Specific antigens in malignancy-associated membranous nephropathy
- Division of Nephrology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Membranous nephropathy (MN) is a glomerular disease mediated by autoimmune complex deposition, with approximately 30% of cases attributed to secondary causes. Among them, malignant tumors are a significant cause of secondary MN. Recent advancements in the identification of MN-specific antigens, such as THSD7A and NELL-1, suggest a potential association with malignant tumors, yet definitive proof of this relationship remains elusive. Therefore, this article aims to review the distribution of MN-specific antigens in patients with MN caused by malignant tumors and the possible role of these antigens in the pathogenesis of the disease.
Introduction
Membranous nephropathy (MN) is the most common pathological type of adult nephrotic syndrome, predominantly occurring in middle-aged and elderly patients. It is caused by the deposition of immune complexes along the glomerular basement membrane (1). Approximately 30% of membranous nephropathy cases are attributed to secondary factors (2), among which malignant tumors are one of the common causes, accounting for about 5%–20% (3). As early as 1966, Lee et al. (4), proposed that there was a high coincidence between MN and malignant tumors, and it is currently considered that the presence of any of the following indicators should prompt an evaluation for malignancy-associated MN: (1) the patient’s malignant tumor and MN must occur within a similar time frame, usually within 5 years before or after the diagnosis of membranous nephropathy; (2) a remission of the malignant tumor accompanied by clinical remission of MN, while the recurrence of MN should occur when the malignant tumor recurs; (3) there is a pathophysiological connection between the two diseases, the same antigens are detected in both diseases. To date, more than a dozen MN-related antigens have been identified, of which THSD7A and NELL-1 are thought to be associated with malignancies. Subsequently, PLA2R, PCDH7, HTRA1 and FAT1 antigens were found to be positive in patients with malignancy-associated MN. However, the relationship between these antigens and malignancies is still unclear. This article will review the distribution and the mechanism of action of antigens in MN.
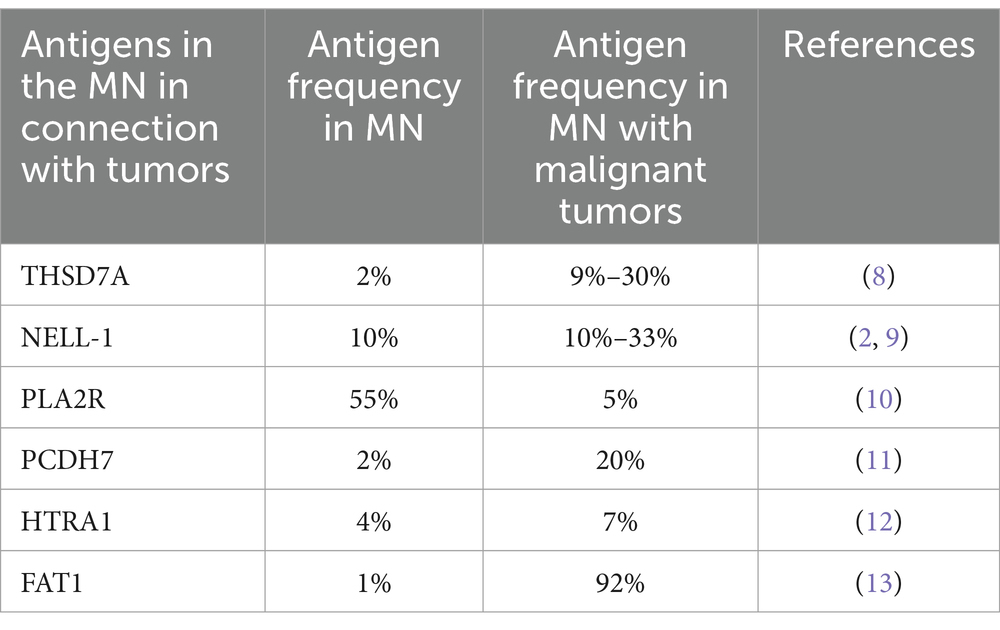
THSD7A
Thrombospondin type 1 domain-containing 7A (THSD7A) is a specific antigen expressed in podocytes, identified after the discovery of PLA2R. Around 1%–3% of patients with MN are THSD7A positive (2), and this accounts for about 5%–10% of patients who do not have PLA2R antibodies (5). In recent years, different studies have shown varying positive rates for THSD7A antibodies in malignancy-associated MN (6, 7). In German and American cohorts, multiple studies indicated that the proportion of patients with THSD7A-positive MN combined with malignant tumors ranged from 9% to 30% (Table 1) (8). However, a Chinese cohort study in 2017 showed a positive rate of 2% for THSD7A antibodies in malignancy-associated MN patients (14). In studies concerning THSD7A-positive malignancy-associated MN, the associated cancers are highly diverse, including breast, lung, and digestive system cancers. Compared to those with THSD7A-positive MN without malignant tumors, patients with THSD7A-positive malignancy-associated MN are older, more often male, have lower serum albumin levels and higher titers of serum THSD7A antibodies (15), as well as more glomerular inflammatory cell infiltration in renal pathology (8). At present, most studies suggest that THSD7A-positive malignancy-associated MN are mainly composed of IgG1, IgG2 and IgG3, while THSD7A-positive kidney tissues in primary MN is mainly composed of IgG4 (5, 6, 10). However, some studies have reported that in renal tissues of patients with THSD7A-positive malignancy-associated MN, IgG4 can also be seen (16, 17). In primary THSD7A-positive MN patients, serum THSD7A antibody IgG subtypes are mainly IgG4; multiple studies have reported that there is no statistically significant difference in the level of THSD7A antibody IgG subtype between sera from THSD7A-positive MN patients with or without malignant tumors (10, 15).
