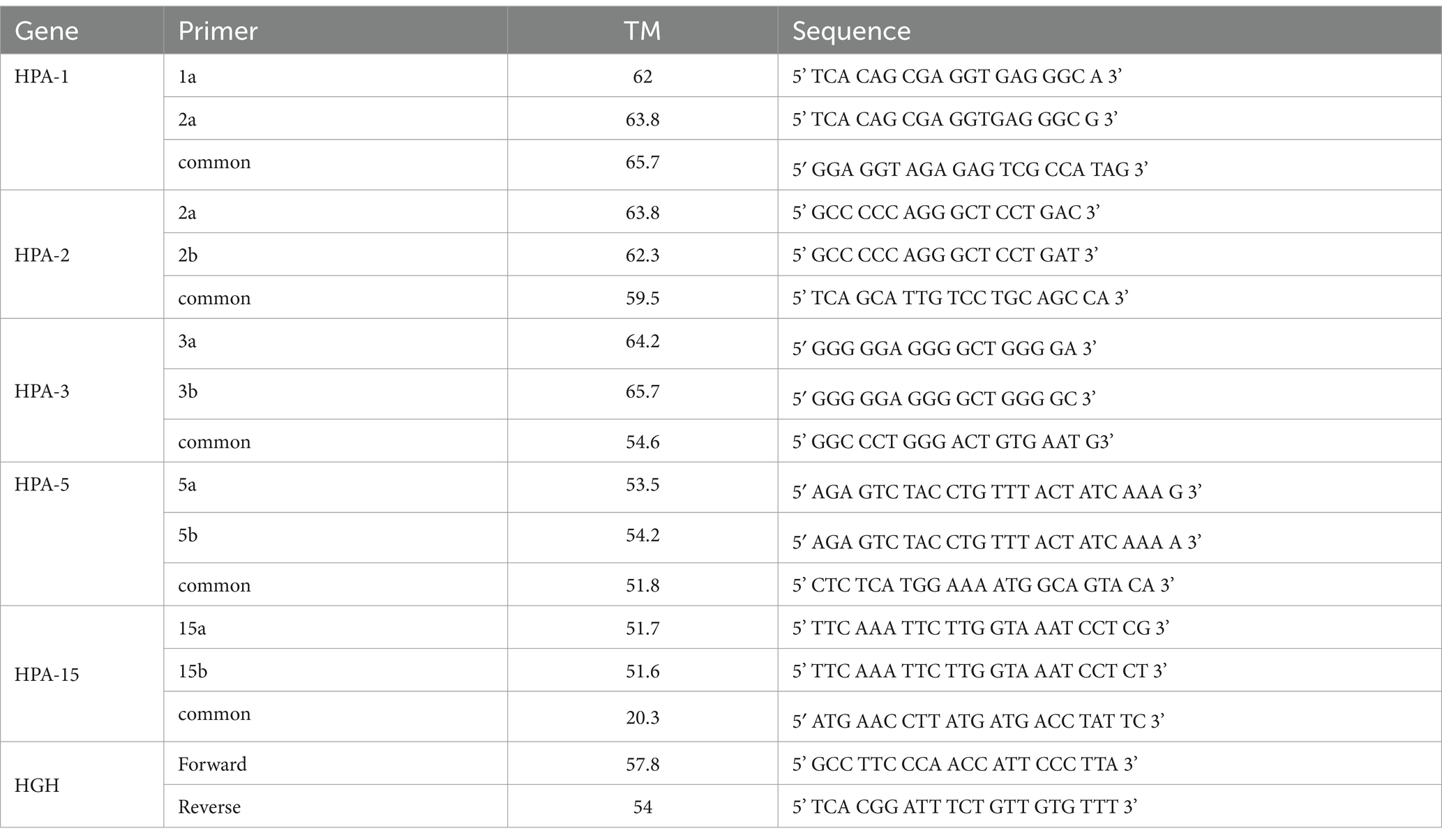
PLT antigen discrepancy pattern among couples with recurrent abortion
- 1Iranian Blood Transfusion Organization Research Center, Tehran, Iran
- 2Department of Biochemistry and Hematology, Faculty of Medicine Semnan University of Medical Sciences, Semnan, Iran
- 3Infectious Disease Specialist, Blood Transfusion Research Center/High Institute for Research & Education in Transfusion Medicine/Microbiology Department, Tehran, Iran
- 4Immunology, Blood Transfusion Research Center /High Institute for Research & Education in Transfusion Medicine, Immunohematology Department, Tehran, Iran
- 5Biochemistry, Iranian Blood Transfusion Organization Research Center, Tehran, Iran
- 6Sarem Fertility and Infertility Research Center, Sarem Cell Research Center, Sarem Hospital, Tehran, Iran
Background: Recurrent abortion refers to a condition of two or more consecutive pregnancies without known etiology affected by miscarriage before the completion of the 20th week of gestational age. However, several hypotheses have been proposed, but not much data are available concerning the relationship between human platelet antigens (HPAs) polymorphisms and recurrent abortion. This study was conducted to evaluate the genetic differences between HPA-1, −2, −3, −5, and − 15 in Iranian couples with a history of recurrent abortion.
Methods: In this cross-sectional study, a total of 74 couples with at least 2 recurrent abortions without any known specified reasons enrolled in the study. HPA polymorphisms genotyping was performed by single-specific primer PCR. Genotype frequency was calculated using the Hardy–Weinberg equation.
Results: A total of 39 couples (52.7%) had HPA genotyping partial mismatches. The most common partial mismatch pairs were found concomitantly on both HPA-15a and HPA-15b in three couples (4%), followed by two (2.7%) on HPA-3a and one (1.3%) in each HPA-2b and HPA-5b. There was a deviation from the Hardy–Weinberg equilibrium in the HPA-2 and -5 systems.
Conclusion: The present study declared that partial mismatches of HPA-3 and -15 genotypes were common among Iranian couples due to the history of recurrent abortion and approximately half of the couples carried at least one HPA gene that was absent in their partners. Further studies might be helpful to clarify the association between HPA polymorphisms and recurrent abortion, such as an investigation into the alloantibodies against HPAs.
