Reversible protein assemblies in the proteostasis network in health and disease
- 1Institute of Molecular Biosciences, University of Graz, Graz, Austria
- 2Department of Molecular Biosciences, Stockholm University, Stockholm, Sweden
While proteins populating their native conformations constitute the functional entities of cells, protein aggregates are traditionally associated with cellular dysfunction, stress and disease. During recent years, it has become clear that large aggregate-like protein condensates formed via liquid-liquid phase separation age into more solid aggregate-like particles that harbor misfolded proteins and are decorated by protein quality control factors. The constituent proteins of the condensates/aggregates are disentangled by protein disaggregation systems mainly based on Hsp70 and AAA ATPase Hsp100 chaperones prior to their handover to refolding and degradation systems. Here, we discuss the functional roles that condensate formation/aggregation and disaggregation play in protein quality control to maintain proteostasis and why it matters for understanding health and disease.
1 Introduction
The cellular proteome encompasses proteins that populate a plethora of conformations, from stably folded proteins to those without fixed tertiary structures, consisting completely of intrinsically-disordered regions (IDRs). For example, in human cells the majority of proteins carry both folded domains and IDRs, while 37% of the proteome is fully folded and only 5% of the proteins occupy entirely disordered conformations (Tsang et al., 2020). The conformational complexity is increased by the occurrence of higher-oligomeric protein assemblies that are formed by both stochastic interactions as well as by direct interactions regulated by internal and external cues. These assemblies may form by liquid-liquid phase separation (LLPS) and it is now established that such biomolecular condensates have functional roles (Banani et al., 2017). In contrast, protein aggregates traditionally have been viewed as disordered structures, spontaneously formed by misfolded proteins (Wang and Roberts, 2018). While biomolecular condensates are essential for cellular regulation, the occurrence of protein aggregates is generally a sign of a structurally compromised proteome, and typically associated with cellular stress (Figure 1A). However, it is nearly impossible to strictly categorize LLPS and protein aggregation as fully separate processes (Lee et al., 2016; Boeynaems et al., 2017). Furthermore, protein aggregation as well as LLPS are accelerated by stress indicating that the processes are closely linked and involved in management of an overloaded protein quality control (PQC) system.
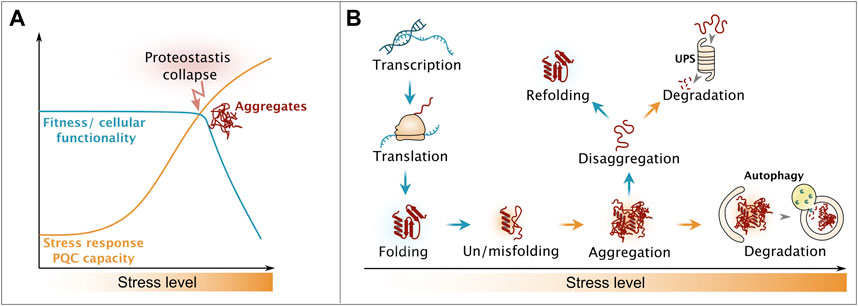
FIGURE 1. Influence of proteostatic stress on cellular fitness. (A) Upon increase of proteotoxic stress, cells activate stress response regimes, ensuring on keeping cellular processes functional. Overburden results in the collapse of the proteostasis system and occurrence of aggregates, leading to drastically decreased cellular healthspan. (B) Strategies of the cellular protein quality control system to counteract proteotoxic stress, end in the triage decision between refolding and degradation. Please see main text for details.
Cellular protein homeostasis (proteostasis) is constantly challenged by the flow of unfolded proteins produced by protein biosynthesis as well as from protein misfolding caused by stochastic errors in gene expression and damage from proteotoxic stressors including but not limited to heat, oxidants and metabolic imbalances (Figure 1B) (Zakariya et al., 2022). Chaperones are in place to assist on-pathway protein folding and thereby to suppress the buildup of aberrant misfolded proteins (Balchin et al., 2020). Here, the class of chaperones belonging to heat shock proteins (Hsps) of the Hsp70 family has a key function. Hsp70s are found across all kingdoms of life and operate in nearly all organelles in eukaryotic cells. Details on their ATPase-driven mode of action is extensively described in recent reviews (Rosenzweig et al., 2019; Kohler and Andréasson, 2020). Stress damage may overwhelm the chaperone folding machinery causing proteotoxic misfolded proteins to assemble into higher-oligomeric states, including aggregates (Mogk et al., 2018). Thus, cells sequester these potentially toxic protein species into quality control compartments by utilizing small heat shock proteins that act as aggregation-promoting factors (aggregases) and thus protect the proteome from aberrant interactions (Haslbeck et al., 2019). During recovery from stress, disaggregation machineries promote the disentanglement of the aggregates and lead to recovery of the constituent proteins (Mogk et al., 2018; Nillegoda et al., 2018). Metazoan cells differ drastically from plant, fungi and bacteria in respect to their disaggregation machinery, which will be explained in greater details in later sections. Once aggregated proteins are disentangled, their refolding is considered to be the preferred pathway, yet alternative pathways involve complete removal from the cell by degradation (Wallace et al., 2015; Määttä et al., 2020). The Hsp70 chaperone network aids both the refolding and targeting to the two major proteolytic systems, the ubiquitin-proteasome system (UPS) and the lysosomal/vacuolar system via autophagy (Figure 1B) (Wallace et al., 2015; Määttä et al., 2020).
In this review, we aim to give an overview on higher-order assembly processes and their interplay. We will discuss the cellular and organismal consequences of disturbances of these intricately balanced systems in response to internal and external cues. A main focus is on the cellular strategies that regulate these processes in metazoan and model organisms, with a special emphasis on disaggregation, a process that determines partitioning between refolding and degradation faiths.
2 Similarities and differences of biomolecular condensates and aggregates
Aiming for a simplified definition, generally accepted features applicable for most biomolecular condensates generated by LLPS include the reversibility of the dynamic phase-separated assemblies at distinct stages and the fact that their protein constituents generally maintain their tertiary structures (Figure 2A) (Alberti and Hyman, 2021; Mehta and Zhang, 2022). On the other hand, aggregated proteins occupy aberrant folds and strictly require the assistance from PQC factors for disentanglement and re-folding (Figure 2B) (Zhao et al., 2020). However, phase-separated assemblies precede aggregate formation and some condensates also require assistance for reversal under specific circumstances, this includes condensate hardening (Lee et al., 2016; Boeynaems et al., 2017), which will be discussed in later sections.
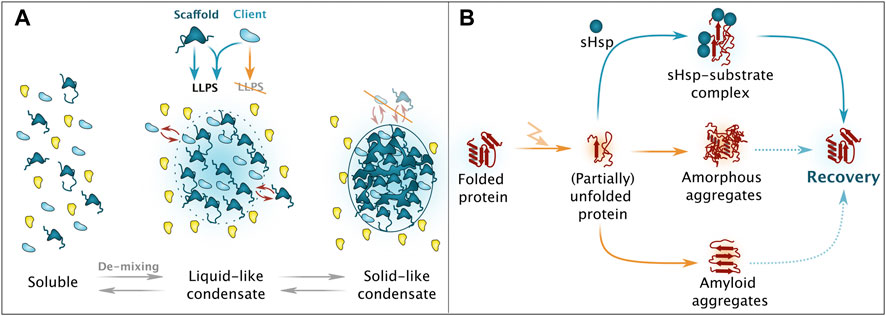
FIGURE 2. Simplified overview on phase separation and aggregation. (A) Upon presence of scaffold proteins with potent liquid-liquid phase separation (LLPS)-enabling features, de-mixing of a protein solution can take place, eventuation in liquid-like condensates, consisting of both scaffold (dark blue) and client (light blue) proteins. Ageing processes result in solid-like condensates that no longer support dynamic exchange of constituents with the surrounding milieu. Please note that also chaperones and protein quality control factors are involved in these processes. (B) Unfolded resp. partially unfolded protein species can adopt different aggregate forms. Depending on the intrinsic nature, amorphous as well as amyloid aggregates can occur, with a limited potential for recovery. Small heats hock proteins (sHsps) are responsible for targeted and regulated sequestration and alleviate subsequent recovery/refolding.
2.1 Phase separation enables dynamic and efficient regulation
Biomolecular condensates or liquid droplets are dynamic and reversible assemblies of molecules that can be dissolved and reused immediately. They assemble via LLPS and the molecules are concentrated in a liquid-like compartment that persist in the surrounding milieu (Brangwynne et al., 2011; Brangwynne et al., 2009; Li et al., 2012; Aggarwal et al., 2013; Feric and Brangwynne, 2013; Wippich et al., 2013). LLPS spatially restricts movement of the molecules without fully trapping them and thus enables controlled recruitment and release kinetics (Zhao et al., 2020).
Their biogenesis and structural integrity is exclusively based on protein-protein or protein-nucleic acid interactions and components of these membrane-less compartments connect and readily exchange with the external environment (Phair and Misteli, 2000). LLPS is driven by the minimization of global free energy, by maximizing weak inter- and intramolecular interactions between constituting macromolecules. This process only occurs at a certain solubility limit with a saturation concentration, which is characteristic for each phase-separating system (Alberti and Hyman, 2021). Whether the system undergoes LLPS not only depends on the molecular identity and solution concentration of the constituents and their post-translational modifications but also on the temperature as well as pH and concentrations of salt, ions, co-solutes, and small metabolites like ATP. RNAs are further key components driving the formation of biomolecular condensates and determine their composition and specificity (Brangwynne et al., 2009; Wolf et al., 2014; Munder et al., 2016; Patel et al., 2017; Hayes et al., 2018; Snead et al., 2019; Adame-Arana et al., 2020).
Condensates are generally composed of scaffolds and client proteins, the first harboring a high number of valences, acting as drivers of LLPS and also playing an essential role in the threshold concentration (Banani et al., 2016). Scaffolds are usually large, abundant proteins without enzymatic activity and condensate assembly depends on their ability to form a dense network of intermolecular interactions (Li et al., 2012). Key for the network are interactions provided by IDRs, which are low-complexity domains with biased amino acid compositions (Molliex et al., 2015; Nott et al., 2015; Patel et al., 2015; Wang et al., 2018). Client proteins have a lower interaction valence and are recruited to condensates formed by the scaffold factors. (Burke et al., 2015; Saha et al., 2016; Franzmann et al., 2018; Murthy et al., 2019; Guillén-Boixet et al., 2020). During LLPS, proteins typically do not undergo extensive structural changes and remain mostly disordered, as observed with NMR (Figure 2A) (Phair and Misteli, 2000).
Due to the lack of membranes, formation and disassembly of condensates via LLPS has the potential to respond rapidly to minor changes in the environment (Lin et al., 2015; Molliex et al., 2015; Nott et al., 2015). Their dynamic nature makes biomolecular condensates ideal compartments as biological reaction centers, signaling hubs and for organizing and regulating key biological processes such as splicing, translation, transcription and chromosome condensation (Su et al., 2016; Gueroussov et al., 2017; Tsang et al., 2019). The reported functions of biomolecular condensates include enhancement or suppression of biochemical reactions, buffering protein concentrations, detecting environmental changes and exertion of mechanical forces (Riback et al., 2017; Franzmann et al., 2018; Shin et al., 2018; Klosin et al., 2020). Biomolecular condensates are abundantly present in the cytoplasm, the nucleus, the mitochondrial matrix, the stroma of chloroplasts and the cytosol of bacteria (Zaslavsky and Uversky, 2018). Some structures are common to all cells, like nuclear speckles, paraspeckles and nucleoli, all found in the cellular nucleus, as well as cytoplasmic P-bodies and stress granules (Frottin et al., 2019; Ilık and Aktaş, 2022; Kedersha et al., 1999; Riggs et al., 2020; Sheth and Parker, 2003; Xing et al., n.d.).
Eukaryotic cells exposed to proteotoxic stressors require mechanisms that guarantee the integrity of the proteome until normal growth conditions are restored. Here, LLPS and molecular condensates play a central role. Key steps include global inhibition of protein synthesis and the selective upregulation of expression of stress response factors (Dever et al., 1992; Brostrom et al., 1996; Harding et al., 2003; Lu et al., 2004). The inhibition of translation and the subsequent rise of ribosome-free RNA in the cytosol is mainly regulated by stress-induced phosphorylation and inactivation of the translation initiation factor eIF2α. The released RNA and eIF2α are sequestered in stress granules via LLPS. When the stress is relieved, the stress granules shrink, eIF2α is dephosphorylated and mRNA translation is resumed. The coordinated reactivation of these abundant protein synthesis components is required for adaptive gene expression that trails minutes to a few hours behind the removal of the stress (Jousse et al., 2003; Wheeler et al., 2016).
2.2 Protein aggregation is a protective response to an overwhelmed proteostasis system
Protein aggregation is the association of misfolded proteins in higher-order assemblies, usually formed in cells with severely perturbed proteostasis, where the build-up of proteins with aberrant conformations exceeds PQC capacity. Aggregates consist of proteins at least in part in non-native states and involve stable intramolecular molecular interactions (Figure 2B). These non-native conformations are often toxic to a cell and are usually recognized by the cellular PQC system (Emma Mee Hayes et al., 2022). Practically all protein species are susceptible to aggregation, yet in the cell the process appears to affect proteins differentially. Proteins especially susceptible to aggregation were found to be large, have a high isoelectric point, disordered regions and hydrophilic amino acids (Kramer et al., 2012; Walther et al., 2015; Hosp et al., 2017; Uemura et al., 2018; Määttä et al., 2020).
Aggregates can be grouped into highly-ordered amyloid fibrils and disordered or amorphous protein aggregates, with alternative classifications based on size, reversibility of the aggregation, tertiary structure, modifications and morphology suggested in (Narhi et al., 2012). Some proteins misfold into a more ordered and less dynamic state such as the rigid amyloid fold (Serio et al., 2000). The amyloid conformation is typically not a functional state but a generic structural motif, consisting of elongated assemblies of nearly identical ß-sheets that are stacked onto each other. Amyloids are thermodynamically extremely stable, thus these conformations challenge the view that natively folded proteins populate the most stable conformations (Raabe et al., 2021). Moreover, amyloids may act as seeds converting other soluble proteins into the amyloid state, thus this aggregate subtype acts as a potentially infectious agent and may carry epigenetic traits (Serio et al., 2000; Raabe et al., 2021).
Small heat shock proteins (sHsps) and disaggregase machineries ensure that the aggregation is reversible despite the stable nature of protein aggregates [please see (Gallardo et al., 2021) for further details]. sHsps are regarded as first line of defense when cells experience proteotoxic stress by interacting with a wide plethora of substrates, forming stable sHsp-substrate complexes leading to their sequestration. These chaperones counteract uncontrolled aggregation of their substrates, but do not facilitate refolding upon stress relieve, a task left for cellular disaggregation machineries (extensively reviewed in (Mogk et al., 2019)). The reversibility is a protective mechanism (Saad et al., 2017) that may involve reactivation of the aggregated protein but extensively damaged proteins that cannot be repaired are ultimately subjected to degradation (Specht et al., 2011; Malinovska et al., 2012; Ungelenk et al., 2016).
