Endocrine system-related adverse events associated with PD-1/PD-L1 inhibitors: data mining from the FDA adverse event reporting system
- 1Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Pharmacy, Sichuan Mianyang 404 Hospital, Mianyang, China
- 3Department of Pharmacy/Evidence-Based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, China
- 4Department of Pharmacology, Faculty of Medicine, University of the Basque Country UPV/EHU, Leioa, Spain
Background: Various immune checkpoint inhibitors, such as programmed cell death protein-1 (PD-1) and its ligand (PD-L1), have been approved for use, but they have side effects on the endocrine glands.
Methods: Adverse event reports related to PD-1/PD-L1 inhibitors from the FDA Adverse Event Reporting System (FAERS) from the first quarter of 2019 to the first quarter of 2023 were extracted, and the reported Odds ratio methods (ROR method) and comprehensive standard methods (MHRA methods) were used for data mining and analysis.
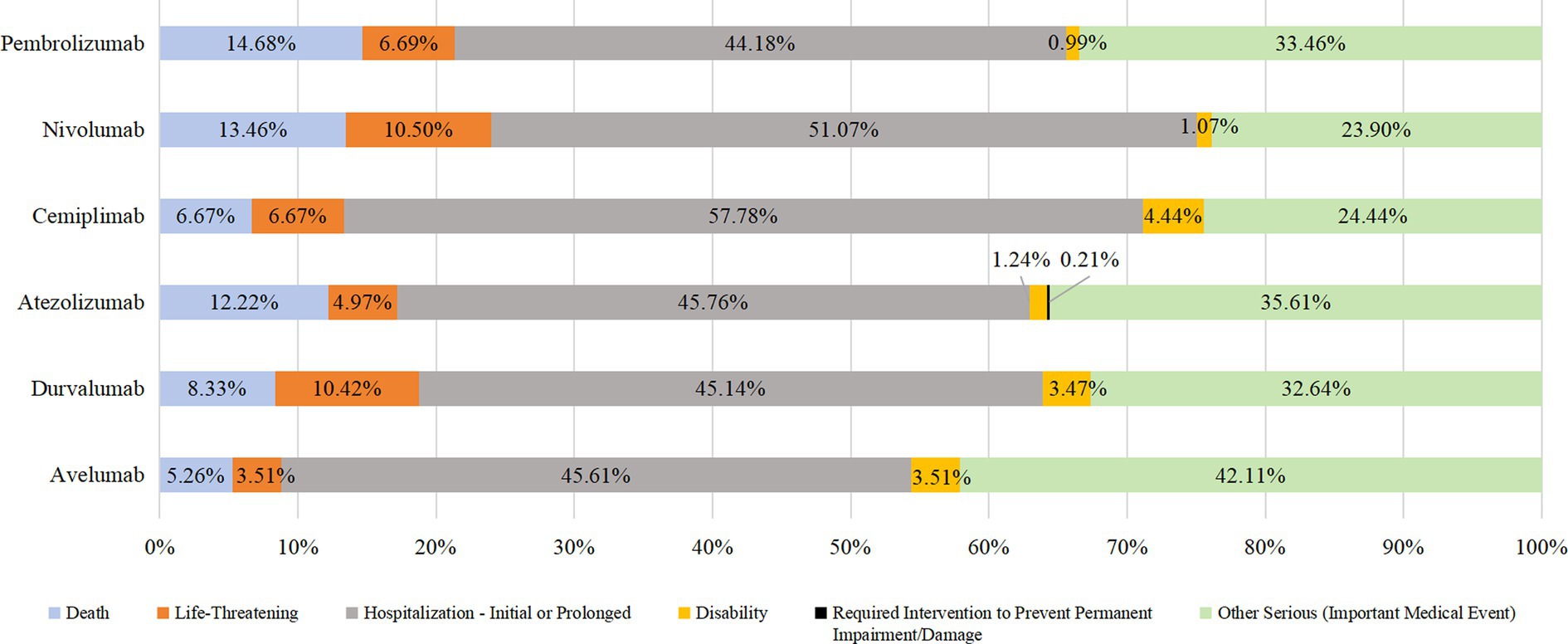
Results: A total of 5,322 reports (accounts for 6.68% of the total reports)of AEs in endocrine system were collected, including 1852 of pabolizumab (34.80%), 2,326 of navuliumab (43.71%), 54 of cimipriliumab (1.01%), 800 of atilizumab (15.03%), 222 of duvariumab (4.17%) and 68 of averumab (1.28%). Endocrine system-related AEs were mainly present in men (excluding those treated with pembrolizumab) aged ≥65 years. The ratio of AEs components in the endocrine system for the six drugs was approximately 3–8%. The main endocrine glands involved in AEs were the thyroid (pembrolizumab), pituitary and adrenal (nivolumab), adrenal (cemiplimab, atezolizumab, and avelumab), and thyroid (durvalumab). Most patients experienced AEs between 30 and 365 (mean, 117) days,the median time was 61d. AEs resulted in prolonged hospitalization in >40% and death in >10% of cases after administration of pembrolizumab, nivolumab, or durvalumab.
Conclusion: Men aged ≥65 years should be concerned about endocrine-related AEs. There was a lengthy interval between the use of PD-1/PD-L1 inhibitors and endocrine system-related AEs, but the outcome was serious. Special attention should be given to endocrine system-related AEs when using pembrolizumab, nivolumab, or durvalumab.