1 Introduction
Recurrent abortion is one of the major concerns in the field of gynecology, which causes a huge amount of distress and economic burden. It is defined as two consecutive spontaneous miscarriages before the 20th week of gestation with no known causative factor and affects approximately 5% of all pregnancies (1, 2). The fundamental pathophysiological mechanisms remain unclear in approximately 50% of cases.
To date, 41 human platelet antigens (HPAs) have been described in the Immuno Polymorphism Database (IPD)1 (3). Several types of HPAs are involved in different clinical conditions, including venous thrombosis (4), myocardial infarction (5), post-transfusion purpura (PTP), platelet (PLT) transfusion refractoriness, alloimmune thrombocytopenia, idiopathic thrombocytopenic purpura (ITP) (6, 7), drug-induced immune thrombocytopenia (DITP), fetal/neonatal alloimmune thrombocytopenia (FNAIT) (8, 9), and susceptibility to HCV infection (10).
FNAIT is the most common cause of intracranial hemorrhage in full-term infants and can also lead to intrauterine growth retardation and miscarriage (11). FNAIT can occur during pregnancy following maternal exposure to paternal alloantigens on fetal PLTs (12).
The maternal IgG-type alloantibodies can cross the placenta and bind to the fetal PLTs. Phagocytic cells can remove these PLTs from the circulation, thus fetal thrombocytopenia occurs (13, 14). Although these results do not include the frequency of miscarriage related to the disease, FNAIT may be much more frequent than is thought, as it has been reported by some groups (15, 16). Not much data are available concerning HPA polymorphisms and recurrent abortion.
This study was conducted to evaluate the distribution pattern of HPA-1, −2, −3, −5, and − 15 [the HPA-4b alleles are rare in the Iranian population (17, 18)] in Iranian couples with a history of recurrent abortion and investigate the presence of alloantibodies in women with two or more HPA system discrepancies versus their partner.
2 Materials and methods
2.1 Study population
Informed consent was obtained from all couples. In this cross-sectional study from February 2016 to December 2017, a total of 74 couples with at least two consecutive pregnancy losses without any specified causes (according to the medical records obtained from the infertility center) were selected at the Sarem Hospital, Tehran, Iran. Ectopic, molar, and biochemical pregnancies were not included in this study.
Couples who had at least two histories of miscarriage without any known etiology were randomly selected and assigned to the present study. Their clinical situation was evaluated by infectious disease screening, e.g., tuberculosis and brucellosis history of TORCH. In addition, screening to rule out genetic disorders and clinical and para-clinical investigations regarding endocrine disease, e.g., thyroid disorders and drug toxicity, were also accomplished. If any of the mentioned diseases exist or were thought to exist, those couples were excluded from the study. The inclusion criteria for the study include the mother with no anatomical abnormality in their genitourinary system, and the father with no hypo, isospermia, or any anatomical or functional abnormal finding in their genitourinary system. After signing an informed consent, 4 mL of blood samples were collected in the EDTA tubes. Approximately at least 6 months gap was maintained between the last miscarriage and sampling.
2.2 HPA genotyping
Genomic DNA was extracted from the EDTA-anticoagulated whole blood samples using a QIAamp DNA blood mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s specifications (extracted DNAs were stored at −20 until processing).
HPA-1, −2, −3, −5, and − 15 were genotyped using sequence-specific primers PCR (SSP-PCR), according to Bhatti et al. and Meyer et al. (19, 20). Sequence-specific primers accompanied by product values are given in Table 1. A pair of primers for the human growth hormone (HGH) was included in the PCR of each allele to serve as an internal control for the PCR. Negative control was carried out in all PCR runs. More than half of the SSP-PCR results were independently confirmed by the following confirmatory tests (RFLP, PCR sequencing, and Inotrain’s exclusive panels). These results are available as internal laboratory documentation.
2.3 PIFT assay
PLT immunofluorescence test (PIFT) is an efficient and sensitive method in the initial screening of PLT antibody detection, but it has low specificity when non-HPA antibodies, especially Class I human leukocyte antigen (HLA) antibodies, are also present (14, 21). In the present study, the PIFT method was performed for the initial screening of PLT alloantibodies, and PRA was a candidate method to rule out non-specific positive results due to the presence of anti-HLA alloantibodies.
Whole blood was collected from six donors with ‘O’ blood group in the EDTA tubes. Then it was pooled and centrifuged at 500 g for 10 min. The supernatant of the PLT-rich plasma was removed, and then the buffer was washed and re-suspended in PBS/EDTA (centrifuged at 2000 g for 5 min and the supernatant was discarded). The washing step was repeated two times, and the final concentration was 100,000 PLTs/ml. In total, 50 μL of PLT concentrate was mixed with patient plasma (50 μL) and incubated for 30 min at 37°C (negative, positive, and isotype controls were added to each test batch).
Three consecutive washes were also performed by adding PBS/EDTA buffer (5 min at 1500 g). Finally, cells were incubated for 30 min in the dark with fluorescein isothiocyanate (FITC) Rabbit anti-human immunoglobulin G (DAKO F018501) at 1:30 dilution. Samples were analyzed after a final wash in a Partec PASII flow cytometer using the FlowMax software (Partec, Germany).
2.4 Panel reactive antibodies (PRAs)
The plasma was analyzed using a panel of mononucleated cells from 40 healthy donors. Briefly, 1 μL of isolated cells at a concentration of 3 × 106 cells/ml were added to plates. The plates were incubated at 37°C for 30 min, and then 5 μL of rabbit complement was added and incubated at 37°C for 90 min. In total, 2 μL of Eosin Y solution (MERK) was added, and after 1–2 min, 10 μL of 73% formalin was added. The plate was visualized under inverted fluorescence microscopy. A PRA result of >10% was considered positive.
2.5 Statistical analysis
The Hardy–Weinberg equilibrium test was carried out to evaluate the distributions of the gene frequencies of HPA-1, HPA-2, HPA-3, HPA-5, and HPA-15.
Data were analyzed using SPSS version 22 software (SPSS Inc., Chicago, IL). The statistical significance of the differences between groups was calculated using Pearsonʼs χ2 test. The level of significance for all statistical tests was a p-value of <0.05.
3 Results
In total, 74 couples were included, with the mean age of women being 32 ± 7 years (ranging from 22 to 46 years), and the mean number of miscarriages being 2.5 ± 0.9. There were six Rh-negative women, and all of them had received an anti-Rh globulin injection.
