- Department of Microbiology and Parasitology, Institute of Aquaculture, Universidade de Santiago de Compostela, Santiago de Compostela, Spain
Vibrio anguillarum causes a hemorrhagic septicemia that affects cold- and warm-water adapted fish species. The main goal of this work was to determine the temperature-dependent changes in the virulence factors that could explain the virulence properties of V. anguillarum for fish cultivated at different temperatures. We have found that although the optimal growth temperature is around 25°C, the degree of virulence of V. anguillarum RV22 is higher at 15°C. To explain this result, an RNA-Seq analysis was performed to compare the whole transcriptome profile of V. anguillarum RV22 cultured under low-iron availability at either 25 or 15°C, which would mimic the conditions that V. anguillarum finds during colonization of fish cultivated at warm- or cold-water temperatures. The comparative analysis of transcriptomes at high- and low-iron conditions showed profound metabolic adaptations to grow under low iron. These changes were characterized by a down-regulation of the energetic metabolism and the induction of virulence-related factors like biosynthesis of LPS, production of hemolysins and lysozyme, membrane transport, heme uptake, or production of siderophores. However, the expression pattern of virulence factors under iron limitation showed interesting differences at warm and cold temperatures. Chemotaxis, motility, as well as the T6SS1 genes are expressed at higher levels at 25°C than at 15°C. By contrast, hemolysin RTX pore-forming toxin, T6SS2, and the genes associated with exopolysaccharides synthesis were preferentially expressed at 15°C. Notably, at this temperature, the siderophore piscibactin system was strongly up-regulated. In contrast, at 25°C, piscibactin genes were down-regulated and the vanchrobactin siderophore system seems to supply all the necessary iron to the cell. The results showed that V. anguillarum adjusts the expression of virulence factors responding to two environmental signals, iron levels and temperature. Thus, the relative relevance of each virulence factor for each fish species could vary depending on the water temperature. The results give clues about the physiological adaptations that allow V. anguillarum to cause infections in different fishes and could be relevant for vaccine development against fish vibriosis.
Introduction
Vibrio anguillarum is a marine bacterium inhabitant of the estuarine and marine coastal ecosystems worldwide. It is an important fish pathogen since it is the etiological agent of classical vibriosis in warm- and cold-water fish species leading to high mortalities and economic losses in aquaculture (Toranzo et al., 2017). Bivalve molluscs and crustaceans are also occasionally affected by this bacterium (Aguirre-Guzman et al., 2004; Paillard et al., 2004). V. anguillarum isolates are classified into 23 different O-serogroups (O1 to O23) (Pedersen et al., 1999), the most virulent strains belong to serotypes O1, O2, and, to a lesser extent, O3. The remaining serotypes are mostly environmental strains isolated from seawater, marine animals, or sediments (Frans et al., 2011; Toranzo et al., 2017).
As a marine bacterium, V. anguillarum must be able to adapt its physiology to environmental fluctuations in salinity, availability of nutrients or temperature. It is an eurythermal bacterium capable of growing in a wide range of temperatures, from 10 to 35–42°C, with its optimal growth temperature around 25–30°C (Guérin-Faublée et al., 1995). Seasonal rising of seawater temperature has been associated with the proliferation of Vibrio species (Maeda et al., 2003), and hence, the occurrence of some fish diseases caused by these bacteria is also increased (Le Roux et al., 2015). V. anguillarum can cause vibriosis at low temperatures (5–18°C) (Ma et al., 2017), with 15°C considered as the optimal temperature to cause vibriosis outbreaks (Austin and Austin, 2007; Bellos et al., 2015). Thus, vibriosis episodes, as most ectothermic aquatic animal infectious diseases (Guijarro et al., 2015), usually occur at a temperature lower than that for the optimal growth of the bacteria. In addition, psychrotrophic V. anguillarum strains were also isolated from farmed fish in Norway causing diseases at water temperatures of 1–4°C (Olafsen et al., 1981). Remarkably, V. anguillarum survives in a cultivable form for more than a year in saline water (Hoff, 1989) where darkness, coldness (around 5°C), and anaerobiosis are environmental factors that help the pathogen to survive (Eguchi et al., 2000). This favors the dissemination and presence of V. anguillarum in seawater and sediments in all seasons (Muroga et al., 1986; Maeda et al., 2003).
The mechanisms of V. anguillarum that cause disease in fish are not yet completely understood; nonetheless, a considerable number of virulence factors have been identified. They include motility, chemotaxis, LPS, extracellular products with hemolytic and proteolytic activities, and multiple iron-uptake systems (Li and Ma, 2017; Toranzo et al., 2017). Exopolysaccharide production is required for biofilm formation and colonization of skin mucosal layer in the initial stages of infection (Croxatto et al., 2007; Weber et al., 2010). To date, the most relevant virulence factors identified in V. anguillarum are the metalloprotease EmpA (Crisafi et al., 2014), hemolysins such as Vah1 (Hirono et al., 1996), Vah2–5 or Rtx (Rodkhum et al., 2006; Li et al., 2008), and a complete set of iron uptake mechanisms including genes for heme utilization (Mouriño et al., 2004), transport of unchelated ferrous iron (feoABC) and, most notably, three siderophore systems vanchrobactin, anguibactin, and piscibactin (Li and Ma, 2017; Balado et al., 2018). Interestingly, we recently found that many highly pathogenic V. anguillarum strains are able to produce two siderophores simultaneously: vanchrobactin and piscibactin (Balado et al., 2018). Since piscibactin has a dual requirement of iron starvation and low temperature to be synthesized, the production of each siderophore is balanced in a temperature-dependent manner. It was postulated that piscibactin production would enhance niche flexibility conferring wide virulence properties even at low temperatures (Balado et al., 2018). In addition, some recent studies have demonstrated that environmental stress could affect the expression of virulence genes that are involved in the ability of V. anguillarum to cause infection (Crisafi et al., 2014; Guanhua et al., 2018).
The ability of a bacterial pathogen to cause disease depends not only on the presence of virulence factors but also, and more importantly, on a tight control of their expression. The main goal of this work was to determine the temperature-dependent changes in the transcriptome that could identify virulence properties of V. anguillarum for fish cultivated at cold- or warm-water temperatures. For this purpose, an RNA-Seq analysis was performed to compare the whole transcriptome profile of V. anguillarum strain RV22 cultured under low-iron availability at either 25 or 15°C, which would mimic the conditions that V. anguillarum finds during host colonization at warm- or cold-water temperatures.
Materials and Methods
Bacterial Strains and Culture Media
We have used V. anguillarum strain RV22 (serotype O2) to study the temperature-dependent virulence properties of V. anguillarum since it is closely related to HI610, a strain that was reported to cause high mortality ratios at temperatures below the optimal growth (Rønneseth et al., 2017). Both strains share 97.6782% of their genome sequence with an identity of 99.9689% according to data from NCBI genome pairwise comparison (accession no.: HI610, GCA_001989835.1; RV22, GCA_000257185.1).
Vibrio anguillarum RV22 was routinely grown at 25°C on tryptic soy agar (TSA) or broth (TSB) (Cultimed) supplemented with 1% NaCl (TSA-1 or TSB-1). Stock cultures were kept frozen at −80°C in TSB-1 with 15% (v/v) glycerol. CM9 minimal medium (Miller, 1992) was also used in some assays.
Fish Virulence Assays
The virulence assay was carried out with Senegalese sole (Solea senegalensis) fingerlings with an average weight of 10 g. Fish were divided into four groups of 30 animals. Two fish groups were maintained in 50-L seawater tanks at 15°C and the others at 25°C with continuous aeration. Fish were inoculated intraperitoneally (ip) with 0.1 ml of bacterial cell suspension of V. anguillarum RV22. The dose used was 2–5 × 105 CFU per fish obtained by 10-fold serial dilutions of a bacterial suspension at an OD600 = 0.5 prepared by resuspending several colonies from a 24-h TSA-1 culture into saline solution (0.85% NaCl). The precise number of injected bacterial cells was determined by plate count of 10-fold serial dilutions on TSA-1. Two control groups (one per each temperature) were inoculated with 0.1 ml of saline solution (0.85% NaCl) and maintained at 15 or 25°C. Mortalities were recorded daily for 10 days after injection and statistical significance of differences in survival curves was determined using the Kaplan–Meier method with Mantel–Cox log-rank test using SPSS (version 20; IBM SPSS Inc., Chicago, IL). P values were considered significant when P < 0.05. The protocol for animal experimentation used in this study followed the Spanish and European Union legislation and has been reviewed and approved by the Bioethics Committee of the University of Santiago de Compostela.
