Most accurate mutations in SARS-CoV-2 genomes identified in Uzbek patients show novel amino acid changes
- 1Center of Genomics and Bioinformatics, Academy of Sciences of Uzbekistan, Tashkent, Republic of Uzbekistan
- 2Department of Chemistry for Natural Substances, National University of Uzbekistan, Tashkent, Uzbekistan
- 3Luxembourg Institute of Health, Strassen, Luxembourg
Purpose: The rapid changes in the coronavirus genomes created new strains after the first variation was found in Wuhan in 2019. SARS-CoV-2 genotypes should periodically undergo whole genome sequencing to control it because it has been extremely helpful in combating the virus. Many diagnoses, treatments, and vaccinations have been developed against it based on genome sequencing. With its practical implications, this study aimed to determine changes in the delta variant of SARS-CoV-2 widespread in Uzbekistan during the pandemic by genome sequencing, thereby providing crucial insights for developing effective control strategies that can be directly applied in the field.
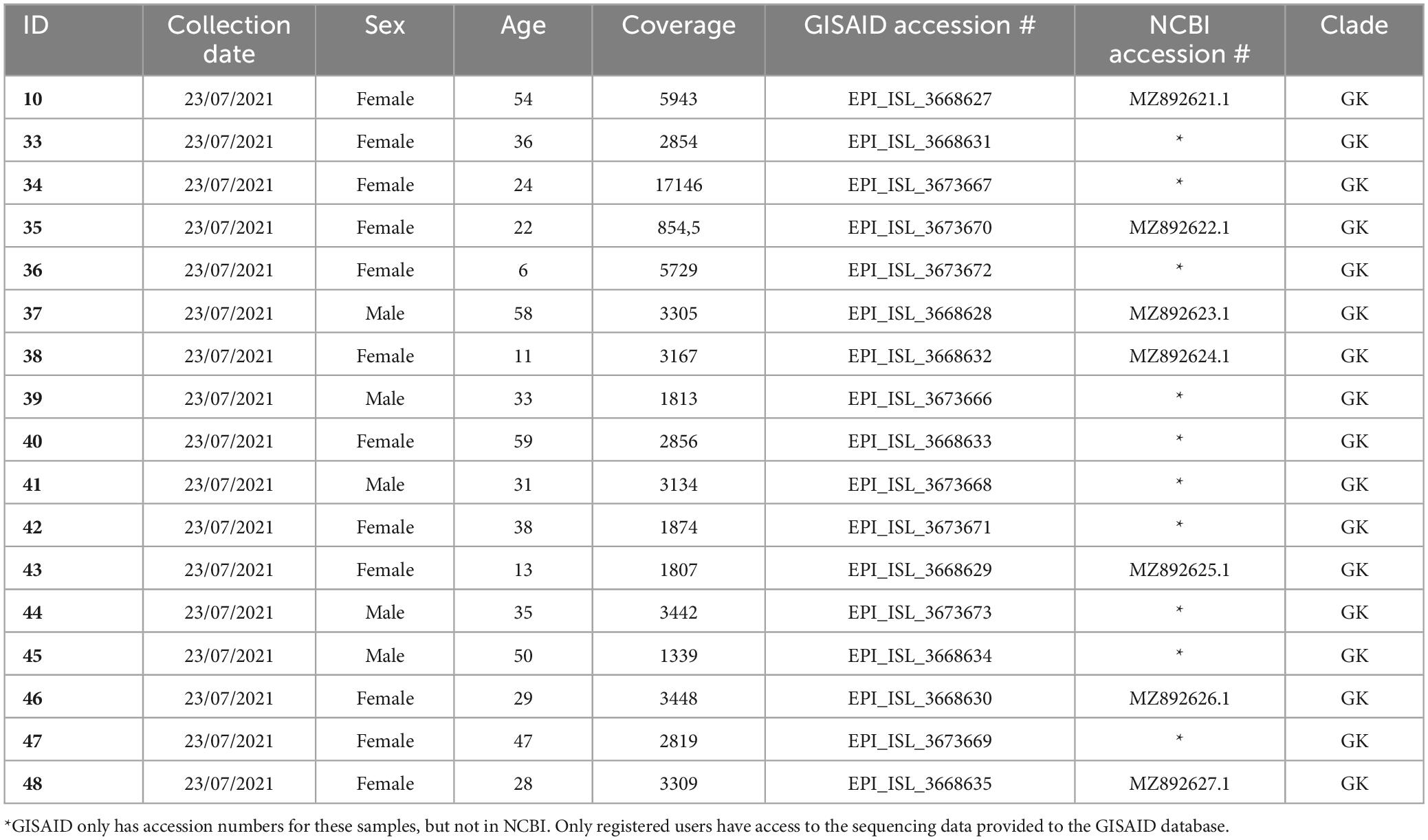
Design: We meticulously generated 17 high-quality whole-genome sequence data from 48 SARS-CoV-2 genotypes of COVID-19 patients who tested positive by PCR in Tashkent, Uzbekistan. Our rigorous approach, which includes stringent quality control measures and multiple rounds of verification, ensures the accuracy and reliability of our findings.
Methods: Our study employed a unique combination of genome sequencing and bioinformatics web tools to analyze amino acid (AA) changes in the virus genomes. This approach allowed us to understand the genetic changes in the delta variant of SARS-CoV-2 widespread in Uzbekistan during the pandemic.
Results: Our study revealed significant nucleotide polymorphisms, including non-synonymous (missense) and synonymous mutations in the coding regions of the sequenced sample genomes. These findings, categorized by phylogenetic analysis into the G clade (or GK sub-clade), contribute to our understanding of the delta variant of SARS-CoV-2 widespread in Uzbekistan during the pandemic. A total of 134 mutations were identified, consisting of 65 shared and 69 unique mutations. These nucleotide changes, including one frameshift mutation, one conservative and disruptive insertion-deletion, four upstream region mutations, four downstream region mutations, 39 synonymous mutations, and 84 missense mutations, are crucial in the ongoing battle against the virus.
Conclusion: The comprehensive whole-genome sequencing data presented in this study aids in tracing the origins and sources of circulating SARS-CoV-2 variants and analyzing emerging variations within Uzbekistan and globally. The genome sequencing of SARS-CoV-2 from samples collected in Uzbekistan in late 2021, during the peak of the pandemic’s second wave nationwide, is detailed here. Following acquiring these sequences, research efforts have focused on developing DNA and plant-based edible vaccines utilizing prevalent SARS-CoV-2 strains in Uzbekistan, which are currently undergoing clinical trials.
Introduction
Considerable research has been conducted on the SARS-Cov-2 genome since 2020. To date, 15,778,185 SARS-CoV-2 genome sequences have been shared via online data platforms such as GISAID (1), which helps track the new variants and mutations. The causative agent of COVID-19, SARS-CoV-2, is constantly evolving as it spreads from human to human (2).
