Intensive Monitoring Studies for Assessing Medicines: A Systematic Review
- 1Centre for Health Evaluation and Research (CEFAR), National Association of Pharmacies, Lisbon, Portugal
- 2Faculty of Pharmacy, University of Lisbon, Lisbon, Portugal
- 3Department of Epidemiology and National Cancer Registry (RON), Portuguese Institute of Oncology, Francisco Gentil, E.P.E., Lisbon, Portugal
- 4Setubal and Santarem Regional Pharmacovigilance Unit, Lisbon, Portugal
- 5Faculty of Medicine, Center for Evidence-Based Medicine, University of Lisbon, Lisbon, Portugal
- 6Division of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, Netherlands
- 7Faculty of Medicine, Institute of Molecular Medicine and Laboratory of Clinical Pharmacology and Therapeutics, University of Lisbon, Lisbon, Portugal
Introduction: Intensive monitoring (IM) is one of the methods of post-marketing active surveillance based upon event monitoring, which has received interest in the current medicines regulatory landscape. For a specific period of time, IM involves primary data collection and is actively focused on gathering longitudinal information, mainly safety, since the first day of drug use.
Objectives: To describe IM systems and studies' data published over 11-years period (2006–2016). Specifically, we reviewed study population/event surveillance, methodological approaches, limitations, and its applications in the real-world evidence generation data.
Methods: We completed a systematic search of MEDLINE and EMBASE to identify studies published from 2006 to 2016, that used IM methodology. We extracted data using a standardized form and results were analyzed descriptively. The methodological quality of selected studies was assessed using the modified Downs and Black checklist.
Results: From 1,400 screened citations, we identified 86 papers, corresponding to 69 different studies. Seventy percent of reviewed studies corresponded to established IM systems, of which, more than half were prescription event monitoring (PEM) and modified-PEM. Among non-established IM systems, vaccines were the most common studied drugs (n = 14). The median cohort size ranged from 488 (hospitals) to 10,479 (PEM) patients. Patients and caregivers were the event data source in 39.1% of studies. The mean overall quality score was similar between established and non-established IM.
Conclusions: Over the study period, IM studies were implemented in 26 countries with different maturity levels of post-marketing surveillance systems. We identified two major limitations: only 20% of studies were conducted at hospital-level, which is a matter of concern, insofar as healthcare systems are facing a lack of access to new medicines at ambulatory care level. Additionally, IM access to data of drug exposure cohorts, either at identification or at follow-up stages, could somehow constitute a barrier, given the complexity of managerial, linkable, and privacy data issues.
Introduction
Bridging the gap between information generated by randomized clinical trials (RCT) and how to interpret different evidence sources to better understand the real-world drug usage is of great importance, since drugs often do not perform as well in RCT as in routine clinical practice, the latter characterized by a variety of sociocultural behaviors and clinical settings (1, 2). Overtime this was clearly a lesson learned and nowadays society, including payers, demands an integrated assessment of benefits and risks under real life conditions as the next logical step after RCT (3, 4). The adoption and use of real-world evidence (RWE), defined as the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of routine care data, is being increasingly important for regulatory decision-making (5, 6). RWE can provide insights into key evidentiary needs by regulators which include: (1) monitoring of medication performance in routine care, including the effectiveness, safety (e.g., labeling changes, withdrawals) and value; (2) identifying new patient strata in which a drug may have added value or unacceptable harms; and (3) monitoring targeted utilization (7).
In the last decades, a tale of withdrawals (8–10) has boosted interest in pharmacovigilance and in response, regulators have started to reform their systems, which have shifted from a largely reactive response, that relied mainly on spontaneous reporting (SR), to a more proactive approach to drug safety issues (11). Specifically, in late 2005, the US Food and Drugs Administration (FDA) and the European Medicines Agency (EMA) issued guidance documents on therapeutic risk management planning aimed at strengthening proactive postmarketing surveillance (12). More recently, the European Union implemented new pharmacovigilance legislation, where regulatory agencies have now extended powers to demand for post-authorization efficacy studies (PAES) in addition to post-authorization safety studies (PASS) (13). Overall, it has been recognized that the knowledge of drugs is no longer restricted to a binary decision at the time of marketing authorization and the prevailing paradigm changed from a risk centered approach to a benefit/risk assessment throughout the medicine entire lifecycle (1, 14).
Framed onto the scope of all these regulatory changes, intensive methods of post-marketing surveillance based on drug event monitoring (15), known as intensive monitoring (IM) methodology has been of interest (16–18). IM established systems were launched in New Zealand [Intensive Medicines Monitoring Program (IMMP)] (19) and in the UK [Prescription Event Monitoring (PEM)] (20, 21), in the late 1970s and early 1980s, respectively. Since then, these systems and its background methodology have evolved and been implemented in several geographies worldwide, such as in the Netherlands [Lareb Intensive Monitoring (LIM)] (11), Japan (22), or in some African countries (23).
As compared to SR system that passively monitors all drugs during their whole life cycle and cover all population (24, 25). IM combines the strengths of pharmacoepidemiological and clinical pharmacovigilance approaches and focuses on specific drugs. For a specific period of time, IM involves primary data collection and is defined as an observational inception cohort of subjects exposed to the drug(s) of interest (26). IM cohorts of drug exposures are identified either through prescribers (e.g., PEM), pharmacies (e.g., IMMP), and national pharmacovigilance systems (e.g., LIM) and followed in a systematic and prospective fashion through a large variety of sources (e.g., patients, prescribers, and hospitals).
Although IM systems were developed more than 30 years ago, there has not been a global comprehensive synthesis of event drug monitoring research studies to date. The purpose of this systematic review is to describe IM systems and studies' data published in the decade following the paradigm shift in medicines regulatory assessment, which was largely characterized by a more proactive approach to drug safety issues. From 2006 to 2016, we reviewed study population/event surveillance, methodological approaches (including data collection sources and analysis), limitations, main outcomes of interest, and IM applications in the real-world evidence generation data.
Materials and Methods
This study followed current guidance of conducting and reporting systematic reviews, including guidance for undertaking reviews in health care on public health intervention reviews by the Center for Reviews and Dissemination of the University of York (27) and recommendations from the PRISMA-P statement regarding reporting items (28). The protocol for this review was registered at PROSPERO (CRD42017069309) available at https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017069309.
For inclusion in the review, papers had to report data on an IM study/system as defined above. RCT, studies conducted through automated databases (e.g., claims or electronic health/medical records), registries, SR schemes and case-reports/series were excluded. No restriction on study population, intervention, outcomes and comparator was imposed for study selection, although we only included studies published in English, Portuguese, Spanish, Italian, or French. Letters to editor and conference proceedings were also excluded, as these materials often reflect preliminary analysis and it is less likely that methods and results are described with the necessary details.
Electronic database identification of reports was undertaken on MEDLINE and EMBASE via OVID SP interface from inception to the 20th of April 2017, to include studies published on the time-frame of interest: January 2006 to December 2016. Complementary searches were made to identify potential additional articles: reference checking and hand-searching. The search strategy was developed after several iterations and it is presented in Additional File 1.
References located and potentially eligible for inclusion were exported to an Excel® file where authors recorded eligibility criteria of selected abstracts and full paper references. The abstracts were independently checked against the inclusion criteria by CT, MC, and PB and classified as include, unclear or exclude. The full reports for all articles that classified as include or unclear were retrieved, and two authors (CT, MC) independently evaluated its eligibility criteria for inclusion. All disagreements were resolved by discussion or, if necessary, by arbitration by a third review author (AM). The main reasons for exclusion, either at the title/abstract or at the full text screening phases were recorded.
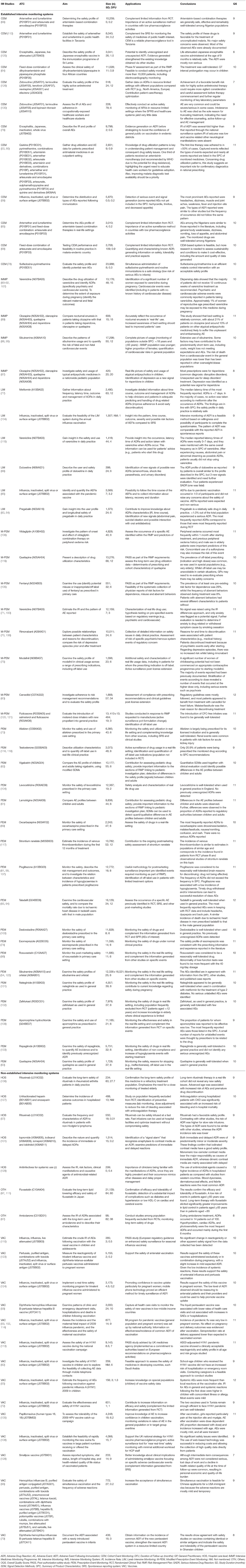
Data from included papers were extracted by three authors (MC, CT, PB) and validated by a fourth author (FB), using a standardized data extraction form designed and pre-piloted for this review. This form was designed to systematically retrieve information from each included study on the following items: (1) general characteristics: title, first author, citation, year of publication and country, (2) type of IM system: (2.1) established systems: Cohort event monitoring (CEM), IMMP, LIM, PEM, or Modified-PEM (M-PEM) or (2.2) non-established systems/single IM studies, (3) background & rationale, (4) research question, (5) setting, (6) study design, (7) population eligibility criteria, (8) drugs studied [classified according to the Anatomical Therapeutic Chemical classification (ATC) from World Health Organization (WHO)] (29), (9) methods and data collection (variables), (10) drug domains studied, (11) data sources of events reporting [patients/caregivers (PCG), healthcare professionals (HCP)], (11) data analysis, (12) duration of follow-up and study time frame, (13) number of patients included, (14) limitations pointed by the authors, (15) authors' conclusions, (16) applications, and (17) sources of funding.
