The Flexible Electrochromic Device That Turns Blue Under Both Positive and Negative Application Voltages
- State Key Laboratory of Luminescent Materials and Devices, Research Institute of Materials Science, South China University of Technology, Guangzhou, China
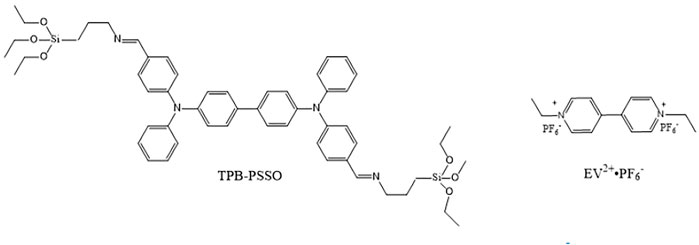
Using the photocurable electrolyte solution containing viologen derivative 1,1′-bis- (4-vinylbenzyl)-4,4′-bipyridinium dihexafluorophosphate (EV2+•2PF6−) and its composite of EV2+•2PF6− and triphenylamine derivative N,N′-di(4-((3-(triethoxysilyl)-propyl)imino)methyl)phenyl-N,N′-diphenyl-4,4′-biphenyldiamine (TPB-PSSO) as the electrochromic active layer, respectively, the flexible electrochromic devices (FECDs) were prepared by photocurable technology. The device structure was PET-ITO/photocurable electrolyte solution/PET-ITO. The electrochromic properties of FECDs were investigated. The results show that the FECDs can all undergo a reversible color change, especially the PET-ITO/photocurable electrolyte solution-composite/PET-ITO can undergo polychromatic transition. The FECD based on EV2+•2PF6− can reversibly change between colorless and deep blue, and the FECD based on the composite of TPB-PSSO-EV2+•2PF6− can reversibly change between black blue, light yellow, and sky blue.
1 Introduction
In recent years, people have paid more and more attention to energy-saving, which means electrochromic materials and corresponding devices have gradually become a research hotspot. The organic molecules can undergo a reversible electrochemical redox under an applied voltage, resulting in a color change known as organic electrochromic materials. Organic electrochromic materials have been studied because of their advantages, such as easy synthesis, modification, and fabrication of flexible devices (Liu et al., 2020a; Liu et al., 2020b; Frolov et al., 2020; Ma et al., 2020; Nad and Malik, 2020; Xiao et al., 2020; Zhang et al., 2020; Kim et al., 2021; Zhang X et al., 2021; Kim et al., 2021; Hao et al., 2021; Huang et al., 2021; Li et al., 2021). Organic electrochromic devices (ECDs) have good application prospects, such as smart windows and displays. In our previous work, a series of triphenylamine (TPA) derivatives were synthesized, and their electrochromic properties were investigated (Liu et al., 2017; Zeng et al., 2017; Zeng et al., 2018; Zeng et al., 2019). As solid films, these derivatives exhibited high optical contrast and a short response time under applied voltages. In addition, a series of conjugated linear and star oligothiophene derivatives and organic conjugated oligomers containing 4-ethylenedioxythiophene were synthesized (La et al., 2008; Yin et al., 2010; Jiang et al., 2012; Zhong et al., 2015; Wan et al., 2018). These organic compounds exhibited reversible and distinct chromatic changes. 4,4-Bipyridine, the core group of viologen derivatives, has a strong electron-acquiring ability. The N atom of TPA derivatives has a lone electron-pair and strong electron-supplying ability, making them prone to electron gain and loss.
In this study, the N,N′-di (4-((3-(triethoxysilyl)propyl) imino)methyl)phenyl-N,N′-diphenyl-4,4′-biphenyldiamine (TPB-PSSO) and a 1,1′-bis (4-vinylbenzyl)-4,4′-bipyridinium dihexafluorophosphate (EV2+•2PF6−) were synthesized. Using the photocurable electrolyte solution containing EV2+•2PF6− and PSSO-EV2+•2PF6− composite as the electrochromic active layer, respectively, the flexible electrochromic devices (FECDs) were prepared, and their electrochromic properties were investigated. The molecular structures of TPB-PSSO and EV2+•2PF6− are presented below (Figure 1).
