Public Database-Driven Insights Into Aging Stress-Associated Defective Gut Barrier With Low SARS-CoV-2 Receptors
- 1Laboratory of Mucosal Exposome and Biomodulation, Department of Convergence Medical Sciences, Pusan National University, Yangsan, South Korea
- 2Graduate Program of Genome Data Sciences, Pusan National University, Yangsan, South Korea
The novel coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a global pandemic, and resulted in high case-fatality rate in the elderly. In addition to typical respiratory responses, ~50% of clinical cases include gastrointestinal symptoms such as diarrhea, vomiting, abdominal pain, and persistent fecal shedding of the virus even after its clearance from the pulmonary system. In the present study, we assessed aging-associated gut transcriptomic responses considering the gastrointestinal symptoms contributing to COVID-19 severity. Intestinal expression of SARS-CoV-2 receptors and defense biomarkers decreased with increasing age. Moreover, aging-associated integrated stress responses (ISR) and mTOR-linked cell metabolic stress signals counteracted gut defense biomarkers. However, SARS-CoV-2 receptor expression was positively associated with gut barrier integrity potently via downregulation of the two stress-responsive signals. Gut transcriptome-based mechanistic prediction implicates that high susceptibility to COVID-19 in the elderly with low SARS-CoV-2 receptors is due to aging stress-associated defective gut defense, providing a new avenue for viral entry receptor-independent interventions.
Introduction
Since the first report on an unknown pneumonia-like disorder caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Wuhan area, the coronavirus disease (COVID-19) has become a worldwide pandemic, and is majorly attributed to zoonotic sources (1–3). Although the common symptoms include fever, cough, dyspnea, fatigue, and sputum production, fatal cases present lymphopenia and severe inflammatory distress such as organ failure in addition to airway dysfunction (4). Such severe complications are prominent in subjects with underlying health conditions including cardiovascular diseases, diabetes, or obesity, requiring hospitalization and intensive care (4–7). Moreover, based on the population-based studies, the elderly group (particularly aged 70 years or older) among the patients with COVID-19 presented high case-fatality rate with severe complications in Italy and China (3, 8, 9). A quantitative systemic review demonstrated that ~25 and 71% of the elderly subjects developed renal injuries and required supplementary oxygen, respectively (8). Although the complications of COVID-19 with aging are evident, its mechanistic assessments are required for developing precise interventions for the susceptible population.
During cellular infection by SARS-CoV-2, the viral spike (S) protein recognizes angiotensin converting enzyme 2 (ACE2) as a viral receptor to enter the host cells. Moreover, this entry requires S protein priming by cellular proteases, which entails S protein cleavage and allows fusion of viral and cellular membranes. SARS-CoV-2 employs the cellular serine protease, transmembrane protease serine 2 (TMPRSS2), which cleaves the S protein of human coronaviruses on the cell membrane for priming (10). Successful viral entry depends on ACE2 and TMPRSS2, which are not only coexpressed in the airway epithelia but also highly expressed in gut cells such as esophageal, ileal, and colonic epithelial cells (11), indicating that the gastrointestinal tract acts as an alternative route for SARS-CoV-2 invasion. Furthermore, for ~50% of COVID-19 clinical cases, SARS-CoV-2 can be detected in fecal samples and gut mucosa of the infected hosts (12–14). In addition, half of infected patients display prolonged fecal shedding of SARS-CoV-2 even after viral clearance from the respiratory tract (15), thereby suggesting the transmission of coronavirus via fecal–oral route. In particular, persistent inflammatory distress in the insulted gut during viral infection may contribute to COVID-19 severity.
In response to viral infection, human cells activate a common adaptive pathway, known as the integrated stress response (ISR), to restore cellular integrity. The core biochemical event in ISR is the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α) by the eIF2α kinase family, leading to global translational arrest, and the induction of specific stress-responsive genes to achieve biological homeostasis in the insulted hosts (16, 17). In the present study, assuming age to be a crucial risk factor of COVID-19 severity, we investigated the transcriptomic features of human gut with aging stress. In particular, the aging stress in association with ISR and other stress signaling was evaluated to predict the defective responses to SARS-CoV-2 in the elderly subjects.
Methods
Age-Linked Transcriptome Data
RNA-seq raw counts and normalized TPM matrices (Illumina paired-end, 76 bp) were downloaded from the Genotype-Tissue Expression (GTEx) Portal (version 8, 17,382 samples from 30 tissue types). All accessed data used in this study are publicly available on the web portal (https://gtexportal.org/home/index.html) and have been deidentified, except for patient age range and gender. Non-diseased transverse colon tissues (n = 937) containing the mucosal parts from the different age groups were selected for the transcriptomic analysis (Supplementary Figure 1A). Samples from the sigmoid colon without the mucosa were excluded.
Genomic Analysis Using Colon Cancer Datasets
Clinical sources of transcriptomic data from colon cancer tissue samples of patients are listed in the dataset (GEO ID: gse39582, n = 566). Among a large series of colon cancer data collected for the Cartes dIdentité des Tumeurs (CIT) program from the French Ligue Nationale Contre le Cancer (http://cit.ligue-cancer.net), 566 were analyzed for mRNA expression profiles using Affymetrix U133plus2 chip and, among these, 463 were analyzed for DNA alteration profiles using the CGH Array (CIT-CGHarray V6). Survival analysis was performed in three datasets of patients with colorectal cancer (gse39582 [n = 566], gse24551 [n = 333], and gse14333 [n = 290]). Dataset gse24551 was derived from genome-wide expression at exon level for two independent series of colorectal cancer tissue biopsies using the Affymetrix Human Exon 1.0 ST platform. Dataset gse14333 was from the expression profiles of surgically resected specimens in 290 patients with colorectal cancer using Affymetrix Human Genome U133Plus 2.0 arrays.
