Independent Risk Factors for Deaths due to AIDS in Chongqing, China: Does Age Matter?
- 1Division of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing, China
- 2National Key Laboratory for Infectious Diseases Prevention and Treatment With Traditional Chinese Medicine, Chongqing Public Health Medical Center, Chongqing, China
Background: People living with HIV (PLWH) are aging worldwide, and different management strategies may be required for older and younger PLWH. However, demographic characteristics, illness distribution, mortality, and independent risk factors in the PLWH population in China are not yet fully understood, especially in patients aged 50 years or older.
Methods: We conducted a retrospective analysis of 4445 HIV-positive Chinese inpatients in Chongqing, China.
Results: The mortality rate in patients 50 years or older (the older group) was significantly higher than that in those under 50 years (the younger group) (p < 0.001). In the younger group, independent risk factors for death included: nadir CD4+ T-cell counts <200 cells/μL, not owning medical healthcare insurance, not being on cART, injection drug use, and having one of the following comorbidities: Pneumocystis pneumonia, cryptococcal meningitis, AIDS malignancy, non-AIDS malignancy, and kidney disease. In the older group, independent predictors of death included: being urban residents, nadir CD4+ T-cell counts <200 cells/μL, not being on cART, and having comorbidities such as Pneumocystis pneumonia, hepatitis C, talaromycosis, non-AIDS malignancy, and kidney disease.
Conclusions: Demographic characteristics, illness distribution, mortality, and independent risk factors for death in HIV-positive patients differ between the older group and the younger group, indicating that a changing suite of medical and allied support services may be required the for management of older PLWH.
Introduction
Since the introduction of combination antiretroviral therapy (cART), the life expectancy of people living with HIV (PLWH) has gradually been approaching that of the general population (1, 2). This improvement in life expectancy and longevity has resulted in an increase in the number of PLWH living into older age, with an estimated 4 million of PLWH being over 50 years old globally. This number has doubled since the introduction of cART in 1996, and continues to increase (3, 4). Also, an increasing proportion of new HIV infections in older adults are contributing to this increase in PLWH aged 50 or more (5–7). This trend is global, and is observable in both high-income, and low- and middle-income countries (LMIC). In high-income countries, half of PLWH are now aged 50 years or older (8, 9). In the United States, for example, the median age of HIV-infected adults has passed 50 years, with Canada, Australia, and most European countries closely following (10). In LMIC, more PLWH are surviving into older age, with a dramatic increase in the number of older PLWH (11). In Latin America, the Caribbean, Sub-Saharan Africa, Asia, the Middle East, and North Africa, emerging trends regarding PLWH survival and aging are similar to that in high-income countries (10). In mainland China, the number of new Acquired Immune Deficiency Syndrome (AIDS) cases among over-50-year-olds is increasing year on year. The number of PLWH in China aged 50 and above in 2014 was 4.2 times the number in 2008 (27,520 vs. 6,599) (12). The proportion of over-50-year-olds increased from 13.7% in 2008 to 26.6% in 2014, representing a 2-fold increase (12–14).
Compared with younger PLWH, older PLWH are more likely to have different demographic, clinical, hematological, and immunological baseline homeostatic conditions, which are likely to affect outcomes of disease (10, 15, 16). Older PLWH face a variety of unique challenges including possible accelerated aging, and higher incident rates of non-AIDS-related co-morbidities, such as stroke, hypertension, heart disease, liver cirrhosis, dyslipidemia, diabetes, kidney diseases, and non-AIDS malignancy (8, 11, 17). In addition, older PLWH face more challenges across stages of the HIV care continuum due to a more rapid decline in immune function (18), and higher mortality rates (7, 11, 16, 19). In high-income settings, the contribution of non-AIDS-related diseases to mortality is increasing in PLWH, particularly in older people (20–23), whereas in sub-Saharan Africa, infectious diseases, especially tuberculosis, fungal, and bacterial infections, remain the main causes of morbidity and mortality (24, 25).
Chongqing is the largest centrally controlled municipality in China, consisting of 26 districts, eight counties, and four autonomous counties, and is spread over an area of 82,402 square kilometers. It is also the most populous Chinese municipality, having a population of over 30 million. Between 2007 and 2012, the number of reported HIV infections in this province grew at an average annual rate of 19.7%, which is substantially higher than the national rate (3.1%) (26). The average proportion of patients who were 50 years or older at HIV diagnosis increased dramatically from 3.6% between 1988 and 2003 to 45.4% between 2013 and 2017 (16). Chongqing is an essential regional hub of south-western China and is more heavily affected by the HIV/AIDS epidemic compared with other regions in China. However, the demographic characteristics, prevalence of comorbidities, and predictors of death in the PLWH population in this region are not fully understood, especially in those who are 50 years or older. In order to investigate the differences between PLWH aged 50 or older and PLWH aged <50 years, we conducted a retrospective analysis on HIV infected patients hospitalized at Chongqing Public Health Medical Center from January 2014 to January 2018, aiming to provide key information about the PLWH in this region, and to improve clinical management and outcomes for older PLWH. This analysis might serve as a template to guide healthcare policy aimed at better meeting the complex healthcare needs of the rapidly increasing number of older PLWH in China.
Materials and Methods
Study Design and Setting
This was a retrospective cohort study of opportunistic infections and non-AIDS co-morbidities among hospitalized PLWH from January 2014 to January 2018. All serologically confirmed HIV-infected patients that were admitted to Chongqing Public Health Medical Center during the study period were enrolled in this study. Where a particular patient was admitted more than once during the study period, these admissions were collated and recorded as one admission, and all clinical laboratory data and diagnoses were also collated as data for the single patient. Unique patient identification numbers were generated to distinguish re-admissions and follow-up visits. In our analysis, we only used data collected from patients entered into the electronic medical record system (27). All eligible hospitalized patients were stratified into two study groups: the older group, consisting of PLWH aged 50 years or older, and the younger group, consisting of PLWH <50 years old.