2 Experimental
2.1 Materials
All solvents were purified and dried using standard methods. Triphenylamine (TPA), phosphorus oxychloride (POCl3), NH4PF6, photocurable resin (PET-400DA), and photoinitiator-184 were purchased from Alfa Aesar Chemical Co., Ltd. Lithium perchlorate (LiClO4), tetrahydrofuran (THF), and potassium carbonate (K2CO3) were purchased from Shanghai RichJoint Chemical Reagents Co., Ltd. 1,2-Dichloroethane, acetonitrile (ACN), and ethanol (EtOH) were purchased from Tianjin Fuyu Fine Chemical Co. Ltd. N,N-Dimethylformamide (DMF) and nitric acid (HNO3, 65%) were purchased from Guangzhou Chemical Reagent Factory. Sodium hydroxide (NaOH) was purchased from Fuchen (Tianjin) Chemical Reagents Co., Ltd. 3-Aminopropyl triethoxysilane (APTES) was purchased from Foshan Daoning Chemicals Ltd. Dichloromethane (DCM) and propylene carbonate (PC) were obtained from Sinopharm Chemical Reagent Co., Ltd. Indium tin oxide (ITO) PET (10 Ω) was purchased from Zhuhai Kaivo Optoelectronic Technology Co., Ltd.
2.2 Instruments
Fourier transform infrared (FTIR) spectroscopy was performed on a Nicolet 6700 (Thermo Fisher Scientific) spectrometer. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE-600 NMR spectrometer. Using a Thermo Helios-g spectrometer, the ultraviolet-visible (UV-vis) spectra were recorded. Using a CHI750A electrochemical workstation (Zhenhua Apparatus Co., Shanghai), the electrochemical properties were measured in a PC solution containing 0.1 M LiClO4. A saturated calomel electrode (SCE), platinum wire, and glassy carbon electrode were used as the reference electrode (RE), counter electrode (CE), and working electrode (WE), respectively.
2.3 Synthesis
2.3.1 Synthesis of N,N′-Di(4-((3-(triethoxysilyl)propyl)imino)methyl)phenyl-N,N′-diphenyl-4,4′-biphenyldiamine (TPB-PSSO)
The TPB-PSSO was synthesized and characterized according to the procedure reported (Zhang X. et al., 2021).
2.3.2 Synthesis of 1,1′-Bis(4-vinylbenzyl)-4,4′-bipyridinium Dihexafluorophosphate (EV2+•2PF6−)
4,4′-Bipyridine of 3.12 g (20 mmol), 60 ml acetonitrile (ACN), and 5 ml (60 mmol) ethane bromide were added into a 100 ml single flask. The solution was stirred under N2 at 80°C for 48 h. Then, the solution was filtrated, and a yellowish crude product was obtained. The yellowish crude product was washed with ACN and dried in a vacuum. The 30 ml NH4PF6 aqueous solution was added to the aqueous solution of the above yellow product, and the mixture solution was stirred quickly for 3 h. Then, the mixture solution was filtrated, and a white crude product was obtained. The white crude product was washed with distilled water and vacuum drying. The 9.3 g white EV2+•2PF6− powder was obtained. 1H NMR (DMSO, 400 MHz, ppm): 9.38 (d, J = 7.2 Hz, 4H), 8.76 (d, J = 6.9 Hz, 4H), 4.73 (m, J = 2.0 Hz, 4H), 1.61 (t, J = 2.0 Hz, 6H). 13C-NMR (DMSO, 400 MHz, ppm); 149.10, 145.96, 127.07, 57.07, 16.85; HRMS (ESI): (C14H18N2+) 214.1417, calcd. for 214.1439.
