Physicochemical Properties of Experimental Resin-Based Materials Containing Fluoridated Calcium Phosphates
- 1Paulo Picanço School of Dentistry, Fortaleza, Brazil
- 2Post-Graduate Program in Dentistry, Department of Restorative Dentistry, Federal University of Ceará, Fortaleza, Brazil
- 3Dental Biomaterials, Departamento de Odontologia, Facultad de Ciencias de la Salud, CEU-Cardenal Herrera University, Valencia, Spain
The aim of this study was to evaluate the physicochemical properties of experimental adhesives containing tailored fluoridated calcium phosphate fillers. Five experimental resins were formulated, one contained no calcium phosphate filler (Control) and the other four resins contained 40 wt% of calcium phosphate fillers doped with increasing concentrations of fluoride: adhesives CaP (no fluoride), CaP-2F (2 wt% F), CaP-4F (4 wt% F), and CaP-8F (8 wt% F). Resin specimens were prepared for a three-point bending test to evaluate the elastic modulus (E) and flexural strength (FS) at 24 h and after 2 months of water storage. Disk-shaped specimens were prepared to analyze the water sorption (Ws) according to ISO 4049. The degree of conversion (DC) of the tested resins was also assessed through FTIR. Statistical analysis was performed with ANOVA and Tukey’s test (p < 0.05). The addition of F-CaP particles at 0 and 2 wt% fluoride had no effect on the DC (p > 0.05) compared to the filler-free resin. However, these same experimental resins showed the highest E after water storage. All resins had a significant reduction of the FS after water storage (p < 0.001), but at a lower percentage rate for those experimental resins containing fluoride-free CaP or fluoride-doped CaP-2F. These latter experimental resins achieved similar Ws to control the filler-free resin. Conversely, those resins containing 4 or 8 wt% fluoride-doped CaP had a significant Ws increase (p < 0.05). In conclusion, the incorporation of calcium phosphate particles without fluoride or with 2 wt% fluoride may represent a promising strategy to generate adhesive resins with specific physicochemical properties. Fluoride incorporation in CaP fillers for dental resin adhesive should be performed at low concentrations in order to avoid excessive water sorption and a decrease of polymerization.
Introduction
Resin composite restorations are the foremost procedure in modern restorative dentistry as such materials present optimal esthetics and appropriate mechanical properties to replace those dental hard tissues that were lost for different causes (e.g., caries and tooth wear). Such a restorative approach allows for more conservative cavity preparations as resin composites can be bonded directly onto dentin and enamel through the use of adhesive systems (Palaniappan et al., 2011; Van Meerbeek et al., 2020). Nevertheless, the bond between the adhesive and dentin is still characterized by issues related to the longevity of simplified adhesives (Hashimoto, 2010; Feitosa et al., 2014); the main cause for frequent failure/replacement of restorations (Ferracane, 2011; Demarco et al., 2017, Feitosa et al., 2019).
The dentin substrate is a highly heterogeneous dental tissue (overall 30 vol% organic components), characterized by differences in wetness and morphology/physiology, depending on its location and/or proximity to the pulp tissue (Marshall et al., 1997; Bertassoni et al., 2012). Bonding to dentin is still an important challenge due to the presence of dentinal tubules as well as the pulpal fluid, which make the use of hydrophilic monomers necessary to accomplish proper resin hybridization (Van Landuyt et al., 2008; Sauro et al., 2009, Van Meerbeek et al., 2020). Such hydrophilic components, if present in high concentration, can decrease the mechanical properties of adhesive resins (Ito et al., 2005), and increase water sorption (Feitosa et al., 2014), permeability (Sauro et al., 2009; Feitosa et al., 2019), and hydrolytic degradation (Ferracane, 2006; Sauro et al., 2019).
Severe degradation of the resin-dentin bonds occurs quite rapidly as it relies on the hydrolysis of unprotected collagen fibrils, principally when the bonding procedures were performed using simplified etch-and-rinse adhesives (Carrilho et al., 2007). In this regard, after finishing a composite restoration, the demineralized collagen that was not perfectly enveloped by the resin polymers of the adhesives is prone to hydrolytic degradation (Sauro and Pashley, 2016). Furthermore, the collagen breakdown might be accelerated by the activation of host-derived matrix metalloproteinases (MMPs) and cysteine cathepsins (Scaffa et al., 2012), thereby promoting significant resin-dentin degradation within a period of a few months after completing the composite restoration (Carrilho et al., 2007).
