Perinatal maternal characteristics predict a high risk of neonatal asphyxia: A multi-center retrospective cohort study in China
- 1Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Beijing, China
- 2Department of Obstetrics and Gynecology, National Clinical Research Center for Obstetrics & Gynecologic Diseases, Peking Union Medical College Hospital (CAMS), Beijing, China
- 3Department of Obstetrics and Gynecology, Hunan Maternal and Child Health Care Hospital, Changsha, China
- 4Department of Obstetrics and Gynecology, Nanfang Hospital Southern Medical University, Guangzhou, China
- 5Department of Obstetrics and Gynecology, Women's Hospital School of Medicine Zhejiang University, Hangzhou, China
- 6Department of Obstetrics and Gynecology, Henan Provincial People's Hospital Zhengzhou, Henan, China
- 7Department of Obstetrics and Gynecology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China
- 8Department of Obstetrics and Gynecology, Sichuan University West China Second Hospital, Chengdu, China
- 9Department of Obstetrics and Gynecology, Northwest Women and Children's Hospital, Xi'an, China
- 10Department of Obstetrics and Gynecology, Shengjing Hospital Affiliated to China Medical University, Shenyang, China
- 11Department of Obstetrics and Gynecology, The First Clinical Hospital Affiliated to Harbin Medical University, Harbin, China
Objective: This study aimed to identify various perinatal maternal characteristics that contributed to neonatal asphyxia (NA) in term and late-preterm newborns based on the data obtained from a Chinese birth registry cohort and to establish an effective model for predicting a high risk of asphyxia.
Method: We retrospectively reviewed and analyzed the birth database from July 1, 2016, to June 30, 2017, in the main economically developed regions of China. Asphyxia was defined as an Apgar score <7 at 5 min post-delivery with umbilical cord arterial blood pH < 7.2 in the infant born after 34weeks. We compared the perinatal maternal characteristics of the newborns who developed asphyxia (NA group, n = 1,152) and those who did not (no NA group, n = 86,393). Candidate predictors of NA were analyzed using multivariable logistic regression. Subsequently, a prediction model was developed and validated by an independent test group.
Result: Of the maternal characteristics, duration of PROM ≥ 48 h, a gestational week at birth <37, prolonged duration of labor, hypertensive disorder, nuchal cord, and birth weight <2,500 or ≥4,000 g, abnormal fetal heart rate, meconium-stained amniotic fluid, and placenta previa were included in the predicting model, which presented a good performance in external validation (c-statistic of 0.731).
Conclusion: Our model relied heavily on clinical predictors that may be determined before or during birth, and pregnant women at high risk of NA might be recognized earlier in pregnancy and childbirth using this methodology, allowing them to avoid being neglected and delayed. Future studies should be conducted to assess its usefulness.
Introduction
Globally, the occurrence of neonatal asphyxia (NA) is between 0.5 and 1.0% in full-term neonates, which is higher in preterm newborns (1), and NA contributes to 23% of the main causes of neonatal death (2). NA is defined as the failure of neonates to initiate and sustain breathing at birth (3), followed by impaired gas exchange leading to progressive hypoxemia, hypercapnia, and significant metabolic acidosis if prolonged (4). Severe NA may cause multiple organ damage, including brain damage, cardiac injury, respiratory distress, renal injury, liver incompetence, and necrotizing enterocolitis, even endangers neonatal survival (4–10). Among these, brain damage, which is also termed hypoxic-ischemic encephalopathy (HIE), is of the greatest concern due to its high lethality and long-term neurological sequelae, like cerebral palsy, epilepsy, intellectual incompetence, cognitive deficits, and motor disability, thereby leaving the family and society a lifetime burden (11–13).
It remains a priority to identify the newborns that may experience NA and minimize the rate of NA. Socioeconomic factors have been demonstrated to be strongly related to NA, like low socioeconomic and educational status, inadequate antenatal care, and poor intrapartum care (14, 15). Moreover, medical factors like maternal obstetrical complications including pregnancy-induced hypertension disease and gestational diabetes, parity, early gestational age, low birth weight, premature rupture of membranes, a prolonged second stage of labor, shoulder dystocia, abnormal fetal heart rate (FHR), and intrauterine meconium staining have already been identified to be risk factors of NA (16–20).
To summarize, several antenatal factors and intrapartum events have been associated with the presence of NA. Nevertheless, it is difficult to identify fetuses at high risk of asphyxia and to classify them into appropriate monitoring and management strategies during prenatal and intrapartum care. This study aimed to develop and evaluate prognosis models for predicting NA by combining multiple predictors, identify women at risk of adverse birth outcomes, and improve maternal and neonatal management.
Materials and methods
Data sources and study design
This retrospective multi-center childbirth registration research analyzed birth data and delivery problems from 14 representative medical institutions (including two secondary and 12 tertiary hospitals) across 10 provinces in China's four major economic regions from October 1, 2016, to September 30, 2017. Clinicians at each hospital gathered and reported complete medical information for each birth into a pre-designed standardized data collecting system that relied on digital and written medical records. The study was authorized by the study centers' Institutional Ethics Committees. All patients consented to and signed a consent form for the gathering of data from their medical records, as well as the publishing of their medical information, at the time of registration.
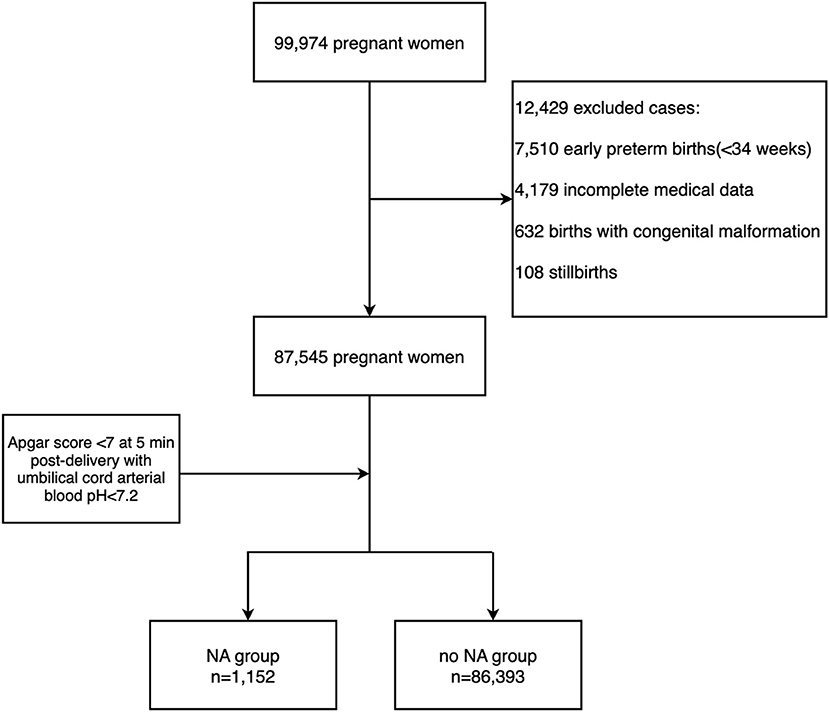
Since it is not recommended to establish the diagnosis of asphyxia by using the Apgar score alone (21, 22), the diagnosis for birth asphyxia was based on the committee opinion of the American College of Obstetrics and Gynecology (ACOG) (23) and World Health Organization (WHO): neonates in the term births and late-preterm births (24) with Apgar score <7 at 5 min post-delivery with umbilical cord arterial blood pH < 7.2 were diagnosed NA (25). Exclusion criteria included early-preterm newborns, stillbirth, congenital malformation, and incomplete medical data. Hence from the data of 99,974 pregnant women, a total of 87,545 pregnancies were selected for evaluation, including 1,152 pregnancies diagnosed with NA and 86,393 pregnancies that didn't complicate with NA. They were with complete basic information and with gestational week ≥34 weeks (Figure 1). In this case, we excluded the early-preterm newborns <34 gestational weeks.