2.4 Preparation of the Photocurable Electrolyte Solution
The LiClO4 and EV2+•2PF6− [or composite of TPB-PSSO-EV2+•2PF6− (w/w = 1/1)] were added to 25 ml of propylene carbonate (PC). The solution was stirred until completely dissolved. The concentration of LiClO4 is 2.83 mol/L, and the concentration of EV2+•2PF6− [or composite of TPB-PSSO-EV2+•2 PF ((w/w = 1/1)] is 0.01 M. Then, 0.80 g of photocurable resin (PET-400DA) and 0.02 g of photoinitiator 184 were added to the abovementioned electrolyte solution and stirred evenly. The prepared photocurable electrolyte was stored in an N2 atmosphere away from light, hence being ready for use.
2.5 Preparation of the Flexible Electrochromic Devices
The FECDs with the sandwich structure were assembled using the two pieces of 2.5 × 5 cm2 ITO-PET substrate as the electrode. A certain amount of the abovementioned two-electrolyte solution was injected into the hollow part of the sandwich structure, respectively. Then, the FECDs were put into the photocuring box for 1 min. The structure of FECDs was PET-ITO/photocurable electrolyte solution/PET-ITO. The FECDs based on the EV2+•2PF6− and the composite of TPB-PSSO/EV2+•2PF6− (W/W = 1:1) were named FECD-EP and FECD-composite, respectively.
3 Results and Discussion
3.1 Optoelectrochemical Properties of TPB-PSSO and EV2+•2PF6−
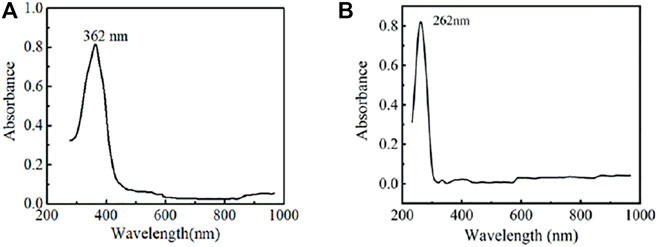
Figure 2 shows the UV-vis spectra of TPB-PSSO and EV2+•2PF6− in PC solution, respectively. The maximum absorption wavelength (λmax) of TPB-PSSO PC solution is 362 nm; that is, the color of the TPB-PSSO is yellow. The maximum absorption wavelength (λmax) of EV2+•2PF6− in the PC solution is 262 nm, and there is no obvious absorption peak in the visible region, which shows that the EV2+•2PF6− solution is colorless and transparent.
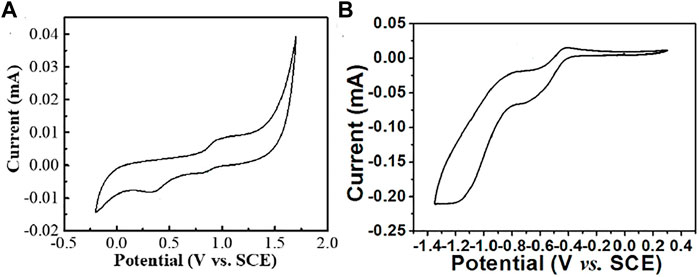
The electrochemical redox ability of organic compounds was tested by cyclic voltammograms (CV). The scanning was carried out at a rate of 50 mV/s. As shown in Figure 3 and Table 1, the onset of oxidation and reduction potential of TPB-PSSO were 0.80 and −0.15 V (vs. SCE), respectively. The onset of reduction potential of EV2+•2PF6− was −0.42 V (vs. SCE). The CV results show that these two compounds have oxidation and reduction abilities and may be used as electrochromic active material.
