- 1Department of Biotechnology, Dr. Harisingh Gour Vishwavidyalaya (A Central University), Sagar, India
- 2Indian Council of Medical Research, Bhopal Memorial Hospital & Research Centre, Bhopal, India
- 3Department of Biomedical and Clinical Technology, Durban University of Technology, Durban, South Africa
- 4School of Life Sciences, University of KwaZulu-Natal, Durban, South Africa
Garlic (Allium sativum), a popular food spice and flavoring agent, has also been used traditionally to treat various ailments especially bacterial infections for centuries in various cultures around the world. The principal phytochemicals that exhibit antibacterial activity are oil-soluble organosulfur compounds that include allicin, ajoenes, and allyl sulfides. The organosulfur compounds of garlic exhibit a range of antibacterial properties such as bactericidal, antibiofilm, antitoxin, and anti-quorum sensing activity against a wide range of bacteria including multi-drug resistant (MDR) strains. The reactive organosulfur compounds form disulfide bonds with free sulfhydryl groups of enzymes and compromise the integrity of the bacterial membrane. The World Health Organization (WHO) has recognized the development of antibiotic resistance as a global health concern and emphasizes antibiotic stewardship along with the urgent need to develop novel antibiotics. Multiple antibacterial effects of organosulfur compounds provide an excellent framework to develop them into novel antibiotics. The review provides a focused and comprehensive portrait of the status of garlic and its compounds as antibacterial agents. In addition, the emerging role of new technologies to harness the potential of garlic as a novel antibacterial agent is discussed.
Introduction
Garlic (Allium sativum), belonging to family Liliaceae, mainly the bulb of garlic, has been used as a spice in cooking worldwide especially in Italy and Southeast Asia. More importantly, garlic has been an ingredient in folk and traditional medicine since ancient times (Rivlin, 2001). Garlic is cultivated all over the world with a per-capita consumption of two pounds per year. As per the Food and Agricultural Organization of the United Nations, China and India are first and second, respectively, in average (1961–2017) garlic production. Health benefits that are associated with the use of garlic are attributed to its anticancer, anti-inflammatory, antifungal, antiviral, and antibacterial activity. Several in vitro, in vivo, and epidemiological studies indicate that garlic exhibits anticancer activity, and the likely mechanism of action is by activating metabolizing enzymes, inhibiting reactive oxygen species, radical scavenging, preventing DNA damage, and tumor inhibition (Cao et al., 2014; Zhang Y. et al., 2019). The immunomodulatory effects of garlic are mediated through its ability to modulate cytokine production as well as activate immune response by stimulating antibody secretion and immune cells (Arreola et al., 2015). Garlic displays anti allergic properties by inhibiting antibody-mediated histamine production and modulates airway allergic response (Kyo et al., 2001; Zare et al., 2008). The anti inflammatory and anti arthritic ability of garlic comes from its ability to inhibit NF-κB signaling (Ban et al., 2009). Garlic oil (GO) exhibits antifungal activity against Candida albicans and Penicillium funiculosum by penetration into cells and organelles and causing differential expression of genes that are critical for cellular metabolism (Li et al., 2016). One of the earliest reports of garlic’s antibacterial activity was by Small et al. (1947) and Stoll and Seebeck (1947). Since then, extensive research has been performed on the antibacterial effects of garlic. The antibacterial activity against various pathogenic and drug-resistant bacteria was tested using crude garlic extracts, garlic powder (GP), garlic extracts using various solvents, GO, and phytochemicals isolated from garlic. The constant and rapid emergence of antimicrobial resistance has been recognized as an alarming threat to human health, which mandates the scientific community to develop novel and effective antibacterial agents (Cheng et al., 2016). Garlic compounds exhibit multiple modes of antibacterial activity and have enormous potential to be developed into novel antibacterial agents. Most reviews about garlic discuss the antibacterial activity of garlic as one of its many health benefits diluting the importance of garlic compounds as potential antibacterial agents. This review exclusively focuses on significant antibacterial studies that were performed with garlic and its phytochemicals.
Active Phytochemicals of Garlic
Most of the health benefits of garlic are attributed to a myriad of cysteine-derived sulfur-containing organic compounds present in garlic (extensively reviewed in Fenwick and Hanley, 1985a,b,c). The organosulfur compounds of intact garlic clove greatly differ from that present in garlic juice obtained after crushing garlic. The intact garlic mainly contains non-volatile γ-glutamyl-S-alk(en)yl-L-cysteines, namely, γ-glutamyl-S-allyl-L-cysteine, γ-glutamyl-S-trans-1-propenyl-L-cysteine, and S-alk(en)yl-L-cysteine sulfoxides such as S-allyl-L-cysteine sulfoxide (alliin), S-(trans-1-propenyl)- L-cysteine sulfoxide (isoalliin), and S-methyl-L- cysteine sulfoxide (methiin) with a small amount of S-allyl cysteine (SAC) (Figure 1A) (Block, 1992). Crushing or cutting garlic cloves releases allinase enzyme sequestered in the vacuoles, which encounters cytosolic alliin to convert it into an array of thoisulfinates of which the most prominent is allicin. The highly reactive, unstable, and volatile allicin decomposes to yield a large number of sulfides such as diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), methyl allyl disulfide (MADS), methyl allyl sulfide, ajoene, and vinyl dithiins (2-vinyl-1,3-dithiin, 3-vinyl-1,2-dithiin) shown in Figure 1B (Brodnitz et al., 1971). The sulfides are oil-soluble compounds that are responsible for the characteristic garlic odor and flavor. Allicin exhibits excellent in vitro antibacterial activity, which resulted in a huge number of studies to evaluate the potential of allicin and oil-soluble organosulfur compounds of garlic as antibacterial agents (Cavallito and Bailey, 1944a). A large body of literature supports the antibacterial potential of garlic organosulfides. The organosulfur compounds present in the aqueous and alcoholic extract of garlic include S-allyl cysteine (SAC), S-allylmercapto-L-cysteine (SAMC), and S-methyl cysteine (Figure 1C). The compounds are non-volatile, non-odiferous, and stable compounds compared to volatile organosulfides. Most health benefits of garlic are largely attributed to these organosulfur compounds present in garlic. However, garlic organosulfur compounds are very unstable with low bioavailability and the presence of these compounds depends on the processing of the garlic during the preparation of garlic supplements (Amagase et al., 2001; Amagase, 2006).
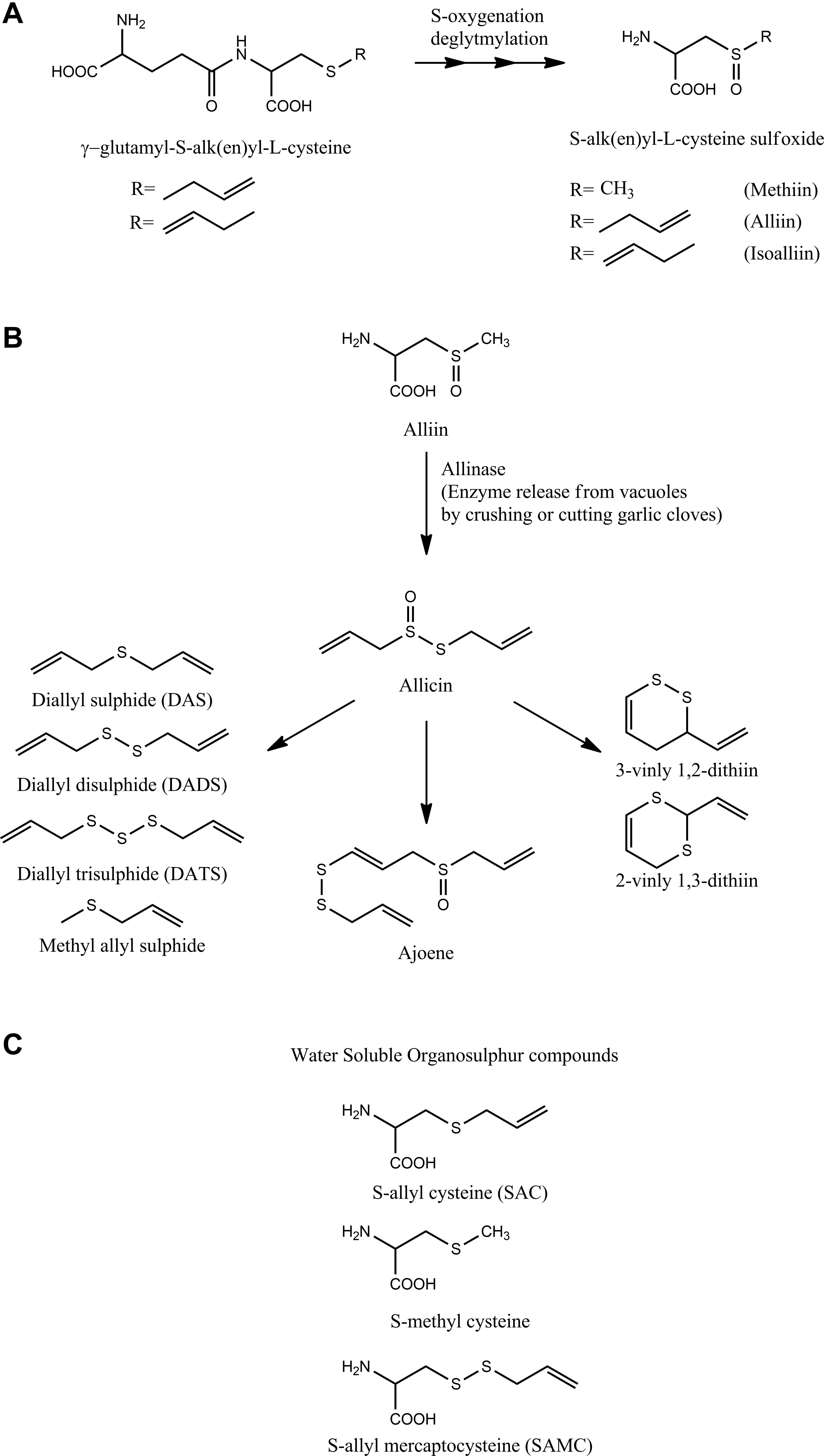
Figure 1. Organosulfur compounds of garlic: The figure shows the major organosulfur compounds present in garlic. (A) The major compounds found in intact garlic cloves. (B) The crushing of garlic clove converts alliin into allicin by the action of allinase enzyme. Allicin is a highly unstable compound that degrades and rearranges itself into different organosulfide compounds shown in the figure. (C) Apart from oil-soluble organosulfur compounds, garlic also has water-soluble organosulfur compounds shown in the figure.
The main antibacterial organosulfur compounds of garlic are allicin, ajoene, and various aliphatic sulfides. The extraction procedure results in concentrating a particular compound rather than providing a pure compound. Extraction of garlic with water or ethanol and concentrating the extract will provide an allicin-rich product. It was noticed that yield with ethanol is better compared to water (Fujisawa et al., 2008). However, extraction of concentrated ethanolic distillate with organic solvent yields a highly concentrated and pure allicin product (Cavallito and Bailey, 1944a; Ratnakar and Murthy, 1995). Later, it was reported that extraction using acetone will yield higher allicin compared to ethanol (Canizares et al., 2004a). Recently, salting-out extraction using ethanol and ammonium sulfate result in effective extraction of allicin (Li et al., 2017). Oil-macerated garlic extract has a very high proportion of ajoene along with other thiosulfinates (Yoshida et al., 1998). Steam distillation of garlic yields GO, which mainly consists of various aliphatic sulfides (Avato et al., 2000). The components of both oil-macerated garlic extract and GO can be separated using chromatographic and distillation techniques (Casella et al., 2013).
PubMed search of “Garlic antibacterial” yields more than 350 research papers. This large body of literature comprises research papers that investigated the antibacterial activity of crude preparation of garlic, various extracts of garlic, and individual organosulfur compounds of garlic against various bacteria including MDR bacteria. Table 1 provides a list of in vitro antibacterial activity of various garlic products and compounds against different bacteria. Similarly, Table 2 provides similar information on in vivo studies. Some of the early research that reported the antibacterial activity of garlic against a wide variety of bacteria has been summarized by Adetumbi and Lau (1983). The present review provides a comprehensive summary of this large body of research.
Antibacterial Activity of Garlic Fresh Extract and Powder
Garlic is one of the popular spices added to food to enhance the flavor, and it has been used in different cultures and traditions around the world to treat bacterial infections for centuries. Several studies have evaluated the antibacterial activity of various garlic preparations such as crude or fresh garlic extract (FGE), and garlic paste. The antibacterial activity of garlic paste and FGE against commensal and pathogen enteric bacteria such as Escherichia coli, E. coli O157:H7, Salmonella species, Shigella species, Vibrio species, Campylobacter species, Listeria monocytogenes, Enterobacter, and Enterococcus species, Lactobacillus acidophilus, Staphylococcus aureus, Streptococcus species, and Clostridium difficile has been reported by various laboratories (Johnson and Vaughn, 1969; Kumar and Berwal, 1998; Ross et al., 2001; Gupta and Ravishankar, 2005; Vuddhakul et al., 2007; Lu et al., 2011b; Jain et al., 2015a; Roshan et al., 2017). These studies suggest that garlic consumption could help in preventing food poisoning. In addition, various studies have evaluated the impact of garlic and its organosulfur compounds on the gut microbiome. Garlic was found to positively influence the gut microbiome and protect the gut microbiome damage from high-fat diet (Chen et al., 2019). Supplementing feed of farrowing sows and European bass with GO decreased pathogenic microbes from the gut microbiome (Rimoldi et al., 2020; Satora et al., 2020). Allicin treatment prevented high carnitine diet-induced dysbiosis to lower the atherosclerosis risk factor trimethylamine N-oxide that is produced by the gut microbiome (Wu W. K. et al., 2015). Oral administration of alliin, precursor of allicin, to rats resulted in decreasing the relative abundance of only Allobaculum genus in the cecum (Zhang C. et al., 2019). The gut microbiome was altered upon intragastric administration of DADS of rat, a low dose of DADS decreased Bacteroidetes phyla but increased Firmicutes phyla bacteria (Yang et al., 2019). Oral administration of propyl propane thiosulfonate restored the richness and evenness of gut microbiome lost due to dextran sodium sulfate-induced colitis in mice (Vezza et al., 2019). In a small-scale clinical trial, aged garlic extract supplementation for 3 months increases the richness and diversity of the gut microbiome with increase in Lactobacillus and Clostridium species (Ried et al., 2018). All the studies indicate that garlic and its compounds have a positive effect on gut microbiome composition and richness. However, the mechanistic details still need to be investigated. In a recent study from our laboratory, FGE exhibited activity against MDR Shiga-toxin producing E. coli (STEC) isolates from clinical and food samples (Bhatwalkar et al., 2019). In addition to antibacterial activity, garlic crude and aqueous extract exhibited anti-adherent activity against the standard strain type of Streptococcus mutans (Jain et al., 2015a). The data suggest that garlic could be used to preserve food and prevent foodborne infections. However, the antibacterial activity was dramatically decreased when experiments were performed with buffered peptone water and ground beef, suggesting that further research is required to utilize garlic as a food/meat-preserving agent (Gupta and Ravishankar, 2005). The causative agent of gastric ulcers, Helicobacter pylori (standard strains types and clinical isolates), was found to be sensitive to GP and 1,000 μg/ml of GP inactivated H. pylori at 6 h in a time course viability assay (O’Gara et al., 2000). Allicin-rich crude extract exhibited better antibacterial activity against Mycobacterium phlei, Mycobacterium smegmatis, and Mycobacterium tuberculosis compared to isoniazid and ethambutol. Also, disk diffusion assay with allicin-rich extract exhibited significant activity against MRSA (Viswanathan et al., 2014). Another study also found that FGE was effective against MDR strains of E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Serratia marcescens, and MRSA in both in vitro and in vivo assays (Farrag et al., 2019).
