Methylene blue in sepsis and septic shock: a systematic review and meta-analysis
- Internal Medicine Department, Medical School, São Paulo State University (UNESP), Botucatu, Brazil
Background: Methylene blue is an interesting approach in reducing fluid overload and vasoactive drug administration in vasodilatory shock. The inhibition of guanylate cyclase induced by methylene blue infusion reduces nitric oxide production and improves vasoconstriction. This systematic review and meta-analysis aimed to assess the effects of methylene blue administration compared to placebo on the hemodynamic status and clinical outcomes in patients with sepsis and septic shock.
Methods: The authors specifically included randomized controlled trials that compared the use of methylene blue with placebo in adult patients with sepsis and septic shock. The outcomes were length of intensive care unit stay, hemodynamic parameters [vasopressor use], and days on mechanical ventilation. We also evaluated the abnormal levels of methemoglobinemia. This systematic review and meta-analysis were recorded in PROSPERO with the ID CRD42023423470.
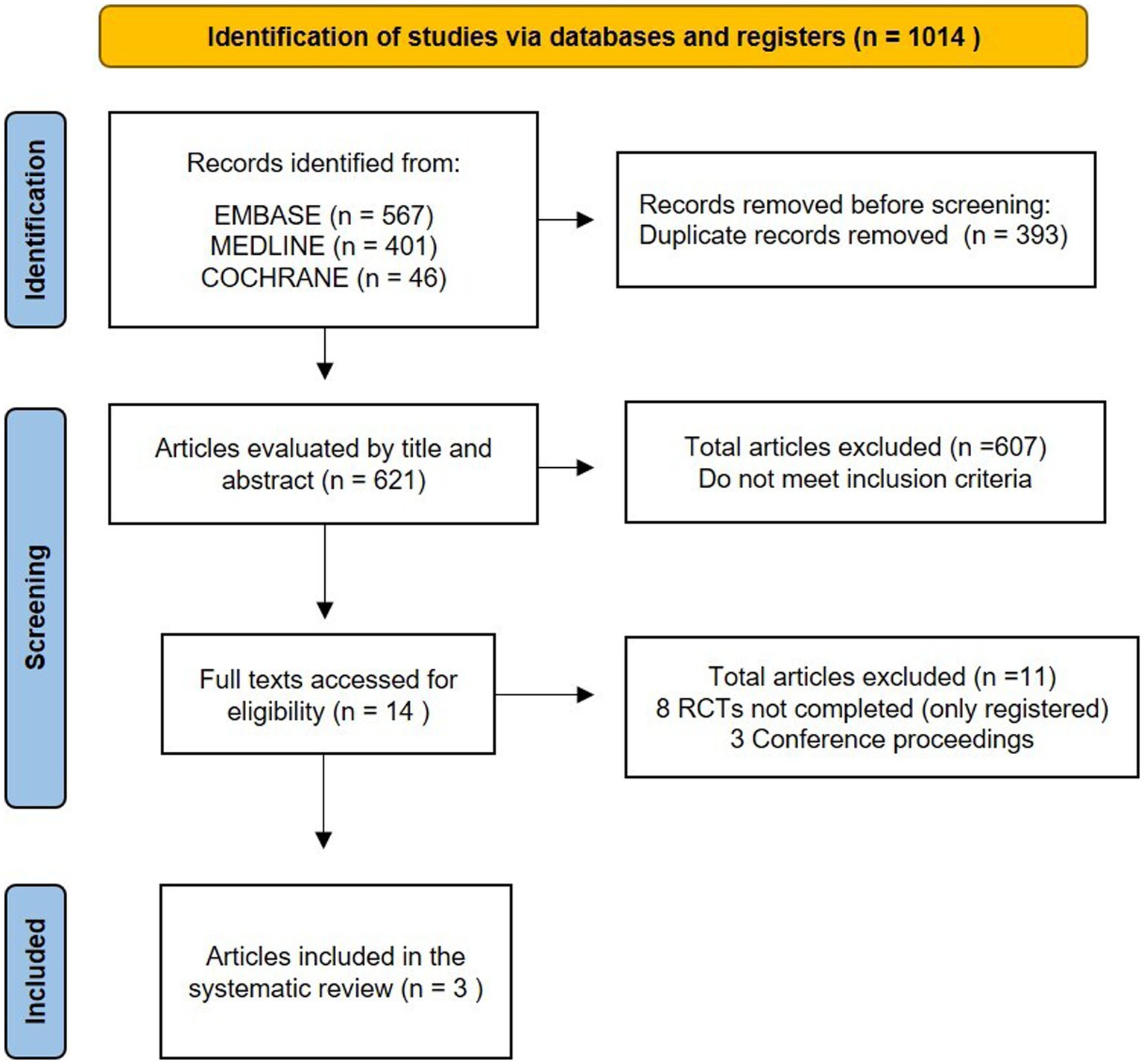
Results: During the initial search, a total of 1,014 records were identified, out of which 393 were duplicates. Fourteen citations were selected for detailed reading, and three were selected for inclusion. The studies enrolled 141 patients, with 70 of them in the methylene blue group and 71 of them in the control group. Methylene blue treatment was associated with a lower length of intensive care unit stay (MD −1.58; 95%CI −2.97, −0.20; I2 = 25%; p = 0.03), decreased days on mechanical ventilation (MD −0.72; 95%CI −1.26, −0.17; I2 = 0%; p = 0.010), and a shorter time to vasopressor discontinuation (MD −31.49; 95%CI −46.02, −16.96; I2 = 0%; p < 0.0001). No association was found with methemoglobinemia.
Conclusion: Administering methylene blue to patients with sepsis and septic shock leads to reduced time to vasopressor discontinuation, length of intensive care unit stay, and days on mechanical ventilation.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023423470, CRD42023423470.
Introduction
Sepsis is an inadequate host response to an infection, resulting in organ dysfunction (1). It involves immunological, hormonal, and circulatory abnormalities such as peripheral vasodilatation. This vasodilatation comprises multifactorial mechanisms that include cytokine stimulation of inducible nitric oxide synthase and activation of various intrinsic vasodilatory pathways. Additionally, there is a diminished responsiveness to vasopressors (2–4). Treatment is mainly based on optimizing circulating volume, administering broad-spectrum antibiotics, and utilizing vasopressors to maintain a suitable mean arterial pressure (MAP) and organ perfusion (5, 6).