Genomic Analysis Using IBD Datasets
Human intestinal tissue datasets were obtained from the gene expression arrays of patients with IBD (gse117993, n = 190). These experiments tested the differential gene expression in these three types of IBD relative to healthy control samples. RNA was isolated from biopsies from 190 pediatric patients undergoing diagnostic colonoscopy for inflammatory bowel diseases, including Crohn's disease (CD) and ulcerative colitis (UC). Single-end, 75-bp sequencing was performed, and raw reads were aligned to the human genome using Gencode v 24 as a reference. We included 14,085 protein-coding mRNA genes in downstream analyses. For clinical dataset, the three major clinical subsets of IBD included only UC, colon-only CD (cCD), and ileocolonic CD (iCD) (Supplementary Figure 1B).
Statistics
Statistical analyses were performed using GraphPad Prism v. 5.01 (La Jolla, CA, USA). For comparative analysis of two groups of data, Student's t-test was performed. For comparative analysis of multiple groups, data were subjected to analysis of variance (ANOVA) with Newman–Keuls method as a post-hoc ANOVA assessment.
Results
Aging Attenuates Expression of SARS-CoV-2 Receptors and Gut Defense Biomarkers
We analyzed aging-associated patterns mainly using the transcriptome dataset of non-diseased tissue from the Genotype-Tissue Expression (GTEx) project. Since the gut is a persistent source of fecal SARS-CoV-2 production, we specifically analyzed colonic RNA-seq transcriptomes from donors of varying ages (aged 20–79 years) (Figures 1A,B). Expression of two SARS-CoV-2 receptors was assessed in different age groups. Compared to the levels in young age group (aged 20–29 years), expression of ACE2 and TMPRSS2 tended to decrease with age, which was prominent in elderly groups (aged 60–79 years) (Figure 1A). For successful viral entry by directly binding to ACE2, other accessary components such as TMPRSS2 and cathepsin L (CTSL) can facilitate S protein priming for receptor binding on the host cell surface (10); however, CTSL without proteolysis activity on the cell surface is dispensable for host cell entry of SARS-CoV-2 (10, 18). In contrast with the levels of ACE2 and TMPRSS2, CTSL expression tended to increase with age in the colon tissue (Figure 1A).
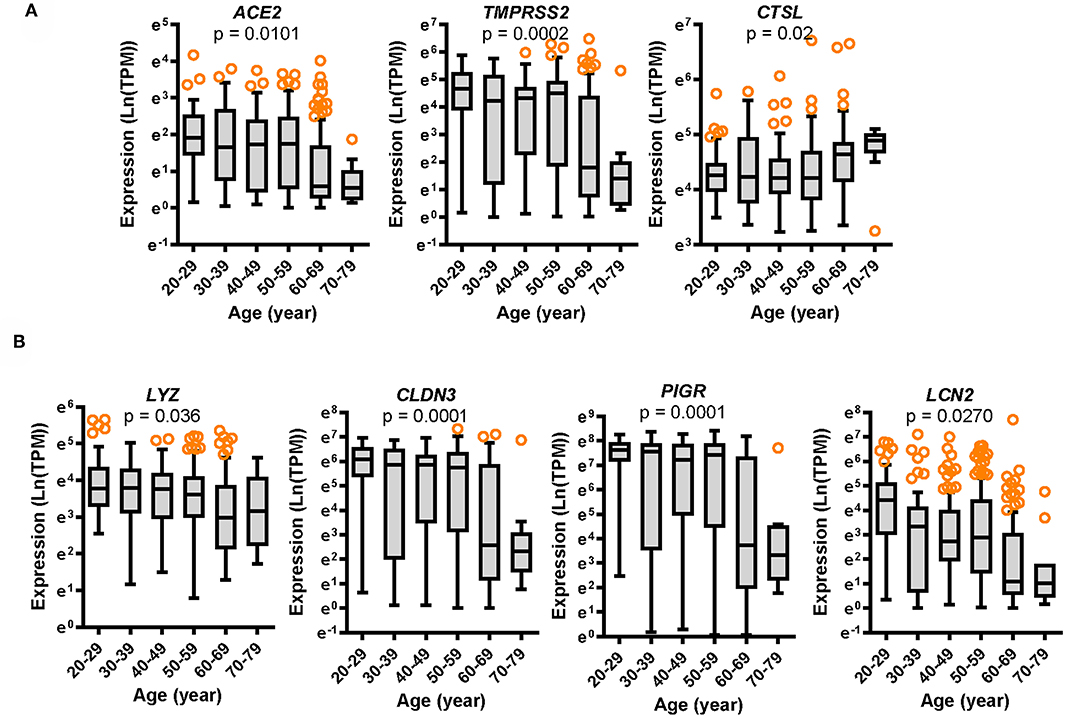
Figure 1. Expression of SARS-CoV-2 receptors and gut defense biomarkers with age. Results are depicted as box-and-whisker plots (Turkey) for the expression of SARS-CoV-2 receptors ACE2, TMPRSS2, and CTSL (A) or gut defense makers LYZ, CLDN3, PIGR, and LCN2 (B) in normal mucosal intestinal tissues (GTEx dataset v8). Values are presented as natural logarithm of transcripts per million (TPM). Statistical significance of the expression variation with age is illustrated on the top of each plot (Kruskal–Wallis test).
