum SST values in coastal waters are 20 – 23°C and 14 – 17°C, respectively (Androulidakis and Krestenitis, 2022).
The low non-native fish species establishment success in the North Aegean Sea could also be driven by basin circulation patterns. It is plausible to assume that the dispersal of Lessepsian species in the Aegean Sea has both reaction-diffusion and directional components (Lockwood et al., 2013): their populations reproduce and disperse northwards along the Asia Minor coasts and the neighboring islands (the reaction-diffusion component), and their propagules (larvae, young and mobile adults) follow the northwards flow of Levantine waters to the Northeastern Aegean Sea (the directional component). Between the Black Sea waters and the Levantine waters emerges a strong, dynamic thermohaline front (Zodiatis and Balopoulos, 1993) that affects biological communities and productivity, separating the more productive North Aegean Sea waters from the oligotrophic waters south of the front (Siokou-Frangou et al., 2002; 2009). A transition between different water bodies is a geographical barrier to the dispersal of non-native species (Ben Rais Lasram et al., 2008), and the aforementioned oceanographic front may constitute an environmental barrier to the further northward dispersal of thermophilic non-native species in the Aegean Sea.
4.2 Trends in the expansion of geographic ranges of non-native fish species in the study area and comparison with the South Aegean Sea and other areas in the Mediterranean
Zenetos et al. (2020) found that during 2012–2017, non-native species introductions in the North Aegean Sea and in the Greek Levantine were decreasing, whereas introductions in the South Aegean Sea were assessed as accelerating. However, the results of this study do not support a slowdown in Lessepsian fish species introductions in the North Aegean Sea in the 2010s, although new introductions do seem to approach a plateau in most subareas in the early 2020s. Moreover, this work revealed that non-native fish species records in the study area increased since the late 1990s at an exponential rate, with almost 90% of the sightings made after 2015, whereas the increases in non-native species and publication numbers were linear. The observed accelerating increase in the number of non-native species records could be the combined effect of the rapid increase in the geographic ranges of certain non-native species in the last few years, especially in the 2020s, and intensified research effort. Notably, it is only recently that studies compiling large numbers of previously unpublished, georeferenced non-native species records have appeared in the literature (Katsanevakis et al., 2020; Ragkousis et al., 2023b; the present study). Nevertheless, it can be inferred from the results of this and previous studies in the area that the “Lessepsian province” is still shifting northwards in the Aegean Sea, representing a dynamic transitional zone in the seascape across which biotic composition is responding to environmental change (Peters et al., 2006; Yarrow and Marín, 2007).
There are clear gradients in the distributions of Lessepsian fishes in the Mediterranean, as records along the longitudinal axis rapidly diminish in the direction of the Aegean Sea and the Strait of Sicily, becoming much less frequent in the western Mediterranean (Azzurro et al., 2022a). However, the biogeographical barriers represented by the Strait of Sicily and the North Aegean Sea exhibit a weakening due to climate change (Azzurro and D’Amen, 2022).
Indeed, the findings of this study regarding the numbers of non-native fish species and the proportion of the established ones contrast with the situation in more heavily invaded areas of the Levantine Sea, where more species, with larger populations, and higher proportions of established species have been reported. For example, eighty-four Indo-Pacific fish species had invaded the Israeli coastal shelf by 2013 (65.5% established), comprising more than half of the total fish abundance and biomass (Edelist et al., 2013). According to Çinar et al. (2021), Turkey has recorded 74 non-native fish species along its Levantine coasts, the majority characterized as established or invasive. The number of non-native fish species in Cyprus has been reported to be comparatively smaller than in other Levantine countries, with 35 species recorded there by 2014 (Iglésias and Frotté, 2015), all Lessepsian. Still, 80% of them had established populations.
Forty-four non-native fish species were recorded by Turkey in the Aegean Sea (Çinar et al., 2021), a number that is comparable to that reported in this study for the North Aegean Sea alone. More species and a higher proportion of established ones have been recorded along the South than in the North Aegean coasts of Turkey.
The Italian seas are less heavily invaded than the North Aegean Sea, with only 28 non-native fish species recorded across Italian waters (Servello et al., 2019; Tiralongo et al., 2020). Seventeen species have been recorded in the Central Mediterranean (6 established), 13 in the Western Mediterranean, most of Indo-Pacific origin (3 possibly established), and only 6 species in the western Adriatic, the majority Lessepsian. The non-native fish species that have been recorded in Spain are even fewer (13), and include four Lessepsian species, all designated as casual (Png-Gonzalez et al., 2023).
Almost 90% of the sightings of non-native fish species in the North Aegean Sea were made after 2015. In comparison, one-third of the species that have invaded the Israeli seas were introduced in the 2000s (Edelist et al., 2013). Furthermore, more than half of the species in Italian waters were detected for the first time during 2012–2017 (Servello et al., 2019). However, the overall number of new non-native species has reportedly decreased in Turkey in the Levantine and Aegean Seas during 2010–2020 (Çinar et al., 2021). Most of the aforementioned trends align with the general trend of massive geographic expansion of the ranges of non-native fish species in the Mediterranean after 1990 (Azzurro et al., 2022a).
