Three-dimensional ultrasound assessment of risk factors for cystocele and Green classification in primipara
- 1Department of Ultrasound, Second People’s Hospital of Wuhu, Wuhu, Anhui, China
- 2Department of Ultrasound, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
Background and aims: The present study aimed to analyze the effects of factors on cystocele and the Green classification.
Materials and methods: We conducted a cross-sectional study on 357 primiparous women examined at our hospital from January 2019 to May 2021. The following data were recorded: maternal characteristics, neonatal characteristics, and factors of childbirth. It was added to the multivariate logistic regression model to determine the independent predictors of the cystocele and the Green classification.
Results: A total of 242 women had cystocele, including 71 women with Green type I cystocele, 134 women with Green type II cystocele, and 37 women with Green type III cystocele. In multivariate logistic regression analysis, body mass index (BMI) at delivery was associated with cystocele, while BMI at delivery and the second stage of labor (SSL) > 1 h were independently with the distance from the symphysis pubis to the bladder neck (SPBN) abnormal (P < 0.05). BMI at examination was associated with the large retrovesical angle (RVA) (P < 0.05). BMI at delivery and the fetal right occiput anterior position (ROA) were independently associated with the distance from the symphysis pubis to the posterior wall of the bladder (SPBP) abnormal (P < 0.05), while epidural anesthesia (EDA) was the protective factor (P < 0.05).
Conclusion: Primipara women should strive to avoid exposure to modifiable risk factors such as controlling weight during pregnancy, reducing weight after delivery, and shortening SSL to reduce the occurrence of cystocele.
Introduction
Pelvic floor dysfunction (PFD) refers to a group of disorders caused by structural defects or degradation, damage, and dysfunction of the pelvic floor support system that severely affects the quality of life. Multiple symptoms of PFD may include stress urinary incontinence (SUI), pelvic organ prolapse (POP), chronic pelvic pain, constipation, and sexual abnormalities (1). Vaginal delivery (VD) significantly increases the possibility of POP in women (2), and it is the most critical epidemiological risk factor for PFD. Most of the damage to a woman’s pelvic floor is likely to occur during the first vaginal birth (3, 4). At present, most studies focus mainly on levator ani muscle (LAM) injury, but there are fewer studies on cystocele and the Green classification. Cystocele is the anterior vaginal wall prolapse accompanied by prolapse of the bladder wall, and it is the most common type of POP and the most prone to recur after surgery (5, 6). The current imaging classification of cystocele is proposed by Green. Cystocele has different clinical manifestations according to the Green’s classification (6). Radiological cystocele type (Green classification) can be distinguished both clinically and on ultrasound, and agreement between methods as well as inter-observer agreement for the clinical diagnosis is moderate to good (7).
In this study, We aim to use three-dimensional ultrasound to diagnosis cystocele and its classification, and analyzed the effects of maternal characteristics, fetal characteristics, and delivery factors on cystocele and its classification. We hypothesized that BMI after 3 months of vaginal delivery will be effect of cystocele and the Green classification.
We used three-dimensional ultrasound to detect cystocele and its type and analyzed the effects of maternal, fetal, and delivery factors on cystocele occurrence and its classification. The objective was to reduce pelvic floor damage in primipara women during childbirth.
Materials and methods
Study design
This was a cross-sectional study of healthy pregnant Chinese women. Details on maternal characteristics and delivery outcomes were obtained from the medical records of the our hospital from January 2019 to May 2021.
The inclusion criteria were nulliparous women with maternal age of ≥18 years, and time of ultrasonography was 42 to 90 days after delivery. Exclusion criteria were delivery at <28 gestational weeks and pre-existing diseases/conditions that are likely to pre-dispose to PFD. This included previous bladder/bowel diseases and chronic kidney disease. Participants did not perform any rehabilitation exercises. The Institutional Review Board approved the study protocol.
Clinical data
The hospital medical record system was checked, and the following details were recorded: maternal characteristics (include maternal age, body mass index (BMI) at delivery, BMI at ultrasound examination, gestational age, hypertension, diabetes), neonatal characteristics (include fetal height, head circumference, chest circumference, birth weight), and factors of childbirth (SSL, episiotomy, perineal tear, forceps use, vacuum use, and fetal orientation).