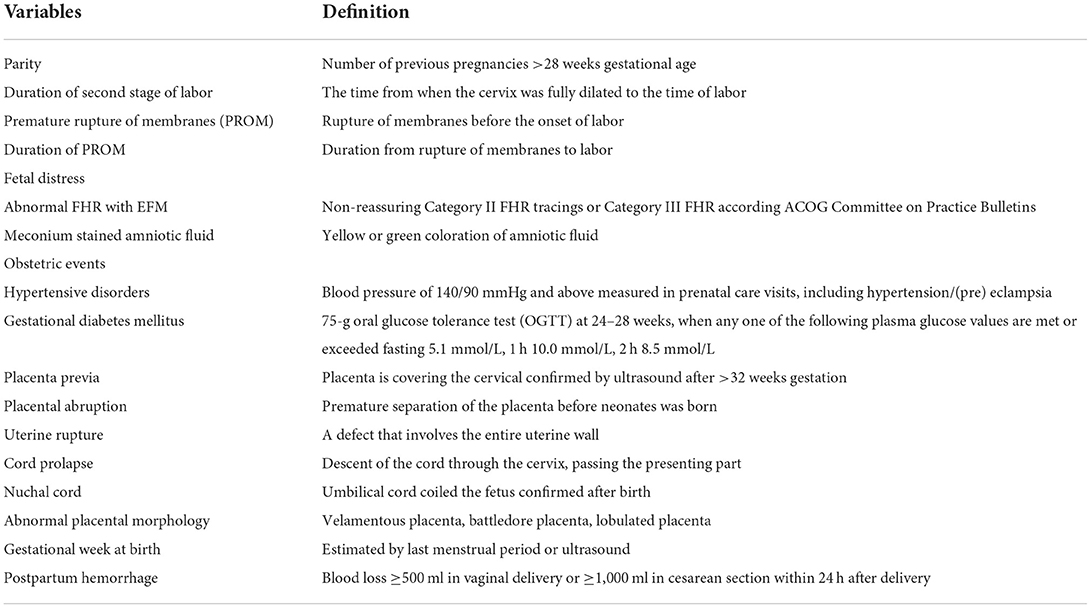
The independent variables included in this study were: (1) maternal basic characteristics and antepartum-related factors: age, medical history, body mass index at birth, weight gain during pregnancy, the way of conception, gravidity and parity, and obstetric complication during pregnancy, (2) maternal intrapartum related factors: type of labor, mode of delivery, augmentation of labor, time of membrane rupture, duration of the second stage, the color of amniotic fluid, (3) neonatal related factors: fetal distress, birth weight, gestational age at birth and nuchal cord. Detailed information on the definition of the variables were shown in Table 1.
Statistical analysis
Variables were compared between two outcomes (NA and no NA) using Python 3.8.5. Normal distributions were tested for all variables. The Student's t-test or Mann-Whitney U rank test was used to compare the numerical variables according to their distribution and the results are shown as means ± standard deviation (range) or as medians and interquartile ranges depending on their distribution. The chi-squared test was used to analyze categorical variables and the results are presented as percentages. Bonferroni-corrected P-values were calculated, and the Bonferroni-corrected alpha was set at 0.05. Variables having corrected P values < 0.05 were included in univariate analysis and multivariate logistic regression. Maternal age was also categorized into <35 and ≥35 y according to the cut-off value of advanced maternal age (26); duration of the second stage of labor was categorized into <2 and ≥2 h according to the definition of the prolonged second stage of labor (27); gestational week at birth and birth weight were categorized according to the definition of preterm/post-term birth and low/high birth weight (24, 28).
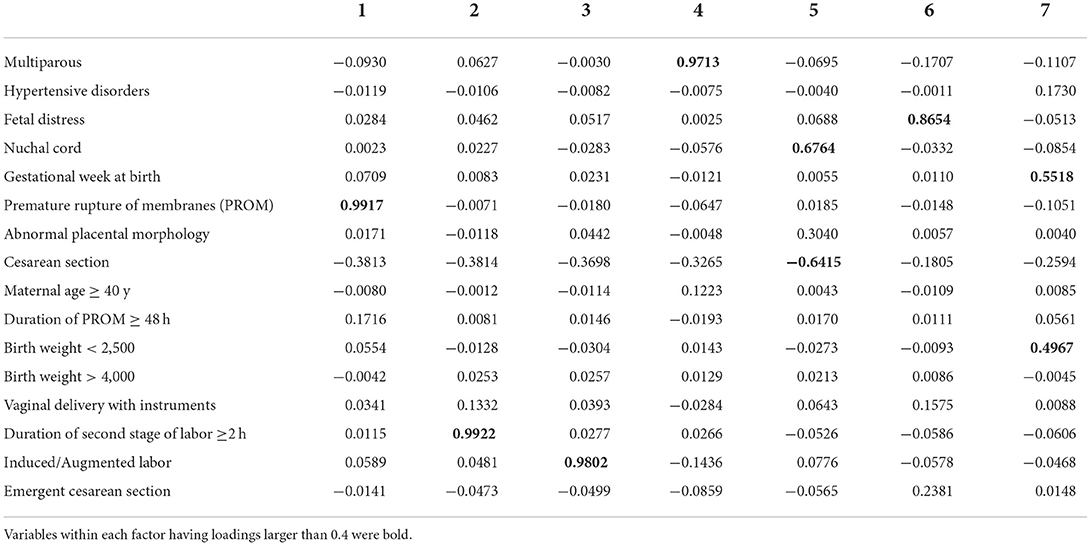
To contemplates the relationships that different variables have with each other, we performed Bartlett's test and the Kaiser-Meyer-Olkin (KMO) test to measure the suitability of data for factor analysis. In Bartlett's test, the P-value was 0, which was statistically significant and indicated that the observed correlation matrix was not an identity matrix. KMO test presented with a result of 0.55, which was not perfect for factorial analysis but would consider applicable. Then we calculated the response value and performed an exploratory factorial analysis by including all the significant variables.
At a 3:1 allocation ratio, a total of 87,545 patients were randomly divided into training and test groups. We verified the statistical significance of combinations of factors while choosing variables for the models. The training group was used to calculate the statistical significance of combinations of variables using univariate and multivariate logistic regression models. We estimated odds ratios (OR) and adjusted ORs with 95 percent confidence intervals (CI) and P values. As a result, the scoring system is formed.
The chi-square values from the logistic regression and C-statistics utilizing the test group from the receiver operating characteristic (ROC) analysis with sensitivity and specificity were used to confirm the scoring system's discriminating capacity. The prediction performance of the identified risk variables and scoring system was assessed using the area under the ROC curve (AUC). Statistical significance was defined as a P value of <0.05.
