Clarifying the chemical reactions of the weakening of adhesion between epoxy resin and aluminum by molecular dynamic simulation and experiment
- 1State Key Laboratory of Alternate Electrical Power System with Renewable Energy Sources, Beijing, China
- 2School of Electrical and Electronic Engineering, North China Electric Power University, Beijing, China
In molecular dynamics simulation, the cross-linked epoxy resin model can well simulate the performance of epoxy resin in actual use. We have established a cross-linked epoxy resin model and a composite model of epoxy resin and aluminum at the molecular level. Based on ReaxFF, the reaction molecular dynamics simulation of pyrolysis aging was carried out by Lammps. The aged epoxy resin is decomposed into H2, CO, H2O, CO2, CH4 and CH2O. Tensile simulation of aged epoxy resin and aluminum system was carried out, and the stress-strain curve was obtained. Compared with non-aging, the maximum stress is reduced by 25.77%. In order to verify the correctness of the simulation results, an epoxy resin with Diglycidyl ether of Bisphenol A (DGEBA) as the resin substrate and 4,4′- Diaminodiphenyl sulfone (44DDS) as the curing agent was prepared, which is coated on the surface of aluminum for curing. In order to explore the change of adhesion during aging, we put the samples in high-temperature vacuum drying oven at 160°C for thermal oxygen aging experiment. The state of epoxy resin at different aging time was studied by SEM, XPS and FTIR. The results showed that the adhesion decreased by 27.16% after aging. The chemical bond in epoxy resin was seriously damaged, the content of the O/C element increased from 15.95% to 22.76%, and the surface cracks of epoxy resin coating increased significantly. Through molecular dynamics simulation and thermal aging experiments, this paper reveals the reasons for the reduction of adhesion between epoxy resin and aluminum caused by thermal aging, which provided theoretical guidance for the aging and falling off of epoxy resin coating in gas-insulated transmission line.
1 Introduction
Epoxy resin has excellent mechanical, electrical, thermal and bonding properties, and is widely used in coatings (Zhang et al., 2015; Waskiewicz et al., 2013; Langer et al., 2019), electronic product packaging (Komoda, 2013; Feng et al., 2018; Li et al., 2019), aerospace (Fischer et al., 2011; Bakis et al., 2021) and electrical equipment (Ramu and Nagamani, 2014; Wang et al., 2019). Because gas-insulated transmission line (GIL) has the advantages of large transmission capacity, low power loss, flexible laying and good reliability, it is suitable for special areas with harsh natural environment and large height drop (Xiao and Yan 2017; Magier et al., 2018). It makes up for the limitations of overhead lines and cables in the field of high voltage direct current transmission (HVDC), and has broad application prospects (Tang et al., 2008). However, during the production, assembly and operation of GIL, the internal metal particles will change the electric field distribution in GIL, resulting in electric field distortion. Under DC voltage, the surface charge accumulation effect will induce surface flashover, which has a great impact on the operation reliability of GIL (Hama et al., 2007; Ma et al., 2015; Ma et al., 2015; Zhou et al., 2017; Xue et al., 2020). The results show that the epoxy coating on the internal electrode of GIL can effectively inhibit the movement of metal particles, thus inhibiting the internal discharge of GIL and improving the operation reliability of GIL (Zhang et al., 2017).
Although the epoxy resin has excellent performance, the epoxy resin will age with the extension of service time during the use process, which shows that the surface is yellow, the gloss is weakened, cracks appear, the mechanical properties and insulation properties are reduced (McGrath et al., 2008; Yang and Jiang, 2015). The epoxy resin coating used in GIL is affected by electric and thermal stress for a long time, which will break the molecular chain of the epoxy resin. After long-time operation, the mechanical properties of the epoxy coating will decline, cracks and even the coating will fall off, which will lead to the complete failure of the epoxy coating (Kim et al., 2005; Zubielewicz and Krolikowska., 2009; Zhang et al., 2014; Mader et al., 2015).
With the rapid development of computer technology, molecular force field and molecular system simulation algorithm have been widely used in the research and application of polymer materials. Molecular dynamics simulation technology can study the physical and chemical changes of materials at the molecular and atomic scales. It has become the third important scientific method after experimental method and theoretical method. Molecular dynamics method can be used to simulate the aging of epoxy resin. Karuth simulated the hydrothermal degradation of cross-linked epoxy resin, and successfully simulated the protonation of water molecules and the nucleophilic attack on the CO bond of ether bond in epoxy amine network (Karuth et al., 2022). At present, many experiment have been carried out on the aging of epoxy resin under different conditions, such as physical aging (Kong et al., 1981; Moosburger-Will et al., 2014), thermal aging (Gao et al., 2011; Chen, 2013), hygrothermal aging (Stocchi et al., 2006; Niu et al., 2021; Gibhardt et al., 2022), photooxidation aging (Zhang et al., 1994; Pan, 2016). In terms of aging experiment, Yang studied the high temperature thermal oxidative aging behavior of bisphenol A epoxy resin fiber reinforced composites cured with anhydride. The results showed that oxidation and molecular rearrangement occurred on the surface of epoxy resin during thermal oxidative aging, and the ester structure on the tertiary carbon atom α、 Hydroxyl and hydrogen are oxidized to carbonyl functional groups. Thermal oxygen aging will significantly reduce the fracture strain, but has little effect on the bending strength (Yang et al., 2015).
