Coronavirus Disease 2019 Vaccinations in Patients With Chronic Liver Disease and Liver Transplant Recipients: An Update
- 1Gastroenterology and Hepatology Unit, Division of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
- 2Department of Biomedical Sciences and Biomedical Engineering, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
- 3Division of Infectious Diseases, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 4Ramathibodi Excellence Center for Organ Transplantation, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Coronavirus disease 2019 (COVID-19) is a current global pandemic associated with an increased mortality, particularly in patients with comorbidities. Patients with chronic liver disease (CLD) and liver transplant (LT) recipients are at higher risk of morbidity and mortality after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Many liver societies have recommended that these patients should receive COVID-19 vaccinations, although there are limited studies assessing risks and benefits in this population. In addition, two doses of mRNA vaccines may not provide sufficient immune response, and booster dose(s) may be necessary, especially in LT recipients. Notably, variants of concern have recently emerged, and it remains unclear whether currently available vaccines provide adequate and durable protective immunity against these novel variants. This review focuses on the role of COVID-19 vaccinations in CLD and LT recipients.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that was first reported to cause a pneumonia outbreak in Wuhan, China, in December 2019 (1) and has resulted in a global pandemic since (2). The disease caused by SARS-CoV-2 is referred to as coronavirus disease 2019 (COVID-19). As of December 19, 2021, COVID-19 has affected more than 273 million people and has a mortality rate of approximately 1.9%, resulting in a substantial global public health issue (2). Since COVID-19 vaccines were launched for general population in late 2020, the overall mortality has tended to decrease. COVID-19 vaccines were developed aim to reduce morbidity, mortality, and prevent transmission. The World Health Organization (WHO) reported that 137 and 194 vaccines have been in clinical and pre-clinical development (3). As of December 30, 2021, vaccination coverage comprises of 62% of the total population and has reached 72.8% among individuals 18 years or older in the United States (4). Patients with chronic liver disease (CLD), including non-cirrhosis diseases, e.g., non-alcoholic fatty liver disease (NAFLD) (5–8), alcoholic-associated liver disease (9), cirrhosis (10–12), and liver transplant (LT) recipients (13) are at risk of increased morbidity and mortality due to liver-related or non-liver-related complications (13). Accordingly, these patients should be prioritized for vaccination (14–16). Nevertheless, there are limited data on COVID-19 vaccines among patients with CLD and LT recipients. The purpose of this review is to discuss the impact of SARS-CoV-2 infections in patients with liver disease and to review evidence-based research on COVID-19 vaccines, particularly in CLD and LT recipients. Further, we highlight extent issues such as booster doses and variants of concern with the goal of providing a reference for vaccinations in patients with CLD and LT recipients.
Search Strategy
We searched the literature using PubMed and Scopus on 1 March 2022 with search terms “COVID-19 vaccine and cirrhosis or liver transplant”. A total of 248 titles and abstracts were retrieved and manual reviewed the abstracts to address the studies of COVID-19 vaccine in patients with CLD and LT recipients. Additional references were reviewed from the literature bibliography to identify the relevant recent data included in this narrative review.
Effects of SARS-CoV-2 on Patients With Liver Disease
Patients With Cirrhosis
Since 2020, there have been numerous reports on the prognosis and outcomes following SARS-CoV-2 infection in patients with cirrhosis. Seminal data were derived from a registry study in Asia (the APCOLIS study) (11) and combined results from the COVIDCirrhosis.org and COVID-Hep.net registries (17) (predominantly from Europe and the United States). Both registry studies reported significantly higher mortality rates in patients with cirrhosis than in control groups comprising non-cirrhotic CLD patients or patients without CLD who had SARS-CoV-2 infection, especially in patients with Child-Turcotte-Pugh B and C. A recent meta-analysis of 16 studies confirmed that in SARS-CoV-2-infected patients, the presence of cirrhosis was significantly associated with increased mortality compared to that in non-cirrhotic patients, with a pooled crude odds ratio (OR) of 2.48 (95% CI: 2.02–3.04) and a pooled adjusted OR of 1.81 (95% CI: 1.36–2.42) (18) (Figure 1). Moreover, the severity of baseline liver cirrhosis status was correlated with higher mortality (18). Cirrhosis-associated immune dysfunction (CAID) is thought to be a major contributor to poorer clinical outcomes in patients with severe liver disease (19). Notably, 25% of cirrhotic patients with SARS-CoV-2 infection only present with hepatic decompensation without any concurrent pulmonary symptoms (17). Thus, cirrhotic patients who present with any type of decompensation should be assessed for SARS-CoV-2 infection regardless of their pulmonary symptoms.
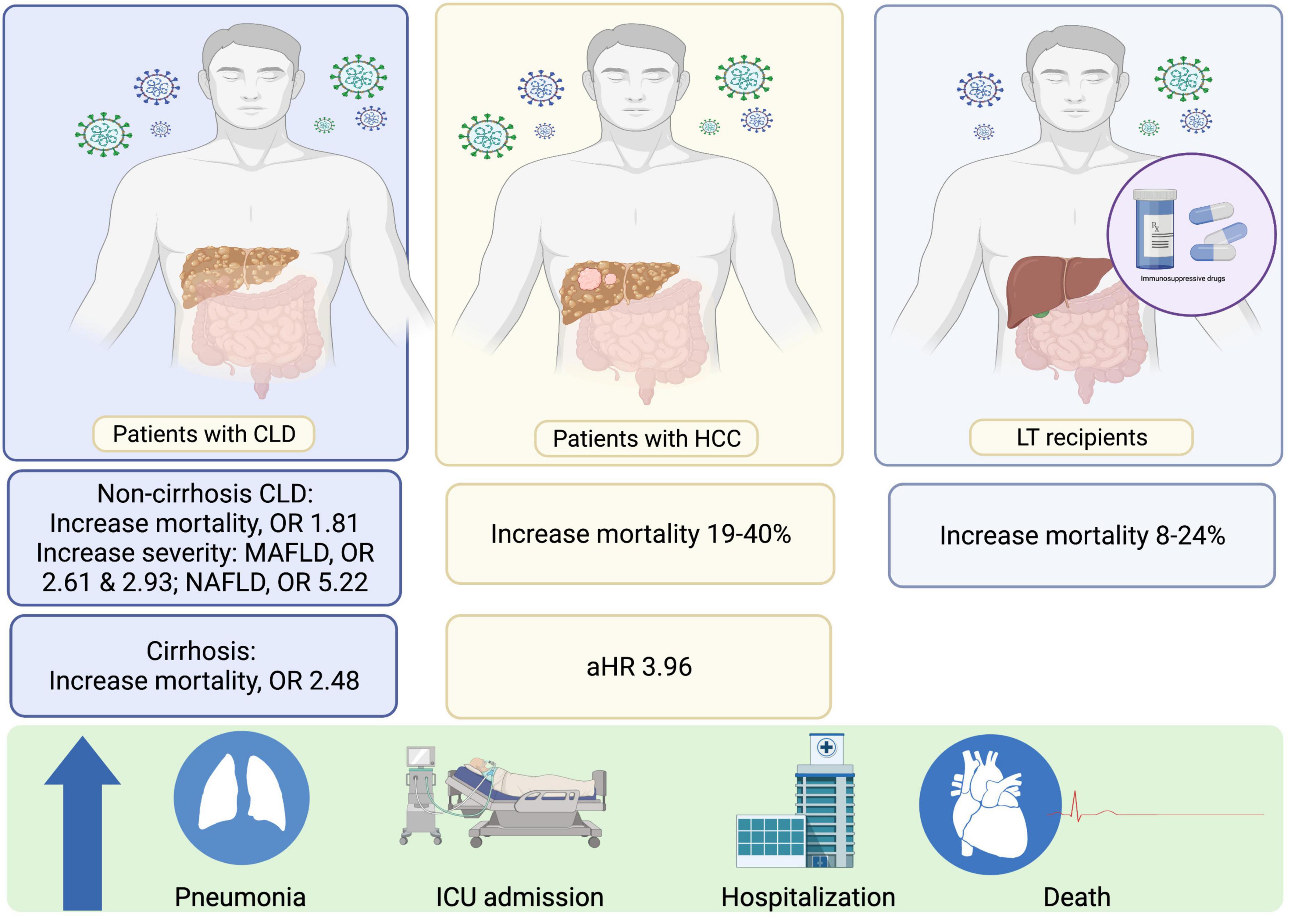
Figure 1. Effects of SARS-CoV-2 in patients with CLD and LT recipients. CLD, chronic liver disease; HCC, HCC, hepatocellular carcinoma; HR, hazard ratios; LT, liver transplant; MAFLD, Metabolic Associated Fatty Liver Disease; NAFLD, Non-alcoholic fatty liver disease; OR, odd ratios; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
Concerns have been raised that all hospitalized cirrhotic patients generally have higher in-hospital mortality compared to patients without cirrhosis. The issue of whether SARS-CoV-2-infected cirrhotic patients have a higher risk of poor outcomes compared to cirrhotic patients hospitalized for other causes is of interest. A small retrospective study from Italy (comprising 50 SARS-CoV-2-positive and 47 SARS-CoV-2-negative patients with cirrhosis) (20) and two national-level studies from the United States (12, 21) (comprising 305 and 8941 SARS-CoV-2-positive and 3301 and 53476 SARS-CoV-2-negative patients with cirrhosis, respectively) reported that SARS-CoV-2 infection was significantly associated with higher all-cause mortality among patients with cirrhosis [adjusted hazard ratios (aHRs): 2.38–3.594]. However, several reports from matched-cohort studies in North America, Canada (22), and Korea (23) demonstrated no significant difference in overall mortality between cirrhotic patients with and those without SARS-CoV-2 infection.
Furthermore, among patients with CLD, NAFLD or the recent nomenclature, Metabolic Associated Fatty Liver Disease (MAFLD) (24), is a rising problem affecting more than one-third of people globally (25). There have been some studies evaluating the role of this particular liver disease in COVID-19 patients. Moctezuma-Velázquez et al. reported that NAFLD is the independent factor associated with the requirement of invasive mechanical ventilator and mortality in COVID-19 patients (26). Subsequently, the association between NAFLD or MAFLD and the increase COVID-19 severity has been confirmed by two meta-analyses (27, 28) (Figure 1). Hegyi et al. showed that different spectrum of the disease has a different impact on COVID-19 outcomes i.e., MAFLD significantly increased the severity of COVID-19 infection with an OR of 2.61 (95%CI: 1.75–3.91), whereas NAFLD showed an OR of 5.22 (95%CI: 1.94–14.03) (27). Similarly, Pan et al. concluded that MAFLD increased the severity of COVID-19 with a pooled OR of 2.93 (CI: 1.87–4.60) (28). In addition, MAFLD patients with high interleukin-6 levels had a higher risk of severe COVID-19 (adjusted OR 1.14, 95%CI 1.05–1.23; P = 0.002) (29).