Ultrasonographic data
An ultrasonographic evaluation was performed by a single examiner with more than 5 years of experience in obstetric ultrasound and with specific training in 3/4D imaging. Women were examined with trans-perineal ultrasound using the GE Voluson E8 system with a 3D/4D RIC 5–9-D probe with an acquisition angle of 180°.
Before the examination, the subject emptied the bladder (urine volume < 50 ml) and rectum and assumed the lithotomy position. The probe was wrapped in a condom and placed above the labia minora. The probe was placed close to the lower edge of the pubic symphysis for a clear display of the midsagittal section of the pelvic floor structure and the position and shape of the bladder were observed under the resting state and Valsalva. Valsalva movement requirements: the duration lasts more than 5 s, while the levator ani muscle hiatus is observed to be dilated, and the pelvic organs are displaced to the dorsal caudal side. The lower edge of the symphysis pubis (SP) was used as the reference level to analyzed the following parameters: the retrovesical angle (RVA), urethral inclination angle, distance from the inferior margin of the symphysis pubis to the bladder neck (SPBN), and the distance from the inferior margin of the symphysis pubis to the posterior wall of the bladder (SPBP) at rest and Valsalva. The urethral rotation angle (URA) was also calculated.
A cystocele was diagnosed on ultrasound if any part of the bladder below the symphysis pubis (8). According to Green classification, the following types of cystocele were diagnosed: Green I cystocele (RVA ≥ 140°, URA < 45°), Green II cystocele (RVA ≥ 140°, URA between 45° and 120°, and Green III (RVA < 140°, the lowest point of the bladder reaching below the symphysis pubis) (9). In Green III cystocele, the lowest point of the bladder is often the posterior wall of the bladder, unlike the bladder neck in other types.
Statistical analyses
Statistical analyses were performed using SPSS v. 20 (SPSS, Chicago, IL, USA). The normality of data was assessed using the Shapiro Wilk method. Normally and non-normally distributed continuous data were expressed as mean ± standard deviation and quartile, respectively. Normally and non-normally distributed continuous data were analyzed by Student’s t-test and Mann-Whitney U test, respectively. Categorical variables were analyzed by the chi-square test. P < 0.05 was considered to be statistically significant. Logistic regression analysis was used to demonstrate independent risk factors for cystocele while controlling for potential confounding factors.
Results
A total of 357 women who had vaginal delivery were enrolled in this study. Of these, 242 (67.8%) women had cystocele, including 71 (19.9%) women with Green type I cystocele, 134 (37.5%) women with Green type II cystocele, and 37 (10.4%) women with Green type III cystocele. Moreover, 281 (78.7%) women had abnormal SPBN, 198 (55.5%) women had increased URA, 240 (67.2%) women had open RVA, and 97 (27.2%) women had abnormal SPBP.
The results for the hospital parameters were as follows: episiotomy: 93 (26.1%) women; second-degree perineal tear: 213 (59.3%) women; epidural anesthesia: 18 (47.1%) women, diabetes: 28 (7.8%) women; hypertension: 4 (1.1%) women; assisted labor: 4 (1.1%) women [including 1 (0.3%) woman with forceps-assisted delivery and 3 (0.8%) women with vacuum-assisted delivery]. The following results were noted for fetal orientation: fetal left occiput anterior (LOA) position in 252 (70.6%) women, fetal right occiput anterior (ROA) position in 81 (22.7%) women, and other fetal positions in 24 (6.72%) women [including occipital posterior (OP) position in 11 (3.1%) women and occipital bone is directly in front of the pubic symphysis position in 13 (3.6%) women].
In the univariate analysis, BMI at delivery, BMI at the examination, and birth weight in patients with cystocele were greater than those in the normal group, and the SSL in these patients was also had an impact on the cystocele group. BMI at delivery, BMI at the examination, and birth weight were greater in the abnormal SPBN group than in the normal group. SSL and different fetal positions also had an impact on SPBN (Table 1). BMI at delivery with increased URA was greater than that in the normal group. BMI at delivery, BMI at the examination, and birth weight were greater in patients with large RVA than in the normal group. The BMI of patients with abnormal SPBP was also higher than that of the normal group. Epidural anesthesia (EDA) and fetal position were found to affect SPBP (Table 2).