3.2 Electrochromic Properties of the FECDs
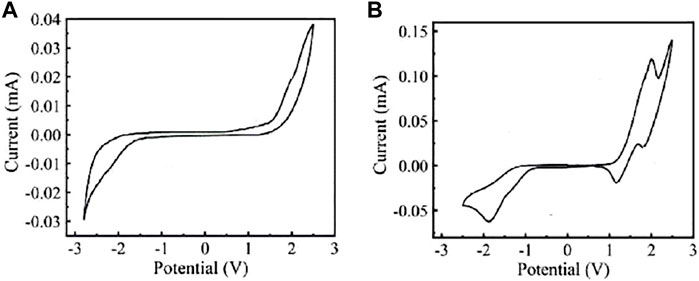
Figure 4 shows the CV curves of FECD-EP and FECD-composite, respectively. The reversible redox process of FECD-EP is the conversion between the EV2+ and radical EV•+ accompanied by the gain and loss of an electron. The FECD-composite has two reversible redox processes: one is the reversible redox process of TPB-PSSO and the other is the reversible redox process of EV2+•2PF6−.
As shown in Figure 5, the FECD-EP and FECD-composite can undergo reversible color change under applied voltage. When the applied voltage is at 0 V, the FECD-EP is colorless, and when the applied voltage is at −3.5 V, the color of the FECD-EP is deep blue. The main reason for this color change is that the EV2+ can get an electron under the applied voltage and be reduced to the monovalent cation radical EV+. When the applied voltage is 0 V, the FECD-composite is light yellow. When the applied voltage is at 2.0 V, the color of the FECD-composite is black blue. When the applied voltage is at −2.0 V, the color of FECD-composite is sky blue.
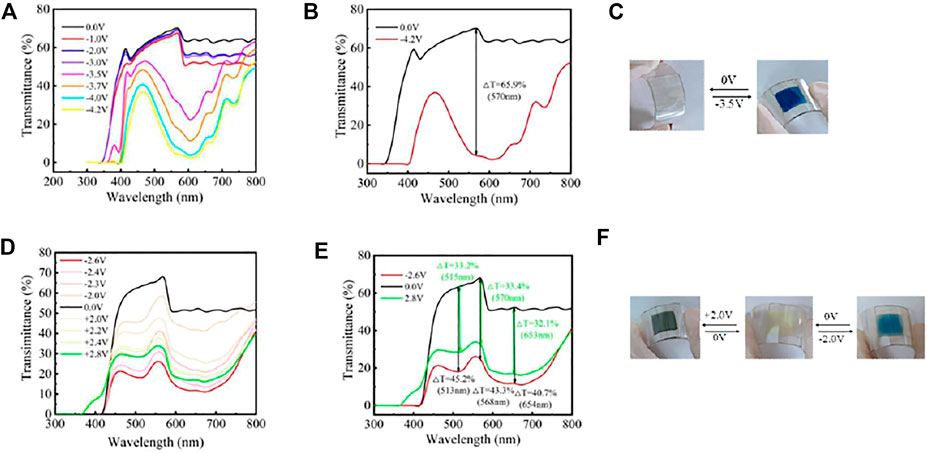
FIGURE 5. Spectroelectrochemistry of FECD-EP (A) and FECD-composite (D). Optical contrast of FECD-EP (B) and FECD-composite (E). Color change images of FECD-EP (C) and FECD-composite (F).
Optical contrast (ΔT) refers to the difference in the optical transmittance of ECD in coloring and bleaching state at a certain wavelength. The ΔT of FECD-EP was 65.9% at 570 nm. The ΔT of the FECD-composite was 33.2%, 33.4%, and 32.1% at 515, 570, and 653 nm, respectively.
