Translational pathway of a novel PFF2 respirator with chitosan nanotechnology: from the concept to the practical applications
- 1Postgraduate Program in Biomedical Engineering, Faculty of Gama, University of Brasilia, Brasilia, Brazil
- 2Postgraduate Program in Collective Health, Faculty of Health Sciences, University of Brasilia, Brasilia, Brazil
- 3Fundação Oswaldo (Fiocruz), Rio de Janeiro, Brazil
- 4Postgraduate Program in Rehabilitation Sciences, Faculty of Ceilandia, University of Brasilia, Brasilia, Brazil
- 5Postgraduate Program in Mechatronic Systems, Faculty of Technology, University of Brasilia, Brasilia, Brazil
- 6Postgraduate Program in Electrical Engineering, University of Brasilia, Brasilia, Brazil
Introduction: Translational Health Research (THR) is a tool aimed at assisting in the transformation of basic and/or applied scientific research into a health technology ready for commercialization. The aim of this study is to present the translational pathway in wich our research group developed a Personal Protective Equipment (PPE) called VESTA® Facial Respirator with chitosan nanotechnology for protection against viruses, bacteria, and fungi. The aim of this study is to present the process of THR applied to a health technology research.
Methods: The theoretical-methodological process of THR was applied to the Research and Development (R&D) of the respirator. This method is characterized by subsequent phases, as follow: T (0)—Concept, T (1) Pre-Clinical, T (2) Clinical, T (3) Industrial Scale Production, and T (4) Characterized by subsequent phases, as follow: Technological Evaluation.
Results: Applying the THR process in the development and production of the Particulate Filtering Facepiece class 2 (PFF2) respirator with chitosan nanotechnology, University of Brasilia was able to transform the research idea into a respirator approved by the National Regulatory Agency for industrial-scale production within 24 months. The THR process is not a linear action; this flexibility allows essential activities for transforming research into a marketable product.
Discussion: The integration among various stakeholders right from the genesis of research is a driving force for the effective utilization of results. The maturity of the country’s industrial sector is crucial for converting university research into a marketable product, and governments need to prioritize these products in healthcare system incorporations. The academic culture of scientific research needs to intensify the technological transfer phase of its inventions. Conclusion: In less than 24 months, the University of Brasilia translated research on a new PPE into the market by applying the THR method.
1 Introduction
In mid-December 2019, the SARS-CoV-2 (COVID-19) pandemic Organization et al. (2020) rapidly tested the capacity of global healthcare systems to manage and treat their populations in the face of its impacts. With the exponential increase in mortality rates, the vulnerability of these sectors became evident Organization et al. (2022). Considering that the health emergency began at the end of January 2020, global estimates indicated over 6.9 million deaths by the year 2023. Approximately 105 countries documented cases of the COVID-19, while 50 countries reported associated deaths. This led to the infection of millions of people, causing substantial concerns among health managers during that period Organization et al. (2023). In Brazil, since 2020, more than 38 million people have been infected, with approximately 708,999 deaths. According to epidemiological data from the first half of January 2024, 34,050 new cases were reported worldwide da Saúde do Brasil (2024).
The global health crisis demanded the immediate implementation of measures to mitigate the effects of the pandemic. Faced with this challenge, the international scientific community mobilized to develop and enhance interventions aimed to tackle the pandemics. These strategies were targeted at containing the virus’s spread and mitigating its health impacts Schuchat (2020). The actions, for the most part, were carried out through the Research, Development, and Innovation (RD&I) process in health. This underscores the crucial importance of the scientific sector, including universities and public research centers, not only globally but also on the national stage. They play a fundamental role in creating scientifically based and safe solutions to meet the needs of the population Rosa et al. (2021); Fernandes et al. (2021).
The specific needs of each region required targeted efforts, which were intrinsically linked to the maturity of their regional public policies. The effectiveness and responsiveness varied among countries, with notable differences in the production and distribution of vaccines, for example. There was a greater focus on seeking and producing current scientific evidence to provide effective solutions for reducing the contagion and deaths caused by SARS-CoV-2. This drove the development and manufacturing of health technologies, such as vaccines, optimized mechanical ventilators, masks, supplies, among other initiatives Jebril (2020); Schaltegger (2020).
Other challenges emerged, such as the need to evaluate treatment proposals for infection while simultaneously assessing the effectiveness of the scientific evidence behind developed health solutions. Therefore, the ability to translate what was produced in the scientific laboratory to hospital beds became an emergent necessity. Hanney et al. (2022) discussed the contributions made and challenges faced by Health Research Systems (HRSs) during the pandemic in countries such as Australia, Brazil, Canada, Germany, New Zealand, the United Kingdom (UK), and the United States (United States of America). This study primarily covers the years 2020 and 2021, with a special emphasis on the response in 2020. The authors explore RD&I processes in health, including health public policies, vaccine development, medical equipment, supplies, and Personal Protective Equipment (PPE), considering not only the capacity to generate scientific evidence but also the implementation of actions aimed at reducing contaminations and deaths Rosa et al. (2021); Hanney et al. (2022).
In the Brazilian context, even in the face of funding shortages, public sectors such as universities, federal institutes, and research centers, through both basic and applied research, have contributed to the battle against the pandemic Rosa et al. (2021); Neiva et al. (2020). As highlighted by Rosa et al. (2021) Brazil has approximately 114 public universities, including federal institutes, distributed across its five regions (Central-West, North, Northeast, Southeast, and South). A survey conducted on 20 May 2020, identified around 894 scientific research projects dedicated to the COVID-19 pandemic, covering the following categories: i. Development and Innovation (D&I); ii. Other types of research projects on COVID-19; iii. Epidemiological Research; and iv. Basic Research (focused on the disease mechanisms) Rosa et al. (2021); Melo et al. (2020).
However, for basic and/or applied research to effectively reach the market and be made available to the population through healthcare systems, it is crucial to address ethical and regulatory aspects Ventura and Oliveira (2022). Therefore, the integration of government, university, society, industry, and the environment becomes necessary, a concept known as the “quintuple helix” Marostica et al. (2021), which requires harmonious operation. The key to transforming basic and/or applied research into technology capable of meeting the needs of the population is the process of Translational Health Research (THR), recognized as the central driver of this transformation Barreto et al. (2020).
1.1 Translational potential of medical equipment in healthcare
Originally referred to as “from bench to bedside”, THR aims to integrate the so-called Phases or Times (T), with the goal of overcoming obstacles and transforming into consumer goods for benefiting the society as a whole Wehling (2006); Khoury et al. (2010). Translation is completed when research, whether basic or applied, evolves into a commercially viable product capable of meeting the population’s demands within healthcare systems Barreto et al. (2020).
Although terms such as translational science and/or translational medicine are often used interchangeably, there are nuances that distinguish them Woolf (2008), particularly in the emphasis on the phases of basic and applied research, the ones linked to ethical and/or clinical aspects Lupatini et al. (2020).
Contemporary perspectives adopt an integrated and non-linear approach to the transformation of scientific research into products that can be used by healthcare systems. THR evolves to encompass elements beyond the evidence synthesis, knowledge translation, technology implementation, assessment, and broader impacts on healthcare systems. It also addresses challenges in the technological transfer process, regulatory registrations, and industrial-scale production. In general, it contributes to ensuring that research not only achieves clinical validations but also enables a specific drug or product to be effectively integrated into the segment’s ecosystem, uniting social, economic, and health benefits Hanney et al. (2022); Barreto et al. (2020); Fort et al. (2017).
When the phases of scientific research are integrated, the chances of overcoming the “valleys of death” and reaching practical implementation Khoury et al. (2010). The expression “Overcoming the valley of death” means that the confirmation of safety and efficacy has progressed to other stages such as industrial-scale production, ensuring the availability of the invention in healthcare. However, more is required; it is necessary for private initiatives or the industry to show interest, especially when the invention originates from the university, making technology transfer necessary. Furthermore, the role of the industry involves making production adjustments to meet regulatory requirements and producing at a scale that assumes market acceptance and, consequently, incorporation into healthcare or health systems Barreto et al. (2020); Rosa (2022). This article presents promising results related to the development of specific Personal Protective Equipment (PPE) for protection against the transmission of SARS-CoV-2 in the context of Translational Health Research (THR).
Currently, Translational Health Research (THR) is subdivided into several categories recognized as stages, phases, or times, each playing a role in the process of transforming scientific knowledge into practical health benefits Rosa (2022). From an epistemological perspective, there are various elements involved in the emergence and consolidation of the THR discipline. When addressing THR, it becomes imperative to consider its gaps, which can be understood as phases, periods, or simply “T” times. The analysis of the evolution from laboratory and/or clinical research - known as basic research - to interventions that enhance individual health, within the scope of THR, is now guided by the times T (0), T (1), T (2), T (3), and T (4). These represent stages or phases to be traversed in the pursuit of translating research into health benefits, a framework stimulated by the U.S. National Institutes of Health (NIH) Roadmap for Medical Research in the early 2000s Wehling (2006); Khoury et al. (2010); Rosa (2022).
1.2 The path for the development of the VESTA® facial respirator
In Brazil, significant efforts have been made to expedite the application of basic research in medical devices. The VESTA® Facial Respirator was developed by incorporating chitosan-based nanotechnology into its filtering element. Chitosan is a natural polysaccharide derived from chitin, a component found in the exoskeleton of crustaceans, and is locally produced. The respirator aimed to be a Personal Protective Equipment (PPE) to improved the efficacy in reducing the transmission of SARS-Cov-2. This initiative resulted in a broad collaboration among academic researchers, a public laboratory, and a private company, achieving rapid progress, as detailed in Hanney et al. (2022). In this context, using a THR model, the University of Brasília (UnB) developed a facial respirator that represents a disruptive innovation. It combines the structure of the N95/PFF2 model with a chitosan nanofilm to increase its protective effectiveness against viruses, bacteria, and fungi. It is important to highlight that both the technology and the production processes are entirely national, with all necessary materials sourced from Brazil and all production stages carried out in the country Wehling (2006); Landim et al. (2023).