One review author (FB) assessed the risk of bias of the included studies using the modified Downs and Black assessment checklist (30), for the risk of bias and the quality of both randomized and non-randomized studies. Data was validated by another reviewer (CT) and the rationale behind assessments was documented. The Downs and Black assessment checklist was selected for the following reasons: (1) in an evaluation by Deeks et al. (31), it was one of the six instruments considered most suitable for use in systematic reviews of non-randomized studies, out of 182 tools identified; (2) it was recommended as one of the most useful tools for assessing risk of bias in non-randomized studies both by Cochrane Collaboration and the Agency for Healthcare Research and Quality (32). As some items of the Downs and Black checklist are only applicable to randomized studies and since the majority of published IM studies are a single-arm design, the Downs and Black checklist was adapted for the purpose of this review as provided in Additional File 2. Our modified checklist included a total of 13 topics out of the 27 of the original version. Consequently, the overall quality score of each study ranged between 0 and 13.
The data synthesis was descriptive as the main aim of this systematic review was to identify methods, not quantify any effect. Data from the included studies were described and presented in text, tables and figures. When multiple papers were retrieved from the same IM study (e.g., results at different follow-up periods or reporting at different outcomes/drug study domains) they were treated as a single study.
Results
Literature Search
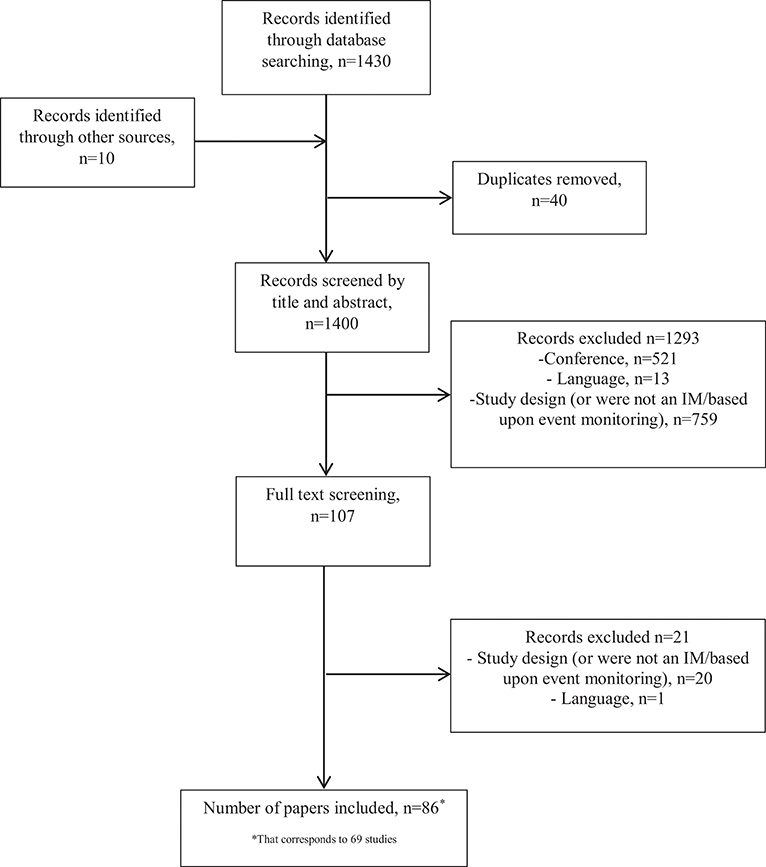
The search and screening process is summarized in Figure 1. A total of 1,430 references were identified through the electronic searches of the databases. Ten additional records were identified through hand searches. After 40 duplicates were removed, we obtained 1,400 citations, which were screened by title and abstract. We excluded 1,293 citations as they did not meet the inclusion criteria, and the remaining 107 were screened full text. Twenty-one citations were further excluded (33–52) and 86 papers were included, corresponding to 69 different studies (53–138).
Overview of Studies
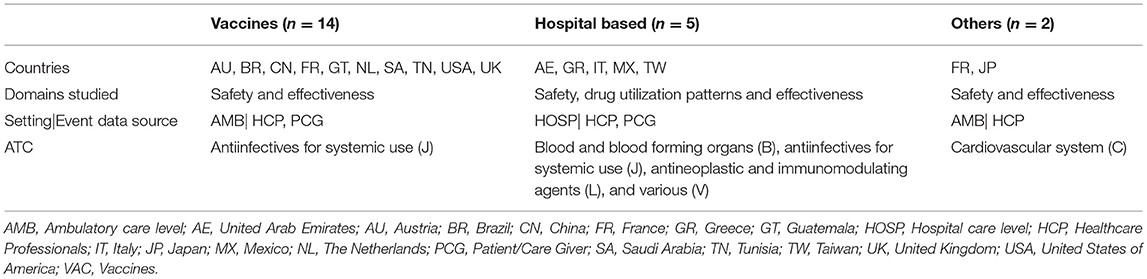
The included studies were conducted in 26 countries. Overall, 70% of studies corresponded to established IM systems: PEM (n = 18), M-PEM (n = 8), CEM (n = 12), LIM (n = 6), and IMMP (n = 4). The remaining (n = 21) were single studies conducted within the IM methodology framework but were not part of any established IM. These studies were grouped in three categories: Vaccines (n = 14), Hospital setting based (n = 5), and Others (n = 2). Tables 1, 2 summarize the main characteristics (drugs monitored ATC, drug domains studied, event data source, methods of data collection and countries where the studies were conducted) of established and non-established IM systems. Data extracted from all included studies are presented in Table 3.
Established IM Systems
PEM and M-PEM represented the majority of the studies included (n = 26). Concerning PEM studies, the median study duration was 35.5 months (range: 8–55) and the duration of patient follow-up varied between 2 and 12 months (median: 6.0). Similar results were found for M-PEM studies. The median number of patients per study was 10479.5 (range: 1,728–28,357) and 7419.5 (range: 551–26,877), for PEM and M-PEM studies, respectively. For both schemes, it was stated that all studies were conducted with unconditional funding from the pharmaceutical industry. The common limitations pointed out by the authors was the non-return by general practitioner (GP) of questionnaires (which might result in non-response bias if the characteristics of patients at responding GP practices differ from those at non-responding GP practices), under-reporting and the restriction to primary care setting. Furthermore, the lack of a concurrent control (single-group cohort design) was also addressed as a limitation, leading to a knowledge gap on the true background incidence for events. Unlike PEM, the M-PEM methodology offered a greater scope to collect information on confounding variables, since a more detailed study-specific questionnaire was used.
Considering CEM studies, the median study and patient follow-up duration, was 10.0 (range: 0.5–109) and 0.7 months (range: 0.2–12), respectively. The median cohort size was 4,789 (range: 228–23,988) patients. Five out of 12 studies were conducted with no sources of funding, 6 studies were financially supported by either governmental institutions (n = 3), non-governmental institutions (n = 2) or both (n = 1) and one study was financed by the pharmaceutical industry. Lack of generalizability (selection bias concerning patients' enrolment and high cohort drop-out rates), baseline events reported as “true” adverse drug events (ADE) (e.g., antimalarials studies with no event collection before vs. after treatment), costly and resource labor intensive for data collection and management were described as limitations of concern.
LIM studies reported the lowest cohort size among the established IM systems. Overall, a median number of 1462.5 (range: 398–3,569) patients were enrolled. The median study duration for the 5 out of 6 studies where this information was available, was 24 months (range: 7–63) and patients' follow-up duration varied between 1 and 12 months (median: 5.0). The majority of the LIM studies (n = 3) did not report the source of funding, 2 studies were conducted with financial support from governmental institutions and one was implemented without any source of funding. Limitations raised were in line with other established IM systems. LIM studies reported event rates rather than true incident rates and no information was provided about the patients that did not accept to participate (e.g., older people might be underrepresented since they do not have access/are not familiar with internet). Furthermore, since the patients were the source of event information, those who experienced an adverse drug reaction (ADR) might be more motivated to fill in a questionnaire than those who did not experience it (reporting bias). It was also stated as a limitation the difficulty in obtaining information about serious and fatal outcomes.
The median number of patients from IMMP studies was 6,891 (range: 420–17,298). The median study duration was similar to PEM studies, however a higher duration of follow-up time period (median: 15 months; range: 2–20) was observed. All studies received funding from governmental institutions and 2 studies were unconditionally co-funded by pharmaceutical industry. Not all IMMP studies reported limitations. From those studies where this information was available, an absence of a comparator group, underestimation of ADE rates and limited clinical detailed information were issues pointed out. Further, in the study of varenicline (92), the “effectiveness assessment” was performed based on information provided by the reporting doctor and for many patients, it was unknown whether varenicline was effective.
Non-established IM Systems
Two-thirds of non-established IM studies reported the IM of vaccines, half of those were related to the influenza H1N1 2009 pandemic vaccine. Almost all vaccines' studies (13 out of 14) targeted vulnerable populations (e.g., children, pregnant women). These studies were carried out using different methods for data collection (HCP face-to-face/web-based/telephone or mobile text messages). The median follow-up time observed was 4.5 months (range: 0.2–10) and the median study duration was 14 months; range: 1–27). The main limitations were non-response bias, non-representativeness, the lack of a control group, small sample size to detect rare outcomes (e.g., autoimmune diseases) and information bias (e.g., recall bias, adverse events following immunization (AEFI) not clinically confirmed).