SARS-CoV-2 Receptors Are Positively Associated With Gut Defense During Aging or Chronic Disease Progression
In response to viral entry, the host epithelial defense is a deterministic factor of the pathogenic outcomes in infected patients. We analyzed the expression of gut barrier defense biomarkers such as lysozyme (LYZ), claudin 3 (CLDN3), polymeric immunoglobulin receptor (PIGR), and lipocalin 2 (LCN2) in the colon. Expression of the corresponding genes LYZ, CLDN3, PIGR, and LCN2 was modestly associated with age (p = 0.036, p = 0.0001, p = 0.0001, and p = 0.0270, respectively) and tended to decrease with increasing age (Figure 1B). Notably, levels of gut defense biomarkers were significantly attenuated in the elderly subjects (aged 60–79 years). Moreover, expression of key SARS-CoV-2 receptors was positively associated with levels of gut defense biomarkers (Figures 2A,B). From the GTEx-based dataset, subjects with high expression of ACE2 or TMPRSS2 presented high levels of LYZ, CLDN3, PIGR, and LCN2 in the intestine, indicating a protective action of SARS-CoV-2 receptors against gut infection.
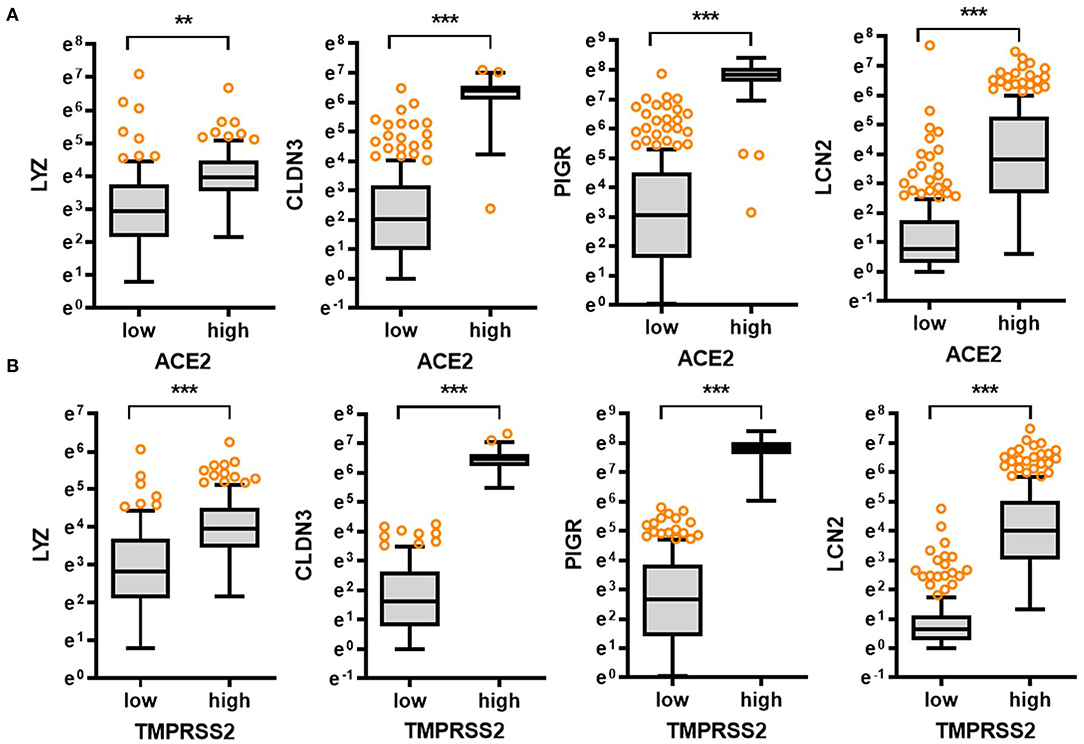
Figure 2. Comparative expression of barrier defense biomarkers with SARS-CoV-2 receptor levels. Based on ACE2 (A) or TMPRSS2 (B) levels in the normal mucosal intestinal tissues (GTEx dataset v8), we selected the 150 highest and 150 lowest level samples, which were further evaluated based on LYZ, CLDN3, PIGR, and LCN2 levels. Values are presented as natural logarithm of transcripts per million (TPM). Asterisks (*) indicate significant differences from the low expression group (**p < 0.01, ***p < 0.001).
In addition to the analyses of the non-diseased tissues from GTEx project, gene expression in biopsies from patients with chronic intestinal distress [colon cancer and inflammatory bowel disease (IBD)] was also evaluated. In patients with colon cancer, ACE2 expression tended to increase with disease progression (Figure 3A), whereas TMPRSS2 levels were not significantly altered in the lesions (Supplementary Figure 2A); however, patients with high levels of ACE2 displayed good prognosis compared to those with low expression (Figure 3B). Moreover, high expression levels of TMPRSS2 are positively associated with good prognoses for CRC patients (Supplementary Figure 2B). Results of survival analyses demonstrate the protective roles of SARS-CoV-2 receptors in oncological disease progress, which were in accordance with the results in non-diseased colonic tissues of the GTEx dataset. Furthermore, the patterns were also verified in the tissue expression from patients with IBD (Figures 3C,D). Expression of ACE2 and TMPRSS2 was markedly elevated in patients with UC and CD, including colon-only CD (cCD) and ileocolonic CD (iCD), when compared to that in the control group (Figure 3A and Supplementary Figure 2C, respectively). Patients with high expression of ACE2 or TMPRSS2 displayed high levels of LYZ and LCN2 in the intestine (Figure 3D), indicating a protective action of SARS-CoV-2 receptors against gut barrier disruption.
