A 26-year-old man with multiple organ failure caused by Aeromonas dhakensis infection: a case report and literature review
- 1Department of Respiratory and Critical Care Medicine, People’s Hospital of Chongqing Liang Jiang New Area, Chongqing, China
- 2Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Infections in humans are mainly caused by Aeromonas hydrophila, Aeromonas caviae, and Aeromonas veronii. In recent years, Aeromonas dhakensis has been recognized as widely distributed in the environment, with strong virulence. However, this bacterial infection usually does not appear in patients with pneumonia as the first symptom.
Case report: We report a 26-year-old man who was admitted to the hospital with community-acquired pneumonia as the first symptom and developed serious conditions such as hemolytic uremic syndrome, multiple organ dysfunction, and hemorrhagic shock within a short period. He died after 13 h of admission, and the subsequent metagenomic-next generation sequencing test confirmed the finally identified pathogen of infection as A. dhakensis.
Conclusion: Aeromonas is a rare pathogen identified in the diagnosis of community-acquired pneumonia. Hence, doctors need to develop their experience in identifying the difference between infections caused by pathogenic microorganisms. Medical attention is essential during the occurrence of respiratory symptoms that could be controlled by empirical drugs, such as cephalosporins or quinolones. When patients with community-acquired pneumonia present hemoptysis and multiple organ dysfunction in clinical treatment, an unusual pathogen infection should be considered, and the underlying etiology should be clarified at the earliest for timely treatment.
Introduction