4.3 Non-native fish species effects on commercial fisheries in the study area
Marine invasive species may have negative impacts on human activities, such as interfering with fishing operations and causing considerable revenue losses in the fisheries sector (Galanidi et al., 2018). On the other hand, several non-native species that have established large populations in their invaded ranges are potential fisheries targets (Van Rijn et al., 2020). Indeed, non-native species have favorably transformed some local fisheries, a foremost example being fisheries in Israel (Van Rijn et al., 2020), and to a lesser extent in Turkey (Gucu et al., 2010), or Cyprus (Michailidis, 2010; Evagelopoulos et al., 2020). At the same time, targeting by fisheries has been proposed as a promising management measure for controlling nuisance populations of non-native species (Nuñez et al., 2012; Kleitou et al., 2021; Ulman et al., 2022; Ofir et al., 2023). Fisheries-based management of non-native species should consider both benefits and costs of each species to ecosystems, ecosystem services and fisheries in a structured, iterative and adaptive framework (Kleitou et al., 2021).
Published information on the effects of non-native fish species on commercial fisheries in Greece is scarce. Only one study reviewed the impact of Lessepsian species on fisheries, reporting two species (F. commersonii and S. diaspros) recorded in boat seine catches in the late 2000s (Lefkaditou et al., 2010). The present study provides comprehensive data on the presence of 14 non-native fish species in commercial catches between 2017 and 2022, including MEDITS samples collected from the North Aegean Sea. Additionally, the field data include isolated records of L. guentheri, L. sceleratus, and S. commerson from the late 2000s.
However, most of the species recorded in commercial fisheries catches occurred infrequently and in low quantities. The Northeastern Aegean Sea stands out as the hotspot area for non-native fish species catches, where more species and larger quantities were recorded than elsewhere in the study area. Siganus luridus, E. golanii, and S. diaspros were the most frequently occurring species, comprising a maximum of 11.23 kg and 23.8% of the total fish biomass per sample. Nevertheless, the numbers of non-native fish species and the quantities caught have, on average, increased with time; pooled samples in 2021 had 3.7 times the number of species, 12.5 times the abundance, and 6.7 times the biomass of non-native fish species compared to 2017.
Higher species numbers and quantities of non-native fish species have been recorded in static nets catches in the South Aegean Sea than in the study area: non-native species represented 12 - 60% of the total fish species richness and 7 - 77% of the total fish biomass in 94 GNS samples collected off Rhodes in 2014–2015 (Corsini-Foka et al., 2017). Moreover, a total of seven non-native fish species in 92 commercial boat seine hauls were reported in the South Aegean Sea during the fishing period 2008–2009, with CPUE reaching, in the case of Siganus rivulatus, a maximum of 3.5 kg per haul (Lefkaditou et al., 2010). In the study area, non-native fish were comparatively less important in static nets catches, rarely exceeding one species per haul, while their mean relative abundance and biomass per haul did not surpass 1.33% and 0.99%, respectively. Moreover, on a boat seine survey that collected a total of 94 samples off Rhodes Island, 11 non-native fish species were recorded, while non-native fish species biomass per year ranged from 2.9 to 37.8 kg during 2002 – 2010 (Corsini-Foka and Kondylatos, 2015). Instead, small quantities of S. luridus and S. diaspros were recorded in a few samples in the monitoring of boat seine catches in the study area. Lastly, the number of species recorded in MEDITS and OTB samples in the study area during 2016 – 2023 (10 species) was similar to those recorded in the South Aegean in MEDITS and other scientific surveys during 1996 – 2014 (nine species) (Peristeraki et al., 2006; Lefkaditou et al., 2010; Peristeraki et al., 2017), but the only species present in bottom trawl catches in both Aegean Sea subbasins were S. pachygaster, S. diaspros, and U. moluccensis. Bottom trawl catches of non-native fish species have been generally small across the Aegean Sea.
The field data revealed that most non-native fish species catches in the study area are discarded, except for E. golanii and S. chrysotaenia, while S. luridus is landed or discarded on a case-by-case basis. It appears that the small catches and the absence of market interest result in low commercial value and thus no incentive to land most non-native fish species. Lefkaditou et al. (2010) commented that discarding was the fate of the major part of Lessepsian fish caught in Greek waters apart from Siganus spp., whereas Corsini-Foka et al. (2010) reported that Siganus spp., S. chrysotaenia, S. commerson, and E. golanii were commercially exploited in the Dodecanese islands in the early 2010s. More recently, F. commersonii was reported to have minor commercial value in the Southeastern Aegean Sea (Corsini-Foka et al., 2017). Currently, the above-mentioned species as well as Upeneus spp., Parupeneus forsskali, Scarus ghobban, Sphyraena flavicauda, and occasionally S. rubrum, are landed in the Southeastern Aegean Sea (Kondylatos G., personal communication).
