Visual Maps Development: Reconsidering the Role of Retinal Efnas and Basic Principle of Map Alignment
- 1Centre National de la Recherche Scientifique, UPR3212 - Institute of Cellular and Integrative Neurosciences, University of Strasbourg, Strasbourg, France
- 2Neuroscience, Department of Biology, University of Virginia, Charlottesville, VA, United States
- 3Donald K. Johnson Eye Institute, Krembil Research Institute, University Health Network, Toronto, ON, Canada
Introduction
Understanding and modeling the formation of neural maps has been a challenging subject in neurobiology, particularly in the visual system. One of the most studied visual mapping model corresponds to the topographic organization of the visual inputs in the superior colliculus -SC (or tectum in non-mammalian vertebrates), a layered structure in the midbrain involved in multisensory processing, controlling visual orientation and attention (May, 2006; Krauzlis et al., 2013). The superficial layers of the SC (stratum griseum superficiale-SGS and stratum opticum) receive organized inputs from ganglion cells in the retina (RGCs) and from neurons in layer V of the primary visual cortex (V1). RGCs extend their axons during embryonic life, forming the optic nerve, and organize in a retino-collicular map in the SC during the first postnatal week. Around post-natal day 6, while retino-collicular mapping is underway, axons from layer V neurons in V1 reach the SC, forming the cortico-collicular map, which aligns with the retino-collicular map in the SGS, so that corresponding sources of visual inputs are mapped topographically and adjusted with respect to the visual field: retinal nasal-temporal and V1 medial-lateral axes project onto the anterior (rostral)-posterior (caudal) axis in the SC (Figure 1A) and dorsal-ventral/anterior-posterior axes in retina/V1 project onto the lateral-medial axis of the SC (Cang and Feldheim, 2013). Evidence suggests that both the retino- and cortico-collicular inputs converge on the same cells in the SC and that spontaneous correlated activity in the retina during development instructs retino/cortico-collicular maps alignment (Boka et al., 2006; Triplett et al., 2009; Phillips et al., 2011). However, the molecular cues, if any, involved in the alignment of the visual maps in the SC are still unknown.
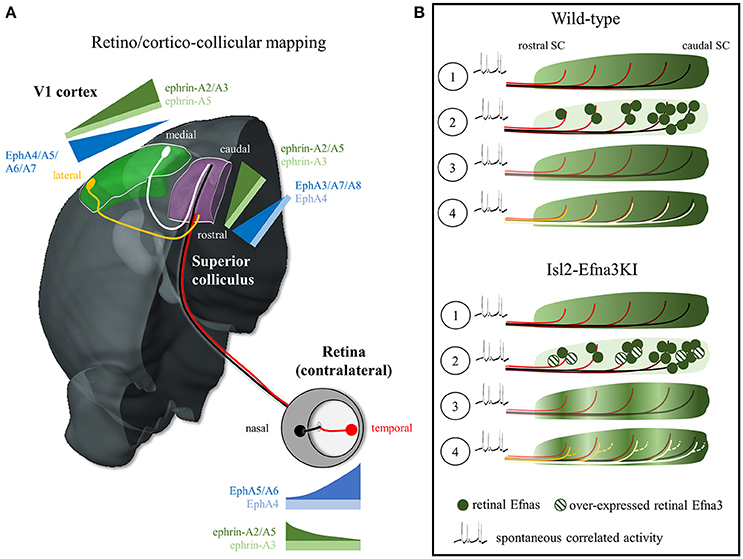
Figure 1. EphA/Efna expression, retino/cortico-collicular projection pattern and map alignment mechanism. (A) Representation of the EphA/Efna gradients of expression within the retino/cortico-collicular projection system in mouse. In the retina, temporal axons (red) expressing high levels of EphA and low levels of Efnas project in the rostral superior colliculus whereas nasal axons (black, low EphAs, high Efnas) project onto the rostral pole of the superior colliculus during the first postnatal week. From P6 to P12, V1 lateral axons (yellow, high EphAs) project to the superior colliculus and align with the temporal retinal projections. V1 medial axons (white, low EphAs) align with nasal retinal projections in the SC. (B) Map alignment principle. ① During retino-collicular projections, RGC fire spontaneous correlated activity and express EphA receptors on their axons that are engaged with collicular Efna gradients for mapping. ② During mapping, RGCs axons, carrying gradients of Efnas, transpose these ligands into the colliculus leading to ③ a smooth low-rostral to high-caudal gradient in Wild-type animals or to an oscillatory gradient in Isl2-Efna3KI animals. ④ Cortico-collicular projecting neurons, carrying EphA receptors, align with the retino-collicular map based on spontaneous correlated activity and V1 EphAs -> transposed retinal Efnas repulsive interactions. In Wild-type, V1 axons are facing a smooth gradient of transposed retinal Efnas leading to a linear homogeneous cortico-collicular map. In Isl2-Efna3KI animals, V1 axons are facing an oscillatory retinal Efnas gradient, leading to local duplications of the cortico-collicular map. SC, superior colliculus; V1, primary visual cortex.
