Semaphorin 3A Contributes to Secondary Blood–Brain Barrier Damage After Traumatic Brain Injury
- 1Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China
- 2Key Laboratory of Injuries, Variations and Regeneration of Nervous System, Tianjin Neurological Institute, Tianjin, China
- 3Tianjin Medical University, Tianjin, China
- 4Department of Clinical Laboratory Diagnostics, Tianjin Medical University General Hospital, Tianjin, China
- 5The Second Hospital of Tianjin Medical University, Tianjin, China
- 6Tianjin Chest Hospital, Tianjin, China
Semaphorin 3A (SEMA3A) is a member of the Semaphorins family, a class of membrane-associated protein that participates in the construction of nerve networks. SEMA3A has been reported to affect vascular permeability previously, but its influence in traumatic brain injury (TBI) is still unknown. To investigate the effects of SEMA3A, we used a mouse TBI model with a controlled cortical impact (CCI) device and a blood–brain barrier (BBB) injury model in vitro with oxygen-glucose deprivation (OGD). We tested post-TBI changes in SEMA3A, and its related receptors (Nrp-1 and plexin-A1) expression and distribution through western blotting and double-immunofluorescence staining, respectively. Neurological outcomes were evaluated by modified neurological severity scores (mNSSs) and beam-walking test. We examined BBB damage through Evans Blue dye extravasation, brain water content, and western blotting for VE-cadherin and p-VE-cadherin in vivo, and we examined the endothelial cell barrier through hopping probe ion conductance microscopy (HPICM), transwell leakage, and western blotting for VE-cadherin and p-VE-cadherin in vitro. Changes in miR-30b-5p were assessed by RT-PCR. Finally, the neuroprotective function of miR-30b-5p is measured by brain water content, mNSSs and beam-walking test. SEMA3A expression varied following TBI and peaked on the third day which expressed approximate fourfold increase compared with sham group, with the protein concentrated at the lesion boundary. SEMA3A contributed to neurological function deficits and secondary BBB damage in vivo. Our results demonstrated that SEMA3A level following OGD injury almost doubled than control group, and the negative effects of OGD injury can be improved by blocking SEMA3A expression. Furthermore, the expression of miR-30b-5p decreased approximate 40% at the third day and 60% at the seventh day post-CCI. OGD injury also exhibited an effect to approximately decrease 50% of miR-30b-5p expression. Additionally, the expression of SEMA3A post-TBI is regulated by miR-30b-5p, and miR-30b-5p could improve neurological outcomes post-TBI efficiently. Our results demonstrate that SEMA3A is a significant factor in secondary BBB damage after TBI and can be abolished by miR-30b-5p, which represents a potential therapeutic target.
Introduction
Traumatic brain injury (TBI) is a high-incidence disease that can harm human health, and with the development of society, the morbidity of this disease has tended to increase (Cope et al., 2012). The mortality of TBI is 10.8%, and the disability rate is 12%. TBI has become the fifth major cause of death among people under 40 years old, and young adults account for approximate 70% of all TBI patients (Sen, 2017).
Due to the comprehensive effect of primary injury and secondary injury, TBI may cause various types of damage in the clinical setting (Blennow et al., 2012; Khalili et al., 2017; Sen, 2017). The primary injury of TBI leads to a series of effects including neural damage, intracerebral hemorrhage and primary blood–brain barrier (BBB) disruption, which result in subsequent pathological events in the central nervous system (CNS) (Blennow et al., 2012; Jiang et al., 2017). BBB consists of various cells (endothelial cell, astrocytes, pericytes, and neurons), and acts as a dynamic interface between circulation and CNS (Alluri et al., 2018). The function of BBB in regulating the exchange of substances protects the CNS from damage caused by pathogenic microorganisms or other macromolecular substances in the circulation (Eliceiri et al., 1999; Marmarou, 2007). Secondary brain damage resulting from the interaction of primary pathological events and products is characterized by secondary BBB damage which occurs from hours to days following TBI (Marmarou, 2007; Ge et al., 2015). The secondary destruction of BBB by reason of the primary pathological events and products results in the further damage including brain edema, intracranial hypertension, inflammation and even poor neurological prognosis (Zweckberger et al., 2006; Ge et al., 2015; Xiong et al., 2017). As a result, improving secondary BBB damage is a significant approach for treatment and improving prognosis after TBI. Currently, clinical therapy focuses mainly on decompressive craniectomy, the evacuation of intracranial hematoma, and dehydrant treatment, which aims to treat the symptoms of brain edema with limited therapeutic effects. The effect of hypothermia therapy on TBI patients is also contradicted by the National Institutes of Health (NIH) (Mena et al., 2011). In conclusion, an efficient therapeutic method that addresses the cause of secondary BBB damage is urgently needed.
Semaphorin 3A (SEMA3A) is a member of a membrane-associated and secreted proteins family with more than 20 types identified (Yang et al., 2018), which participate in the construction of nerve networks. SEMA3A has long been known in nervous system as an axonal guidance factor to guide these growing axons by repelling them or preventing them from entering certain regions (Yazdani and Terman, 2006). A wide range of studies on SEMA3A also have been performed in different physiological and pathological processes, including cardiogenesis, angiogenesis, vasculogenesis, tumor metastasis, osteoclastogenesis and immune regulation (Takamatsu et al., 2010). Duh (2011) and Joyal et al. (2011) have reported that SEMA3A prevents angiogenesis in the retina and destructs the blood–eye barrier. The antiangiogenic effect is associated with the integrity of endothelial cells and SEMA3A alters the integrity of these cells through VE-cadherin serine phosphorylation and internalization (Le Guelte et al., 2012). The integrity of BBB depends on inter-endothelial-cell junction and its complex structures (Sayeed et al., 2018). The complex structures of inter-endothelial-cell junction which include microvascular endothelium, tight junctions and adherens junctions, can help to regulate BBB permeability and functional activities (Bazzoni et al., 2000; Zlokovic, 2008). However, whether SEMA3A participates in TBI and how SEMA3A carries out its biological functions remain unknown.
Based on the database analysis of TargetScan, Miranda and miRDB, we found that miRNA-30 was closely related to SEMA3A. Previous research indicated that miR-30c was related to SEMA3A in neurogenesis (Sun et al., 2016) and MiR-30b promotes axon outgrowth of retinal ganglion cells by inhibiting SEMA3A expression (Han et al., 2015). Furthermore, miR-30a-5p was also reported to significantly suppress the inflammation responses following spinal cord injury (Fu et al., 2018). In circulation system and urinary system, miR-30 family was also demonstrated the positive effect following ischemia injury (Shen et al., 2015; Gu et al., 2016). However, which factors can regulate the secretion of SEMA3A in brain tissue following TBI needs further investigation.
We investigated the alterations in SEMA3A after TBI to determine whether SEMA3A can act as a factor in secondary BBB damage and regulate neurological function post-TBI. Furthermore, our results indicated OGD injury can induce the secretion of SEMA3A in vitro through imitating hypoxic pathological status. However, miR-30b-5p was confirmed to regulate SEMA3A expression efficiently and abolished the effect of SEMA3A on the integrity of the BBB in vivo and in vitro.
Materials and Methods
Animals
Adult male C57BL/6 mice weighing 20–25 g were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. All mice were maintained in the animal facilities of Tianjin Medical University General Hospital with free access to food and water in a temperature-controlled (20 ± 2°C) and humidity-controlled (55 ± 5%) vivarium under a 12 h light/dark cycle. All animal experiments were approved by the Ethics Committee of Tianjin Medical University (Tianjin, China). Furthermore, all the experimental protocols for this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Tianjin Medical University Animal Care and Use Committee.
Experimental Design
Five experimental procedures were performed to investigate the influence of SEMA3A on secondary BBB damage post-TBI and its regulation factor.
In experiment 1, the post-TBI changes in the expression level of SEMA3A and its related receptors were determined by western blotting. The mice used for this experiment were divided into five groups: sham group, TBI 1Day group, TBI 3Day group, TBI 7Day group, and TBI 14Day group, with each group consisting of six mice. The concentrated area was determined by Double-Immunofluorescence Staining and the mice were assigned to two groups of six: sham group and TBI group (third Day post-TBI).
In experiment 2, we studied the effect of SEMA3A on neurological deficits and BBB damage post-TBI. The TBI mice were divided into five groups, each comprising six mice: the Sham group; the PBS group, in which PBS (2 μl) was injected into the lateral ventricle; the siRNA-control group, which was transfected with siRNA-control; the SEMA3A group, in which the SEMA3A protein (200 ng/μl) was injected into the lateral ventricle; and the siRNA group, which was transfected with siRNA-SEMA3A. mNSSs and modified beam-walking test were used to evaluate the neurological function and tested at 1st, 3rd, 7th, and 14th days post-CCI. EB dye extravasation assay, brain water content and BBB tight junction protein western blotting test were used to evaluate BBB damage post-TBI and tested at third day following TBI (SEMA3A group served as the positive control and the administered dose was 200 ng/μl).
In experiment 3, the OGD injury’s effect on the expression of SEMA3A was studied with western blotting in vitro, and bEnd.3 cells were divided into sham group and OGD group. The influence of SEMA3A on endothelial cell barrier was evaluated by FITC-Dextran transport studies, HPICM and BBB tight junction protein western blotting test. In FITC-Dextran transport studies and BBB tight junction protein western blotting test, bEnd.3 cells were divided into Control group, OGD+PBS group, OGD+siRNA-C group, OGD+siRNA-SEMA3A group and OGD+SEMA3A group. In HPICM test, bEnd.3 cells were divided into control group, SEMA3A group, OGD group, OGD+siRNA-C group, OGD+siRNA-SEMA3A group (OGD+SEMA3A group and SEMA3A group served as positive control, and the administered dose was 200 ng/μl).
In experiment 4, we used RT-PCR to study the expression level change of miR-30b-5p post-TBI in vivo and in vitro. The mice were divided into Sham 1D group, Sham 3D group, Sham 7D group, Sham 14D group, TBI 1D group, TBI 3D group, TBI 7D group, and TBI 14D group, with each group consisting of six mice. Then, the association between SEMA3A and miR-30b-5p was measured by western blotting and Luciferase reporter assay.
In experiment 5, we used brain water content, mNSSs and modified beam-walking test to measure the neuroprotective effect of miR-30b-5p during TBI. The mice were divided into the Sham group, TBI+PBS group, TBI+agomir-C group and TBI+agomir group. Furthermore, we also detected whether miR-30b-5p protected brain following TBI by regulating SEMA3A. The mice were divided into TBI+agomir-C+SEMA3A group and TBI+agomir+SEMA3A group. SEMA3A was injected on second day post-TBI with the dose of 200 ng/μl.