As discussed by Rosa (2022), the group responsible for the development of the VESTA® Respirator relied on the process of Translational Health Research (THR) to organize its activities. This approach was adopted with the purpose of ensuring the effective transfer of knowledge generated at the university to benefit society during the critical phases of contagion established by the pandemic. Scientific and technological advancement is recognized as a continuous initiative that influences and has lasting impacts on nations around the contemporary world Khoury et al. (2010). In pandemic periods, such as the between 2020 and 2023, it becomes essential for countries to have a flexible health industrial sector, capable of adapting and optimizing collaboration with universities and public research centers. This aims to facilitate the systematic and agile transfer of research to the healthcare system Lupatini et al. (2020); Khoury et al. (2010).
However, for this transformation to occur, favorable conditions are necessary. Translational Health Research (THR) focused on medical equipment requires a mature ecosystem of innovation and production that is interconnected, with important aspects of the quintuple helix well-defined and articulated. In addition to generating quality scientific evidence, a Health Industrial/Economic Complex (CEIS) must be prepared to industrially scale the scientific discovery in a coordinated and rapid manner. Social and economic demands, according to Fernandes et al. (2022), are two sides of the same coin. The CEIS becomes a vector of social development as it encourages scientific development to meet health needs while simultaneously acting as an economic vector by supporting the industrial sector in the production of medicines, equipment, and supplies to meet the health needs of the population Woolf (2008); Gadelha (2022).
1.3 Chitosan-based nanotechnologies: evidence and perspectives
Natural polymers (or biopolymers), those directly sourced from nature, have been undergoing a considerable increase in research for biomedical applications, as exemplified by chitosan, due to its attractive characteristics, including renewability, wide availability, biodegradability, biocompatibility, and high functionality for the development of various materials, as well as safety and low toxicity Guo et al. (2024). Chitosan, a natural cationic polymer derived from chitin, has thus emerged as a multifaceted and promising solution and is Generally Recognized as Safe (GRAS) by the United States Food and Drug Administration (US FDA) Dash et al. (2011); Rinaudo (2006); Duan et al. (2018); Boroumand et al. (2021).
Exploratory studies on chitosan have revealed its virucidal and antimicrobial activity and effectiveness in inactivating a wide range of viruses, including enteric viruses, respiratory syncytial virus, plant viruses, feline calicivirus (FCV-F9), bacteriophage MS2, human papillomavirus (HPV), human immunodeficiency virus (HIV), and different types of coronaviruses Landim et al. (2023); Du et al. (2018); Kumar et al. (2004). The bactericidal, fungicidal, and virucidal properties of chitosan result from its interaction with negatively charged residues through the adsorption of macromolecules present on its surfaces Dash et al. (2011). Other studies have also highlighted its potential in various regenerative therapies, such as tissue engineering and wound healing. Its mucoadhesive capacity allows chitosan nanoparticles to be administered via transmucosal routes, such as intranasal, intraocular, intravaginal, intratracheal, or intrapulmonary routes in microencapsulated drug delivery systems Boroumand et al. (2021); Burdueel et al. (2018); Hamdi et al. (2020); Morris et al. (2019).
Recently, researchers at the University of Brasília have expanded studies on the potential of chitosan for developing masks capable of filtering other types of viruses, such as H1N1 and coronavirus variants. This expansion is based on the virucidal and antimicrobial properties already demonstrated by chitosan. Chitosan’s ability to inactivate a wide range of viruses has sparked interest in its application for protection against other infectious agents. Additionally, its biocompatibility and low toxicity are characteristics that make it promising for the development of effective and safe personal protective materials. This line of research aims to further explore the potential of chitosan as a multifunctional solution to combat various viral threats, thereby expanding its biomedical applications and its relevance in public health.
This environment shared by the University of Brasília (UnB), in collaboration with CERTBIO - Laboratory for Evaluation and Development of Biomaterials in the Northeast and Life Care Medical Industry and Commerce, to transform basic research into a marketable product, ready to be integrated into healthcare systems. Translational Health Research (THR) played a crucial role in visualizing the stages, institutions, and, especially, the requirements of the Brazilian health regulatory framework as an essential component of the scientific process. This is because outlining these phases of the Research, Development, and Innovation (RD&I) process in health represents an improvement in basic or applied research Felipe et al. (2020). Translational governance in the field of RD&I in health facilitates integration between essential sectors of society and accelerates the introduction of innovations for the use of the population Barreto et al. (2020).
2 Materials and methods
The codification and presentation of Translational Health Research (THR), originating from initiatives outlined in the Roadmap of the National Institutes of Health (NIH), represent the diversity of the RD&I process in health. Simultaneously, they refine scientific practice based on empirical evidence. Capturing the complex nuances of research allows for the delineation and characterization of stages, essential funding, calculation of average durations, and identification of key contributors. This understanding sheds light on the mechanisms of knowledge generation and its applications for the benefit of society, aiming to avoid temporal and material waste, but primarily playing a role in delivering tangible results that meet the population’s expectations for the sector. This non-linear approach, as highlighted in Rosa (2022), ensures a smooth and natural transition between phases, promoting an RD&I process in health without setbacks. Hence, allows activities related to the phases to be executed simultaneously and even to regress from one phase to another.
The foundation of the theoretical-methodological process of Translational Health Research (THR) is linked to identifying, consolidating, and overcoming its Times, which traditionally aim to prevent scientific research from getting lost in the Valley of Death before being assimilated into healthcare systems. The central objective of THR is, therefore, to effectively overcome this challenge, enabling the successful transition from research to practical application in healthcare systems (Figure 1).
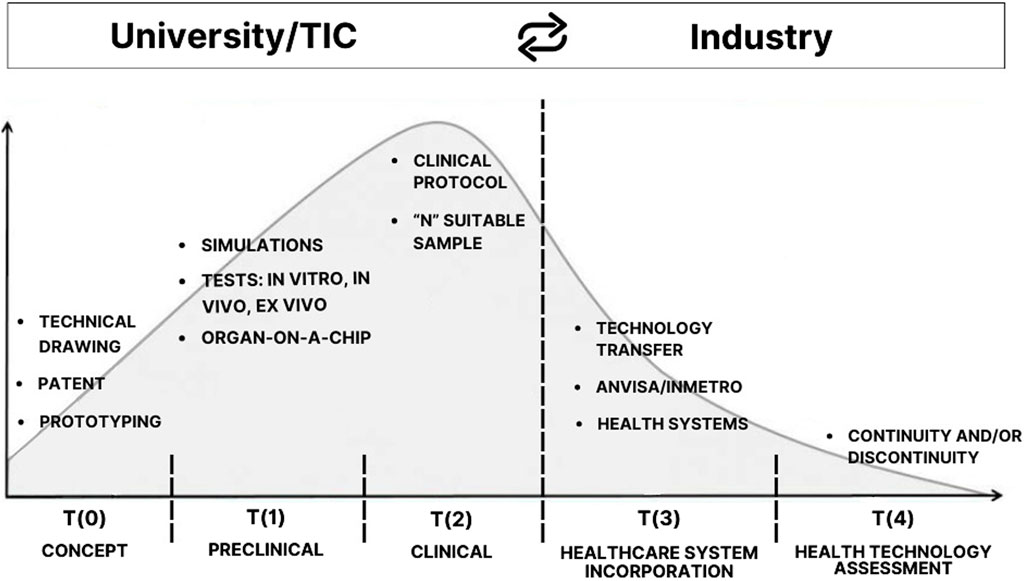
Figure 1. Translational Research maps the path from academia to industry and is marked by the “Valley of Death”. This critical point, at the border between T (2) and T (3) phases, is where promising discoveries commonly encounter obstacles and fail to cross the valley to reach industry. The authors adapted from Seyhan (2019).
As described by Seyhan (2019), the Valley of Death is a gap that has opened between basic research (bench), clinical research, and patients (bedside) in need of new treatments, diagnostics, and prevention. This gap widens and deepens, justifying the analogy with the term. Studies demonstrate that the concept of the Valley of Death is used to describe situations where technology has failed to reach clinical implementation, i.e., to have evidence that allows application in healthcare systems Rosa (2022); Fernandez-Moure (2016).
The anticipation and preparation for potential failures in the RD&I process in health, with a translational perspective, have become essential to prevent promising research projects from facing significant impediments before their practical implementation, thus avoiding premature failures. Therefore, the theoretical-methodological framework of THR represented by the Times (T (0)) to (T (4)) has gradually been systematically operated by major scientific conglomerates such as the NIH. Gradually, other important centers of scientific development have adopted this theoretical-methodological framework.
Table 1 illustrates the epistemology of the T phases, which were progressively adapted and applied to a variety of research objects as they were made public.

Table 1. Example of a translation model. The sequencing of translation from T (0) to T (4) was an advancement for THR but does not imply linearity in research. Difficulties in the ANVISA registration and/or registration phase T (3) may require a return to phases T (0), T (1), or T (2)—depending on the issue. Source: Rosa (2022).
Initially, the Times for THR were restricted to T (1) and T (2), essentially representing the pre-clinical and clinical research phases Khoury et al. (2007). However, the proposal presented in the figure above is a development of the earlier approach by Khoury et al. (2007), which already employed the mapping of THR through its times. The translational model, with the five phases T (0) to T (4), came to guide the THR process, and Khoury et al. (2010) played a crucial role in this transformation.
Specifically for the translation faced by the RD&I in health of the VESTA® Facial Respirator, the initial theoretical-methodological process presented by Khoury et al. (2010) was used. This was adapted taking into account the specificities involved in this technology. The methodological appropriation of THR and its Times for the VESTA® Facial Respirator can be observed in Figure 2.
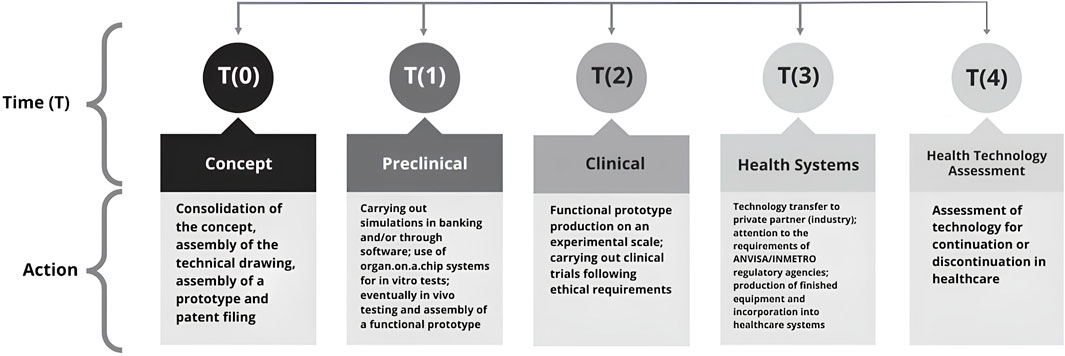
Figure 2. Methodological model of Translational Health Research (THR) applied to the VESTA® facial respirator, demonstrating the Phases or Time (T) and their respective Actions. Phases T (0) to T (4) represent the R&D ecosystem in health necessary to transform basic and/or applied research into a marketable product. Adapted from Khoury et al. (2010). For the VESTA® device.