Potential candidates are the Eph tyrosine kinase receptors and their membrane-bound ligands, the Efns (ephrins), key players in the formation of visual maps (Flanagan and Vanderhaeghen, 1998; Lemke and Reber, 2005; McLaughlin and O'Leary, 2005; Scicolone et al., 2009; Triplett and Feldheim, 2012; Cang and Feldheim, 2013). They are divided in two sub-families, the EphB/Efnbs and the EphA/Efnas. Receptors and ligands are expressed in complementary gradients between interconnected structures (i.e., retina and SC/tectum or V1 and SC/tectum) and as reciprocal gradients within a structure (i.e., retina). Here we will focus on the EphA/Efna signaling system. During mapping, in mouse RGCs, EphA4/5/6 run from low-nasal to high-temporal, whereas Efna2/3/5 are expressed from high-nasal to low-temporal. In the SC EphA3/4/7/8 run from high-rostral to low-caudal and in V1 EphA4/5/6/7 show a high-lateral to low-medial gradient. Efna2/3/5 present a graded rostral low-caudal high expression in the SC and a lateral low-medial high gradient in V1 (Figure 1A; Park et al., 1997; Feldheim et al., 1998; Reber et al., 2004; Cang et al., 2005; Rashid et al., 2005). Most of the work addressing the role of EphA/Efna signaling in visual map formation focused on the activation of retinal EphAs by collicular Efnas (forward signaling). However clear answers about the function of the countergradients (retinal Efnas and collicular EphAs) have remained elusive.
Hypotheses of the Role of Retinal Efnas in Retino-Collicular Mapping
Several hypotheses have been raised concerning the role of retinal Efnas. In vitro and in vivo evidence in chick suggested that Efnas present on RGCs modulate the activity of the retinal EphAs through interactions triggering phosphorylation of the receptor tyrosine kinase domain of the receptor or by silencing of the EphAs through inhibition of tyrosine phosphorylation. This mechanism generates a desensitization/masking of EphA receptors toward trans-binding of the Efna ligands in the SC/tectum, therefore sharpening the functional EphA gradients in the RGCs (Connor et al., 1998; Dütting et al., 1999; Hornberger et al., 1999; Carvalho et al., 2006). However, it is not clear whether EphAs desensitization in the retina occurs by cis-interaction, in the same RGCs or by trans-interaction, between neighboring RGCs in the retina/optic nerve, or both (Weth et al., 2014). Another hypothesis, based on in vitro and in vivo experiments in chick and mouse, suggested that retinal Efnas directly participate to the mapping process in the SC. Retinal Efnas are activated by collicular EphAs leading to axon branching inhibition posterior and anterior to the correct location of the termination zone in the SC (Yates et al., 2001; Rashid et al., 2005; Lim et al., 2008; Yoo et al., 2011).