Although several enzyme inhibitors may be employed in order to increase the durability of dentin bonds (Scaffa et al., 2012; Van Meerbeek et al., 2020), one of the most promising strategies to decrease the degradation of the dentin collagen is based on a biomimetic remineralization of the dentine substrate (Tay and Pashley, 2008; Brackett et al., 2011, Sauro and Pashley, 2016). Such a technique may restore demineralized dentin collagen fibrils back to their natural (mineralized) stage through the deposition of nanophases of amorphous calcium phosphate (ACP) (Tay and Pashley, 2008; Sauro and Pashley, 2016). Several ion-releasing fillers have been incorporated to adhesive resins for this purpose, such as modified Portland cement (Profeta et al., 2012), bioactive glass (Sauro et al., 2012), and calcium silicate/polycarboxylate (Sauro and Pashley, 2016). Nevertheless, none of these fillers are able to form an acid-resistant fluoride-containing calcium phosphate (Xu et al., 2010). It has been advocated that the addition of fluoride may improve the anti-caries properties, thereby avoiding or reducing the incidence of secondary caries; it is one of the major reasons for the replacement of composite restorations (Andrade Neto et al., 2016).
The aim of this study was to evaluate some specific physicochemical properties, such as degree of conversion, water sorption, elastic modulus, and flexural strength of experimental resin adhesives containing tailored calcium phosphate fillers doped with fluoride at different concentrations (F-CaP). The hypothesis tested was that the incorporation of the experimental fillers tested in this study would not interfere with the physicochemical properties of the experimental resin adhesives.
Materials and Methods
Experimental Adhesive and Inorganic Particles
A resin blend (control) was prepared by mixing bisphenol-A-glycidyl-dimethacrylate (BisGMA 20 wt%), urethane-dimethacrylate (UDMA, 27 wt%), triethylene-glycol-dimethacrylate (TEGDMA 25 wt%), hydroxy-ethyl-methacrylate (HEMA 5 wt%), and 20 wt% ethanol. After the mixture of monomers and solvent, a ternary photoinitiator system was dissolved comprising 0.5 wt% camphorquinone (photosensitizer), 1 wt% ethyl-dimethylamine-benzoate (coinitiator), and 1.5 wt% diphenyliodonium hexafluorophophate (accelerator). All reagents were mixed in a dark room at 25 °C and ultrasonicated for 10 min at the end to warrant adequate homogeneity to the blend.
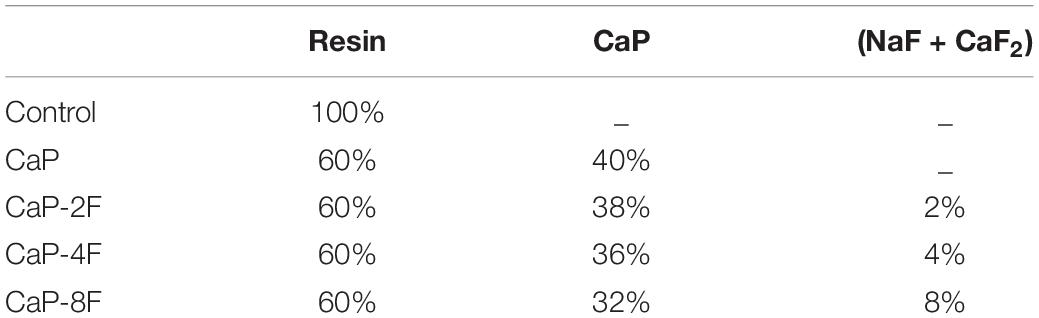
The inorganic filler (CaP-F) was obtained by a specific reaction as previously reported (Sauro and Feitosa, 2019), which is a precursor of fluorapatite. In brief, a 1:1 molar ratio of beta-tricalcium phosphate (β-TCP) and monocalcium phosphate monohydrate (MCPM) were mixed with a 1:1 weight ratio of calcium and sodium fluorides in final concentrations of 5, 10, or 20%, to attain fillers with increasing concentration of fluoride. Four different micro fillers were incorporated (40 wt%) into the resin blend to create four experimental resins to obtain the materials with compositions that had different final concentrations of fluoride (0, 2, 4, 8 wt%) except for the control filler-free adhesive (Control). The calcium phosphate mixture without fluoride addition (CaP) was also tested. Fluoride containing groups were designated as CaP-2F, CaP-4F, and CaP-8F, according to the final concentration of fluoride (Table 1). All reagents were purchased from Sigma Aldrich Chemicals (Milwaukee, United States).
