Crosstalk Between ATP-P2X7 and Adenosine A2A Receptors Controlling Neuroinflammation in Rats Subject to Repeated Restraint Stress
- 1CNC—Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal
- 2Department of Life Sciences, Faculty of Sciences and Technology, University of Coimbra, Coimbra, Portugal
- 3Faculty of Medicine, University of Coimbra, Coimbra, Portugal
Depressive conditions precipitated by repeated stress are a major socio-economical burden in Western countries. Previous studies showed that ATP-P2X7 receptors (P2X7R) and adenosine A2A receptors (A2AR) antagonists attenuate behavioral modifications upon exposure to repeated stress. Since it is unknown if these two purinergic modulation systems work independently, we now investigated a putative interplay between P2X7R and A2AR. Adult rats exposed to restraint stress for 14 days displayed an anxious (thigmotaxis, elevated plus maze), depressive (anhedonia, increased immobility), and amnesic (modified Y maze, object displacement) profile, together with increased expression of Iba-1 (a marker of microglia “activation”) and interleukin-1β (IL1β) and tumor necrosis factor α (TNFα; proinflammatory cytokines) and an up-regulation of P2X7R (mRNA) and A2AR (receptor binding) in the hippocampus and prefrontal cortex. All these features were attenuated by the P2X7R-preferring antagonist brilliant blue G (BBG, 45 mg/kg, i.p.) or by caffeine (0.3 g/L, p.o.), which affords neuroprotection through A2AR blockade. Notably, BBG attenuated A2AR upregulation and caffeine attenuated P2X7R upregulation. In microglial N9 cells, the P2X7R agonist BzATP (100 μM) or the A2AR agonist CGS26180 (100 nM) increased calcium levels, which was abrogated by the P2X7R antagonist JNJ47965567 (1 μM) and by the A2AR antagonist SCH58261 (50 nM), respectively; notably JNJ47965567 prevented the effect of CGS21680 and the effect of BzATP was attenuated by SCH58261 and increased by CGS21680. These results provide the first demonstration of a functional interaction between P2X7R and A2AR controlling microglia reactivity likely involved in behavioral adaptive responses to stress and are illustrative of a cooperation between the two arms of the purinergic system in the control of brain function.
Introduction
Depression represents the major burden of disease in Europe (Andlin-Sobocki et al., 2005) and the constellation of mood alterations associated with depression can be recapitulated in animal models repeatedly exposed to different stressors (de Kloet et al., 2005; Berton et al., 2012). The use of animal models converges with imaging studies to identify modifications of different brain regions, such as the hippocampus, prefrontal, and limbic cortices, that are associated with mood dysfunction (de Kloet et al., 2005) and provide compelling evidence for the involvement of neuroinflammation (Rial et al., 2016; Deng et al., 2020; Troubat et al., 2021) and of synaptic dysfunction (Duman and Aghajanian, 2012; Vose and Stanton, 2017) as key processes in the etiology of major depression. However, the identification of molecular systems that may be targeted to correct depressive symptoms has still failed to yield novel and effective anti-depressants (Ménard et al., 2016).
One candidate system is operated by purines, which fulfill numerous roles controlling neuronal communication, neuron-glia communication, and neuroinflammation (Agostinho et al., 2020). ATP is a danger signal in the brain (Rodrigues et al., 2015) and one of its receptors, P2X7 receptors (P2X7R), has been associated with mood dysfunction (reviewed in Ribeiro et al., 2019; Illes et al., 2020), based on the association of particular P2X7R haplotypes with depression (Czamara et al., 2018) and with the ability of genetic deletion or pharmacological antagonism of P2X7R to control mood dysfunction in different animal models of repeated stress (Iwata et al., 2016; Yue et al., 2017; Farooq et al., 2018; Aricioglu et al., 2019). The mechanism underlying the impact of P2X7R on mood is still undefined, but the control of glia, mainly microglia, which contributes to the build-up of neuroinflammation, stems as a promising candidate mechanism (Yue et al., 2017; Bhattacharya and Jones, 2018). Together with possible neuronal effects of P2X7R, the control of neuroinflammation can account for the general neuroprotective properties of P2X7R antagonists, such as the blood-brain barrier-permeant drug, brilliant blue G (BBG; Díaz-Hernández et al., 2009, 2012; Arbeloa et al., 2012; Carmo et al., 2014; Wang et al., 2015; Yue et al., 2017; Farooq et al., 2018; Aricioglu et al., 2019).
The purinergic system is particularly enticing since it encompasses two parallel signaling systems: one involving ATP and P2R and the other involving the dephosphorylation product of ATP, adenosine, which acts on P1 or adenosine receptors, mainly inhibitory A1 receptors and facilitatory A2A receptors (A2AR) in the brain (Fredholm et al., 2005). The extracellular conversion of ATP into adenosine is mediated by ectonucleotidases (Cunha, 2001; Zimmermann et al., 2012) and we have shown that the extracellular formation of ATP-derived adenosine is selectively associated with the activation of neuronal A2AR (Rebola et al., 2008; Augusto et al., 2013; Carmo et al., 2019; Gonçalves et al., 2019), as well as with A2AR located in other cell types (e.g., Deaglio et al., 2007; Flögel et al., 2012; Flores-Santibáñez et al., 2015; Mahmut et al., 2015; Meng et al., 2019). A2AR are mainly located in synapses (Rebola et al., 2005), but also control microglia and neuroinflammation (Orr et al., 2009; Rebola et al., 2011; Madeira et al., 2016; Duarte et al., 2019) to robustly impact neurodegeneration (reviewed in Cunha, 2016). Both selective A2AR antagonists and the non-selective adenosine receptor antagonist caffeine (Fredholm et al., 1999), can control mood and memory alterations in rodents exposed to repeated stress (Yamada et al., 2013; Kaster et al., 2015), as per the mood normalizing properties afforded by the intake of caffeine in humans (reviewed in Grosso et al., 2016) and the association of A2AR polymorphisms with anxiety and depression (Hamilton et al., 2004; Hohoff et al., 2010; Oliveira et al., 2019).
