Metagenome next-generation sequencing plays a key role in the diagnosis and selection of effective antibiotics on the treatment of Nocardia pneumonia: a case report
- 1Department of Respiratory Oncology, Renmin Hospital of Qingxian, Cangzhou, China
- 2Department of Neurology, General Hospital of Central Theater Command of the People’s Liberation Army, Wuhan, China
- 3Department of Laboratory Medicine, Renmin Hospital of Qingxian, Cangzhou, China
- 4Department of Infectious Diseases, Hospital 902 of Joint Logistics Support Force of People’s Liberation Army, Bengbu, China
Nocardia disease is an opportunistic infection, the occurrence is rare and mostly occurs in patients with immune deficiency. Even if the patient is immunocompetent, it can still be life-threatening. This case report describes a previously healthy 78-year-old male farmer with lung lesions discovered on a computerized tomography scan. Combined with the patient’s history of fever and the results of elevated laboratory markers associated with inflammation, the patient was diagnosed with a lung infection. After escalating empirical broad-spectrum antibiotics, antiviral and antifungal therapy, the patient continued to deteriorate to septic shock. In the meanwhile, the patient’s sputum was cultured repeatedly, and no obvious positive pathogenic bacteria were found. Considering the patient was elderly and that these lesions were solid with burr signs, as well as the progression after antimicrobial therapy cancer was considered in the differential diagnosis. Artificial intelligence (YITU, Hangzhou Yitu Medical Technology Limited Company) was also applied, and it also calculated that these lesions were cancerous. The patient received a puncture biopsy of the largest lung lesion. During the puncture pus was withdrawn from largest lung lesion. Culture and metagenome next-generation sequencing (mNGS) detection performed on pus indicated Nocardia otitidiscaviarum. The test report of the mNGS is also attached with a susceptibility report of commonly used clinical antibiotics to this Nocardia spp. Using this result, the patient’s disease was quickly controlled after selecting the targeted drug compound sulfamethoxazole and intravenous meropenem for treatment. In view of the high misdiagnosis rate and poor sensitivity of culture for Nocardia spp., this case emphasized mNGS playing a key role in the diagnosis and selection of effective antibiotics for the treatment of Nocardia spp. lung infections.
Introduction
Nocardia spp. is an aerobic species of prokaryotic microorganism that is widely found in soil, air, spoilage plants, and other organic matter (1). To date, 791 Nocardia spp. isolates were identified, of 119 recognized Nocardia spp. species with valid names, 54 were related to human infection among the existing literatures (2). The Nocardia nova complex, Nocardia cyriacigeorgica, Nocardia farcinica are the most common pathogenic bacteria, and the cases of Nocardia otitidiscaviarum as pathogenic bacteria are few (3). Nocardia is generally considered to be an opportunistic pathogen that causes infection in people with immune dysfunction, such as long-term oral hormone medications, diseases associated with malignant tumors and/or organ transplantation, and HIV patients (4). Infections caused by Nocardia spp. often manifest as subacute, chronic localized, or disseminated suppurative infections (5, 6). Infection by Nocardia spp. is most commonly acquired by inhalation of bacteria into the lungs (5, 6). Subsequent haematogenous spread to the central nervous system and other sites can then occur (7). A low index of clinical suspicion and the low percentage of positive cultures for Nocardia spp. infection may lead to a misdiagnosis (8). The resultant delayed appropriate treatment may have disastrous consequences. Nocardia lung abscess is easily misdiagnosed as lung cancer in clinical practice, especially when the disease continues to progress after antibiotic treatment in some patients (9, 10). We report a case of a feverish patient with lung lesions that were suspected to be lung cancer after the disease progressed despite anti-inflammatory and antibiotic therapy. Enlightening, metagenome next-generation sequencing (mNGS) indicated Nocardia otitidiscaviarum, which provided an accurate result for the differential diagnosis.