Degree of Conversion
The polymerization of each resin was assessed by means of a Fourier-transform infrared spectroscopy (FTIR Bruker Spectrometer, Bruker, Bremen, Germany), equipped with a crystal of attenuated total reflectance (ATR). The distance between the light-curing tip and the adhesive drop was standardized at 2 mm. Each drop (0.3 μL) was dispensed directly onto the ATR crystal, providing a similar height of adhesive resin and the spectra (1580–1660 cm–1) of uncured resins were detected at 4 cm–1 resolution and 32 scans. After light-curing using a LED unit (DB 685, Dabi Atlante, São Paulo, Brazil) for 40 s at 1100 mW/cm2 irradiance, the spectra of the polymerized adhesives were also evaluated. Each material (Control, CaP, CaP-2F, CaP-4F, and CaP-8F) was surveyed in triplicate. The degree of conversion was obtained by calculating the ratio between the peak (height) 1635 cm–1 (aliphatic C = C double bond) and 1608 cm–1 (aromatic C = C double bond as internal reference) (Andrade Neto et al., 2016). Data were statistically analyzed by a Shapiro-Wilk normality test (p > 0.05) and after the normalization of the data was confirmed, these were analyzed by one-way ANOVA and Tukey’s test (p < 0.05).
Specimens Preparation
Bar-shaped specimens (2 mm width, 1 mm thickness, and 7 mm length) were prepared for a three-point bending test, whilst disk-shaped specimens (1 mm thickness and 7 mm diameter) were prepared for the assessment of the water sorption of the tested materials. All specimens were dispensed in pre-fabricated silicone molds following the indication of ISO 4049, except for the dimensions of the specimens (Lopes et al., 2009). Each specimen was light-cured for 40 s using the LED curing system (DB 685, Dabi Atlante). All specimens were carefully removed from the molds and checked for defects using a stereomicroscope; those that presented voids, bubbles, or structural defects were discarded. Eight specimens per group (n = 8) were prepared for each test. They were kept dried for 24 h at 37°C to ensure a proper polymerization reaction.
Three-Point Bending Test
After 24 h storage at 37°C, the flexural strength and modulus of the experimental bar-shaped specimens were obtained by three-point bending (5 mm distance between the supports) in a universal testing machine (Instron 3345, Canton, United States) using a 500 N load cell. The test was monitored by the Bluehill 2 software (Instron), which generated a stress/strain graph that allowed for the detection of an elastic (flexural) modulus and maximum flexural strength. Specimens were prepared and tested after 24 h (dried) and further specimens were immersed for 2 months in distilled water and tested as described earlier after water storage. Data attained were statistically analyzed by a Shapiro-Wilk normality test and after confirming the normalization of the data, a two-way ANOVA as well as a Tukey’s test was performed (α = 5%).
Water Sorption
The protocol for water sorption assessment was in accordance with the ISO 4049. Disk-shaped specimens were measured using a digital caliper and the volume of each specimen was calculated. Thereafter, they were dehydrated in a vacuum chamber with silica gel. They were weighed daily in a precision balance (0.01 mg, Mitutoyo, Tokyo, Japan) until the weight stabilized with no change within the next 6 days. This initial weight (M1) was established and the specimens were individually immersed in 1.5 mL of distilled water at 37°C for one week. The weight after immersion (M2) was rapidly detected avoiding dehydration. Following, the specimens were kept again in the drying chamber for a period of approximately three weeks until stabilization of the weight (M3). The water sorption results were obtained using the formula (M2-M3)/V. Data were statistically analyzed by a Shapiro-Wilk normality test. After normal data was confirmed, the results were analyzed by a one-way ANOVA and a Tukey’s test (p < 0.05).
Results
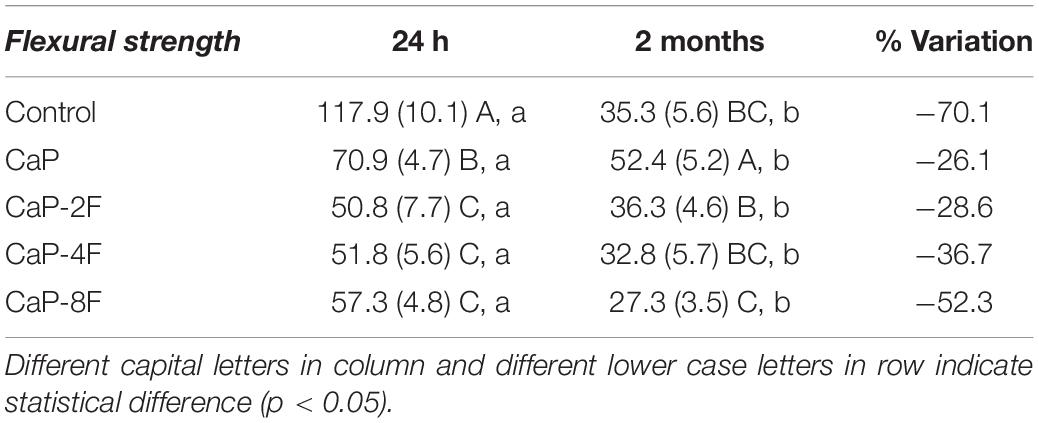
The results of the degree of conversion (DC) are presented in Figure 1A. The highest DC was attained with the adhesive resin containing no filler (78.1%), and it was significantly different (p < 0.001) compared to CaP-4F and CaP-8F; these latter two materials attained the lowest degree of conversion. The adhesive resin containing the fluoride-free calcium phosphates (CaP) and that doped with 2 wt% fluoride (CaP-2F) achieved similar DC values to the control filler-free resin (p = 0.439 and p = 0.596, respectively). The results of maximum flexural strength are presented in Table 2. The statistical interaction between factors was significant (p < 0.001). After water storage, the flexural strength of all adhesive resins presented a significant reduction (p < 0.001). However, a lower reduction (%) was observed in the groups CaP and CaP-2F, while a greater reduction was observed in the control group (filler-free adhesive resin) (Table 2). Regarding the initial flexural strength, the incorporation of calcium phosphate fillers with and without fluoride induced an overall decrease compared to the control filler-free resin (p < 0.05). In particular, there was a greater reduction in the resins with fillers containing fluoride, rather than the resin containing the fluoride-free calcium phosphate fillers (CaP). Moreover, after prolonged water storage, the highest flexural strength was detected with the resin containing fluoride-free CaP (52.4 MPa), while the lowest values were observed in the group CaP-8F (27.3 MPa).