Genotype frequencies for the HPA-1, HPA-2, −3, −5, and − 15 systems among studied couples were as follows: HPA-1aa: 100%, HPA-1bb: 0%, HPA-2aa: 0.6%, HPA-2ab: 96.6%, HPA-2bb: 2.8%, HPA-3aa: 24.3%, HPA-3ab: 55.5%, HPA-3bb: 20.1%, HPA-5aa: 98.6%, HPA-5bb: 1.3%, HPA-15aa: 24.3%, HPA-15ab: 46.5%, and HPA-15bb: 29.1%.
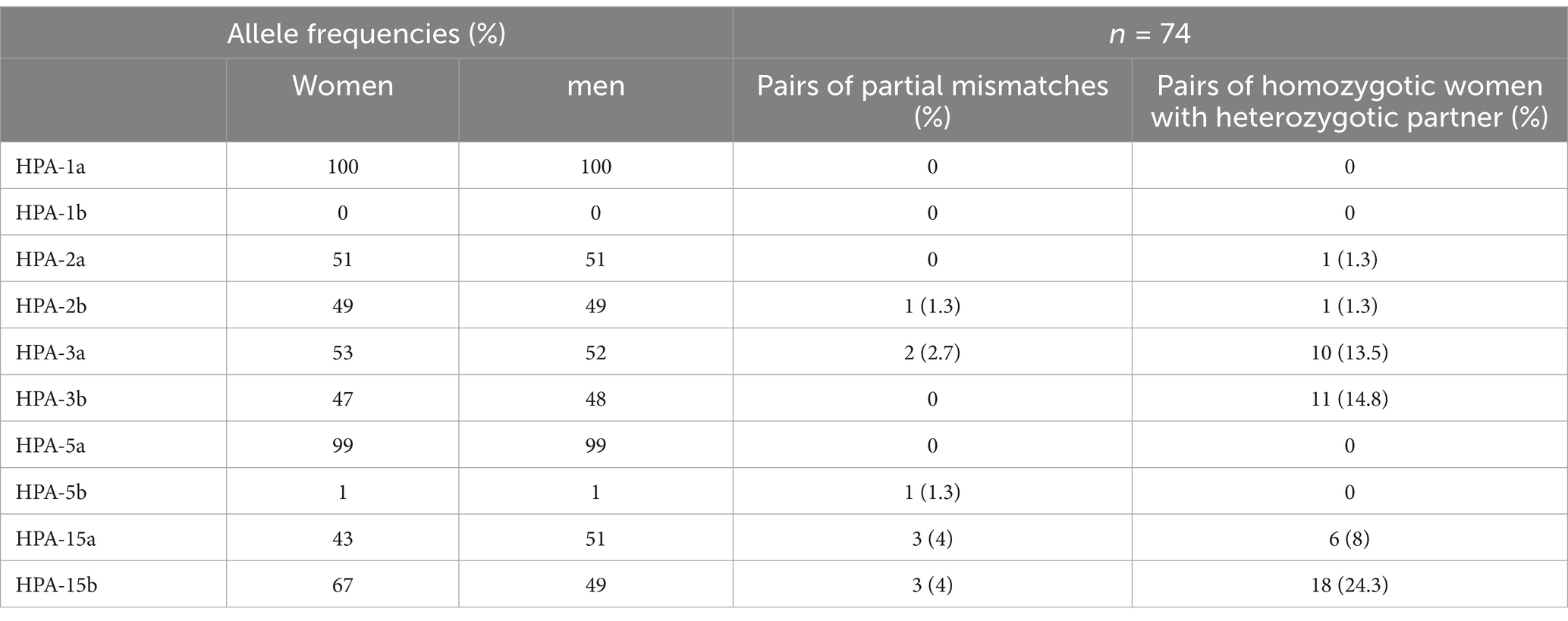
A total of 39 couples (52.7%) had partial mismatches of HPAs. Mismatches in the distribution of HPA-2, −3, −5, and − 15 among couples with a history of recurrent abortion are shown in Table 2.
Among the studied couples with a history of recurrent abortion, we have noted that in HPA-1, −2, −3, and − 5, the ‘a’ allele was more prevalent than the b allele. In the HPA-1 and -5 systems, the most frequent genotype was the homozygous a/a genotype, and the homozygous b/b genotype was very rare. In HPA-2 and -15 systems, the most frequent genotype was the heterozygous a/b genotype, less frequent was the homozygous a/a genotype, and the homozygous b/b genotype was intermittent. In the HPA-3 system, the most frequent genotype was the heterozygous a/b genotype, less frequent was the homozygous b/b genotype, and the homozygous a/a genotype was intermittent.
The frequency of HPA-3a in women and men was 53 and 52%, respectively, whereas HPA-3b was 47 and 48%, respectively. The frequency of HPA-15a in women and men was 43 and 51%, respectively, whereas HPA-15b was 67 and 49%, respectively.
The common partial mismatch pairs between couples were seen as follows: 3 (4%) in both HPA-15a and HPA-15b, 2 (2.7%) in HPA-3a, and 1 (1.3%) in HPA-2b and HPA-5b. In total, 45 men (60.8%) had the heterozygote genotype of the particular HPAs, whereas their partner carried the homozygote genotype (data not shown). The frequencies of homozygote women with heterozygote partners were 24.3% in HPA-15b, 14.8% in HPA-3b, 13.5% in HPA-3a, 8% in HPA-15a, and 1.3% in both HPA-2a and HPA-2b. In 25 (33.7%) couples, mismatches were observed simultaneously for both the HPA-15 and HPA-3 systems. There was a deviation from the Hardy–Weinberg equilibrium in the HPA-2 and -5 systems (p: <0.0001, Table 3).