Results
Maternal and prenatal events related factors
Of the retrospective cohort consisting of 87,545 pregnancies, 1,152(1.3%) births complicated with NA were identified. The comparisons of the maternal characteristics of the NA group and the no NA group is presented in Table 2. The mean age of the pregnant women with and without NA were 30.05 ± 4.94 and 30.72 ± 4.5 years, respectively, which showed no differences between the two groups (P = 0.628), as well as the proportion of advanced maternal age. Among study participants, 733(63.6%) of the mothers with NA and 51,775(59.9%) of the mothers without NA were primiparous. There was a significant difference in parities of mothers between the two groups (P = 0.003). In addition, the body mass index at birth, weight gain during pregnancy, and the rate of assisted reproductive technology did not differ between the two groups, and the proportion of multiple births was also similar (P = 0.08).
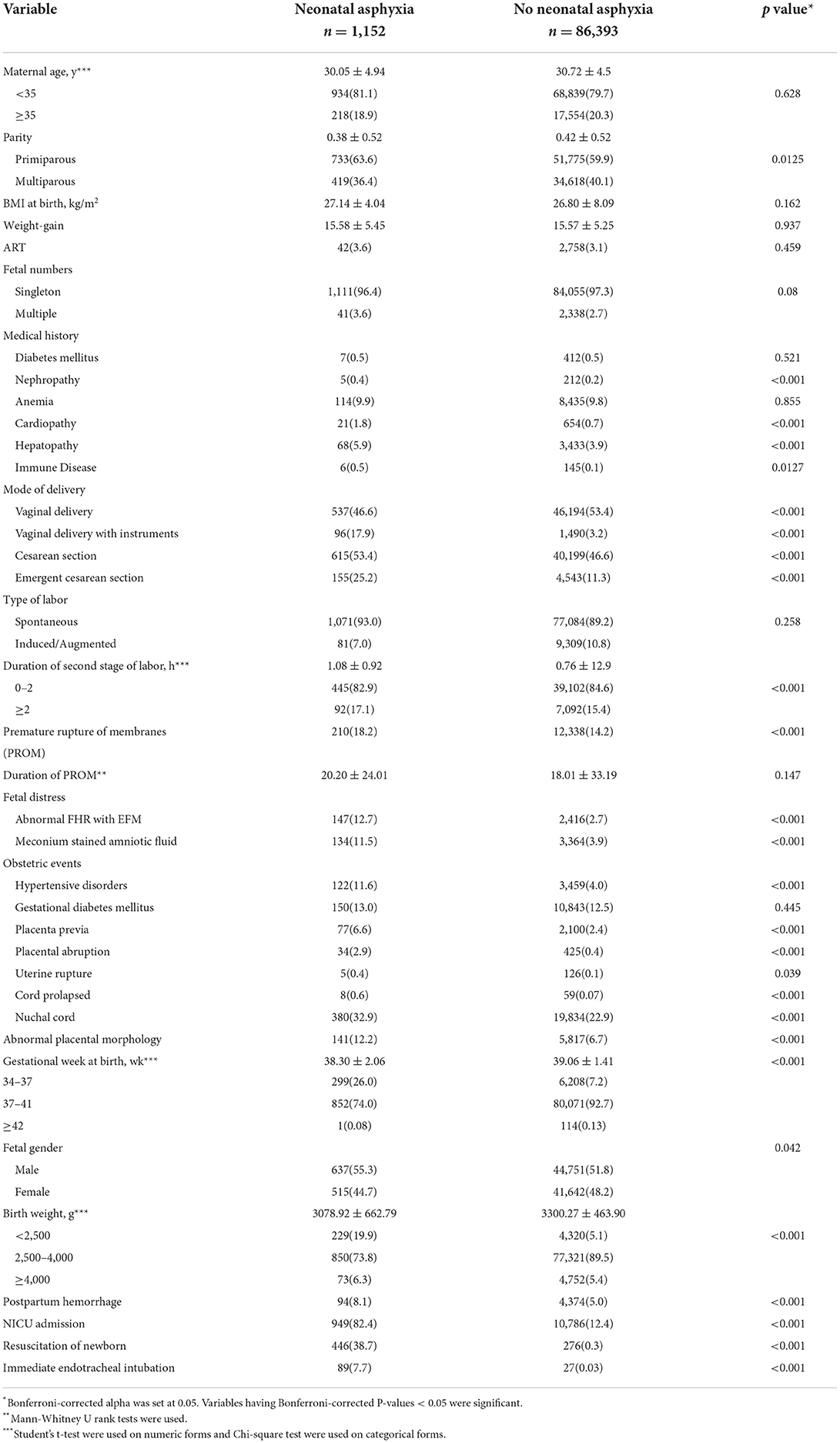
Table 2. Maternal characteristics and perinatal events established for neonatal asphyxia [Data was given as mean ± SD or n (%)].
Pre-pregnancy complications are shown in Table 2. The mothers complicated with heart disease, kidney disease, liver complications, and immune disease before getting pregnant were predisposed to arise NA. However, the occurrences of pre-existing diabetes mellitus and anemia weren't significantly higher in the NA group. Perinatal obstetric complications are also presented in Table 2. Mothers complicated with hypertensive disorders (11.6%NA vs. 2.7% no NA, P < 0.001), placenta previa (6.6%NA vs. 2.4% no NA, P < 0.001), placental abruption (2.9%NA vs. 0.4% no NA, P < 0.001), uterine rupture (0.4%NA vs. 0.1% no NA, P < 0.001), cord prolapse (11.6%NA vs. 2.7% no NA, P < 0.001), nuchal cord (11.6%NA vs. 2.7% no NA, P = 0.039), and abnormal placental morphology (12.2%NA vs. 6.7% no NA, P < 0.001) were more likely to develop NA. Surprisingly, the presences of gestational diabetes mellitus were similar in the two groups (13.0%NA vs. 12.5% no NA, P = 0.445).
The maternal intrapartum characteristics are also shown in Table 2. Labor started spontaneously in 1,071(93.0%) of the NA group compared with 7,084(89.2%) no NA group (P = 0.258). The median duration of the second stage of labor was 0.77 ± 0.92 in the NA group and was 0.55 ± 12.9 in the group without NA, which were statistically different among the two groups (P < 0.001). Premature rupture of membranes (PROM) was presented in 210(18.2%) of the NA compared to 12,338(14.2%) of the no NA group (P < 0.001), but the duration of PROM showed no differences (P = 0.147). Of the enrolled pregnant women, cesarean sections were performed in 615(53.4%) patients from the NA group as compared to 40,199(46.6%) of the no NA group, the rate was significantly higher in the NA group (P < 0.001). The proportion of vaginal delivery with instruments among vaginal delivery and emergent cesarean section among cesarean section were 17.9 and 25.2% in the NA group, respectively, the rates of which were significantly lower in the no NA group, only 3.2 and 11.3% of the patients accepted instrumented vaginal delivery and emergent cesarean section, respectively (P < 0.001). Consistent with previous research, the two groups had significant differences in the presence of fetal distress, including abnormal fetal heart rate (FHR) monitored by an electrical fetal monitor (EFM) device (12.7% NA vs. 2.7% no NA, P < 0.001) and meconium-stained amniotic fluid (11.5% NA vs. 3.9% no NA, P < 0.001), which were more commonly seen in mothers with NA.
As for the neonatal characteristics, Table 2 also showed the detailed results of the gestational week at birth and birth weight, of which the two groups were significantly different not only calculated as numerical variables but also when categorized into three groups (P < 0.001). The neonates complicated with asphyxia tended to be smaller in gestational week and birth weight. Interestingly, our study showed that male newborns seem to be more likely to develop NA (55.3% NA vs. 51.8% no NA, P = 0.042). Of the patients who had NA, 949(82.4%) of the neonates were admitted to NICU, and only 12.4% of neonates without NA were transferred to NICU (P < 0.001). Furthermore, the use of resuscitation of newborns (38.7% NA vs. 0.3% no NA, P < 0.001) and immediate endotracheal intubation (7.7% NA vs. 0.03% no NA, P < 0.001) were more common in the NA group. Of the maternal outcomes, the occurrence of postpartum bleeding was also significantly higher in the NA group (8.1% NA vs. 5.0% no NA, P < 0.001).