In recent years, multiple studies have reported that THSD7A-positive malignancy-associated MN patients are positive for THSD7A staining in both renal and tumor tissues. Chen et al. (16) reported the expression of THSD7A antigen in renal tissue and lymph node tissue with tumor metastasis in a patient with non-small cell lung cancer, and the patient’s serum THSD7A antibody was positive. In patients with tumors such as rectal cancer, NF1-related neurofibromas and endometrial cancer combined with MN, THSD7A was also found to be positive in the kidney and tumor tissue (17, 18). In addition, one patient with MN who underwent multiple renal biopsies initially showed negative THSD7A staining for the first renal biopsy. However, after 1 year, upon the reoccurrence of the kidney disease, the THSD7A staining was positive. Two years later, the patient was diagnosed with bladder cancer, and THSD7A staining was also positive in the tumor tissue (19). Simultaneous positivity for THSD7A in renal and tumor tissue suggests that THSD7A may play an important role in pathogenic mechanism leading to MN associated with tumors. However, Liu et al. found that only one (16%) of nine patients with malignancy-associated MN had positive staining for THSD7A in their tumor tissues (20). Hara et al. (21) also reported negative staining for THSD7A in two cases of THSD7A-positive malignancy-associated MN. Therefore, tumors and MN antigens may not be always consistent. Although THSD7A-positive malignancy-associated MN patients with serum THSD7A antibody positivity are still rare (14, 20), Chen et al. (16) reported a lung cancer patient who developed MN during targeted drug therapy and whose serum THSD7A antibody was positive. When the kidney disease was alleviated, the patient’s serum THSD7A antibody turned negative, and the patient’s tumor was also under control. These results suggest that the correlation between THSD7A antibody and disease changes is related to the pathogenesis of THSD7A-positive malignancy-associated MN.
THSD7A is a transmembrane N-glycoprotein expressed in normal glomerular podocytes, endothelial cells and mesangial cells (22), while human placental vascular endothelial cells also express THSD7A (23). In recent years, THSD7A has been studied not only in MN but also in malignant tumors. Research has explored the expression of THSD7A in more than 70 types of malignant tumor tissues and found that THSD7A expresses differently in various tumors. Among them, THSD7A is relatively commonly expressed in colorectal cancer, renal cancer, breast cancer and prostate cancer (24), and it was recently found that the transcription level and protein level of THSD7A were significantly increased in gastric cancer (25). In the study of tumor pathogenesis, uncontrolled angiogenesis is considered a hallmark of malignant tumors (26), and studies have confirmed that THSD7A participates in the invasion, metastasis and generation of tumor vessels (27). In vitro cell culture experiments knocking out THSD7A can inhibit cancer cell proliferation and migration also confirm this mechanism (25). For MN, it is currently believed that its pathogenesis is mainly attributed to the immune system’s response to antibodies, in which B cells (plasma cells) play a major role, but T-B cells also make a significant contribution to autoimmune diseases (28). Therefore, it is speculated that THSD7A may play a role in the pathogenesis of tumor-associated MN through T cells (Figure 1). However, the mechanism of immune system abnormalities in malignant tumors is very complex considering the involvement of various genetic and environmental factors in tumor and MN onset. Therefore, there is currently no research to confirm the clear relationship between THSD7A-MN and malignant tumors. The immunological pathogenesis of THSD7A-positive malignancy-associated MN remains to be studied.