Controlled Cortical Impact (CCI) Model
The TBI model was established in C57BL/6 mice by a CCI device (eCCI-6.3 device, Custom Design and Fabrication, United States). The mice adapted to the environment for 1 week before the experiments. The mice were anesthetized with 10% chloral hydrate (3 mg/kg) via an intraperitoneal injection before surgery. The mice were then placed in a stereotaxic frame. After the surgical site was clipped and cleaned, to expose the dura, a 4.0-mm diameter hole was drilled in the right parietal bone (2.0 mm lateral to the sagittal sutures, 2.0 mm posterior to the bregma). To induce moderate TBI, the CCI device was set to a depth of 2 mm, a velocity of 4.5 m/s and a dwell time of 200 ms (Ge et al., 2018; Xu et al., 2018). Immediately after the injury, the skull was closed by sterilized medical bone wax and the incision was sutured with 6-0 silk sutures. The mice were placed on a heating pad to recover from anesthesia and moving normally (the waking time post-CCI is 0.5–1 h). And then, the mice were removed back to the animal facilities of Tianjin Medical University General Hospital and set in independent cages. After that, the mice were monitored the incision (redness, swelling, discharge), appetite, fecal, urine production and specific signs related to the CCI every day. The mice in the sham group were subjected to all procedures except CCI. All the animal experimental protocols for this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Tianjin Medical University Animal Care and Use Committee.
Cell Culture of the Mouse Brain Endothelial Cell Line (bEnd.3) and Oxygen-Glucose Deprivation (OGD) Cell Injury Model
The bEnd.3 cells were purchased from the BeNa Culture Collection (Suzhou, China) and cultured in DMEM-basic medium (Gibco, United States) with 10% FBS and 1% penicillin/streptomycin (Thermo Fisher Scientific). The cells were maintained in a culture chamber (Thermo Fisher Scientific) at 37°C with 5% CO2.
To imitate BBB injury in vitro, OGD was carried out. The culture medium was replaced with glucose-free endothelial cell medium (ECM), and the cells were placed into an anaerobic chamber (HERACELL 150i, Thermo Fisher Scientific) for 4 h at 37°C with the CO2 level at 5% and the O2 level at 1%. The cell viability was evaluated by Cell Counting Kit-8 (Dojindo, Shanghai, China) and maintained above 65%.
Western Blot Analysis
The mice were anesthetized with 10% chloral hydrate (3 mg/kg) via an intraperitoneal injection and sacrificed through transcardiac perfusion to eliminate test error caused by proteins expressed by blood cells. The brains were removed from the mice and homogenized in radioimmunoprecipitation assay (RIPA) buffer (Beyotime) mixed with phenylmethylsulfonyl fluoride (PMSF) (1 mM) for 30 min. The homogenates were then centrifuged for 10 min (12,000 rpm, 4°C), and the supernatants were collected. The supernatants were mixed with 4X loading buffer and boiled at 95°C for 10 min. The total protein content of the protein samples was determined through a bicinchoninic acid (BCA) protein assay kit (Thermo), and the proteins were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE). Next, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Roche, Canada) and blocked with 5% non-fat dry milk in Tris-buffered saline (TBS) for 2 h at room temperature. The blots were then incubated with primary antibodies overnight at 4°C and with the appropriate horseradish peroxidase (HRP)-conjugated secondary IgG for 1 h at room temperature. Finally, the blots were developed with an enhanced chemiluminescence (ECL) system (Millipore, Billerica, MA, United States), and the expression levels of protein samples were quantified with ImageJ software. β-Actin served as a loading control (Sun et al., 2017). More information about the antibodies is presented in Table 1.
Double-Immunofluorescence Staining
The mice were grouped into a TBI group and a sham group, which were anesthetized with 10% chloral hydrate (3 mg/kg) through an intraperitoneal injection and sacrificed through transcardiac perfusion [cold phosphate-buffered saline (PBS) and 4% paraformaldehyde] 72 h after TBI. The brains were removed and embedded in optimal cutting temperature (OCT) medium (Sakura, Oakland, CA, United States). The brains were subsequently sliced into 8-mm-thick coronal sections. After blocking with 3% bovine serum albumin (BSA) for 30 min at 37°C, the sections were incubated with the appropriate primary antibody mixture, which was mixed with an anti-SEMA3A antibody (1:75, Abcam, ab23393) and an anti-CD31 antibody (1:100, Abcam, Ab24590), overnight at 4°C. The samples were then incubated with Alexa Fluor-conjugated anti-rabbit IgG (1:500, Molecular Probes) for 1 h at room temperature, and the nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) for 5 min.
siRNA Transfection
siRNA transfection was carried out in vivo with the method reported previously (Chen et al., 2009). siRNA-SEMA3A (0.5 nmol, RiboBio, Guangzhou, China) and siRNA-control (0.5 nmol, RiboBio, Guangzhou, China) were diluted with the same volume of Entranster TM -in vivo transfection reagent (Engreen, Beijing, China). The mixed solutions were injected intracerebroventricularly (i.c.v.) into the lateral ventricle by using stereotactic coordinates (1.5 mm posterior to the bregma, 1.0 mm right lateral to the sagittal suture, and 2 mm in depth). The CCI treatment was administered immediately post-transfection.
siRNA transfection was carried out in vitro through Lipofectamine-3000 (Thermo Fisher Scientific). siRNA-SEMA3A (1 nmol, RiboBio Biotechnology, Guangzhou, China) and siRNA-control (1 nmol, RIBOBIO, Guangzhou, China) were diluted with the same volume of Lipofectamine-3000 and added to the culture medium for 5 h. The OGD treatment was performed at 36 h post-transfection.
Modified Neurological Severity Scores (mNSS)
mNSSs were carried out as previously reported (Chen et al., 2001) to evaluate neurological function. The observer was blinded to the experimental conditions and treatments. All the mice were tested at 1, 3, 7, and 14 days post-CCI. Lower scores indicated better neurological function. If the mice died before 14 days post-CCI, their data was not included in the statistical analysis.
Motor Function Testing
Motor function was evaluated through a modified beam-walking task, as previously reported (Feeney et al., 1982). Before TBI induction, we trained the mice to walk along a narrow beam and enter a darkened box at the end of the beam using stimulation with bright light and loud noise. As the mouse entered the goal box, all the stimulations were stopped immediately. The tests were carried out on the 1st, 3rd, 7th, and 14th days post-TBI and recorded by an observer who was blinded to the experimental conditions and treatments. Shorter times indicated better motor function.
Evans Blue (EB) Dye Extravasation Assay
The mice were injected with a 2% EB solution (Sigma-Aldrich, 5 ml/kg) through the femoral vein on the third day after CCI, as previously reported (Tchantchou and Zhang, 2013; Xu et al., 2018). After 2 h, the mice were transcardially perfused with PBS and sacrificed. The lesioned hemispheres were dissected, weighed and incubated in N,N-dimethylformamide (1 ml/100 mg) at room temperature for 48 h. Then, the mixtures were centrifuged at 1,000 rpm for 5 min. The supernatants were collected, and optical density (OD) values were determined by a spectrophotometer (λ = 632 nm). The quantity of extravasated EB dye was measured according to the standard curve.
Brain Water Content
Brain water content was evaluated through the wet–dry weight method on the third day post-CCI, as previously reported (Yan et al., 2011; Sun et al., 2017; Xu et al., 2018). Briefly, the mice were sacrificed, and the brains were obtained without transcardiac perfusion. The lesioned hemispheres were isolated and weighed directly. Then, the lesioned hemispheres were dried in an electrothermostatic blast oven at 80°C for 72 h. Brain water content (%) was calculated using the following formula: (wet brain weigh-dry brain weight)/wet brain weight × 100%.
FITC-Dextran Transport Studies
The bEnd.3 cells were cultured in transwells (Corning, 0.4 μm) and divided into a negative control group, an OGD+PBS group, an OGD+siRNA-C group, an OGD+siRNA-SEMA3A group and an OGD+SEMA3A group, which served as a positive control. After the different groups had been treated separately, we added FITC-dextran (Sigma-Aldrich, 4 kDa, 2 mg/ml) into the medium in the apical chamber and collected the medium in the basolateral chamber after 1 h. Then, we tested the OD value of the collected medium with a spectrophotometer.
Hopping Probe Ion Conductance Microscopy (HPICM) Scanning
The HPICM system consisted of an ICnano scanner controller (Ionscope) and a sample scan head SH01 (Ionscope, Melbourn, Cambridgeshire, United Kingdom), which was placed on an inverted TiU microscope (Nikon, Tokyo, Japan). Cell movement in the horizontal X–Y–Z direction was measured by a 25-μm LISA piezo stage (P-753.21C, Physik Instrumente) and two 100-μm PIHera piezo stages (P-621.2C, Physik Instrumente, Karlsruhe, Germany), which were managed by the ICnano controller. The ion current between the nanopipette tip and the cell surface was monitored by an external Axon Multi Clamp700B amplifier (Molecular Devices, Sunnyvale, CA, United States). The nanopipettes, which were filled with Leibovitz’s L15 medium (Thermo Fisher Scientific), were pulled from the borosilicate glass (OD 1.00 mm, I.D. 0.59 mm, VitalSense Scientific Instruments, Wuhan, China) with a laser-based puller (Model P-2000, Sutter Instruments, Novato, CA, United States) (Chen et al., 2013). First, we continuously scanned the cells, to which we added PBS or the SEMA3A protein during the scanning procedure, for 2 h. The OGD group, OGD+siRNA-C group and OGD+siRNA-SEMA3A group were scanned after they had been treated separately. All the primary data was analyzed by SICM Image Viewer software (Ionscope).
Quantitative Real-Time PCR
RNA was harvested from the acquired tissues on the 1st, 3rd, 7th, and 14th days post-TBI and from the cultured cells post-OGD with TRIzol reagent (Invitrogen). The RNA was reverse transcribed into single-stranded complementary DNA with the PrimeScript RT Reagent Kit (TaKaRa). The single-stranded complementary DNA was amplified with SYBR Premix Ex Taq II (TaKaRa) and calculated by an MJ Research real-time PCR system (Bio-Rad, Hercules, CA, United States). The reaction was carried out under the following conditions: 95°C for 2 min, followed by 45 cycles of 95°C for 15 s and 62°C for 1 min. All the primers are presented in Table 2. U6 was used as an endogenous control.
Transfection of miR-30b-5p in vivo and in vitro
To downregulate or upregulate the expression level of miR-30b-5p in mice, the mice were randomly divided into the TBI+PBS group, the TBI+antagomir control group, the TBI+antagomir group, the TBI+agomir control group, and the TBI+agomir group. The oligomers were transfected at a dose of 800 ng per mouse by the Entranster TM-in vivo transfection reagent (Engreen, Beijing, China). The mixed solutions were injected i.c.v. into the lateral ventricle by using stereotactic coordinates (1.5 mm posterior to the bregma, 1.0 mm right lateral to the sagittal suture, and 2 mm in depth). The CCI treatment was administered 24 h post-transfection.
In in vitro experiments, the culture cells were randomly divided into the OGD+PBS group the OGD+inhibitor control group, the OGD+inhibitor group, the OGD+mimic control group, and the OGD+mimic group. The solutions were transfected into the cells separately by Lipofectamine-3000 (Thermo Fisher Scientific) at a dose of 100 nM. The OGD treatment was performed at 36 h post-transfection.
To evaluate the transfection efficacy of miR-30b-5p, real-time PCR was carried out to detect changes in the expression levels of miR-30b-5p in mouse brain tissue and bEnd.3 cells. Oligomers, mimic, inhibitor and control reagent were all purchased from RiboBio Biotechnology.
Luciferase Reporter Assay
To determine whether miR-30b-5p directly targeted the Sema3a gene, a luciferase reporter assay was performed. Luciferase reporter constructs were created by ligating Sema3a 3′ untranslated region (UTR) fragments containing the predicted binding sites. The pGL3- Sema3a -3′UTR construct was then inserted into the pGL3 control vector containing the SV40 promoter (Promega, Madison, WI, United States) using the XbaI enzyme. In addition, a mutant (Mut) luciferase reporter was generated from the wild-type (WT) luciferase reporter by deleting the binding site for miR-30b-5p.
