- 1Division of Virology, Department of Microbiology and Immunology, The Institute of Medical Science, University of Tokyo, Tokyo, Japan
- 2Department of Pathology, The National Institute of Infectious Diseases, Tokyo, Japan
- 3Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin-Madison, Madison, WI, United States
- 4Aaron Diamond AIDS Research Center, Affiliate of the Rockefeller University, New York, NY, United States
- 5Department of Special Pathogens, International Research Center for Infectious Diseases, Institute of Medical Science, University of Tokyo, Tokyo, Japan
Vaccination is an effective strategy to control influenza disease. Adjuvants enhance the efficacy of vaccines, but few adjuvants are approved for human use, so novel, safe, and effective adjuvants are urgently needed. The glycolipid adjuvant 7DW8-5 has shown adjuvanticity to malaria vaccine; however, its adjuvant effect for seasonal influenza vaccine remains unknown. Here, we evaluated the adjuvanticity of 7DW8-5 to a quadrivalent split influenza vaccine in a mouse model. 7DW8-5 significantly enhanced virus-specific antibody production when administrated with influenza vaccine compared with that of vaccine alone; 10 μg of 7DW8-5 induced similar antibody levels to those induced by alum. Mouse body weight loss was reduced and, notably, the survival rate was increased in the vaccine plus 7DW8-5 group compared with that in the vaccine plus alum group. Our results indicate that the glycolipid 7DW8-5 is a promising adjuvant for influenza vaccine.
Introduction
Influenza virus causes seasonal influenza epidemics every winter and influenza pandemics every few decades, leading to serious economic and social disruption (Cauchemez et al., 2009; Gasparini et al., 2012). Annual vaccination is a useful means to control seasonal influenza (Houser and Subbarao, 2015; Rajao and Perez, 2018; Yamayoshi and Kawaoka, 2019). Currently, three types of licensed seasonal influenza vaccines are available: live attenuated, recombinant HA, and inactivated vaccines (Grohskopf et al., 2018). These vaccines are composed of three or four different types of influenza viruses (i.e., H1N1, H3N2, and one or two influenza B viruses), which are updated annually by the World Health Organization (WHO) to reflect the most recent circulating strains1. The inactivated influenza vaccines, including whole inactivated virus vaccine, split virus vaccine, and subunit vaccine, are widely used throughout the world; however, their efficacy is suboptimal, especially in the elderly2 (Wood and Siegrist, 2011; Choi et al., 2015; Dominguez et al., 2016; Flannery et al., 2018).
To improve vaccine efficacy, the addition of adjuvants is one of the most effective strategies. Adjuvants are substances that enhance the immune response to an antigen, and the ideal adjuvant would maximize vaccine efficacy and a strong safety profile (Petrovsky, 2015). Although several adjuvants are in clinical trials, many have failed to progress to approval for human use with vaccines due to toxicity, stability, biocompatibility, cost, and availability (Petrovsky and Aguilar, 2004; Sivakumar et al., 2011). Given that only a few adjuvants in combination with vaccines are currently approved for use in humans [e.g., alum, MF59, AS03, and AS04 (Mbow et al., 2010; Di Pasquale et al., 2015)], the development of novel and safe adjuvants is urgently needed.
The glycolipid alpha-Galactosylceramide (α-GalCer) binds CD1d, an MHC I-like molecule (Blumberg et al., 1991), which is primarily expressed by antigen-presenting cells (APC) and is presented to invariant natural killer T (iNKT) cells (Girardi and Zajonc, 2012). The adjuvant effect of α-GalCer has been investigated for vaccines against tumors and various infectious diseases, including influenza (Gonzalez-Aseguinolaza et al., 2002; Ko et al., 2005; Youn et al., 2007; Choi et al., 2008; Huang et al., 2008, 2013; Guillonneau et al., 2009; Kopecky-Bromberg et al., 2009; Kim et al., 2010; Miller et al., 2011; Lu et al., 2014; Artiaga et al., 2016). In fact, α-GalCer has been tested for several types of influenza vaccines (i.e., inactivated vaccines, live attenuated vaccines, and a DNA vaccine) in different animal models such as pigs and mice, and has been shown to enhance virus-specific antibody production and/or the protective efficacy of influenza vaccines, suggesting that α-GalCer is a promising adjuvant for influenza vaccines (Kamijuku et al., 2008; Guillonneau et al., 2009; Kopecky-Bromberg et al., 2009; Lee et al., 2011; Artiaga et al., 2016; Dwivedi et al., 2016; Fotouhi et al., 2017). Recently, Li et al. (2010) screened a library of 25 synthetic analog of α-GalCer, and identified a lead candidate, named 7DW8-5, that displayed a superior adjuvant effect for HIV and malaria vaccines in mice compared with the parental α-GalCer. For influenza vaccines, the adjuvanticity of 7DW8-5 has been tested for a DNA vaccine against H5N1 influenza virus (Hung et al., 2014); however, its adjuvant effects for other types of influenza vaccines are unknown.
In this study, we evaluated the adjuvant effect of 7DW8-5 on the commercial HA split vaccine in a mouse model. We found that 7DW8-5 enhanced the protective efficacy of the commercial HA split vaccine in mice, thereby demonstrating its promise as an adjuvant for the HA split influenza vaccine in humans.
Materials and Methods
Cells and Viruses
Madin-Darby canine kidney (MDCK) cells were maintained in minimum essential medium (MEM) (Gibco) supplemented with 5% newborn calf serum (Sigma) at 37°C in 5% CO2. MDCK cells were used for plaque assays to determine virus titers.
Mouse-adapted A/California/04/2009 virus (H1N1; MA-CA04), generated in our laboratory as previously described (Sakabe et al., 2011), was used to challenge mice. A/California/07/2009 virus (H1N1; CA07), which was isolated early in the 2009 pandemic, was used as an antigen for the ELISA after purification and inactivation to determine the virus-specific antibody titers of sera obtained from the immunized mice.
Influenza Vaccine and Adjuvants
Quadrivalent split influenza HA vaccines were obtained from DENKA SEIKEN Co., Ltd. (Japan). The quadrivalent split influenza HA vaccine (for the 2016–2017 season), which contains the HA proteins (equivalent to 30 μg of HA protein for each virus included in a vaccine vial) of CA07 (H1N1), A/Hong Kong/4801/2014 (H3N2), B/Phuket/3073/2013 (Yamagata lineage), and B/Texas/2/2013 (Victoria lineage), was used. Aluminum hydroxide gel Alhydrogel® adjuvant 2% (alum), purchased from InvivoGen, was used as a positive control [antigen: alum = 1:1 (v/v)] (approximately equal to 500 μg of alum/dose). The 7DW8-5 was purchased from Funakoshi Co., Ltd. (endotoxin level, <100 EU/mg), suspended in sterile water at a concentration of 1 mg/ml, heated at 80°C for 10 min, and then sonicated in a water bath for 15 min at room temperature. Stocks were stored at −20°C until use. Before being mixed with the split influenza HA vaccine, the 7DW8-5 suspension was sonicated again for 5 min after thawing. The 7DW8-5 and the HA split vaccine were diluted with the endotoxin-free D-PBS (Millipore, Cat. TMS-012-A, Endotoxin <0.005 EU/mL).
