Clinical characteristics and the risk factors for the exacerbation of symptoms in patients with inflammatory bowel disease during the COVID-19 pandemic
- Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Background: In 2023, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant caused a large-scale outbreak of coronavirus disease 2019 (COVID-19) in China. It is not clear the risk factors that lead to the exacerbation of symptoms in patients with inflammatory bowel disease (IBD) after COVID-19 infection. Our study aims to find out the risk factors for the exacerbation of IBD-related symptoms in IBD patients with COVID-19 infection and to provide guidance for the clinical management of IBD.
Methods: This is a retrospective, observational study. The online questionnaire was distributed to conduct a survey to collect demographic, clinical, and IBD related characteristics in IBD patients. Univariate and multivariate regression analyses were conducted to assess the independent effects.
Results: In total, 534 cases of IBD patients were analyzed in our study. Among them, 466 (87.3%) cases diagnosed with COVID-19, 160 (34.3%) cases experienced exacerbation of IBD symptoms, and 84 (18.0%) patients opted for medication discontinuation. Male sex (OR 2.04, 95% CI 1.34–3.49, p = 0.001), and the decrease in body mass index (BMI) (OR 0.93, 95% CI 0.87–1.00, p = 0.035) were positively correlated with the exacerbation of IBD symptoms. Furthermore, the medication discontinuation (OR 2.60, 95% CI 1.58–4.30, p < 0.001) was strongly positively correlated with the exacerbation of IBD symptoms. No significant association was seen between age, comorbidities, smoking, disease activity, vaccination, therapy for COVID-19 and the worsening of IBD symptoms.
Conclusion: This study confirms that the infection rate of COVID-19 in China IBD patients was comparable to the general population. Male sex, the decrease in BMI and medication discontinuation are significant risk factors for the exacerbation of IBD-related symptoms in IBD patients with COVID-19 infection.
1 Introduction
Since the outbreak of coronavirus disease 2019 (COVID-19), the global spread of this pandemic has been swift and far-reaching (1, 2). On 11 March 2020, the World Health Organization officially declared the outbreak a pandemic, marking a significant turning point. With the relaxation of the COVID-19 pandemic in China, the country has experienced a large-scale outbreak of the virus in the past year. Although the majority of COVID-19 cases present with mild symptoms, a small proportion of patients may progress to severe pneumonia, necessitating hospitalization and potentially leading to respiratory failure or fatality (3, 4). Existing clinical evidence showed that people of all age groups were susceptible to contracting COVID-19, however elderly adults and people with underlying diseases were more likely to develop severe pneumonia (5). The individuals who have contracted COVID-19 do not possess durable immunity and are susceptible to recurrent infections (6). Thus, the battle against COVID-19 is a protracted one.
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is an immune-mediated inflammatory gastrointestinal chronic disease with increasing prevalence in China (7). Patients with IBD require more elaborate management (7, 8). Most IBD patients are treated with immunomodulators, which can increase the risk of infection. With the spread of the COVID-19 pandemic, there is growing concern about the impact of COVID-19 on patients with IBD. Due to the unique nature of IBD treatment and individualized approach to therapy, managing patients with IBD poses significant challenges during this pandemic (9). Current evidence suggests that there is no difference in COVID-19 incidence and primary clinical outcomes (i.e., hospitalization rate, need for respiratory assistance, and death) between patients with IBD and the general population, but the risk factors that affect the exacerbation of IBD symptoms after COVID-19 infection are unknown (10, 11). The aim of this study is to investigate the impact of COVID-19 on symptoms and medication use in patients with IBD, and to analyze the potential risk factors for the exacerbation of symptoms in IBD patients with COVID-19 infection.
2 Methods
2.1 Study design
This observational and cross-sectional study was conducted at the Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University (Hefei, China), from January 4, 2023 to April 17, 2023.
The self-reported questionnaire survey was conducted via Wenjuanxing, which is an online survey platform. Questionnaires were distributed to patients attending the First Affiliated Hospital of Anhui Medical University from January 2023 to March 2023. As the most representative IBD center in Anhui Province, we had a sufficient number of patients. Most of the patients (97.2%) had a long history of residence in Anhui province. The participants were requested to document whether they experienced COVID-19 infection, medication discontinuation, and the subjective exacerbation of IBD symptoms subsequent to contracting COVID-19. Medication discontinuation was defined as no medication up to 2 consecutive weeks in all patients except those treated with infliximab (IFX) and other biologics. For patients treated with IFX and other biologics, medication discontinuation refers to delayed medication for more than 1 week in the induction period and more than 2 weeks in the maintenance period (12). Clinical characteristics and the risk factors for the exacerbation of symptoms in patients with IBD after COVID-19 infection were analyzed.
This study was approved by the Ethics Committee of Anhui Medical University (protocol number: 2023-0134). All patients provided the agreement statement that the participation was voluntary on the first page of the questionnaire.
2.2 Participants
Inflammatory bowel disease was diagnosed based on the Chinese consensus on diagnosis and treatment of inflammatory bowel disease (Beijing, 2018). COVID-19 diagnosis was based on viral tests (by nucleic acid or rapid antigen tests, irrespective of symptoms). The exclusion criteria were as follows: (1) The patients had difficulty in understanding the questionnaire. (2) The patients who did not undergo any nucleic acid or rapid antigen tests for COVID-19. (3) The IBD unclassified (IBDU) patients who defined as those with colon IBD but unable to distinguish UC or CD.