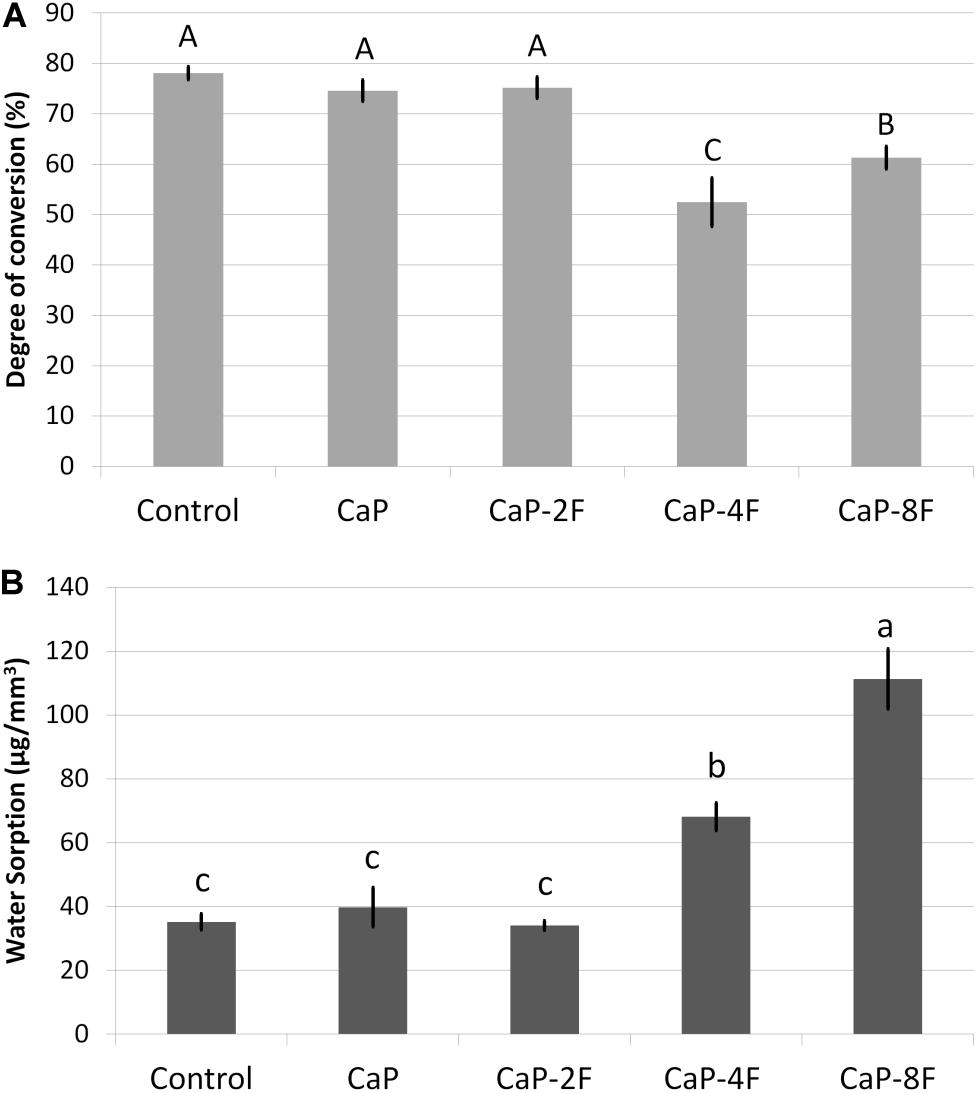
Figure 1. Means (standard deviations) of the degree of conversion (%) in (A) and water sorption (μg/mm3) in (B). Different letters indicate statistical difference (p < 0.05).