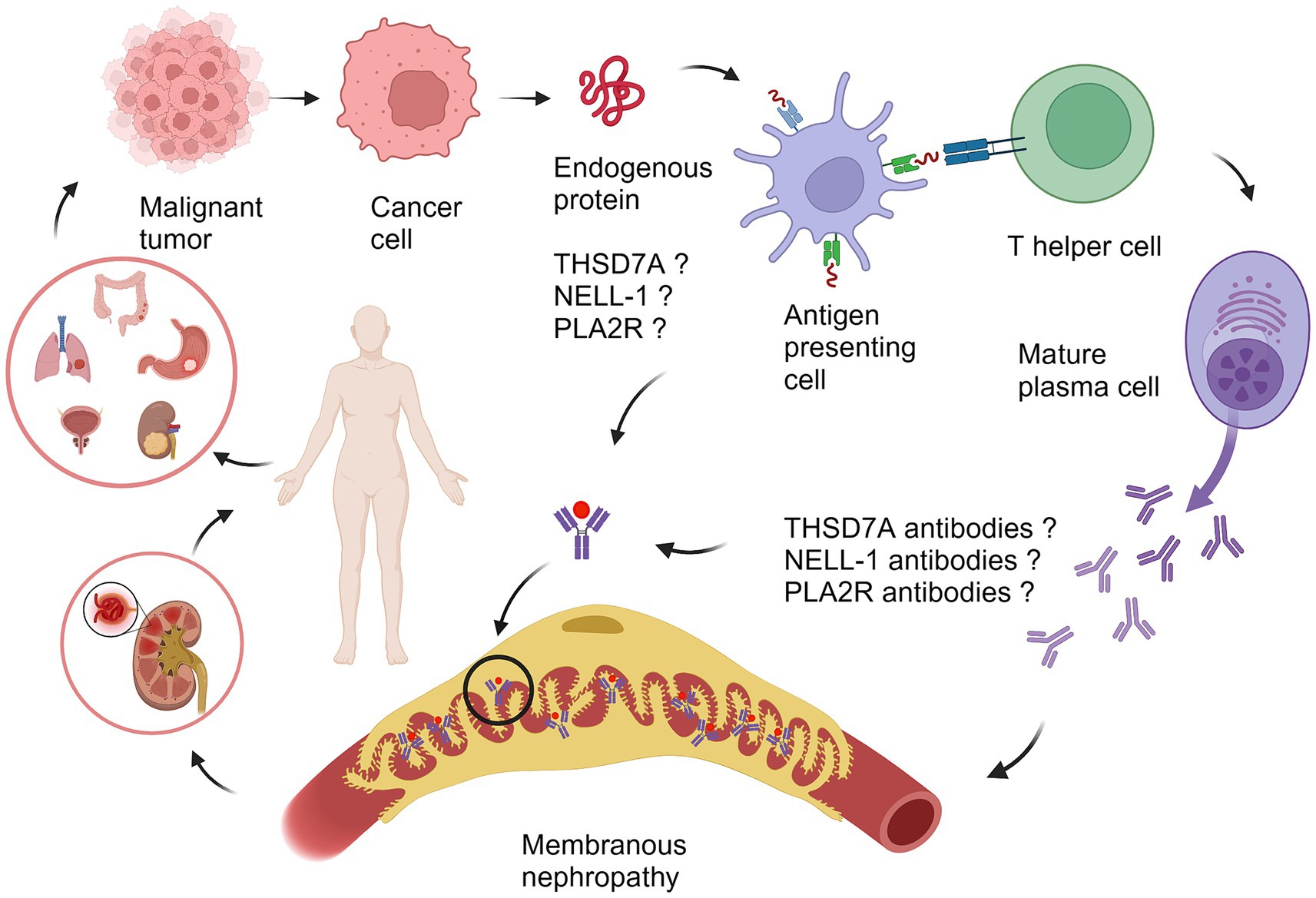
Figure 1. The proposed pathogenesis of malignancy-associated membranous nephropathy. Different types of tumors secrete tumor-associated antigens such as THSD7A, NELL-1, and PLA2R. The antigen is ingested by an antigen-presenting cell (APC), cut into peptide segments, presented to the APC surface, interacts with helper T cells (Th cell), specifically recognizes B cells, promotes the differentiation of B cells into mature plasma cells, secretes antibodies related to THSD7A, NELL-1, and PLA2R, and forms an antigen–antibody immune complex with the tumor antigen, which circulates to the glomerulus and deposits on the basement membrane of the glomerulus, causing MN. PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin domain-containing 7A; NELL-1, neural epidermal growth factor-like 1 protein. The illustration was created with BioRender.com.
NELL-1
Following the discovery of PLA2R and THSD7A, Sethi et al. (29) found neural epidermal growth factor-like 1 protein (NELL-1) through laser microdissection and mass spectrometry analysis of renal biopsy tissues from patients with MN who were negative for PLA2R, accounting for approximately 10% of cases of MN (2). At the same time, in this cohort, 11.7% of NELL-1 positive MN were combined with malignant tumors. Subsequently, Caza et al. (9) reported that among patients with NELL-1 positive MN in the cohort, the proportion of those combined with malignant tumors was as high as 30%, suggesting that NELL-1 is a target antigen for MN associated with malignant tumors. In our center’s cohort of 832 patients with membranous nephropathy and Japan’s cohort of 104 patients, NELL-1 positive MN patients did not combine with tumors (30). The reason for large differences between the cohorts, which may be related to geography and ethnicity. Compared with MN patients without malignant tumors (9), NELL-1 positive malignancy-associated MN patients were clinically characterized by older age and more males. No significant difference of serum creatinine and 24-h urinary protein quantification levels was found between these patients and those PLA2R-MN and THSD7A-MN patients (9). It has been reported that there was a significant increase in the number of inflammatory cells infiltrating glomeruli in malignancy-associated MN patients (31), but this feature was not observed in NELL-1 positive malignancy-associated MN patients. NELL-1 positive malignancy-associated MN glomerular capillary loop immune complex shows segmental deposition or combined mesangial region immune complex deposition, renal tissue IgG subclass mainly by IgG1, pathological performance similar to NELL-1 positive MN without combination of malignant tumors (9).