This investigation unfolds as a qualitative study, as outlined by Poupart et al. (2008), supported by the unique approach of the case study proposed by and is grounded in the singular approach of the case study proposed by Ginzburg (1990). The core of the analysis lies in the interpretation of the translational research of the VESTA® respirator, which materializes through the intricate process of RD&I, originating from the University. The ability to identify relevant points, considered as revealing clues during the investigation, not only enriches the interpretation but also enhances the analysis by eliminating elements that may distort the desired course. This meticulous approach incorporates elements of micro-history, as advocated by Ginzburg (1990), providing a more refined and insightful dimension to the understanding of the phenomenon in question.
Thus, this study is not limited to the surface of the health innovation process but delves into complex interpretative layers, utilizing a unique approach that highlights not only events but also subtle clues outlining the pathway of the VESTA® respirator, traversing all phases or stages of Translational Health Research. The exploration of the theme of funding and the pattern of interaction among stakeholders linked to the national Science, Technology, and Innovation (ST&I) system in the context of VESTA®, focusing on the models of the Quintuple Helix; the analysis and description of the steps taken by the VESTA® Respirator Project, based on the conventional phases of RD&I in health; and the proposition of a translational model for health devices originating from the university, grounded in scientific and technological activities carried out during the RD&I process of VESTA®, are some of the guiding objectives touched upon in this extensive theoretical-methodological framework presented here.
3 Results
3.1 Concept–phase T (0)
3.1.1 The case of the VESTA® facial respirator
The RD&I process in the health area of VESTA®, from the beginning, encompassed the theoretical and methodological foundation of Translational Health Research (THR). Initially, there was the organization of the research group and the start of building the scientific concept of the technology. In response to the health emergency caused by COVID-19 on 20 March 2020. The research group lead by the Biomedical Engineering at the Gama Faculty (FGA), in collaboration with researchers from the Ceilândia UnB Campus (FCE), Planaltina UnB Campus (FUP), Campus Darcy Ribeiro (UnB), and the Federal University of Campina Grande (UFCG), represented by the Laboratory for Evaluation and Development of Biomaterials in the Northeast (Certbio), performed clinical and enhancement investigations on the PFF2 respirator the PFF2-type respirator. The technology involved adding a layer of protection with chitosan nanotechnology (biological layer), thereby increasing the effectiveness in retaining and filtering microorganisms Rosa (2022).
The respirator is a dynamic respiratory device with multifunctional properties designed to prevent infectious diseases (Figure 3). It features self-cleaning characteristics and drug delivery capabilities. It was developed with a dynamic mechanical structure, incorporating a dual-flow filtration system that allows for a higher air flow. The filtering element performs self-cleaning both at the inlet and outlet, inactivating viruses through a nanolayer that acts as a dual-phase filtering stage, forming nanopores that also enable controlled drug delivery. The scheme presented below illustrates the scientific conception of this technology, i.e., the idea behind its development.
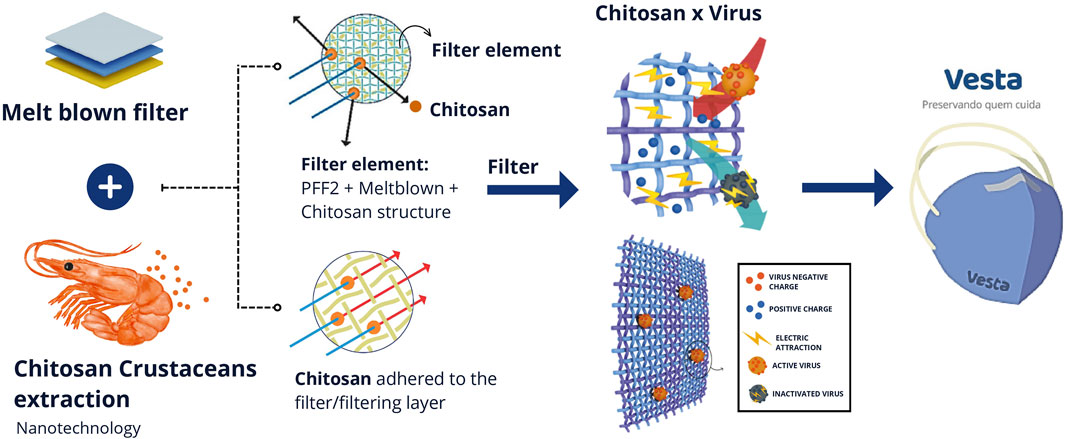
Figure 3. Illustration of the scientific conception of VESTA®. The VESTA® respirator is composed of the deposition of chitosan nanoparticles, derived from the exoskeleton of crustaceans, onto the pre-existing melt-blown (filtering) technology in PFF2 masks. The chitosan nanoparticle is positively electrostatically charged, attracting viruses, bacteria, and fungi that are negatively charged. Upon contact, virus inactivation occurs. Source: the authors.
The SARS-CoV-2 virus exhibits a spherical structure with a diameter ranging from 50 to 200 nm. Its configuration includes an external envelope with a negative electric charge, measuring about 20 nm in length, giving it a crown-like appearance Lopes and Carneiro (2022).
The chitosan polymer chain, due to its chemical characteristics, carries an overall positive charge with manifold possibilities Ng et al. (2016); Felipe et al. (2017). Due to the electric field produced by the differences in their charges, the virus and chitosan are attracted to each other, resulting in the disorganization of the viral structure. Additionally, the manufacturing process of the PFF2, which interleaves the meltblown with the other layers of the mask, induces the generation of static energy that enhances the inactivation of viruses, bacteria, and fungi Bandi (2020).
3.1.2 Clinical protocol and patent application text
In the context of R&D in health for VESTA®, the strategy was adopted to initiate the development of intellectual property protection protocols, encompassing the patenting process, preclinical, and clinical trials, already during phase T (0). Traditionally, preclinical and clinical stages are associated with phases T (1) and T (2), respectively. However, in this case, these phases were brought forward to identify limitations and expedite necessary activities. This was done to explore the nonlinear nature of Health Technological Protection (HTP) and ensure timely submissions.
The intellectual property protection, named “Dynamic Respirator with Multifunctional Properties to Prevent Infectious Diseases with Autocleaning and Drug Delivery Properties”, was filed with the National Institute of Industrial Property (INPI) on 23/04/2021, under process number BR 10 2021 007808. The patent filing involved various stages, including prior state of the art analysis for the proposed technology, drafting of the patent text, and collaborations between the inventors and the Technological Innovation Nucleus, Technological Development Center, and Projects and Innovation Directorate (NIT/CDT/DPI/UnB), requiring time and resources.
While the patent filing process represents a significant investment in time and resources, it opens up discussions about technology transfer with industry and/or private enterprises, being essential for translation. In the Brazilian context, scientific development carried out in a public university is typically only discussable with the private sector after the completion of the intellectual property protection process.
The drafting of the clinical protocol, which also represented the T (0) for VESTA®, was titled: “Controlled, randomized, and pragmatic clinical trial to assess the effectiveness of a respirator with chitosan nanoparticles in reducing the incidence of contamination and infection by SARS-CoV-2 in healthcare professionals”. It was submitted to research ethics committees registered in the Registry of Promoting Entities of the Research Ethics Committee (CEP) in early October 2020 and received approval at the end of March 2021.
3.1.3 Production of the mockup and prototyping: pilot batch
When scientists or researchers dedicate themselves to the development of health technologies, as is the case with VESTA®, it is crucial to consider a series of aspects to ensure the production of the mockup and its compliance with the established objectives. The mockup represents the conceptual version of the prototyped technology, ready to support activities in the T (1) and T (2) stages of translational research. This prototype should incorporate the scientific characteristics of the invention and form the pilot batch of the technology, intended to be tested in the pre-clinical and clinical phases. However, a respirator is classified as personal protective equipment (PPE) and is subject to various national and international standards, imposing considerable limitations on the design processes. Often, solutions are compelled to conform to these specifications. Thus, the deconstruction process becomes a form of reverse engineering, serving as the basis for the development of the VESTA® respirator Rosa et al. (2021).
Any endeavor dedicated to the production of health devices for the prevention and treatment of COVID-19, especially during the early stages of contagion, faced significant challenges, such as shortages of supplies and logistical issues, among other gaps. In the T (0) phase of translation, professionals in product design and engineering associated with the VESTA® R&D took on the task of developing a plan that would facilitate understanding of national and international standards. This proved crucial to optimize the mapping of technology production, with the aim of reducing gaps between the T phases Rosa (2022); Rosa et al. (2021).
The knowledge of the productive ecosystem involving the product life cycle Rosa et al. (2021); Felipe et al. (2020); Rosa (2022), and the levels of technological maturity: TRL/MRL scale Rosa et al. (2021); Felipe et al. (2020); Rosa (2022), was essential for the development of the VESTA® R&D. During the initial and challenging phase of the COVID-19 pandemic, the interdisciplinary group in Biomedical Engineering at the University of Brasília (UnB) succeeded in incorporating such activities in the T (0) stage, ensuring the production of the pilot batch that underpinned the implementation of subsequent T (1) and T (2) phases (Figure 4). Images of the initial batches of the VESTA® Facial Respirator are available in the Supplementary Material.