Thus, the available evidence indicates P2X7R as well as A2AR as major players in the control of mood dysfunction, with both receptors systems undergoing an up-regulation in animal models exposed to repeated stress (Cunha et al., 2006; Kongsui et al., 2014; Kaster et al., 2015; Aricioglu et al., 2019). However, it has never been explored if there is any interplay between both receptors systems in the control of mood dysfunction. As a first step to test the existence of such an interplay, we now exploited a rat model of repeated restraint stress to test if P2X7R blockade with BBG would impact A2AR up-regulation and, conversely, if caffeine blockade of A2AR could interfere with P2X7R up-regulation.
Materials and Methods
Animals
Male Wistar rats (adults, 220–250 g, n = 78: 18 controls treated with vehicle, nine controls treated with BBG, nine controls treated with caffeine; 18 stressed treated with vehicle, nine stressed treated with BBG, nine stressed treated with caffeine, six for electrophysiology) were obtained from Charles River (Barcelona, Spain) and were maintained at 23–25°C, with 12 h light / 12 h dark cycle and standard chow and tap water ad libitum. All procedures in this study were conducted following the principles and procedures outlined as “3Rs” in the guidelines of the European Union (2010/63/EU), FELASA, and ARRIVE, and were approved by the Portuguese Ethical Committee (DGAV) and by the Institution’s Ethics’ Committee (ORBEA 238-2019/14102019). Since the behavioral alterations caused by this protocol of restraint stress were so far only validated in male rats, the “3Rs” guidelines imposed the use of only male rats to obtain the first proof-of-concept supporting the existence of any interaction between P2X7R and A2AR.
In vivo Drug Treatments
As done previously (Carmo et al., 2014), the blood-brain barrier-permeant and efficacious P2X7R antagonist brilliant blue G (BBG, 45 mg/kg dissolved in saline; from Sigma–Aldrich, Portugal) or saline were administered intraperitoneally every 48 h at 7 PM, starting 3 days before the protocol of restraint stress, until the sacrifice of the animals. The tested dose of BBG has previously been shown to yield a brain concentration of 200–220 nM (Díaz-Hernández et al., 2012), which is within the effective and selective range of BBG towards central P2X7R and is without evident side-effects in control rodents (Donnelly-Roberts and Jarvis, 2007).
Caffeine (Sigma, Portugal) was administered through the drinking water as previously reported (Duarte et al., 2009; Cognato et al., 2010) at a dose (0.3 g/L) estimated to correspond to a daily intake of 3–4 cups of coffee by humans (Fredholm et al., 1999), which rodents consume without modification of their water intake (Duarte et al., 2009, 2012; Silva et al., 2013). This yields a concentration of circa 30 μM in the brain parenchyma (Costenla et al., 2010; Silva et al., 2013), which selectively targets adenosine receptors (Lopes et al., 2019) and mimics the neuroprotective impact of A2AR antagonists, rather than of A1R (Cunha et al., 2006; Dall’Igna et al., 2007), namely in animal models of stress and depression (Kaster et al., 2015; Machado et al., 2017). Caffeine intake was allowed only overnight (7 PM-7 AM), starting 3 days before the protocol of restraint stress, until the sacrifice of the animals and this repeated exposure to caffeine is expected to afford neuroprotection without major modification of behavioral or physiological parameters in control rodents (Duarte et al., 2009; Yang et al., 2009; Cognato et al., 2010).
Restraint Stress
The stress model used consisted of a repeated physical restraint of rats, as done previously (Cunha et al., 2006). The rats were individually placed in a room adjacent to their colony in an independent plastic compartment and immobilized in a 25 × 7 cm plastic bottle, with a plastic taper on the outside and a 1 cm hole at one end for breathing. After the termination of each daily restraint stress session, the rats were returned to their home cages. The schedule of sub-chronic restraint stress consisted of a daily 4 h immobilization period (between 10 AM and 4 PM) during 14 consecutive days, the time previously defined to be required to cause stable behavioral modifications for at least 1 week in adult male rats (Cunha et al., 2006). Control age-matched rats were handled as their tested littermates except that they were not isolated or immobilized.