1 Introduction
The programmed death-1 and programmed cell death-ligand 1 (PD-1/PD-L1) were major immune checkpoint inhibitors. The PD-1/PD-L1 derived drugs were specifically recognizing and blocking immunosuppressive molecules to achieve anti-tumor response, namely enhancing anti-tumor immune response, inhibiting immune evasion, inducing tumor cell death,It’s called immunotherapy for tumors (1–3). The immunotherapy is another important therapy strategy after surgery, chemotherapy and radiotherapy, which has been widely applied in the treatment of melanoma, lung, lymphoma, kidney, endometrial and other tumors (2, 4–6). However, PD-1/PD-L1 inhibitor will enhance over-activated immune cells response to normal cells, resulting in immune-related adverse events (irAEs) in organs or tissues.
Therefore, while benefiting from treatment, cancer patients will also be troubled by irAEs, such as gastrointestinal toxicity, skin toxicity, endocrine toxicity, immune-associated pneumonia, etc. (2), involving multiple systems. Treatment can trigger autoimmune reactions in various ways (e.g., increasing the level of autoantibodies (1)) and then involve multiple glands (e.g., pituitary, thyroid, and adrenal) to cause the corresponding functional disorders. Recent studies have shown that endocrine toxicity is irreversible in 50% of cases (7) and can be life-threatening if not identified and treated appropriately (8–10). The disproportional reporting is most usually employed in adverse drug events signal monitoring, which containing reporting odds ratio (ROR), comprehensive standard (MHRA) and proportional reporting ratio (PRR) methods. Up to date, there are numerous studies have been applied these methods in drug safety investigation (11–13).
Therefore, the proportional imbalance method was adopted in this study, we conducted data mining through the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database. We focused on AEs reported after the use of PD-1/PD-L1 inhibitors in the endocrine system. We concentrated on the risks and characteristics of AEs caused by these drugs and provided references for further prevention and management.
2 Methods
2.1 Data sources
FAERS is an open database of anonymous patient health and treatment information that contains information on adverse event and medication error reports submitted to FDA. We used data from the FAERS database. The present study did not involve therapeutic interventions or the collection of human samples and, as such, was exempt from approval from an institutional review board approval. There are seven tables: patient demographic and administrative information, medication information, adverse drug reaction information, information on reporting sources, start and end dates of drug therapy, indications for use/diagnosis, and case deletions.
There are numerous types of PD-1/PD-L1 inhibitors. We included only single-agent preparations and excluded varieties available only on the market in China. As a result, we included six drugs for analyses. Pembrolizumab is a PD-1 inhibitor. Nivolumab is a PD-1 inhibitor. Cemiplimab is a PD-L1 inhibitor of atezolizumab, durvalumab, and avelumab.
2.2 Data processing
Cemiplimab was launched in the USA relatively recently (September 2018) compared with the other drugs. Hence, the time range of data extraction in the present study was the first quarter of 2019 to the first quarter of 2023 (17 quarters in total). The search terms (including the generic name and trade name of the drug) we used were “pembrolizumab/Keytruda,” “nivolumab/opdivo/opdualag,” “cemiplimab/libtayo,” “atezolizumab/tecentriq,” “durvalumab/imfinzi,” and “avelumab/bavencio.” The FAERS database is updated each quarter, so published reports will inevitably be duplicated. Hence, reprocessing was done using MySQL 8.0,1 as recommended by the FDA. If the CASEID and FDA_DT were identical, then the latest PRIMARYID was selected. If the CASEID and FDA_DT were identical, then the DELETE report was selected from the DELETE table. The FAERS database is encoded using the Medical Dictionary for Regulatory Activities (MedDRA) of the International Council for Organizations of Medical Sciences. Therefore, systematic organ classification (SOC) and the preferred term (PT) in the latest edition of the MedDRA Glossary of Adverse Drug Reactions (MedDRA 25.1) were used in the present study. MedDRA 25.1 standardizes and minimizes international terminology of terms to describe AEs (14, 15). According to PUBMED age grouping standard (16), the entire population was categorized into three distinct age groups: minors (age < 18 years), adults (18 ≤ age < 65 years), and senior citizens (≥65 years).
2.3 Data analyses
Data (e.g., target number of AEs reports and background number of AEs occurrences of the primary suspected drug) were obtained. Potential AE signals were screened based on a four-cell table (14, 17) of the proportional imbalance method (See Supplementary Table S1). Adopt the ROR and MHRA method, the ROR, proportional reporting ratio (PRR), and X2 equivalents are calculated, respectively. To avoid false-positive signals, the calculated corresponding values should reach the set threshold and be defined as the PT of valid signals (See Supplementary Table S2) (18–20). The larger the calculated value, the stronger the signal, indicating that the target drug is more likely to be associated with the target AEs, but this does not mean that there is a causal relationship between the two (21). All statistical analyses were undertaken using Prism 8 (GraphPad, La Jolla, CA, United States), SPSS29 and Excel® (Microsoft, Redmond, WA, United States).