For the reporter assay, 293T cells were cultured in 24-well plates. The WT or Mut Sema3a-3′UTR was co-transfected with miR-21-3p agomir or the agomir negative control (sequences listed in Table 2; GenePharma) into 293T cells with Lipofectamine-3000. The cells were harvested, and luciferase activity was evaluated with a dual-luciferase reporter system (Promega, Madison, WI, United States) after 48 h of incubation.
Statistical Analysis
All data were based on the group sizes of n = 6 and every sample was tested three times to confirm. Data of the mNSS test and beam-walking test were analyzed using repeated measures ANOVA followed by LSD post hoc analysis. For other data, statistical comparisons were analyzed using one-way ANOVA followed by LSD post hoc analysis or Student’s t-test. All data were expressed as the mean ± SEM and normally distributed which were analyzed by SPSS statistical software (version 22.0, IBM). A p-value less than 0.05 was considered significant.
Results
SEMA3A Expression Levels After TBI
To determine if SEMA3A is associated with TBI, biochemical assays were performed in the above groups from 1st day to 14th day after TBI (Figure 1A). We found that the expression of SEMA3A and related receptors, Plexin-A1 and Nrp-1, were dramatically elevated on the first day after TBI and peaked on the third day (Figures 1B–F). Double-immunofluorescence staining for SEMA3A and endothelial cells was used to investigate the difference in expression levels between the lesion boundary and the contralateral tissue, which revealed a concentrated expression of SEMA3A at the lesion boundary after TBI (Figures 1G–L). These data may demonstrate that SEMA3A is associated with a destructive process or a reparative process after TBI.
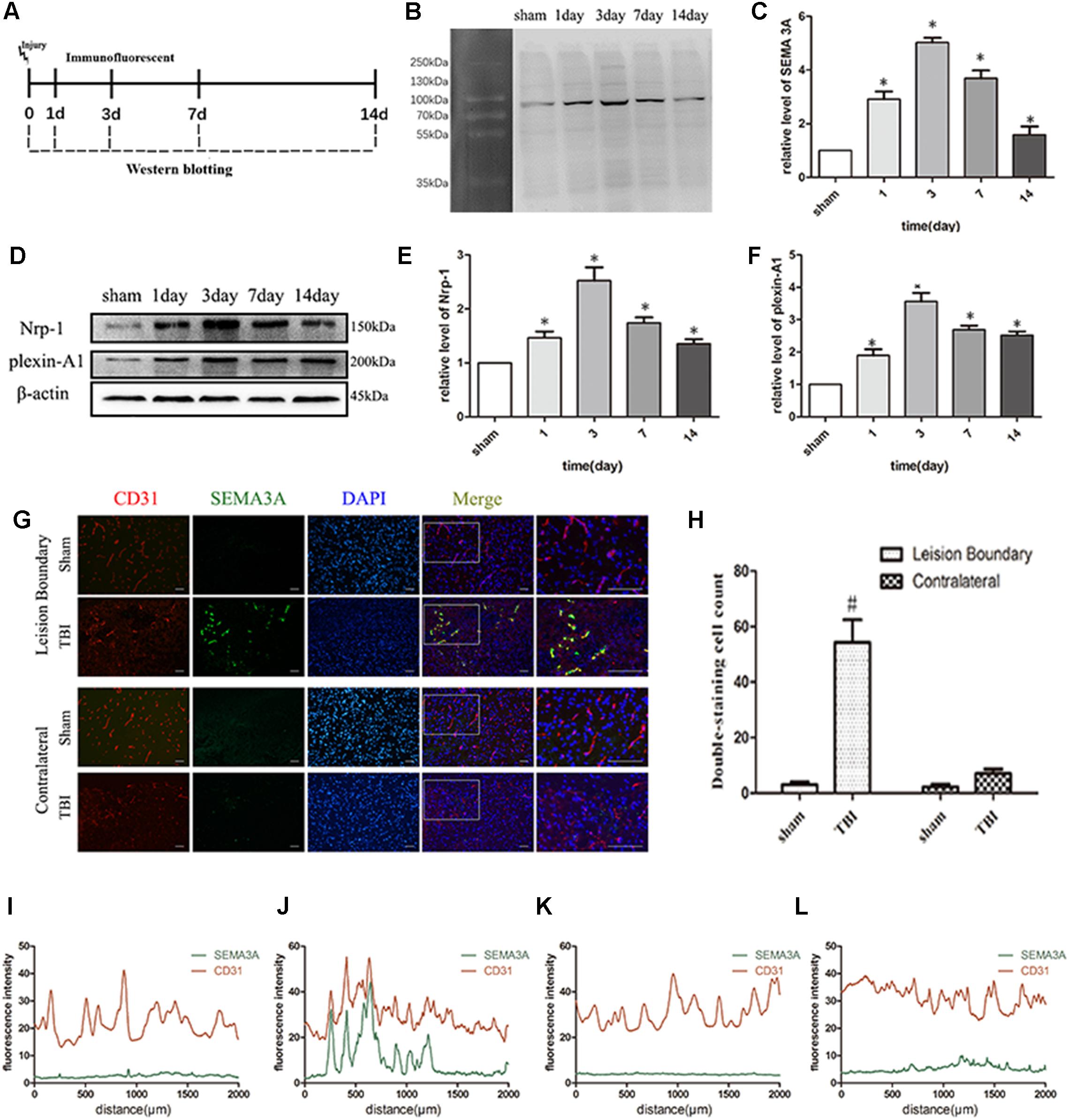
Figure 1. TBI altered the expression of SEMA3A and its related receptors, Nrp-1 and plexin-A1. (A) Schematic diagram of the experimental design. (B,C) Quantitative data from western blotting illustrating the time course of SEMA3A following TBI, with an increase on the first day post-TBI and peaked on the third day. (D–F) Quantitative data from western blotting illustrating the time course of the related receptors of SEMA3A following TBI, with an increase on the first day post-TBI and peaked on the third day. (G–L) Double-immunostaining data indicating that SEMA3A was mainly secreted and concentrated at the lesion boundary after TBI. (I) Fluorescence intensity of lesion boundary of sham group. (J) Fluorescence intensity of lesion boundary of TBI group. (K) Fluorescence intensity of contralateral of sham group. (L) Fluorescence intensity of contralateral of TBI group. Scale bar = 50 μm. The data are expressed as the mean ± SEM, and n = 6 for each group. ∗p < 0.05 vs. sham groups, #p < 0.01 vs. sham group and TBI contralateral group.
SEMA3A Contributes to the Neurological Deficits Induced by TBI
To evaluate the effect of SEMA3A on neurological deficits after TBI, we compared the neurological function of TBI mice with mNSS scores and beam-walking test from the 1st day to the 14th day post-TBI (Figure 2A) and decrease SEMA3A expression with siRNA (Figures 2B,C).
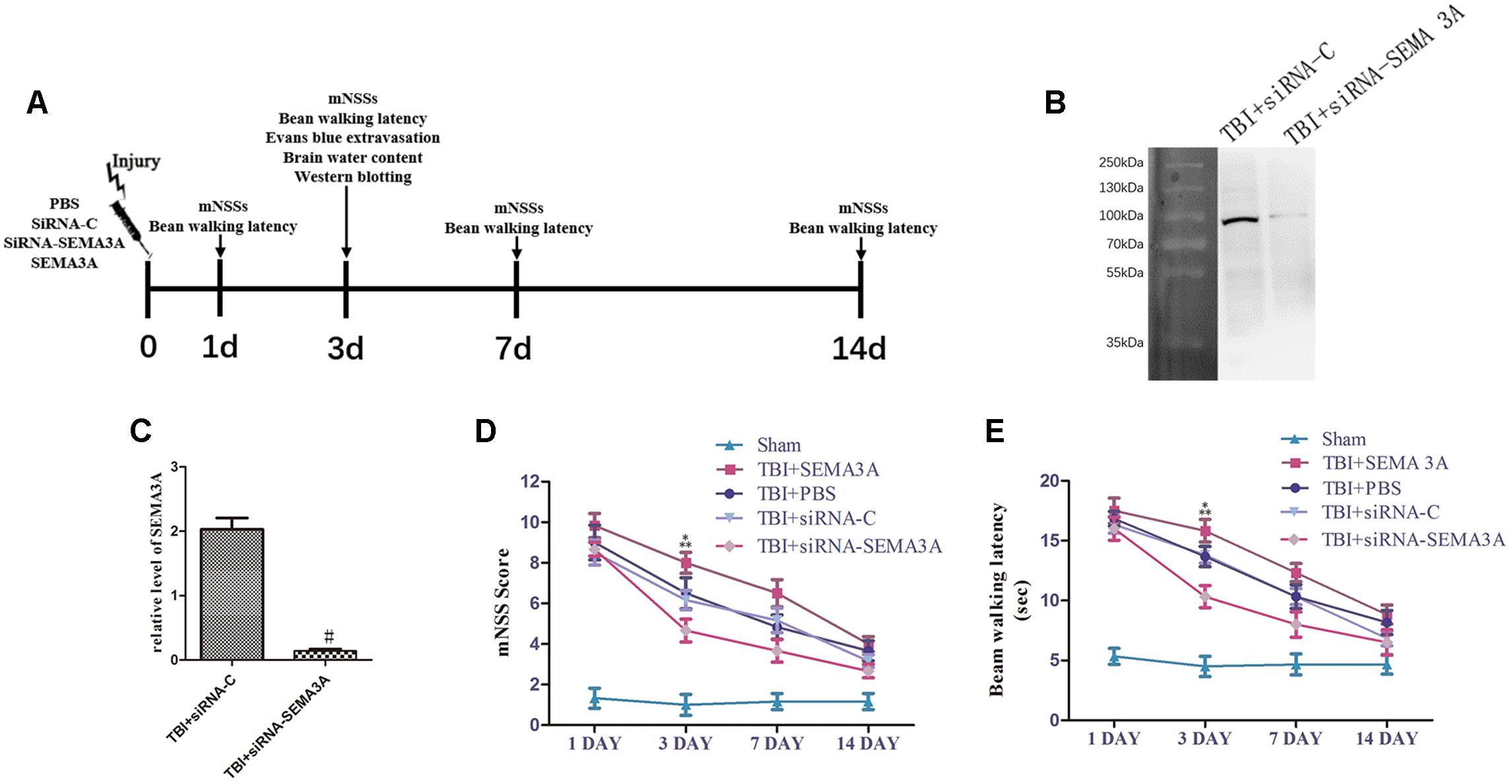
Figure 2. Influence of SEMA3A on neurological outcomes following TBI. (A) Schematic diagram of the experimental design. (B,C) The expression of SEMA3A was blocked by siRNA-SEMA3A in vivo. (D,E) We used mNSSs to test neurological function (D) and beam balance tests (E) to evaluate motor function on the 1st, 3rd, 7th, and 14th days post-CCI. The downregulation of SEMA3A significantly decreased mNSS scores and reduced the times on the beam balance test on the third day after injury. The data are expressed as the mean ± SEM, and n = 6 for each group. #p < 0.01 vs. control groups, ∗p < 0.05 TBI+siRNA-SEMA groups vs. siRNA-control groups, ∗∗p < 0.05 TBI+SEMA3A groups vs. TBI+PBS groups.