Following the research conducted by Rivers et al. and the sepsis and septic shock treatment guided by early goal-directed therapy, there has been little progress in the therapeutic arsenal of this condition (4). The initial approach is fluid administration, but this is often insufficient to improve organ perfusion substantially. In such cases, vasopressor administration becomes necessary. Although norepinephrine is the first-choice vasoactive agent, there are some concerns with high doses that may cause potential adverse effects, such as increased myocardial oxygen consumption, tachyarrhythmias, myocardial ischemia, decreased regional blood flow, hyperglycemia, and hypercoagulability (7–10). Moreover, norepinephrine disrupts the immune response by reducing the production of proinflammatory mediators and reactive oxygen species while increasing the production of anti-inflammatory mediators. This compromise in host defense potentially contributes to the observed immunoparalysis in patients with septic shock (11). Furthermore, with prolonged exposure to high-dose catecholamines, adrenergic receptors experience downregulation and desensitization, and consequently, some patients may exhibit inadequate responsiveness (7, 12, 13).
In this context, strategies that prioritize decatecholaminization have been proposed for the management of patients with septic shock; however, as additional vasopressor agents, including vasopressin, terlipressin, and angiotensin-2, are similarly linked to an increased likelihood of tachyarrhythmias, organic ischemia, and immune dysfunction (9, 10), methylene blue (MB) has been explored as a potential alternative to reach the hemodynamic targets (14). MB acts by blocking the enzyme guanylate cyclase, reducing excessive nitric oxide production, and alleviating its vasorelaxant effect in vascular smooth muscle, restoring vascular tone and increasing blood pressure (10, 15, 16).
Although MB effectively increases vascular tone and arterial pressure, its affordability and widespread availability notwithstanding, the absence of randomized clinical trials poses challenges in evaluating its effectiveness in patients with sepsis (10). While several systematic reviews have previously been published on this subject matter, recently, a randomized controlled trial (RCT) with a larger sample was published (17). Hence, we conducted an updated systematic review and meta-analysis with the aim of assessing the impact of MB administration, in comparison to a placebo, on both hemodynamic status and clinical outcomes in patients with sepsis and septic shock.
