Corrigendum: The disease associated Tau35 fragment has an increased propensity to aggregate compared to full-length tau
- 1Department of Basic and Clinical Neuroscience, King’s College London, London, United Kingdom
- 2European Molecular Biology Laboratory, Hamburg Site, Hamburg, Germany
- 3Sussex Neuroscience, School of Life Sciences, University of Sussex, Brighton, United Kingdom
- 4London Centre for Nanotechnology, University College London, London, United Kingdom
A Corrigendum on
The disease associated Tau35 fragment has an increased propensity to aggregate compared to full-length tau
by Lyu C, Da Vela S, Al-Hilaly Y, Marshall KE, Thorogate R, Svergun D, Serpell LC, Pastore A and Hanger DP (2021). Front. Mol. Biosci. 8:779240. doi: 10.3389/fmolb.2021.779240
In the published article, there was an error in the caption for Figure 1. The column used for size-exclusion chromatography was incorrectly stated. The corrected caption appears below:
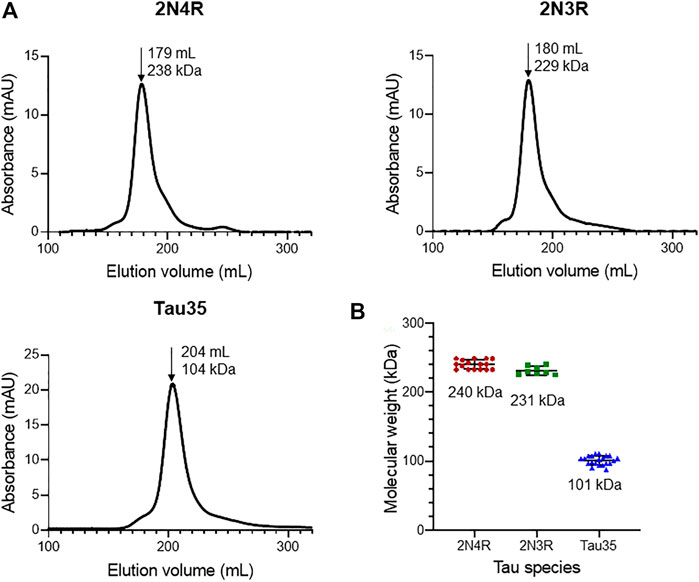
FIGURE 1. Size-exclusion chromatography of recombinant tau species. Gel filtration (HiLoad 26/600 Superdex 200 pg column) of recombinant 2N4R tau, 2N3R tau, and Tau35. (A) Representative elution profiles showing apparent molecular weights calculated from the elution volumes (arrows) of each tau species in comparison to a standard calibration curve. (B) Scatter plots showing the range of apparent molecular weights determined for each tau species. Bars indicate mean ± SD, n ≥ 8.
In the published article, there was an error. The column used for size-exclusion chromatography was incorrectly stated. A correction has been made to Results, Characterizing the Tau35 Conformation Ensemble by Small-Angle X-Ray Scattering, paragraph 1. This sentence previously stated:
“The apparent molecular weights of the tau species in phosphate buffered saline (PBS) were estimated from their elution volumes from a Superdex 200 10/300 column (Figure 1A) in comparison to globular protein standards.”
The corrected sentence appears below:
“The apparent molecular weights of the tau species in phosphate buffered saline (PBS) were estimated from their elution volumes from a HiLoad 26/600 Superdex 200 pg column (Figure 1A) in comparison to globular protein standards.”
A second correction has been made to Experimental Procedures, Size-Exclusion Chromatography Measurements. This sentence previously stated:
“Analytical SEC was carried out using a Superdex 200 10/300 GL column (Cytiva), pre-calibrated using a gel filtration marker kit (WMGF200) based on globular proteins.”
The corrected sentence appears below:
“Analytical SEC was carried out using a HiLoad 26/600 Superdex 200 pg column (Cytiva), pre-calibrated using a gel filtration marker kit (WMGF200) based on globular proteins.”
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: biophysical studies, hybrid methods, tauopathy, tau truncation, small angle x-ray scattering, intrinsically disordered proteins, neurodegeneration, dementia
Citation: Lyu C, Da Vela S, Al-Hilaly Y, Marshall KE, Thorogate R, Svergun D, Serpell LC, Pastore A and Hanger DP (2023) Corrigendum: The disease associated Tau35 fragment has an increased propensity to aggregate compared to full-length tau. Front. Mol. Biosci. 10:1276677. doi: 10.3389/fmolb.2023.1276677
Received: 12 August 2023; Accepted: 17 August 2023;
Published: 15 September 2023.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2023 Lyu, Da Vela, Al-Hilaly, Marshall, Thorogate, Svergun, Serpell, Pastore and Hanger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diane P. Hanger, diane.hanger@kcl.ac.uk; Annalisa Pastore, annalisa.1.pastore@kcl.ac.uk
†Present address: Youssra Al-Hilaly, Chemistry Department, College of Sciences, Mustansiriyah University, Baghdad, Iraq
 Chen Lyu
Chen Lyu Stefano Da Vela2
Stefano Da Vela2  Youssra Al-Hilaly
Youssra Al-Hilaly Karen E. Marshall
Karen E. Marshall Richard Thorogate
Richard Thorogate Louise C. Serpell
Louise C. Serpell Annalisa Pastore
Annalisa Pastore Diane P. Hanger
Diane P. Hanger