In the mNSS test (Figure 2D), there was no significant difference between the PBS group and the siRNA-control group, which exhibited a decreasing trend from the 1st day to the 14th day post-TBI, illustrating that all mice sustained relatively comparable injuries. The SEMA3A group, which served as the positive control group, was compared with the PBS group on the third day post-TBI, which showed that SEMA3A could effectively aggravate neurological deficits. In addition, in a comparison of the siRNA-control group and the siRNA group on the third day post-TBI, neurological function began to recover, with an obvious decrease in mNSSs. All these tendencies were echoed in the beam-walking test (Figure 2E), which indicated that SEMA3A was a significant factor that participated in the process associated with the neurological deficits induced by TBI.
SEMA3A Contributes to BBB Leakage in vivo
We investigated whether SEMA3A can carry out the same function in the context of BBB leakage after TBI with Evans Blue (EB) dye extravasation assay and brain water content measurement at 72 h post-injury according to the previous research (Li et al., 2011; Sun et al., 2017; Xu et al., 2018). The results demonstrated that SEMA3A can increase the EB leakage from blood vessels into the brain tissue; however, this expression was effectively blocked when SEMA3A was blocked by siRNA on the third day after TBI (Figures 3A,B). Following the appearance of this discrepancy, brain edema was obviously decreased due to the decline in SEMA3A levels (Figure 3C). It was previously reported that the serine phosphorylation and internalization of the adherens junction molecule VE-cadherin upon SEMA3A stimulation play key roles in controlling endothelial barrier function. We aimed to detect variations in brain endothelial junctions after TBI. We found that the siRNA group had a decreased p-VE-cadherin/VE-cadherin ratio (Figures 3D,E), which indicated that SEMA3A may act as a factor that can alter the barrier function of brain endothelial junctions and contribute to the destruction of the BBB post-TBI.
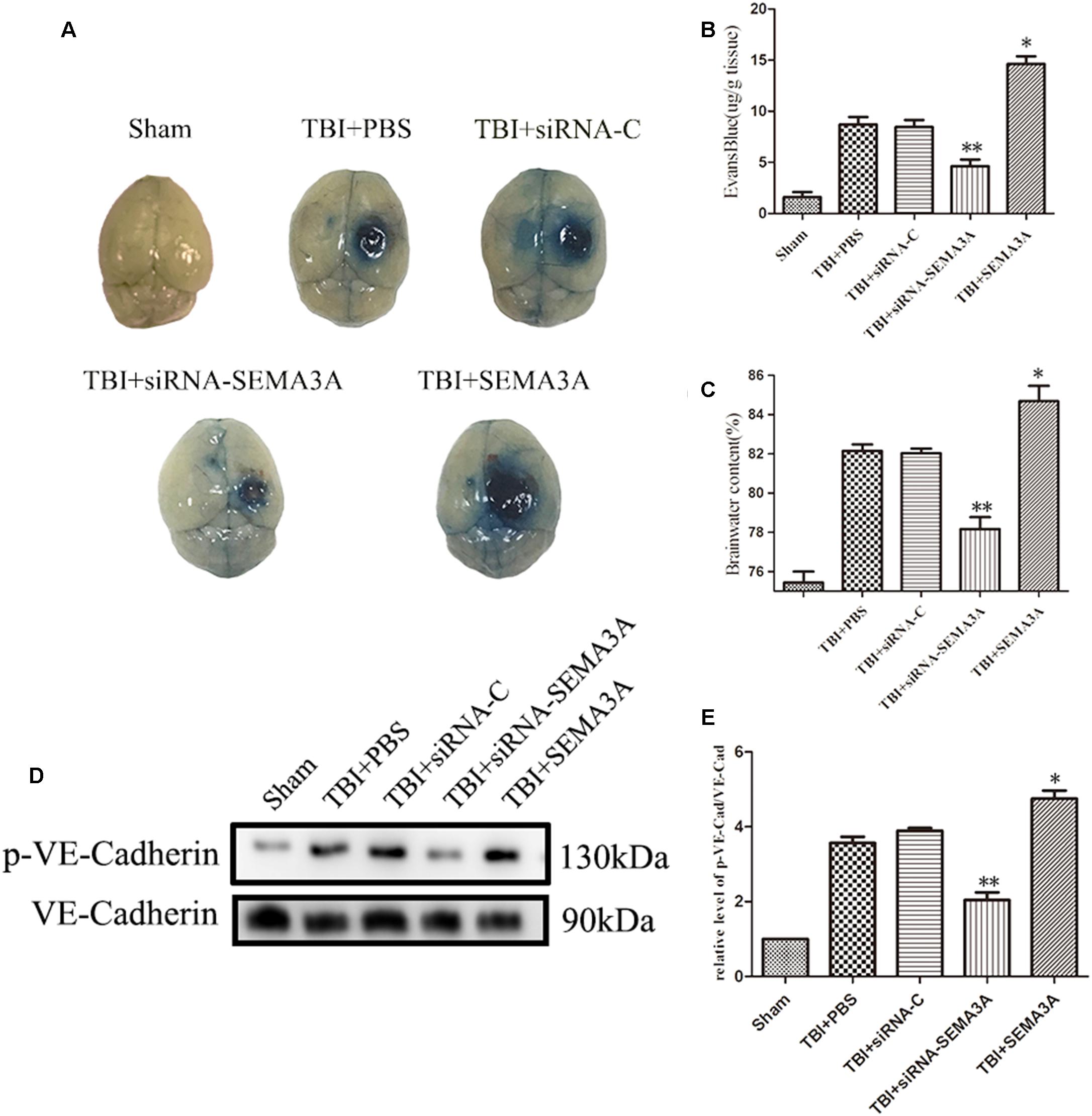
Figure 3. The impact of SEMA3A on BBB leakage after TBI. (A) The general view of the EB dye extravasation assay in brains of injured mice on the third day after injury. (B) Quantitative analysis of the EB dye extravasation assay presented in (A). (C) The brain water content was measured on the third day after injury. SEMA3A can significantly increase BBB leakage and edema, and all these negative effects post-TBI can be efficiently decreased by blocking the expression of SEMA3A. (D,E) The p-VE-cadherin/VE-cadherin ratio was examined on the third day after injury through western blotting, and the results illustrated that the downregulation of SEMA3A can decrease VE-cadherin serine phosphorylation post-TBI. The data are expressed as the mean ± SEM, and n = 6 for each group. ∗p < 0.05 TBI+SEMA3A groups vs. TBI+PBS groups, ∗∗p < 0.05 TBI+siRNA-SEMA groups vs. siRNA-control groups.
OGD Induces Changes of SEMA3A Expression in Endothelial Cells and Contributes to BBB Leakage Induced by OGD
Oxygen-glucose deprivation is the most common way to imitate hypoxic injury in vitro (Beckmann et al., 2018), and Hypoxic injury generally occurs after TBI (Salehi et al., 2017). Furthermore, OGD is also a recognized method to establish BBB injury model in vitro (Ge et al., 2018). To investigate the changes in SEMA3A expression after trauma injury in vitro, we treated the endothelial cells (bEnd.3) with OGD and then tested the expression levels of SEMA3A, plexin-A1 and Nrp-1. OGD could efficiently increase the expression levels of SEMA3A and its related receptors (Figures 4A–E), which indicated that SEMA3A is associated with a destructive process after OGD injury in vitro.
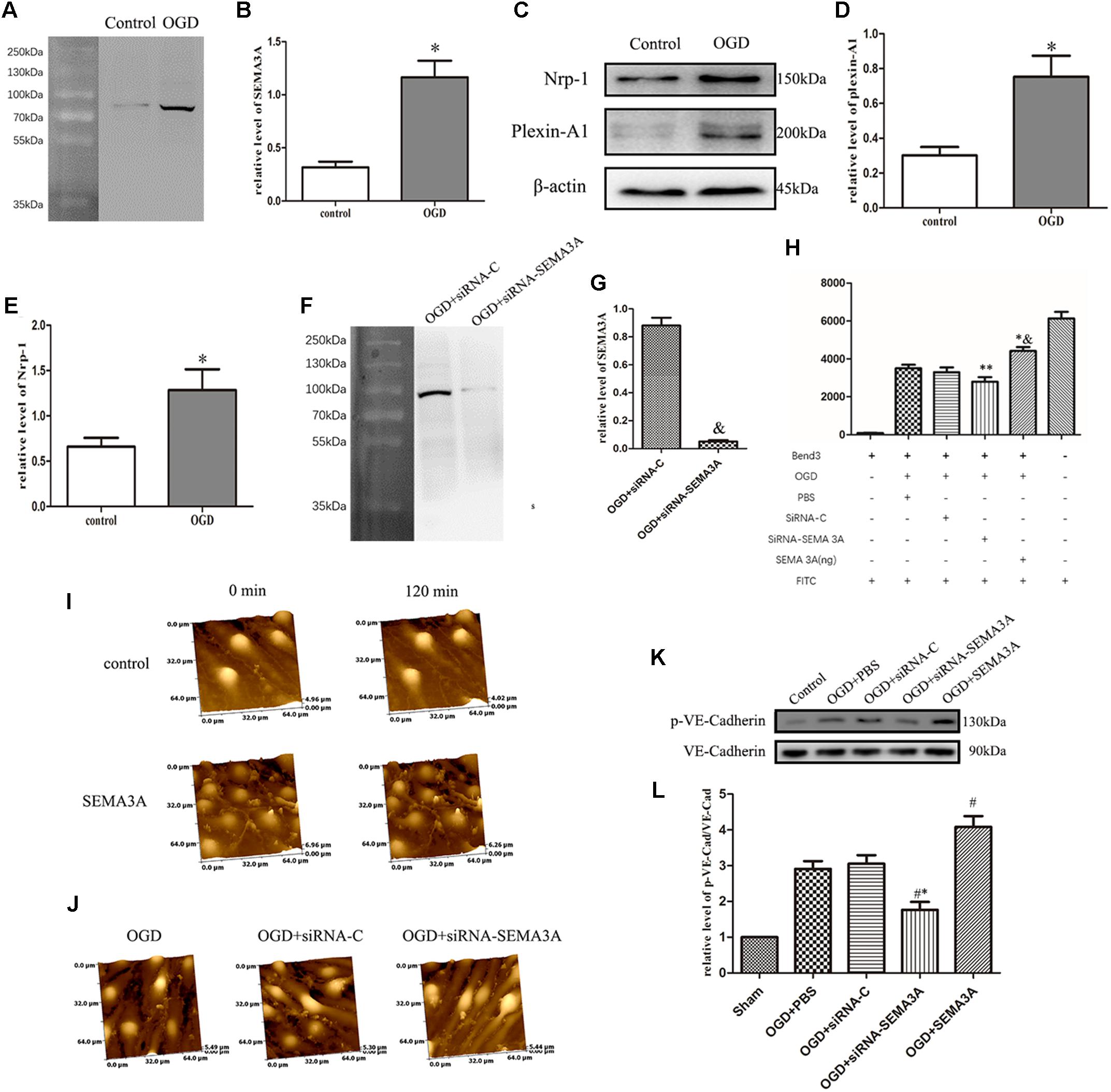
Figure 4. OGD induced changes in SEMA3A expression in endothelial cells and contributes to BBB leakage induced by OGD. (A–E) To investigate the causes of the alterations of SEMA3A post-injury, we treated endothelial cells (bEnd.3) with OGD for 4 h and tested protein expression levels with western blotting. The results indicated that compared with those in the control group, which was cultured under normoxic conditions, the expression levels of SEMA3A (A,B), Nrp-1 and plexin-A1 (C–E) in the OGD group significantly increased. (F,G) The expression of SEMA3A was blocked by siRNA-SEMA3A in vitro. (H) FITC-dextran transport studies showed that the amount of FITC-dextran that was transported through the transwell into the lower chamber dramatically increased in the OGD group and the SEMA3A group. This amount decreased when SEMA3A was blocked. (I) We treated bEnd.3 cells with the SEMA3A protein and observed the morphological changes by HPICM. The results illustrated that SEMA3A can efficiently increase defects of the BBB. (J) Observations of the integrity of the barrier by HPICM indicated that the destruction of the barrier that is induced by OGD is efficiently impaired by the downregulation of SEMA3A expression. (K,L) Testing of tight junction changes post-OGD with western blotting revealed that SEMA3A contributes to VE-cadherin serine phosphorylation. The data are expressed as the mean ± SEM, and n = 6 for each group. ∗p < 0.01 vs. control group, &p < 0.01 vs. control group, ∗∗p < 0.05 vs. control groups, ∗&p < 0.05 vs. OGD+PBS group, #∗p < 0.01 vs. siRNA-control group, #p < 0.05 vs. OGD+PBS group.