3 Results
3.1 Primary characteristics of AEs reported in the endocrine system
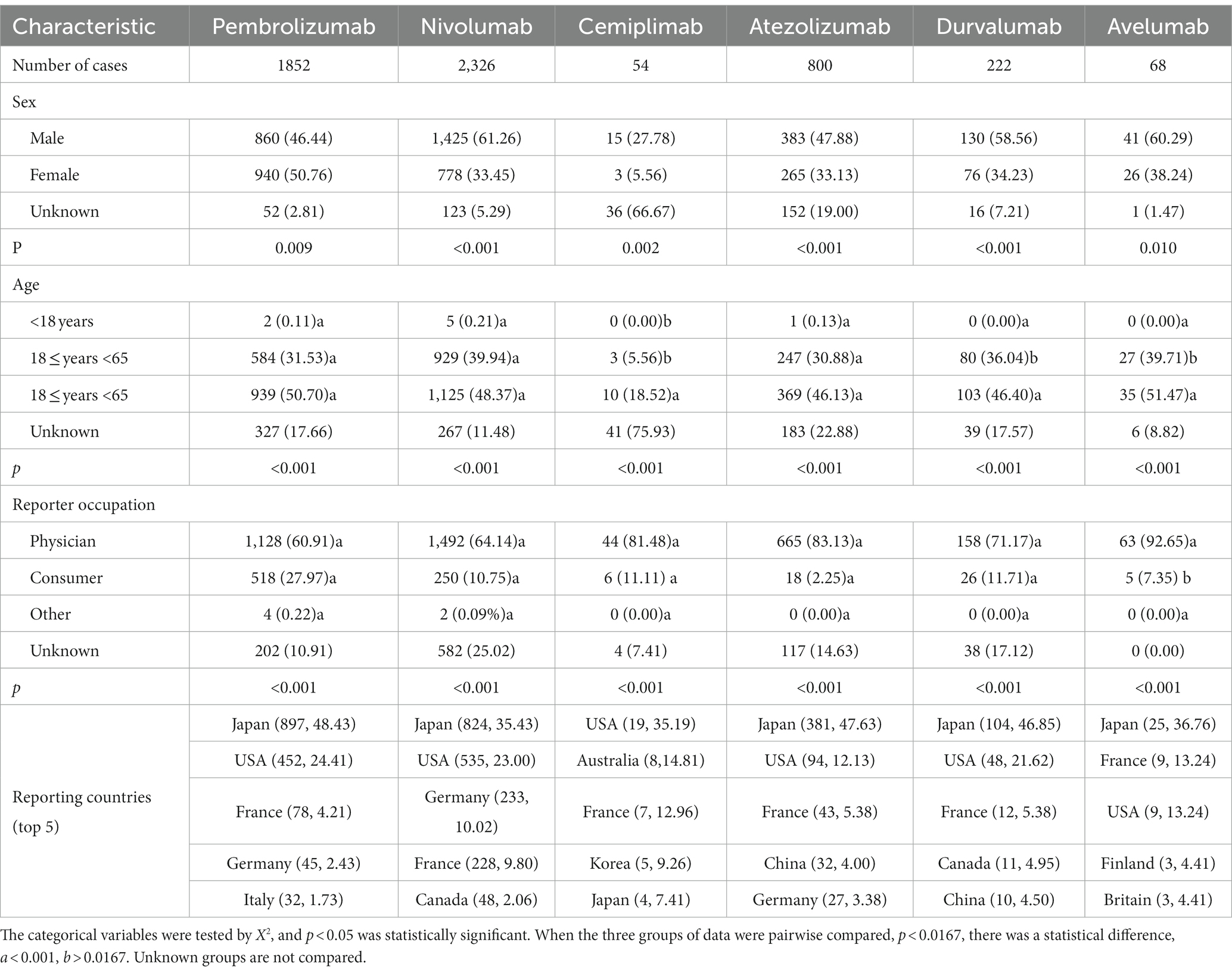
As of the first quarter of 2023, the FAERS database collected 79,700 AEs reports. Among them, there were 22,918, 34,267, 1,239, 13,862, 6,014, and 1,300 AEs reports involving pembrolizumab, nivolumab, cemiplimab, atezolizumab, durvalumab, and avelumab as the primary suspected drug, respectively. Among them, there were 5,322 reports related to the endocrine system (accounting for 6.68% of the total reports), with the aforementioned six drugs accounting for 34.80, 43.71, 1.01, 15.03, 4.17 and 1.28%, respectively. Patients who suffered PD-1/PD-L1 inhibitor-associated AEs were predominantly male (except for those who had AEs after taking pembrolizumab) and most of the population is over aged >65 years. The occupations at the top of the list were physician and consumers. The countries with the largest number of AEs reports by drug were mostly Japan. The specific number and proportion of reports are shown in Table 1.
3.2 Proportion of AEs in the endocrine system
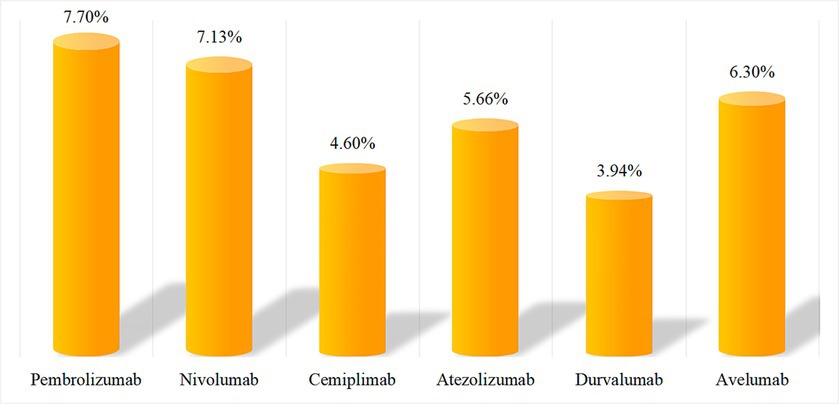
After SOC classification of excavated effective signals, we found no significant difference in the ratio of AEs components associated with PD-1/PD-L1 inhibitors in the endocrine system (approximately 3–8%). The specific numbers of cases and composition ratios are shown in Figure 1.