Antibacterial Activity of Garlic Aqueous Extract
There are several reports of antibacterial activity of aqueous garlic extract (AGE) against a variety of bacteria. In vitro assay with AGE (10%) showed complete inhibition of Bacillus cereus and the activity varies upon the storage conditions and heat treatment of the aqueous extract (Saleem and Al-Delaimy, 1982). AGE exhibited in vitro antibacterial activity against various pathogenic bacteria including Shigella and Salmonella species and enterotoxigenic E. coli (Arora and Kaur, 1999). In addition, AGE fully cured the rabbits that were challenged with Sh. flexneri Y by completely clearing them of bacteria with no significant side effects (Chowdhury et al., 1991).
Supporting the results obtained with GP, in vitro assays indicated that H. pylori is sensitive to AGE, and the sensitivity was more compared to S. aureus (Cellini et al., 1996; Sivam, 2001). In vitro antibacterial assays report that AGE is effective against various Gram-positive and Gram-negative oral bacteria, which include periodontal pathogenic bacteria Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and S. mutans (Bakri and Douglas, 2005; Fani et al., 2007; Velliyagounder et al., 2012). Different studies reported that AGE exhibited activity against a large variety of Gram-positive and Gram-positive pathogenic bacteria including MDR strains and isolates such as MDR M. tuberculosis showing not only the effectiveness of garlic against drug-resistant bacteria but also its broad spectrum (Iwalokun et al., 2004; Gupta et al., 2010; Gull et al., 2012; Meriga et al., 2012). In an interesting study, it was found that counts of S. aureus in hamburger upon addition of AGE reduced in a dose-dependent manner during storage for different time points in the fridge and freezer, supporting the idea of using garlic for meat preservation (Mozaffari Nejad et al., 2014). To compare the antibacterial activity of various garlic health products, aqueous extracts of different products that included GP, GO, gelatinous GP suspension, aged garlic extract, and gelatinous suspension of aged garlic extract were prepared along with fresh garlic. All the extracts exhibited activity against Neisseria gonorrhoeae, S. aureus, and Enterococcus faecalis. The activity was correlated to the amount of fresh garlic constituents, namely, allicin and SAC, present in the products (Ruddock et al., 2005). The Burkholderia cepacia complex (Bcc) consists of 17 different species of soil bacteria that are pathogenic to allium species. These bacteria cause life-threatening lung infections in patients suffering from cystic fibrosis. AGE exhibited activity against Bcc, and this activity correlated with the allicin content of the extract (Wallock-Richards et al., 2014). In a recent study, non-aged and aged garlic cloves were pressed to remove their juices, dried, and powdered before extracted with water, ethanol, and chloroform. All three extracts of aged garlic exhibited antibacterial activity while only chloroform extract of non-aged garlic had activity against B. cereus (Jang et al., 2018). All these studies indicate that allicin is the main phytochemical responsible for the antibacterial activity of AGE. Although the ethanol extract of garlic also has allicin, AGE is more effective due to the presence of other antibacterial chemicals, which might result in a synergistic or additive effect.
Antibacterial Activity of Garlic Ethanolic Extract
HPLC analysis of ethanolic extract of garlic (EGE) revealed that it contains various thoisulfinates, the major one being allicin. The anti-H. pylori activity of this extract decreased with the decrease in the concentration of allicin. Furthermore, it was seen that the maturation of garlic increases the allicin yield and extract with acetone yielding a higher percentage of allicin compared to ethanol (Canizares et al., 2004a, b). In vitro studies have reported that EGE was found to show antibacterial activity against various pathogenic bacteria including MDR bacteria, MDR M. tuberculosis isolates, and vancomycin-resistant S. aureus (VRSA) isolates (Hannan et al., 2011; Karuppiah and Rajaram, 2012; Snowden et al., 2014; Liaqat et al., 2015). The antibacterial and antiadherence activity of organic solvent (chloroform, acetone, and ethanol) extracts of garlic was least compared to crude and aqueous extract against S. mutans (Jain et al., 2015a). The leaves of wild garlic (Allium ursinum subsp. ucrainicum) found in Serbia were extracted with 70 and 96% ethanol and 80% and absolute methanol, and the S-alk(en)ylcysteines (alliin, isoalliin, and methiin) content of the extracts was analyzed using NMR studies. The extracts exhibited some degree of antibacterial activity against test enteropathogenic bacterial strains with Salmonella enteritidis being the most sensitive. The tested bacteria were more sensitive to ethanolic extract compared to other extracts (Pavlovic et al., 2017). However, the study should have determined the amount of allicin in the extracts for better interpretation of the results instead of alliin, which is a precursor of allicin. Ethanolic (30%) extract of fermented black garlic exhibited antibacterial activity against 11 bacterial strains that cause oral diseases. Short and long incubation with this extract inhibited the growth of more than 90% of salivary bacteria (Vlachojannis et al., 2018). Water extract of the Toluene extract of garlic has been reported to decrease the mortality of Caenorhabditis elegans from P. aeruginosa infections (Rasmussen et al., 2005) and clear the lungs of mice of P. aeruginosa by modulating inflammation (Bjarnsholt et al., 2005). Allicin along with other thoisulfinates present in EGE seems to be responsible for its antibacterial activity. Other than ethanol and methanol extract, the chloroform extract of both aged and non-aged garlic exhibited activity against B. cereus by disk diffusion assay (Jang et al., 2018). The hexane extract of solid waste of the GO extraction process exhibited activity against various bacteria including S. aureus and MRSA. DASs present in the extract were responsible for this activity (Nakamoto et al., 2020).
Antibacterial Activity of Garlic Oil
Garlic oil is obtained by steam distillation of macerated or mashed garlic. Reverse-phase high-performance liquid chromatography (HPLC) studies have determined that the GO consists of a large variety of diallyl sulphides and other sulfides (O’Gara et al., 2000; Kim et al., 2004). A recent study has performed an exhaustive analysis of the content of GO and reported that the majority of GO is composed of diallyl and allyl methyl sulfides (Mnayer et al., 2014). The anti-mycobacterium effect of GO was demonstrated using in vitro and in vivo studies (Jain, 1998; Viswanathan et al., 2014). The anti-H. pylori effect of GO was many folds greater than that of GP. This could be because allicin is the only antibacterial thiosulfinate found in GP whereas GO has many organosulfides. The time course viability studies showed concentration-dependent inhibition of H. pylori by GO with 64 μg/ml resulting in complete inhibition in 4.5 h (O’Gara et al., 2000). However, two independent clinical studies indicated that administration of garlic GO was unable to ameliorate the H. pylori infection (Graham et al., 1999; Aydin et al., 2000). GO has been reported to exhibit antibacterial activity against 14 enteric pathogens and 11 commensal enteric bacteria with commensal bacteria being more sensitive. In time course viability studies, the inhibition of Enterobacter aerogenes growth increased with an increase in the concentration of GO, and complete killing was noticed at 22 mg/ml in 1 h (Ross et al., 2001). In another study, different GOs with varying percentages of DDS and DTS along with pure DDS were tested against Gram-positive (S. aureus and Bacillus subtilis) and Gram-negative (E. coli and P. aeruginosa). The antibacterial activity was not significant; however, the little activity that was exhibited was found with GO with a higher percentage of DDS. Interestingly, pure DDS showed little activity against only selected tested bacteria (Avato et al., 2000). However, disk diffusion assay found GO to be effective against S. aureus, E. coli, P. aeruginosa, B. subtilis, and MRSA (Casella et al., 2013; Viswanathan et al., 2014). An in vitro study tested the activity of GO against 40 S. aureus and 60 MRSA isolates and found that GO was more effective against S. aureus compared to MRSA, although this activity was significantly less than standard antibiotics (Tsao S.M. and Yin, 2001). Another study by the same group has reported that GO is effective against 237 clinical isolates of P. aeruginosa and K. pneumoniae, which also included drug-resistant strains. The minimum inhibitory concentration (MIC) values for P. aeruginosa were smaller compared to K. pneumoniae, and four times MIC of GO eliminated P. aeruginosa and K. pneumoniae in 16 and 24 h, respectively, in kill curve assays (Tsao S. and Yin, 2001). However, weak antibacterial activity of GO against six different bacteria has been reported using in vitro assays (Kim et al., 2004). Other bacteria that were reported to be sensitive to GO are Salmonella typhi, L. monocytogenes, and Campylobacter jejuni (Robyn et al., 2013; Mnayer et al., 2014). The discrepancy in the antibacterial activity of GO among various in vitro studies could be due to the solubility and volatile nature of GO.
Antibacterial Activity of Ajoene
Allicin can react with itself to yield ajoene, which is found abundantly in oil-macerated garlic. Besides, two ajoene-related compounds Z-10-devinylajoene and iso-E-10-devinylajoene were also isolated from oil-macerated garlic extract. Studies from the Fujino group reported that ajoene and its related compounds were found to display antibacterial activity against several Gram-positive and Gram-negative bacteria including Mycobacterium species (Naganawa et al., 1996; Yoshida et al., 1998, 1999). In all these studies, it was noticed that these compounds were more active against Gram-positive bacteria compared to Gram-negative. The same group also reported the antibacterial activity of ajoene and its related compounds against H. pylori (Ohta et al., 1999). Mice challenged with P. aeruginosa cleared the infection rapidly when treated with ajoene compared to the control group (Jakobsen et al., 2012). Pure ajoene exhibited antibacterial activity against Cronobacter sakazakii in a concentration-, time-, and temperature-dependent manner (Feng et al., 2014). More studies testing the activity of ajoene against more bacteria, especially clinical isolates and MDR strains, along with stability and pharmacokinetic studies are needed to better understand and utilize ajoene and its related compounds as antibacterial agents.
Antibacterial Activity of Garlic Organosulfides
The major constituents of GO are various aliphatic disulfides. DADS, which is the most abundant allyl sulfides in GO. DAS exhibits poor anti-H. pylori effect, but this activity increased as the number of sulfurs increased (O’Gara et al., 2000). A study from the same laboratory reported that a mixture of diallyl sulfides (DASS) and dimethyl trisulfide (DMTS) exhibits activity against six enteric pathogens with DMTS being several folds effective compared to DADS (Ross et al., 2001). The activity of DADS and DATS against antibiotic-sensitive and -resistant isolates of H. pylori was confirmed by in vitro studies (Liu et al., 2008). In vitro studies indicated that DAS, DADS, DATS, and diallyl tetrasulfide (DATTS) were effective against S. aureus, where DAS was being least effective and the activity increases with the increase in the number of sulfurs. The activity of DATS and DATTS was comparable to standard antibiotics. It was interesting that MRSA was sensitive to all the DASs that were tested (Tsao S.M. and Yin, 2001). Similar results were reported with 237 clinical isolates of P. aeruginosa and K. pneumoniae including drug-resistant isolates (Tsao S. and Yin, 2001). The addition of DAS and DADS to meat significantly reduced the growth of aerobes and inhibited the pathogenic bacteria (Yin and Cheng, 2003). In line with the above reports, an in vitro study investigated the antibacterial activity of not only DASs but also dimethyl sulfides and dipropyl disulfide. The results indicate that they have moderate antibacterial activity against test bacteria, and this activity improves with an increase in the number of sulfurs (Kim et al., 2004). A later study using disk diffusion assay reported that DADS was effective, whereas dipropyl disulfide was not effective against S. aureus, E. coli, and P. aeruginosa (Casella et al., 2013). Administration of DAS and DADS to diabetic mice infected with MRSA significantly protected the mice by lowering bacteria load in the kidneys. In addition, inflammatory cytokines, namely, IL-6 and TNF-alpha, and coagulation factors C-reactive protein, fibronectin, and fibrinogen were decreased while anticoagulation factors antithrombin III (AT-III) and protein C were increased by DAS and DADS treatment. Moreover, malondialdehyde was decreased upon DAS and DADS, indicating protection from lipid peroxidation by MRSA infection (Tsao et al., 2007). Ally methyl sulfide (AMS) was shown to retard the growth of pleuropneumoniae causing pig pathogen Actinobacillus pleuropneumonia and protected the pigs by reducing the lung lesions by 20% (Becker et al., 2012). DAS exhibited concentration-dependent antibacterial activity against A. actinomycetemcomitans with and without heat treatment, indicating that DAS is heat stable (Velliyagounder et al., 2012). In vitro studies reported that DAS, DADS, and DATS exhibit activity against C. jejuni (Lu et al., 2011a; Robyn et al., 2013). The in vitro assay treatment of DAS also displays antibacterial activity against C. sakazakii and E. coli O157:H7 (Lu et al., 2011b; Feng et al., 2014). The enzymatic degradation of alliin, an organosulfur compound that alliinase enzyme converts into allicin and that is further degraded into a variety of organosulfides, resulted in higher percentage of DADS and diethenes showed better antibacterial activity against tested bacteria compared to alkali degradation products of alliin (Wu et al., 2017). Mixtures of DASs with various amounts of mono- to hexasulfides were prepared, and their anti-mycobacterial activity was evaluated. It was found that while all the combinations exhibited some activity, the most potent combination was the one that had higher quantity of DATS (Oosthuizen et al., 2017). Different studies, in vitro and in vivo, suggest that using GO, combinations, or individual aliphatic disulfides exhibited antibacterial activity against a wide range of microorganisms. It was noticed that the antibacterial activity increases with the increase in the number of sulfur, suggesting that antibacterial activity is mediated by formation of disulfide bonds between the compounds and bacterial protein, mainly enzymes.