Immunization and Protection
Five-week-old female BALB/c mice were purchased from Japan SLC Inc. After one week of adaptation, the mice (10 mice per group) were immunized with a suboptimal dose of influenza HA vaccine [0.001 μg/dose (2016–2017 season) calculated on the basis of the amount of HA from CA07] with or without 7DW8-5 into the gastrocnemius muscle. Two weeks later, the mice were boost immunized (second immunization) intramuscularly. On day 14 after the boost-immunization, blood was collected via the facial vein by using a goldenrod animal lancet (5 mm), and sera were obtained to measure virus-specific antibody titers. Three weeks after the boost-immunization, the immunized mice were challenged intranasally, under anesthesia, with 10 MLD50 (Fifty Percent Mouse Lethal Dose) of MA-CA04 virus. Body weight and survival of four mice from each group were monitored daily for 14 days after virus challenge. Mice that lost more than 25% of their original body weight were euthanized.
The remaining mice (6 mice per group) were used to assess virus replication. To determine virus titers in mice, organ samples were harvested on days 3 and 6 post-challenge and were homogenized and titrated on MDCK cells by using a plaque assay.
Measurement of Virus-Specific Antibody Titers
Virus-specific antibody titers in the sera were determined by using a modified ELISA as previously described (Uraki et al., 2014). Briefly, 96-well ELISA plates (IWAKI) were coated with 6 μg/ml of inactivated, purified CA07 virus solution overnight at 4°C (50 μl/well). The plates were then blocked with 200 μl of 20% Blocking One (Nacalai) in water at room temperature for 1 h. After blocking, the plates were washed once with PBS containing 0.05% Tween-20 (PBS-T), and then 2-fold serially diluted serum samples were added to the plates, followed by a 1-h incubation at room temperature. Bound total IgG was detected by using peroxidase-labeled goat anti-mouse IgG (gamma) antibody, F (ab′) 2 fragment (Kirkegaard & Perry Laboratory Inc.) and horseradish peroxidase-conjugated anti-mouse IgG1, or IgG2a antibodies (Southern Biotech). After the plates were washed four times with PBS-T, 100 μl of 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt substrate solution was added to each well to initiate the color reaction, and the OD was measured at a wavelength of 405 nm. The antibody titer was defined as the reciprocal of the highest serum dilution that produced an OD405 > 0.1 after correcting for the negative serum control.
Pathological Examination
Animal tissues were fixed in 4% paraformaldehyde phosphate (PFA) buffer solution for pathologic examination. They were then processed for paraffin embedding and cut into 3 μm-thick serial sections. The sections were stained using a standard hematoxylin and eosin procedure, and serial sections were processed for immunohistological staining with a rabbit polyclonal antibody for type A influenza virus nucleoprotein (prepared in the Department of Pathology, National Institute of Infectious Diseases, Tokyo, Japan) or a rat monoclonal antibody for mouse F4/80 antigen (CL: A3-1, Bio Rad). Specific antigen-antibody reactions were visualized with 3, 3′-Diaminobenzidine (DAB) staining by using a Dako Envision system (Dako Cytomation).
Statistics
We used R3 and lme4 (Bates et al., 2015) to perform a linear mixed effects analysis of the body weight data, which were normalized to the initial weight of each individual animal. As fixed effects, we used the different treatment groups (i.e., vaccine alone, vaccine plus 7DW8-5 and vaccine plus alum), and the time of the measurement (with an interaction term between those fixed effects). As random effects, we used the intercepts for the individual animals. We used the lsmeans (Lenth, 2016) package to compare the groups at different time points for each model separately, and the p-values were adjusted using Holm’s method. For the comparisons of antibody titers and virus titers, we used a one-way ANOVA, followed by Tukey’s Post Hoc tests. The data were log-transformed before the comparisons were made to stabilize the variance. For the virus titer data, each timepoint was analyzed separately. For the antibody titers, values <10 were arbitrarily set to 5, because it was not possible to determine whether antibodies were completely absent or were present below the detection limit. For the analysis of the survival data, we used the Log-rank test, comparing the vaccine plus 7DW8-5 or alum to the vaccine alone group. We used OASIS 2 (Han et al., 2016) software for this analysis. P values of <0.05 were considered statistically significant.
Ethics Statement
All experiments with mice were performed in the biosafety level 2 containment laboratory in the Institute of Medical Science, the University of Tokyo (Tokyo, Japan) in accordance with the Regulations for Animal Care of the University of Tokyo and the Guidelines for Proper Conduct of Animal Experiments by the Science Council of Japan, and were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval no. PA 14-38).
Results
7DW8-5 Significantly Enhances Influenza Virus-Specific Antibody Production in Mice
To evaluate the adjuvant effect of the glycolipid 7DW8-5 on a commercial HA split vaccine, mice were immunized with PBS, 7DW8-5 (1 μg/dose or 10 μg/dose), HA vaccine alone (0.001 μg of HA for each virus/dose), or HA vaccine plus 7DW8-5 (1 μg/dose or 10 μg/dose) via intramuscular administration in a 100 μl volume twice with a 2-week interval between the vaccinations. Commercially available alum adjuvant was used as a positive control, as described in the Materials and Methods, because alum is the most frequently used adjuvant worldwide and has been used in many clinical studies (Tetsutani and Ishii, 2012). Two weeks after the boost immunization, sera samples were obtained from the immunized mice and examined for the presence of virus-specific antibody in an ELISA. No antibody against CA07 virus was detected in the groups of mice that receive PBS only or 7DW8-5 only. Most mice immunized with the HA vaccine alone produced no or very low levels of virus-specific antibodies except for one mouse whose virus-specific IgG titer was 1280 (Figure 1). The mean antibody titer in the sera of the group of mice immunized with the HA vaccine plus 1 μg of 7DW8-5 (i.e., 1440.0) was significantly higher than that in the vaccine alone group (i.e., 240.5) (Figure 1). The vaccine plus 10 μg of 7DW8-5 also induced much higher levels of virus-specific antibody compared with the vaccine alone group, and the mean antibody titer was comparable to that induced by HA vaccine plus alum (Figure 1). These results demonstrate that 7DW8-5 significantly enhances the immunogenicity of the HA split vaccine.