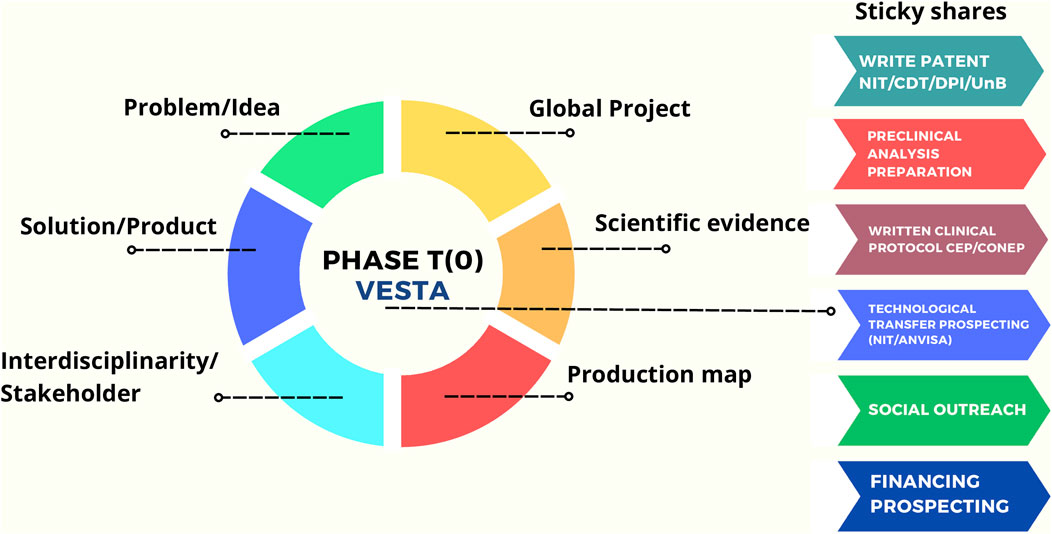
Figure 4. Comprehensive model of Translational Phase T (0) for VESTA®, with an emphasis on integration and maximization of available university tools to transform the idea into healthcare access. The model highlights the importance of an interdisciplinary approach among stakeholders, with a evidence-based perspective on the issue and justification of the Project. The Global Project is the element that connects all stakeholders and requires actions aligned with its development and implementation. Source: Rosa (2022).
With the effective planning and consolidation of the global project, as outlined in the model presented earlier, the search for funding to support the translational phase budget began. This approach was designed to maximize the use of tools available at the university, with the goal of turning the idea into improvements in access to healthcare. In this way, the T (0) stage of VESTA® was successful, providing a framework for submitting funding proposals, drafting patents, guiding the pre-clinical phase, and contributing to the development of the project associated with the Research Ethics Committee (CEP) and the National Research Ethics Commission (CONEP). In the context of the T (0) phase model of VESTA®, prospecting partners in the private sector and collaborating with the NIT/UnB proved crucial in achieving the desired results.
3.3 Preclinical VESTA®—phase T (1)
3.3.1 Mechanical and biological activities
The pre-clinical phase of VESTA® is closely related to the conceptual phase of the product (T (0)). It became evident that the interface between the areas of product design and engineering and biology and mechanical engineering played a significant role in formalizing T (1) VESTA®. Throughout the conceptual development of the product, various regulatory elements contributing to the formalization of the T (1) phase plan were identified. Pre-clinical tests were guided by Mechanical and Biological segments, complying with regulatory requirements for testing respiratory protection equipment, including the ABNT NBR 13698:2011 standard for mechanical tests and ISO 18184:2019 for biological tests Landim et al. (2023). The following tests were outlined to be conducted: i. mechanical evaluations, ii. antiviral tests, iii. microbiological tests, and iv. cytotoxicity tests. In these tests, the control group consisted of the commercial PFF2 model, while the experimental group adopted the VESTA® model. Since the focus of this study is on the translational research process in health, a detailed analysis is recommended for the pre-clinical tests of mechanical evaluations, antiviral, microbiological, and cytotoxicity assessments conducted earlier in our study Landim et al. (2023).
In comparative tests, VESTA® demonstrated superiority in tear resistance and met the safety requirements of the National Health Regulatory Agency (ANVISA); it also showed significant antibacterial and antiviral capacity without toxicity to HaCaT and VERO CCL-81/E6 cells Landim et al. (2023). The pre-clinical results, in addition to validating the technology in regulatory aspects, meeting the prescribed standards for this type of medical product, supported the writing of the clinical protocol and later the request for ANVISA registration and approval (the national agency that authorizes the production of the technology on a commercial scale). ANVISA registration corresponds to phase T (3) of translational research. The schematic representation of Phase (1) VESTA® is provided below (Figure 5).
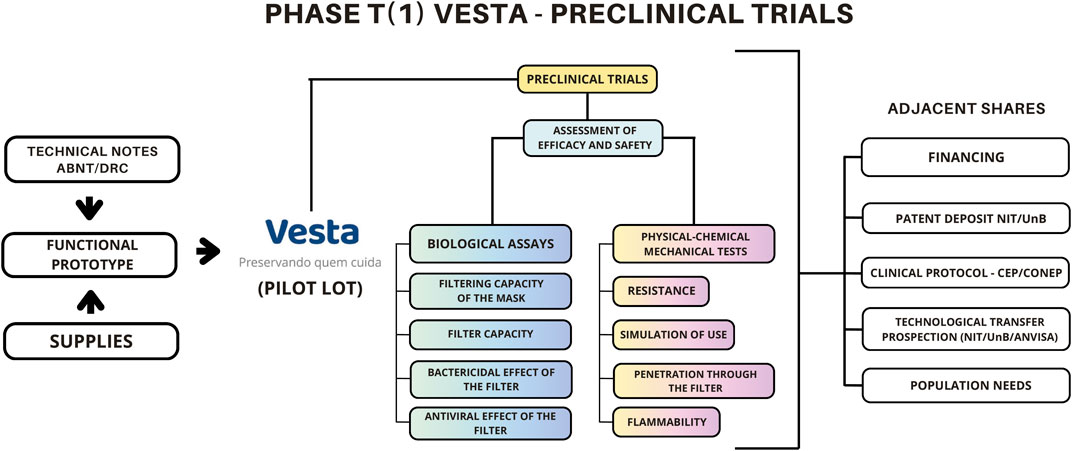
Figure 5. Translational Phase T (1) of the VESTA® Model. This stage aims primarily at evaluating the effectiveness and safety of the technology through pre-clinical trials and pilot bench tests. This phase requires a precise assessment of Standards and Technical Notes from regulatory bodies, as well as the development of a functional prototype. Source: Rosa (2022).
The previous representation highlights the fundamental steps of the technology’s efficacy and safety evaluation process, including related actions that clearly laid the groundwork for the translation of the technology to the current phase. Activities such as patent writing and seeking funding permeated both T (0) and T (1) phases. Specific aspects, such as identifying accredited and equipped laboratories for conducting pre-clinical tests, covering both the biological and mechanical-physical-chemical bases, posed a significant challenge during the execution of the VESTA® R&D project.
Limitations, such as the absence of equipped laboratories within the University of Brasília (UnB) and the lack of financial resources to hire external laboratories and specialized workforce, particularly for large-scale biological assays, were obstacles encountered in the T (1) phase. However, in the context of the translational perspective, the T (1) phase of the VESTA® R&D project was successful, as it managed to incorporate data obtained from pre-clinical tests into the clinical protocol. This protocol, in turn, was later approved by the CEP/CONEP.
3.4 Clinical trial VESTA®—phase T (2)
The T (2) phase of translational research involves conducting tests on human subjects, a stage that, in Brazil, requires prior authorization from the CEP and CONEP. During the COVID-19 emergency period, CONEP issued guidance specifying that research protocols related to SARS-CoV-2 should be submitted by the CEP to CONEP for evaluation. Following this process, the clinical protocol for the VESTA® Respirator was submitted [CAAE number 39177620.5.0000.8093 (Supplementary Material)]. The clinical study plays a crucial role in transitioning from research to practical application, determining the progression from T (2) to T (3) or even the return from T (2) to T (1) and/or T (0), depending on the circumstances.
It is important to highlight that the clinical study, or simply T (2), in the translational research perspective, should be considered in conjunction with the subsequent stages. The scenario where clinical research is conducted, inheriting the continuity of the pre-clinical study, must align with T (3), particularly regarding the regulatory requirements of ANVISA. Alternatively, it must be adequately prepared to meet the demands imposed by T (3). Ongoing dialogue with the private sector is crucial as it plays a fundamental role in translating the knowledge generated at the university to meet market demands. Furthermore, in the case of VESTA®, stages T (0), T (1), and T (2) were executed by the university. It is worth noting that the responsibility for registering the technology with ANVISA falls on the private sector, making it essential for patent filing and technology transfer stages to be completed effectively.
3.4.1 Clinical study–controlled and randomized
The VESTA® trial obtained definitive approval in April 2021, with the CAAE number 39177620.5.0000.8093. The approved protocol is titled “Efficacy of a facial respirator with chitosan nanoparticles (VESTA®) to reduce the incidence of contamination and infection by SARS-CoV-2 in healthcare professionals”. Throughout the process, the clinical protocol underwent improvements until it was considered approved by CONEP. The stages of the pilot phase of this clinical trial are detailed in the table below:
Table 2 synthesizes crucial information about the pilot clinical trial conducted by the VESTA® PD&I development group. Due to confidentiality issues related to the technology transfer process and the publication of unpublished results, the raw research database and its statistical analyses are not available. However, the results indicate the equivalence of the tested technology, without demonstrating inferiority. The information presented in this context is essential to highlight the successful execution of the T (2) phase in the translational perspective.