Although there are many studies on the aging behavior of epoxy resin, the properties of epoxy resin after aging can still meet the needs of Engineering in many cases. The main material of GIL inner electrode is aluminum, and epoxy resin is attached to the electrode to inhibit the movement and placement of particles. However, once the adhesion between the epoxy resin coating and aluminum decreases to a certain extent or even falls off, the epoxy resin coating will be completely ineffective. More importantly, the coating falling off will become a foreign matter of GIL, which will reduce the insulation reliability of Gil and easily lead to discharge. At present, there is no clear study on the problem of epoxy resin coating falling off in GIL, and the change of interfacial adhesion between epoxy resin and aluminum involved in this study is not clear. Therefore, the problem of epoxy resin coating falling off from aluminum in GIL needs further study.
In this paper, the epoxy resin model and the composite model of epoxy resin and aluminum were established by molecular dynamics simulation. The adhesion between epoxy resin and aluminum was studied by molecular simulation. Through experiments, epoxy resin was prepared with diglycidyl ether of bisphenol A (DGEBA) and 4,4′- diaminodiphenyl sulfone (44DDS) as raw materials, and attached to aluminum alloy after curing for thermal aging test. The samples with different aging time were tested by pull-out test, and the microstructure of the coating was tested by scanning electron microscope (SEM), and the microstructure of the coating was analyzed by fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectrometer (XPS). The change of adhesion between epoxy resin and aluminum alloy in GIL during aging was explored, and the reason for the failure of epoxy resin coating on GIL electrode was explained.
2 Molecular dynamics simulation
2.1 Build model
Epoxy resin is crosslinked by 44DDS and DGEBA. The chemical molecular structure of epoxy monomers DGEBA and 44DDS is shown in Figure 1A. N is the polymerization degree of the resin. For the DER332 epoxy resin used in the actual industry, the average polymerization degree n is 0.03, that is, it is a mixture of 97 mol% DGEBA with n = 0 and 3 mol% DGEBA with n = 1 (Wu., 2007). In order to reasonably simplify the calculation, the degree of polymerization n is set to 0 when constructing the DGEBA molecular model. According to the chemical formulas of DGEBA and 44DDS, the models of DGEBA and 44DDS were built, and the reasonable molecular structure was obtained by geometry optimization, as shown in the Figure 1B and Figure 1C.
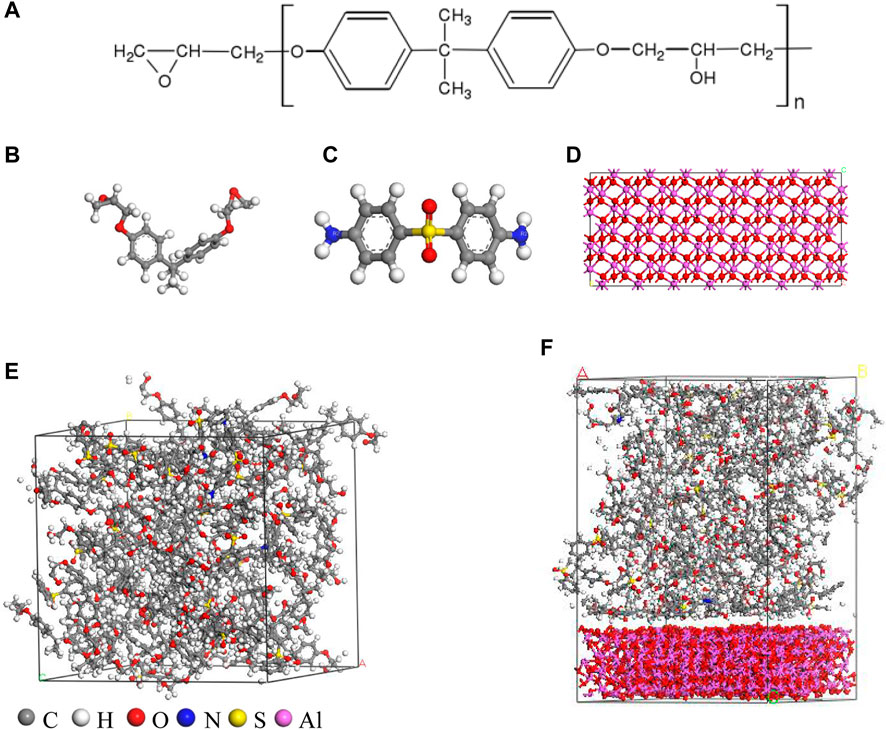
FIGURE 1. (A) Molecular formula of DGEBA; (B) molecular structures of DGEBA; (C) molecular structures of 44DDS; (D) alumina structure after supercell; (E) Crosslinked epoxy resin model; (F) composite model of epoxy resin and alumina.
The crosslinking reaction is realized by the reaction of amino group (-NH2) in 44DDS and epoxy group (-CH(O)CH-) in DGEBA. The reaction is divided into two steps. First, the oxygen in the epoxy group in DGEBA combines with the hydrogen of -NH2 in 44DDS to form -OH, and -NH2 becomes -NH after losing a hydrogen atom. Then, -NH forms covalent bonds with the broken carbon at the end of the epoxy monomer. In this way, DGEBA and 44DDS are connected. Then, the hydrogen in -NH- continues to react with the oxygen in the epoxy ring, and C in the epoxy and N in 44DDS form a new covalent bond. In this way, an amino group in 44DDS reacts with only two epoxy groups in DGEBA, and two amino groups in a 44DDS can react with two DGEBA. Therefore, the molar ratio of DGEBA to 44DDS should be 2:1 (Wu and Xu, 2007).