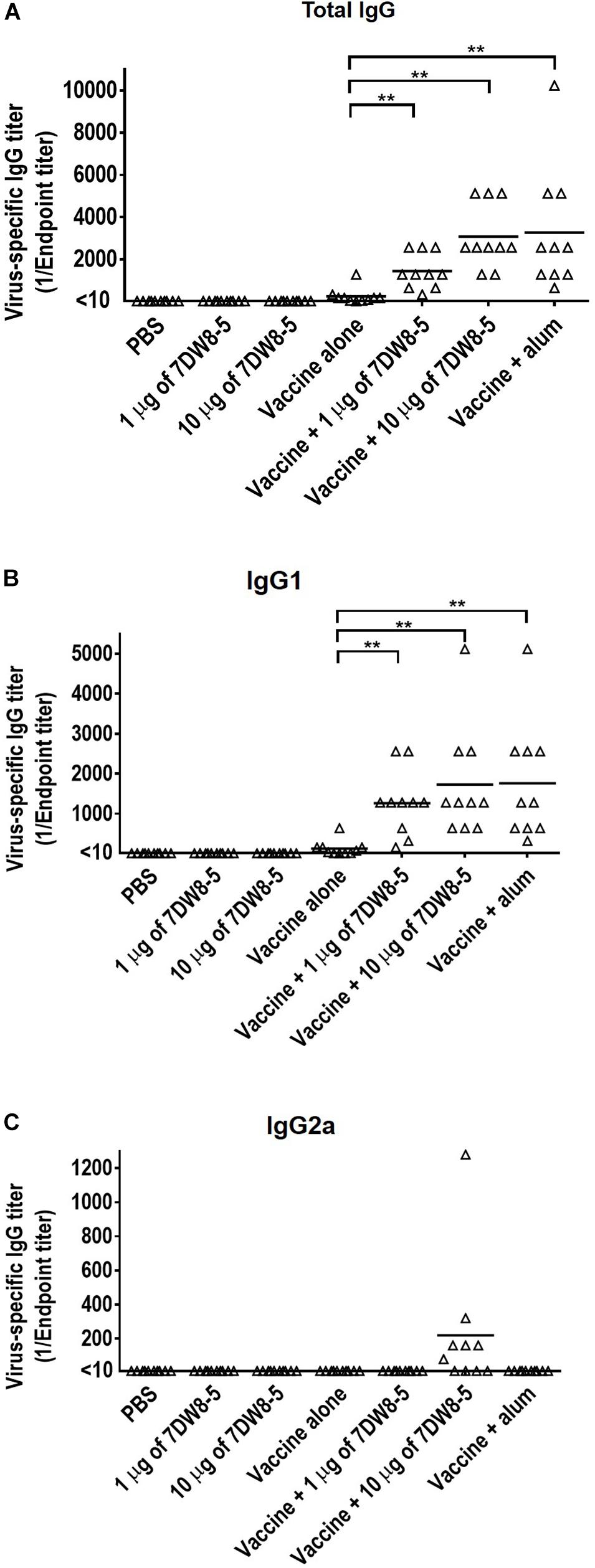
Figure 1. Virus-specific antibody titers induced by 7DW8-5 in combination with HA vaccine in mice. Six-week-old BALB/c mice (n = 10) were immunized with a commercial influenza HA vaccine with or without adjuvant twice with a 2-week interval between the vaccinations. Blood samples were collected 2 weeks after the second immunization. Virus-specific antibodies were measured by using an ELISA with inactivated and purified CA07 virus as the coating antigen. (A) Virus-specific total IgG antibody titers; (B) virus-specific IgG1 antibody titers; (C) virus-specific IgG2a antibody titers. The data were analyzed by using a one-way ANOVA followed by Tukey’s Post Hoc tests. The data were log2 transformed before the comparisons to stabilize the variance. The lines indicate the means of the antibody titers (n = 10). Values <10 were assigned the value 5 as described in the section “Materials and Methods.” ∗∗P < 0.01.
We also measured the influenza virus-specific IgG1 and IgG2a titers in the sera by using an ELISA because IgG1 and IgG2a are stimulated during Th2-type and Th1-type immune responses, respectively (Stevens et al., 1988; Mosmann and Coffman, 1989; Hauge et al., 2007). The IgG1 titers in the HA vaccine plus 1 μg of 7DW8-5, the HA vaccine plus 10 μg of 7DW8-5, and the HA vaccine plus alum groups were significantly higher than that in the HA vaccine alone group (Figure 1B). Seven of the ten mice immunized with the HA vaccine plus 10 μg of 7DW8-5 expressed a high level of IgG2a antibodies, whereas no IgG2a antibody was detected in the other groups (Figure 1C). These results suggest that the HA vaccine plus 10 μg of 7DW8-5 induced both Th1-type and Th2-type immune responses, whereas the HA vaccine plus 1 μg of 7DW8-5 and the HA vaccine plus alum induced only the Th2-type immune response.
7DW8-5 Enhances the Protective Efficacy of Influenza Vaccine Against Lethal Virus Challenge in Mice
To examine whether 7DW8-5 enhances the protective efficacy of the HA split vaccine, we challenged the immunized mice with 10 MLD50 of MA-CA04 virus 3 weeks after the boost-immunization and monitored their body weight changes and survival for 14 days. All mice given PBS or 7DW8-5 alone experienced body weight loss upon virus challenge and died by 6 days post-challenge, whereas 2 of 4 mice were protected from lethal challenge in the vaccine plus alum group (Figure 2 and Supplementary Table 1). More importantly, 3 of 4 mice immunized with the vaccine plus 1 μg of 7DW8-5 survived and all four mice that received the vaccine plus 10 μg of 7DW8-5 were protected from the lethal infection (Figure 2 and Supplementary Table 1). These results demonstrate that the adjuvanticity of the glycolipid adjuvant 7DW8-5 was sufficient to protect mice from lethal challenge with MA-CA04 virus.