2.3 Data collection
Sociodemographic information of IBD patients mainly included age, gender, weight, height, occupation, place of residence during the pandemic, underlying diseases, and smoking status. IBD-related data included disease duration and disease activity. The COVID-19-related information included COVID-19 diagnosis status, COVID-19 symptoms, medication, and vaccinations. In addition, we also collected the medication regimens for IBD before and after COVID-19 infection and whether medications were discontinued.
2.4 Statistical analysis
We summarized the clinical characteristics of the study population using descriptive statistics, followed by unpaired t tests and Mann–Whitney U tests when appropriate. Univariate and multivariable regression analyses were conducted to assess the independent effects of age, gender, comorbidities, smoking, disease activity, COVID-19 vaccine, COVID-19 treatment, BMI, and medication discontinuation on the exacerbation of symptoms in IBD. Statistical analysis were performed using SPSS-20 software. The goodness of fit of all regression models was evaluated using the Hosmer-Lemeshow test, with a p value>0.05 indicating a good fit. A significance level of p < 0.05 was considered statistically significant. Finally, the results were plotted using GraphPad Prism software.
3 Results
3.1 Study population
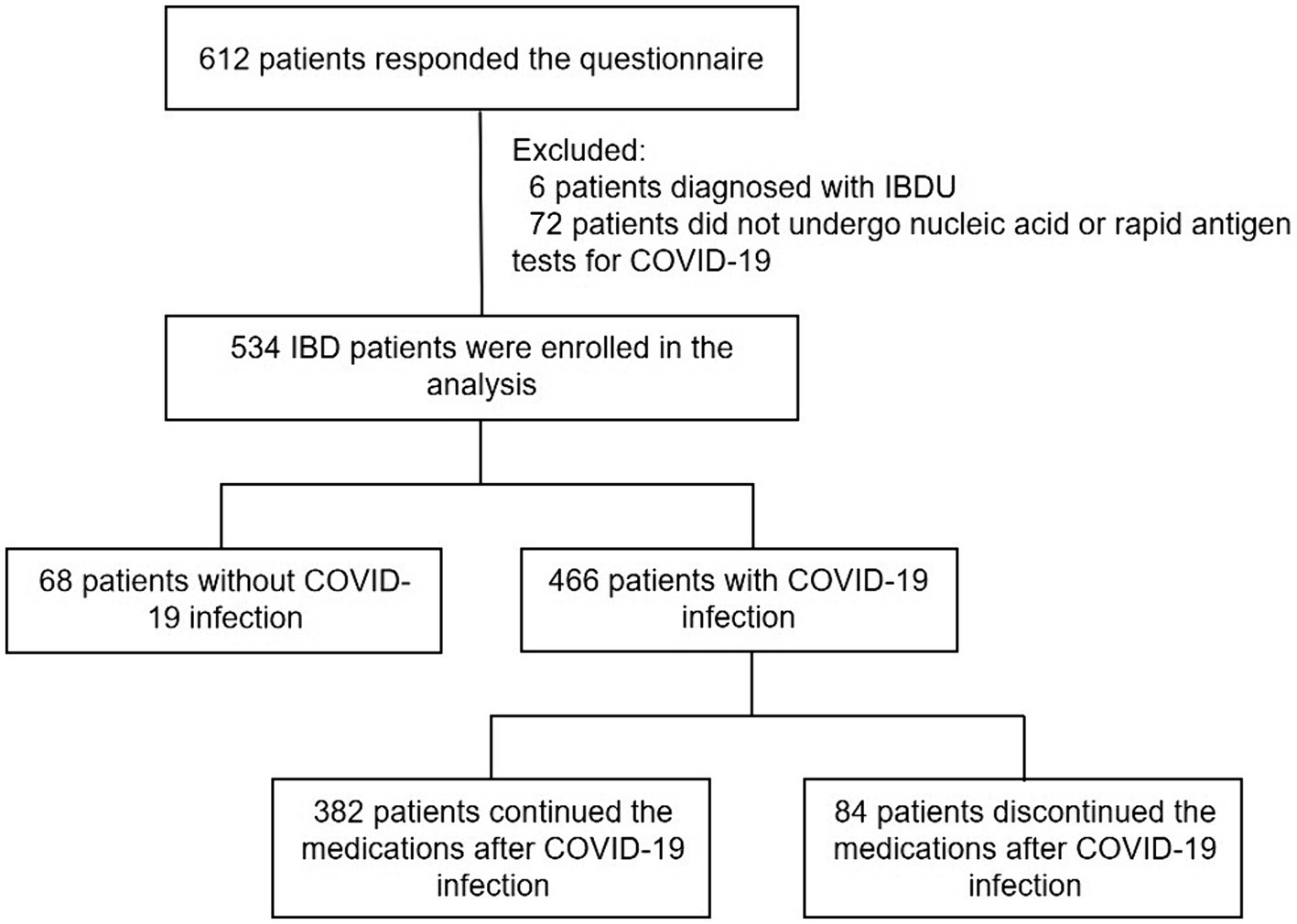
A total of 612 IBD patients participated in the survey. 78 patients were excluded due to diagnosed with IBDU (n = 6) or did not undergo nucleic acid or rapid antigen tests (n = 72). Finally, 534 patients were analyzed, including 466 (87.3%) patients who were diagnosed with COVID-19 infection and 68 (12.7%) patients without COVID-19 infection (Figure 1).