NELL-1 is a 140 kDa modular glycoprotein normally expressed in neural tissues with lower expression levels in non-neural tissues such as the kidney and liver (31, 32). NELL-1 has higher expression in renal tubules than glomeruli (33). NELL-1 has been studied in numerous types of tumors prior to the discovery of NELL-1 as a MN-specific antigen. Expression levels of NELL-1 vary across tumor types. It has been reported that NELL-1 was overexpressed at both transcriptional and protein levels in neuroblastoma and osteosarcoma (34, 35), as well as prostate cancer, lung cancer and breast cancer (36). However, NELL-1 was downregulated at the gene, transcript and protein levels in renal cancer, gastric cancer and lymphoma (37–39). In previous studies on the pathogenesis of malignant tumors, abnormal CpG island and promoter methylation of genes were closely related to the occurrence of tumors. Studies on the pathogenic mechanism of NELL-1 in malignant tumors show that abnormal CpG islands and promoter methylation lead to downregulation of NELL-1 expression, thereby regulating the malignant behavior of renal cancer cells (39). In addition to renal cancer, NELL-1 gene promoter methylation can also be detected in colorectal cancer (40), esophageal cancer (41). Therefore, NELL-1 may participate in the cell growth, differentiation and tumorigenesis of different tumors. However, despite the high expression of NELL-1 found in different malignancies, only a few patients with malignancies will continue to develop into MN. In recent years, studies on NELL-1 positive malignancy-associated MN have found that the expression of NELL-1 was observed in tumor and kidney specimens from patients with breast cancer, lymphoid follicle carcinoma and esophageal cancer (9, 42), but no study has confirmed a clear mechanism of action for NELL-1 in MN associated with malignant tumors. Although NELL-1 has been proven to play a role in tumorigenesis and development, its pathophysiological mechanism in malignant tumors remains to be further studied.
PLA2R
It is widely acknowledged that PLA2R is the most important antigen in primary membranous nephropathy, but a study has revealed that 16 (5.3%) of the 302 patients with PLA2R antibody-positive MN were found to have malignant tumors within 24 months after the diagnosis of MN (10). There are also reports show that 3 (25%) of the 12 patients with malignancy-associated MN were positive for PLA2R, suggesting that PLA2R may be associated with malignancy-associated MN. However, in the study conducted by Uhlen M, no remission of MN was observed after anti-tumor treatment in patients with PLA2R-positive MN combined with malignant tumors. It is considered that the relationship between PLA2R-positive MN and malignant tumors may be a coincidence (43). Therefore, the relationship between PLA2R and malignancy-associated MN needs further clarification. Compared with PLA2R-positive MN without malignancy, patients with PLA2R-positive malignancy-associated MN were older, had more proteinuria and worse renal function, and showed heavier interstitial fibrosis pathologically (10). Zhao et al. found that IgG subclass IgG3 was mainly positive (14) in renal tissues of PLA2R-positive malignancy-associated MN, while it was mainly positive for IgG4 (44) in primary MN with PLA2R positivity. It is considered that negativity of PLA2R and IgG4 in renal tissue are indicators of malignancy-associated MN (45, 46). In patients with PLA2R-positive malignancy-associated MN, serum PLA2R antibody IgG subtypes were found to be consistent with those of primary MN (10, 46), suggesting that the pathogenesis of PLA2R-positive malignancy-associated MN and PLA2R-positive primary MN may have pathways in common.
PLA2R is a type I transmembrane receptor glycoprotein expressed on the surface of glomerular podocytes (44). PLA2R expression has been found in both kidney and malignant tumor tissues, but the role of PLA2R positivity in the pathogenesis of malignancy-associated MN remains unclear. In recent years, the research on PLA2R in tumors has gradually increased. Through the analysis of tumor gene chip database, it was found that the expression level of PLA2R varied in different tumor tissues, and the expression level of PLA2R mRNA was decreased in breast cancer (47) and kidney cancer (48), but not in pancreatic cancer and gastric cancer (47). In addition, there are controversies about the role of PLA2R in tumor cells. Some studies have found that PLA2R can promote apoptosis and inhibit cell transformation in tumor tissues (47, 49, 50). Vindrieux et al. (48) found that knockdown of PLA2R promotes the formation of cancer cell colonies in renal cancer cell lines. In addition, the death of cancer cells was observed in PLA2R-expressing cancer cells due to an increase in intracellular reactive oxygen species (ROS), suggesting that PLA2R promotes tumor cell death. However, research by Jones et al. found that both renal cancer (48) and breast cancer tissues were observed to have PLA2R promoter methylation phenomenon. The methylation of PLA2R promoter would inhibit the expression of tumor suppressor genes in cancer cells, thus promoting cancer cell proliferation (51). Since there are still few case reports of PLA2R-positive MN patients with tumors in which PLA2R is expressed simultaneously in tumor tissues, the role of PLA2R in the pathogenesis of kidney and malignant tumors is not clear, which warrants further research.