3.3 AEs signals and correlation with the endocrine system
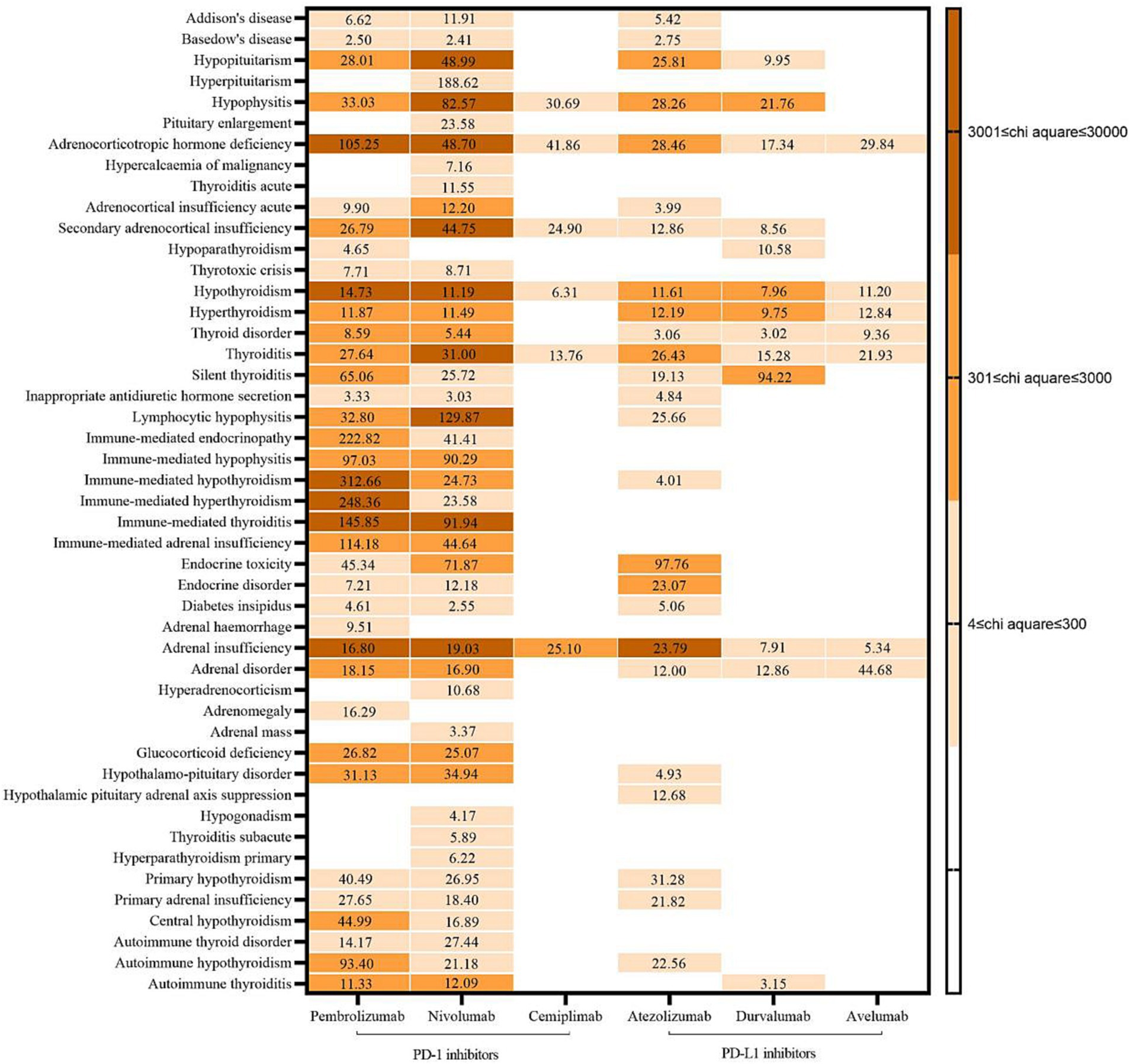
A total of 131 AEs signals were detected in the endocrine system: 37 for pembrolizumab, 43 for nivolumab, six for cemiplimab, 25 for atezolizumab, 13 for durvalumab, and seven for avelumab. Endocrine system-related AEs had a strong correlation with pembrolizumab, including immune-mediated hypothyroidism (X2 = 27,216.81, ROR = 312.66), adrenocorticotropin deficiency (10,269.46, 105.25), and hypothyroidism (6307.58, 14.73). AEs closely associated with nivolumab were pituitary inflammation (X2 = 14,605.84, ROR = 82.57), adrenal insufficiency (6549.80, 19.03), and hypopituitarism (5874.78, 48.99). Adrenal dysfunction was the main factor in AEs attributed to a strong correlation between the use of cemiplimab and atezolizumab (X2 = 497.78, ROR = 25.10; X2 = 4582.73, ROR = 23.79). The AEs with a strong correlation with durvalumab use was silent thyroiditis (X2 = 508.62, ROR = 94.22). The AEs with strong association of avilumab was adrenal disease (X2: 252.79, ROR: 44.68). See Figure 2 for details.