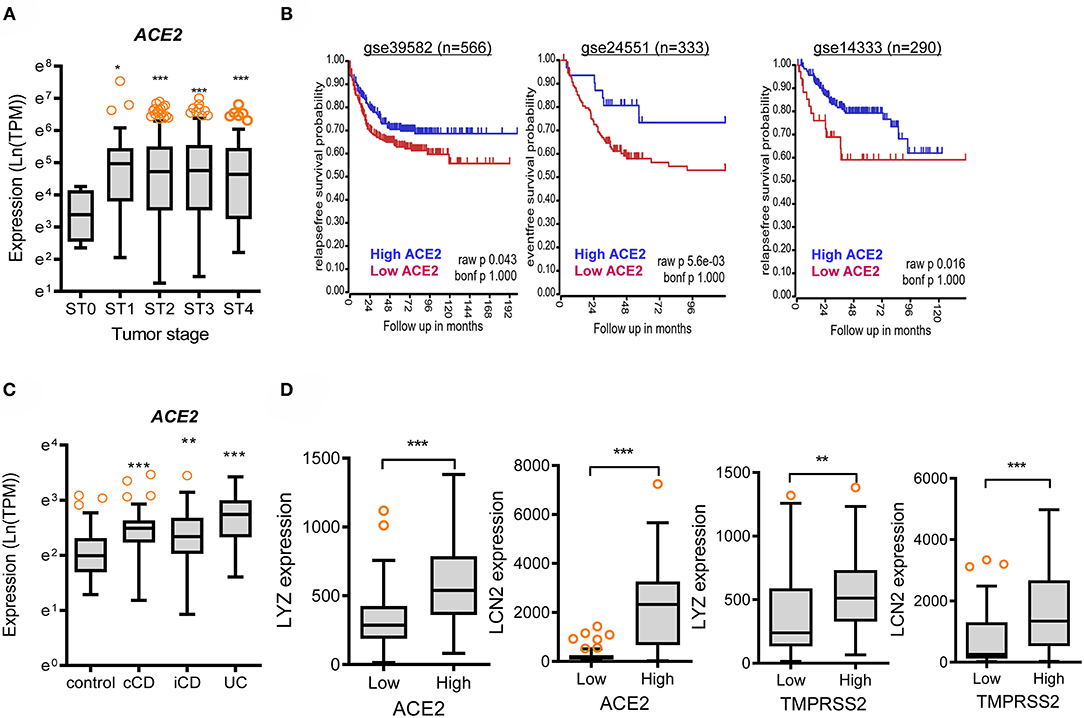
Figure 3. Involvement of SARS-CoV-2 receptors in chronic intestinal diseases. (A) ACE2 expression in different tumor stages from the transcriptome dataset in patients with colon cancer (GEO ID: gse39582, n = 566). Values are presented as natural logarithm of transcripts per million (TPM). Asterisks (*) indicate significant differences from levels at Stage 0 (*p < 0.05, **p < 0.01, ***p < 0.001 using two-tailed unpaired Student's t-test). (B) Kaplan–Meier plot of survival analysis based on tissue ACE2 transcript levels in patients with CRC from three datasets (gse39582 [n = 566, expression cutoff 150.5], gse24551 [n = 333, expression cutoff 444.6], and gse14333 [n = 290, expression cutoff 44.5]). (C) Intestinal expression of ACE2 was compared in patients with different IBD types from datasets gse117993 (n = 190). UC, ulcerative colitis; cCD, colon-only CD; iCD, ileocolonic CD. Values are presented as natural logarithm of transcripts per million (TPM). Results are depicted as box-and-whisker plots (Turkey). Asterisks (*) indicate significant differences from the control group (*p < 0.05, **p < 0.01, ***p < 0.001 using two-tailed unpaired Student's t-test). (D) Based on ACE2 or TMPRSS2 levels from datasets gse117993 (n = 190), we selected the 150 highest and 150 lowest level samples, which were further evaluated based on LYZ and LCN2 levels. Values are presented as natural logarithm of TPM. Asterisks (*) indicate significant differences from the low expression group (*p < 0.05, **p < 0.01, ***p < 0.001).
Aging-Associated ISR Potently Counteracts Levels of Gut Defense Biomarkers
To elucidate the molecular mechanisms of gastrointestinal distress, eIF2α kinase-mediated ISR was evaluated as the common adaptive pathway in response to the external insults including viral infection. The alpha subunit of eIF2 is targeted by four different stress-related mammalian protein kinases, namely, heme-regulated eIF2α kinase (HRI, EIF2AK1), double-stranded RNA-dependent protein kinase R (PKR, EIF2AK2), RNA-dependent protein kinase-like ER kinase (PERK, EIF2AK3), and eIF2α kinase general control non-repressed 2 (GCN2, EIF2AK4) (16, 17). In particular, SARS-CoV-2-infected cells display a PKR-linked pathogenesis including specific 28S rRNA cleavage (19–21). Expression of four eIF2α kinases was assessed in different age groups. Expression of EIF2AK2 and EIF2AK4 was significantly associated with age (p = 0.0012, and p = 0.0003, respectively) and tended to increase with age (Figure 4A). Notably, the levels of EIF2AK2 and EIF2AK4 were significantly elevated in elderly subjects (aged 60–79 years) when compared to those in the young group (aged 20–29 years). Furthermore, we evaluated whether eIF2α kinases are involved in regulation of gut barrier integrity. Expression of EIF2AK2 or EIF2AK4 was positively associated with the levels of gut defense biomarkers (Figures 4B,C). Subjects with high expression of EIF2AK2 or EIF2AK4 displayed low levels of LYZ, CLDN3, PIGR, and LCN2 in the intestine, thereby suggesting a negative regulation of gut defense by eIF2α kinase-linked signaling.