PCDH7, HTRA1, FAT1
In addition to the MN specific antigens mentioned above, several newly discovered MN antigens may also be associated with malignant tumors. Approximately 20% of patients with Protocadherin 7 (PCDH7)-MN have malignant tumors (11). Previous studies have shown that PCDH7 is involved in tumorigenesis in bladder cancer, renal cancer, lung cancer and gastric cancer (52–57), but its pathogenesis in MN remains unclear. Approximately 7% of patients with high-temperature requirement A1 (HTRA1)-MN have concomitant malignant tumors (12). Although HTRA1 has not been fully studied in the kidney, previous studies on malignant tumors suggest that HTRA1 has tumor suppressive effects (58–60). The loss of expression of HTRA1 leads to an increase in tumor invasiveness, enhanced metastatic ability and chemotherapeutic resistance (60). Protocadherin FAT1 (FAT1) is associated with hematopoietic stem cell transplant (HSCT)-related MN (0.6%) (13). As one of the most common mutant genes in a variety of cancers (61), whether FAT1 has a clear mechanism of action in HSCT-related MN requires further investigation.
It is necessary to acknowledge the limitations of this review. For example, we did not delve into the specific pathogenic mechanisms of antigens associated with malignancies in MN, as there is currently a lack of research on the pathogenic mechanisms in these patients. The second restraint is the limited number of cases of tumor-associated MN, especially those with immunostaining of antigens in both renal and tumor tissues. Therefore, further understanding of the characteristics of antigens in tumor-associated MN requires studies with larger sample sizes and longer follow-up time.
Conclusion
In this article, we summarize the clinical and pathological characteristics of antigen-positive malignancy-associated MN reported in recent years. We also summarize the possible role of MN-specific antigens in the pathogenesis of malignancy-associated MN. Due to the complexity of the pathogenesis of malignant tumors, further explorations of the role of antigens in the pathogenesis will provide therapeutic targets for diseases in the future.
Author contributions
XH: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. GW: Project administration, Supervision, Validation, Visualization, Writing – review & editing. HC: Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of Beijing grant 7192050 and Capital’s Funds for Health Improvement and Research (2022-2-2066).
Acknowledgments
The authors would like to express our appreciation to everyone who was involved in the drafting and preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hoxha, E, Reinhard, L, and Stahl, R. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol. (2022) 18:466–78. doi: 10.1038/s41581-022-00564-1
2. Sethi, S, Glassock, RJ, Haas, M, Vriese, ASD, Caza, TN, Hoxha, E, et al. Mayo Clinic consensus report on membranous nephropathy: proposal for a novel classification. Mayo Clin Proc. (2023) 98:1671–84. doi: 10.1016/j.mayocp.2023.08.006
3. Leeaphorn, N, Kue, APP, Thamcharoen, N, Ungprasert, P, Stokes, MB, and Knight, EL. Prevalence of cancer in membranous nephropathy: a systematic review and meta-analysis of observational studies. Am J Nephrol. (2014) 40:29–35. doi: 10.1159/000364782
4. Lee, CJ, Yamauchi, H, and Hopper, J. The association of cancer and the nephrotic syndrome. Ann Intern Med. (1966) 64:41–51. doi: 10.7326/0003-4819-64-1-41
5. Tomas, NM, Beck, LH, Meyer-Schwesinger, C, Seitz-Polski, B, Ma, H, Zahner, G, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. (2014) 371:2277–87. doi: 10.1056/NEJMoa1409354
6. Xu, QQ, Zou, GM, Zhuo, L, Gao, HM, and Li, WG. Lung cancer patients with nephropathy as the first manifestation: literature review and clinical study report. Front Oncol. (2022) 12:1002155. doi: 10.3389/fonc.2022.1002155
7. Iwakura, T, Ohashi, N, Kato, A, Baba, S, and Yasuda, H. Prevalence of enhanced granular expression of thrombospondin Type-1 domain-containing 7A in the glomeruli of Japanese patients with idiopathic membranous nephropathy. PLoS One. (2015) 10:e0138841. doi: 10.1371/journal.pone.0138841