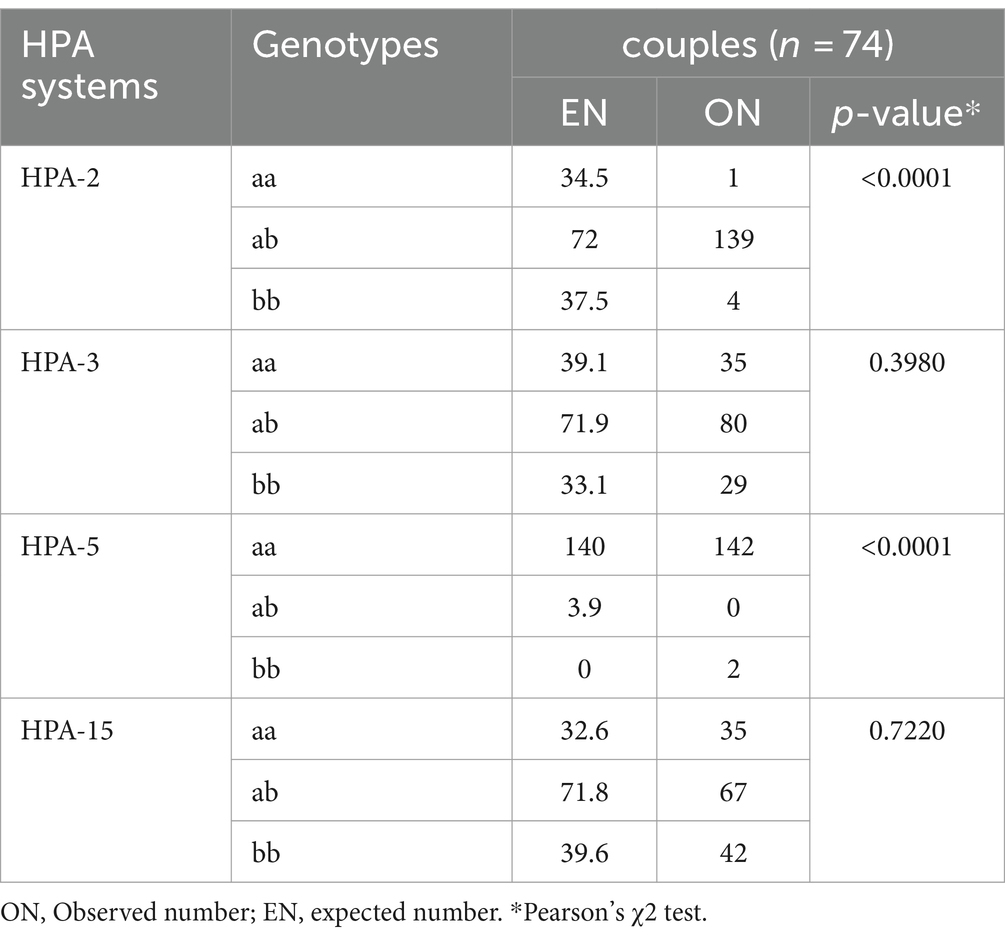
Table 3. Evaluation of the distribution of gene frequencies of HPA-2, −3, −5, and − 15 by the Hardy–Weinberg equilibrium test.
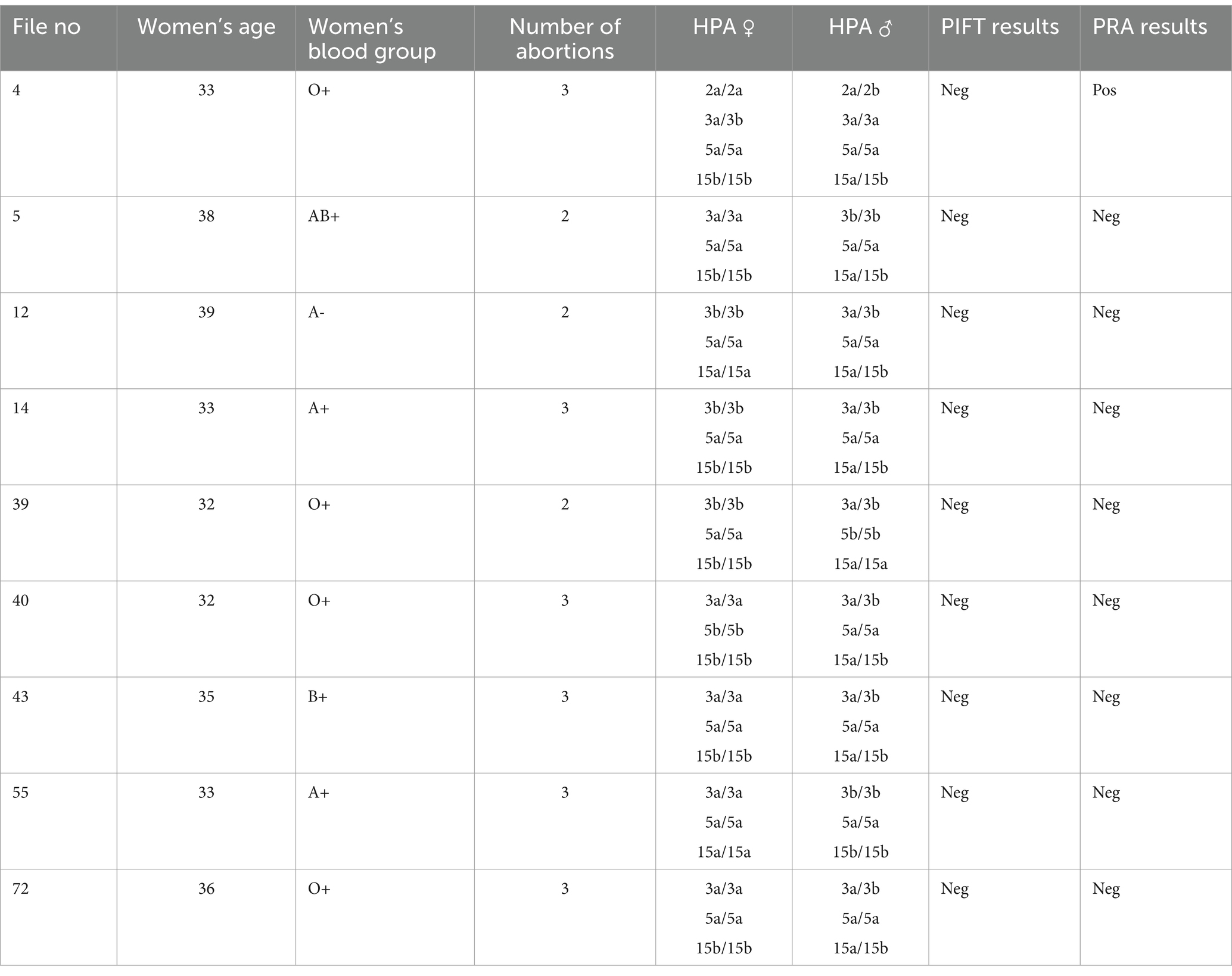
Antibody screening was conducted for nine women who have had two or more discrepancies in HPAs with their partners to amplify the chance of alloimmunization. The PIFT and PRA results are shown in Table 4.