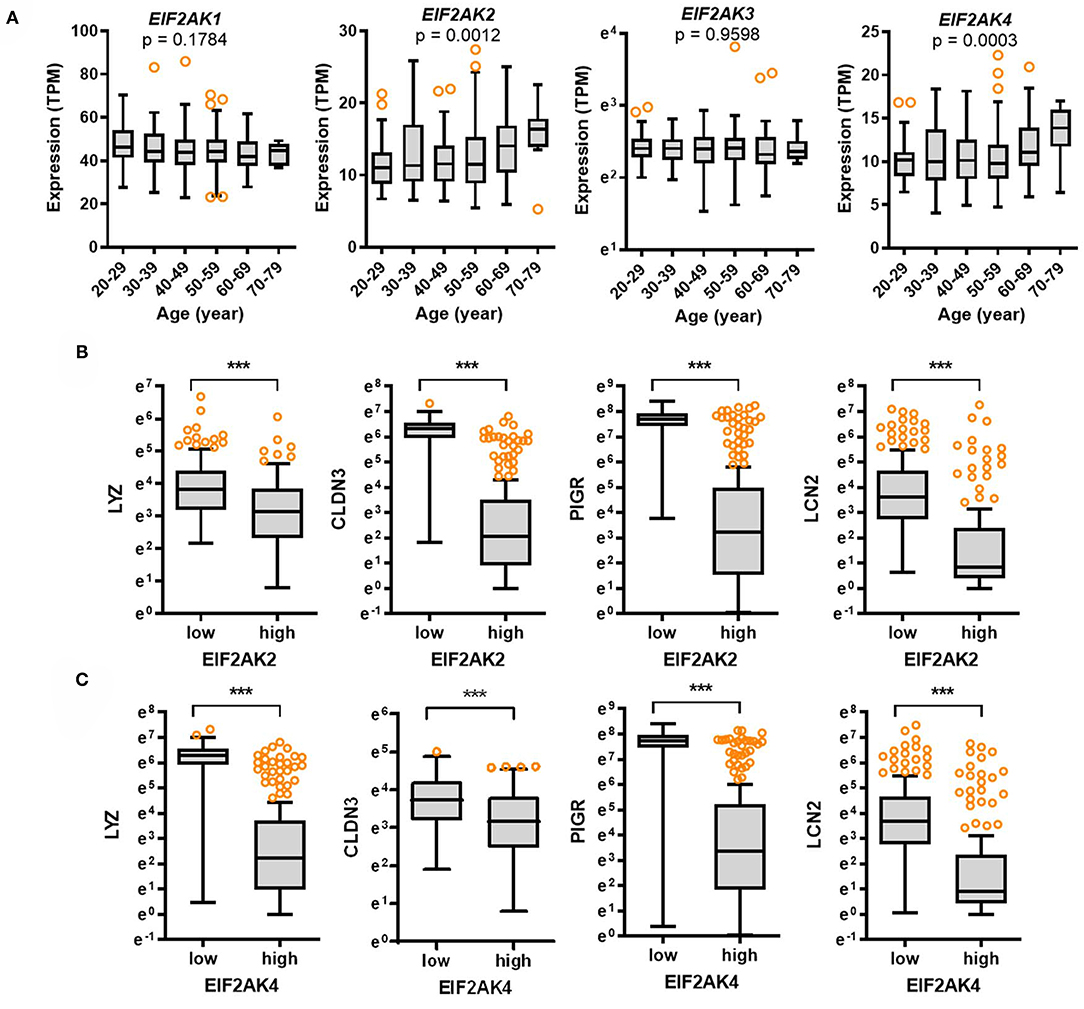
Figure 4. Expression of eIF2α kinases and their association with gut defense biomarkers with age. (A) Results are depicted as box-and-whisker plots (Turkey) for expression of eIF2α kinases (EIF2AK1, EIF2AK2, EIF2AK3, or EIF2AK4) in normal mucosal intestinal tissues (GTEx dataset v8). Values are presented as transcripts per million (TPM). Statistical significance of the expression variation with age is illustrated on the top of each plot (Kruskal–Wallis test). (B,C) Considering the EIF2AK2 (B) or EIF2AK4 (C) levels, we selected the 150 highest and 150 lowest level samples, which were further evaluated based on LYZ, CLDN3, PIGR, and LCN2 levels. Values are presented as natural logarithm of TPM. Asterisks (*) indicate significant differences from the low expression group (***p < 0.001).
Aging-Associated Cell Metabolic Stress Downregulates Levels of Gut Defense Biomarkers
The mammalian target of rapamycin (mTOR) is a central sentinel component of cellular metabolism that regulates the key aging processes including nutrient availability, energy homeostasis, cellular senescence, cell stemness, and proteostasis (22, 23). Although the expression of mTOR was not significantly associated with age (p = 0.353), there was an association between age and levels of ribosomal protein S6 kinase beta 1 (RPS6KB1) as a hallmark of activation by mTOR (p = 0.0001), which tended to increase with age (Figure 5A). Notably, expression of RPS6KB1 was significantly elevated in the elderly subjects (60–79 years) when compared to those in the young group (aged 20–29 years). Furthermore, we verified whether mTOR-S6 kinase signaling module as the key aging-regulator is involved in gut barrier defense by analyzing the GTEx dataset. Expression of mTOR or RPS6KB1 was associated with levels of gut defense biomarkers (Figures 5B,C). Subjects with high expression of mTOR or RPS6KB1 presented low levels of LYZ, CLDN3, PIGR, and LCN2 in the intestine, thereby indicating a negative regulation of gut defense by mTOR-S6 kinase signaling module. Since mTOR-S6 kinase signaling facilitates processes that fuel cell growth and proliferation, the signaling module counteracts cell differentiation to polarized enterocytes and other specialized intestinal epithelial cells such as goblet cells and Paneth cells, which is crucial for maintaining the gut epithelial barrier integrity (24). As a key intestinal differentiation factor, Krüppel-like factor 4 (KLF4) expression tended to decrease with age (Supplementary Figure 2D). Moreover, subjects with high expression of mTOR or RPS6KB1 displayed low levels of KLF4 in the intestine (Figures 5B,C), indicating insufficient differentiation and immature gut barrier by mTOR-S6 kinase signaling activation with age.