8. Hoxha, E, Beck, LH, Wiech, T, Tomas, NM, Probst, C, Mindorf, S, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol. (2017) 28:520–31. doi: 10.1681/ASN.2016010050
9. Caza, TN, Hassen, SI, Dvanajscak, Z, Kuperman, M, Edmondson, R, Herzog, C, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. (2021) 99:967–76. doi: 10.1016/j.kint.2020.07.039
10. Haxthausen, F, Reinhard, L, Pinnschmidt, HO, Rink, M, Soave, A, Hoxha, E, et al. Antigen-specific IgG subclasses in primary and malignancy-associated membranous nephropathy. Front Immunol. (2018) 9:3035. doi: 10.3389/fimmu.2018.03035
11. Sethi, S, Madden, B, Debiec, H, Morelle, J, Charlesworth, MC, Gross, L, et al. Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrol. (2021) 32:1249–61. doi: 10.1681/ASN.2020081165
12. Al-Rabadi, LF, Caza, T, Trivin-Avillach, C, Rodan, AR, Andeen, N, Hayashi, N, et al. Serine protease HTRA1 as a novel target antigen in primary membranous nephropathy. J Am Soc Nephrol. (2021) 32:1666–81. doi: 10.1681/ASN.2020101395
13. Sethi, S, Madden, B, Moura, MC, Nasr, SH, Klomjit, N, Gross, LA, et al. Hematopoietic stem cell transplant-membranous nephropathy is associated with Protocadherin FAT1. J Am Soc Nephrol. (2022) 33:1033–44. doi: 10.1681/ASN.2021111488
14. Wang, J, Cui, Z, Lu, J, Probst, C, Zhang, YM, Wang, X, et al. Circulating antibodies against thrombospondin type-I domain-containing 7A in Chinese patients with idiopathic membranous nephropathy. Clin J Am Soc Nephrol. (2017) 12:1642–51. doi: 10.2215/CJN.01460217
15. Zaghrini, C, Seitz-Polski, B, Justino, J, Dolla, G, Payré, C, Jourde-Chiche, N, et al. Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Kidney Int. (2019) 95:666–79. doi: 10.1016/j.kint.2018.10.024
16. Chen, MJ, Zhang, L, Zhong, W, Zheng, K, Ye, W, and Wang, MZ. Case report: THSD7A-positive membranous nephropathy caused by Tislelizumab in a lung Cancer patient. Front Immunol. (2021) 12:619147. doi: 10.3389/fimmu.2021.619147
17. Taguchi, S, Koshikawa, Y, Ohyama, S, Miyachi, H, Ozawa, H, and Asada, H. Thrombospondin type-1 domain-containing 7A-associated membranous nephropathy after resection of rectal cancer: a case report. BMC Nephrol. (2019) 20:43. doi: 10.1186/s12882-019-1236-y
18. Lin, FJ, Zhang, D, Chang, J, Tang, XL, Guan, WB, Jiang, GR, et al. THSD7A-associated membranous nephropathy in a patient with neurofibromatosis type 1. Eur J Med Genet. (2018) 61:84–8. doi: 10.1016/j.ejmg.2017.10.014
19. Matsumoto, A, Matsui, I, Mano, K, Mizuno, H, Katsuma, Y, Yasuda, S, et al. Recurrent membranous nephropathy with a possible alteration in the etiology: a case report. BMC Nephrol. (2021) 22:253. doi: 10.1186/s12882-021-02457-0
20. Zhang, CM, Zhang, MC, Chen, DC, Ren, Q, Xu, WW, Zeng, CH, et al. Features of phospholipase A2 receptor and thrombospondin type-1 domain-containing 7A in malignancy-associated membranous nephropathy. J Clin Pathol. (2019) 72:705–11. doi: 10.1136/jclinpath-2019-205852
21. Hara, S, Tsuji, T, Fukasawa, Y, Hisano, S, Morito, S, Hyodo, T, et al. Clinicopathological characteristics of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. Virchows Arch. (2019) 474:735–43. doi: 10.1007/s00428-019-02558-0
22. Ju, WJ, Greene, CS, Eichinger, F, Nair, V, Hodgin, JB, Bitzer, M, et al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res. (2013) 23:1862–73. doi: 10.1101/gr.155697.113
23. Wang, CH, Su, PT, Du, XY, Kuo, MW, Lin, CY, Yang, CC, et al. Thrombospondin type I domain-containing 7A (THSD7A) mediates endothelial cell migration and tube formation. J Cell Physiol. (2010) 222:685–94. doi: 10.1002/jcp.21990
24. Stahl, PR, Hoxha, E, Wiech, T, Schröder, C, Simon, R, and Stahl, RA. THSD7A expression in human cancer. Genes Chromosomes Cancer. (2017) 56:314–27. doi: 10.1002/gcc.22440
25. Shen, KY, Chen, BY, Yang, L, and Gao, WC. Integrated analysis of single-cell and bulk RNA-sequencing data reveals the prognostic value and molecular function of THSD7A in gastric cancer. Aging. (2023) 15:11940–69. doi: 10.18632/aging.205158
