Association of T Stage and Serum CEA Levels in Determining Survival of Rectal Cancer
- 1Department of General Surgery, The Affiliated Jianhu Hospital of Nantong University, Jianhu People's Hospital, Jianhu, China
- 2Department of Medical Oncology, The Affiliated Jianhu Hospital of Nantong University, Jianhu People's Hospital, Jianhu, China
Purpose: To investigate the association of T stage and serum carcinoembryonic antigen (CEA) levels in determining oncologic outcomes of rectal cancer.
Methods: Patients diagnosed with stage I–II rectal cancer patients were identified from the Surveillance, Epidemiology, and End Results database.
Results: In stage T1N0M0 disease, elevated level of serum CEA (C1) was associated with 227.6% increased risk of mortality compared to normal level of serum CEA (C0; hazard ratio = 3.276, 95% confidence interval = 2.781–3.858, P < 0.001).
Conclusions: Stage T1N0M0 rectal cancer, when involved in preoperative serum CEA elevation, may be a surrogate of biologically aggressive disease and correlate with unfavorable oncologic outcomes. Moreover, this subgroup of rectal cancer deserves more clinical attention of oncologists.
Introduction
Rectal cancer is one of the most common malignancies worldwide (1). According to the American Joint Committee on Cancer (AJCC) staging system, the anatomic extent of primary tumor (T stage) is one of the most important prognostic predictors. However, patients with the same T stage of rectal cancer may show considerably different clinical outcomes.
First reported in 1965, carcinoembryonic antigen (CEA) is a 180–200 kDa glycoprotein and a member of the immunoglobulin superfamily (2). CEA is secreted by a variety of solid tumors, including 90% of colorectal cancers (3). As the single most important and reliable serum prognostic biomarker in colorectal cancer, elevated preoperative CEA levels are found to be associated with worse prognosis of colorectal cancer (4–9).
Previous studies have demonstrated that CEA was associated with cancer cell adhesion and innate immunity in colorectal cancer. In addition, CEA was also reported to facilitate attachment of colorectal cancer cells to sites of metastasis and support tumor progression (10–12).
Traditionally, the distant spread of tumor cells has been considered a late event, yet findings of several previous studies indicated that acquisition of metastatic potential could occur in the very early stage of tumor progression (13–16). Wo et al. (17) reported that a very small tumor size involved in lymph node positivity may be a surrogate for aggressive biology. We then suspect that very early stage rectal cancer with serum CEA elevation could suggest the early acquisition of metastatic potential and predict a very poor survival of rectal cancer.
However, to our knowledge, few studies were reported to investigate the association of T stage and serum CEA levels (C0 and C1) in determining prognosis of rectal cancer. Therefore, we conduct this large population-based study to examine whether very early T stage in the context of serum CEA elevation may be a surrogate for biologically aggressive disease and predict for poor cause-specific survival (CSS) of rectal cancer. To remove the effect of lymph node positivity on our research, we then excluded node-positive patients and focused the analysis on stage I–II patients.