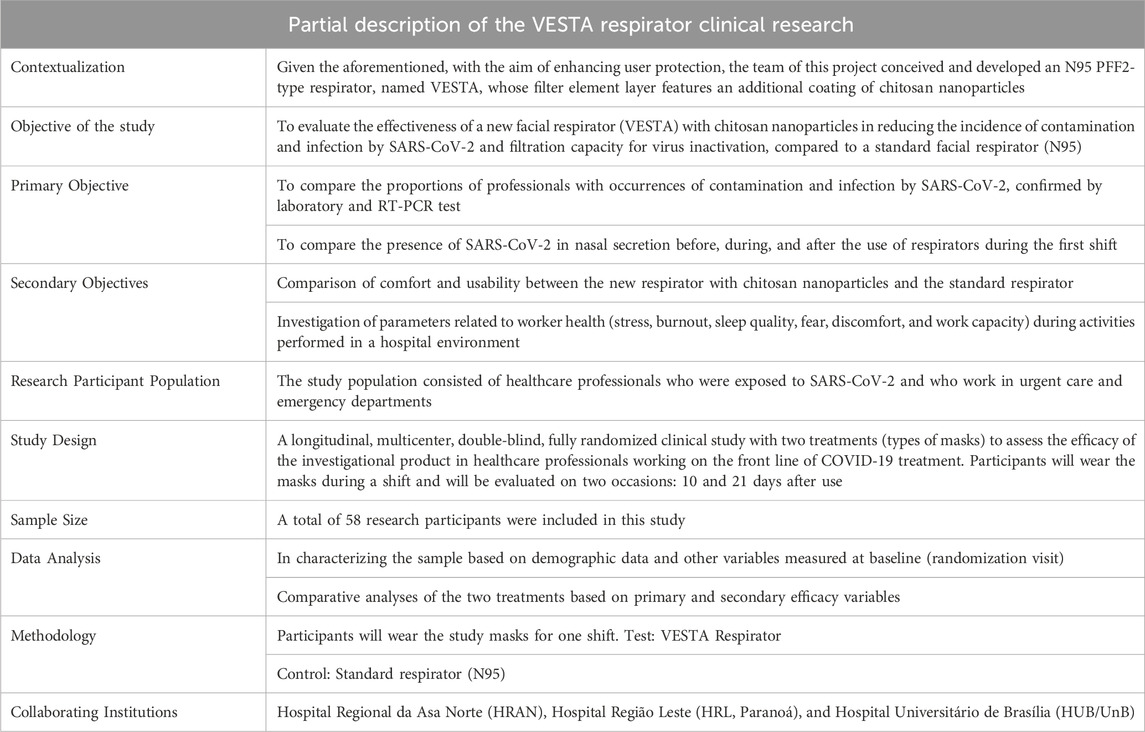
Table 2. Data from the clinical protocol of the VESTA research and research database. Source: Adapted from Rosa (2022).
3.4.2 Planning and clinical design
It is worth noting that the VESTA® Trial adopted a sound methodology with proper control, such as blinding of the outcome assessors and statistical analyst; concealed allocation using opaque and sealed envelopes; and randomization using a random number generator. This methodology aimed to provide valid results and to reduce the risk of bias. In the approximately 40-day period, from May 2 to 10 June 2021, during what can be considered one of the most critical moments of the COVID-19 pandemic in Brazil, data activities for the clinical study were carried out. These activities were organized as demonstrated in the flowchart in Figure 6, which details the division of tasks within the group: I) Participant selection; II) Grouping of collected data; III) Monitoring participants during the study; IV) Sending samples as necessary; and V) Thorough analysis of the obtained data.
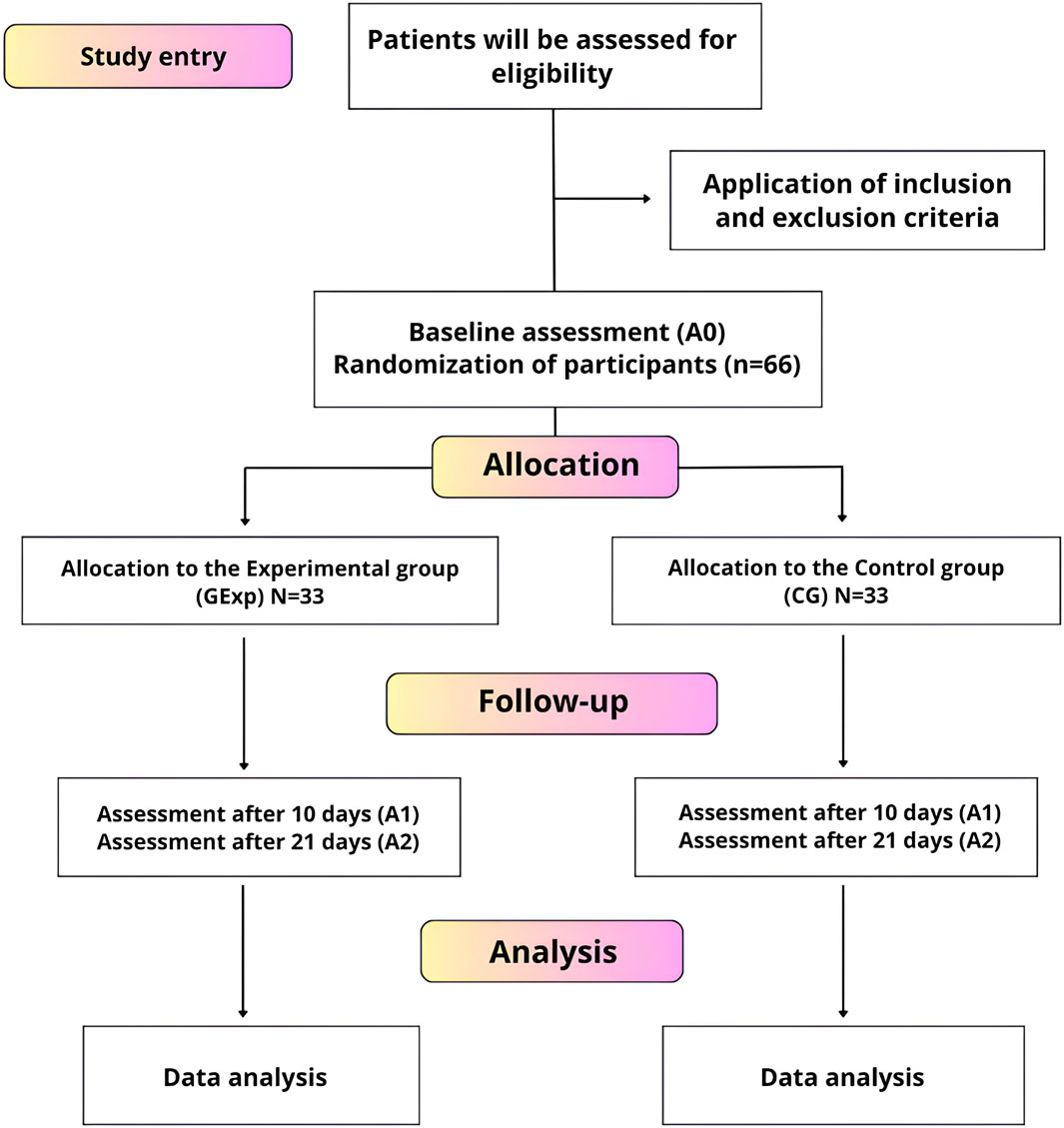
Figure 6. Flowchart of the controlled and randomized clinical trial of VESTA®. The diagram provides a detailed overview of the process by which participants are recruited, randomized into experimental and control groups, followed up, and assessed throughout the study. Source: Adapted from Rosa (2022).
The clinical study started with 66 research participants, and there was a loss of 8 participants during the study, concluding with 58 participants at the end. During this period, approximately 180 blood tests and 180 RT-PCR tests were collected. In addition, about 80 biological analyses of the masks used by the participants were performed, both in the experimental and control groups (estimates). During this stage, various researchers, healthcare professionals, private sector partners, collaborators from other higher education institutions, and the participants themselves dedicated themselves intensively to the T2 phase of the VESTA® PD&I project.
3.4.3 Regulations and contributions from the private sector in phase T (2) of the VESTA® translation
The regulatory elements associated with ANVISA, especially in the context of clinical trials for market-oriented technologies, go beyond the usual considerations of basic research. When the intention is to influence healthcare systems, clinical trials must not only ensure the ethical and statistical requirements of safety and efficacy but also incorporate the complexities inherent in large-scale production, which are intrinsic to private sector involvement. We advocate for the promotion of the concurrent adoption of an integrated approach that addresses both ethical, statistical, and regulatory aspects as well as commercial considerations. Additionally, we emphasize that the VESTA® PD&I, based on the presented and discussed results, fully completed the T (2) phase of translation. Finally, it is imperative to recognize the importance of entrepreneurial thinking among researchers at the forefront of scientific research, always in compliance with technical standards and current legislation. The integration of the university’s NIT plays an essential role in this context.
Technology transfer offices (NITs) possess the necessary expertise to assist researchers in navigating the intricate landscape of industrial regulations and in the effective management of intellectual property. Moreover, they can facilitate strategic partnerships with the industry, contributing to the practical application of research results and the commercial viability of developed products. Thus, collaboration between researchers, NITs, and industries becomes indispensable for overcoming the intrinsic challenges associated with conducting clinical research involving equipment manufactured according to Good Manufacturing Practices (GMP), all while safeguarding the associated intellectual property.
Conducting clinical research involving equipment manufactured in compliance with Good Manufacturing Practices (GMP) introduces a significant layer of complexity to the process. GMP [40] constitutes rigorous standards that industries must adhere to in order to ensure the quality and safety of their products. Integrating these practices with the preservation of the intellectual property of the invention emerges as an additional challenge, demanding a strategic and meticulous approach. Researchers face the responsibility of ensuring that the devices used in the research comply with the standards stipulated by GMP, all while safeguarding the intellectual property associated with their creation.
3.4.4 Technical notes and collegiate board resolution (RDC)
The application of technical standards, such as Technical Note No. 004/2016/GGTPS/DIREG/ANVISA ANVISA (2020b) and the Resolution of the Collegiate Board of Directors (RDC) No. 448, dated 15 December 2020, from ANVISA ANVISA (2020a), was fundamental for the completion of phase T (2) in the development of the VESTA® Respirator. The incorporation of these standards enabled the formalization of a more robust clinical trial protocol. Technical Note No. 004/2016 provided detailed guidance on the requirements for conducting clinical trials, including guidelines for the collection and presentation of data related to the safety and efficacy of the product. This approach allowed us to structure the trial to meet the standards required by ANVISA, ensuring the quality and reliability of the obtained results ANVISA (2020b).
The RDC No. 448 ANVISA (2020a), in turn, established specific requirements for the manufacturing, import, and commercialization of Personal Protective Equipment (PPE) during the public health emergency related to SARS-CoV-2. By incorporating these requirements into our clinical trial protocol and by preparing the statistical analysis and the Final Study Report following the current ANVISA clinical reporting guidelines, it was possible to ensure that the VESTA® Respirator also met the urgent demands of the health market.