Case report
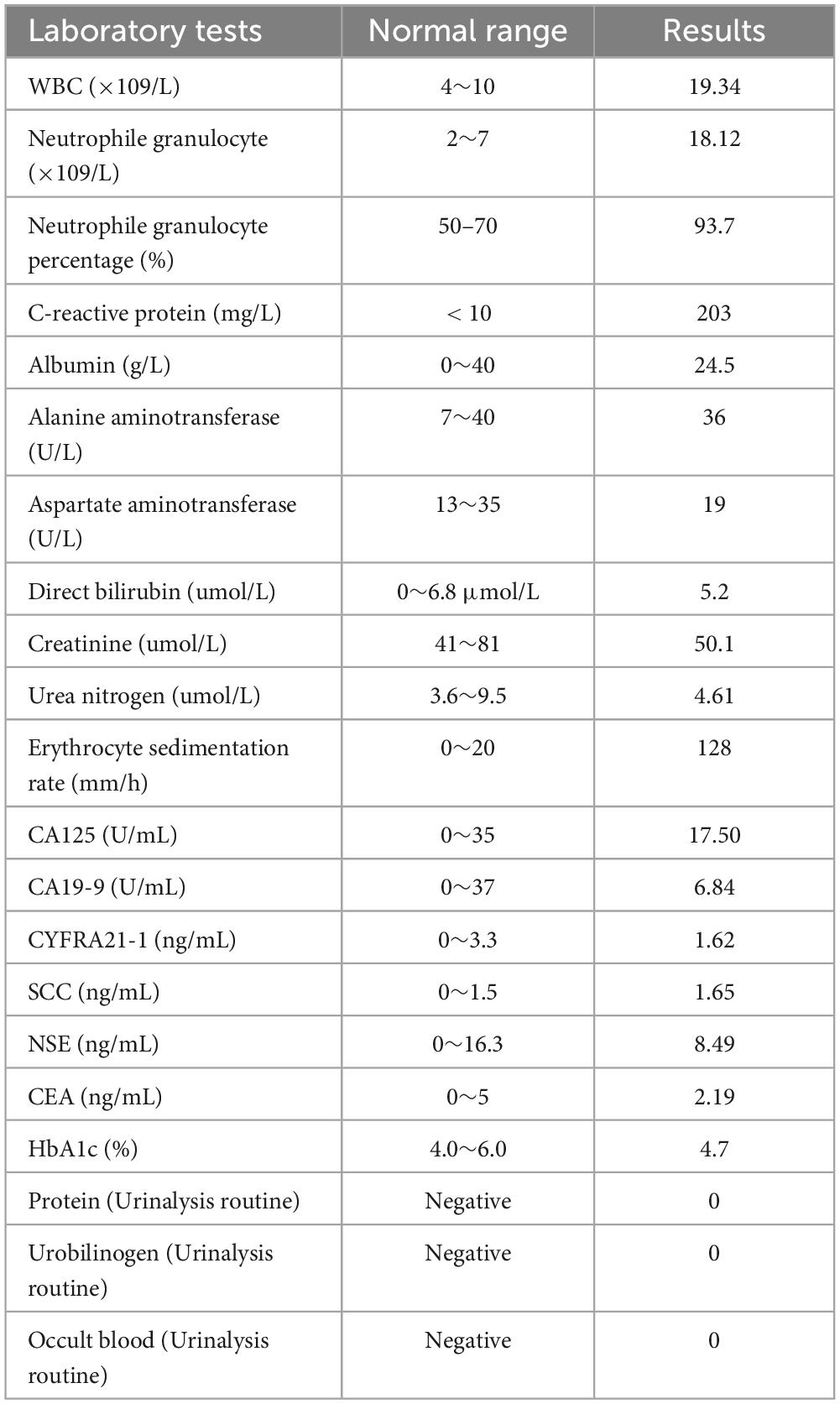
A 78-year-old male farmer was previously in good health. This admission to hospital was due to fever with cough and wheezing for two weeks. The patient denied a history of diabetes mellitus or immune system disease, and had no history of food or drug allergy, travel, or pet contact. Two weeks prior, he had a cold with a fever. His highest body temperature was 38.5°C. Subsequently, the patient developed a cough with sputum, which was yellow and easily produced at a rate of about 10 ml per day. Physical examination at hospital presentation showed that the temperature was 37.7°C, the pulse rate was 110 beats/min, and the respiration rate was 20 breaths/min, the blood pressure was 143/94 mmHg. The patient was alert and wheezing. The superficial lymph nodes in the axilla, groin, neck, and clavicle regions were not large, and a few moist rales could be heard in both lungs. The rhythm of the heart was irregular, and premature beats could be heard. The abdomen was flat and soft, the liver and spleen were not palpable, and there was no edema of both lower limbs. The patient received a computerized tomography (CT) scan examination of chest, which revealed multiple solid lesions in both lungs (the largest is about 4 cm in diameter), small pleural effusion in the left thorax, and mediastinal adenopathy (see Figure 1A). The results displayed elevated laboratory markers (white blood cell count (neutrophile granulocyte), C-reactive protein, erythrocyte sedimentation rate) associated with inflammation (see Table 1). The other laboratory results such as blood biochemical tests and blood lung cancer markers showed no special anomalies (see Table 1).