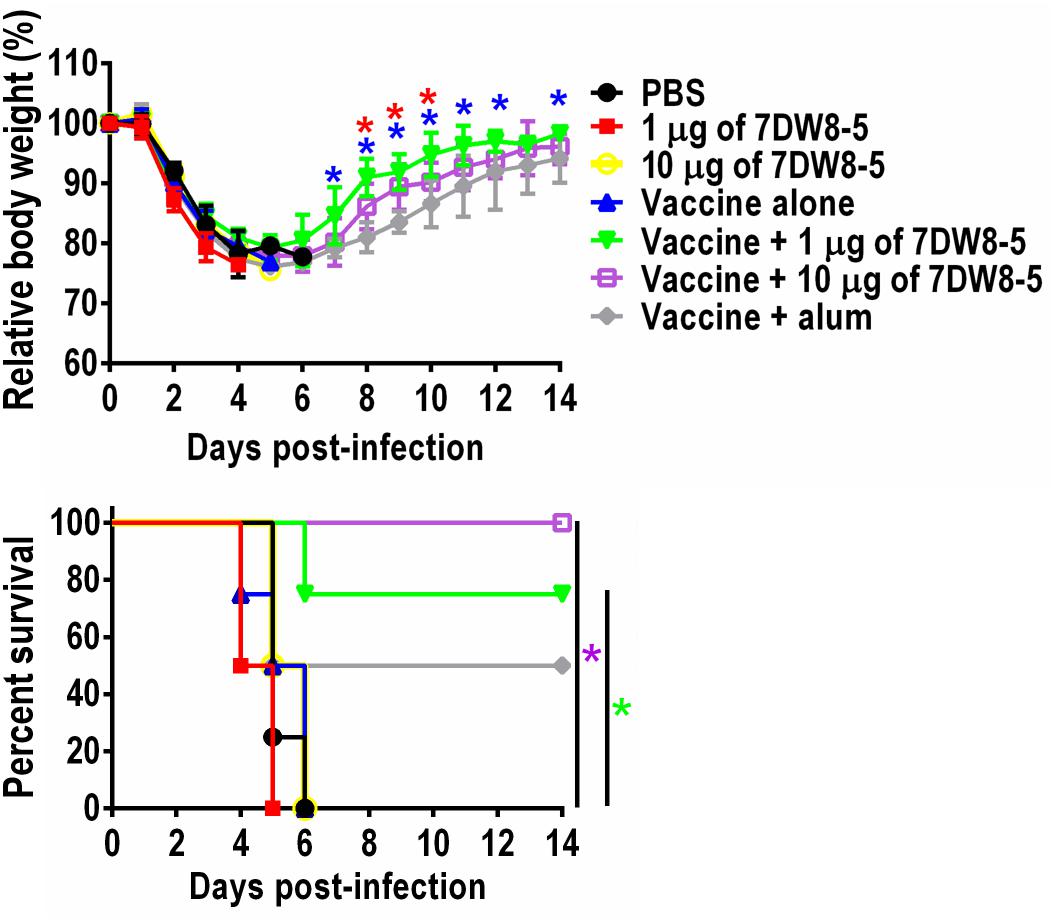
Figure 2. Protective efficacy of 7DW8-5 plus vaccine against lethal challenge. Six-week-old BALB/c mice (n = 4) were mock-immunized with PBS or 7DW8-5 alone, immunized with HA vaccine alone or 7DW8-5-adjuvanted HA vaccine twice with a 2-week interval between vaccinations. Mice were intranasally challenged with 10 MLD50 of MA-CA04 virus 3 weeks after the second immunization. Body weight and survival were monitored daily for 14 days. The body weight data shown are means ± standard deviation (SD). Green asterisks indicate a significant difference between the vaccine alone group and the vaccine plus 1 μg of 7DW8-5 group; purple asterisks indicate a significant difference between the vaccine alone group and the vaccine plus 10 μg of 7DW8-5 group; blue asterisks indicate a significant difference between the vaccine plus 1 μg of 7DW8-5 group and the vaccine plus alum group; red asterisks indicate a significant difference between the vaccine plus 10 μg of 7DW8-5 group and the vaccine plus alum group. ∗P < 0.05.
HA Vaccine Plus 7DW8-5 Did Not Completely Prevent Challenge Virus Replication in Immunized Mice
To examine the effect of 7DW8-5 on virus replication in the immunized mice after challenge, the mice immunized with HA vaccine plus 7DW8-5 were challenged with 10 MLD50 of MA-CA04 virus 3 weeks after the second immunization, and organ samples (i.e., nasal turbinates and lungs) were collected from the sacrificed mice on days 3 and 6 post-infection for virus titration. On day 3 post-challenge, over 106 PFU of virus was detected in both the nasal turbinates and lungs of mice in all of the groups (Table 1). In contrast, on day 6 post-challenge, the mean virus titers in the lungs for the vaccine plus 7DW8-5 and the vaccine plus alum groups were lower than that of the vaccine alone group, although the difference was not statistically significant (Table 1).
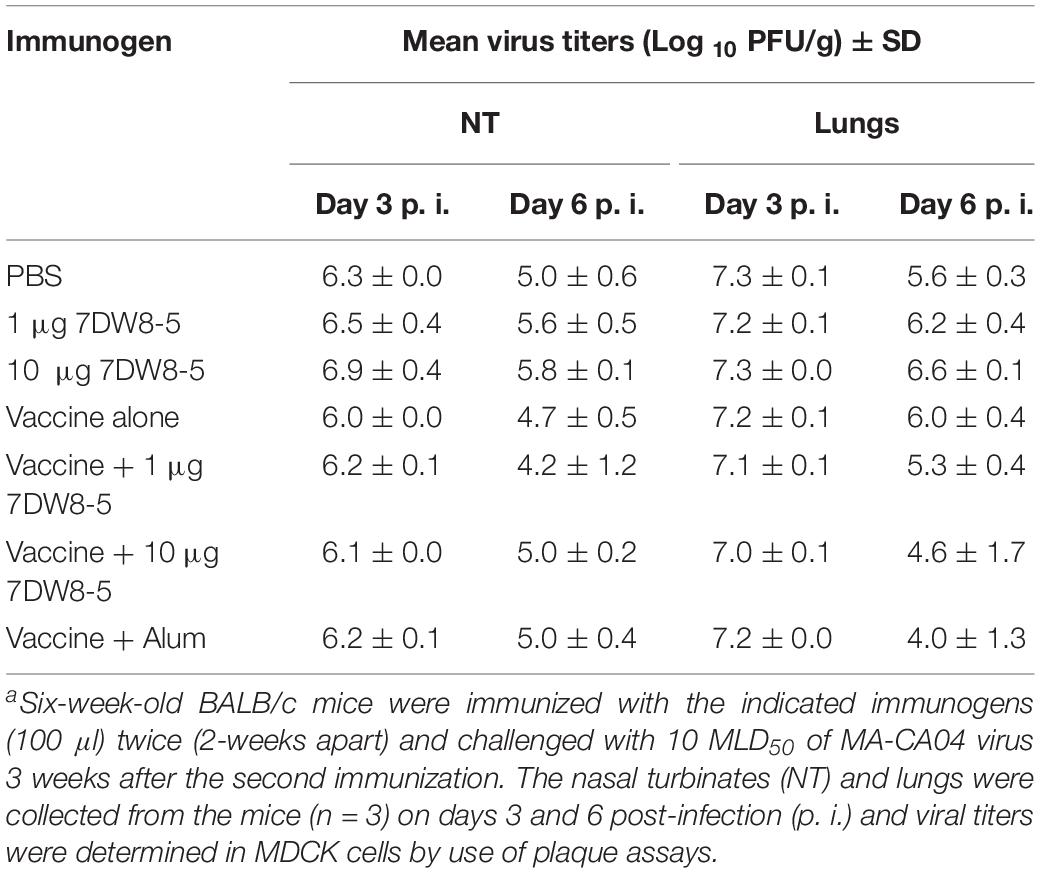
Table 1. Virus replication in the respiratory tract of immunized mice challenged with MA-CA04 virusa.
Pathological analysis of mice immunized with PBS, 7DW8-5 (10 μg/dose), HA vaccine alone (0.001 μg/dose), or HA vaccine plus 7DW8-5 (10 μg/dose) after challenge with 10 MLD50 MA-CA04 virus revealed that infection with MA-CA04 resulted in detectable viral antigens in bronchial epithelial cells and in infiltration of inflammatory cells into the lungs of all mice tested on day 6 post-challenge (Figure 3A). No apparent difference in inflammation, viral antigen distribution pattern, or the number of viral antigen-positive cells was observed across all groups (Figure 3A). Moderate inflammation, including infiltration of neutrophils, monocytes/macrophages, or lymphocytes, was observed in more than half of the sections from each mouse across all groups; however, we detected more F4/80-positive cells in the lungs of immunized mice after virus challenge in the HA vaccine alone and the 7DW8-5 plus HA vaccine groups compared with the PBS and 7DW8-5 groups (Figure 3B). These results suggest that immunization of mice with the HA vaccine induces infiltration of macrophages into the lungs after virus infection, although no difference in the number of F4/80-positive cells was observed between the HA vaccine alone and the 7DW8-5 plus HA vaccine groups.