Regarding the previous results, we predicted that SEMA3A may act as a significant factor in BBB injury. To determine the function of SEMA3A in BBB leakage induced by OGD, we established a BBB model with endothelial cells (bEnd.3) in vitro and evaluated BBB leakage with FITC-dextran transport studies and HPICM. We found that SEMA3A can efficiently open the BBB model and increase leakage. From morphological observations through HPICM, we found that SEMA3A led to the formation of cavities in the BBB model in vitro. However, the leakage induced by OGD can be efficiently decreased by blocking the expression of SEMA3A (Figures 4F–J). Next, we examined the expression of p-VE-cadherin and VE-cadherin and calculated the p-VE-cadherin/VE-cadherin ratio. The results revealed that blocking the expression of SEMA3A can decrease VE-cadherin serine phosphorylation in bEnd.3 cells post-OGD (Figures 4K,L) and demonstrated that SEMA3A affected BBB integrity after OGD by changing the function of tight junctions.
miR-30b-5p Regulates SEMA3A Expression Following TBI in vivo and in vitro
To study whether miR-30b-5p could regulate SEMA3A expression in central nerve system, we first carried out RT-PCR to assess the miRNA level of miR-30b-5p in brain tissue on the 1st, 3rd, 7th, and 14th days after TBI and in bEnd.3 cells at 4 h post-OGD. We found that the miRNA level of miR-30b-5p in brain tissue decreased after TBI and reached its lowest value on the seventh day (Figure 5A). In addition, the miRNA level of miR-30b-5p in bEnd.3 cells also decreased following OGD (Figure 5B). We then downregulated and upregulated the level of miR-30b-5p in vivo and in vitro. We found that the level of SEMA3A changed in response to variations in the miR-30b-5p level. miR-30b-5p helped to reduce SEMA3A expression post-injury (Figures 5C–F). Furthermore, to study the sites in the SEMA3A 3′UTR to which miR-30b-5p binds, we used miRDB to find the potential binding site and conducted a luciferase reporter assay. The results showed that miR-30b-5p inhibited the luciferase activity of the WT construct but not the Mut 3′UTR reporter construct (Figures 5G,H), indicating that miR-30b-5p directly targets SEMA3A and downregulates its expression by binding to sites in the 3′UTR of Sema3a.
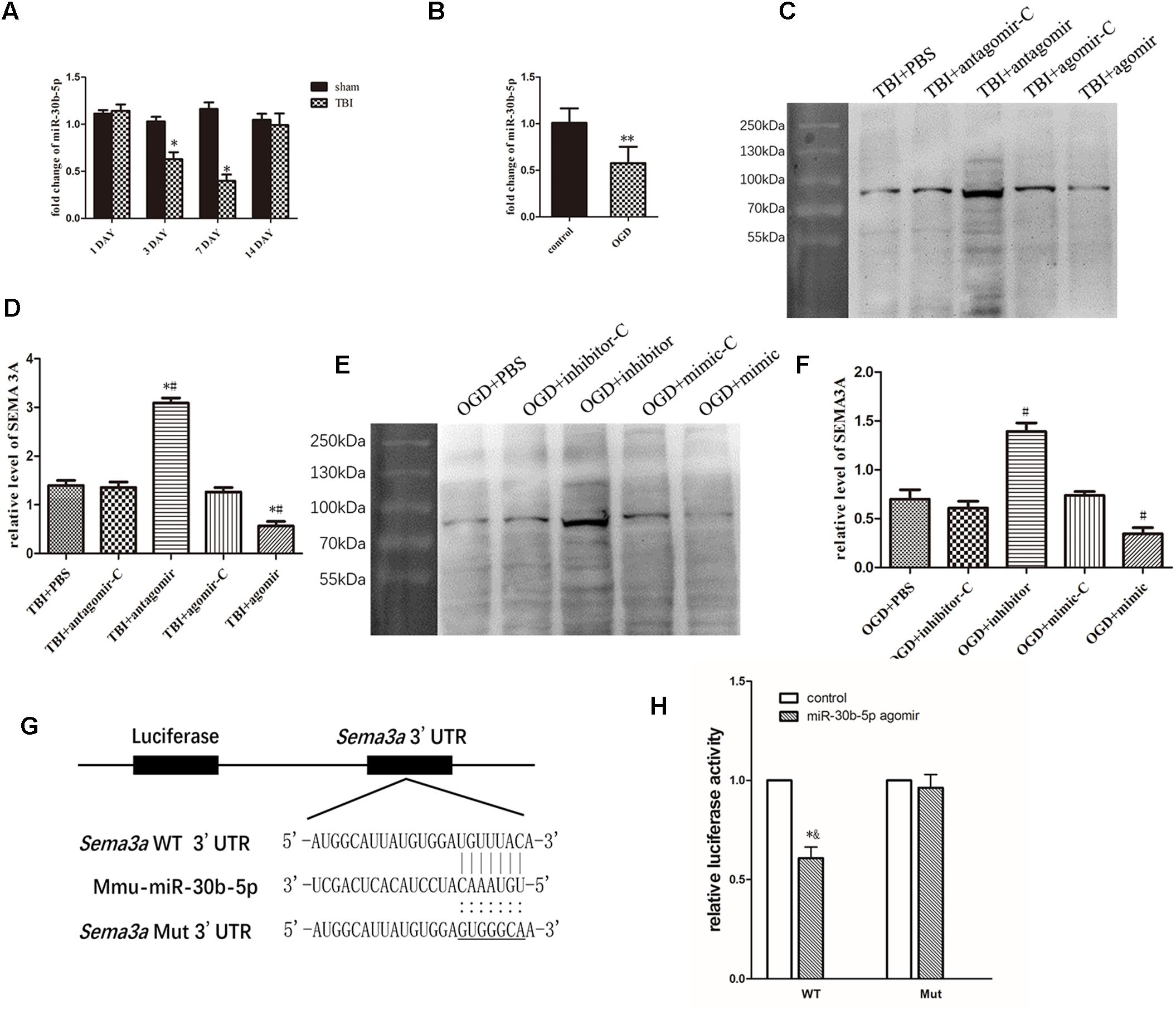
Figure 5. miR-30b-5p regulated SEMA3A expression following injury in vivo and in vitro. (A) Compared with those of the sham groups, the RT-PCR results indicated that the expression level of miR-30b-5p in CCI mice decreased and reached its lowest value on the seventh day post-injury. (B) In an in vitro experiment, the expression level of miR-30b-5p decreased in bEnd.3 cells post-OGD. (C,D) In the CCI mouse model, the downregulation of miR-30b-5p increased the expression level of SEMA3A, and the upregulation of miR-30b-5p decreased SEMA3A levels. (E,F) These results were repeated in the OGD model in bEnd.3 cells. (G) Schematic representation of the potential binding sites for miR-30b-5p in the Sema3a 3′UTR. Seed sequences of the WT (Sema3a WT 3′UTR) and mutant (Sema3a Mut 3′UTR) luciferase reporters are shown in the binding site. (H) Data from the luciferase assay indicated that miR-30b-5p inhibited the luciferase activity of the WT but not the Mut 3′UTR reporter construct, suggesting that miR-30b-5p could directly target Sema3a and downregulate its expression by binding to the 3′UTR sites. The data are expressed as the mean ± SEM, and n = 6 for each group. ∗p < 0.05 vs. sham group, ∗∗p < 0.05 vs. control group, ∗#p < 0.01 vs. control groups, #p < 0.01 vs. control groups, and ∗&p < 0.05 vs. control groups.
The Neuroprotective Effect of miR-30b-5p in Mice Following TBI
To investigate whether miR-30b-5p could improve neurological outcomes after TBI by regulating SEMA3A (Figure 6A), we carried out brain water content, mNSSs and beam-walking test on the third day post-TBI in vivo. Firstly, compared with control groups, miR-30b-5p agomir group could alleviate brain edema following TBI (Figure 6B). Furthermore, according to the results of mNSSs (Figure 6C) and beam-walking test (Figure 6D), we found that the neurological deficits post-TBI could be efficiently improved. Besides, we compared the TBI+agomir+SEMA3A group and TBI+agomir-C+SEMA3A group. SEMA3A recombinant protein were injected on the second day post TBI. The results indicated that upregulation of miR-30b-5p could attenuate the negative effect of SEMA3A on neurological outcomes (Figures 6B–D). All these results echoed the negative influence of SEMA3A, and indicated that miR-30b-5p could be a potential therapy after TBI by inhibiting SEMA3A expression.