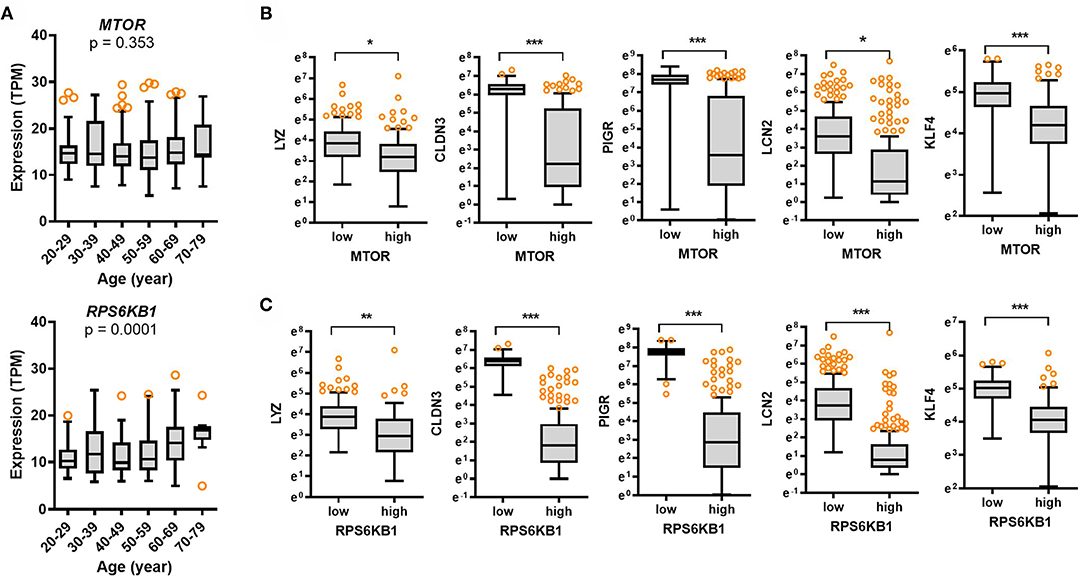
Figure 5. Expression of cell metabolic stress markers and their association with gut defense biomarkers with age. (A) Results are depicted as box-and-whisker plots (Turkey) for expression of cellular metabolic stress markers (mTOR or RPS6KB1) in normal mucosal intestinal tissues (GTEx dataset v8). Values are presented as transcripts per million (TPM). Statistical significance of the expression variation with age is illustrated on the top of each plot (Kruskal–Wallis test). (B,C) Considering the mTOR (B) or RPS6KB1 (C) levels, we selected the 150 highest and 150 lowest level samples, which were further evaluated based on LYZ, CLDN3, PIGR, and LCN2 levels. Values are presented as natural logarithm of TPM. Asterisks (*) indicate significant differences from the low expression group (*p < 0.05, **p < 0.01, ***p < 0.001).
We studied two stress signaling modules (eIF2α kinase and mTOR-S6 kinase) counteracting gut barrier integrity via clinical transcriptome analysis. Moreover, eIF2α kinase and mTOR-S6 kinase signaling modules were assessed for their association with levels of SARS-CoV-2 receptors. Subjects with high expression of ACE2 or TMPRSS2 displayed low levels of EIF2AK2, EIF2AK4, mTOR, or RPS6KB1 (Figures 6A,B), thereby supporting negative regulation of eIF2α kinase and mTOR-S6 kinase signaling by SARS-CoV-2 receptors in the gastrointestinal tract.

Figure 6. Regulation of stress responsive biomarkers by SARS-CoV-2 receptor. Considering the ACE2 or TMPRSS2 levels in normal mucosal intestinal tissues (GTEx dataset v8), we selected the 150 highest and 150 lowest level samples, which were further evaluated based on the expression of cell metabolic stress markers (A, mTOR or RPS6KB1) or eIF2α kinases (B, EIF2AK2 or EIF2AK4). Values are presented as transcripts per million (TPM). Asterisks (*) indicate significant differences from the low expression group (***p < 0.001). (C) A putative scheme for SARS-CoV-2 receptor-mediated modulation of gut barrier defense with age. In response to SARS-CoV-2 infection, SARS-CoV-2-responsive receptors positively contribute to maintenance of gut barrier defense via suppressing two stress signaling pathways including eIF2α kinase and mTOR-S6 kinase.
Discussion
As a prediction model from the results, SARS-CoV-2-responsive receptors positively contribute to maintenance of gut barrier defense via attenuation of two stress signaling pathways of eIF2α kinase and mTOR-S6 kinase (Figure 6C). Nevertheless, the expression of viral receptors diminishes with age, thereby elevating two stress signaling modules and subsequently weakening the gut barrier defense in elderly subjects. The gut acts as an alternative source of SARS-CoV-2 infection, leading to symptoms such as diarrhea and prolonged fecal shedding of the virus, which potently occurs due to high levels of SARS-CoV-2 receptors in the gastrointestinal tract. High expression of ACE2 in the intestinal epithelial cells implicates two potent routes of infection into the gastrointestinal tract. First, the well-known airway infection via human-to-human transmission presumably spreads via circulation to the rest of the body including gut and liver. The second route of gastrointestinal infection is airway-bypassing fecal–oral transmission from infected water or food. In particular, ACE2 acts as a coreceptor for nutrient uptake and particularly amino acid absorption from food (25), thereby indicating that SARS-CoV-2 in the contaminated food utilizes the receptor for its entry into the human body. Based on recent clinical evidences, ~50% of the COVID-19 patients present detectable levels of fecal SARS-CoV-2 RNA even after its clearance from the respiratory tract (11–13, 15, 25, 26), indicating that the digestive tract may act as a major site of viral replication and activity. Moreover, the infected gastrointestinal tract can be a crucial source of proinflammatory mediators such as bacterial products, metabolites, and gut-derived immune components which reversely aggravate the disease severity in the respiratory tract and other organs in infected hosts. This gut-to-airway infection supports the recent experimental evidence that intragastric inoculation of SARS-CoV-2 causes productive infection and leads to pulmonary pathological changes (27). Collectively, the enteric entry and replication of SARS-CoV-2 can be one of pivotal pathogenic pathways in addition to the airway infection.