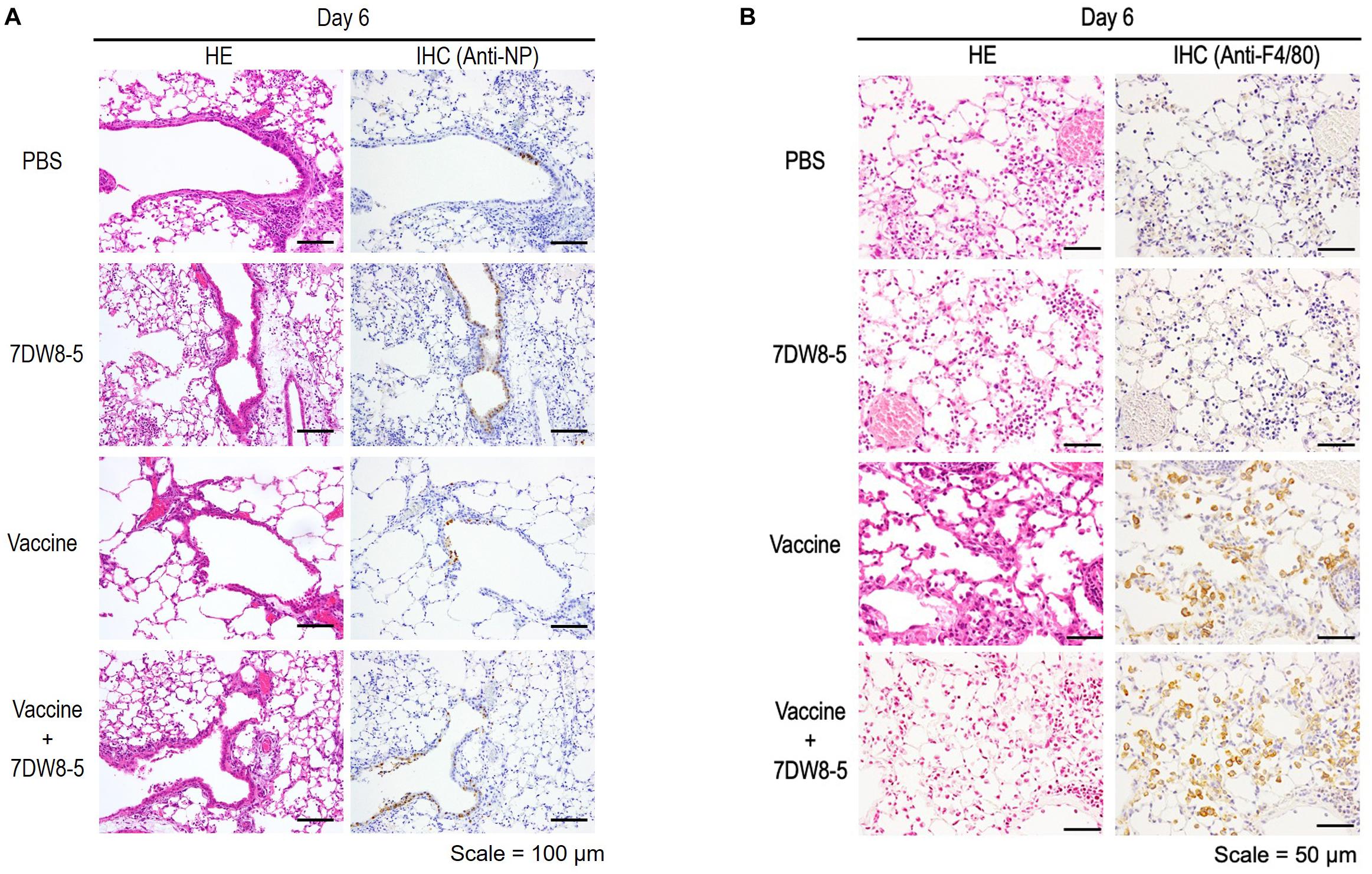
Figure 3. Pathological analysis of the lungs of immunized mice after challenge. Six-week-old BALB/c mice (n = 3) were immunized with PBS, 7DW8-5 (10 μg/dose), HA vaccine alone, or HA vaccine plus 7DW8-5 (10 μg/dose) twice with a 2-week interval between vaccinations. The immunized mice were intranasally challenged with 10 MLD50 of MA-CA04 virus 3 weeks after the second immunization. The lungs were fixed with 4% PFA buffer solution on day 6, and stained as described in the Materials and Methods section. HE, hematoxylin and eosin staining; IHC, immunohistochemistry for the detection of influenza virus NP antigen (A) or anti-F4/80 antibody (B). Scale bars: 100 μm (A) and 50 μm (B).
Taken together, our results demonstrate that although 7DW8-5 did not facilitate a reduction in virus replication by the HA vaccine, it did improve vaccine efficacy as evaluated by lethality.
Discussion
The use of adjuvants is an effective approach to improve vaccine efficacy. The CD1d-binding glycolipid α-GalCer is recognized as a member of a new class of adjuvants and its adjuvanticity has been investigated for vaccines against tumors and various infectious diseases, including influenza (Gonzalez-Aseguinolaza et al., 2002; Ko et al., 2005; Youn et al., 2007; Choi et al., 2008; Huang et al., 2008, 2013; Guillonneau et al., 2009; Kopecky-Bromberg et al., 2009; Kim et al., 2010; Miller et al., 2011; Lu et al., 2014; Artiaga et al., 2016). In this study, we evaluated the adjuvanticity of the glycolipid 7DW8-5, a novel analog of α-GalCer that showed a superior adjuvant effect compared with that of α-GalCer in malaria and HIV vaccines (Li et al., 2010), for a commercial influenza HA split vaccine in a mouse model. Although the adjuvant effect of 7DW8-5 on the DNA vaccine for H5N1 influenza virus was less than that of the parental compound α-GalCer (Hung et al., 2014), 7DW8-5 did enhance virus-specific antibody production and the protective efficacy of a commercial HA split vaccine against a lethal challenge of influenza A virus in mice. The safety and adjuvanticity of α-GalCer have been demonstrated in humans in Phase I clinical trials (Giaccone et al., 2002; Ishikawa et al., 2005; Nicol et al., 2011). In addition, 7DW8-5 has been shown to have a good safety profile and potent immune-enhancing activity in a non-human primate model (Padte et al., 2013). Additionally, we found that 7DW8-5 has a dose-sparing effect on the HA vaccine because the mean titer of the virus-specific antibody in mice immunized with 0.03 μg of HA vaccine alone was 2560, as described in our previous study (Feng et al., 2019), which is comparable to that in mice immunized with 0.001 μg of HA vaccine plus 10 μg of 7DW8-5 (mean antibody titer: 3072) in this study. These findings, coupled with our results, suggests that the glycolipid 7DW8-5 could be a promising adjuvant for the commercial HA split influenza vaccine.