Behavioral Evaluation
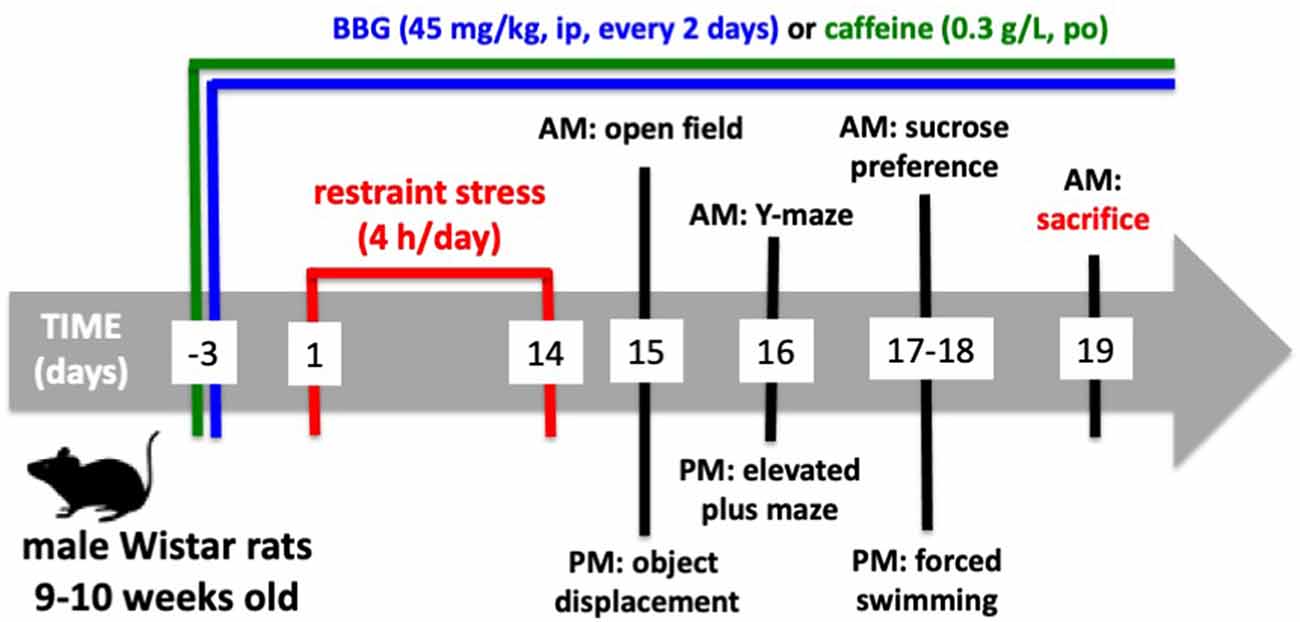
Behavioral tests were carried out from 9 AM until 4 PM on the 15th until the 18th day after beginning the restraint stress protocol (Figure 1). As shown in Figure 1, the animals were subject to a tight schedule of behavioral characterization, with a minimal time interval between each test, which could lead to cross-testing interferences. However, the analysis of the performance of control animals in the successive tests did not show evident differences from historic controls where rats of the same age and strain were tested in each different test with wider time gaps between the different tests (Cunha et al., 2006; Cognato et al., 2010; Carmo et al., 2014; Coelho et al., 2014; Matheus et al., 2016). All behavior tests were carried out by two experimenters who were unaware of the phenotypes or drug treatments, in a sound-attenuated room with an eight lux illumination and visual cues on the walls, to which the animals were previously habituated. The apparatuses were cleaned with 20% ethyl alcohol to remove any odors after testing each animal.
Locomotion and exploratory behavior were monitored using an open-field arena made of dark gray PVC measuring 100 × 100 cm2 (divided by white lines into 25 squares of 20 × 20 cm2) and was surrounded by 40-cm high walls. Each rat was placed in the center of the open field and the following variables were recorded for 10 min: number of peripheral squares (adjacent to the walls) crossed (peripheral locomotion), number of central squares (away from the walls) crossed (central locomotion) and total locomotion (peripheral locomotion plus central locomotion).
Anxiety was further assessed using the elevated plus-maze, which consisted of four arms of the same size (40 cm × 5 cm) arranged in the form of a cross and raised 50 cm above the floor. Two opposed arms were surrounded by 30 cm high opaque black Plexiglas walls, except for the entrance (closed arms) while the other two had no walls (open arms). Each animal was placed on the central square of the maze facing an enclosed arm and was allowed to explore the maze for 5 min. The number of entries and the time spent in both open and closed arms were recorded, considering an entry only when the whole body and four paws were inside an arm.
The depressive-like behavior was evaluated in the forced swimming test, where rats were placed in individual glass cylinders (40 cm in height and 17 cm in diameter) containing water (water depth was 30 cm, kept at 25 ± 1°C) to measure the total duration of immobility, climbing, and swimming during a 10-min session. A rat was regarded as immobile when floating motionless or making only those movements necessary to keep its head above the water. The climbing behavior was defined as upward-directed movements of the forepaws usually along the side of the swimming chamber and the swimming behavior is defined as movement (usually horizontal) throughout the swimming chamber; diving and face shaking behaviors were not considered.
Anhedonic-like behavior was evaluated with the sucrose preference test, where rats were first single-housed in a cage with two bottles and free access to food. After 4 h of habituation, one bottle was randomly switched to contain 1.2% sucrose solution and the total consumption of water and sucrose solution was measured at the end of a 16 h test period (12 h dark phase plus 4 h light phase). Sucrose preference was calculated as the ratio of sucrose vs. total intake.
Spatial memory was evaluated using a 2-trials Y-maze paradigm (Dellu et al., 1997). The test was carried out in a Plexiglas apparatus with equal three arms (10 cm wide, 35 cm long, and walls of 25 cm height) in a Y-shape, separated by equal angles. The test consists of two sessions of 5 min duration separated by a 2-h inter-trial interval. During the first session, the rat was placed at the end of one arm and allowed to explore the two available arms since the third arm (the novel arm) was blocked by a guillotine door. During the second session, the “‘novel”’ arm was opened and the rat was again placed in the start arm and allowed to explore the three arms. Memory performance was evaluated by measuring the time spent exploring the “novel” arm compared to the exploration of the other two arms. An entry into an arm was defined as the placement of all four paws into the arm.