4 Discussion
Recurrent abortion is a complex phenomenon that involves a wide range of possible causes. Genetic elements, such as chromosomal irregularities and parental karyotype anomalies, are frequently linked to this condition (22). Immune factors, such as autoimmune and alloimmune antibodies, can also play a significant role (23). Environmental factors, including pollution, may contribute to the risk of recurrent abortion. The interaction between genetic and immunological factors is particularly important, with immunological treatment showing promising results in preventing recurrent abortions (24).
The results of this study are valuable, as little information is available about the association between HPA polymorphisms and recurrent abortion. The present study is the first report of the gene frequencies of PLT antigens among couples with a history of recurrent miscarriage in Iran to investigate the alleles a and b antigens of HPA-1, HPA-2, HPA-3, HPA-5, and HPA-15 by the PCR-SSP method. The results showed that HPA-3 and -15 partial mismatch was common among Iranian couples with a history of recurrent abortion, and approximately half of the couples carried at least one HPA system that was absent in their partners. Primarily, partial mismatches were found on the HPA-15b, HPA-3b, HPA-3a, and HPA-15a systems. The genetic variations in HPA-3 and HPA-15 were higher than those of other HPAs, which correlated with the gene frequencies in the Iranian population.
The presence of the anti-PLT antibodies was investigated by the PIFT and PRA methods for nine women with a higher degree of HPA mismatch. The PIFT test results were negative in all tested cases, but the PRA test was positive in one case.
PLT-specific antigens have different patterns of frequency in different ethnic groups and genotyping of HPAs is important in the diagnosis of alloimmune thrombocytopenic syndromes, improving the treatment of patients with PLT auto/alloantibodies, genetic counseling for the prevention of FNAIT, etc.
Goodman C.S. et al. showed that HPA-1 polymorphism (a/b9L33P) significantly increased the prevalence of recurrent abortion compared to the control group (25). In the European white population, antibodies against HPA-1a, HPA-5b, and HPA-15b antigens were reported as the main causes of FNAIT (26). Bleeding risk may be significant throughout the severe thrombocytopenia phase, but the main risk was reported to be intracranial hemorrhage (ICH) before 20 weeks of gestation (27). In the present study, the HPA-2 heterozygous was observed in approximately 96.5% of cases, thus the possibility of alloimmunization with the HPA-2 was rare, which was consistent with the reports from Iranian blood donors by 92% heterozygosity in the HPA-2 system (17). However, Iman et al. reported an infant with FNAIT that was caused by a maternal and neonatal discrepancy in HPA-2b (28). This antigen was absent on the mother’s PLTs but it was expressed on both the father’s and baby’s PLTs, and the presence of antibody against HPA-2b in the mother’s serum was confirmed (28). The probability of FNAIT occurrence in Iran is very low due to heterozygosity in the HPA-2 system.
However, only a few cases of NAIT caused by anti-HPA-3a or anti-HPA-3b have been reported in the past (29–32). Schallmoser et al. described a term newborn with FNAIT caused by anti-HPA-3a (30). Kataoka et al. reported a case of neonatal alloimmune thrombocytopenia due to an anti-HPA-3b antibody. This infant was severely affected by ICH (32). Furthermore, Ertel et al. indicated that alloimmunization against HPA-15a and HPA-15b should be considered a cause of immune thrombocytopenia (33). In the Iranian population, Shaiegan et al. (17) revealed a distribution of 0.47.5 and 0.52.5 for HPA-15a and -15b alleles, respectively, which was not consistent with reports in Caucasian populations (34). Matsuhashi et al. reported the first case of anti-HPA-15 in Japan (35).
We also found an HPA-5b partial mismatch in this study. There is limited data on the immune response against HPA-5b, the second most frequent antigen associated with FNAIT after HPA-la. In Caucasians (36), the allelic frequencies are 0.99 for HPA-5a and 0.10 for HPA-5b, which is similar to that of the Iranian population (17). Semana et al. suggested a strong association of alloimmunization HPA-5b with a cluster of HLA (HLA DR) molecules (37). In a Caucasoid population, maternal antibodies to HPA-5b are detected in approximately 15% of serologically confirmed cases (38). Karami et al. showed that there is a relationship between rs5918 T > C polymorphism of the HPA-1 gene and recurrent abortion among the Iranian population.
HPAs are components of PLT glycoprotein (GP) complexes GPIIb/IIIa, GPIb/IX, and GPIa/IIa (3) and might be contributing to the production of autoantibodies. In addition, these antigens show a different pattern of distribution among different ethnic groups and diseases (39). Animal studies have shown that anti-GPIb alpha antibodies may increase the incidence of recurrent abortion (40). It has also been shown that trophoblasts isolated from the human placenta express αIIbβ3 integrin (41, 42). Therefore, it could be hypothesized that during pregnancy, maternal anti-αIIb and anti-β3 integrin antibodies target human villous trophoblasts, which leads to placental perfusion impairment and recurrent abortion.
As previously mentioned, there are three clinical conditions that can be brought on by PLT-specific antibodies: FNAIT, PTP, and post-transfusion refractoriness to PLTs (PTR). In short, people who lack a specific HPA allo epitope are immunized against it after receiving PLT transfusions or while carrying an antigen-positive fetus. PTR results from receiving allogeneic PLT transfusions. Class I HLA molecules typically cause these antibodies, whereas HPA molecules do so less frequently. Approximately 50–90% of patients develop HLA antibodies after receiving repeated transfusions of blood components containing leukocytes. HPA antibodies are present concurrently in 17–25% of these cases, which reduce the transfusion-associated PLT survival time (43, 44). In FNAIT, the characteristic of anti-PLT antibodies is usually anti-HLA I and, to a lesser extent, HPA antibodies, which are created during pregnancy and are based on the genetic immunity difference between the fetus and the mother (45). PTP is also closely related to immunogenicity through PLT-specific antigens. Antibodies with specificity against platelet GPs, such as GPIIb-IIIa, GPIb-IX, and GPIa-IIa, along with anti-HPA alloantibodies, are responsible for platelet destruction in PTP (46, 47).