50 DGEBA and 25 44DDS were crosslinked. Based on the crosslinking reaction mechanism, perl script was used for modeling. The force field used in the modeling process is CompassII. The cross-linking reaction is carried out at 0.1MPa and 500 K. The initial close contacts cutoff is set to 3.5Å. When there are pairs of reaction atoms within the reaction distance, the cross-linking reaction is carried out. After the cross-linking reaction within the reaction distance is completed, the kinetic relaxation is carried out, and then the reaction distance of 0.5Å is increased. This process is repeated. The maximum reaction distance is set to 7Å (Wu and Xu., 2006). After the crosslinking reaction, the crosslinking degree of pure epoxy resin model can reach 90%. Then, 1ns molecular dynamics optimization was carried out under the pressure of 0.1MPa, the temperature of 298 K and NPT ensemble to obtain a crosslinked epoxy resin model with a size of 31.95 Å × 31.95 Å x31.95Å and a density of 1.182g/cm3. The epoxy resin model is shown in Figure 1E. In the experiment, the density of cured epoxy resin is 1.1∼1.2g/cm3, and the crosslinked epoxy resin model after structural optimization is consistent with the actual curing situation.
In Figure 1D, the molecular structure of alumina was expanded to 33.31 Å × 33.31 Å x12.99Å. By building layered structure as a crystal, the cross-linked epoxy resin is placed on the alumina model in Figure 1F. After exporting the model file, use atomsk to convert the file into the data file of lammps. Based on ReaxFF, the model is subject to energy minimization and equalization at 300K and 1 atm. After this process, a new chemical bond will be formed at the interface between alumina and epoxy resin. At the interface between the two materials,the type of chemical bond will be manually adjusted to make the bonding conform to the chemical principle.
2.2 Electrothermal cracking simulation
2.2.1 ReaxFF
ReaxFF determines the connection relationship between atoms based on the bond order, bond distance and the relationship between bond order and energy, which can characterize the chemical reactions between atoms and molecules through the breaking of chemical bonds. The energy expression of ReaxFF is:
Where Esystem is the total energy of the system. Among them, based on the interaction between chemical bonds: Ebond is the bond energy, Eover is the supersaturated bond energy, Eunder is the unsaturated bond energy, Eval is the bond angular energy, Epen is the penalty energy term, Etors is the dihedral angular energy, Econj is the conjugation term of the molecule. Non chemical bond interaction: EvdiWaaals is the van der Waals force interaction energy, and ECoulomb is the electrostatic interaction energy. In recent years, ReaxFF has been widely used in the study of complex chemical reaction systems.
2.2.2 Simulation condition setting
Based on ReaxFF, this paper simulates the actual working conditions of insulators in DC GIL through Lammps, and applies 3kV/mm electric field to the simulation system. During the aging process of epoxy resin, bond fracture and product formation will occur. The research shows that the results obtained by high temperature simulation in ReaxFF are basically consistent with the experimental test. The change of temperature only affects the cracking rate and degree, and the reflected path and final product remain unchanged. In this paper, the pre simulation was carried out from 2300 K to 6300 K. It was found that the epoxy resin coating began to decompose seriously from about 5000 K. In order to shorten the simulation time, molecular dynamics simulation was carried out at 6300 K. The simulation duration is 100 ps, the step size is 0.25 fs, and the reaction state of the current system is output every 1,000 steps. NVT ensemble was used in the simulation process. First, a dynamic relaxation of 4ps was carried out at 300 K, and then an electric field of 3kV/mm was applied. The system temperature increased to 6300 K at the rate of 500k/ps, and maintained this state until the end of the simulation (Ni et al., 2018).
2.2.3 Result
The main pyrolysis products of epoxy resin are shown in the Figure 2A. The main products are H2, CO, CH4, CH2O, H2O, and CO2. This is the same as the experimental result (Zhou et al.,2011). The first 4ps of the simulation process is the dynamic relaxation of epoxy resin, and no product is produced. Then, with the rapid rise of simulation temperature, the chemical bond of epoxy resin began to break and began to produce products. Within 12ps of temperature rise, the product increased gradually. When the aging time reached 16 ps, the molecular numbers of various products no longer increased and began to fluctuate up and down.
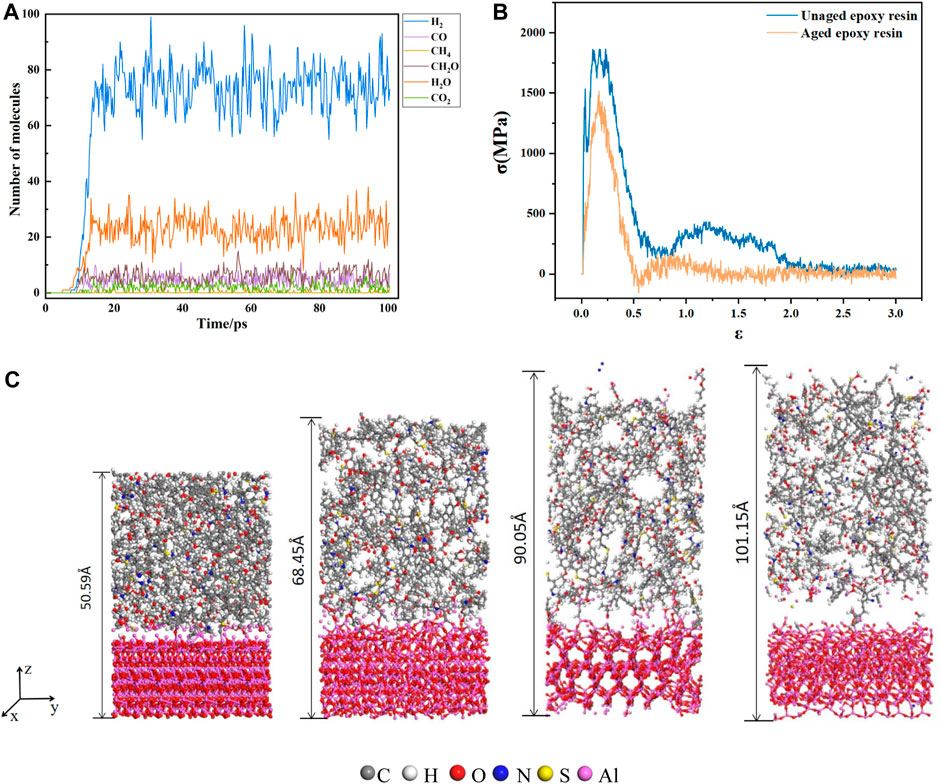
FIGURE 2. (A) The variation curve of main products with time in electrothermal cracking simulation; (B) stress strain curve of epoxy resin aluminum composite model; (C) model picture of epoxy resin and aluminum composite model at 0, 36000, 60000, 81000 steps.