The persistence of aberrantly folded and aggregated proteins is linked to cytotoxicity (Figure 1A). The proteotoxicity may involve loss of function of the trapped protein but has also been shown to be caused by co-sequestration and depletion of PQC factors essential for proteostasis, the disruption of cell membrane integrity due to aberrant interactions and perturbation of interactions/trafficking (Goggin et al., 2008; Olzscha et al., 2011; Arlet et al., 2014; Yu et al., 2014; Cenini et al., 2016; Grima et al., 2017). The aggregation may in turn be driven by pathological mutations, rendering proteins prone for misfolding, or accelerated by aging that leads to a global decline in PQC capacity due to decreased expression of chaperones and degradation factors and the overall accumulation of misfolded proteins caused by summation of external/internal cues (Hipp et al., 2019; Stein et al., 2022).
2.3 Biomolecular condensates fluently transition into aggregates
Biomolecular condensates initially show liquid-like properties and some of these phase-separated compartments age towards less dynamic gel-like or inert solid states (Molliex et al., 2015; Harmon et al., 2017; Riback et al., 2017; Iserman et al., 2020). Protein concentration, the absence of binding partners and a low water content enhance condensate aging and vice versa increased protein compositional heterogeneity in biomolecular condensates inhibits condensate hardening (Patel et al., 2015; Maharana et al., 2018; Majumdar et al., 2019). Condensate aging influences protein function, as a more liquid phase allows increased and more dynamic molecular interactions, while a more solid phase is difficult to reverse and sequesters proteins from the cytosol (Hernández-Vega et al., 2017). In yeast, the formation of solid-like condensates has been shown to be a physiological process serving an adaptive function. During stressful conditions, including heat shock, the downregulation of housekeeping protein synthesis allows yeast to reduce the burden of newly produced misfolding-prone proteins (Kroschwald et al., 2018; Kroschwald et al., 2015; Riback et al., 2017; Iserman et al., 2020). The translation initiation factor and stress granule component Ded1 is required for translation of mRNAs that encode housekeeping genes and is disposable from mRNAs encoding PQC factors. In response to heat stress, Ded1 phase-separates to form biomolecular condensates that harden quickly, leading to a situation where Ded1 is trapped inside stress granules. This in turn leads to changes in mRNA translation resulting in reduced expression of housekeeping genes and favored expression of stress factors. Dissipation of the hardened Ded1-condensates during stress recovery requires assistance from PQC factors (Kroschwald et al., 2018; Kroschwald et al., 2015; Riback et al., 2017; Iserman et al., 2020). Hence, the temperature sensitivity of Ded1 itself is suggested to determine global temperature sensitivity and the point, where yeast stops growing and instead invests into stress factor production.
LLPS is sensitive to stress-provoked changes, thus stress may lead to conformational alterations and/or reveal new interaction sites, potentially promoting condensate aging (Alberti and Hyman, 2021). Condensate-forming proteins carry long segments of IDRs that on the one hand function as scaffolds for condensate assembly and on the other hand render these proteins more misfolding- and aggregation-prone (Hegyi and Tompa, 2008; Määttä et al., 2020). Condensate hardening is associated with protection of biological function during stress, as the hardened state is proposed to act as kinetic trap for potentially aggregation-prone proteins, and thus hinders further damage caused by soluble misfolded proteins. Further, it relieves the burden from the PQC system (Riback et al., 2017; Franzmann et al., 2018; Kroschwald et al., 2018; Iserman et al., 2020).
Misfolding-prone proteins accumulate in biomolecular condensates and promote their hardening by interacting with other proteins in the condensates and by establishing long-lived physical crosslinks that also may include RNA (Choi et al., 2008; Lee et al., 2016; Boeynaems et al., 2017). Interestingly, defective ribosomal products, originating from ongoing translation and increasing upon stress and with age, are the main source of misfolding-prone proteins in cells and are also implicated in condensate aging (Schubert et al., 2000). They tend to accumulate in stress granules, nucleoli and PML bodies, another phase-separated compartment, and thus these assemblies are suggested to present overflow compartments that sequester misfolded proteins when their concentration reaches a critical level (Audas et al., 2016; Ganassi et al., 2016; Frottin et al., 2019; Mediani et al., 2019). In this line, occurence, formation and dissolution of biomolecular condensates like stress granules, have dual functions, they can serve as regulatory compartment to stop/re-activate translation according to cellular needs during exposure to stress (mentioned earlier in the text), but also represent storage compartments for unfolded proteins for easier chaperone-mediated downstream processes. Ubc9ts is a misfolded model protein with a weakly destabilizing amino acid substitution and has been shown to become massively enriched in reconstituted condensates causing condensate aging and hardening (Mateju et al., 2017; Guillén-Boixet et al., 2020). Consistently, Ubc9ts, SOD1 and other misfolding-prone proteins were shown to accumulate in stress granules, changing their properties and dynamics (Ganassi et al., 2016; Lee et al., 2016; Boeynaems et al., 2017; Mateju et al., 2017; Apicco et al., 2018). Under native conditions, stress granules require RNA for assembly and enzymatic RNA removal leads to their disintegration (Bounedjah et al., 2014). Intriguingly, stress granules containing mutated SOD1 are resistant to RNA removal (Mateju et al., 2017). It was shown that misfolded proteins enter LLPS compartments to prevent their irreversible aggregation (Nollen et al., 2001) (Frottin et al., 2019). Biomolecular condensates might also change the kinetics of protein aggregation by promoting the formation of a rate-limiting nucleation point for protein misfolding and aggregation (Weber et al., 2019).
2.4 Disease-associated mutations are drivers of misfolding and aggregation
As biomolecular condensates play essential roles in cellular organization and physiology, failed LLPS can culminate in protein misfolding and aggregation (Babinchak and Surewicz, 2023). In line, anomalous LLPS and aberrant membrane-less compartments are associated with the pathogenesis of multiple human diseases. There is a strong link between condensate forming-proteins and age-related diseases such as neurodegeneration and cancer (Alberti and Hyman, 2021; Wang et al., 2021; Uversky, 2022). Proteins linked to neurodegeneration form condensates that promote protein aggregation and amyloid formation (Molliex et al., 2015; Patel et al., 2015; Ambadipudi et al., 2017; Wegmann et al., 2018; Babinchak et al., 2019; Ray et al., 2020). Many mutations are thought to change the conformational landscape of proteins, promoting amyloid-like interactions culminating in aggregation (Mackenzie et al., 2017; Murray et al., 2017; Qamar et al., 2018). For instance, mutations in proteins associated with Amyotrophic Lateral Sclerosis (ALS) lead to altered rates of condensate hardening (Midic et al., 2009; Murakami et al., 2015; Patel et al., 2015; Wang et al., 2018; Tsang et al., 2020). In pathological conditions, it is speculated that LLPS may even favor the formation of aggregates (Zbinden et al., 2020).
Insoluble protein deposits are hallmarks of neurodegenerative diseases, e.g., Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and ALS. Aberrant aggregation involves a cascade of events and requires extended periods of time and eventually manifests in the clinical phase of neurodegeneration, thus the decline of neuronal health correlates with accumulation of aggregates (Hou et al., 2019). The disease-linked proteins Tau and α-Synuclein are inherently disordered and only obtain a defined structure when they associate with their binding partners (Avila et al., 2016; Stephens et al., 2019). Expanded repeat sequences such as polyQ, HTT and polyGA introduce unfolded protein stretches that promote protein aggregation (Nonaka et al., 2018; Bonfanti et al., 2019). During aggregation, the misfolded protein monomers come into contact, forming soluble conglomerates that further aggregate via structural rearrangements into stable and highly organized fibrils or amorphous aggregates without higher-ordered structure. Some studies ascribe oligomers to be the more toxic proteins species as they were shown to penetrate lipid bilayers and cause stress (Wegmann et al., 2016; De et al., 2019a; De et al., 2019b; Lobanova et al., 2022; Meng et al., 2022). Thus, the formation of aggregate fibrils, such as neurofibrillary tangles and Lewy-bodies, and the consequent sequestration is proposed to delay toxicity (Cowan and Mudher, 2013; Chartier and Duyckaerts, 2018). Interestingly, it was demonstrated that aggregate formation and progressive motor decline in a mouse model of Huntington’s disease depend on continuous expression of polyQ and thus can be reversible (Yamamoto et al., 2000).
3 Disaggregation is a decision point for refolding and degradation
To counteract aberrant condensate or aggregate formation, PQC factors are required to reset the stressed system. The transcriptional stress response system driven by heat shock transcription factors (HSFs) plays a key role. HSFs induce gene regulatory programs that support the removal of misfolded and aggregated proteins, thereby counteracting proteostasis collapse (Joutsen and Sistonen, 2019). In yeast it has been firmly established that the activity of Hsf1 is negatively regulated by levels of free Hsp70, with the result that misfolded proteins titrate Hsp70 to activate Hsf1 (Masser et al., 2020). Similarly, human HSF1 is negatively regulated by Hsp70 (Kmiecik et al., 2020; Masser et al., 2020). Thus, HSFs function as a proteostasis-sensitive mechanism that control PQC factor levels before and after stress.
Disaggregation is an essential first step to disentangle the aggregated protein and thus to either enable its subsequent rescue by refolding or removal by degradation. Most proteins in aggregates have been found to undergo disaggregation proportional to their aggregation propensity, i.e., a more severe loss in solubility is counteracted by faster disaggregation. It was recently demonstrated that proteins carrying IDRs are disaggregated faster in vivo (Määttä et al., 2020). On the molecular level, the explanation may simply be found in that weaker intramolecular interactions permit faster disentanglement. Alternatively, disordered regions that drive aggregation might also speed up subsequent extraction from aggregates by presenting flexible loop regions that the disaggregase machinery can bind. In this line, it was recently shown that in vitro disassembly of α-Synuclein fibrils requires N- and C-terminal extensions allowing chaperone binding cycles to facilitate the generation of power strokes (Gao et al., 2015).
The disaggregation machinery itself is based on a conserved bi-chaperone disaggregase centered around Hsp100 family members and Hsp70. While plants, fungi and even bacteria carry the complete system, metazoans have lost the Hsp100 component and rely on a system powered by Hsp70 working in concert with J-domain protein (JDP) cochaperones and Hsp110 nucleotide exchange factors (NEFs) (simplified overview found in Figure 3). See brief description below, or for an in-depth description of different disaggregation mechanisms, see recent reviews (Nillegoda et al., 2018; Shorter and Southworth, 2019).
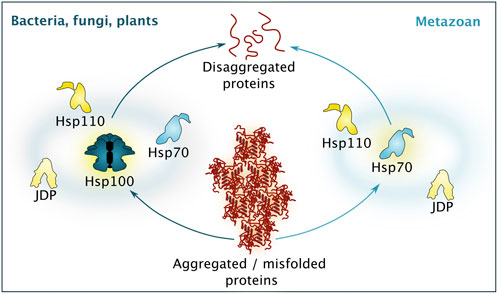
FIGURE 3. Simplified comparison between different disaggregation machineries. While bacteria, fungi and plants rely of disaggregation based on the activity of a Hsp100 isoform, supported by Hsp70, Hsp110 and a diverse set of J-domain proteins (JDP), metazoan disaggregation is based on Hsp70 activity, supported by co-chaperones belonging to the Hsp110 and JDP class. Please note that additional chaperones and protein quality control factors are involved to guarantee efficient disaggregation.
3.1 Cellular disaggregation and refolding strategies
Disaggregation and refolding of proteins trapped in aggregates is central to resetting the proteostasis system during proteotoxic stress recovery. This task is a complex and energy-consuming process, which involves unfolding of the aggregated species and then re-folding of the polypeptide into the native state. The process has parallels to de novo folding that occurs at the ribosomal tunnel exit but is not impacted by the strict directionality of mRNA translation since disaggregation can proceed from any end of a substrate. Briefly, the aggregates are handled by chaperone disaggregase machineries, initially characterized in yeast (Parsell et al., 1994; Glover and Lindquist, 1998; Mogk et al., 1999; Weibezahn et al., 2004). Notably, while, for example, bacteria, fungi, plants and protists possess a powerful bi-chaperone disaggregase centered around the ring-shaped AAA+ -chaperone Hsp100 (ClpB in bacteria, Hsp104 in yeast, Hsp101 in plants) and Hsp70, there is no cytosolic Hsp104 paralogue in metazoans (Sanchez and Lindquist, 1990; Parsell et al., 1994; Glover and Lindquist, 1998; Goloubinoff et al., 1999; Hong and Vierling, 2001; Mosser et al., 2004; Shorter, 2011).
Yeast Hsp104 cooperates with Hsp70 assisted by its JDP co-chaperones and Hsp110 class nucleotide exchange factors (NEFs) to thread trapped polypeptides in an ATP-dependent manner through its central pore, resolving a wide range of protein aggregates and hardened condensates (simplified overview found in Figure 3) (Deville et al., 2017; Gates et al., 2017; Kaimal et al., 2017). The role of Hsp70 is to recruit Hsp104 to the surface of the aggregate and to initiate disaggregation, likely by modifying the aggregate surface. Hsp70 in turn depends on its JDP to find its bindings sites and together they have the potential to disaggregation. Even though not required for disaggregation in vitro (Tessarz et al., 2008), yeast Hsp110 (Sse1 and Sse2) NEFs have been shown to be strictly required for this process in vivo, likely representing an indirect mechanism by resetting Hsp70 via nucleotide exchange and substrate release so that the Hsp70 chaperone can cycle on and off the aggregate surface (Kaimal et al., 2017). Intriguingly, yeast JDP Apj1 together with Hsp70 was shown to support disaggregation of intra-nuclear aggregates independent of Hsp104 (den Brave et al., 2020). In that line, bacterial Hsp70 was also shown to possess limited disaggregation activity, especially on large aggregates, (Ben-Zvi et al., 2004), thus it will be an exciting task to determine the mechanistic fundamentals how Hsp70s differ in their disaggregation capacities in different species.
Metazoans lack cytosolic/nuclear Hsp100 orthologs and thus rely on the disaggregation activity provided by Hsp70 itself in cooperation with a specific subset of JDP co-chaperones and NEFs from the Hsp110 family (simplified overview found in Figure 3). Together, these factors are capable of dissolving a wide range of aggregates in vitro and in vivo (Shorter, 2011; Rampelt et al., 2012; Mattoo et al., 2013; Nillegoda et al., 2017; Nillegoda et al., 2015; Gao et al., 2015; Nillegoda and Bukau, 2015; Kirstein et al., 2017). The promiscuity towards aggregated substrates is configured by different JDPs and the formation of mixed-class JDP complexes guarantees fine-tuning of the selection of aggregated proteins (Duennwald et al., 2012; Mattoo et al., 2013; Nillegoda et al., 2017; Nillegoda et al., 2015; Gao et al., 2015; Kirstein et al., 2017). The Hsp110 NEF enhances protein disaggregation activity and appears to function primarily by resetting the disaggregase machinery for new rounds of polypeptide extractions via substrate release. Hsp110 has also been proposed to take part in the disaggregation process by providing a holdase activity and hence to directly interact with substrate polypeptides (Mattoo et al., 2013; Gao et al., 2015; Nillegoda and Bukau, 2015; Nillegoda et al., 2015).