LT Recipients
The presentation of LT recipients with SARS-CoV-2 infection is distinct to that of cirrhotic patients, with fewer detrimental effects observed in LT recipients. Of note, LT recipients often share common demographic characteristics and comorbidities associated with known risk factors for unfavorable outcomes in SARS-CoV-2-infected patients. For instance, LT recipients are more likely to have renal impairment and diabetes compared to the general population. Hence, risk adjustments incorporating these factors are crucial when determining whether LT itself is a risk factor for higher mortality in patients with SARS-CoV-2 infection (19). The overall mortality of SARS-CoV-2 infection in LT recipients is approximately 20% (range: 8–24% in reports which included at least 10 LT recipients) (19, 30) (Figure 1). Despite this high percentage, LT itself is not associated with a higher mortality rate in patients with SARS-CoV-2 infection after risk adjustment (19). In SARS-CoV-2-infected LT recipients that are symptomatic, respiratory symptoms are the main presenting symptom; the difference in clinical manifestation between LT recipients and non-LT recipients is that gastrointestinal symptoms are more common and are observed in up to 42% of LT recipients (19, 30).
LT recipients are typically on immunosuppressive therapy, which may impact the course of disease in the case of SARS-CoV-2 infection. Patients on immunosuppressive agents may be more vulnerable to severe infection, and the management of immunosuppressive agents during the treatment of SARS-CoV-2 infection may be problematic. A recent systematic review and meta-analysis of 509 LT recipients with SARS-CoV-2 infection reported that the use of immunosuppressants was significantly more common in non-severe cases and in patients who survived from infection, with pooled ORs of 10.7 (95% CI: 3.11–36.94) and 16.0 (95% CI: 11.48–22.30), respectively (31). The results demonstrated that continuation of immunosuppressive agents was not harmful and may be associated with more favorable outcomes following SARS-CoV-2 infection. Therefore, withdrawal of immunosuppressants in LT recipients with COVID-19 should be discouraged. Nonetheless, distinct outcomes of different immunosuppressive therapies have been reported. A nationwide study of 111 LT recipients in Spain demonstrated that baseline immunosuppression containing mycophenolate was an independent predictor of a severe disease course, especially at doses >1,000 mg/day (32). In contrast, a multicenter study from Europe reported that tacrolimus use was independently associated with a lower mortality (33). However, these results were derived from observational studies, which precluded definitive recommendations regarding types of immunosuppressive therapy for the management of LT recipients with SARS-CoV-2 infection. Notably, although immunosuppression may not be associated with more severe disease or higher case mortality rates among patients who have already been infected with SARS-CoV-2, immunosuppression may affect immunogenicity following COVID-19 vaccination, which will be discussed later in this review.
Patients With Hepatocellular Carcinoma
The direct impact of SARS-CoV-2 infection in patients with hepatocellular carcinoma (HCC) with regard to disease severity and mortality are not well established. To date, there have only been two small reports of patients with HCC who were infected with SARS-CoV-2, comprising 21 and 22 patients with HCC, respectively. The mortality rates in these reports varied from 19 to 40% (9, 34). The second report, which originally included 867 patients with CLD of any severity, demonstrated that among patients with CLD, decompensated cirrhosis and presence of HCC were independent predictors of mortality due to SARS-CoV-2 infection, with adjusted HRs of 2.41 (1.34–4.32), and 3.96 (1.74–8.98), respectively (9).
In different circumstances, the SARS-CoV-2 pandemic has indirectly impacted the HCC management cascade. Several studies have compared the management of HCC between 2020 and the same period in 2019 and reported a significant reduction in HCC surveillance completion rates and the number of new HCC cases (35, 36). In patients already diagnosed with HCC, delayed treatment rates were significantly higher and the overall response rate based on post-treatment imaging was lower in 2020 than in 2019 (34, 37).
Immunogenicity of COVID-19 Vaccines
Vaccination is an effective measure to resolve a pandemic (38). Although different vaccine platforms induce distinct targets of host immunity (39–41), most vaccines induce adaptive immune responses. Vaccines induce B cells and T cells to respond specifically to a target pathogen upon re-exposure (42). These specific immune responses can be enhanced using the same or different vaccines (43). Booster vaccines operate on the selection and expansion of memory cells to increase the quantity and specificity of the immune responses against the pathogen (44, 45).
Immune Responses to COVID-19 Vaccines
With regard to COVID-19 vaccines, the entire virus or components of the virus are used to design and construct the vaccines (46, 47). Immunodominant antigens are present on spike proteins of SARS-CoV-2, particularly on the receptor binding domain (RBD) (48, 49). The binding domain plays a crucial role in viral invasion by interaction with the angiotensin-converting enzyme 2 receptor on host cells (50). Therefore, most of the advanced vaccine platforms such as mRNA, viral vectors, and protein subunits employ this antigen for vaccine construction (41, 47, 51). After being primed and boosted by vaccines, B cells differentiate into plasma cells and secrete specific antibodies, predominantly comprising immunoglobulin G (IgG) (49). RBD-specific IgG can neutralize the virus and prevent it from attaching to host cells (52).
Antibody responses are used as the main laboratory parameter for measuring immune responses pre- and post-vaccination (53, 54). High antibody titers are used as a proxy of vaccine effectiveness (55, 56). However, neutralizing antibodies (NAs) are a more accurate measure of the effectiveness of antibodies against novel variants (57–59). Indeed, NA levels are an immune correlate of protection used to predict vaccine efficacy without conducting efficacy trials (56, 60). There are no definite thresholds for NA which can assess the clinical protection. Relative to other vaccine platforms, mRNA vaccines have been demonstrated to provide excellent antibody responses (55). Additionally, viral vector vaccines provide good antibody responses and T cell responses after one or two vaccine doses (40, 44, 61). Inactivated SARS-CoV-2 vaccines, which are a classic platform, have been reported to provide minimal antibody responses (39, 62) but can reduce disease severity (63). Different essays have been used in clinical practice to measure level and functions of the antibodies. Chemiluminescence immunoassay (CLIA) is applied to quantify the antigen specific immunoglobulins (64) while virus neutralization test (VNT) can analyze the neutralizing activity using the live virus or the surrogates (65, 66).
Immunogenicity of COVID-19 Vaccines Among Patients With CLD and LT Recipients
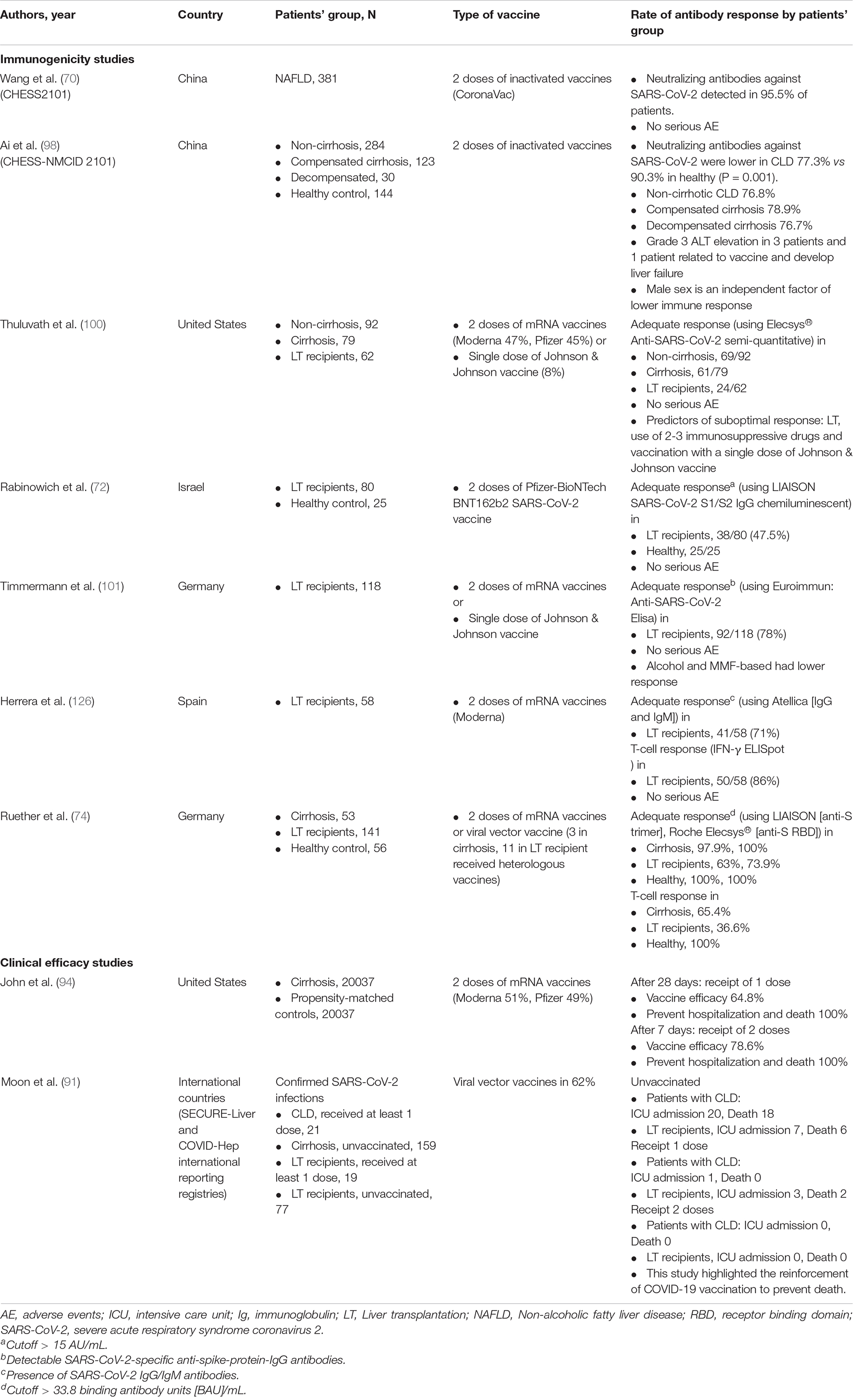
CLD and cirrhotic patients typically present with impairments in immunological status (67) (Figure 2). Despite reports of the immunocompromised status of patients with chronic hepatitis B (CHB) (68), a recent study in Wuhan demonstrated that inactivated SARS-CoV-2 vaccines were safe and induced comparable immunogenicity in patients with CHB when compared to existing clinical trial data (69). Inactivated SARS-CoV-2 vaccines have also been reported to induce NAs in a large population of patients with NAFLD (70). Preliminary data have suggested that LT recipients are less likely to reach seroconversion after SARS-CoV-2 vaccination (71, 72) and antibody responses are reduced in LT patients after mRNA vaccination (72). However, the reactogenicity in LT patients is comparable with current safety data in the general population (73). To date, limited data are available on vaccines immunogenicity in patients with cirrhosis. A German study reported that seroconversion was achieved in 63% of LT recipients and 100% of cirrhotic patients and controls based on an anti-S trimer assay. However, no significant difference was observed in antibody responses between cirrhotic patients and healthy controls (74). With regard to T cell immunity, antigen-specific T cell responses against the SARS-CoV-2 spike protein were lower in patients with LT (36.6%) and cirrhosis (65.4%) than in controls (74). To date, there has been a paucity of immunogenicity studies, and immunological factors underlying inadequate vaccination responses in these populations have not been comprehensively explored. Relevant studies will be discussed in more detail in the sections on humoral-mediated immunity (HMI) and cellular-mediated immunity (CMI) in patients with CLD and LT recipients (Table 1).