IM non-established system studies classified as “Others” covered only drugs from cardiovascular system ATC main group. Regarding hospital-based studies, a wide range of drugs were monitored, although the median number of patients included was lowest (488) within all reviewed studies. Regarding funding sources, 8 out of the 21 studies did not mention the source of funding, 7 were supported by governmental institutions, 3 from the pharmaceutical industry, 1 from a non-governmental organization, and 2 reported no sources of funding.
Overall Quality Score
The mean overall quality score (OQS) was 9.7 out of 13 (range: 7–12), being similar between established (9.9; range: 6–12) and non-established (9.7; range: 7–12) IM studies. Among established IM studies, M-PEM and PEM presented the highest mean OQS (10.5 and 10.3, respectively). Detailed results about OQS of each reviewed study are shown in Additional File 3.
Discussion
In the decade following the paradigm shift in medicines regulatory systems, from a largely reactive response to a more proactive approach to drug safety issues (2006–2016), we thorough examined IM methodological features for data collection and analysis, population surveilled, limitations and its applications in the daily practice environment. IM studies reviewed were implemented in 26 countries with different maturity levels of post-marketing surveillance systems. IM systems operated either in countries with non-existing or weak monitoring SR schemes, such as sub-Saharan African countries (23, 139), or in countries that have the most widely used record-linkage databases in the world for drug research, such as the UK (e.g., Clinical Practice Research Datalink) (140) or the Netherlands (e.g., PHARMO) (141)—picturing the contribution of IM systems in the real-world evidence generation data. Regardless the differences found within the methodologies used, these schemes were developed with the purpose of filling the gap between RCT (high internal validity and low external validity) (142, 143), SR data (limited by under and selective reporting) (25, 144) and automated database studies (their large size and their longer follow-up times and representativeness make it possible to study real-world effectiveness and safety, but they are usually poor in detailed covariate data) (145, 146). Based on event monitoring and by tracking patients and drug use in a life-cycle based fashion, the results originating from IM studies encompasses the identification/quantification of factors that possibly negatively affect the benefit/risk balance, including (new) adverse events (identification and strengthening of signals), increase of knowledge of drug utilization patterns, identification of off-label use, among others. Moreover, by collecting longitudinal data since the first day of drug use, it allows to follow the time course (latency time and duration), outcome and management (to help clinicians and patients to adequate predicting with handling ADE, improving adherence and avoid early-discontinuation) of ADE; information that very few post-authorization methods can provide.
In the beginning of the century, Waller and Evans (147) argued that pharmacovigilance should be less focused on finding harm and more focused on extending knowledge of safety. Since then, the regulatory landscape has evolved and in parallel, an endeavor of post-marketing active surveillance schemes to meet the new regulatory challenges was witnessed. IM systems were no exception. For example, in the UK, PEM moved toward a more target surveillance: M-PEM. In the latter, efforts are done to better understand known or partially known drug risks (e.g., target analysis of events requiring special monitoring, more detailed characterization of drug usage, adherence to prescribing guidelines) and an alignment with regulatory requirements (e.g., PASS as part of RMP), is explicitly described as applications of this scheme. Further, the target sample size of 10,000 patients in conventional PEM-studies, which was driven by sensitivity assumption to detect rare and uncommon events was abandoned in M-PEM studies, where a specific sample size is calculated depending on the research question of interest (18). Some authors argue that IM is not an efficient way to detect these frequency-type events and for that purpose, other methods should be considered. For example, SR would probably be a more suitable method followed by an analytical study to confirm the signal (85). Likewise, the limited follow-up time duration does not allow for the detection of long-term events (e.g., cancer).
On the whole, drugs monitored through the reviewed studies were in the early post-marketing phase or were characterized by uncertainties concerning specific safety issues, namely those identified in the RMP (safety concerns raised from RCT, post-marketing experience and/or suspicion of inappropriate drug use). This was generally in line with IM drug entry decision criteria previously described by Coulter (19) and more recently by Harrison-Woolrych (148). Also, noteworthy that older drugs can be studied within this methodology. This was the case of metformin, marketed 60 years ago, where relevant information from the daily practice perspective, such as the outcome, management and the time course of metformin related ADE was lacking (77). We also observed that two-thirds of CEM studies were launched in resource-constrained settings and developed for monitoring artemisinin-based combination therapy for malaria treatment, aiming to complement information from RCT. In recent years, CEM was adapted and covered other drugs, such as antiretrovirals (126), vaccines (76), among others. Overtime, some practical handbooks have been issued by the WHO to support the implementation of specific programs [malaria (149), HIV/AIDS (150), and tuberculosis (151)]. The experiences of countries that have implemented CEM indicate that this was a key opportunity to raise awareness and to build pharmacovigilance capacity in these settings, which can be expected to have a positive effect on SR activities in the long run (23). The latter is of importance, since there is a need to strengthen ADR reporting rates in low-income countries and IM studies could be used in national pharmacovigilance systems (152).
Despite IM features found worldwide, the majority of monitored drugs were prescribed at the primary care level, highlighting the limited research in hospital and other secondary settings, either among established or non-established IM studies. At hospital level, where the drug market is rapidly changing, with more and more new drugs being introduced (e.g., cancer, autoimmune diseases, infectious diseases, etc.) (153), it seems that automated databases or often registries (drug registries or frequently disease registries) supplement IM systems. This might be partially due to efficiency reasons tied with decisions taken at an early stage dialogue with regulatory agencies. A recent study (154) revealed that one third of drugs approved in Europe (2007–2010), were coupled with a requirement for a registry, mainly with the purpose of gathering additional safety data. Most of the registries involved were derived from existing disease registries, i.e., designed for other purposes. The latter feature is seen as an advantage of this source due to efficiency reasons. However, it could also represent a weakness, since the multipurpose nature of registries frequently means that they are often organized for broader questions and therefore are limited by their heterogeneity in safety data collection and reporting (155). In other words, they may lack a focused hypothesis since they are viewed as a data collection structure within which studies can be performed rather than a study aimed at answering a specific research question (16, 17, 153). It is also important to cover drugs prescribed by specialists, where patients are frequently more complex in terms of underlying disease and co-morbidities. This drawback was not a reality within LIM studies, where the inclusion point was commonly the community pharmacy, but was the case of PEM/M-PEM. In the UK, to overcome this, a new IM system is being developed: the Specialist Cohort Event Monitoring (SCEM). A few SCEM studies are ongoing: OBSERVA—Observational Safety Evaluation of Asenapine and ROSE—Rivaroxaban Observational Safety Evaluation, both in response to post-authorization commitments requested by the European Medicines Agency (156).
Over the study period, the reviewed IM studies were not restricted to safety data collection. Other domains of drug outcomes, such as drug utilization patterns (both in terms of prescriber characteristics and patient population) and in a less extent, effectiveness (“therapeutic response”) were studied. Concerning safety, our review illustrated a high degree of variability and a lack of standardization. Regardless of causality assessment, terms such as “adverse event” and “adverse reaction” were often used interchangeably, without explicit definitions to ensure consistency of use. In PEM and IMMP methodology the reported information was treated as adverse events. However, in LIM studies it was stated that although a causality assessment was not performed, the term ADR was used for the reactions reported as the authors claimed that patients were asked only to report symptoms that they believed to be associated with the use of the monitored drug. In this review, we used the terms reported by the authors but we encourage developing methodological and guidance safety reporting standards, for example through scientific and collaborative working groups at international level (e.g., International Society of Pharmacovigilance and International Society for Pharmacoepidemiology).
Patients and caregivers were the event data source in 39.1% of the studies. Overtime, the evolving regulatory landscape has heightened the recognition of patients as important players in clinical practice (157). Since 2012, in the European Union, patients can report ADE directly to competent authorities. Nevertheless, the concept of patient reporting schemes is far from new—it has been around for more than 50 years (158). Studies on patient reporting have demonstrated the ability of early identification of new and strengthening potential safety signals (159–161). Moreover, reports of symptomatic non-serious ADE from PCG are of great importance, since these events are often systematically downgraded by HCP, though they play a negative role on patients' quality of life and adherence to treatment, and ultimately on the benefit-risk of a drug. On the contrary, PCG could be less valuable to detect asymptomatic or serious or fatal events (162–164).
As any other primary data collection study, IM schemes are costlier and labor intensive. In a recent survey documenting the experiences of four African countries with CEM programmes (23), limited/inadequate funding was often considered as a challenge to deal with. This constraint was also reported in the New Zealand, where due to funding cessation, IMMP was disestablished in 2013 (148). It also seems that Japan-PEM (J-PEM) is no longer operational, since no published study from this scheme was found within the timeframe of our study. The J-PEM was launched in 1997 (165) and at least two pilot studies were conducted: troglitazone (166) and losartan (167). Although, J-PEM employed the method of a concurrent-control, which represented an advantage when compared with the majority of the reviewed IM studies, it appeared to be rather complex concerning data protection and managerial issues (22).