The importance of non-native fish species for commercial fisheries in the study area is currently negligible in comparison with the situation in countries of the Levantine. For example, non-native species became a preferable target for Israeli commercial fishers due to their stable catch, high market value, and proximity to the shore, which reduces fuel costs (Van Rijn et al., 2020), while many species have become an important part of the catch also for recreational fishers (Frid et al., 2023). The CPUE of non-native fish species increased dramatically in Israel between 1996 and 2002 and continued to slowly increase up to 2013. At least a dozen non-native fish species are exploited by fisheries in Turkey, some of which have been target species since the 1970s (Bilecenoğlu, 2010). Finally, in Cyprus, in terms of commercial landings, 6 non-native taxa contributed to 29% of the total landings weight and 28% of the total landings value between 2017–2019 (Kleitou et al., 2022).
The analysis of the fisheries monitoring data revealed new (first records), more accurate (systematically collected across space and time), and more comprehensive (in terms of horizontal and bathymetric extent) information than the existing scientific literature, highlighting their significance as an important source for biological invasions research. However, monitoring commercial fisheries operations comes with several inherent limitations regarding data collection for non-native species. The locations sampled often align with fishing grounds that are regularly frequented by fishers, who primarily target commercially valuable species or those with high densities and marketable sizes (Pennino et al., 2016). Indeed, non-native species are typically not targeted by fishers in areas where they hold little to no value or are rare (Lefkaditou et al., 2010). Additionally, some non-native species are actively avoided by fishers due to the damage they cause to fishing gear or increased handling time (Galanidi et al., 2018; Huseyinoglu et al., 2023). While the use of on-board observers helps mitigate some of the drawbacks of fisheries-dependent data, their coverage of fishing activity may be limited (Pennino et al., 2016). Furthermore, the taxonomic skills of observers in identifying non-native species may sometimes be inadequate, and time constraints may lead to misidentifications or the discarding of specimens. Finally, fishers too may discard catches of non-native species with no commercial value before landing their catches, thus preventing their detection by onshore observers.
Potential adaptations or improvements in fisheries data collection regarding a more effective monitoring of non-native species could involve enhancing the training of observers to improve their ability to identify such species. Periodic training would allow them to adapt their knowledge to new and incoming species. Furthermore, the collection of official fisheries data could be reformed to include disaggregated information on the catches of non-native species, a need that has been acknowledged across the Eastern Mediterranean (e.g., Van Rijn et al., 2020; Kleitou et al., 2022). Finally, information should be collected in the framework of national data collection programs regarding non-native species catches by recreational fishers (Kleitou et al., 2022).
The relatively restricted temporal extent of the field data and the exclusion of unpublished data from citizen science initiatives are further significant limitations of this study. Moreover, as the numbers of the commercial fisheries monitoring samples for the PS, LLS, FPO and SB-SV gear were much smaller than for GNS, GTR and OTB, the results of any comparisons made in this study between gear need to be used with caution. However, this is a baseline study that can be periodically updated with new information from the ongoing Greek multiannual program on fisheries data collection, the international scientific literature, and any additional sources that may become available in the future. We believe that monitoring the spread of non-native species in relation to their interactions with fisheries and the changing physical environment in the North Aegean Sea is important and will provide valuable insights regarding the evolution of the cumulative effects of these major stressors of the Mediterranean Sea in the 21st century.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because the study used retrospectively collected data.
Author contributions
AE: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. DK: Writing – review & editing, Data curation, Investigation. AC: Writing – review & editing, Investigation. CG: Funding acquisition, Formal analysis, Writing – review & editing. AS: Writing – review & editing. AA: Writing – review & editing. NK: Writing – review & editing, Project administration. EK: Writing – review & editing, Project administration, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The commercial fisheries and MEDITS data analyzed in this article were collected in the framework of the Greek multiannual program according to DCF, for which the Ministry of Agriculture and Food is responsible. The publication of this article was funded by the Greek multiannual program according to DCF and the Ministry of Agriculture and Food. CG was supported by the Operational Programme of Fisheries and Sea (OPFS) 2014-2020, Greece, and the European Maritime and Fisheries Fund (EMFF) and the project “Monitoring and control of invasive alien species in Greece using innovative techniques under current and future climate conditions – INVASION” (MIS 5049543).
Acknowledgments
The authors thank the Ministry of Agriculture and Food, which is responsible for the Multiannual Union Programme for the Data Collection in the Fisheries and Aquaculture sectors (EU MAP) and the staff involved in commercial fisheries sampling and the scientific survey MEDITS, which is part of the EU MAP programme. The authors also thank the reviewers for their careful reading of the manuscript and their many insightful comments and suggestions. A prior version of this article has appeared online as preprint on Research Square (Evangelopoulos et al., 2024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1398037/full#supplementary-material
Supplementary Data Sheet 1 | PRISMA notes and tables.