Our study was conducted in accordance with the Declaration of Helsinki, and prior approval was obtained from the institutional review board of Chongqing Public Health Medical Center. The institutional review board waived the requirement for written informed consent, since this study was retrospective in nature, and all patient data were analyzed in anonymity.
Disease Diagnosis
For our study, AIDS malignancy was defined as non-Hodgkin lymphoma, cervical premalignant lesions, and Kaposi sarcoma. Malignancies other than non-Hodgkin lymphoma, cervical premalignant lesions, and Kaposi sarcoma were defined as non-AIDS malignancy. Heart disease included the following diagnoses: myocardial infarction, angina pectoris, coronary artery bypass grafting, and angioplasty. Diagnostic criteria for hypertension, diabetes, and kidney disease included two or more consecutive measurements of blood pressure measurements >140/90 mm Hg, fasting serum glucose levels >126 mg/dL, and estimated glomerular filtration rate (eGFR) <60 mL/min by use of the modification of diet in kidney disease estimating equation, respectively. All patients were tested for syphilis, hepatitis C, hepatitis B, and fasting lipids, and diagnoses were confirmed according to testing results. A large fraction of the diagnoses of Pneumocystis pneumonia, oral candidiasis, and toxoplasma encephalopathy were presumptive due to easier establishment of a clinical diagnosis and limited availability of pathogenetic testing for these specific diseases.
Statistical Analysis
Patient information was anonymized and de-identified prior to analysis. The following variables were extracted from the medical records of Chongqing Public Health Medical Center: age, gender, medical healthcare insurance, urban clinic location, nadir CD4+ T-cell count, injection drug use, cART initiation, and diagnoses. For deceased patients, duration of hospitalization was recorded and causes of death were extracted from death certificates. For discharged patients, duration of hospitalization and diagnoses on discharge were extracted from the discharge certificate of the medical records. All analyses were performed using SPSS software Version 18.0 (IBM-SPSS, Chicago, IL, USA). Differences in proportions were compared by the Chi-squared test or Fisher's Exact test as appropriate. P < 0.05 were considered statistically significant. Strength of association was determined by calculating adjusted odds ratios (aORs) and their 95% confidence intervals (CIs). In identifying independent factors associated with deaths, variables were initially analyzed using a bivariate model, and subsequently independent risk factors were identified by means of a logistic regression model using a forward, stepwise approach, which began with inclusion of all variables associated with deaths on bivariate analysis (p ≤ 0.1), and subsequently included only those variables with p ≤ 0.05 in the final model. The sensitivity, specificity, area under the receiver operating characteristic curve, Nagelkerke R2, and Hosmer-Lemeshow tests were used to assess the quality of the logistic regression model.
Results
Study Flow and Characteristics of the Study Population
From January 2014 to January 2018, there were 6,065 AIDS-associated admissions to Chongqing Public Health Medical Center. After collation of multi-admissions and exclusion of cases with incomplete data and length of hospital stay ≤ 24 h, 4,445 hospitalized patients with AIDS were enrolled in this study. These patients were stratified into an older group (50 years or older), and a younger group (<50 years old). Among these 4,445 patients, 1,866 (42.0%) were 50 years or older and 2579 (58.0%) were <50 years old.
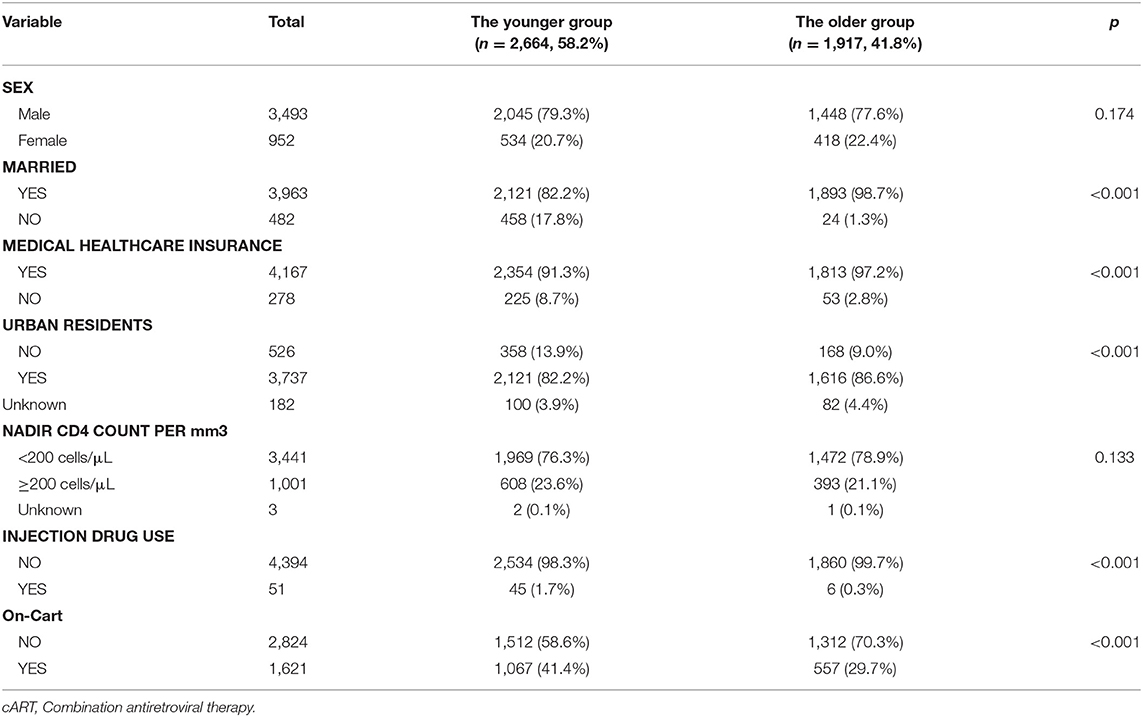
Among the data of 4,445 patients eligible for analysis, 78.6% were male and 21.4% were female; 89.2% were married, and 93.7% owned medical healthcare insurance (Table 1).