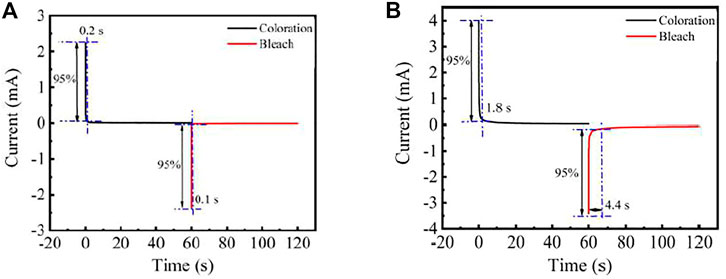
The response time of FECD-EP and FECD-composite is measured by chronoamperometry. The time required for the current change to reach 95% during coloring and bleaching is denoted as coloring response time (tc) and bleaching response time (tb), respectively. The timing-current spectra of FECD-EP and FECD-composite are shown in Figure 6. When FECD-EP alternatively switches the voltage of −3.0 and 1.0 V, the Tc is 0.2 s and Tb is 0.1 s. When the applied voltage of −2.5 and 2.5 V is alternately switched with FECD-composite, the tc and tb are 1.8 and 4.4 s. Comparing the tc and tb of FECD-EP, it can be found that the coloring and bleaching time of the FECD-composite is longer.
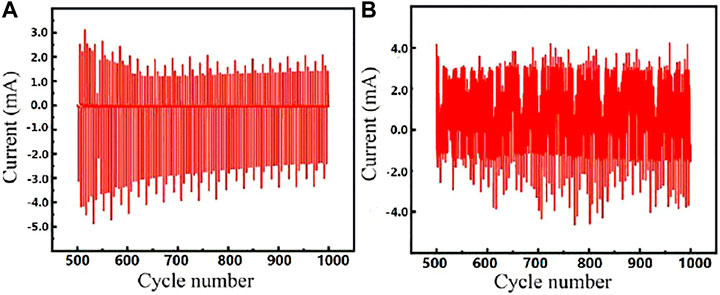
Figure 7 shows the cyclic stability curves of FECD-EP and FECD-composite. The FECD-EP retained 67.1% of their original charge after 1,000 cycles. However, the timing-current curve of the FECD-composite fluctuated greatly, and the amount of charge at the 1,000th cycle was slightly higher than the initial charge. The reason might be that excessively high driving voltage may easily cause over-oxidation or over-reduction of electrochromic materials in the electrochromic process.
The coloration efficiency of an electrochromic device refers to the change value of the optical density of the device when the unit charge is injected or extracted during the coloration process. The coloration efficiency of FECD-EP and FECD-composite can be calculated according to Eq. (1):
The η is the coloration efficiency (cm2/C), ΔOD is the change of optical density, Q is the amount of charge injected (or withdrawn) (cm2/C) of unit area, and Tc and Tb are, respectively, the optical transmittance of colored state (%) and bleached state (%) at a specific wavelength. The η of FECD-EP at 570 nm was 151.50 cm2/C. When the positive applied voltage was applied, the η of FECD-composite at 515, 570, and 653 nm was 74.15 cm2/C, 68.16 cm2/C, and 104.86 cm2/C, respectively. When the reverse applied voltage was applied, the η of FECD-composite at 513, 568, and 654 nm was 116.70 cm2/C, 93.25 cm2/C, and 140.28 cm2/C.
4 Conclusion
Using the photocurable electrolyte solution containing EV2+•2PF6− and its composite of EV2+•2PF6− and TPB-PSSO as the electrochromic active layer, respectively, the FECDs were prepared by photocurable technology. The FECD based on EV2+•2PF6− can reversibly change between colorless transparent and deep blue, and the FECD based on the composite of TPB-PSSO-EV2+•2PF6− can reversibly change from light yellow to sky blue and dark blue under both positive and negative application voltages.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation.
Author Contributions
SJ: methodology, formal analysis, investigation, writing—original draft. XZ: validation. XG: conceptualization, software, data curation. MZ: visualization. PL: resources, writing—review and editing, supervision, project administration, funding acquisition.