Expression of the SARS-CoV-2 receptor is high in the gut; however, it decreases with age according to our transcriptomic analysis of the clinical dataset (Figure 1A). Nevertheless, the elderly subjects are more susceptible to COVID-19 than the younger groups in the recent global pandemic. In the present study, we propose mechanistic links of high disease severity in the elderly patients with low level of SARS-CoV-2 receptors. In addition to the SARS-CoV-2 receptors, the virus can impact host physiology via ISR. In case of SARS-CoV-2 infection, cells display EIF2AK2 (PKR)-linked pathogenesis including the ribosomal stress response via specific cleavage of 28S rRNA (19–21). Even though levels of the virus entry receptors decrease with age, the viral RNA triggers ribosomal stress leading to PKR activation and ISR via pattern recognition receptors, which can contribute to SARS-CoV-2-induced mucosal pathogenesis. Epithelial PKR activation plays a pivotal role in gut barrier disruption by regulating the lipid raft including caveolae (28). Moreover, lipid rafts contribute to SARS-CoV-2 infection in the early replication process (29, 30). Notably, ACE2 is located in the lipid rafts, which potently plays a pivotal role in the initial step of the virus entry-triggered signaling. PKR activation-induced structural alterations in lipid rafts facilitate caveolae-mediated degradation of epidermal growth factor receptor that is a crucial signaling mediator for maintaining the gut epithelial barrier integrity (28). PKR-linked molecular events during virus entry are well consistent with the patterns in clinical transcriptome analyses in the present study. Elevated levels of PKR signaling were associated with deterioration of gut defense with age despite attenuated ACE2 expression in the elderly subjects. Therefore, ISR-linked disruption of gut defense may be an important mechanism of COVID-19 severity in elderly groups with low level of SARS-CoV-2 receptors.
We propose that mTOR-S6 kinase signaling is inversely associated with the expression of KLF4, a key intestinal differentiation factor. Due to insufficient cues for differentiation, formation of goblet and Paneth cells can be retarded in the gut barrier, which results in deficiencies in mucus and lysozyme secretion (24). Therefore, aging-associated increase of mTOR-S6 kinase signaling potently counteracts KLF4-mediated differentiation of the gut barrier cells, which can account for reduced mucosal defense against SARS-CoV-2 infection in the elderly population. Moreover, mTOR-S6 kinase signaling directly inhibits adenosine monophosphate-activated protein kinase (AMPK), the key regulator of energy metabolism, to promote cell proliferation under nutrient stress (31). Since AMPK improves gut epithelial differentiation and barrier function (32), mTOR may downregulate gut defense via attenuation of AMPK pathway. Mechanistically, AMPK inactivation is associated with reduced expression of caudal type homeobox 2 (CDX2), the key transcription factor for intestinal epithelium maturity and Paneth cell development (33). Detailed molecular epigenetic machinery of CDX2 expression can be associated with polycomb repressive complex 2-regulated enrichment of H3K27me3 and lysine-specific histone demethylase-1-mediated reduction of H3K4me3 (32). Collectively, cell metabolic stress signaling of mTOR-S6 kinase potently attenuates AMPK activation, thus contributing to immature epithelial barrier via insufficient cellular differentiation with age. Although the expression of SARS-CoV-2 receptors is inversely associated with two stress signaling modules (eIF2α kinase and mTOR-S6 kinase), the levels of SARS-CoV-2 receptors diminish with age. Instead, elevated two stress signaling modules were positively involved in defective gut defense in the elderly subjects. Furthermore, disrupted gut barrier may increase the exposure to infectious agents and subsequently excessive inflammatory responses, which could be a crucial step of COVID-19 severity associated with age; however, extensive experimental evidences are still warranted to support the clinical transcriptome-based predictions of age-associated responses to COVID-19 and future interventions with the planet disorder.
Data Availability Statement
The datasets generated for this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Project design and hypotheses were developed by YM. YM analyzed the data, prepared the manuscript, and supervised the overall project.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A3B05041889).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.606991/full#supplementary-material
References
1. Contini C, Di Nuzzo M, Barp N, Bonazza A, De Giorgio R, Tognon M, et al. The novel zoonotic COVID-19 pandemic: an expected global health concern. J Infect Dev Ctries. (2020) 14:254–64. doi: 10.3855/jidc.12671
2. Mushi V. The holistic way of tackling the COVID-19 pandemic: the one health approach. Trop Med Health. (2020) 48:69. doi: 10.1186/s41182-020-00257-0
3. Wu JT, Leung K, Bushman M, Kishore N, Niehus R, de Salazar PM, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. (2020) 26:506–10. doi: 10.1038/s41591-020-0822-7
4. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. (2020) 20:e276–88. doi: 10.1016/S1473-3099(20)30651-4
5. El-Sayed Moustafa JS, Jackson AU, Brotman SM, Guan L, Villicana S, Roberts AL, et al. ACE2 expression in adipose tissue is associated with COVID-19 cardio-metabolic risk factors and cell type composition. medRxiv. (2020). doi: 10.1101/2020.08.11.20171108