2.3 Stretch simulation
The uncracked cross-linked epoxy resin and the aged 10ps epoxy resin were combined with alumina respectively. At a pressure of 1 atm and a temperature of 298K, the unaged epoxy resin and alumina composite model and the aged and cracked alumina composite model were stretched along the z-axis under the same conditions. The stretching rate was 0.001/fs, the timestep was 0.001 fs, and 300000 steps were run.
In the tensile process, under the action of external force, bond fracture occurs at the interface and inside the epoxy resin with the increase of the distance between atoms. The change of the model at different tensile moments is shown in Figure 2C. The length of the model is from 50.59 Å to 101.15 Å. The relationship between stress and strain in the tensile process is output as the stress-strain curve, as shown in Figure 2B. The
3 Experiment materials and method
3.1 Samples
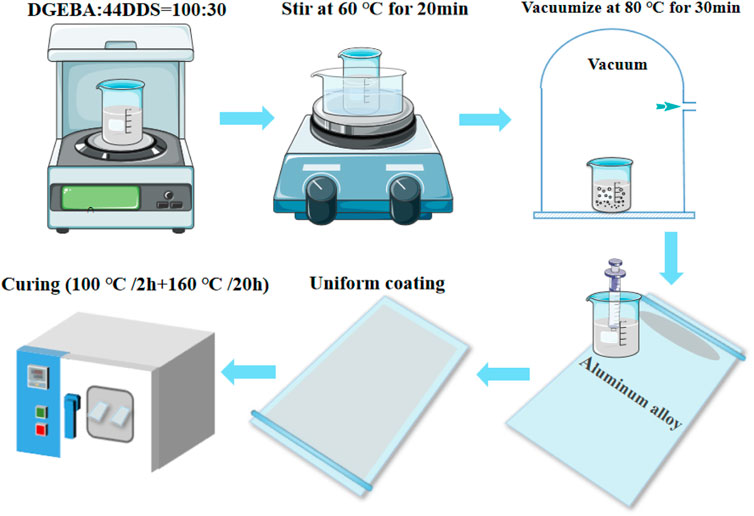
The sample preparation process is shown in Figure 3. The resin substrate is DGEBA and the curing agent is 44DDS. First, mix the DGEBA and 44DDS in a beaker at a mass ratio of 100:30. Because the mixture of epoxy resin and curing agent has a high viscosity at room temperature, it is very difficult to stir. Therefore, it should be stirred at a higher temperature. Under the condition of 60°C water bath, use a magnetic stirrer to stir for 20 min, place the beaker in an electric vacuum drying oven, and vacuumize at 80°C for 30 min. High temperature resistant paper is used to stick on both sides of the aluminum alloy to make the height difference between the edge and the middle of the aluminum alloy, and a groove is formed in the middle of the aluminum alloy. After vacuum pumping, use a straw to adsorb a small amount of epoxy resin solution to one side of the aluminum alloy at 60°C, and push the glass rod to the other side at a uniform speed, so that the epoxy resin can be uniformly coated on the 7,075 aluminum alloy. Then, the aluminum alloy coated with epoxy resin is placed in the oven for curing. Curing procedure: 100°C/2 h + 160°C/20 h. After curing, stop heating and slowly cool the temperature in the oven to room temperature to obtain the epoxy resin sample attached to the aluminum alloy.
The thickness of the final epoxy coating can be controlled by controlling the thickness of the tape. Select a thickness of 50 ± 5 μm of epoxy resin coating samples for the pull-off test. The samples were characterized after aging test at 160°C for 600 h.
3.2 Testing and characterization
3.2.1 Pull-off test
The Positest at-A adhesion tester produced by Defelso company is used. The spindle used for bonding the coating is 20mm in diameter. The Araldite 2015 adhesive divided into two parts A and B is mixed in 1:1 and stirred evenly. The spindle is bonded to the surface of the epoxy resin coating within 3 h. After 12 h, the adhesive adhesion performance is the best. The tensile force is applied uniformly to separate the coating from the aluminum alloy. This force is the adhesion. Select three samples for each aging time to reduce the experimental error.
3.2.2 Characterization
The spotlight 400 and Frontier fourier transform infrared spectroscopy (FTIR) produced by PerkinElmer company in Germany was used to observe the changes of functional groups in the range of 4,000 ∼ 400 cm−1 on the surface of composite samples before and after aging.
AXIS-Ultra DDD-600 W X-ray photoelectron spectrometer (XPS) from Shimadzu-Kratos, Japan, was used. The excitation source was Mg Ka target, and its photoelectron energy was 1,253.6eV with a take-off Angle of 45°. The bond energy is corrected by the C (1s) primary peak of 284.6 eV.
Scanning electron microscope (SEM) was used to test the morphology of the coating under different aging time. The magnification of SEM is 5,000 times.