Funding
This research was financially supported by the NSFC (Grant nos. 20674022, 20774031, and 21074039), the Natural Science Foundation of Guangdong (Grant nos. 2014A030313241, 2014B090901068, and 2016A010103003), and the Ministry of Education of the People’s Republic of China (Grant no. 20090172110011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Frolov, D. G., Khorova, A. I., Kharitonova, E. P., Keshtov, M. L., and Makhaeva, E. E. (2020). Electrochromicbehavior of Poly(amine-Amide) with Pendant N-Phenylcarbaz- Ole and Triphenylamine Units and its Composite with Multiwalled Carbon Nanotubes. Mater. Today Commun. 25, 101369. doi:10.1016/j.mtcomm.2020.101369
Hao, Q., Li, Z. J., Bai, B., Zhang, X., Zhong, Y. W., Wan, L. J., et al. (2021). A Covalent Organic Framework Film for Three‐State Near‐Infrared Electrochromism and a Molecular Logic Gate. Angew. Chem. Int. Ed. 60 (22), 12498–12503. doi:10.1002/anie.202100870
Huang, Z.-J., Li, F., Xie, J.-P., Mou, H.-R., Gong, C.-B., and Tang, Q. (2021). Electrochromic Materials Based on Tetra-Substituted Viologen Analogues with Broad Absorption and Good Cycling Stability. Solar Energ. Mater. Solar Cell 223, 110968. doi:10.1016/j.solmat.2021.110968
Jiang, Y., Wang, J., Guan, L., Zhong, Y., Liu, P., and Deng, W. (2012). Preparation and Electrochromic Properties of Polythiophene Derivative Films Based on Oligothiophene Derivative Monomers. Acta Chim. Sinica 70, 103–106. doi:10.6023/A1109093
Kim, K.-W., Lee, J. K., Tang, X., Lee, Y., Yeo, J., Moon, H. C., et al. (2021). Novel Triphenylamine Containing Poly-Viologen for Voltage-Tunable Multi-Colorelectrochromic Device. Dyes Pigm. 190, 109321. doi:10.1016/j.dyepig.2021.109321
La, M., Liu, M.-M., Liu, P., Deng, W.-J., and Tong, Z. (2008). Electrochromic Properties Based on Oligothiophene Derivatives. Chin. J. Chem. 26, 1523–1526. doi:10.1002/cjoc.200890276
Li, F.-W., Yen, T.-C., and Liou, G.-S. (2021). Synthesis of High-Performance Electrochromic Material for Facile Fabrication of Truly Black Electrochromic Devices. Electrochimica Acta 367, 137474. doi:10.1016/j.electacta.2020.137474
Liu, W., Zhang, X., LiuMa, J., Ma, X., Zeng, J., Liu, P., et al. (2017). Electrochromic Properties of Organic-Inorganic Composite Materials. J. Alloys Compd. 718, 379–385. doi:10.1016/j.jallcom.2017.05.222
Liu, Y., Liu, T., Pang, L., Guo, J., Wang, J., Qi, D., et al. (2020). Novel Triphenylamine Polyamides Bearing Carbazole and Aniline Substituents for Multi-Coloredelectrochromic Applications. Dyes Pigm. 173, 107995. doi:10.1016/j.dyepig.2019.107995
Liu, Y., Pang, L., Liu, T., Guo, J., Wang, J., and Li, W. (2020). Novel Triphenylaminepolyazomethines Bearing Carbazole and Trifluoromethyl Substituents: Preparation and Electrochromicbehavior. Dyes Pigm. 173, 107921. doi:10.1016/j.dyepig.2019.107921
Ma, F., Liu, F., Hou, Y., Niu, H., and Wang, C. (2020). Electrochromic Materials Based on Novel Polymers Containing Triphenylamine Units and Benzoc 1,2,5 Thiadiazole Units. Synth. Met. 259, 116235. doi:10.1016/j.synthmet.2019.116235