On the other hand, it has been shown that during pregnancy, the uterine natural killer (uNK) and trophoblasts are functional partners, and the importance of uNK cells during a normal pregnancy has been determined (48). Eksteen et al. reported that anti-HPA-1a may target trophoblast function and cause trophoblast damage (49). Therefore, another hypothesis in relation to LRP is the formation of immune complexes (antibody-HPAs) on trophoblasts that trigger NK antibody-dependent cellular cytotoxicity (ADCC) on trophoblasts and as a result lead to their damage.
Deviation from the Hardy–Weinberg equilibrium regarding HPA-2 and -5 in couples was principally due to the higher number of observed HPA-2ab and -5aa alleles when compared with their expected numbers. One reason for deviating from the Hardy–Weinberg equilibrium was consanguineous marriages. This study was conducted as a pilot study, and the main limitation of this study was the small size of the study population.
It is important to note that fetal HPA genotyping is not the only consideration in the management of FNAIT and recurrent abortions. Other factors, such as maternal antibody level, maternal medical history, and fetal ultrasound evaluation, can be taken into account for comprehensive and individualized management. We then emphasize the importance of taking into consideration that decisions regarding fetal HPA genotyping and interventions to prevent recurrent abortions should be made in collaboration with a medical team specializing in maternal-fetal medicine or medical genetics, which can evaluate the specific case and provide appropriate advice based on clinical guidelines and current best practices.
In conclusion, this study revealed that HPA-3a, −3b, −15a, −15b gene polymorphisms may be involved in recurrent abortion, and further studies are essential to clarify the role of HPA polymorphisms in miscarriage, including the production of alloantibodies against HPAs.
Fetal HPA genotyping using maternal plasma cell-free DNA is recommended for alloimmunized pregnant women to determine whether their fetuses are at risk for FNAIT and whether interventions are needed to prevent recurrent abortion.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by this study was approved by the Ethics Committee of the High Institute for research and education in Transfusion medicine (ethical committee code number: IR.TMI.REC.1395.002). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
GS: Data curation, Funding acquisition, Investigation, Writing – original draft. AG: Conceptualization, Formal analysis, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. MZ: Methodology, Supervision, Validation, Writing – original draft. MS: Formal analysis, Funding acquisition, Methodology, Resources, Writing – original draft. SS: Formal Analysis, Funding acquisition, Software, Validation, Writing – original draft. AZ: Formal analysis, Software, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the High Institute for Research and Education in Transfusion Medicine, Tehran, Iran.
Acknowledgments
The authors wish to thank Ataee, Mostakhdemin, Dadashi, and Sarem Hospital staff for their technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Primary Immune thrombocytopenic purpura, ITP; Human platelet antigens, HPA; Interleukin, IL; Post-transfusion purpura, PTP; Platelet transfusion refractoriness, PTR; Fetal/neonatal alloimmune thrombocytopenia, FNAIT; Human growth hormone, HGH; Odds ratio, OR; Immuno Polymorphism Database, IPD; Drug-induced immune thrombocytopenia, DITP; Hemolytic disease of the fetus and newborn, HDFN; Sequence-specific primers PCR, SSP-PCR; Platelet Immuno Florescent Test, PIFT.
Footnotes
References
1. Stirrat, GM. Recurrent miscarriage I: definition and epidemiology. Lancet. (1990) 336:673–5. doi: 10.1016/0140-6736(90)92159-F
2. Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. (2012) 98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048
3. Al-Ouda, S, Al-Banyan, A, Abdel Gader, A, Bayoumy, N, and Al-Gahtani, F. Gene frequency of human platelet alloantigens-1 to-6 and-15 in Saudi blood donors. Transfus Med. (2016) 26:220–4. doi: 10.1111/tme.12297
4. Ridker, PM, Hennekens, CH, Schmitz, C, Stampfer, MJ, and Lindpaintner, K. PIA1/A2 polymorphism of platelet glycoprotein IIIa and risks of myocardial infarction, stroke, and venous thrombosis. Lancet. (1997) 349:385–8. doi: 10.1016/S0140-6736(97)80010-4
5. Rosenberg, N, Zivelin, A, Chetrit, A, Dardik, R, Kornbrot, N, and Inbal, A. Effects of platelet membrane glycoprotein polymorphisms on the risk of myocardial infarction in young males. Isr Med Assoc J. (2002) 4:411–4.