The Hsp70 machinery is central to all fates of resolubilized polypeptides. NEFs, driving the release of substrates, presumably play decisive roles in discrimination between refolding and degradation (Brehmer et al., 2001; Bracher and Verghese, 2015; Rosenzweig et al., 2019). As the Hsp70 disaggregase has inherent refolding activity, the machine is primed for efficient refolding after aggregate extraction and a handover mechanism to Hsp90 chaperones ensures a strong bias towards refolding over degradation (Nillegoda et al., 2018; Kohler and Andréasson, 2020). Yet, the integrated nature of the system with Hsp70 involved both in the disaggregation step as well as in downstream folding pathways has made it difficult study these separate functions. Likely, the nature of the substrate determines just how dependent its refolding is on Hsp70 following disaggregation. Based on the insight from studying de novo folding of proteins at ribosomes, small fast-folding substrates may fold completely without the aid of Hsp70 while more complex multidomain proteins are likely to depend of the chaperone (Komar, 2009; Kramer et al., 2009; Fedyukina and Cavagnero, 2011; Oh et al., 2011; Han et al., 2012).
Hsp70 was shown to protect stress granules from accumulation of misfolding-prone proteins like SOD1 and is required for dissolution of SOD1-containing stress granules that transitioned from liquid-like to solid-like state (Mateju et al., 2017). Further, the Hsp70 disaggregation machinery governs dissolution of stress granules containing defective ribosome products and associates with ribonuclear granules to maintain them in liquid-like states (Ganassi et al., 2016; Mateju et al., 2017). Similarly, yeast Hsp104 is essential for disaggregation of solid-like stress granules, originally assumed to be aggregates (Cherkasov et al., 2013; Kroschwald et al., 2015; Wallace et al., 2015; Riback et al., 2017; Franzmann et al., 2018). For example, this chaperone is required for the release of phase-separated Ded1 and other condensate-forming proteins like Pab1 from aged stress granules to facilitate re-entry into the cell cycle by starting translation of housekeeping changes during recovery after heat stress (Kroschwald et al., 2018; 2015; Riback et al., 2017; Yoo et al., 2022). Hsp104 and Hsp70 co-localize with stress granules, which is essential for post-stress recovery (Cherkasov et al., 2013).
Alternative disaggregation machineries like RuvBL1/2, Cyp40, HTRA1 or VCP/p97 have been discussed in recent years, some of them favoring degradation after extraction of misfolded proteins from aggregates, (Ratajczak et al., 1993; Hirabayashi et al., 2001; Tennstaedt et al., 2012; Poepsel et al., 2015; Zaarur et al., 2015; Baker et al., 2017; Darwich et al., 2020). RuvBL1/2 is described to exhibit a general, yet limited disaggregase activity and is proposed to function as chaperone in complex with Hsp90 to regulate assembly of nucleolar ribonucleoprotein complexes. RuvBL1/2 was shown to suppress seeding of amyloids and co-sediments with amyloid assemblies in human cells (Olzscha et al., 2011; Zaarur et al., 2015).
Perhaps of physiological relevance, disaggregation in metazoans appears to be slower than in yeast, as 5 h after heat shock most of the aggregates were still on their way to be fully disaggregated in human cells (Rampelt et al., 2012; Wallace et al., 2015; Määttä et al., 2020). Similarly, a C. elegans study showed that while there is a minute-scale disaggregation rate for misfolded luciferase in vitro, traces of luciferase aggregates were found days after heat shock in vivo (Kirstein et al., 2017). It should be noted in this regard that in vitro disaggregation models using firefly luciferase are very sensitive to the aggregation conditions employed in the experiments and not every type of aggregate can be disaggregated in vitro. Thus, it is likely that the cell offers an environment and factors that greatly impact on the physiological states of the aggregates and hence how efficiently they can be disaggregated. For example, orchestrated aggregation likely depends on LLPS as well as specific aggregation factors, for example, sHSPs, that ensure that the aggregates/condensates downstream are compatible with efficient and quantitative disaggregation (Mogk et al., 2019).
3.2 The sequential actions of disaggregation and degradation
Terminally damaged or irretrievably misfolded proteins are extracted from aggregates and targeted for degradation via one of two dominant degradative pathways in cells, the ubiquitin-proteasome system (UPS) and autophagy (Figure 4) (Wang and Le, 2019). Similar to the scenario of refolding, UPS degradation of aggregates typically requires disentanglement of the substrate by disaggregation. The scenario of autophagy may or may not involve such previous disaggregation depending on if the pathway involved belongs to macro- or microautophagy (Saha et al., 2018; Wang et al., 2022). Chaperone-mediated autophagy, a selective form of microautophagy relying on the Hsp70 system for its specificity, takes care of soluble substrates and has been described only in metazoan cells so far (reviewed in (Kaushik and Cuervo, 2018)).
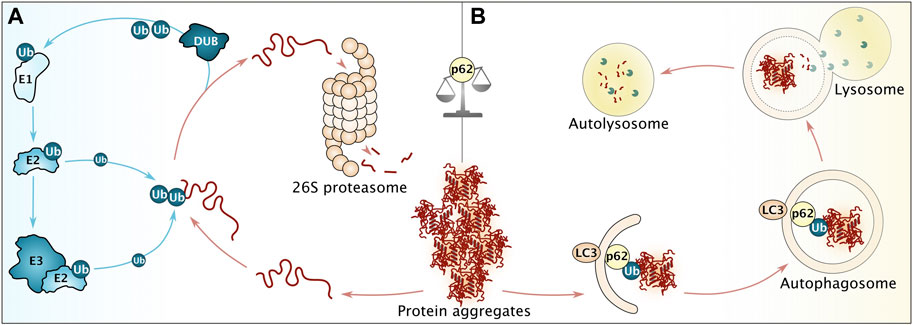
FIGURE 4. Overview of major cellular degradation pathways. (A) Before degradation via the ubiquitin-proteasome system (UPS), aggregated proteins require disaggregation, followed by decoration with poly-ubiquitin (Ub) moieties. This reaction is facilitated by ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2) and ubiquitin ligases (E3). Prior to degradation, ubiquitin moieties are removed by deubiquitinating enzymes (DUB). (B) Persistent aggregates are subjected towards autophagosomal degradation. To that end, p62 recognizing ubiquitin moieties facilitates the interaction of aggregates with LC3 on the growing autophagosome membrane. Please note that more proteins are involved to ensure efficient autophagy. After membrane closure, the autophagosome fuses with lysosomes in metazoan, leading to degradation of proteinaceous content (in autolysosomes). p62 (among other factors) serves to balance degradation via UPS and autophagy.
Metazoan VCP/p97 (homologous to yeast and plant Cdc48) and ATP-independent HTRA1 can execute disaggregation (Nillegoda et al., 2018). Human HTRA1 is a serine protease and is implicated in diseases involving proteostasis imbalances and was shown to efficiently degrade neurodegenerative proteins Aβ and Tau, in vitro and in vivo. Furthermore, protease-inactivated HTRA1 converts fibrillar tau to soluble species, substantiating its disaggregation activity (Wilken et al., 2004; Yang et al., 2006; Tennstaedt et al., 2012; Poepsel et al., 2015). Interestingly, the combination of disaggregation and immediate proteolytic degradation of substrates in HTRA1 functionality, guarantees uni-directionality of this process, thus elimintes the requirement of ATP-dependent substrate release. The generation of short peptides with proposedly lower affinity to respective binding sites, ensures the energy-independent dissociation of degraded substrates from HTRA1 (Poepsel et al., 2015).
Another disaggregase system tightly associated with subsequent degradation is VCP/p97 belonging to the AAA+ superfamily. It consists of a hexameric ring, coupling ATP hydrolysis with mechanical work. Its structural similarity to yeast Hsp104 and bacterial ClpB made it an early focus of interest when hunting for metazoan disaggregase machineries (Parsell et al., 1994; Hirabayashi et al., 2001; Kobayashi et al., 2007). In contrast to HTRA1, VCP/p97 collaborates with the UPS for proteolysis of its substrates. VCP/p97 is involved in PQC, ribosome-associated quality control as well as ER-associated degradation (ERAD) and has been shown to reduce cytotoxicity associated with polyQ aggregates in Drosophila melanogaster and C. elegans (Ballar et al., 2011; Bodnar and Rapoport, 2017; Defenouillère et al., 2013; Nishikori et al., 2008; Verma et al., 2013). It further plays a role in aggregate clearance from the nucleus after heat shock and engages with tau and huntingtin in post-mortem brain tissue (Hirabayashi et al., 2001; Gallagher et al., 2014; Darwich et al., 2020). Thus, cells harbor machineries that directly link disaggregation to degradation using specialized proteases or the UPS system.
UPS targets the majority of cellular proteins and ubiquitin conjugation is facilitated by an enzymatic cascade, modulating protein half-lives from seconds to days (Figure 4A) (Galves et al., 2019). Ubiquitin is activated by ubiquitin-activating enzymes (E1), handed over to ubiquitin-conjugating enzymes (E2) and transferred either directly onto the substrate or via ubiquitin ligases (E3). Prior to degradation, ubiquitin is removed by deubiquitinating enzymes (DUB) (Hoeller et al., 2007; Pohl and Dikic, 2019). Ubiquitinated proteins are recognized and degraded by the 26S proteasome holoenzyme, consisting of a barrel-shaped 20S core particle with proteolytic activity, capped on both ends by 19S regulatory particles, responsible for recognition and regulatory processes (Lu et al., 2017; Coleman et al., 2021).
The function of the proteasome has been suggested to be regulated at the subcellular level. Its localization is highly dynamic, most recent studies point towards predominant nuclear localization in metazoan, similar as observed in yeast (Lehmann et al., 2002; Marshall et al., 2016; Nemec et al., 2017; Tomita et al., 2019). Interestingly, starvation and nutrient depletion in plants lead to proteasome disassembly and thus inactivation into storage granules (Gu et al., 2017). VCP/p97, proteasome particles, E1-, E2-and E3-enzymes as well as DUBs are found in biomolecular condensates in the nucleus and p62, an adaptor protein linking UPS and autophagy, was shown to be an essential component of these droplets (Fu et al., 2021). LLPS properties is brought on by p62 self-interaction as well as interaction with poly-ubiquitin chains (Sun et al., 2018; Zaffagnini et al., 2018). These foci have been proposed to function as catalytic centers for degradation of misfolded proteins that are assembled by proteotoxic cues, for example, heat and oxidative stress. The droplets are likely only acceptable under transient stress conditions, since prolonged engagement of disaggregases and proteasomes would hinder them from performing their canonical roles in proteostasis. Empirically, inhibition of VCP/p97 has been shown to increase foci size, while suppression of ubiquitination activity prevents foci formation (Yasuda et al., 2020). Also stress granules show a related behavior. Ubiquitinated proteins, DUBs and several ubiquitin-binding proteins have been shown to accumulate in these condensates, with DUBs adopting a regulatory role in stress granule dynamics. The VCP/p97 disaggregation machinery contributes to clearing stress granules and mutations of VCP/p97 delay disappearance of stress granules and cellular recovery (Kwon et al., 2007; Buchan et al., 2013; Ganassi et al., 2016; Kedersha et al., 2016; Mateju et al., 2017; Dao et al., 2018; Turakhiya et al., 2018; Xie et al., 2018; Youn et al., 2018; Wang et al., 2019). Interestingly, cytosolic stress granules were shown to relieve the nuclear UPS by trapping misfolding-prone defective ribosomal products via LLPS and thus hindering their accumulation in the nucleus (Xu et al., 2022).
Proteins that fail to undergo efficient disaggregation remain as large and persistent protein aggregates and are ultimately directed towards autophagy for bulk destruction (Figure 4B). This self-eating mechanism involves de novo membrane synthesis around the aggregate which results in engulfment of the cargo into autophagosomes, followed by their subsequent delivery to and fusion with proteolytic lysosomes (autolysosomes) and involves a plethora of receptors and other regulatory proteins (Melentijevic et al., 2017; Runwal et al., 2019). Targeting works via cargo recognition by adaptor proteins with p62 (Cue5 in yeast) playing a key role by interacting with ubiquitin moieties on the protein aggregates, and linking them to LC3 (or Atg8 in yeast) on the autophagosome membranes. Selective degradation of aggregates via autophagy (aggrephagy) plays a critical role in limiting their accumulation in cells (Kraft et al., 2009; Sun et al., 2020). Stress granules associated with ubiquitinated proteins recruit p62, thus triggering degradation of ubiquitin-positive stress granules by autophagy (Ganassi et al., 2016; Mateju et al., 2017). Thus, cells are equipped with regulatory mechanisms, ensuring the beneficial role of stress granules on the UPS system as dumping ground for aggregation-prone proteins but at the same time prompting their removal, if these condensates become oversaturated with ubiquitinated proteins.
Intriguingly, there is a substantial crosstalk between the UPS and autophagy. In this regard, p62 orchestrates the UPS and autophagy with a mechanistic base in its innate ability to physically associate with both systems. It either escorts ubiquitinated proteins to the proteasome as shuttling factor or to autophagosomes as autophagy receptor (Seibenhener et al., 2004; Pankiv et al., 2007; Wurzer et al., 2015). Autophagy is upregulated in conditions of deficient degradation of ubiquitinated proteins by UPS, either via genetic or pharmacological proteasome inhibition (Pandey et al., 2007; Wu et al., 2008; Zhu et al., 2010; Laussmann et al., 2011; Selimovic et al., 2013; Li et al., 2019). Furthermore, loss of Hsp70 or its NEF Hsp110 also reduces proteasome activity and simultaneously induces autophagy (Feleciano et al., 2019). Proteasome inhibition leads to its ubiquitination in yeast, initiating the first steps towards selective degradation of inactivated proteasomes via autophagy (Marshall et al., 2016; Waite et al., 2016; Nemec et al., 2017). Crosstalk also goes the other way if UPS gets overwhelmed. In that scenario, misfolded or damaged proteins form large insoluble aggregates that cannot rapidly be removed by the proteasome, thus requiring degradation via autophagy (Hyun et al., 2003). Moreover, protein aggregates were shown to inhibit the proteasome, also requiring the activation of autophagy (Bence et al., 2001; Lindersson et al., 2004; Díaz-Hernández et al., 2006; Myeku et al., 2016; Sun-Wang et al., 2020). In summary, the UPS and autophagy complement each other and share both substrates and common factors.