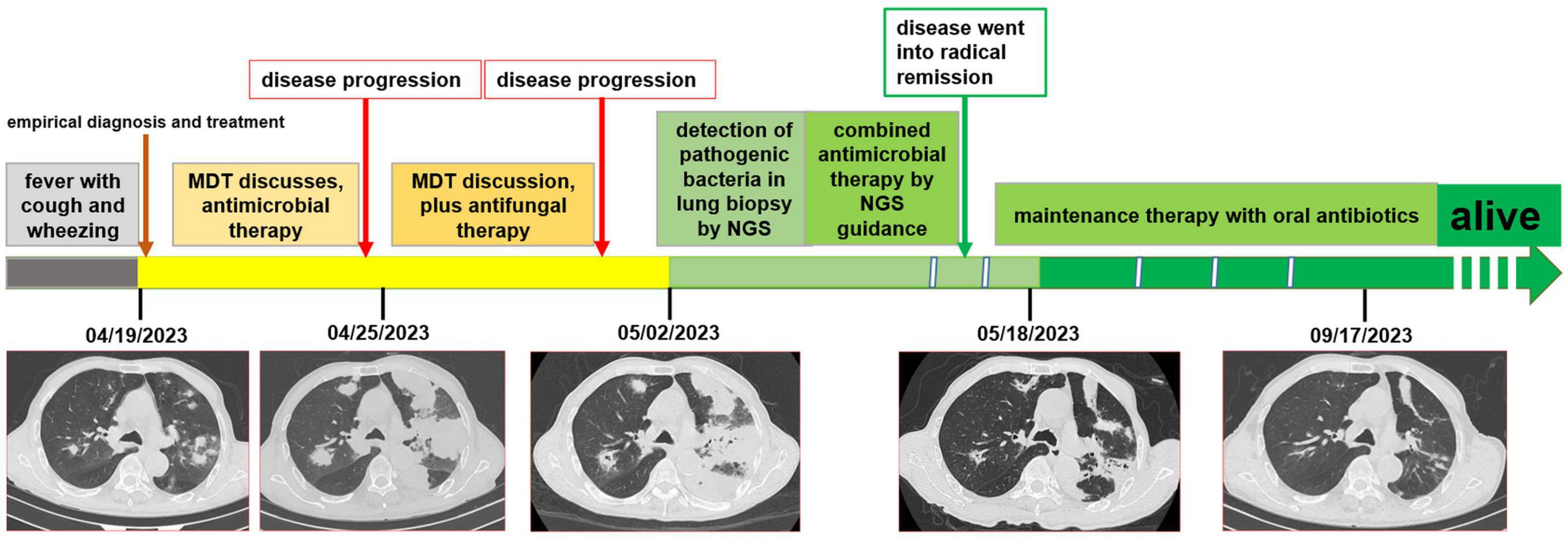
Figure 1. The timeline of the patient’ disease evolution and treatments. (A) On admission; (B) six days after admission; (C) thirteen days after admission; (D) one month after admission; (E) five months after admission.
Combined with the patient’s history of fever and the results of elevated laboratory markers associated with inflammation, the patient was diagnosed with a lung infection by the multidisciplinary team (MDT) discussion. Therefore, the patient was treated with intravenous moxalactam sodium as antibiotic treatment and intravenous diprophylline for antiasthmatic treatment and symptomatic supportive treatment. One week after the treatment, the patient’s symptoms worsened and thick yellow sputum appeared. In the meanwhile, the patient’s sputum was cultured repeatedly, and no obvious positive pathogenic bacteria were found. After the second MDT discussion it was suggested to perform G-(1,3)-β-D -glucan test (G test), galactomannan test (GM test), and Cryptococcus testing. It was suggested that pulmonary fungal infection or viral infection should be considered in the case of poor response to antibacterial therapy. Intravenous meropenem combined with voriconazole and ribavirin were then given. Subsequent tests, such as G test, GM test, cryptococcal antigen test were all negative.
Chest CT reexamination was performed, which revealed a markedly enlarged bilateral area of patchy hyperdense opacities, with large patches of ground-glass opacities surrounding the lesions (see Figure 1B). Considering the patient was elderly and that these lesions were solid with burr signs, as well as the progression after antimicrobial therapy cancer was considered in the differential diagnosis. Artificial intelligence (YITU, Hangzhou Yitu Medical Technology Limited Company) was also applied, and it also determined that these lesions were cancerous. The third MDT discussion resulted in the suggestion that these lesions may be lung cancer with intrapulmonary metastasis. The infection was considered to be part of obstructive pneumonia caused by cancer. The patient underwent fiberoptic bronchoscopy, which revealed no abnormalities in the bronchi other than thick sputum. The pathogen detection by cultured of duct lavage fluid also did not obtain significant results. After one week of treatment and failure of tests to find the cause, the patient continued to progress to septic shock.