4 Analysis of test results
4.1 Adhesion analysis
The relation of adhesion between epoxy resin and aluminum alloy after different aging time is shown in the Figure 4A. After 600 h aging experiment, the adhesion between epoxy resin and aluminum alloy decreases from 2.253MPa to 1.641MPa, which decreases by 27.16%. In the early and middle stages of aging, adhesion decreases rapidly. When aged to 300 h, the adhesion between epoxy resin and aluminum alloy decreases to 1.745MPa, which is 22.54% lower than that without aging. In the middle and late stage of aging experiment, the adhesion still decreases, but the rate of decline obviously slows down. During the aging time of 300 h at the late aging stage, the adhesion decreases from 1.745MPa to 1.641MPa, which only decreases by 4.62% compared with that without aging. The adhesion between epoxy resin and aluminum alloy tends to be stable.
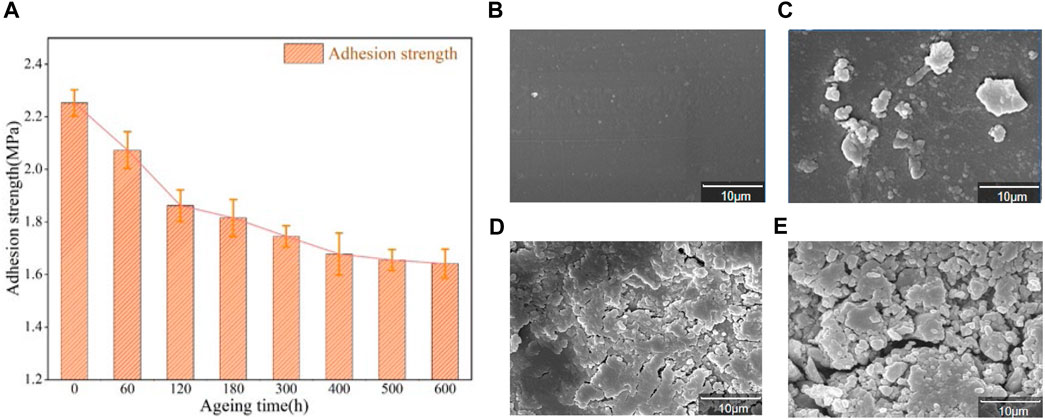
FIGURE 4. (A) The relationship between adhesion and time between epoxy resin and aluminum alloy; Scanning electron microscope of epoxy resin with magnification of 5,000 times (B) 0 h;(C) 120 h; (D) 400 h; (E) 600 h.
4.2 Surface morphology analysis
SEM images of epoxy coating surfaces aged at 160 °C for different times (0 h,120 h,400 h, 600 h) are shown in Figure 4B, Figure 4C,D and Figure 4E. As can be seen from Figure 4B, the surface of the unaged epoxy resin coating is very smooth. After thermal aging at 160°C, the surface of the coating becomes uneven and the roughness gradually increases. After aging for 600 h, cracks in the coating also increase significantly. As shown in Figure 4C, after aging for 120 h, the surface of the coating began to become no longer smooth, with small bumps. With the increase of aging time, the bumps on the surface of the coating are increased, some of the bumps are enlarged and tend to spread into cracks. When aging for 400 h, the number of surface cracks is significantly increased and particles are produced. When the coating is aged for 600 h, the surface cracks deepen and expand, and the particles are more and more dense.
4.3 Fourier transform infrared spectroscopy analysis
The Figure 5A shows the FTIR spectra of epoxy resin before and after thermal oxygen aging test at 160°C. From the figure, we can see the effect of thermal oxygen aging on epoxy resin coating.
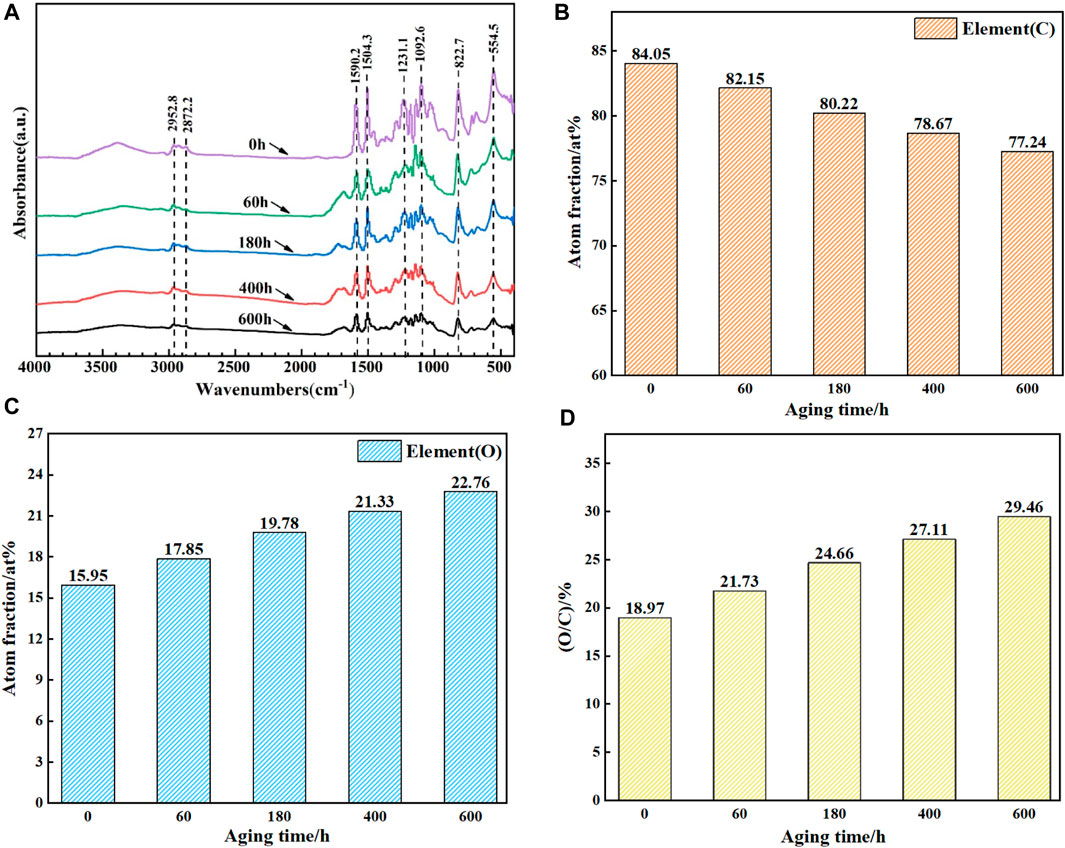
FIGURE 5. (A) FTIR diagram of epoxy resin with different aging time; (B) (C) and (D) are the relative atomic content of O and C in epoxy resin and the value of C/O respectively.