3.3 Interplay between refolding and degradation
Proteins that adopt an aberrant fold are rapidly recognized by chaperones that prevent aggregation, facilitate folding, and coordinate the interaction with E3 ubiquitin ligases, thus coupling protein refolding with degradation. The chaperone machinery interacts only transiently with the aberrantly folded substrate, yet it will rebind in repeated cycles if the protein does not reach its native state and remains misfolded (Kohler and Andréasson, 2020). The key chaperone Hsp70 employs NEFs that ensure efficient release and thus cycling in the chaperone network (Rosenzweig et al., 2019; Kohler and Andréasson, 2020). For persistently misfolded proteins, release from Hsp70 is mediated by dedicated armadillo and BAG-type NEFs that carry specialized substrate release domains that compete the substrate off the chaperone substrate binding site (Rauch et al., 2016; Gowda et al., 2018; Rosam et al., 2018). This orchestrated release enables other factors such as ubiquitin E3 ligases to get access to the hydrophobic peptides that constitute the Hsp70 binding site and also function as degrons (Rauch et al., 2016; Gowda et al., 2018; Rosam et al., 2018; Abildgaard et al., 2023). For example, the metazoan E3 ligase CHIP associates with Hsp70 and will eventually ubiquitinate persistently misfolded proteins that undergo repeated Hsp70 interaction cycles (Murata et al., 2001). CHIP mediates substrate ubiquitination through its C-terminal catalytic domain and its N-terminus interacts with Hsp70. The interaction with the disaggregation machinery is essential for CHIP activity, as aggregate-bound chaperones are required for efficient ubiquitination (Qian et al., 2006; Tetzlaff et al., 2008; Kalia et al., 2011). Whether misfolded proteins are subjected towards refolding or degradation has been suggested to depend on the ratio between disaggregation machinery and CHIP. Elevated CHIP expression and prolonged Hsp70-substrate interaction lead to a larger fraction of substrates becoming ubiquitinated by CHIP and thus targeted for degradation (Qian et al., 2006). Hsp70 and other chaperones are expressed at significantly higher levels than CHIP, suggesting a potential cellular preference for refolding over degradation (Meacham et al., 2001). Regulation of proteasomal targeting of Hsp70-disentangled substrates is also assisted by the NEF Bag1, that carries a ubiquitin-binding domain for specific recognition of modified substrates as well as Hsp70 substrate release domains (Lüders et al., 2000; Tsukahara and Maru, 2010; Rauch et al., 2016; Hantouche et al., 2017). In line, BAG3:BAG1 ratio determines degradation via UPS or autophagy. Proteotoxic stress and cellular ageing lead to BAG3 upregulation and outcompete BAG1, redirecting Hsp70-CHIP-associated substrates towards autophagy-mediated degradation pathways (e.g., chaperone-assisted selective autophagy) (Carra et al., 2008; Gamerdinger et al., 2011; Gamerdinger et al., 2009; Minoia et al., 2014; Rapino et al., 2014). A related role is played by Hsp70 NEFs of the Hsp110 class that interact with the proteasome as well as with Hsp70 as a substrate releasing NEF. Thus, Hsp110 functions as an Hsp70-misfolded protein receptor at the proteasome that fast-tracks misfolded protein associated with Hsp70 for degradation and ensures the timely release from the chaperone close to the proteolytic chamber (Kandasamy and Andréasson, 2018; Gersing et al., 2021). These functions appear to be conserved between yeast and man (Rauch et al., 2016). The intricate interdependency between Hsp70-dependent refolding, aggregate sequestration by sHsps and the UPS has been recently tested by genetic analysis in yeast. Imbalances between the activity systems rather than the processes themselves were found to severely affect protein homeostasis and cellular fitness (Jawed et al., 2022).
When assessing the ratio between refolding and degradation rates, PQC has a preference towards rescuing misfolded proteins with only little degradation of reporter and bulk proteins, as observed both in yeast and in mammalian cell culture during recovery from mild cellular stresses (Hageman et al., 2007; Medicherla and Goldberg, 2008; Wallace et al., 2015; Määttä et al., 2020). Refolding of aggregated proteins spares the cell the burden of novel biosynthesis of metabolic and regulatory proteins, which is vital to rapid recovery of cellular functions after stress (Medicherla and Goldberg, 2008). However, the interrelation of refolding and degradation networks ensures that the occurrence and persistence of proteotoxic species is prevented by all means, even if one subsystem malfunctions.
3.4 PQC failure due to disease
Increasing age decreases the functionality of PQC, resulting, e.g., from lower expression and/or activity of proteasome subunits and ubiquitin-related enzymes, and thus leads to occurrence and accumulation of toxic protein aggregates (Lee et al., 2000; Lee et al., 1999; Ly et al., 2000; Petropoulos et al., 2000; Ferrington et al., 2005; Tonoki et al., 2009). Mutations in genes encoding for chaperones and PQC-related factors further accelerate the decline leading to age-associated disorders (H et al., 2022; Macario et al., 2005; Tittelmeier et al., 2020), as has been seen for mutations in VCP/p97 that are linked to neurodegenerative disorders (Watts et al., 2004; Johnson et al., 2010; Meyer and Weihl, 2014). Similarly, alterations of degradation pathways are linked to the onset of human age-related diseases including cancer and neurodegeneration. Additionally, autophagy is shown to be dysfunctional in neurodegenerative diseases and loss-of-function mutations in the autophagy machinery leads to an early onset of neurodegenerative phenotypes in mice (Hara et al., 2006; Komatsu et al., 2006; Corti et al., 2020; Rana et al., 2021). Neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease involve loss of proteasomal degradation activity (Keller et al., 2000; Furukawa et al., 2002; McNaught et al., 2002; Seo et al., 2004; Díaz-Hernández et al., 2006; Thibaudeau et al., 2018), while UPS-associated enzymes, ubiquitin moieties and chaperones are abundantly detected in insoluble aggregate structures such as Lewy bodies and neurofibrillary tangles, which further underpins the abortive attempt of PQC to counteract pathological aggregation processes (García-Sierra et al., 2012; Zhang et al., 2019; Arakhamia et al., 2020; Schweighauser et al., 2020).
4 Outlook and open questions
The strict distinction between biomolecular condensates as easily reversible and beneficial vs. aggregates as irreversibly, toxic compartment is not reflecting the fact that aging condensates can adopt solid-like, irreversible properties, while especially protein-disorder diseases show that aggregation processes producing almost inert compartments can indeed be the less toxic species compared to soluble oligomers. Thus, a more nuanced way of interpretation is required. Especially substantiated by the fact that distinction between phase-separated condensates and aggregates formed by misfolded protein species is not always the easiest task, as seen in yeast, where stress granules were originally identified as aggregated compartments. However, as discussed above, biomolecular condensates can incorporate misfolded proteins and it is hypothesized that phase separation might be a transition state preceding aggregate formation. Traditionally, LLPS has been widely studied in vitro under controlled and predictable conditions, a valuable tool gathering basic knowledge on these processes, nevertheless, in vivo data reflecting the dynamic nature of this process is inevitable for a complete picture. The facts that biomolecular condensates can adopt different states ranging from liquid-like to solid-like, harbor different scaffold and client proteins depending on cellular conditions and govern essential cellular processes make their full potential hard to understand. Research has just begun to explore aging as additional complexity factor, as traditionally PQC studies, especially those in model systems like yeast and bacteria, were conducted in young, immaculately working systems that instantly react to proteotoxic cues to safeguard cellular proteostasis. In light of our perpetually aging society and the fact that PQC capacity decreases over time (Hipp et al., 2019), it is vital to understand the parallels and differences between young and aged proteostasis systems. We already know that several PQC factors, including the proteasome, loose their competence over time. Interestingly, while some organisms develop age-associated diseases like cancer and neurodegeneration, others remain healthy. Not taking heritability and external triggers into account, it might additionally be feasible that other PQC branches take over to guarantee a balanced and healthy proteome. While rapidly-growing cells are especially challenged by the flood of ribosomal products prone to misfolding and subsequent aggregation, non-dividing cells like neurons or modelled by stationary post-diauxic yeast supposedly face different problems. As nuclear-localized proteins involved in DNA binding, chromatin organization and transcriptional activity were recently shown to be especially vulnerable to heat shock, substantiated by the fact that topoisomerases and proteins involved in DNA replication had the lowest melting points in bacteria (Mateus et al., 2018; Määttä et al., 2020), it seems intuitive that PQC forces need to be consolidated to counteract sudden proteotoxic impact protecting the weakest members of the cellular proteome. Thus, it is feasible that specific PQC components alter their localization or expression patterns with increasing age. While several studies focused on gene expression upon proteotoxic cues and age, especially potential distribution patterns of major chaperones and folding factors represent exciting research questions for future studies.
Author contributions
VK and CA wrote the manuscript. VK designed the figures. Both authors read and approved the final version of the manuscript.
Funding
VK was supported by the Austrian Science Fund (FWF, Grant No. J4342-B21) and CA was supported by Swedish Research Council (project grant 2019-04052), Swedish Cancer Society (project grant 20 1045) and Knut and Alice Wallenberg Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abildgaard, A. B., Voutsinos, V., Petersen, S. D., Larsen, F. B., Kampmeyer, C., Johansson, K. E., et al. (2023). HSP70-binding motifs function as protein quality control degrons. Cell. Mol. Life Sci. CMLS 80, 32. doi:10.1007/s00018-022-04679-3
Adame-Arana, O., Weber, C. A., Zaburdaev, V., Prost, J., and Jülicher, F. (2020). Liquid phase separation controlled by pH. Biophys. J. 119, 1590–1605. doi:10.1016/j.bpj.2020.07.044
Aggarwal, S., Snaidero, N., Pähler, G., Frey, S., Sánchez, P., Zweckstetter, M., et al. (2013). Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLOS Biol. 11, e1001577. doi:10.1371/journal.pbio.1001577
Alberti, S., and Hyman, A. A. (2021). Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213. doi:10.1038/s41580-020-00326-6
Ambadipudi, S., Biernat, J., Riedel, D., Mandelkow, E., and Zweckstetter, M. (2017). Liquid–liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat. Commun. 8, 275. doi:10.1038/s41467-017-00480-0
Ambadipudi, S., Reddy, J. G., Biernat, J., Mandelkow, E., and Zweckstetter, M. (2019). Residue-specific identification of phase separation hot spots of Alzheimer’s-related protein tau. Chem. Sci. 10, 6503–6507. doi:10.1039/C9SC00531E
Apicco, D. J., Ash, P. E. A., Maziuk, B., LeBlang, C., Medalla, M., Al Abdullatif, A., et al. (2018). Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo. Nat. Neurosci. 21, 72–80. doi:10.1038/s41593-017-0022-z
Arakhamia, T., Lee, C. E., Carlomagno, Y., Kumar, M., Duong, D. M., Wesseling, H., et al. (2020). Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell 180, 633–644. doi:10.1016/j.cell.2020.01.027
Arlet, J.-B., Ribeil, J.-A., Guillem, F., Negre, O., Hazoume, A., Marcion, G., et al. (2014). HSP70 sequestration by free α-globin promotes ineffective erythropoiesis in β-thalassaemia. Nature 514, 242–246. doi:10.1038/nature13614
Audas, T. E., Audas, D. E., Jacob, M. D., David Ho, J. J., Ho, J. J. D., Khacho, M., et al. (2016). Adaptation to stressors by systemic protein amyloidogenesis. Dev. Cell 39, 155–168. doi:10.1016/j.devcel.2016.09.002
Avila, J., Jiménez, J. S., Sayas, C. L., Bolós, M., Zabala, J. C., Rivas, G., et al. (2016). Tau structures. Front. Aging Neurosci. 8, 262. doi:10.3389/fnagi.2016.00262
Babinchak, W. M., Haider, R., Dumm, B. K., Sarkar, P., Surewicz, K., Choi, J.-K., et al. (2019). The role of liquid-liquid phase separation in aggregation of the TDP-43 low-complexity domain. J. Biol. Chem. 294, 6306–6317. doi:10.1074/jbc.RA118.007222
Babinchak, W. M., and Surewicz, W. K. (2023). Biophysical studies of LLPS and aggregation of TDP-43 LCD. Methods Mol. Biol. Clifton N. J. 2551, 497–513. doi:10.1007/978-1-0716-2597-2_31
Baker, J. D., Shelton, L. B., Zheng, D., Favretto, F., Nordhues, B. A., Darling, A., et al. (2017). Human cyclophilin 40 unravels neurotoxic amyloids. PLOS Biol. 15, e2001336. doi:10.1371/journal.pbio.2001336
Balchin, D., Hayer-Hartl, M., and Hartl, F. U. (2020). Recent advances in understanding catalysis of protein folding by molecular chaperones. FEBS Lett. 594, 2770–2781. doi:10.1002/1873-3468.13844
Ballar, P., Pabuccuoglu, A., and Kose, F. A. (2011). Different p97/VCP complexes function in retrotranslocation step of mammalian ER-associated degradation (ERAD). Int. J. Biochem. Cell Biol. 43, 613–621. doi:10.1016/j.biocel.2010.12.021