6. Moula AI, Micali LR, Matteucci F, Luca F, Rao CM, Parise O, et al. Quantification of death risk in relation to sex, pre-existing cardiovascular diseases and risk factors in COVID-19 patients: let's take stock and see where we are. J Clin Med. (2020) 9:2685. doi: 10.3390/jcm9092685
7. Valerio A, Nisoli E, Rossi AP, Pellegrini M, Todesco T, Ghoch ME. Obesity and higher risk for severe complications of Covid-19: what to do when the two pandemics meet. J Popul Ther Clin Pharmacol. (2020) 27(S Pt 1):e31–6. doi: 10.15586/jptcp.v27iSP1.708
8. Neumann-Podczaska A, Al-Saad SR, Karbowski LM, Chojnicki M, Tobis S, Wieczorowska-Tobis K. COVID 19 - clinical picture in the elderly population: a qualitative systematic review. Aging Dis. (2020) 11:988–1008. doi: 10.14336/AD.2020.0620
9. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
10. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80 e8. doi: 10.1016/j.cell.2020.02.052
11. Zhang H, Kang ZJ, Gong HY, Xu D, Wang J, Li ZX, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. (2020) 69:1010–8. doi: 10.1136/gutjnl-2020-320953
12. Santos VS, Gurgel RQ, Cuevas LE, Martins-Filho PR. Prolonged fecal shedding of SARS-CoV-2 in pediatric patients: a quantitative evidence synthesis. J Pediatr Gastroenterol Nutr. (2020) 71:150–2. doi: 10.1097/MPG.0000000000002798
13. Wang X, Zhou Y, Jiang N, Zhou Q, Ma WL. Persistence of intestinal SARS-CoV-2 infection in patients with COVID-19 leads to re-admission after pneumonia resolved. Int J Infect Dis. (2020) 95:433–5. doi: 10.1016/j.ijid.2020.04.063
14. Song Y, Liu P, Shi XL, Chu YL, Zhang J, Xia J, et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. (2020) 69:1143–4. doi: 10.1136/gutjnl-2020-320891
15. Wu YJ, Guo C, Tang LT, Hong ZS, Zhou JH, Dong X, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. (2020) 5:434–5. doi: 10.1016/S2468-1253(20)30083-2
16. Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. (2016) 17:1374–95. doi: 10.15252/embr.201642195
17. Park SH, Moon Y. Integrated stress response-altered pro-inflammatory signals in mucosal immune-related cells. Immunopharmacol Immunotoxicol. (2013) 35:205–14. doi: 10.3109/08923973.2012.742535
18. Zhang X, Zhou Y, Yu X, Huang Q, Fang W, Li J, et al. Differential roles of cysteinyl cathepsins in TGF-beta signaling and tissue fibrosis. iScience. (2019) 19:607–22. doi: 10.1016/j.isci.2019.08.014
19. Banerjee S, An S, Zhou A, Silverman RH, Makino S. RNase L-independent specific 28S rRNA cleavage in murine coronavirus-infected cells. J Virol. (2000) 74:8793–802. doi: 10.1128/JVI.74.19.8793-8802.2000
20. de Wilde AH, Wannee KF, Scholte FE, Goeman JJ, Ten Dijke P, Snijder EJ, et al. A kinome-wide small interfering RNA screen identifies proviral and antiviral host factors in severe acute respiratory syndrome coronavirus replication, including double-stranded RNA-activated protein kinase and early secretory pathway proteins. J Virol. (2015) 89:8318–33. doi: 10.1128/JVI.01029-15
21. Krahling V, Stein DA, Spiegel M, Weber F, Muhlberger E. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J Virol. (2009) 83:2298–309. doi: 10.1128/JVI.01245-08
22. Papadopoli D, Boulay K, Kazak L, Pollak M, Mallette F, Topisirovic I, et al. mTOR as a central regulator of lifespan and aging. F1000Res. (2019) 8:998. doi: 10.12688/f1000research.17196.1
23. Escobar KA, Cole NH, Mermier CM, VanDusseldorp TA. Autophagy and aging: maintaining the proteome through exercise and caloric restriction. Aging Cell. (2019) 18:e12876. doi: 10.1111/acel.12876
24. Zhou Y, Rychahou P, Wang Q, Weiss HL, Evers BM. TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium. Cell Death Dis. (2015) 6:e1631. doi: 10.1038/cddis.2014.588
25. Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. (2012) 487:477–81. doi: 10.1038/nature11228
26. Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut. (2020) 69:973–4. doi: 10.1136/gutjnl-2020-321195
27. Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. (2020) 28:124–33 e4. doi: 10.1016/j.chom.2020.05.020
28. Park SH, Kim J, Moon Y. Caveolar communication with xenobiotic-stalled ribosomes compromises gut barrier integrity. Commun Biol. (2020) 3:270. doi: 10.1038/s42003-020-0994-1
29. Fecchi K, Anticoli S, Peruzzu D, Iessi E, Gagliardi MC, Matarrese P, et al. Coronavirus interplay with lipid rafts and autophagy unveils promising therapeutic targets. Front Microbiol. (2020) 11:1821. doi: 10.3389/fmicb.2020.01821
30. Li GM, Li YG, Yamate M, Li SM, Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. (2007) 9:96–102. doi: 10.1016/j.micinf.2006.10.015
31. Ling NXY, Kaczmarek A, Hoque A, Davie E, Ngoei KRW, Morrison KR, et al. mTORC1 directly inhibits AMPK to promote cell proliferation under nutrient stress. Nat Metab. (2020) 2:41–9. doi: 10.1038/s42255-019-0157-1
32. Sun X, Yang Q, Rogers CJ, Du M, Zhu MJ. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression Cell Death Differ. (2017) 24:819–31. doi: 10.1038/cdd.2017.14
Keywords: SARS-CoV-2, gut barrier, integrated stress responses, metabolic stress, aging
Citation: Moon Y (2020) Public Database-Driven Insights Into Aging Stress-Associated Defective Gut Barrier With Low SARS-CoV-2 Receptors. Front. Med. 7:606991. doi: 10.3389/fmed.2020.606991
Received: 16 September 2020; Accepted: 30 November 2020;
Published: 22 December 2020.
Edited by:
Hu Zhang, West China Hospital, Sichuan University, ChinaReviewed by:
Roberto Gramignoli, Karolinska Institutet (KI), SwedenXiaofei Sun, University of California, San Francisco, United States
Copyright © 2020 Moon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuseok Moon, moon@pnu.edu
 Yuseok Moon
Yuseok Moon