Previous studies have shown that the induction of IgG2a antibodies, which occurs during the Th1-type immune response, is associated with increased efficacy of influenza vaccines (Huber et al., 2001, 2006; Proietti et al., 2002; Hovden et al., 2005). In contrast, inactivated influenza vaccines and subunit vaccines induce the Th2-type immune response, which is associated with the stimulation of IgG1 antibodies in BALB/c mice (Hocart et al., 1989; Benne et al., 1997; Moran et al., 1999). In the current study, we found that mice immunized with the HA vaccine plus 10 μg of 7DW8-5 had increased titers of both IgG1 and IgG2a antibodies (Figure 1C), suggesting that both Th1-type and Th2-type immune responses were induced by vaccination with the HA vaccine plus 10 μg of 7DW8-5. Additionally, pathological analysis showed that immunization of mice with the HA vaccine or with 7DW8-5 plus the HA vaccine induced macrophage infiltration into the lungs after virus challenge (Figure 3B). The induction of both Th1- and Th2-type immune responses in addition to macrophage infiltration may have facilitated viral clearance from the lungs of the infected mice immunized with the HA vaccine plus 10 μg of 7DW8-5, resulting in 100% protection (Figure 2).
The current influenza vaccines induce strain-specific antibody responses against viral HA proteins; however, strain-specific antibodies can only provide a reasonable measure of protection if the vaccine strains match the antigenicity of the current circulating strains (Pica and Palese, 2013; Khurana, 2018). Therefore, an approach aimed at broadening the cross-reactivity of influenza vaccines by targeting T cell responses is of considerable interest. The glycolipid α-GalCer activates iNKT cells, leading to the production of Th1 and Th2 cytokines (Bendelac et al., 2007), and the subsequent stimulation of various immune cells including dendritic cells (Fujii et al., 2003, 2006), natural killer (NK) cells (Kawano et al., 1997; Brossay et al., 1998), B cells (Kitamura et al., 2000), and CD4+ and CD8+ T cells (Singh et al., 1999; Hermans et al., 2003). Guillonneau et al. showed that α-GalCer enhanced T cell-mediated immune responses in mice immunized with an inactivated influenza virus vaccine and protected the immunized mice from heterologous influenza A virus challenge (Guillonneau et al., 2009). Therefore, the use of α-GalCer, and its analogs such as 7DW8-5, as an adjuvant could be an effective strategy to compel vaccines to elicit broader immune responses.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
All experiments with mice were performed in the biosafety level 2 containment laboratory in the Institute of Medical Science, the University of Tokyo (Tokyo, Japan) in accordance with the Regulations for Animal Care of the University of Tokyo and the Guidelines for Proper Conduct of Animal Experiments by the Science Council of Japan, and were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval no. PA 14-38).
Author Contributions
HF, MY, TW, and YK designed the experiments. HF and LW performed most of the experiments. NN and HH performed the pathological analysis. MT provided material for the pilot experiments. HF, TL, TW, and YK analyzed the data. TW and YK oversaw the study. HF, TW, and YK wrote the manuscript. All authors reviewed and approved the manuscript.
Funding
This research was supported by the Leading Advanced Projects for Medical Innovation (LEAP) from the Japan Agency for Medical Research and Development (AMED) (JP18am001007), Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (Nos. 16H06429, 16K21723, and 16H06434), Research Program on Emerging and Re-emerging Infectious Diseases from AMED (JP19fk0108056 and JP19fk0108104), the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from MEXT and AMED (JP19fm0108006), JSPS KAKENHI Grant Number 19K06399, and the NIAID-funded Center for Research on Influenza Pathogenesis (CRIP, HHSN272201400008C).
Conflict of Interest Statement
YK has received speaker’s honoraria from Toyama Chemical and Astellas and grant support from Chugai Pharmaceuticals, Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Otsuka Pharmaceutical, and Kyoritsu Seiyaku; and is a founder of FluGen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Susan Watson for editing the manuscript. We also thank Denka Seiken Co., Ltd. for providing the influenza vaccines. We also thank Mutsumi Ito, Izumi Ishikawa, Yuriko Tomita, Naomi Fujimoto, Fujimi Arai, Mikiko Tanaka, Yuko Sato, and Izumi Fujimori for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02157/full#supplementary-material
Footnotes
- ^ https://www.who.int/influenza/vaccines/virus/recommendations/en/
- ^ https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm
- ^ www.r-project.org
References
Artiaga, B. L., Yang, G., Hackmann, T. J., Liu, Q., Richt, J. A., Salek-Ardakani, S., et al. (2016). Alpha-Galactosylceramide protects swine against influenza infection when administered as a vaccine adjuvant. Sci. Rep. 6:23593. doi: 10.1038/srep23593
Bates, D., Machler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48.
Bendelac, A., Savage, P. B., and Teyton, L. (2007). The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336. doi: 10.1146/annurev.immunol.25.022106.141711
Benne, C. A., Harmsen, M., van der Graaff, W., Verheul, A. F., Snippe, H., and Kraaijeveld, C. A. (1997). Influenza virus neutralizing antibodies and IgG isotype profiles after immunization of mice with influenza A subunit vaccine using various adjuvants. Vaccine 15, 1039–1044. doi: 10.1016/s0264-410x(96)00287-3
Blumberg, R. S., Terhorst, C., Bleicher, P., McDermott, F. V., Allan, C. H., Landau, S. B., et al. (1991). Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J. Immunol. 147, 2518–2524.
Brossay, L., Chioda, M., Burdin, N., Koezuka, Y., Casorati, G., Dellabona, P., et al. (1998). CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188, 1521–1528. doi: 10.1084/jem.188.8.1521
Cauchemez, S., Ferguson, N. M., Wachtel, C., Tegnell, A., Saour, G., Duncan, B., et al. (2009). Closure of schools during an influenza pandemic. Lancet Infect. Dis. 9, 473–481. doi: 10.1016/S1473-3099(09)701768
Choi, W. S., Noh, J. Y., Baek, J. H., Seo, Y. B., Lee, J., Song, J. Y., et al. (2015). Suboptimal effectiveness of the 2011-2012 seasonal influenza vaccine in adult korean populations. PLoS One 10:e0098716. doi: 10.1371/journal.pone.0098716
Choi, Y. S., Hoory, T., Monie, A., Wu, A., Connolly, D., and Hung, C. F. (2008). Alpha-Galactosylceramide enhances the protective and therapeutic effects of tumor cell based vaccines for ovarian tumors. Vaccine 26, 5855–5863. doi: 10.1016/j.vaccine.2008.08.027
Di Pasquale, A., Preiss, S., Tavares Da Silva, F., and Garcon, N. (2015). Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines 3, 320–343. doi: 10.3390/vaccines3020320
Dominguez, A., Godoy, P., and Torner, N. (2016). The effectiveness of influenza vaccination in different groups. Expert. Rev. Vaccin. 15, 751–764. doi: 10.1586/14760584.2016.1142878
Dwivedi, V., Manickam, C., Dhakal, S., Binjawadagi, B., Ouyang, K., Hiremath, J., et al. (2016). Adjuvant effects of invariant NKT cell ligand potentiates the innate and adaptive immunity to an inactivated H1N1 swine influenza virus vaccine in pigs. Vet. Microbiol. 186, 157–163. doi: 10.1016/j.vetmic.2016.02.028
Feng, H. P., Yamashita, M., Lopes, T. J. D., Watanabe, T., and Kawaoka, Y. (2019). Injectable Excipients as novel influenza vaccine adjuvants. Front. Microbiol. 10:19. doi: 10.3389/fmicb.2019.00019
Flannery, B., Chung, J. R., Belongia, E. A., McLean, H. Q., Gaglani, M., Murthy, K., et al. (2018). Interim estimates of 2017-18 seasonal influenza vaccine effectiveness - United States. MMWR Morb. Mortal. Wkly. Rep. 67, 180–185. doi: 10.15585/mmwr.mm6706a2
Fotouhi, F., Shaffifar, M., Farahmand, B., Shirian, S., Saeidi, M., Tabarraei, A., et al. (2017). Adjuvant use of the NKT cell agonist alpha-galactosylceramide leads to enhancement of M2-based DNA vaccine immunogenicity and protective immunity against influenza A virus. Arch. Virol. 162, 1251–1260. doi: 10.1007/s00705-017-3230-7
Fujii, S., Shimizu, K., Hemmi, H., Fukui, M., Bonito, A. J., Chen, G., et al. (2006). Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc. Natl. Acad. Sci. U.S.A. 103, 11252–11257. doi: 10.1073/pnas.0604812103
Fujii, S., Shimizu, K., Smith, C., Bonifaz, L., and Steinman, R. M. (2003). Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198, 267–279. doi: 10.1084/jem.20030324
Gasparini, R., Amicizia, D., Lai, P. L., and Panatto, D. (2012). Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum. Vaccin. Immunother. 8, 21–28. doi: 10.4161/hv.8.1.17622
Giaccone, G., Punt, C. J., Ando, Y., Ruijter, R., Nishi, N., Peters, M., et al. (2002). A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 8, 3702–3709.