Limitations of Previous Experimental Approaches
As summarized above, strong evidence from in vitro and in vivo analyses in chick and mouse unambiguously demonstrated the role of EphA/Efna signaling in retino-collicular mapping. However, their mechanism of action has remained controversial for several reasons. First, their presence in both projecting and target structures only allowed the characterization of their function at a system level in null-animals, precluding the assessment of the relative contribution of collicular, retinal and cortical gradients. Second, the co-expression of several members of both receptors and ligands made almost impossible the identification of a member-specific function. Finally, EphAs and Efnas are expressed as gradients, suggesting that growth cones detect variations of EphA/Efna concentrations over a given distance, indicating a quantitative mechanism (Goodhill, 1998; Goodhill and Baier, 1998). The homogeneous removal of EphAs or Efnas, as performed in knock-out approaches, provides qualitative information -whether this molecule is involved in mapping- but will not tell how it works. To address such a mechanism, a quantitative perturbation of the gradient should be performed by, for example, changing the orientation or the periodicity.
New Approaches for Better Insights Into Mapping Mechanisms
In 2000, Brown and colleagues circumvent these constraints by developing an original gain-of-function approach, the Isl2-EphA3 knock-in mouse model (Isl2-EphA3KI), which presents a quantitative perturbation of the EphA receptor gradients in the retina (Brown et al., 2000). Such approach revealed to be extraordinarily informative in addressing the mechanism of action of the retinal EphAs because the perturbation of their expression occurred in the RGCs only (cell-specificity) and because the graded expression of EphAs was quantitatively altered, from a smooth to an oscillating gradient (change in periodicity). This approach, and subsequent work using the Isl2-EphA3KI model, yielded crucial findings about the mechanisms of retino-collicular and cortico-collicular mapping which have been impacting both experimental (Brown et al., 2000; Reber et al., 2004; Triplett et al., 2009; Bevins et al., 2011; Owens et al., 2015) and theoretical neurosciences (Koulakov and Tsigankov, 2004; Reber et al., 2004; Tsigankov and Koulakov, 2006, 2010; Willshaw, 2006; Grimbert and Cang, 2012; Sterratt, 2013; Sterratt and Hjorth, 2013; Hjorth et al., 2015; Owens et al., 2015). It is now widely acknowledged that competition and relative EphA signaling between RGCs control early steps of retino-collicular map formation.
Recently, tissue/cell-specific in vivo approaches were developed to address the role of retinal Efnas. Work from Drescher's lab, using a conditional deletion of retinal Efna5, suggested that the high expression of Efna5 on nasal RGC axons repels temporal RGCs axons, expressing high levels of EphAs, therefore preventing them to invade the caudal SC (Suetterlin and Drescher, 2014). This principle is based on a target-independent axon-axon interaction mechanism between RGCs, originally hypothesized by Bonhoeffer (Bonhoeffer and Huf, 1985; Weth et al., 2014). In a work recently published (Savier et al., 2017) we analyzed visual maps formation and alignment in the SC in Isl2-Efna3KI animals. Similar to the Isl2-EphA3KI model, the Isl2-Efna3KI mouse over-express the Efna3 ligand in 50% of the RGCs only, therefore creating an oscillatory gradient of Efnas in the RGCs (cell-specificity and quantitative perturbation). The analysis of the retino-collicular mapping in these animals showed no targeting perturbation, suggesting that Efna3 is not directly involved in retino-collicular mapping (Savier et al., 2017), further confirming results obtained from Efna3−/− animals (Pfeiffenberger et al., 2006). Moreover, our results also suggested that the axon-axon interaction principle (Suetterlin and Drescher, 2014; Weth et al., 2014) does not seem to apply to Efna3, raising the question of member-specific function of Efnas in the visual system. Unlike the retinal EphAs (Bevins et al., 2011), the retinal Efnas do not seem to be functionally interchangeable.
A surprising observation was made when we analyzed cortico-collicular mapping in the Isl2-Efna3KI animals. In ~50% of the heterozygous and homozygous mutants, the cortico-collicular map was disrupted, presenting local duplications of the projections from V1 generating a misalignment with the retino-collicular map. This was confirmed when genetic inactivation of the over-expressed retinal Efna3 in the Isl2-Efna3KI mutants restored a wild-type cortico-collicular map (Savier et al., 2017). Although unexpected, this result is in line with previous studies demonstrating that retinal inputs are required for cortico-collicular map formation and alignment (Khachab and Bruce, 1999; Triplett et al., 2009) however, the mechanisms were unclear.