3.2 Characteristics of the IBD patients responded to the questionnaire
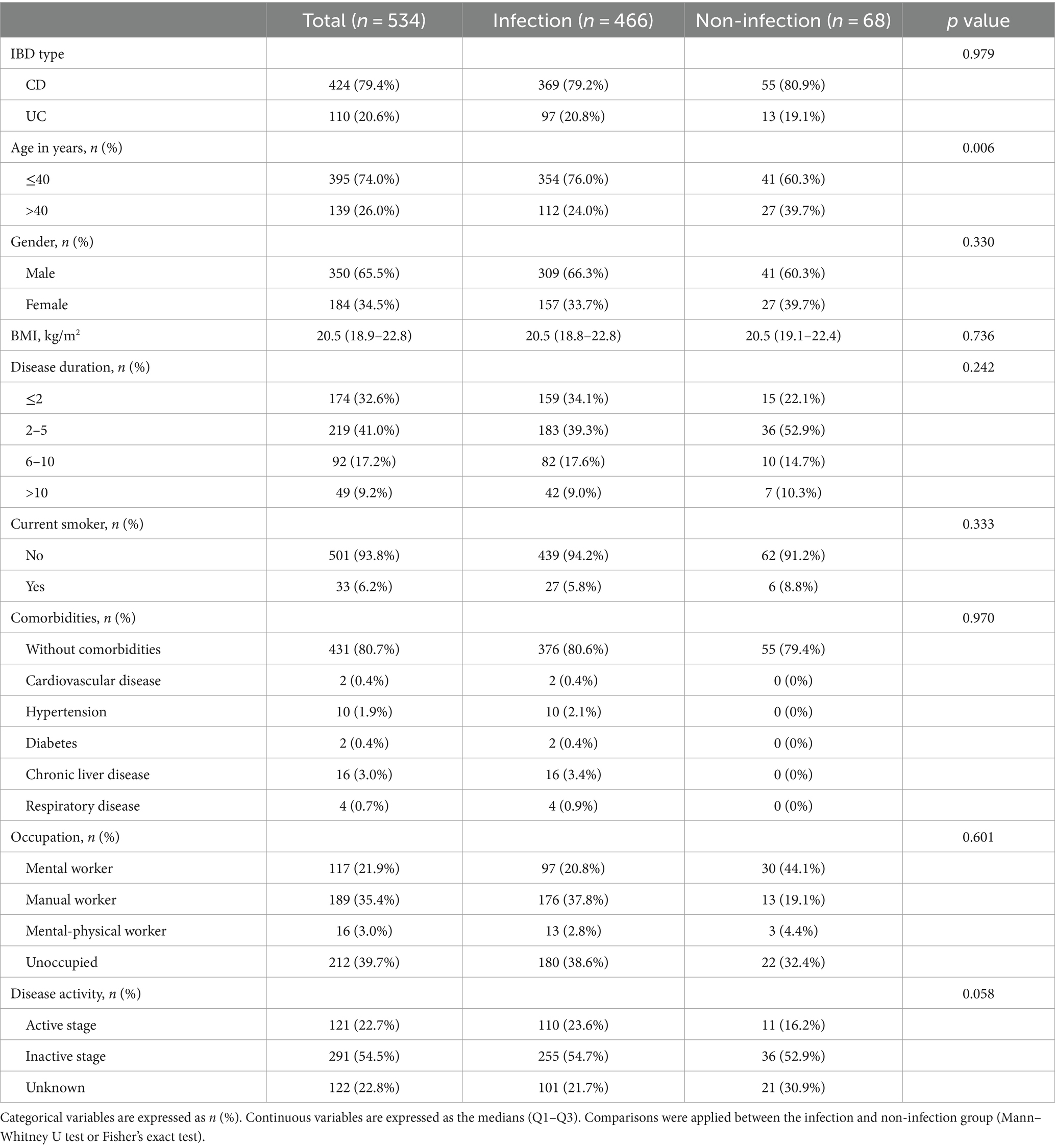
Demographic, clinical and IBD related characteristics were summarized in Table 1. Among them, there were 424 (79.4%) cases of CD, and 110 (20.6%) cases of UC. 350 (65.5%) patients were male. Also, 395 (74.0%) patients were aged≤40 and 139 (26.0%) aged>40. 33 (6.2%) patients were current smokers. The mean BMI of these patients was 20.5 (18.9–22.8).
Among these patients, 431 (80.7%) did not have comorbidity disease, and the most common comorbidity were hypertension (1.9%) and chronic liver disease (3.0%). Of all the patients, 121 (22.7%) were in the active stage of IBD, 291 (54.5%) were in remission, and 122 (22.8%) were uncertain.
Comparative analysis between the infection and non-infection groups showed no difference in IBD type, gender, BMI, disease duration, current smoker, comorbidities, occupation, or disease activity. There was a significant difference in age (p = 0.006) between infected and non-infected individuals.