Methods
Inclusion criteria
The protocol for this review was recorded in the Prospective International Register of Systematic Reviews (PROSPERO) with the ID CRD42023423470. We conducted our study following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). The search strategy was based on the PICO (P: Patient; I: Intervention; C: Comparison; O: Outcome) methodology. The PICO framework is as follows: Patient—adult (more than 18 years old) patients with sepsis and septic shock (Sepsis or septic shock was defined according to the current consensus criteria at the time the studies were performed) (1, 19); Intervention—the use of MB; Comparison—the control group receiving standard treatment without MB; and Outcome—mortality rate, length of intensive care unit (ICU) stay, hemodynamic parameters [MAP and vasopressor use], and days on mechanical ventilation. We also evaluated the side-effect levels of methemoglobinemia (MHb). Only RCT designs were considered for inclusion.
Search strategy
Two researchers independently searched three electronic databases, including MEDLINE, EMBASE, and the Cochrane Library, for studies assessing the MB role in sepsis or septic shock until May 2023. Language or period limitations were not applied during the search process. Our search terms were selected according to the following terms: “methylene blue,” “sepsis,” “septic shock,” and their respective synonyms. The complete search strategy for each database can be found in the Supplementary Table S1.
Study selection and data extraction
The search results were entered into Rayyan software (20). After removing the duplicates, two reviewers (R.S.B. and T.L.) independently screened all titles and abstracts and selected full articles for review. Disagreements on study selection were settled by a third reviewer (M.F.M.). Two authors independently gathered data from all eligible trials, encompassing study design and methodology, patient characteristics, and descriptions of interventions. To perform statistical analysis, medians and interquartile ranges were transformed into means and standard deviations using an online calculator (21, 22).
Risk of bias
Two researchers (R.S.B. and T.L.) independently evaluated the risk of bias (RoB) in the studies, and disagreements were resolved in consultation with the third researcher (M.F.M.). The Cochrane Risk of Bias (RoB) 2.0 tool (23) was used to determine the adequacy of the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and the overall RoB for each study.
Certainty of evidence assessment
We utilized the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) (24) system to evaluate the quality of evidence. This system assesses methodological limitations, inconsistency, imprecision, indirectness, and publication bias to categorize the quality of evidence as very low, low, moderate, or high. The summary of the table was created using GRADEpro GDT software.
Synthesis of results and analysis
The statistical analyses were conducted using Review Manager 5.4.1, and a p-value of < 0.05 was defined as statistical significance. Dichotomous outcomes were reported as the risk ratio (RR) and continuous outcomes as the mean difference (MD), both with a 95% confidence interval (CI). The weight of each study was calculated using the inverse variance method, and a random-effects model was used to explore MB intervention, as this considers the heterogeneity between RCTs. The heterogeneity between RCTs was assessed using I2, with substantial heterogeneity identified as I2 > 50%. Due to the small sample size of RCTs, no subgroup analysis was performed.