Patients and Methods
Patient Selection in the Surveillance, Epidemiology, and End Results Database
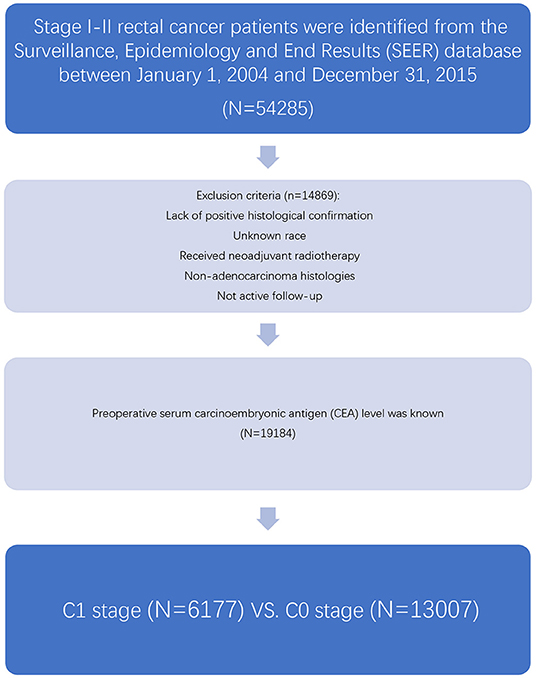
As an authoritative source of information on cancer incidence and survival in the USA and a comprehensive source of population-based information including all the newly diagnosed cancer cases occurring in Surveillance, Epidemiology, and End Results (SEER)-participating areas, the SEER database encompasses ~28% of the American population. The SEER database did not contain any identifiers and was publicly available for researchers. SEER*Stat is a software provided by the SEER program to obtain patient information using online access. At first, the case-listing session of the SEER*Stat software (SEER*Stat 8.3.5) was used to list all patient-related information, and patients diagnosed with stage I–II (node-negative) rectal cancer between January 1, 2004 and December 31, 2015 were identified from the SEER database (Figure 1). Rectal cancer patients were identified by the ICD-O-3 site codes C199 and C209 and behavior code 3 (NAACCR Items 522 and 523) (9). We chose to include these years because the information of preoperative serum CEA was recorded starting from 2004 and SEER follow-up ended in 2015.
Then, those received neoadjuvant radiotherapy, lack of positive histological confirmation, with unknown race, non-adenocarcinoma histologies, or not active follow-up were excluded from our study. Patients with preoperative serum CEA level available were included in our study, and we grouped “positive/elevated” and “negative/normal; within normal limits” as C1 and C0 (C-stage information). The cutoff values of CEA were 2.5 ng/ml for non-smokers and 5 ng/ml for smokers, respectively. In addition, the following patient clinicopathological variables were also retrieved from the SEER database: T stage, race, gender, tumor location, age at diagnosis, year of diagnosis, grade, and histology.
Statistical Analysis
In the present study, Pearson's chi-squared test was used to compare all the patients' clinicopathological variables retrieved from the SEER database between the normal and elevated preoperative serum CEA levels. Some multivariable Cox proportional hazard models were built to identify independent prognostic variables of rectal cancer in our analyses. Survival curves were generated using Kaplan–Meier method, and differences between the curves were analyzed by log-rank test. The primary outcomes of interest in the present study were CSS and overall survival (OS). CSS was calculated from the date of diagnosis to the date of cancer-specific death. Deaths attributed to the rectal cancer were treated as events, and deaths from other causes were treated as censored observations at the date of death. To determine whether there was a significant interaction between the preoperative serum CEA level and T stage in predicting CSS, we also defined an interaction variable (T stage and serum CEA level). Two-sided P < 0.05 was considered statistically significant. Statistical analyses were mainly performed using the SPSS version 22 (IBM Corporation, Armonk, NY, USA).
Results
Patient Characteristics
Overall, 19,184 patients diagnosed with stage I–II rectal cancer patients were identified from the SEER database between January 1, 2004 and December 31, 2015 (Figure 1). Of these patients, 13,007 (67.8%) patients were assigned to C0 stage, and 6,177 (32.2%) patients were assigned to C1 stage. AJCC staging system suggested that 6,077 patients were in the T1 stage, 4,783 patients in the T2 stage, 7,021 in the T3 stage, and 1,303 were in the T4 stage. A total of 2,560 (13.3%) patients died of rectal cancer at the end of the follow-up time. The median follow-up time of the whole cohort was 44 months (0–143 months). Patients' baseline characteristics are summarized in Table 1.