Hippocampal-dependent memory was also evaluated using the object displacement test, where rats were exposed to two identical objects in the same open field apparatus in which they were habituated and were allowed to explore for 5 min the objects fixed in opposite corners 10 cm away from walls and 70 cm apart from each other. In the test trial, carried out 2-h after, rats were again placed for 5 min in the open field arena, except that one of the objects was moved to a novel position. Memory performance was quantified with an object displacement index defined as the ratio between the time exploring the object in the novel location over the total time exploring both objects. Exploration of an object is defined as directing the nose to the object at a distance equal to or less than 2 cm from the object and/or touching it with the nose; rearing on to the object was not considered exploratory behavior.
The sequence of the tests is indicated in Figure 1.
mRNA Expression
After completion of the battery of behavior analysis, rats were sacrificed by decapitation under deep anesthesia upon exposure to a halothane-saturated atmosphere. One hippocampus or part of the prefrontal cortex of each rat was used to extract total RNA with a MagNA Lyser Instrument and a MagNA Pure Compact RNA Isolation kit (Roche, Portugal), according to the manufacturer’s instructions. The integrity, quantity, and purity of the RNA yields were checked by electrophoresis and spectrophotometry. Reverse transcription for first-strand cDNA synthesis from each sample was performed using a random hexamer primer with the Transcriptor First Strand cDNA Synthesis kit (Roche), according to the manufacturer’s instructions. The resulting cDNAs were used as templates for real-time PCR, which was carried out on the LightCycler instrument (Roche) using the FastStart DNA Master SYBR Green I kit (Roche). The mRNA expression of the marker of microglia “activation” Iba1 (ionized calcium-binding adaptor molecule 1), of the pro-inflammatory cytokines interleukin-1β (IL1β) and tumor necrosis factor α (TNFα) and of P2X7R, was calculated relative to GADPH (glyceraldehyde 3-phosphate dehydrogenase) mRNA expression, using the following primers (from Tib MolBiol, Germany): Iba1 (forward: 5′-TGC GCA AGA GAT CTG CCA TC-3′; reverse: 5′-ACC AGT TGG CTT CTG GTG TT-3′); IL1β (forward: 5′-ATG AGA GCA TCC AGC TTC AAA TC-3′; reverse: 5′-CAC ACT AGC AGG TCG TCA TCA TC-3′); TNFα (forward: 5′-CGA GAT GTG GAA CTG GCA GA-3′; reverse: 5′-CTA CGG GCT TGT CAC TCG A-3′); P2rx7 (forward: 5′-CTG CCT CCC GTC TCA ACT AC-3′; reverse: 5′-GCC TCT CTG GAT AGC ACG AT-3′); GAPDH (forward: 5′-CCC TTC ATT GAC CTC AAC TAC-3′; reverse: 5′-CTT CTC CAT GGT GGT GAA GAC-3′). Quantification was carried out based on standard curves run simultaneously with the test samples generated by conventional PCR amplification, as previously described (Costenla et al., 2011; Rebola et al., 2011). The purity and specificity of the resulting PCR products were assessed by melting curve analysis and electrophoresis. Control reactions were performed to verify that no amplification occurred without cDNA.
Receptor Binding Assay
The binding assays were performed as previously described (Cunha et al., 2006), using the second hippocampus and the rest of the prefrontal cortex from each rat. After purifying whole membranes by centrifugation-based fractionation (Rebola et al., 2005), the membranes were resuspended in Tris-Mg solution (containing 50 mM Tris and 10 mM MgCl2, pH 7.4) with 4 U/ml of adenosine deaminase (to remove endogenous adenosine). Binding with 2 nM of 3H-SCH58261 (specific activity of 77 Ci/mmol; prepared by GE Healthcare and offered by E.Ongini, Schering-Plough, Italy), a supramaximal concentration of this selective A2AR ligand (Lopes et al., 2004), was performed for 1 h at room temperature with 286–343 (hippocampus) or 54–71 μg of protein (prefrontal cortex), with constant swirling. The binding reactions were stopped by the addition of 4 ml of ice-cold Tris-Mg solution and filtration through Whatman GF/C filters (GE Healthcare). The radioactivity was measured with 2 ml of scintillation liquid (AquaSafe 500 Plus, Zinsser Analytic). The specific binding was expressed as fmol/mg protein and was estimated by subtraction of the non-specific binding, which was measured in the presence of 12 μM of xanthine amine congener (XAC; Sigma), an antagonist of adenosine receptors. All binding assays were performed in duplicate.
Calcium Transients in N9 Microglial Cells
A murine microglial cell line, N9 (a kind gift from Professor Claudia Verderio, CNR Institute of Neuroscience, Milan, Italy), was grown as previously described (Gomes et al., 2013) in an RPMI medium supplemented with 30 mM glucose (Sigma), 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO, Invitrogen, Portugal) and maintained at 37°C in an incubator with a humidified atmosphere with 5% CO2, until reaching confluence. N9 cells were then detached using 0.05% trypsin (T3924, Sigma) for 5 min, resuspended in RPMI after washing and centrifugation, and counted using a hemocytometer with trypan blue. N9 cells were then seeded in a 48-multiwell at a density of 0.02 × 106 cells and remained in culture for 48 h. Then, cells were incubated for 45 min with Fluo-4-AM (4 μM; Life Technologies) dissolved in recording buffer (132 mM NaCl, 4 mM KCl, 1.4 mM MgCl2, 6 mM glucose, 10 mM HEPES, 1.8 mM CaCl2; pH 7.4) with 0.05% bovine serum albumin to facilitate probe entry into the cells, as previously described (Simões et al., 2012). The cells were then washed and left in a recording buffer for 15 min to allow complete Fluo-4 AM de-esterification. In some experimental conditions, the following modifiers of the evoked signals were added to the recording buffer during the de-esterification and kept until the end of the experiment: 1 μM JNJ47965567 (2-(phenylthio)-N-[[tetrahydro-4-(4-phenyl-1-piperazinyl)-2H-pyran-4-yl]methyl-3-pyridinecarboxamide, a selective P2X7R antagonist from Tocris), 50 nM SCH58261 (2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine, a selective A2AR antagonist from Tocris) or 100 nM CGS21680 (4-[2-[[6-amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid, a selective A2AR agonist from Tocris).