Aeromonas dhakensis is a Gram-negative bacillus that is widely distributed in water environments. The mortality rate caused by infection with A. dhakensis is higher than that of other Aeromonas infections due to the abundance of virulence genes. It causes gastroenteritis, wound infection, sepsis, respiratory tract infection, hepatobiliary disease, urinary tract infection, muscle necrosis, rhabdomyolysis, necrotizing fasciitis, and the rare
hemolytic uremic syndrome. If an acute infection is not treated promptly, it may develop rapidly and lead to serious consequences. In this case study, we reported the onset and treatment of a 26-year-old patient infected with A. dhakensis.
Case report
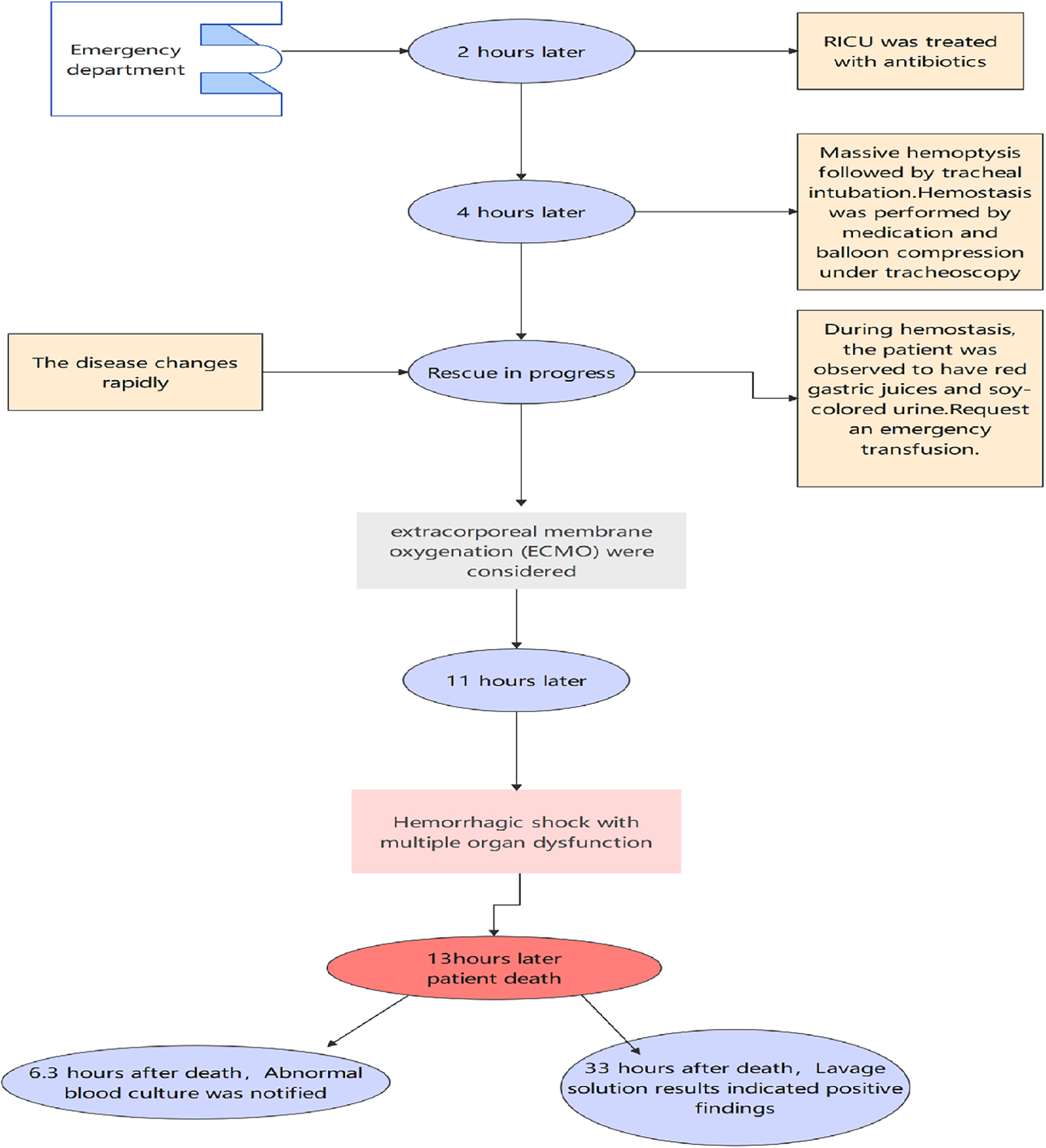
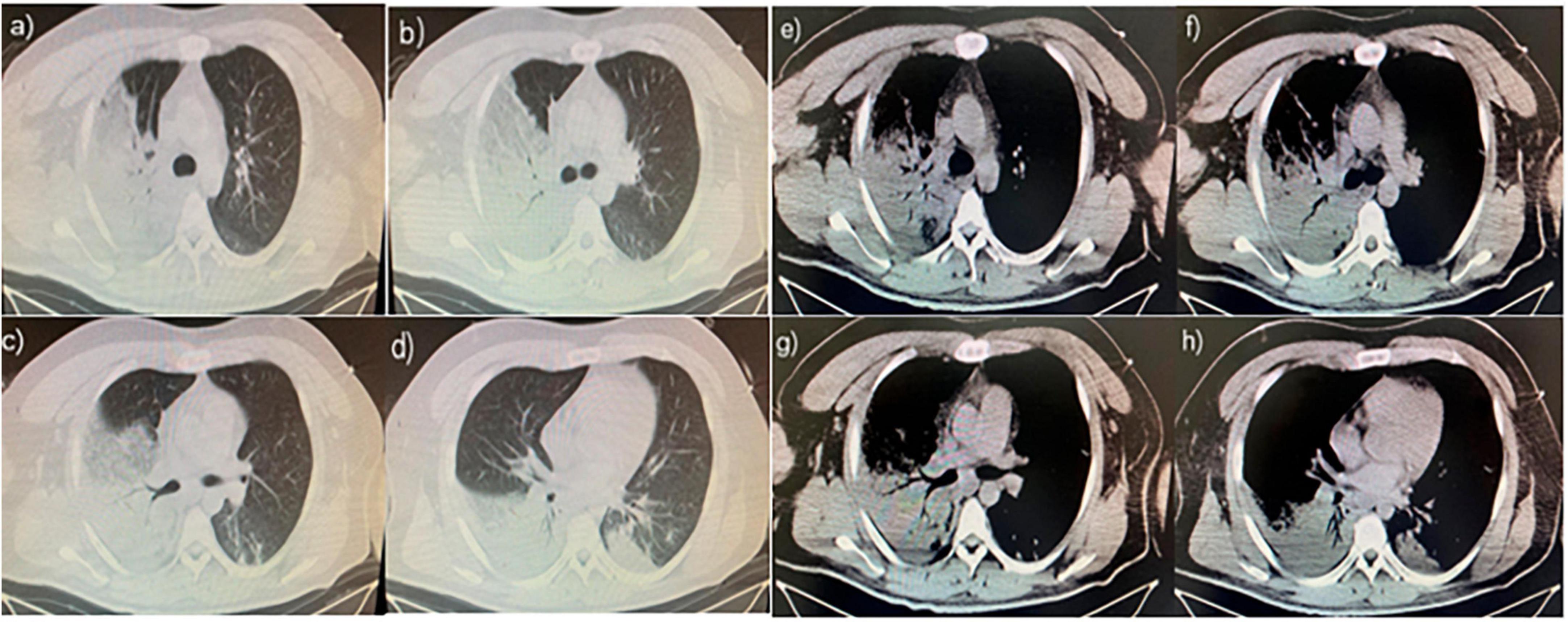
A 26-year-old man with no history of lung diseases or other disorders was admitted to the local hospital due to cough and fatigue for 3 days, hemoptysis, dyspnea, fever, chest pain, and wheezing for a day. The patient was admitted to our emergency department. A blood routine examination showed that the white blood cell count was 7.06 × 109/L, and the percentage of neutrophils was 69.9%. The liver function showed alanine aminotransferase at 150 U/L and aspartate transferase at 84 U/L, and the kidney function-related creatinine level was 197 mmol/L, and the uric acid level was 607 mmol/L. The coagulation function D-dimer level was 3730 ng/mL. Blood gas analysis (without oxygen) revealed the following: pH 7.32, PCO2 43 mmHg, PO2 37 mmHg, BE −3.9 mmol/L, HCO3 22.21 mmol/L, Lac 4.8 mmol/L, SO2 65%, Na+ 132 mmol/L, K+ 3.8 mmol/L, and Glu tendency of 7.1 mmol/L. The Chest computer tomography (CT) scan displayed a double lung infection (Figure 1). A physical examination revealed a body temperature of 37.5°C, and vital signs were within normal range. Lucid, poor spirit, and wet rales could be heard in both lungs. After 2 h, the patient was transferred to the Respiratory Intensive Care Unit (RICU).