This way, by adhering to the technical standards of ANVISA, we were able to establish a comprehensive clinical trial protocol for the VESTA® Respirator, ensuring its efficacy, safety, and regulatory compliance. This approach allowed us to move forward with confidence in the T (2) phase of product development, contributing to delivering to the industry a reliable result to meet the registration and registration procedures of ANVISA.
3.5 ANVISA registrations and/or records and health systems—phase T (3)
In Brazil, the request for registration of health technology with ANVISA is made by the industry responsible for manufacturing the technology, not by the university that conducted the research for its development. This dynamic occurs because the industry generally takes on the responsibility for large-scale production and commercialization of the product in the market. The university, on the other hand, often plays a crucial role in the early stage of technology development, conducting pre-clinical and clinical studies to assess its safety and efficacy.
However, for the technology to be commercially available and used in clinical practice, it is imperative that a company takes responsibility for registration with ANVISA. This manufacturing company is tasked with compiling all technical documentation, clinical study results, test reports, quality certificates, and other requirements established by ANVISA for product registration. Once all these requirements are met and approved by ANVISA, the health technology can be marketed in Brazil. In the specific case of the VESTA® Respirator, the manufacturing company mentioned in the registration and/or registration request to ANVISA was Life Care Medical Indústria Comércio EIRELI.
To understand the R&D routine of the VESTA® Respirator in phase T (3), we can consider the following fundamental information: the intellectual property protection through patenting the invention; the technology transfer agreement to the industry; the registration and/or approval with ANVISA and, finally, the incorporation into healthcare systems.
3.5.1 Technology transfer
The legal agreement, known as the Confidentiality and Non-Disclosure Agreement, signed between the University of Brasília (UnB) and the company LIFE CARE, was formalized in March 2021, followed by the preparation of the licensing contract by the Technology Innovation Center (NIT)/Technology Cooperation Advisory (ACT)/Intellectual Property Department (DPI) of UnB in December of the same year. This agreement aims to establish that the involved parties are interested in exploring possible future collaborations that may require the exchange of confidential and exclusive information between them. The subject of this agreement relates to the “Technology” named “Dynamic respirator with multifunctional properties to prevent infectious diseases, with self-cleaning and drug delivery protection properties”—VESTA® Facial Respirator. To facilitate the understanding of the technology transfer process, a Table 3 has been prepared that lists the titles of the documents exchanged between UnB and the company, accompanied by a column listing the documents that the company needs to present.
The University of Brasília (UnB) and Life Care developed strategies to meet mutual interests and established criteria for technology transfer, including the definition of terms related to royalty payments and other activities outlined in a work plan. In this specific context, royalties represent periodic payments made by the licensee (Life Care) to the licensor (UnB) in exchange for the right to use intellectual property, such as an invention, patent, trademark, copyright, or technical know-how [43]. These payments serve as compensation for the use or commercial exploitation of the intellectual property protected by the licensor.
With the formalization of Technology Licensing Agreement No. 23106.091134/2021-81 in December 2021, Life Care, as the licensee, initiated the process of registration and/or certification with ANVISA. The mentioned contract, executed between the University of Brasília (UnB) through the Center for Development Support (CDT) and the company Life Care Medical Indústria Comércio Eirelli, enabled the use and commercial exploitation of the creation embodied in the patent application No. BR 10 2021 007808 1.
3.5.2 ANVISA registrations and records
The preparation of the product registration dossier for ANVISA, as well as the registration process with the agency, are crucial steps that precede large-scale industrial production, particularly in the case of Personal Protective Equipment (PPE) like VESTA® (The registration number is 8233240002 and refers to the Disposable Facial Protection Mask–Life Care (Supplementary Material)). ANVISA establishes specific requirements that manufacturers must meet to register and commercialize these products in the country, including Complete Technical Documentation, Safety and Efficacy Studies, Certificates of Conformity, Labeling and Packaging Information, and Test Reports and Quality Certification.
The Life Care organized the protocol and met the requirements, providing all necessary information. They prepared a complete registration dossier and submitted the product registration to ANVISA, demonstrating that VESTA® meets the quality, safety, and efficacy standards required for its commercialization in the country. These steps are crucial to ensure that only safe and effective products reach the market, aiming to protect the health and safety of users.
The submission of VESTA®’s registration occurred shortly after the technological transfer. The petitioning was done in March 2022, marking the notification of VESTA® Facial Respirator to ANVISA. In practical terms, this means that the research project initiated by the University of Brasília (UnB), Federal University of Campina Grande (UFCG), and other partners, driven by the phenomenon of the COVID-19 health emergency, began the PD&I process in March 2020, culminating in the notification of health technology registration in March 2022. The request was made by the company LIFE CARE MEDICAL INDÚSTRIA COMERCIO EIRELI, corresponding to Notification of Medical Device Class I–low risk, according to the risk associated with its use. The registration number is 8233240002 and refers to the Disposable Facial Protection Mask–Life Care.
However, it is a fact that the research project of the VESTA® Respirator, guided by Translational Health Research, was in accordance with the regulatory requirements of ANVISA. Furthermore, it established a technology translation model, classified as low risk, belonging to Class I, which encompasses from the beginning of the research project to the petitioning and registration with ANVISA, completed within a period of 24 months. The course from the start of the research to the authorization for industrial-scale production unfolded over a period of 2 years.
3.5.3 Industrial-scale production
Use and indications: This product has undergone tests in accordance with ABNT/NBR 13698 standards and has been approved by the SECRETARY OF LABOR OF THE MINISTRY OF ECONOMY as a semi-facial particle-filtering piece Class PFF2 de Normas Técnicas (2024). It is intended for the protection of the respiratory tract against dust and non-oily mists, free from the emission of gases and/or vapors, such as metal or plastic fumes, silica, textile fibers, refined cement (Portland), iron ore, coal ore, aluminum ore, powdered soap, talcum powder, lime, caustic welding, vegetable dust (such as wheat, rice, corn, sugarcane bagasse, etc.), aviary dust containing feed residues, feces, bird feathers and down, sanding and grinding dust, sulfuric acid mists (when used with suitable protective eyewear), COVID-19 virus, bacteria, among others.
The use of this respirator should be carried out with the knowledge and approval of the occupational health, safety, and medical areas and/or the person responsible for the company. It can be employed to reduce occupational exposure to aerosols containing potentially pathogenic biological agents (Figure 7 Medical (2024).
Figure 7. The figure depicts the final stage in the translational process of the VESTA® respirator, where the product is publicly available for purchase on the Life Care company’s website. Source: Medical (2024).
Absolutely, transitioning scientific research into a commercially viable product, as seen with the VESTA® Respirator, involves a unique set of challenges. Beyond the scientific and technical aspects, additional hurdles include securing funding for development, compliance with government regulations, navigating market competition, and ensuring product quality and safety. This transition from research to a market-ready product often requires collaboration between research institutions, private industries, and regulatory bodies to address these multifaceted challenges effectively.
Indeed, navigating the commercial landscape post-approval introduces a new set of challenges. While obtaining approval for industrial production marks a significant milestone, the subsequent competition in the market can be formidable. In the case of VESTA®, despite its innovative extra layer of chitosan in a disposable PFF2 mask, it competes with various other similar products in the market. The completion of the T (3) phase by PD&I VESTA®, securing approval for industrial production and making the technology available to the public, signifies an important step in providing access to society. However, as of early 2024, specific data on the quantity of units produced and sold is not available. It will be essential for ongoing monitoring and analysis to understand the market reception and impact of the VESTA® Respirator.
The T (4) phase, involving the assessment of the ongoing production or potential discontinuation of the technology, has not been executed in this context. However, it is crucial to highlight that preserving the culture of translational research, deeply rooted in research groups associated with the university, plays a fundamental role in enabling effective translation from science developed within the academic institution.
4 Discussion
4.1 Stakeholders
A synergy among various key actors, forming the “quintuple helix” composed of academia, industry, government, civil society, and environment, played an essential role in consolidating the RD&I of the VESTA® Facial Respirator.
The university takes on the responsibility of leading and organizing the research and development process, providing a conducive environment for the generation of scientific knowledge, especially in the pre-clinical and clinical phases. On the other hand, the industry possesses the expertise necessary to translate validated scientific models into marketable products, driving innovation to the market. The government often plays an essential role as a funder of scientific development, promoting investments and policies that stimulate innovation. Civil society plays a fundamental role in identifying and directing health needs to be addressed, ensuring that innovations are truly useful and relevant to the community. Finally, the environment provides the conditions to leverage scientific development, as exemplified by the VESTA® project, which integrates natural resources like chitosan with sustainable economic activities such as fishing and shrimp shell processing.
4.2 To fund: a fundamental issue
The research aimed at adapting the VESTA® Facial Respirator to meet ethical and regulatory requirements, as in any scientific endeavor, required funding to address challenges in conducting tests, clinical trials, prototyping, and other crucial stages leading to the practical application of research results. During the COVID-19 pandemic, various sources of funding emerged to boost the progress of VESTA®’s Research, Development, and Innovation (RD&I).
Typically, funding for scientific research, whether basic or applied, comes from the public sector, and in the case of VESTA®, it was no different. Publicly affiliated institutions such as the National Council for Scientific and Technological Development (CNPq), the Foundation for Research Support of the Federal District (FAPDF), and the Federal Institute of Education, Science, and Technology of Brasília - Ceilândia Campus (IFT), which traditionally provide public funding for scientific research, contributed to VESTA®’s advancement. Some offered more substantial amounts, while others provided smaller supports.
Additionally, there was modest support from the private sector of civil society, such as the Brasília Section of the Institute of Electrical and Electronics Engineers (IEEE) group. However, it is important to highlight the fundamental role of individuals who, moved by the research group’s efforts, individually contributed through online donations using crowdfunding platforms like Vakinha. These donations were essential for the VESTA® Project to quickly respond to its financial needs. Unlike the other mentioned forms of support, which went through extensive bureaucratic processes, individual contributions allowed for more agile access to resources for research advancement.
Due to the specific nature of the COVID-19 pandemic, civil society, through individual donations, played a significant role in driving VESTA®’s research forward. We dare to say that if the Vakinha donation had not been made, VESTA®’s research might not have overcome the valley of death of Phases T (0) and T (1).