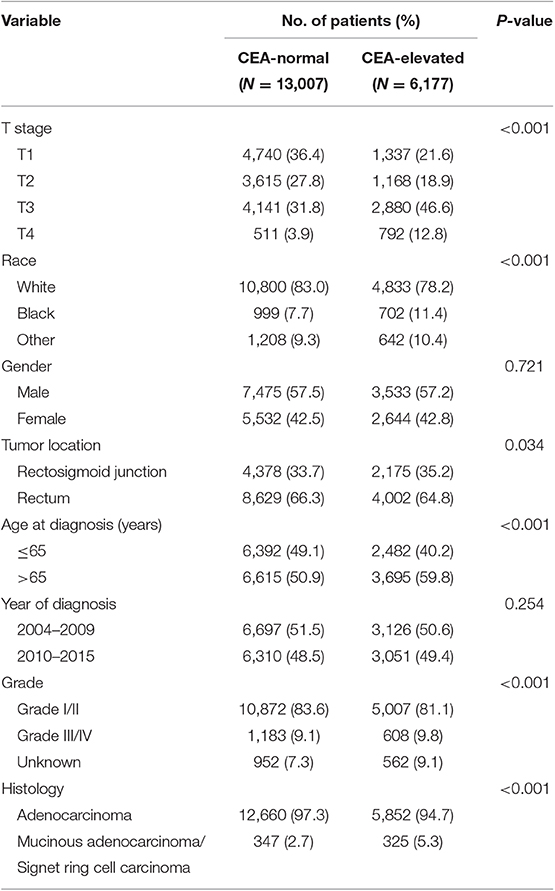
Table 1. Comparison of baseline characteristics of stage I–II rectal cancer by the serum carcinoembryonic antigen (CEA) level.
Improved Risk of T1 Stage Compared With Other T Stages in the Context of Serum CEA Elevation
It was found that C1 stage was more likely to correlate with higher T stage, black, rectosigmoid junction, older age, higher grade, and mucinous adenocarcinoma/Signet ring cell carcinoma (Table 1, P < 0.05). As shown in Table 2, race, gender, tumor location, age at diagnosis, year of diagnosis, grade, histology, T stage, and serum CEA level were included in the multivariate Cox analysis. When multivariate Cox analysis was performed, we convinced the following clinicopathological characteristics as independent prognostic factors in stage I–II rectal cancer; these included race, gender, tumor location, age at diagnosis, tumor grade, T stage, and serum CEA level, while the risk between T2,C0 and T1,C0 was not statistical difference (P = 0.925). It was shown that, in the context of serum CEA elevation, T1 stage presented unexpected higher risk of rectal-cancer-specific mortality