Compared with the younger group, more patients in the older group were married (98.7 vs. 82.2%, p < 0.001), had medical healthcare insurance (97.2 vs. 91.3%, p < 0.001), and were urban residents (86.6 vs. 82.2%, p < 0.001), and fewer patients in the older group had a history of injection drug use (0.3 vs. 1.7%, p < 0.001), or had received antiretroviral treatment (29.7 vs. 41.4%, p < 0.001) (Table 1).
Difference in the Prevalence of Diseases Between the Two Groups
Among the 4,445 patients, 3,852 (86.7%) were admitted to hospital due to the presence of an overt opportunistic infection. There was no statistical difference in the prevalence of opportunistic infections between the older group and the younger group (86.2 vs. 87.0%, p = 0.471). Compared with those in the younger group, patients in the older group had a significantly lower prevalence of tuberculosis, syphilis, hepatitis C, cryptococcal meningitis, toxoplasma encephalopathy, and talaromycosis. In addition, patients in the older group had a significantly higher prevalence of Pneumocystis pneumonia, non-AIDS malignancy, stroke, heart disease, diabetes, hypertension, and kidney disease, compared with those in the younger group (Table 2).
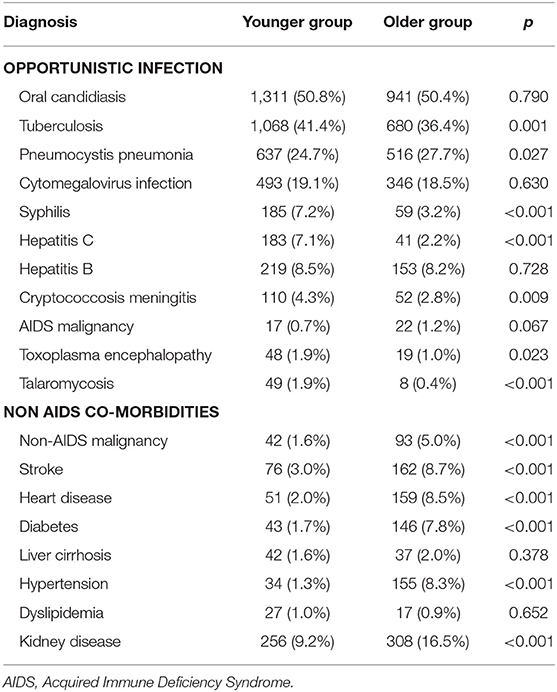
Table 2. Distribution of opportunistic infections and non-AIDS co-morbidities in 4,445 hospitalized PLWH stratified by age.
Difference in Mortality Between the Two Groups
The overall mortality rate for 4,445 hospitalized PLWH was 9.2%. Mortality of patients in the older group was significantly higher compared to the younger group, viz. 11.3 and 7.7%, respectively (p < 0.001). Table 3 shows the distribution of opportunistic infections and non-AIDS comorbidities in the two groups. Compared with the younger group, patients who died during hospitalization in the older group had a higher prevalence of oral candidiasis, tuberculosis, Pneumocystis pneumonia, cytomegalovirus infection, and hepatitis C.
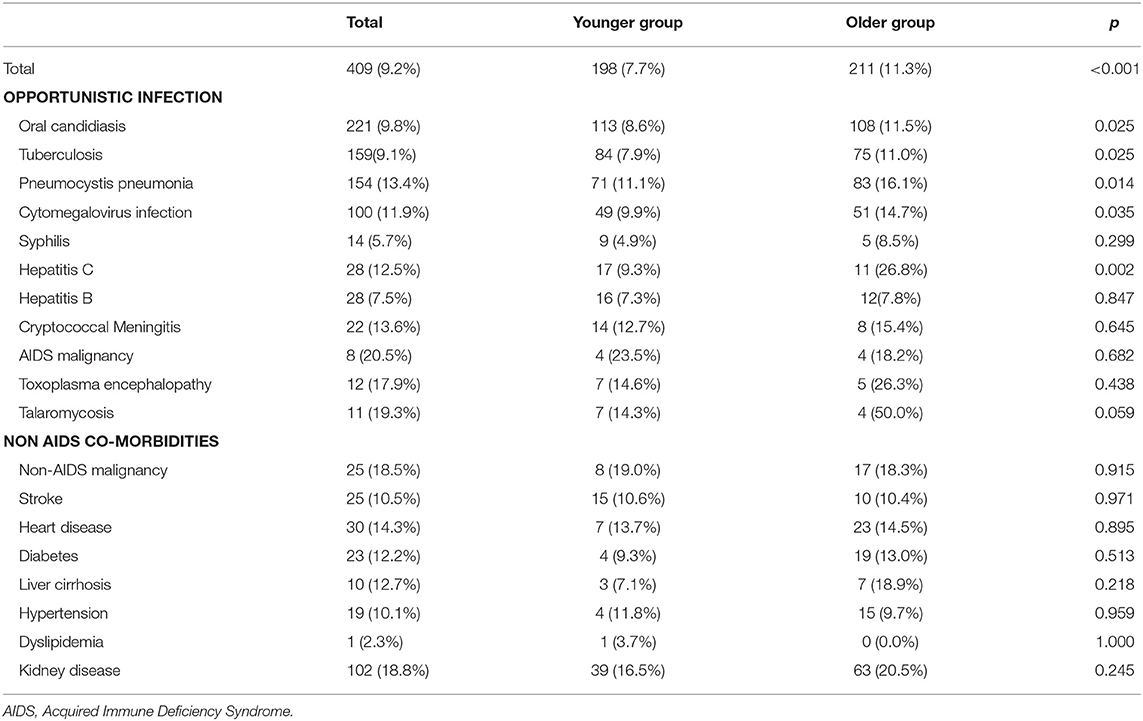
Table 3. Distribution of mortality in two age groups stratified by opportunistic infections and non-AIDS comorbidities.