Supplementary Data Sheet 2 | Fisheries data analysis.
Supplementary Data Sheet 3 | Data tables.
Supplementary Data Sheet 4 | Species photos.
Supplementary Data Sheet 5 | DNA barcoding analysis.
References
Akyol O., Ünal V., Ceyhan T. (2006). Occurrence of Two Lessepsian Migrant Fish, Oxyurichthys petersi (Gobiidae) and Upeneus pori (Mullidae), from the Aegean Sea. Cybium 30, 389–390.
Albano P. G., Steger J., Bošnjak M., Dunne B., Guifarro Z., Turapova E., et al. (2021). Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc B. 288, 20202469. doi: 10.1098/rspb.2020.2469
Albouy C., Guilhaumon F., Leprieur F., Ben Rais Lasram F., Somot S., Aznar R., et al. (2013). Projected climate change and the changing biogeography of coastal Mediterranean fishes. J. Biogeogr. 40, 534–547. doi: 10.1111/jbi.12013
Ananiadis C. (1952). On the appearance of the fish Tetrodon spadiceus (Rich.) in Greek seas. Proc. Hellenic Hydrobiolo institute. 6, 73–74.
Androulidakis Y. S., Krestenitis Y. N. (2022). Sea surface temperature variability and marine heat waves over the aegean, ionian, and cretan seas from 2008–2021. J. Mar. Sci. Eng. 10, 42. doi: 10.3390/jmse10010042
Apostolopoulos G., Karachle P. K. (2016). “New records of two fishes in Hellenic Waters (Kastellorizo Island): Scarus ghobban (Forsskål, 1775) and Oxyurichthys petersi (Klunzinger, 1871),” in Karachle P. K., Angelidis A., Apostolopoulos G., Ayas D., Ballesteros M., Bonnici C., et al (2016). New mediterranean biodiversity records (July 2019). Mediterr. Mar. Sci. 17 (1), 230–252. doi: 10.12681/mms.1684
Azzurro E., D’Amen M. (2022). Climate change paves the way for a new inter-ocean fish interchange. Front. Ecol. Environ. 20, 558–563. doi: 10.1002/fee.2459
Azzurro E., Maynou F., Belmaker J., Golani D., Crooks J. A. (2016). Lag times in Lessepsian fish invasion. Biol. Invasions 18, 2761–2772. doi: 10.1007/s10530-016-1184-4
Azzurro E., Smeraldo S., D’Amen M. (2022a). Spatio-temporal dynamics of exotic fish species in the Mediterranean Sea: Over a century of invasion reconstructed. Glob. Change Biol. 28, 6268–6279. doi: 10.1111/gcb.16362
Azzurro E., Smeraldo S., Minelli A., D’Amen M. (2022b). ORMEF: a Mediterranean database of exotic fish records. Sci. Data 9, 363. doi: 10.1038/s41597-022-01487-z
Ben Rais Lasram F., Guilhaumon F., Albouy C., Somot S., Thuiller W., Mouillot D. (2010). The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob. Change Biol. 16, 3233–3245. doi: 10.1111/j.1365-2486.2010.02224.x
Ben Rais Lasram F., Tomasini J., Guilhaumon F., Romdhane M., Do Chi T., Mouillot D. (2008). Ecological correlates of dispersal success of Lessepsian fishes. Mar. Ecol. Prog. Ser. 363, 273–286. doi: 10.3354/meps07474
Bianchi C. N., Morri C. (2003). Global sea warming and “tropicalization” of the Mediterranean Sea: biogeographic and ecological aspects. Biogeographia 24, 319–328. doi: 10.21426/B6110129
Bilecenoğlu M. (2010). “Alien marine fishes of Turkey – an updated review,” in Fish invasions of the mediterranean sea: change and renewal. Eds. Golani D., Appelbaum-Golani B. (Pensoft Publishers, Sofia–Moscow), 189–217.