Mechanistic Models of Retino/Cortico-Collicular Maps Alignment in the SC
Two alternative hypotheses have been suggested to account for the alignment of the cortico-collicular projections onto the retino-collicular map: a gradient-matching model and a retinal-matching model (Cang and Feldheim, 2013). The gradient-matching hypothesis suggests that V1 axons carrying EphAs are activated by collicular Efnas leading to the formation and alignment of the cortico-collicular map through forward signaling. Although forward EphA signaling is relevant, positional information provided by collicular Efnas to V1 ingrowing axons seems unlikely regarding the results obtained with the Isl2-EphA3KI and Isl2-Efna3KI models. In Isl2-EphA3KI, the collicular expression of Efnas is unaltered; however, a duplication of the cortico-collicular projections can be observed ruling out the gradient-matching model (Triplett et al., 2009). Further refutation of this model came from the results observed in the Isl2-Efna3KI mouse. A duplication of the cortico-collicular projections is observed in 50% of the mutants whereas the collicular expression of Efnas is normal (Savier et al., 2017).
What is the direct evidence supporting a retinal-matching model and what are the molecular players? A first indication came from work in anophthalmic mice that showed dispersed organization of the cortico-collicular projections suggesting that retinal inputs are required for the establishment of a precise topographic organization (Khachab and Bruce, 1999). This was confirmed in enucleated and Math5-null mice (lacking 90–95% of the RGCs) which showed enlarged cortico-collicular termination zones (Triplett et al., 2009). In the Isl2-EphA3 homozygote KI, both retino- and cortico-collicular maps are fully duplicated but aligned (Triplett et al., 2009). The colllicular duplication of the V1 projections copies the duplication of the retino-collicular map. Triplett and collaborators demonstrated that this duplication is due to correlated spontaneous activity, a stochastic process originating in the retina and propagating to the SC, which instructs the alignment of the cortico-collicular projections onto the retino-collicular map (Triplett et al., 2009). Further confirmation of the retinal-matching model and identification of the molecular effectors came from our analysis of the Isl2-Efna3KI animals (Savier et al., 2017). Here, the oscillatory expression of retinal Efna3, due to the over-expressed Efna3 in 50% of the RGCs, is transposed to the SC during retino-collicular mapping and provides positional information for incoming V1 axons carrying EphAs. The presence of an oscillatory Efna3 expression of retinal origin in the SC generates local duplications of V1 projections (Figure 1B). However, Efna3 by itself cannot provide positional information as it is not graded. Therefore, together with Efna3, other Efnas presenting a graded expression must be involved in cortico-collicular map alignment in the SC. Retinal Efna2 and Efna5 are good candidates and computational modeling supports this hypothesis (Savier et al., 2017) although some controversies appeared as nasal Efna5 has been shown to be involved in axon-axon interaction during retino-collicular mapping (Suetterlin and Drescher, 2014). Residual endogenous collicular Efnas, previously engaged during retino-collicular mapping, could also participate in the alignment of the V1 projections, although it has been shown that upon binding to EphAs, the EphA/Efna complexes are cleaved and internalized, making EphAs and Efnas unavailable for further guidance (Janes et al., 2005; Yoo et al., 2011).
Altogether, these results support a retinal-matching model for the alignment of the retino- and cortico-collicular maps. A recent view favors a mechanism based on spontaneous correlated activity (Triplett et al., 2009) and (partial) Efnas transposition from the RGCs into the SC during retino-collicular mapping (Savier et al., 2017). These Efnas of retinal origin then provide positional information, through forward signaling, to the ingrowing V1 axons bearing EphAs (Figure 1B, Movie 1). The variable penetrance of the cortico-collicular duplication observed in Isl2-Efna3KI mutants is likely to be the consequence of the stochastic nature of the mapping process where spontaneous correlated activity tends to associate neighboring projections in the SC whereas local oscillatory Efna3 tends to separate them through repulsion (Triplett et al., 2009; Savier et al., 2017).