Independent Risks of Death
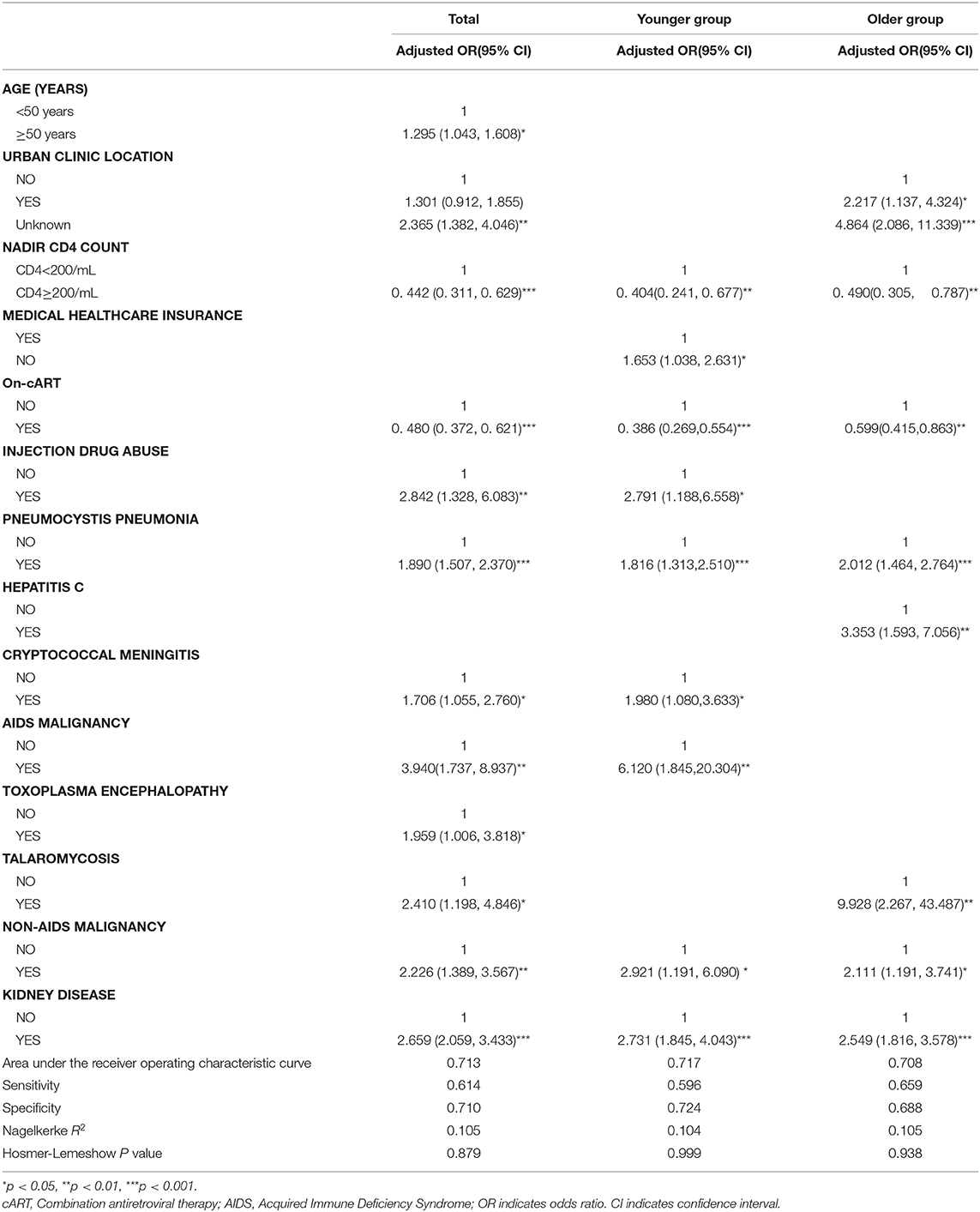
For the entire cohort, we conducted a multivariate logistic regression analysis of factors that may have had an impact on the risk of death. All variables with a p ≤ 0.1 in the univariate analysis were included in a logistic regression model using a forward stepwise approach, and adjusted for age (years), urban clinic location, nadir CD4+ T-cell count, whether on- cART, injection drug use, and having one of the following comorbidities: Pneumocystis pneumonia, Cytomegalovirus disease, syphilis, hepatitis C, cryptococcal meningitis, AIDS malignancy, toxoplasma encephalopathy, talaromycosis, non-AIDS malignancy, stroke, heart disease, and kidney disease. We observed that the following factors had significantly increased the risk of death in our hospitalized patients: being aged 50 years or older, being urban residents, nadir CD4+ T-cell counts being <200 cells/μL, not being on cART, being injection drug users, and having one of the following comorbidities: Pneumocystis pneumonia, cryptococcal meningitis, AIDS-related malignancy, toxoplasma encephalopathy, talaromycosis, non-AIDS malignancy, and kidney disease (Table 4). The−2Loglikelihood of the logistic regression was 2499.863, with a correct classification rate of 90.8% (Hosmer-Lemeshow test p = 0.879 and Nagelkerke's R2 = 0.105). The area under the receiver operating characteristic curve was 0.713. We selected the numerical value with the highest Youden index {(sensitivity+specificity)-1} as our cut-off point for the predicted probability (cut-off for probability = 9.05%) (Table 4).