Growth Conditions and Total RNA Extraction
Vibrio anguillarum RV22 was grown aerobically in CM9 minimal medium under iron-deficient (2,2′-dipyridyl 50 μM) conditions at two different temperatures: 25 and 15°C. These culture conditions would mimic those that the pathogen encounters during the infection of warm- and cold-water fishes. As reference controls, V. anguillarum was grown in CM9 supplemented with iron (Fe2SO4 10 μM) at the same temperatures. Bacterial cells were harvested at the mid-log phase (OD600 ∼ 0.8) by centrifugation at 10,000 × g for 10 min and total RNA was isolated with RNAwiz (Ambion) following the manufacturer’s recommendations. RNA was isolated from three independent samples at each experimental condition (biological replicates). The quality and concentration of RNA were determined by 1% agarose gel visualization and by using the RNA 6000 NanoKit on the Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA). RNA samples were stored at −80°C until further utilization.
cDNA Library Construction and Sequencing
RNA extraction from triplicate biological samples was used to construct independent cDNA libraries for whole-transcriptome sequencing. Elimination of residual DNA and bacterial rRNA depletion was done before library construction. Each biological replicate was represented in an independent library that consisted of ca. 20 M of 2 × 150 bp reads. Massive sequencing was performed in an Illumina MiSeq sequencing machine using NextSeq High Output 1 × 150 pb kit. Construction of cDNA Libraries and Illumina Sequencing was carried out by FISABIO Sequencing and Bioinformatics Service (Valencia, Spain). RNAseq reads were deposited at NCBI Sequence Read Archive (SRA) under accession no. SRP213600. Reads mapped statistics and accession numbers of different samples are shown in Supplementary Table S1.
Bioinformatic Analysis and Gene Expression Quantification
RNA-Seq data analysis and validation were performed using the set of open source software programs included in Tuxedo suite (Ghosh and Chan, 2016). Briefly, reads were aligned to chromosomes I and II of V. anguillarum RV22 strain (GenBank accession no. GCA_000257185.1) using Tophat. Then, Cufflinks was used to assemble these mapped reads into possible transcripts and generate a final transcriptome assembly. Cuffdiff was used to identify differentially expressed genes and transcripts between each biological condition. The complete data set is shown in Supplementary Table S2. Finally, the R package CummeRbund was used to process the output files of Cuffdiff, resulting in an output in the form of quality plots and figures. Blast2GO was used for functional annotation, enzyme code mapping, and analysis of pathway maps. COG database and KEGG were used to DEGs functional classification. Subcellullar localization prediction was done using PSORTb v3.0 (Yu et al., 2010).
Phenotypic Characterization
In order to validate some of the results obtained through RNA-Seq analysis, some phenotypic tests were evaluated under the same conditions used for RNA-Seq. We chose three traits that have been reported to be involved in the virulence of V. anguillarum: motility, formation of biofilm, and hemolytic activity.
Motility was measured by the soft agar method. An inoculum in CM9 at mid-log phase (OD600 ∼ 0.8) was stabbed into CM9 soft agar (CM9 supplemented with 0.4% agar) containing either Fe2SO4 10 μM or 2,2′-dipyridyl 50 μM. Motility was evaluated by observing cloudiness after incubation at 15 or 25°C during 48 h. The test was repeated three times. Photobacterium damselae subsp. piscicida DI21 was used as a non-motile control.
Biofilm formation was evaluated by the crystal violet staining assay. Glass tubes containing 10 ml of CM9 containing either Fe2SO4 10 μM or 2,2′-dipyridyl 50 μM were incubated at 25 or 15°C until mid-log phase (OD600 ∼ 0.8). The content of each tube was gently removed, and the remaining attached bacteria were fixed with 10 ml of methanol 99% (Panreac). The tubes were then stained for 5 min with 2 ml of crystal violet 2% (bioMérieux). Excess stain was rinsed off by placing the tubes under running tap water. After washing with PBS and air drying, the dye bound to the biofilm was solubilized with 10 ml of glacial acetic acid 33% (v/v) (Panreac). The absorbance was measured at 570 nm in a spectrophotometer (Hitachi U-2000). The assay was repeated three times. Statistical significance was determined by Student’s t test with a threshold p value < 0.05.
Hemolytic activity was detected in Columbia Agar plates (Oxoid). V. anguillarum RV22 was cultured in CM9 plates containing 2,2′-dipyridyl 50 μM at either 25 or 15°C for 24 and 48 h, respectively. A loopful of cell biomass, scratched from the surface of CM9 plates, were deposited on the surface of Columbia Agar plates. Hemolytic halos were inspected after 48 h of incubation at 25 or 15°C.
β-Galactosidase Assay
The V. anguillarum RV22 strain carrying either plasmid pMB276 (PfrpA:lacZ in pHRP309) or plasmid pMB277 (ParaC1:lacZ) (Balado et al., 2018) was grown in CM9 minimal medium under iron starvation (2,2′-dipyridyl 50 μM) at 10, 15, or 25°C up to OD600 ∼ 0.3–0.4. Then, β-galactosidase activities were measured by the method of Miller (1992) as previously described (Balado et al., 2018). The results shown are the means of three independent experiments, with each measure being performed in triplicate. LacZ fusion of housekeeping gene proC (pML263) (Balado et al., 2018) was used as constitutive control.
Results
Growth Kinetics and Virulence of V. anguillarum RV22 at Cold (15°C) and Warm (25°C) Temperatures
Temperature is a determining factor that could have effects on immune response of ectotherms, especially if fish are exposed to temperatures outside their thermal limits (Fletcher and Secombes, 2015). Thereby, in order to assay the virulence properties of V. anguillarum at two different water temperatures (15 and 25°C), S. senegalensis was chosen as a fish model, since it is a species that is naturally exposed to water temperatures from 12 to 26°C (Arjona et al., 2010). Then, survival curves at each temperature were compared to the growth ability of V. anguillarum when it is cultivated in CM9 medium (Figure 1A). Groups of 30 fish were acclimated at water temperature of 15°C (cold) or 25°C (warm) and infected with the same dose of V. anguillarum RV22 (2–5 × 105 CFU per fish). No mortality was observed in fish control groups inoculated with 0.1 ml of saline solution (0.85% NaCl). The results show that the fish mortality observed after infection with V. anguillarum RV22 significantly increase (p = 0.006) at cold-water temperature (Figure 1B). Mortality observed at 15°C was higher than that found at 25°C, reaching 95% 7 days post-infection. On the contrary, when the fish were maintained at a temperature of 25°C, a 63% mortality was reached 9 days after challenge. Thus, the slow growth of V. anguillarum when cultivated in vitro at 15°C (Figure 1A) greatly contrasts with the substantial increase in its virulence at the same temperature compared with the virulence at 25°C (Figure 1B).