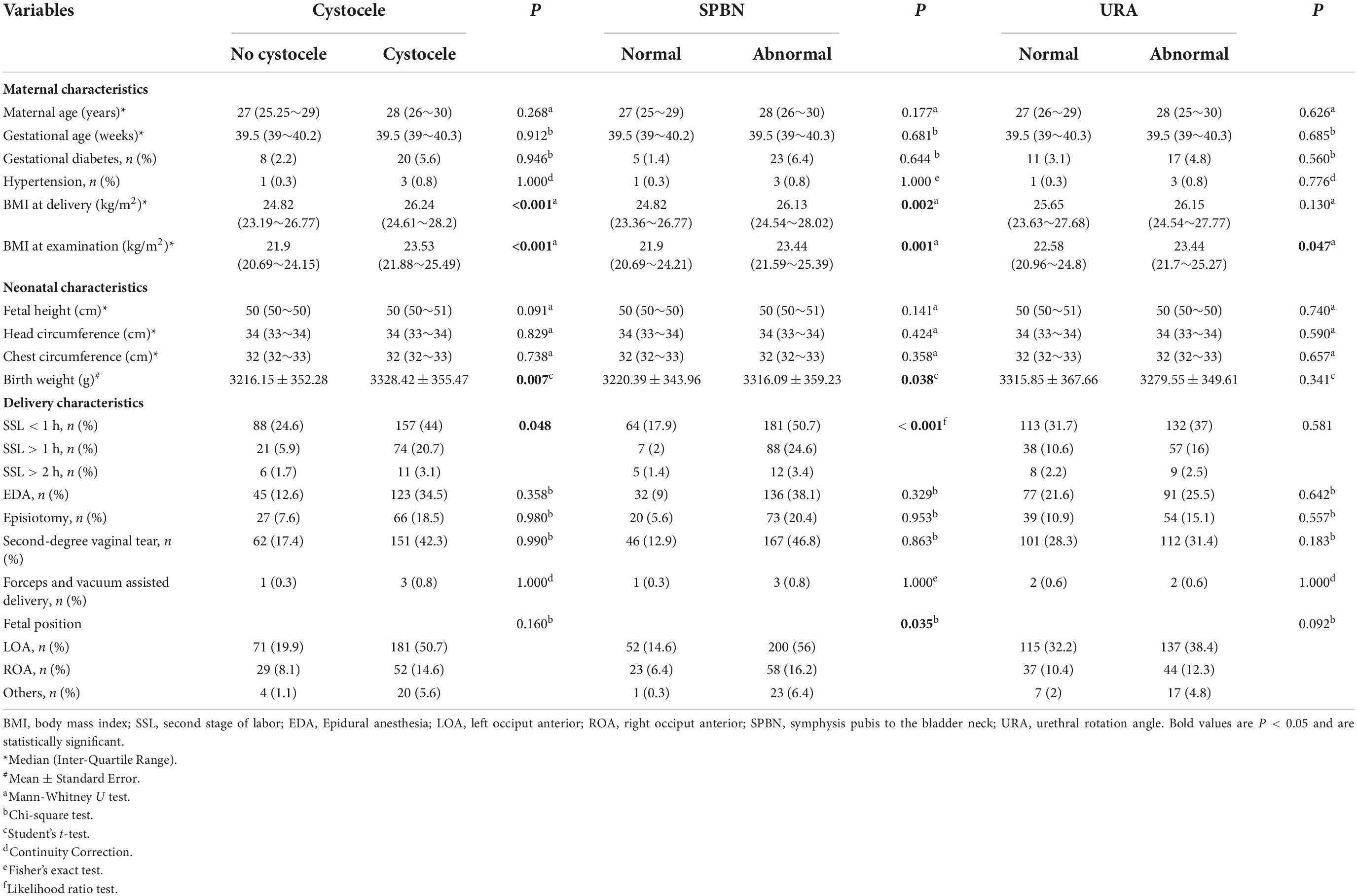
Table 1. Analysis of factors associated with cystocele, symphysis pubis to the bladder neck (SPBN), and urethral rotation angle (URA).