compared with stages T2 and T3. In stage T1 disease, elevated level of serum CEA was associated with 227.6% increased risk of mortality compared to normal level of serum CEA.
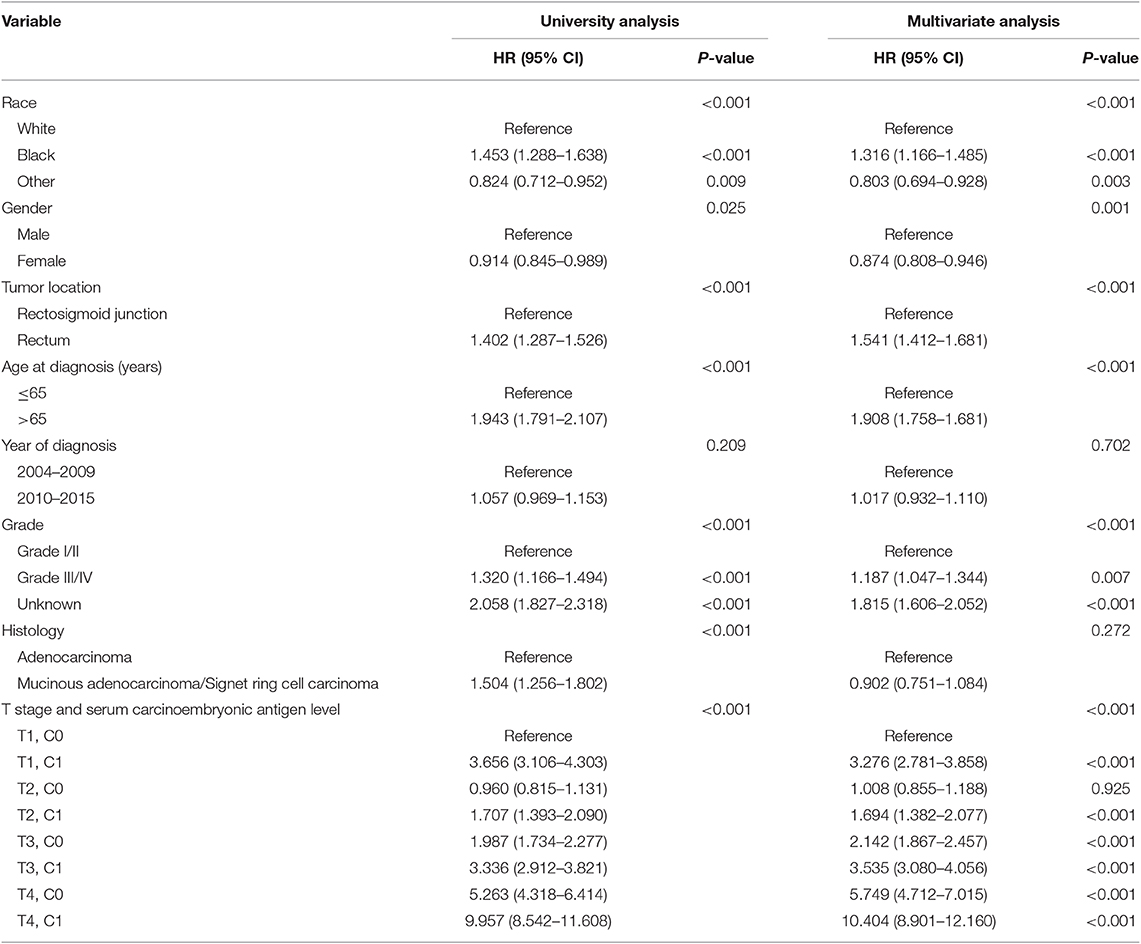
Table 2. Multivariate Cox regression analyses of cause-specific survival (CSS) in stage I–II rectal cancer.
Kaplan–Meier survival curves are plotted in Figures 2, 3. The 5-year CSS rate was 92.2% in T1C0, 75.2% in T1C1, 93.1% in T2C0, 86.7% in T2C1, 84.6% in T3C0, 76.3% in T3C1, 62.9% in T4C0, and 43.6% in T4C1 (Figure 2, P < 0.001). Therefore, T1C1 presented a similar 5-year CSS rate compared with T3C1 (75.2 vs. 76.3%, P = 0.238). In addition, the finding was even more pronounced in OS. The 5-year OS rate was 78.6% in T1C0, 48.3% in T1C1, 77.1% in T2C0, 62.3% in T2C1, 68.5% in T3C0, 54.5% in T3C1, 45.3% in T4C0, and 30.0% in T4C1 (Figure 3, P < 0.001). T1C1 presented significantly lower 5-year CSS rate compared with T3C1 (48.3 vs. 54.5%, P < 0.001).
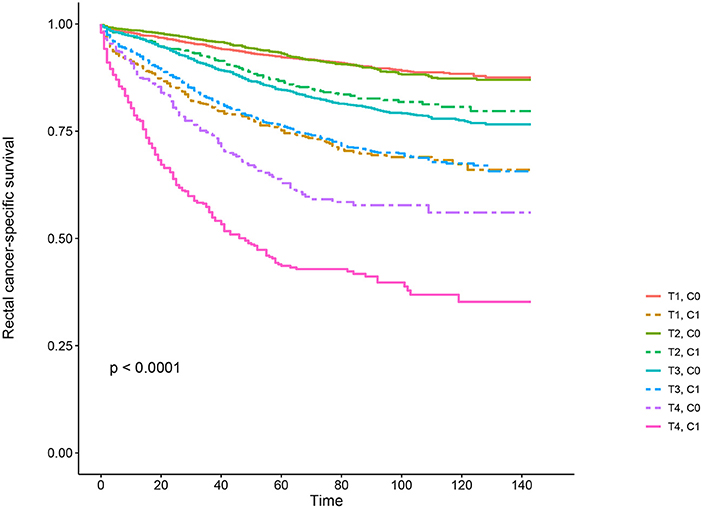
Figure 2. Kaplan–Meier cause-specific survival (CSS) curves of T stage combined with the serum carcinoembryonic antigen (CEA) level.