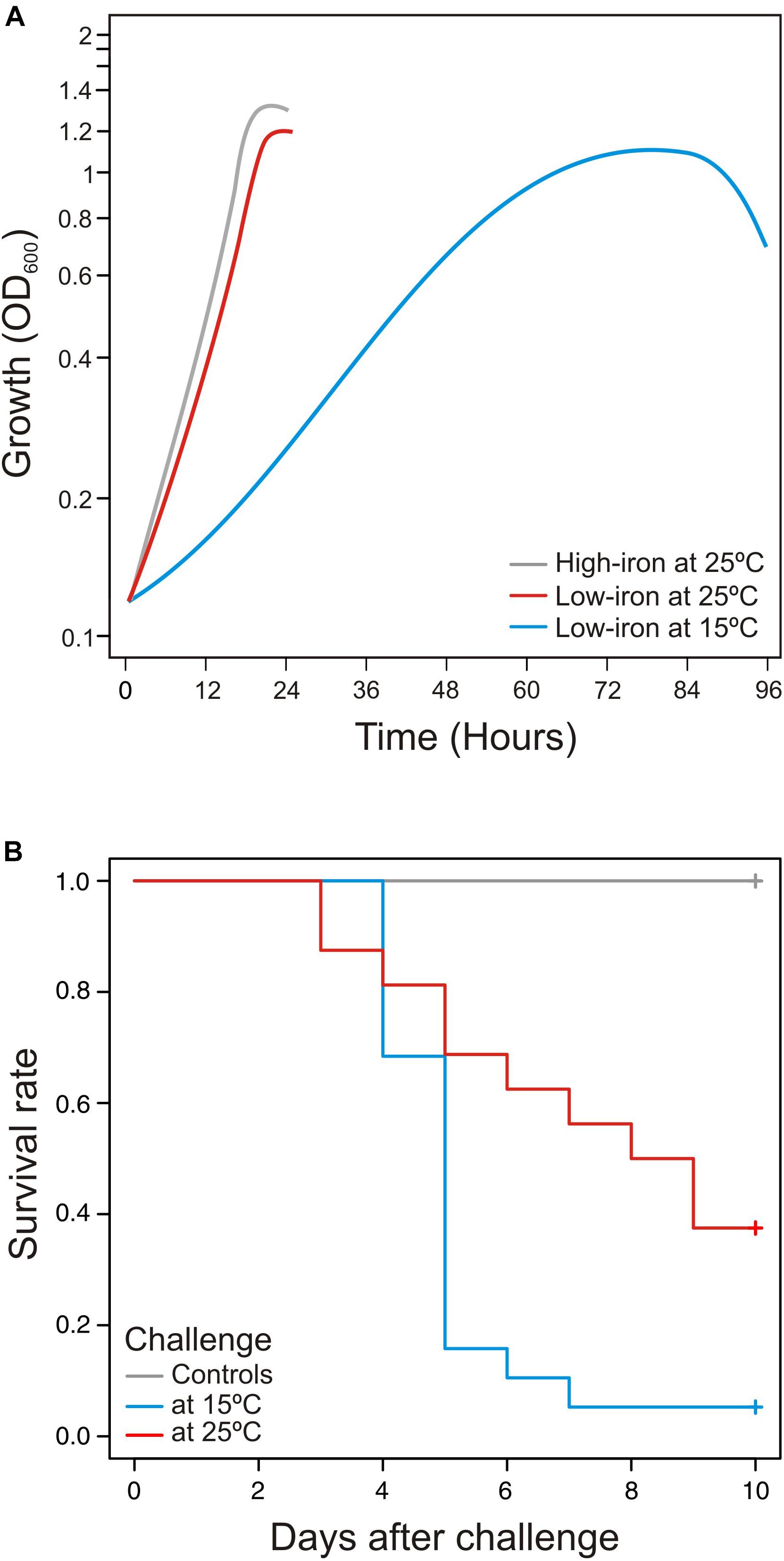
Figure 1. (A) Influence of temperature in growth dynamics and (B) virulence of Vibrio anguillarum at cold-water (15°C) and warm-water (25°C) temperature. Growth (A) and virulence (B) of V. anguillarum RV22 strain assayed at two different temperatures: 15 and 25°C. Growth was assayed in CM9 minimal medium supplemented with Fe2SO4 10 μM (high-iron conditions) or 2,2′-dipyridyl 50 μM (low-iron conditions) by measuring OD600 for 24 h (25°C) or 96 h (15°C). Senegalese sole fingerlings were experimentally infected with the same dose of V. anguillarum RV22 (2–5 × 105 CFU per fish) and kept at 15 or 25°C for 10 days, recording mortalities daily.
Analysis of Transcriptome Changes at 25 and 15°C Under Low-Iron Conditions
To analyze the expression pattern of V. anguillarum during iron starvation at cold- and warm-water temperature, an RNA-Seq assay was performed in cells of V. anguillarum RV22 grown in CM9 minimal medium, supplemented with 50 μM of the iron chelator 2,2′-dipyridyl (low-iron conditions), at 15 and 25°C (Figure 2A). The addition of 2,2′-dipyridyl reduces the availability of iron, mimicking the iron-deprived conditions that bacterial pathogens find during host colonization (Skaar, 2010). In addition, we used as a control the expression pattern of V. anguillarum RV22 grown at 25°C in CM9 supplemented with iron (high-iron conditions).
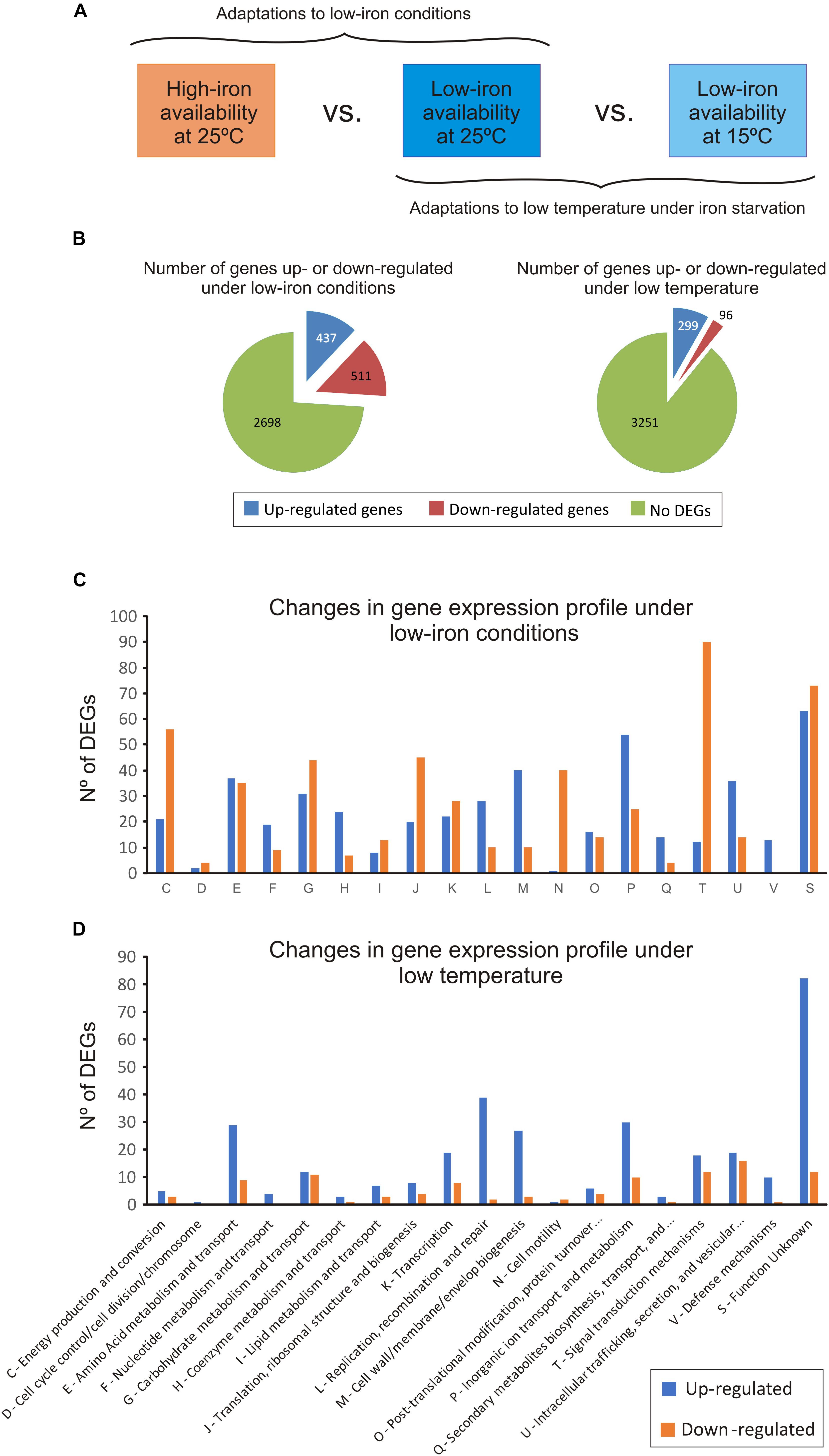
Figure 2. Changes in the transcriptome of Vibrio anguillarum RV22 between low- and high-iron conditions at 25°C (to test the effect of iron) and between 25 and 15°C under iron starvation (to test the effect of temperature). (A) General overview of the experimental design. (B) Summary of the number of differentially expressed genes (DEGs), up-regulated and down-regulated, under iron limitation and at low temperature. (C) Number of DEGs under iron limitation classified under the KEGG categories. (D) Number of DEGs, up-regulated and down-regulated, at 15°C classified under the KEGG categories.