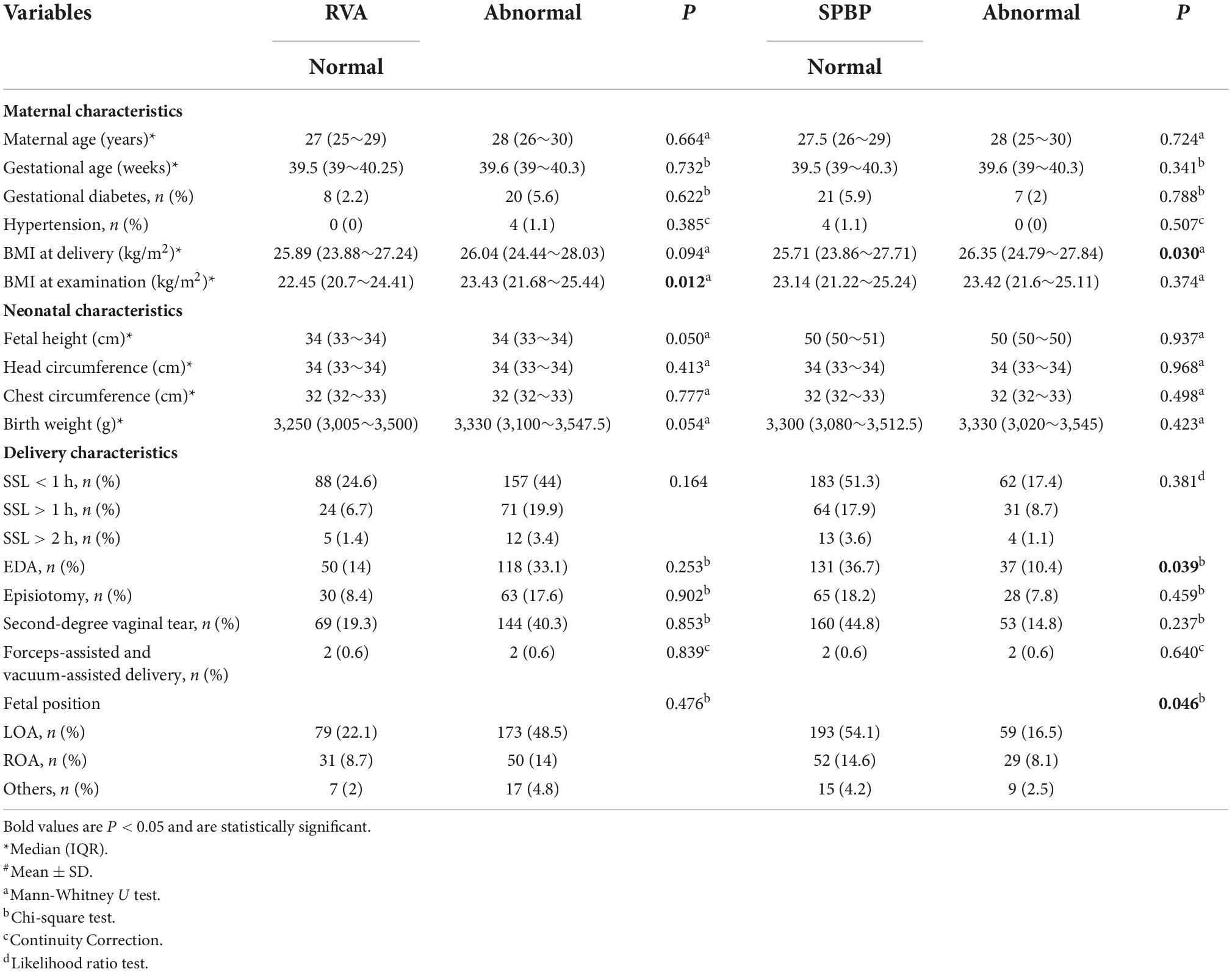
Table 2. Analysis of factors associated with retrovesical angle (RVA) and symphysis pubis to the posterior wall of the bladder (SPBP).
According to the results of the univariate analysis, the above factors were entered into the multivariate logistic regression model (forward, conditional). BMI at delivery was an independent risk factor of cystocele (OR = 1.145, 95% CI: 1.053∼1.245); BMI at delivery (OR = 1.18, 95% CI: 1.065∼1.307) was independent risk factors of SPBN (P < 0.05). The SSL > 1 h compared to the SSL < 1 h (OR = 4.10, 95% CI: 1.786∼1.562) was the risk factors of SPBN (P < 0.05). BMI at examination (OR = 1.103, 95% CI: 1.018∼1.196) was the risk factor for the large RVA (P < 0.05). BMI at delivery (OR = 1.091, 95% CI: 1.006∼1.183) and the fetal ROA position compared to the LOA were the risk factors of SPBP (P < 0.05), while EDA (OR = 0.582, 95% CI: 0.358∼0.947) was the protective factor (P < 0.05). In the logistic regression analysis, all factors had no significant association with URA (P > 0.05) (Figure 1).