In the younger group, we conducted a multivariate logistic regression analysis of factors that may have had an impact on the risk of death. All variables with a p ≤ 0.1 in the univariate analysis were included in a logistic regression model using a forward stepwise approach, and adjusted for nadir CD4+ T-cell count, possession of medical healthcare insurance, on-cART, injection drug use, and having one of the following comorbidities: oral candidiasis, Pneumocystis pneumonia, Cytomegalovirus disease, cryptococcal meningitis, AIDS malignancy, toxoplasma encephalopathy, talaromycosis, non-AIDS malignancy, and kidney disease. The factors that independently significantly increased the risk of death were nadir CD4+ T-cell counts being <200 cells/μL, not having medical healthcare insurance, not being on cART, injection drug use, and having one of the following comorbidities: Pneumocystis pneumonia, cryptococcal meningitis, an AIDS malignancy, a non-AIDS malignancy, and kidney disease. The−2Loglikelihood of the logistic regression was 1282.751, with a correct classification rate of 92.4% (Hosmer-Lemeshow test p = 0.999 and Nagelkerke's R2 = 0.104). The area under the receiver operating characteristic curve was 0.717. We selected the numerical value with the highest Youden index as our cut-off point for the predicted probability (cut-off for probability = 7.7%) (Table 4).
In the older group, we conducted a multivariate logistic regression analysis of factors that may have had an impact on the risk of death. All variables with a p ≤ 0.1 in the univariate analysis were included in a logistic regression model using a forward stepwise approach, and were adjusted for urban residents, nadir CD4+ T-cell count, whether on-cART, and having one of the following comorbidities: Pneumocystis pneumonia, Cytomegalovirus disease, hepatitis C, toxoplasma encephalopathy, talaromycosis, non-AIDS malignancy, and kidney disease. Independent risk factors for death included: being urban residents, nadir CD4+ T-cell count being <200 cells/μL, not being on cART, and having comorbidities such as Pneumocystis pneumonia, hepatitis C, talaromycosis, non-AIDS malignancy, and kidney disease. The−2Loglikelihood of the logistic regression was 1215.188, with a correct classification rate of 88.6% (Hosmer-Lemeshow test p=0.938 and Nagelkerke's R2 = 0.105). The area under the receiver operating characteristic curve was 0.708. We selected the numerical value with the highest Youden index as our cut-off point for the predicted probability (cut-off for probability = 10.3%) (Table 4).
The common risk factors in the two groups were nadir CD4+ T-cell counts, whether on-cART, and having one of the following comorbidities: Pneumocystis pneumonia, non-AIDS malignancy, and kidney disease. However, urban clinic location, hepatitis C, and talaromycosis were risk factors applicable to just the older group (Table 4).
Discussion
Chongqing Public Health Medical Center, the largest government-funded tertiary referral infectious disease hospital in south-western China, serves the municipality of Chongqing and surrounding provinces, and has had over 2000 HIV-associated medical admissions annually for the past several years.
In the USA, the overall rate of AIDS-related illnesses decreased between 2001 and 2008 (35). Reductions in AIDS-related infections were reported over a similar period in patients in Brazil and France (36), and in children in the USA (37). In our study, opportunistic infections remained the leading causes of hospital admission (86.7%) regardless of age, which is similar to global data (23). This finding may partly be explained by the low nadir CD4+ T-cell counts and the low cART coverage in our cohort of patients, less than half of whom were receiving cART at admission. This explanation is particularly true of patients in the older group, with over 70% of these patients not being on cART, and nearly 80% having nadir CD4+ T-cell counts <200 cells/μL. As shown in Table 1, the proportion of on-cART patients in the older group was also significantly lower, indicating that increased cART coverage and cART adherence support are highly warranted in this population.
In older individuals, delay in HIV diagnosis is not unusual in Chongqing, primarily due to unwillingness in this population to be tested, and difficulty in identifying individuals who do not have commonly recognized risk factors for infection, who thus avoid or bypass routine screening tests. An encouraging social factor, however, is that the majority of those aged 50 years or older had medical healthcare insurance in Chongqing, which makes it feasible to integrate health examinations and HIV screening tests in this population. If this group has access to early diagnosis and treatment, their overall disease situation would be greatly improved, since cART use has been demonstrated to be associated with improved survival, increased CD4+ T-cell counts, and reduced numbers of HIV-associated hospital admissions (23, 38).