3.4 Time of occurrence of AEs in the endocrine system
The onset time of AEs in the endocrine system was more distributed between 30–365 days, the median time was 61d, the median onset time of AEs in the endocrine system was 42d for pembrolizumab, 63 days for nivolumab, 161 days for cemiplimab, 73.5 days for atezolizumab, and 42 days for durvalumab. Avelumab was 56 days. Time of adverse endocrine system events with the use of six drugs. See Figure 3 for details.
3.5 PD-1/PD-L1 inhibitors produce AEs in the endocrine system
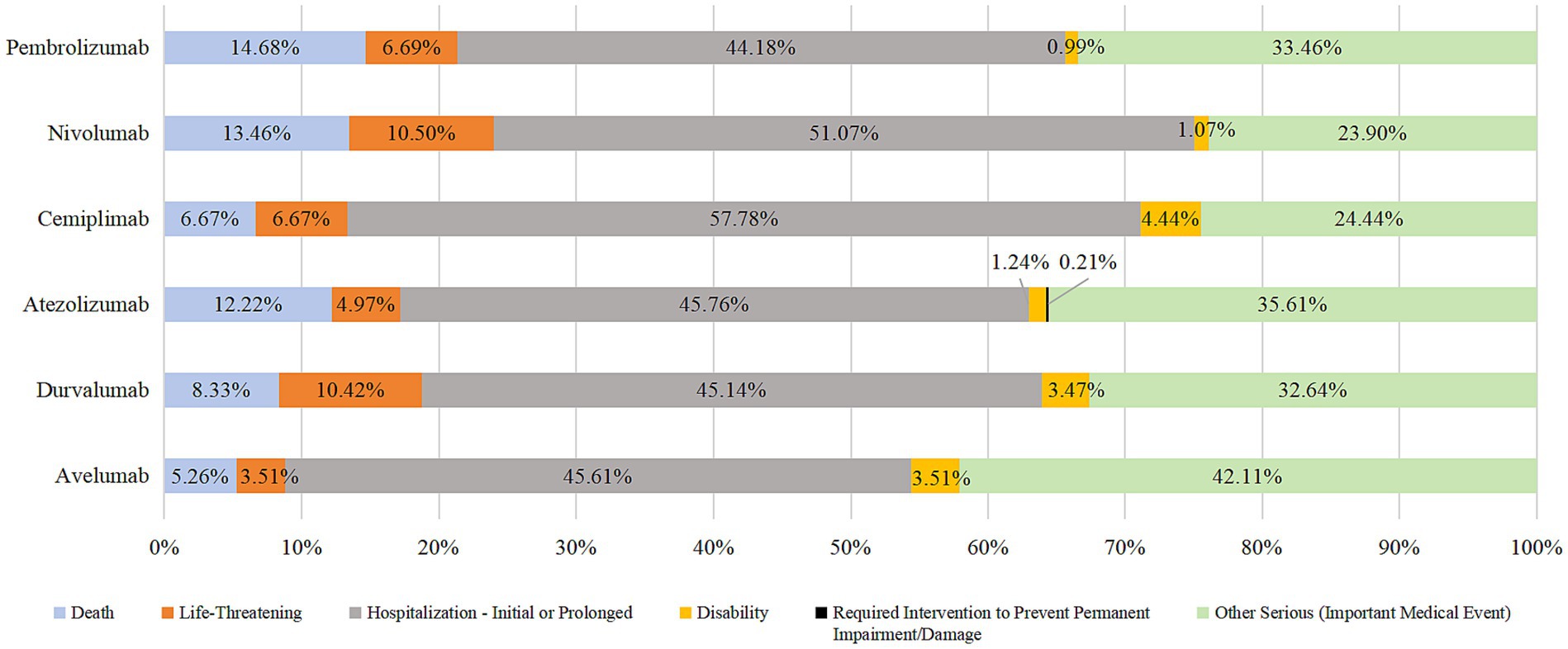
In addition to unknown serious medical events, the most prevalent outcome of endocrine system AEs due to the use of PD-1/PD-L1 inhibitors was prolonged hospital stay (44.18–57.78%). The other most prevalent outcomes were death, life-threatening injury, and disability. Death due to taking pembrolizumab, nivolumab, or durvalumab accounted for >10% of cases. See Figures 3, 4 for details.
4 Discussion
4.1 Basic characteristics of reported cases of PD-1/PD-L1 inhibitor-associated endocrine system AEs
We found that, except for pembrolizumab, five PD-1/PD-L1 inhibitors associated with AEs occurred mainly in men, a result which is consistent with those of other studies (22, 23). Our finding is probably related to the completeness of the data. Compared with the data for the other five drugs, the data size for pembrolizumab was relatively large. The proportion of cases in which the sex of the patient was not known was relatively low (2.81%). Results can vary if data on sex are missing. All six PD-1/PD-L1 inhibitors were used for patients aged >65 years, which may have been related to the age when the disease was diagnosed (24, 25), however, elderly men over 65 years of age should be especially aware of the occurrence of relevant AEs. Most of the reporters are medical personnel, indicating that the AEs reported to the database has strong reliability. The countries that reported the most AEs were Japan and the USA. This finding suggests that other countries may be paying less attention to AEs in the endocrine system, but this may also be related to the number of people taking these drugs in such countries.