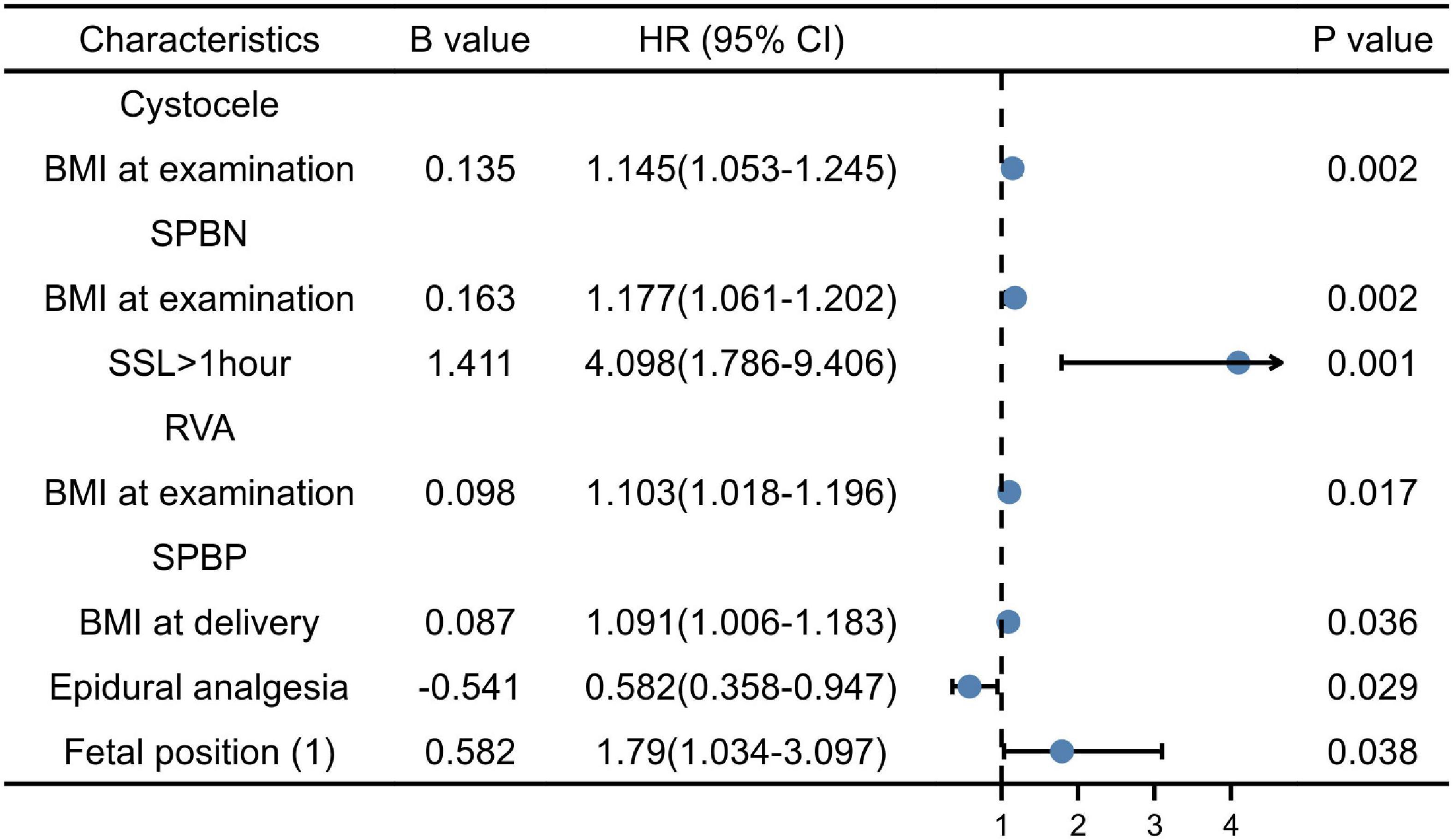
Figure 1. Multiple factor analysis of cystocele, symphysis pubis to the bladder neck (SPBN), retrovesical angle (RVA), and symphysis pubis to the posterior wall of the bladder (SPBP).