The comparative transcriptome analysis under low- and high-iron at 25°C identified 948 differentially expressed genes (DEGs) (Figure 2B): 511 genes were down-regulated and 437 were up-regulated under low-iron conditions. The complete data set is shown in Supplementary Table S2. For a better interpretation of the low-iron induced adaptations, the predicted products of DEGs were grouped in 13 functional KEGG categories (Figure 2C). The results showed profound changes in cellular metabolism. Most of the down-regulated DEGs encode functions related to signal transduction (category T), energy metabolism (C), cell motility (N), and translation and ribosome structure (J). By contrast, functions related to DNA replication, recombination and repair (L), cell wall/membrane biogenesis (M), and inorganic ion transport (P) were up-regulated under low iron. In addition, the number of DEGs down- and up-regulated related to amino acids (E) and carbohydrate metabolism (G) functions, as well as some transcriptional factors (K) were equilibrate, which would suggest that the adaptation to grow under iron deprivation implies changes in amino acid and carbohydrate requirements (Figure 2C). Specific aspects of low-iron adaptation are detailed below.
Most DEGs at low temperature were up-regulated (299 DEGs) (Figures 2B,D). They were mostly related with amino acids metabolism and transport (E), DNA replication recombination and repair (L), cell wall/membrane biogenesis (M), and inorganic ion transport (P) (Figure 2D). Despite the significantly slow growth rate at 15°C, the expression pattern of the genes related to the energetic metabolism was quite similar. There was a down-regulation of some metabolic genes including the regulator HexR, a protein that mediates the control of the central carbon metabolism by repression (Leyn et al., 2011). In addition, a pronounced down-regulation of genes involved in the synthesis of large and small subunits of ribosomes and tRNA biogenesis (translation, J) was observed. Thus, these limiting conditions required a low level of ribosomal proteins for the bacterial cell and demand a low rate of polypeptide synthesis (Dennis and Bremer, 2008). Some components of the carbohydrate and amino acid transport systems (ABC transporters and phosphotransferase systems) were also down-regulated at 15°C (Supplementary Table S2). The results suggest that the decrease of nutrient import to the cell under the conditions tested negatively affects the ability of V. anguillarum RV22 to grow at low temperatures, resulting in a slower growth kinetics.
Iron Starvation Redirects Central Metabolism and Induces the Expression of Virulence Factors
The analysis of metabolic genes up- and down-regulated under iron starvation showed global metabolic adaptations and the induction of most virulence factors (Supplementary Table S3). In particular, genes encoding functions of TCA cycle and cytochromes were down-regulated extensively. The ATP synthase was also down-regulated. By contrast, alternative members of the respiratory chain were up-regulated. Simultaneously, seven DEGs coding enzymes of the glycolytic pathway and most genes coding pentose phosphate pathway functions were up-regulated. Under iron starvation genes encoding functions for valine, leucine and isoleucine degradation were down-regulated. The genes hutGHIU involved in histidine metabolism were also down-regulated. By contrast, biosynthesis of the aromatic amino acids phenylalanine, tyrosine, and tryptophan were up-regulated since the operon trpBCGE was 4- to 6-fold induced. However, conversion of phenylalanine to tyrosine was repressed since transcription of phhA decreased. Additionally, the synthesis pathway of arginine (arginine/ornithine), which is one of the amino acids required to synthesize the siderophore vanchrobactin (Soengas et al., 2006), was induced 2-fold.
The virulence-related factors identified to date in V. anguillarum include LPS, motility and chemotaxis, multiple iron-uptake systems, and the secretion of extracellular products with hemolytic and proteolytic activities (Li and Ma, 2017; Toranzo et al., 2017). Most genes involved in polysaccharide production (VAR_RS0112290–VAR_RS0112465), transport and assemble (wza–wzc) (VAR_RS0107285–VAR_RS0107295), and wbfB–wbfD (VAR_RS0107305–VAR_RS0107315) (Croxatto et al., 2007; Naka et al., 2011) were significantly up-regulated during iron starvation (Supplementary Table S4). In this regard, we tested biofilm formation under the same conditions used for RNA-Seq, and no significant differences were detected between cells grown under iron starvation or under iron excess at 25°C (Supplementary Figure S1). Hemolytic activity is a major virulence factor in V. anguillarum and contributes to the hemorrhagic septicemia characteristic of vibriosis (Hirono et al., 1996). Some cytotoxic proteins have been characterized to date including EmpA, Vah1 cluster (vah1 and plp genes), Vah2–5 hemolysins, and the multifunctional autoprocessing Rtx toxin (MARTX, rtxACHBDE) (Li et al., 2008). The genome of V. anguillarum RV22 contains vah1–3 and MARTX toxins (does not contain either empA, vah4, or vah5 gene). It is noteworthy that Vah1 and Vah2 hemolysins showed high expression levels under high-iron conditions, which greatly contrast with the low expression level of MARTX and Vah3 under the same condition. While Vah2 was almost constitutively expressed in all assayed conditions, Vah1, Vah3, and MARTX were induced when V. anguillarum was grown under low iron (Table 1 and Supplementary Table S5). Some DEGs up-regulated under low iron were identified as outer membrane transporters. They include genes encoding non-specific outer membrane proteins (OMP proteins) and two complete Type VI Secretion Systems (T6SS).
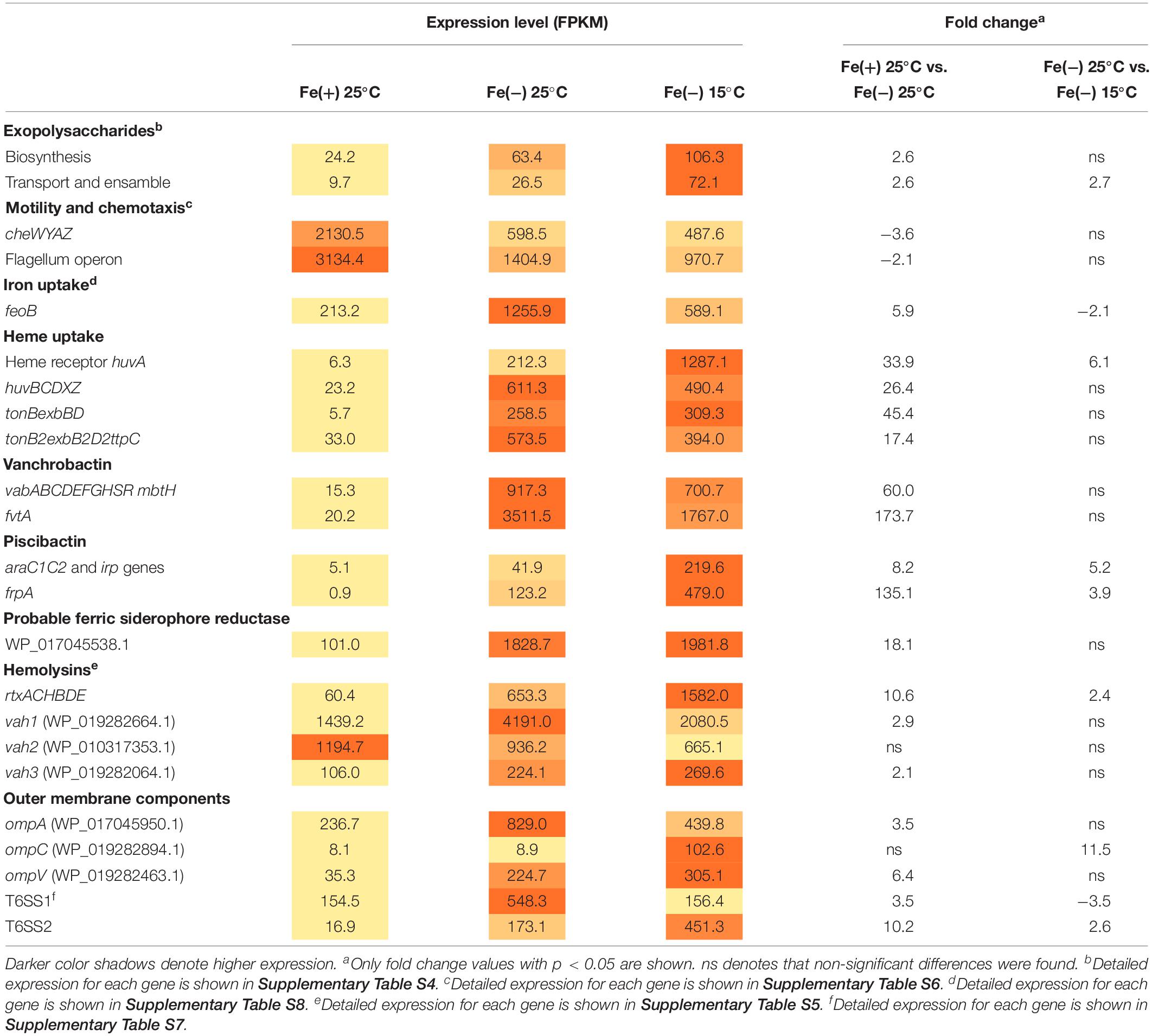
Table 1. Expression of most relevant virulence-related factors at 25 and 15°C and under iron deficiency.