The patient received a chest CT re-examination (see Figure 1C) and a puncture biopsy of the largest lung lesion under CT guidance. During the puncture pus was withdrawn from largest lung lesion. Culture and metagenome next-generation sequencing (mNGS) were performed on the pus and the puncture fluid. Next-generation sequencing (NGS) was conducted on the pus specimen. Various microbes were detected by mNGS (see Figure 2), which showed Nocardia otitidiscaviarum was the pathogenic bacterium (the Nocardia otitidiscaviarum probe sequence is F- ACCGACCGAGATATTCAAATGATGA, R- TTCCACGAAAGGAAAGAACAATGTT, Probes- CGAGTCCAGCTGCGCCGAGAGT). Culture of the puncture fluid was positive and displayed dry chalky white colonies (see Figure 3). At this moment, the patient was diagnosed with pulmonary infection by Nocardia otitidiscaviarum.
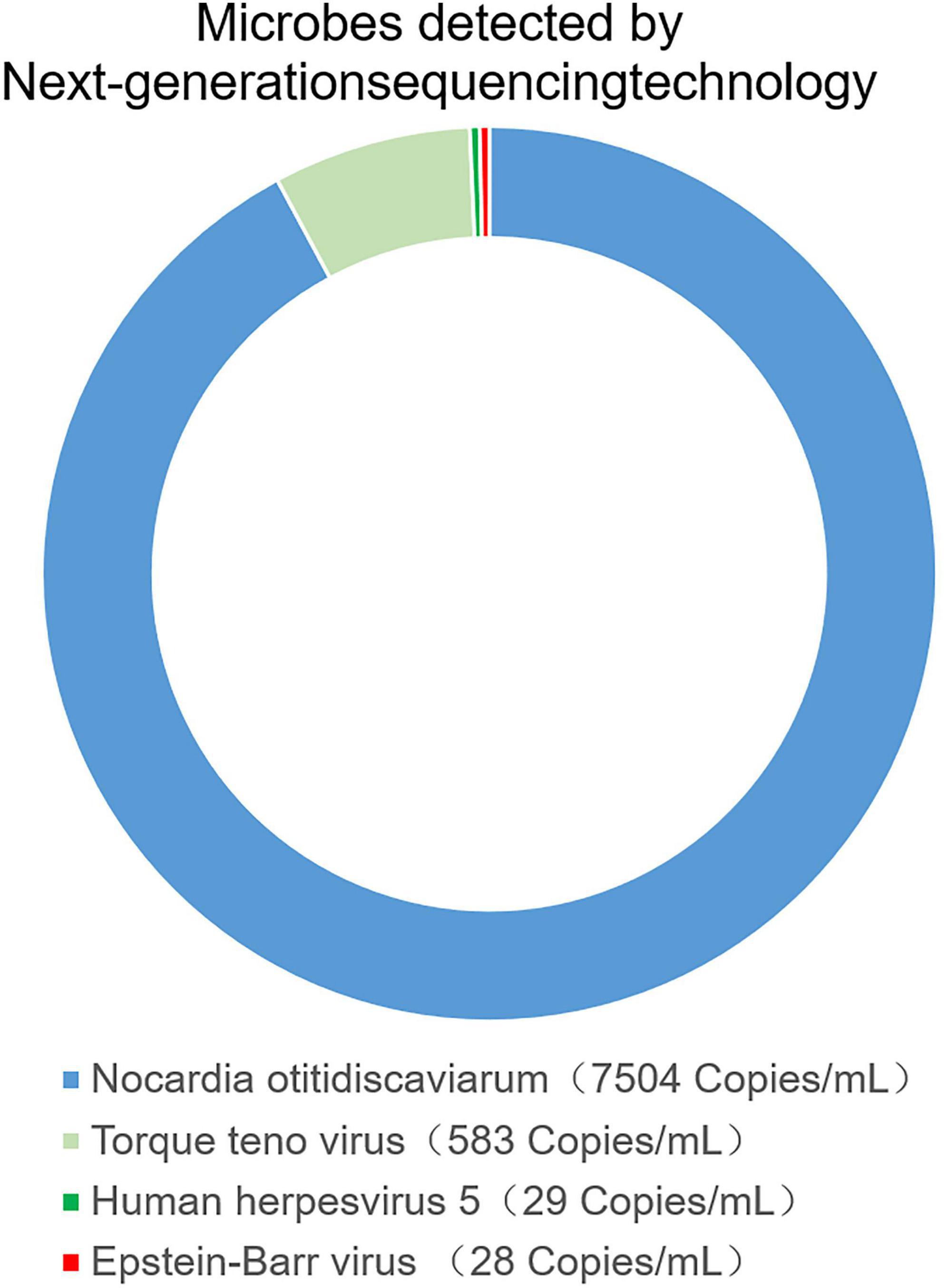
Figure 2. The pus was found in percutaneous lung neoplasm biopsy puncture, which was detected microbes by metagenome next-generation sequencing technology (mNGS).