Low response rate and/or non-response bias was frequently mentioned as a limitation of both established and non-established IM system studies. A postal survey aiming to identify reasons for non-response in PEM studies (168), found workload and lack of payment, as the main reasons for non-response. In M-PEM studies, GP were offered a modest reimbursement for completion of questionnaires, which had a positive impact on the response rate (the median response rate increased from 50% in PEM to 64% in M-PEM) (18). Moreover, unforeseen challenges when conducted CEM studies were found, namely socio-cultural reasons that led to selective/non-participation (e.g., in Kenya some women could not give informed consent without permission from their husbands) (23). In LIM studies, non-response bias was also investigated (169). The major reason for non-response raised by patients was the fact that the study was not (properly) informed in the pharmacy. Further reasons, such as time-consuming, no-access to internet or being too ill to participate, were also pointed out (170). For external validity purposes, it is important to know whether IM population is comparable to the whole population using the monitored drug. Härmark et al. (171) found that LIM population were more often male, younger and healthier (higher percentage of de novo treated patients, shorter disease treatment duration and less co-medication) than the reference population. The authors concluded that these differences might lead to an underestimation of events, however it was not clear whether this influenced their time-course.
Our systematic review is subject to some limitations. Firstly, unpublished research (gray literature, reports) was not captured by our search strategy and therefore not included in this study. Secondly, we acknowledge that our review is limited by what authors have reported or presented in their studies. However, an assessment of quality was performed for all reviewed studies. Despite these limitations, we believe that our results are relevant and represent the first systematic review with the most comprehensive information available of IM systems implemented worldwide.
Conclusions
Over the study period, IM studies were implemented in 26 countries with different maturity levels of post-marketing surveillance systems, picturing the contribution of IM schemes in the real-world evidence generation data. Based on event monitoring and by tracking patients and drug use in a life-cycle based fashion, specific applications of the reviewed studies covered the following: increase of knowledge of drug safety data profile (outcome, time-course and management of ADE) identification of potential unrecognized and unsuspected ADE (tool for signal generation), gathering ADE data in resource limiting settings from populations frequently excluded from RCT (pregnant women, pediatrics and elderly), increase of knowledge of drug utilization patterns, and identification of off-label use. Overtime, an alignment with regulatory requirements was observed, where some studies have been undertaken to address specific questions related to safety concerns and drug utilization patterns (e.g., phase IV assessment as part of the RMP).
Framed onto the scope of IM systems implementation criteria, we identified two major limitations. Unexpectedly, only 20% of reviewed studies were conducted at hospital-level, which is a matter of concern, insofar as healthcare systems are facing a lack of access to new medicines at ambulatory care level (e.g., issues concerning pricing/reimbursement), and there has been a shift of new drugs introduction to hospital setting. Additionally, IM access to data of (new) drug exposure cohorts, either at identification or at follow-up stages, could somehow constitute a barrier, given the complexity of managerial, linkable and privacy data issues.
Data Availability
All datasets analyzed for this study are included in the manuscript and/or the Supplementary Files.
Author Contributions
MC and CT were the guarantors. All authors contributed to the study protocol, the development of the selection criteria, the risk of bias assessment strategy, and data extraction criteria. CT, MC, and JA developed the search strategy. CT, MC, and PF examined compliance of studies with eligibility criteria, with a fourth author acting as an arbiter (AM). CT, MC, and PF extracted data from reports of all included studies which was validated by a third author (FB). FB performed quality assessments which were validated by CT, with a third reviewer serving as the final arbitrator (MC). AM, HL, JA, and JC contributed to the result interpretation and discussion of results. All authors read, provided feedback and approved the final manuscript. All authors had full access to all data in the study and take responsibility for its integrity and the accuracy of the data analysis.
Funding
The publication fee was supported by the Center for Health Evaluation and Research (CEFAR), National Association of Pharmacies, Lisbon, Portugal.
Conflict of Interest Statement
CT was working at CEFAR/ANF when the study was performed and is currently employed by the Faculty of Pharmacy and has no conflict of interest to declare. MC is currently employed/receive support from CEFAR/ANF and has no conflict of interest to declare. HL reports that he is the past chairman of Dutch Medicines Evaluation Board and past-member of the EMA CHMP, and Scientific Director of the Utrecht WHO Collaborating Centre for Pharmaceutical Policy and Regulation. This centre accepts no direct funding or donations from the pharmaceutical industry or other private parties.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2019.00147/full#supplementary-material
References
1. Eichler H, Abadie E, Breckenridge A, Flamion B, Gustafsson LL, Leufkens H, et al. Bridging the efficacy-effectiveness gap: a regulator's perspective on addressing variability of drug response. Nat Rev Drug Discov. (2011) 10:495–506. doi: 10.1038/nrd3501
2. Ligthelm R, Borz V, Gumprecht J, Kawamori R, Wenying Y, Valensi P, et al. Importance of observational studies in clinical practice. Clin Ther. (2007) 29:1284–92. doi: 10.1016/j.clinthera.2007.07.004
3. Avorn J. In defense of pharmacoepidemiology—embracing the Yin and Yang of drug research. N Engl J Med. (2007) 357:2219–21. doi: 10.1056/NEJMp0706892
4. Garattini S, Chalmers I. Patients and the public deserve big changes in evaluation of drugs. Br Med J. (2009) 338:804–6. doi: 10.1136/bmj.b1025
5. Klonoff DC, Gutierrez A, Fleming A, Kerr D. Real-world evidence should be used in regulatory decisions about new pharmaceutical and medical device products for diabetes. J. Diabetes Sci. Technol. (2019). doi: 10.1177/1932296819839996
6. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. (2016) 375:2293–7. doi: 10.1056/NEJMsb1609216
7. Schneeweiss S, Glynn RJ. Real-world data analytics fit for regulatory decision-making. Am J Law Med. (2018) 44:197–216. doi: 10.1177/0098858818789429
8. Avorn J. Two centuries of assessing drug risks. N Engl J Med. (2012) 367:193–7. doi: 10.1056/NEJMp1206652
9. Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med. (2016) 14:1–11. doi: 10.1186/s12916-016-0553-2
10. Qureshi ZP, Vazquez ES, Monguio RR, Stevenson KB, Szeinbach SL. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol Drug Saf. (2011) 20:772–7. doi: 10.1002/pds.2155
11. Härmark L, van Grootheest ACK. Web-based intensive monitoring: from passive to active drug surveillance. Expert Opin Drug Saf. (2012) 11:45–51. doi: 10.1517/14740338.2012.629184
12. Lis Y, Roberts MH, Kamble S, Guo JJ, Raisch DW. Comparisons of food and drug administration and european medicines agency risk management implementation for recent pharmaceutical approvals: report of the international society for pharmacoeconomics and outcomes research risk benefit management working. Value Health. (2012) 15:1108–18. doi: 10.1016/j.jval.2012.06.019
13. European Parliament Council. Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards Pharmacovigilance. Directive 2001/83/EC of the European Parliament and of the Council (2001).
14. Eichler H, Pignatti F, Flamion B, Leufkens H, Breckenridge A. Balancing early market access to new drugs with the need for benefit/risk data: a mounting dilemma. Nat Rev Drug Discov. (2008) 7:818–26. doi: 10.1038/nrd2664
15. Finney DJ. The design and logic of a monitor of drug use. J Chronic Dis. (1965) 18:77–98. doi: 10.1016/0021-9681(65)90054-8
16. EMA. Guideline on Good Pharmacovigilance Practices (GVP) Module VIII—Post-authorisation Safety Studies (Rev 3). London: EMA (2017).
17. ENCePP. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Guide on Methodological Standards in Pharmacoepidemiology (Revision 6). EMA/95098/2010 (2018).
18. Layton D, Hazell L, Shakir SAW. Modified prescription-event monitoring studies: a tool for pharmacovigilance and risk management. Drug Saf. (2011) 34:e1–9. doi: 10.2165/11593830-000000000-00000
19. Coulter D. The New Zealand intensive medicines monitoring programme. Pharmacoepidemiol Drug Saf. (1998) 7:79–90.
20. Inman W. Postmarketing surveillance of adverse drug reactions in general practice. II: prescription-event monitoring at the University of Southampton. Br Med J. (1981) 282:1216–7. doi: 10.1136/bmj.282.6271.1216
21. Inman W. Prescription-event monitoring. Br Med J. (1982) 285:809–10. doi: 10.1136/bmj.285.6344.809-b
22. Kubota K. Prescription-event monitoring in Japan (J-PEM). Drug Saf. (2002) 25:441–4. doi: 10.2165/00002018-200225060-00008
23. Suku CK, Hill G, Sabblah G, Darko M, Muthuri G, Abwao E, et al. Experiences and lessons from implementing cohort event monitoring programmes for antimalarials in four African countries: results of a questionnaire-based survey. Drug Saf. (2015) 38:1115–26. doi: 10.1007/s40264-015-0331-7
24. Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. (2006) 29:385–96. doi: 10.2165/00002018-200629050-00003
25. Wise L, Parkinson J, Raine J, Breckenridge A. New approaches to drug safety: a pharmacovigilance tool kit. Nat Rev Drug Discov. (2009) 8:779–82. doi: 10.1038/nrd3002
26. Härmark L, van Grootheest AC. Pharmacovigilance: methods, recent developments and future perspectives. Eur J Clin Pharmacol. (2008) 64:743–52. doi: 10.1007/s00228-008-0475-9
27. Centre for Reviews and Dissemination. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York: CRD (2008).
28. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1–9. doi: 10.1186/2046-4053-4-1
29. Guidelines for ATC Classification and DDD Assignment. WHO Collaborating Centre for Drug Statistics Methodology. Oslo: WHO (2018).
30. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. (1998) 52:377–84. doi: 10.1136/jech.52.6.377
31. Deeks J, Dinnes J, D'Amico R, Sowden A, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. (2003) 7:1–179. doi: 10.3310/hta7270
32. Viswanathan M, Ansari M, Berkman N, Chang S, Hartling L, McPheeters L, et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Rockville, MD: Agency for Healthcare Research and Quality (2012).