Table 2 shows that compared with the younger group, patients in the older group were more likely to have Pneumocystis pneumonia, a non-AIDS malignancy, stroke, heart disease, diabetes, hypertension, and kidney disease, and were less likely to have tuberculosis, syphilis, hepatitis C, cryptococcal meningitis, toxoplasma encephalopathy, and talaromycosis. This observation suggests that different patterns of medical and allied support services may be needed for different age groups. For example, opioid substitution therapy, and hepatitis C screening and treatment are more applicable to PLWH <50 years of age, since a larger proportion of this population have a history of injection drug use. This is contrary to observations made in New York City (39). Sexually transmitted disease (STD) screening also needs to be enhanced because of the higher prevalence of syphilis in this population. Unfortunately, the older cohort in our study experiences a double burden of disease, having a higher prevalence of both communicable diseases and non-communicable chronic diseases, according to our results. This finding indicates that medical services based on a multidisciplinary team may be indicated for these older patients, since a larger proportion in this cohort have strokes, heart disease, diabetes, hypertension, and kidney disease, and some general physicians may not be well-enough equipped with the specialist knowledge and skills required to adequately and comprehensively manage other communicable and non-communicable chronic diseases and conditions occurring in tandem with overt HIV infection and AIDS.
Our results showed that the overall mortality in our hospitalized patients was 9.2%, which is lower than that in Malawi (22.7%) (11), and in Bangladesh (19.5%) (28). We also found that similar to Malawi (11) and other low-income countries (28, 29), older patients in our cohort had higher mortality rates than younger adults (p < 0.001), and most deaths were associated with opportunistic infections. This is in contrast to reports from high–income countries (30, 31), where deaths in HIV-infected patients occur mainly secondary to non-AIDS-related medical conditions.
It is true that older people have higher mortality than younger ones. However, the reasons of mortality in older people living with HIV may differ with HIV-uninfected older people, and thus, it is necessary to understand risk factors for death in HIV-infected older individuals and provide targeted evidence for healthcare professionals (32). In our study, we not only found that the older people have higher mortality than younger ones, but also found that demographic characteristics, illness distribution, mortality, and independent risk factors for death differ between the older group and the younger group.
Overall, our results showed that the prevalence of opportunistic infections was higher in the younger group, whereas the prevalence of non-AIDS malignancy, stroke, heart disease, diabetes, hypertension, and kidney disease was higher in the older group. Our study results also showed that factors such as being aged 50 years or older, being urban residents, nadir CD4+ T-cell counts being <200 cells/μL, not being on cART, being injection drug users, and having one of the following comorbidities: Pneumocystis pneumonia, hepatitis C, talaromycosis, non-AIDS malignancy, and kidney disease were independent risk factors for death. Among these factors, and concordant with observations in Bangladesh (28), patients in the older group had a significantly higher prevalence of, and mortality from, Pneumocystis pneumonia compared with the younger group, indicating that Pneumocystis pneumonia prophylaxis awareness, delivery, and utilization should be a priority in older PLWH.
We stratified the entire cohort by age and found that older patients and younger patients had common, as well as differing risk factors for mortality. As shown in Table 4, we found that both groups shared common risk factors for death, and these factors included nadir CD4+ T-cell counts, whether on-cART, and having one of the following comorbidities: Pneumocystis pneumonia, non-AIDS malignancy, and kidney disease. However, urban residential status, presence of co-infection with hepatitis C, and talaromycosis comorbidity in the older group bestowed significantly higher mortality risks, whereas in patients in the younger group, a concomitant diagnosis of cryptococcal meningitis imparted significantly higher mortality risk. The most likely explanation for this is that cryptococcal meningitis was more likely to occur in younger patients, and the elderly were not predisposed to cryptococcal meningitis, mostly because of older Chongqing patient's limited environmental exposure (33).
Interestingly, older patients who were urban residents tended to have a higher mortality in our cohort. The most likely explanation for this is that older people may be more willing to choose small clinics within their urban neighborhood for examination, assessment, and treatment due to factors related to patient convenience. In addition, more older rural patients may choose staying at home when becoming seriously ill, because dying at home is considered to be psychologically more auspicious and comfortable for patients facing an impending death, since it gives family members and friends more time to be with the person, and grants them more autonomy, privacy, and dignity (34). If this is indeed the case, it would thus be more difficult for healthcare providers and researchers to collect and collate information related to these deaths.
It is possible, also, that attending doctors at the small clinics attended by older patients lack specialist training in HIV-associated illnesses, and thereby may miss diagnoses, or diagnose HIV-associated illnesses much later in the course of these diseases. In contrast, younger people are likely to be more willing to travel longer distances to access specialized HIV clinics and tertiary level care, in order to seek more comprehensive medical attention and care.
There are limitations to this study. Firstly, this was a retrospective, observational study, and study data may have been subject to incorrect interpretation, and other general limitations related to retrospective observational investigations. Secondly, data for some variables were incomplete, and this may result in a degree of bias. Thirdly, although the diagnoses of most diseases were definitive, a small number of diagnoses in our study were presumptive. Fourthly, sample size was also limited as we have analyzed only 409 cases of death due to AIDS out of 4,445 hospitalized PLWH. Finally, this report reflects the experience of just one specialist hospital that primarily provides care for Chinese patients with HIV, and therefore the results may not be generalizable to all HIV-infected patients admitted to other hospitals in China, or to different populations elsewhere in the world.
In conclusion, this study observed that opportunistic infections remain the dominant reason for admission to hospital in south western China, and the demographic characteristics, illness distribution, mortality, and independent risk factors for death differ between patients aged 50 years or older, and those under 50 years. Our age-based analysis demonstrates the evolution and complexity of health needs of older PLWH in China. Our results suggest that a changing set of medical and allied support services may be required for older PLWH, such as cART adherence support, a requirement for specialist physicians trained in both HIV-related diseases and non-HIV related chronic diseases, enhanced national screening services, and targeted early intervention programs.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Our study was conducted in accordance with the Declaration of Helsinki and prior approval was obtained from the institutional review board of Chongqing Public Health Medical Center. The institutional review board waived the requirement for written informed consent, since this study was retrospective and all patient data were analyzed in anonymity.