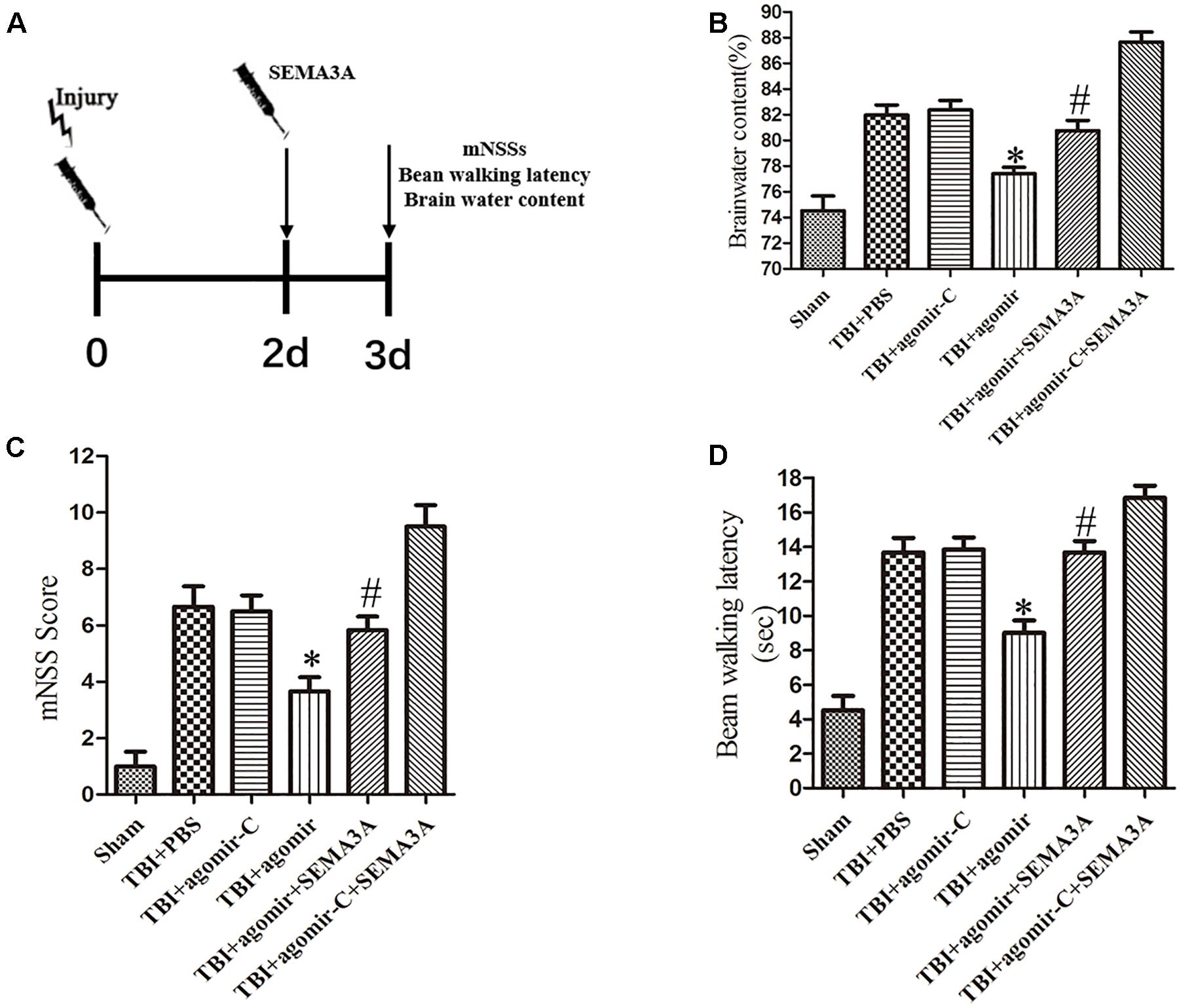
Figure 6. The neuroprotective effect of miR-30b-5p in mice following TBI by regulating SEMA3A. (A) Schematic diagram of the experimental design. (B–D) The brain water content (B), mNSS score (C) and beam balance test (D) were measured on the third day after injury. Upregulation of miR-30b-5p could efficiently decrease brain edema, and improve neurological outcomes post-TBI. The negative effect of recombinant SEMA3A protein also can be improved by upregulation of miR-30b-5p. The data are expressed as the mean ± SEM, and n = 6 for each group, ∗p < 0.05 vs. control groups, #p < 0.05 vs. TBI+agomir-C+SEMA3A group.
Discussion
The effect of SEMA3A on vascular permeability has been proven in a previous report (Acevedo et al., 2008), but neurological research on SEMA3A is very limited, mainly focusing on ischemia stroke, multiple sclerosis lesions, and particularly the functions of SEMA3A in suppressing axon formation and promoting dendrite growth (Shelly et al., 2011; Costa et al., 2015; Hou et al., 2015; Gutierrez-Franco et al., 2016). However, nothing is known about the effects of SEMA3A during TBI and how it is regulated. Thus, we investigated the effects of SEMA3A on secondary BBB injury after TBI in this study. We found that the expression level of SEMA3A significantly changed in a mouse TBI model. SEMA3A induced by TBI could affect neurologic outcomes. OGD injury can induce the secretion of SEMA3A through imitating hypoxic pathological status. Furthermore, we uncovered convincing evidence that SEMA3A expression can be regulated by miR-30b-5p and the neurological deficits post-TBI could be efficiently improved through upregulation of miR-30b-5p in brain tissue.
Blood–brain barrier is formed by the endothelial cells lining the brain microvessels, under the inductive influence of neighboring cell types including astrocytes and pericytes. The endothelium forms the major interface between the blood and the CNS, and by a combination of low passive permeability and presence of specific transport systems, enzymes and receptors regulates molecular and cellular traffic across the barrier layer (Abbott, 2013). Most of pathological changes on CNS are resulting from the damage of BBB. SEMA3A is widely known as a neuronal guidance factor in CNS, which can participate in the construction of nerve networks by regulating not only growth cone motility but also dendritic development and maturation via Nrp-1/Plexin-A1 receptor complex (Campbell et al., 2001), whereby Plexin-A1 acts as the signal transducer (Acevedo et al., 2008).
To investigate the potential effect of SEMA3A on secondary BBB damage after TBI, we employed a CCI mouse model and observed an increasing trend for the expression levels of SEMA3A, Nrp-1 and plexin-A1 after TBI, and peaked on the third day post-injury. Moreover, Nrp-1 is necessary for vessel development, since Nrp-1 knockouts could impair angiogenesis and cardiovascular development (Takashima et al., 2002; Gu et al., 2003). Together with plexins (plexin-A1) and neuropilins (Nrp-1 and Nrp-2), semaphorins (including SEMA3A) have the ability to interrupt vascular formation and tumor vascularization (Gaur et al., 2009). According to our results, we have sufficient reason to predict that SEMA3A may be responsible for secondary BBB damage post-TBI. BBB disruption is considered to be one of the most significant factors associated with pathological changes post-TBI (Wood, 2018) and has been widely demonstrated to be a transient process that can lead to poor lifelong outcomes (Alluri et al., 2015). We confirmed that SEMA3A contributes to the neurological deficits and BBB leakage post-TBI. All of these negative effects following TBI could be improved by blocking SEMA3A expression. Furthermore, the serine phosphorylation of VE-cadherin due to SEMA3A efficiently led to the dysfunction of tight junctions (Le Guelte et al., 2012). TBI always leads to alterations of VE-cadherin (Hue et al., 2014), and the dysfunction of tight junctions contributes to damage to the integrity of the BBB (Goncalves et al., 2012). In our experiments, we confirmed that SEMA3A could increase VE-cadherin serine phosphorylation post-TBI and weaken cell–cell adhesion, contributing to BBB leakage as a vascular permeability factor after TBI. Brain edema is one of the most serious pathological changes that leads to mortality and disability. Vasogenic brain edema is caused by the destruction of BBB integrity (Blixt et al., 2015). We found that the increase in brain water content post-TBI can be effectively decreased by downregulating the expression of SEMA3A.
Oxygen-glucose deprivation is currently the most frequently used in vitro model to imitate BBB injury in vitro (Ge et al., 2018) through hypoxic pathological damage (Salvador et al., 2015). Hypoxia is significantly associated with negative effects following TBI and contributes to morbidity and mortality (Chesnut et al., 1993). Some compounds induced by hypoxemia, such as extracellular glutamate, can increase damage following primary damage (Bullock et al., 1998). Endothelial cells (bEnd.3) account for the characteristics of the BBB and are regarded as a suitable model for the BBB in vitro (Forster et al., 2005). We first demonstrated that OGD injury triggers the release of SEMA3A and its related receptors (Nrp-1 and plexin-A1). Furthermore, the disruption of BBB integrity and BBB leakage induced by OGD can be significantly repaired by blocking the expression of SEMA3A. From morphological observations through HPICM, we found that SEMA3A led to the formation of cavities in the BBB model in vitro. The leakage of the BBB model induced by SEMA3A probably resulted from these cavities. Decreasing the expression level of SEMA3A in bEnd.3 cells can efficiently reduce leakage post-OGD, and fewer cavities can be observed in HPICM. The enhancement of the VE-cadherin function promotes endothelial protection and helps to overcome the defects that result from OGD stress in brain endothelial cells (Ishiguro et al., 2011). The VE-cadherin serine phosphorylation following injury was also confirmed to have reduced by SiRNA-SEMA3A.
miRNAs play crucial roles in physiological and pathological processes post-TBI. Redell et al. (2009) first reported the expression changes and pleiotropy of miRNAs in the pathophysiological processes following TBI. Our group also reported a microarray analysis of miRNA expression in the rat’s cerebral cortex post-TBI (Lei et al., 2009). In previous studies, we demonstrated that the neurological outcomes following TBI could be improved through targeting miRNA-21 and miRNA-124 (Ge et al., 2015, 2016, 2018; Huang et al., 2018). In the previous study, several miRNAs were reported to regulate SEMA3A expression. Rezaeepoor et al. (2018) confirmed that SEMA3A serve as an immune modulator is suppressed by miR-145-5p. Furthermore, Liu et al. (2019) also reported that miR-145-5p suppresses osteogenic differentiation of adipose-derived stem cells by targeting SEMA3A. miR-362 can downregulate the expression of SEMA3A in non-small-cell lung carcinoma (NSCLC) and promote lung cancer metastasis (Luo et al., 2018). In the study of hepatocellular carcinoma, SEMA3A can serve as a target regulated by miR-192-5p to promote the proliferation and metastasis of hepatocellular carcinoma cell (Yan-Chun et al., 2017). The research on microRNA regulating SEMA3A was also pretty extensive in optic nervous system (Baudet et al., 2011; Han et al., 2015; Villain et al., 2018). According to the analysis of TargetScan, Miranda and miRDB, we predicted that miRNA-30 is closely related to SEMA3A. miR-30 has been widely discovered, and it has been proven to act as tumor suppressor in the development of various cancers (Cheng et al., 2012; Zhong et al., 2014; Singh et al., 2017; Zhang et al., 2017). Some studies have also confirmed that miR-30 family inhibited tumor cell growth by changing the tumor cell autophagy. For example, Zhang et al. (2017) found that miR-30d could inhibit autophagy of colon cancer cells and promote cell apoptosis through targeting PI3K and ATG5 (Singh et al., 2017). Furthermore, miRNA-30 has also been reported to change its expression in Alzheimer’s progression (Liu et al., 2014). However, in a recent study, miRNA-30c was confirmed to regulate neurogenesis resulting from SEMA3A in the subventricular zones (Sun et al., 2016). Similarly, miR-30b-5p, a mature miRNA derived from pre-miR-30b, caught our attention. miR-30b was confirmed to contribute to proliferation, migration, and invasion in glioblastoma (Li et al., 2018). Whether miR-30b-5p could also be a regulation factor in CNS, particularly following TBI is still unknown. Based on the results exhibited above, we demonstrated a decreasing trend in the miR-30b-5p expression level, which reached its lowest value on the seventh day following injury in the CCI mouse injury model and the OGD-treated cell injury model. Moreover, we also found that the upregulation or downregulation of miR-30b-5p can decrease or increase the expression of SEMA3A post-injury in vivo and in vitro, which demonstrated that SEMA3A could be abolished by miR-30b-5p in brain tissue. miR-30b-5p inhibited the luciferase activity of the WT, but not the Mut 3′UTR reporter construct, also suggesting that miR-30b-5p could directly target SEMA3A and downregulate its expression by binding to the 3′UTR sites. In addition, upregulation of miR-30b-5p level in the injured brain by intracerebroventricular infusion of miR-30b-5p agomir acted a better neurological outcome after TBI through regulating SEMA3A. The negative effect of SEMA3A on neurological outcomes could be attenuated by upregulation of miR-30b-5p which indicated that miR-30b-5p could be the potential therapeutic treatment for regulating SEMA3A following TBI.