On 11 March 2020, the World Health Organization (WHO) formally declared a pandemic (3). SARS-CoV-2 has scattered globally and is currently a significant problem. More people have suffered in Europe and Latin America than in other countries, especially during the early phases of the coronavirus outbreak.
Scientists are currently using the established nomenclature systems for naming and tracking genetic lineages of SARS-CoV-2 through GISAID, Nextstrain, and Pango in their research. The WHO called for the creation of the Technical Advisory Group on Virus Evolution, which suggested labeling the virus strains with Greek alphabet letters, such as Alpha, Beta, Gamma, Delta, etc., to facilitate more accessible and more helpful discussion among audiences outside of the scientific community (3).
SARS-CoV-2 is classified within the β-coronavirus genus lineage B. It is characterized by possessing large enveloped, positive-sense, single-stranded RNAs with a length of 30 kilobases. The viral genome encodes four structural proteins and various accessory and non-structural proteins. Notable among these are the viral pp1a-pp1ab replicase, the 3C-like protease (3CLpro), the papain-like protease (PLpro), and the RNA-dependent RNA polymerase (RdRp) (4, 5).
Finding relevant information on viral lineages, variants of interest, and variants of concern requires SARS-CoV2 whole-genome sequencing (6). Global databases of the SARS-CoV-2 genome have been mentioned in our previous paper (7).
The virus genomes sequenced were used to create the global phylogenetic tree of SARS-Cov-2. The sequences are categorized into multiple clades. Our previous study examined the earliest clades and their characteristics (7). Many viral variants were later discovered and uploaded to global databases. The Delta variant was first reported in India at the end of 2020, but it has since spread worldwide to 135 countries and continues growing. Delta shared some mutations with other variants and possessed unique mutations on spike proteins, which may be responsible for its rapid spread and increased virulence (8). Significantly more transmissible delta variants have been connected to important S-protein mutations like D614G, L452R, P681R, and T478K (9). The spike protein promotes virus-cell membrane fusion and attachment to host cell-surface receptors. Additionally, it serves as the main target for neutralizing antibodies made following infection (10).
For the first time, an infectious epidemic was found in Uzbekistan in March 2020. The government decided to conduct public testing for COVID-19. This incident signaled the beginning of the pandemic in Uzbekistan, necessitating accurate identification and monitoring of coronavirus genotypes that were rapidly spreading throughout the population (7, 11). The SARS-CoV-2 genome should be sequenced at the population level to identify virus strains and investigate their local and worldwide dissemination. In addition, the comprehensive sequencing of the entire viral genome associated with infection is a valuable tool in elucidating outbreak dynamics. This includes investigating variations in pandemic size over time, spatial dissemination patterns, and transmission routes, as emphasized in the WHO COVID Report of 2021.
The global distribution of the virus was caused by worldwide travel. We started sequencing the whole genome of COVID-19 samples to primarily identify virus genotypes spread in our territory and analyze genomic diversity, types of mutations, and the emergence of new variations of SARS-CoV-2 (7, 11). In this study, we described the following whole-genome sequence data from individuals in the Republic of Uzbekistan who were infected with COVID-19. We evaluated 134 mutations, including non-synonymous and synonymous, and effectively reconstructed 17 high-quality sample genome sequences for COVID-19 genotypes. In the global phylogenetic tree, comparative analysis utilizes SARS-CoV-2 genomes known as the Uzbekistan sample genomes in the GK subclade. The S region of the sequenced genomic data of one of the coronavirus genotypes was used to develop the edible vaccine against coronavirus threats in Uzbekistan (12).
Materials and methods
Gathering samples
Using the technique from our earlier research (7), samples were taken from 100 symptomatic patients with a high temperature and an intermittent cough. The samples were then immediately put in a viral transport medium. Patients suspected of having COVID-19 infection were referred to the diagnostics laboratories of the private BiogenMed COVID-19 testing laboratory in Tashkent, Republic of Uzbekistan. After SARS-CoV-2 testing in the laboratory, specimens of biological origin were randomly collected from PCR-positive individuals.
The scientific inquiry was approved by the Ethics Committee of the Ministry of Health of the Republic of Uzbekistan (#6/20-1582). All experiments were conducted in conformity with the applicable standards and legislation. Samples were renumbered and de-identified so that no one, not even researchers, knew who the patients were. Only anonymized data, including age and biological sex, were preserved for reporting purposes. All patients in the sample collection provided verbal consent for voluntary participation. We stated to all participants that the collected samples would be used for a sequencing project exclusively, without disclosing their identities or disturbing them in the future. In this study, verbal consent was preferred over written consent since patients were hesitant to sign any written document due to their concern about COVID-19 infection at the time and a lack of knowledge of the genome sequencing investigation. No minors were involved in the sample collection. There was no need to do so because the sequencing experiment was non-invasive, participants were not subjected to further downstream clinical procedures, and the identity of samples was anonymized in this investigation.
RNA extraction, Real-Time PCR, and sample evaluation were carried out after our previous work (7). Among all evaluated patients, 48 PCR-positive samples (28 females and 20 men) were randomly chosen for further research.