Author Contributions
YZ: patients' enrollment, data generation and collection, statistical analysis, and writing of the manuscript. ZY: data generation and collection, statistical analysis, and writing of the manuscript. ML: patient enrollment, data generation, and analysis of data. YL: analysis of the data and writing of the manuscript. YQ: data generation and collection. XH: patient enrollment, data generation and collection. YZ: patient enrollment, data generation and collection. VH: manuscript revision. YC: study design, clinical data discussion, and manuscript revision. All authors approved the final version of the manuscript.
Funding
This study was supported by the National Science and Technology Major Project of China during the 13th Five-year Plan period (2018ZX10302104), Joint Medical Research Project of Chongqing Science and Technology Commission (2018QNXM012, 2020MSXM097), Chongqing Municipal Committee Unit Capacity Improvement Plan Project (2019NLTS003), Scientific Research Fund of the Infectious Disease Alliance of Beijing You'an Hospital (LM202021), Chongqing Natural Science Foundation (cstc2018jcyjAX0428), Chongqing Public Health Medical Center Youth Innovation Program (2019QNKYXM04, 2019QNKYXM09). The funding bodies have had no role in the trial design, trial execution, collection and interpretation of data, manuscript writing and editing, or decision to submit for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our sincere thanks and gratitude to all medical personnel including clinical fellows, nurses, and cleaners of Chongqing Public Health Medical Center, and the non-governmental organizations that provide care and support for HIV-infected persons, for their valuable support and contribution during data collection.
Abbreviations
AIDS, Acquired Immune Deficiency Syndrome; cART, Combination antiretroviral therapy; PLWH, people living with HIV; STDs, Sexually transmitted diseases; eGFR, estimated glomerular filtration rate; aORs, adjusted odds ratios; CIs, confidence intervals.
References
1. Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. (2017) 4:e349–e56. doi: 10.1016/S2352-3018(17)30066-8
2. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. (2013) 382:1525–33. doi: 10.1016/S0140-6736(13)61809-7
3. McGettrick P, Barco EA, Mallon PWG. Ageing with HIV. Healthcare. (2018) 6:17. doi: 10.3390/healthcare6010017
4. Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS. (2014) 28(Suppl 4):S453–9. doi: 10.1097/QAD.0000000000000479
5. Tavoschi L, Gomes Dias J, Pharris A. New HIV diagnoses among adults aged 50 years or older in 31 European countries, 2004-15: an analysis of surveillance data. Lancet HIV. (2017) 4:e514–e21. doi: 10.1016/S2352-3018(17)30155-8
6. Chen H, Wu X, Chen L, Lu H, Tang Z, Shen Z, et al. Rapidly spreading human immunodeficiency virus epidemic among older males and associated factors: a large-scale prospective cohort study in rural southwest China. Sex Transm Dis. (2019) 46:234–9. doi: 10.1097/OLQ.0000000000000957
7. Asher I, Guri KM, Elbirt D, Bezalel SR, Maldarelli F, Mor O, et al. Characteristics and outcome of patients diagnosed with hiv at older age. Medicine. (2016) 95:e2327. doi: 10.1097/MD.0000000000002327
8. Croxford S, Kitching A, Desai S, Kall M, Edelstein M, Skingsley A, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health. (2017) 2:e35–e46. doi: 10.1016/S2468-2667(16)30020-2
9. Chambers LA, Wilson MG, Rueda S, Gogolishvili D, Shi MQ, Rourke SB. Evidence informing the intersection of HIV, aging and health: a scoping review. AIDS Behav. (2014) 18:661–75. doi: 10.1007/s10461-013-0627-5
10. Althoff KN, Smit M, Reiss P, Justice AC. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS. (2016) 11:527–36. doi: 10.1097/COH.0000000000000305
11. Allain TJ, Aston S, Mapurisa G, Ganiza TN, Banda NP, Sakala S, et al. Age related patterns of disease and mortality in hospitalised adults in Malawi. PLoS ONE. (2017) 12:e0168368. doi: 10.1371/journal.pone.0168368
12. Wang LY, Qin QQ, Ge L, Ding ZW, Cai C, Guo W, et al. Characteristics of HIV infections among over 50-year-olds population in China. Zhonghua Liu Xing Bing Xue Za Zhi. (2016) 37:222–6. doi: 10.3760/cma.j.issn.0254-6450.2016.02.015
13. NCAIDS N, China CDC. Update on the AIDS/STD epidemic in China and main response in control and prevention in December, 2014. Chin J AIDS STD. (2015) 21:87. doi: 10.13419/j.cnki.aids.2015.02.01
14. Qin QQ, Wang L, Ding Z-W. Quality analysis of National HIV /AIDS online case reporting in 2008. Pract Prevent Med. (2009) 16:1785–7.