Results
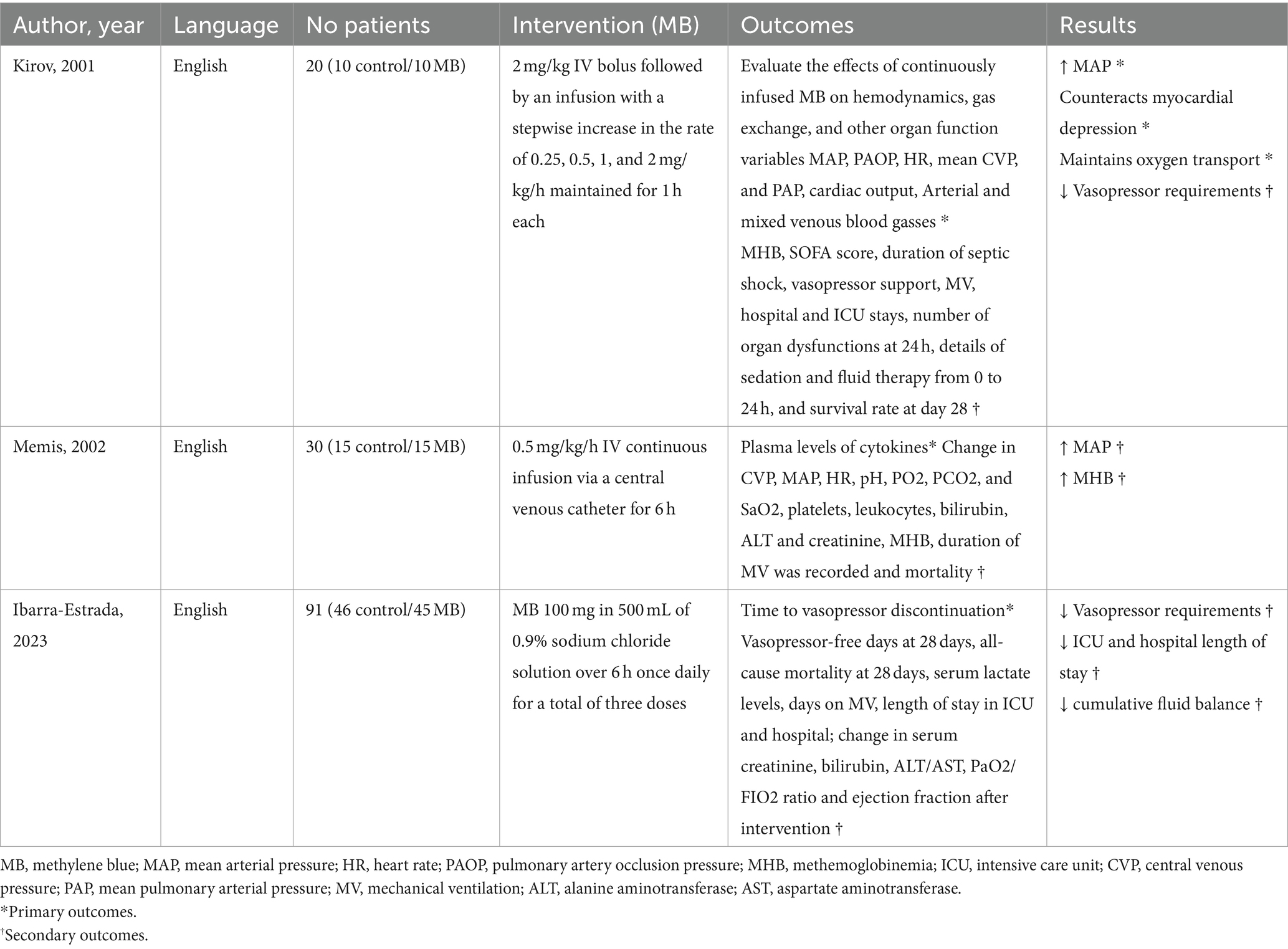
We identified 1,014 records during the initial search, out of which 393 were duplicates. Thus, the total number of abstracts and titles screened was 621. It led to 607 records being excluded and 14 full texts being screened for eligibility. Out of these 14 studies, eight trials were excluded because they are still in progress (five of them with unclear status), and three were conference abstract publications (Figure 1; Supplementary Table S2). Therefore, three studies were included (Table 1) (17, 25, 26). All studies in the data synthesis were RCTs with two arms (MB and control groups), including 141 patients, with 70 of them in the MB group and 71 of them in the control group. Of these, 111 patients had septic shock and 30 had sepsis (severe sepsis, the terminology prevailing at the time, more specifically). One of them was a pilot study (26). Details of these trials and MB infusion are available in Table 1.
The evaluation of the bias risk was conducted using the Cochrane Collaboration tool (Supplementary Figure S1). No study scored a low RoB in all domains. However, all studies manifest some concerns about the domain selection of the reported results due to the absence of a pre-specified protocol before the analysis was performed.
MB treatment was associated with decreased days on mechanical ventilation (MD, −0.72; 95%CI, −1.26 to −0.17; I2 = 0%; p = 0.010), a lower length of ICU stay (MD, −1.58; 95%CI, −2.97 to −0.20; I2 = 25%; p = 0.03), and less time to vasopressor discontinuation (MD, −31.49; 95%CI, −46.02 to −16.96; I2 = 0%; p < 0.0001) (Figure 2).