SARS-CoV-2 sequencing
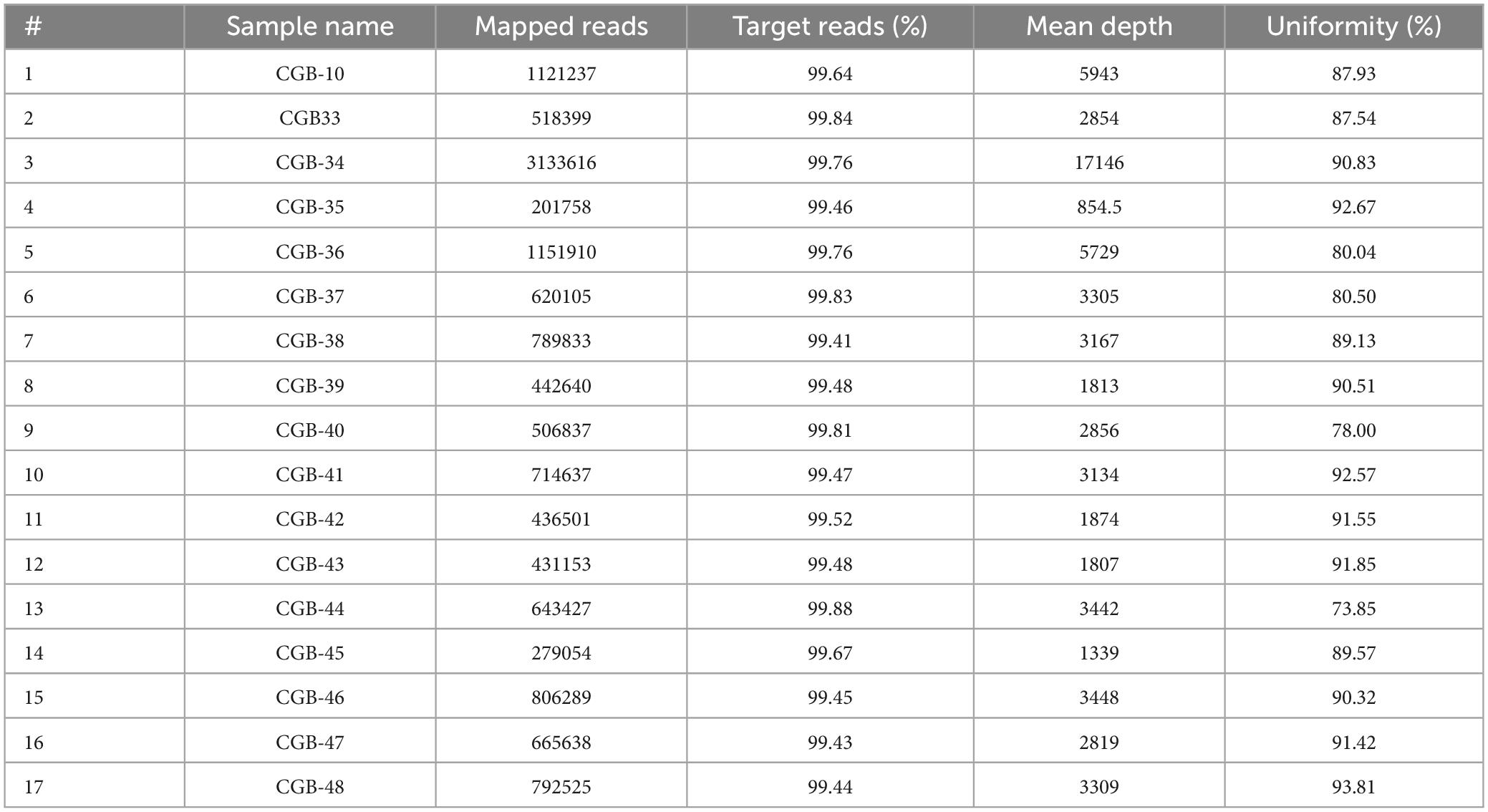
SARS-CoV-2 sequencing and data analysis were carried out as in our previous study (8). We used coverage analysis (v5.12.0.0) and variant caller (v5.12.0.4) while estimating the probability of the data belongings. Variant callers were filtered to retain only dependable variants and eliminate variants with reading depths of less than 1000 and ion torrent quality scores of less than 400 (Table 2 and Supplementary Table 1). We utilized the Maximum Likelihood Tree in the Molecular Evolutionary Genetics Analysis (MEGA)1 program to cluster samples for the filtered variants. The consensus for each SARS-CoV-2 genome sequence was then submitted to the NCBI under accession numbers GI:2085183815 to GI:2085183892 (or MZ892621.1 to MZ892627.1; NCBI database; Table 1) and to GISAID2 under accession numbers EPI_ISL_3668627 to EPI_ISL_3673673 (available for registered users).
Results and discussion
Sample selection for sequencing
More than a hundred patients suffering from high fever and sporadic cough symptoms were randomly selected in this work from a Tashkent-based private COVID-19 testing laboratory. Forty-eight PCR-positive samples were chosen for sequencing. Only 17 high-quality sequences were selected to be submitted to worldwide databases (twelve women and five men with an average age of 34, Table 1).
The results in this work show twice the higher infectivity level of women after random analysis. Twelve of seventeen patients were women among those subjected to SARS-CoV-2 infection during random selection. Thus, we expect greater sensitivity of women among Uzbekistani patients than men to SARS-CoV-2 infection. We did not observe the tendency of older people to be more sensitive to coronavirus infection. Among the studied patients, only four out of seventeen people were >50 years old after random selection. The Delta variant is notably more transmissible, estimated to have a 50–80% increase in transmissibility compared to the Alpha variant (13). In addition, the Delta variant is believed to have higher transmissibility compared to earlier strains of SARS-CoV-2, particularly among children and adolescents. According to a decision analytic model analyzing 106,866 confirmed COVID-19 infections, susceptibility to the Delta variant was significantly higher among the 10 to 15-year-old age group, showing a 1.92-fold increase compared to the pre-Delta variant (14). This corresponds with our results: seven patients were <30 years old after random selection.
There are numerous gaps in the consensus sequence as a result of 31 SARS-CoV-2 samples being disqualified from additional analysis due to inadequate sequencing coverage. The average number of mapped reads for the remaining 17 samples (12 women and five men) was 779 739 (Table 1). The target reads were 99.61%, and the mean read depth was 3814. The average coverage consistency in the chosen samples was 87.8% (Table 2).
Identifying unique and reliable mutations in all high-quality sequenced samples
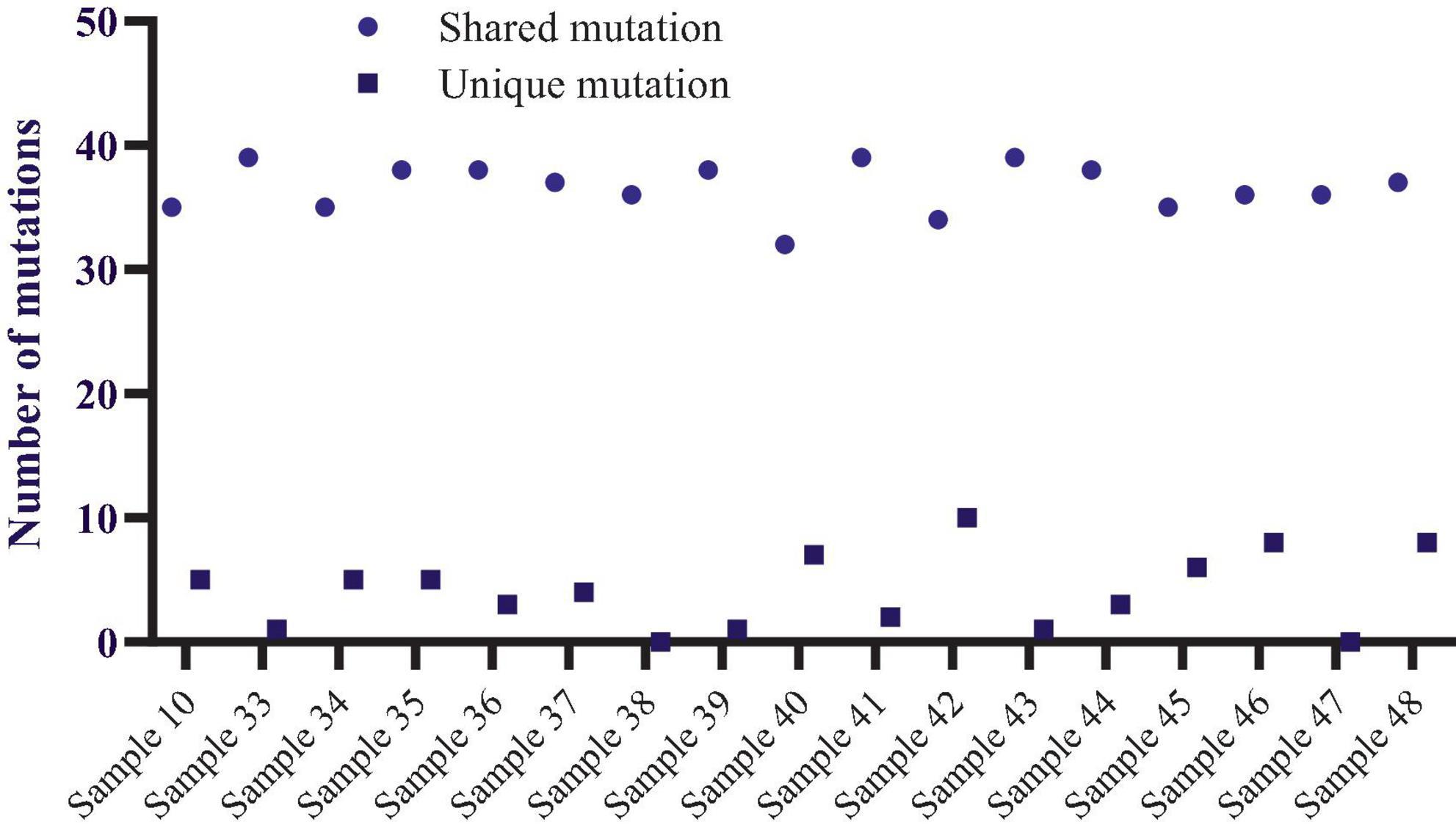
Using the maximum likelihood method, the MEGA X program generated a phylogenetic tree based on the 17 viral sequences of SARS-CoV-2 taken from samples of COVID-19 patients in Tashkent city. The Wuhan reference strain NC 045512.2 was the root of this maximum likelihood tree, which was constructed using 134 mutations found in the 17 sequences (Supplementary Table 1 and Figure 1). Bootstrap values are illustrated.