Selection of variables for discriminating the high-risk group and scoring system
According to the exploratory factorial analysis, seven factors were used for hypothesis testing since their eigenvalues were over 1 (Table 3). The interactions were not important by analyzing the loadings within each factor, except for the factors 5 and 7. There was a connection between the nuchal cord and cesarean section within factor 5 and a connection between the gestational week at birth and birth weight within factor 7. However, the variables didn't cluster, so we limit ourselves to comparing the main effects within each factor.
Univariate and multivariate logistic regression analyses were performed in the training group to evaluate the risk factors associated with NA (Table 4). After adjustments for possible effects of confounding variables, age ≥40 y (aOR 1.159, 95% CI 0.977–1.375), nulliparity (aOR 1.138, 95% CI 1.059–1.221), vaginal delivery with a normal duration of the second stage of labor (aOR 1.077, 95% CI 1.001–1.159), PROM (aOR 1.196, 95% CI 0.761–1.315), and a gestational week at birth ≥42 wk (aOR 1.128, 95% CI 0.738–1.467) didn't increase the risk of NA significantly.
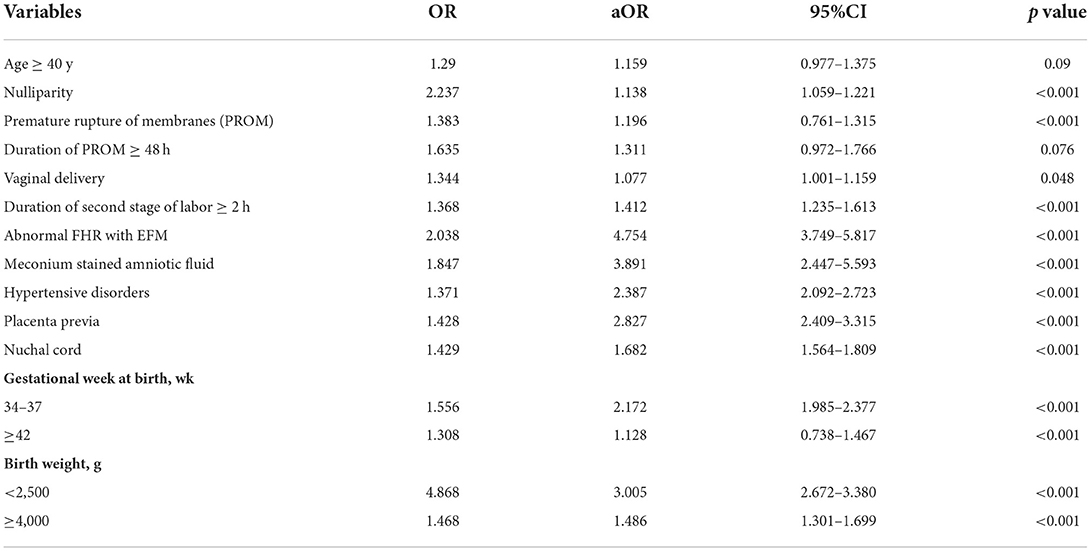
Table 4. Univariate and multivariate logistic regression to evaluate the impact of variables on the presence of neonatal asphyxia.
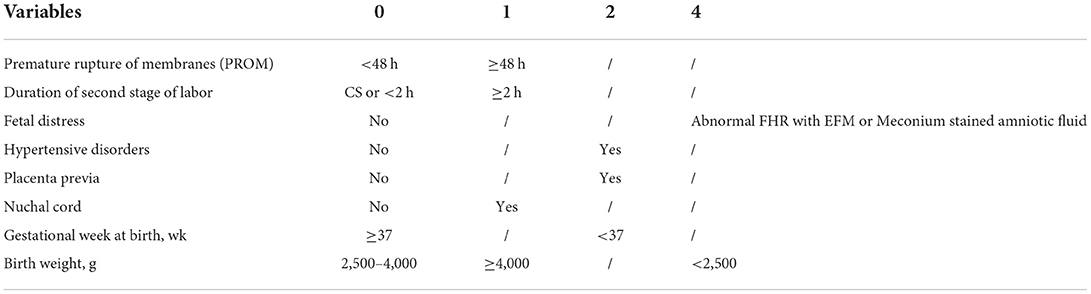
To estimate a more precise model for predicting, we included eight variables with higher aOR to be determinants of NA and assigned scores based on aOR values of the factors. The cut-off value for a prolonged duration of the second stage and a prolonged time of membrane rupture was set at 2 h (27) and 48 h (29), respectively. A scoring system as shown in Table 5, duration of PROM ≥48 h (aOR 1.311, 95% CI 0.972–1.766), vaginal delivery with a prolonged duration of the second stage (aOR 1.412, 95% CI 1.235–1.613), nuchal cord (aOR 1.682, 95% CI 1.564–1.809), and birth weight ≥4,000 (aOR 1.486, 95% CI 1.301–1.699) were assigned one point. While hypertensive disorder (aOR 2.387, 95% CI 2.092–2.723), gestational week <37 wk (aOR 2.172, 95% CI 1.985–2.377), and placenta previa (aOR 2.827, 95% CI 2.409–3.315) were assigned two points. Abnormal FHR with EFM (aOR 4.754, 95% CI 3.749–5.817) or meconium-stained amniotic fluid (aOR 3.891, 95% CI 2.447–5.593), and birth weight <2,500 g (aOR 3.005, 95% CI 2.672–3.380) were assigned four points due to their high coefficient. Each patient was evaluated according to the scoring system.
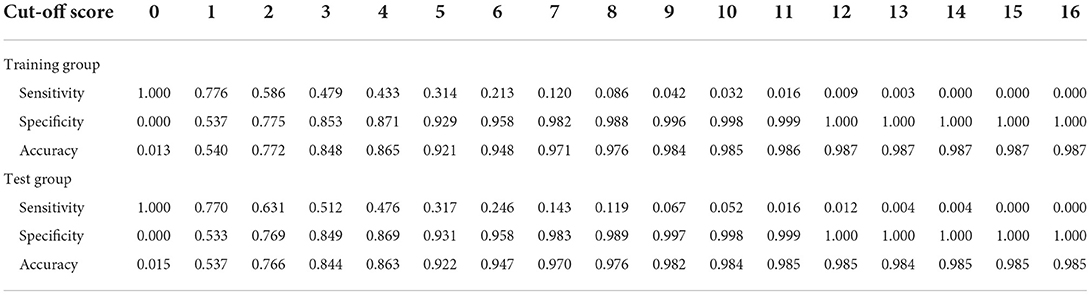
Table 6 showed the different cut-off score's sensitivity and specificity, in distinguishing patients from the NA group and no NA group. During the performance study, the scoring system performed best when the cut-off value was set at four points, with a specificity of 0.871, a sensitivity of 0.433, and an accuracy of 0.865 in predicting NA. The scoring system was validated to perform also well in the independent testing group, with a specificity of 0.869, a sensitivity of 0.476, and an accuracy of 0.863. The AUC of the training group and the test group were 0.724 and 0.731, respectively, as indicated in the ROC curve of the predictive model in Figure 2.