Nad, S., and Malik, S. (2020). Design, Synthesis, and Electrochromic Behaviors of Donor‐Acceptor‐Donor Type Triphenylamine‐ Iso ‐Naphthalenediimide Derivatives. Chemelectrochem 7 (19), 4144–4152. doi:10.1002/celc.202001114
Wan, Z., Zeng, J., Li, H., LiuLow-VoltageDriven, P., and Deng, W. (2018). Multicolored, Low-Voltage-Driven, Flexible Organic Electrochromic Devices Based on Oligomers. Macromol. Rapid Commun. 39, 1700886. doi:10.1002/marc.201700886
Xiao, S., Zhang, Y., and Xiao, D. (2020). Latent Fingermarks on Copperplate Paper: Facile Visualization via Electrochromismof 1,1 '-Bis(3-Sulfonatopropyl) Viologen. Electr. Ochimica Acta 363, 137186. doi:10.1016/j.electacta.2020.137186
Yin, B., Jiang, C., Wang, Y., La, M., Liu, P., and Deng, W. (2010). Synthesis and Electrochromic Properties of Oligothiophene Derivatives. Synth. Met. 160, 432–435. doi:10.1016/j.synthmet.2009.11.025
Zeng, J., Li, H., Wan, Z., Ai, L., Liu, P., and Deng, W. (2019). Colorless-to-black Electrochromic Materials and Solid-State Devices with High Optical Contrast Based on Cross-Linked Poly(4-Vinyltriphenylamine). Solar Energ. Mater. Solar Cell 195, 89–98. doi:10.1016/j.solmat.2019.02.037
Zeng, J., Wan, Z., Li, H., Liu, P., and Deng, W. (2018). Visible and Near-Infrared Electrochromic Properties of Polymers Based on Triphenylamine Derivatives with Acceptor Groups. Solar Energ. Mater. Solar Cell 178, 223–233. doi:10.1016/j.solmat.2018.01.024
Zeng, J., Zhang, X., Zhu, X., and Liu, P. (2017). Synthesis and Electrochromic Properties of star-shaped Oligomers with Phenyl Cores. Chem. Asian J. 12, 2202–2206. doi:10.1002/asia.201700890
Zhang, W.-j., Lin, X.-c., Li, F., Huang, Z.-j., Gong, C.-b., and Tang, Q. (2020). Multicolored Electrochromic and Electrofluorochromic Materials Containing Triphenylamine and Benzoates. New J. Chem. 44 (38), 16412–16420. doi:10.1039/d0nj03666h
Zhang, X., Zeng, J., Xu, Z., Zhu, M., and Liu, P. (2021). A Fast-Response Electrochromic Device Based on a Composite Gel Film Comprising Triphenylamine Derivatives and WO3. New J. Chem. 45, 5503–5508. doi:10.1039/d1nj00113b
Zhang, Y., Shi, X., Xiao, S., and Xiao, D. (2021). Visible and Infrared Electrochromism of Bis(2-(2-(2-Hydroxyethoxy)ethoxy)ethyl) Viologen with Sodium Carboxymethyl Chitosan-Based Hydrogel Electrolytes. Dyes Pigm. 185, 108893. doi:10.1016/j.dyepig.2020.108893
Keywords: viologen derivative, triphenylamine derivative, composite, photocurable electrolyte solution, flexible electrochromic devices
Citation: Jin S, Zhang X, Guo X, Zhu M and Liu P (2022) The Flexible Electrochromic Device That Turns Blue Under Both Positive and Negative Application Voltages. Front. Nanotechnol. 4:887442. doi: 10.3389/fnano.2022.887442
Received: 01 March 2022; Accepted: 21 March 2022;
Published: 21 April 2022.
Edited by:
Haizeng Li, Shandong University, ChinaCopyright © 2022 Jin, Zhang, Guo, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Liu, mcpliu@scut.edu.cn
 Shiqi Jin
Shiqi Jin  Ping Liu
Ping Liu