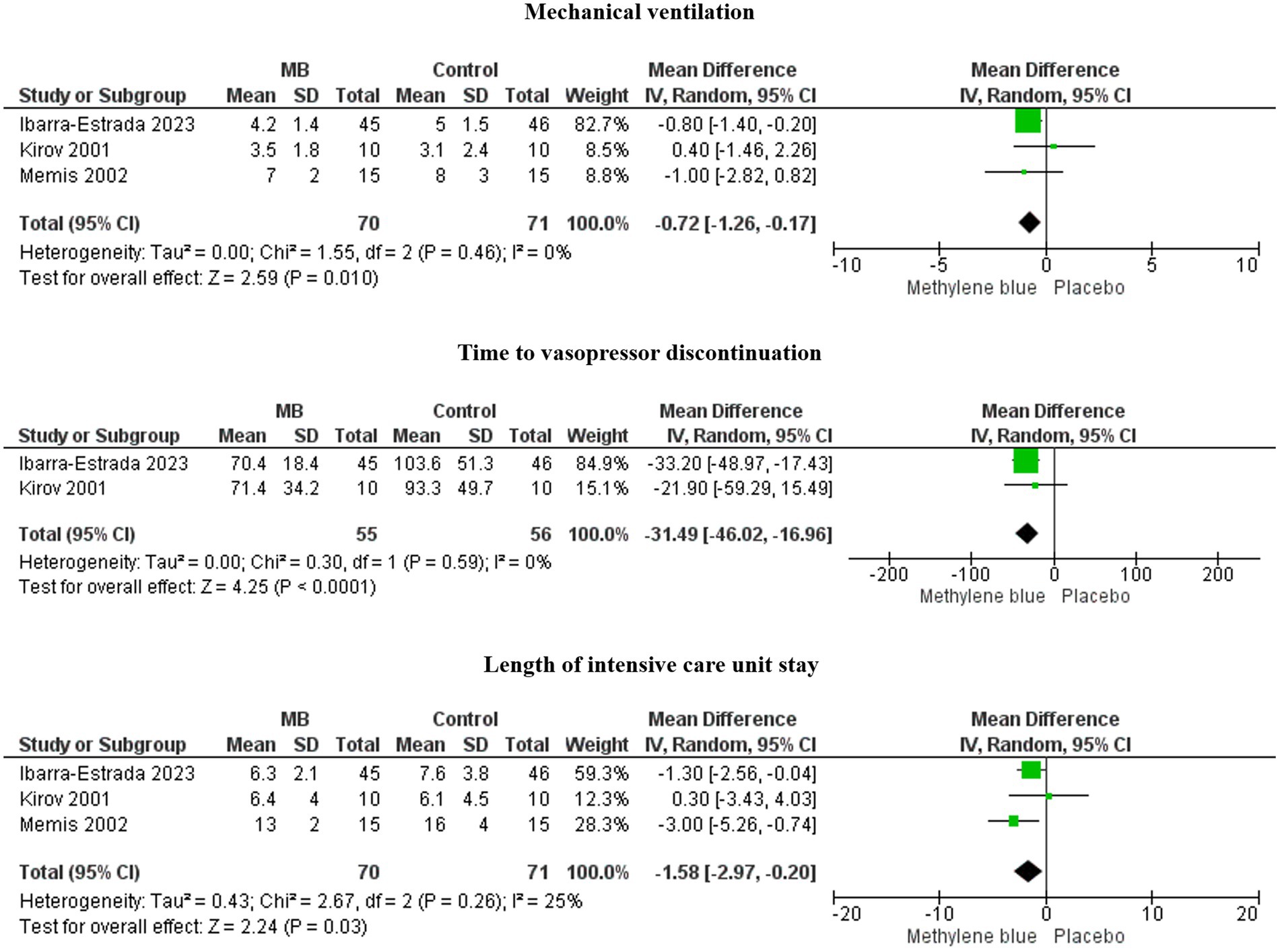
Figure 2. Forests plots of time to vasopressor discontinuation, length of ICU stay, and days on mechanical ventilation.