According to the examination, the patient was diagnosed with severe pneumonia and abnormal liver function. While being kept on a non-invasive ventilator, 1,000 mg of imipenem was given intravenously every 8 h, 600 mg of linezolid was given intravenously every 6 h, and reduced glutathione, polyene phosphatidylcholine, and carlo sulfonyl sodium were used. After transferring the patient to RICU post 2 h, the hemoptysis level increased to almost 100 ml, and blood gas analysis indicated respiratory failure. Thus, the patient received endotracheal intubation. Then, tracheoscopy was performed and active hemorrhage was observed in the right upper lobe opening. The lavage fluid was collected and cultured. The right upper lobe bronchus was blocked by a bronchoscope balloon, pituitrin, carlo sulfonyl sodium, and hemagglutinin to stop the bleeding.
Typically, the bleeding could be controlled, but it was counterproductive. The blood was drained from the stomach tube, the urine turned to a soy sauce color, and blood pressure dropped. Considering the patient had gastrointestinal bleeding and hemolysis, emergency blood transfusion measures were initiated, and vasoactive drugs were administered. To date, 1,000 ml of blood was aspirated under a bronchoscope, 300 ml from a gastric tube, and 150 ml was drained by the urinary tube, and the patient went into hemorrhagic shock. In addition, antishock treatment and extracorporeal membrane oxygenation (ECMO) were considered.
These measures stabilized the patient’s blood pressure at 80–95/50–55 mmHg, and the oxygen saturation was stabilized at 80%–90%. Indubitably, the patient’s condition deteriorated rapidly, leading to multiple organ failures before the initiation of ECMO therapy and eventually leading to death. At 6.3 h after death, the blood cultures suggested the presence of Aeromonas hydrophila/Aeromonas caviae (Figure 2). At 33 h, irrigation fluid culture also signaled the presence of A. hydrophila/A. caviae after the patient’s death. This is the first case of Aeromonas infection that we have encountered and also a rare case of multiple organ failure reported in the literature.
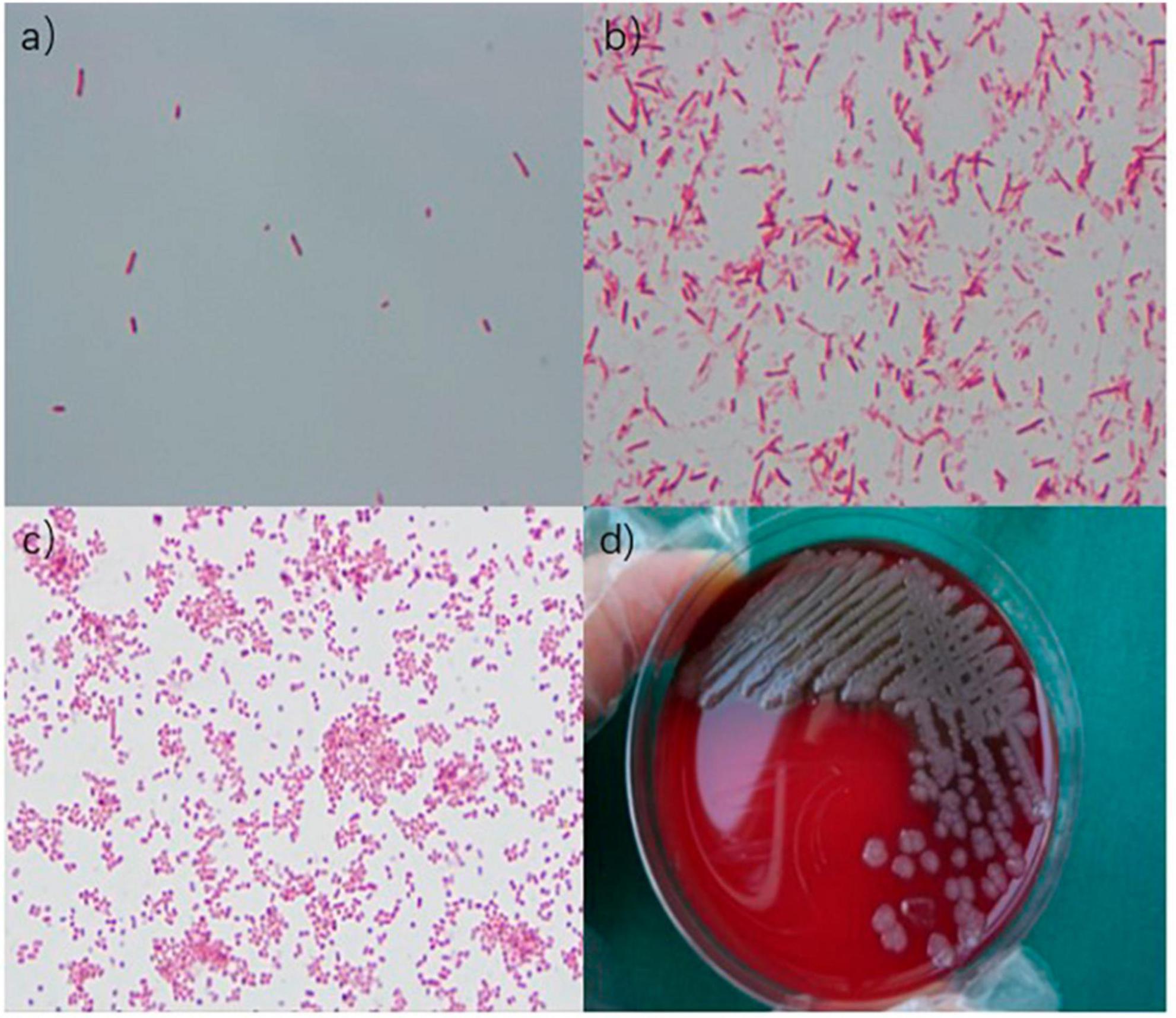
Figure 2. (a) Gram-negative bacilli were observed under an oil immersion lens. (b) Gram-negative bacilli were observed under an oil immersion lens. (c) Gram-negative bacilli were observed under a microscope. (d) Medium colony.
