- Institute of Physiology, University Medical Center of the Johannes Gutenberg University, Mainz, Germany
A commentary on
Comparison of spike parameters from optically identified GABAergic and glutamatergic neurons in sparse cortical cultures
by Becchetti, A., and Wanke, E. (2015). Front. Cell. Neurosci. 9:157. doi: 10.3389/fncel.2015.00157
We are pleased to note that our publication “Comparison of spike parameters from optically identified GABAergic and glutamatergic neurons in sparse cortical cultures” by Weir et al. (2015) raised some discussion on the feasibility of solely electrophysiological discrimination of distinct neuronal subpopulations in vitro. We agree with Becchetti and Wanke (2015) that their report and our study on the same question were conducted with different technical approaches and that this may explain the observed differences between both studies. Although we obviously recorded a reduced spontaneous neuronal activity under our sparse culture conditions, these conditions were necessary to enable the unequivocal identification of single units in recordings with extracellular electrodes. Our combined approach of extracellular single unit recordings and cellular calcium imaging analyses in sparse neuronal cultures from GAD67-GFP transgenic mice clearly allowed the definite identification of inhibitory GAD67-positive interneurons and excitatory GAD67-negative neurons. The ratio of excitatory to inhibitory neurons and thus also the relative density of inhibitory connections was carefully assessed in our study and comparable to data from the cerebral cortex in vivo. For technical reasons this unambiguous assignment of a single neuron to one recording electrode is not possible at high cellular densities.
We performed additional experiments and now also analyzed Fano factors of spike counts recorded in culture medium and under different culture conditions (low density culture 15.6 ± 1.8 recorded neurons; medium density culture 54.3 ± 3.1 recorded neurons). Fano factors of these cultures were compared to those of clearly identified GFP-positive, inhibitory and GFP-negative, excitatory units published in our previous study (Weir et al., 2015). Fano factors were calculated for all units by dividing the variation by the average spike counts within a time window of 800 ms. Note, that Becchetti et al. previously used a time window of 6 s for calculation of Fano factors (Becchetti et al., 2012). However, our additional analyses revealed that this methodological difference in the bin width does not account for the different experimental outcome, since Fano factors calculated with 6 s and 800 ms bins were strongly correlated (Low density culture R2 = 0.596, p < 0.0001; High density culture R2 = 0.649, p < 0.0001).
As shown in Figure 1 below, all four experimental groups show a similar distribution of Fano factors and no significant differences in average Fano factor in different conditions (low density cultures 2.91 ± 0.16, n = 204 cells; medium dense cultures 2.6 ± 0.17, n = 152 cells; GFP-negative neurons 2.83 ± 0.3, n = 81 cells: GFP-positive interneurons 3.23 ± 1.03, n = 20 cells; One-Way ANOVA R2 = 0.005, p = 0.54). Single unit recordings at culture conditions with 1000 cells per mm2 as suggested by Becchetti and Wanke are not possible because spike sorting algorithms under this condition revealed a high failure rate.
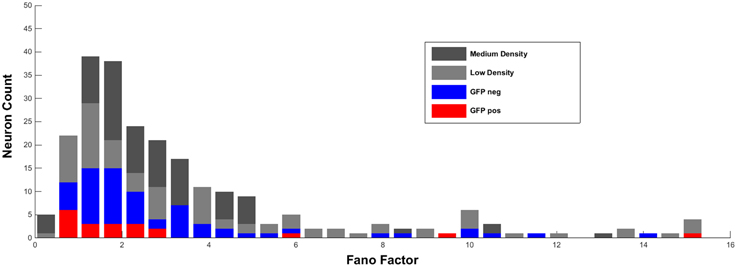
Figure 1. Distribution of Fano factors (calculated as described in Weir et al., 2015) of single units in low and medium dense cultures recorded in culture medium. For comparison, Fano factors of visually-identified GFP-positive and GFP-negative units recorded in ACSF are added.
Thus, on the basis of our new and additional experiments and analyses we cannot confirm that the Fano factor represents a sufficient parameter to reliably discriminate under in vitro conditions between excitatory and GABAergic inhibitory neurons.
However, we would like to point out that a number of previous studies (Barthó et al., 2004; Sirota et al., 2008; Sakata and Harris, 2009) and also our own analyses in adult rat cerebral cortex (Reyes-Puerta et al., 2014) were able to identify inhibitory interneurons recorded under in vivo conditions on the basis of their extracellular firing properties. Therefore, it cannot be excluded that a more immature functional state of neurons cultured under in vitro conditions hamper the identification of inhibitory interneurons on the basis of the extracellular spiking properties.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was supported by funding from the DAAD to KW, the DFG (PAK 520 and SFB 1080) to HL and the Ministerium für Bildung, Wissenschaft, Weiterbildung to AS.
References
Barthó, P., Hirase, H., Monconduit, L., Zugaro, M., Harris, K. D., and Buzsáki, G. (2004). Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 92, 600–608. doi: 10.1152/jn.01170.2003
Becchetti, A., Gullo, F., Bruno, G., Dossi, E., Lecchi, M., and Wanke, E. (2012). Exact distinction of excitatory and inhibitory neurons in neural networks: a study with GFP-GAD67 neurons optically and electrophysiologically recognized on multielectrode arrays. Front. Neural Circuits 6:63. doi: 10.3389/fncir.2012.00063
Becchetti, A., and Wanke, E. (2015). A Commentary on “Comparison of spike parameters from optically identified GABAergic and glutamatergic neurons in sparse cortical cultures.” Front. Cell. Neurosci. 9:157. doi: 10.3389/fncel.2015.00157
Reyes-Puerta, V., Sun, J. J., Kim, S., Kilb, W., and Luhmann, H. J. (2014). Laminar and columnar structure of sensory-evoked multineuronal spike sequences in adult rat barrel cortex in vivo. Cereb. Cortex. doi: 10.1093/cercor/bhu007. [Epub ahead of print].
Sakata, S., and Harris, K. D. (2009). Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron 64, 404–418. doi: 10.1016/j.neuron.2009.09.020
Sirota, A., Montgomery, S., Fujisawa, S., Isomura, Y., Zugaro, M., and Buzsáki, G. (2008). Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697. doi: 10.1016/j.neuron.2008.09.014
Keywords: neuronal culture, multi-electrode array, imaging, interneurons, network activity, spike waveform
Citation: Weir K, Blanquie O, Kilb W, Luhmann HJ and Sinning A (2015) Response: “Commentary: Comparison of spike parameters from optically identified GABAergic and glutamatergic neurons in sparse cortical cultures.” Front. Cell. Neurosci. 9:224. doi: 10.3389/fncel.2015.00224
Received: 12 May 2015; Accepted: 27 May 2015;
Published: 09 June 2015.
Edited by:
Enrico Cherubini, International School for Advanced Studies, ItalyReviewed by:
Rustem Khazipov, Institut National de la Santé et de la Recherche Médicale, FranceCopyright © 2015 Weir, Blanquie, Kilb, Luhmann and Sinning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heiko J. Luhmann,bHVobWFubkB1bmktbWFpbnouZGU=
 Keiko Weir
Keiko Weir Oriane Blanquie
Oriane Blanquie Werner Kilb
Werner Kilb Heiko J. Luhmann
Heiko J. Luhmann Anne Sinning
Anne Sinning