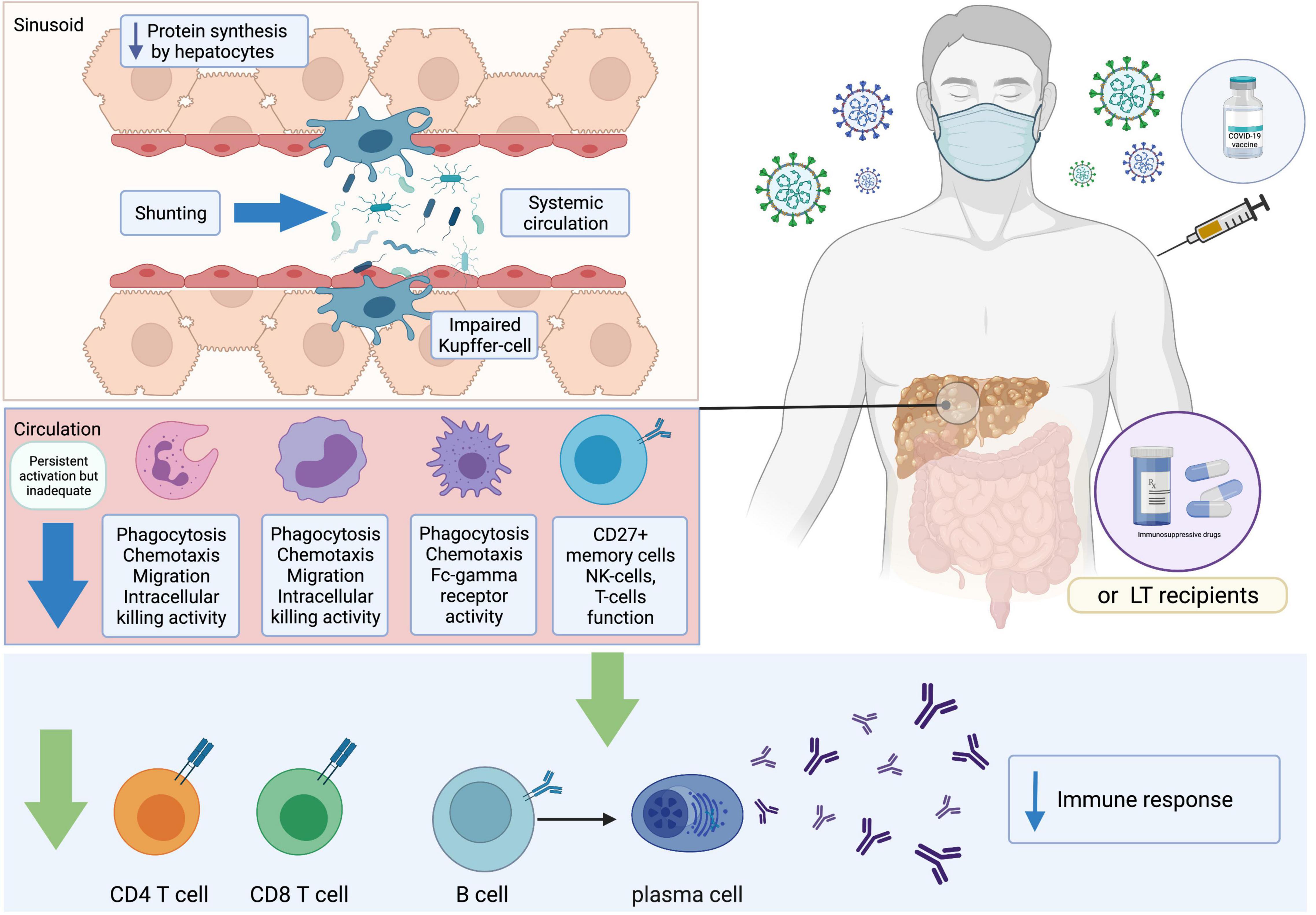
Figure 2. Immune responses after receiving COVID-19 vaccines. COVID-19, coronavirus disease 2019; LT, liver transplant.
Phase III Clinical Trials of COVID-19 Vaccine Subgroups in Patients With CLD and LT Recipients
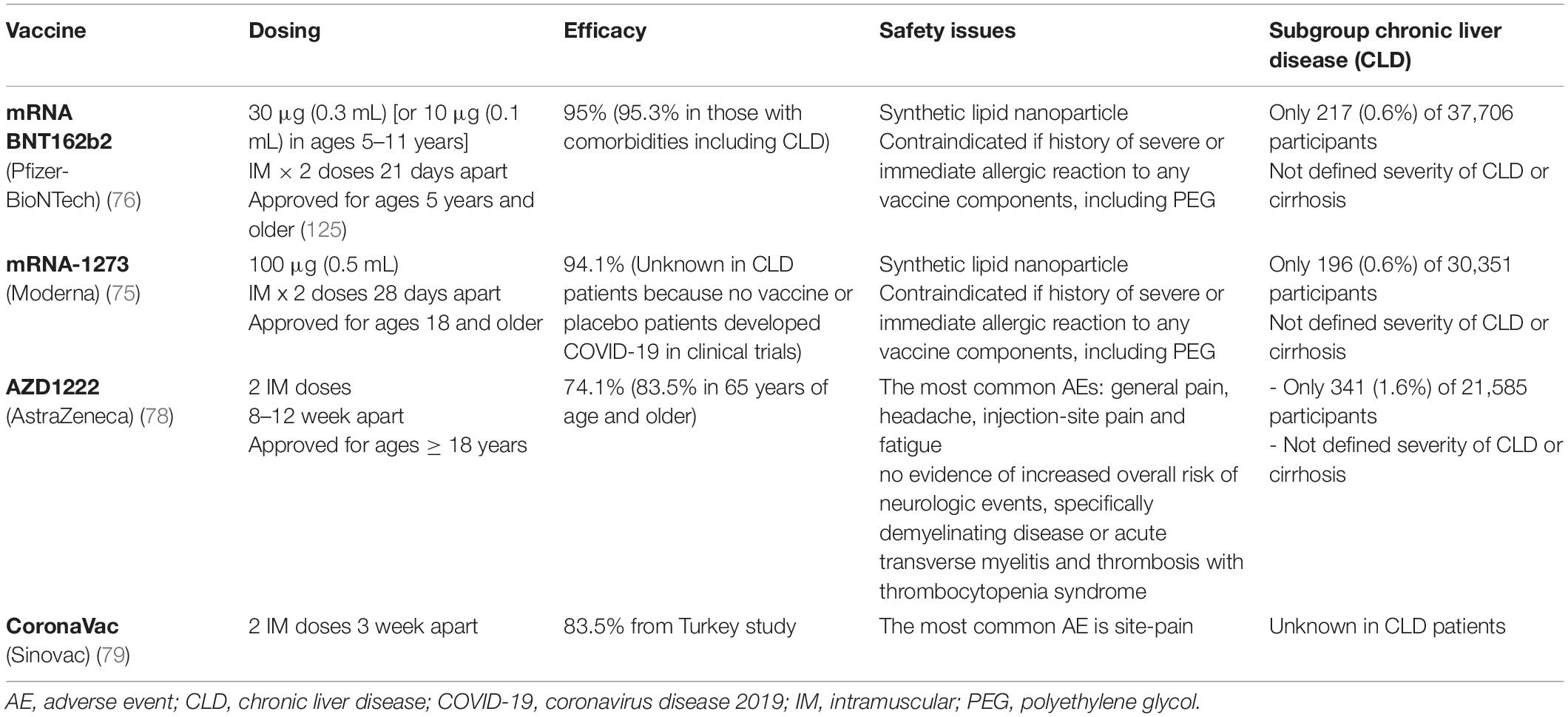
Patients with CLD or cirrhosis and LT recipients are strongly recommended to receive vaccinations against SARS-CoV-2 to prevent severe COVID-19 and unfavorable consequences. Several SARS-CoV-2 vaccine platforms have been utilized among these populations. However, the immunogenicity, efficacy, and safety data are variable and inconsistent due to a limited number of CLD, cirrhosis, and LT patients included in phase III studies (Table 2). Only 217 (0.6%) of 37,706 participants and 196 (0.6%) of 30,351 participants received the Pfizer and Moderna vaccines, respectively (75, 76). Nevertheless, trials of the Oxford/AstraZeneca vaccine excluded patients with liver disease (except Gilbert disease) in an initial study (77), with only 1.6% of patients with liver diseases enrolled in a subsequent study (78). In addition, there is a lack of clinical data on the CoronaVac vaccine among patients with liver diseases (79). Collectively, this highlights the scarcity of data on vaccine efficacy and immune responses in cirrhotic patients who have received SARS-CoV-2 vaccines.
A suboptimal immune response is of concern, because suboptimal immunogenicity following vaccination against other infections in such patients is well-established and has been reported in the literature. Crucially, LT recipients who must be maintained on immunosuppressants may still be vulnerable to infection even after vaccination (80, 81). Furthermore, vaccine efficacy and safety issues still remain insufficiently defined. Only a small proportion of patients with underlying liver disease, especially those with CLD, were recruited for the BNT162b2 (0.6%), mRNA-1273 (0.6%), ChAdOx1-nCoV-19 (1.6%), and Ad26.COV2-S studies (3 out of 12 SOT recipients) (75–77, 82) (Table 2). Moreover, LT recipients considered to be immunocompromised were excluded from these studies. Therefore, a degree of hesitancy to receive vaccines among LT recipients is inevitable owing to their complex medical conditions and multiple immunosuppressants. However, COVID-19 vaccinations are strongly recommended for LT recipients based on the relative benefits compared to the risks. A survey in Italy revealed that most LT recipients accepted COVID-19 vaccines, with only a few (6%) reporting hesitancy and favorably deferring vaccination owing to concerns of unknown adverse reactions from the vaccine (83).
mRNA-Based Vaccines
The effects of mRNA-based vaccines have been more widely evaluated in solid organ transplant (SOT) recipients. Two currently available mRNA vaccines that are widely utilized include the mRNA-1273 (Moderna) vaccine and BNT162b2 (BioNTech and Pfizer) vaccine.