Discussion
Aeromonas is a Gram-negative bacillus widely distributed in freshwater, river/estuarine water (brackish water), surface water, drinking water, polluted water bodies, and sewage sludge (1–5). The classification includes A. hydrophila, A. caviae, Aeromonas veronii biovar sobria, Aeromonas milmiliae, Aeromonas salmonicides, Aeromonas intermedialis, Aeromonas janeii, Aeromonas shuiensis, and Aeromonas fragilis (6). Human infections are commonly caused by A. hydrophila, A. veronii biovar sobria, and A. caviae (7). The most common route of infection is contact with fresh or brackish water, which is usually stagnant in warm months (May–October in the Northern Hemisphere), its bacterial count reaches a peak value, thereby elevating the incidence of Aeromonas infection in summer (8, 9). Patients with chronic underlying diseases, such as nephritis, diabetes, tumors, leukemia, and hepatobiliary pancreas, have low immunity and are at a high risk of Aeromonas infection. It directly enters the blood through the peritoneal barrier and reaches the thoracic tissue, pelvic tissue, lymph, gallbladder, and other parts. In severe cases, it may be life-threatening (10). The patient, in this case, was a 26-year-old man with no underlying diseases or low immunity. However, he had embarked on self-driving trips for 3 days before the onset of the disease. En route, he swam in a lake, which could be the cause of Aeromonas infection; the onset was rapid, and the clinical manifestations were critical.
Aeromonas cause a variety of human diseases such as diarrhea, wound infection, bacteremia, respiratory tract infection, eye infection, osteomyelitis, meningitis, pelvic abscess, otitis, cystitis, endocarditis, peritonitis, cholecystitis, joint infection, necrotizing fasciitis, and folliculitis (11–16). Diarrhea is the most common clinical manifestation. Different from the typical infection symptoms, this patient had respiratory symptoms of cough, expectoration, and hemoptysis as initial presentation, without diarrhea.
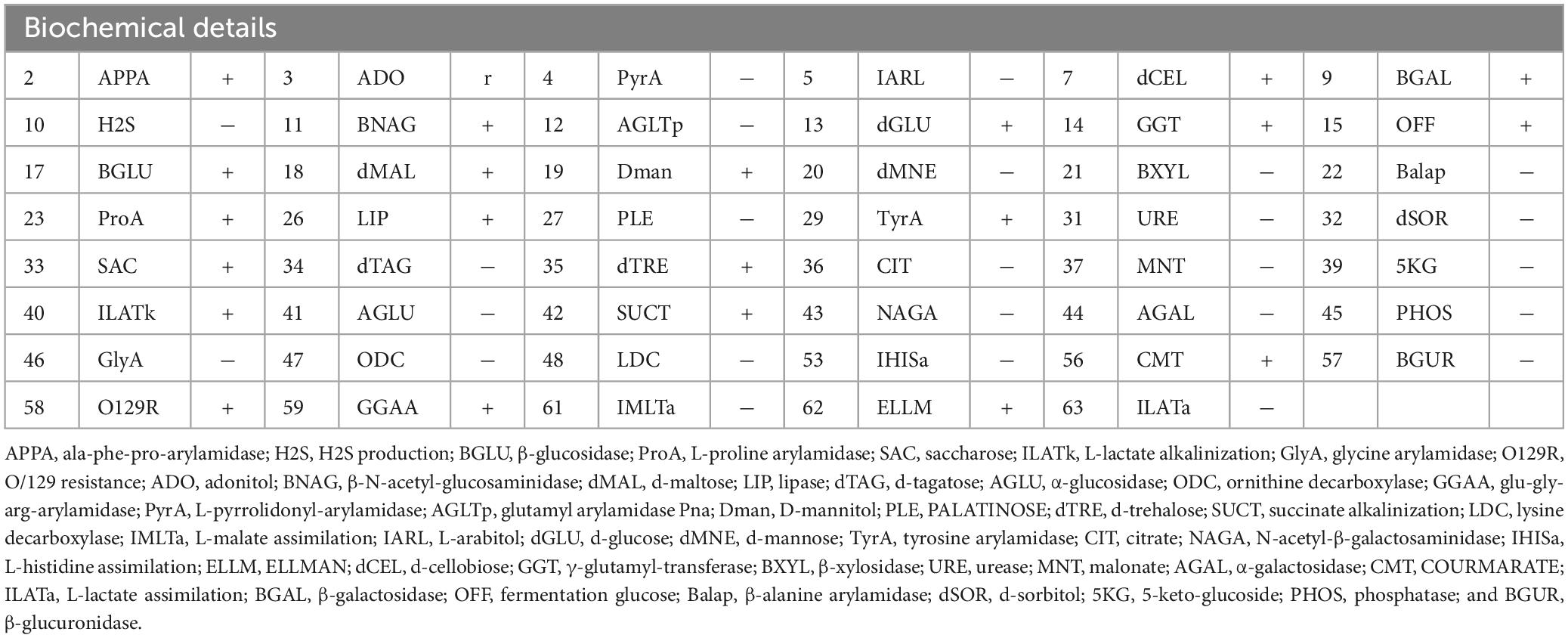
Within 11 h of admission, 1,000 ml of blood was aspirated under a bronchoscope, 300 ml of blood from the gastric tube, and 150 ml of blood was drained by the urinary tube. Subsequently, Aeromonas was detected in blood culture and bronchoscopic lavage fluid culture, and the biochemical results are shown in Table 1. The results indicated that the patient was infected