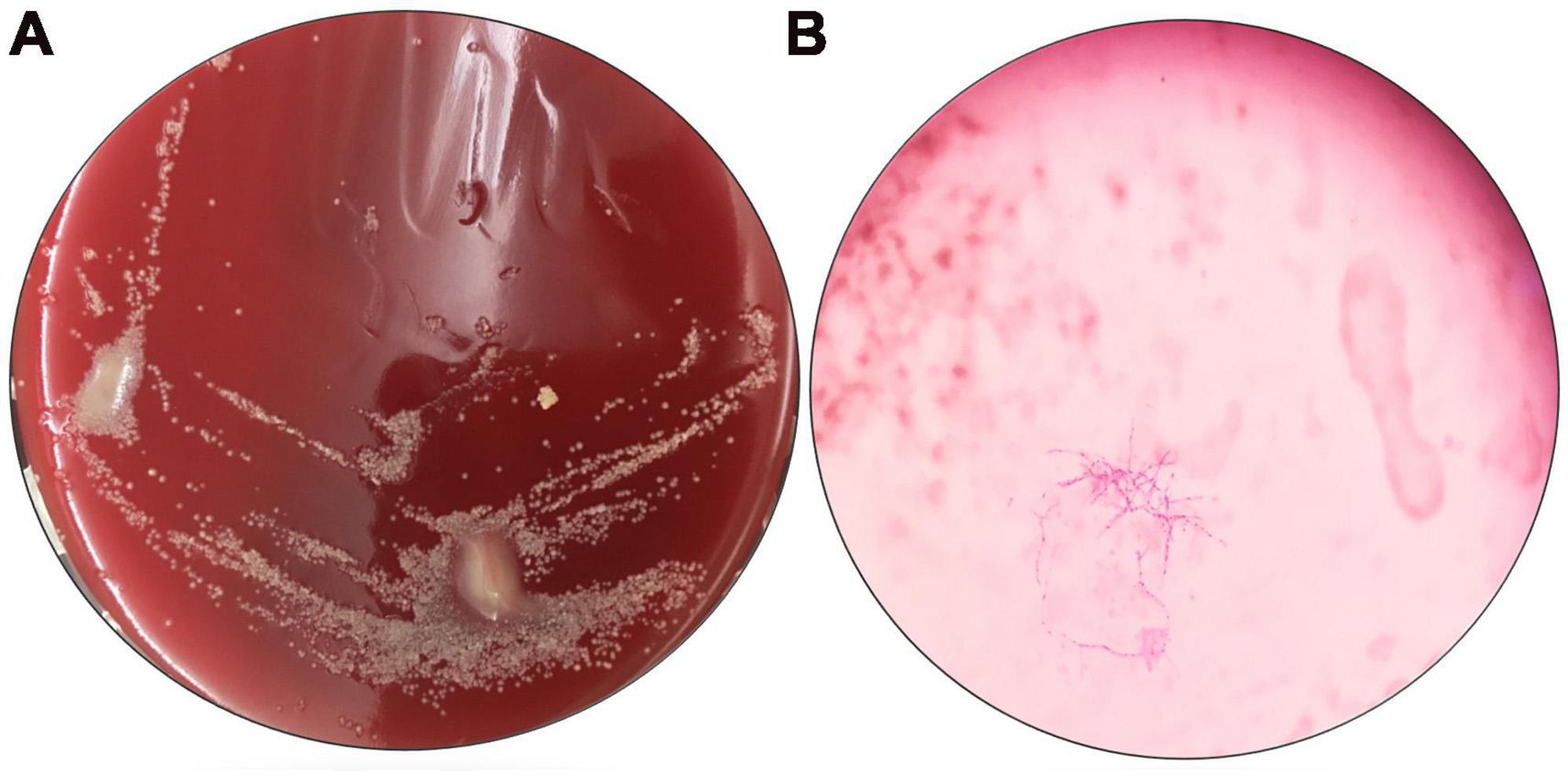
Figure 3. Results of pathogen culture in the pus. (A) Culture original by nutrient agar; (B) oil mirror of pathogenic bacteria by weak anti-acid staining, 1000×.