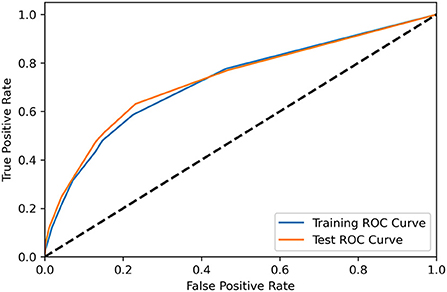
Figure 2. The receiver operating characteristic curve demonstrated the performance of the scoring system for predicting NA in the training group (blue) and test group (orange).
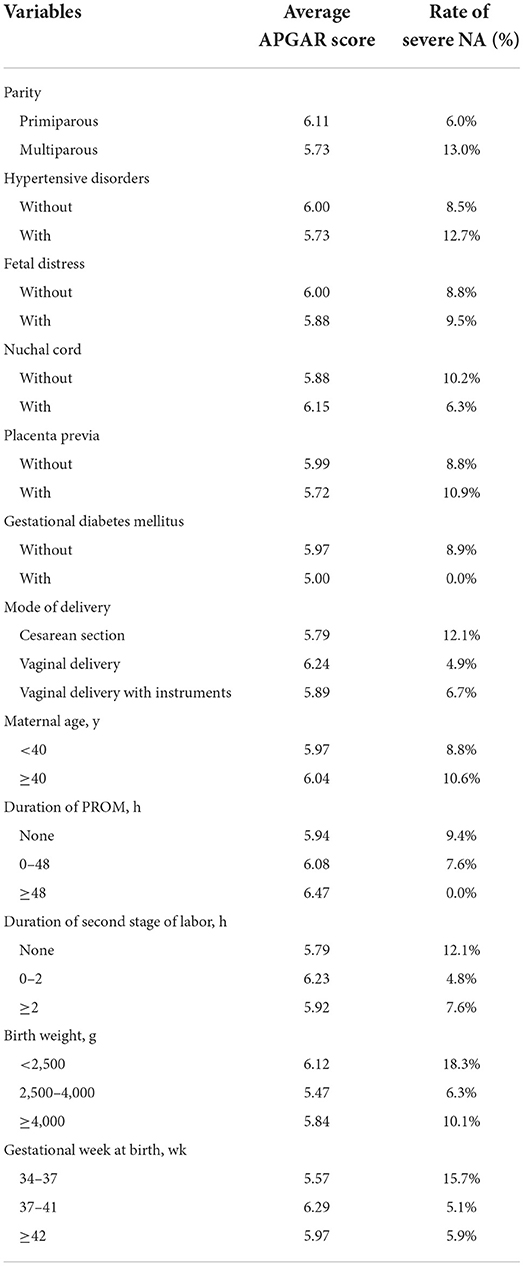
Using exploratory data analysis, we analyzed the influence of the variables on the APGAR scores among the NA group, from both the average APGAR score and the rate of severe NA, defined by an APGAR score of <3 at 5 min after birth. As shown in Table 7, the variables of multiparous, hypertensive disorders, placenta previa, cesarean section, maternal age ≥40 y, duration of the second stage of labor ≥2 h, birth weight <2,500 g, and the gestational week at birth <37 weeks were associated with lower APGAR score and a higher rate of severe NA.
Discussion
NA may lead to severe hypoxic-ischemic organ damage especially encephalopathy in newborns followed by severe long-term sequelae, even perinatal death. Hence, we conducted a nationwide multi-center retrospective cohort study of 87,545 pregnancies to develop a model for predicting NA in women who delivered in major economic regions of China. The overall prevalence of NA among newborns was 1.3% in these middle-high income regions. Compared with previous studies conducted in low- and middle-income countries, our database was collected from developed regions, where neonates are less likely to be born with NA (30, 31). A predictive model was developed by using both clinical prenatal, pre-delivery, and delivery variables in the training group and showed a good discriminative ability validated by the independent test group (c-statistic 0.731).
According to earlier research, nulliparity may be a risk factor for birth asphyxia and poor newborn outcomes (32, 33), though the association was not obvious (aOR 1.138, 95% CI 1.059–1.221) in our study. It is generally agreed that primiparous pregnant women had longer labor than multiparous pregnant women during vaginal birth (34). Hence parity and labor duration were mixed in contributing to the risk of NA. In low- and middle-income nations, prolonged duration of the second stage has been shown to be substantially linked to an increased risk of asphyxia (25, 35–38). Prolonged labor, especially in the second stage, may indicate that there exists an abnormal fetal position or cephalopelvic disproportion, which increases the chance of birth trauma. And the attempt to speed up delivery by using oxytocin may also cause fetal distress due to the stress of too many uterine contractions (39, 40). The forceps delivery or vacuum extraction were also predisposed to be applied when the labor fails to progress, which exerts pressure on the newborn's brain and increases the risk of NA (37, 41). Our study also revealed a significantly higher rate of cesarean sections in the NA group in our cohort, the percentage of emergent cesarean sections in cesarean sections was significantly higher in the NA group (25.2% vs. 11.3%). However, the multivariate logistic regression didn't show a significant relationship between the mode of delivery and the risk of NA (vaginal delivery, aOR 1.077, 95% CI 1.001–1.159). In previous studies, NA is more associated with birth via cesarean section, of which the explanations were the lack of squeezing to fetal lungs by the vaginal canal and less surfactant secreted to the alveolar surface during delivery (42). Though in a clinical scenario of a neonate born with NA caused by birth trauma, it is inevitable for obstetricians to reconsider that the outcome would have been different if a cesarean had been performed instead of vaginal delivery, or performed earlier. However, it is unrealistic and unnecessary to predict whether the fetus will be damaged by labor and performing the cesarean in advance. On the one hand, the newborn outcomes of cesareans performed for emergency reasons conducted over 30 min after the decision to operate weren't worse than those performed sooner (43). On the other hand, more cesareans in first labors to prevent NA may actually increase the rate of uterine rupture for the subsequent pregnancy, hence increasing the overall risk of NA (44).
Prolonged duration from PROM to labor was found to be positively linked with NA in our study, which has been confirmed by multiple previous studies (20, 45, 46). The umbilical cord is no longer surrounded by the amniotic fluid following a PROM and might get squeezed directly by the uterine contractions. Because the flow of oxygen-rich blood to the newborn is halted when the cord is compressed, the baby may suffer from birth asphyxia. Another explanation might be that without the protection of the membrane, external bacteria could easily be passed to the infant, potentially resulting in neonatal infection, followed by NA. Preeclampsia was proved to be strongly associated with an elevated risk of NA, as indicated in other studies (47–49). This impact is mediated by fetal feeding and oxygenation being reduced as a result of uteroplacental vascular insufficiency, as well as an increased chance of delivering preterm (48). According to our study, NA was linked to the nuchal cord, which was analogous to Ethiopia's research (35, 49). One reasonable explanation is that a tight nuchal cord constricts umbilical arteries, resulting in hypoxia.