Variant Caller produced all evaluated mutations in these selected samples. The number of mutations ranged from 36 (samples 38,47, and 36) to 45 (sample 48), altogether unique and shared mutations (Figure 1). Compared to the NC_045512.2 reference genome, most viral genomes represented between 39 and 44 mutations. These included thirty-nine mutations in samples 39 and 40, forty mutations in samples 10, 33, 34, and 43, forty-one mutations in samples 37, 41, 44, and 45, and forty-three mutations in only samples 35 and forty-four mutations in samples 42 and 46. The mutations mentioned above were calculated together with shared and unique mutations. Samples 38 and 46 do not carry unique mutations (Figure 1).
The total number of shared mutations and unique mutations is calculated for each sample.
The most commonly occurring nucleotide substitution found was cytosine to thymine (51/134 mutations), then guanine to thymine (28/134 mutations), thymine to cytosine and guanine to adenine (12/134 mutations), and then adenine to guanine (10/134 mutations). The Supplementary Table 1 shows the breakdown of these mutations. Every mutation had homozygosity. In the gene encoding the S protein we identified one shared disruptive inframe deletion (c.467_472delAGTTCA), two unique (c.*4300A>G, c.*4352G>T), two shared (c.*4308G>T, c.*4358G>T) downstream gene variant mutations, four unique (c.646C>T, c.2533G>T, c.3761G>T and c.3790G>T) and nine shared missense variant mutations, four unique (c.936C>T, c.1995C>T, c.2259G>A and c.2388T>C) and one shared (c.3183C>T) synonymous variant mutations. In the M (matrix) region one unique (c.100C>T and c.463C>T) and one shared (c.245T>C) missense mutation was observed; in N (nucleocapsid) region one shared (c.-3delA) upstream gene variant mutation, three unique (c.200C>T, c.518C>T and c.1085C>T) and six shared (c.188A>G, c.608G>T, c.643G>T, c.1129G>T, c.1152G>C and c.1154G>A) missense mutations and four unique (c.105G>A, c.123G>A, c.987G>A and c.1080C>T) synonymous mutations were identified. No mutations were found in the E (envelope) region (Supplementary Tables 1, 2). The nucleotide substitution C>T was established to enhance the viral adaptation in hosts (15). The reason was linked with the occurrence of amino acids of a hydrophobic nature that could contribute to better cell penetration. This change was reported as evidence of host-dependence and approach to combat coronavirus (16, 17).
In addition, our analysis revealed one frameshift mutation, 41 missense mutations, 27 synonymous mutations, and two upstream gene mutations within the ORF1ab region. Within the ORF3a region, one synonymous mutation and ten missense mutations were identified. In the ORF6 region, one missense mutation was observed, while the ORF7a region displayed six missense mutations and one synonymous mutation. Additionally, the ORF8a region exhibited one missense mutation, one upstream gene mutation, and one conservative insertion-deletion mutation. Finally, one synonymous mutation (c.57C>T) was found in the ORF10 region. We found 134 mutations in total, 65 shared and 69 unique, representing one frameshift mutation, one conservative and disruptive inframe deletion, four upstream region mutations, four downstream region mutations, 39 synonymous mutations, and 84 missense mutations (Supplementary Table 1).
The construction of the phylogenetic tree was derived from viral sequences
We wanted to examine the significant alterations in all sequences to understand what distinguished our cases from others worldwide. The software MEGA X was employed to construct a phylogenetic tree utilizing the maximum likelihood method, incorporating the analysis of 17 viral sequences (Figure 1). The variant caller identified one hundred thirty-four mutations from the abovementioned sequences (v.5.12.0.4) (Supplementary Table 1).
Most of the shared mutations with more sequences, for example, eighteen mutations (C3037T, G15451A, C16466T, C21618G, GAGTTCA22028G, T22917G, C22995A, A23403G, C23604G, G24410A, C25469T, AGATTTC28247A, T26767C, TA28270T, A28461G, G28881T, G29402T, and G29742T) with 17 sequences and four mutations (G210T, C241T, C14408T, T27638C, C27752T) with 16 samples, seven mutations (C6402T, C8986T, G9053T, A11201G, A11332G, C19220T and G28916T) with 12 sequences and four mutations (C14925T, A21137G, A24110C and A25439C) with six sequences, accordingly (Supplementary Table 1).