4.3 Scientific entrepreneurship
Translational Research (THR) and interdisciplinarity are two complementary and inseparable aspects. The VESTA® project illustrates how, in all phases of translation, from the initial conception stage (T (0)) to the large-scale production (T (3)) of the technology, various scientific areas and sectors of civil society collaborated in an integrated manner. This multidisciplinary and collaborative approach was crucial for the successful progress of the project, allowing the merging of knowledge, experiences, and resources from different fields to achieve significant and impactful results.
The scientific development originating from universities often faces challenges that may not be immediately apparent. One of the most challenging to identify is the lack of scientific entrepreneurship, especially among researchers in the fields of biomedical engineering and health, who may not be familiar with technical standards and regulatory agency regulations for the production of medical equipment. Basic and/or applied research conducted at universities that do not incorporate pilot batch production in accordance with technical standards faces significant challenges in overcoming the early stages of development, known as “valleys of death”. In the case of the VESTA® THR project, the integration of essential elements for translation, such as compliance with technical standards, was carried out from the conceptual phase, i.e., from the project’s inception. This demonstrates a proactive and strategic approach to ensure that regulatory and quality requirements are considered from the beginning, maximizing the chances of success in transitioning from concept to clinical practice. One of the motivations for the UnB group to embrace the translation of VESTA® was precisely to identify that the technology is classified as low-risk Class I, where regulatory requirements were easier to meet.
4.4 Innovation culture
Researchers affiliated with universities often show low engagement with the services offered by TTOs, which are responsible for promoting intellectual property protection and supporting technology transfer. This lack of engagement can be attributed to various factors, including a lack of awareness about the services offered, institutional disincentives to pursue intellectual property protection and technology transfer, and even bureaucratic barriers that hinder access to TTO services. Any research with ambitions of becoming a marketable product needs to align seamlessly with TTO guidelines. Understanding the role of TTOs in the translational process was crucial for VESTA® as it ensured that activities such as patent filing and technology transfer were integral to the research activities from T (0) onwards. Despite all limitations, without integration between researchers and TTOs, research loses traction in overcoming the valleys of death.
4.5 Non-linearity of the THR
From another perspective, Translational Health Research, as analyzed, is a non-linear process. Despite the sequence suggested by the translational phases, crucial activities are needed from the very beginning, in the (T (0)) stage, also known as the conceptual phase. These activities, which used to be associated only with later translational phases like T (2) and T (3), include patent filings, production in compliance with good manufacturing practices, and technology transfers. It is imperative that, from the conception of the scientific or technological idea T (0), the involvement of a company or private initiative that will commercialize the technology is planned. Otherwise, the chances of achieving technology transfer in the T (3) phase are significantly reduced. Even with promising results from the T (2) phase or successful clinical trials, the absence of business partnerships hinders translation, thus increasing the risk of failure at this crucial stage.
According to the findings of this study, it is part of the university’s responsibility to provide practical solutions ready to be used by society. To address the challenges of technology transfer, it is essential for the university, through its research groups, to establish a strong integration with NITs. Furthermore, working collaboratively to overcome the “valley of death” in technology transfer by actively seeking partnerships in the private sector is crucial. By adopting this coordinated and proactive approach, the gap between T (2) (clinical research) and T (3) (ANVISA registration and/or incorporation into healthcare services) phases can be significantly reduced.
4.6 Incorporation into healthcare systems
To what extent is the industry willing to receive technological transfer from the university? This question demands a particularly important reflection as it is key to transforming scientific development into a health solution for the population. When considering the perspective of the Quintuple Helix, each stakeholder plays a crucial role in this dynamic. The university, through its researchers, acts as developers, the industry as producers, the state as a financier, civil society, and environmental aspects included. However, in the specific case of the VESTA® Facial Respirator, which has overcome the valleys of death and elevated scientific research to the level of a product ready to be commercialized, what will happen? Will international competition for PFF2 masks emerge as a new valley of death? Questions like these place us in the perspective of the epistemology of science, as VESTA® may become a commercially discarded option for not being able to compete with international competition.
The Quintuple Helix worked for the inventive and innovative process of VESTA®, but it still does not address aspects of incorporation. Often, the same state that finances scientific development does not have well-defined instruments for incorporation, thus leaving the innovation it funded outside its purchasing power or even its power of incorporation into the healthcare system. In Brazil, the Unified Health System (SUS) is free and universal, providing medications, equipment, and procedures to serve a large portion of the Brazilian population. Furthermore, SUS has historically played a role in the social and economic development of Brazil, as it enhances the Social Innovation Ecosystem (CEIS).
5 Conclusion
The University of Brasília (UnB) and its partners, in alignment with the industry, implemented the Translational Science and Technology (TST) process for the development of the VESTA® Facial Respirator. They successfully overcame the valleys of death, reaching industrial-scale production within 24 months. They developed a translational model for medical equipment that addressed the demands initially presented during the health emergency caused by the SARS-CoV-2 pandemic, from the technological concept to market availability.
The trajectory of the project emphasizes the need for an integrated approach from the early stages, highlighting the transversality of translational research and its non-linearity. Interdisciplinarity, scientific entrepreneurship, and a culture of innovation proved crucial in overcoming challenges, particularly by anticipating potential obstacles in the process of PD&I in health. The incorporation into healthcare systems, while still presenting challenges, is a crucial step in transforming scientific achievements into tangible benefits for society, especially in countries with universal healthcare systems like Brazil.
This study provides valuable insights for researchers, innovation managers, and policymakers, reinforcing the need for a comprehensive and proactive approach in conducting PD&I projects, highlighting the essential role of each component of the quintuple helix to achieve impactful and sustainable results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MF: Writing–original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization. LS: Writing–review and editing, Conceptualization. CG: Writing–review and editing, Methodology. AM: Writing–review and editing, Methodology, Investigation. RC: Writing–review and editing, Methodology, Investigation. AS: Writing–review and editing, Methodology. LC: Writing–review and editing, Methodology. AR: Writing–review and editing, Methodology. SR: Writing–review and editing, Writing–original draft, Methodology, Investigation, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was funded by the following stakeholders: i. National Council for Scientific and Technological Development (CNPq): process number: 307885/2020-8 and COVID-19 ORDER process number 403472/2020-2; ii. Foundation for Research Support of the Federal District (FAPDF): process number 00193-00000736/2021-64 and UnB/DPI/FAP/DF (COVID-19) process number 6913; iii. Online crowdfunding (VAKINHA). ID 964530. VESTA®—the mask from the University of Brasília. Civil society participation in the R&D process in healthcare through online donations; iv. Brasília Section of the Institute of Electrical and Electronics Engineers (IEEE); v. Federal Institute of Education, Science, and Technology of Brasília—Ceilândia Campus (IFT); vi. National Conference of Bishops of Brazil (CNBB). The funding source had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Acknowledgments
We are very grateful to all those who kindly contributed to the favorable research environment, particularly: Dr. Fátima Mrué, former Secretary of Health of the Municipality of Goiânia/Goiás/Brazil; Mr. Manoel Clemente from MCI Indústria e Comércio de Produtos Eletrônicos Ltda; Professor Marcus Vinícius Lia Fook and his collaborators from the Center for Research in Bioproducts and Biomaterials (CertBio/UFCG). We thank the collaborators from CDT/UnB (Technological Development Center of the University of Brasília); from the City Hall of Goiás/Brazil; from HRAN (Regional Hospital of Asa Norte); from Inovatie Health Services; As always, the responsibility for the final version remains with the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnano.2024.1384775/full#supplementary-material
References
ANVISA (2020a). Resolution No. 448, of December 15. Available at: https://antigo.anvisa.gov.br/documents/10181/6156982/RDC_448_2020_.pdf/7daf7838-fb07-4a1f-8b7dQ14 5425df7c2ccd (Accessed February 02, 2024).
ANVISA (2020b). Clinical evaluation of medical devices. Available at: hhttps://antigo.anvisa.gov.br/documents/10181/5736483/31V2.pdf/3ce3507c-4bc7-409d-a558-99b7be25da19 (Accessed February 02, 2024).
Bandi, M. M. (2020). N95-electrocharged filtration principle based face mask design using common materials. Okinawa Institute of Science and Technology Graduate University.
Barreto, J. O. M., Silva, E. N., Gurgel-Gonçalves, R., Rosa, S. S. R. F., Felipe, M. S. S., and Santos, L. M. P. (2020). Translational research in public health: challenges of an evolving field.
Boroumand, H., Badie, F., Mazaheri, S., Seyedi, Z. S., Nahand, J. S., Nejati, M., et al. (2021). Chitosan-based nanoparticles against viral infections. Front. Cell. Infect. Microbiol. 11, 643953. doi:10.3389/fcimb.2021.643953
Burduel, A. C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., and Andronescu, E. (2018). Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials 8, 681. doi:10.3390/nano8090681
CDC Covid-19 Research Team (2020). Public health response to the initiation and spread of pandemic covid-19 in the United States, february 24–april 21, 2020. Morb. Mortal. Wkly. Rep. 69, 551–556. doi:10.15585/mmwr.mm6918e2
da Saúde do Brasil M (2024). Casos de covid-19 e influenza crescem no brasil nas primeiras semanas de 2024: semana epidemiológica 2. Available at: https://www.gov.br/saude/pt-br/assuntos/coronavirus/atualizacao-de-casos/informe-se-2-de-2024-vigilancia-das-sindromes-gripais-influenza-covid-19-e-outros-virus-respiratorios-de-importancia-em-saude-publica#:∼:text=Nesse3lica (Accessed January 02, 2024)
Dash, M., Chiellini, F., Ottenbrite, R. M., and Chiellini, E. (2011). Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 36, 981–1014. doi:10.1016/j.progpolymsci.2011.02.001
de Normas Técnicas AB (2024). Abnt nbr 13698-2011 - equipamento de proteção respiratória — peça semifacial filtrante para partículas. Available at: https://www.zambini.org.br/pdfs/ABNT%20NBR%2013698-2011%20%20-%20Equipamento%20de%20prote%C3%A7%C3%A3o%20respirat%C3%B3ria%20%E2%80%94%20Pe%C3%A7a%20semifacial%20filtrante%20para%20part%C3%ADculas.pdf (Accessed February 02, 2024).