6. Shaiegan, M, Ghasemi, A, Zadsar, M, Ahmadi, J, Samiee, S, and Madani, T. Human platelet antigens polymorphisms: association to primary immune thrombocytopenia in the Iranian patients. Iran J Pediatric Hematol Oncol. (2020) 11:41–50. doi: 10.18502/ijpho.v11i1.5006
7. Ghaffari, K, Rad, MA, Hasan-Abad, AM, Khosravi, M, Benvidi, A, Iraji, M, et al. Association of the human platelet antigens polymorphisms with platelet count in patients with COVID-19. Front Med. (2023) 10:10. doi: 10.3389/fmed.2023.1265568
8. Curtis, BR. Genotyping for human platelet alloantigen polymorphisms: applications in the diagnosis of alloimmune platelet disorders In: Seminars in thrombosis and hemostasis. (2008) 34:539–48. doi: 10.1055/s-0028-1103365
9. Kupatawintu, P, Nathalang, O, O-Charoen, R, and Patmasiriwat, P. Gene frequencies of the HPA-1 to 6 and Gov human platelet antigens in Thai blood donors. Immunohematology. (2005) 21:5–9. doi: 10.21307/immunohematology-2019-385
10. Ghasemi, A, Zadsar, M, Shaiegan, M, Samiei, S, Namvar, A, Rasouli, M, et al. Human platelet antigens polymorphisms; association to the development of liver fibrosis in patients with chronic hepatitis C. J Med Virol. (2020) 92:45–52. doi: 10.1002/jmv.25423
11. Vadasz, B, Chen, P, Yougbaré, I, Zdravic, D, Li, J, Li, C, et al. Platelets and platelet alloantigens: lessons from human patients and animal models of fetal and neonatal alloimmune thrombocytopenia. Genes Dis. (2015) 2:173–85. doi: 10.1016/j.gendis.2015.02.003
12. Peterson, JA, McFarland, JG, Curtis, BR, and Aster, RH. Neonatal alloimmune thrombocytopenia: pathogenesis, diagnosis and management. Br J Haematol. (2013) 161:3–14. doi: 10.1111/bjh.12235
13. Bayat, B, Traum, A, Berghöfer, H, Werth, S, Zhu, J, Bein, G, et al. Current anti-HPA-1a standard antibodies react with the β3 integrin subunit but not with αIIbβ3 and αvβ3 complexes. Thromb Haemost. (2019) 119:1807–15. doi: 10.1055/s-0039-1696716
14. Matsuhashi, M, and Tsuno, NH. Laboratory testing for the diagnosis of immune-mediated thrombocytopenia. Ann Blood. (2018) 3:41. doi: 10.21037/aob.2018.09.02
15. Bertrand, G, Drame, M, Martageix, C, and Kaplan, C. Prediction of the fetal status in noninvasive management of alloimmune thrombocytopenia. Blood. (2011) 117:3209–13. doi: 10.1182/blood-2010-08-302463
16. Murphy, M, Hambley, H, Nicolaides, K, and Waters, A. Severe fetomaternal alloimmune thrombocytopenia presenting with fetal hydrocephalus. Prenat Diagn. (1996) 16:1152–5. doi: 10.1002/(SICI)1097-0223(199612)16:12<1152::AID-PD8>3.0.CO;2-J
17. Shaiegan, M, Ghasemi, A, Madani, T, Samiee, S, Bayat, B, Mohammadi, S, et al. Gene frequencies of human platelet antigen-1 to-5 and-15 in the Iranian population. ISBT Sci Ser. (2020) 15:273–80. doi: 10.1111/voxs.12532
18. Nozarimirarkolaei, M, Dadashi, M, Ghasemi, A, Samiee, S, Shaeigan, M, and Zadsar, M. Frequency of human platelet antigens− 1 to− 5 and− 15 in Turkmen blood donors. Adv Biomed Res. (2023) 12:47. doi: 10.4103/abr.abr_282_21
19. Bhatti, F, Uddin, M, Ahmed, A, and Bugert, P. Human platelet antigen polymorphisms (HPA-1,-2,-3,-4,-5 and-15) in major ethnic groups of Pakistan. Transfus Med. (2010) 20:78–87. doi: 10.1111/j.1365-3148.2009.00982.x
20. Meyer, O, Hildebrandt, M, Schulz, B, Blasczyk, R, and Salama, A. Simultaneous genotyping of human platelet antigens (HPA) 1 through 6 using new sequence-specific primers for HPA-5. Transfusion. (1999) 39:1256–8. doi: 10.1046/j.1537-2995.1999.39111256.x
21. Bub, CB, Martinelli, BM, Avelino, TM, Gonçalez, AC, Barjas-Castro, ML, and Castro, V. Platelet antibody detection by flow cytometry: an effective method to evaluate and give transfusional support in platelet refractoriness. Revista brasileira de hematología e hemoterapia. (2013) 35:252–5. doi: 10.5581/1516-8484.20130062
22. Vaiman, D. Genetic regulation of recurrent spontaneous abortion in humans. Biom J. (2014) 38:11. doi: 10.4103/2319-4170.133777
23. Pandey, MK, Rani, R, and Agrawal, S. An update in recurrent spontaneous abortion. Arch Gynecol Obstet. (2005) 272:95–108. doi: 10.1007/s00404-004-0706-y
24. Mowbray, J. Genetic and immunological factors in human recurrent abortion. Am J Reprod Immunol Microbiol. (1987) 15:138–40.
25. Goodman, CS, Coulam, CB, Jeyendran, RS, Acosta, VA, and Roussev, R. Which thrombophilic gene mutations are risk factors for recurrent pregnancy loss? Am J Reprod Immunol. (2006) 56:230–6. doi: 10.1111/j.1600-0897.2006.00419.x
26. Ghevaert, C, Campbell, K, Walton, J, Smith, GA, Allen, D, Williamson, LM, et al. Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion. (2007) 47:901–10. doi: 10.1111/j.1537-2995.2007.01208.x
27. Bertrand, G, Martageix, C, Jallu, V, Vitry, F, and Kaplan, C. Predictive value of sequential maternal anti-HPA-1a antibody concentrations for the severity of fetal alloimmune thrombocytopenia. J Thromb Haemost. (2006) 4:628–37. doi: 10.1111/j.1538-7836.2006.01809.x
28. Al-Sheikh, IH, Khalifa, M, Rahi, A, Qadri, MI, and Al, AK. A rare case of neonatal alloimmune thrombocytopenia due to anti-HPA-2b. Ann Saudi Med. (1998) 18:547–9. doi: 10.5144/0256-4947.1998.547
29. Hopkins, M, Lucas, G, Calvert, A, Bendukidze, N, Green, F, Kotecha, K, et al. Human platelet antigen (HPA)-specific immunoglobulin M antibodies in neonatal alloimmune thrombocytopenia can inhibit the binding of HPA-specific immunoglobulin G antibodies. Transfusion. (2017) 57:1267–71. doi: 10.1111/trf.14047
30. Schallmoser, K, Kutschera, J, Macher, S, Ulrich, S, Eichler, P, Panzer, S, et al. Delayed detectability of anti-HPA-3a by the MAIPA assay in a severe neonatal alloimmune thrombocytopenia, but successful transfusion of incompatible donor platelets: a case report. Vox Sang. (2006) 91:181–3. doi: 10.1111/j.1423-0410.2006.00809.x