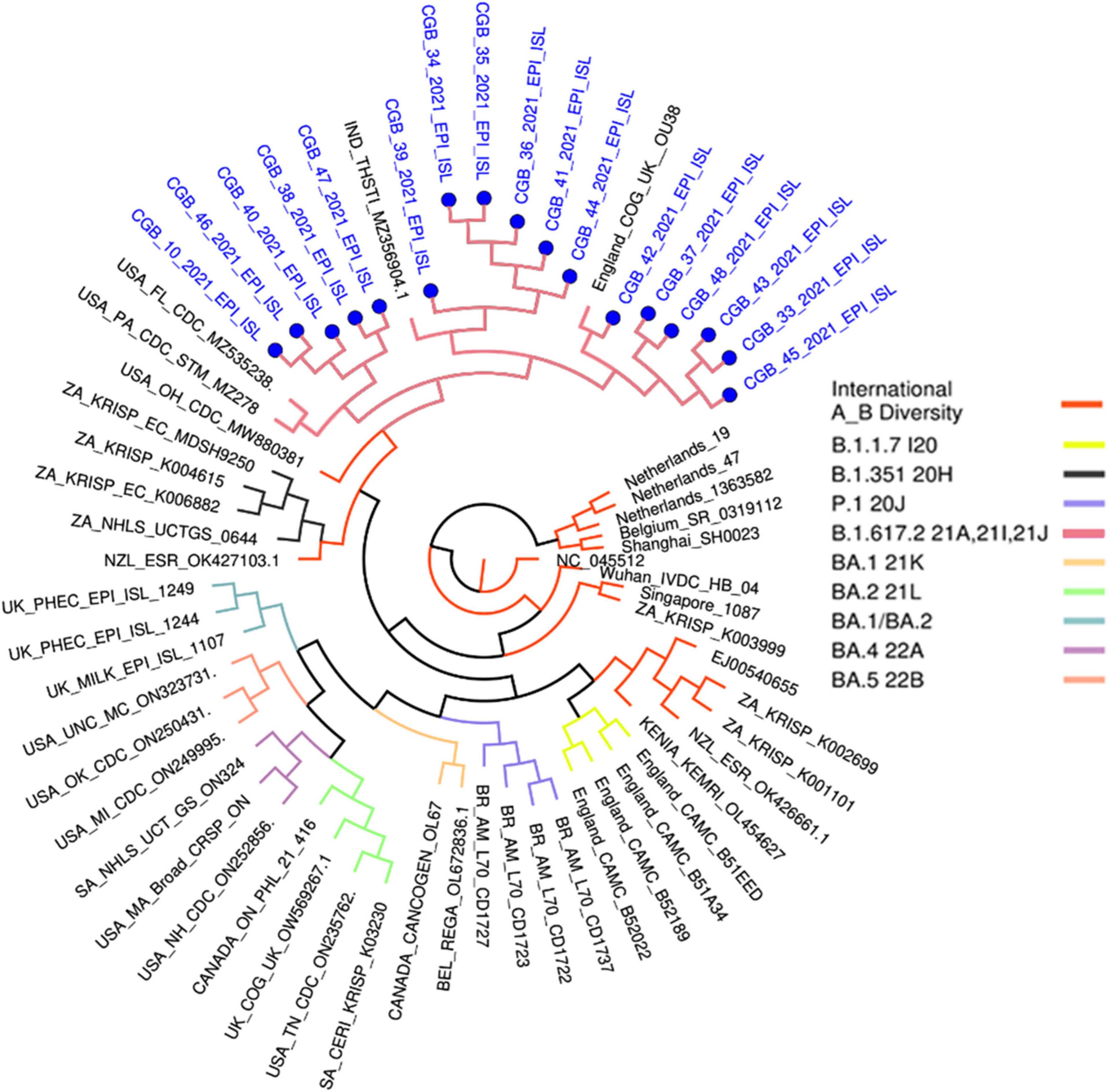
These variants have been grouped into the USA, Indian, and England variants. All are considered Delta strains (B.1.617.2) in the phylogenetic tree provided by genomedetective.com. Interestingly, Beta variant samples are located in the Neighbor Cluster in the tree, whereas Alpha, Gamma, and Omicron variant samples were found in the further branches (Figure 3).
This phylogenetic tree was generated in www.genomedetective.com using our sequenced samples.
*B.1.1.7 I20 belongs to the Alpha variant. B.1.351 20H belongs to the Beta variant. P.1 20J belongs to the Gamma variant. B.1.617.2 21A, 21I, 21J belong to the Delta variant. BA.1 21K, BA.2 21L, BA. 1/BA.2, BA.4 22A, and BA.5 22B belong to Omicron variant of SARS-CoV-2.
Our findings show that the whole-genome sequences obtained from the 17 symptomatic COVID-19 patients represent significant nucleotide diversity (Table 1 and Supplementary Tables 1, 2). Based on the similarity of mutations observed in the Delta strain, the 17 sequenced samples have been clustered into a predominant clade of SARS-CoV-2 within the GISAID public database, designated as clade G (or GK subclade). The G clade has been known for S-protein mutations such as D614G, L452R, P681R, and T478K [11]. These mutations were also found in all 17 samples using the viralvar.org website. However, some other mutations in this region were found in each sample. For example, 34 S-protein mutations were identified in sample 39, while 23 mutations were found in sample 47 (Supplementary Table 3).
In accordance with the findings of Cherian et al. (18), the recently identified lineages B.1.617.1 and B.1.617.2 were prominently represented within the phylogenetic tree. These lineages were characterized by signature mutations, including L452R, T478K, E484Q, D614G, and P681R, within the spike protein, notably within the receptor-binding domain (RBD) (18). Those mutations, except E484Q, were also shared with all 17 samples in our result. In addition, mutation T19R was shared with all samples, whereas G142D (in 15 samples), D950N (in 12 samples), I850L (in 6 samples), T95I (in 5 samples), A222V and F797C (in 2 samples) were found (Supplementary Table 3).
The structural analysis of the receptor-binding domain (RBD) mutations, specifically L452R, T478K, and E484Q, indicated a potential enhancement in ACE2 binding affinity. Conversely, the presence of P681R within the furin cleavage site suggested a possible acceleration in the rate of S1-S2 cleavage, thereby facilitating heightened transmissibility. Moreover, the RBD mutations L452R and E484Q were found to diminish the binding affinity to specific monoclonal antibodies (mAbs), potentially impacting their neutralization efficacy (18). Interestingly, the E484Q mutation did not appear in our sequenced samples.
Most of the unique amino acid changes were found in the S-spike region in Sample 39 (26 mutations; F106L, G107V, T108L, T109L, L110stop, D111I, S112R, K113R, T114P, Q115S, S116P, L117Y, I119L, V120L, N121I, N122T, A123L, T124L, N125M, V126L, V127L, I128L, V130S, C131V, E132N, and Q134N) and in Sample 47 (16 mutations; A260V, G261W, A262C, A264S, Y265L, Y266L, V267C, Y269L, L270S, A263C, L277I, Q271S, P272T, R273stop, T274D, and L276S). In contrast, five AA changes were found in Sample 33 (Supplementary Table 3). The virus genome must be sequenced to identify SARS-CoV-2 strains and investigate local and worldwide dissemination. In addition, the comprehensive sequencing of the entire viral genome associated with infection is a valuable tool in elucidating outbreak dynamics. This includes investigating variations in pandemic size over time, spatial dissemination patterns, and transmission routes, as emphasized in the WHO COVID Report of 2021. Indeed, this investigation revealed that many diseases originated in the United States, India, and England (GK subclade) (Figure 2). The global distribution of the virus was caused by worldwide travel.