26. Yu, J, Zhang, A, Gu, X, Hua, Y, Yang, LD, Ge, SF, et al. Nuclear PD-L1 promotes EGR1-mediated angiogenesis and accelerates tumorigenesis. Cell Discov. (2023) 9:33. doi: 10.1038/s41421-023-00521-7
27. Flockerzi, FA, Hohneck, J, Langer, F, Bohle, RM, and Stahl, PR. THSD7A positivity predicts poor survival and is linked to high FAK expression and FGFR1-wildtype in female patients with squamous cell carcinoma of the lung. Int J Mol Sci. (2023) 24:639. doi: 10.3390/ijms241310639
28. Zhao, QH, Dai, HR, Liu, XL, Jiang, HX, Liu, WB, Feng, ZD, et al. Helper T cells in idiopathic membranous nephropathy. Front Immunol. (2021) 12:665629. doi: 10.3389/fimmu.2021.665629
29. Sethi, S, Debiec, H, Madden, B, Charlesworth, MC, Morelle, J, Gross, L, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. (2020) 97:163–74. doi: 10.1016/j.kint.2019.09.014
30. Wang, GQ, Sun, LJ, Dong, HR, Wang, YY, Xu, XY, Zhao, ZR, et al. Neural epidermal growth factor-like 1 protein-positive membranous nephropathy in Chinese patients. Clin J Am Soc Nephrol. (2021) 16:727–35. doi: 10.2215/CJN.11860720
31. Lefaucheur, C, Stengel, B, Nochy, D, Martel, P, Hill, GS, Jacquot, C, et al. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. (2006) 70:1510–7. doi: 10.1038/sj.ki.5001790
32. Matsuhashi, S, Noji, S, Koyama, E, Myokai, F, Ohuchi, H, Taniguchi, S, et al. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn. (1995) 203:212–22. doi: 10.1002/aja.1002030209
33. Watanabe, TK, Katagiri, T, Suzuki, M, Shimizu, F, Fujiwara, T, Kanemoto, N, et al. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. (1996) 38:273–6. doi: 10.1006/geno.1996.0628
34. Maeda, K, Matsuhashi, S, Tabuchi, K, Watanabe, T, Katagiri, T, Oyasu, M, et al. Brain specific human genes, NELL1 and NELL2, are predominantly expressed in neuroblastoma and other embryonal neuroepithelial tumors. Neurol Med Chir. (2001) 41:582–9. doi: 10.2176/nmc.41.582
35. Shen, J, LaChaud, G, Khadarian, K, Shrestha, S, Zhang, XL, Soo, C, et al. NELL-1 expression in benign and malignant bone tumors. Biochem Biophys Res Commun. (2015) 460:368–74. doi: 10.1016/j.bbrc.2015.03.040
36. Uhlen, M, Zhang, C, Lee, S, Sjostedt, E, Fagerberg, L, Bidkhori, G, et al. A pathology atlas of the human cancer transcriptome. Science. (2017) 357:2507. doi: 10.1126/science.aan2507
37. Gao, CL, Zhang, Q, Kong, DY, Wu, D, Su, CL, Tong, JX, et al. MALDI-TOF mass Array analysis of Nell-1 promoter methylation patterns in human gastric Cancer. Biomed Res Int. (2015) 2015:136941. doi: 10.1155/2015/136941
38. Slovak, ML, Bedell, V, Hsu, YH, Estrine, DB, Norma, NJ, Delioukina, ML, et al. Molecular karyotypes of Hodgkin and reed-Sternberg cells at disease onset reveal distinct copy number alterations in chemosensitive versus refractory Hodgkin lymphoma. Clin Cancer Res. (2011) 17:3443–54. doi: 10.1158/1078-0432.CCR-10-1071
39. Nakamura, R, Oyama, T, Tajiri, R, Mizokami, A, Namiki, M, Nakamoto, M, et al. Expression and regulatory effects on cancer cell behavior of NELL1 and NELL2 in human renal cell carcinoma. Cancer Sci. (2015) 106:656–64. doi: 10.1111/cas.12649
40. Mori, Y, Cai, K, Cheng, Y, Wang, S, Paun, B, Hamilton, JP, et al. A genome-wide search identifies epigenetic silencing of somatostatin, tachykinin-1, and 5 other genes in colon cancer. Gastroenterology. (2006) 131:797–808. doi: 10.1053/j.gastro.2006.06.006
41. Jin, Z, Mori, Y, Yang, J, Sato, F, Ito, T, Cheng, Y, et al. Hypermethylation of the nel-like 1 gene is a common and early event and is associated with poor prognosis in early-stage esophageal adenocarcinoma. Oncogene. (2007) 26:6332–40. doi: 10.1038/sj.onc.1210461
42. Zhou, S, Meng, FL, Yue, SL, Li, H, Zhang, LH, and Wang, T. Backtracking cryptic recurrence of esophageal cancer from membranous nephropathy: the detection of glomerular NELL-1 and IgG4. Clin Kidney J. (2023) 16:756–9. doi: 10.1093/ckj/sfac261
43. Timmermans, S, Ayalon, R, Paassen, P, Beck, LH, Rie, H, Wirtz, J, et al. Anti-phospholipase A2 receptor antibodies and malignancy in membranous nephropathy. Am J Kidney Dis. (2013) 62:1223–5. doi: 10.1053/j.ajkd.2013.07.019