3.3 Characteristics of IBD patients with COVID-19 infections
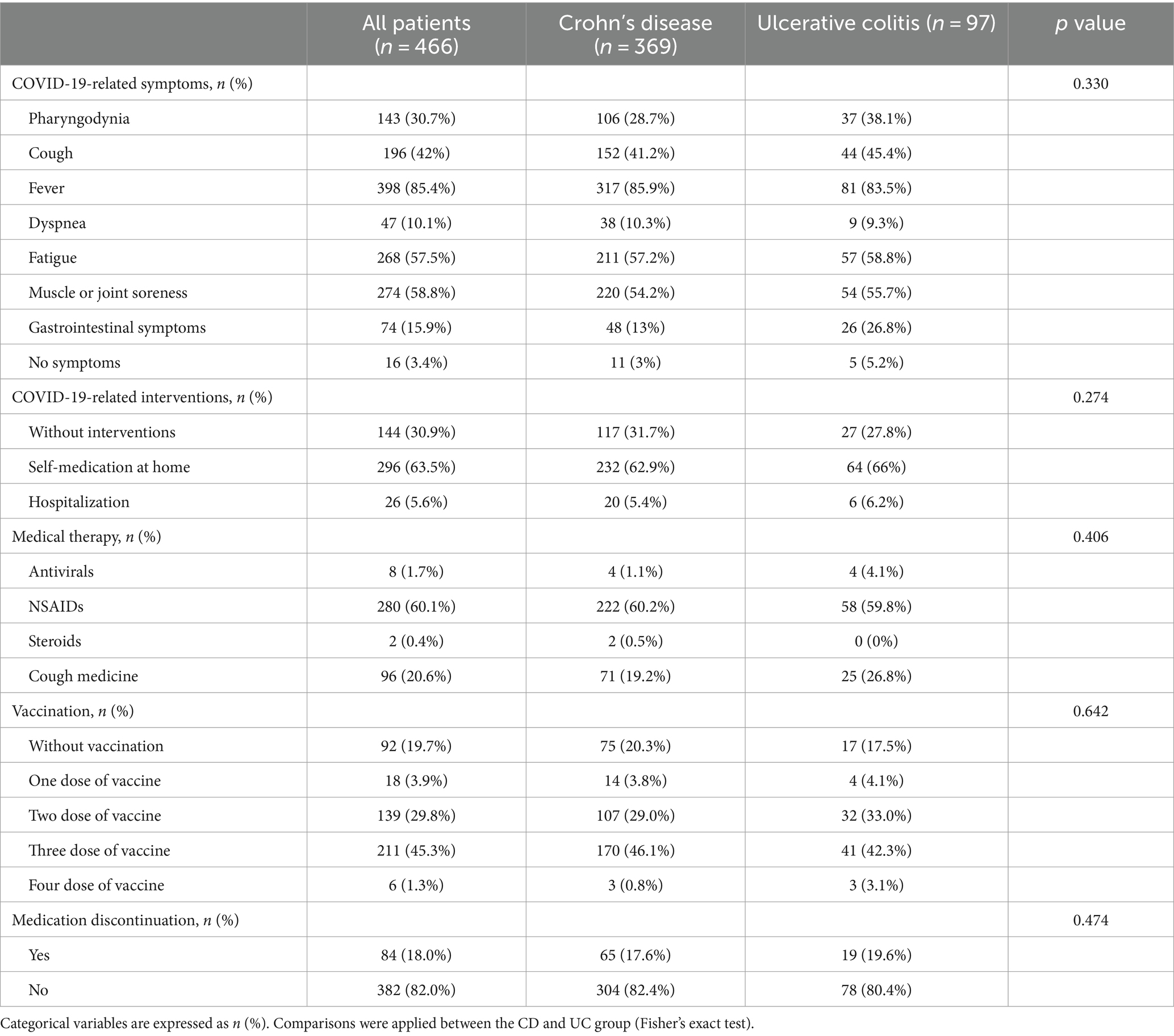
The characteristics of patients with COVID-19 infections were summarized in Table 2. A total of 466 patients were infected with COVID-19, including 369 (79.2%) cases of CD and 97 (20.8%) cases of UC. Most patients had multiple symptoms, with fever (85.4%), muscle or joint soreness (58.8%), and fatigue (57.5%) being the most common. However, gastrointestinal symptoms were less common, including diarrhea (15.1%), abdominal pain (4.46%), and hematochezia (0.2%). After being infected with COVID-19, 144 (30.9%) patients did not take any intervention measures, 296 (63.5%) cases chose to self-medicate at home, and 26 (5.6%) cases opted for hospitalization.
Among the medications used in the treatment of COVID-19, 280 (60.1%) patients took non-steroidal anti-inflammatory drugs (NSAIDs), 96 (20.6%) patients took cough medicine, 8 (1.7%) patients took antivirals, and 2 (0.4%) patients took steroids. Regarding vaccinations, 92 (19.7%) patients did not receive any vaccine, 18 (3.8%) patients received one dose, 139 (29.8%) patients received two doses, 211 (45.2%) patients received three doses, and 6 (1.2%) patients received four doses.
Among the 466 patients infected with COVID-19, 84 (18.0%) individuals discontinued their medication treatment. We further inquired the reasons for medication discontinuation. Among them, 44 (52.4%) patients concerned about drug adverse reactions, which was the primary reason for medication discontinuation. 31 (36.9%) patients were due to transportation inconvenience caused by the COVID-19 pandemic, 20 (23.8%) were limited by inpatient bed shortage, and some other reasons.