Not surprisingly, iron acquisition systems were strongly up-regulated under iron starvation. The feoB that mediates ferrous iron acquisition (Li and Ma, 2017) was 5.9-fold induced. huvABCDZX genes encoding heme uptake and utilization system (Mouriño et al., 2004) increased the transcription level more than 30-fold and, more notably, the genes encoding siderophore systems, vanchrobactin and piscibactin, were among the top up-regulated genes since they were induced more than 60-fold. Nonetheless, at 25°C, the vanchrobactin system is expressed more than 20-fold compared to piscibactin. Both ferri-siderophore complexes and heme group are internalized via specific outer membrane receptors in a process that is driven by the inner membrane potential and mediated by the energy-transducing systems TonB1 and TonB2, whose genes were up-regulated 46- and 18-fold under iron starvation. Interestingly, locus VAR_RS0103565 was induced 18.1-fold under iron starvation and it could code a putative ferric-siderophore transport protein uncharacterized to date.
Overall, virulence-related factors were up-regulated under low iron compared to high iron either at 25 or 15°C, although two exceptions were found. Chemotaxis-related (cheWYAZ) and motility-related (fli and flg) genes decreased their expression 2.3- and 5.8-fold when V. anguillarum was grown under low iron (Supplementary Table S6). In agreement with this observation, the evaluation of motility in soft agar showed that V. anguillarum RV22 is less motile under iron starvation (Supplementary Figure S2).
V. anguillarum Expresses a Specific Cocktail of Virulence Factors at Each Temperature, Either 25 or 15°C
The main focus of the present work was to characterize the changes in expression of virulence factors after temperature drop that would allow the understanding of the enhanced virulence shown by V. anguillarum at 15°C compared to 25°C water temperature. RNA-Seq results showed that, although the expression of LPS biosynthetic genes did not show statistically significant differences, genes encoding functions related to exopolysaccharide synthesis showed maximal expression at low temperature (Supplementary Table S4). In fact, biofilm formation showed a statistically significant increase at 15°C (Supplementary Figure S1). As shown above, MARTX is almost silenced under high-iron conditions, increasing 10-fold the operon transcription when V. anguillarum senses low-iron levels. Besides, the expression of MARTX was 2.4-fold up-regulated at 15°C. Thus, MARTX would be preferentially expressed at low temperatures and under iron starvation (Table 1 and Supplementary Table S5). The effect of temperature on hemolytic phenotype could be detected on Columbia Agar plates, where V. anguillarum RV22 cells caused a higher translucency of the hemolytic halo when it was grown at 15°C (Supplementary Figure S3), indicating production of different hemolysins at either 25 or 15°C.
While major porines OmpA and OmpV were 3.5- and 6.4-fold induced under iron starvation at 25°C, transcription of ompC increased only at low temperature (11.5-fold). Vibrio anguillarum genome harbors two T6SS (Guanhua et al., 2018). T6SS1 and T6SS2 were induced 3- and 10-fold, respectively, under iron starvation. However, T6SS1 was ca. 3-fold more expressed at 25°C than T6SS2 (Table 1 and Supplementary Table S7). It is noteworthy that each T6SS responds to low temperature in opposite ways. While T6SS1 was 3.5-fold down-regulated (decrease from 550 to 156), T6SS2 was 2.6-fold induced (Table 1). Thus, both systems are up-regulated during iron starvation, but T6SS1 is preferentially expressed at 25°C and T6SS2 at 15°C. Overall, the results showed that T6SS1 has a high basal expression level since it was significantly expressed in all the assayed conditions. By contrast, T6SS2 required low iron and cold temperature to be induced.
Piscibactin biosynthetic and uptake genes as well as heme receptor HuvA were greatly up-regulated at low temperature. Regarding the piscibactin system, the AraC-like transcriptional regulator araC1 (VAR_RS0103450) and the putative piscibactin outer membrane receptor frpA (VAR_RS0103460) were two of the most up-regulated genes at 15°C since their transcription showed a 6.8- and 3.9-fold increase, respectively (Table 1 and Supplementary Table S8). To confirm whether temperature acts as an environmental regulatory signal that modulates piscibactin gene transcription, the transcriptional activities of frpA (PfrpA) and araC1 (ParaC1) (Balado et al., 2018) promoters were measured at three temperatures (10, 15, and 25°C). The results indicate that the β-galactosidase activity of both piscibactin promoters dramatically decreased as temperature was increased (Figure 3), denoting a strong negative correlation between temperature and the transcriptional activity of piscibactin promoters. These results confirm the transcriptomic data and demonstrate that piscibactin operon is repressed at 25°C and that it is highly expressed at low temperatures.
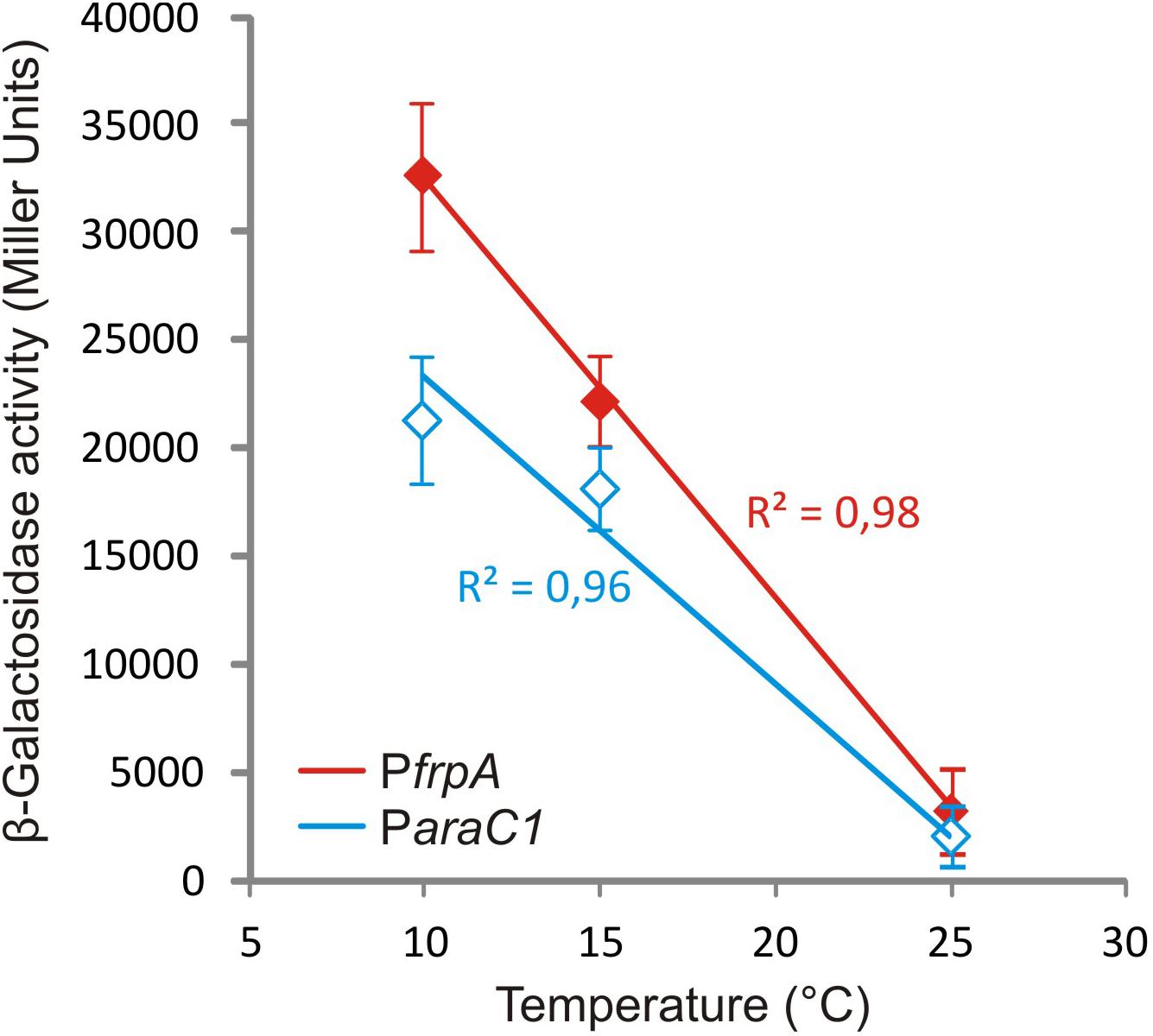
Figure 3. Plot of β-galactosidase activity of transcriptional fusions between two promoters from siderophore piscibactin gene cluster (PfrpA and ParaC1) of Vibrio anguillarum RV22 and lacZ at increasing temperatures. All measures were carried out in triplicate, and standard deviations for each point are indicated.