After de-esterification, cytosolic Ca2+-dependent fluorescence was recorded using a VICTOR3 Multiplate reader (Perkin Elmer) with Wallac 1420 software, using an exciting wavelength of 485 nm and recording the emission wavelength at 530 nm, close to the ideal wavelength to monitor Fluo-4 fluorescence (494/506 nm). The baseline fluorescence was recorded at 0.2 Hz. [Ca2+]i transients were triggered by the application of different stimuli, either 100 μM BzATP [2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate, a selective P2X7R agonist from Sigma], 100 nM CGS21680 or 100 μM glutamate (to mimic excitotoxic conditions, from Sigma) and fluorescence was recorded for 5 min at 0.6 Hz (Janks et al., 2018). When glutamate was used as a trigger of Ca2+ transients, experiments were performed with the recording buffer without MgCl2 and with 133.4 mM NaCl. After recording the stimulus-induced [Ca2+]i transient response, cells were exposed to ionomycin (10 μM, Tocris) to induce a steep increase of extracellular Ca2+ influx and consequently a maximum fluorescence response.
The fluorescence data were background-corrected by subtracting the mean fluorescence value of N9 cells that were not incubated with Fluo-4-AM. Intracellular calcium concentration was estimated for each time point using the formula: [Ca2+] = Kd × (F − Fmin)/(Fmax - F), in which Kd is the dissociation constant of Fluo-4 (345 nM), F is the fluorescence recorded at each time point, Fmax is the maximal fluorescence, obtained upon ionomycin application, and Fmin is the minimal fluorescence. The magnitude of [Ca2+]i transients evoked by each stimulus (Δ[Ca2+]i) was obtained subtracting the mean of basal levels from the maximum value after stimulus application.
Electrophysiological Recordings
Rats were decapitated after anesthesia and the brain was quickly removed and placed in ice-cold, oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF; in mM: 124.0 NaCl, 4.4 KCl, 1.0 Na2HPO4, 25.0 NaHCO3, 2.0 CaCl2, 1.2 MgCl2, 10.0 glucose). Using a McIlwain tissue chopper (Brinkmann Instruments, NY, USA), slices (400 μm-thick) from the dorsal hippocampus were cut transverse to its long axis and placed in a holding chamber with oxygenated ACSF. Slices were allowed to recover for at least 1 h before being transferred to a submerged recording chamber and superfused at 3 mL/min with oxygenated ACSF kept at 30.5°C.
Extracellular field excitatory post-synaptic potential (fEPSP) were recorded as previously described (Costenla et al., 2011) with the stimulating bipolar concentric electrode placed in the proximal CA1 stratum radiatum for stimulation of the Schaffer collaterals and the recording electrode, filled with 4 M NaCl (2–5 MΩ resistance), placed in the CA1 stratum radiatum targeting the distal dendrites of pyramidal neurons. Stimulation was delivered every 20 s with rectangular pulses of 0.1 ms duration using either a Grass S44 or a Grass S48 square pulse stimulator (Grass Technologies, RI, USA). After amplification (ISO-80, World Precision Instruments, Hertfordshire, UK), the recordings were digitized (BNC-2110, National Instruments, Newbury, UK), averaged in groups of three, and analyzed using the WinLTP version 2.10 software (WinLTP Limited, Bristol, UK; Anderson and Collingridge, 2007). The intensity of stimulation was chosen between 30–50% of maximal fEPSP response, determined based on input/output curves in which the fEPSP slope was plotted vs. stimulus intensity. Alterations of basal synaptic transmission were quantified as the percentage change of the average value of the fEPSP slope taken from 15–20 min after beginning exposure to the tested drug applied through the superfusion medium, relative to the average value of the fEPSP slope during the 5 min that preceded the application of each modifying drug. Long-term potentiation (LTP) was induced by a high-frequency stimulation (HFS) train (100 Hz for 1 s). LTP was quantified as the percentage change of the average fEPSP slope taken between 55 and 60 min after LTP induction relative to the average slope of the fEPSP measured during the 10 min that preceded LTP induction. The effect of drugs on LTP was assessed by comparing LTP magnitude in the absence and presence of the drug in experiments carried out in different slices from the same animal.
Statistics
Data are presented as the mean ± SEM of n experiments (i.e., n independent rats or cell cultures). The comparison of control and stressed rats and the effect of drugs was analyzed using a two-tailed unpaired Student’s t-test. When testing the impact of a drug on the effects of stress, the data were first analyzed with a two-way ANOVA followed by a Newman–Keuls post hoc test. The comparison between the effect of multiple drugs was carried out using a Dunnett’s test. All tests were performed using Prism 6.0 software (GraphPad, San Diego, CA, USA) considering significance at a 95% confidence interval.