These three essential outcomes, with low heterogeneity, were statistically significant in the overall effect analysis.
As there is no consistent standardization among studies for assessing mortality [one study (25) used hospital mortality and others (17, 26) used 28-day mortality], and since there was difficulty in extracting data on MAP in one of the studies (17), we opted not to utilize this information to prevent potential bias.
Additionally, we performed a meta-analysis to evaluate the levels of MHb, the main side-effect event related to MB infusion. No statistical difference was found in MHb, including all trials that evaluated MB (MD, 0.85; CI95%, −0.44 to 2.15; I2 = 99%; p = 0.20) (Supplementary Figure S2).
We assessed the certainty of evidence using GRADE (24) and the funnel plot for each outcome (Supplementary Figure S3; Supplementary Table S3). Regarding vasopressor use, there was moderate certainty evidence that MB decreases the time to vasopressor discontinuation compared to usual care. There is low certainty evidence that MB reduces the length of ICU stay and days on mechanical ventilation due to inconsistency and imprecision. The evidence was downgraded because of variation in the estimated treatment effects, no optimal sample size information, a large CI, and heterogeneity.
Discussion
This systematic review and meta-analysis evaluated the effect of MB in three RCTs. They found that MB treatment significantly reduced time to vasopressor discontinuation, days on mechanical ventilation, and length of ICU stay.
MB is an interesting approach in reducing fluid overload and vasoactive drug administration. The inhibition of guanylate cyclase induced by MB infusion reduces nitric oxide production and improves vasoconstriction. Based on this rationale, MB was administered in different scenarios, mainly in patients with vasodilatory shock. However, contradictory results were observed regarding improving systemic vascular resistance and mortality (27, 28). Moreover, MB was only administered to patients with sepsis and septic shock in the three selected RCTs for this study.
Kirov et al. (26) performed a randomized, controlled, open-label pilot study that randomized 20 patients with septic shock to receive either MB or isotonic saline adjunctive to sepsis conventional treatment. MB was administered through a bolus injection of 2 mg/kg, followed 2 h later by increasing rates of 0.25, 0.5, 1, and 2 mg/kg/h, sustained for 1 h each. Hemodynamic and organ function variables were evaluated continuously for 24 h, and the survival rate on day 28 was documented. The MB infusion had no adverse effects. There was also improvement in myocardial depression, oxygen delivery, and reduced vasoactive drug support (26).
Memis et al. (25) performed a prospective, randomized, double-anonymized, placebo-controlled study to evaluate the effect of MB infusion on plasma levels of cytokines in 30 patients with severe sepsis. Patients were randomly assigned to receive either an MB infusion of 0.5 mg/kg/h or a similar volume of isotonic saline for 6 h. Plasma concentrations of inflammatory biomarkers, hemodynamics, and biochemical parameters were evaluated at baseline, immediately after MB infusion, and at 24 and 48 h after MB infusion. In addition, the duration of mechanical ventilation and hospital survival were recorded. MB infusion did not change cytokine levels or outcomes in severe sepsis; however, it resulted in a transient increase in arterial pressure.
Ibarra-Estrada et al. (17) performed the largest RCT with MB in patients with septic shock. They randomized 91 patients with septic shock to receive an intravenous infusion of 500 mL of 0.9% sodium chloride solution with or without 100 mg of MB over 6 h once daily for three doses. The primary outcome was time to vasopressor discontinuation at 28 days, while secondary outcomes included vasopressor-free days at 28 days, duration of mechanical ventilation, length of stay in the ICU and the hospital, and mortality at 28 days. Administering methylene blue infusion within 24 h of initiating norepinephrine resulted in a shorter time to vasopressor discontinuation, increased vasopressor-free days, and a reduction in the length of stays in the ICU and the hospital without adverse effects.
Summarizing these data, MB infusion improved hemodynamic status, decreased length of ICU stay, and days on mechanical ventilation. In addition, there was no effect on MHb levels, suggesting that this intervention is safe. The different populations, methods, dosages, and timing of MB administration can explain divergent results in some studies. Early MB start, in the first 8 h of sepsis, and continuous infusion for a longer time, due to a half-life of 5–6 h, are probably more effective (12, 28). A recent cohort study demonstrated that the method of MB administration may impact its efficacy in patients experiencing shock, with a reduction in 28-day mortality observed in the group receiving MB through bolus injection followed by continuous infusion (29).