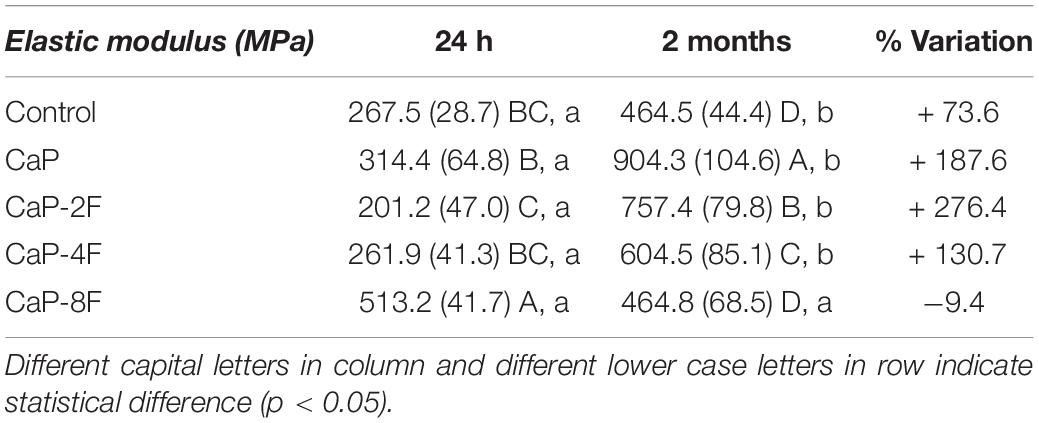
The results of the flexural modulus are presented in Table 3. The interaction between factors was statistically significant (p < 0.001). All the tested adhesive resins, except the specimens in group CaP-8F (p = 0.139), depicted an increase in the elastic modulus after prolonged water storage (p < 0.001). Moreover, an increase (%) of the modulus was evident in those experimental resins containing CaP-2F and CaP (Table 3). The initial highest flexural modulus was attained with the resin CaP-8F (513.2 MPa), whilst the lowest was observed with the 2 wt% resin (201.2 MPa). After water storage, the highest modulus was detected in the CaP group, followed by the specimens in group CaP-2F.
The outcomes of water sorption are depicted in Figure 1B. Higher water sorption was observed in the resin adhesive CaP-8F (111.3 μg/mm3), followed by the resin CaP-4F; both presented values significantly higher than the control filler-free resin, CaP, and CaP-2F (p = 0.0154, p = 0.0308, and p = 0.0127, respectively). The incorporation of calcium phosphate fillers (CaP) and those with only 2 wt% fluoride had no influence on the water sorption compared to the filler-free control resin (p = 0.693 and p = 0.915, respectively).
Discussion
An optimal degree of conversion is essential for dental resins to obtain adequate mechanical properties as well as chemical stability. Several properties such as compressive strength, wear resistance, fracture toughness, water sorption, and solubility may be affected by “poor” polymerization; this can also affect the clinical longevity of resin-based restorative materials (Calheiros et al., 2008). In the present study, experimental adhesives containing bioactive fillers with high concentrations of fluoride (CaP-4F and CaP-8F) presented a lower degree of conversion in comparison with those containing a filler with a lower concentration of fluoride (CaP-2F, CaP) and the control filler-free adhesive. These findings are in agreement with those of Shinohara et al. (2009) who demonstrated a similar degree of conversion between the fluoride-containing Clearfil Protect Bond and the fluoride-free Clearfil SE Bond. Both adhesives possess very similar chemical compositions, but the Protect Bond releases fluoride (from inorganic particles) and contains an antibacterial monomer. Indeed, the outcomes reported by Shinohara et al. (2009) showed a significant reduction of the degree of conversion between the adhesive resin containing fluoride (DC: 50-60%) and the fluoride-free adhesive, which achieved a degree of conversion of 70-80%.
Fluorine is a well-known highly electronegative chemical element; in other words, fluoride has a great potential to attract electrons. In this regard, during the polymerization reaction, fluoride ions may promote a certain inhibition (Tjaderhane et al., 2013) on the free-radical polymerization reaction due to partial inactivation of unpaired electrons. Based on the present results, it was observed that only fluoride concentrations higher than 2 wt% were able to induce significant inhibition of the polymerization in the experimental adhesives tested in this study (Figure 1A).