33. Alaei M, Najmi AK, Kausar H, Akhtar M. A prospective research study of anti-glaucoma drugs prescribing, utilization pattern and adverse drug reaction recording in a university hospital. Drug Res. (2015) 65:164–8. doi: 10.1055/s-0034-1376964
34. Menzaghi B, Ricci E, Vichi F, De Sociod GV, Carenzi L, Martinelli C, et al. Gender differences in HIV infection: is there a problem? Analysis from the SCOLTA cohorts. Biomed Pharmacother. (2014) 68:385–90. doi: 10.1016/j.biopha.2014.01.007
35. Bernabeu I, Pico A, Venegas E, Aller J, Alvarez-Escolá C, García-Arnés JA, et al. Safety of long-term treatment with Pegvisomant: analysis of Spanish patients included in global ACROSTUDY. Pituitary. (2016) 19:127–37. doi: 10.1007/s11102-015-0691-0
36. Burton DC, Bigogo GM, Audi AO, Williamson J, Munge K, Wafula J, et al. Risk of injection-site abscess among infants receiving a preservative-free, two-dose vial formulation of pneumococcal conjugate vaccine in Kenya. PLoS ONE. (2015) 10:1–18. doi: 10.1371/journal.pone.0141896
37. Layton D, Marshall V, Boshier A, Friedmann P, Shakir SA. Serious skin reactions and selective COX-2 inhibitors: a case series from prescription-event monitoring in England. Drug Saf. (2006) 29:687–96. doi: 10.2165/00002018-200629080-00005
38. De Socio G, Bonfanti P, Ricci E, Orofino G, Madeddu G, Penco G, et al. Cholesterol levels in HIV-HCV infected patients treated with lopinavir: results from the SCOLTA project. Biomed Pharmacother. (2008) 62:16–20. doi: 10.1016/j.biopha.2007.07.012
39. Madeddu G, Quirino T, Carradori S, Ricci E, Grosso C, Penco G, et al. Renal toxicity in HIV-infected patients receiving HAART including tenofovir. Infez Med. (2006) 14:125–34.
40. Ortin Castano A, Otero MJ. Adverse drug events in out-patients as the cause of an initial consultation to neurology. Neurologia. (2006) 21:232–8.
41. Oshikoya K. Adverse event monitoring of artemesinin combination therapy in Nigeria: the challenges and limitations of the study. West Afr J Med. (2011) 29:221–4. doi: 10.4314/wajm.v29i4.68230
42. Pandey K, Ravidas V, Siddiqui NA, Sinha SK, Verma RB, Singh TP, et al. Pharmacovigilance of miltefosine in treatment of visceral leishmaniasis in endemic areas of Bihar, India. Am J Trop Med Hyg. (2016) 95:1100–5. doi: 10.4269/ajtmh.16-0242
43. Patel KK, Patel AK, Ranjan RR, Patel AR, Patel JK. Tenofovir-associated renal dysfunction in clinical practice: an observational cohort from western India. Indian J Sex Transm Dis. (2010) 31:30–4. doi: 10.4103/0253-7184.68998
44. Paterson H, Clifton J, Miller D, Ashton J, Harrison-Woolrych M. Hair loss with use of the levonorgestrel intrauterine device. Contraception. (2007) 76:306–9. doi: 10.1016/j.contraception.2007.06.015
45. Pourseyed S, Fattahi F, Pourpak Z. Adverse drug reactions in patients in an Iranian department of internal medicine. Pharmacoepidemiol Drug Saf. (2009) 18:188–91. doi: 10.1002/pds.1663
46. Pregnancy TE. Safety of zidovudine/lamivudine scored tablets in children with HIV infection in Europe and Thailand. Eur J Clin Pharmacol. (2017) 73:463–8. doi: 10.1007/s00228-016-2182-2
47. Schulz M, Hämmerlein A, Hinkel U, Weis G, Gillissen A. Safety and usage pattern of an over-the-counter ambroxol cough syrup: a community pharmacy-based cohort study. Int J Clin Pharmacol Ther. (2006) 44:409–21. doi: 10.5414/CPP44409
48. Tinto H, Sevene E, Dellicour S, Calip GS, D'Alessandro U, Macete E, et al. Assessment of the safety of antimalarial drug use during early pregnancy (ASAP): protocol for a multicenter prospective cohort study in Burkina Faso, Kenya and Mozambique. Reprod Health. (2015) 12:1–9. doi: 10.1186/s12978-015-0101-0
49. Tobaiqy M, Stewart D, Helms PJ, Bond C, Lee AJ, Bateman N, et al. A pilot study to evaluate a community pharmacy-based monitoring system to identify adverse drug reactions associated with paediatric medicines use. Eur J Clin Pharmacol. (2010) 66:627–32. doi: 10.1007/s00228-010-0790-9
50. Vannice KS, Keita M, Sow SO, Durbin AP, Omer SB, Moulton LH, et al. Active surveillance for adverse events after a mass vaccination campaign with a group a meningococcal conjugate vaccine (PsA-TT) in Mali. Clin Infect Dis. (2015) 61:S493–500. doi: 10.1093/cid/civ497
51. Warren RB, Smith CH, Yiu ZZN, Ashcroft DM, Barker JNWN, Burden AD, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. (2015) 135:2632–40. doi: 10.1038/jid.2015.208
52. Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H. Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Saf. (2008) 31:789–98. doi: 10.2165/00002018-200831090-00007
53. Dodoo AN, Fogg C, Nartey ET, Ferreira GL, Adjei GO, Kudzi W, et al. Profile of adverse events in patients receiving treatment for malaria in urban Ghana: a cohort-event monitoring study. Drug Saf. (2014) 37:433–48. doi: 10.1007/s40264-014-0164-9
54. Acharya NV, Wilton LV, Shakir SAW. Safety profile of orlistat: results of a prescription-event monitoring study. Int J Obes. (2006) 30:1645–52. doi: 10.1038/sj.ijo.0803323
55. Andrade GN, Pimenta AM, Silva DA, Madeira AMF. Eventos adversos pós-vacinação contra influenza pandêmica A (H1N1) 2009 em crianças. Cad Saúde Públ. (2012) 28:1713–24. doi: 10.1590/S0102-311X2012000900010
56. Ankrah D, Mantel-Teeuwisse AK, De Bruin ML, Amoo PK, Ofei-Palm CN, Agyepong I, et al. Incidence of adverse events among healthcare workers following H1N1 mass immunization in Ghana: a prospective study. Drug Saf. (2013) 36:259–66. doi: 10.1007/s40264-013-0037-7
57. Arredondo-Garza T, Majluf-Cruz A, Vela-Ojeda J, Mariscal-Ramírez I, Solis-Anaya L, Lopez-Gutiérrez JR, et al. Peri-infusional adverse reactions to Rituximab in patients with non-Hodgkin's lymphoma. Arch Med Res. (2013) 44:549–54. doi: 10.1016/j.arcmed.2013.09.011
58. Asturias EJ, Contreras-Roldan IL, Ram M, Garcia-Melgar AJ, Morales-Oquendo V, Hartman K, et al. Post-authorization safety surveillance of a liquid pentavalent vaccine in Guatemalan children. Vaccine. (2013) 31:5909–14. doi: 10.1016/j.vaccine.2013.09.015
59. Auffret M, Béné J, Gautier S, Moreau-Crépeaux S, Caron J. Pharmacovigilance monitoring of a cohort of pregnant women vaccinated against influenza A(H1N1) variant virus in the Nord-Pas de Calais region of northern France. Eur J Obstet Gynecol Reprod Biol. (2013) 170:114–8. doi: 10.1016/j.ejogrb.2013.05.025
60. Aurich-Barrera B, Wiltone LV, Shakir SAW. Use and risk management of carvedilol for the treatment of heart failure in the community in England: results from a modified prescription-event monitoring study. Drug Saf. (2009) 32:43–54. doi: 10.2165/00002018-200932010-00004
61. Aurich-Barrera B, Wilton L, Brown D, Shakir S. Paediatric postmarketing pharmacovigilance using prescription-event monitoring: comparison of the adverse event profiles of lamotrigine prescribed to children and adults in England. Drug Saf. (2010) 33:751–63. doi: 10.2165/11536830-000000000-00000
62. Aurich-Barrera B, Wilton L, Brown D, Shakir S. Paediatric post-marketing pharmacovigilance: comparison of the adverse event profile of vigabatrin prescribed to children and adults. Pharmacoepidemiol Drug Saf. (2011) 20:608–18. doi: 10.1002/pds.2105
63. Twaites BR, Wilton LV, Shakir SA. The safety of quetiapine: results of a post-marketing surveillance study on 1728 patients in England. J Psychopharmacol. (2007) 21:392–9. doi: 10.1177/0269881107073257
64. Baiden R, Oduro A, Halidou T, Gyapong M, Sie A, Macete E, et al. Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania. Malar J. (2015) 14:160. doi: 10.1186/s12936-015-0664-9
65. Bassi PU, Osakwe AI, Isah A, Suku C, Kalat M, Jalo I, et al. Safety of artemisinin-based combination therapies in Nigeria: a cohort event monitoring study. Drug Saf. (2013) 36:747–56. doi: 10.1007/s40264-013-0044-8
66. Bassi P, Osakwe A, Suku C, Kalat M, Elagbaje C, Isah A, et al. Cohort event monitoring of patients treated for uncomplicated malaria with artemisinin-based combination therapies in selected hospitals and community pharmacies in Nigeria. Niger Postgrad Med J. (2016) 23:172–81. doi: 10.4103/1117-1936.196246