As shown in Figure 5A, compared with the non-aged samples, the FTIR spectra of the epoxy resin aged at 160°C for 600 h have changed significantly. Firstly, the peaks of 2952 cm−1 and 2872 cm−1 were related to C-H stretching. After thermal oxygen aging, the peaks decreased by 35.67% and 46.27% respectively, which proved that the hydrocarbon units in the epoxy resin were seriously aged after thermal oxygen aging test. The breaking of -C=C-C=O at 1,590.2 cm−1 and C-C chemical bond at 1,504.3 resulted in the decrease of absorption peak. In addition, with the extension of aging time, the absorption peak at 1,630.5 cm−1 increases at the aging time of 60 h, which proves that -CH2- in the cured epoxy is oxidized and combined with benzene ring to form C=O. after aging for a longer time, C=O decomposes and the absorption peak gradually weakens. In addition, the absorption peaks at 1,231.1 cm−1, 1,092.6 cm−1, 822.7 cm−1 and 554.5 cm−1 were weakened due to thermal oxygen decomposition. After thermal oxygen aging, the intensity of all absorption peaks decreased significantly, indicating that the structural damage of epoxy resin caused by thermal oxygen aging was very obvious.
4.4 X-ray photoelectron spectrometer analysis
In order to understand the changes of micro elements in epoxy resin coating during thermal oxygen aging, the chemical components of epoxy resin coating during 600 h thermal oxygen aging at 160°C were analyzed by XPS. The two most important elements in the epoxy resin coating are analyzed. Figure 5B, Figure 5C and Figure 5D lists the relative atomic fraction of C and O and the value of O/C atomic ratio. It can be seen from the figure that with the increase of aging time, the relative atomic content of C atoms in the epoxy coating gradually decreases, from 84.05% to 77.24%. The relative atomic content of O atom gradually increased from 15.95% to 22.76%. The O/Cvalue increased from 18.97% without aging to 29.46%. Consistent with the FTIR spectrum analysis, the reason for the increase of O/C relative atomic content is that some C-containing functional groups are oxidized by O2, and the increase of O element content will reduce the relative C element content. In addition, it may be caused by the combination of C and O elements in epoxy to form CO2 volatilization (Cysne Barbosa et al., 2016).
5 Discussion
In the molecular dynamics simulation part, after the tensile test of the model of epoxy resin and aluminum with different aging degrees, we get the conclusion that aging will reduce the adhesion between epoxy resin and aluminum. In order to verify the correctness of this model and the simulation results, the thermal aging experiment of aluminum based epoxy resin corresponding to the simulation was carried out, and the samples were pulled off. The experimental results show that the adhesion between the aged epoxy resin and aluminum is also weakened. In order to explore the changes in the morphology, internal chemical bonds and element content of epoxy resin during the experiment, we carried out SEM, FTIR and XPS experiments. During the aging experiment,the epoxy resin coating is dehydrated due to thermal aging, the molecular chain is broken, and the resin volume gradually shrinks and decomposes. In addition, due to the different coefficient of thermal expansion between epoxy resin and aluminum alloy, local thermal stress will be generated at the interface between epoxy resin and aluminum alloy during thermal aging, which will lead to cracks. The cracks on the surface of the aged epoxy resin provide a channel for oxygen to enter the interior of the sample, so that the interior of the coating is also eroded by oxygen, resulting in a large number of cracks in the coating. The precipitation of crystal water and the generation of cracks in the coating, the fracture of chemical bonds and the change of the relative element content of epoxy resin will have a great impact on the mechanical properties of epoxy resin coating and the adhesion between epoxy resin and aluminum alloy, resulting in the continuous decline of adhesion.
Both simulation and experiment show that aging will break the internal chemical bond of epoxy resin and reduce the adhesion between epoxy resin and aluminum. It is proved that the simulation model and conclusion are correct. In the future research, based on this model, aluminum surface treatment or nano modification of epoxy resin can be carried out, so as to further explore the methods to improve the interfacial adhesion and the aging cracking resistance of the coating.
6 Conclusion
In this paper, a cross-linked epoxy resin model is constructed. After electrothermal cracking aging simulation, the main products of epoxy resin decomposition are H2, CO, H2O, CO2, CH4, and CH2O. After the cross-linked epoxy resin and the aged epoxy resin were constructed with alumina respectively, the tensile simulation was carried out. The maximum stress after aging was 25.77% lower than that of the non-aging model. We verify the correctness of the simulation through aging experiments. After DGEBA and 44DDS are mixed and coated on the aluminum alloy, the epoxy resin is cured after heating 100°C/2 h + 160°C/20 h. After 600 h and 160°C aging experiment, the adhesion between epoxy resin and aluminum alloy decreased by 27.16%. A large number of cracks appeared on the surface of the epoxy resin coating, and the internal chemical bond was broken in a large range. The content of C element in the epoxy resin decreased and the content of O element increased. This experiment can provide guidance for the aging failure of epoxy resin coating during GIL operation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JW contributes to the design and implementation of the research. JW contributes to the model design. ZW contributes to the model validation. NY contributes to the debonding simulations. ZH contributes to validation. YC contributes to the data analysis. JW contributes to the draft review. QL contributes to supervision, conceptualization, and writing.