In our study, low-birth-weight and preterm delivery neonates were shown to be at a higher risk of having NA compared with normal-birth-weight and full-term birth babies, which was consistent with earlier research (35, 37, 50). We didn't include the early-preterm newborns in analyses, because our initial data processing suggested that gestational week at birth was a strong self-predicator of NA, especially in newborns smaller than 34 weeks. Additionally, premature and low-birth-weight neonates may suffer from multiple organ immaturity and complications, especially in the respiratory system (51), which may obscure other underlying risk factors causing NA in statistical analyses. Post-term pregnancy (49) and macrosomia (52) were also demonstrated to be correlated to adverse neonatal outcomes due to placental insufficiency and increased risk of shoulder dystocia during labor, respectively. We identified a higher risk of NA in male newborns when evaluating the neonatal data. This finding was also reported by Sunny et al. (53). Studies have been conducted in China (54) and Israel (55, 56) to investigate the association between fetal gender and adverse pregnancy outcomes. Women carrying male fetuses were at increased risk for operative vaginal delivery, non-reassuring FHR, and lower Apgar scores. One cause may be those male infants are more likely to be macrosomia (57). More diggings on the underlying causes need to be performed.
As mentioned above, the risk factors were analyzed by factorial analysis but they didn't cluster well. It is hard to discriminate among different types of variables by analyzing the raw database. Clinically, some intrapartum events are direct causes of NA such as placental abruption, uterine rupture, cord prolapse, and severe shoulder dystocia, which might result in an abruptly disturbed blood supply to the fetus, since the placenta and umbilical cord are the key points of fetal feeding and oxygenation. However, most of these clinical episodes lack a sentinel event and are unexpected and unpredictable, necessitating urgent resuscitation (44). Nevertheless, these intrapartum events are related to some maternal comorbidities and complications. For instance, pregnancies complicated with placenta previa and hypertension disorder are at high risk of the presentation of placental abruption (58, 59), macrosomia is a risk factor for shoulder dystocia (60), and PROM may increase the potential of cord prolapse (61). So we included these “not so urgent” perinatal events in our scoring system to get a more advanced evaluation. When the fetal suffers from an oxygen deficit in the uterine, the first clinical presentation might be an abnormal FHR monitored by an electrical device or auscultation (62, 63). Though a marked increase in the cesarean delivery rate was related to the high false-positive rate of abnormal FHR (64), it was still well established that a non-reassuring category II is associated with low Apgar scores and neonatal intensive care unit (NICU) admission (65). Pruksanusak et al. (66) demonstrated a higher ability in predicting peripartum asphyxia of the combination of an abnormal five-tier FHR classification (67) and maternal-associated risk factors than the three-tier system (68). A longstanding non-reassuring fetal oxygen deficit may cause a release of intrauterine meconium, and the presence of meconium in the amniotic fluid increases the likelihood of meconium aspiration during intrauterine gasping or the first few breaths after delivery (69). In this case, the meconium amniotic fluid was widely identified as a risk factor for NA in former research (36, 38, 46, 49, 50, 70). We did not include vaginal delivery with instruments or emergent cesarean section in the scoring system because these two modes of labor may not be causal but merely associated with NA, which may be performed for a concerning fetal indication or obstetrical events that are themselves the key risk factor for NA, such as prolonged labor, non-reassuring FHR, meconium-stained amniotic fluid, placental and umbilical abnormalities.
Most previous studies were conducted in low- and middle-income countries. Our research was the first based on a database collected from the main economically developed regions of China as socioeconomic status plays an important role in the presence of NA. This is one of the few multi-center cohort studies focusing on predicting NA by including both characteristics during pre-delivery and delivery. Our model relied heavily on clinical predictors that may be determined before or during birth, such as a gestational week, fetal weight (which can be assessed before birth), hypertensive disorders, and the other risk factors described above. Compared with other predictive models from previous studies (37, 53, 70, 71), our predictive model is more clinically applicable, since the risk factors were quantified precisely by specifically assigned scores. And we accomplished a pretty high sensitivity by using as minimal variables as possible, making the model concise to the greatest extent possible. Our predictive scoring system could identify certain neonates that will experience NA prospectively, and could be the proper triaging of the obstetric risk assessment instrument. Pregnant women at high risk of NA might be recognized earlier in pregnancy and childbirth using this methodology, allowing them to avoid being neglected and delayed. Our study methods have several limitations. This was a retrospective cohort study, which limited the information of the generalized data. To be specific, the reasons for applying instruments during vaginal delivery or emergent cesarean section were not able to obtain from the raw data and the neonatal follow-ups, especially on the severity of HIE and other complications were absent. So this clinical score could not predict the severity of NA since it was not able to be quantified. This limitation can be resolved in a prospective study with a pre-designed protocol and detailed recording.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Peking Union Medical College Hospital Review Board (reference number: JS-1151). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YY wrote the manuscript and participated in data analysis. YT, MZ, JH, SL, XW, XL, YC, CL, and JS conducted data collection and quality control at their medical centers. JG conceived and designed the study. JL participated in designing the study and revising the language. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the CAMS Innovation Fund for Medical Science (CIFMS) (2020-I2M-C&T-B-046) and Beijing Municipal Natural Science Foundation (No. 7212072).
Acknowledgments
We thank the following hospitals, Beijing Haidian Maternal and Child Health Hospital, Sichuan University West China Second Hospital, Southern Medical University Nanfang Hospital, Northwest Women and Children's Hospital, Ruijin Maternal and Child Health Hospital, Hunan Maternal and Child Health Care Hospital, and Shengjing Hospital Affiliated to China Medical University, where the register work was conducted for this program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.944272/full#supplementary-material
Supplementary Data Sheet 1. Coding of t test/Mann-Whitney U rank test on numeric variables and Chi-squared Test/ Fisher Exact Test.
Supplementary Data Sheet 2. Coding of logistic regression.
Supplementary Data Sheet 3. Coding of factorial analysis.
Supplementary Data Sheet 4. Coding of scoring system.
Supplementary Data Sheet 5. Coding of exploratory data analysis.
References
1. Dare S, Oduro AR, Owusu-Agyei S, Mackay DF, Gruer L, Manyeh AK, et al. Neonatal mortality rates, characteristics, and risk factors for neonatal deaths in Ghana: analyses of data from two health and demographic surveillance systems. Glob Health Action. (2021) 14:1938871. doi: 10.1080/16549716.2021.1938871
2. Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet. (2005) 365:891–900. doi: 10.1016/S0140-6736(05)71048-5
3. World Health Organization. Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization (2012).
4. Rainaldi MA, Perlman JM. Pathophysiology of birth asphyxia. Clin Perinatol. (2016) 43:409–22. doi: 10.1016/j.clp.2016.04.002