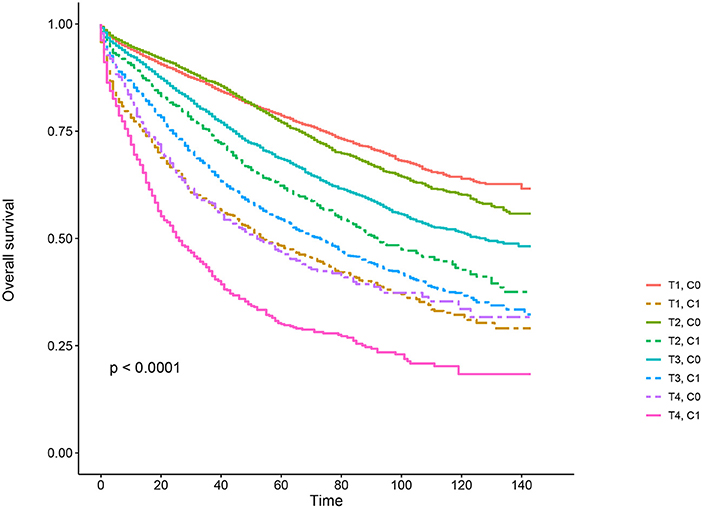
Figure 3. Kaplan–Meier overall survival (OS) curves of T stage combined with the serum carcinoembryonic antigen (CEA) level.
Effect of Preoperative Serum CEA Elevation in T Stage
The forest plot was drawn to show the hazard ratios (HRs) to compare CSS between normal- and elevated-CEA groups in respective T stages (Figure 4). Compared with normal level of serum CEA, in stage T2, elevated level of serum CEA was associated with 69.0% increased risk of rectal-cancer-specific mortality [HR = 1.690, 95% confidence interval (CI) = 1.366–2.090, P < 0.001]; in stage T3, elevated level of serum CEA was associated with 67.2% increased risk of rectal-cancer-specific mortality (HR = 1.672, 95% CI = 1.486–1.882, P < 0.001); in stage T4, elevated level of serum CEA was associated with 81.3% increased risk of rectal-cancer-specific mortality (HR = 1.813, 95% CI = 1.485–2.213, P < 0.001); by contrast, however, in stage T1, elevated level of serum CEA presented even up to 211.6% increased risk of rectal-cancer-specific mortality (HR = 3.116, 95% CI = 2.639–3.679, P < 0.001).
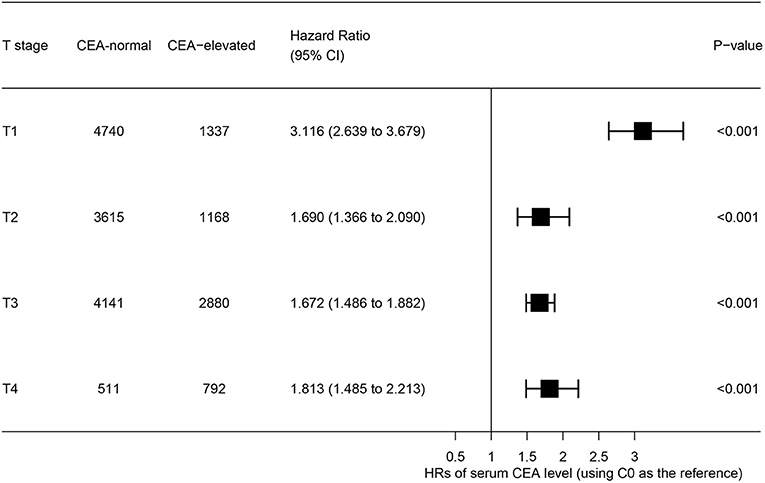
Figure 4. Hazard ratios (HRs) of cause-specific survival (CSS) between normal and elevated level of serum carcinoembryonic antigen (CEA) according to the T stage.
Discussion
We aimed to investigate the association of T stage and serum CEA levels in determining CSS of rectal cancer. We hypothesized that rectal cancer with very early T stage (stage T1) and serum CEA elevation may be a biological surrogate for aggressive disease, thus predicting a poor oncological outcome. With ~20,000 patients diagnosed with stage I–II rectal cancer included in our study.