4.2 AEs distribution and correlation in endocrine system associated PD-1/PD-L1 inhibitors
The findings of this study, the number of AEs signals associated with the endocrine system was higher for PD-1 inhibitors than for PD-L1 inhibitors and the degree of correlation was also larger, especially pembrolizumab and nivolumab. The above findings are similar to the results of previous relevant studies (26), and this kind of ADR should be paid attention to when using related drugs. The results also suggest that, which should be considered (especially for pembrolizumab and nivolumab). The main organs involved in AEs associated with pembrolizumab use were the thyroid gland and adrenal glands, whereas they were the pituitary gland and adrenal glands for nivolumab, the adrenal glands for cemiplimab, the adrenal glands for atezolizumab, and the thyroid gland for avelumab. According to the Clinical Application Guide of Immune Checkpoint Inhibitors for Gynecological Tumors (2), the common types of endocrine toxicity caused by ICIs are dysfunction in the thyroid gland and acute pituitary inflammation, these data are consistent with the results of our study. As mentioned above, endocrine glands contain rich blood flow and may trigger autoimmune reactions through various ways such as the activation of autoimmune cells, thus involving multiple glands (1). However, the mechanism of adverse reactions in different glands may be different. In addition to immune-mediated mechanisms (3), thyroid injury may also be associated with the upregulation of PD-1 receptors in the thyroid gland (1). Furthermore, elevated levels of serum IL-1β, IL-2, and GM-CSF at baseline, as well as decreased levels of serum IL-8, G-CSF, and MCP-1 at an early stage are correlated with thyroid dysfunction (27, 28). Pituitary is also associated with humoral immunity, usually involving the anterior pituitary, which results in permanent dysfunction of one or more pituitary endocrine axes (29, 30). Combined with the results of our study, the use of PD-1/PD-L1 inhibitors seems to result in additional abnormalities in the functions of the thyroid gland and pituitary gland. These data suggest that monitoring abnormalities in the function of these two organs is important. In the event of a serious acute reaction, immunotherapy should be stopped promptly, and the corresponding drug treatment and symptomatic treatment should be applied (1).
In addition, the above guidelines mentioned that adrenal diseases were rare endocrine toxicity, but the results of this study found that the use of PD-1/PD-L1 inhibitors had a large association with adrenal diseases, which is worth noting. The mechanism of this glandular disease may be related to the infiltration of CD4 + t cells, especially Th1 and Th17 cells (3). Therefore, in clinical practice, medical personnel should still pay attention to the occurrence of such AEs. If patients have abnormal adrenal function and indicators, physicians and clinical pharmacists should judge whether it is caused by such drugs according to the baseline assessment, so as to correctly handle adverse drug reaction (ADR), it also provides reference for whether to adjust the anti-tumor therapy regimen in the future.
4.3 Occurrence time and outcome of AEs in endocrine system associated with PD-1/PD-L1 inhibitors
Our data suggested that AEs in the endocrine system occurred between 30 and 365 (mean, 117) days after the use of PD-1/PD-L1 inhibitors, the median time was 61d, which was consistent with Viola suggestion (31). The above results indicate that this type of AEs occurs slowly, and long-term follow-up monitoring is needed for patients using this type of drugs.
Hospitalization/prolonged hospitalization was the most prevalent outcome of AEs, followed by death, life-threatening illness, and disability. According to the Common Terminology Criteria for Adverse Events (CTCAE), patients who reach grade 3 or above will be hospitalized for intravenous hormone therapy (2), especially if there is a corresponding emergency/crisis, Additional treatments, such as anti-infection therapy, blood purification, and ventilatory support, are needed (1), if the treatment is not timely and incorrect, it can endanger life or even death (33), indicating the severity of the AEs, it is necessary to do a baseline assessment before the use of this type of drug in clinical practice, and close monitoring during use to identify ADR as early as possible and timely intervention to reduce or even avoid the occurrence of adverse outcomes. It is worth noting that the death outcome of pembrolizumab, nivolumab and durvalumab accounted for more than 10%. When patients use the above three drugs, clinical pharmacists should set them as key monitoring objects, closely monitor the corresponding indicators and changes in symptoms and signs, pay attention to the suitability of medication, and cooperate with the clinic to improve the prognosis.
5 Research limitations
First, the FAERS database has a large amount of data, there are also a lot of missing data information such as gender, age and adverse event occurrence time, especially the time is not accurate enough. Secondly, the accuracy and professionalism of the information of “adverse events” in the database need to be improved. Reporters from different occupational backgrounds may use different descriptions of endocrine toxicity and endocrine disorders, which may lead to deviations in the equivalence calculation of ROR and PRR for a single PT. Despite these limitations, spontaneous reporting may be the best way to collect more AEs that might otherwise be overlooked (34). Third, the AEs signal detected in this study indicates that the drug is statistically correlated with the AEs, but it does not mean that there is a causal link in biology, and further clinical trials are needed to explore (35, 36). In addition, sensitivity analysis cannot be performed in the current proportion imbalance method, and the impact of combined drug use on the outcome is difficult to predict,other research methods can be explored to evaluate the impact in the future. Fourth, due to a lack of information on the total number of people used, the incidence of specific adverse events cannot be calculated (37), therefore, the intensity of the association between drugs and adverse events was measured.
6 Conclusion
Men aged ≥65 years should be concerned about endocrine-related AEs. The use of different PD-1/PD-L1 inhibitors mainly involves interaction with the endocrine glands, so physicians should be careful when prescribing drugs for patients with associated underlying diseases. There was a lengthy interval between the use of PD-1/PD-L1 inhibitors and endocrine system-related AEs, but the outcome was serious. Therefore, long-term, meticulous monitoring and appropriate treatment are necessary. Special attention should be given to endocrine system-related AEs when using pembrolizumab, nivolumab, or durvalumab.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
HS: Conceptualization, Investigation, Validation, Writing – original draft. YH: Visualization, Writing – original draft. SD: Data curation, Writing – original draft. LY: Data curation, Writing – original draft. JW: Data curation, Formal analysis, Writing – review & editing. LC: Methodology, Supervision, Writing – review & editing. ZC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the National Natural Science Foundation of China (82073921) and the National Key Clinical Specialties Construction Program.