Çinar M. E., Bilecenoğlu M., Yokeş M. B., Öztrk B., Taşkin E., Bakir K., et al. (2021). Current status (as of end of 2020) of marine alien species in Turkey. PloS One 16, e0251086. doi: 10.1371/journal.pone.0251086
Corrales X., Katsanevakis S., Coll M., Heymans J. J., Piroddi C., Ofir E., et al. (2020). Advances and challenges in modelling the impacts of invasive alien species on aquatic ecosystems. Biol. Invasions 22, 907–934. doi: 10.1007/s10530-019-02160-0
Corrales X., Ofir E., Coll M., Goren M., Edelist D., Heymans J. J., et al. (2017). Modeling the role and impact of alien species and fisheries on the Israeli marine continental shelf ecosystem. J. Mar. Syst. 170, 88–102. doi: 10.1016/j.jmarsys.2017.02.004
Corsini-Foka M., Kalogirou S. (2008). On the finding of the Indo-Pacific fish Scomberomorus commerson in Rhodes (Greece). Mediterr. Mar. Sci. 9, 167. doi: 10.12681/mms.147
Corsini-Foka M., Kondylatos G. (2015). Native and alien ichthyofauna in coastal fishery of Rhodes (eastern Mediterranean) (2002-2010). Front. Mar. Sci. Conference Abstract: XV European Congress Ichthyol. doi: 10.3389/conf.fmars.2015.03.00069
Corsini-Foka M., Mastis S., Kondylatos G., Batjakas I. E. (2017). Alien and native fish in gill nets at Rhodes, eastern Mediterranean, (2014–2015). J. Mar. Biol. Ass. 97, 635–642. doi: 10.1017/S0025315417000467
Corsini-Foka M., Pancucci-Papadopoulou M. A., Kalogirou S. (2010). “Is the Lessepsian Province in expansion? The Aegean Sea experience,” in Report of the Sub-Regional Technical meeting on the Lessepsian migration and its impact on Eastern Mediterranean fishery, Nicosia, Cyprus (AO-EastMed Project, Athens), 50–59.
Corsini-Foka M., Zenetos A., Crocetta F., Cinar M., Kocak F., Golani D., et al. (2015). Inventory of alien and cryptogenic species of the Dodecanese (Aegean Sea, Greece): Collaboration through COST Action training school. Manag. Biol. Invasions 6, 351–366. doi: 10.3391/mbi.2015.6.4.04
D’Amen M., Smeraldo S., Azzurro E. (2023). Salinity, not only temperature, drives tropical fish invasions in the Mediterranean Sea, and surface-only variables explain it better. Coral Reefs 42, 467–472. doi: 10.1007/s00338-023-02357-9
Edelist D., Rilov G., Golani D., Carlton J. T., Spanier E. (2013). Restructuring the S ea: profound shifts in the world’s most invaded marine ecosystem. Divers. Distrib 19, 69–77. doi: 10.1111/ddi.12002
ELSTAT (2023) Hellenic statistical authority (Athens: Greece). Available at: https://www.statistics.gr/en/ (Accessed November 3, 2023).
ESRI (2023) ArcGIS pro (Redlands, CA, USA: Environmental Systems Research Institute). Available at: https://www.esri.com/en-us/arcgis/products/arcgis-pro/ (Accessed November 3, 2023).
Evagelopoulos A., Nikolaou A., Michailidis N., Kampouris T. E., Batjakas I. E. (2020). Progress of the dispersal of the alien goatfish Parupeneus forsskali (Fourmanoir & Guézé, 1976) in the Mediterranean, with preliminary information on its diet composition in Cyprus. Bioinvasions Rec 9, 209–222. doi: 10.3391/bir.2020.9.2.06
Evangelopoulos A., Karampetsis D., Christidis A., Gubili C., Sapounidis A., Adamidou A., et al. (2024)Alien fish species in the North Aegean Sea: a review of their distributions integrating unpublished fisheries data (Accessed March 7, 2024). doi: 10.21203/rs.3.rs-3766127/v1
Evans J., Arndt E., Schembri P. J. (2020). Atlantic fishes in the Mediterranean: using biological traits to assess the origin of newcomer fishes. Mar. Ecol. Prog. Ser. 643, 133–143. doi: 10.3354/meps13353
Frid O., Gavriel T., Ben-Ari Y., Weinberger A., Yancovich-Shalom H., Belmaker J. (2023). Catch estimates and species composition of recreational fishing in Israel. Fishes 8, 69. doi: 10.3390/fishes8020069
Froese R., Pauly D. (2023). FishBase (World Wide Web electronic publication). Available at: www.fishbase.org. 06/2023.
Galanidi M., Aissi M., Ali M., Bakalem A., Bariche M., Bartolo A. G., et al. (2023). Validated inventories of non-indigenous species (NIS) for the mediterranean sea as tools for regional policy and patterns of NIS spread. Diversity 15, 962. doi: 10.3390/d15090962
Galanidi M., Zenetos A., Bacher S. (2018). Assessing the socio-economic impacts of priority marine invasive fishes in the Mediterranean with the newly proposed SEICAT methodology. Mediterr. Mar. Sci. 19, 107. doi: 10.12681/mms.15940
Galil B. S., Danovaro R., Rothman S. B. S., Gevili R., Goren M. (2019). Invasive biota in the deep-sea Mediterranean: an emerging issue in marine conservation and management. Biol. Invasions 21, 281–288. doi: 10.1007/s10530-018-1826-9
Galil B., Marchini A., Occhipinti-Ambrogi A., Ojaveer H. (2017). The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manage. Biol. Invasions 8, 141–152. doi: 10.3391/mbi.2017.8.2.02
Golani D., Azzurro E., Jakov D., Massuti E., Relini L. O., Briand F. (2021). Atlas of exotic fishes in the mediterranean sea. 2nd Edition (Paris: CIESM Publishers).