Antibacterial Activity of Allicin
It is an established fact that allicin is an effective, broad-spectrum, and principal antibacterial component of garlic (Ankri and Mirelman, 1999). Allicin was identified as the principal ingredient of garlic that is responsible for the antibacterial activity of a wide variety of bacteria (Cavallito and Bailey, 1944a). Allicin was found to exhibit activity against M. tuberculosis including drug-resistant strains (Rao et al., 1946a; Delaha and Garagusi, 1985; Ratnakar and Murthy, 1995). Vancomycin-sensitive and -resistant clinical isolates and standard strains of Enterococci species were sensitive to allicin (Jonkers et al., 1999a). Allicin exhibited the best anti-H. pylori activity against three strains compared to DASs (O’Gara et al., 2000). A meta-analysis of clinical data indicated that adding allicin to conventional therapy improves the eradication of H. pylori infections (Si et al., 2019). Allicin along with related thoisulfinates, allyl methyl, and methyl allyl thiosulfinate were found and purified from acetone garlic extract. Allicin along with allyl methyl and methyl allyl mixture exhibited activity against H. pylori and showed synergy when used together (Canizares et al., 2004b). In a recent report, allicin was found to be active against C. difficile and other commensal gut bacteria, and no significant synergy was observed when allicin was tested with standard antibiotics (Roshan et al., 2017). The same group reported that allicin did not affect spore germination, but significantly inhibited spore outgrowth of C. difficile spores (Roshan et al., 2017). In vitro assay found that allicin was effective against 30 strains of Staphylococcus epidermidis including methicillin-resistant strains (Perez-Giraldo et al., 2003). A stable aqueous extract of allicin was found to be effective against 30 clinical MRSA isolates, some of which were mupirocin resistant. Aqueous cream of allicin also exhibited activity against tested MRSA strains (Cutler and Wilson, 2004). Similarly, aqueous allicin extract and cream demonstrated anti-Lancefield group B streptococci clinical isolate using in vitro assays (Cutler et al., 2009). A comparative in vitro study of antibacterial activity against S. aureus and E. coli activity of FGE, allicin, and clinically used antibiotics was performed. The results of the study indicated that fresh garlic was more potent against S. aureus compared to allicin and not much difference in activity was noticed against E. coli while both bacteria were more sensitive to antibiotics than garlic extract or allicin (Fujisawa et al., 2009). The administration of allicin to rainbow trout through its diet almost eliminated mortality when infected with Aeromonas hydrophila, a fish pathogen. In addition, in vitro studies also indicated that this bacterium was sensitive to allicin (Nya et al., 2010). It was found that A. actinomycetemcomitans was sensitive to allicin, and this activity disappeared upon heating, indicating that allicin is thermolabile (Velliyagounder et al., 2012). Although an in vitro assay found that C. jejuni was sensitive to allicin, in vivo studies indicated that allicin had no significant effect on colonization of C. jejuni in broilers. The possible explanation for this could be that the presence of mucus inhibited the activity of allicin in vitro (Robyn et al., 2013). In addition to AGE, allicin also exhibits dose-dependent antibacterial activity against Bcc (Wallock-Richards et al., 2014). In an interesting study, allicin vapors were able to exhibit bactericidal activity against MDR lung pathogenic bacteria such as P. aeruginosa and Streptococcus pyogenes (Reiter et al., 2017). It was found that the active ingredient of Bald’s eyeslave, an Anglo-Saxon medical remedy made up of garlic, onions, bovine bile, and brass effective against S. aureus and P. aeruginosa, was allicin (Fuchs et al., 2018). Allicin is the most potent antibacterial organosulfur compound found in garlic. The higher activity is thought to be due to the highly reactive sulfoxide group of allicin. However, the stability and solubility of allicin are the challenges in its clinical use. Animal studies highlight the reduced bioavailability and toxicity associated with allicin administration (Amagase et al., 2001).
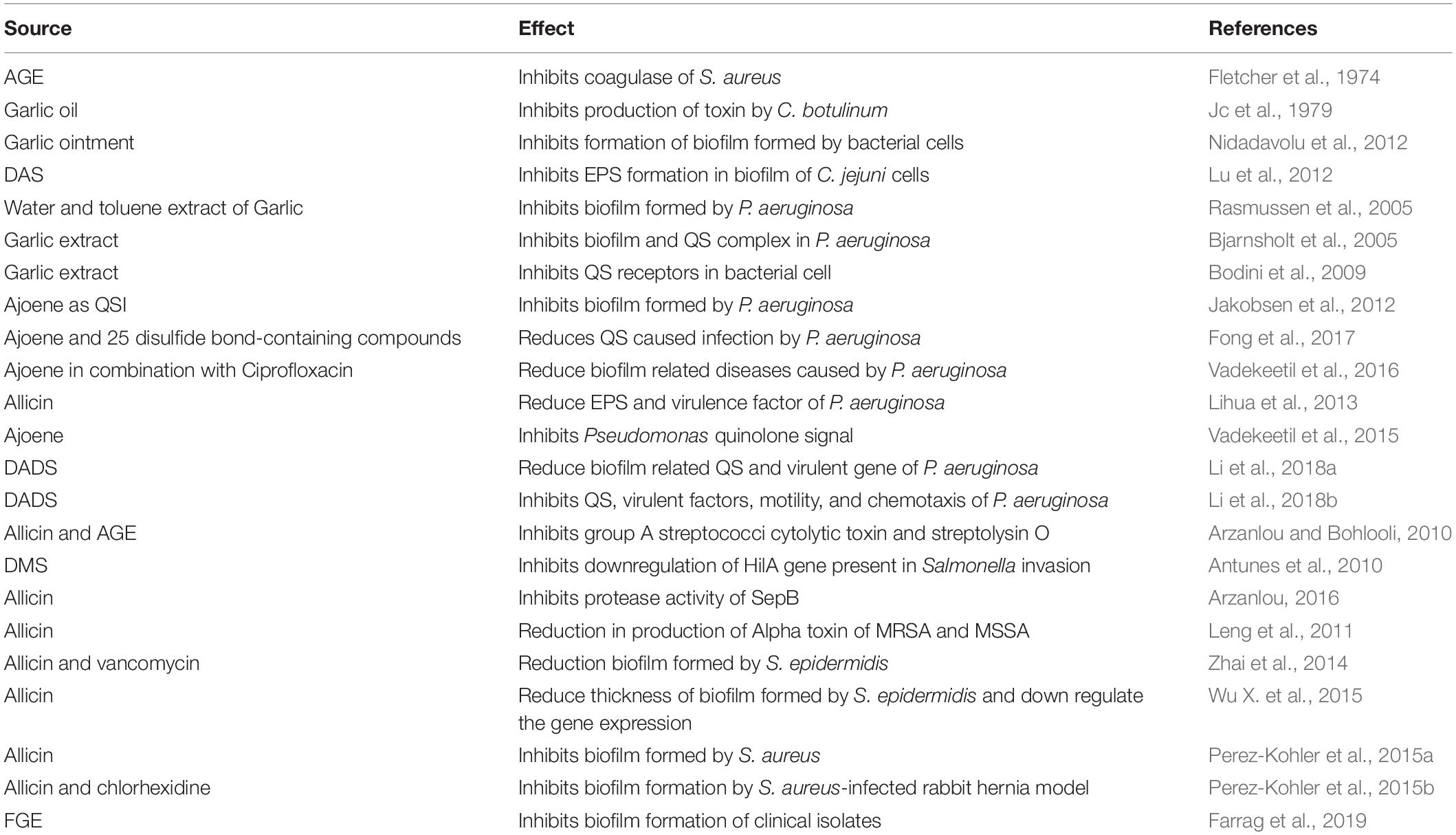
Antibiofilm and Antivirulence Properties of Garlic and Its Organosulfur Compounds
Bacterial biofilms are aggregations of bacterial cells in a matrix of extracellular polymeric substances (EPS) that include proteins, nucleic acids, polysaccharides, and lipids that are secreted by the bacteria. The formation of biofilm is a complex process that involves quorum sensing (QS) signaling. QS is also associated with the expression and release of various virulence factors that play a major role in pathogenesis. The formation of biofilm has been strongly associated with bacterial pathogenesis and antibiotic resistance. Therefore, developing strategies to inhibit biofilm formation has been a major area of research for many years. In addition to using synthetic antibiofilm agents, the use of many phytochemicals including garlic and its organosulfur compounds has gained a lot of interest. Table 3 lists the antibiofilm and anti-QS studies that have been performed using garlic and its compounds. AGE was found to inhibit the coagulase activity of S. aureus using in vitro assays (Fletcher et al., 1974). GO was found to inhibit toxin production by Clostridium botulinum type A (Jc et al., 1979). Garlic ointment made by mixing GP with petroleum jelly not only prevented the formation of biofilm but also disrupted the already formed biofilm of bacteria that were isolated from burn wounds (Nidadavolu et al., 2012). DAS was found to kill both planktonic and sessile C. jejuni cells in the biofilm much better than ciprofloxacin and erythromycin. FTIR and Raman spectroscopy revealed that DAS treatment altered the proteins and polysaccharides of biofilm and damaged the EPS, which was visualized by electron microscopy (Lu et al., 2012). A genetic screening system was utilized to screen many herbal and pure compounds for their QS inhibition activity, and it was found that garlic exhibited significant inhibition of QS. Microarray transcriptome analysis indicated that the water extract of toluene extract of garlic affected the expression of virulence genes that were controlled by QS. In addition, garlic altered the in vitro biofilm to increase the penetration and killing of P. aeruginosa in the biofilms by tobramycin (Rasmussen et al., 2005). It was also found that pretreatment of P. aeruginosa biofilm to this extract made it more susceptible to tobramycin and polymorphonuclear leukocytes (Bjarnsholt et al., 2005). QS strains were used to identify that the water extract of toluene extract of garlic inhibited LuxR, AhyR, and TarR QS receptors (Bodini et al., 2009). Bioactivity-guided fractionation of garlic extract identified ajoene as quorum sensing inhibition (QSI) and microarray studies revealed that it reduced the expression of few QS-controlled virulence genes of P. aeruginosa such as lasB and rhlA, which increase the production of protease and rhamnolipid, respectively. Similar to previous observations, pretreatment of biofilms with ajoene increased the antibacterial activity of tobramycin on biofilm-associated P. aeruginosa (Jakobsen et al., 2012). The QSI activity of ajoene encouraged the screening of a library of compounds to identify a couple of sulfur-containing compounds that were similar to ajoene with QSI activity. Twenty-five disulfide bond-containing compounds were synthesized based on a quantitative structure–activity relationship (QSAR) study. These compounds could reduce the production of virulence factors, which included elastase, rhamnolipid, and pyocyanin. Besides, they were also able to inhibit the infection of P. aeruginosa in the murine implant infection model (Fong et al., 2017). The motility and biofilm formation of P. aeruginosa was significantly decreased when treated with a combination of ajoene and ciprofloxacin compared to independent treatment with each agent. Ajoene alone and in combination with ciprofloxacin significantly increased the serum sensitivity, phagocytic uptake, and killing of P. aeruginosa compared to no treatment. Furthermore, in the P. aeruginosa infection-associated murine pyelonephritis model, the combination of ajoene with ciprofloxacin significantly reduced the bacterial load of kidneys and bladder with reduced tissue damage compared to control and individual treatment of ajoene and ciprofloxacin (Vadekeetil et al., 2016). In addition to inhibiting production of long-chain acyl homoserine lactones, ajoene was also found to inhibit Pseudomonas quinolone signal (PQS) (Vadekeetil et al., 2015).
Allicin was also found to not only reduce biofilm formation of P. aeruginosa by reducing attachment and EPS production but also reduce the production of virulence factors such as exotoxin A, elastase, pyoverdine, and rhamnolipid (Lihua et al., 2013). Recently, DADS was found to decrease in vitro biofilm formation and swarming motility of P. aeruginosa. Relative gene expression studies indicated that it reduced the expression of many important QS and virulent genes (Li et al., 2018a). An interesting follow-up study performed an RNA transcriptome and proteome analysis on P. aeruginosa upon DADS treatment. The result indicated that all the three QS systems and virulent factors were downregulated by DADS treatment. Also, DADS treatment inhibited systems involved in motility and chemotaxis of P. aeruginosa (Li et al., 2018b). In vitro studies found that allicin and aqueous garlic (fresh and aged) extract inhibited production of streptolysin O, a cytolytic toxin by all strains of group A streptococci (GAS) (Arzanlou and Bohlooli, 2010). The transcription regulator HilA plays a crucial role in regulating the complex mechanism of Salmonella invasion, and it was found that dimethyl sulfide (DMS) downregulates the expression of the hilA gene and multiple virulent genes (Antunes et al., 2010). Another toxin produced by GAS is streptococcal pyrogenic exotoxin B (SpeB). The protease activity of SpeB was inhibited by allicin in vitro, and it is due to inhibition of truncation of SpeBm, the precursor protein of SpeB (Arzanlou, 2016). The reduction in the production of alpha-toxin by methicillin-susceptible and -resistant S. aureus upon treatment with allicin was confirmed by hemolysis and Western blot analysis. In addition, hla and agrA genes that regulate the production of alpha-toxin were downregulated by allicin (Leng et al., 2011). Administration of allicin alone or with vancomycin significantly reduced biofilm formation by S. epidermidis compared to vancomycin or saline treatment in a rabbit prosthetic joint infection model (Zhai et al., 2014). In vitro studies indicate that allicin exhibits antibiofilm property against S. epidermidis; however, this activity was less compared to water or ethanolic garlic extract. Allicin, water, and ethanolic extract of garlic exhibited antibacterial activity on biofilm-associated bacteria. Allicin decreased the thickness of the biofilm in a concentration-dependent manner. Gene expression studies indicated that allicin treatment of biofilm-associated bacteria resulted in downregulation of app and icaA genes that are associated with bacterial adhesion whereas only icaA was downregulated in planktonic cells (Wu X. et al., 2015). In vitro biofilm formation of S. aureus on reticular polypropylene mesh used in hernia was partially inhibited by allicin, but this effect diminished over time and a combination of allicin with chlorhexidine had no synergistic effect on the activity. However, allicin and chlorhexidine were cytotoxic individually, but the cytotoxicity was significantly reduced when cells were treated with the combination of both (Perez-Kohler et al., 2015a). To evaluate the effect of presoaking polypropylene mesh in allicin with chlorhexidine on biofilm formation in vivo, rabbit hernia model was infected with S. aureus, and it was found that combining allicin resulted in lower bacterial clearance and formation of biofilm compared to chlorhexidine treatment (Perez-Kohler et al., 2015b). The spectrum of antibiofilm property of FGE was tested against strong biofilm forming MDR clinical isolates of P. aeruginosa, K. pneumoniae, S. marcescens, and MRSA using in vitro and in vivo assays. In vitro assay indicated that FGE not only inhibited the formation of biofilm but also eradicated biofilms by these isolates on various surfaces. In vivo mice infection studies with P. aeruginosa and MRSA studies indicated that FGE significantly improved the survival of the animals and bacteria were not detected in different organs compared to control (Farrag et al., 2019).
The major organosulfur compounds of garlic, namely, allicin, ajoene, and aliphatic sulfides, pose QSI and antibiofilm activity. These activities are most explored in P. aeruginosa compared to other bacteria. It is evident from the data that garlic compounds downregulate QS and biofilm-associated genes. However, the precise mechanism in terms of whether the compounds bind and modify the transcription factor or interact with promoters of these genes is yet to be investigated. In vivo studies are encouraging to test the use of these compounds in clinical testing.