67. Bongard V, Marc D, Philippe V, Jean-Louis M, Maryse LM. Incidence rate of adverse drug reactions during long-term follow-up of patients newly treated with amiodarone. Am J Ther. (2006) 13:315–9. doi: 10.1097/00045391-200607000-00007
68. Brasseur P, Vaillant MT, Olliaro PL. Anti-malarial drug safety information obtained through routine monitoring in a rural district of South-Western Senegal. Malar J. (2012) 11:1–13. doi: 10.1186/1475-2875-11-402
69. Buggy Y, Layton D, Fogg C, Shakir SAW. Safety profile of oxcarbazepine: results from a prescription-event monitoring study. Epilepsia. (2010) 51:818–29. doi: 10.1111/j.1528-1167.2009.02489.x
70. Buggy Y, Cornelius V, Wilton L, Shakir SAW. Risk of depressive episodes with rimonabant: a before and after modified prescription event monitoring study conducted in England. Drug Saf. (2011) 34:501–9. doi: 10.2165/11588510-000000000-00000
71. Buggy Y, Cornelius V, Fogg C, Kasliwal R, Layton D, Shakir SAW. Neuropsychiatric events with varenicline: a modified prescription-event monitoring study in general practice in England. Drug Saf. (2013) 36:521–31. doi: 10.1007/s40264-013-0046-6
72. Chaabane A, Aouam K, Fredj N, Ben Toumi A, Braham D, Boughattas NA, et al. Les vaccins anti A(H1N1) en Tunisie: efficacité et tolérance. Therapie. (2011) 66:281–9. doi: 10.2515/therapie/2011028
73. Coughtrie AL, Doe C, Layton D, Shakir SAW. Utilisation and tolerability of aliskiren in the primary care setting in England. J Clin Hypertens. (2016) 18:1237–43. doi: 10.1111/jch.12852
74. Davies M, Wilton LV, Shakir SAW. Safety profile of esomeprazole: results of a prescription-event monitoring study of 11,595 patients in England. Drug Saf. (2008) 31:313–23. doi: 10.2165/00002018-200831040-00005
75. Davies M, Wilton L, Shakir S. Safety profile of modafinil across a range of prescribing indications, including off-label use, in a primary care setting in England: results of a modified prescription-event monitoring study. Drug Saf. (2013) 36:237–46. doi: 10.1007/s40264-013-0025-y
76. De Alwis KNLSK, Abeysinghe MRN, Wickramesinghe AR, Wijesinghe PR. A cohort event monitoring to determine the adverse events following administration of mouse brain derived, inactivated Japanese Encephalitis vaccine in an endemic district in Sri Lanka. Vaccine. (2014) 32:924–30. doi: 10.1016/j.vaccine.2013.12.047
77. de Jong L, Härmark L, van Puijenbroek E. Time course, outcome and management of adverse drug reactions associated with metformin from patient' s perspective: a prospective, observational cohort study in the Netherlands. Eur J Clin Pharmacol. (2016) 72:615–22. doi: 10.1007/s00228-016-2019-z
78. De Sousa A, Rabarijaona LP, Tenkorang O, Inkoom E, Ravelomanantena HV, Njarasoa S, et al. Pharmacovigilance of malaria intermittent preventive treatment in infants coupled with routine immunizations in 6 African countries. J Infect Dis. (2012) 205:S82–90. doi: 10.1093/infdis/jir799
79. Dodoo ANO, Renner L, van Grootheest AC, Labadie J, Antwi-Agyei KO, Hayibor S, et al. Safety monitoring of a new pentavalent vaccine in the expanded programme on immunisation in Ghana. Drug Saf. (2007) 30:347–56. doi: 10.2165/00002018-200730040-00007
80. Dodoo ANO, Fogg C, Asiimwe A, Nartey ET, Kodua A, Tenkorang O, et al. Pattern of drug utilization for treatment of uncomplicated malaria in urban Ghana following national treatment policy change to artemisinin-combination therapy. Malar J. (2009) 8:2. doi: 10.1186/1475-2875-8-2
81. Fogg C, Kasliwal R, Shakir SA. Risk management and outcomes of adverse events to pioglitazone in primary care in the UK: an observational study. Drug Saf. (2009) 32:229–37. doi: 10.2165/00002018-200932030-00005
82. Aljadhey H, Alyabsi M, Alrwisan A, Alqahtani N, Almutairi R, Al Tawil E, et al. The safety of H1N1 vaccine in children in Saudi Arabia: a cohort study using modern technology in a developing country. Drug Saf. (2012) 35:555–61. doi: 10.2165/11597450-000000000-00000
83. Härmark L, van Puijenbroek E, Straus S, van Grootheest K. Intensive monitoring of pregabalin: results from an observational, Web-based, prospective cohort study in the Netherlands using patients as a source of information. Drug Saf. (2011) 34:221–31. doi: 10.2165/11585030-000000000-00000
84. Härmark L, van Puijenbroek E, van Grootheest K. Longitudinal monitoring of the safety of drugs by using a web-based system: the case of pregabalin. Pharmacoepidemiol Drug Saf. (2011) 20:591–7. doi: 10.1002/pds.2135
85. Härmark L, van Hunsel F, Hak E, van Grootheest ACK. Monitoring the safety of influenza A (H1N1) vaccine using web-based intensive monitoring. Vaccine. (2011) 29:1941–7. doi: 10.1016/j.vaccine.2010.12.123
86. Härmark L, van Puijenbroek E, van Grootheest K. Intensive monitoring of duloxetine: results of a web-based intensive monitoring study. Eur J Clin Pharmacol. (2013) 69:209–15. doi: 10.1007/s00228-012-1313-7
87. Harrison-Woolrych M, Garcia-Quiroga J, Ashton J, Herbison P. Safety and usage of atypical antipsychotic medicines in children: a nationwide prospective cohort study. Drug Saf. (2007) 30:569–79. doi: 10.2165/00002018-200730070-00002
88. Harrison-Woolrych M, Ashton J, Herbison P. Fatal and non-fatal cardiovascular events in a general population prescribed sibutramine in New Zealand: a prospective cohort study. Drug Saf. (2010) 33:605–13. doi: 10.2165/11532440-000000000-00000
89. Harrison-Woolrych M, Skegg K, Ashton J, Herbison P, Skegg DCG. Nocturnal enuresis in patients taking clozapine, risperidone, olanzapine and quetiapine: comparative cohort study. Br J Psychiatry. (2011) 199:140–4. doi: 10.1192/bjp.bp.110.087478
90. Harrison-Woolrych M, Maggo S, Tan M, Savage R, Ashton J. Cardiovascular events in patients taking varenicline: a case series from intensive postmarketing surveillance in New Zealand. Drug Saf. (2012) 35:33–43. doi: 10.2165/11597690-000000000-00000
91. Harrison-Woolrych M, Paterson H, Tan M. Exposure to the smoking cessation medicine varenicline during pregnancy: a prospective nationwide cohort study. Pharmacoepidemiol Drug Saf. (2013) 22:1086–92. doi: 10.1002/pds.3489
92. Harrison-Woolrych M, Ashton J. Utilization of the smoking cessation medicine varenicline: an intensive post-marketing study in New Zealand. Pharmacoepidemiol Drug Saf. (2010) 19:949–53. doi: 10.1002/pds.2003
93. Hazell L, Boshier A, Harris S, Wilton LV, Shakir SAW. An observational cohort study investigating the cardiovascular safety of tadalafil when prescribed in primary care in England: mortality due to ischaemic heart disease. BJU Int. (2007) 99:387–93. doi: 10.1111/j.1464-410X.2007.06581.x
94. Hazell L, Cornelius V, Wilton LV, Shakir SAW. The safety profile of tadalafil as prescribed in general practice in England: results from a prescription-event monitoring study involving 16 129 patients. BJU Int. (2009) 103:506–14. doi: 10.1111/j.1464-410X.2008.08000.x
95. Hill GR, Ashton J, Harrison-Woolrych M. Sibutramine usage in New Zealand: an analysis of prescription data by the Intensive Medicines Monitoring Programme. Pharmacoepidemiol Drug Saf. (2007) 16:1217–26. doi: 10.1002/pds.1447
96. Hua L, Hongtao H, Shunqin W, Jinping G, Jiandong C, Zhaoliang L, et al. Simultaneous vaccination of Chinese applicants for a United States immigrant visa. Travel Med Infect Dis. (2008) 6:130–6. doi: 10.1016/j.tmaid.2008.03.004
97. Itakura H, Nakaya N, Kusunoki T, Shimizu N, Hirai S, Mochizuki S, et al. Long-term event monitoring study of fluvastatin in Japanese patients with hypercholesterolemia: efficacy and incidence of cardiac and other events in elderly patients (≥65 years old). J Cardiol. (2011) 57:77–88. doi: 10.1016/j.jjcc.2010.09.003
98. Kasliwal R, Wilton LV, Cornelius V, Aurich-barrera B, Shakir SAW. Safety profile of rosuvastatin: results of a prescription-event monitoring study of 11,680 patients. Drug Saf. (2007) 30:157–70. doi: 10.2165/00002018-200730020-00005