15. Carriquiry G, Giganti MJ, Castilho JL, Jayathilake K, Cahn P, Grinsztejn B, et al. Virologic failure and mortality in older ART initiators in a multisite Latin American and Caribbean Cohort. J Int AIDS Soc. (2018) 21:e25088. doi: 10.1002/jia2.25088
16. Wu G, Zhou C, Zhang X, Zhang W, Lu R, Ouyang L, et al. Higher risks of virologic failure and all-cause deaths among older people living with HIV in Chongqing, China. AIDS Res Human Retroviruses. (2019) 35:1095–102. doi: 10.1089/aid.2019.0096
17. Cordey S, Hartley MA, Keitel K, Laubscher F, Brito F, Junier T, et al. Detection of novel astroviruses MLB1 and MLB2 in the sera of febrile Tanzanian children. Emerg Microbes Infect. (2018) 7:1–3. doi: 10.1038/s41426-018-0025-1
18. Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med. (2007) 23:567–83. doi: 10.1016/j.cger.2007.02.004
19. Kiplagat J, Mwangi A, Keter A, Braitstein P, Sang E, Negin J, et al. Retention in care among older adults living with HIV in western Kenya: A retrospective observational cohort study. PLoS ONE. (2018) 13:e0194047. doi: 10.1371/journal.pone.0194047
20. Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. (2014) 384:241–8. doi: 10.1016/S0140-6736(14)60604-8
21. Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV cohort study. HIV Med. (2013) 14:195–207. doi: 10.1111/j.1468-1293.2012.01051.x
22. Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. (2006) 43:27–34. doi: 10.1097/01.qai.0000233310.90484.16
23. Ford N, Shubber Z, Meintjes G, Grinsztejn B, Eholie S, Mills EJ, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. (2015) 2:e438–44. doi: 10.1016/S2352-3018(15)00137-X
24. Wong EB, Omar T, Setlhako GJ, Osih R, Feldman C, Murdoch DM, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS ONE. (2012) 7:e47542. doi: 10.1371/journal.pone.0047542
25. Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d'Ivoire. Clin Infect Dis. (2011) 54:714–23. doi: 10.1093/cid/cir898
26. Zhang Y, Xiao Q, Zhou L, Ma D, Liu L, Lu R, et al. The AIDS epidemic and economic input impact factors in Chongqing, China, from 2006 to 2012: a spatial-temporal analysis. BMJ Open. (2015) 5:e006669. doi: 10.1136/bmjopen-2014-006669
27. World Health Organization. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children. (2018). Available online at: https://www.who.int/hiv/pub/toolkits/cryptococcal-disease-policy/en (accessed May 23, 2020).
28. Shahrin L, Leung DT, Matin N, Pervez MM, Azim T, Bardhan PK, et al. Characteristics and predictors of death among hospitalized HIV-infected patients in a low HIV prevalence country: Bangladesh. PLoS ONE. (2014) 9:e113095. doi: 10.1371/journal.pone.0113095
29. Agaba PA, Digin E, Makai R, Apena L, Agbaji OO, Idoko JA, et al. Clinical characteristics and predictors of mortality in hospitalized HIV-infected Nigerians. J Infect Dev Countr. (2011) 5:377–82. doi: 10.3855/jidc.1096
30. Krentz HB, Dean S, Gill MJ. Longitudinal assessment (1995-2003) of hospitalizations of HIV-infected patients within a geographical population in Canada. HIV Med. (2006) 7:457–66. doi: 10.1111/j.1468-1293.2006.00408.x
31. Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquired Immune Deficiency Syndromes. (2006) 41:194–200. doi: 10.1097/01.qai.0000179459.31562.16
33. Zheng H, Li M, Luo Y, Wang D, Yang J, Chen Q, et al. A retrospective study of contributing factors for prognosis and survival length of cryptococcal meningoencephalitis in Southern part of China (1998-2013). BMC Infect Dis. (2015) 15:77. doi: 10.1186/s12879-015-0826-y
34. Yeh ST, Ng YY, Wu SC. Hospital and patient characteristics regarding the place of death of hospitalized impending death patients: a multilevel analysis. Int J Environ Res Public Health. (2019) 16:4609. doi: 10.3390/ijerph16234609
35. Berry SA, Fleishman JA, Moore RD, Gebo KA. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001-2008. J Acquired Immune Deficiency Syndromes. (2012) 59:368. doi: 10.1097/QAI.0b013e318246b862
36. Luz PM, Bruyand M, Ribeiro S, Bonnet F, Moreira RI, Hessamfar M, et al. AIDS and non-AIDS severe morbidity associated with hospitalizations among HIV-infected patients in two regions with universal access to care and antiretroviral therapy, France and Brazil, 2000–2008: hospital-based cohort studies. BMC Infect Dis. (2014) 14:278. doi: 10.1186/1471-2334-14-278
37. Berry SA, Gebo KA, Rutstein RM, Althoff KN, Korthuis PT, Gaur AH, et al. Trends in hospitalizations among children and young adults with perinatally acquired HIV. Pediatr Infect Dis J. (2014) 33:488. doi: 10.1097/INF.0000000000000126
38. Adlakha A, Pavlou M, Walker D, Copas A, Dufty N, Batson S, et al. Survival of HIV-infected patients admitted to the intensive care unit in the era of highly active antiretroviral therapy. Int J STD & AIDS. (2011) 22:498–504. doi: 10.1258/ijsa.2011.010496
Keywords: aging, HIV, AIDS, death, opportunistic infections
Citation: Zhou Y, Yang Z, Liu M, Lu Y, Qin Y, He X, Zeng Y, Harypursat V and Chen Y (2021) Independent Risk Factors for Deaths due to AIDS in Chongqing, China: Does Age Matter? Front. Med. 7:586390. doi: 10.3389/fmed.2020.586390
Received: 23 July 2020; Accepted: 07 December 2020;
Published: 17 February 2021.
Edited by:
Harapan Harapan, Syiah Kuala University, IndonesiaReviewed by:
Evy Yunihastuti, University of Indonesia, IndonesiaMohammad Bellal Hossain, University of Dhaka, Bangladesh
Copyright © 2021 Zhou, Yang, Liu, Lu, Qin, He, Zeng, Harypursat and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaokai Chen, yaokaichen@hotmail.com
†These authors have contributed equally to this work
 Yihong Zhou
Yihong Zhou Zhongping Yang2†
Zhongping Yang2†  Yanqiu Lu
Yanqiu Lu Yaokai Chen
Yaokai Chen