with Aeromonas, leading to Aeromonas hemolytic-uremic syndrome, which is a rare manifestation caused by this bacterium. Since A. hydrophila/A. caviae is often confused with A. dhakensis due to similar homology, conventional automatic laboratory biochemical identification cannot distinguish the species type effectively (17). Hence, metagenomic-next generation sequencing was recommended for species identification by whole genome sequencing. The results finally confirmed A. dhakensis infection in the patient.
It is a subspecies of A. hydrophila, also known as Aquariumonas. It was originally isolated from children with diarrhea in Bangladesh during the period of 1993–1994 (18). In Beaz-Hidalgo et al. (19), ascertained that the Dakar subspecies of A. hydrophila and A. aquarium were the same species and that the Dakar subspecies were different from other subspecies of A. hydrophila. Therefore, the Dakar subspecies of A. hydrophila and A. aquarium were merged into a new species of A. dhakensis (19). Accumulating evidence shows that A. dhakensis is widely distributed in the environment, primarily in coastal areas, and can cause various human and animal infections, including gastroenteritis, wound infection, sepsis, respiratory tract infection, hepatobiliary disease, urinary tract infection, muscle necrosis, rhabdomyolysis, and necrotizing fasciitis (20). The reported mortality rate among patients with A. dhakensis extraintestinal infection varies from 25.5% to 37.5%, which is much higher than in those infected with other Aeromonas species (0%–14%) (20, 21). Previous studies reported that A. dhakensis carries several virulence factors and exerts high toxicity on human blood cell lines (22–25) via exotoxins (act, aerA, hlyA, alt, ast, and other genes), type III secretion system (aexT, aopP, ascF-G, ascV, and other genes), extracellular enzymes (gcat, exu, ahyB, lip, ser, epr CAI, and other genes), adhesion factors associated with invasion (tapA gene), and flagella (laf and fla genes) (7). The high mortality rate and the abundance of virulence genes make it a crucial pathogenic species. However, the pathogenesis mechanism and regulation of toxicity remain unclear (26). The majority of Aeromonas bacteria carry at least one virulence gene. Act, hlyA, aerA, gcat, and lip genes related to cytolysis were detected in both enteric Aeromonas and exenteric Aeromonas to varying degrees, which cause hemolysis in the body.