99. Kasliwal R, Wilton LV, Shakir SAW. Monitoring the safety of pioglitazone: results of a Prescription-Event Monitoring study of 12 772 patients in England. Drug Saf. (2008) 31:839–50. doi: 10.2165/00002018-200831100-00003
100. Kasliwal R, Wilton LV, Shakir SAW. Safety and drug utilization profile of varenicline as used in general practice in England: interim results from a prescription-event monitoring study. Drug Saf. (2009) 32:499–507. doi: 10.2165/00002018-200932060-00006
101. Lapi F, Cecchi E, Pedone C, Attanasio F, Banchelli G, Vannacci A, et al. Safety aspects of iodinated contrast media related to their physicochemical properties: a pharmacoepidemiology study in two Tuscany hospitals. Eur J Clin Pharmacol. (2008) 64:723–37. doi: 10.1007/s00228-008-0477-7
102. Launay O, Krivine A, Charlier C, Truster V, Tsatsaris V, Lepercq J, et al. Low rate of pandemic A/H1N1 2009 influenza infection and lack of severe complication of vaccination in pregnant women: a prospective cohort study. PLoS ONE. (2012) 7:1–9. doi: 10.1371/journal.pone.0052303
103. Layton D, Wilton L, Shakir SAW. Examining the tolerability of the non-sedating antihistamine desloratadine: a Prescription-Event Monitoring study in England. Drug Saf. (2009) 32:169–79. doi: 10.2165/00002018-200932020-00009
104. Layton D, Osborne V, Gilchrist A, Shakir SAW. Examining the utilization and tolerability of the non-sedating antihistamine levocetirizine in England using prescription-event monitoring data. Drug Saf. (2011) 34:1177–89. doi: 10.2165/11593930-000000000-00000
105. Layton D, Osborne V, Al-Shukri M, Shakir SAW. Indicators of drug-seeking aberrant behaviours: the feasibility of use in observational post-marketing cohort studies for risk management. Drug Saf. (2014) 37:639–50. doi: 10.1007/s40264-014-0193-4
106. Layton D, Coughtrie AL, Qayum N, Shakir SAW. Pattern of onset and risk factors for peripheral oedema during vildagliptin use: analysis from the vildagliptin prescription-event monitoring study in England. Drug Saf. (2016) 39:1093–104. doi: 10.1007/s40264-016-0451-8
107. Harrison-Woolrych M, Ashton J. Psychiatric adverse events associated with varenicline: an intensive postmarketing prospective cohort study in New Zealand. Drug Saf. (2011) 34:763–72. doi: 10.2165/11594450-000000000-00000
108. Mackenzie IS, Macdonald TM, Shakir S, Dryburgh M, Mantay BJ, Mcdonnell P, et al. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes. Br J Clin Pharmacol. (2011) 73:801–11. doi: 10.1111/j.1365-2125.2011.04142.x
109. MacLennan KM, Boshier A, Wilton LV, Shakir SAW. Examination of the safety and use of apomorphine prescribed in general practice in England as a treatment for erectile dysfunction. BJU Int. (2006) 98:125–31. doi: 10.1111/j.1464-410X.2006.06253.x
110. McNaughton R, Lynn E, Osborne V, Coughtrie A, Layton D, Shakir S. Safety of intranasal quadrivalent live attenuated influenza vaccine (QLAIV) in children and adolescents: a pilot prospective cohort study in England. Drug Saf. (2016) 39:323–33. doi: 10.1007/s40264-015-0384-7
111. Morishita R, Itakura H, Nakaya N, Yoshida M, Odawara M, Ichihara A, et al. Risk factors for cardiovascular events in Japanese patients treated with fluvastatin from the Long-Term Event Monitoring (LEM) study. Curr Vasc Pharmacol. (2012) 10:178–86. doi: 10.2174/157016112799305049
112. Mssusa AK, Fimbo AM, Nkayamba AF, Irunde HF, Sillo HB, Shewiyo DH, et al. Safety profile of artemether-lumefantrine: a cohort event monitoring study in public health facilities in Tanzania. Clin Drug Invest. (2016) 36:401–11. doi: 10.1007/s40261-016-0385-z
113. Nazareth I, Tavares F, Rosillon D, Haguinet F, Bauchau V. Safety of AS03-adjuvanted split-virion H1N1 (2009) pandemic influenza vaccine: a prospective cohort study. BMJ Open. (2013) 3:1–12. doi: 10.1136/bmjopen-2012-001912
114. Omon E, Damase-Michel C, Hurault-Delarue C, Lacroix I, Montastruc JL, Oustric S, et al. Non-adjuvanted 2009 influenza A (H1N1)v vaccine in pregnant women: the results of a French prospective descriptive study. Vaccine. (2011) 29:9649–54. doi: 10.1016/j.vaccine.2011.10.034
115. Oosterhuis I, Härmark L, van Puijenbroek E. Experiences with the use of varenicline in daily practice in the Netherlands: a prospective, observational cohort study. Drug Saf. (2014) 37:449–57. doi: 10.1007/s40264-014-0166-7
116. Osborne V, Hazell L, Layton D, Shakir SAW. Drug utilization of intrinsa (testosterone patch) in England: interim analysis of a prescription-event monitoring study. Drug Saf. (2010) 33:213–21. doi: 10.2165/11533720-000000000-00000
117. Osborne V, Layton D, Perrio M, Wilton L, Shakir SAW. Incidence of venous thromboembolism (VTE) in users of strontium ranelate: an analysis of data from a Prescription-Event Monitoring Study in England. Drug Saf. (2010) 33:579–91. doi: 10.2165/11533770-000000000-00000
118. Osborne V, Layton D, Shakir SAW. Implications of off-label use: an example from the final results of an observational cohort study on intrinsa® (testosterone patch). Post Reprod Health. (2014) 20:48–54. doi: 10.1177/2053369114535762
119. Osborne V, Davies M, Layton D, Shakir SAW. Utilisation of extended release quetiapine (Seroquel XLTM): results from an observational cohort study in England. Eur Psychiatry. (2016) 33:61–7. doi: 10.1016/j.eurpsy.2015.12.004
120. Perrio MJ, Wilton LV, Shakir SAW. A modified prescription-event monitoring study to assess the introduction of Seretide EvohalerTM in England: an example of studying risk monitoring in pharmacovigilance. Drug Saf. (2007) 30:681–95. doi: 10.2165/00002018-200730080-00005
121. Perrio MJ, Wilton LV, Shakir SAW. A modified prescription-event monitoring study to assess the introduction of Flixotide EvohalerTM into general practice in England: an example of pharmacovigilance planning and risk monitoring. Pharmacoepidemiol Drug Saf. (2007) 16:969–78. doi: 10.1002/pds.1411
122. Perrio MJ, Wilton LV, Shakir SAW. The safety profiles of orlistat and sibutramine: results of Prescription-Event Monitoring studies in England. Obesity. (2007) 15:2712–22. doi: 10.1038/oby.2007.323
123. Regan AK, Tracey LE, Blyth CC, Richmond PC, Effler PV. A prospective cohort study assessing the reactogenicity of pertussis and influenza vaccines administered during pregnancy. Vaccine. (2016) 34:2299–304. doi: 10.1016/j.vaccine.2016.03.084
124. Regan AK, Blyth CC, Mak DB, Richmond PC, Effler PV. Using SMS to monitor adverse events following trivalent influenza vaccination in pregnant women. Aust New Zeal J Obstet Gynaecol. (2014) 54:522–8. doi: 10.1111/ajo.12266
125. Sanchayan K, Fernandopulle R, Amarasinghe A, Thiyahiny SN, Ranganathan S. Safety of live attenuated Japanese encephalitis vaccine given at the age of 9 months in National Immunisation Programme of Sri Lanka. Ceylon Med J. (2016) 61:99–105. doi: 10.4038/cmj.v61i3.8344
126. Setkina S, Dotsenko M, Bondar S, Charnysh I, Kuchko A, Kaznacheeva A, et al. Safety and effectiveness of highly active antiretroviral therapy in treatment-naive HIV patients: preliminary findings of a cohort event monitoring study in Belarus. Drug Saf. (2015) 38:365–72. doi: 10.1007/s40264-015-0279-7
127. Sharif-Askari FS, Sulaiman SAS, Sharif-Askari NS, Hussain AAS, Railey MJ. Adverse outcomes of anticoagulant use among hospitalized patients with chronic kidney disease: a comparison of the rates of major bleeding events between unfractionated heparin and enoxaparin. PLoS ONE. (2014) 9:1–9. doi: 10.1371/journal.pone.0106517
128. Sniadack MM, Neff LJ, Swerdlow DL, Schieber RA, McCauley MM, Mootrey GT. Follow-up of cardiovascular adverse events after smallpox vaccination among civilians in the United States, 2003. Clin. Infect. Dis. (2008) 46 (Suppl. 3):S251–7. doi: 10.1086/524741
129. Sun H-Y, Chen Y-C, Wang Y-W, Gau C-S, Chang S-C. A prospective study of antimicrobial-related adverse drug reactions in hospitalized patients. J Microbiol Immunol Infect. (2008) 41:151–9.