Discussion
Mechanical injury to the pelvic floor support system, denervation, ischemia and reperfusion injury, and defective soft tissue remodeling are some of the underlying mechanisms of injury for the development of PFDs (10). During pregnancy and after VD, pelvic organ support changes, and area of levator hiatus (HA) increases; thus, indicating a decrease in pelvic organ support (11, 12), This may increase the risk of PFD. The present study found that many factors influence the development of maternal cystocele during delivery.
Maternal and neonatal characteristics
Similar to previous studies, we found that the increase in BMI at delivery was significantly associated with abnormal SPBP (OR = 1.091, 95% CI: 1.006∼1.183). Not only does BMI during pregnancy increase the risk of pelvic floor disease, but BMI after delivery also affects the occurrence of cystocele. The increase in BMI at examination was significantly associated with abnormal SPBN (OR = 1.18, 95% CI: 1.065∼1.307) and open RVA (OR = 1.103, 95% CI: 1.018∼1.196). An obvious potential explanation for the increased prevalence of cystocele in obese women may be the long-term increase in intra-abdominal pressure in these individuals. Noblett et al. (13) found a strong correlation between BMI and intra-abdominal pressure, and between BMI and intravesical pressure, with Pearson’s correlation coefficient of 0.76 and 0.71, respectively. The chronic increased pressure may lead to pelvic floor muscle fatigue and/or a chronic stretch on the pudendal nerve, which in turn may lead to pelvic floor muscle weakness. Although the decrease in pelvic muscle strength after VD will lead to POP, the correlation between them will weaken after a reduction in BMI (14). For primipara women, early weight loss can also help to reduce cystocele.
In other studies on the pelvic floor, age was found to be an important factor for cystocele (15). In the present study, no significant association was found between age and Green’s classification of cystocele. This may be because all the primipara women in this study were young. In addition, there were fewer cases of gestational diabetes mellitus and gestational hypertension in this study, neither hypertension nor GDM showed a statistically significant association with cystocele.
The most important risk factor for avulsion injuries during natural delivery is the birth weight (16). The increase in birth weight has an important effect on the time of labor and subsequently has a series of effects on the pelvic floor muscles. In our study, it had no significant in the multivariate binary logistic analysis on cystocele, only appeared as a significant variable in the Mann-Whitney U test.
Delivery characteristics
Prolonged labor may exceed the stretch limit of soft tissues, resulting in an imbalance in the repair and degradation process. When used for a long time, stress may cause temporary or permanent physical and/or functional damage through hypoxia, ischemia, and other harmful processes, leading to PFD (17). We found that SSL > 1 h was 4.10 times higher than SSL < 1 h for primary maternal of abnormal SPBN. Reports on the effects of episiotomy and perineal tear on pelvic floor function are not completely consistent (18, 19). But most studies believe that the use of episiotomy during vacuum-assisted delivery or forceps-assisted delivery of primipara women can reduce perineal tear or OASIS (20, 21). We did not confirm that episiotomy and tear have a significant effect on cystocele, which is consistent with the report of Ruan et al. (22). This may be because of the low episiotomy rate or the short observation period, or it may be because of only first-and second-degree tears in our study. Studies have shown that third-degree perineal tear is a significant risk factor for postpartum PFD (23).
Schiessl B (24) found that EDA can prolong SSL, thereby increasing the risk of UI; however, in our study we think that EDA is a protective factor of SPBP (OR = 0.582, 95% CI: 0.358∼0.947). The EDA effect could be explained by the resulting muscle relaxation. It is plausible that active pushing in labor distends and compresses the pelvic floor more forcefully, resulting in neuromuscular or vascular injury. Intrapartum epidural analgesia may be beneficial by preventing premature pushing. Another potential explanation may be the degree of levator relaxation in women with dense epidurals because a paralyzed muscle is less likely to suffer trauma, given a certain degree of distension (25).
The occipital position (OP) is the most common malposition with a prevalence of 5–13% at delivery (26). It increases the risk of LAM injury (27). In the present study, there is no difference in statistics due to the low numbers of OP. However, to our surprise, the results showed that ROA was more prone to SPBP abnormalities than LOA (OR = 1.79, 95% CI: 1.034∼3.097).
Strengths and limitations
All women underwent delivery in the same hospital following similar obstetric approaches. Moreover, all parturients had complete delivery information, which largely reduced the recall bias. Previous studies on postpartum pelvic floor injury mostly focused on factors such as birth weight, weight gain during pregnancy, and the labor process. In our study, we found that not only BMI at delivery has an important effect on cystocele in primipara women, but BMI within 42∼90 days postpartum is also an independent risk factor for abnormal SPBP and open RVA. Early postpartum weight loss can reduce pelvic floor injury.