An immunogenicity study using two doses of the BNT162b2 mRNA vaccine in LT recipients reported significantly weaker immune responses (72%) than that in age-matched immunocompetent individuals (94%). The geometric mean of RBD IgG and NA titers were also significantly lower among LT recipients. A combination of immunosuppressive therapy and impaired renal function were identified as risk factors for poor immune responses. Although approximately half of the participants experienced some adverse events (AEs), all AEs were mild and were more frequently observed in male participants (84). A prospective study in SOT (including LT) recipients who received the mRNA-1273 vaccine revealed discordance between HMI and CMI. CMI was elicited in approximately one-third of the patients, but no NAs were detected. CMI responses were decreased in patients who underwent kidney transplantation or those maintained on tacrolimus and prednisone regimens and more immunosuppressive drugs (85). Several predictors of poor HMI responses in LT recipients that received two doses of mRNA vaccines were reported among recipients with high tacrolimus levels, those receiving more than two immunosuppressive agents, and those with obesity. However, no significant associations were observed with recipients’ age and time from LT (86). Moderate HMI and CMI responses of the two-dose mRNA-1273 vaccine in SOT (including LT) recipients have been reported. Positive anti-RBD antibodies were detected in only one-third of the patients, and only a subset of patients produced robust NAs and CD4+ T cell responses (87).
Adenovirus Vector-Based Vaccines
Currently available adenovirus vector-based vaccines for COVID-19 include the ChAdOx1-nCoV-19 vaccine (AstraZeneca and University of Oxford), Ad26.COV2-S vaccine (Johnson & Johnson), and Sputnik V (Gamaleya Research Institute). Immunogenicity and safety data of adenovirus vector-based vaccines in SOT recipients are limited. A small case series of 12 SOT recipients who underwent Ad26.COV2-S vaccination reported a significantly lower rate of detectable anti-RBD antibodies (17%) compared to 59% obtained with the mRNA vaccine series, which is in accordance with the trends of titer magnitude (88). Nonetheless, there has limited data in patients with CLD, only 1.6% of such patients in phase III clinical trial.
Inactivated Vaccines
Although inactivated SARS-CoV-2 vaccines are considered safe for patients with CLD and LT recipients, results from phase III trials remain to be confirmed. Immunogenicity, efficacy, and safety data focusing on CLD and LT patients are thus limited. Among the different vaccine platforms, inactivated vaccines may offer long-term safety but with potential suboptimal immune responses. Studies on BBIBP-CoV (Sinopharm) in LT recipients are scarce. There had a cross-sectional study reported the seropositivity in kidney transplant recipients only 43% after receiving the second dose of Sinopharm vaccine (89). A prospective study that included 10 liver and 38 kidney transplant recipients receiving CoronaVac (Sinovac) revealed a significantly lower seropositivity rate compared to that in healthy individuals; moreover, antibody levels were significantly higher in patients that received BioNTech than in those that received CoronaVac (90).
Protein Subunit Vaccines
Data regarding immunogenicity after vaccination with protein subunit vaccines are limited, and further investigations are warranted.
Clinical Efficacy of COVID-19 Vaccines
Unvaccinated CLD and LT patients are anticipated to experience greater severity and mortality due to severe COVID-19. Vaccination is an essential tool that may alleviate these events. The efficacies of mRNA vaccines and Ad26.COV2.S were reported to be greater than 95 and 66%, respectively, in phase III studies (75, 76, 82); however, data on vaccine efficacy and clinical outcomes in CLD patients and LT recipients are limited due to the small number of participants in the studies. A recent efficacy study extracted data from the SECURE-Liver and COVID-Hep international database of 21 CLD patients that predominantly had cirrhosis and 19 LT recipients (91). Of patients with CLD, one-third were admitted to hospital, and only 5% were admitted to the intensive care unit without any fatalities. Among LT recipients, approximately the same proportion of patients underwent hospital admission; however, 16% required mechanical ventilation support, and 11% succumbed to death. All patients with severe disease received only a single vaccine dose 1–2 weeks prior. Based on these data, COVID-19 vaccination of patients with CLD and LT recipients should be encouraged to avoid unfavorable consequences (91). A single transplant retrospective study of 557 SOT (105 LT) recipients in the United States revealed that breakthrough infection after 2 weeks of full vaccination with either BNT162b2, mRNA-1273 or Ad26.COV2.S vaccines occurred in only 3 of 459 (0.65%) fully vaccinated SOT recipients. All cases were mild infections without mortality (92). A national registry in England comparing unvaccinated SOT recipients and those vaccinated with either ChAdOx1-S or BNT162b2 vaccines revealed that vaccination was not associated with a reduction in infection risk. However, vaccines offered a 20% reduction in the risk of death within 28 days after infection. Subgroup analysis revealed that both ChAdOx1-S and BNT162b2, provided protection against death with a statistically significant risk reduction was observed only in those who received ChAdOx1-S. Therefore, vaccination of SOT recipients confers some protection against SARS-CoV-2-related mortality (93). However, the efficacy of the vaccine likely depends on concurrently circulating variants of the strain in the community, and the outcomes are also subject to access to healthcare and respiratory support.
Recently, the large propensity-match controls with patients with cirrhosis study indicated that COVID-19 vaccine could prevent the hospitalization as high as 100% after obtained mRNA vaccine at least 1 dose (94). In addition, completion of 2 doses could reduce the SARS-CoV-2 infection from 64.8% (receipt of 1 dose) to 78.6% (94) (Table 1). Nevertheless, breakthrough COVID-19 infection can occur despite vaccination but not fatal. John et al. (95) reported COVID-19 vaccination can reduce the mortality of COVID-19 infection post-vaccination by approximately 80% in patients received either partial or full vaccination. Of interest, this benefit was generalized in any types of mRNA vaccine and cirrhosis severity. This study highlighted the value of COVID-19 vaccination despite the breakthrough infection can develop.
Current Recommendations of Liver Societies
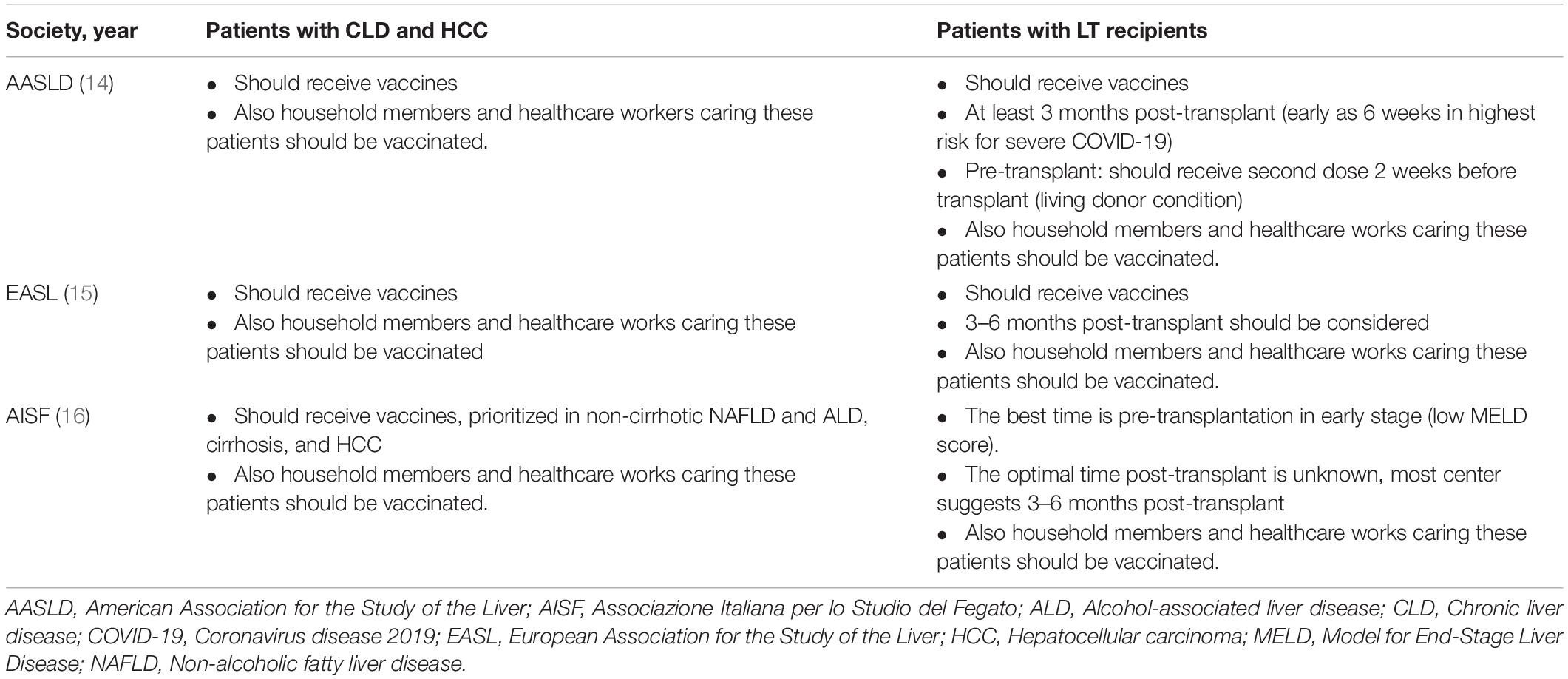
Current recommendations of renowned international liver societies indicate that patients with CLD and LT recipients should receive COVID-19 vaccines (14–16) (Table 3).
Patients With CLD and HCC
All patients with CLD and HCC are advised to receive COVID-19 vaccines. Patients should not discontinue their medications for underlying diseases including immunosuppressive agents in patients with autoimmune hepatitis (AIH). Interruption of immunosuppressants may be harmful, such as the occurrence of hepatitis flares that eventually lead to liver failure in patients with AIH. In patients with HCC who may undergo resection, locoregional treatments (such as radiofrequency ablation, microwave ablation, and transarterial chemoembolization) and systemic therapy should not be delayed due to vaccination. In this regard, vaccinations should be administered as early as possible.
LT Recipients and Living Donors
LT recipients and living donors should also receive COVID-19 vaccines. During the pre-transplant period, patients on waiting lists for transplantation and living donors should be prioritized for vaccination (14, 16), and vaccinations should be completed within 2 weeks prior to LT (14). If vaccinations are not completed before LT, the second dose can be postponed to 6 weeks post-transplantation (14). For LT recipients who have not received the COVID-19 vaccine, the optimal timing is 3 months post-LT, as this is the timepoint of the lowest dose of immunosuppressive drugs (96). Patients that are prone to SARS-CoV-2 infection, especially patients at risk for severe COVID-19 infection, may be vaccinated at 6 weeks post-transplantation (14); however, the immune response to COVID-19 vaccines may be suboptimal.