Gucu M., Ok M., Sakman S. (2010). “Past and present of fish fauna in the NE Levant Sea and factor facilitating the colonization by Lessepsian fishes,” in Report of the Sub-Regional Technical meeting on the Lessepsian migration and its impact on Eastern Mediterranean fishery, Nicosia, Cyprus (FAO-EastMed Project, Athens), 88–108.
Huseyinoglu M. F., Arda Y., Jiménez C. (2023). Manual of invasive alien species in the Eastern Mediterranean (Gland: IUCN).
Iglésias S. P., Frotté L. (2015). Alien marine fishes in Cyprus: update and new records. Aquat. Invasions 10, 425–438. doi: 10.3391/ai.2015.10.4.06
Johnstone I. M. (1986). Plant invasion windows: A time-based classification of invasion potential. Biol. Rev. 61, 369–394. doi: 10.1111/j.1469-185X.1986.tb00659.x
Juza M., Fernández-Mora À., Tintoré J. (2022). Sub-regional marine heat waves in the mediterranean sea from observations: long-term surface changes, sub-surface and coastal responses. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.785771
Katsanevakis S., Poursanidis D., Hoffman J., Rizgalla J., Bat-Sheva Rothman S., Levitt-Barmats Y., et al. (2020). Unpublished Mediterranean records of marine alien and cryptogenic species. Bioinvasions Rec. 9, 165–182. doi: 10.3391/bir.2020.9.2.01
Kleitou P., Crocetta F., Giakoumi S., Giovos I., Hall-Spencer J. M., Kalogirou S., et al. (2021). Fishery reforms for the management of non-indigenous species. J. Environ. Manage. 280, 111690. doi: 10.1016/j.jenvman.2020.111690
Kleitou P., Moutopoulos D. K., Giovos I., Kletou D., Savva I., Cai L. L., et al. (2022). Conflicting interests and growing importance of non-indigenous species in commercial and recreational fisheries of the Mediterranean Sea. Fish. Manage. Ecol. 29, 169–182. doi: 10.1111/fme.12531
Kondylatos G., Nikolidakis S. (2021). “First record of Fistularia petimba Lacepède, 1803 in Greece,” in Crocetta F., Al-Mabruk S. A. A. A., Azzurro E., Bakiu R., Bariche M., Batjakas I. E., et al. (2021). New alien mediterranean biodiversity records (November 2021). Mediterr. Mar. Sci. 22 (3), 724–746. doi: 10.12681/mms.26668
Kourafalou V. H., Barbopoulos K. (2003). High resolution simulations on the North Aegean Sea seasonal circulation. Ann. Geophys. 21, 251–265. doi: 10.5194/angeo-21-251-2003
Lefkaditou E., Vassilopoulou V., Petrakis G., Peristeraki P., Kavadas S., Politou C. -Y. (2010). “The effect of Lessepsian immigrants to the Hellenic Fisheries,” in Report of the Sub-Regional Technical meeting on the Lessepsian migration and its impact on Eastern Mediterranean fishery, Nicosia, Cyprus (FAO-EastMed Project, Athens), 60–73.
Libralato S., Caccin A., Pranovi F. (2015). Modeling species invasions using thermal and trophic niche dynamics under climate change. Front. Mar. Sci. 2. doi: 10.3389/fmars.2015.00029
Lockwood J. L., Hoopes M. F., Marchetti M. P. (2013). Invasion ecology. 2nd ed (Chichester: Wiley-Blackwell).
Machias A., Giannoulaki M., Somarakis S., Siapatis A. (2007). “Small pelagic fish,” in State of hellenic fisheries. Eds. Papaconstantinou C., Zenetos A., Vassilopoulou V., Tserpes G. (Athens: HCMR Publications), 192–207.
Michailidis N. (2010). “Study on the lessepsian migrant Lagocephalus sceleratus in Cyprus,” in eport of the Sub-Regional Technical meeting on the Lessepsian migration and its impact on Eastern Mediterranean fishery, Nicosia, Cyprus (FAO-EastMed Project, Athens), 74–87.