The limitations of our study are a small sample size and relatively inadequate data. In this study, all primipara women were younger, with less degree of hypertension and diabetes. However, in future research, we will collect more samples for a more comprehensive analysis.
Conclusion
During delivery, the pelvic floor muscles of pregnant women will continue to be affected by mechanical injury and physiological changes after delivery. Some potential risk factors are uncontrollable, such as maternal age, fetal position, and fetal weight. However, according to the currently available data, efforts should be made to avoid exposure to modifiable risk factors, such as controlling weight during pregnancy, reducing weight after delivery, shortening the SSL, and reducing the occurrence of cystocele.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WY: project development, manuscript writing, data analysis, and data collection. QM: project development, data analysis, and manuscript editing. WX: data collection and manuscript editing. YZ: data analysis. JW: project development, data collection, and manuscript editing. YZ: statistics and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dwyer PL. Female pelvic floor dysfunction and estrogen therapy. Climacteric. (2001) 4:179–80. doi: 10.1080/cmt.4.3.179.180
2. Hallock JL, Handa VL. The epidemiology of pelvic floor disorders and childbirth: an update. Obstet Gynecol Clin North Am. (2016) 43:1–13. doi: 10.1016/j.ogc.2015.10.008
3. Kamisan Atan I, Lin S, Dietz HP, Herbison P, Wilson PD, ProLong Study G. It is the first birth that does the damage: a cross-sectional study 20 years after delivery. Int Urogynecol J. (2018) 29:1637–43. doi: 10.1007/s00192-018-3616-4
4. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the women’s health initiative: gravity and gravidity. Am J Obstet Gynecol. (2002) 186:1160–6. doi: 10.1067/mob.2002.123819
5. Miedel A, Tegerstedt G, Morlin B, Hammarstrom M. A 5-year prospective follow-up study of vaginal surgery for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. (2008) 19:1593–601. doi: 10.1007/s00192-008-0702-z
6. Bu L, Yang D, Nie F, Li Q, Wang YF. Correlation of the type and degree of cystocele with stress urinary incontinence by transperineal ultrasound. J Med Ultrason. (2020) 47:123–30. doi: 10.1007/s10396-019-00972-0
7. Chantarasorn V, Dietz HP. Diagnosis of cystocele type by clinical examination and pelvic floor ultrasound. Ultrasound Obstet Gynecol. (2012) 39:710–4. doi: 10.1002/uog.10156
8. Dietz HP. Ultrasound in the assessment of pelvic organ prolapse. Best Pract Res Clin Obstet Gynaecol. (2019) 54:12–30. doi: 10.1016/j.bpobgyn.2018.06.006
9. Eisenberg VH, Chantarasorn V, Shek KL, Dietz HP. Does levator ani injury affect cystocele type? Ultrasound Obstet Gynecol. (2010) 36:618–23. doi: 10.1002/uog.7712
10. Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Womens Health. (2013) 9:265–77; quiz 276–7. doi: 10.2217/whe.13.17
11. Reimers C, Staer-Jensen J, Siafarikas F, Saltyte-Benth J, Bo K, Ellstrom Engh M. Change in pelvic organ support during pregnancy and the first year postpartum: a longitudinal study. BJOG. (2016) 123:821–9. doi: 10.1111/1471-0528.13432
12. Staer-Jensen J, Siafarikas F, Hilde G, Benth JS, Bo K, Engh ME. Postpartum recovery of levator hiatus and bladder neck mobility in relation to pregnancy. Obstet Gynecol. (2015) 125:531–9. doi: 10.1097/AOG.0000000000000645
13. Noblett KL, Jensen JK, Ostergard DR. The relationship of body mass index to intra-abdominal pressure as measured by multichannel cystometry. Int Urogynecol J Pelvic Floor Dysfunct. (1997) 8:323–6. doi: 10.1007/BF02765589
14. Blomquist JL, Carroll M, Munoz A, Handa VL. Pelvic floor muscle strength and the incidence of pelvic floor disorders after vaginal and cesarean delivery. Am J Obstet Gynecol. (2020) 222:62.e1–62.e8. doi: 10.1016/j.ajog.2019.08.003