Acknowledgments
We sincerely thank everyone who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1366691/full#supplementary-material
Footnotes
References
1. Yongzhong, W, Qilan, W, Danlan, P, Danlan, P, Bing, C, Danbo, B, et al. Consensus of Chinese experts on emergency management of major endocrine adverse reactions of immune checkpoint inhibitors. J Chongqing Med Univ. (2023) 48:1–12. doi: 10.13406/j.cnki.cyxb.002758 (in Chinese)
2. Beihua, K, Jihong, L, Aijun, Y, Yun, Z, Xiaoping, L, Qinglei, G, et al. Clinical application guide of immunological checkpoint inhibitors for gynecological tumors. J Integr Cancer Ther Electr. (2023) 9:67–98. doi: 10.13283/j.cnki.xdfckjz.2023.05.001 (in Chinese)
3. Okiyama, N, and Tanaka, R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol Int. (2022) 71:169–78. doi: 10.1016/j.alit.2022.01.001
4. Xiaoli, L, Zhenyan, LI, and Lili, W. Progress and challenge of radiotherapy combined with immunotherapy for esophageal cancer. [J]. J Oncol (2023) 29:1–7. (in Chinese)
5. Dongli, Z, Shen, C, Weichuan, Z, et al. Efficacy and safety of programmed death factor-1 / programmed death factor-1 ligand inhibitors in treatment of renal cell carcinoma. Chin Gen Pract (2023) 1–9. (in Chinese)
6. Chenxi, Z, Yi, X, Yingying, H, Dandan, L, Yawei, L, Wei, Z, et al. Effect of smoking on the efficacy of immune checkpoint inhibitors in patients with driver-negative non-small cell lung cancer. Chin J Clin Pharmacol. (2023) 39:1397–401. doi: 10.13699/j.cnki.1001-6821.2023.10.007. (in Chinese)
7. Ferrari, SM, Fallahi, P, Elia, G, et al. Autoimmune endocrine dysfunctions associated with cancer immunotherapies. Int J Mol Sci. (2019) 20:2560. doi: 10.3390/ijms20102560
8. Torino, F, Barnabei, A, Paragliola, RM, Marchetti, P, Salvatori, R, and Corsello, SM. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: clinical evidence and pathogenic hypotheses. Eur J Endocrinol. (2013) 169:R153–64. doi: 10.1530/EJE-13-0434
9. Hughes, J, Vudattu, N, Sznol, M, Gettinger, S, Kluger, H, Lupsa, B, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy [J]. Diabetes Care. (2015) 38:e55–7. doi: 10.2337/dc14-2349
10. Orlov, S, Salari, F, Kashat, L, and Walfish, PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. (2015) 100:1738–41. doi: 10.1210/jc.2014-4560
11. Jain, D, Sharma, G, and Kumar, A. Adverse effects of proton pump inhibitors (PPIs) on the renal system using data mining algorithms (DMAs). Expert Opin Drug Saf. (2023) 22:741–52. doi: 10.1080/14740338.2023.2189698
12. Faiza, J, and Anoop, K. Identification of signal of clindamycin associated renal failure acute: a disproportionality analysis. Curr Drug Saf. (2023) 19:123–8. doi: 10.2174/1574886318666230228142856
13. Shu, Y, He, X, Liu, Y, Wu, P, and Zhang, Q. A real-world disproportionality analysis of Olaparib: data Mining of the Public Version of FDA adverse event reporting system. Clin Epidemiol. (2022) 14:789–802. doi: 10.2147/CLEP.S365513
14. Sakaeda, T, Tamon, A, Kadoyama, K, and Okuno, Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. (2013) 10:796–803. doi: 10.7150/ijms.6048
15. Omar, NE, Fahmy Soliman, AI, Eshra, M, Saeed, T, Hamad, A, and Abou-Ali, A. Postmarketing safety of anaplastic lymphoma kinase (ALK) inhibitors: an analysis of the FDA adverse event reporting system (FAERS). ESMO Open. (2021) 6:100315. doi: 10.1016/j.esmoop.2021.100315
16. National Library of Medicine. Age Groups. [EB/OL] (1998–2024–01–30]. Available at: https://www.ncbi.nlm.nih.gov/mesh/68009273
17. Lin, L, Jiaying, Z, Li, C, Enwei, L, et al. Adverse event signal mining of tocilizumab based on FAERS database in the United States. Chin J Pharm. (2021) 32:1874–9. (in Chinese)
18. Chen, JJ, Huo, XC, Wang, SX, Wang, F, and Zhao, Q. Data mining for adverse drug reaction signals of daptomycin based on real-world data: a disproportionality analysis of the US Food and Drug Administration adverse event reporting system [J]. Int J Clin Pharm. (2022) 44:1351–60. doi: 10.1007/s11096-022-01472-x
19. Sakaeda, T, Kadoyama, K, and Okuno, Y. Adverse event profiles of platinum agents: data mining of the public version of the FDA adverse event reporting system, AERS, and reproducibility of clinical observations. Int J Med Sci. (2011) 8:487–91. doi: 10.7150/ijms.8.487
20. Ziyan, J, Chen, C, Yufan, D, Bing, W, Ting, X, et al. Study on signal mining of benzodiazepine-related dementia events based on FAERS. Herald Med. (2021) 40:1356–60. (in Chinese)