The disparities observed in virus structure among the samples illustrate the evolution of viral morphology over time in response to varying environmental conditions. The genomic sequence data derived from these 17 samples, subsequently deposited in global databases such as NCBI and GISAID, hold significant promise for public health and research entities in elucidating patterns of disease dissemination within the region. Moreover, these findings are anticipated to inform the development of diagnostic assays, therapeutic interventions, and vaccine candidates. Several institutions, including our center, have initiated efforts toward developing a national vaccine based on the SARS-CoV-2 genome sequences identified in symptomatic patients from Uzbekistan. The sequence information presented herein enriches the COVID sequence database (GISAID) with updated sequence data and mutational profiles specific to our geographical region. This contribution holds significance for forthcoming molecular epidemiological investigations and evolutionary phylogenetic studies conducted by health and scientific organizations.
The structural map of SARS-CoV-2 proteins was developed based on the one by Jamison et al. (19). The proteins’ 3D structures were developed by modeling their sequences in Swissmodel.3 The ones with no crystals obtained yet were not modeled in the database; thus, we did not include their models.
The mutations observed in this work belonged to both structural and non-structural proteins (Figure 3). Among non-structural proteins, we did not determine changes in NSP1, NSP7, NSP8, NSP9, NSP10, and NSP11. The number of mutations observed in this work coincides with proteins’ molecular parameters; the highest number of mutations were determined in NSP3 and spike protein. NSP3, the largest coronavirus protein, is one of the essential proteins involved in replication/transcription processes (20). NSP3 is one of the well-established proteases in SARS; NSP3 is responsible for papain-like functions (21) and NSP5 is known as 3CL (chymotrypsin-like main) protease (22).
Compared to protein length, the highest numbers of mutations in the genome were observed in N protein (9 mutations) and ORF3a (10 mutations). Even though the highest numbers (12 and 13) of mutations were found in NSP3 and spike proteins, respectively, their frequencies were intermediate relative to protein length. Compared to previous variants, the delta variant has higher transmissibility, and the efficacy of vaccines was lower against it (9). We needed to carry out whole genome reads to understand the genome changes better and fight those new variants. Our results demonstrated twice higher missense mutations than synonymous ones.
Spike protein
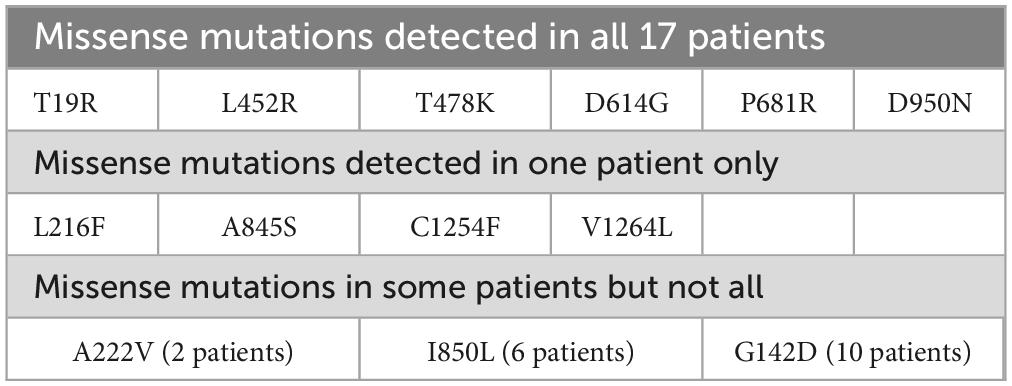
The high pathogenic effects of coronavirus during the pandemic were linked to many features. The affinity receptor-binding domain (RBD) of spike protein to human angiotensin-converting enzyme 2 (ACE2) was one of the most cited points contributing to the deadly effects of the virus (23). Mutations in the RBD in various virus strains determined its impact on ACE2, leading to reduced or enhanced binding affinity (24). In this work, we observed six mutations (plus one deletion) in all studied 17 patients (Table 1). Only four changes were determined in one patient. Two mutations were determined in 2 and 6 patients (Table 3).
The changes D614G, L452R, P681R, and T478K were earlier detected in other works (25, 26). Among these changes, the ones causing the changes in electrostatic interactions on the surface can be considered the most significant. Surface charge is regarded as one of the points that could contribute to spike protein binding efficacy based on electrostatic interaction that might facilitate conformational changes (27, 28). Among the patients studied in this work, we observed several changes that did not affect the affinity of the RBD of the spike protein to ACE2. Most of the changes either belonged to sites other than RBD or were not included in RBD-ACE2 interactions. Three of six mutations detected in all patients in this work were changes to either Arg or Lys. The significant change belonged to pLeu452Arg, where the Leu at 452 position changed to Arg. Aggarwal et al. (29) established enhanced binding affinity resulting from the pLeu452Arg mutation (29). This mutation was earlier found in Indian patients (18, 28). The location of Leu at the 452 position is described in Figure 4, together with an indication of spike protein RBD and ACE2.