According to NGS testing guidance, compound sulfamethoxazole 1.5 g every 6 h was given orally combined with intravenous meropenem. Two days later, the patient’s temperature returned to normal, his spirits gradually improved, his cough decreased, and his sputum volume decreased. Ten days after this treatment, chest CT re-examination was performed, which revealed a reduction in the extent of bilateral lung shadow (see Figure 1D). The patient was scheduled to be discharged with oral trimethoprim-sulfamethoxazole for another 4 months. Subsequent follow-up re-examination of the chest CT showed that the lesions were absorbed in both lungs (see Figure 1E).
Discussion
Nocardia spp. is filamentous actinobacteria of the order corynebacteriales and mostly known for their ability to cause life-threatening infections in humans (11). Nocardia spp. are responsible for a range of diseases that affect various organ systems, including the lungs, skin, soft tissue and the intracranial- and abdominal cavity (12, 13). The lungs are the primary site of infection, accounting for 75–80% of the total infection (14). With Nocardia spp. invading and causing pulmonary infections known as pulmonary nocardiosis (PN). PN is characterized by suppurative inflammation and necrosis within the lungs, triggered by the inhalation of Nocardia spp. spores or fragmented hyphal elements through the respiratory tract (1, 14).
PN often manifests as cough, dyspnea, fever, night sweats, anorexia, fatigue, weight loss, hemoptysis, and pleuritic chest pain (15). The imaging manifestations of pulmonary Nocardia spp. infection lack specificity and can be manifested as single or multiple nodules, pulmonary mass (with or without cavity), reticular nodular infiltration, interstitial infiltration, lobar consolidation, subpleural plaque and pleural effusion (16, 17). Imaging features like these can easily be misdiagnosed as lung cancer or viral pneumonia (18–20). This misdiagnosis is more common when empirical antimicrobial treatments have failed (21). Even if the diagnosis is more accurate with AI, there are still misdiagnoses of such lesions. Therefore, the diagnosis of PN is difficult.
At present, it is recognized that Nocardia spp. isolated from clinical specimens is the gold standard for the diagnosis of PN (22). However, due to its special biological characteristics, the positive detection rate of Nocardia spp. is low. And the positive detection rate of Nocardia spp. detected from sputum is low in PN patients (23). In addition, when the results of the culture are negative, they may have to be repeated multiple times (22, 23). Even so, no positive results were obtained from culture of peripheral blood, sputum and duct lavage fluid in this case. As mNGS does not require traditional microbial culture, nor does it require specific amplification, it is theoretically able to detect any pathogen with a known genome sequence. In addition to its high sensitivity, mNGS can detect pathogens even when their numbers are low due to its ability to sequence DNA at a sufficient depth. Compared to culture-based methods requiring an average feedback time of 7–14 days, mNGS provides pathogenic evidence within 2–3 days (21). Its ability to directly type pathogens without the need for further strain identification enables immediate initiation of targeted treatment based on the type of pathogen identified (24). As a result of this characteristic of mNGS more precise clinical use of drugs can be achieved and pathogen treatment can be optimized (25). Therefore, mNGS has transformed the diagnostic landscape of lower respiratory tract infections (LRIs) and the selection of effective antibiotics to treat pathogenic bacteria (26).
Treatment of this disease should follow the principles of adequate dose, adequate course of treatment and individualization. Drug susceptibility testing is particularly important as a guide. Literature data showed that PN is generally effective to sulfonamides antibiotics, and effective to linezolid, imipenem and amikacin (16, 17). Antimicrobial susceptibilities for Nocardia spp. have been shown to be species specific and may differ geographically, which highlights the importance of susceptibility testing (17, 25, 26). Therefore, this knowledge of antimicrobial susceptibilities for Nocardia spp. may highlight the utility of mNGS, which could suggest susceptibility and drugs that need to be excluded from use. The bacteria detected by mNGS can directly provide accurate clinical drug guidance, achieve targeted treatment of infected bacteria, and optimize the treatment plan (27). Combination therapy can enhance the efficacy and should be used as the initial treatment (28, 29). Common regimens include sulfamethoxazole combined with imipenem or amikacin, imipenem combined with cefotaxime or amikacin, and amikacin combined with cefotaxime. Triple therapy should be considered for immunocompromised patients with disseminated infection, and the course of treatment should be 6–12 months (30, 31).