The dose of immunosuppressants should not be reduced or discontinued during vaccination, as this may increase the risk of rejection. LT recipients with abnormal liver biochemistry tests should be assessed for acute graft rejection (14). Of note, if patients have respiratory tract symptoms despite receiving vaccines, further evaluation of SARS-CoV-2 infection is warranted. Vaccinations may be postponed in patients with an unstable medical condition or those receiving treatment with high dose immunosuppressants or corticosteroids owing to acute cellular rejection post-transplantation. Additionally, all household contacts and healthcare workers should be vaccinated against SARS-CoV-2 infection and other transmissible diseases to prevent transmission to patients (14–16, 96). Critically, other behavioral measures such as social distancing, wearing masks, and washing hands are necessary in addition to vaccinations.
Novel Perspectives of COVID-19 Vaccines in Patients With CLD and LT Recipients
Studies on HMI and CMI in Patients With CLD and LT Recipients
A study of non-cirrhotic patients with NAFLD [median age: 39 years, interquartile range (IQR): 33–48 years] who obtained two doses of inactivated vaccines reported that NA positivity in the serum of patients was 95.5% compared to 97.6% in the general population (97). Moreover, no serious AEs were observed, and only mild and self-limited symptoms were reported, such as injection site pain (18.4%), muscle pain (5.5%), and headache (5.2%). The CHESS-NMCID 2101 study by the same group, receipt of two doses of inactivated vaccines predominantly comprising CHB patients (87.8%), reported that NAs were detected in the same proportion of patients with CLD (76.8% in non-cirrhosis, 78.9% in compensated cirrhosis, and 76.7% in decompensated cirrhosis) (98) in contrast to the higher levels of NAs (90.3%) in healthy controls. This study highlighted the lower immune response in patients with CLD compared to that in the healthy population. Similarly, the most common side effect was pain at the injection site (8.2%). In addition, male sex was an independent predictive factor of negative serological responses to the vaccine [OR: 1.86 (1.12–3.90)]. The mechanisms underlying this effect are not fully understood but may be partly due to androgen-modulating genes associated with low antibody production (99).
Three other prospective studies confirmed the poor antibody responses in patients with cirrhosis and even greater hyporesponsiveness in LT recipients (72, 100, 101). A study by Thuluvath et al. (100) reported that 22.8% of patients with cirrhosis and 61.3% of LT recipients exhibited poor antibody responses after receiving two doses of mRNA vaccines or a single dose of the Johnson & Johnson vaccine. Similarly, a study in Israel reported lower immune responses in patients with LT, whereby only 47.5% of patients had a positive serological response and patients had a low antibody titer (95.4 vs. 200.5 AU/mL in LT recipients and healthy control, respectively) (72). The risk factors for negative serology were older age, renal failure, receiving high-dose steroids in the last 12 months, use of mycophenolate mofetil, and use of triple immunosuppressants. A summary of the risk factors for poor vaccine responses are presented in Figure 3. A study by Timmermann et al. (101) reported lower immune responses among LT recipients. A recent study conducted in Germany reported that patients with cirrhosis had adequate antibody responses when compared to healthy individuals with 100% positivity (74). However, the T cell response was lower in cirrhotic patients compared with that in the healthy population (65% vs. 100%). Of note, LT recipients had low or absent vaccine responses in terms of both antibody and cellular responses. A summary of relevant studies is presented in Table 1.
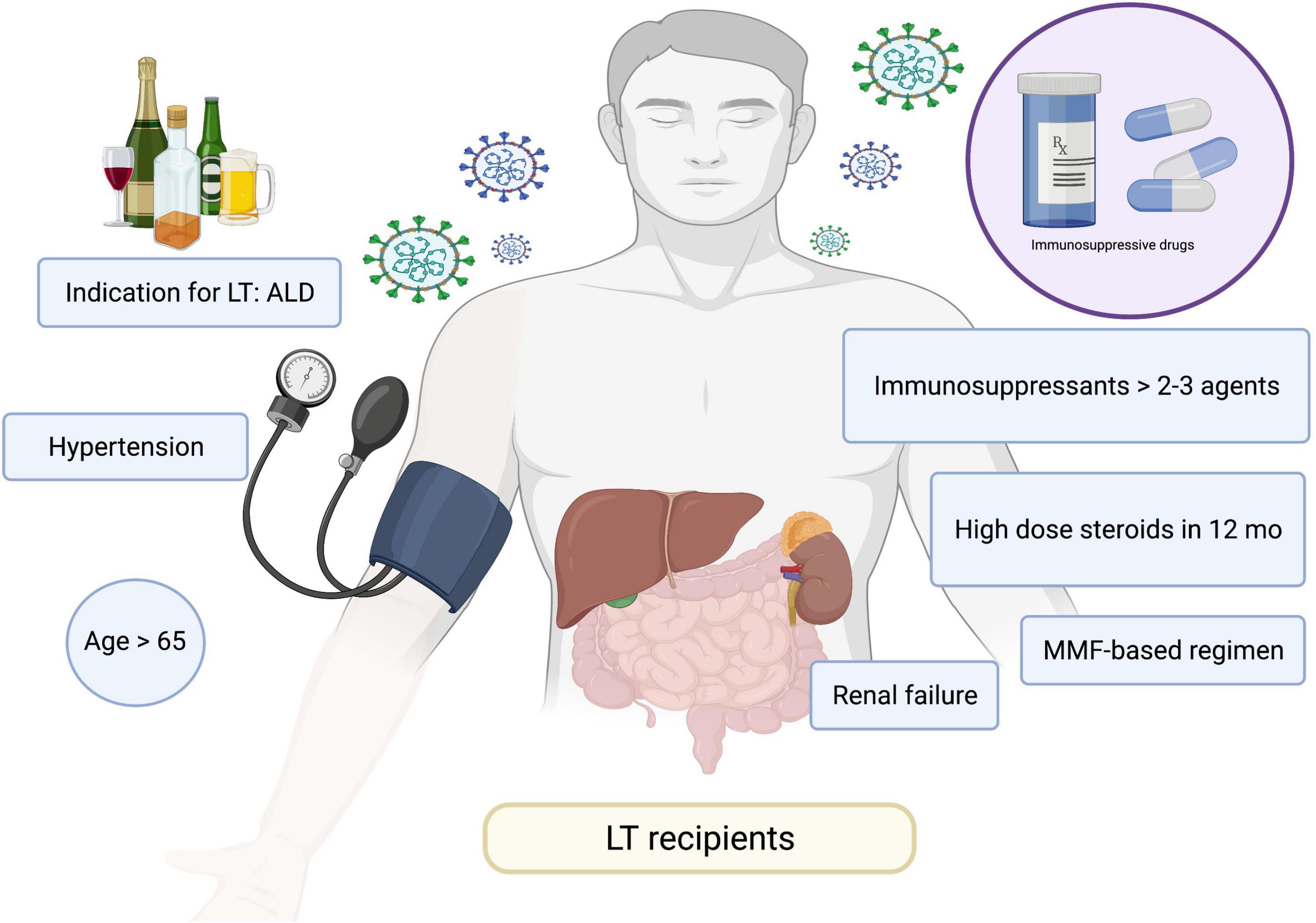
Figure 3. Risk factors for poor responses to COVID-19 vaccines. ALD, alcohol-associated liver disease; COVID-19, coronavirus disease 2019; MMF, mycophenolate mofetil; LT, liver transplant.
Booster Vaccines in Patients With CLD and LT Recipients
Immunity derived from COVID-19 vaccines decreases in parallel with the time elapsed after vaccination. Accordingly, the Centers for Disease Control and Prevention have suggested that individuals receive a booster shot at least 5 months after receiving two doses of mRNA vaccines or at least 2 months after receiving the Johnson & Johnson vaccine (102). Nevertheless, there have been limited studies in patients with CLD and LT recipients. A proposed scheme of COVID-19 vaccination in patients with CLD and LFT recipients is presented in Figure 4.
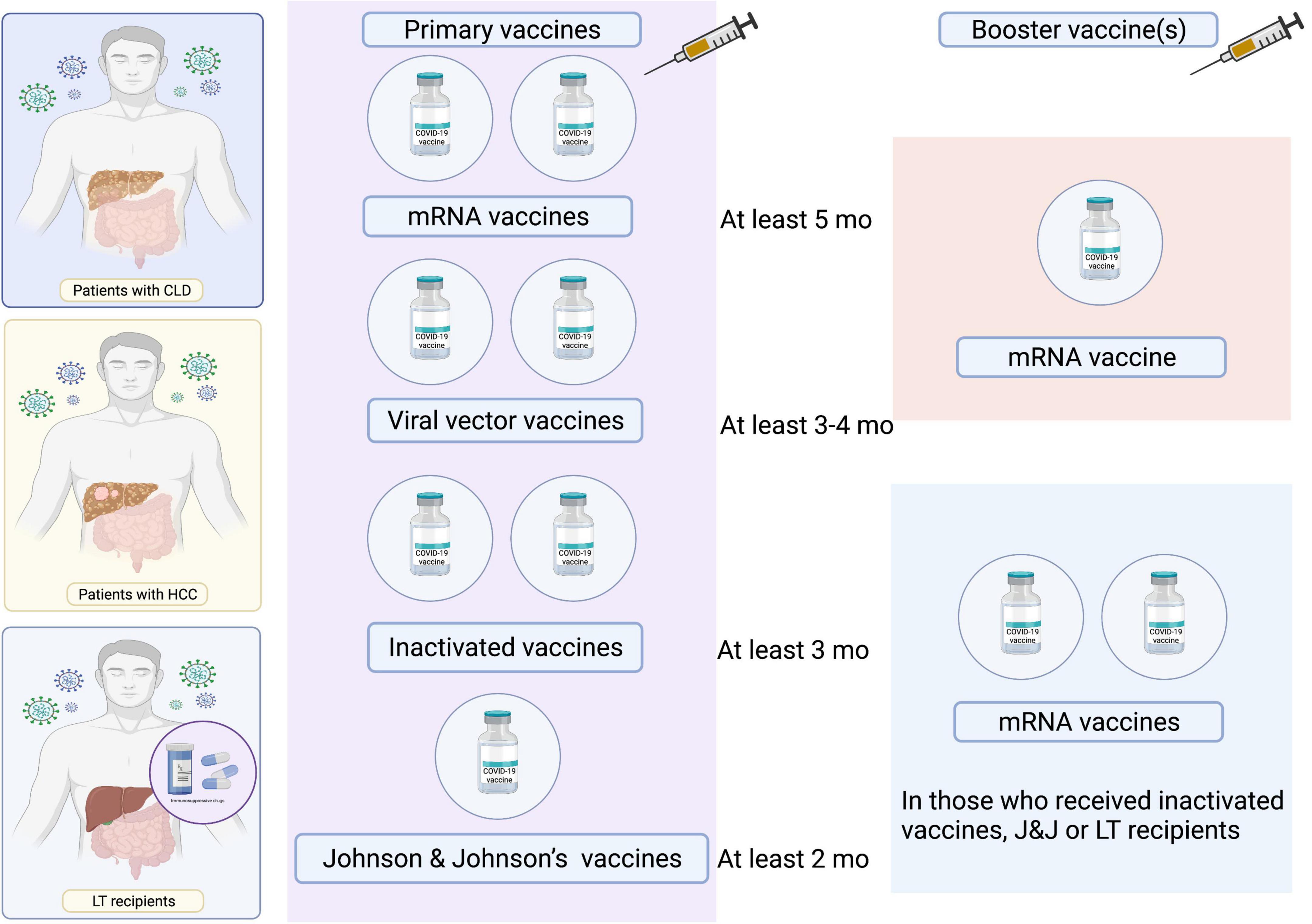
Figure 4. Proposed scheme of COVID-19 vaccination in patients with CLD and LT recipients. LT, liver transplant.