When interpreting the findings of this study, it is important to take into account several limitations. Despite including only RCTs, the study has a small sample size. Another practical limitation to be considered is the availability of MB in some European countries. In addition, the degree of certainty of most outcomes was considered low by the GRADE tool (24). Despite these limitations, our analysis adds information about a potential new approach for treating sepsis and septic shock patients. In addition, this meta-analysis could help physicians make decisions in clinical practice.
In this context, considering its established safety profile and affordability, MB can be viewed as a viable option for reducing the use of catecholamines. Importantly, other RCTs of MB administration in patients with sepsis and septic shock are needed to define the ideal dose, timing, duration of treatment, and the best subgroup of patient’s candidates for its use.
Conclusion
Although more studies are necessary in the future, the findings of this meta-analysis suggest that MB could be a promising sparing vasopressor agent in patients with sepsis and septic shock.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RB: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. TL: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. LZ: Writing – original draft, Writing – review & editing. PA: Writing – original draft, Writing – review & editing. FP: Writing – original draft, Writing – review & editing. ST: Writing – original draft, Writing – review & editing. MFM: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MFM declared that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1366062/full#supplementary-material
Abbreviations
MB, Methylene blue; RCTs, Randomized controlled trials; ICU, Intensive care unit; MAP, Mean arterial pressure; MHb, Methemoglobinemia; RR, Risk ratio; MD, Mean difference; CI, Confidence interval.
References
1. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Hosseinian, L, Weiner, M, Levin, MA, and Fischer, GW. Methylene blue: magic bullet for Vasoplegia? Anesth Analg. (2016) 122:194–201. doi: 10.1213/ANE.0000000000001045
3. Evans, T, Carpenter, A, Kinderman, H, and Cohen, J. Evidence of increased nitric oxide production in patients with the sepsis syndrome. Circ Shock. (1993) 41:77–81.
4. Rivers, E, Nguyen, B, Havstad, S, Ressler, J, Muzzin, A, Knoblich, B, et al. Early goal-directed therapy in the treatment of severe Sepsis and septic shock. N Engl J Med. (2001) 345:1368–77. doi: 10.1056/NEJMoa010307
5. Puntillo, F, Giglio, M, Pasqualucci, A, Brienza, N, Paladini, A, and Varrassi, G. Vasopressor-sparing action of methylene blue in severe Sepsis and shock: a narrative review. Adv Ther. (2020) 37:3692–706. doi: 10.1007/s12325-020-01422-x
6. Evans, L, Rhodes, A, Alhazzani, W, Antonelli, M, Coopersmith, CM, French, C, et al. Surviving Sepsis campaign: international guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. (2021) 49:e1063–143. doi: 10.1097/CCM.0000000000005337
7. Dünser, MW, and Hasibeder, WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. (2009) 24:293–316. doi: 10.1177/0885066609340519
8. Schmittinger, CA, Torgersen, C, Luckner, G, Schröder, DCH, Lorenz, I, and Dünser, MW. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. (2012) 38:950–8. doi: 10.1007/s00134-012-2531-2
9. Russell, JA. Vasopressor therapy in critically ill patients with shock. Intensive Care Med. (2019) 45:1503–17. doi: 10.1007/s00134-019-05801-z
10. Arias-Ortiz, J, and Vincent, JL. Administration of methylene blue in septic shock: pros and cons. Crit Care. (2024) 28:46. doi: 10.1186/s13054-024-04839-w
11. Stolk, RF, Van Der Pasch, E, Naumann, F, Schouwstra, J, Bressers, S, Van Herwaarden, AE, et al. Norepinephrine dysregulates the immune response and compromises host defense during Sepsis. Am J Respir Crit Care Med. (2020) 202:830–42. doi: 10.1164/rccm.202002-0339OC
12. Belletti, A, Musu, M, Silvetti, S, Saleh, O, Pasin, L, Monaco, F, et al. Non-adrenergic vasopressors in patients with or at risk for vasodilatory shock. A systematic review and Meta-analysis of randomized trials. PLoS One. (2015) 10:e0142605. doi: 10.1371/journal.pone.0142605
13. Scheeren, TWL, Bakker, J, De Backer, D, Annane, D, Asfar, P, Boerma, EC, et al. Current use of vasopressors in septic shock. Ann Intensive Care. (2019) 9:20. doi: 10.1186/s13613-019-0498-7
14. Rudiger, A, and Singer, M. Decatecholaminisation during sepsis. Crit Care. (2016) 20:309. doi: 10.1186/s13054-016-1488-x
15. Aguilar Arzapalo MF. Methylene blue effectiveness as contributory treatment in patients with septic shock. Intensive care med Exp [internet]. 2016;4((Aguilar Arzapalo M.F.) SSA UADY, Mérida, Mexico). (2016) Available at: https://www.embase.com/search/results?subaction=viewrecord&id=L617955446&from=export.