In conclusion, PN is rare and occurs mostly in immunocompromised patients. The diagnosis of Nocardia spp. infection in immunocompetent individuals is often difficult. NGS of sputum, alveolar lavage fluid and lung tissue can improve the detection rate of Nocardia spp. infection and avoid catastrophic consequences. This case report has certain reference significance for the diagnosis and treatment of special diseases in the department of respiratory medicine and critical care medicine.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The patient provided written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
NF: Data curation, Formal analysis, Writing – original draft. HF: Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. FH: Data curation, Methodology, Writing – review & editing. JZ: Project administration, Validation, Writing – review & editing. PL: Data curation, Formal analysis, Project administration, Validation, Writing – original draft. M-JL: Data curation, Methodology, Writing – original draft. Y-YD: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the patient who agreed to be included in this report for his cooperation and support for academic communication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tajima Y, Tashiro T, Furukawa T, Murata K, Takaki A, Sugahara K, et al. Pulmonary nocardiosis with endobronchial involvement caused by Nocardia araoensis. Chest. (2024) 165:e1–4. doi: 10.1016/j.chest.2023.07.067
2. Wang H, Zhu Y, Cui Q, Wu W, Li G, Chen D, et al. Epidemiology and antimicrobial resistance profiles of the Nocardia species in China, 2009 to 2021. Microbiol Spectr. (2022) 10:e0156021. doi: 10.1128/spectrum.01560-21
3. Conville PS, Brown-Elliott BA, Smith T, Zelazny AM. The complexities of Nocardia taxonomy and identification. J Clin Microbiol. (2017) 56:e1419–1417. doi: 10.1128/JCM.01419-17
4. Wang X, Yang H, Xie Y, Xian X. Severe disseminated Nocardia brasiliensis pneumonia with normal immune function: A case report. Medicine. (2024) 103:e36402. doi: 10.1097/MD.0000000000036402
5. O’Brien A, Hart J, Higgins A, Arthur I, Lee GH, Leung M, et al. Nocardia species distribution and antimicrobial susceptibility within Australia. Intern Med J. (2023) 54:613–9. doi: 10.1111/imj.16234
6. García Vicente AM, Noriega Álvarez E, González Rodríguez JC, Soriano Castrejón ÁM. Brain nocardiosis defined by 18 F-fluorocholine PET/CT. Clin Nucl Med. (2023) 48:327–9. doi: 10.1097/RLU.0000000000004564
7. Ninan MM, Venkatesan M, Balaji V, Rupali P, Michael JS. Pulmonary nocardiosis: Risk factors and species distribution from a high burden centre. Indian J Med Microbiol. (2022) 40:582–4. doi: 10.1016/j.ijmmb.2022.08.010
8. Stellern JJ, Plaisted J, Welles C. Disseminated nocardiosis with persistent neurological disease. BMJ Case Rep. (2024) 17:e257935. doi: 10.1136/bcr-2023-257935
9. Kesim S, Oksuzoglu K, Ozguven S. Nocardia infection with adrenal gland abscess mimicking metastatic lung cancer on FDG PET/CT. Clin Nucl Med. (2023) 48:e24–5. doi: 10.1097/RLU.0000000000004446
10. Lopes K, Montenegro C, Vìlchez J, Camacho ME, Marques HG. IMAGES: Nocardia pneumonia – A surprising and rare pulmonary infection mimicking lung cancer. Pulmonology. (2022) 28:415–7. doi: 10.1016/j.pulmoe.2022.03.013
11. Sheikh-Taha M, Corman LC. Pulmonary Nocardia beijingensis infection associated with the use of alemtuzumab in a patient with multiple sclerosis. Mult Scler. (2017) 23:872–4. doi: 10.1177/1352458517694431
12. Ma H, Wang X, Yan H, Liu Q, Yang D, Bian T. Dual intracranial infection with Nocardia farcinica and Cryptococcus neoformans diagnosed by next-generation sequencing in a patient with nephrotic syndrome: A case report. Medicine. (2022) 101:e30325. doi: 10.1097/MD.0000000000030325
13. Wang X, Liang Y, Cheng Q, Nong W, Hu L. Abdominal abscesses caused by Nocardia farcinica in an immunocompromised patient: A case report and literature review. Infect Drug Resist. (2023) 16:7447–54. doi: 10.2147/IDR.S441117
14. Huang L, Chen X, Xu H, Sun L, Li C, Guo W, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. (2019) 94:165–72. doi: 10.1016/j.diagmicrobio.2018.12.007
15. Mehrabadi SM, Taraghian M, Pirouzi A, Khaledi A, Neshani A, Rashki S. Pulmonary nocardiosis in suspected tuberculosis patients: A systematic review and meta-analysis of cross-sectional studies. Ethiop J Health Sci. (2020) 30:293–300. doi: 10.4314/ejhs.v30i2.17
16. Kanne JP, Yandow DR, Mohammed TL, Meyer CA. CT findings of pulmonary nocardiosis. AJR Am J Roentgenol. (2011) 197:W266–72. doi: 10.2214/AJR.10.6208
17. Al Umairi RS, Pandak N, Al Busaidi M. The findings of pulmonary nocardiosis on chest high resolution computed tomography: Single centre experience and review of literature. Sultan Qaboos Univ Med J. (2022) 22:357–61. doi: 10.18295/squmj.9.2021.131
18. Wu XY, Ding F, Li K, Huang WC, Zhang Y, Zhu J. Analysis of the causes of solitary pulmonary nodule misdiagnosed as lung cancer by using artificial intelligence: A retrospective study at a single center. Diagnostics. (2022) 12:2218. doi: 10.3390/diagnostics12092218
19. Zhu J, Huang WC, Huang B, Zhu Y, Jiang XJ, Zou JN, et al. Clinical characteristics and prognosis of COVID-19 patients with initial presentation of lung lesions confined to a single pulmonary lobe. Am J Transl Res. (2020) 12:7501–9.