Conclusion
New genetic approaches recently challenged previous hypotheses about the role of retinal Efnas in visual map formation and uncovered novel principle and mechanism. This new concept suggests that a leading sensory map (the retino-collicular map) carries molecular cues (retinal Efnas) allowing subsequent sensory map alignment (cortico-collicular map) (Movie 1). In support of this concept, recent evidence suggests that sonic hedgehog is transported by contra-lateral RCGs axons to the optic chiasm to repel ipsi-lateral RGC axons (Peng et al., 2018). Such a principle could be relevant for sensory map formation allowing fine adjustments compensating for intrinsic variability. A similar process has been shown in the lateral geniculate nucleus, where V1 cortical inputs are required for the proper targeting of RCGs (Shanks et al., 2016). However, such new principle needs to be further challenged both experimentally and theoretically using appropriate animal models presenting tissue/cell-specific rearrangement.
Author Contributions
ES and MR wrote and edited the manuscript and designed the figure and the movie.
Funding
This work was supported by CNRS, University of Strasbourg and University of Strasbourg Institute for Advanced Study (USIAS).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank our colleagues Dr. Frank Pfrieger and Amélie Barthélémy for their scientific and technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2018.00077/full#supplementary-material
References
Bevins, N., Lemke, G., and Reber, M. (2011). Genetic dissection of EphA receptor signaling dynamics during retinotopic mapping. J. Neurosci. 31, 10302–10310. doi: 10.1523/JNEUROSCI.1652-11.2011
Boka, K., Chomsung, R., Li, J., and Bickford, M. E. (2006).Comparison of the ultrastructure of cortical and retinal terminals in the rat superior colliculus. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288, 850–858. doi: 10.1002/ar.a.20359
Bonhoeffer, F., and Huf, J. (1985). Position-dependent properties of retinal axons and their growth cones. Nature 315, 409–410. doi: 10.1038/315409a0
Brown, A., Yates, P. A., Burrola, P., Ortuño, D., Vaidya, A., Jessell, T. M., et al. (2000). Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell 102, 77–88. doi: 10.1016/S0092-8674(00)00012-X
Cang, J., and Feldheim, D. A. (2013). Developmental mechanisms of topographic map formation and alignment. Annu. Rev. Neurosci. 36, 51–77. doi: 10.1146/annurev-neuro-062012-170341
Cang, J., Kaneko, M., Yamada, J., Woods, G., Stryker, M. P., and Feldheim, D. A. (2005). Ephrin-as guide the formation of functional maps in the visual cortex. Neuron 48, 577–589. doi: 10.1016/j.neuron.2005.10.026
Carvalho, R. F., Beutler, M., Marler, K. J. M., Knöll, B., Becker-Barroso, E., Heintzmann, R., et al. (2006). Silencing of EphA3 through a cis interaction with ephrinA5. Nat. Neurosci. 9, 322–330. doi: 10.1038/nn1655
Connor, R. J., Menzel, P., and Pasquale, E. B. (1998). Expression and tyrosine phosphorylation of Eph receptors suggest multiple mechanisms in patterning of the visual system. Dev. Biol. 193, 21–35. doi: 10.1006/dbio.1997.8786
Dütting, D., Handwerker, C., and Drescher, U. (1999). Topographic targeting and pathfinding errors of retinal axons following overexpression of ephrinA ligands on retinal ganglion cell axons. Dev. Biol. 216, 297–311. doi: 10.1006/dbio.1999.9489
Feldheim, D. A., Vanderhaeghen, P., Hansen, M. J., Frisén, J., Lu, Q., Barbacid, M., et al. (1998). Topographic guidance labels in a sensory projection to the forebrain. Neuron 21, 1303–1313. doi: 10.1016/S0896-6273(00)80650-9