15. Karjalainen PK, Gillor M, Dietz HP. Predictors of occult stress urinary incontinence. Aust N Z J Obstet Gynaecol. (2021) 61:263–9. doi: 10.1111/ajo.13290
16. Garcia-Mejido JA, Gutierrez-Palomino L, Borrero C, Valdivieso P, Fernandez-Palacin A, Sainz-Bueno JA. Factors that influence the development of avulsion of the levator ani muscle in eutocic deliveries: 3-4d transperineal ultrasound study. J Matern Fetal Neonatal Med. (2016) 29:3183–6. doi: 10.3109/14767058.2015.1118041
17. Nygaard IE, Clark E, Clark L, Egger MJ, Hitchcock R, Hsu Y, et al. Physical and cultural determinants of postpartum pelvic floor support and symptoms following vaginal delivery: a protocol for a mixed-methods prospective cohort study. BMJ Open. (2017) 7:e014252. doi: 10.1136/bmjopen-2016-014252
18. Leombroni M, Buca D, Liberati M, Falo E, Rizzo G, Khalil A, et al. Post-partum pelvic floor dysfunction assessed on 3d rotational ultrasound: a prospective study on women with first- and second-degree perineal tears and episiotomy. J Matern Fetal Neonatal Med. (2021) 34:445–55. doi: 10.1080/14767058.2019.1609932
19. Shmueli A, Gabbay Benziv R, Hiersch L, Ashwal E, Aviram R, Yogev Y, et al. Episiotomy - risk factors and outcomes(). J Matern Fetal Neonatal Med. (2017) 30:251–6. doi: 10.3109/14767058.2016.1169527
20. van Bavel J, Hukkelhoven C, de Vries C, Papatsonis DNM, de Vogel J, Roovers JWR, et al. The effectiveness of mediolateral episiotomy in preventing obstetric anal sphincter injuries during operative vaginal delivery: a ten-year analysis of a national registry. Int Urogynecol J. (2018) 29:407–13. doi: 10.1007/s00192-017-3422-4
21. Frenette P, Crawford S, Schulz J, Ospina MB. Impact of episiotomy during operative vaginal delivery on obstetrical anal sphincter injuries. J Obstet Gynaecol Can. (2019) 41:1734–41. doi: 10.1016/j.jogc.2019.02.016
22. Ruan L, Xu X, Wu H, Xiao Y, Li W, Lin H, et al. Painless labor with patient-controlled epidural analgesia protects against short-term pelvic floor dysfunction: a retrospective cohort study. Ann Palliat Med. (2020) 9:3326–31. doi: 10.21037/apm-20-1430
23. Durnea CM, Khashan AS, Kenny LC, Durnea UA, Dornan JC, O’Sullivan SM, et al. What is to blame for postnatal pelvic floor dysfunction in primiparous women-pre-pregnancy or intrapartum risk factors? Eur J Obstet Gynecol Reprod Biol. (2017) 214:36–43. doi: 10.1016/j.ejogrb.2017.04.036
24. Schiessl B, Janni W, Jundt K, Rammel G, Peschers U, Kainer F. Obstetrical parameters influencing the duration of the second stage of labor. Eur J Obstet Gynecol Reprod Biol. (2005) 118:17–20. doi: 10.1016/j.ejogrb.2004.01.045
25. Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG. (2010) 117:1485–92. doi: 10.1111/j.1471-0528.2010.02704.x
26. Simkin P. The fetal occiput posterior position: state of the science and a new perspective. Birth. (2010) 37:61–71. doi: 10.1111/j.1523-536X.2009.00380.x
Keywords: ultrasound, vaginal delivery, cystocele, Green classification, pelvic floor dysfunction
Citation: Yin W, Ma Q, Xie W, Zhu Y and Wang J (2022) Three-dimensional ultrasound assessment of risk factors for cystocele and Green classification in primipara. Front. Med. 9:979989. doi: 10.3389/fmed.2022.979989
Received: 28 June 2022; Accepted: 08 November 2022;
Published: 30 November 2022.
Edited by:
Simcha Yagel, Hadassah Medical Center, IsraelReviewed by:
Ali Çetin, University of Health Sciences (Turkey), TurkeyDulce Oliveira, University of Porto, Portugal
Copyright © 2022 Yin, Ma, Xie, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junli Wang, wjl980134@163.com
†These authors have contributed equally to this work
 Weiwei Yin1†
Weiwei Yin1†  Qianqing Ma
Qianqing Ma Junli Wang
Junli Wang