There were 306 (65.7%) patients reported no IBD-related symptom exacerbation and 160 (34.3%) patients reported IBD-related symptom exacerbation. 73 (45.6%) patients had IBD-related symptom exacerbation within 1 week after infection, and 37 (23.1%) patients had IBD-related symptom exacerbation within 2 weeks. We found that IBD patients with COVID-19 infection had a greater probability of IBD-related symptom exacerbation within the first 2 weeks.
After COVID-19 infection, 33 patients changed their treatment plans, while 433 patients did not change the treatment plans. In the subset of 33 patients who changed their treatment plans, 23 patients with CD were treated with adalimumab in seven cases, enteral nutrition in nine cases, ustekinumab in four cases, glucocorticoid in two cases, and infliximab in one case; among the 10 patients with UC, seven cases were treated with glucocorticoids, and three cases were treated with traditional Chinese medicine.
3.4 Factors related to the exacerbation of IBD-related symptoms
In this study, we performed univariate and subsequent multivariate regression analyses to explore factors that were more likely to be related to the exacerbation of IBD-related symptoms (Table 3; Figure 2). The univariate analysis showed that male sex (OR 2.22, 95% CI 1.49–3.31, p < 0.001), age > 40 (OR 1.70, 95% CI 1.10–2.63, p = 0.017), comorbidities (OR 1.81, 95% CI 1.13–2.89, p = 0.013), BMI (OR 0.92, 95% CI 0.86–0.98, p = 0.013), disease activity (OR 1.63, 95% CI 1.08–2.47, p = 0.019), and medication discontinuation (OR 2.85, 95% CI 1.76–4.61, p < 0.001) were significantly associated with the exacerbation of IBD-related symptoms. However, in a subsequent multivariate analysis, male sex (OR 2.04, 95% CI 1.34–3.49, p = 0.001) and the decrease in BMI (OR 0.93, 95% CI 0.87–1.00, p = 0.035) were positively correlated with the exacerbation of IBD symptoms. Furthermore, medication discontinuation (OR 2.60, 95% CI 1.58–4.30, p < 0.001) was strongly positively correlated with the exacerbation of IBD-related symptoms.
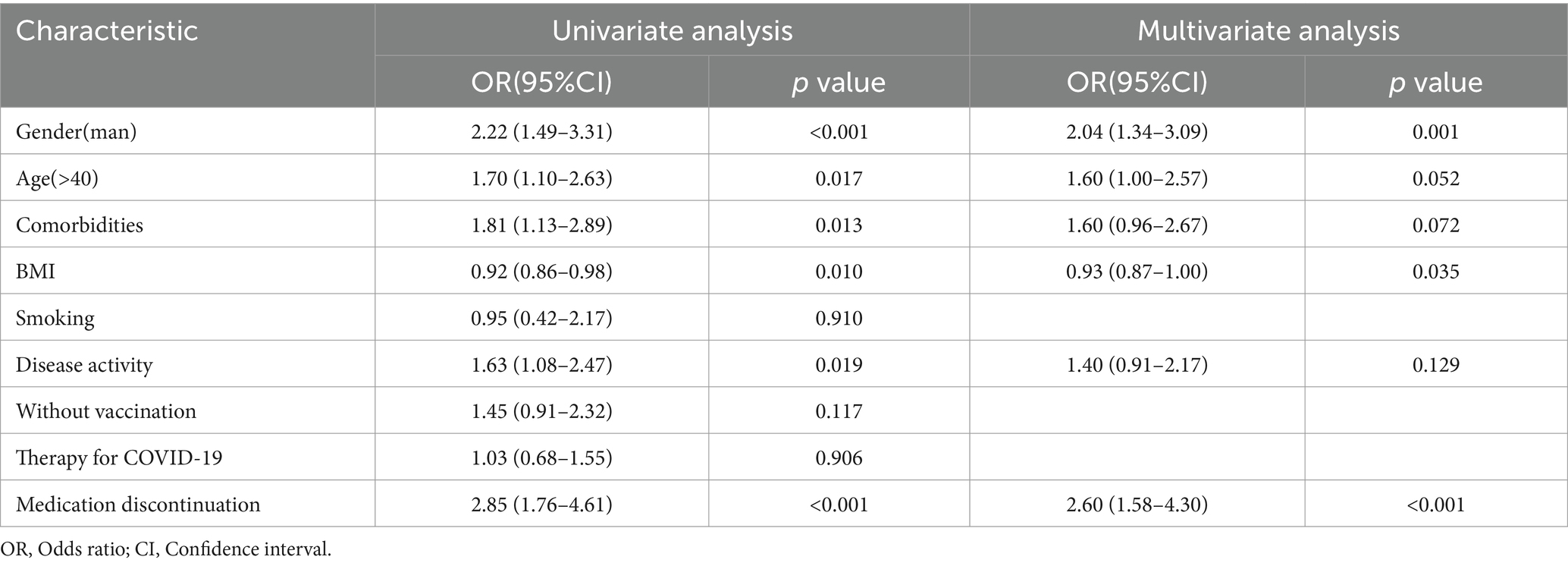
Table 3. Univariate and multivariate regression analysis of risk factors for exacerbating IBD-related symptoms.