Flexural strength is comprised of a wide variety of tensions such as compression, shear, and tensile. The clinical relevance of this mechanical property relies on different tensions that dental materials undergo during chewing. The results of the current investigation demonstrated a decrease in flexural strength after 2 months of water storage for all the resin-based materials tested in this study. However, such a degradation rate was notably lower for those resins containing fluoride-free calcium phosphates (CaP) or 2 wt% fluoride (CaP-2F). Conversely, the greatest degradation was observed in the control filler-free resin and the resin containing calcium phosphates with 8 wt% fluoride (CaP-8F) (Table 2). The initial high flexural strength of CaP-8F, even with a lower degree of conversion, may be related to the high strength of fillers containing high amount of fluoride. Nevertheless, after water storage and a loss of fluoride from these fillers, the drop in flexural strength was expected, as the fillers become more porous.
A possible explanation to justify the reduction in the flexural strength of the tested materials might be in part related to the DC of the polymeric chain, as well as to the hydrolytic degradation of polymers (Ferracane, 2006; Feitosa et al., 2013, 2019) due to breakdown of ester bonds in methacrylate monomers. Such a situation may have been caused by water uptake, which is usually triggered by the presence of hydrophilic monomers such as hydroxyl-ethyl-methacrylate (HEMA) (Van Landuyt et al., 2008; Van Meerbeek et al., 2020). However, the inorganic chemical reaction between the water and the bioactive calcium phosphate fillers may have reduced the polymer degradation. The rationale behind this hypothesis is that the water may have been absorbed by the filler for a successive bioactive reaction [Ca(H2PO4)2.H2O + Ca3(PO4)2 + 7H2O - > 4 CaHPO4.2H2O], rather than leaving it available for polymer chains breakdown (Sauro et al., 2012).
In this study, we intentionally added small amounts of HEMA to promote water uptake and demonstrate the protective effect of the calcium phosphate particles. Nevertheless, with the addition of a high percentage of fluoride (i.e., 8 wt%) a higher release of this ion might be expected, but with a concomitant intense drop in mechanical properties (Table 2).
Regarding the elastic (flexural) modulus, only the resin with calcium phosphates and 8 wt% fluoride (CaP-8F) presented a reduction after two-month water storage. We speculate that such a situation may have been caused by high solubility of those specific resins, which in turn may have jeopardized the mechanical properties of the experimental resins (Table 3). Conversely, the increase of the elastic modulus observed in the other tested resin-based materials could be due to the fact that the fillers used in those materials transformed into more complex forms of calcium phosphates such as fluorapatite and hydroxyapatite (Sauro and Feitosa, 2019). Such a crystallization process may have contributed to the increase of the elastic modulus after water storage; this was significantly higher for CaP and CaP-2F than the control filler-free resin (Table 3).
Moreover, it was also found in a previous investigation (Ito et al., 2005) that the leaching of unreacted monomers and water uptake may provide the formation of hydrogen bonds, particularly with BisGMA (Stansbury, 2012), leading to higher rigidity of the polymer after water storage.
Water sorption in resin composites is a process of water diffusion principally through the organic resin matrix (Ferracane, 2006). Although water sorption is characterized by a multifactorial process, water uptake occurs mostly due to the hydrophilic nature of monomeric units (Ito et al., 2005; Sauro et al., 2019; Van Meerbeek et al., 2020). When one analyzes the results in Figure 1, it is possible to note that the higher the degree of conversion, the lower the water sorption. Therefore, also in this case, the extent of polymerization may play an important role on the water sorption of an adhesive resin. Control resin, CaP, and CaP-2F presented the highest degree of conversion (Figure 1A) and, consequently, they depicted the lowest water sorption (Figure 1B). Even though control, CaP, and CaP-2F demonstrated similar water sorption (p < 0.05), adhesives containing calcium phosphate particles did uptake more water due to the aforementioned inorganic reaction. Conversely, the water absorbed by the control group is the actual water sorption that might induce degradation and jeopardize mechanical properties. Thus, although the measured water sorption was similar, lower degradation and reduction on mechanical properties could be expected with the addition of these particular calcium phosphate fillers, with or without fluoride (Tables 2, 3). Since higher concentrations of fluoride (8 wt%) were detrimental to the physicochemical properties of experimental adhesives, the study hypothesis needs to be rejected.