Discussion
The water temperature is a risk factor that enhances the occurrence of disease outbreaks caused by Vibrio species (Le Roux et al., 2015). V. anguillarum is able to cause disease outbreaks in a wide temperature range, since it can infect many different farmed fish species at both cold- and warm-water temperatures (Bellos et al., 2015; Ma et al., 2017; Rønneseth et al., 2017; Toranzo et al., 2017). On the other hand, pathogenic bacteria face a drastic shift in iron availability upon host encounter (Skaar, 2010). Low-iron adaptations were described in most bacterial pathogens, and it is well established that low-iron conditions up-regulate the expression of most virulence factors (Andrews et al., 2003; Mey et al., 2005; Friedman et al., 2006). Our results show that both environmental signals, iron and temperature, dually regulate the expression of virulence factors and other key components of the cell. We have shown that although a low temperature reduces the growth of V. anguillarum, the virulence achieved for fish kept in cold water (15°C) is significantly higher than that observed in warmer water (25°C). Although low temperature can have negative effects on the fish immune system, especially on the adaptive immunity (Fletcher and Secombes, 2015), the observation that, at 15°C, there is an increase in the expression of some relevant virulence factors such as the heme receptor HuvA, siderophore piscibactin, MARTX toxins, and T6SS2, could explain in part the higher virulence at this temperature. However, other important factors like hemolysin Vah1, T6SS1, ferrous iron transport, and vanchrobactin siderophore system showed higher expression at the optimal growth temperature (25°C). Thus, all these suggest that V. anguillarum produces a specific cocktail of virulence factors at each temperature and that its virulence at a particular temperature would be more related to the modulation of expression of virulence factors than to the higher or lower capacity to grow.
Numerous virulence factors have been described in V. anguillarum (Toranzo et al., 2017; Hickey and Lee, 2018), and although most studies were performed in strains from serotype O1, some discrepancies exist among serotypes (Tang et al., 2016). V. anguillarum LPS have endotoxic activity and allow the pathogen to adhere and to persist within the host by evading the immune system response (Boesen et al., 1999; Lindell et al., 2012). We found a significant up-regulation of genes involved in LPS biosynthesis, assembly, and export under iron starvation conditions, but additionally, their expression increased at low temperature (Supplementary Table S4). Chemotaxis and motility have been recognized as virulence-related factors (Larsen, 2004; Guanhua et al., 2018). Although V. anguillarum RV22 is motile under the three conditions tested (Supplementary Figure S2), our results showed that chemotaxis and flagellum-related genes were significantly down-regulated under both low iron availability and low temperature. Low-iron conditions also reduced motility of Vibrio vulnificus (Pajuelo et al., 2016). Nevertheless, although genes encoding structural components of flagellum were down-regulated, their basal expression was relatively high, and some proteins of the flagellum motor were even up-regulated under those conditions. Congruently, it was reported that V. anguillarum is chemotactic and motile at the temperature range of 5–25°C, with the maximal motility achieved at 25°C (Larsen, 2004). Active motility is needed for entry into the fish host (Ormonde et al., 2000), but once the bacterium has invaded the fish, motility is no longer needed for the progression of vibriosis (Milton et al., 1996; O’Toole et al., 1996).
Hemolysin Vah1 and MARTX toxin are mainly responsible for the hemolytic and cytotoxic activities of V. anguillarum in fish (Hirono et al., 1996; Li et al., 2008). Vah1 and RtxA have cytotoxic effects on Atlantic salmon kidney cells and only a vah1 rtxA double mutant was no longer cytotoxic. However, only rtxA mutants had reduced virulence in Atlantic salmon (Li et al., 2008). Our results revealed that MARTX is preferentially produced at 15°C. Consequently, the implication of MARTX in the virulence assays previously reported could be overestimated, since the implication of MARTX in virulence would vary according to the environment temperature.
Three non-specific outer membrane proteins (OMP proteins) were differentially expressed at 15 and 25°C. While OmpA and OmpV were induced under iron starvation, expression of OmpC significantly increased under iron deficiency but only at 15°C (Table 1). OmpA plays a crucial role in the attachment of bacterial cells to the host cells during the invasion process and evasion of host defenses (Confer and Ayalew, 2013). OmpV is one of the major outer membrane proteins in V. cholerae and it is highly immunogenic (Stevenson et al., 1985). OmpC is involved in membrane integrity maintenance and resistance to antibiotics (Choi and Lee, 2019) and it has been reported that its synthesis is favored in high osmolarity conditions and warm temperatures (25°C) (Davey et al., 1998).
Vibrio anguillarum has two T6SS that respond to iron deprivation by increasing their expression. In many bacteria, two or more T6SS gene clusters are found in a single bacterial genome (Bingle et al., 2008), often possessing different functions and regulation mechanisms (Cascales, 2008; Bernard et al., 2010; Schwarz et al., 2010). Interestingly, our results show that while T6SS1 is preferentially expressed at 25°C, T6SS2 shows the highest expression at 15°C. Although no significant loss of virulence was observed in V. anguillarum when T6SS2 was inactivated (Weber et al., 2009; Tang et al., 2016), recent works showed that inactivation of either T6SS1 or T6SS2 decreased the fitness of the bacterium within the fish (Guanhua et al., 2018). It was also reported that T6SS2 would regulate the expression of extracellular proteases via the stress-response regulator RpoS and the quorum sensing regulator VanT (Weber et al., 2009). Notably, the expression pattern of T6SS2 genes showed large discrepancies between O1 and non-O1 strains harboring 100% identical gene clusters. In some strains, their expression was not detected, while in other strains, its expression was more active under warm marine-like conditions (Tang et al., 2016). These findings suggest that unidentified expression factor(s) encoded outside the T6SS cluster might modulate its expression. The functional impact of T6SS in V. anguillarum fitness needs to be elucidated in further studies.
The genes encoding vanchrobactin and piscibactin siderophore systems are among the most up-regulated genes under iron starvation, increasing more than 60-fold their transcription. Nonetheless, the vanchrobactin system is significantly more expressed than piscibactin at 25°C. The results proved that the expression of ParaC1 and PfrpA promoters, which control the transcription of piscibactin genes (Balado et al., 2018), is proportional to the temperature decrease. Thus, while piscibactin genes are almost silenced at 25°C, they are significantly transcribed at 15°C. This result reinforces previous work showing that piscibactin siderophore has a dual requirement of low iron and low temperature to be synthesized (Balado et al., 2018). Since piscibactin is more relevant for virulence than vanchrobactin (Balado et al., 2018), the piscibactin transcription increment at 15°C would partially explain the higher degree of virulence of V. anguillarum at this temperature.
Conclusion
Knowledge on virulence factors regulation is essential for the development of new immunoprophylactic and chemotherapeutic treatments against the bacterial infections (Cole et al., 2009). Overall, the results obtained in the present work showed that disease severity caused by V. anguillarum is multifactorial and context dependent. V. anguillarum modulates the expression of most virulence factors in a temperature-dependent manner and in response to low iron levels. Thus, each virulence factor is favored at a specific temperature range, which implies that its relevance could greatly vary among the different fish species that this bacterium can infect, and even among different geographic locations. Current fish vaccines against vibriosis are usually produced by growing the bacterium at 25°C and in rich media with an excess of iron. Under these conditions, according to our results, some relevant cell components may not be adequately expressed, resulting in a vaccine that lacks some important antigens that are produced by V. anguillarum during an infection, which could result in vaccines with a lower degree of protection. Altogether, our results give clues about the physiological adaptations that allow V. anguillarum to cause infections in different fishes and could be useful to implement novel strategies to combat the incidence of vibriosis outbreaks by optimization of vaccine preparations.
Data Availability Statement
The datasets generated for this study can be found in the NCBI Sequence Read Archive (SRA). Accession Number: SRP213600.
Ethics Statement
The animal study was reviewed and approved by Bioethics Committee of the University of Santiago de Compostela.