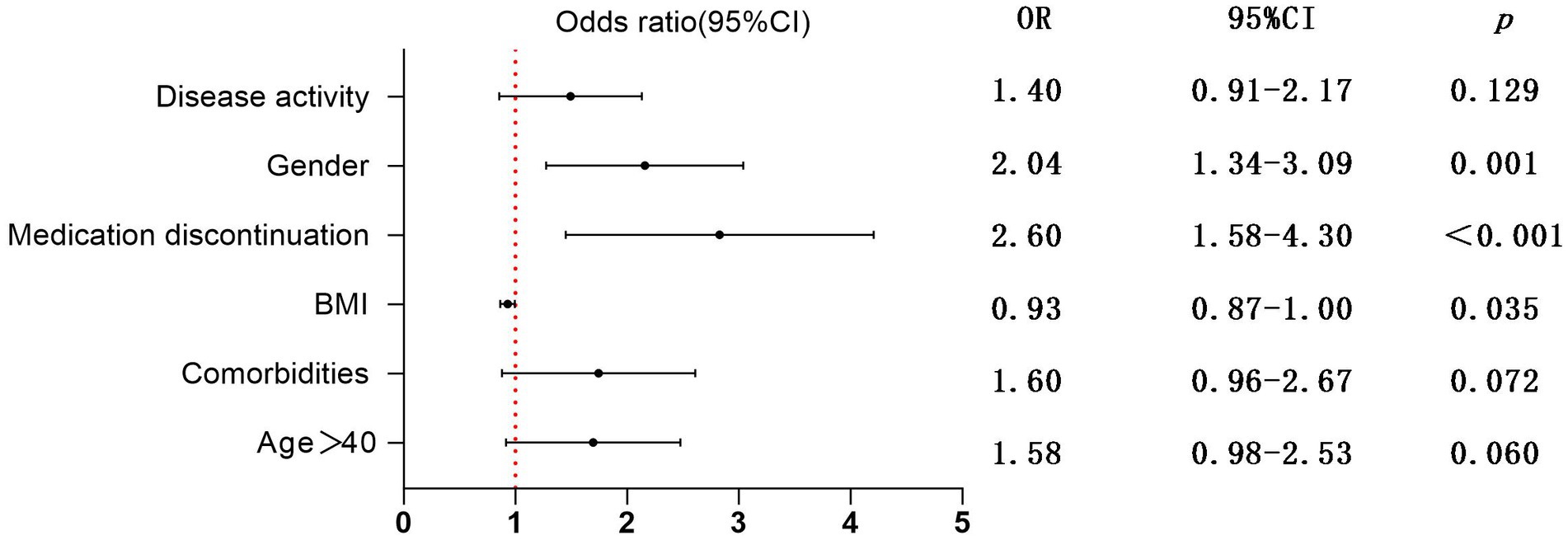
Figure 2. Multivariate regression analysis of risk factors for exacerbating IBD-related symptoms; OR, Odds ratio; CI, Confidence interval.
4 Discussion
The COVID-19 pandemic poses the most challenging issue in the era of IBD biology, which has caused a lot of concern and attention among clinical physicians (9, 13). In our study, the infection rate of COVID-19 in IBD patients was 87.3%, comparable to the infection rate among the general population during the same period (13). The data suggests that individuals with IBD are not more susceptible to COVID-19 infection, which is consistent with previous literature reports (14).
We found that patients aged≤40 have significantly higher COVID-19 infection rates than patients aged>40. Comparative analysis between the infection and non-infection groups showed no difference in age in a recent study (11). We consider that young patients had more contact with people for reasons such as work and study, which increased the infection rate of COVID-19. However, elderly patients mostly stayed at home during the COVID-19 pandemic.
Several studies have reported that COVID-19 primarily presented with respiratory symptoms, but can also involve the gastrointestinal tract in some cases (11, 15, 16). However, in our study, gastrointestinal symptoms did not have a higher incidence rate, indicating that the intestines were not the primary target of the SARS-CoV-2 virus. Therefore, patients with IBD do not need to be overly concerned about contracting COVID-19.
It was reported that NSAIDs use had been associated with an increased risk of clinical relapse in IBD patients. However, short-term use of NSAIDs appears to be safe, and the data available suggest that selective COX-2 inhibitors are the safer option. NSAIDs should be avoided as long-term treatment or with high doses, especially in patients with active inflammation (17). In our study, patients with COVID-19 infection usually took NSAIDs in small doses for short periods of time. Meanwhile, we did not find the significant association between therapy for COVID-19 and the exacerbation of IBD symptoms in univariate regression analysis (p = 0.906).
The vaccination rate was 80.3% in our patients. Some patients remained skeptical about COVID-19 vaccination. The most common concerns given by patients were the vaccine safety and efficacy (18). Several studies revealed that the COVID-19 vaccine was safe and effective in IBD patients (19–21). Garrido et al. found a high acceptance rate and a good safety profile of SARS-CoV-2 vaccination in IBD patients treated with biologics. Indeed, adverse events were common but overall mild and transitory. These data supported the prioritization and rapid vaccination of these individuals (22). A recent study found that the three vaccination doses were associated with reduced gastrointestinal (GI) symptoms after infection compared with the unvaccinated group (23). There was room for targeted education to improve COVID-19 vaccine uptake in patients with IBD.