Michailidis N., Corrales X., Karachle P. K., Chartosia N., Katsanevakis S., Sfenthourakis S. (2019). Modelling the role of alien species and fisheries in an Eastern Mediterranean insular shelf ecosystem. Ocean Coast. Manage. 175, 152–171. doi: 10.1016/j.ocecoaman.2019.04.006
Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. 6, e1000097. doi: 10.1371/journal.pmed.1000097
Nittis K., Perivoliotis L. (2002). Circulation and hydrological characteristics of the North Aegean Sea: a contribution from real-time buoy measurements. Mediterr. Mar. Sci. 3, 21. doi: 10.12681/mms.255
Nuñez M. A., Kuebbing S., Dimarco R. D., Simberloff D. (2012). Invasive Species: to eat or not to eat, that is the question. Conserv. Lett. 5, 334–341. doi: 10.1111/j.1755-263X.2012.00250.x
O’Dea R. E., Lagisz M., Jennions M. D., Koricheva J., Noble D. W. A., Parker T. H., et al. (2021). Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: a PRISMA extension. Biol. Rev. 96, 1695–1722. doi: 10.1111/brv.12721
Ofir E., Corrales X., Coll M., Heymans J. J., Goren M., Steenbeek J., et al. (2023). Evaluation of fisheries management policies in the alien species-rich Eastern Mediterranean under climate change. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1155480
Oliverio M., Taviani M. (2003). The Eastern Mediterranean Sea: tropical invasions and niche opportunities in a “Godot Basin”. Biogeographia 24, 313–318. doi: 10.21426/B6110004
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br. Med. J. 372, n71. doi: 10.1136/bmj.n71
Pennino M. G., Conesa D., López-Quílez A., Munõz F., Fernández A., Bellido J. M. (2016). Fishery-dependent and -independent data lead to consistent estimations of essential habitats. ICES J. Mar. Sci. 73, 2302–2310. doi: 10.1093/icesjms/fsw062
Peristeraki P., Lazarakis G., Skarvelis C., Georgiadis M., Tserpes G. (2006). Additional records on the occurrence of alien fish species in the eastern Mediterranean Sea. Mediterr. Mar. Sci. 7, 61. doi: 10.12681/mms.170
Peristeraki P., Tserpes G., Lampadariou N., Stergiou K. I. (2017). Comparing demersal megafaunal species diversity along the depth gradient within the South Aegean and Cretan Seas (Eastern Mediterranean). PloS One 12, e0184241. doi: 10.1371/journal.pone.0184241
Peters D. P. C., Gosz J. R., Pockman W. T., Small E. E., Parmenter R. R., Collins S. L., et al. (2006). Integrating patch and boundary dynamics to understand and predict biotic transitions at multiple scales. Landsc. Ecol. 21, 19–33. doi: 10.1007/s10980-005-1063-3
Pisano A., Marullo S., Artale V., Falcini F., Yang C., Leonelli F. E., et al. (2020). New evidence of mediterranean climate change and variability from sea surface temperature observations. Remote Sens. 12, 132. doi: 10.3390/rs12010132
Png-Gonzalez L., Comas-González R., Calvo-Manazza M., Follana-Berná G., Ballesteros E., Díaz-Tapia P., et al. (2023). Updating the national baseline of non-indigenous species in spanish marineWaters. Diversity 15, 630. doi: 10.3390/d15050630
Ragkousis M., Sini M., Koukourouvli N., Zenetos A., Katsanevakis S. (2023a). Invading the greek seas: spatiotemporal patterns of marine impactful alien and cryptogenic species. Diversity 15, 353. doi: 10.3390/d15030353
Ragkousis M., Zenetos A., Ben Souissi J., Tsiamis K., Ferrario J., Marchini A., et al. (2023b). Unpublished Mediterranean and Black Sea records of marine alien, cryptogenic, and neonative species. Bioinvasions Rec. 12, 339–369. doi: 10.3391/bir.2023.12.2.01
Raitsos D. E., Beaugrand G., Georgopoulos D., Zenetos A., Pancucci-Papadopoulou A. M., Theocharis A., et al. (2010). Global climate change amplifies the entry of tropical species into the eastern Mediterranean Sea. Limnol. Oceanogr. 55, 1478–1484. doi: 10.4319/lo.2010.55.4.1478
R Core Team (2023) R: A language and environment for statistical computing. Available at: https://www.R-project.org/ (Accessed November 3, 2023).