Conclusion
Calcium phosphate fillers without fluoride and with 2 wt% fluoride may attain the best physicochemical property outcomes for experimental adhesives. These particles present a lower reduction in flexural strength after water storage, did not interfere on the degree of conversion, and presented the best elastic modulus and similar water sorption to the control filler-free resin. Therefore, within the limitations of this investigation, we can affirm that our calcium phosphate fillers with low concentrations of fluoride may be the ideal choice to create a bioactive adhesive system, without impairing the physicochemical properties of the resin-based material.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
VF performed project supervision, funding acquisition, formal analysis, and drafting the manuscript. LP performed experiments and drafted the manuscript. MM performed investigation, methodology, and data analysis. DD-P performed methodology and degree of conversion investigation. AA performed funding acquisition and experimental analyses. LR wrote and reviewed and edited the manuscript. SS performed project administration, writing up, and review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andrade Neto, D. M., Carvalho, E. V., Rodrigues, E. A., Feitosa, V. P., Sauro, S., Mele, G., et al. (2016). Novel hydroxyapatite nanorods improve anti-caries efficacy of enamel infiltrants. Dent. Mater. 32, 784–793. doi: 10.1016/j.dental.2016.03.026
Bertassoni, L. E., Orgel, J. P., Antipova, O., and Swain, M. V. (2012). The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 8, 2419–2433. doi: 10.1016/j.actbio.2012.02.022
Brackett, M. G., Li, N., Brackett, W. W., Sword, R. J., Qi, Y. P., Niu, L. N., et al. (2011). The critical barrier to progress in dentin bonding with the etch-and-rinse technique. J. Dent. 2011, 238–248. doi: 10.1016/j.jdent.2010.12.009
Calheiros, F. C., Daronch, M., Rueggeberg, F. A., and Braga, R. R. (2008). Degree of conversion and mechanical properties of a BisGMA:TEGDMA composite as a function of the applied radiant exposure. J. Biomed. Mater. Res. B. Appl. Biomater. 84, 503–509. doi: 10.1002/jbm.b.30897
Carrilho, M. R., Geraldeli, S., Tay, F. R., Goes, M. F., Carvalho, R. M., Tjaderhane, L., et al. (2007). In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 86, 529–533. doi: 10.1177/154405910708600608
Demarco, F. F., Collares, K., Correa, M. B., Cenci, M. S., Moraes, R. R., and Opdam, N. J. (2017). Should my composite restorations last forever? Why are they failing?. Braz. Oral. Res. 31(Suppl. 1):e56. doi: 10.1590/1807-3107BOR-2017.vol31.0056
Demarco, F. F., Corrêa, M. B., Cenci, M. S., Moraes, R. R., and Opdam, N. J. (2012). Longevity of posterior composite restorations: not only a matter of materials. Dent. Mater. 28, 87–101. doi: 10.1016/j.dental.2011.09.003
Feitosa, V. P., Bazzocchi, M. G., Putignano, A., Orsini, G., Luzi, A. L., Sinhoreti, M. A. C., et al. (2013). Dicalcium phosphate (CaHPO4⋅2H2O) precipitation through ortho- or meta-phosphoric acid-etching: effects on the durability and nanoleakage/ultra-morphology of resin-dentine interfaces. J. Dent. 41, 1068–1080. doi: 10.1016/j.jdent.2013.08.014
Feitosa, V. P., Sauro, S., Ogliari, F. A., Stansbury, J. W., Carpenter, G. H., Watson, T. F., et al. (2014). The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J. Dent. 42, 565–574. doi: 10.1016/j.jdent.2014.02.009
Feitosa, V. P., Sauro, S., Zenobi, W., Silva, J. C., Abuna, G., Van Meerbeek, B., et al. (2019). Degradation of adhesive-dentin interfaces created using different bonding strategies after five-year simulated pulpal pressure. J. Adhes. Dent. 21, 199–207. doi: 10.3290/j.jad.a42510
Ferracane, J. L. (2006). Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 22, 211–22. doi: 10.1016/j.dental.2005.05.005
Ferracane, J. L. (2011). Resin composite – State of the art. Dent. Mater. 27, 29–38. doi: 10.1016/j.dental.2010.10.020
Hashimoto, M. (2010). A review: micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. J. Biomed. Mater. Res. B. Appl. Biomater. 92, 268–280. doi: 10.1002/jbm.b.31535
Ito, S., Hashimoto, M., Wadgaonkar, B., Svizero, N., Carvalho, R. M., Yiu, C., et al. (2005). Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26, 6449–6459. doi: 10.1016/j.biomaterials.2005.04.052
Lopes, M. B., Moraes, R. R., Gonini-Junior, A., and Piva, E. (2009). Impact of curing protocol on the selected properties of a model bis-GMA/TEGDMA dental resin composite. Biomed. Mater. 4:025014. doi: 10.1088/1748-6041/4/2/025014
Marshall, G. W., Marshall, S. J., Kinney, J. H., and Salooch, M. (1997). The dentin substrate: structure and properties related to bonding. J. Dent. 25, 441–458. doi: 10.1016/s0300-5712(96)00065-6
Palaniappan, S., Bharadwaj, D., Mattar, D. L., Peumans, M., Van Meerbeek, B., and Lambrechts, P. (2011). Nanofilled and microhybrid composite restorations: Five-year clinical wear performances. Dent. Mater. 27, 692–700. doi: 10.1016/j.dental.2011.03.012
Profeta, A. C., Mannocci, F., Foxton, R. M., Thompson, I., Watson, T. F., and Sauro, S. (2012). Bioactive effects of a calcium/sodium phosphosilicate on the resin-dentin interface: a microtensile bond strength, scanning electron microscopy, and confocal microscopy study. Eur. J. Oral Sci. 120, 353–362. doi: 10.1111/j.1600-0722.2012.00974.x
Sauro, S., and Feitosa, V. P. (2019). Preparation, Composition and Application of a Bioactive Fluoride-Doped Calcium Phosphate Able to Induce Controlled Deposition of Fluorapatite. W.O. Patent No 2019/243592 A1. Geneva: World Intellectual Property Organization.