Results
The model of repeated restraint stress triggers robust and reproducible behavioral alterations of mood and memory in adult rats (see Figure 2). Thus, whereas there was no significant change of spontaneous locomotion (n = 18; t = 0.991, p = 0.328, unpaired Student’s t-test; Figure 2A), stressed rats displayed a thigmotaxic behavior indicative of an increased anxiety-like profile, as indicated by the decreased number of crossings in the center of the open field (n = 18; t = 7.229, p < 0.001, unpaired Student’s t-test; Figure 2B). This was confirmed in the elevated plus-maze where stressed rats displayed a decreased number of entries in the open arms (n = 18; t = 9.002, p < 0.001, unpaired Student’s t-test; Figure 2C) and decreased time in the open arms (n = 18; t = 8.628, p < 0.001, unpaired Student’s t-test). Stressed rats also displayed anhedonic behavior in a sucrose preference test (n = 18; t = 5.673, p < 0.001, unpaired Student’s t-test; Figure 2D) and an increased immobility time in the forced swimming test (n = 18; t = 9.959, p < 0.001, unpaired Student’s t-test; Figure 2E), as well as a decreased time spent climbing the walls of the swimming container (n = 18; t = 7.069, p < 0.001, unpaired Student’s t-test; Figure 2F), indicative of depressive-like behavior. Short-term memory was also deteriorated in stressed compared to control rats, as observed by a decreased time searching the novel (previously hidden) arm of a Y-maze (n = 18; t = 6.033, p < 0.001, unpaired Student’s t-test; Figure 2G) and a decreased preference to explore the displaced object (t = 8.009, p < 0.001 between displaced and non-displaced object in control rats and t = 1.885, p = 0.069 between displaced and non-displaced object in stressed rats, unpaired Student’s t-test; Figure 2H).
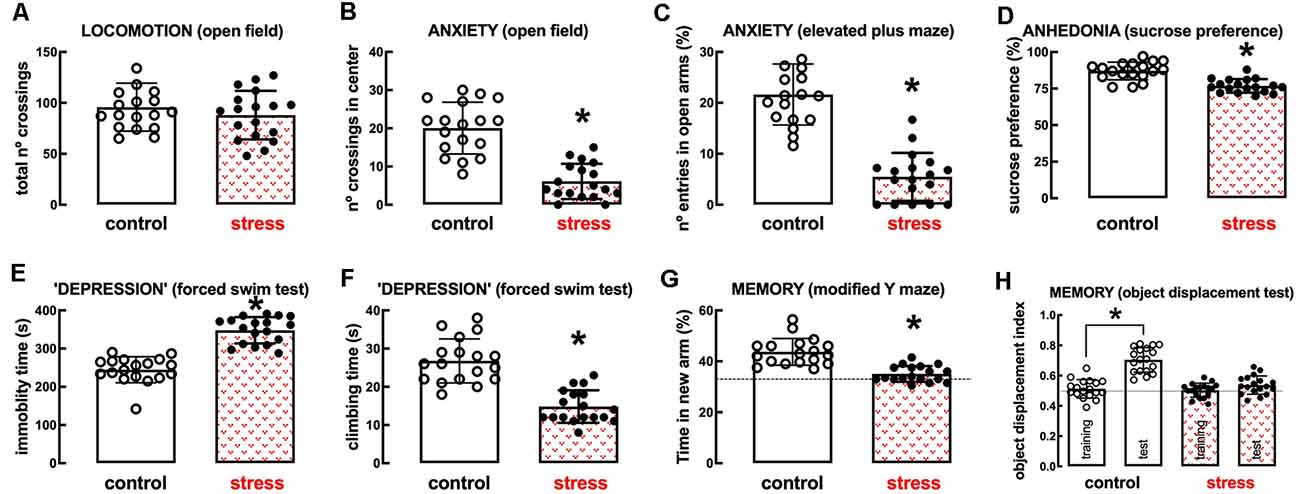
Figure 2. Male adult Wistar rats (8–10 weeks old) subject to a protocol of restraint stress (4 h/day) during 14 days display the expected features of depressed rats. Compared with non-stressed control rats (open bars), stressed rats (red checkered bars) displayed a preserved locomotor activity as evaluated in the open field (A), anxiety-like behavior as evaluated in the open field (B), and in the elevated plus-maze (C) tests, anhedonia as evaluated in the sucrose preference test (D), helpless-like behavior as evaluated by the forced-swimming test (E,F) and impaired memory performance as evaluated by a modified Y maze test (G) and an object-displacement test (H). Data are shown as mean ± SEM; n = 16–18 rats per group. *P < 0.001 using a Student’s t-test.
In line with the involvement of the hippocampus and prefrontal cortex in processing mood and memory-related information (de Kloet et al., 2005) and the association of a heightened inflammatory status in these brain regions in mood impaired animals (Troubat et al., 2021), repeated restraint stress increased the expression of inflammatory markers in the hippocampus and prefrontal cortex (Figures 3A–C). Thus, the hippocampus of stressed rats displayed increased mRNA levels of the marker of microglia “activation” Iba1 (n = 12; t = 9.095, p < 0.001, unpaired Student’s t-test; Figure 3A) and of the pro-inflammatory cytokines interleukin-1β (IL1β; n = 12; t = 7.194, p < 0.001, unpaired Student’s t-test; Figure 3B) and tumor necrosis factor α (TNFα; n = 12; t = 11.46, p < 0.001, unpaired Student’s t-test; Figure 3C). Likewise, the prefrontal cortex of stressed rats also displayed increased mRNA levels of Iba1 (n = 12; t = 4.928, p < 0.001, unpaired Student’s t-test; Figure 3A), IL1β (n = 12; t = 6.028, p < 0.001, unpaired Student’s t-test; Figure 3B) and TNFα (n = 12; t = 20.01, p < 0.001, unpaired Student’s t-test; Figure 3C).