20. Zhu J, Zhang Y, Gao XH, Xi EP. Coronavirus disease 2019 or lung cancer: A differential diagnostic experience and management model from Wuhan. J Thorac Oncol. (2020) 15:e141–2. doi: 10.1016/j.jtho.2020.04.030
21. Li M, Wang B, Liu P, Wang H, Zhu J. Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report. Open Life Sci. (2023) 18:20220596. doi: 10.1515/biol-2022-0596
22. Weng SS, Zhang HY, Ai JW, Gao Y, Liu YY, Xu B, et al. Rapid detection of Nocardia by next-generation sequencing. Front Cell Infect Microbiol. (2020) 10:13. doi: 10.3389/fcimb.2020.00013
23. Xing F, Xia Y, Lu Q, Lo SKF, Lau SKP, Woo PCY. Rapid diagnosis of fatal Nocardia kroppenstedtii bacteremic pneumonia and empyema thoracis by next-generation sequencing: A case report. Front Med (Lausanne). (2023) 10:1226126. doi: 10.3389/fmed.2023.1226126
24. Wang S, Xing L. Metagenomic next-generation sequencing assistance in identifying non-tuberculous mycobacterial infections. Front Cell Infect Microbiol. (2023) 13:1253020. doi: 10.3389/fcimb.2023.1253020
25. Zheng Y, Qiu X, Wang T, Zhang J. The diagnostic value of metagenomic next-generation sequencing in lower respiratory tract infection. Front Cell Infect Microbiol. (2021) 11:694756. doi: 10.3389/fcimb.2021.694756
26. Yu J, Diaz JD, Goldstein SC, Patel RD, Varela JC, Reyenga C, et al. Impact of next-generation sequencing cell-free pathogen DNA test on antimicrobial management in adults with hematological malignancies and transplant recipients with suspected infections. Transplant Cell Ther. (2021) 27:500.e1–500.e6. doi: 10.1016/j.jtct.2021.02.025
27. Steinbrink J, Leavens J, Kauffman CA, Miceli MH. Manifestations and outcomes of Nocardia infections: Comparison of immunocompromised and nonimmunocompromised adult patients. Medicine. (2018) 97:e12436. doi: 10.1097/MD.0000000000012436
28. Gandham N, Kannuri S, Gupta A, Mukhida S, Das N, Mirza S. A post-transplant infection by Nocardia cyriacigeorgica. Access Microbiol. (2023) 5:000569.v3. doi: 10.1099/acmi.0.000569.v3
29. Horino T, Ohnishi H, Komori M, Terada Y. Pulmonary nocardiosis in a patient with rheumatoid arthritis. Intern Med. (2023). doi: 10.2169/internalmedicine.2910-23 [Epub ahead of print].
30. Margalit I, Lebeaux D, Tishler O, Goldberg E, Bishara J, Yahav D, et al. How do i manage nocardiosis? Clin Microbiol Infect. (2021) 27:550–8. doi: 10.1016/j.cmi.2020.12.019
Keywords: Nocardia, otitidiscaviarum, immunocompetent patient, next-generation sequencing (NGS), pulmonary co-infection
Citation: Fan N, Fang H, Huang F, Zhou J, Liu P, Li M-J and Ding Y-Y (2024) Metagenome next-generation sequencing plays a key role in the diagnosis and selection of effective antibiotics on the treatment of Nocardia pneumonia: a case report. Front. Med. 11:1373319. doi: 10.3389/fmed.2024.1373319
Received: 29 January 2024; Accepted: 09 May 2024;
Published: 27 May 2024.
Edited by:
Mohamed Said, University of Pretoria, South AfricaReviewed by:
Yutaka Yoshii, The Jikei University School of Medicine, JapanRosemary Griessel, University of Cape Town, South Africa
Copyright © 2024 Fan, Fang, Huang, Zhou, Liu, Li and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye-Ying Ding, dyybb1987@163.com
†These authors have contributed equally to this work and share first authorship
 Na Fan1†
Na Fan1†  Meng-Jie Li
Meng-Jie Li