However, this study had some limitations. In this study, we mainly focused on the effect of SEMA3A on secondary BBB damage following TBI and its regulation factors. In addition, destruction of the BBB always leads to the invasion of hazardous substances into the brain tissue. Several pathological events post-TBI are resulting from the damage of BBB. For our future study, downstream signaling of SEMA3A will be studied to clarify its mechanism in other types of pathological damage induced by TBI. We are studying the mechanism of SEMA3A and miR-30b-5p in regulating BBB damage from two aspects: apoptosis and inflammation. Even though the results of this study were not influenced, the effective time point of knockdown group and recombinant SEMA3A protein group following TBI also need to be considered and regulated to make these them comparable in the further mechanism research.
In summary, the major discovery of this study is that the expression level of SEMA3A increased and miR-30b-5p decreased following TBI induced by CCI treatment in mice and OGD treatment in bEnd.3 cells. The downregulation of SEMA3A levels could alleviate BBB damage post-TBI in vivo and in vitro, and improve the neurological outcomes of CCI mice. Furthermore, the expression level of miR-30b-5p was altered following injury, and miR-30b-5p could regulate SEMA3A expression post-TBI in vivo and in vitro. miRNA-30b-5p has also been confirmed to improve neurological outcomes following TBI through regulating SEMA3A. Therefore, SEMA3A is a promising therapeutic target for TBI, and miR-30b-5p could be a potential treatment for the downregulation of SEMA3A.
Author Contributions
JZ and YY designed the experiments. MY, XW, and YF carried out the experiments, analyzed the experimental results, wrote the manuscript. YC, DS, XX, JW, GG, RP, XL, FL, TS, YW, DW, HR, ZH, XG, QL, and KF took part in the experiments and proposed some suggestions.
Funding
This research was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81700403, 81501057, 81671380, 81502173, 81501069, 81330029, and 81720108015), Tianjin Municipal Science and Technology Commission (Grant Nos. 16PTSYJC00180, 15ZXLCSY00060, and 15ZXJZSY00040), Tianjin Natural Science Foundation (Grant No. 17JCZDJC35900), the Tianjin Medical University General Hospital Funding (Grant No. ZYYFY201616), the Tianjin Research Program of Application Foundation and Advanced Technology (Grant No. 15JCQNJC11300), the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (Grant No. 2016YD02), National Key R&D Program of China (Grant No. 2017YFC1104004), the Technology Program Fund of Tianjin Health and Family Planning Commission for the Key Field of Traditional Chinese Medicine (Grant No. 2018001).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciate Li Liu, Weiyun Cui, Fanglian Chen, Liqun He, Ying Li, Shu Zhang, and Lei Zhou from Tianjin Neurological Institute for their technical support.
References
Abbott, N. J. (2013). Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 36, 437–449. doi: 10.1007/s10545-013-9608-0
Acevedo, L. M., Barillas, S., Weis, S. M., Gothert, J. R., and Cheresh, D. A. (2008). Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 111, 2674–2680. doi: 10.1182/blood-2007-08-110205
Alluri, H., Shaji, C. A., Davis, M. L., and Tharakan, B. (2018). a mouse controlled cortical impact model of traumatic brain injury for studying blood-brain barrier dysfunctions. Methods Mol. Biol. 1717, 37–52. doi: 10.1007/978-1-4939-7526-6_4
Alluri, H., Wiggins-Dohlvik, K., Davis, M. L., Huang, J. H., and Tharakan, B. (2015). Blood-brain barrier dysfunction following traumatic brain injury. Metab. Brain Dis. 30, 1093–1104. doi: 10.1007/s11011-015-9651-7
Baudet, M. L., Zivraj, K. H., Abreu-Goodger, C., Muldal, A., Armisen, J., Blenkiron, C., et al. (2011). miR-124 acts through CoREST to control onset of Sema3A sensitivity in navigating retinal growth cones. Nat. Neurosci. 15, 29–38. doi: 10.1038/nn.2979
Bazzoni, G., Martinez-Estrada, O. M., Orsenigo, F., Cordenonsi, M., Citi, S., and Dejana, E. (2000). Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 275, 20520–20526. doi: 10.1074/jbc.M905251199
Beckmann, A., Grissmer, A., Wolf, S., Recktenwald, J., and Meier, C. (2018). Oxygen-glucose deprivation in mouse astrocytes is associated with ultrastructural changes in connexin 43 gap junctions. Neuroscience 397, 67–79. doi: 10.1016/j.neuroscience.2018.11.043
Blennow, K., Hardy, J., and Zetterberg, H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899. doi: 10.1016/j.neuron.2012.11.021
Blixt, J., Svensson, M., Gunnarson, E., and Wanecek, M. (2015). Aquaporins and blood-brain barrier permeability in early edema development after traumatic brain injury. Brain Res. 1611, 18–28. doi: 10.1016/j.brainres.2015.03.004
Bullock, R., Zauner, A., Woodward, J. J., Myseros, J., Choi, S. C., Ward, J. D., et al. (1998). Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 89, 507–518. doi: 10.3171/jns.1998.89.4.0507
Campbell, D. S., Regan, A. G., Lopez, J. S., Tannahill, D., Harris, W. A., and Holt, C. E. (2001). Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J. Neurosci. 21, 8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001
Chen, C., Hu, Q., Yan, J., Yang, X., Shi, X., Lei, J., et al. (2009). Early inhibition of HIF-1alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol. Dis. 33, 509–517. doi: 10.1016/j.nbd.2008.12.010
Chen, J., Sanberg, P. R., Li, Y., Wang, L., Lu, M., Willing, A. E., et al. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688. doi: 10.1161/hs1101.098367
Chen, X., Zhu, H., Liu, X., Lu, H., Li, Y., Wang, J., et al. (2013). Characterization of two mammalian cortical collecting duct cell lines with hopping probe ion conductance microscopy. J. Membr. Biol. 246, 7–11. doi: 10.1007/s00232-012-9495-6
Cheng, C. W., Wang, H. W., Chang, C. W., Chu, H. W., Chen, C. Y., Yu, J. C., et al. (2012). MicroRNA-30a inhibits cell migration and invasion by downregulating vimentin expression and is a potential prognostic marker in breast cancer. Breast Cancer Res. Treat. 134, 1081–1093. doi: 10.1007/s10549-012-2034-4
Chesnut, R. M., Marshall, S. B., Piek, J., Blunt, B. A., Klauber, M. R., and Marshall, L. F. (1993). Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir. Suppl. 59, 121–125. doi: 10.1007/978-3-7091-9302-0_21
Cope, E. C., Morris, D. R., and Levenson, C. W. (2012). Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr. Rev. 70, 410–413. doi: 10.1111/j.1753-4887.2012.00486.x
Costa, C., Martinez-Saez, E., Gutierrez-Franco, A., Eixarch, H., Castro, Z., Ortega-Aznar, A., et al. (2015). Expression of semaphorin 3A, semaphorin 7A and their receptors in multiple sclerosis lesions. Mult. Scler. 21, 1632–1643. doi: 10.1177/1352458515599848
Duh, E. J. (2011). Sema3A [corrected] resists retinal revascularization. Blood 117, 5785–5786. doi: 10.1182/blood-2011-03-343228
Eliceiri, B. P., Paul, R., Schwartzberg, P. L., Hood, J. D., Leng, J., and Cheresh, D. A. (1999). Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 4, 915–924. doi: 10.1016/S1097-2765(00)80221-X
Feeney, D. M., Gonzalez, A., and Law, W. A. (1982). Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217, 855–857. doi: 10.1126/science.7100929
Forster, C., Silwedel, C., Golenhofen, N., Burek, M., Kietz, S., Mankertz, J., et al. (2005). Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 565, 475–486. doi: 10.1113/jphysiol.2005.084038
Fu, X., Shen, Y., Wang, W., and Li, X. (2018). MiR-30a-5p ameliorates spinal cord injury-induced inflammatory responses and oxidative stress by targeting Neurod 1 through MAPK/ERK signalling. Clin. Exp. Pharmacol. Physiol. 45, 68–74. doi: 10.1111/1440-1681.12856
Gaur, P., Bielenberg, D. R., Samuel, S., Bose, D., Zhou, Y., Gray, M. J., et al. (2009). Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin. Cancer Res. 15, 6763–6770. doi: 10.1158/1078-0432.CCR-09-1810
Ge, X., Han, Z., Chen, F., Wang, H., Zhang, B., Jiang, R., et al. (2015). MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain Res. 1603, 150–157. doi: 10.1016/j.brainres.2015.01.009
Ge, X., Huang, S., Gao, H., Han, Z., Chen, F., Zhang, S., et al. (2016). miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res. 1650, 31–40. doi: 10.1016/j.brainres.2016.07.015
Ge, X., Li, W., Huang, S., Yin, Z., Yang, M., Han, Z., et al. (2018). Increased miR-21-3p in injured brain microvascular endothelial cells following traumatic brain injury aggravates blood-brain barrier damage by promoting cellular apoptosis and inflammation through targeting MAT2B. J. Neurotrauma doi: 10.1089/neu.2018.5728 [Epub ahead of print].