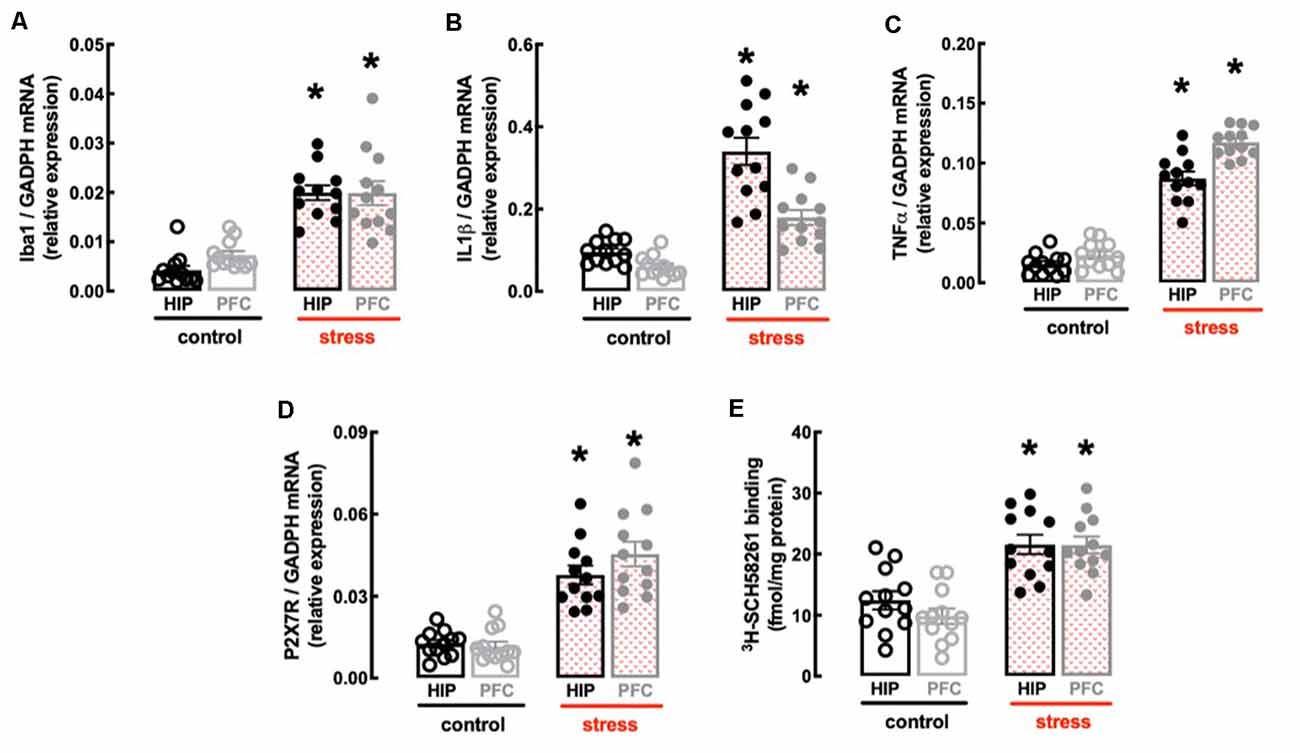
Figure 3. Male adult Wistar rats (8–10 weeks old) subject to a protocol of restraint stress (4 h/day) during 14 days display an increased expression of inflammatory markers and an up-regulation of P2X7 and A2A receptors in the hippocampus (black) and prefrontal cortex (gray). Compared with non-stressed control rats (open bars), stressed rats (red checkered bars) displayed an increased expression of the microglia marker Iba1 (A), of interleukin 1β (IL1β; B), of tumor necrosis factor α (TNFα; C), and P2X7 receptors (P2X7R; D) as well as an increased density of A2A receptors (A2AR; E) as assessed by the binding density of a supramaximal concentration of the selective A2AR antagonist 3H-SCH58261 (2 nM). Data are shown as mean ± SEM; n = 11–12 rats per group. *P < 0.001 vs. control using a Student’s t-test.
Finally, the protocol of restraint stress triggered an up-regulation of P2X7R and of A2AR (Figures 3D,E), two purinergic receptor systems that have been implicated in mood alterations caused by stressful conditions (e.g., Kaster et al., 2015; Iwata et al., 2016). Thus, stressed rats displayed an increased expression of P2X7R mRNA in the hippocampus (n = 12; t = 6.82, p < 0.001, unpaired Student’s t-test; Figure 3D) and prefrontal cortex (n = 12; t = 6.967, p < 0.001, unpaired Student’s t-test; Figure 3D), as well as an increased binding density of the selective A2AR antagonist 3H-SCH58261 in the hippocampus (n = 12; t = 4.212, p < 0.001, unpaired Student’s t-test; Figure 3E) and prefrontal cortex (n = 12; t = 6.181, p < 0.001, unpaired Student’s t-test; Figure 3E).