In this study, it was found that serum CEA elevation was more likely to correlate with higher T stage, black, rectosigmoid junction, older age, and higher grade and mucinous adenocarcinoma/Signet ring cell carcinoma. Kaplan–Meier survival analyses showed that, in the context of serum CEA elevation, 5-year CSS rate of stage T1 was 75.2%, which did not achieve statistical difference from stage T3 (76.3%). When it comes to overall survival, 5-year OS rate of stage T1 with preoperative serum CEA elevation (48.3%) was even lower than stage T3 involved in CEA elevation (54.5%) and did not achieve statistical difference from stage T4 (45.3%), meaning elevated preoperative serum level of CEA could identify a subgroup of stage T1 rectal cancers with similar CSS compared with some stage T3 diseases and with similar OS compared with some stage T4 diseases.
After adjusting for known rectal cancer prognostic factors (race, gender, tumor location, age at diagnosis, year of diagnosis, grade, and histology), the interaction variable (T stage and serum CEA level) we defined was demonstrated to be an independent prognostic factor of rectal cancer. Multivariate Cox analysis showed that, in the context of serum CEA elevation, T1 stage presented unexpected higher risk of rectal-cancer-specific mortality compared with stages T2 and T3. In stage T1 disease, elevated level of serum CEA was associated with 227.6% increased risk of mortality compared to normal level of serum CEA. In addition, race, gender, tumor location, age at diagnosis, and tumor grade were also identified as independent prognostic factors of stage I–II rectal cancer. Apart from T1, it was found that preoperative serum CEA elevation presented ~75% higher risks of rectal-cancer-specific mortality in respective T stage, yet the number greatly increased to 211.6% in T1 stage.
In 2000, the Colorectal Working Group of the AJCC proposed the inclusion of serum level of CEA (C stage) into the conventional AJCC TNM staging system of colorectal cancer (18). Moreover, the American Society of Clinical Oncology and the European Group on Tumor Markers have both supported the inclusion of preoperative serum CEA level as a prognostic tool in colorectal cancer (19–21).
Several previous researches reported the serum CEA level as a strong prognostic role in colon cancer (4–7, 22–26). In 2011, Thirunavukarasu et al. (25) reported that preoperative serum CEA level was an independent prognostic biomarker of colon cancer, and prognosis was worse in high CEA patients with a lower stage compared with low CEA patients with a higher stage. In that study, high CEA was even deemed as strong as node positivity for predicting poor oncologic outcomes of colon cancer.
Yet, few studies focus on elucidating the prognostic role of serum CEA level in rectal cancer. In 2016, also using the SEER database, Tarantino et al. (9, 27) conducted the two large population-based investigations, which provided compelling evidence that elevated level of preoperative serum CEA was a strong predictor of worse overall and cancer-specific survival in rectal cancer. In 2018, Liu et al. (8) demonstrated that preoperative serum CEA was an independent prognostic factor of rectal cancer, and elevated serum CEA level presented evidently poorer survival compared with normal serum CEA level in stages I–IV. However, the only two previous studies focused on investigating the prognostic role of preoperative serum CEA level did not examine the association of CEA level and T stage in predicting the tumor outcomes of rectal cancer.
Although the conventional shows that cancer acquires the metastatic potential step by step as they grow to a large size (28), however, some previous studies indicated that acquisition of metastatic potential could occur very early in tumor progression. A previous research demonstrated the extremely poor survival of very small tumor size when involved in lymph node positivity (17). In addition, findings of our research show that elevation of preoperative serum CEA level in very early stage (stage T1) rectal cancer is associated with very poor OS and CSS and may be a surrogate of aggressive biology. We believe that both lymph node involvement and serum CEA elevation are deemed as the acquisition of metastatic ability. Our study, combined with that of Wo et al. consistently support the aforementioned idea that acquisition of metastatic potential could occur very early in tumor progression, thus associated with poor oncologic outcomes, and the initial biological feature are more likely to determine the potential of distant metastasis during cancer progression, rather than the accumulated metastatic ability (29).