Fluctuation of IBD-related symptoms was a concern for most patients and physicians during the infection. Hu et al. (11) reported that over 40% of patients experienced fluctuations in CD-related symptoms after COVID-19 infection, including common GI symptoms and CD-specific manifestations. In our study, 160 (34.3%) patients reported IBD-related symptom exacerbation. On multivariate regression analysis, we found that male sex and low BMI were risk factors for the exacerbation of symptoms in patients with IBD after contracting COVID-19. On the contrary, age, comorbidities, disease activity, vaccination, and therapy for COVID-19 were not significantly related. We also found 84 patients chose to discontinue medication therapy, and multivariate regression analysis showed a strong positive correlation between medication discontinuation and symptom exacerbation. Sahyoun et al. demonstrated that patient factors, including age, sex, race, obesity, and current use of advanced IBD therapies, in the setting of COVID-19 infection were not predictors of post-infectious IBD fares. They observed that the management of COVID-19 had an impact on the temporary interruption of therapy for IBD, but without impact on the exacerbation of IBD symptoms (24).
We further analyzed and revealed that the main reasons for patients discontinuing medication were concerns about drug adverse reactions and transportation inconvenience caused by the COVID-19 pandemic. Most patients experienced symptom exacerbation within 2 weeks after COVID-19 infection, which suggested to clinicians that even if infected with COVID-19, IBD treatment should not be stopped. Furthermore, if there is a delay in medication for some reason, it is also advisable to resume treatment within 2 weeks if possible.
It appears that biologic therapy is not associated with severe COVID-19, providing reassurance that patients can continue biologic therapy (25, 26). Zhang et al. reported that anti-TNF monotherapy, IL12/23 antagonists, and tofacitinib are associated with reduced odds of hospitalization, possibly through abrogation of the cytokine storm induced with severe COVID-19 infection (27). In our study, the majority of the patients did not change their treatment plans due to COVID-19 infection. In the subset of 33 patients who altered their treatment plans, there was an observed increase in the utilization of adalimumab and enteral nutrition among CD patients, as well as an increase in glucocorticoid usage among UC patients. However, due to the small sample size, there may be a significant random element.
Our study has some limitations. Firstly, the study population primarily consisted of individuals from Anhui Province, resulting in a limited sample size. Secondly, the assessment of symptom exacerbation in inflammatory bowel disease heavily relies on patients’ subjective judgment, introducing a certain degree of error. Furthermore, future research should aim to include a more diverse and larger sample size to enhance the robustness of the results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JW: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft. YF: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft. BB: Data curation, Writing – original draft. YW: Data curation, Writing – original draft. QL: Data curation, Writing – original draft. JH: Data curation, Visualization, Writing – original draft. NH: Conceptualization, Methodology, Supervision, Writing – review & editing. QM: Methodology, Project administration, Supervision, Writing – review & editing. WH: Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the 2022 Provincial Quality Project of higher education in Anhui Province (2022jyxm762).
Acknowledgments
We acknowledge the friendly contribution of Li Ang on the statistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhu, N, Zhang, D, Wang, W, Li, X, Yang, B, Song, J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Tripathi, K, Godoy Brewer, G, Thu Nguyen, M, Singh, Y, Saleh Ismail, M, Sauk, JS, et al. COVID-19 and outcomes in patients with inflammatory bowel disease: systematic review and Meta-analysis. Inflamm Bowel Dis. (2022) 28:1265–79. doi: 10.1093/ibd/izab236
3. Onder, G, Rezza, G, and Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
4. Wu, Z, and McGoogan, JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
5. Li, R, Pei, S, Chen, B, Song, Y, Zhang, T, Yang, W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. (2020) 368:489–93. doi: 10.1126/science.abb3221
6. Bhattacharya, D. Instructing durable humoral immunity for COVID-19 and other vaccinable diseases. Immunity. (2022) 55:945–64. doi: 10.1016/j.immuni.2022.05.004
7. Ma, T, Wan, M, Liu, G, Zuo, X, Yang, X, and Yang, X. Temporal trends of inflammatory bowel disease burden in China from 1990 to 2030 with comparisons to Japan, South Korea, the European Union, the United States of America, and the world. Clin Epidemiol. (2023) 15:583–99. doi: 10.2147/CLEP.S402718
8. Rubin, DT, Sninsky, C, Siegmund, B, Sans, M, Hart, A, Bressler, B, et al. International perspectives on Management of Inflammatory Bowel Disease: opinion differences and similarities between patients and physicians from the IBD GAPPS survey. Inflamm Bowel Dis. (2021) 27:1942–53. doi: 10.1093/ibd/izab006