31. Lin, M, Shieh, SH, Liang, DC, Yang, TF, and Shibata, Y. Neonatal Alloimmune thrombocytopenia in Taiwan due to an antibody against a labile component of HPA-3a (Bak). Vox Sang. (1995) 69:336–40. doi: 10.1111/j.1423-0410.1995.tb00369.x
32. Kataoka, S, Kobayashi, H, Chiba, K, Nakamura, M, Shinada, S, Morita, S, et al. Neonatal alloimmune thrombocytopenia due to an antibody against a labile component of human platelet antigen-3b (Bakb). Transfus Med. (2004) 14:419–23. doi: 10.1111/j.1365-3148.2004.00537.x
33. Ertel, K, Al-Tawil, M, Santoso, S, and Kroll, H. Relevance of the HPA-15 (Gov) polymorphism on CD109 in alloimmune thrombocytopenic syndromes. Transfusion. (2005) 45:366–73. doi: 10.1111/j.1537-2995.2005.04281.x
34. Randen, I, Sørensen, K, Killie, MK, and Kjeldsen-Kragh, J. Rapid and reliable genotyping of human platelet antigen (HPA)-1,-2,-3,-4, and-5 a/b and Gov a/b by melting curve analysis. Transfusion. (2003) 43:445–50. doi: 10.1046/j.1537-2995.2003.00354.x
35. Matsuhashi, M, Tsuno, NH, Kawabata, M, Yokoyama, T, Tazaki, Y, Takashima, T, et al. The first case of alloantibody against human platelet antigen-15b in Japan: possible alloimmunization by a hydatidiform mole. Transfusion. (2010) 50:1126–30. doi: 10.1111/j.1537-2995.2009.02537.x
36. Kiefel, V, Santoso, S, Katzmann, B, and Mueller-Eckhardt, C. The bra/brb alloantigen system on human platelets. Blood. (1989) 73:2219–23. doi: 10.1182/blood.V73.8.2219.2219
37. Semana, G, Zazoun, T, Alizadeh, M, Morel-Kopp, M-C, Genetet, B, and Kaplan, C. Genetic susceptibility and anti-human platelet antigen 5b alloimmunization role of HLA class II and TAP genes. Hum Immunol. (1996) 46:114–9. doi: 10.1016/0198-8859(96)00019-5
38. Mueller-Eckhardt, C, Grubert, A, Weisheit, M, Mueller-Eckhardt, G, Kiefel, V, Kroll, H, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. (1989) 333:363–6. doi: 10.1016/S0140-6736(89)91733-9
39. Ghevaert, C, Rankin, A, Huiskes, E, Porcelijn, L, Javela, K, Kekomaki, R, et al. Alloantibodies against low-frequency human platelet antigens do not account for a significant proportion of cases of fetomaternal alloimmune thrombocytopenia: evidence from 1054 cases. Transfusion. (2009) 49:2084–9. doi: 10.1111/j.1537-2995.2009.02246.x
40. Bussel, J, and Clines, D. Immune thrombocytopenic purpura, neonatal alloimmune thrombocytopenia, and posttransfusion purpura. In: Hematology basic principles and practice. Ed. R. Hoffman. Philadelphia: Churchill Livingstone. (2000) 2096–114.
41. Snir, A, Brenner, B, Paz, B, and Lanir, N. Presence of integrin alpha (IIb) beta3 in early gestation human trophoblasts: possible involvement of fibrin as a matrix ligand. Thromb Res. (2010) 125:253–6. doi: 10.1016/j.thromres.2009.11.022
42. Kabir-Salmani, M, Fukuda, MN, Kanai-Azuma, M, Ahmed, N, Shiokawa, S, Akimoto, Y, et al. The membrane-spanning domain of CD98 heavy chain promotes αvβ3 integrin signals in human extravillous trophoblasts. Mol Endocrinol. (2008) 22:707–15. doi: 10.1210/me.2007-0243
43. Aster, RH. New approaches to an old problem: Refractoriness to platelet transfusions. Transfusio. (1988) 4:95–6. doi: 10.1046j.1537-2995.1988.28288179038.x
44. Langenscheidt, F, Kiefel, V, Santoso, S, and Mueller-Eckhardt, C. Platelet transfusion refractoriness associated with two rare platelet-specific alloantibodies (anti-Baka and anti-PlA2) and multiple HLA antibodies. Transfusion. (1988) 28:597–600. doi: 10.1046/j.1537-2995.1988.28689059040.x
45. Blanchette, VS, and Rand, ML. Platelet disorders in newborn infants: diagnosis and management. Semin Perinatol. (1997) 21:53–62. doi: 10.1016/s0146-0005(97)80020-1
46. Guz, K, Uhrynowska, M, Kopeć, I, Dębska, M, Husebekk, A, and Brojer, E. Recent advances in understanding the clinical relevance of antiplatelet alloantibodies. Pol Arch Intern Med. (2017) 127:190–4. doi: 10.20452/pamw.3932
47. Wool, GD, and Brown, N. Alloantibodies and platelets In: Immunologic Concepts in Transfusion Medicine. Ed. RW Maitta. London: Elsevier (2020). 117–48. doi: 10.1016/C2018-0-02699-7
48. Yougbaré, I, Zdravic, D, and Ni, H. Fetal and neonatal alloimmune thrombocytopenia: novel mechanisms of miscarriage learned from placental pathology in animal models. J Pediatr Pediatr Med. (2018) 2:28–33. doi: 10.29245/2578-2940/2018/1.1113
49. Eksteen, M, Heide, G, Tiller, H, Zhou, Y, Nedberg, NH, Martinez-Zubiaurre, I, et al. Anti-human platelet antigen (HPA)-1a antibodies may affect trophoblast functions crucial for placental development: a laboratory study using an in vitro model. Reprod Biol Endocrinol. (2017) 15:1–8. doi: 10.1186/s12958-017-0245-6
Keywords: antigens, human platelet, recurrent abortion, pregnancy, gynecology
Citation: Shad GA, Ghasemi A, Zadsar M, Shaeigan M, Samiee S and Zare A (2024) PLT antigen discrepancy pattern among couples with recurrent abortion. Front. Med. 11:1291779. doi: 10.3389/fmed.2024.1291779
Edited by:
Abraham A. Pouliakis, National and Kapodistrian University of Athens, GreeceReviewed by:
Giuseppe Basile, IRCCS Istituto Ortopedico Galeazzi, ItalyIoulia Magaliou, University of Thessaly, Greece
Copyright © 2024 Shad, Ghasemi, Zadsar, Shaeigan, Samiee and Zare. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryam Zadsar, maryam.zad@gmail.com; Ali Ghasemi, a.qasemi2012@yahoo.com
†ORCID: Ali Ghasemi, https://orcid.org/0000-0002-4996-7656
Maryam zadsar, http://orcid.org/0000-0001-9519-4853
 Ghazal Ahmadzadeh Shad1
Ghazal Ahmadzadeh Shad1  Ali Ghasemi
Ali Ghasemi Ahad Zare
Ahad Zare