Moreover, findings of our study are of clinical significance. Currently, stage I rectal cancer is treated with radical surgical resection alone because of relatively favorable oncologic outcomes. However, approximately 10–15% of patients will develop tumor recurrence after radical resection (30, 31). In the present study, we happen to identify a subgroup of stage T1 rectal cancer with very poor prognosis compared with the rest of stage I rectal cancer, meaning that those stage T1 rectal cancer with involvement of preoperative serum CEA elevation should catch more attention of oncologists.
However, there are two limitations in our study. On the one hand, this study did not include some known prognostic factors of rectal cancer in our analyses, including microsatellite instability status and postoperative complications, which were not available from the SEER database and might introduce biases to some extent. We cannot provide ranges and mean values of CEA in both groups. On the other hand, the present study was retrospective rather than based on prospective data. Therefore, our findings still need to be validated in other cohorts, especially in large prospective clinical studies.
In conclusion, our study demonstrates that stage T1 rectal cancer, when involved in preoperative serum CEA elevation, may be a surrogate of biologically aggressive disease and correlate with unfavorable OS and CSS. Moreover, this subgroup of rectal cancer deserves more clinical attention of oncologists. Our findings, if validated in future database studies, would provide a new therapy idea for early stage rectal cancer.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Patients in this study were identified from the SEER database, and the approval for use of the data was obtained through a request submitted to the SEER program. The approval by the institutional review board was not needed because SEER database is publicly available.
Author Contributions
SW and WG have made a substantial and direct contribution to this study, and approved it for submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by Medical Science and Technology Development Plan Project of Yancheng (YK2015075).
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. (1965) 121:439–62. doi: 10.1084/jem.121.3.439
3. Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. (2005) 23:338–51. doi: 10.1081/CNV-58878
4. Becerra AZ, Probst CP, Tejani MA, Aquina CT, González MG, Hensley BJ, et al. Evaluating the prognostic role of elevated preoperative carcinoembryonic antigen levels in colon cancer patients: results from the national cancer database. Ann Surg Oncol. (2016) 23:1554–61. doi: 10.1245/s10434-015-5014-1
5. Ogata Y, Murakami H, Sasatomi T, Ishibashi N, Mori S, Ushijima M, et al. Elevated preoperative serum carcinoembrionic antigen level may be an effective indicator for needing adjuvant chemotherapy after potentially curative resection of stage II colon cancer. J Surg Oncol. (2010) 99:65–70. doi: 10.1002/jso.21161
6. Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. (2008) 51:503–7. doi: 10.1007/s10350-008-9246-z
7. Spindler BA, Bergquist JR, Thiels CA, Habermann EB, Kelley SR, Larson DW, et al. Incorporation of CEA improves risk stratification in stage II colon cancer. J Gastrointest Surg. (2017) 21:770–7. doi: 10.1007/s11605-017-3391-4
8. Liu Q, Lian P, Luo D, Cai S, Li Q, Li X. Combination of carcinoembryonic antigen with the American Joint Committee on Cancer TNM staging system in rectal cancer: a real-world and large population-based study. Onco Targets Ther. (2018) 11:5827–34. doi: 10.2147/OTT.S171433
9. Tarantino I, Warschkow R, Schmied BM, Güller U, Mieth M, Cerny T, et al. Predictive value of CEA for survival in stage I rectal cancer: a population-based propensity score-matched analysis. J Gastrointest Surg. (2016) 20:1213–22. doi: 10.1007/s11605-016-3137-8
10. Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. (2013) 32:643–71. doi: 10.1007/s10555-013-9444-6