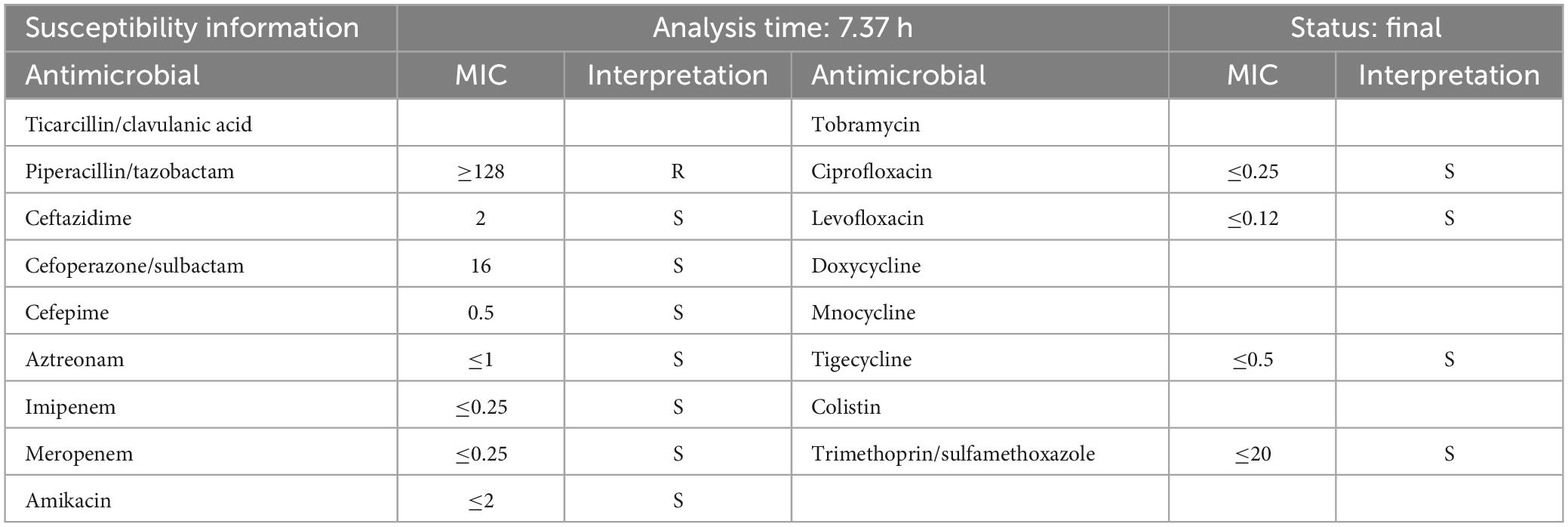
Aeromonas dhakensis is susceptible to cefepime, aminoglycosides, fluoroquinolones, and tetracyclines, and hence, these drugs can be used for the treatment of the infection (22, 27). Chao et al. (28) indicated that more than 80% of clinical strains are sensitive to third- or fourth-generation cephalosporins, aminoglycosides, fluoroquinolones, and imipenems. Therefore, the above drugs are still the first choice for treatment. However, A. dhakensis intrinsically harbors class B (metallo-β-lactamases, MBLs; CphA), C (AmpC cephalosporinase; AQU-1), and D β-lactamases (penicillinases). CphA has a specific substrate profile for hydrolyzing carbapenems; however, carbapenem therapy using CphA on Aeromonas infections remains controversial (29, 30). It is notable that ertapenem is a breakthrough in the treatment of bacteremia caused by A. dhakensis (29). The best antibacterial options for treating A. dhakensis infection include fluoroquinolone or cefepime until susceptibility results are available (20). For severe pneumonia cases, we chose imipenem and linezolid for anti-infection treatment. Combined with blood culture and drug sensitivity test of the patient as shown in Table 2, A. dhakensis was found to be susceptible to ceftazidime (MIC = 2 μg/ml), cefoperazone/sulbactam (MIC = 16 μg/ml), cefepime (MIC = 0.5 μg/ml), aztreonam (MIC ≤ 1 μg/ml), imipenem (MIC ≤ 0.25 μg/ml), meropenem (MIC ≤ 0.25 μg/ml), amikacin (MIC ≤ 2 μg/ml), ciprofloxacin (MIC ≤ 0.25 μg/ml), levofloxacin (MIC ≤ 0.12 μg/ml), tigecycline (MIC ≤ 0.5 μg/ml), and trimethoprim/sulfamethoxazole (MIC ≤ 20 μg/ml). A. dhakensis was found to be resistant to piperacillin/tazobactam (MIC ≥ 128 μg/ml). The drug resistance genes of the bacteria could not be ascribed to any carbapenemase genes (CphA, KPC, IMP, VIM, NDM, and OXA-48-like), extended-spectrum β-lactamase genes (CTX-M-1, CTX-M-9, CTX-M-15-like, TEM, and SHV), and Ampc enzyme genes (ACC and FOX). The pathogenic bacterial infection was susceptible to imipenem cilastatin sodium. Unfortunately, the patient died due to rapid disease progression.
Conclusion
Based on the diagnosis and treatment process of this patient, we realized that Aeromonas is not a common bacterium in doctors’ empirical diagnoses of community-acquired pneumonia patients. First, the pathogenic microorganisms responsible for hemoptysis in community-acquired pneumonia are Staphylococcus aureus, Mycobacterium tuberculosis, Streptococcus pneumoniae, Klebsiella pneumoniae, Aspergillus, and Mucor. Aeromonas, and infection with community-acquired pneumonia as the initial symptom and hemolysis lead to pulmonary hemorrhage, multiple organ dysfunction, and hemorrhagic shock, is rare. Second, the patient was admitted to the hospital with respiratory symptoms rather than the common symptom of diarrhea caused by Aeromonas infection, which is a rare manifestation of Aeromonas infection, and the occurrence of hemolytic uremic syndrome is again a rare clinical symptom. Third, based on the literature review, Aeromonas infection occurs in individuals with low immunity to the disease (31); however, this patient was young with no history of basic diseases or low immunity situation. In the present case, Aeromonas caused rapid progression, indicating that it could also infect the normal immune population and can also appear in severe manifestations. Fourth, although the patient had a clear manifestation of hemolytic uremic syndrome caused by Aeromonas infection, Escherichia coli is the most common pathogen causing the syndrome, followed by Shigella dysentery, Salmonella, Campylobacter, Yersinia, and enterovirus (32, 33). Only two cases of Aeromonas causing hemolytic uremic syndrome have been reported to date (33–35), and hence, the clinical presentation of this patient is rare. Fifth, if the patient was admitted to the hospital when respiratory symptoms occurred, cephalosporins or quinolones were administered according to the experience of doctors in the treatment of community-acquired pneumonia in order to make a life-saving treatment attempt.