Banani, S. F., Lee, H.-O. K., Hyman, A. A., and Rosen, M. K. (2017). Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298. doi:10.1038/nrm.2017.7
Banani, S. F., Rice, A. M., Peeples, W. B., Lin, Y., Lin, Y., Jain, S., et al. (2016). Compositional control of phase-separated cellular bodies. Cell 166, 651–663. doi:10.1016/j.cell.2016.06.010
Ben-Zvi, A., De Los Rios, P., Dietler, G., and Goloubinoff, P. (2004). Active solubilization and refolding of stable protein aggregates by cooperative unfolding action of individual hsp70 chaperones. J. Biol. Chem. 279, 37298–37303. doi:10.1074/jbc.M405627200
Bence, N. F., Sampat, R. M., and Kopito, R. R. (2001). Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555. doi:10.1126/science.292.5521.1552
Bodnar, N., and Rapoport, T. (2017). Molecular mechanism of substrate processing by the Cdc48 ATPase complex. Cell 169, 722–735. doi:10.1016/j.cell.2017.04.020
Boeynaems, S., Bogaert, E., Kovacs, D., Konijnenberg, A., Timmerman, E., Volkov, A., et al. (2017). Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol. Cell 65, 1044–1055. doi:10.1016/j.molcel.2017.02.013
Bonfanti, S., Lionetti, M. C., Fumagalli, M. R., Chirasani, V. R., Tiana, G., Dokholyan, N. V., et al. (2019). Molecular mechanisms of heterogeneous oligomerization of huntingtin proteins. Sci. Rep. 9, 7615. doi:10.1038/s41598-019-44151-0
Bounedjah, O., Desforges, B., Wu, T.-D., Pioche-Durieu, C., Marco, S., Hamon, L., et al. (2014). Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res. 42, 8678–8691. doi:10.1093/nar/gku582
Bracher, A., and Verghese, J. (2015). The nucleotide exchange factors of Hsp70 molecular chaperones. Front. Mol. Biosci. 2, 10. doi:10.3389/fmolb.2015.00010
Brangwynne, C. P., Eckmann, C. R., Courson, D. S., Rybarska, A., Hoege, C., Gharakhani, J., et al. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732. doi:10.1126/science.1172046
Brangwynne, C. P., Mitchison, T. J., and Hyman, A. A. (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. 108, 4334–4339. doi:10.1073/pnas.1017150108
Brehmer, D., Rüdiger, S., Gässler, C. S., Klostermeier, D., Packschies, L., Reinstein, J., et al. (2001). Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat. Struct. Biol. 8, 427–432. doi:10.1038/87588
Brostrom, C. O., Prostko, C. R., Kaufman, R. J., and Brostrom, M. A. (1996). Inhibition of translational initiation by activators of the glucose-regulated stress protein and heat shock protein stress response systems. Role of the interferon-inducible double-stranded RNA-activated eukaryotic initiation factor 2alpha kinase. J. Biol. Chem. 271, 24995–25002. doi:10.1074/jbc.271.40.24995
Buchan, J. R., Kolaitis, R.-M., Taylor, J. P., and Parker, R. (2013). Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153, 1461–1474. doi:10.1016/j.cell.2013.05.037
Burke, K. A., Janke, A. M., Rhine, C. L., and Fawzi, N. L. (2015). Residue-by-Residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol. Cell 60, 231–241. doi:10.1016/j.molcel.2015.09.006
Carra, S., Seguin, S. J., and Landry, J. (2008). HspB8 and Bag3: A new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy 4, 237–239. doi:10.4161/auto.5407
Cenini, G., Rüb, C., Bruderek, M., and Voos, W. (2016). Amyloid β-peptides interfere with mitochondrial preprotein import competence by a coaggregation process. Mol. Biol. Cell 27, 3257–3272. doi:10.1091/mbc.E16-05-0313
Chartier, S., and Duyckaerts, C. (2018). Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity? Cell Tissue Res. 373, 149–160. doi:10.1007/s00441-018-2854-6
Cherkasov, V., Hofmann, S., Druffel-Augustin, S., Mogk, A., Tyedmers, J., Stoecklin, G., et al. (2013). Coordination of translational control and protein homeostasis during severe heat stress. Curr. Biol. CB 23, 2452–2462. doi:10.1016/j.cub.2013.09.058
Choi, S. I., Han, K. S., Kim, C. W., Ryu, K.-S., Kim, B. H., Kim, K.-H., et al. (2008). Protein solubility and folding enhancement by interaction with RNA. PloS One 3, e2677. doi:10.1371/journal.pone.0002677
Coleman, R. A., Mohallem, R., Aryal, U. K., and Trader, D. J. (2021). Protein degradation profile reveals dynamic nature of 20S proteasome small molecule stimulation. RSC Chem. Biol. 2, 636–644. doi:10.1039/D0CB00191K
Corti, O., Blomgren, K., Poletti, A., and Beart, P. M. (2020). Autophagy in neurodegeneration: New insights underpinning therapy for neurological diseases. J. Neurochem. 154, 354–371. doi:10.1111/jnc.15002
Cowan, C., and Mudher, A. (2013). Are tau aggregates toxic or protective in tauopathies? Front. Neurol. 4, 114. doi:10.3389/fneur.2013.00114
Dao, T. P., Kolaitis, R.-M., Kim, H. J., O’Donovan, K., Martyniak, B., Colicino, E., et al. (2018). Ubiquitin modulates liquid-liquid phase separation of UBQLN2 via disruption of multivalent interactions. Mol. Cell 69, 965–978. doi:10.1016/j.molcel.2018.02.004
Darling, A. L., Liu, Y., Oldfield, C. J., and Uversky, V. N. (2018). Intrinsically disordered proteome of human membrane-less organelles. PROTEOMICS 18, 1700193. doi:10.1002/pmic.201700193
Darwich, N. F., Phan, J. M., Kim, B., Suh, E., Papatriantafyllou, J. D., Changolkar, L., et al. (2020). Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau. Science 370, eaay8826. doi:10.1126/science.aay8826
De, S., Whiten, D. R., Ruggeri, F. S., Hughes, C., Rodrigues, M., Sideris, D. I., et al. (2019a). Soluble aggregates present in cerebrospinal fluid change in size and mechanism of toxicity during Alzheimer’s disease progression. Acta Neuropathol. Commun. 7, 120. doi:10.1186/s40478-019-0777-4
De, S., Wirthensohn, D. C., Flagmeier, P., Hughes, C., Aprile, F. A., Ruggeri, F. S., et al. (2019b). Different soluble aggregates of Aβ42 can give rise to cellular toxicity through different mechanisms. Nat. Commun. 10, 1541. doi:10.1038/s41467-019-09477-3
Defenouillère, Q., Yao, Y., Mouaikel, J., Namane, A., Galopier, A., Decourty, L., et al. (2013). Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products. Proc. Natl. Acad. Sci. 110, 5046–5051. doi:10.1073/pnas.1221724110
den Brave, F., Cairo, L. V., Jagadeesan, C., Ruger-Herreros, C., Mogk, A., Bukau, B., et al. (2020). Chaperone-mediated protein disaggregation triggers proteolytic clearance of intra-nuclear protein inclusions. Cell Rep. 31, 107680. doi:10.1016/j.celrep.2020.107680
Dever, T. E., Feng, L., Wek, R. C., Cigan, A. M., Donahue, T. F., and Hinnebusch, A. G. (1992). Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68, 585–596. doi:10.1016/0092-8674(92)90193-g
Deville, C., Carroni, M., Franke, K. B., Topf, M., Bukau, B., Mogk, A., et al. (2017). Structural pathway of regulated substrate transfer and threading through an Hsp100 disaggregase. Sci. Adv. 3, e1701726. doi:10.1126/sciadv.1701726
Díaz-Hernández, M., Valera, A. G., Morán, M. A., Gómez-Ramos, P., Alvarez-Castelao, B., Castaño, J. G., et al. (2006). Inhibition of 26S proteasome activity by huntingtin filaments but not inclusion bodies isolated from mouse and human brain. J. Neurochem. 98, 1585–1596. doi:10.1111/j.1471-4159.2006.03968.x
Duennwald, M. L., Echeverria, A., and Shorter, J. (2012). Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans. PLOS Biol. 10, e1001346. doi:10.1371/journal.pbio.1001346
Emma Mee Hayes Sirvio, L., and Ye, Y. (2022). A potential mechanism for targeting aggregates with proteasomes and disaggregases in liquid droplets. Front. Aging Neurosci. 14, 854380. doi:10.3389/fnagi.2022.854380
Fedyukina, D. V., and Cavagnero, S. (2011). Protein folding at the exit tunnel. Annu. Rev. Biophys. 40, 337–359. doi:10.1146/annurev-biophys-042910-155338
Feleciano, D. R., Juenemann, K., Iburg, M., Brás, I. C., Holmberg, C. I., and Kirstein, J. (2019). Crosstalk between chaperone-mediated protein disaggregation and proteolytic pathways in aging and disease. Front. Aging Neurosci. 11, 9. doi:10.3389/fnagi.2019.00009
Feric, M., and Brangwynne, C. P. (2013). A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat. Cell Biol. 15, 1253–1259. doi:10.1038/ncb2830
Ferrington, D. A., Husom, A. D., and Thompson, L. V. (2005). Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 19, 644–646. doi:10.1096/fj.04-2578fje
Franzmann, T. M., Jahnel, M., Pozniakovsky, A., Mahamid, J., Holehouse, A. S., Nüske, E., et al. (2018). Phase separation of a yeast prion protein promotes cellular fitness. Science 359, eaao5654. doi:10.1126/science.aao5654
Frottin, F., Schueder, F., Tiwary, S., Gupta, R., Körner, R., Schlichthaerle, T., et al. (2019). The nucleolus functions as a phase-separated protein quality control compartment. Science 365, 342–347. doi:10.1126/science.aaw9157
Fu, A., Cohen-Kaplan, V., Avni, N., Livneh, I., and Ciechanover, A. (2021). p62-containing, proteolytically active nuclear condensates, increase the efficiency of the ubiquitin-proteasome system. Proc. Natl. Acad. Sci. U. S. A. 118, e2107321118. doi:10.1073/pnas.2107321118
Furukawa, Y., Vigouroux, S., Wong, H., Guttman, M., Rajput, A. H., Ang, L., et al. (2002). Brain proteasomal function in sporadic Parkinson’s disease and related disorders. Ann. Neurol. 51, 779–782. doi:10.1002/ana.10207
Gallagher, P., Candadai, S., and Gardner, R. (2014). The requirement for Cdc48/p97 in nuclear protein quality control degradation depends on the substrate and correlates with substrate insolubility. J. Cell Sci. 127, 1980–1991. doi:10.1242/jcs.141838
Gallardo, P., Salas-Pino, S., and Daga, R. R. (2021). Reversible protein aggregation as cytoprotective mechanism against heat stress. Curr. Genet. 67, 849–855. doi:10.1007/s00294-021-01191-2
Galves, M., Rathi, R., Prag, G., and Ashkenazi, A. (2019). Ubiquitin signaling and degradation of aggregate-prone proteins. Trends biochem. Sci. 44, 872–884. doi:10.1016/j.tibs.2019.04.007
Gamerdinger, M., Hajieva, P., Kaya, A. M., Wolfrum, U., Hartl, F. U., and Behl, C. (2009). Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 28, 889–901. doi:10.1038/emboj.2009.29
Gamerdinger, M., Kaya, M., Wolfrum, U., Clement, A., and Behl, C. (2011). BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 12, 149–156. doi:10.1038/embor.2010.203
Ganassi, M., Mateju, D., Bigi, I., Mediani, L., Poser, I., Lee, H. O., et al. (2016). A surveillance function of the HSPB8-BAG3-HSP70 chaperone complex ensures stress granule integrity and dynamism. Mol. Cell 63, 796–810. doi:10.1016/j.molcel.2016.07.021
Gao, X., Carroni, M., Nussbaum-Krammer, C., Mogk, A., Nillegoda, N. B., Anna, S., et al. (2015). Human Hsp70 disaggregase reverses Parkinson’s-linked α-synuclein amyloid fibrils. Mol. Cell 59, 781–793. doi:10.1016/j.molcel.2015.07.012
García-Sierra, F., Jarero-Basulto, J. J., Kristofikova, Z., Majer, E., Binder, L. I., and Ripova, D. (2012). Ubiquitin is associated with early truncation of tau protein at aspartic Acid421 during the maturation of neurofibrillary tangles in Alzheimer’s disease. Brain Pathol. 22, 240–250. doi:10.1111/j.1750-3639.2011.00525.x
Gates, S. N., Yokom, A. L., Lin, J., Jackrel, M. E., Rizo, A. N., Kendsersky, N. M., et al. (2017). Ratchet-like polypeptide translocation mechanism of the AAA+ disaggregase Hsp104. Science 357, 273–279. doi:10.1126/science.aan1052
Gersing, S. K., Wang, Y., Grønbæk-Thygesen, M., Kampmeyer, C., Clausen, L., Willemoës, M., et al. (2021). Mapping the degradation pathway of a disease-linked aspartoacylase variant. PLoS Genet. 17, e1009539. doi:10.1371/journal.pgen.1009539
Glover, J. R., and Lindquist, S. (1998). Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 94, 73–82. doi:10.1016/S0092-8674(00)81223-4
Goggin, K., Beaudoin, S., Grenier, C., Brown, A.-A., and Roucou, X. (2008). Prion protein aggresomes are poly(A)+ ribonucleoprotein complexes that induce a PKR-mediated deficient cell stress response. Biochim. Biophys. Acta BBA - Mol. Cell Res. 1783, 479–491. doi:10.1016/j.bbamcr.2007.10.008
Goloubinoff, P., Mogk, A., Zvi, A. P. B., Tomoyasu, T., and Bukau, B. (1999). Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc. Natl. Acad. Sci. U. S. A. 96, 13732–13737. doi:10.1073/pnas.96.24.13732
Gowda, N. K. C., Kaimal, J. M., Kityk, R., Daniel, C., Liebau, J., Öhman, M., et al. (2018). Nucleotide exchange factors Fes1 and HspBP1 mimic substrate to release misfolded proteins from Hsp70. Nat. Struct. Mol. Biol. 25, 83–89. doi:10.1038/s41594-017-0008-2
Grima, J. C., Daigle, J. G., Arbez, N., Cunningham, K. C., Zhang, K., Ochaba, J., et al. (2017). Mutant huntingtin disrupts the nuclear pore complex. Neuron 94, 93–107. doi:10.1016/j.neuron.2017.03.023
Gu, Z. C., Wu, E., Sailer, C., Jando, J., Styles, E., Eisenkolb, I., et al. (2017). Ubiquitin orchestrates proteasome dynamics between proliferation and quiescence in yeast. Mol. Biol. Cell 28, 2479–2491. doi:10.1091/mbc.e17-03-0162
Gueroussov, S., Weatheritt, R. J., O’Hanlon, D., Lin, Z.-Y., Narula, A., Gingras, A.-C., et al. (2017). Regulatory expansion in mammals of multivalent hnRNP assemblies that globally control alternative splicing. Cell 170, 324–339. doi:10.1016/j.cell.2017.06.037
Guillén-Boixet, J., Kopach, A., Holehouse, A. S., Wittmann, S., Jahnel, M., Schlüßler, R., et al. (2020). RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181, 346–361. doi:10.1016/j.cell.2020.03.049
Hageman, J., Vos, M. J., van Waarde, M. A. W. H., and Kampinga, H. H. (2007). Comparison of intra-organellar chaperone capacity for dealing with stress-induced protein unfolding. J. Biol. Chem. 282, 34334–34345. doi:10.1074/jbc.M703876200
Han, Y., David, A., Liu, B., Magadán, J. G., Bennink, J. R., Yewdell, J. W., et al. (2012). Monitoring cotranslational protein folding in mammalian cells at codon resolution. Proc. Natl. Acad. Sci. 109, 12467–12472. doi:10.1073/pnas.1208138109