Funding
This research was funded by the National Natural Science Foundation of China (52177140), and the Fundamental Research Funds for the Central Universities (2022JG003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bakis, G., Wendel, J. F., Zeiler, R., Aksit, A., Haublein, M., Demleitner, M., et al. (2021). Mechanical properties of the carbon nanotube modified epoxy-carbon fiber unidirectional prepreg laminates. Polymers 13, 770. doi:10.3390/polym13050770
Chen, H. (2013). Effect of thermal oxidative aging on Breakdown Properties of epoxyresin/Montmorillonite Nanocomposites. Heilongjiang, China: Harbin University of Science and Technology. doi:10.7666/d.Y2412966
Cysne Barbosa, A. P., Fulco, A. P. P., Guerra, E. S. S., Arakaki, F. K., Tosatto, M., Costa, M. C. B., et al. (2016). Accelerated aging effects on carbon fiber/epoxy composites. Compos. Part B Eng. 110, 298–306. doi:10.1016/j.compositesb.2016.11.004
Feng, Y., Hu, J., Wang, F., Huang, Q., Peng, C., and Xu, Z. (2018). Synthesizing promising epoxy acrylate prepolymers applied in ultraviolet cured adhesives based on esterification reaction. Mat. Res. Express 5, 065321. doi:10.1088/2053-1591/aacd3f
Fischer, F., Beier, U., Wolff-Fabris, F., and Altstadt, V. (2011). Toughened high performance epoxy resin system for aerospace applications. Sci. Eng. Compos. Mat. 18, 209–215. doi:10.1515/SECM.2011.042
Gao, Y., Cui, W., and Mu, W. (2011). Study on thermal oxidative aging behavior of epoxy resin adhesive. New Chem. Mater. 39, 72–74.
Gibhardt, D., Buggisch, C., Meyer, D., and Fiedler, B. (2022). Hygrothermal aging history of amine-epoxy resins: Effects on thermo-mechanical properties. Front. Mat. 9. doi:10.3389/fmats.2022.826076
Hama, H., Hikosaka, T., Okabe, S., and Okubo, H. (2007). Cross-equipment study on charging phenomena of solid insulators in high voltage equipment. IEEE Trans. Dielectr. Electr. Insul. 14, 508–519. doi:10.1109/TDEI.2007.344633
Karuth, A., Alesadi, A., Vashisth, A., Xia, W. J., and Rasulev, B. (2022). Reactive molecular dynamics study of hygrothermal degradation of crosslinked epoxy polymers. ACS Appl. Polym. Mat. 4, 4411–4423. doi:10.1021/acsapm.2c00383
Kim, J, K., Lebbai, M., and Woo, R. S. (2005). Effects of hygrothermal ageing and thermal shock on reliability of interfacial adhesion between black oxide coated copper substrate and epoxy resin. Compos. Interfaces 12, 739–756. doi:10.1163/156855405774984020
Kong, S. W., Garth, L. W., McGrath, J. E., Banthia, A. K., and Tant, M. R. (1981). Physical aging of linear and network epoxy resins. Polym. Eng. Sci. 21, 943–950. doi:10.1002/pen.760211413
Langer, E., Waskiewicz, S., and Kuczynska, H. (2019). Application of new modified Schiff base epoxy resins as organic coatings. J. Coat. Technol. Res. 16, 1109–1120. doi:10.1007/s11998-019-00185-7
Li, K., Zhang, L., and Xu, Z. (2019). Decomposition behavior and mechanism of epoxy resin from waste integrated circuits under supercritical water condition. J. Hazard. Mat. 374, 356–364. doi:10.1016/j.jhazmat.2019.04.028
Ma, G., Zhou, H., Li, C., Jiang, J., and Chen, X. (2015). Designing epoxy insulators in SF6-filled DC-GIL with simulations of ionic conduction and surface charging. IEEE Trans. Dielectr. Electr. Insul. 22, 3312–3320. doi:10.1109/TDEI.2015.005031
Mader, E., Liu, J., Hiller, J., Lu, W., Li, Q., Zhandarov, S., et al. (2015). Coating of carbon nanotube fibers: Variation of tensile properties, failure behavior, and adhesion strength. Front. Mat. 2. doi:10.3389/fmats.2015.00053
Magier, T., Tenzer, M., and Koch, H. (2018). Direct current gas-insulated transmission lines. IEEE Trans. Power Deliv. 33, 440–446. doi:10.1109/TPWRD.2017.2716182
McGrath, L. M., Parnas, R. S., King, S. H., Schroeder, J. L., Fischer, D. A., and Lenhart, J. L. (2008). Investigation of the thermal, mechanical, and fracture properties of alumina-epoxy composites. Polymer 49, 999–1014. doi:10.1016/j.polymer.2007.12.014
Moosburger-Will, J., Greisel, M., and Horn, S. (2014). Physical aging of partially crosslinked RTM6 epoxy resin. J. Appl. Polym. Sci. 131, 72–74. doi:10.1002/app.41121
Ni, X., Wang, J., Wang, J., Li, Q., and Han, Z. (2018). Micro-control simulation of electro-thermal dissociation characteristics of carbon nanotubes/epoxy resin composites. Diangong Jishu Xuebao 33, 5159–5167. doi:10.19595/j.cnki.1000-6753.tces.L80834
Niu, Y., Yan, Y., and Yao, J. (2021). Hygrothermal aging mechanism of carbon fiber/epoxy resin composites based on quantitative characterization of interface structure. Polym. Test. 94 (12), 107019. doi:10.1016/j.polymertesting.2020.107019
Pan, Y. (2016). Degradation of epoxy resin adhesive in UV/O3 environment, 2016. Qinhuangdao, Hebei, China: Yanshan University, 26–38.