Author Contributions
MAL and MB performed the lab experiments, analyzed the data, and wrote the first draft of the manuscript. MB and MLL corrected the draft and built the final version of the manuscript. All authors conceived and designed the study, contributed to manuscript revision, and read and approved the submitted version.
Funding
This work was supported by grants AGL2015-63740-C2-1-R and RTI2018-093634-B-C21 (AEI/FEDER, EU) from the State Agency for Research (AEI) of Spain and cofunded by the FEDER Programme from the European Union. The support of Xunta de Galicia (Spain) with grant GRC2018/018 is also acknowledged.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02335/full#supplementary-material
References
Aguirre-Guzman, G., Mejia Ruiz, H., and Ascencio, F. (2004). A review of extracellular virulence product of Vibrio species important in diseases of cultivated shrimp. Aquac. Res. 35, 1395–1404. doi: 10.1111/j.1365-2109.2004.01165.x
Andrews, S. C., Robinson, A. K., and Rodriguez-Quinones, F. (2003). Bacterial iron homeostasis. FEMS Microbiol. Rev. 27, 215–237. doi: 10.1016/s0168-6445(03)00055-x
Arjona, F. J., Ruiz-Jarabo, I., Vargas-Chacoff, L., Martín Del Río, M. P., Flik, G., Mancera, J. M., et al. (2010). Acclimation of Solea senegalensis to different ambient temperatures: implications for thyroidal status and osmoregulation. Mar. Biol. 157, 1325–1335. doi: 10.1007/s00227-010-1412-x
Austin, B., and Austin, D. A. (2007). Epizootiology: Gram-Negative Bacteria in Bacterial Fish Pathogens: Disease of Farmed and Wild Fish. Berlin: Springer.
Balado, M., Lages, M. A., Fuentes-Monteverde, J. C., Martínez-Matamoros, D., Rodríguez, J., Jiménez, C., et al. (2018). The siderophore piscibactin is a relevant virulence factor for Vibrio anguillarum favored at low temperatures. Front. Microbiol. 9:1766. doi: 10.3389/fmicb.2018.01766
Bellos, G., Angelidis, P., and Miliou, H. (2015). Effect of temperature and seasonality principal epizootiological risk factor on vibriosis and photobacteriosis outbreaks for european sea bass in greece (1998-2013). J. Aquac. Res. Dev. 6:338. doi: 10.4172/2155-9546.1000338
Bernard, C. S., Brunet, Y. R., Gueguen, E., and Cascales, E. (2010). Nooks and crannies in type VI secretion regulation. J. Bacteriol. 192, 3850–3860. doi: 10.1128/JB.00370-310
Bingle, L. E. H., Bailey, C. M., and Pallen, M. J. (2008). Type VI secretion: a beginner’s guide. Curr. Opin. Microbiol. 11, 3–8. doi: 10.7150/thno.23085
Boesen, H. T., Pedersen, K., Larsen, J. L., Koch, C., and Ellis, A. E. (1999). Vibrio anguillarum resistance to rainbow trout (Oncorhynchus mykiss) serum: role of O-antigen structure of lipopolysaccharide. Infect. Immun. 67, 294–301.
Cascales, E. (2008). The type VI secretion toolkit. EMBO Rep. 9, 735–741. doi: 10.1038/embor.2008.131
Choi, U., and Lee, C.-R. (2019). Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 10:953. doi: 10.3389/fmicb.2019.00953
Cole, D. W., Cole, R., Gaydos, S. J., Gray, J., Hyland, G., Jacques, M. L., et al. (2009). Aquaculture: environmental, toxicological, and health issues. Int. J. Hyg. Environ. Health 212, 369–377. doi: 10.1016/j.ijheh.2008.08.003
Confer, A. W., and Ayalew, S. (2013). The OmpA family of proteins: roles in bacterial pathogenesis and immunity. Vet. Microbiol. 163, 207–222. doi: 10.1016/j.vetmic.2012.08.019
Crisafi, F., Denaro, R., Genovese, M., Yakimov, M., and Genovese, L. (2014). Application of relative real-time PCR to detect differential expression of virulence genes in Vibrio anguillarum under standard and stressed growth conditions. J. Fish Dis. 37, 629–640. doi: 10.1111/jfd.12158
Croxatto, A., Lauritz, J., Chen, C., and Milton, D. L. (2007). Vibrio anguillarum colonization of rainbow trout integument requires a DNA locus involved in exopolysaccharide transport and biosynthesis. Environ. Microbiol. 9, 370–382. doi: 10.1111/j.1462-2920.2006.01147.x
Davey, M. L., Hancock, R. E., and Mutharia, L. M. (1998). Influence of culture conditions on expression of the 40-kilodalton porin protein of Vibrio anguillarum serotype O2. Appl. Environ. Microbiol. 64, 138–146.
Dennis, P. P., and Bremer, H. (2008). Modulation of chemical composition and other parameters of the cell at different exponential growth rates. EcoSal Plus 3, 1–49. doi: 10.1128/ecosal.5.2.3
Eguchi, M., Fujiwara, E., and Miyamoto, N. (2000). Survival of Vibrio anguillarum in freshwater environments: adaptation or debilitation? J. Infect. Chemother. 6, 126–129. doi: 10.1007/PL00012152
Frans, I., Michiels, C. W., Bossier, P., Willems, K. A., Lievens, B., and Rediers, H. (2011). Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J. Fish Dis. 34, 643–661. doi: 10.1111/j.1365-2761.2011.01279.x
Friedman, D. B., Stauff, D. L., Pishchany, G., Whitwell, C. W., Torres, V. J., and Skaar, E. P. (2006). Staphylococcus aureus redirects central metabolism to increase iron availability. PLoS Pathog. 2:e87. doi: 10.1371/journal.ppat.0020087
Ghosh, S., and Chan, C.-K. K. (2016). Analysis of RNA-Seq data using TopHat and cufflinks. Methods Mol. Biol. 1374, 339–361. doi: 10.1007/978-1-4939-3167-5-18
Guanhua, Y., Wang, C., Wang, X., Ma, R., Zheng, H., Liu, Q., et al. (2018). Complete genome sequence of the marine fish pathogen Vibrio anguillarum and genome-wide transposon mutagenesis analysis of genes essential for in vivo infection. Microbiol. Res. 216, 97–107. doi: 10.1016/J.MICRES.2018.08.011
Guérin-Faublée, V., Rosso, L., Vigneulle, M., and Flandrois, J. P. (1995). The effect of incubation temperature and sodium chloride concentration on the growth kinetics of Vibrio anguillarum and Vibrio anguillarum-related organisms. J. Appl. Bacteriol. 78, 621–629. doi: 10.1111/j.1365-2672.1995.tb03108.x
Guijarro, J. A., Cascales, D., García-Torrico, A. I., García-Domínguez, M., and Méndez, J. (2015). Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Front. Microbiol. 6:700. doi: 10.3389/fmicb.2015.00700
Hickey, M. E., and Lee, J.-L. (2018). A comprehensive review of Vibrio (Listonella) anguillarum: ecology, pathology and prevention. Rev. Aquac. 10, 585–610. doi: 10.1111/raq.12188
Hirono, I., Masuda, T., and Aoki, T. (1996). Cloning and detection of the hemolysin gene of Vibrio anguillarum. Microb. Pathog. 21, 173–182. doi: 10.1006/mpat.1996.0052
Hoff, K. A. (1989). Survival of Vibrio anguillarum and Vibrio salmonicida at different salinities. Appl. Environ. Microbiol. 55, 1775–1786.