5. Witt CL. Neonatal consequences of asphyxia. NAACOGS Clin Issu Perinat Womens Health Nurs. (1991) 2:48–77.
6. Durkan AM, Alexander RT. Acute kidney injury post neonatal asphyxia. J Pediatr. (2011) 158:e29–33. doi: 10.1016/j.jpeds.2010.11.010
7. Bhatti A, Kumar P. Systemic effects of perinatal asphyxia. Indian J Pediatr. (2014) 81:231–3. doi: 10.1007/s12098-013-1328-9
8. Lu Q, Cheng S, Zhou M, Yu J. Risk factors for necrotizing enterocolitis in neonates: a retrospective case-control study Pediatr Neonatol. (2017) 58:165–70. doi: 10.1016/j.pedneo.2016.04.002
9. Ananthan A, Wagh D, Minutillo C. Intestinal perforation associated with perinatal asphyxia and anaemia. J Pediatr Gastroenterol Nutr. (2018) 66:e83–5. doi: 10.1097/MPG.0000000000001818
10. Bozkurt O, Yucesoy E. Acute kidney injury in neonates with perinatal asphyxia receiving therapeutic hypothermia. Am J Perinatol. (2021) 38:922–9. doi: 10.1055/s-0039-1701024
11. Garcias Da Silva LF, Nunes ML, Da Costa JC. Risk factors for developing epilepsy after neonatal seizures. Pediatr Neurol. (2004) 30:271–7. doi: 10.1016/j.pediatrneurol.2003.09.015
12. Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. (2012) 366:2085–92. doi: 10.1056/NEJMoa1112066
13. Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes N Engl J Med. (2014) 371:140–9. doi: 10.1056/NEJMoa1315788
14. Perveen F, Tayyab S, Zuberi BF. Risk factors for perinatal deaths in Pakistan. J Obstet Gynaecol Res. (2011) 37:1359–64. doi: 10.1111/j.1447-0756.2011.01536.x
15. Rani S, Chawla D, Huria A, Jain S. Risk factors for perinatal mortality due to asphyxia among emergency obstetric referrals in a tertiary hospital. Indian Pediatr. (2012) 49:191–4. doi: 10.1007/s13312-012-0058-9
16. Barton DP, Turner MJ, Boylan PC, MacDonald D, Stronge JM. Fetal acidosis in labour a prospective study on the effect of parity. Eur J Obstet Gynecol Reprod Biol. (1991) 39:93–8. doi: 10.1016/0028-2243(91)90070-2
17. Milsom I, Ladfors L, Thiringer K, Niklasson A, Odeback A, Thornberg E. Influence of maternal, obstetric and fetal risk factors on the prevalence of birth asphyxia at term in a Swedish urban population. Acta Obstet Gynecol Scand. (2002) 81: 909–17. doi: 10.1034/j.1600-0412.2002.811003.x
18. Lee AC, Mullany LC, Tielsch JM, Katz J, Khatry SK, LeClerq SC, et al. Risk factors for neonatal mortality due to birth asphyxia in southern Nepal: a prospective, community-based cohort study. Pediatrics. (2008) 121:e1381–90. doi: 10.1542/peds.2007-1966
19. Nayeri F, Shariat M, Dalili H, Bani Adam L, Zareh Mehrjerdi F, Shakeri A. Perinatal risk factors for neonatal asphyxia in Vali-e-Asr hospital, Tehran-Iran. Iran J Reprod Med. (2012) 10:137–40.
20. Nauman Kiyani A, Khushdil A, Ehsan A. Perinatal factors leading to birth asphyxia among term newborns in a tertiary care hospital. Iran J Pediatr. (2014) 24:637–42.
21. Committee Opinion No. 644 The Apgar Score. Obstet Gynecol. (2015) 126, e52–5. doi: 10.1097/AOG.0000000000001108
23. Herrera CA, Silver RM. Perinatal asphyxia from the obstetric standpoint: diagnosis and interventions. Clin Perinatol. (2016) 43:423–38. doi: 10.1016/j.clp.2016.04.003
24. ACOG Committee Opinion No. 579: Definition of term pregnancy. Obstet Gynecol. (2013) 122:1139–40. doi: 10.1097/01.AOG.0000437385.88715.4a
25. Sendeku FW, Azeze GG, Fenta SL. Perinatal asphyxia and its associated factors in Ethiopia: a systematic review and meta-analysis. BMC Pediatr. (2020) 20:135. doi: 10.1186/s12887-020-02039-3
26. Frick AP. Advanced maternal age and adverse pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. (2021) 70:92–100. doi: 10.1016/j.bpobgyn.2020.07.005
27. American College of Obstetrics and Gynecology Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. (2003) 102:1445–54. doi: 10.1016/j.obstetgynecol.2003.10.011
28. Hughes MM, Black RE, Katz J. 2500-g low birth weight cutoff: history and implications for future research and policy. Matern Child Health J. (2017) 21:283–9. doi: 10.1007/s10995-016-2131-9
29. Krispin E. Management of premature rupture of membranes at term: the need to correct a recurring mistake in articles, chapters, and recommendations of professional organizations. Am J Obstet Gynecol. (2017) 217:661e1–3. doi: 10.1016/j.ajog.2017.08.111
30. Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. (2005) 115:519–617. doi: 10.1542/peds.2004-1441
31. Usman F, Imam A, Farouk ZL, Dayyabu AL. Newborn mortality in sub-Saharan Africa: why is perinatal asphyxia still a major cause? Ann Glob Health. (2019) 85. doi: 10.5334/aogh.2541
32. Locatelli A, Lambicchi L, Incerti M, Bonati F, Ferdico M, Malguzzi S, et al. Is perinatal asphyxia predictable? BMC Pregnancy Childbirth. (2020) 20:186. doi: 10.1186/s12884-020-02876-1
33. Xu EH, Mandel V, Huet C, Rampakakis E, Brown RN, Wintermark P. Maternal risk factors for adverse outcome in asphyxiated newborns treated with hypothermia: parity and labor duration matter. J Matern Fetal Neonatal Med. (2021) 34:4123–31. doi: 10.1080/14767058.2019.1706472
34. Laughon SK, Berghella V, Reddy UM, Sundaram R, Lu Z, Hoffman MK. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. (2014) 124:57–67. doi: 10.1097/AOG.0000000000000278
35. Abdo RA, Halil HM, Kebede BA, Anshebo AA, Gejo NG. Prevalence and contributing factors of birth asphyxia among the neonates delivered at Nigist Eleni Mohammed memorial teaching hospital, Southern Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. (2019) 19:536. doi: 10.1186/s12884-019-2696-6
36. Gebregziabher GT, Hadgu FB, Abebe HD. Prevalence and associated factors of perinatal asphyxia in neonates admitted to Ayder Comprehensive Specialized Hospital, Northern Ethiopia: a cross-sectional study. Int J Pediatr. (2020) 2020:4367248. doi: 10.1155/2020/4367248
37. Kune G, Oljira H, Wakgari N, Zerihun E, Aboma M. Determinants of birth asphyxia among newborns delivered in public hospitals of West Shoa Zone, Central Ethiopia: a case-control study. PLoS ONE. (2021) 16:e0248504. doi: 10.1371/journal.pone.0248504
38. Nadeem G, Rehman A, Bashir H. Risk factors associated with birth asphyxia in term newborns at a tertiary care hospital of Multan, Pakistan. Cureus. (2021) 13:e18759. doi: 10.7759/cureus.18759
39. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. (2009) 114(2 Pt 1):386–97. doi: 10.1097/AOG.0b013e3181b48ef5
40. Simpson KR. Clinicians' guide to the use of oxytocin for labor induction and augmentation. J Midwifery Womens Health. (2011) 56: 214–21. doi: 10.1111/j.1542-2011.2011.00052.x
41. Altman MR, Lydon-Rochelle MT. Prolonged second stage of labor and risk of adverse maternal and perinatal outcomes: a systematic review. Birth. (2006) 33:315–22. doi: 10.1111/j.1523-536X.2006.00129.x
42. Ahmed R, Mosa H, Sultan M, Helill SE, Assefa B, Abdu M, et al. Prevalence and risk factors associated with birth asphyxia among neonates delivered in Ethiopia: a systematic review and meta-analysis. PLoS ONE. (2021) 16:e0255488. doi: 10.1371/journal.pone.0255488