Flanagan, J. G., and Vanderhaeghen, P. (1998). The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309–345. doi: 10.1146/annurev.neuro.21.1.309
Goodhill, G. J. (1998). Mathematical guidance for axons. Trends Neurosci. 21, 226–231. doi: 10.1016/S0166-2236(97)01203-4
Goodhill, G. J., and Baier, H. (1998). Axon guidance: stretching gradients to the limit. Neural Comput. 10, 521–527. doi: 10.1162/089976698300017638
Grimbert, F., and Cang, J. (2012). New model of retinocollicular mapping predicts the mechanisms of axonal competition and explains the role of reverse molecular signaling during development. J. Neurosci. 32, 9755–9768. doi: 10.1523/JNEUROSCI.6180-11.2012
Hjorth, J. J., Savier, E., Sterratt, D. C., Reber, M., and Eglen, S. J. (2015). Estimating the location and size of retinal injections from orthogonal images of an intact retina. BMC Neurosci. 16:80. doi: 10.1186/s12868-015-0217-8
Hornberger, M. R., Dütting, D., Ciossek, T., Yamada, T., Handwerker, C., Lang, S., et al. (1999). Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22, 731–742. doi: 10.1016/S0896-6273(00)80732-1
Janes, P. W., Saha, N., Barton, W. A., Kolev, M. V., Wimmer-Kleikamp, S. H., Nievergall, E., et al. (2005). Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123, 291–304. doi: 10.1016/j.cell.2005.08.014
Khachab, M. Y., and Bruce, L. L. (1999). The development of corticocollicular projections in anophthalmic mice. Brain Res. Dev. Brain Res. 114, 179–192. doi: 10.1016/S0165-3806(99)00020-6
Koulakov, A. A., and Tsigankov, D. N. (2004). A stochastic model for retinocollicular map development. BMC Neurosci. 5:30. doi: 10.1186/1471-2202-5-30
Krauzlis, R. J., Lovejoy, L. P., and Zénon, A. (2013). Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36, 165–182. doi: 10.1146/annurev-neuro-062012-170249
Lemke, G., and Reber, M. (2005). Retinotectal mapping: new insights from molecular genetics. Annu. Rev. Cell Dev. Biol. 21, 551–580. doi: 10.1146/annurev.cellbio.20.022403.093702
Lim, Y. S., McLaughlin, T., Sung, T. C., Santiago, A., Lee, K. F., and O'Leary, D. D. M. (2008). p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron 59, 746–758. doi: 10.1016/j.neuron.2008.07.032
May, P. J. (2006). The mammalian superior colliculus: laminar structure and connections. Prog. Brain Res. 151, 321–378. doi: 10.1016/S0079-6123(05)51011-2
McLaughlin, T., and O'Leary, D. D. (2005). Molecular gradients and development of retinotopic maps. Annu. Rev. Neurosci. 28, 327–355. doi: 10.1146/annurev.neuro.28.061604.135714
Owens, M. T., Feldheim, D. A., Stryker, M. P., and Triplett, J. W. (2015). Stochastic interaction between neural activity and molecular cues in the formation of topographic maps. Neuron 87, 1261–1273. doi: 10.1016/j.neuron.2015.08.030
Park, S., Frisén, J., and Barbacid, M. (1997). Aberrant axonal projections in mice lacking EphA8 (Eek) tyrosine protein kinase receptors. EMBO J. 16, 3106–3114. doi: 10.1093/emboj/16.11.3106
Peng, J., Fabre, P. J., Dolique, T., Swikert, S. M., Kermasson, L., Shimogori, T., et al. (2018). Sonic hedgehog is a remotely produced cue that controls axon guidance trans-axonally at a midline choice point. Neuron 97, 326–340.e4. doi: 10.1016/j.neuron.2017.12.028
Pfeiffenberger, C., Yamada, J., and Feldheim, D. A. (2006). Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J. Neurosci. 26, 12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006
Phillips, M. A., Colonnese, M. T., Goldberg, J., Lewis, L. D., Brown, E. N., and Constantine-Paton, M. (2011). A synaptic strategy for consolidation of convergent visuotopic maps. Neuron 71, 710–724. doi: 10.1016/j.neuron.2011.06.023