Sagarin R., Pauchard A. (2010). Observational approaches in ecology open new ground in a changing world. Front. Ecol. Environ. 8, 379–386. doi: 10.1890/090001
Saygu İ., Heymans J. J., Fox C. J., Özbilgin H., Eryaşar A. R., Gökçe G. (2020). The importance of alien species to the food web and bottom trawl fisheries of the Northeastern Mediterranean, a modelling approach. J. Mar. Syst. 202, 103253. doi: 10.1016/j.jmarsys.2019.103253
Servello G., Andaloro F., Azzurro E., Castriota L., Catra M., Chiarore A. (2019). Marine alien species in Italy: a contribution to the implementation of descriptor D2 of the Marine Strategy Framework Directive. Medit. Mar. Sci. 20, 1–48. doi: 10.12681/mms.18711
Siokou-Frangou I., Bianchi M., Christaki U., Christou E. D., Giannakourou A., Gotsis O., et al. (2002). Carbon flow in the planktonic food web along a gradient of oligotrophy in the Aegean Sea (Mediterranean Sea). J. Mar. Syst., 33–34335–353. doi: 10.1016/S0924-7963(02)00065-9
Siokou-Frangou I., Zervoudaki S., Christou E. D., Zervakis V., Georgopoulos D. (2009). Variability of mesozooplankton spatial distribution in the North Aegean Sea, as influenced by the Black Sea waters outflow. J. Mar. Syst. 78, 557–575. doi: 10.1016/j.jmarsys.2008.12.025
Stern N., Gouws G., Golani D., Goren M., Gon O. (2019). Champsodontidae (Pisces: Trachinoidei) in the Eastern Mediterranean: how many species are there? J. Nat. Hist. 53, 2869–2881. doi: 10.1080/00222933.2020.1758820
Thomaz S. M., Kovalenko K. E., Havel J. E., Kats L. B. (2015). Aquatic invasive species: general trends in the literature and introduction to the special issue. Hydrobiologia 746, 1–12. doi: 10.1007/s10750-014-2150-8
Tiralongo F., Crocetta F., Riginella E., Lillo A. O., Tondo E., Macali A., et al. (2020). Snapshot of rare, exotic and overlooked fish species in the Italian seas: A citizen science survey. J. Sea Res. 164, 101930. doi: 10.1016/j.seares.2020.101930
Tsagarakis K., Coll M., Giannoulaki M., Somarakis S., Papaconstantinou C., Machias A. (2010). Food-web traits of the North Aegean Sea ecosystem (Eastern Mediterranean) and comparison with other Mediterranean ecosystems. Estuar. Coast. Shelf. Sci. 88, 233–248. doi: 10.1016/j.ecss.2010.04.007
Ulman A., Ali F. Z., Harris H. E., Adel M., Mabruk S. A. A. A., Bariche M., et al. (2022). Lessons from the western atlantic lionfish invasion to inform management in the mediterranean. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.865162
Van Rijn I., Kiflawi M., Belmaker J. (2020). Alien species stabilize local fisheries catch in a highly invaded ecosystem. Can. J. Fish. Aquat. 77, 752–761. doi: 10.1139/cjfas-2019-0065
Vaz A. S., Kueffer C., Kull C. A., Richardson D. M., Schindler S., Muñoz-Pajares A. J. (2017). The progress of interdisciplinarity in invasion science. Ambio 46, 428–442. doi: 10.1007/s13280-017-0897-7
WoRMS Editorial Board (2023) World register of marine species. Available at: https://www.marinespecies.org (Accessed November 3, 2023).
Yarrow M. M., Marín V. H. (2007). Toward conceptual cohesiveness: a historical analysis of the theory and utility of ecological boundaries and transition zones. Ecosystems 10, 462–476. doi: 10.1007/s10021-007-9036-9
Zenetos A., Gofas S., Verlaque M., Cinar M. E., Garcia Raso J. E., Bianchi C. N., et al. (2010). Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterr. Mar. Sci. 11, 381. doi: 10.12681/mms.87
Zenetos A., Karachle P. K., Corsini-Foka M., Gerovasileiou V., Simboura N., Xentidis N. J., et al. (2020). Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? The case study of Greece. Mediterr. Mar. Sci. 21, 775. doi: 10.12681/mms.25136
Zervakis V., Georgopoulos D. (2002). Hydrology and circulation in the North Aegean (eastern Mediterranean) throughout 1997 and 1998. Mediterr. Mar. Sci. 3, 5. doi: 10.12681/mms.254
Keywords: Lessepsian species, non-indigenous species, alien species, biological invasions, geographic range, commercial fisheries
Citation: Evangelopoulos A, Karampetsis D, Christidis A, Gubili C, Sapounidis A, Adamidou A, Kamidis N and Koutrakis E (2024) Non-native fish species in the North Aegean Sea: a review of their distributions integrating unpublished fisheries data. Front. Mar. Sci. 11:1398037. doi: 10.3389/fmars.2024.1398037
Received: 08 March 2024; Accepted: 07 May 2024;
Published: 24 May 2024.
Edited by:
Jasmine Ferrario, University of Pavia, ItalyReviewed by:
Periklis Kleitou, Marine and Environmental Research Lab (MER), CyprusFrancesco Tiralongo, University of Catania, Italy
Copyright © 2024 Evangelopoulos, Karampetsis, Christidis, Gubili, Sapounidis, Adamidou, Kamidis and Koutrakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athanasios Evangelopoulos, a.evangelopoulos@inale.gr
 Athanasios Evangelopoulos
Athanasios Evangelopoulos Dimitrios Karampetsis
Dimitrios Karampetsis Aris Christidis
Aris Christidis  Chrysoula Gubili
Chrysoula Gubili Argyrios Sapounidis
Argyrios Sapounidis Angeliki Adamidou
Angeliki Adamidou Nikolaos Kamidis
Nikolaos Kamidis Emmanouil Koutrakis
Emmanouil Koutrakis