16. Carrillo-Esper, R, Sosa-García, JO, Carrillo-Córdova, JR, and Carrillo-Córdova, LD. Methylene blue for the management of septic shock refractory to vasopressors. Rev Mex Anestesiol. (2010) 33:214–9.
17. Ibarra-Estrada, M, Kattan, E, Aguilera-González, P, Sandoval-Plascencia, L, Rico-Jauregui, U, Gómez-Partida, CA, et al. Early adjunctive methylene blue in patients with septic shock: a randomized controlled trial. Crit Care. (2023) 27:110. doi: 10.1186/s13054-023-04397-7
18. Shamseer, L, Moher, D, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 349:g7647. doi: 10.1136/bmj.g7647
19. Bone, RC, Balk, RA, Cerra, FB, Dellinger, RP, Fein, AM, Knaus, WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. (1992) 101:1644–55. doi: 10.1378/chest.101.6.1644
20. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
21. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
22. Luo, D, Wan, X, Liu, J, and Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
23. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
24. Guyatt, G, Oxman, AD, Akl, EA, Kunz, R, Vist, G, Brozek, J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
25. Memis, D, Karamanlioglu, B, Yuksel, M, Gemlik, I, and Pamukcu, Z. The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care. (2002) 30:755–62. doi: 10.1177/0310057X0203000606
26. Kirov, MY, Evgenov, OV, Evgenov, NV, Egorina, EM, Sovershaev, MA, Sveinbjørnsson, B, et al. Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med. (2001) 29:1860–7. doi: 10.1097/00003246-200110000-00002
27. Kwok, ESH, and Howes, DW. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. (2006) 21:359–63. doi: 10.1177/0885066606290671
28. Zhao, CC, Zhai, YJ, Hu, ZJ, Huo, Y, Li, ZQ, and Zhu, GJ. Efficacy and safety of methylene blue in patients with vasodilatory shock: a systematic review and meta-analysis. Front Med (Lausanne). 9:950596. doi: 10.3389/fmed.2022.950596
Keywords: sepsis, septic shock, methylene blue, meta-analysis, systematic review
Citation: Ballarin RS, Lazzarin T, Zornoff L, Azevedo PS, Pereira FWL, Tanni SE and Minicucci MF (2024) Methylene blue in sepsis and septic shock: a systematic review and meta-analysis. Front. Med. 11:1366062. doi: 10.3389/fmed.2024.1366062
Edited by:
Mikhail Kirov, Northern State Medical University, RussiaReviewed by:
Mathieu Jozwiak, Centre Hospitalier Universitaire de Nice, FranceEvgeny Suborov, Ministry of Emergency Situations, Russia
Copyright © 2024 Ballarin, Lazzarin, Zornoff, Azevedo, Pereira, Tanni and Minicucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raquel Simões Ballarin, raquel.ballarin@unesp.br
†These authors have contributed equally to this work and share first authorship
 Raquel Simões Ballarin
Raquel Simões Ballarin Taline Lazzarin†
Taline Lazzarin†  Leonardo Zornoff
Leonardo Zornoff Paula Schmidt Azevedo
Paula Schmidt Azevedo Filipe Welson Leal Pereira
Filipe Welson Leal Pereira Marcos Ferreira Minicucci
Marcos Ferreira Minicucci