11. Arabzadeh A, Chan C, Nouvion AL, Breton V, Benlolo S, DeMarte L, et al. Host-related carcinoembryonic antigen cell adhesion molecule 1 promotes metastasis of colorectal cancer. Oncogene. (2013) 32:849–60. doi: 10.1038/onc.2012.112
12. Cai Z, Xiao J, He X, Ke J, Zou Y, Chen Y, et al. Accessing new prognostic significance of preoperative carcinoembryonic antigen in colorectal cancer receiving tumor resection: more than positive and negative. Cancer Biomark. (2017) 19:161–8. doi: 10.3233/CBM-160287
13. Engel J, Eckel R, Kerr J, Schmidt M, Fürstenberger G, Richter R, et al. The process of metastasisation for breast cancer. Eur J Cancer. (2003) 39:1794–806. doi: 10.1016/S0959-8049(03)00422-2
14. Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell. (2008) 13:58–68. doi: 10.1016/j.ccr.2007.12.003
15. Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. (2008) 321:1841–4. doi: 10.1126/science.1161621
16. Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. (2003) 100:7737–42. doi: 10.1073/pnas.1331931100
17. Wo JY, Chen K, Neville BA, Lin NU, Punglia RS. Effect of very small tumor size on cancer-specific mortality in node-positive breast cancer. J Clin Oncol. (2012) 23:32–3. doi: 10.1016/j.breastdis.2012.01.026
18. Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American joint committee on cancer prognostic factors consensus conference: colorectal working group. Cancer. (2015) 88:1739–57. doi: 10.1002/(SICI)1097-0142(20000401)88:7<1739::AID-CNCR30>3.0.CO;2-T
19. Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. (2007) 43:1348–60. doi: 10.1016/j.ejca.2007.03.021
20. Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. (2006) 24:5313–27. doi: 10.1200/JCO.2006.08.2644
21. Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. (2014) 134:2513–22. doi: 10.1002/ijc.28384
22. Harrison LE, Guillem JG, Paty P, Cohen AM. Preoperative carcinoembryonic antigen predicts outcomes in node-negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg. (1997) 185:55–9. doi: 10.1016/S1072-7515(97)00012-4
23. Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. (2010) 101:396–400. doi: 10.1002/jso.21495
24. Ozawa H, Kotake K, Hosaka M, Hirata A, Nakagawa Y, Fujita S, et al. Incorporation of serum carcinoembryonic antigen levels into the prognostic grouping system of colon cancer. Int J Colorect Dis. (2017) 32:1–9. doi: 10.1007/s00384-017-2772-1
25. Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. (2011) 103:689–97. doi: 10.1093/jnci/djr078
26. Margalit O, Mamtani R, Yang YX, Reiss KA, Golan T, Halpern N, et al. Assessing the prognostic value of carcinoembryonic antigen levels in stage I and II colon cancer. Eur J Cancer. (2018) 94:1–5. doi: 10.1016/j.ejca.2018.01.112
27. Tarantino I, Warschkow R, Worni M, Merati-Kashani K, Köberle D, Schmied BM, et al. Elevated preoperative CEA is associated with worse survival in stage I-III rectal cancer patients. Br J Cancer. (2012) 107:266. doi: 10.1038/bjc.2012.267
28. Norton L, Massague J. Is cancer a disease of self-seeding? Nat Med. (2006) 12:875–8. doi: 10.1038/nm0806-875
29. Yu KD, Jiang YZ, Chen S, Cao ZG, Wu J, Shen ZZ, et al. Effect of large tumor size on cancer-specific mortality in node-negative breast cancer. Mayo Clin Proc. (2012) 87:1171–80. doi: 10.1016/j.mayocp.2012.07.023
30. Blumberg D, Paty PB, Picon AI, Guillem JG, Klimstra DS, Minsky BD, et al. Stage I rectal cancer: identification of high-risk patients. J Am Coll Surg. (1998) 186:574–9; discussion:579–80. doi: 10.1016/S1072-7515(98)00018-0
Keywords: T stage, carcinoembryonic antigen, rectal cancer, survival, SEER
Citation: Wu S and Gu W (2020) Association of T Stage and Serum CEA Levels in Determining Survival of Rectal Cancer. Front. Med. 6:270. doi: 10.3389/fmed.2019.00270
Received: 12 July 2019; Accepted: 04 November 2019;
Published: 10 January 2020.
Edited by:
Julian Pardo, Fundacion Agencia Aragonesa para la Investigacion y el Desarrollo, SpainReviewed by:
Rebeca Sanz-Pamplona, Biomedical Research Institute of Bellvitge, SpainRoberto Pazo, Hospital Universitario Miguel Servet, Spain
Copyright © 2020 Wu and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenlong Gu, guwenlongmark1@163.com
 Shengwen Wu1
Shengwen Wu1  Wenlong Gu
Wenlong Gu