43. Bloom SL, Leveno KJ, Spong CY, Gilbert S, Hauth JC, Landon MB, et al. Decision-to-incision times and maternal and infant outcomes. Obstet Gynecol. (2006) 108:6–11. doi: 10.1097/01.AOG.0000224693.07785.14
44. Hill MG, Reed KL, Brown RN, Newborn Brain Society Guidelines and Publications Committee. Perinatal asphyxia from the obstetric standpoint Semin Fetal Neonatal Med. (2021) 26:101259. doi: 10.1016/j.siny.2021.101259
45. Woday A, Muluneh A, St Denis C. Birth asphyxia and its associated factors among newborns in public hospital, northeast Amhara, Ethiopia. PLoS ONE. (2019) 14:e0226891. doi: 10.1371/journal.pone.0226891
46. Bayih WA, Yitbarek GY, Aynalem YA, Abate BB, Tesfaw A, Ayalew MY, et al. Prevalence and associated factors of birth asphyxia among live births at Debre Tabor General Hospital, North Central Ethiopia. BMC Pregnancy Childbirth. (2020) 20:653. doi: 10.1186/s12884-020-03348-2
47. Locatelli A, Incerti M, Paterlini G, Doria V, Consonni S, Provero C, et al. Antepartum and intrapartum risk factors for neonatal encephalopathy at term. Am J Perinatol. (2010) 27:649–54. doi: 10.1055/s-0030-1249761
48. Khader YS, Batieha A, Al-Njadat RA, Hijazi SS. Preeclampsia in Jordan: incidence, risk factors, and its associated maternal and neonatal outcomes J Matern Fetal Neonatal Med. (2018) 31:770–6. doi: 10.1080/14767058.2017.1297411
49. Berhe YZ, Kebedom AG, Gebregziabher L, Assefa NE, Berhe LZ, Mohammednur SA, et al. Risk factors of birth asphyxia among neonates born in public hospitals of Tigray, Northern Ethiopia. Pediatric Health Med Ther. (2020) 11:13–20. doi: 10.2147/PHMT.S231290
50. Mulugeta T, Sebsibe G, Fenta FA, Sibhat M. Risk factors of perinatal asphyxia among newborns delivered at public hospitals in Addis Ababa, Ethiopia: case-control study. Pediatric Health Med Ther. (2020) 11:297–306. doi: 10.2147/PHMT.S260788
51. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. (2008) 371:261–9. doi: 10.1016/S0140-6736(08)60136-1
52. Rowe R, Soe A, Knight M, Kurinczuk JJ. UK Midwifery Study System, Neonatal admission and mortality in babies born in UK alongside midwifery units: a national population-based case-control study using the UK Midwifery Study System (UKMidSS). Arch Dis Child Fetal Neonatal Ed. (2021) 106:194–203. doi: 10.1136/archdischild-2020-319099
53. Sunny AK, Paudel P, Tiwari J, Bagale BB, Kukka A, Hong Z, et al. A multicenter study of incidence, risk factors and outcomes of babies with birth asphyxia in Nepal. BMC Pediatr. (2021) 21:394. doi: 10.1186/s12887-021-02858-y
54. Hou L, Wang X, Li G, Zou L, Chen Y, Zhang W. Cross sectional study in China fetal gender has adverse perinatal outcomes in mainland China. BMC Pregnancy Childbirth. (2014) 26:372. doi: 10.1186/s12884-014-0372-4
55. Melamed N, Yogev Y, Glezerman M. Fetal gender and pregnancy outcome. J Matern Fetal Neonatal Med. (2010) 23:338–44. doi: 10.3109/14767050903300969
56. Weissmann-Brenner A, Simchen MJ, Zilberberg E, Kalter A, Dulitzky M. Combined effect of fetal sex and advanced maternal age on pregnancy outcomes. Med Sci Monit. (2015) 21:1124–30. doi: 10.12659/MSM.893057
57. Ju H, Chadha Y, Donovan T, O'Rourke P. Fetal macrosomia and pregnancy outcomes. Aust N Z J Obstet Gynaecol. (2009) 49:504–9. doi: 10.1111/j.1479-828X.2009.01052.x
58. Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS ONE. (2017) 12:e0175914. doi: 10.1371/journal.pone.0175914
59. Anderson E, Raja EA, Shetty A, Gissler M, Gatt M, Bhattacharya S, et al. Changing risk factors for placental abruption: a case crossover study using routinely collected data from Finland, Malta and Aberdeen. PLoS ONE. (2020) 15:e0233641. doi: 10.1371/journal.pone.0233641
60. Kadji C, Cannie MM, Carlin A, Jani JC. Protocol for the prospective observational clinical study: estimation of fetal weight by MRI to PREdict neonatal MACROsomia (PREMACRO study) and small-for-gestational age neonates. BMJ Open. (2019) 9:e027160. doi: 10.1136/bmjopen-2018-027160
61. Kaymak O, Iskender C, Ibanoglu M, Cavkaytar S, Uygur D, Danisman N. Retrospective evaluation of risk factors and perinatal outcome of umbilical cord prolapse during labor. Eur Rev Med Pharmacol Sci. (2015) 19:2336–9.
62. Sheen TC, Lu M-H, Lee MY, Chen SR. Nonreassuring fetal heart rate decreases heart rate variability in newborn infants. Ann Noninvasive Electrocardiol. (2014) 19:273–8. doi: 10.1111/anec.12139
63. Gravett C, Eckert LO, Gravett MG, Dudley DJ, Stringer EM, Mujobu TB, et al. Non-reassuring fetal status: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2016) 34:6084–92. doi: 10.1016/j.vaccine.2016.03.043
64. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. (1996) 334:613–8. doi: 10.1056/NEJM199603073341001
65. Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. (2011) 118:803–8. doi: 10.1097/AOG.0b013e31822f1b50
66. Pruksanusak N, Thongphanang P, Suntharasaj T, Suwanrath C, Geater A. Combined maternal-associated risk factors with intrapartum fetal heart rate classification systems to predict peripartum asphyxia neonates. Eur J Obstet Gynecol Reprod Biol. (2017) 218:85–91. doi: 10.1016/j.ejogrb.2017.09.008
67. Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. (2007) 197:26e1–6. doi: 10.1016/j.ajog.2007.03.037
68. Macones GA, Hankins GDV, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. (2008) 112:661–6. doi: 10.1097/AOG.0b013e3181841395
69. Kopincova J, Calkovska A. Meconium-induced inflammation and surfactant inactivation: specifics of molecular mechanisms. Pediatr Res. (2016) 79:514–21. doi: 10.1038/pr.2015.265
70. Torres-Muñoz J, Fonseca-Perez JE, Laurent K. Biological and psychosocial factors, risk behaviors, and perinatal asphyxia in a university hospital: matched case-control study, Cali, Colombia (2012-2014). Front Public Health. (2021) 9:535737. doi: 10.3389/fpubh.2021.535737
Keywords: predictive model, neonatal asphyxia, risk factors, fetal distress, cohort study
Citation: Yu Y, Gao J, Liu J, Tang Y, Zhong M, He J, Liao S, Wang X, Liu X, Cao Y, Liu C and Sun J (2022) Perinatal maternal characteristics predict a high risk of neonatal asphyxia: A multi-center retrospective cohort study in China. Front. Med. 9:944272. doi: 10.3389/fmed.2022.944272
Received: 15 May 2022; Accepted: 15 July 2022;
Published: 08 August 2022.
Edited by:
Marco La Verde, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyReviewed by:
Sebastian Isac, Carol Davila University of Medicine and Pharmacy, RomaniaFatemeh Yarmahmoodi, Shiraz University of Medical Sciences, Iran
Pablo Vázquez, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2022 Yu, Gao, Liu, Tang, Zhong, He, Liao, Wang, Liu, Cao, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsong Gao, gaojsong@163.com; Juntao Liu, 13901365269@163.com
 Yi Yu1,2
Yi Yu1,2  Mei Zhong
Mei Zhong Xinghui Liu
Xinghui Liu Caixia Liu
Caixia Liu