Hantouche, C., Williamson, B., Valinsky, W. C., Solomon, J., Shrier, A., and Young, J. C. (2017). Bag1 Co-chaperone promotes TRC8 E3 ligase-dependent degradation of misfolded human ether a go-go-related gene (hERG) potassium channels. J. Biol. Chem. 292, 2287–2300. doi:10.1074/jbc.M116.752618
Hara, T., Nakamura, K., Matsui, M., Yamamoto, A., Nakahara, Y., Suzuki-Migishima, R., et al. (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889. doi:10.1038/nature04724
Harding, H. P., Zhang, Y., Zeng, H., Novoa, I., Lu, P. D., Calfon, M., et al. (2003). An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633. doi:10.1016/s1097-2765(03)00105-9
Harmon, T. S., Holehouse, A. S., Rosen, M. K., and Pappu, R. V. (2017). Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife 6, e30294. doi:10.7554/elife.30294
Haslbeck, M., Weinkauf, S., and Buchner, J. (2019). Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 294, 2121–2132. doi:10.1074/jbc.REV118.002809
Hayes, M. H., Peuchen, E. H., Dovichi, N. J., and Weeks, D. L. (2018). Dual roles for ATP in the regulation of phase separated protein aggregates in Xenopus oocyte nucleoli. eLife 7, e35224. doi:10.7554/eLife.35224
Hegyi, H., and Tompa, P. (2008). Intrinsically disordered proteins display no preference for chaperone binding in vivo. PLoS Comput. Biol. 4, e1000017. doi:10.1371/journal.pcbi.1000017
Hernández-Vega, A., Braun, M., Scharrel, L., Jahnel, M., Wegmann, S., Hyman, B. T., et al. (2017). Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Rep. 20, 2304–2312. doi:10.1016/j.celrep.2017.08.042
Hipp, M. S., Kasturi, P., and Hartl, F. U. (2019). The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435. doi:10.1038/s41580-019-0101-y
Hirabayashi, M., Inoue, K., Tanaka, K., Nakadate, K., Ohsawa, Y., Kamei, Y., et al. (2001). VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 8, 977–984. doi:10.1038/sj.cdd.4400907
Hoeller, D., Hecker, C.-M., Wagner, S., Rogov, V., Dötsch, V., and Dikic, I. (2007). E3-Independent monoubiquitination of ubiquitin-binding proteins. Mol. Cell 26, 891–898. doi:10.1016/j.molcel.2007.05.014
Hong, S. W., and Vierling, E. (2001). Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant J. Cell Mol. Biol. 27, 25–35. doi:10.1046/j.1365-313x.2001.01066.x
Hosp, F., Gutiérrez-Ángel, S., Schaefer, M. H., Cox, J., Meissner, F., Hipp, M. S., et al. (2017). Spatiotemporal proteomic profiling of Huntington’s disease inclusions reveals widespread loss of protein function. Cell Rep. 21, 2291–2303. doi:10.1016/j.celrep.2017.10.097
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. doi:10.1038/s41582-019-0244-7
Hyun, D.-H., Lee, M., Halliwell, B., and Jenner, P. (2003). Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J. Neurochem. 86, 363–373. doi:10.1046/j.1471-4159.2003.01841.x
Ilık, İ. A., and Aktaş, T. (2022). Nuclear speckles: Dynamic hubs of gene expression regulation. FEBS J. 289, 7234–7245. doi:10.1111/febs.16117
Iserman, C., Desroches Altamirano, C., Jegers, C., Friedrich, U., Zarin, T., Fritsch, A. W., et al. (2020). Condensation of Ded1p promotes a translational switch from housekeeping to stress protein production. Cell 181, 818–831. doi:10.1016/j.cell.2020.04.009
Jawed, A., Ho, C.-T., Grousl, T., Shrivastava, A., Ruppert, T., Bukau, B., et al. (2022). Balanced activities of Hsp70 and the ubiquitin proteasome system underlie cellular protein homeostasis. Front. Mol. Biosci. 9, 1106477. doi:10.3389/fmolb.2022.1106477
Johnson, J. O., Mandrioli, J., Benatar, M., Abramzon, Y., Van Deerlin, V. M., Trojanowski, J. Q., et al. (2010). Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68, 857–864. doi:10.1016/j.neuron.2010.11.036
Jousse, C., Oyadomari, S., Novoa, I., Lu, P., Zhang, Y., Harding, H. P., et al. (2003). Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 163, 767–775. doi:10.1083/jcb.200308075
Joutsen, J., and Sistonen, L. (2019). Tailoring of proteostasis networks with heat shock factors. Cold Spring Harb. Perspect. Biol. 11, a034066. doi:10.1101/cshperspect.a034066
Kaimal, J. M., Kandasamy, G., Gasser, F., and Andréasson, C. (2017). Coordinated Hsp110 and Hsp104 activities power protein disaggregation in Saccharomyces cerevisiae. Mol. Cell. Biol. 37, 000277–e117. doi:10.1128/MCB.00027-17
Kalia, L., Kalia, S., Chau, H., Lozano, A., Hyman, B., and McLean, P. (2011). Ubiquitinylation of α-synuclein by carboxyl terminus hsp70-interacting protein (CHIP) is regulated by bcl-2-associated athanogene 5 (BAG5). PloS One 6, e14695. doi:10.1371/journal.pone.0014695
Kandasamy, G., and Andréasson, C. (2018). Hsp70-Hsp110 chaperones deliver ubiquitin-dependent and -independent substrates to the 26S proteasome for proteolysis in yeast. J. Cell Sci. 131, jcs210948. doi:10.1242/jcs.210948
Kaushik, S., and Cuervo, A. M. (2018). The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381. doi:10.1038/s41580-018-0001-6
Kedersha, N. L., Gupta, M., Li, W., Miller, I., and Anderson, P. (1999). RNA-binding proteins tia-1 and tiar link the phosphorylation of eif-2α to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442. doi:10.1083/jcb.147.7.1431
Kedersha, N., Panas, M. D., Achorn, C. A., Lyons, S., Tisdale, S., Hickman, T., et al. (2016). G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J. Cell Biol. 212, 845–860. doi:10.1083/jcb.201508028
Keller, J. N., Hanni, K. B., and Markesbery, W. R. (2000). Impaired proteasome function in Alzheimer’s disease. J. Neurochem. 75, 436–439. doi:10.1046/j.1471-4159.2000.0750436.x
Kioka, H, H., H., Yohei, M., Shunsuke, N., Kato, K, M., Osamu, T., Yuki, K., et al. (2022). Loss-of-function mutations in the co-chaperone protein BAG5 cause dilated cardiomyopathy requiring heart transplantation. Sci. Transl. Med. 14, eabf3274. doi:10.1126/scitranslmed.abf3274
Kirstein, J., Arnsburg, K., Scior, A., Anna, S., Szlachcic, A., Guilbride, D. L., et al. (2017). In vivo properties of the disaggregase function of J-proteins and Hsc70 in Caenorhabditis elegans stress and aging. Aging Cell 16, 1414–1424. doi:10.1111/acel.12686
Klosin, A., Oltsch, F., Harmon, T., Honigmann, A., Jülicher, F., Hyman, A. A., et al. (2020). Phase separation provides a mechanism to reduce noise in cells. Science 367, 464–468. doi:10.1126/science.aav6691
Kmiecik, S. W., Le Breton, L., and Mayer, M. P. (2020). Feedback regulation of heat shock factor 1 (Hsf1) activity by Hsp70-mediated trimer unzipping and dissociation from DNA. EMBO J. 39, e104096. doi:10.15252/embj.2019104096
Kobayashi, T., Manno, A., and Kakizuka, A. (2007). Involvement of valosin-containing protein (VCP)/p97 in the formation and clearance of abnormal protein aggregates. Genes cells. 12, 889–901. doi:10.1111/j.1365-2443.2007.01099.x
Kohler, V., and Andréasson, C. (2020). Hsp70-mediated quality control: should I stay or should I go? Biol. Chem. 401, 1233–1248. doi:10.1515/hsz-2020-0187
Komar, A. A. (2009). A pause for thought along the co-translational folding pathway. Trends biochem. Sci. 34, 16–24. doi:10.1016/j.tibs.2008.10.002
Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J., Tanida, I., et al. (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884. doi:10.1038/nature04723
Kraft, C., Reggiori, F., and Peter, M. (2009). Selective types of autophagy in yeast. Biochim. Biophys. Acta - Mol. Cell Res. 1793, 1404–1412. doi:10.1016/j.bbamcr.2009.02.006
Kramer, G., Boehringer, D., Ban, N., and Bukau, B. (2009). The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat. Struct. Mol. Biol. 16, 589–597. doi:10.1038/nsmb.1614
Kramer, R. M., Shende, V. R., Motl, N., Pace, C. N., and Scholtz, J. M. (2012). Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. Biophys. J. 102, 1907–1915. doi:10.1016/j.bpj.2012.01.060
Kroschwald, S., Maharana, S., Mateju, D., Malinovska, L., Nüske, E., Poser, I., et al. (2015). Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife 4, e06807. doi:10.7554/eLife.06807
Kroschwald, S., Munder, M. C., Maharana, S., Franzmann, T. M., Richter, D., Ruer, M., et al. (2018). Different material states of Pub1 condensates define distinct modes of stress adaptation and recovery. Cell Rep. 23, 3327–3339. doi:10.1016/j.celrep.2018.05.041
Kwon, S., Zhang, Y., and Matthias, P. (2007). The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 21, 3381–3394. doi:10.1101/gad.461107
Laussmann, M. A., Passante, E., Düssmann, H., Rauen, J. A., Würstle, M. L., Delgado, M. E., et al. (2011). Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 18, 1584–1597. doi:10.1038/cdd.2011.27
Lee, C.-K., Klopp, R. G., Weindruch, R., and Prolla, T. A. (1999). Gene expression profile of aging and its retardation by caloric restriction. Science 285, 1390–1393. doi:10.1126/science.285.5432.1390
Lee, C. K., Weindruch, R., and Prolla, T. A. (2000). Gene-expression profile of the ageing brain in mice. Nat. Genet. 25, 294–297. doi:10.1038/77046
Lee, K.-H., Zhang, P., Kim, H. J., Mitrea, D. M., Sarkar, M., Freibaum, B. D., et al. (2016). C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles. Cell 167, 774–788. doi:10.1016/j.cell.2016.10.002
Lehmann, A., Janek, K., Braun, B., Kloetzel, P.-M., and Enenkel, C. (2002). 20 S proteasomes are imported as precursor complexes into the nucleus of yeast. Huber. J. Mol. Biol. 317, 401–413. doi:10.1006/jmbi.2002.5443
Li, C., Wang, X., Li, X., Qiu, K., Jiao, F., Liu, Y., et al. (2019). Proteasome inhibition activates autophagy-lysosome pathway associated with TFEB dephosphorylation and nuclear translocation. Front. Cell Dev. Biol. 7, 170. doi:10.3389/fcell.2019.00170
Li, P., Banjade, S., Cheng, H.-C., Kim, S., Chen, B., Guo, L., et al. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340. doi:10.1038/nature10879
Lin, Y., Lin, Y., Protter, D. S. W., Rosen, M. K., and Parker, R. (2015). formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219. doi:10.1016/j.molcel.2015.08.018
Lindersson, E., Beedholm, R., Højrup, P., Moos, T., Gai, W., Hendil, K. B., et al. (2004). Proteasomal inhibition by α-synuclein filaments and oligomers. J. Biol. Chem. 279, 12924–12934. doi:10.1074/jbc.M306390200
Lobanova, E., Whiten, D., Ruggeri, F. S., Taylor, C. G., Kouli, A., Xia, Z., et al. (2022). Imaging protein aggregates in the serum and cerebrospinal fluid in Parkinson’s disease. Brain 145, 632–643. doi:10.1093/brain/awab306
Lu, P. D., Harding, H. P., and Ron, D. (2004). Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33. doi:10.1083/jcb.200408003
Lu, Y., Wu, J., Dong, Y., Chen, S., Sun, S., Ma, Y.-B., et al. (2017). Conformational landscape of the p28-bound human proteasome regulatory particle. Mol. Cell 67, 322–333. doi:10.1016/j.molcel.2017.06.007
Lüders, J., Demand, J., and Höhfeld, J. (2000). The ubiquitin-related BAG-1 provides a link between the molecular chaperones hsc70/hsp70 and the proteasome. J. Biol. Chem. 275, 4613–4617. doi:10.1074/jbc.275.7.4613
Ly, D. H., Lockhart, D. J., Lerner, R. A., and Schultz, P. G. (2000). Mitotic misregulation and human aging. Science 287, 2486–2492. doi:10.1126/science.287.5462.2486
Määttä, T. A., Rettel, M., Sridharan, S., Helm, D., Kurzawa, N., Stein, F., et al. (2020). Aggregation and disaggregation features of the human proteome. Mol. Syst. Biol. 16, e9500. doi:10.15252/msb.20209500
Macario, A. J. L., Grippo, T. M., and de Macario, E. C. (2005). Genetic disorders involving molecular-chaperone genes: A perspective. Genet. Med. 7, 3–12. doi:10.1097/01.GIM.0000151351.11876.C3
Mackenzie, I. R., Nicholson, A. M., Sarkar, M., Messing, J., Purice, M. D., Pottier, C., et al. (2017). TIA1 mutations in amyotrophic lateral Sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95, 808–816. doi:10.1016/j.neuron.2017.07.025
Maharana, S., Wang, J., Papadopoulos, D. K., Richter, D., Pozniakovsky, A., Poser, I., et al. (2018). RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 360, 918–921. doi:10.1126/science.aar7366
Majumdar, A., Dogra, P., Maity, S., and Mukhopadhyay, S. (2019). Liquid-liquid phase separation is driven by large-scale conformational unwinding and fluctuations of intrinsically disordered protein molecules. J. Phys. Chem. Lett. 10, 3929–3936. doi:10.1021/acs.jpclett.9b01731
Malinovska, L., Kroschwald, S., Munder, M. C., Richter, D., and Alberti, S. (2012). Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol. Biol. Cell 23, 3041–3056. doi:10.1091/mbc.E12-03-0194
Marshall, R. S., McLoughlin, F., and Vierstra, R. D. (2016). Autophagic turnover of inactive 26S proteasomes in yeast is directed by the ubiquitin receptor Cue5 and the Hsp42 chaperone. Cell Rep. 16, 1717–1732. doi:10.1016/j.celrep.2016.07.015
Masser, A. E., Ciccarelli, M., and Andréasson, C. (2020). Hsf1 on a leash - controlling the heat shock response by chaperone titration. Exp. Cell Res. 396, 112246. doi:10.1016/j.yexcr.2020.112246
Mateju, D., Franzmann, T. M., Patel, A., Kopach, A., Boczek, E. E., Maharana, S., et al. (2017). An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 36, 1669–1687. doi:10.15252/embj.201695957
Mateus, A., Bobonis, J., Kurzawa, N., Stein, F., Helm, D., Hevler, J., et al. (2018). Thermal proteome profiling in bacteria: Probing protein state in vivo. Mol. Syst. Biol. 14, e8242. doi:10.15252/msb.20188242
Mattoo, R. U. H., Sharma, S. K., Priya, S., Finka, A., and Goloubinoff, P. (2013). Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J. Biol. Chem. 288, 21399–21411. doi:10.1074/jbc.m113.479253
McNaught, K. St.P., Belizaire, R., Jenner, P., Olanow, C. W., and Isacson, O. (2002). Selective loss of 20S proteasome α-subunits in the substantia nigra pars compacta in Parkinson’s disease. Neurosci. Lett. 326, 155–158. doi:10.1016/S0304-3940(02)00296-3