Synergistic Effect of Garlic and Its Compounds
The combination therapy sometimes leads to a synergistic effect, which effectively lowers the dose of individual drugs. Similarly, synergistic antibacterial effects were noticed when garlic and its compounds were used in combination with other phytochemicals and antibiotics. Both garlic crude extract and pure allicin exhibited strong synergy with vancomycin against 11 VRE clinical isolates with bacteriostatic action (Jonkers et al., 1999a). Raw garlic extract and commercial garlic tablets displayed synergistic effects against H. pylori when used along with omeprazole whereas no such effect was noticed when used in combination with amoxicillin, clarithromycin, and metronidazole (Jonkers et al., 1999b). In a clinical study, administration of allicin along with standard treatment (lansoprazole, clarithromycin, and amoxicillin) improved the percentage of H. pylori eradication by 23% in patients (Kockar et al., 2001). The combination of DATS and DATTS was either additive or synergistic when tested in combination with ceftazidime, gentamicin, imipenem, and meropenem except for DAT when used in combination with ceftazidime and gentamicin against ceftazidime and gentamicin-resistant K. pneumoniae, respectively (Tsao S. and Yin, 2001). Gentamicin administration induces nephrotoxicity and few reports have indicated that co-administration of aged garlic extract, garlic, S-allyl cysteine, DAS, and DADS ameliorates this nephrotoxicity. It was demonstrated that none of these agents decreased the activity of gentamicin; moreover, SAC, DAS, and DADS have enhanced the antibacterial activity of gentamicin, which makes them safe to use along with gentamicin to protect from nephrotoxicity (Maldonado et al., 2005). The MIC90 (concentration of the drug at which 90% of the growth is inhibited) against S. aureus of cefazolin and oxacillin were reduced by 128- and 64-fold, respectively, in the presence of 1/4 MIC90 of allicin. In the case of Staphylococcus epidermidis, MIC90 of cefazolin and oxacillin were reduced by 4- and 32-fold, respectively, in the presence of 1/4 and 1/8 MIC90 of allicin, respectively. The MIC90 of cefoperazone decreased by 16- and 8-fold in the presence of 1/2 and 1/4 MIC90 of allicin, respectively, against the cefoperazone-sensitive and -resistant strain of P. aeruginosa. The results indicate that allicin in combination with beta-lactam antibiotics results in synergy (Cai et al., 2007). The synergy of allicin with cefoperazone was confirmed against P. aeruginosa using the kill curve assay. In the case of tobramycin, certain synergy was observed against P. aeruginosa when used in combination with allicin, whereas no synergy was observed in the case of ciprofloxacin (Cai et al., 2008). It is reported that serum of patients that were administered garlic extract with standard antituberculosis had increased antitubercular activity compared to the control group, suggesting a synergistic effect when garlic is given in combination with antituberculosis drugs (Gupta et al., 1999). In the P. aeruginosa foreign body infection mouse model, it was noticed that in short-term infection, the bacterial clearance with the combination of ajoene and tobramycin was significantly better compared to placebo or individual treatments. The clearance of bacteria with ajoene alone was also significantly better compared to placebo. In case of long-term infection, the clearing of bacterial cells was significantly improved with ajoene and tobramycin treatment compared to control. However, there was no advantage of combining ajoene to tobramycin in clearing the bacteria (Christensen et al., 2012). Disk diffusion assay results indicated that the crude extract of garlic showed a synergistic effect when used in combination with gentamicin against E. coli, and it is interesting to find out that it was the only one out of the tested herbs that did not show any antagonistic effects with any test antibiotics (Ushimaru et al., 2012). In a similar study, antibiotic-resistant P. aeruginosa showed sensitivity to cefotaxime and ceftriaxone when FGE was added; this activity was better than FGE alone (Li et al., 2015). Another study reported that the ethyl acetate extract of garlic was antagonistic to the activity of chloramphenicol (Mahomoodally et al., 2018).
MDR E. coli isolated from drinking water in Bangladesh were not susceptible to AGE but were found to be sensitive to a combination of 1:1:1 combination of lime juice, garlic, and ginger extract (Rahman et al., 2011). The zone of inhibitions of Tazma honey and garlic crude extract combination were higher than when these were used individually against common pathogenic bacteria (Andualem, 2013). Garlic essential oil in combination with essential oils from several other species did not result in any synergy against tested pathogenic bacteria (Bag and Chattopadhyay, 2015). The in vitro interaction studies of a combination of AGE and Manuka honey against extended-spectrum beta-lactamase-producing E. coli showed different effects on different isolates ranging from synergy, additive, and indifferent to antagonistic effects (Idris and Afegbua, 2017). Mechanistic studies to understand the synergistic effects of garlic with antibiotics or other chemicals are lacking. Such studies are required for encouraging the use of garlic compounds to complement conventional medicine.
Mechanisms of Antibacterial Activity of Garlic Organosulfur Compounds
The principal active ingredient responsible for the antibacterial activity of garlic was identified to be allicin (Cavallito and Bailey, 1944a). This finding was immediately followed by the observation that cysteine and other sulfhydryl-containing compounds inhibited the antibacterial activity of allicin, leading to the hypothesis that allicin might exert its antibacterial effect by reacting with sulfhydryl groups of the bacterial proteins (Cavallito and Bailey, 1944b; Cavallito et al., 1945). Allicin would react to sulfhydryl groups of cysteines irreversibly and will not be available to react with the sulfhydryl groups of the enzymes. The ability of allicin to inhibit various sulfhydryl enzymes indicates that the mechanism of antibacterial action of allicin is by reacting with the sulfhydryl groups of the many metabolically important bacterial enzymes (Wills, 1956). The action of allicin is mostly non-specific as it is found to inhibit urease, papain, amylase, and alcohol dehydrogenase. NMR experiments identified S-allylmercaptocysteine, confirming the reaction between allicin and sulfhydryl group of cysteine (Rabinkov et al., 1998). The rapid permeability of allicin through lipid bilayers supports the idea of allicin able to reach and react with sulfhydryl groups of bacterial proteins (Miron et al., 2000). The treatment with reducing agents such as β-mercaptoethanol and dithiothreitol resulted in the loss of allicin’s antibacterial activity, indicating that allicin forms disulfide bonds with the sulfhydryl groups of enzymes (Rabinkov et al., 1998; Jonkers et al., 1999a). Mass spectrometry and Raman spectrum analysis confirm that allicin enters the cell rapidly and reacts with cysteine and glutathione sulfhydryl groups (Fujisawa et al., 2009; Miron et al., 2010; Lu et al., 2011a). The enzymatic activity of bacterioferritin comigratory protein (BCP) from B. cepacia, which has two catalytically cysteines, was inhibited by allicin and mass spectrometry analysis confirmed that S-allyl thiol groups were added to these cysteines by allicin (Wallock-Richards et al., 2014). The inhibition of trypsin-like protease and general protease activity of P. gingivalis cell extract by AGE suggests that the antibacterial activity of garlic could be due to inhibition of proteolysis (Bakri and Douglas, 2005). Streptolysin O and mature Streptococcal pyrogenic exotoxin B of Streptococci that contain functionally important cysteines were inhibited by allicin and the addition of DTT reversed the inhibition (Arzanlou and Bohlooli, 2010; Arzanlou, 2016). Mass spectrometric proteomic analysis of cytoplasm of E. coli treated with allicin revealed 73 S-thioallylated proteins including some essential metabolic enzymes. It was shown that allicin reacts with low-molecular-weight cellular thiols such as glutathione (GSH) causing oxidative stress (Muller et al., 2016). Overall, these studies establish that the mechanism of antibacterial activity of allicin is by reacting with the sulfhydryl attaching allythio group through a disulfide bond. However, it should be noted that the mechanism of action of allicin is non-specific, which could make it cytotoxic. Alternatively, it has been reported that treatment of allicin inhibits DNA, RNA, and protein synthesis in bacteria, and the inhibition of RNA synthesis is more profound (Feldberg et al., 1988). In vivo study revealed that allicin modulates immunological parameters such as increased phagocytic and serum lysozyme activity to protect rainbow trout fish from A. hydrophila infection (Nya et al., 2010).
Allicin is highly unstable and is degraded into various organosulfide compounds (Figure 1B). The organosulfides also have been reported to show activity against a wide range of bacteria. Different studies have shown that organosulfides constitute a majority of GO. The mechanism of action of organosulfides, like allicin, is to react with free sulfhydryl groups of enzymes. However, organosulfides are not as reactive as allicin due to the absence of oxygen that is bound to sulfur in allicin. The activity of H. pylori arylamine N-acetyltransferase was inhibited in the presence of DAS and DADS, suggesting that these compounds exert antibacterial activity by inhibiting bacterial enzymes (Chung et al., 1998). In a more recent report, DAS was also found to inhibit the activity of the GST enzyme (Velliyagounder et al., 2012). Like allicin, addition of cysteine has reduced the antibacterial activity of GO against E. aerogenes, suggesting that sulfides also react with free sulfhydryl groups of enzymes (Ross et al., 2001). A recent study established that the antibacterial activity of diallyl polysulfides is due to their ability to react with sulfhydryl groups of various enzymes of B. subtilis such as bacillithiol and CoA and with amino acid cysteine (Kyo et al., 2001).
FTIR and Raman spectroscopy analysis revealed that treatment of C. jejuni, C. sakazakii, E. coli O157:H7, L. monocytogenes, and Bifidobacterium species with garlic crude extract and allyl sulfides causes many spectral changes that indicate the interaction of these agents with various cellular components; most notable changes indicate reaction with sulfhydryl groups, modification of cell membrane, and wall components to damage and destroy the integrity of the cell. These observations were consistent with the electron microscopy data that showed damage to cell wall and membrane (Lu et al., 2011a, b, 2012; Booyens et al., 2014; Booyens and Thantsha, 2014; Feng et al., 2014). Treatment of C. jejuni with DAS decreased the cellular ATP levels and increased the level of cellular protein in the culture, suggesting loss of cell membrane integrity (Lu et al., 2012).
A global proteomic analysis was performed to determine the mechanism of the anti-H. pylori effect of DATS. The results of 2D gel electrophoresis of proteins showed that upon treatment with DATS, proteins involved in metabolism, biosynthesis, bacterial virulence, and redox reactions were downregulated while stress response chaperon proteins were upregulated. The production of CagA and VacA virulent protein was decreased due to DATS treatment (Zare et al., 2008). A study performed RNA sequencing to study the changes in the global transcriptome of C. sakazakii upon treatment with DAS. Although there were a large number of genes that were up- and downregulated by the DAS treatment, clusters of genes that are related to cell shape and wall maintenance and lipopolysaccharide synthesis were upregulated while RNA and amino acid biosynthetic genes were downregulated. This indicates that DAS causes injury to the cell wall and membrane and decreases general metabolism (Feng et al., 2014).
The anti-C. sakazakii activity of ajoene was also diminished by the addition of cysteines, indicating that its antibacterial activity also involves reacting with sulfhydryl groups of bacterial enzymes. The transcriptome analysis of C. sakazakii treated with ajoene showed that the NADH expression factor and nitrate reductase gene were downregulated, which is related to reactive oxygen species reactions. In addition, flagellum and bacterial motility genes were downregulated, suggesting that ajoene negatively impacts biofilm formation (Feng et al., 2014).
In summary, two main mechanisms of action of garlic organosulfur compounds emerged from the reported studies: (1) the reaction of garlic compounds to the free sulfhydryl group on the proteins and/or enzymes to inactivate them, and (2) the disruption of composition and integrity of bacterial cell membrane and/or cell wall. Besides, some work also suggests that garlic compounds could also have a global effect on DNA, RNA, and protein synthesis. These mechanisms are observed in both Gram-positive and Gram-negative bacteria, suggesting that garlic and its compounds use similar antibacterial mechanisms for both groups of bacteria. However, the activity of the compounds is not specific, which could restrict their clinical application. The mechanism of action of garlic and its compound has been summarized in Figure 2.
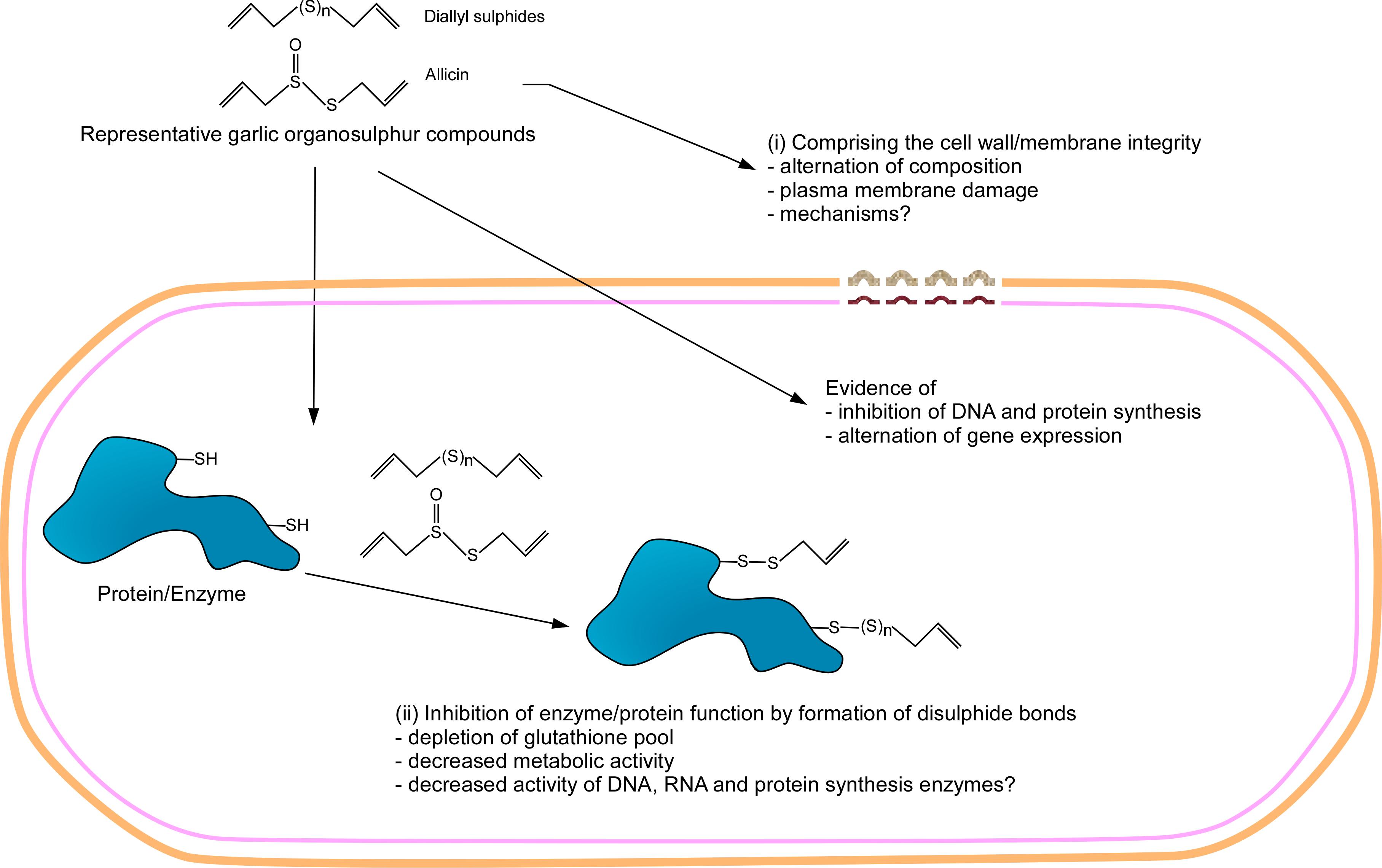
Figure 2. Illustration of the mechanism of action of garlic organosulfur compounds. Garlic organosulfur compounds exert their antibacterial activity mainly through two mechanisms: (i) The organosulfur compounds are highly reactive with sulfur having the capability to form disulfide bonds with the free sulfhydryl groups of proteins including enzymes. The formation of disulfide bonds renders the enzyme inactive, resulting in the death of bacteria. (ii) The organosulfur compound interacts with the cell membrane of bacteria. This interaction compromises the integrity of the cell membranes of the bacteria leading to leakage of cell content leading to death. In addition, it is also thought that garlic organosulfur compounds interfere with protein production, DNA replication, and alter gene expression.