Data availability statement
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the First Affiliated Clinical Research Ethics Review Committee of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the relation for the publication of any potentially identifiable images or data included in this article.
Author contributions
DL: Writing – original draft, Writing – review & editing. LD: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hazen TC, Fliermans CB, Hirsch RP, Esch G. Prevalence and distribution of Aeromonas hydrophila in the United States. Appl Environ Microbiol. (1978) 36:731–8. doi: 10.1128/aem.36.5.731-738.1978
2. Seidler RJ, Allen DA, Colwell R, Joseph S, Daily O. Isolation, enumeration, and characterization of Aeromonas from polluted waters encountered in diving operations. Appl Environ Microbiol. (1980) 39:1010–8. doi: 10.1128/aem.39.5.1010-1018.1980
3. Kaper JB, Lockman H, Colwell RR, Joseph S. Aeromonas hydrophila: Ecology and toxigenicity of isolates from an estuary. J Appl Bacterial. (1981) 50:359–77. doi: 10.1111/j.1365-2672.1981.tb00900.x
4. van der Kooij D. Properties of aeromonads and their occurrence and hygienic significance in drinking water. Zentralbl Bakteriol Mikrobiol Hyg B Umwelthyg Krankenhaushyg Arbeitshyg Prev Med. (1988) 187:1–17.
5. Monfort P, Baleux B. Distribution and survival of motile Aeromonas spp. in brackish water receiving sewage treatment effluent. Appl Environ Microbiol. (1991) 57:2459–67. doi: 10.1128/aem.57.9.2459-2467.1991
6. Martinez-Murcia AJ, Benlloch S, Collins MD. Phylogenetic interrelationships of members of the genera Aeromonas and Plesiomonas as determined by 16S ribosomal DNA sequencing: Lack of congruence with results of DNA-DNA hybridizations. Int J Syst Bacterial. (1992) 42:412–21. doi: 10.1099/00207713-42-3-412
7. Janda JM, Abbott SL. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin Microbiol Rev. (2010) 23:35–73. doi: 10.1128/CMR.00039-09
8. Burke V, Robinson J, Gracey M, Peterson D, Meyer N, Haley V. Isolation of Aeromonas spp. from an unchlorinated domestic water supply. Appl Environ Microbiol. (1984) 48:367–70. doi: 10.1128/aem.48.2.367-370.1984
9. Burke V, Robinson J, Gracey M, Peterson D, Partridge K. Isolation of Aeromonas hydrophila from a metropolitan water supply: Seasonal correlation with clinical isolates. Appl Environ Microbiol. (1984) 48:361–6. doi: 10.1128/aem.48.2.361-366.1984
10. Patil SM, Hilker ED. Aeromonas hydrophila community-acquired bacterial pneumonia with septic shock in a chronic lymphocytic leukemia patient due to absolute neutropenia and lymphopenia. Cureus. (2022) 14:e23345. doi: 10.7759/cureus.23345
11. Janda J, Abbott S. Human pathogens. 1st ed. In: Austin B, Altwegg M, Gosling P, Joseph S editors. The genus: Aeromonas. Chicester: John Wiley & Sons, Ltd (1996). p. 175.
12. Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: An expanding Panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. (1998) 27:332–44. doi: 10.1086/514652
13. Janda JM. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin Microbiol Rev. (1991) 4:397–410. doi: 10.1128/CMR.4.4.397
14. Altwegg M. Aeromonas. 7th ed. In: Murray P, Baron E, Pfaller M, et al. et al. editors. Manual of clinical microbiology. Washington, DC: ASM Press (1999). 507 p.
15. Ouderkirk JP, Bekhor D, Turett GS, Murali R. Aeromonas meningitis complicating medicinal leech therapy. Clin Infect Dis. (2004) 38:e36–7. doi: 10.1086/381438
16. Choi JP, Lee SO, Kwon HH, Kwak Y, Choi S, Lim S, et al. Clinical significance of spontaneous Aeromonas bacterial peritonitis in cirrhotic patients: A matched case-control study. Clin Infect Dis. (2008) 47:66–72. doi: 10.1086/588665
17. Figueras MJ, Alperi A, Saavedra MJ, Ko W, Gonzalo N, Navarro M, et al. Clinical relevance of the recently described species Aeromonas aquariorum. J Clin Microbiol. (2009) 47:3742–6. doi: 10.1128/JCM.02216-08
18. Huys G, Kämpfer P, Albert MJ, Kühn I, Denys R, Swings J. Aeromonas hydrophila subsp. dhakensis subsp. nov., isolated from children with diarrhoea in Bangladesh, and extended description of Aeromonas hydrophila subsp. hydrophila (Chester 1901) Stanier 1943 (approved lists 1980). Int J Syst Evol Microbiol. (2002) 52:705–12. doi: 10.1099/00207713-52-3-705
19. Beaz-Hidalgo R, Martínez-Murcia A, Figueras M. Reclassification of Aeromonas hydrophila subsp. dhakensis Huys et al. 2002 and Aeromonas aquariorum Martínez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst Appl Microbiol. (2013) 36:171–6.