Meacham, G. C., Patterson, C., Zhang, W., Younger, J. M., and Cyr, D. M. (2001). The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat. Cell Biol. 3, 100–105. doi:10.1038/35050509
Mediani, L., Guillén-Boixet, J., Vinet, J., Franzmann, T. M., Bigi, I., Mateju, D., et al. (2019). Defective ribosomal products challenge nuclear function by impairing nuclear condensate dynamics and immobilizing ubiquitin. EMBO J. 38, e101341. doi:10.15252/embj.2018101341
Medicherla, B., and Goldberg, A. L. (2008). Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J. Cell Biol. 182, 663–673. doi:10.1083/jcb.200803022
Mehta, S., and Zhang, J. (2022). Liquid–liquid phase separation drives cellular function and dysfunction in cancer. Nat. Rev. Cancer 22, 239–252. doi:10.1038/s41568-022-00444-7
Melentijevic, I., Toth, M. L., Arnold, M. L., Guasp, R. J., Harinath, G., Nguyen, K. C., et al. (2017). C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542, 367–371. doi:10.1038/nature21362
Meng, J. X., Zhang, Y., Saman, D., Haider, A. M., De, S., Sang, J. C., et al. (2022). Hyperphosphorylated tau self-assembles into amorphous aggregates eliciting TLR4-dependent responses. Nat. Commun. 13, 2692. doi:10.1038/s41467-022-30461-x
Meyer, H., and Weihl, C. C. (2014). The VCP/p97 system at a glance: Connecting cellular function to disease pathogenesis. J. Cell Sci. 127, 3877–3883. doi:10.1242/jcs.093831
Midic, U., Oldfield, C. J., Dunker, A. K., Obradovic, Z., and Uversky, V. N. (2009). Protein disorder in the human diseasome: Unfoldomics of human genetic diseases. BMC Genomics 10, S12. doi:10.1186/1471-2164-10-S1-S12
Minoia, M., Boncoraglio, A., Vinet, J., Morelli, F. F., Brunsting, J. F., Poletti, A., et al. (2014). BAG3 induces the sequestration of proteasomal clients into cytoplasmic puncta: Implications for a proteasome-to-autophagy switch. Autophagy 10, 1603–1621. doi:10.4161/auto.29409
Mogk, A., Mogk, A., Bukau, B., and Kampinga, H. H. (2018). Cellular handling of protein aggregates by disaggregation machines. Mol. Cell 69, 214–226. doi:10.1016/j.molcel.2018.01.004
Mogk, A., Ruger-Herreros, C., and Bukau, B. (2019). Cellular functions and mechanisms of action of small heat shock proteins. Annu. Rev. Microbiol. 73, 89–110. doi:10.1146/annurev-micro-020518-115515
Mogk, A., Tomoyasu, T., Goloubinoff, P., Rüdiger, S., Röder, D., Langen, H., et al. (1999). Identification of thermolabile Escherichia coli proteins: Prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18, 6934–6949. doi:10.1093/emboj/18.24.6934
Molliex, A., Temirov, J., Lee, J., Coughlin, M., Kanagaraj, A. P., Kim, H. J., et al. (2015). Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. doi:10.1016/j.cell.2015.09.015
Mosser, D. D., Ho, S., and Glover, J. R. (2004). Saccharomyces cerevisiae Hsp104 enhances the chaperone capacity of human cells and inhibits heat stress-induced proapoptotic signaling. Biochemistry 43, 8107–8115. doi:10.1021/bi0493766
Munder, M. C., Midtvedt, D., Franzmann, T., Nüske, E., Otto, O., Herbig, M., et al. (2016). A pH-driven transition of the cytoplasm from a fluid-to a solid-like state promotes entry into dormancy. eLife 5, e09347. doi:10.7554/eLife.09347
Murakami, T., Qamar, S., Lin, J. Q., Schierle, G. S. K., Rees, E., Miyashita, A., et al. (2015). ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88, 678–690. doi:10.1016/j.neuron.2015.10.030
Murata, S., Minami, Y., Minami, M., Chiba, T., and Tanaka, K. (2001). CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2, 1133–1138. doi:10.1093/embo-reports/kve246
Murray, D. T., Kato, M., Lin, Y., Thurber, K. R., Hung, I., McKnight, S. L., et al. (2017). Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 171, 615–627. doi:10.1016/j.cell.2017.08.048
Murthy, A. C., Dignon, G. L., Kan, Y., Zerze, G. H., Parekh, S. H., Mittal, J., et al. (2019). Molecular interactions underlying liquid-liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol. 26, 637–648. doi:10.1038/s41594-019-0250-x
Myeku, N., Clelland, C. L., Emrani, S., Kukushkin, N. V., Wai Haung Yu, Yu, W. H., Yu, W. H., et al. (2016). Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat. Med. 22, 46–53. doi:10.1038/nm.4011
Narhi, L. O., Schmit, J., Bechtold-Peters, K., and Sharma, D. (2012). Classification of protein aggregates. J. Pharm. Sci. 101, 493–498. doi:10.1002/jps.22790
Nemec, A. A., Howell, L. A., Peterson, A. K., Murray, M. A., and Tomko, R. J. (2017). Autophagic clearance of proteasomes in yeast requires the conserved sorting nexin Snx4. J. Biol. Chem. 292, 21466–21480. doi:10.1074/jbc.M117.817999
Nillegoda, N. B., and Bukau, B. (2015). Metazoan hsp70-based protein disaggregases: Emergence and mechanisms. Front. Mol. Biosci. 2, 57. doi:10.3389/fmolb.2015.00057
Nillegoda, N. B., Kirstein, J., Anna, S., Szlachcic, A., Berynskyy, M., Stank, A., et al. (2015). Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524, 247–251. doi:10.1038/nature14884
Nillegoda, N. B., Stank, A., Malinverni, D., Alberts, N., Szlachcic, A., Barducci, A., et al. (2017). Evolution of an intricate J-protein network driving protein disaggregation in eukaryotes. eLife 6, e24560. doi:10.7554/eLife.24560
Nillegoda, N. B., Wentink, A. S., and Bukau, B. (2018). Protein disaggregation in multicellular organisms. Trends biochem. Sci. 43, 285–300. doi:10.1016/j.tibs.2018.02.003
Nishikori, S., Yamanaka, K., Sakurai, T., Esaki, M., and Ogura, T. (2008). p97 Homologs from Caenorhabditis elegans, CDC-48.1 and CDC-48.2, suppress the aggregate formation of huntingtin exon1 containing expanded polyQ repeat. Genes Cells Devoted Mol. Cell. Mech. 13, 827–838. doi:10.1111/j.1365-2443.2008.01214.x
Nollen, E. A. A., Salomons, F. A., Brunsting, J. F., Van Der Want, J. J. L., Sibon, O. C. M., and Kampinga, H. H. (2001). Dynamic changes in the localization of thermally unfolded nuclear proteins associated with chaperone-dependent protection. Proc. Natl. Acad. Sci. U. S. A. 98, 12038–12043. doi:10.1073/pnas.201112398
Nonaka, T., Masuda-Suzukake, M., Hosokawa, M., Shimozawa, A., Hirai, S., Okado, H., et al. (2018). C9ORF72 dipeptide repeat poly-GA inclusions promote intracellular aggregation of phosphorylated TDP-43. Hum. Mol. Genet. 27, 2658–2670. doi:10.1093/hmg/ddy174
Nott, T. J., Nott, T. J., Petsalaki, E., Farber, P., Jervis, D., Fussner, E., et al. (2015). Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936–947. doi:10.1016/j.molcel.2015.01.013
Oh, E., Becker, A. H., Sandikci, A., Huber, D., Chaba, R., Gloge, F., et al. (2011). Selective ribosome profiling reveals the cotranslational chaperone action of trigger factor in vivo. Cell 147, 1295–1308. doi:10.1016/j.cell.2011.10.044
Olzscha, H., Schermann, S. M., Woerner, A. C., Pinkert, S., Hecht, M. H., Tartaglia, G. G., et al. (2011). Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144, 67–78. doi:10.1016/j.cell.2010.11.050
Pandey, U., Nie, Z., Batlevi, Y., Mccray, B., Ritson, G., Nedelsky, N., et al. (2007). HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863. doi:10.1038/nature05853
Pankiv, S., Clausen, T. H., Lamark, T., Brech, A., Bruun, J.-A., Outzen, H., et al. (2007). p62/SQSTM1 binds directly to atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145. doi:10.1074/jbc.M702824200
Parsell, D. A., Kowal, A. S., Singer, M. A., and Lindquist, S. (1994). Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372, 475–478. doi:10.1038/372475a0
Patel, A., Lee, H. O., Jawerth, L., Maharana, S., Jahnel, M., Hein, M. Y., et al. (2015). A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077. doi:10.1016/j.cell.2015.07.047
Patel, A., Malinovska, L., Saha, S., Wang, J., Alberti, S., Krishnan, Y., et al. (2017). ATP as a biological hydrotrope. Science 356, 753–756. doi:10.1126/science.aaf6846
Petropoulos, I., Conconi, M., Wang, X., Hoenel, B., Brégégère, F., Milner, Y., et al. (2000). Increase of oxidatively modified protein is associated with a decrease of proteasome activity and content in aging epidermal cells. J. Gerontol. Ser. A 55, B220–B227. doi:10.1093/gerona/55.5.B220
Phair, R. D., and Misteli, T. (2000). High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609. doi:10.1038/35007077
Poepsel, S., Sprengel, A., Sacca, B., Kaschani, F., Kaiser, M., Gatsogiannis, C., et al. (2015). Determinants of amyloid fibril degradation by the PDZ protease HTRA1. Nat. Chem. Biol. 11, 862–869. doi:10.1038/nchembio.1931
Pohl, C., and Dikic, I. (2019). Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 366, 818–822. doi:10.1126/science.aax3769
Qamar, S., Wang, G., Randle, S. J., Ruggeri, F. S., Varela, J. A., Lin, J. Q., et al. (2018). FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell 173, 720–734. doi:10.1016/j.cell.2018.03.056
Qian, S.-B., McDonough, H., Boellmann, F., Cyr, D. M., and Patterson, C. (2006). CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature 440, 551–555. doi:10.1038/nature04600
Raabe, D., Willbold, D., Willbold, D., Strodel, B., Schröder, G. F., Hoyer, W., et al. (2021). Amyloid-type protein aggregation and prion-like properties of amyloids. Chem. Rev. 121, 8285–8307. doi:10.1021/acs.chemrev.1c00196
Rampelt, H., Kirstein-Miles, J., Nillegoda, N. B., Chi, K., Scholz, S. R., Morimoto, R. I., et al. (2012). Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 31, 4221–4235. doi:10.1038/emboj.2012.264
Rana, T., Behl, T., Sehgal, A., Mehta, V., Singh, S., Bhatia, S., et al. (2021). Exploring the role of autophagy dysfunction in neurodegenerative disorders. Mol. Neurobiol. 58, 4886–4905. doi:10.1007/s12035-021-02472-0
Rapino, F., Jung, M., and Fulda, S. (2014). BAG3 induction is required to mitigate proteotoxicity via selective autophagy following inhibition of constitutive protein degradation pathways. Oncogene 33, 1713–1724. doi:10.1038/onc.2013.110
Ratajczak, T., Carrello, A., Mark, P. J., Warner, B. J., Simpson, R. J., Moritz, R. L., et al. (1993). The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59). J. Biol. Chem. 268, 13187–13192. doi:10.1016/S0021-9258(19)38636-3
Rauch, J. N., Zuiderweg, E. R. P., and Gestwicki, J. E. (2016). Non-canonical interactions between heat shock cognate protein 70 (Hsc70) and bcl2-associated anthanogene (BAG) Co-chaperones are important for client release. J. Biol. Chem. 291, 19848–19857. doi:10.1074/jbc.M116.742502
Ray, S., Sandipan, R., Shashikant, R., Singh, N., Singh, N., Kumar, R., et al. (2020). α-Synuclein aggregation nucleates through liquid-liquid phase separation. Nat. Chem. 12, 705–716. doi:10.1038/s41557-020-0465-9
Riback, J. A., Katanski, C. D., Kear-Scott, J. L., Pilipenko, E. V., Rojek, A. E., Sosnick, T. R., et al. (2017). Stress-Triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168, 1028–1040. doi:10.1016/j.cell.2017.02.027
Riggs, C. L., Kedersha, N., Ivanov, P., and Anderson, P. (2020). Mammalian stress granules and P bodies at a glance. J. Cell Sci. 133, jcs242487. doi:10.1242/jcs.242487
Rosam, M., Krader, D., Nickels, C., Hochmair, J., Back, K. C., Agam, G., et al. (2018). Bap (Sil1) regulates the molecular chaperone BiP by coupling release of nucleotide and substrate. Nat. Struct. Mol. Biol. 25, 90–100. doi:10.1038/s41594-017-0012-6
Rosenzweig, R., Nillegoda, N. B., Mayer, M. P., and Bukau, B. (2019). The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 20, 665–680. doi:10.1038/s41580-019-0133-3
Runwal, G., Stamatakou, E., Siddiqi, F. H., Puri, C., Zhu, Y., and Rubinsztein, D. C. (2019). LC3-positive structures are prominent in autophagy-deficient cells. Sci. Rep. 9, 10147. doi:10.1038/s41598-019-46657-z
Ryan, V. H., Dignon, G. L., Zerze, G. H., Chabata, C. V., Silva, R., Conicella, A. E., et al. (2018). Mechanistic view of hnRNPA2 low-complexity domain structure, interactions, and phase separation altered by mutation and arginine methylation. Mol. Cell 69, 465–479. doi:10.1016/j.molcel.2017.12.022
Saad, S., Cereghetti, G., Feng, Y., Picotti, P., Peter, M., and Dechant, R. (2017). Reversible protein aggregation is a protective mechanism to ensure cell cycle restart after stress. Nat. Cell Biol. 19, 1202–1213. doi:10.1038/ncb3600
Saha, S., Panigrahi, D. P., Patil, S., and Bhutia, S. K. (2018). Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 104, 485–495. doi:10.1016/j.biopha.2018.05.007
Saha, S., Weber, C. A., Nousch, M., Adame-Arana, O., Hoege, C., Hein, M. Y., et al. (2016). Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166, 1572–1584. doi:10.1016/j.cell.2016.08.006
Sanchez, Y., and Lindquist, S. L. (1990). HSP104 required for induced thermotolerance. Science 248, 1112–1115. doi:10.1126/science.2188365
Schubert, U., Antón, L. C., Gibbs, J., Norbury, C. C., Yewdell, J. W., and Bennink, J. R. (2000). Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404, 770–774. doi:10.1038/35008096
Schweighauser, M., Shi, Y., Tarutani, A., Kametani, F., Murzin, A. G., Ghetti, B., et al. (2020). Structures of α-synuclein filaments from multiple system atrophy. Nature 585, 464–469. doi:10.1038/s41586-020-2317-6
Seibenhener, M. L., Babu, J. R., Geetha, T., Wong, H. C., Krishna, N. R., and Wooten, M. W. (2004). Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol. Cell. Biol. 24, 8055–8068. doi:10.1128/MCB.24.18.8055-8068.2004
Selimovic, D., Porzig, B. B. O. W., El-Khattouti, A., Badura, H. E., Ahmad, M., Ghanjati, F., et al. (2013). Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell. Signal. 25, 308–318. doi:10.1016/j.cellsig.2012.10.004
Seo, H., Sonntag, K.-C., and Isacson, O. (2004). Generalized brain and skin proteasome inhibition in Huntington’s disease. Ann. Neurol. 56, 319–328. doi:10.1002/ana.20207
Serio, T. R., Cashikar, A. G., Kowal, A. S., Sawicki, G. J., Moslehi, J. J., Serpell, L., et al. (2000). Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science 289, 1317–1321. doi:10.1126/science.289.5483.1317