Third Dose of mRNA-Based Vaccines
Based on several studies that have reported weak immune responses after two standard doses of mRNA-based vaccines in SOT recipients maintained on immunosuppressants, the WHO has recommended the administration of an additional dose of COVID-19 vaccines for a primary series of immunocompromised patients, including SOT recipients (103). This was supported by a study investigating immunogenicity after an additional dose of the BNT162b2 vaccine, which was offered 2 months after complete vaccination in 101 types of SOT (including the liver) recipients. The prevalence of anti–SARS-CoV-2 antibodies was increased from 40% to 68% after the third dose. Approximately 44% of seronegative patients were seroconverted after the third dose. Patients who remained seronegative were more likely to be older, maintained on a higher dose of immunosuppression, and have poor renal function. No serious AEs were reported, although allograft rejection was observed (104). Another study randomly assigned SOT recipients (including LT recipients who received two doses of the mRNA-1273 vaccine 2 months prior) to receive either a third dose of the mRNA-1273 vaccine or a placebo. This study revealed substantially higher anti-RBD antibody level responses and rates of neutralization inhibition and CMI, with minimal side effects (105).
Fourth Dose of mRNA-Based Vaccines
A recent case series of the fourth dose of mRNA-based vaccines in 37 SOT recipients, 4 of whom were LT recipients, has been reported. Participants with a weak response (14%) and no response (84%) after three doses of the BNT162b2 vaccine were vaccinated with a fourth dose and appeared to exhibit a slightly increased HMI response; however, these effects were only observed in participants with previously developed weak immune responses. Nevertheless, no improvements were noted among participants that lacked an initial response. Additionally, the NA titers and CMI responses of both groups were low (106). Another study examined antibody responses to the fourth dose of the SARS-CoV-2 vaccine in 18 SOT recipients and reported similar results (107); in this study, 50% of participants were negative and all participants with low-positive titers were included. These findings suggest that immunogenic potential exists in poor responders, and further interventions such as monoclonal antibody administration post-exposure or temporary reduction of immunosuppressants after each vaccination may be warranted. In addition, the balance between allograft rejection and immunity protection should be taken into consideration.
Additional doses, including the third or fourth dose of the two vaccines in LT recipients, may improve vaccine immunogenicity. However, a significant proportion of patients may remain vulnerable to COVID-19. Therefore, compliance with hygiene measures and avoidance of disease exposure should be maintained. Furthermore, due to the relatively short follow-up period in previous studies, the occurrence of allograft rejection and other indirect AEs will need to be monitored in more long-term studies.
Variants of Concern
Delta Variant (B.1.617.2)
The effectiveness of vaccines against the SARS-CoV-2 Delta variant has been examined for the mRNA-based vaccine (BNT162b2) produced by Pfizer Inc. and BioNTech SE and a replication-deficient simian adenovirus vector ChAdOx1 nCoV-19 (Vaxzevria) from Oxford University and AstraZeneca. The results revealed modest differences in effectiveness with the Delta variant as compared with the Alpha variant. The effectiveness of two doses of the BNT162b2 vaccine was 93.7% (95% CI: 91.6–95.3) while that of the ChAdOx1 nCoV-19 vaccine was 74.5% (95% CI: 68.4–79.4) (60). In addition, the estimated neutralization capacity of the Pfizer BioNTech vaccine against the Delta variant was 5.8-fold lower (108). The neutralizing activity against the Delta variant induced by CoronaVac (Sinovac) vaccination was lower than that due to natural infection (58).
Omicron Variant (B.1.1.529)
Recent data indicate that levels of NAs against the novel Omicron variant are lower, especially in viral vector-vaccinated individuals (65). Several reports have demonstrated decreased immunity against SARS-CoV-2 in long-term cohorts after vaccination (109–111). Thus, the existing immunity induced by conventional vaccination may be insufficient to protect against emerging SARS-CoV-2 variants of concern (65, 110, 112, 113).
Safety of COVID-19 Vaccine for CLD and LT Recipients
The AEs post-vaccination usually were mild, self-limited and non-fatal including the local AEs such as pain at injection site and systemic events such as fever, fatigue, flu-like symptoms and myalgia (75–78). There have been case report and case series of acute liver injury after mRNA vaccination (114–117). Shroff et al. (114) reported 10 of 16 patients required hospitalization and therapy with good recovery. The authors concluded that some patients may experience liver injury post-vaccination from autoimmune triggering particular in who had pre-existing AIH (6 out of 16 patients). Additionally, two cases of AIH had been reported after receiving viral vector vaccines (118). None of the patients developed liver failure. At present, there has been only one case report of acute hepatic artery thrombosis from vaccine-induced prothrombotic immune thrombocytopenia that presented with acute liver failure (119). However, this is a very rare adverse event following by adenovirus vector ChAdOx1 nCoV-19 vaccination which usually occurred in younger woman. In summary, the benefit of vaccination outweighs the risk of the rare incident severe AEs.
Future Directions and Unmet Needs
There are scarce data regarding vaccine effectiveness and immune responses including humoral and cellular immunity following COVID-19 vaccination in CLD patients and LT recipients. Challenging issues that warrant further evaluation include the effective durability of vaccine protection against SARS-CoV-2 infection, need for booster vaccines (such as fourth doses particularly in LT recipients), requirement for serologic testing to guide the need for booster vaccines, and effectiveness and safety of heterologous vaccines. Recently, variants of concern, including Omicron, have emerged as pertinent challenges. Future research is needed to verify the immunity conferred by currently available vaccines in patients with CLD and LT recipients.
Furthermore, as patients with CLD and LT recipients, considered one of the immunosuppressed populations, are at risk for developing COVID-19, the role of pre-exposure prophylaxis with monoclonal-antibody such as tixagevimab and cilgavimab in which the interim analysis showed promising results should be further investigated (120). In addition, early administration of the monoclonal antibody e.g., sotrovimab (121) and antiviral agents such as molnupiravir (122), nirmetralvir (123) may benefit in terms of prevent progression to severe COVID-19 in patients with CLD and LT recipients, nonetheless, the data in these specific groups of patients are still warrant. It is also important to note that the use of particular agents should be aware in these patients’ population e.g., remdesivir (124) should not be prescribed in those with AST/ALT above 5 times upper limit of normal, and nirmatrelvir is contraindicated in Child C cirrhosis patients (123).
Conclusion
Patients with CLD and LT recipients constitute immunocompromised populations owing to CAID or administration of immunosuppressants. These patient populations are thus more prone to developing severe SARS-CoV-2 infection with high mortality rates when compared with the healthy population. A key preventative measure is COVID-19 vaccination, and several platforms are currently available. Several international liver societies have recommended that these patients receive vaccination owing to the greater benefits over the potential risks, despite limited studies to date. Studies have suggested that these patients are hyporesponsive to COVID-19 vaccines, and booster shots are required. In conclusion, large knowledge gaps remain regarding the effects of vaccination in patients with CLD and LT recipients, and future research is warranted. In addition to vaccination, cautionary measures such as social distancing, washing hands, and wearing masks should be practiced as part of standard lifestyle behaviors.
Author Contributions
AK contributed substantially to the conceptualization, drafting, and critical revision of the manuscript. All authors made a substantial contribution to the literature research and drafting of the manuscript and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AASLD, American Association for the Study of Liver Diseases; ACE2, Angiotensin I Converting Enzyme 2; Ad26.COV2-S, vaccine (Johnson & Johnson); AEs, adverse events; AIH, autoimmune hepatitis; ALD, alcohol-associated liver disease; ALT, alanine aminotransferase; APASL, Asian-Pacific Association for the Study of the Liver; BNT162b2, Pfizer-BioNTech mRNA vaccine; CAID, Cirrhosis-associated immune dysfunction; CHB, chronic hepatitis B; ChAdOx1-nCoV-19, vaccine (AstraZeneca and University of Oxford); CI, Confidence interval; CLD, chronic liver disease; CMI, cellular mediated immunity; COVID-19, coronavirus disease 2019; EASL, European Association for the Study of the Liver; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HMI, humoral mediated immunity; HR, hazard ratios; IgG, immunoglobulin G; IM, intramuscular; IQR, Interquartile range; LT, Liver transplantation; MAFLD, Metabolic Associated Fatty Liver Disease; MELD, Model for End-Stage Liver Disease; MMF, mycophenolate mofetil; mRNA-1273, Moderna mRNA vaccine; NA, neutralizing antibody; NAFLD, Non-alcoholic fatty liver disease; OR, odd ratios; PEG, polyethylene glycol; RBD, receptor binding domain; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant Sputnik V (Gamaleya Research Institute); WHO, World health organization.
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506.
2. WHO. Weekly Epidemiological Update on COVID-19 - 21 December 2021. (2021). Available online at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—21-december-2021 (accessed January 4, 2022)
3. WHO. COVID-19 Vaccine Tracker and Landscape. (2022). Available online at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed January 4, 2022)
4. CDC. COVID Data Tracker. Atlanta, GA: Centers for Disease Control and Prevention (2020). Available online at: https://covid.cdc.gov/covid-data-tracker (accessed January 4, 2022).