44. Beck, LH, Bonegio, RG, Lambeau, G, Beck, DM, Powell, DW, Cummins, TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. (2009) 361:11–21. doi: 10.1056/NEJMoa0810457
45. Lonnbro-Widgren, J, Ebefors, K, Molne, J, Nystrom, J, and Haraldsson, B. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous nephropathy. Clin Kidney J. (2015) 8:433–9. doi: 10.1093/ckj/sfv049
46. Qu, Z, Liu, G, Li, J, Wu, LH, Tan, Y, Zheng, X, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant. (2012) 27:1931–7. doi: 10.1093/ndt/gfr534
47. Vindrieux, D, Augert, A, Girard, CA, Gitenay, D, Lallet-Daher, H, Wiel, C, et al. PLA2R1 mediates tumor suppression by activating JAK2. Cancer Res. (2013) 73:6334–45. doi: 10.1158/0008-5472.CAN-13-0318
48. Vindrieux, D, Devailly, G, Augert, A, Calvé, BL, Ferrand, M, Pigny, P, et al. Repression of PLA2R1 by c-MYC and HIF-2alpha promotes cancer growth. Oncotarget. (2014) 5:1004–13. doi: 10.18632/oncotarget.1681
49. Augert, A, Vindrieux, D, Girard, CA, Calve, BL, Gras, B, Ferrand, M, et al. PLA2R1 kills cancer cells by inducing mitochondrial stress. Free Radic Biol Med. (2013) 65:969–77. doi: 10.1016/j.freeradbiomed.2013.08.177
50. Augert, A, Payre, C, de Launoit, Y, Gil, J, Lambeau, G, and Bernard, D. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. (2009) 10:271–7. doi: 10.1038/embor.2008.255
51. Jones, PA . DNA methylation and cancer. Oncogene. (2002) 21:5358–60. doi: 10.1038/sj.onc.1205597
52. Zhou, E, Wu, F, Guo, MF, Yin, ZR, Li, YM, Li, ML, et al. Identification of a novel gene signature of lung adenocarcinoma based on epidermal growth factor receptor-tyrosine kinase inhibitor resistance. Front Oncol. (2022) 12:1008283. doi: 10.3389/fonc.2022.1008283
53. Li, SC, Sun, J, Ma, JJ, Zhou, CX, Yang, X, Zhang, S, et al. LncRNA LENGA acts as a tumor suppressor in gastric cancer through BRD7/TP53 signaling. Cell Mol Life Sci. (2022) 80:5. doi: 10.1007/s00018-022-04642-2
54. Wang, Y, Zhang, YJ, Su, XW, Qiu, QZ, Yuan, Y, Weng, CH, et al. Circular RNA circDVL1 inhibits clear cell renal cell carcinoma progression through the miR-412-3p/PCDH7 axis. Int J Biol Sci. (2022) 18:1491–507. doi: 10.7150/ijbs.69351
55. Zhao, ZH, Zhang, YJ, Li, C, Li, XW, Chu, YC, Guo, Q, et al. Microenvironment-tailored micelles restrain carcinoma-astrocyte crosstalk for brain metastasis. J Control Release. (2022) 349:520–32. doi: 10.1016/j.jconrel.2022.07.009
56. Beukers, W, Hercegovac, A, Vermeij, M, Kandimalla, R, Blok, AC, Madelon, M, et al. Hypermethylation of the polycomb group target gene PCDH7 in bladder tumors from patients of all ages. J Urol. (2013) 190:311–6. doi: 10.1016/j.juro.2013.01.078
57. Zhou, XR, Updegraff, BL, Guo, YB, Peyton, M, Girard, L, Larsen, JE, et al. PROTOCADHERIN 7 acts through SET and PP2A to potentiate MAPK signaling by EGFR and KRAS during lung tumorigenesis. Cancer Res. (2017) 77:187–97. doi: 10.1158/0008-5472.CAN-16-1267-T
58. Chien, J, Aletti, G, Baldi, A, Catalano, V, Muretto, P, Keeney, GL, et al. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest. (2006) 116:1994–2004. doi: 10.1172/JCI27698
59. Chien, J, Staub, J, Hu, SI, Erickson-Johnson, MR, Couch, FJ, Smith, DI, et al. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene. (2004) 23:1636–44. doi: 10.1038/sj.onc.1207271
60. Bowden, MA, Nezza-Cossens, LA, Jobling, T, Salamonsen, LA, and Nie, GY. Serine proteases HTRA1 and HTRA3 are down-regulated with increasing grades of human endometrial cancer. Gynecol Oncol. (2006) 103:253–60. doi: 10.1016/j.ygyno.2006.03.006
Keywords: membranous nephropathy, malignant tumor, THSD7A, NELL-1, PLA2R
Citation: Hu X, Wang G and Cheng H (2024) Specific antigens in malignancy-associated membranous nephropathy. Front. Med. 11:1368457. doi: 10.3389/fmed.2024.1368457
Edited by:
Piergiorgio Messa, University of Milan, ItalyReviewed by:
Hans-Joachim Paust, University Medical Center Hamburg-Eppendorf, GermanyCopyright © 2024 Hu, Wang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Cheng, drchengh@163.com
 Xiaoying Hu
Xiaoying Hu  Guoqin Wang
Guoqin Wang Hong Cheng
Hong Cheng