Girardi, E., and Zajonc, D. M. (2012). Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol. Rev. 250, 167–179. doi: 10.1111/j.1600-065X.2012.01166.x
Gonzalez-Aseguinolaza, G., Van Kaer, L., Bergmann, C. C., Wilson, J. M., Schmieg, J., Kronenberg, M., et al. (2002). Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 195, 617–624. doi: 10.1084/jem.20011889
Grohskopf, L. A., Sokolow, L. Z., Broder, K. R., Walter, E. B., Fry, A. M., and Jernigan, D. B. (2018). Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 influenza season. MMWR Recomm. Rep. 67, 1–20. doi: 10.15585/mmwr.rr6703a1
Guillonneau, C., Mintern, J. D., Hubert, F. X., Hurt, A. C., Besra, G. S., Porcelli, S., et al. (2009). Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc. Natl. Acad. Sci. U.S.A. 106, 3330–3335. doi: 10.1073/pnas.0813309106
Han, S. K., Lee, D., Lee, H., Kim, D., Son, H. G., Yang, J. S., et al. (2016). OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 7, 56147–56152. doi: 10.18632/oncotarget.11269
Hauge, S., Madhun, A. S., Cox, R. J., Brokstad, K. A., and Haaheim, L. R. (2007). A comparison of the humoral and cellular immune responses at different immunological sites after split influenza virus vaccination of mice. Scand. J. Immunol. 65, 14–21. doi: 10.1111/j.1365-3083.2006.01862.x
Hermans, I. F., Silk, J. D., Gileadi, U., Salio, M., Mathew, B., Ritter, G., et al. (2003). NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 171, 5140–5147. doi: 10.4049/jimmunol.171.10.5140
Hocart, M. J., Mackenzie, J. S., and Stewart, G. A. (1989). The immunoglobulin G subclass responses of mice to influenza A virus: the effect of mouse strain, and the neutralizing abilities of individual protein A-purified subclass antibodies. J. Gen. Virol. 70(Pt 9), 2439–2448. doi: 10.1099/0022-1317-70-9-2439
Houser, K., and Subbarao, K. (2015). Influenza vaccines: challenges and solutions. Cell Host Microbe 17, 295–300. doi: 10.1016/j.chom.2015.02.012
Hovden, A. O., Cox, R. J., Madhun, A., and Haaheim, L. R. (2005). Two doses of parenterally administered split influenza virus vaccine elicited high serum IgG concentrations which effectively limited viral shedding upon challenge in mice. Scand. J. Immunol. 62, 342–352. doi: 10.1111/j.1365-3083.2005.01666.x
Huang, Y., Chen, A., Li, X., Chen, Z., Zhang, W., Song, Y., et al. (2008). Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine 26, 1807–1816. doi: 10.1016/j.vaccine.2008.02.002
Huang, Y. L., Hung, J. T., Cheung, S. K., Lee, H. Y., Chu, K. C., Li, S. T., et al. (2013). Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 2517–2522. doi: 10.1073/pnas.1222649110
Huber, V. C., Lynch, J. M., Bucher, D. J., Le, J., and Metzger, D. W. (2001). Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 166, 7381–7388. doi: 10.4049/jimmunol.166.12.7381
Huber, V. C., McKeon, R. M., Brackin, M. N., Miller, L. A., Keating, R., Brown, S. A., et al. (2006). Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccin. Immunol. 13, 981–990. doi: 10.1128/CVI.00156-06
Hung, J. T., Tsai, Y. C., Lin, W. D., Jan, J. T., Lin, K. H., Huang, J. R., et al. (2014). Potent adjuvant effects of novel NKT stimulatory glycolipids on hemagglutinin based DNA vaccine for H5N1 influenza virus. Antiviral. Res. 107, 110–118. doi: 10.1016/j.antiviral.2014.04.007
Ishikawa, A., Motohashi, S., Ishikawa, E., Fuchida, H., Higashino, K., Otsuji, M., et al. (2005). A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 11, 1910–1917. doi: 10.1158/1078-0432.CCR-04-1453
Kamijuku, H., Nagata, Y., Jiang, X., Ichinohe, T., Tashiro, T., Mori, K., et al. (2008). Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal. Immunol. 1, 208–218. doi: 10.1038/mi.2008.2
Kawano, T., Cui, J., Koezuka, Y., Toura, I., Kaneko, Y., Motoki, K., et al. (1997). CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278, 1626–1629. doi: 10.1126/science.278.5343.1626
Khurana, S. (2018). Development and regulation of novel influenza virus vaccines: a United States young scientist perspective. Vaccines 6:E24. doi: 10.3390/vaccines6020024
Kim, D., Hung, C. F., Wu, T. C., and Park, Y. M. (2010). DNA vaccine with alpha-galactosylceramide at prime phase enhances anti-tumor immunity after boosting with antigen-expressing dendritic cells. Vaccine 28, 7297–7305. doi: 10.1016/j.vaccine.2010.08.079
Kitamura, H., Ohta, A., Sekimoto, M., Sato, M., Iwakabe, K., Nakui, M., et al. (2000). alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 199, 37–42. doi: 10.1006/cimm.1999.1602
Ko, S. Y., Ko, H. J., Chang, W. S., Park, S. H., Kweon, M. N., and Kang, C. Y. (2005). Alpha-galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 175, 3309–3317. doi: 10.4049/jimmunol.175.5.3309
Kopecky-Bromberg, S. A., Fraser, K. A., Pica, N., Carnero, E., Moran, T. M., Franck, R. W., et al. (2009). Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine 27, 3766–3774. doi: 10.1016/j.vaccine.2009.03.090
Lee, Y. S., Lee, K. A., Lee, J. Y., Kang, M. H., Song, Y. C., Baek, D. J., et al. (2011). An alpha-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine 29, 417–425. doi: 10.1016/j.vaccine.2010.11.005
Lenth, R. V. (2016). Least-squares means: the R Package lsmeans. J. Stat. Softw. 69, 1–33. doi: 10.18637/jss.v069.i01