5. Targher G, Mantovani A, Byrne CD, Wang X-B, Yan H-D, Sun Q-F, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. (2020) 69:1545–7. doi: 10.1136/gutjnl-2020-321611
6. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. (2020) 73:451–3. doi: 10.1016/j.jhep.2020.03.044
7. Younossi ZM, Stepanova M, Lam B, Cable R, Felix S, Jeffers T, et al. Independent predictors of mortality among patients with NAFLD hospitalized With COVID-19 Infection. Hepatol Commun. (2021) 2021:1802. doi: 10.1002/hep4.1802
8. Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int. (2020) 40:2515–21. doi: 10.1111/liv.14583
9. Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. (2021) 19:1469.e–79.e. doi: 10.1016/j.cgh.2020.09.027
10. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the united states: a multicenter research network study. Gastroenterology. (2020) 159:768.e–71.e. doi: 10.1053/j.gastro.2020.04.064
11. Sarin SK, Choudhury A, Lau GK, Zheng M-H, Ji D, Abd-Elsalam S, et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. (2020) 14:690–700. doi: 10.1007/s12072-020-10072-8
12. Ge J, Pletcher MJ, Lai JC. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a national COVID cohort collaborative study. Gastroenterology. (2021) 161:1487.e–501.e. doi: 10.1053/j.gastro.2021.07.010
13. Elhence A, Vaishnav M, Biswas S, Chauhan A, Anand A, Shalimar Coronavirus disease-2019 (COVID-19) and the liver. J Clin Translat Hepatol. (2021) 9:247–55. doi: 10.14218/JCTH.2021.00006
14. Fix OK, Blumberg EA, Chang K-M, Chu J, Chung RT, Goacher EK, et al. AASLD expert panel consensus statement: vaccines to prevent COVID-19 infection in patients with liver disease. Hepatology. (2021) 74:1049–64. doi: 10.1002/hep.31751
15. Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. (2021) 74:944–51. doi: 10.1016/j.jhep.2021.01.032
16. Russo FP, Piano S, Bruno R, Burra P, Puoti M, Masarone M, et al. Italian association for the study of the liver position statement on SARS-CoV2 vaccination. Dig Liver Dis. (2021) 53:677–81. doi: 10.1016/j.dld.2021.03.013
17. Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. (2021) 74:567–77. doi: 10.1016/j.jhep.2020.09.024
18. Middleton P, Hsu C, Lythgoe MP. Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ Open Gastroenterol. (2021) 8:e000739. doi: 10.1136/bmjgast-2021-000739
19. Marjot T, Webb GJ, Barritt AS, Moon AM, Stamataki Z, Wong VW, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. (2021) 18:348–64. doi: 10.1038/s41575-021-00426-4
20. Iavarone M, D’Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. (2020) 73:1063–71.
21. Ioannou GN, Liang PS, Locke E, Green P, Berry K, O’Hare AM, et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infection in US veterans: risk of infection, hospitalization, ventilation, and mortality. Hepatology. (2021) 74:322–35. doi: 10.1002/hep.31649
22. Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. (2021) 70:531–6. doi: 10.1136/gutjnl-2020-322118
23. Jeon D, Son M, Choi J. Impact of liver cirrhosis on the clinical outcomes of patients with COVID-19: a nationwide cohort study of Korea. Kor J Intern Med. (2021) 36:1092–101. doi: 10.3904/kjim.2020.486
24. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9.
25. Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, et al. Global prevalence and clinical characteristics of metabolic associated fatty liver disease. A meta-analysis and systematic review of 10,739,607 individuals. J Clin Endocrinol Metabol. (2022):dgac321. [Online ahead of print]. doi: 10.1210/clinem/dgac321
26. Moctezuma-Velázquez P, Miranda-Zazueta G, Ortiz-Brizuela E, Garay-Mora JA, González-Lara MF, Tamez-Torres KM, et al. NAFLD determined by Dallas Steatosis Index is associated with poor outcomes in COVID-19 pneumonia: a cohort study. Intern Emerg Med. (2022). [Online ahead of print]. doi: 10.1007/s11739-022-02933-x
27. Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, et al. Metabolic associated fatty liver disease is associated with an increased risk of severe COVID-19: a systematic review with meta-analysis. Front Med. (2021) 8:626425. doi: 10.3389/fmed.2021.626425
28. Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: a meta-analysis. Dig Liver Dis. (2021) 53:153–7. doi: 10.1016/j.dld.2020.09.007
29. Gao F, Zheng KI, Yan H-D, Sun Q-F, Pan K-H, Wang T-Y, et al. Association and interaction between serum interleukin-6 levels and metabolic dysfunction-associated fatty liver disease in patients with severe Coronavirus Disease 2019. Front Endocrinol. (2021) 12:604100. doi: 10.3389/fendo.2021.604100
30. Becchetti C, Gschwend SG, Dufour J-F, Banz V. COVID-19 in liver transplant recipients: a systematic review. J Clin Med. (2021) 10:4015.
31. Yadav DK, Adhikari VP, Ling Q, Liang T. Immunosuppressants in liver transplant recipients with coronavirus disease 2019: capability or catastrophe?—a systematic review and meta-analysis. Front Med. (2021) 8:756922. doi: 10.3389/fmed.2021.756922
32. Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. (2021) 74:148–55. doi: 10.1016/j.jhep.2020.07.040
33. Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology. (2021) 160:1151.e–63.e. doi: 10.1053/j.gastro.2020.11.045
34. Amaddeo G, Brustia R, Allaire M, Lequoy M, Hollande C, Regnault H, et al. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. (2020) 3:100199. doi: 10.1016/j.jhepr.2020.100199
35. Mahmud N, Kaplan DE, Goldberg DS, Taddei TH, Serper M. Changes in hepatocellular carcinoma surveillance and risk factors for noncompletion in the veterans health administration cohort during the coronavirus disease 2019 pandemic. Gastroenterology. (2021) 160:2162.e–4.e. doi: 10.1053/j.gastro.2021.01.007
36. Gandhi M, Ling W-H, Chen C-H, Lee JH, Kudo M, Chanwat R, et al. Impact of COVID-19 on hepatocellular carcinoma management: a multicountry and region study. J Hepatocell Carcinoma. (2021) 8:1159–67. doi: 10.2147/JHC.S329018
37. Jin Z-C, Chen L, Zhong B-Y, Zhu H-D, Zeng C-H, Li R, et al. Impact of COVID-19 pandemic on intervals and outcomes of repeated transarterial chemoembolization in patients with hepatocellular carcinoma. Front Oncol. (2021) 11:602700. doi: 10.3389/fonc.2021.602700
38. Katz IT, Weintraub R, Bekker L-G, Brandt AM. From vaccine nationalism to vaccine equity - finding a path forward. N Engl J Med. (2021) 384:1281–3. doi: 10.1056/NEJMp2103614
39. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. (2021) 21:181–92. doi: 10.1016/S1473-3099(20)30843-4
40. Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. (2021) 27:270–8.
41. Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. (2020) 383:1544–55. doi: 10.1056/NEJMoa2024671
42. Elsevier. Cellular and Molecular Immunology. 10th ed. (2022). Available online at: https://www.elsevier.com/books/cellular-and-molecular-immunology/abbas/978-0-323-75748-5 (accessed January 3, 2022)
43. He Q, Mao Q, An C, Zhang J, Gao F, Bian L, et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg Microbes Infect. (2021) 10:629–37. doi: 10.1080/22221751.2021.1902245
44. Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. (2021) 27:279–88.
45. Graham SP, McLean RK, Spencer AJ, Belij-Rammerstorfer S, Wright D, Ulaszewska M, et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines. (2020) 5:1–6. doi: 10.1038/s41541-020-00221-3
46. Kandeil A, Mostafa A, Hegazy RR, El-Shesheny R, El Taweel A, Gomaa MR, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine: preclinical studies. Vaccines. (2021) 9:214. doi: 10.3390/vaccines9030214
47. van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. (2020) 586:578–82.
48. Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. (2020) 21:1336–45. doi: 10.1038/s41590-020-0782-6
49. Dejnirattisai W, Zhou D, Ginn HM, Duyvesteyn HME, Supasa P, Case JB, et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. (2021) 184:2183.e–200.e. doi: 10.1016/j.cell.2021.02.032
50. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. (2020) 581:215–20. doi: 10.1038/s41586-020-2180-5
51. Tian J-H, Patel N, Haupt R, Zhou H, Weston S, Hammond H, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. (2021) 12:372. doi: 10.1038/s41467-020-20653-8
52. Supasa P, Zhou D, Dejnirattisai W, Liu C, Mentzer AJ, Ginn HM, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. (2021) 184:2201.e–11.e. doi: 10.1016/j.cell.2021.02.033
53. Galipeau Y, Greig M, Liu G, Driedger M, Langlois M-A. Humoral responses and serological assays in SARS-CoV-2 infections. Front Immunol. (2020) 11:610688. doi: 10.3389/fimmu.2020.610688
54. Collier A-RY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N Engl J Med. (2021) 385:2010–2. doi: 10.1056/NEJMc2115596
55. Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. (2021) 6:1140–9. doi: 10.1038/s41564-021-00947-3
56. Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. (2021) 27:2032–40. doi: 10.1038/s41591-021-01540-1
57. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. (2021) 27:1205–11. doi: 10.1038/s41591-021-01377-8
58. Vacharathit V, Aiewsakun P, Manopwisedjaroen S, Srisaowakarn C, Laopanupong T, Ludowyke N, et al. CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis. (2021) 21:1352–4. doi: 10.1016/S1473-3099(21)00568-5
59. Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. (2022) 3:e52–61. doi: 10.1016/S2666-5247(21)00267-6
60. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. (2021) 385:585–94.
61. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. (2020) 396:467–78. doi: 10.1016/S0140-6736(20)31604-4
62. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. (2021) 21:39–51. doi: 10.1016/S1473-3099(20)30831-8
63. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. (2021) 398:213–22. doi: 10.1016/S0140-6736(21)01429-X
64. Ma H, Zeng W, He H, Zhao D, Jiang D, Zhou P, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. (2020) 17:773–5. doi: 10.1038/s41423-020-0474-z
65. Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart AS, Pollard AJ, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. (2022) 399:234–6. doi: 10.1016/S0140-6736(21)02844-0
66. Tan CW, Chia WN, Qin X, Liu P, Chen MI-C, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. (2020) 38:1073–8. doi: 10.1038/s41587-020-0631-z
68. Liu N, Liu B, Zhang L, Li H, Chen Z, Luo A, et al. Recovery of circulating CD56dim NK cells and the balance of Th17/Treg after nucleoside analog therapy in patients with chronic hepatitis B and low levels of HBsAg. Int Immunopharmacol. (2018) 62:59–66. doi: 10.1016/j.intimp.2018.06.043
69. Xiang T, Liang B, Wang H, Quan X, He S, Zhou H, et al. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell Mol Immunol. (2021) 18:2679–81. doi: 10.1038/s41423-021-00795-5
70. Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. (2021) 75:439–41. doi: 10.1016/j.jhep.2021.04.026
71. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. (2021) 325:2204–6. doi: 10.1001/jama.2021.7489
72. Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. (2021) 75:435–8. doi: 10.1016/j.jhep.2021.04.020
73. Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. (2021) 105:2170–4. doi: 10.1097/TP.0000000000003780
74. Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. (2021) 20:162.e–72.e. doi: 10.1016/j.cgh.2021.09.003
75. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. (2021) 384:403–16.
76. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. (2020) 383:2603–15.
77. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. (2021) 397:99–111.
78. Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. (2021) 385:2348–60. doi: 10.1056/NEJMoa2105290
79. Simões E. New Brazil Data Shows Disappointing 50.4% Efficacy for China’s CoronaVac Vaccine. (2021). Available online at: https://www.reuters.com/article/us-health-coronavirus-brazil-coronavirus-idUSKBN29H2CE (accessed May 23, 2021)
80. Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. (2017) 27:1942. doi: 10.1002/rmv.1942
81. Härmälä S, Parisinos CA, Shallcross L, O’Brien A, Hayward A. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis. BMJ Open. (2019) 9:e031070. doi: 10.1136/bmjopen-2019-031070
82. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. (2021) 384:2187–201.
83. Costantino A, Invernizzi F, Centorrino E, Vecchi M, Lampertico P, Donato MF. COVID-19 vaccine acceptance among liver transplant recipients. Vaccines. (2021) 9:1314. doi: 10.3390/vaccines9111314
84. Davidov Y, Tsaraf K, Cohen-Ezra O, Likhter M, Ben Yakov G, Levy I, et al. Immunogenicity and adverse effects of the 2-Dose BNT162b2 messenger RNA vaccine among liver transplantation recipients. Liver Transpl. (2021) 28:215–23. doi: 10.1002/lt.26366
85. Fernández-Ruiz M, Almendro-Vázquez P, Carretero O, Ruiz-Merlo T, Laguna-Goya R, San Juan R, et al. Discordance between SARS-CoV-2–specific cell-mediated and antibody responses elicited by mRNA-1273 vaccine in kidney and liver transplant recipients. Transplant Direct. (2021) 7:e794. doi: 10.1097/TXD.0000000000001246
86. Cholankeril G, Al-Hillan A, Tarlow B, Abrams D, Jacobs JS, Flores NP, et al. Clinical factors associated with lack of serological response to SARS-CoV-2 messenger RNA vaccine in liver transplantation recipients. Liver Transpl. (2022) 28:123–6. doi: 10.1002/lt.26351
87. Hall VG, Ferreira VH, Ierullo M, Ku T, Marinelli T, Majchrzak-Kita B, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. (2021) 21:3980–9. doi: 10.1111/ajt.16766
88. Boyarsky BJ, Chiang TP-Y, Ou MT, Werbel WA, Massie AB, Segev DL, et al. Antibody response to the janssen COVID-19 vaccine in solid organ transplant recipients. Transplantation. (2021) 105:e82–3. doi: 10.1097/TP.0000000000003850
89. Nafar M, Chehrazi S, Dalili N, Firouzan A, Poorrezagholi F, Samadian F, et al. Humoral immunity of BBIBP-CorV (Sinopharm) in kidney transplant recipients: is it time to revise vaccination strategies. Transpl Infect Dis. (2022) 24:e13798. doi: 10.1111/tid.13798
90. Erol Ç, Yanık Yalçın T, Sarı N, Bayraktar N, Ayvazoğlu Soy E, Yavuz Çolak M, et al. Differences in antibody responses between an inactivated SARS-CoV-2 Vaccine and the BNT162b2 mRNA vaccine in solid-organ transplant recipients. Exp Clin Transplant. (2021) 19:1334–40. doi: 10.6002/ect.2021.0402
91. Moon AM, Webb GJ, García-Juárez I, Kulkarni AV, Adali G, Wong DK, et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol Commun. (2021) 6:889–97. doi: 10.1002/hep4.1853
92. Malinis M, Cohen E, Azar MM. Effectiveness of SARS-CoV-2 vaccination in fully vaccinated solid organ transplant recipients. Am J Transplant. (2021) 21:2916–8. doi: 10.1111/ajt.16713
93. Callaghan CJ, Mumford L, Curtis RMK, Williams SV, Whitaker H, Andrews N, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. (2022) 106:436–46. doi: 10.1097/TP.0000000000004059
94. John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines With COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med. (2021) 181:1306–14. doi: 10.1001/jamainternmed.2021.4325
95. John BV, Deng Y, Schwartz KB, Taddei TH, Kaplan DE, Martin P, et al. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. (2022): [Online ahead of print]. doi: 10.1002/hep.32337
96. Danziger-Isakov L, Kumar D, Ast Id Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33:e13563. doi: 10.1111/ctr.13563
97. Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. (2020) 324:951–60. doi: 10.1001/jama.2020.15543
98. Ai J, Wang J, Liu D, Xiang H, Guo Y, Lv J, et al. Safety and immunogenicity of SARS-CoV-2 vaccines in patients with chronic liver diseases (CHESS-NMCID 2101): a multicenter study. Clin Gastroenterol Hepatol. (2021). [Online ahead of print]. doi: 10.1016/j.cgh.2021.12.022
99. Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA. (2014) 111:869–74. doi: 10.1073/pnas.1321060111
100. Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. (2021) 75:1434–9. doi: 10.1016/j.jhep.2021.08.008
101. Timmermann L, Globke B, Lurje G, Schmelzle M, Schöning W, Öllinger R, et al. Humoral immune response following SARS-CoV-2 vaccination in liver transplant recipients. Vaccines. (2021) 9:1422. doi: 10.3390/vaccines9121422
102. CDC. COVID-19 Booster Shot. Atlanta, GA: Centers for Disease Control and Prevention (2022). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed January 9, 2022)
103. World Health Organization. Interim Recommendations for an Extended Primary Series with an Additional Vaccine dose for COVID-19 Vaccination in Immunocompromised Persons: Interim Guidance, 26 October 2021. Geneva: World Health Organization (2021). 15 p. Available online at: https://apps.who.int/iris/handle/10665/347079 (accessed January 6, 2022).
104. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. (2021) 385:661–2.
105. Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. (2021) 385:1244–6. doi: 10.1056/NEJMc2111462
106. Kamar N, Abravanel F, Marion O, Romieu-Mourez R, Couat C, Del Bello A, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Netw Open. (2021) 4:e2136030. doi: 10.1001/jamanetworkopen.2021.36030
107. Alejo JL, Mitchell J, Chiang TP-Y, Abedon AT, Boyarsky BJ, Avery RK, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. (2021) 105:e280–1.
108. Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. (2021) 397:2331–3. doi: 10.1016/S0140-6736(21)01290-3
109. Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. (2021) 9:999–1009. doi: 10.1016/S2213-2600(21)00220-4
110. Xiang T, Liang B, Fang Y, Lu S, Li S, Wang H, et al. Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset. Front Immunol. (2021) 12:708523. doi: 10.3389/fimmu.2021.708523
111. Lo Sasso B, Giglio RV, Vidali M, Scazzone C, Bivona G, Gambino CM, et al. Evaluation of anti-SARS-Cov-2 S-RBD IgG antibodies after COVID-19 mRNA BNT162b2 vaccine. Diagnostics. (2021) 11:1135. doi: 10.3390/diagnostics11071135
112. Tea F, Stella AO, Aggarwal A, Darley DR, Pilli D, Vitale D, et al. SARS-CoV-2 neutralizing antibodies: longevity, breadth, and evasion by emerging viral variants. PLoS Med. (2021) 18:e1003656. doi: 10.1371/journal.pmed.1003656
113. Liu Z, Wu H, Egland KA, Gilliland TC, Dunn MD, Luke TC, et al. Human immunoglobulin from transchromosomic bovines hyperimmunized with SARS-CoV-2 spike antigen efficiently neutralizes viral variants. Hum Vaccin Immunother. (2021) 18:1940652. doi: 10.1080/21645515.2021.1940652
114. Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: a multicenter case series. J Hepatol. (2022) 76:211–4. doi: 10.1016/j.jhep.2021.07.024
115. Dumortier J. Liver injury after mRNA-based SARS-CoV-2 vaccination in a liver transplant recipient. Clin Res Hepatol Gastroenterol. (2022) 46:101743. doi: 10.1016/j.clinre.2021.101743
116. Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, Cerny A, Dayer E, Vergani D, et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. (2021) 123:102706. doi: 10.1016/j.jaut.2021.102706
117. Garrido I, Lopes S, Simões MS, Liberal R, Lopes J, Carneiro F, et al. Autoimmune hepatitis after COVID-19 vaccine - more than a coincidence. J Autoimmun. (2021) 125:102741. doi: 10.1016/j.jaut.2021.102741
118. Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. (2021) 123:102688. doi: 10.1016/j.jaut.2021.102688
119. Sohrabi M, SobheRakhshankhah E, Ziaei H, AtaeeKachuee M, Zamani F. Acute liver failure after vaccination against of COVID-19; a case report and review literature. Respir Med Case Rep. (2022) 35:101568. doi: 10.1016/j.rmcr.2021.101568
120. Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for prevention of Covid-19. N Engl J Med. (2022). [Online ahead of print]. doi: 10.1056/NEJMoa2116620
121. Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. (2021) 385:1941–50. doi: 10.1056/NEJMoa2107934
122. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. (2022) 386:509–20. doi: 10.1056/NEJMoa2116044
123. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. (2022) 386:1397–408. doi: 10.1056/NEJMoa2118542
124. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. (2020) 383:1813–26. doi: 10.1056/NEJMoa2007764
125. FDA. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. FDA (2021). Available online at: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed January 6, 2022)
Keywords: COVID-19, SARS-CoV-2, vaccine, cirrhosis, liver transplantation, immune
Citation: Sripongpun P, Pinpathomrat N, Bruminhent J and Kaewdech A (2022) Coronavirus Disease 2019 Vaccinations in Patients With Chronic Liver Disease and Liver Transplant Recipients: An Update. Front. Med. 9:924454. doi: 10.3389/fmed.2022.924454
Received: 20 April 2022; Accepted: 03 June 2022;
Published: 22 June 2022.
Edited by:
Diego Ripamonti, Papa Giovanni XXIII Hospital, ItalyReviewed by:
Nicola Squillace, San Gerardo Hospital, ItalyBenedetto Maurizio Celesia, UOC Infectious Diseases ARNAS Garibaldi, Italy
Copyright © 2022 Sripongpun, Pinpathomrat, Bruminhent and Kaewdech. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Apichat Kaewdech, apichat.ka@psu.ac.th
†ORCID: Pimsiri Sripongpun, orcid.org/0000-0003-0007-8214; Nawamin Pinpathomrat, orcid.org/0000-0002-0776-595X; Jackrapong Bruminhent, orcid.org/0000-0003-0930-8936; Apichat Kaewdech, orcid.org/0000-0002-4058-5977
 Pimsiri Sripongpun
Pimsiri Sripongpun Nawamin Pinpathomrat
Nawamin Pinpathomrat Jackrapong Bruminhent
Jackrapong Bruminhent Apichat Kaewdech
Apichat Kaewdech