Emerging Novel Techniques and Opportunities to Use Garlic as Novel Antibacterial Agent
The emergence of various technologies such as nanotechnology, refined organic synthesis methods, and specialized drug delivery methods provide ample opportunities to use garlic organosulfur compounds as novel antibacterial agents. Green nanoparticle synthesis in recent times has emerged as a powerful tool to use phytochemicals to not only synthesize nanoparticles but also improve the antibacterial functions of these chemicals and particles (Wang and Vermerris, 2016).
Garlic extract has been used for the green synthesis and stabilization of silver nanoparticles. Eco-friendly garlic-silver nanoparticles synthesized using garlic clove extract displayed a greater antibacterial and antibiofilm activity on clinically important pathogens such as MRSA and P. aeruginosa compared to garlic extract or silver nitrate (Vijayakumar et al., 2019). A novel highly active molecule, (2E, 2E)-4,4-trisulfanediylbis (but-2-enoic acid) (TSDB) was synthesized through comparative molecular field analysis (COMFA) using the structure of DATS. TSDB displayed a robust inhibitory effect against S. aureus at low concentration. TSDB treatment increased the conductivity better than DATS, indicating better membrane penetration. The increase in the levels of protein and no change in the levels of alkaline phosphate in culture upon treatment with TSDB and DATS compared to control suggest damage to the cell membrane but not so much to the cell wall (Wu et al., 2018). Solgel prepared using tetraethyl orthosilicate was loaded with 20% ethanol extract of garlic, which displayed controlled release and stability of garlic components with increased antibacterial and antibiofilm activity against MRSA (Girish et al., 2019). GO microspheres were monodispersed in water by microemulsion technique to overcome its volatile characteristics and poor aqueous solubility. The study specified that the water-dilutable microemulsion that is formed by GO encapsulated in a nanoparticle vector is effective in preventing S. aureus than E. coli (Zheng et al., 2013). In another study, wild garlic (Allium ursinum L.) extract was encapsulated using spray congealing technology to shield its valued active compounds and expand its oral bioavailability. The encapsulation led to an enhancement of the extract dissolution performance as well as an improvement in the solubility of more than 18-fold compared to the pure extract. Microparticles were stable over a 3-month period, showing only a minor decrease in the content of active compounds (allicin and S-methyl methane thiosulfonate) and upholding a good antimicrobial activity. The study suggests that such spray congealing technology can be used to improve the solubility, bioavailability, and stability of the garlic active ingredients including allicin without affecting their antibacterial properties (Tomsik et al., 2019). The stability of phytochemicals present in GO, mainly allicin, was improved when biogenic nanoscale mesoporous silicon derived from the silicon-accumulator plant Tabasheer (Bambuseae) was used as a potential carrier as the antibacterial activity of this material was better than GO only control (Le et al., 2017). More research should be focused to make similar nanoparticles, emulsions, and novel formulations using pure garlic compounds, mainly allicin, to improve their stability. In vitro studies with allicin aerosol and vapors using a lung model demonstrated the antibacterial efficacy of allicin with a correlation between aerosol deposition pattern and bacterial growth inhibition. Interesting synergy was observed with allicin that was administered with ethanol against E. coli (Reiter et al., 2020). It was interesting to note that DAS is not a strong antibacterial compound, but when given in combination with zinc oxide nanorods as an emulsion, it displayed a synergistic effect against S. aureus and MRSA bacteria under in vitro and in vivo conditions (Rauf et al., 2018). In a study, SAC, which is not antibacterial by itself, exhibited antibacterial activity when in complex with palladium (II) against E. coli, P. aeruginosa, and S. aureus (Spera et al., 2011). Organosulfides were converted into nano-iron sulfides with 500-fold superior antibacterial activity against pathogenic and drug-resistant bacteria compared to compounds themselves. The nano-iron sulfides released hydrogen polysulfanes and topical application in animal models resulted in reduced biofilm formation and accelerated wound healing (Xu et al., 2018).
Leontiev et al. (2018) developed a series of allicin analogs and evaluated their antimicrobial properties and thermal stability against bacteria and the model fungus Saccharomyces cerevisiae. Here, dimethyl-, diethyl-, diallyl-(allicin), dipropyl-, and dibenzyl-thiosulfinates form a series of molecules with increasing molecular mass and hydrophobicity, which would be anticipated to affect physical characteristics such as rate of diffusion, volatility, and membrane permeability, all of which are expected to affect the antibacterial properties of the molecules. In this study, the more volatile compounds showed noteworthy antimicrobial properties via the gas phase. Thiosulfinates differed in their effectivity against specific organisms, and some were thermally more stable than allicin. These results encourage the application of garlic-based compounds in medicine and agriculture either singly or in combination with other antimicrobials (Leontiev et al., 2018). Attaching N-propylthiol (similar chemistry to allicin) to ciprofloxacin increased the sensitivity of MRSA toward ciprofloxacin, suggesting that combination chemistry with garlic organosulfur could potentiate existing antibiotics (Sheppard and Long, 2016). Another study screened a chemical library composed of 19 synthesized pyridyl disulfides that emulate the chemical reactivity of allicin for antimicrobial activity against Gram-positive species including VRSA. The study identified pyridyl disulfides as stable alternatives to allicin with a similar narrow-spectrum profile and are thought to function as pro-oxidants like that of allicin (Sheppard et al., 2018).
As garlic is consumed regularly all over the world, it is considered non-toxic without any side effects. However, there are limited reports of toxic side effects of garlic and its constituents. In some individuals, contact with garlic and its constituents (especially oil-soluble sulfur compounds) leads to skin irritation and dermatitis (Jappe et al., 1999). In vivo studies administering garlic juice resulted in stomach damage. Garlic juice rich in allicin and allicin itself cause damage to the intestinal epithelial mucosa (Kodera, 1997). Allicin was also reported to immobilize sperms in vitro (Qian et al., 1986). In vitro cytotoxicity studies showed that DAS did not affect the cell growth or viability, whereas both DAS and allicin changed the morphology of cells. Allicin also significantly decreased the metabolic activity of cells (Velliyagounder et al., 2012; Perez-Kohler et al., 2015a). The concentration at which these effects are noticed are relatively high and more studies need to be done to evaluate the toxicity of garlic and its compounds at concentrations that exhibit antibacterial effects.
Conclusion
The extensive research strongly indicates that garlic organosulfur compounds exhibit strong antibacterial activity against a wide range of bacteria including MDR strains. Although garlic organosulfur compounds have been known to be excellent antibacterial compounds, not much progress has been made in the direction of utilizing them clinically to tackle the problem of antibiotic resistance. The toxicity data of garlic and its compounds from animal studies are inconsistent with some studies reporting no toxic effect, whereas some report inflammation and toxic effects. The lower stability, solubility, and bioavailability of these compounds have hindered their use in the clinical setting. However, the organosulfur compounds are attractive because their bactericidal activity is exerted through multiple mechanisms, making it difficult for bacteria to develop resistance. Another concern that should be addressed for the use of garlic compounds is their toxicity and specificity to use them as antibacterial agents. Although a great deal of research has been done on the antibacterial potential of garlic and its compounds, there are recent gaps that need to be filled to utilize them as antibacterial agents in clinical settings. The first major area where more research should be focused is to develop robust and economical extraction or synthesis procedures that would yield pure garlic compounds. Besides, most of the organosulfur compounds of garlic are not water soluble and are unstable. Thus, formulations using advanced nanoparticle or emulsion techniques should be developed with improved solubility and self-life. A database should be created to curate the antibacterial data of garlic compounds by themselves and in combination with other antibiotics, which should be performed under pre-established guidelines. Such a database can be used by artificial intelligence tools to predict the most effective combinations for testing or treatment. Most of the research reports the in vitro antibacterial activity of garlic compounds that are important but not necessarily translate to in vivo conditions. Therefore, future research should be focused on validating the antibacterial activity of garlic compounds in animal infection models. Furthermore, elaborate toxicology and pharmacokinetic studies with respect to different pure organosulfur compounds, administered amount, and route should be performed. Clinical studies of garlic compounds except for anti-H. pylori activity has not been performed. As garlic is approved by FDA as “generally recognized as safe,” garlic products should be administered in addition to standard antibiotics in the clinical setting to establish the role of garlic in complementary medicine. All these gaps in the research have been summarized in a cartoon (Figure 3). Recent advances in science and technology such as combinatorial and refined chemical synthesis techniques, nanotechnology, bioinformatics, computation tools, and advanced formulation can effectively overcome the challenges to develop the garlic organosulfur-based novel antibiotics. In the light of rapid emergence of antibiotic resistance, it is warranted that more research should be focused to understand the precise mechanism of action at a molecular level of organosulfur compounds. The organosulfur compounds have the potential to make a huge impact on human health by decreasing the mortality associated with bacterial infections.
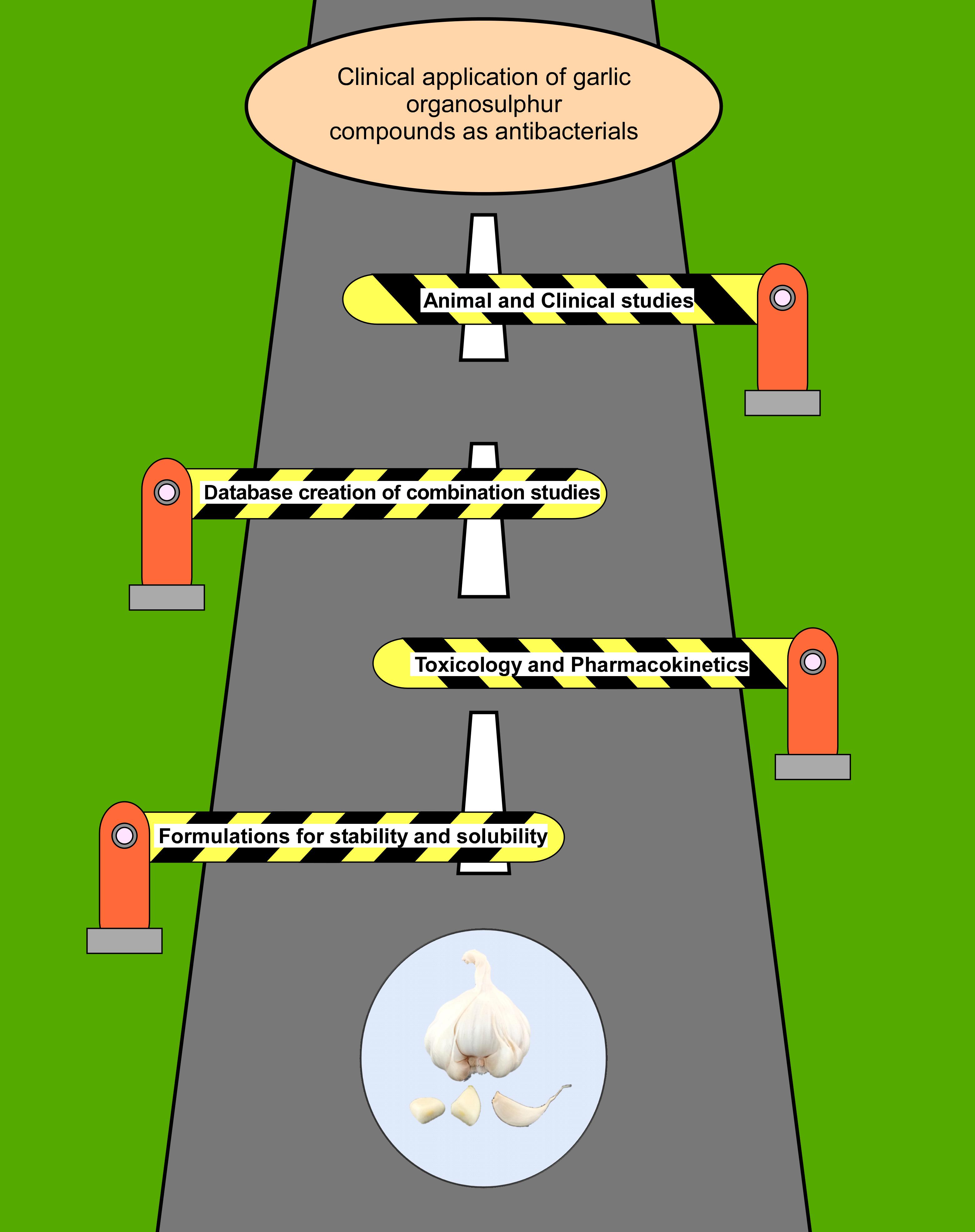
Figure 3. Cartoon representation of the challenges that should be crossed for garlic/garlic compounds to be used in the clinical setting as antibacterials. The road to developing garlic/garlic compounds into novel clinically relevant antibiotics is subject to crossing the barrier shown in the cartoon, which are as follows: (1) the development of new formulations that would improve the specificity, stability, and solubility of garlic organosulfur compounds; (2) to access the safety and bioavailability of organosulfur compounds by performing toxicology and pharmacokinetic studies with various compounds administered through different routes; (3) to avoid repetition and encourage in vivo studies, creation of a database to record the in vivo and in vitro antibacterial studies of garlic compounds alone and along with other antibiotics or phytochemicals; and (4) more pre-clinical and clinical studies should be performed to access the safety and efficacy of these compounds. Research in these areas will ensure the use of garlic-based novel antibacterials in the clinical setting.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
The open access publications funds were provided by Durban University of Technology.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
SK is thankful to Research Fellowship by Durban University of Technology.
Abbreviations
MDR, multi-drug-resistant; FGE, fresh garlic extract; GP, garlic powder; GO, garlic oil; AGE, aqueous garlic extract; QS, quorum sensing; SAC, S-allyl cysteine; STEC, Shiga toxin-producing Escherichia coli; MRSA, methicillin-resistant Staphylococcus aureus; VRSA, vancomycin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; DAS, diallyl sulfide; DADS, diallyl disulfide; DATS, diallyl trisulfide; DATTS, diallyl tetrasulfide; MIC, minimum inhibitory concentration; Bcc, Burkholderia cepacia complex; EPS, extrapolysaccharide; QSI, quorum sensing inhibition.
References
Adetumbi, M. A., and Lau, B. H. (1983). Allium sativum (garlic)–a natural antibiotic. Med. Hypotheses 12, 227–237.