20. Chen PL, Lamy B, Ko WC. Aeromonas dhakensis, an increasingly recognized human pathogen. Front Microbiol. (2016) 7:793. doi: 10.3389/fmicb.2016.00793
21. Chen PL, Wu CJ, Tsai PJ, Tang H, Chuang Y, Lee N, et al. Virulence diversity among bacteremic Aeromonas isolates: Ex vivo, animal, and clinical evidences. PLoS One. (2014) 9:e111213. doi: 10.1371/journal.pone.0111213
22. Chen PL, Wu CJ, Chen CS, Tsai P, Tang H, Ko W. A comparative study of clinical Aeromonas dhakensis and Aeromonas hydrophila isolates in southern Taiwan: A. dhakensis is more predominant and virulent. Clin Microbiol Infect. (2014) 20:O428–34. doi: 10.1111/1469-0691.12456
23. Morinaga Y, Yanagihara K, Eugenin FL, Beaz-Hidalgo R, Kohno S, Figueras Salvat M. Identification error of Aeromonas aquariorum: A causative agent of septicemia. Diagn Microbiol Infect Dis. (2013) 76:106–9. doi: 10.1016/j.diagmicrobio.2013.01.019
24. Tomás JM. The main Aeromonas pathogenic factors. ISRN Microbiol. (2012) 2012:256261. doi: 10.5402/2012/256261
25. Rasmussen-Ivey CR, Figueras MJ, McGarey D, Liles M. Virulence factors of Aeromonas hydrophila: In the wake of reclassification. Front Microbiol. (2016) 7:1337. doi: 10.3389/fmicb.2016.01337
26. Kitagawa H, Ohge H, Yu L, Kayama S, Hara T, Kashiyama S, et al. Aeromonas dhakensis is not a rare cause of Aeromonas bacteremia in Hiroshima, Japan. J Infect Chemother. (2020) 26:316–20. doi: 10.1016/j.jiac.2019.08.020
27. Wu C, Chen P, Hsueh P, Chang M, Tsai P, Shih H, et al. Clinical implications of species identification in monomicrobial Aeromonas bacteremia. PLoS One. (2015) 10:e0117821. doi: 10.1371/journal.pone.0117821
28. Chao CM, Lai CC, Tsai HY, Wu C, Tang H, Ko W, et al. Pneumonia caused by Aeromonas species in Taiwan, 2004-2011. Eur J Clin Microbiol Infect Dis. (2013) 32:1069–75. doi: 10.1007/s10096-013-1852-6
29. Wu CJ, Chen PL, Wu JJ, Yan J, Lee C, Lee H, et al. Distribution and phenotypic and genotypic detection of a metallo-β-lactamase, CphA, among bacteraemic Aeromonas isolates. J Med Microbiol. (2012) 61:712–9. doi: 10.1099/jmm.0.038323-0
30. Wu CJ, Wang HC, Chen PL, Chang M, Sunny Sun H, Chou P, et al. AQU-1, a chromosomal class C β-lactamase, among clinical Aeromonas dhakensis isolates: Distribution and clinical significance. Int J Antimicrob Agents. (2013) 42:456–61. doi: 10.1016/j.ijantimicag.2013.08.002
31. Tang HJ, Lai CC, Lin HL, Chao C. Clinical manifestations of bacteremia caused by Aeromonas species in southern Taiwan. PLoS One. (2014) 9:e91642. doi: 10.1371/journal.pone.0091642
32. Figueras MJ, Horneman AJ, Martinez-Murcia A, Guarro J. Controversial data on the association of Aeromonas with diarrhoea in a recent Hong Kong study. J Med Microbiol. (2007) 56:996–8. doi: 10.1099/jmm.0.47062-0
33. Bogdanović R, Cobeljić M, Marković M, Nikolić V, Ognjanović M, Sarjanović L, et al. Haemolytic-uraemic syndrome associated with Aeromonas hydrophila enterocolitis. Pediatr Nephrol. (1991) 5:293–5. doi: 10.1007/BF00867480
34. Robson WL, Leung AK, Trevenen CL. Haemolytic-uraemic syndrome associated with Aeromonas hydrophila enterocolitis. Pediatr Nephrol. (1992) 6:221. doi: 10.1007/BF00866324
Keywords: community-acquired pneumonia, hemoptysis, Aeromonas, hemolytic uremic syndrome, multiple organ dysfunction
Citation: Luo D and Dai L (2024) A 26-year-old man with multiple organ failure caused by Aeromonas dhakensis infection: a case report and literature review. Front. Med. 11:1289338. doi: 10.3389/fmed.2024.1289338
Received: 05 September 2023; Accepted: 29 February 2024;
Published: 17 April 2024.
Edited by:
Awdhesh (Dash) Kalia, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Claudia R. Libertin, Mayo Clinic Florida, United StatesSteven E. Fiester, Furman University, United States
Copyright © 2024 Luo and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liwan Dai, jojolovely@163.com
 Dan Luo
Dan Luo Liwan Dai
Liwan Dai