Rashid, T., Upton, A. L., Blentic, A., Ciossek, T., Knöll, B., Thompson, I. D., et al. (2005). Opposing gradients of ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron 47, 57–69. doi: 10.1016/j.neuron.2005.05.030
Reber, M., Burrola, P., and Lemke, G. (2004). A relative signalling model for the formation of a topographic neural map. Nature 431, 847–853. doi: 10.1038/nature02957
Savier, E., Eglen, S. J., Bathélémy, A., Perraut, M., Pfrieger, F. W., Lemke, G., et al. (2017). A molecular mechanism for the topographic alignment of convergent neural maps. eLife 6:e20470. doi: 10.7554/eLife.20470
Scicolone, G., Ortalli, A. L., and Carri, N. G. (2009). Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Res. Bull. 79, 227–247. doi: 10.1016/j.brainresbull.2009.03.008
Shanks, J. A., Ito, S., Schaevitz, L., Yamada, J., Chen, B., Litke, A., et al. (2016). Corticothalamic axons are essential for retinal ganglion cell axon targeting to the mouse dorsal lateral geniculate nucleus. J. Neurosci. 36, 5252–5263. doi: 10.1523/JNEUROSCI.4599-15.2016
Sterratt, D. C. (2013). On the importance of countergradients for the development of retinotopy: insights from a generalised gierer model. PLoS ONE 8:e67096. doi: 10.1371/journal.pone.0067096
Sterratt, D. C., and Hjorth, J. J. J. (2013). Retinocollicular mapping explained? Vis. Neurosci. 30, 125–128. doi: 10.1017/S0952523813000254
Suetterlin, P., and Drescher, U. (2014). Target-independent ephrina/EphA-mediated axon-axon repulsion as a novel element in retinocollicular mapping. Neuron 84, 740–752. doi: 10.1016/j.neuron.2014.09.023
Triplett, J. W., and Feldheim, D. A. (2012). Eph and ephrin signaling in the formation of topographic maps. Semin. Cell Dev. Biol. 23, 7–15. doi: 10.1016/j.semcdb.2011.10.026
Triplett, J. W., Owens, M. T., Yamada, J., Lemke, G., Cang, J., Stryker, M. P., et al. (2009). Retinal input instructs alignment of visual topographic maps. Cell 139, 175–185. doi: 10.1016/j.cell.2009.08.028
Tsigankov, D., and Koulakov, A. A. (2010). Sperry versus Hebb: topographic mapping in Isl2/EphA3 mutant mice. BMC Neurosci. 11:155. doi: 10.1186/1471-2202-11-155
Tsigankov, D. N., and Koulakov, A. A. (2006). A unifying model for activity-dependent and activity-independent mechanisms predicts complete structure of topographic maps in ephrin-A deficient mice. J. Comput. Neurosci. 21, 101–114. doi: 10.1007/s10827-006-9575-7
Weth, F., Fiederling, F., Gebhardt, C., and Bastmeyer, M. (2014). Chemoaffinity in topographic mapping revisited–is it more about fiber-fiber than fiber-target interactions? Semin. Cell Dev. Biol. 35, 126–135. doi: 10.1016/j.semcdb.2014.07.010
Willshaw, D. (2006). Analysis of mouse EphA knockins and knockouts suggests that retinal axons programme target cells to form ordered retinotopic maps. Development 133, 2705–2717. doi: 10.1242/dev.02430
Yates, P. A., Roskies, A. L., McLaughlin, T., and O'Leary, D. D. (2001). Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. J. Neurosci. 21, 8548–8563.
Keywords: EphA, ephrin-As, retina, superior colliculus, visual cortex, visual mapping
Citation: Savier E and Reber M (2018) Visual Maps Development: Reconsidering the Role of Retinal Efnas and Basic Principle of Map Alignment. Front. Cell. Neurosci. 12:77. doi: 10.3389/fncel.2018.00077
Received: 24 January 2018; Accepted: 06 March 2018;
Published: 21 March 2018.
Edited by:
Xin Qi, School of Medicine, Case Western Reserve University, United StatesReviewed by:
Alexandra Rebsam, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2018 Savier and Reber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Reber, michael.reber@uhnresearch.ca; reberm@inci-cnrs.unistra.fr
 Elise Savier
Elise Savier Michael Reber
Michael Reber