Ramu, T. S., and Nagamani, H. N. (2014). Alumina and silica based epoxy nano-composites for electrical insulation. IEEE Trans. Dielectr. Electr. Insul. 21, 236–243. doi:10.1109/TDEI.2013.003555
Stocchi, A., Pellicano, A., Rossi, J. P., Bernal, C., and Montemartini, P. (2006). Physical and water aging of glass fiber-reinforced plastic pipes. Compos. Interfaces 13, 685–697. doi:10.1163/156855406779366831
Tang, H., Wu, G., Fan, J., Li, J., and Wang, H. (2008). Insulation design of gas insulated HVDC transmission line. Power Syst. Technol. 32, 65–70. CNKI:SUN:DWJS.0.2008-06-017.
Wang, Y., Li, Y., Zhang, Z., Zhao, H., and Zhang, Y. (2019). Repair performance of self-healing microcapsule/epoxy resin insulating composite to physical damage. Appl. Sci. (Basel). 9, 4098. doi:10.3390/app9194098
Waskiewicz, S., Zenkner, K., Langer, E., Lenartowicz, M., and Gajlewicz, I. (2013). Organic coatings based on new Schiff base epoxy resins. Prog. Org. Coat. 76, 1040–1045. doi:10.1016/j.porgcoat.2013.02.017
Wu, C. (2007). Atomistic molecular simulations of crosslinked epoxy resins. [dissertation/doctoral thesis]. Hunan: Hunan University.
Wu, C., and Xu, W. (2006). Atomistic molecular modelling of crosslinked epoxy resin. Polymer 47, 6004–6009. doi:10.1016/j.polymer.2006.06.025
Wu, C., and Xu, W. (2007). Atomistic simulation study of absorbed water influence on structure and properties of crosslinked epoxy resin. Polymer 48, 5440–5448. doi:10.1016/j.polymer.2007.06.038
Xiao, D., and Yan, J. (2017). Application and development of gas insulated transmission line (GIL). High. Volt. Eng. 43, 699–707. doi:10.13336/j.1003-6520.hve.20170303002
Xue, J., Chen, J., Dong, J., Sun, G., Deng, J., and Zhang, G. (2020). A novel sight for understanding surface charging phenomena on downsized HVDC GIL spacers with non-uniform conductivity. Int. J. Electr. Power & Energy Syst. 120, 105979. doi:10.1016/j.ijepes.2020.105979
Yang, J., and Jiang, B. (2015). Long-term moisture effects on the interfacial shear strength between surface treated carbon fiber and epoxy matrix. Compos. Part A Appl. Sci. Manuf. 78, 311–317. doi:10.1016/j.compositesa.2015.08.027
Yang, Y., Xian, G., Li, H., and Sui, L. (2015). Thermal aging of an anhydride-cured epoxy resin. Polym. Degrad. Stab. 118, 111–119. doi:10.1016/j.polymdegradstab.2015.04.017
Zhang, C., Lin, H., Zhang, S., Xie, Q., Ren, C., and Shao, T. (2017). Plasma surface treatment to improve surface charge accumulation and dissipation of epoxy resin exposed to DC and nanosecond-pulse voltages. J. Phys. D. Appl. Phys. 50, 405203–405214. doi:10.1088/1361-6463/aa829b
Zhang, G., Pitt, W. G., Goates, S. R., and Owen, N. L. (1994). Studies on oxidative photodegradation of epoxy resins by IR-ATR spectroscopy. J. Appl. Polym. Sci. 54, 419–427. doi:10.1002/app.1994.070540402
Zhang, X., Zhong, Y., Li, L., and Yan, Y. (2014). Adhesion failure of antiscratch coatings on polycarbonate under UV irradiation. J. Appl. Polym. Sci. 131. doi:10.1002/app.40507
Zhang, Y., Zhang, W., Li, D., Sun, Y., Wang, Z., Hou, C., et al. (2015). Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method. Int. J. Mol. Sci. 16, 2239–2251. doi:10.3390/ijms16012239
Zhou, H., Ma, G., Li, C., Shi, C., and Qin, S. (2017). Impact of temperature on surface charges accumulation on insulators in SF6-filled DC-GIL. IEEE Trans. Dielectr. Electr. Insul. 24, 601–610. doi:10.1109/TDEI.2016.005838
Zhou, Y., Wu, W., and Qiu, K. (2011). Recycling of organic materials and solder from waste printed circuit boards by vacuum pyrolysiscentrifugation coupling technology. Waste Manag. 31, 2569–2576. doi:10.1016/j.wasman.2011.07.002
Keywords: epoxy resin, adhesion, molecular dynamic simulation, thermal aging experiment, aging mechanism
Citation: Wang J, Wang Z, Yan N, Han Z, Chang Y, Wang J and Li Q (2022) Clarifying the chemical reactions of the weakening of adhesion between epoxy resin and aluminum by molecular dynamic simulation and experiment. Front. Mater. 9:985758. doi: 10.3389/fmats.2022.985758
Received: 04 July 2022; Accepted: 21 July 2022;
Published: 11 August 2022.
Edited by:
Jiefeng Liu, Guangxi University, ChinaReviewed by:
Yunqi Xing, Hebei University of Technology, ChinaXianhao Fan, Guangxi University, China
Copyright © 2022 Wang, Wang, Yan, Han, Chang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, wangjian31791@ncepu.com
 Jian Wang
Jian Wang Zhe Wang
Zhe Wang Ningning Yan2
Ningning Yan2  Qingmin Li
Qingmin Li