Amagase, H. (2006). Clarifying the real bioactive constituents of garlic. J. Nutr. 136(3 Suppl.), 716S–725S. doi: 10.1093/jn/136.3.716S
Amagase, H., Petesch, B. L., Matsuura, H., Kasuga, S., and Itakura, Y. (2001). Intake of garlic and its bioactive components. J. Nutr. 131, 955S–962S. doi: 10.1093/jn/131.3.955S
Andualem, B. (2013). Combined antibacterial activity of stingless bee (Apis mellipodae) honey and garlic (Allium sativum) extracts against standard and clinical pathogenic bacteria. Asian Pac. J. Trop. Biomed. 3, 725–731. doi: 10.1016/S2221-1691(13)60146-X
Ankri, S., and Mirelman, D. (1999). Antimicrobial properties of allicin from garlic. Microbes Infect. 1, 125–129. doi: 10.1016/s1286-4579(99)80003-3
Antunes, L. C., Buckner, M. M., Auweter, S. D., Ferreira, R. B., Lolic, P., and Finlay, B. B. (2010). Inhibition of Salmonella host cell invasion by dimethyl sulfide. Appl. Environ. Microbiol. 76, 5300–5304. doi: 10.1128/AEM.00851-10
Arora, D. S., and Kaur, J. (1999). Antimicrobial activity of spices. Int. J. Antimicrob. Agents 12, 257–262.
Arreola, R., Quintero-Fabian, S., Lopez-Roa, R. I., Flores-Gutierrez, E. O., Reyes-Grajeda, J. P., Carrera-Quintanar, L., et al. (2015). Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015:401630. doi: 10.1155/2015/401630
Arzanlou, M. (2016). Inhibition of streptococcal pyrogenic exotoxin B using allicin from garlic. Microb. Pathog. 93, 166–171. doi: 10.1016/j.micpath.2016.02.010
Arzanlou, M., and Bohlooli, S. (2010). Inhibition of streptolysin O by allicin - an active component of garlic. J. Med. Microbiol. 59(Pt. 9), 1044–1049. doi: 10.1099/jmm.0.019539-0
Avato, P., Tursil, E., Vitali, C., Miccolis, V., and Candido, V. (2000). Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 7, 239–243. doi: 10.1016/s0944-7113(00)80010-0
Aydin, A., Ersoz, G., Tekesin, O., Akcicek, E., and Tuncyurek, M. (2000). Garlic oil and Helicobacter pylori infection. Am. J. Gastroenterol. 95, 563–564. doi: 10.1111/j.1572-0241.2000.t01-1-01812.x
Bag, A., and Chattopadhyay, R. R. (2015). Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS One 10:e0131321. doi: 10.1371/journal.pone.0131321
Bakri, I. M., and Douglas, C. W. (2005). Inhibitory effect of garlic extract on oral bacteria. Arch. Oral Biol. 50, 645–651. doi: 10.1016/j.archoralbio.2004.12.002
Ban, J. O., Oh, J. H., Kim, T. M., Kim, D. J., Jeong, H. S., Han, S. B., et al. (2009). Anti-inflammatory and arthritic effects of thiacremonone, a novel sulfur compound isolated from garlic via inhibition of NF-kappaB. Arthritis Res. Ther. 11:R145. doi: 10.1186/ar2819
Becker, P. M., van Wikselaar, P. G., Mul, M. F., Pol, A., Engel, B., Wijdenes, J. W., et al. (2012). Actinobacillus pleuropneumoniae is impaired by the garlic volatile allyl methyl sulfide (AMS) in vitro and in-feed garlic alleviates pleuropneumonia in a pig model. Vet. Microbiol. 154, 316–324. doi: 10.1016/j.vetmic.2011.07.011
Bhatwalkar, S. B., Gound, S. S., Mondal, R., Srivastava, R. K., and Anupam, R. (2019). Anti-biofilm and antibacterial activity of allium sativum against drug resistant shiga-toxin producing Escherichia coli (STEC) isolates from patient samples and food sources. Indian J. Microbiol. 59, 171–179. doi: 10.1007/s12088-019-00784-3
Bjarnsholt, T., Jensen, P. O., Rasmussen, T. B., Christophersen, L., Calum, H., Hentzer, M., et al. (2005). Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 151(Pt. 12), 3873–3880. doi: 10.1099/mic.0.27955-0
Block, E. (1992). The organosulfur chemistry of the genus azzium - implications for the organic chemistry of sulfur. Angew. Chem. Int. Ed. 31, 1135–1178.
Bodini, S. F., Manfredini, S., Epp, M., Valentini, S., and Santori, F. (2009). Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett. Appl. Microbiol. 49, 551–555. doi: 10.1111/j.1472-765X.2009.02704.x
Booyens, J., and Thantsha, M. S. (2014). Fourier transform infra-red spectroscopy and flow cytometric assessment of the antibacterial mechanism of action of aqueous extract of garlic (Allium sativum) against selected probiotic bifidobacterium strains. BMC Complement Altern. Med. 14:289. doi: 10.1186/1472-6882-14-289
Booyens, J., Labuschagne, M. C., and Thantsha, M. S. (2014). In vitro antibacterial mechanism of action of crude garlic (Allium sativum) clove extract on selected probiotic bifidobacterium species as revealed by SEM, TEM, and SDS-PAGE analysis. Probiotics Antimicrob. Proteins 6, 82–87. doi: 10.1007/s12602-013-9145-z
Brodnitz, M. H., Pascale, J. V., and Van Derslice, L. (1971). Flavor components of garlic extract. J. Agric. Food Chem. 19, 273–275. doi: 10.1021/jf60174a007
Cai, Y., Wang, R., An, M. M., Liang, B. B., and Fang, Y. (2008). In vitro bactericidal activity of allicin combined with cefoperazone, tobramycin and ciprofloxacin. Int. J. Antimicrob. Agents 31, 179–180. doi: 10.1016/j.ijantimicag.2007.10.009
Cai, Y., Wang, R., Pei, F., and Liang, B. B. (2007). Antibacterial activity of allicin alone and in combination with beta-lactams against Staphylococcus spp. and Pseudomonas aeruginosa. J. Antibiot. 60, 335–338. doi: 10.1038/ja.2007.45
Canizares, P., Gracia, I., Gomez, L. A., Garcia, A., Martin De Argila, C., Boixeda, D., et al. (2004a). Thermal degradation of allicin in garlic extracts and its implication on the inhibition of the in-vitro growth of Helicobacter pylori. Biotechnol. Prog. 20, 32–37. doi: 10.1021/bp034135v
Canizares, P., Gracia, I., Gomez, L. A., Martin de Argila, C., Boixeda, D., Garcia, A., et al. (2004b). Allyl-thiosulfinates, the bacteriostatic compounds of garlic against Helicobacter pylori. Biotechnol. Prog. 20, 397–401. doi: 10.1021/bp034143b
Cao, H. X., Zhu, K. X., Fan, J. G., and Qiao, L. (2014). Garlic-derived allyl sulfides in cancer therapy. Anticancer Agents Med. Chem. 14, 793–799. doi: 10.2174/1871520614666140521120811
Casella, S., Leonardi, M., Melai, B., Fratini, F., and Pistelli, L. (2013). The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother. Res. 27, 380–383. doi: 10.1002/ptr.4725
Cavallito, C. J., and Bailey, J. H. (1944a). Allicin, the antibacterial principle of Allium sativum. I. isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 66, 1950–1951. doi: 10.1021/ja01239a048
Cavallito, C. J., and Bailey, J. H. (1944b). Preliminary note on the inactivation of antibiotics. Science 100:390. doi: 10.1126/science.100.2600.390
Cavallito, C. J., Bailey, J. H., Haskell, T. H., McCormick, J. R., and Warner, W. F. (1945). The inactivation of antibacterial agents and their mechanism of action. J. Bacteriol. 50, 61–69. doi: 10.1128/JB.50.1.61-69.1945
Cellini, L., Di Campli, E., Masulli, M., Di Bartolomeo, S., and Allocati, N. (1996). Inhibition of Helicobacter pylori by garlic extract (Allium sativum). FEMS Immunol. Med. Microbiol. 13, 273–277.
Chen, K., Xie, K., Liu, Z., Nakasone, Y., Sakao, K., Hossain, A., et al. (2019). Preventive effects and mechanisms of garlic on dyslipidemia and gut microbiome dysbiosis. Nutrients 11:1225. doi: 10.3390/nu11061225
Cheng, G., Dai, M., Ahmed, S., Hao, H., Wang, X., and Yuan, Z. (2016). Antimicrobial drugs in fighting against antimicrobial resistance. Front. Microbiol. 7:470. doi: 10.3389/fmicb.2016.00470
Chowdhury, A. K., Ahsan, M., Islam, S. N., and Ahmed, Z. U. (1991). Efficacy of aqueous extract of garlic & allicin in experimental shigellosis in rabbits. Indian J. Med. Res. 93, 33–36.
Christensen, L. D., van Gennip, M., Jakobsen, T. H., Alhede, M., Hougen, H. P., Hoiby, N., et al. (2012). Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J. Antimicrob. Chemother. 67, 1198–1206. doi: 10.1093/jac/dks002
Chung, J. G., Chen, G. W., Wu, L. T., Chang, H. L., Lin, J. G., Yeh, C. C., et al. (1998). Effects of garlic compounds diallyl sulfide and diallyl disulfide on arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Am. J. Chin. Med. 26, 353–364. doi: 10.1142/S0192415X98000397
Cutler, R. R., and Wilson, P. (2004). Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 61, 71–74. doi: 10.1080/09674845.2004.11732646
Cutler, R. R., Odent, M., Hajj-Ahmad, H., Maharjan, S., Bennett, N. J., Josling, P. D., et al. (2009). In vitro activity of an aqueous allicin extract and a novel allicin topical gel formulation against Lancefield group B streptococci. J. Antimicrob. Chemother. 63, 151–154. doi: 10.1093/jac/dkn457
Dakka, D. (2011). Antibacterial effect of garlic (Allium sativum) on Staphyloccus aureus: an in vitro study. Afr. J. Biotech. 10, 666–669.
Delaha, E. C., and Garagusi, V. F. (1985). Inhibition of mycobacteria by garlic extract (Allium sativum). Antimicrob. Agents Chemother. 27, 485–486. doi: 10.1128/aac.27.4.485
Dikasso, D., Lemma, H., Urga, K., Debella, A., Addis, G., Tadele, A., et al. (2002). Investigation on the antibacterial properties of garlic (Allium sativum) on pneumonia causing bacteria. Ethiop. Med. J. 40, 241–249.
Fani, M. M., Kohanteb, J., and Dayaghi, M. (2007). Inhibitory activity of garlic (Allium sativum) extract on multidrug-resistant Streptococcus mutans. J. Indian Soc. Pedod. Prev. Dent. 25, 164–168.
Farrag, H. A., Hosny, A., Hawas, A. M., Hagras, S. A. A., and Helmy, O. M. (2019). Potential efficacy of garlic lock therapy in combating biofilm and catheter-associated infections; experimental studies on an animal model with focus on toxicological aspects. Saudi Pharm. J. 27, 830–840. doi: 10.1016/j.jsps.2019.05.004
Feldberg, R. S., Chang, S. C., Kotik, A. N., Nadler, M., Neuwirth, Z., Sundstrom, D. C., et al. (1988). In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 32, 1763–1768. doi: 10.1128/aac.32.12.1763
Feng, S., Eucker, T. P., Holly, M. K., Konkel, M. E., Lu, X., and Wang, S. (2014). Investigating the responses of Cronobacter sakazakii to garlic-drived organosulfur compounds: a systematic study of pathogenic-bacterium injury by use of high-throughput whole-transcriptome sequencing and confocal micro-raman spectroscopy. Appl. Environ. Microbiol. 80, 959–971. doi: 10.1128/AEM.03460-13
Fenwick, G. R., and Hanley, A. B. (1985a). The genus allium–part 1. Crit. Rev. Food Sci. Nutr. 22, 199–271. doi: 10.1080/10408398509527415
Fenwick, G. R., and Hanley, A. B. (1985b). The genus allium. part 2. Crit. Rev. Food Sci. Nutr. 22, 273–377. doi: 10.1080/10408398509527417
Fenwick, G. R., and Hanley, A. B. (1985c). The genus allium–part 3. Crit. Rev. Food Sci. Nutr. 23, 1–73. doi: 10.1080/10408398509527419
Fletcher, R. D., Parker, B., and Hassett, M. (1974). Inhibition of coagulase activity and growth of Staphylococcus aureus by garlic extracts. Folia Microbiol. 19, 494–497. doi: 10.1007/BF02872915
Fong, J., Yuan, M., Jakobsen, T. H., Mortensen, K. T., Delos Santos, M. M., Chua, S. L., et al. (2017). Disulfide bond-containing ajoene analogues as novel quorum sensing inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 60, 215–227. doi: 10.1021/acs.jmedchem.6b01025
Fuchs, A. L., Weaver, A. J. Jr., Tripet, B. P., Ammons, M. C. B., Teintze, M., and Copie, V. (2018). Characterization of the antibacterial activity of Bald’s eyesalve against drug resistant Staphylococcus aureus and Pseudomonas aeruginosa. PLoS One 13:e0208108. doi: 10.1371/journal.pone.0208108
Fujisawa, H., Suma, K., Origuchi, K., Kumagai, H., Seki, T., and Ariga, T. (2008). Biological and chemical stability of garlic-derived allicin. J. Agric. Food Chem. 56, 4229–4235. doi: 10.1021/jf8000907
Fujisawa, H., Watanabe, K., Suma, K., Origuchi, K., Matsufuji, H., Seki, T., et al. (2009). Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci. Biotechnol. Biochem. 73, 1948–1955. doi: 10.1271/bbb.90096
Girish, V. M., Liang, H., Aguilan, J. T., Nosanchuk, J. D., Friedman, J. M., and Nacharaju, P. (2019). Anti-biofilm activity of garlic extract loaded nanoparticles. Nanomedicine 20:102009. doi: 10.1016/j.nano.2019.04.012
Graham, D. Y., Anderson, S. Y., and Lang, T. (1999). Garlic or jalapeno peppers for treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 94, 1200–1202. doi: 10.1111/j.1572-0241.1999.01066.x
Gull, I., Saeed, M., Shaukat, H., Aslam, S. M., Samra, Z. Q., and Athar, A. M. (2012). Inhibitory effect of Allium sativum and zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann. Clin. Microbiol. Antimicrob. 11:8. doi: 10.1186/1476-0711-11-8
Gupta, R. L., Jain, S., Talwar, V., Gupta, H. C., and Murthy, P. S. (1999). Antitubercular activity of garlic (Allium sativum) extract on combination with conventional antitubercular drugs in tubercular lymphadenitis. Indian J. Clin. Biochem. 14, 12–18. doi: 10.1007/bf02869146
Gupta, R., Thakur, B., Singh, P., Singh, H. B., Sharma, V. D., Katoch, V. M., et al. (2010). Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Indian J. Med. Res. 131, 809–813.
Gupta, S., and Ravishankar, S. (2005). A comparison of the antimicrobial activity of garlic, ginger, carrot, and turmeric pastes against Escherichia coli O157:H7 in laboratory buffer and ground beef. Foodborne Pathog. Dis. 2, 330–340. doi: 10.1089/fpd.2005.2.330
Hannan, A., Ikram Ullah, M., Usman, M., Hussain, S., Absar, M., and Javed, K. (2011). Anti-mycobacterial activity of garlic (Allium sativum) against multi-drug resistant and non-multi-drug resistant mycobacterium tuberculosis. Pak. J. Pharm. Sci. 24, 81–85.