21. Zhou, Y, Chen, M, Liu, L, and Chen, Z. Difference in gastrointestinal risk associated with use of GLP-1 receptor agonists: a real-world pharmacovigilance study. Diabetes Metab Syndr Obes. (2022) 15:155–63. doi: 10.2147/DMSO.S348025
22. Ling, L, Anqi, L, and Junxian, Y. Data analysis of pneumonia induced by PD-1/PD-L1 inhibitors based on FAERS. Chin J Hosp Pharm. (2021) 41:1288–92. doi: 10.13286/j.1001-5213.2021.13.02. (in Chinese)
23. Bai, S, Tian, T, Pacheco, JM, Tachihara, M, Hu, P, and Zhang, J. Immune-related adverse event profile of combination treatment of PD-(L)1 checkpoint inhibitors and bevacizumab in non-small cell lung cancer patients: data from the FDA adverse event reporting system. Transl Lung Cancer Res. (2021) 10:2614–24. doi: 10.21037/tlcr-21-464
24. National Health Commission of the People's Republic of China Medical Administration. Guidelines for diagnosis and treatment of esophageal cancer. Chin J Dig Surg. (2022) 21:1247–68. (in Chinese)
25. National Comprehensive Cancer Network. Hepatocellular Carcinoma (Version 1.2023). [EB/OL]. (2023-09-14)[2024-01-30]. Available at: https://www.nccnchina.org.cn/guide/detail/443
26. Sonpavde, GP, Petros, G, and Yushun, L. Immune-related adverse events with PD-1 versus PD-L1 inhibitors: a meta-analysis of 8730 patients from clinical trials [J]. Future Oncol. (2021):2545–58.
27. Kurimoto, C, Inaba, H, Ariyasu, H, Iwakura, H, Ueda, Y, Uraki, S, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. (2020) 111:1468–77. doi: 10.1111/cas.14363
28. Chennamadhavuni, A, Abushahin, L, Jin, N, Presley, CJ, and Manne, A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and Rechallenging immune checkpoint inhibitors&13. Front Immunol. (2022) 13:779691. doi: 10.3389/fimmu.2022.779691
29. Chang, LS, Barroso-Sousa, R, Tolaney, SM, Hodi, FS, Kaiser, UB, and Min, L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. (2019) 40:17–65. doi: 10.1210/er.2018-00006
30. Scott, ES, Long, GV, Guminski, A, Clifton-Bligh, RJ, Menzies, AM, and Tsang, VH. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol. (2018) 178:173–80. doi: 10.1530/EJE-17-0810
31. Trevisani, V, Iughetti, L, Lucaccioni, L, and Predieri, B. Endocrine immune-related adverse effects of immune-checkpoint inhibitors. Expert Rev Endocrinol Metab. (2023) 18:441–51. doi: 10.1080/17446651.2023.2256841
32. de Filette, J, Jansen, Y, Schreuer, M, Everaert, H, Velkeniers, B, Neyns, B, et al. Incidence of thyroid-related adverse events in melanoma patients treated with Pembrolizumab. J Clin Endocrinol Metab. (2016) 101:4431–9. doi: 10.1210/jc.2016-2300
33. Iglesias, P . Cancer immunotherapy-induced endocrinopathies: clinical behavior and therapeutic approach. Eur J Intern Med. (2018) 47:6–13. doi: 10.1016/j.ejim.2017.08.019
34. Yang, Z, Lv, Y, Yu, M, Mei, M, Xiang, L, Zhao, S, et al. GLP-1 receptor agonist-associated tumor adverse events: a real-world study from 2004 to 2021 based on FAERS. Front Pharmacol. (2022) 13:925377. doi: 10.3389/fphar.2022.925377
35. Jiaying, Z, Xxiangdan, C, Li, C, et al. Application of ratio imbalance method in signal mining of adverse reactions of nivolumab. Oncol Pharm. (2019) 9:798–803. (in Chinese)
36. Xiaowen, H, Liu, L, Yiwen, R, et al. Adverse event signal mining of platinum drugs based on FAERS. Oncol Pharm. (2020) 10:608–16. (in Chinese)
Keywords: PD-1/PD-L1 inhibitor, endocrine system, adverse events, FAERS, data mining
Citation: Shi H, He Y, Dan S, Yang L, Wang J, Chen L and Chen Z (2024) Endocrine system-related adverse events associated with PD-1/PD-L1 inhibitors: data mining from the FDA adverse event reporting system. Front. Med. 11:1366691. doi: 10.3389/fmed.2024.1366691
Edited by:
J. Francis Borgio, Imam Abdulrahman Bin Faisal University, Saudi ArabiaReviewed by:
Hanqing Liu, Renmin Hospital of Wuhan University, ChinaJeehee Yoon, Chonnam National University Bitgoeul Hospital, Republic of Korea
Anoop Kumar, Delhi Pharmaceutical Sciences and Research University, India
Weimin Zhong, Xiamen Fifth Hospital, China
Copyright © 2024 Shi, He, Dan, Yang, Wang, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Wang, 1040110291@qq.com: Li Chen, chenl_hxey@scu.edu.cn
 Hongxia Shi
Hongxia Shi Yunhua He1
Yunhua He1  Lin Yang
Lin Yang Jing Wang
Jing Wang Li Chen
Li Chen