Goncalves, A., Leal, E., Paiva, A., Teixeira Lemos, E., Teixeira, F., Ribeiro, C. F., et al. (2012). Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obesity Metab. 14, 454–463. doi: 10.1111/j.1463-1326.2011.01548.x
Gu, C., Rodriguez, E. R., Reimert, D. V., Shu, T., Fritzsch, B., Richards, L. J., et al. (2003). Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45–57. doi: 10.1016/S1534-5807(03)00169-2
Gu, D., Zou, X., Ju, G., Zhang, G., Bao, E., and Zhu, Y. (2016). Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016:2093940. doi: 10.1155/2016/2093940
Gutierrez-Franco, A., Costa, C., Eixarch, H., Castillo, M., Medina-Rodriguez, E. M., Bribian, A., et al. (2016). Differential expression of sema3A and sema7A in a murine model of multiple sclerosis: implications for a therapeutic design. Clin. Immunol. 163, 22–33. doi: 10.1016/j.clim.2015.12.005
Han, F., Huo, Y., Huang, C. J., Chen, C. L., and Ye, J. (2015). MicroRNA-30b promotes axon outgrowth of retinal ganglion cells by inhibiting Semaphorin3A expression. Brain Res. 1611, 65–73. doi: 10.1016/j.brainres.2015.03.014
Hou, S. T., Nilchi, L., Li, X., Gangaraju, S., Jiang, S. X., Aylsworth, A., et al. (2015). Semaphorin3A elevates vascular permeability and contributes to cerebral ischemia-induced brain damage. Sci. Rep. 5:7890. doi: 10.1038/srep07890
Huang, S., Ge, X., Yu, J., Han, Z., Yin, Z., Li, Y., et al. (2018). Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 32, 512–528. doi: 10.1096/fj.201700673R
Hue, C. D., Cao, S., Dale Bass, C. R., Meaney, D. F., and Morrison, B. III (2014). Repeated primary blast injury causes delayed recovery, but not additive disruption, in an in vitro blood-brain barrier model. J. Neurotrauma 31, 951–960. doi: 10.1089/neu.2013.3149
Ishiguro, M., Suzuki, Y., Mishiro, K., Kakino, M., Tsuruma, K., Shimazawa, M., et al. (2011). Blockade of phosphodiesterase-III protects against oxygen-glucose deprivation in endothelial cells by upregulation of VE-cadherin. Curr. Neurovasc. Res. 8, 86–94. doi: 10.2174/156720211795495385
Jiang, L., Hu, Y., He, X., Lv, Q., Wang, T. H., and Xia, Q. J. (2017). Breviscapine reduces neuronal injury caused by traumatic brain injury insult: partly associated with suppression of interleukin-6 expression. Neural Regen. Res. 12, 90–95. doi: 10.4103/1673-5374.198990
Joyal, J. S., Sitaras, N., Binet, F., Rivera, J. C., Stahl, A., Zaniolo, K., et al. (2011). Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 117, 6024–6035. doi: 10.1182/blood-2010-10-311589
Khalili, H., Paydar, S., Safari, R., Arasteh, P., Niakan, A., and Abolhasani Foroughi, A. (2017). Experience with traumatic brain injury: Is early tracheostomy associated with better prognosis? World Neurosurg. 103, 88–93. doi: 10.1016/j.wneu.2017.02.060
Le Guelte, A., Galan-Moya, E. M., Dwyer, J., Treps, L., Kettler, G., Hebda, J. K., et al. (2012). Semaphorin 3A elevates endothelial cell permeability through PP2A inactivation. J. Cell Sci. 125, 4137–4146. doi: 10.1242/jcs.108282
Lei, P., Li, Y., Chen, X., Yang, S., and Zhang, J. (2009). Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 1284, 191–201. doi: 10.1016/j.brainres.2009.05.074
Li, M., Li, F., Luo, C., Shan, Y., Zhang, L., Qian, Z., et al. (2011). Immediate splenectomy decreases mortality and improves cognitive function of rats after severe traumatic brain injury. J. Trauma 71, 141–147. doi: 10.1097/TA.0b013e3181f30fc9
Li, Z., Guo, J., Ma, Y., Zhang, L., and Lin, Z. (2018). Oncogenic role of MicroRNA-30b-5p in glioblastoma through targeting proline-rich transmembrane protein 2. Oncol. Res. 26, 219–230. doi: 10.3727/096504017X14944585873659
Liu, Q. Y., Chang, M. N., Lei, J. X., Koukiekolo, R., Smith, B., Zhang, D., et al. (2014). Identification of microRNAs involved in Alzheimer’s progression using a rabbit model of the disease. Am. J. Neurodegen. Dis. 3, 33–44.
Liu, X., Zhu, W., Wang, L., Wu, J., Ding, F., and Song, Y. (2019). miR-145-5p suppresses osteogenic differentiation of adipose-derived stem cells by targeting semaphorin 3A. In Vitro Cell Dev. Biol. Anim. 55, 189–202. doi: 10.1007/s11626-019-00318-7
Luo, D., Zhang, Z., Zhang, Z., Li, J. Y., Cui, J., Shi, W. P., et al. (2018). Aberrant expression of miR-362 promotes lung cancer metastasis through downregulation of Sema3A. J. Immunol. Res. 2018:1687097. doi: 10.1155/2018/1687097
Marmarou, A. (2007). A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus 22:E1. doi: 10.3171/foc.2007.22.5.2
Mena, J. H., Sanchez, A. I., Rubiano, A. M., Peitzman, A. B., Sperry, J. L., Gutierrez, M. I., et al. (2011). Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: comparing classic and modified Glasgow Coma Scale score model scores of 13. J. Trauma 71, 1185–1192; discussion 1193. doi: 10.1097/TA.0b013e31823321f81185-1192;
Redell, J. B., Liu, Y., and Dash, P. K. (2009). Traumatic brain injury alters expression of hippocampal microRNAs: potential regulators of multiple pathophysiological processes. J. Neurosci. Res. 87, 1435–1448. doi: 10.1002/jnr.21945
Rezaeepoor, M., Ganjalikhani-Hakemi, M., Shapoori, S., Eskandari, N., Sharifi, M., Etemadifar, M., et al. (2018). Semaphorin-3A as an immune modulator is suppressed by MicroRNA-145-5p. Cell J. 20, 113–119. doi: 10.22074/cellj.2018.4842
Salehi, A., Zhang, J. H., and Obenaus, A. (2017). Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow Metab. 37, 2320–2339. doi: 10.1177/0271678X17701460
Salvador, E., Burek, M., and Forster, C. Y. (2015). Stretch and/or oxygen glucose deprivation (OGD) in an in vitro traumatic brain injury (TBI) model induces calcium alteration and inflammatory cascade. Front. Cell. Neurosci. 9:323. doi: 10.3389/fncel.2015.00323
Sayeed, I., Turan, N., Stein, D. G., and Wali, B. (2018). Vitamin D deficiency increases blood-brain barrier dysfunction after ischemic stroke in male rats. Exp. Neurol. 312, 63–71. doi: 10.1016/j.expneurol.2018.11.005
Sen, N. (2017). An insight into the vision impairment following traumatic brain injury. Neurochem. Int. 111, 103–107. doi: 10.1016/j.neuint.2017.01.019
Shelly, M., Cancedda, L., Lim, B. K., Popescu, A. T., Cheng, P. L., Gao, H., et al. (2011). Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron 71, 433–446. doi: 10.1016/j.neuron.2011.06.041
Shen, Y., Shen, Z., Miao, L., Xin, X., Lin, S., Zhu, Y., et al. (2015). miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-gamma-lyase expression. Antioxid. Redox Signal. 22, 224–240. doi: 10.1089/ars.2014.5909
Singh, S. V., Dakhole, A. N., Deogharkar, A., Kazi, S., Kshirsagar, R., Goel, A., et al. (2017). Restoration of miR-30a expression inhibits growth, tumorigenicity of medulloblastoma cells accompanied by autophagy inhibition. Biochem. Biophys. Res. Commun. 491, 946–952. doi: 10.1016/j.bbrc.2017.07.140
Sun, D., Gu, G., Wang, J., Chai, Y., Fan, Y., Yang, M., et al. (2017). Administration of tauroursodeoxycholic acid attenuates early brain injury via Akt pathway activation. Front. Cell. Neurosci. 11:193. doi: 10.3389/fncel.2017.00193
Sun, T., Li, W., and Ling, S. (2016). miR-30c and semaphorin 3A determine adult neurogenesis by regulating proliferation and differentiation of stem cells in the subventricular zones of mouse. Cell Prolif. 49, 270–280. doi: 10.1111/cpr.12261
Takamatsu, H., Okuno, T., and Kumanogoh, A. (2010). Regulation of immune cell responses by semaphorins and their receptors. Cell Mol. Immunol. 7, 83–88. doi: 10.1038/cmi.2009.111
Takashima, S., Kitakaze, M., Asakura, M., Asanuma, H., Sanada, S., Tashiro, F., et al. (2002). Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 99, 3657–3662. doi: 10.1073/pnas.022017899
Tchantchou, F., and Zhang, Y. (2013). Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 30, 565–579. doi: 10.1089/neu.2012.2647
Villain, G., Poissonnier, L., Noueihed, B., Bonfils, G., Rivera, J. C., Chemtob, S., et al. (2018). miR-126-5p promotes retinal endothelial cell survival through SetD5 regulation in neurons. Development 145:dev156232. doi: 10.1242/dev.156232
Wood, H. (2018). Traumatic brain injury: evidence of blood-brain barrier disruption after concussion. Nat. Rev. Neurol. 14:254. doi: 10.1038/nrneurol.2018.29
Xiong, Y., Mahmood, A., and Chopp, M. (2017). Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen. Res. 12, 19–22. doi: 10.4103/1673-5374.198966
Xu, X., Yin, D., Ren, H., Gao, W., Li, F., Sun, D., et al. (2018). Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol. Dis. 117, 15–27. doi: 10.1016/j.nbd.2018.05.016
Yan, E. B., Hellewell, S. C., Bellander, B. M., Agyapomaa, D. A., and Morganti-Kossmann, M. C. (2011). Post-traumatic hypoxia exacerbates neurological deficit, neuroinflammation and cerebral metabolism in rats with diffuse traumatic brain injury. J. Neuroinflammation 8:147. doi: 10.1186/1742-2094-8-147
Yan-Chun, L., Hong-Mei, Y., Zhi-Hong, C., Qing, H., Yan-Hong, Z., and Ji-Fang, W. (2017). MicroRNA-192-5p Promote the Proliferation and Metastasis of Hepatocellular Carcinoma Cell by Targeting SEMA3A. Appl. Immunohistochem. Mol. Morphol. 25, 251–260. doi: 10.1097/PAI.0000000000000296
Yang, K., Miron, R. J., Bian, Z., and Zhang, Y. F. (2018). A bone-targeting drug-delivery system based on Semaphorin 3A gene therapy ameliorates bone loss in osteoporotic ovariectomized mice. Bone 114, 40–49. doi: 10.1016/j.bone.2018.06.003
Yazdani, U., and Terman, J. R. (2006). The semaphorins. Genome Biol. 7:211. doi: 10.1186/gb-2006-7-3-211
Zhang, R., Xu, J., Zhao, J., and Bai, J. (2017). Mir-30d suppresses cell proliferation of colon cancer cells by inhibiting cell autophagy and promoting cell apoptosis. Tumour Biol. 39:1010428317703984. doi: 10.1177/1010428317703984
Zhong, Z., Xia, Y., Wang, P., Liu, B., and Chen, Y. (2014). Low expression of microRNA-30c promotes invasion by inducing epithelial mesenchymal transition in non-small cell lung cancer. Mol. Med. Rep. 10, 2575–2579. doi: 10.3892/mmr.2014.2494
Zlokovic, B. V. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. doi: 10.1016/j.neuron.2008.01.003
Keywords: traumatic brain injury, blood–brain barrier, Semaphorin 3A, miRNA-30b-5p, oxygen-glucose deprivation
Citation: Yang M, Wang X, Fan Y, Chen Y, Sun D, Xu X, Wang J, Gu G, Peng R, Shen T, Liu X, Li F, Wang Y, Wang D, Rong H, Han Z, Gao X, Li Q, Fan K, Yuan Y and Zhang J (2019) Semaphorin 3A Contributes to Secondary Blood–Brain Barrier Damage After Traumatic Brain Injury. Front. Cell. Neurosci. 13:117. doi: 10.3389/fncel.2019.00117
Received: 20 December 2018; Accepted: 11 March 2019;
Published: 26 March 2019.
Edited by:
Silvia Sánchez-Ramón, Universidad Complutense de Madrid, SpainReviewed by:
Farida Hellal, Institute for Stroke and Dementia Research (ISD), GermanyYaohui Tang, Shanghai Jiao Tong University, China
Copyright © 2019 Yang, Wang, Fan, Chen, Sun, Xu, Wang, Gu, Peng, Shen, Liu, Li, Wang, Wang, Rong, Han, Gao, Li, Fan, Yuan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhua Yuan, yyhxxx39@sina.com Jianning Zhang, jianningzhang@hotmail.com
†These authors have contributed equally to this work
 Mengchen Yang
Mengchen Yang Xiaoxue Wang3,4†
Xiaoxue Wang3,4†  Xin Xu
Xin Xu Jianning Zhang
Jianning Zhang