Sauro, S., Makeeva, I., Faus-Matoses, V., Foschi, F., Giovarruscio, M., Pires, P. M., et al. (2019). Effects of ions-releasing restorative materials on the dentine bonding longevity of modern universal adhesives after load-cycle and prolonged artificial saliva aging. Materials 12:722. doi: 10.3390/ma12050722
Sauro, S., Mannocci, F., Toledano, M., Osorio, R., Thompson, I., and Watson, T. F. (2009). Influence of the hydrostatic pulpal pressure on droplets formation in current etch-and-rinse and self-etch adhesives: a video rate/TSM microscopy and fluid filtration study. Dent. Mater. 25, 1392–1402. doi: 10.1016/j.dental.2009.06.010
Sauro, S., Osorio, R., Watson, T. F., and Toledano, M. (2012). Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentin interface. J. Mater. Sci. Mater. Med. 23, 1521–1532. doi: 10.1007/s10856-012-4606-6
Sauro, S., and Pashley, D. H. (2016). Strategies to stabilise dentine-bonded interfaces through remineralising operative approaches – State of the art. Int. J. Adhesion Adhes. 69, 39–57. doi: 10.1016/j.ijadhadh.2016.03.014
Scaffa, P. M., Vidal, C. M., Barros, N., Gesteira, T. F., Carmona, A. K., Breschi, L., et al. (2012). Chlorhexidine inhibits the activity of dental cysteine cathepsins. J. Dent. Res. 91, 420–425. doi: 10.1177/0022034511435329
Shinohara, M. S., De Goes, M. F., Schneider, L. F., Ferracane, J. L., Pereira, P. N., Di Hipólito, V., et al. (2009). Fluoride-containing adhesive: durability on dentin bonding. Dent. Mater. 25, 1383–1391. doi: 10.1016/j.dental.2009.06.011
Stansbury, J. W. (2012). Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 28, 13–22. doi: 10.1016/j.dental.2011.09.005
Tay, F. R., and Pashley, D. H. (2008). Guided tissue remineralisation of partially demineralised human dentin. Biomaterials 29, 1127–1137. doi: 10.1016/j.biomaterials.2007.11.001
Tjaderhane, L., Nascimento, F. D., Breschi, L., Mazzoni, A., Tersariol, I. L., Geraldeli, S., et al. (2013). Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine. Dent. Mater. 29, 116–135. doi: 10.1016/j.dental.2012.08.004
Van Landuyt, K. L., Snauwaert, J., Peumans, M., De Munck, J., Lambrechts, P., and Van Meerbeek, B. (2008). The role of HEMA in one-step self-etch adhesives. Dent. Mater. 24, 1412–1419. doi: 10.1016/j.dental.2008.02.018
Van Meerbeek, B., Yoshihara, K., Van Landuyt, K., Yoshida, Y., and Peumans, M. (2020). From Buonocore’s pioneering acid-etch technique to self-adhering restoratives. a status perspective of rapidly advancing dental adhesive technology. J. Adhes. Dent. 22, 7–34. doi: 10.3290/j.jad.a43994
Keywords: fluoride, calcium phosphate, polymerization, water sorption, resin systems, mechanical properties, dental materials
Citation: Feitosa VP, Pinheiro LS, Moura MEM, De-Paula DM, Alves AH, Rodrigues LK and Sauro S (2020) Physicochemical Properties of Experimental Resin-Based Materials Containing Fluoridated Calcium Phosphates. Front. Mater. 7:582395. doi: 10.3389/fmats.2020.582395
Received: 11 July 2020; Accepted: 13 August 2020;
Published: 15 October 2020.
Edited by:
Luis Alberto Loureiro Dos Santos, Federal University of Rio Grande do Sul, BrazilReviewed by:
Vicente Leitune, Federal University of Rio Grande do Sul, BrazilJoanna Mystkowska, Bialystok University of Technology, Poland
Copyright © 2020 Feitosa, Pinheiro, Moura, De-Paula, Alves, Rodrigues and Sauro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salvatore Sauro, salvatore.sauro@uchceu.es
 Victor P. Feitosa
Victor P. Feitosa Levy S. Pinheiro2
Levy S. Pinheiro2  Lidiany Karla Rodrigues
Lidiany Karla Rodrigues Salvatore Sauro
Salvatore Sauro