9. Allocca, M, Fiorino, G, Zallot, C, Furfaro, F, Gilardi, D, Radice, S, et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol. (2020) 18:2134–5. doi: 10.1016/j.cgh.2020.04.071
10. Macaluso, FS, Giuliano, A, Fries, W, Viola, A, Abbruzzese, A, Cappello, M, et al. Severe activity of inflammatory bowel disease is a risk factor for severe COVID-19. Inflamm Bowel Dis. (2023) 29:217–21. doi: 10.1093/ibd/izac064
11. Hu, W, Li, X, Yan, Z, Wang, Q, Luo, J, Yu, Q, et al. Impact of the first wave of COVID-19 on Crohn’s disease after the end of “zero-COVID” policy in China. Front Public Health. (2023) 11:1186275. doi: 10.3389/fpubh.2023.1186275
12. Chen, J, Peng, X, Zhang, M, and Zhi, M. Impact of medication discontinuation on patients with inflammatory bowel disease during the COVID-19 outbreak. Gastroenterology. (2021) 160:2223. doi: 10.1053/j.gastro.2020.05.087
13. China Centers for Disease Control and Prevention (2023). The national situation of the COVID-19 infection epidemic. Available at: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202301/t20230125_263519.html
14. Hadi, Y, Dulai, PS, Kupec, J, Mohy-Ud-Din, N, Jairath, V, Farraye, FA, et al. Incidence, outcomes, and impact of COVID-19 on inflammatory bowel disease: propensity matched research network analysis. Aliment Pharmacol Ther. (2022) 55:191–200. doi: 10.1111/apt.16730
15. Kim, GU, Kim, MJ, Ra, SH, Lee, J, Bae, S, Jung, J, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. (2020) 26:948.e1–3. doi: 10.1016/j.cmi.2020.04.040
16. Nalbandian, A, Sehgal, K, Gupta, A, Madhavan, MV, McGroder, C, Stevens, JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
17. Hijos-Mallada, G, Sostres, C, and Gomollón, F. NSAIDs, gastrointestinal toxicity and inflammatory bowel disease. Gastroenterol Hepatol. (2022) 45:215–22. doi: 10.1016/j.gastrohep.2021.06.003
18. Clarke, K, Pelton, M, Stuart, A, Tinsley, A, Dalessio, S, Bernasko, N, et al. COVID-19 vaccine hesitancy in patients with inflammatory bowel disease. Dig Dis Sci. (2022) 67:4671–7. doi: 10.1007/s10620-021-07377-5
19. Feng, J, Yang, T, Yao, R, Feng, B, Hao, R, Qiao, Y, et al. Low vaccination and infection rate of omicron in patients with inflammatory bowel disease: a comparative study of three unique cohorts. Front Public Health. (2023) 11:1115127. doi: 10.3389/fpubh.2023.1115127
20. Pellegrino, R, Pellino, G, Selvaggi, L, Selvaggi, F, Federico, A, Romano, M, et al. BNT162b2 mRNA COVID-19 vaccine is safe in a setting of patients on biologic therapy with inflammatory bowel diseases: a monocentric real-life study. Expert Rev Clin Pharmacol. (2022) 15:1243–52. doi: 10.1080/17512433.2022.2120466
21. Bhurwal, A, Mutneja, H, Bansal, V, Goel, A, Arora, S, Attar, B, et al. Effectiveness and safety of SARS-CoV-2 vaccine in inflammatory bowel disease patients: a systematic review, meta-analysis and meta-regression. Aliment Pharmacol Ther. (2022) 55:1244–64. doi: 10.1111/apt.16913
22. Garrido, I, Lopes, S, and Macedo, G. Safety of COVID-19 vaccination in inflammatory bowel disease patients on biologic therapy. J Crohns Colitis. (2022) 16:687–8. doi: 10.1093/ecco-jcc/jjab189
23. Hong, Y, Che, T, Shen, X, Chen, J, Wang, K, Zhao, L, et al. The association of three vaccination doses with reduced gastrointestinal symptoms after severe acute respiratory syndrome coronavirus 2 infections in patients with inflammatory bowel disease. Front Med. (2024) 11:1377926. doi: 10.3389/fmed.2024.1377926
24. Sahyoun, LC, Fetene, J, McMillan, C, Protiva, P, Al Bawardy, B, Gaidos, JKJ, et al. Impact of COVID-19 treatment on real-world outcomes in inflammatory bowel disease. Dig Dis Sci. (2024) 69:1654–60. doi: 10.1007/s10620-024-08355-3
25. Brenner, EJ, Ungaro, RC, Gearry, RB, Kaplan, GG, Kissous-Hunt, M, Lewis, JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. (2020) 159:481–91.e3. doi: 10.1053/j.gastro.2020.05.032
26. Macaluso, FS, and Orlando, A. Could patients with inflammatory bowel disease treated with Immunomodulators or biologics be at lower risk for severe forms of COVID-19? Gastroenterology. (2021) 160:1877–8. doi: 10.1053/j.gastro.2020.05.026
Keywords: coronavirus disease-19 (COVID-19), inflammatory bowel disease (IBD), exacerbation, symptoms, medication discontinuation
Citation: Wu J, Fang Y, Bai B, Wu Y, Liu Q, Hu J, Hu N, Mei Q and Han W (2024) Clinical characteristics and the risk factors for the exacerbation of symptoms in patients with inflammatory bowel disease during the COVID-19 pandemic. Front. Med. 11:1404880. doi: 10.3389/fmed.2024.1404880
Edited by:
Faris Lami, University of Baghdad, IraqReviewed by:
Igor Brasil-Costa, Evandro Chagas Institute, BrazilAntonietta G. Gravina, University of Campania Luigi Vanvitelli, Italy
Copyright © 2024 Wu, Fang, Bai, Wu, Liu, Hu, Hu, Mei and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiao Mei, meiqiao@hotmail.com; Wei Han, hwbb1016@aliyun.com
†These authors have contributed equally to this work and share first authorship
 Juan Wu
Juan Wu Yuanyuan Fang
Yuanyuan Fang Bingqing Bai
Bingqing Bai Yumei Wu
Yumei Wu  Qiao Mei
Qiao Mei Wei Han
Wei Han