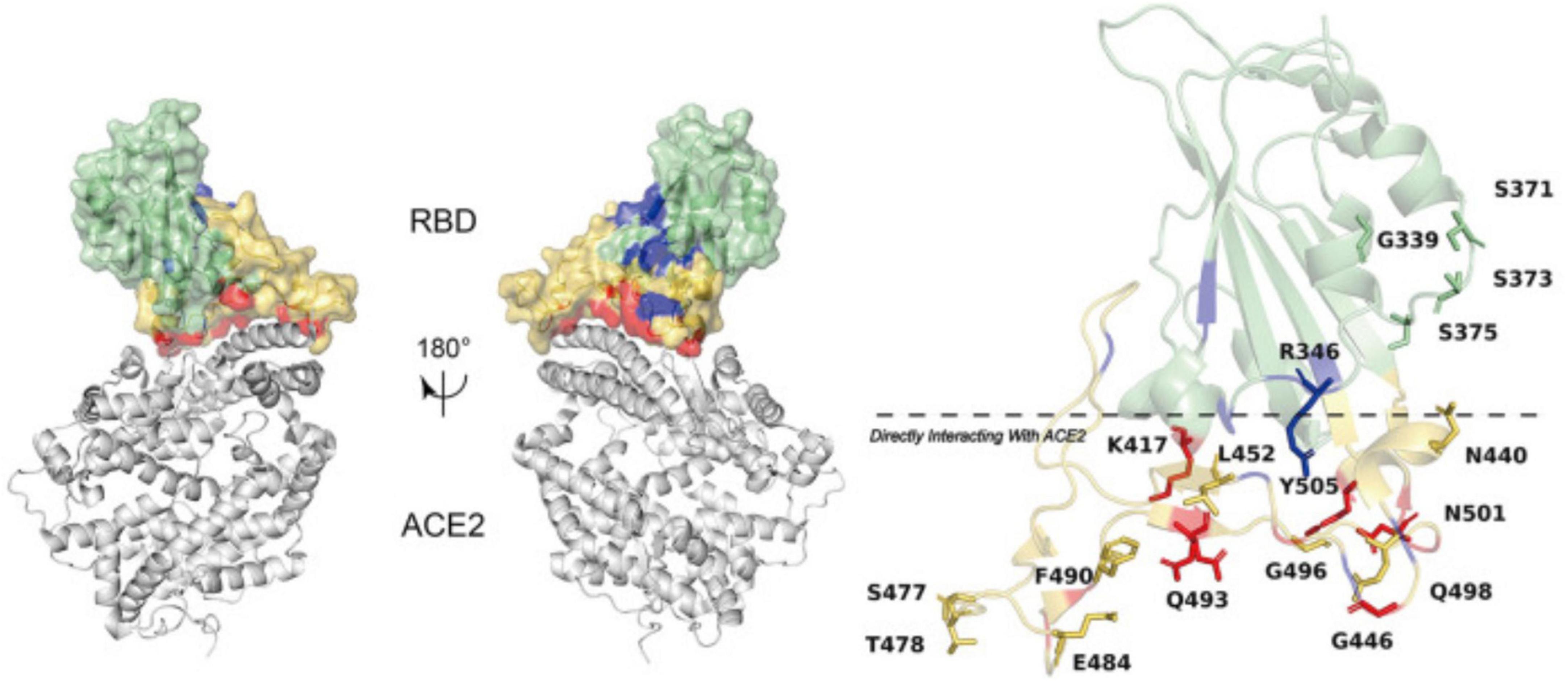
Figure 4. Amino acid mutations established in spike protein RBD and the RBD affinity with ACE2. The figure has been re-used from (28) with the permission of Frontiers.
Another significant amino acid change was T478K, the one observed in all patients, leading to the substitution of hydrophobic threonine (T) to charged lysine (K), which was established to enhance spike protein surface electrostatic potential that might affect spike RBD affinity with human ACE-2 (30).
Studying the consequences of patients’ health conditions if infected with various mutated/unmutated SARS-CoV-2 would result in a deeper understanding of the outcomes of multiple mutations. However, we did not study any clinical consequences due to anonymized samples ruled out by the ethical committee. In such cases, using computer software is another reliable approach (31). We will use computational analysis, such as molecular docking or molecular dynamics simulations, to predict the consequences. Because of the large number of mutations in this work, we planned to conduct computational calculations as separate research for several mutations. For now, our study is limited to the outcomes and discussions obtained.
Conclusion
This study’s comprehensive whole-genome sequence data facilitates tracing the origins and sources of circulating SARS-CoV-2 variants and the identification and comparative analysis of emerging variations within Uzbekistan and beyond. The genome sequencing of SARS-CoV-2, derived from specimens collected from infected individuals in Uzbekistan towards the conclusion of 2021, has been outlined in this study. This timeframe corresponds to the second wave of the coronavirus disease pandemic, which had attained nationwide dissemination. After acquiring these genomic sequences, efforts have been directed toward developing DNA-based and plant-based edible vaccines utilizing the identified SARS-CoV-2 strains prevalent in Uzbekistan. Presently, these vaccine candidates are undergoing clinical trial evaluations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Dr. Kamal Rizaev, Chairman of the Ethics Committee of the Ministry of Health of the Republic of Uzbekistan; Mrs. Larisa Alieva, Private Clinic of BiogenMed. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because In this study, verbal consent to participate in sample collection was preferred over written consent since patients were hesitant to sign any written document due to concerns about COVID-19 infection in their illness condition as well as a lack of knowledge of the genome sequencing investigation. Participants were explained that anonymized data, including age and biological sex, would be preserved for scientific reporting purposes. We informed all participants that the collected samples would be used for a “research purpose DNA sequencing project” exclusively, without disclosing their identities, following a scientific article publication without participants’ IDs. All patients in the sample collection provided verbal consent for voluntary participation. Moreover, in the internal government regulations, there was no demand to collect informed written consent from participants to publish results in a scientific article due to using samples taken for only a DNA sequencing experiment, which was non-invasive, and not related to any further downstream clinical procedures. We assure you that the samples were renumbered and de-identified so that no one, not even researchers knew the participant’s identity.
Author contributions
MA: Conceptualization, Data curation, Funding acquisition, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Methodology, Visualization. AY: Methodology, Visualization. AA: Methodology, Visualization, Writing – original draft, Writing – review & editing. BN: Methodology. DU: Methodology. SS: Methodology. KU: Methodology. AA: Methodology. ZB: Conceptualization, Data curation, Funding acquisition, Methodology, Visualization. IA: Supervision, Writing – original draft, Writing – review & editing.
Funding
The research was funded with the project “M-2021-2 – Production of vaccines using plant cells based on DNA and gene- knockout vectors”. Grant holder – ZB.
Acknowledgments
We thank the private clinics of BiogenMed, Tashkent, and Uzbekistan, including Larisa E. Alieva, for supporting our collection of samples from symptomatic patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1401655/full#supplementary-material
Footnotes
References
1. World Health Organization. hCoV-19 Data Sharing Via GISAID (2023). Available online at: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed July 20, 2023).
2. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. (2019) 17(3):181–92.
3. Tulimilli SV, Dallavalasa S, Basavaraju CG, Kumar Rao V, Chikkahonnaiah P, Madhunapantula SV, et al. Variants of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) and vaccine effectiveness. Vaccines. (2022) 10(10):1751.
4. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. (2015) 1282:1–23.
5. Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang WA. pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579(7798):270–3.
6. Yavarian J, Nejati A, Salimi V, Shafiei Jandaghi NZ, Sadeghi K, Abedi A. Whole genome sequencing of SARS-CoV2 strains circulating in Iran during five waves of pandemic. PLoS One. (2022) 17(5):e0267847. doi: 10.1371/journal.pone.0267847
7. Ayubov MS, Buriev ZT, Mirzakhmedov MK, Yusupov AN, Usmanov DE, Shermatov SE. Profiling of the most reliable mutations from sequenced SARS-CoV-2 genomes scattered in Uzbekistan. PLoS One. (2022) 17(3):e0266417. doi: 10.1371/journal.pone.0266417
8. Zhan Y, Yin H, Yin JYB. 1.617.2 (Delta) variant of SARS-CoV-2: features, transmission and potential strategies. Int J Biol Sci. (2022) 18(5):1844–51. doi: 10.7150/ijbs.66881
9. Dhawan M, Sharma A, Priyanka Thakur N, Rajkhowa TK, Choudhary OP. Delta variant (B.1.617.2) of SARS-CoV-2: mutations, impact, challenges and possible solutions. Hum Vaccines Immunother. (2022) 18(5):2068883. doi: 10.1080/21645515.2022.2068883
10. Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EMSARS-. CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. (2021) 19(7):409–24.