130. Tetteh RA, Nartey ET, Lartey M, Mantel-Teeuwisse AK, Leufkens HGM, Nortey PA, et al. Adverse events and adherence to HIV post-exposure prophylaxis: a cohort study at the Korle-Bu Teaching Hospital in Accra, Ghana. BMC Public Health. (2015) 15:1–13. doi: 10.1186/s12889-015-1928-6
131. Twaites B, Wilton LV, Layton D, Shakir SAW. Safety of nateglinide as used in general practice in England: results of a prescription-event monitoring study. Acta Diabetol. (2007) 44:233–9. doi: 10.1007/s00592-007-0010-y
132. Twaites BR, Wilton LV, Shakir SA. Safety of zafirlukast: results of a postmarketing surveillance study on 7976 patients in England. Drug Saf. (2007) 30:419–29. doi: 10.2165/00002018-200730050-00005
133. Marshall V, Wilton L, Shakir S. Safety profile of repaglinide as used in general practice in England: results of a prescription-event monitoring study. Acta Diabetol. (2006) 43:6–13. doi: 10.1007/s00592-006-0203-9
134. van Balveren-Slingerland L, Kant A, Härmark L. Web-based intensive monitoring of adverse events following influenza vaccination in general practice. Vaccine. (2015) 33:2283–8. doi: 10.1016/j.vaccine.2015.03.014
135. van Klooster TM, Kemmeren JM, van der Maas NAT, de Melker HE. Reported adverse events in girls aged 13-16 years after vaccination with the human papillomavirus (HPV)-16/18 vaccine in the Netherlands. Vaccine. (2011) 29:4601–7. doi: 10.1016/j.vaccine.2011.04.050
136. Vassilopoulos D, Delicha EM, Settas L, Andrianakos A, Aslanidis S, Boura P, et al. Safety profile of repeated rituximab cycles in unselected rheumatoid arthritis patients: a long-term, prospective real-life study. Clin Exp Rheumatol. (2016) 34:893–900.
137. Vlckova V, Cornelius V, Kasliwal R, Wilton L, Shakir S. Hypoglycaemia with pioglitazone: analysis of data from the Prescription-Event Monitoring study. J Eval Clin Pract. (2010) 16:1124–8. doi: 10.1111/j.1365-2753.2009.01280.x
138. Willemen MJC, Mantel-teeuwisse AK, Buggy Y, Layton D, Straus SMJM, Leufkens HGM, et al. Reasons for and time to discontinuation of rimonabant therapy: a modified prescription-event monitoring study. Drug Saf. (2012) 35:1147–58. doi: 10.1007/BF03262000
139. Ampadu HH, Hoekman J, Bruin ML, Pal SN, Olsson S, Sartori D, et al. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports. Drug Saf. (2016) 39:335–45. doi: 10.1007/s40264-015-0387-4
140. Parkinson J. The clinical practice research datalink: the new 54 million fully integrated research data and clinical trial system. In: Andrews EB, Moore N, editors. Mann's Pharmacovigilance. Chichester: John Wiley & Sons Ltd (2014). p. 421–8. doi: 10.1002/9781118820186.ch26
141. Herings R, Pedersen L. Pharmacy-based medical record linkage systems. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. Chichester: John Wiley & Sons Ltd. (2012). p. 2701–86.
142. Roehr B. Trial participants need to represent patients better. BMJ. (2008) 336:737. doi: 10.1136/bmj.39539.578507.DB
143. Stricker B, Psaty B. Detection, verification, and quantification of adverse drug reactions. BMJ. (2004) 329:44–7. doi: 10.1136/bmj.329.7456.44
144. Heeley E, Riley J, Layton D, Wilton LV, Shakir SAW. Prescription-event monitoring and reporting of adverse drug reactions. Lancet. (2001) 358:1872–3. doi: 10.1016/S0140-6736(01)06898-2
145. Park BJ, Stergachis A. Automated databases in pharmacoepidemiologic studies. In: Hartzema AG, Tilson HH, Chan KA, editors. Pharmacoepidemiology and Therapeutic Management. Incinnati, OH: Whitney Books (2008) 519–544.
146. Torre C, Martins AP. Overview of pharmacoepidemiological databases in the assessment of medicines under real-life conditions. In: Lunet N, editor. Epidemiology—Current Perspectives on Research and Practice. Croatia: In Tech (2012). p. 131–54. doi: 10.5772/35318
147. Waller PC, Evans SJW. A model for the future conduct of pharmacovigilance. Pharmacoepidemiol Drug Saf. (2003) 12:17–29. doi: 10.1002/pds.773
148. Harrison-Woolrych M, Tatley M, Kunac D. Pharmacovigilance in New Zealand. In: Andrews EB, Moore N, editors. Mann's Pharmacovigilance. Chichester: John Wiley & Sons Ltd. (2014). p. 291–4.
151. WHO. A Practical Handbook on the Pharmacovigilance of Medicines Used in the Treatment of Tuberculosis: Enhancing the Safety of the TB Patient (2012).
152. Aagaard L, Strandell J, Melskens L, Petersen P, Hansen EH. Global patterns of adverse drug reactions over a decadee. Drug Saf. (2012) 35:1171–82. doi: 10.1007/BF03262002
153. Bouvy JC, Blake K, Slattery J, Bruin ML, Arlett P, Kurz X. Registries in European post-marketing surveillance: a retrospective analysis of centrally approved products, 2005–2013. Pharmacoepidemiol Drug Saf. (2017) 26:1442–50. doi: 10.1002/pds.4196
154. Jonker CJ, van den Berg HM, Kwa MSG, Hoes AW, Mol PGM. Registries supporting new drug applications. Pharmacoepidemiol. Drug Saf. (2017) 26:1451–7. doi: 10.1002/pds.4332
155. Dixon WG, Carmona L, Finckh A, Hetland ML, Kvien TK, Landewe R, et al. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann Rheum Dis. (2010) 69:1596–602. doi: 10.1136/ard.2009.125526
156. Layton D, Shakir SAW. Specialist cohort event monitoring studies: a new study method for risk management in pharmacovigilance. Drug Saf. (2015) 38:153–63. doi: 10.1007/s40264-014-0260-x
157. Scurti V, Romero M, Tognoni G. A plea for a more epidemiological and patient-oriented pharmacovigilance. Eur J Clin Pharmacol. (2012) 68:11–9. doi: 10.1007/s00228-011-1096-2
158. Hunsel F, van Härmark L, Pal S, Olsson S, van Grootheest K. Experiences with adverse drug reaction reporting by patients. Drug Saf. (2012) 35:45–60. doi: 10.2165/11594320-000000000-00000
159. Egberts T, Smulders M, Koning F, Meyboom R, Leuflens H. Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. BMJ. (1996) 313:530–1. doi: 10.1136/bmj.313.7056.530
160. Hunsel F, Van Waal S, Härmark L. The contribution of direct patient reported ADRs to drug safety signals in the Netherlands from 2010 to 2015. Pharmacoepidemiol. Drug Saf. (2017) 26:977–83. doi: 10.1002/pds.4236
161. Inácio P, Cavaco A, Airaksinen M. The value of patient reporting to the pharmacovigilance system: a systematic review. Br J Clin Pharmacol. (2017) 83:227–46. doi: 10.1111/bcp.13098
162. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. (2010) 362:865–9. doi: 10.1056/NEJMp0911494
163. Foster JM, Van Der Molen T, de Jong L, van den Berg H. Patient-reporting of side effects may provide an important source of information in clinical practice. Eur. J. Cancer. (2007) 63:979–80. doi: 10.1007/s00228-007-0339-8
164. Hakobyan L, Haaijer-Ruskamp FM, Zeeuw D, Dobre D, Denig P. A review of methods used in assessing non-serious adverse drug events in observational studies among type 2 diabetes mellitus patients. Health Qual Life Outcomes. (2011) 9:1–9. doi: 10.1186/1477-7525-9-83
165. Kubota K. A design for prescription-event monitoring in japan (J-PEM). Pharmacoepidemiol Drug Saf. (1999) 8:447–56.
166. Kubota K, Kawabe E, Hinotsu S, Hamada C, Ohashi Y, Kurokawa K. Pilot study of prescription-event monitoring in Japan comparing troglitazone with alternative oral hypoglycemics. Eur J Clin Pharmacol. (2001) 56:831–9. doi: 10.1007/s002280000232
167. Samizo K, Kawabe E, Hinotsu S, Sato T, Kageyama S, Hamada C, et al. Comparison of losartan with ACE inhibitors and dihydropyridine calcium channel antagonists: a pilot study of prescription-event monitoring in Japan. Drug Saf. (2002) 25:811–21. doi: 10.2165/00002018-200225110-00005
168. Key C, Layton D, Shakir SAW. Results of a postal survey of the reasons for non-response by doctors in a Prescription Event Monitoring study of drug safety. Pharmacoepidemiol Drug Saf. (2002) 11:143–8. doi: 10.1002/pds.690
169. Härmark LVD, Huls HJ, Gier JJH, van Grootheest ACK. Non-response in a pharmacy and patient-based intensive monitoring system: a quantitative study on non-response bias and reasons for non-response. Int J Pharm Pract. (2014) 22:159–62. doi: 10.1111/ijpp.12038
170. Härmark L, Lie-kwie M, Berm L, Gier H, van Grootheest ACK. Patients' motives for participating in active post-marketing surveillance. Pharmacoepidemiol. Drug Saf. (2013) 22:70–6. doi: 10.1002/pds.3327
Keywords: adverse drug reaction reporting systems, clinical practice pattern, drug monitoring, pharmacovigilance, systematic review
Citation: Torre C, Cary M, Borges FC, Ferreira PS, Alarcão J, Leufkens HG, Costa J and Martins AP (2019) Intensive Monitoring Studies for Assessing Medicines: A Systematic Review. Front. Med. 6:147. doi: 10.3389/fmed.2019.00147
Received: 20 April 2019; Accepted: 12 June 2019;
Published: 19 July 2019.
Edited by:
Steffen Thirstrup, NDA Advisory Services Ltd, United KingdomCopyright © 2019 Torre, Cary, Borges, Ferreira, Alarcão, Leufkens, Costa and Martins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Torre, carla.torre@ff.ulisboa.pt
 Carla Torre
Carla Torre Maria Cary1
Maria Cary1  Hubert G. Leufkens
Hubert G. Leufkens Ana Paula Martins
Ana Paula Martins