Li, X., Fujio, M., Imamura, M., Wu, D., Vasan, S., Wong, C. H., et al. (2010). Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc. Natl. Acad. Sci. U.S.A. 107, 13010–13015. doi: 10.1073/pnas.1006662107
Lu, G., Zhou, A., Meng, M., Wang, L., Han, Y., Guo, J., et al. (2014). Alpha-galactosylceramide enhances protective immunity induced by DNA vaccine of the SAG5D gene of Toxoplasma gondii. BMC Infect. Dis. 14:3862. doi: 10.1186/s12879-014-0706-x
Mbow, M. L., De Gregorio, E., Valiante, N. M., and Rappuoli, R. (2010). New adjuvants for human vaccines. Curr. Opin. Immunol. 22, 411–416. doi: 10.1016/j.coi.2010.04.004
Miller, D. S., Finnie, J., Bowden, T. R., Scholz, A. C., Oh, S., Kok, T., et al. (2011). Preclinical efficacy studies of influenza A haemagglutinin precursor cleavage loop peptides as a potential vaccine. J. Gen. Virol. 92, 1152–1161. doi: 10.1099/vir.0.028985-0
Moran, T. M., Park, H., Fernandez-Sesma, A., and Schulman, J. L. (1999). Th2 responses to inactivated influenza virus can Be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J. Infect. Dis. 180, 579–585. doi: 10.1086/314952
Mosmann, T. R., and Coffman, R. L. (1989). Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 46, 111–147. doi: 10.1016/s0065-2776(08)60652-5
Nicol, A. J., Tazbirkova, A., and Nieda, M. (2011). Comparison of clinical and immunological effects of intravenous and intradermal administration of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells. Clin. Cancer Res. 17, 5140–5151. doi: 10.1158/1078-0432.CCR-10-3105
Padte, N. N., Boente-Carrera, M., Andrews, C. D., McManus, J., Grasperge, B. F., Gettie, A., et al. (2013). A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS One 8:e78407. doi: 10.1371/journal.pone.0078407
Petrovsky, N. (2015). Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug. Saf. 38, 1059–1074. doi: 10.1007/s40264-015-03504
Petrovsky, N., and Aguilar, J. C. (2004). Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 82, 488–496. doi: 10.1111/j.0818-9641.2004.01272.x
Pica, N., and Palese, P. (2013). Toward a universal influenza virus vaccine: prospects and challenges. Annu. Rev. Med. 64, 189–202. doi: 10.1146/annurev-med-120611-145115
Proietti, E., Bracci, L., Puzelli, S., Di Pucchio, T., Sestili, P., De Vincenzi, E., et al. (2002). Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169, 375–383. doi: 10.4049/jimmunol.169.1.375
Rajao, D. S., and Perez, D. R. (2018). Universal vaccines and vaccine platforms to protect against influenza viruses in humans and agriculture. Front. Microbiol. 9:123. doi: 10.3389/fmicb.2018.00123
Sakabe, S., Ozawa, M., Takano, R., Iwastuki-Horimoto, K., and Kawaoka, Y. (2011). Mutations in PA. NP, and HA of a pandemic (H1N1) 2009 influenza virus contribute to its adaptation to mice. Virus. Res. 158, 124–129. doi: 10.1016/j.virusres.2011.03.022
Singh, N., Hong, S., Scherer, D. C., Serizawa, I., Burdin, N., Kronenberg, M., et al. (1999). Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163, 2373–2377.
Sivakumar, S. M., Safhi, M. M., Kannadasan, M., and Sukumaran, N. (2011). Vaccine adjuvants - current status and prospects on controlled release adjuvancity. Saudi Pharm. J. 19, 197–206. doi: 10.1016/j.jsps.2011.06.003
Stevens, T. L., Bossie, A., Sanders, V. M., Fernandez-Botran, R., Coffman, R. L., Mosmann, T. R., et al. (1988). Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334, 255–258. doi: 10.1038/334255a0
Tetsutani, K., and Ishii, K. J. (2012). Adjuvants in influenza vaccines. Vaccine 30, 7658–7661. doi: 10.1016/j.vaccine.2012.10.007
Uraki, R., Das, S. C., Hatta, M., Kiso, M., Iwatsuki-Horimoto, K., Ozawa, M., et al. (2014). Hemozoin as a novel adjuvant for inactivated whole virion influenza vaccine. Vaccine 32, 5295–5300. doi: 10.1016/j.vaccine.2014.07.079
Wood, N., and Siegrist, C. A. (2011). Neonatal immunization: where do we stand? Curr. Opin. Infect. Dis. 24, 190–195. doi: 10.1097/QCO.0b013e328345d563
Yamayoshi, S., and Kawaoka, Y. (2019). Current and future influenza vaccines. Nat. Med. 25, 212–220. doi: 10.1038/s41591-018-0340-z
Youn, H. J., Ko, S. Y., Lee, K. A., Ko, H. J., Lee, Y. S., Fujihashi, K., et al. (2007). A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine 25, 5189–5198. doi: 10.1016/j.vaccine.2007.04.081
Keywords: glycolipid, 7DW8-5, influenza vaccine, adjuvants, mice
Citation: Feng H, Nakajima N, Wu L, Yamashita M, Lopes TJS, Tsuji M, Hasegawa H, Watanabe T and Kawaoka Y (2019) A Glycolipid Adjuvant, 7DW8-5, Enhances the Protective Immune Response to the Current Split Influenza Vaccine in Mice. Front. Microbiol. 10:2157. doi: 10.3389/fmicb.2019.02157
Received: 10 May 2019; Accepted: 02 September 2019;
Published: 18 September 2019.
Edited by:
Akio Adachi, Kansai Medical University, JapanReviewed by:
Weiming Yuan, University of Southern California, United StatesEtsushi Kuroda, National Institutes of Biomedical Innovation, Health and Nutrition, Japan
David J. Dowling, Boston Children’s Hospital, United States
Copyright © 2019 Feng, Nakajima, Wu, Yamashita, Lopes, Tsuji, Hasegawa, Watanabe and Kawaoka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tokiko Watanabe, tokikow@ims.u-tokyo.ac.jp; Yoshihiro Kawaoka, yoshihiro.kawaoka@wisc.edu
 Huapeng Feng
Huapeng Feng Noriko Nakajima2
Noriko Nakajima2 Li Wu
Li Wu Moriya Tsuji
Moriya Tsuji Hideki Hasegawa
Hideki Hasegawa Tokiko Watanabe
Tokiko Watanabe