11. Abdullaev A, Abdurakhimov A, Mirakbarova Z, Ibragimova S, Tsoy V, Nuriddinov S. Genome sequence diversity of SARS-CoV-2 obtained from clinical samples in Uzbekistan. PLoS One. (2022) 17(6):e0270314. doi: 10.1371/journal.pone.0270314
12. Buriev ZT, Shermatov SE, Usmanov DE, Mirzakhmedov MK, Ubaydullaeva KA, Kamburova VS. Tomato-made edible COVID-19 vaccine TOMAVAC induces neutralizing IgGs in the blood sera of mice and humans. Front Nutrition. (2023) 10:1275307. doi: 10.3389/fnut.2023.1275307
13. Chen Z, Azman AS, Chen X, Zou J, Tian Y, Sun R. Global landscape of SARS-CoV-2 genomic surveillance and data sharing. Nat Genet. (2022) 54(4):499–507.
14. Chun JY, Jeong H, Kim Y. Age-varying susceptibility to the delta variant (B.1.617.2) of SARS-CoV-2. JAMA Netw Open. (2022) 5(3):e223064. doi: 10.1001/jamanetworkopen.2022.3064
15. Matyášek R, Kovařík A. Mutation patterns of human SARS-CoV-2 and bat RaTG13 coronavirus genomes are strongly biased towards C >U transitions, indicating rapid evolution in their hosts. Genes. (2020) 11(7):761. doi: 10.3390/genes11070761
16. Di Giorgio S, Martignano F, Torcia MG, Mattiuz G, Conticello SG. Evidence for host-dependent RNA editing in the transcriptome of SARS-CoV-2. Sci Adv. (2020) 6(25):eabb5813.
17. Ratcliff J, Simmonds P. Potential APOBEC-mediated RNA editing of the genomes of SARS-CoV-2 and other coronaviruses and its impact on their longer term evolution. Virology. (2021) 556:62–72. doi: 10.1016/j.virol.2020.12.018
18. Cherian S, Potdar V, Jadhav S, Yadav P, Gupta N, Das MSARS-. CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms. (2021) 9(7):1542. doi: 10.3390/microorganisms9071542
19. Jamison DA Jr., Anand Narayanan S, Trovão NS, Guarnieri JW, Topper MJ, Moraes-Vieira PMA. comprehensive SARS-CoV-2 and COVID-19 review, Part 1: Intracellular overdrive for SARS-CoV-2 infection. Eur J Hum Genet. (2022) 30(8):889–98. doi: 10.1038/s41431-022-01108-8
20. Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral Res. (2018) 149:58–74. doi: 10.1016/j.antiviral.2017.11.001
21. Gao X, Qin B, Chen P, Zhu K, Hou P, Wojdyla JA, et al. Crystal structure of SARS-CoV-2 papain-like protease. Acta Pharmaceutica Sin B. (2021) 11(1):237–45.
22. Al Adem K, Ferreira JC, Fadl S, Rabeh WM. pH profiles of 3-chymotrypsin-like protease (3CLpro) from SARS-CoV-2 elucidate its catalytic mechanism and a histidine residue critical for activity. J Biol Chem. (2023) 299(2):102790. doi: 10.1016/j.jbc.2022.102790
23. Lu J, Sun PD. High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity. J Biol Chem. (2020) 295(52):18579–88.
24. Borkotoky S, Dey D, Hazarika Z. Interactions of angiotensin-converting enzyme-2 (ACE2) and SARS-CoV-2 spike receptor-binding domain (RBD): a structural perspective. Mol Biol Rep. (2023) 50(3):2713–21.
25. Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the delta variant B.1.617.2 COVID-19. Clin Pract. (2021) 11(4):778–84.
26. Gobeil SM, Janowska K, McDowell S, Mansouri K, Parks R, Stalls V. Effect of natural mutations of SARS-CoV-2 on spike structure, conformation, and antigenicity. Science. (2021) 373(6555):eabi6226.
27. Areo O, Joshi PU, Obrenovich M, Tayahi M, Heldt CL. Single-particle characterization of SARS-CoV-2 isoelectric point and comparison to variants of interest. Microorganisms. (2021) 9(8):1606. doi: 10.3390/microorganisms9081606
28. Zhang Z, Zhang J, Wang J. Surface charge changes in spike RBD mutations of SARS-CoV-2 and its variant strains alter the virus evasiveness via HSPGs: a review and mechanistic hypothesis. Front Public Health. (2022) 10:952916. doi: 10.3389/fpubh.2022.952916
29. Aggarwal A, Naskar S, Maroli N, Gorai B, Dixit NM, Maiti PK. Mechanistic insights into the effects of key mutations on SARS-CoV-2 RBD-ACE2 binding. Phys Chem Chem Phys. (2021) 23(46):26451–8. doi: 10.1039/d1cp04005g
30. Di Giacomo S, Mercatelli D, Rakhimov A, Giorgi FM. Preliminary report on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Spike mutation T478K. J Med Virol. (2021) 93(9):5638–43. doi: 10.1002/jmv.27062
Keywords: SARS-CoV-2, virus, transmission, AA mutations, delta strain
Citation: Ayubov MS, Mirzakhmedov MK, Yusupov AN, Asrorov AM, Nosirov BV, Usmanov DE, Shermatov SE, Ubaydullaeva KA, Abdukarimov A, Buriev ZT and Abdurakhmonov IY (2024) Most accurate mutations in SARS-CoV-2 genomes identified in Uzbek patients show novel amino acid changes. Front. Med. 11:1401655. doi: 10.3389/fmed.2024.1401655
Received: 15 March 2024; Accepted: 14 May 2024;
Published: 31 May 2024.
Edited by:
Pragya Dhruv Yadav, ICMR-National Institute of Virology, IndiaReviewed by:
Md Maruf Ahmed Molla, Upstate Medical University, United StatesAles Kovarik, Academy of Sciences of the Czech Republic (ASCR), Czechia
Copyright © 2024 Ayubov, Mirzakhmedov, Yusupov, Asrorov, Nosirov, Usmanov, Shermatov, Ubaydullaeva, Abdukarimov, Buriev and Abdurakhmonov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirzakamol S. Ayubov, mirzo.ayubov@gmail.com; Ibrokhim Y. Abdurakhmonov, ibrokhimabdurakhmonov@hotmail.com
 Mirzakamol S. Ayubov
Mirzakamol S. Ayubov Mukhammadjon K. Mirzakhmedov
Mukhammadjon K. Mirzakhmedov Abdurakhmon N. Yusupov
Abdurakhmon N. Yusupov Akmal M. Asrorov
Akmal M. Asrorov Bakhtiyor V. Nosirov3
Bakhtiyor V. Nosirov3  Shukhrat E. Shermatov
Shukhrat E. Shermatov Ibrokhim Y. Abdurakhmonov
Ibrokhim Y. Abdurakhmonov