Larsen, M. H. (2004). Influences of temperature, salinity and starvation on the motility and chemotactic response of Vibrio anguillarum. Microbiology 150, 1283–1290. doi: 10.1099/mic.0.26379-26370
Le Roux, F., Wegner, K. M., Baker-Austin, C., Vezzulli, L., Osorio, C. R., Amaro, C., et al. (2015). The emergence of Vibrio pathogens in Europe: ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front. Microbiol. 6:830. doi: 10.3389/fmicb.2015.00830
Leyn, S. A., Li, X., Zheng, Q., Novichkov, P. S., Reed, S., Romine, M. F., et al. (2011). Control of proteobacterial central carbon metabolism by the HexR transcriptional regulator: a case study in Shewanella oneidensis. J. Biol. Chem. 286, 35782–35794. doi: 10.1074/jbc.M111.267963
Li, L., Rock, J. L., and Nelson, D. R. (2008). Identification and characterization of a repeat-in-toxin gene cluster in Vibrio anguillarum. Infect. Immun. 76, 2620–2632. doi: 10.1128/IAI.01308-1307
Li, Y., and Ma, Q. (2017). Iron acquisition strategies of Vibrio anguillarum. Front. Cell. Infect. Microbiol. 7:342. doi: 10.3389/fcimb.2017.00342
Lindell, K., Fahlgren, A., Hjerde, E., Willassen, N.-P., Fällman, M., and Milton, D. L. (2012). Lipopolysaccharide O-antigen prevents phagocytosis of Vibrio anguillarum by rainbow trout (Oncorhynchus mykiss) skin epithelial cells. PLoS One 7:e37678. doi: 10.1371/journal.pone.0037678
Ma, Y., Wang, Q., Gao, X., and Zhang, Y. (2017). Biosynthesis and uptake of glycine betaine as cold-stress response to low temperature in fish pathogen Vibrio anguillarum. J. Microbiol. 55, 44–55. doi: 10.1007/s12275-017-6370-6372
Maeda, T., Matsuo, Y., Furushita, M., and Shiba, T. (2003). Seasonal dynamics in a coastal Vibrio community examinedby a rapid clustering method based on 16S rDNA. Fish. Sci. 69, 385–394. doi: 10.1046/j.1444-2906.2003.00633.x
Mey, A. R., Wyckoff, E. E., Kanukurthy, V., Fisher, C. R., and Payne, S. M. (2005). Iron and Fur regulation in Vibrio cholerae and the role of Fur in virulence. Infect. Immun. 73, 8167–8178. doi: 10.1128/IAI.73.12.8167-8178.2005
Miller, J. H. (1992). A Short Course in Bacterial Genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press.
Milton, D. L., O’Toole, R., Horstedt, P., and Wolf-Watz, H. (1996). Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178, 1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996
Mouriño, S., Osorio, C. R., and Lemos, M. L. (2004). Characterization of heme uptake cluster genes in the fish pathogen Vibrio anguillarum. J. Bacteriol. 186, 6159–6167. doi: 10.1128/jb.186.18.6159-6167.2004
Muroga, K., Lidia, M., Matsumoto, H., and Nakai, T. (1986). Detection of Vibrio anguillarum from water. Fish. Sci. 52, 641–647.
Naka, H., Dias, G. M., Thompson, C. C., Dubay, C., Thompson, F. L., and Crosa, J. H. (2011). Complete genome sequence of the marine fish pathogen Vibrio anguillarum harboring the pJM1 virulence plasmid and genomic comparison with other virulent strains of V. anguillarum and V. ordalii. Infect. Immun. 79, 2889–2900. doi: 10.1128/IAI.05138-5111
Olafsen, J. A., Christie, M., and Raa, J. (1981). Biochemical ecology of psychrotrophic strains of Vibrio anguillarum isolated from outbreaks of vibriosis at low temperature. Zentralblatt für Bakteriol. Mikrobiol. Hyg. I. Abt. Orig. C Allg. Angew. Ökologische Mikrobiol. 2, 339–348. doi: 10.1016/S0721-9571(81)80027-80020
Ormonde, P., Hörstedt, P., O’Toole, R., and Milton, D. L. (2000). Role of motility in adherence to and invasion of a fish cell line by Vibrio anguillarum. J. Bacteriol. 182, 2326–2328. doi: 10.1128/jb.182.8.2326-2328.2000
O’Toole, R., Milton, D. L., and Wolf-Watz, H. (1996). Chemotactic motility is required for invasion of the host by the fish pathogen Vibrio anguillarum. Mol. Microbiol. 19, 625–637. doi: 10.1046/j.1365-2958.1996.412927.x
Paillard, C., Le Roux, F., and Borrego, J. J. (2004). Bacterial disease in marine bivalves, a review of recent studies: trends and evolution. Aquat. Living Resour. 17, 477–498. doi: 10.1051/alr:2004054
Pajuelo, D., Hernández-Cabanyero, C., Sanjuan, E., Lee, C.-T., Silva-Hernández, F. X., Hor, L.-I., et al. (2016). Iron and Fur in the life cycle of the zoonotic pathogen Vibrio vulnificus. Environ. Microbiol. 18, 4005–4022. doi: 10.1111/1462-2920.13424
Pedersen, K., Grisez, L., van Houdt, R., Tiainen, T., Ollevier, F., and Larsen, J. L. (1999). Extended serotyping scheme for Vibrio anguillarum with the definition and characterization of seven provisional O-serogroups. Curr. Microbiol. 38, 183–189. doi: 10.1007/pl00006784
Rodkhum, C., Hirono, I., Stork, M., Di Lorenzo, M., Crosa, J. H., and Aoki, T. (2006). Putative virulence-related genes in Vibrio anguillarum identified by random genome sequencing. J. Fish Dis. 29, 157–166. doi: 10.1111/j.1365-2761.2006.00692.x
Rønneseth, A., Castillo, D., D’Alvise, P., Tønnesen, Ø, Haugland, G., Grotkjaer, T., et al. (2017). Comparative assessment of Vibrio virulence in marine fish larvae. J. Fish Dis. 40, 1373–1385. doi: 10.1111/jfd.12612
Schwarz, S., West, T. E., Boyer, F., Chiang, W.-C., Carl, M. A., Hood, R. D., et al. (2010). Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6:e1001068. doi: 10.1371/journal.ppat.1001068
Skaar, E. P. (2010). The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6:e1000949. doi: 10.1371/journal.ppat.1000949
Soengas, R. G., Anta, C., Espada, A., Paz, V., Ares, I. R., Balado, M., et al. (2006). Structural characterization of vanchrobactin, a new catechol siderophore produced by the fish pathogen Vibrio anguillarum serotype O2. Tetrahedron Lett. 47, 7113–7116. doi: 10.1016/j.tetlet.2006.07.104
Stevenson, G., Leavesley, D. I., Lagnado, C. A., Heuzenroeder, M. W., and Manning, P. A. (1985). Purification of the 25-kDa Vibrio cholerae major outer-membrane protein and the molecular cloning of its gene: ompV. Eur. J. Biochem. 148, 385–390. doi: 10.1111/j.1432-1033.1985.tb08850.x
Tang, L., Yue, S., Li, G.-Y., Li, J., Wang, X.-R., Li, S.-F., et al. (2016). Expression, secretion and bactericidal activity of type VI secretion system in Vibrio anguillarum. Arch. Microbiol. 198, 751–760. doi: 10.1007/s00203-016-1236-1232
Toranzo, A. E., Magariños, B., and Avendaño-Herrera, R. (2017). “Vibriosis: Vibrio anguillarum, V. ordalii and Aliivibrio salmonicida”, in Fish Viruses and Bacteria: Pathobiology and Protection, eds P. T. K. Woo, and R. C. Cipriano, (Wallingford: CABI), 314–333. doi: 10.1079/9781780647784.0314
Weber, B., Chen, C., and Milton, D. L. (2010). Colonization of fish skin is vital for Vibrio anguillarum to cause disease. Environ. Microbiol. Rep. 2, 133–139. doi: 10.1111/j.1758-2229.2009.00120.x
Weber, B., Hasic, M., Chen, C., Wai, S. N., and Milton, D. L. (2009). Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ. Microbiol. 11, 3018–3028. doi: 10.1111/j.1462-2920.2009.02005.x
Yu, N. Y., Wagner, J. R., Laird, M. R., Melli, G., Rey, S., Lo, R., et al. (2010). PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26, 1608–1615. doi: 10.1093/bioinformatics/btq249
Keywords: Vibrio anguillarum, fish pathogens, RNA-Seq, transcriptome, virulence factors, piscibactin, vanchrobactin
Citation: Lages MA, Balado M and Lemos ML (2019) The Expression of Virulence Factors in Vibrio anguillarum Is Dually Regulated by Iron Levels and Temperature. Front. Microbiol. 10:2335. doi: 10.3389/fmicb.2019.02335
Received: 02 August 2019; Accepted: 25 September 2019;
Published: 15 October 2019.
Edited by:
Eckhard Strauch, Federal Institute for Risk Assessment (BfR), GermanyReviewed by:
Hiroaki Naka, The University of Utah, United StatesChang Chen, South China Sea Institute of Oceanology (CAS), China
Copyright © 2019 Lages, Balado and Lemos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel L. Lemos, manuel.lemos@usc.es
 Marta A. Lages
Marta A. Lages Miguel Balado
Miguel Balado Manuel L. Lemos
Manuel L. Lemos