Du, T., Lu, J., Liu, L., Dong, N., Fang, L., Xiao, S., et al. (2018). Antiviral activity of graphene oxide–silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Appl. Bio Mater. 1, 1286–1293. doi:10.1021/acsabm.8b00154
Duan, B., Huang, Y., Lu, A., and Zhang, L. (2018). Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 82, 1–33. doi:10.1016/j.progpolymsci.2018.04.001
Felipe, L., Rabello, L., Júnior, E., and Santos, I. (2017). Quitosana: da química básica à bioengenharia. Quím. Nova na Esc. 39, 312–320. doi:10.21577/0104-8899.20160089
Felipe, M. S. S., Rezende, K. S., Rosa, M. F. F., and Gadelha, C. A. G. (2020). Um olhar sobre o complexo econômico industrial da saúde e a pesquisa translacional. Saúde em Debate 43, 1181–1193. doi:10.1590/0103-1104201912316
Fernandes, D. R. A., Cag, G., and Maldonado, J. M. S. V. (2021). Vulnerabilities of Brazil’s domestic pharmaceutical and biotech industry in the context of the covid-19 pandemic. Cad. Saúde Pública (Online). e00254720–e00254720.
Fernandes, D. R. A., et al. (2022). Desafios e estratégias das instituições farmacêuticas públicas no contexto do CEIS e da pandemia: uma discussão sobre acesso. in Inovação e desenvolvimento. Ph.D. thesis.
Fernandez-Moure, J. S. (2016). Lost in translation: the gap in scientific advancements and clinical application. Front. Bioeng. Biotechnol. 4, 43. doi:10.3389/fbioe.2016.00043
Fort, D. G., Herr, T. M., Shaw, P. L., Gutzman, K. E., and Starren, J. B. (2017). Mapping the evolving definitions of translational research. J. Clin. Transl. Sci. 1, 60–66. doi:10.1017/cts.2016.10
Gadelha, C. A. G. (2022). Complexo econômico-industrial da saúde: a base econômica e material do sistema único de saúde. Cad. Saúde Pública 38, e00263321. doi:10.1590/0102-311X00263321
Guo, Y., Qiao, D., Zhao, S., Liu, P., Xie, F., and Zhang, B. (2024). Biofunctional chitosan–biopolymer composites for biomedical applications. Mater. Sci. Eng. R Rep. 159, 100775. doi:10.1016/j.mser.2024.100775
Hamdi, M., Feki, A., Bardaa, S., Li, S., Nagarajan, S., Mellouli, M., et al. (2020). A novel blue crab chitosan/protein composite hydrogel enriched with carotenoids endowed with distinguished wound healing capability: in vitro characterization and in vivo assessment. Mater. Sci. Eng. C 113, 110978. doi:10.1016/j.msec.2020.110978
Hanney, S. R., Straus, S. E., and Holmes, B. J. (2022). Saving millions of lives but some resources squandered: emerging lessons from health research system pandemic achievements and challenges. Health Res. Policy Syst. 20, 99. doi:10.1186/s12961-022-00883-6
Jebril, N. (2020). World health organization declared a pandemic public health menace: a systematic review of the coronavirus disease 2019 covid-19. Available at SSRN 3566298.
Khoury, M. J., Gwinn, M., and Ioannidis, J. P. (2010). The emergence of translational epidemiology: from scientific discovery to population health impact. Am. J. Epidemiol. 172, 517–524. doi:10.1093/aje/kwq211
Khoury, M. J., Gwinn, M., Yoon, P. W., Dowling, N., Moore, C. A., and Bradley, L. (2007). The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet. Med. 9, 665–674. doi:10.1097/gim.0b013e31815699d0
Kumar, M. R., Muzzarelli, R., Muzzarelli, C., Sashiwa, H., and Domb, A. (2004). Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 104, 6017–6084. doi:10.1021/cr030441b
Landim, M. G., Carneiro, M. L. B., Joanitti, G. A., Anflor, C. T. M., Marinho, D. D., Rodrigues, J. F. B., et al. (2023). A novel n95 respirator with chitosan nanoparticles: mechanical, antiviral, microbiological and cytotoxicity evaluations. Discov. Nano 18, 118. doi:10.1186/s11671-023-03892-8
Lopes, C. M. Z., and Carneiro, M. R. (2022). Análise do respirador vesta–validação das características intrínsecas ao produto final.
Lupatini, E. O., Barreto, J. O. M., Zimmermann, I. R., and Silva, E. N. (2020). Medicamentos e pesquisa translacional: etapas, atores e políticas de saúde no contexto brasileiro. Saúde em Debate 43, 181–199. doi:10.1590/0103-11042019s214
Marostica, S. J. F., de Souza Corrêa, J., and da Silva, C. M. F. (2021). Tendências da incorporação da quádrupla e quíntupla hélices em pesquisas sobre ecossistemas de inovação. An. do Congr. Int. Conhecimento Inovação–CIKI 1.
Medical LC (2024). Respirador facial vesta – life care medical. Available at: https://lcmed.com.br/produto/mascara-vesta/ (Accessed February 02, 2024).
Melo, C. M. D., Silva, G. A., Melo, A. R., and Freitas, A. C. (2020). Covid-19 pandemic outbreak: the brazilian reality from the first case to the collapse of health services. An. Acad. Bras. Ciências 92, e20200709. doi:10.1590/0001-3765202020200709
Morris, D., Ansar, M., Speshock, J., Ivanciuc, T., Qu, Y., Casola, A., et al. (2019). Antiviral and immunomodulatory activity of silver nanoparticles in experimental rsv infection. Viruses 11, 732. doi:10.3390/v11080732
Neiva, M. B., Carvalho, I., Costa, F., Barbosa-Junior, F., Bernardi, F. A., Sanches, T. L. M., et al. (2020). Brazil: the emerging epicenter of covid-19 pandemic. Rev. Soc. Bras. Med. Trop. 53, e20200550. doi:10.1590/0037-8682-0550-2020
Ng, W. L., Yeong, W. Y., and Naing, M. W. (2016). Polyelectrolyte gelatin-chitosan hydrogel optimized for 3d bioprinting in skin tissue engineering. Int. J. Bioprint 2, 53–62. doi:10.18063/ijb.2016.01.009
of Health (NIH) NI (2024). National institutes of health (nih) — turning discovery into health. Available at: https://www.nih.gov/ (Accessed February 02, 2024).
Organization, W. H., et al. (2020). Origin of sars-cov-2, 26 march 2020 (no. who/2019-ncov/faq/virus_origin/2020.1). World Health Organization.
Organization, W. H., et al. (2022). “Third round of the global pulse survey on continuity of essential health services during the covid-19 pandemic: november–december 2021: interim report, 7 february 2022,” in Tech. rep. (World Health Organization).
Organization, W. H., et al. (2023). Covid-19 epidemiological update, 22 december 2023. COVID-19 epidemiological update.
Poupart, J., Deslauriers, J. P., Groulx, L. H., Laperrière, A., Mayer, R., and Pires, A. (2008). A pesquisa qualitativa. in Enfoques epistemológicos e metodológicos, 2.
Rinaudo, M. (2006). Chitin and chitosan: properties and applications. Prog. Polym. Sci. 31, 603–632. doi:10.1016/j.progpolymsci.2006.06.001
Rosa, M. F. F. (2022). Pesquisa, desenvolvimento e inovação (PD&I) do respirador VESTA: universidade de brasília (UnB) como vetor da pesquisa translacional em saúde. Bibl. Cent. UnB. Available at: http://repositorio.unb.br/jspui/handle/10482/43558.
Rosa, M. F. F., da Silva, E. N., Pacheco, C., Diógenes, M. V. P., Millett, C., Gadelha, C. A. G., et al. (2021). Direct from the covid-19 crisis: research and innovation sparks in Brazil. Health Res. policy Syst. 19, 10–17. doi:10.1186/s12961-020-00674-x
Schaltegger, S. (2020). Unsustainability as a key source of epi-and pandemics: conclusions for sustainability and ecosystems accounting. J. Account. Organ. Change 16, 613–619. doi:10.1108/jaoc-08-2020-0117
Seyhan, A. A. (2019). Lost in translation: the valley of death across preclinical and clinical divide–identification of problems and overcoming obstacles. Transl. Med. Commun. 4, 18–19. doi:10.1186/s41231-019-0050-7
Wehling, M. (2006). Translational medicine: can it really facilitate the transition of research “from bench to bedside”. Eur. J. Clin. Pharmacol. 62, 91–95. doi:10.1007/s00228-005-0060-4
Keywords: COVID-19, SARS-CoV-2, organic controller, vaccination, respirator, infection
Citation: Fleury Rosa MF, Santos LMP, Grabois Gadelha CA, Martins de Toledo A, Carregaro RL, Almeida da Silva AK, Mota da Costa LB, Ferreira da Rocha A and de Siqueira Rodrigues Fleury Rosa S (2024) Translational pathway of a novel PFF2 respirator with chitosan nanotechnology: from the concept to the practical applications. Front. Nanotechnol. 6:1384775. doi: 10.3389/fnano.2024.1384775
Received: 14 February 2024; Accepted: 29 March 2024;
Published: 22 April 2024.
Edited by:
Reza Rastmanesh, American Physical Society, United StatesReviewed by:
Iram Maqsood, University of Maryland, United StatesMyles Joshua Toledo Tan, University of Florida, United States
Copyright © 2024 Fleury Rosa, Santos, Grabois Gadelha, Martins de Toledo, Carregaro, Almeida da Silva, Mota da Costa, Ferreira da Rocha and de Siqueira Rodrigues Fleury Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mário Fabrício Fleury Rosa, mariorosafleury@gmail.com
 Mário Fabrício Fleury Rosa
Mário Fabrício Fleury Rosa Leonor Maria Pacheco Santos
Leonor Maria Pacheco Santos Carlos Augusto Grabois Gadelha2,3
Carlos Augusto Grabois Gadelha2,3  Aline Martins de Toledo
Aline Martins de Toledo Ana Karoline Almeida da Silva
Ana Karoline Almeida da Silva Lindemberg Barreto Mota da Costa
Lindemberg Barreto Mota da Costa Adson Ferreira da Rocha
Adson Ferreira da Rocha Suélia de Siqueira Rodrigues Fleury Rosa
Suélia de Siqueira Rodrigues Fleury Rosa