Idris, A. R., and Afegbua, S. L. (2017). Single and joint antibacterial activity of aqueous garlic extract and Manuka honey on extended-spectrum beta-lactamase-producing Escherichia coli. Trans. R. Soc. Trop. Med. Hyg. 111, 472–478. doi: 10.1093/trstmh/trx084
Iwalokun, B. A., Ogunledun, A., Ogbolu, D. O., Bamiro, S. B., and Jimi-Omojola, J. (2004). In vitro antimicrobial properties of aqueous garlic extract against multidrug-resistant bacteria and Candida species from Nigeria. J. Med. Food 7, 327–333. doi: 10.1089/jmf.2004.7.327
Jain, I., Jain, P., Bisht, D., Sharma, A., Srivastava, B., and Gupta, N. (2015a). Comparative evaluation of antibacterial efficacy of six indian plant extracts against streptococcus mutans. J. Clin. Diagn. Res. 9, ZC50–ZC53. doi: 10.7860/JCDR/2015/11526.5599
Jain, I., Jain, P., Bisht, D., Sharma, A., Srivastava, B., and Gupta, N. (2015b). Use of traditional Indian plants in the inhibition of caries-causing bacteria–Streptococcus mutans. Braz. Dent. J. 26, 110–115. doi: 10.1590/0103-6440201300102
Jakobsen, T. H., van Gennip, M., Phipps, R. K., Shanmugham, M. S., Christensen, L. D., Alhede, M., et al. (2012). Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 56, 2314–2325. doi: 10.1128/AAC.05919-11
Jang, H. J., Lee, H. J., Yoon, D. K., Ji, D. S., Kim, J. H., and Lee, C. H. (2018). Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 27, 219–225. doi: 10.1007/s10068-017-0246-4
Jappe, U., Bonnekoh, B., Hausen, B. M., and Gollnick, H. (1999). Garlic-related dermatoses: case report and review of the literature. Am. J. Contact Dermat. 10, 37–39. doi: 10.1016/s1046-199x(99)90092-1
Jc, D. E. W., Notermans, S., Gorin, N., and Kampelmacher, E. H. (1979). Effect of garlic oil or onion oil on toxin production by clostridium botulinum in meat slurry. J. Food Prot. 42, 222–224. doi: 10.4315/0362-028X-42.3.222
Johnson, M. G., and Vaughn, R. H. (1969). Death of Salmonella typhimurium and Escherichia coli in the presence of freshly reconstituted dehydrated garlic and onion. Appl. Microbiol. 17, 903–905.
Jonkers, D., Sluimer, J., and Stobberingh, E. (1999a). Effect of garlic on vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:3045.
Jonkers, D., van den Broek, E., van Dooren, I., Thijs, C., Dorant, E., Hageman, G., et al. (1999b). Antibacterial effect of garlic and omeprazole on Helicobacter pylori. J. Antimicrob. Chemother. 43, 837–839.
Karuppiah, P., and Rajaram, S. (2012). Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac. J. Trop. Biomed. 2, 597–601. doi: 10.1016/S2221-1691(12)60104-X
Kim, J. W., Kim, Y. S., and Kyung, K. H. (2004). Inhibitory activity of essential oils of garlic and onion against bacteria and yeasts. J. Food Prot. 67, 499–504. doi: 10.4315/0362-028x-67.3.499
Kockar, C., Ozturk, M., and Bavbek, N. (2001). Helicobacter pylori eradication with beta carotene, ascorbic acid and allicin. Acta Medica 44, 97–100.
Kodera, Y. (1997). “Dietary tolerance/absorption/metabolism of garlic,” in Nutraceuticals: Desinger Foods III Garlic, Soy and Licorice, ed. P. Lanchance (Trumbell, CT: Food & Nutrition Press), 95–105.
Kumar, M., and Berwal, J. S. (1998). Sensitivity of food pathogens to garlic (Allium sativum). J. Appl. Microbiol. 84, 213–215.
Kyo, E., Uda, N., Kasuga, S., and Itakura, Y. (2001). Immunomodulatory effects of aged garlic extract. J. Nutr. 131, 1075S–1079S. doi: 10.1093/jn/131.3.1075S
Le, N. T., Kalluri, J. R., Loni, A., Canham, L. T., and Coffer, J. L. (2017). Biogenic nanostructured porous silicon as a carrier for stabilization and delivery of natural therapeutic species. Mol. Pharm. 14, 4509–4514. doi: 10.1021/acs.molpharmaceut.7b00638
Leng, B. F., Qiu, J. Z., Dai, X. H., Dong, J., Wang, J. F., Luo, M. J., et al. (2011). Allicin reduces the production of alpha-toxin by Staphylococcus aureus. Molecules 16, 7958–7968. doi: 10.3390/molecules16097958
Leontiev, R., Hohaus, N., Jacob, C., Gruhlke, M. C. H., and Slusarenko, A. J. (2018). A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Sci. Rep. 8:6763. doi: 10.1038/s41598-018-25154-9
Li, F., Li, Q., Wu, S., and Tan, Z. (2017). Salting-out extraction of allicin from garlic (Allium sativum L.) based on ethanol/ammonium sulfate in laboratory and pilot scale. Food Chem. 217, 91–97. doi: 10.1016/j.foodchem.2016.08.092
Li, G., Ma, X., Deng, L., Zhao, X., Wei, Y., Gao, Z., et al. (2015). Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J. Microbiol. 8:e14814. doi: 10.5812/jjm.14814
Li, W. R., Ma, Y. K., Shi, Q. S., Xie, X. B., Sun, T. L., Peng, H., et al. (2018a). Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 102, 7555–7564. doi: 10.1007/s00253-018-9175-2
Li, W. R., Ma, Y. K., Xie, X. B., Shi, Q. S., Wen, X., Sun, T. L., et al. (2018b). Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa quorum sensing systems and corresponding virulence factors. Front. Microbiol. 9:3222. doi: 10.3389/fmicb.2018.03222
Li, W-R., Shi, Q-S., Dai, H-Q., Liang, Q., Xie, X-B., Huang, X-M., et al. (2016). Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 6:22805. doi: 10.1038/srep22805
Liaqat, F., Sheikh, A. A., Nazir, J., Hussain, T., Rabbani, M., Shaheen, A. Y., et al. (2015). Report-isolation identification and control of vancomycin resistant Staphylococcus aureus. Pak. J. Pharm. Sci. 28, 997–1004.
Lihua, L., Jianhuit, W., Jialini, Y., Yayin, L., and Guanxin, L. (2013). Effects of allicin on the formation of Pseudomonas aeruginosa biofinm and the production of quorum-sensing controlled virulence factors. Pol. J. Microbiol. 62, 243–251.
Liu, W. H., Hsu, C. C., and Yin, M. C. (2008). In vitro anti-Helicobacter pylori activity of diallyl sulphides and protocatechuic acid. Phytother. Res. 22, 53–57. doi: 10.1002/ptr.2259
Lu, X., Rasco, B. A., Jabal, J. M., Aston, D. E., Lin, M., and Konkel, M. E. (2011a). Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl. Environ. Microbiol. 77, 5257–5269. doi: 10.1128/AEM.02845-10
Lu, X., Rasco, B. A., Kang, D. H., Jabal, J. M., Aston, D. E., and Konkel, M. E. (2011b). Infrared and Raman spectroscopic studies of the antimicrobial effects of garlic concentrates and diallyl constituents on foodborne pathogens. Anal. Chem. 83, 4137–4146. doi: 10.1021/ac2001498
Lu, X., Samuelson, D. R., Rasco, B. A., and Konkel, M. E. (2012). Antimicrobial effect of diallyl sulphide on Campylobacter jejuni biofilms. J. Antimicrob. Chemother. 67, 1915–1926. doi: 10.1093/jac/dks138
Mahomoodally, F., Ramcharun, S., and Zengin, G. (2018). Onion and garlic extracts potentiate the efficacy of conventional antibiotics against standard and clinical bacterial isolates. Curr. Top. Med. Chem. 18, 787–796. doi: 10.2174/1568026618666180604083313
Maldonado, P. D., Chanez-Cardenas, M. E., and Pedraza-Chaverri, J. (2005). Aged garlic extract, garlic powder extract, S-allylcysteine, diallyl sulfide and diallyl disulfide do not interfere with the antibiotic activity of gentamicin. Phytother. Res. 19, 252–254. doi: 10.1002/ptr.1674
Meriga, B., Mopuri, R., and MuraliKrishna, T. (2012). Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac. J. Trop. Med. 5, 391–395. doi: 10.1016/S1995-7645(12)60065-0
Miron, T., Listowsky, I., and Wilchek, M. (2010). Reaction mechanisms of allicin and allyl-mixed disulfides with proteins and small thiol molecules. Eur. J. Med. Chem. 45, 1912–1918. doi: 10.1016/j.ejmech.2010.01.031
Miron, T., Rabinkov, A., Mirelman, D., Wilchek, M., and Weiner, L. (2000). The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta 1463, 20–30. doi: 10.1016/s0005-2736(99)00174-1
Mnayer, D., Fabiano-Tixier, A. S., Petitcolas, E., Hamieh, T., Nehme, N., Ferrant, C., et al. (2014). Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 19, 20034–20053. doi: 10.3390/molecules191220034
Mohsenipour, Z., and Hassanshahian, M. (2015). The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J. Microbiol. 8:e18971. doi: 10.5812/jjm.18971v2
Mozaffari Nejad, A. S., Shabani, S., Bayat, M., and Hosseini, S. E. (2014). Antibacterial effect of garlic aqueous extract on Staphylococcus aureus in hamburger. Jundishapur J. Microbiol. 7:e13134. doi: 10.5812/jjm.13134
Muller, A., Eller, J., Albrecht, F., Prochnow, P., Kuhlmann, K., Bandow, J. E., et al. (2016). Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 291, 11477–11490. doi: 10.1074/jbc.M115.702308
Naganawa, R., Iwata, N., Ishikawa, K., Fukuda, H., Fujino, T., and Suzuki, A. (1996). Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl. Environ. Microbiol. 62, 4238– 4242.
Nakamoto, M., Ohishi, K., Kunimura, K., Amano, H., and Wakamatsu, J. (2020). Identification and determination of antibacterial constituents in residue discharged from garlic-processing plant. Eur Food Res Technol 246, 1041–1049.
Nidadavolu, P., Amor, W., Tran, P. L., Dertien, J., Colmer-Hamood, J. A., and Hamood, A. N. (2012). Garlic ointment inhibits biofilm formation by bacterial pathogens from burn wounds. J. Med. Microbiol. 61(Pt. 5), 662–671. doi: 10.1099/jmm.0.038638-0
Nya, E. J., Dawood, Z., and Austin, B. (2010). The garlic component, allicin, prevents disease caused by Aeromonas hydrophila in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 33, 293–300. doi: 10.1111/j.1365-2761.2009.01121.x
O’Gara, E. A., Hill, D. J., and Maslin, D. J. (2000). Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 66, 2269–2273.
Ohta, R., Yamada, N., Kaneko, H., Ishikawa, K., Fukuda, H., Fujino, T., et al. (1999). In vitro inhibition of the growth of Helicobacter pylori by oil-macerated garlic constituents. Antimicrob. Agents Chemother. 43, 1811–1812. doi: 10.1128/aac.43.7.1811
Oosthuizen, C., Arbach, M., Meyer, D., Hamilton, C., and Lall, N. (2017). Diallyl polysulfides from Allium sativum as immunomodulators, hepatoprotectors, and antimycobacterial agents. J. Med. Food 20, 685–690. doi: 10.1089/jmf.2016.0137
Pavlovic, D. R., Veljkovic, M., Stojanovic, N. M., Gocmanac-Ignjatovic, M., Mihailov-Krstev, T., Brankovic, S., et al. (2017). Influence of different wild-garlic (Allium ursinum) extracts on the gastrointestinal system: spasmolytic, antimicrobial and antioxidant properties. J. Pharm. Pharmacol. 69, 1208–1218. doi: 10.1111/jphp.12746
Perez-Giraldo, C., Cruz-Villalon, G., Sanchez-Silos, R., Martinez-Rubio, R., Blanco, M. T., and Gomez-Garcia, A. C. (2003). In vitro activity of allicin against Staphylococcus epidermidis and influence of subinhibitory concentrations on biofilm formation. J. Appl. Microbiol. 95, 709–711. doi: 10.1046/j.1365-2672.2003.02030.x
Perez-Kohler, B., Garcia-Moreno, F., Bayon, Y., Pascual, G., and Bellon, J. M. (2015a). Inhibition of Staphylococcus aureus adhesion to the surface of a reticular heavyweight polypropylene mesh soaked in a combination of chlorhexidine and allicin: an in vitro study. PLoS One 10:e0126711. doi: 10.1371/journal.pone.0126711
Perez-Kohler, B., Garcia-Moreno, F., Brune, T., Pascual, G., and Bellon, J. M. (2015b). Preclinical bioassay of a polypropylene mesh for hernia repair pretreated with antibacterial solutions of chlorhexidine and allicin: an in vivo study. PLoS One 10:e0142768. doi: 10.1371/journal.pone.0142768
Qian, Y. X., Shen, P. J., Xu, R. Y., Liu, G. M., Yang, H. Q., Lu, Y. S., et al. (1986). Spermicidal effect in vitro by the active principle of garlic. Contraception 34, 295–302. doi: 10.1016/0010-7824(86)90010-7
Rabinkov, A., Miron, T., Konstantinovski, L., Wilchek, M., Mirelman, D., and Weiner, L. (1998). The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta 1379, 233–244. doi: 10.1016/s0304-4165(97)00104-9
Rahman, S., Parvez, A. K., Islam, R., and Khan, M. H. (2011). Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Ann. Clin. Microbiol. Antimicrob. 10:10. doi: 10.1186/1476-0711-10-10
Rao, R. R., Rao, S. S., Natarajan, S., and Venkataraman, P. R. (1946a). Inhibition of Mycobacterium tuberculosis by garlic extract. Nature 157:441. doi: 10.1038/157441b0
Rao, R. R., Rao, S. S., Natarajan, S., and Venkataranam, P. R. (1946b). Investigation on plant antibiotics. I. studies on allicin, the antibacterial principle of Allium sativum, (garlic). J. Sci. Industr. Res. 18, 31–33.