Sheth, U., and Parker, R. (2003). Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300, 805–808. doi:10.1126/science.1082320
Shin, Y., Chang, Y.-C., Lee, D. S. W., Berry, J., Sanders, D. W., Ronceray, P., et al. (2018). Liquid nuclear condensates mechanically sense and restructure the genome. Cell 175, 1481–1491. doi:10.1016/j.cell.2018.10.057
Shorter, J., and Southworth, D. R. (2019). Spiraling in control: Structures and mechanisms of the Hsp104 disaggregase. Cold Spring Harb. Perspect. Biol. 11, a034033. doi:10.1101/cshperspect.a034033
Shorter, J. (2011). The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLOS ONE 6, e26319. doi:10.1371/journal.pone.0026319
Snead, Wilton T., Snead, W. T., and Gladfelter, A. S. (2019). The control centers of biomolecular phase separation: How membrane surfaces, PTMs, and active processes regulate condensation. Mol. Cell 76, 295–305. doi:10.1016/j.molcel.2019.09.016
Specht, S., Miller, S. B. M., Mogk, A., and Bukau, B. (2011). Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J. Cell Biol. 195, 617–629. doi:10.1083/jcb.201106037
Stein, K. C., Morales-Polanco, F., van der Lienden, J., Rainbolt, T. K., and Frydman, J. (2022). Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis. Nature 601, 637–642. doi:10.1038/s41586-021-04295-4
Stephens, A. D., Zacharopoulou, M., and Kaminski Schierle, G. S. (2019). The cellular environment affects monomeric α-synuclein structure. Trends biochem. Sci. 44, 453–466. doi:10.1016/j.tibs.2018.11.005
Su, X., Ditlev, J. A., Hui, E., Xing, W., Banjade, S., Okrut, J., et al. (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599. doi:10.1126/science.aad9964
Sun, D., Rongbo, W., Li, P., and Yu, L. (2020). Phase separation in regulation of aggrephagy. J. Mol. Biol. 432, 160–169. doi:10.1016/j.jmb.2019.06.026
Sun, D., Wu, R., Zheng, J., Li, P., and Yu, L. (2018). Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 28, 405–415. doi:10.1038/s41422-018-0017-7
Sun-Wang, J. L., Ivanova, S., and Zorzano, A. (2020). The dialogue between the ubiquitin-proteasome system and autophagy: Implications in ageing. Ageing Res. Rev. 64, 101203. doi:10.1016/j.arr.2020.101203
Tennstaedt, A., Pöpsel, S., Truebestein, L., Hauske, P., Brockmann, A., Schmidt, N., et al. (2012). Human high temperature requirement serine protease A1 (HTRA1) degrades tau protein aggregates. J. Biol. Chem. 287, 20931–20941. doi:10.1074/jbc.M111.316232
Tessarz, P., Mogk, A., and Bukau, B. (2008). Substrate threading through the central pore of the Hsp104 chaperone as a common mechanism for protein disaggregation and prion propagation. Mol. Microbiol. 68, 87–97. doi:10.1111/j.1365-2958.2008.06135.x
Tetzlaff, J. E., Putcha, P., Outeiro, T. F., Ivanov, A., Berezovska, O., Hyman, B. T., et al. (2008). CHIP targets toxic α-synuclein oligomers for degradation. J. Biol. Chem. 283, 17962–17968. doi:10.1074/jbc.M802283200
Thibaudeau, T. A., Anderson, R. T., and Smith, D. M. (2018). A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat. Commun. 9, 1097. doi:10.1038/s41467-018-03509-0
Tittelmeier, J., Nachman, E., and Nussbaum-Krammer, C. (2020). Molecular chaperones: A double-edged sword in neurodegenerative diseases. Front. Aging Neurosci. 12, 581374. doi:10.3389/fnagi.2020.581374
Tomita, T., Hirayama, S., Sakurai, Y., Ohte, Y., Yoshihara, H., Saeki, Y., et al. (2019). Specific modification of aged proteasomes revealed by tag-exchangeable knock-in mice. Mol. Cell. Biol. 39, 004266–e518. doi:10.1128/MCB.00426-18
Tonoki, A., Kuranaga, E., Tomioka, T., Hamazaki, J., Murata, S., Tanaka, K., et al. (2009). Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol. Cell. Biol. 29, 1095–1106. doi:10.1128/MCB.01227-08
Tsang, B., Arsenault, J., Vernon, R. M., Lin, H., Sonenberg, N., Wang, L.-Y., et al. (2019). Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proc. Natl. Acad. Sci. 116, 4218–4227. doi:10.1073/pnas.1814385116
Tsang, B., Pritišanac, I., Scherer, S. W., Moses, A. M., and Forman-Kay, J. D. (2020). Phase separation as a missing mechanism for interpretation of disease mutations. Cell 183, 1742–1756. doi:10.1016/j.cell.2020.11.050
Tsukahara, F., and Maru, Y. (2010). Bag1 directly routes immature BCR-ABL for proteasomal degradation. Blood 116, 3582–3592. doi:10.1182/blood-2009-10-249623
Turakhiya, A., Meyer, S. R., Marincola, G., Böhm, S., Vanselow, J. T., Schlosser, A., et al. (2018). ZFAND1 recruits p97 and the 26S proteasome to promote the clearance of arsenite-induced stress granules. Mol. Cell 70, 906–919. doi:10.1016/j.molcel.2018.04.021
Uemura, E., Niwa, T., Minami, S., Takemoto, K., Fukuchi, S., Machida, K., et al. (2018). Large-scale aggregation analysis of eukaryotic proteins reveals an involvement of intrinsically disordered regions in protein folding. Sci. Rep. 8, 678. doi:10.1038/s41598-017-18977-5
Ungelenk, S., Moayed, F., Ho, C.-T., Grousl, T., Scharf, A., Mashaghi, A., et al. (2016). Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat. Commun. 7, 13673. doi:10.1038/ncomms13673
Uversky, V. N. (2022). State without borders: Membrane-less organelles and liquid–liquid phase transitions. Biochim. Biophys. Acta Mol. Cell Res. 1869, 119251. doi:10.1016/j.bbamcr.2022.119251
Verma, R., Oania, R. S., Kolawa, N. J., and Deshaies, R. J. (2013). Cdc48/p97 promotes degradation of aberrant nascent polypeptides bound to the ribosome. eLife 2, e00308. doi:10.7554/eLife.00308
Waite, K. A., Mota-Peynado, A. D.-L., Vontz, G., and Roelofs, J. (2016). Starvation induces proteasome autophagy with different pathways for core and regulatory particles. J. Biol. Chem. 291, 3239–3253. doi:10.1074/jbc.M115.699124
Wallace, E. W. J., Kear-Scott, J. L., Pilipenko, E. V., Schwartz, M. H., Laskowski, P. R., Rojek, A. E., et al. (2015). Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162, 1286–1298. doi:10.1016/j.cell.2015.08.041
Walther, D. M., Kasturi, P., Zheng, M., Pinkert, S., Vecchi, G., Ciryam, P., et al. (2015). Widespread proteome remodeling and aggregation in aging C. elegans. Cell 161, 919–932. doi:10.1016/j.cell.2015.03.032
Wang, B., Maxwell, B. A., Joo, J. H., Gwon, Y., Messing, J., Mishra, A., et al. (2019). ULK1 and ULK2 regulate stress granule disassembly through phosphorylation and activation of VCP/p97. Mol. Cell 74, 742–757. doi:10.1016/j.molcel.2019.03.027
Wang, B., Wang, B., Zhang, Y., Zhang, L., Lei, Z., Dai, T., et al. (2021). Liquid-liquid phase separation in human health and diseases. Signal Transduct. Target. Ther. 6, 290. doi:10.1038/s41392-021-00678-1
Wang, J., Choi, J.-M., Holehouse, A. S., Lee, H. O., Zhang, X., Jahnel, M., et al. (2018). A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699. doi:10.1016/j.cell.2018.06.006
Wang, L., Klionsky, D. J., and Shen, H.-M. (2022). The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 24, 186–203. doi:10.1038/s41580-022-00529-z
Wang, W., and Roberts, C. J. (2018). Protein aggregation - mechanisms, detection, and control. Int. J. Pharm. 550, 251–268. doi:10.1016/j.ijpharm.2018.08.043
Wang, Y., and Le, W.-D. (2019). Autophagy and ubiquitin-proteasome system. Adv. Exp. Med. Biol. 1206, 527–550. doi:10.1007/978-981-15-0602-4_25
Watts, G. D. J., Wymer, J., Kovach, M. J., Mehta, S. G., Mumm, S., Darvish, D., et al. (2004). Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381. doi:10.1038/ng1332
Weber, C., Michaels, T., and Mahadevan, L. (2019). Spatial control of irreversible protein aggregation. eLife 8, e42315. doi:10.7554/eLife.42315
Wegmann, S., Eftekharzadeh, B., Tepper, K., Zoltowska, K. M., Bennett, R. E., Dujardin, S., et al. (2018). Tau protein liquid–liquid phase separation can initiate tau aggregation. EMBO J. 37, e98049. doi:10.15252/embj.201798049
Wegmann, S., Nicholls, S., Takeda, S., Fan, Z., and Hyman, B. T. (2016). Formation, release, and internalization of stable tau oligomers in cells. J. Neurochem. 139, 1163–1174. doi:10.1111/jnc.13866
Weibezahn, J., Tessarz, P., Schlieker, C., Zahn, R., Maglica, Z., Lee, S., et al. (2004). Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 119, 653–665. doi:10.1016/j.cell.2004.11.027
Wheeler, J. R., Matheny, T., Jain, S., Abrisch, R., and Parker, R. (2016). Distinct stages in stress granule assembly and disassembly. eLife 5, e18413. doi:10.7554/eLife.18413
Wilken, C., Kitzing, K., Kurzbauer, R., Ehrmann, M., and Clausen, T. (2004). Crystal structure of the DegS stress sensor: How a PDZ domain recognizes misfolded protein and activates a protease. Cell 117, 483–494. doi:10.1016/S0092-8674(04)00454-4
Wippich, F., Bodenmiller, B., Trajkovska, M. G., Wanka, S., Aebersold, R., and Pelkmans, L. (2013). Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152, 791–805. doi:10.1016/j.cell.2013.01.033
Wolf, M., Roosen-Runge, F., Zhang, F., Roth, R., Skoda, M. W. A., Jacobs, R. M. J., et al. (2014). Effective interactions in protein–salt solutions approaching liquid–liquid phase separation. J. Mol. Liq., Proc. 7th Mini-Symposium Liq. “Liquid-Liquid Phase Sep. Relat. Top. Liquids” 200 200, 20–27. doi:10.1016/j.molliq.2014.08.006
Wu, W. K. K., Wu, Y. C., Yu, L., Li, Z. J., Sung, J. J. Y., and Cho, C. H. (2008). Induction of autophagy by proteasome inhibitor is associated with proliferative arrest in colon cancer cells. Biochem. Biophys. Res. Commun. 374, 258–263. doi:10.1016/j.bbrc.2008.07.031
Wurzer, B., Zaffagnini, G., Fracchiolla, D., Turco, E., Abert, C., Romanov, J., et al. (2015). Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. eLife 4, e08941. doi:10.7554/elife.08941
Xie, X., Matsumoto, S., Endo, A., Fukushima, T., Kawahara, H., Saeki, Y., et al. (2018). Deubiquitylases USP5 and USP13 are recruited to and regulate heat-induced stress granules through their deubiquitylating activities. J. Cell Sci. 131, jcs210856. doi:10.1242/jcs.210856
Xing, W., Muhlrad, D., Parker, R., and Rosen, M. K. (2020). A quantitative inventory of yeast P body proteins reveals principles of composition and specificity. eLife 9, e56525. doi:10.7554/eLife.56525
Xu, S., Gierisch, M. E., Schellhaus, A. K., Poser, I., Alberti, S., Salomons, F. A., et al. (2022). Cytosolic stress granules relieve the ubiquitin-proteasome system in the nuclear compartment. EMBO J. 42, e111802. doi:10.15252/embj.2022111802
Yamamoto, A., Lucas, J. J., and Hen, R. (2000). Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell 101, 57–66. doi:10.1016/S0092-8674(00)80623-6
Yang, Z., Camp, N. J., Sun, H., Tong, Z., Gibbs, D., Cameron, D. J., et al. (2006). A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 314, 992–993. doi:10.1126/science.1133811
Yasuda, S., Tsuchiya, H., Kaiho, A., Guo, Q., Ikeuchi, K., Endo, A., et al. (2020). Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 578, 296–300. doi:10.1038/s41586-020-1982-9
Yoo, H., Bard, J. A. M., Pilipenko, E. V., and Drummond, D. A. (2022). Chaperones directly and efficiently disperse stress-triggered biomolecular condensates. Mol. Cell 82, 741–755.e11. doi:10.1016/j.molcel.2022.01.005
Youn, J.-Y., Dunham, W. H., Hong, S. J., Knight, J. D. R., Bashkurov, M., Chen, G. I., et al. (2018). High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell 69, 517–532. doi:10.1016/j.molcel.2017.12.020
Yu, A., Shibata, Y., Shah, B., Calamini, B., Lo, D. C., and Morimoto, R. I. (2014). Protein aggregation can inhibit clathrin-mediated endocytosis by chaperone competition. Proc. Natl. Acad. Sci. 111, E1481–E1490. doi:10.1073/pnas.1321811111
Zaarur, N., Xu, X., Lestienne, P., Meriin, A. B., McComb, M., Costello, C. E., et al. (2015). RuvbL1 and RuvbL2 enhance aggresome formation and disaggregate amyloid fibrils. EMBO J. 34, 2363–2382. doi:10.15252/embj.201591245
Zaffagnini, G., Savova, A., Danieli, A., Romanov, J., Tremel, S., Ebner, M., et al. (2018). p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 37, e98308. doi:10.15252/embj.201798308
Zakariya, S. M., Zehra, A., and Khan, R. H. (2022). Biophysical insight into protein folding, aggregate formation and its inhibition strategies. Protein Pept. Lett. 29, 22–36. doi:10.2174/0929866528666211125114421
Zaslavsky, B. Y., and Uversky, V. N. (2018). In aqua veritas: The indispensable yet mostly ignored role of water in phase separation and membrane-less organelles. Biochemistry 57, 2437–2451. doi:10.1021/acs.biochem.7b01215
Zbinden, A., Pérez-Berlanga, M., De Rossi, P., De Rossi, P., and Polymenidou, M. (2020). Phase separation and neurodegenerative diseases: A disturbance in the force. Dev. Cell 55, 45–68. doi:10.1016/j.devcel.2020.09.014
Zhang, J., Li, X., and Li, J.-D. (2019). The roles of post-translational modifications on α-synuclein in the pathogenesis of Parkinson’s diseases. Front. Neurosci. 13, 381. doi:10.3389/fnins.2019.00381
Zhao, Y. G., Zhao, Yan G., and Zhang, H. (2020). Phase separation in membrane biology: The interplay between membrane-bound organelles and membraneless condensates. Dev. Cell 55, 30–44. doi:10.1016/j.devcel.2020.06.033
Keywords: phase separation, biomolecular condensate, aggregate, Hsp70, Hsp100, disaggregation, refolding, degradation
Citation: Kohler V and Andréasson C (2023) Reversible protein assemblies in the proteostasis network in health and disease. Front. Mol. Biosci. 10:1155521. doi: 10.3389/fmolb.2023.1155521
Received: 31 January 2023; Accepted: 09 March 2023;
Published: 20 March 2023.
Edited by:
Kaye N. Truscott, La Trobe University, AustraliaReviewed by:
Harm Kampinga, University Medical Center Groningen, NetherlandsAxel Mogk, Heidelberg University, Germany
Copyright © 2023 Kohler and Andréasson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claes Andréasson, claes.andreasson@su.se
 Verena Kohler
Verena Kohler Claes Andréasson
Claes Andréasson