Rasmussen, T. B., Bjarnsholt, T., Skindersoe, M. E., Hentzer, M., Kristoffersen, P., Kote, M., et al. (2005). Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 187, 1799–1814. doi: 10.1128/JB.187.5.1799-1814.2005
Ratnakar, P., and Murthy, P. S. (1995). Purification and mechanism of action of antitubercular principle from garlic (Allium sativum) active against isoniazid susceptible and resistantMycobacterium tuberculosis H37Rv. Indian J. Clin. Biochem. 10, 34–38. doi: 10.1007/BF02873666
Rauf, M. A., Zubair, S., Ateeq, H., Dabeer, K., Pachauri, S., Ajmal, M., et al. (2018). Synergistic effect of diallyl sulfide with zinc oxide nanorods: a novel and effective approach for treatment of acute dermatitis in model animals. Front. Microbiol. 9:586. doi: 10.3389/fmicb.2018.00586
Reiter, J., Borlinghaus, J., Dorner, P., Schroder, W., Gruhlke, M. C. H., Klaas, M., et al. (2020). Investigation of the deposition behaviour and antibacterial effectivity of allicin aerosols and vapour using a lung model. Exp. Ther. Med. 19, 1541–1549. doi: 10.3892/etm.2019.8387
Reiter, J., Levina, N., van der Linden, M., Gruhlke, M., Martin, C., and Slusarenko, A. J. (2017). Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 22:1711. doi: 10.3390/molecules22101711
Ried, K., Travica, N., and Sali, A. (2018). The effect of kyolic aged garlic extract on gut microbiota, inflammation, and cardiovascular markers in hypertensives: the GarGIC trial. Front. Nutr. 5:122. doi: 10.3389/fnut.2018.00122
Rimoldi, S., Torrecillas, S., Montero, D., Gini, E., Makol, A., Valdenegro, V. V., et al. (2020). Assessment of dietary supplementation with galactomannan oligosaccharides and phytogenics on gut microbiota of European sea bass (Dicentrarchus Labrax) fed low fishmeal and fish oil based diet. PLoS One 15:e0231494. doi: 10.1371/journal.pone.0231494
Rivlin, R. S. (2001). Historical perspective on the use of garlic. J. Nutr. 131, 951S–954S. doi: 10.1093/jn/131.3.951S
Robyn, J., Rasschaert, G., Hermans, D., Pasmans, F., and Heyndrickx, M. (2013). Is allicin able to reduce Campylobacter jejuni colonization in broilers when added to drinking water? Poult. Sci. 92, 1408–1418. doi: 10.3382/ps.2012-02863
Roshan, N., Riley, T. V., and Hammer, K. A. (2017). Antimicrobial activity of natural products against Clostridium difficile in vitro. J. Appl. Microbiol. 123, 92–103. doi: 10.1111/jam.13486
Roshan, N., Riley, T. V., and Hammer, K. A. (2018). Effects of natural products on several stages of the spore cycle of Clostridium difficile in vitro. J. Appl. Microbiol. 125, 710–723. doi: 10.1111/jam.13889
Ross, Z. M., O’Gara, E. A., Hill, D. J., Sleightholme, H. V., and Maslin, D. J. (2001). Antimicrobial properties of garlic oil against human enteric bacteria: evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl. Environ. Microbiol. 67, 475–480. doi: 10.1128/AEM.67.1.475-480.2001
Ruddock, P. S., Liao, M., Foster, B. C., Lawson, L., Arnason, J. T., and Dillon, J. A. (2005). Garlic natural health products exhibit variable constituent levels and antimicrobial activity against Neisseria gonorrhoeae, Staphylococcus aureus and Enterococcus faecalis. Phytother. Res. 19, 327–334. doi: 10.1002/ptr.1667
Saleem, Z. M., and Al-Delaimy, K. S. (1982). Inhibition of Bacillus cereus by garlic extracts. J. Food Prot. 45, 1007–1009. doi: 10.4315/0362-028X-45.11.1007
Satora, M., Magdziarz, M., Rzasa, A., Rypula, K., and Ploneczka-Janeczko, K. (2020). Insight into the intestinal microbiome of farrowing sows following the administration of garlic (Allium sativum) extract and probiotic bacteria cultures under farming conditions. BMC Vet. Res. 16:442. doi: 10.1186/s12917-020-02659-y
Sheppard, J. G., and Long, T. E. (2016). Allicin-inspired thiolated fluoroquinolones as antibacterials against ESKAPE pathogens. Bioorg. Med. Chem. Lett. 26, 5545–5549. doi: 10.1016/j.bmcl.2016.10.002
Sheppard, J. G., McAleer, J. P., Saralkar, P., Geldenhuys, W. J., and Long, T. E. (2018). Allicin-inspired pyridyl disulfides as antimicrobial agents for multidrug-resistant Staphylococcus aureus. Eur. J. Med. Chem. 143, 1185–1195. doi: 10.1016/j.ejmech.2017.10.018
Si, X. B., Zhang, X. M., Wang, S., Lan, Y., Zhang, S., and Huo, L. Y. (2019). Allicin as add-on therapy for Helicobacter pylori infection: a systematic review and meta-analysis. World J. Gastroenterol. 25, 6025–6040. doi: 10.3748/wjg.v25.i39.6025
Sivam, G. P. (2001). Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr. 131, 1106S–1108S.
Sivam, G. P., Lampe, J. W., Ulness, B., Swanzy, S. R., and Potter, J. D. (1997). Helicobacter pylori–in vitro susceptibility to garlic (Allium sativum) extract. Nutr. Cancer 27, 118–121. doi: 10.1080/01635589709514512
Small, L. D., Bailey, J. H., and Cavallito, C. J. (1947). Alkyl thiolsulfinates. J. Am. Chem. Soc. 69, 1710–1713. doi: 10.1021/ja01199a040
Snowden, R., Harrington, H., Morrill, K., Jeane, L., Garrity, J., Orian, M., et al. (2014). A comparison of the anti-Staphylococcus aureus activity of extracts from commonly used medicinal plants. J. Altern. Complement. Med. 20, 375–382. doi: 10.1089/acm.2013.0036
Spera, M. B., Quintao, F. A., Ferraresi, D. K., Lustri, W. R., Magalhaes, A., Formiga, A. L., et al. (2011). Palladium(II) complex with S-allyl-L-cysteine: new solid-state NMR spectroscopic measurements, molecular modeling and antibacterial assays. Spectrochim. Acta A Mol. Biomol. Spectrosc. 78, 313–318. doi: 10.1016/j.saa.2010.10.012
Stoll, A., and Seebeck, E. (1947). Über alliin, die genuine muttersubstanz des Knoblauchöls. Experientia 3:114. doi: 10.1007/bf02137698
Tomsik, A., Saric, L., Bertoni, S., Protti, M., Albertini, B., Mercolini, L., et al. (2019). Encapsulations of wild garlic (Allium ursinum L.) extract using spray congealing technology. Food Res. Int. 119, 941–950. doi: 10.1016/j.foodres.2018.10.081
Tsao, S., and Yin, M. (2001). In vitro activity of garlic oil and four diallyl sulphides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J. Antimicrob. Chemother. 47, 665–670.
Tsao, S. M., and Yin, M. C. (2001). In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J. Med. Microbiol. 50, 646–649. doi: 10.1099/0022-1317-50-7-646
Tsao, S. M., Liu, W. H., and Yin, M. C. (2007). Two diallyl sulphides derived from garlic inhibit meticillin-resistant Staphylococcus aureus infection in diabetic mice. J. Med. Microbiol. 56(Pt. 6), 803–808. doi: 10.1099/jmm.0.46998-0
Ushimaru, P. I., Barbosa, L. N., Fernandes, A. A., Di Stasi, L. C., and Fernandes, A. Jr. (2012). In vitro antibacterial activity of medicinal plant extracts against Escherichia coli strains from human clinical specimens and interactions with antimicrobial drugs. Nat. Prod. Res. 26, 1553–1557. doi: 10.1080/14786419.2011.568943
Vadekeetil, A., Kaur, G., Chhibber, S., and Harjai, K. (2015). Applications of thin-layer chromatography in extraction and characterisation of ajoene from garlic bulbs. Nat. Prod. Res. 29, 768–771. doi: 10.1080/14786419.2014.981815
Vadekeetil, A., Saini, H., Chhibber, S., and Harjai, K. (2016). Exploiting the antivirulence efficacy of an ajoene-ciprofloxacin combination against Pseudomonas aeruginosa biofilm associated murine acute pyelonephritis. Biofouling 32, 371–382. doi: 10.1080/08927014.2015.1137289
Velliyagounder, K., Ganeshnarayan, K., Velusamy, S. K., and Fine, D. H. (2012). In vitro efficacy of diallyl sulfides against the periodontopathogen Aggregatibacter actinomycetemcomitans. Antimicrob. Agents Chemother. 56, 2397–2407. doi: 10.1128/AAC.00020-12
Vezza, T., Algieri, F., Garrido-Mesa, J., Utrilla, M. P., Rodriguez-Cabezas, M. E., Banos, A., et al. (2019). The immunomodulatory properties of propyl-propane thiosulfonate contribute to its intestinal anti-inflammatory effect in experimental colitis. Mol. Nutr. Food Res. 63:e1800653. doi: 10.1002/mnfr.201800653
Vijayakumar, S., Malaikozhundan, B., Saravanakumar, K., Durán-Lara, E. F., Wang, M. H., and Vaseeharan, B. (2019). Garlic clove extract assisted silver nanoparticle - antibacterial, antibiofilm, antihelminthic, anti-inflammatory, anticancer and ecotoxicity assessment. J. Photochem. Photobiol. B 198:111558. doi: 10.1016/j.jphotobiol.2019.111558
Viswanathan, V., Phadatare, A. G., and Mukne, A. (2014). Antimycobacterial and antibacterial activity of Allium sativum bulbs. Indian J. Pharm. Sci. 76, 256–261.
Vlachojannis, C., Chrubasik-Hausmann, S., Hellwig, E., Vach, K., and Al-Ahmad, A. (2018). Activity of preparations from Spilanthes oleracea, propolis, Nigella sativa, and black garlic on different microorganisms involved in oral diseases and on total human salivary bacteria: a pilot study. Phytother. Res. 32, 1992–2001. doi: 10.1002/ptr.6129
Vuddhakul, V., Bhoopong, P., Hayeebilan, F., and Subhadhirasakul, S. (2007). Inhibitory activity of Thai condiments on pandemic strain of Vibrio parahaemolyticus. Food Microbiol. 24, 413–418. doi: 10.1016/j.fm.2006.04.010
Wallock-Richards, D., Doherty, C. J., Doherty, L., Clarke, D. J., Place, M., Govan, J. R., et al. (2014). Garlic revisited: antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS One 9:e112726. doi: 10.1371/journal.pone.0112726
Wang, J., and Vermerris, W. (2016). Antimicrobial nanomaterials derived from natural products-a review. Materials 9:255. doi: 10.3390/ma9040255
Wills, E. D. (1956). Enzyme inhibition by allicin, the active principle of garlic. Biochem. J. 63, 514–520. doi: 10.1042/bj0630514
Wu, T., Chen, N., Liu, C., Liu, R., Zhang, J., Xie, X., et al. (2017). Investigating the chemical constituent and the suppressive effects of alliin hydrolysate on E.coli. Nat. Prod. Res. 31, 2814–2817. doi: 10.1080/14786419.2017.1297440
Wu, T., Huang, Y., Chen, Y., and Zhang, M. (2018). Antibacterial effect of (2E,2E)-4,4-trisulfanediylbis(but-2-enoic acid) against Staphylococcus aureus. PLoS One 13:e0197348. doi: 10.1371/journal.pone.0197348
Wu, W. K., Panyod, S., Ho, C. T., Kuo, C. H., Wu, M. S., and Sheen, L. Y. (2015). Dietary allicin reduces transformation of L-carnitine to TMAO through impact on gut microbiota. J. Funct. Foods 15, 408–417. doi: 10.1016/j.jff.2015.04.001
Wu, X., Santos, R. R., and Fink-Gremmels, J. (2015). Analyzing the antibacterial effects of food ingredients: model experiments with allicin and garlic extracts on biofilm formation and viability of Staphylococcus epidermidis. Food Sci. Nutr. 3, 158–168. doi: 10.1002/fsn3.199
Xu, Z., Qiu, Z., Liu, Q., Huang, Y., Li, D., Shen, X., et al. (2018). Converting organosulfur compounds to inorganic polysulfides against resistant bacterial infections. Nat. Commun. 9:3713. doi: 10.1038/s41467-018-06164-7
Yang, Y., Yang, F., Huang, M., Wu, H., Yang, C., Zhang, X., et al. (2019). Fatty liver and alteration of the gut microbiome induced by diallyl disulfide. Int. J. Mol. Med. 44, 1908–1920. doi: 10.3892/ijmm.2019.4350
Yin, M. C., and Cheng, W. S. (2003). Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 63, 23–28. doi: 10.1016/s0309-1740(02)00047-5
Yoshida, H., Iwata, N., Katsuzaki, H., Naganawa, R., Ishikawa, K., Fukuda, H., et al. (1998). Antimicrobial activity of a compound isolated from an oil-macerated garlic extract. Biosci. Biotechnol. Biochem. 62, 1014–1017. doi: 10.1271/bbb.62.1014
Yoshida, H., Katsuzaki, H., Ohta, R., Ishikawa, K., Fukuda, H., Fujino, T., et al. (1999). An organosulfur compound isolated from oil-macerated garlic extract, and its antimicrobial effect. Biosci. Biotechnol. Biochem. 63, 588–590. doi: 10.1271/bbb.63.588
Zare, A., Farzaneh, P., Pourpak, Z., Zahedi, F., Moin, M., Shahabi, S., et al. (2008). Purified aged garlic extract modulates allergic airway inflammation in BALB/c mice. Iran J. Allergy Asthma Immunol. 7, 133–141.
Zhai, H., Pan, J., Pang, E., and Bai, B. (2014). Lavage with allicin in combination with vancomycin inhibits biofilm formation by Staphylococcus epidermidis in a rabbit model of prosthetic joint infection. PLoS One 9:e102760. doi: 10.1371/journal.pone.0102760
Zhang, C., Xie, J., Li, X., Luo, J., Huang, X., Liu, L., et al. (2019). Alliin alters gut microbiota and gene expression of colonic epithelial tissues. J. Food Biochem. 43:e12795. doi: 10.1111/jfbc.12795
Zhang, Y., Liu, X., Ruan, J., Zhuang, X., Zhang, X., and Li, Z. (2019). Phytochemicals of garlic: promising candidates for cancer therapy. Biomed. Pharmacother. 123:109730. doi: 10.1016/j.biopha.2019.109730
Keywords: garlic (A. sativum), organosulfur compounds, antibiofilm, antibacterial, multi-drug resistance (MDR)
Citation: Bhatwalkar SB, Mondal R, Krishna SBN, Adam JK, Govender P and Anupam R (2021) Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 12:613077. doi: 10.3389/fmicb.2021.613077
Received: 11 October 2020; Accepted: 14 June 2021;
Published: 27 July 2021.
Edited by:
Santi M. Mandal, Indian Institute of Technology Kharagpur, IndiaReviewed by:
Ranjith Kumavath, Central University of Kerala, IndiaSivakumar S., Pusan National University, South Korea
Piyush Baindara, University of Missouri, United States
Copyright © 2021 Bhatwalkar, Mondal, Krishna, Adam, Govender and Anupam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajaneesh Anupam, ranupam@dhsgsu.edu.in
 Sushma Bagde Bhatwalkar
Sushma Bagde Bhatwalkar Rajesh Mondal
Rajesh Mondal Suresh Babu Naidu Krishna
Suresh Babu Naidu Krishna Jamila Khatoon Adam3
Jamila Khatoon Adam3 Patrick Govender
Patrick Govender Rajaneesh Anupam
Rajaneesh Anupam