Impact of the P2X7R Antagonist BBG
The P2X7R-prefering antagonist Brillant Blue G (BBG, 45 mg/kg) was devoid of effects in control rats but attenuated or prevented the behavioral and neurochemical alterations caused by repeated stress (Figures 4, 5). Thus, BBG prevented the stress-induced decrease of the number of crossings in the central area of the open field (effect of stress F(1,32) = 22.13, p < 0.001; effect of BBG F(1,32) = 20.41, p < 0.001; interaction F(1,32) = 17.66, p < 0.001; two-way ANOVA; Figure 4B), the stress-induced decrease of the number of entries in the open arms of the elevated plus maze (effect of stress F(1,32) = 17.96, p < 0.001; effect of BBG F(1,32) = 6.248, p = 0.018; interaction F(1,32) = 23.89, p < 0.001; two-way ANOVA; Figure 4C), the stress-induced decrease of the time spent in the open arms of the elevated plus maze (effect of stress F(1,32) = 23.28, p < 0.001; effect of BBG F(1,32) = 4.187, p = 0.044; interaction F(1,32) = 25.61, p < 0.001; two-way ANOVA), the stress-induced decrease of sucrose preference (effect of stress F(1,32) = 8.737, p = 0.006; effect of BBG F(1,32) = 4.753, p = 0.037; interaction F(1,32) = 6.044, p = 0.019; two-way ANOVA; Figure 4D), the stress-induced increase of immobility in the forced swimming test (effect of stress F(1,32) = 39.91, p < 0.001; effect of BBG F(1,32) = 181.4, p < 0.001; interaction F(1,32) = 13.02, p = 0.001; two-way ANOVA; Figure 4E), the stress-induced decrease of the time climbing the wall in the forced swimming test (effect of stress F(1,32) = 16.35, p < 0.001; effect of BBG F(1,32) = 36.11, p < 0.001; interaction F(1,32) = 12.87, p = 0.001; two-way ANOVA; Figure 4F), the stress-induced decrease of the time spent in the novel arm of the Y-maze (effect of stress F(1,32) = 9.243, p = 0.005; effect of BBG F(1,32) = 6.434, p = 0.016; interaction F(1,32) = 3.596, p = 0.067; two-way ANOVA; Figure 4G), and the stress-induced decrease of the relative time exploring the displaced object (t = 1.928, p = 0.072 between displaced and non-displaced object in stressed rats treated with vehicle and t = 6.246, p < 0.001 between displaced and non-displaced object in stressed rats treated with BBG, unpaired Student’s t-test; Figure 4H).
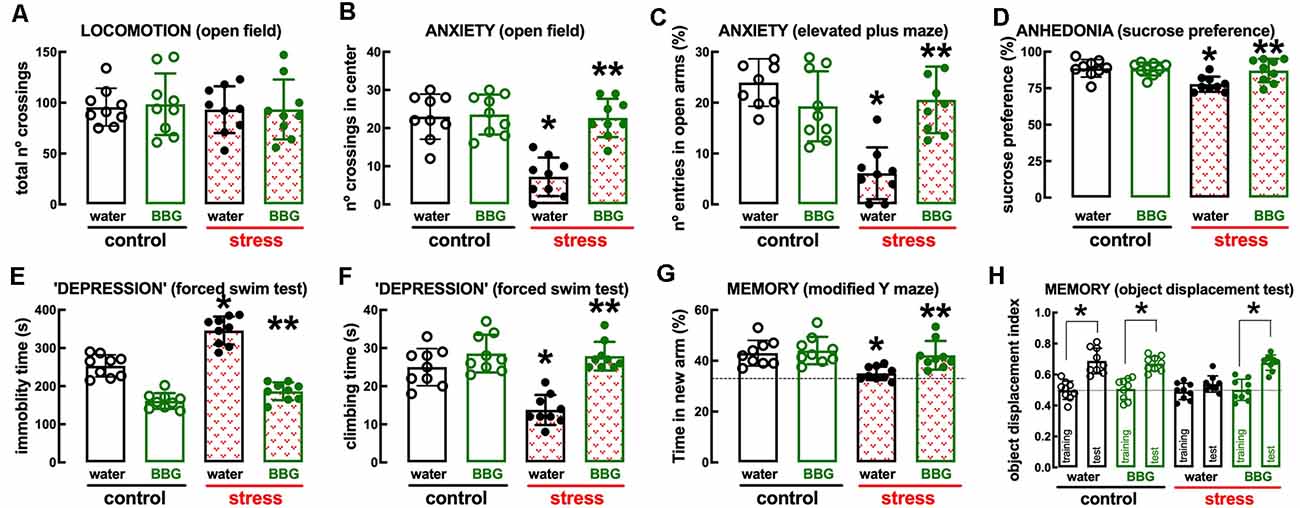
Figure 4. Male adult Wistar rats (8–10 weeks old) subject to a protocol of restraint stress (4 h/day) during 14 days display the expected features of depressed rats, which were prevented by the P2X7 receptor antagonist Brillant Blue G (BBG). Whereas BBG treatment (45 mg/kg, ip, daily, beginning 3 days before the stress protocol and until the sacrifice of the animals; green) was devoid of effects in non-stressed control rats (open bars), BBG prevented all behavioral modifications of stressed rats (red checkered bars): without modification of locomotor activity as evaluated in the open field (A), BBG prevented anxiety-like behavior as evaluated in the open field (B) and in the elevated plus-maze (C) tests, anhedonia as evaluated in the sucrose preference test (D), helpless-like behavior as evaluated by the forced-swimming test (E,F) and impaired memory performance as evaluated by a modified Y maze test (G) and an object-displacement test (H). Data are shown as mean ± SEM; n = 8–9 rats per group. *P < 0.05 vs. control-water, **P < 0.05 vs. stress-water using a Tukey’s multiple comparisons post hoc test after a two-way ANOVA.
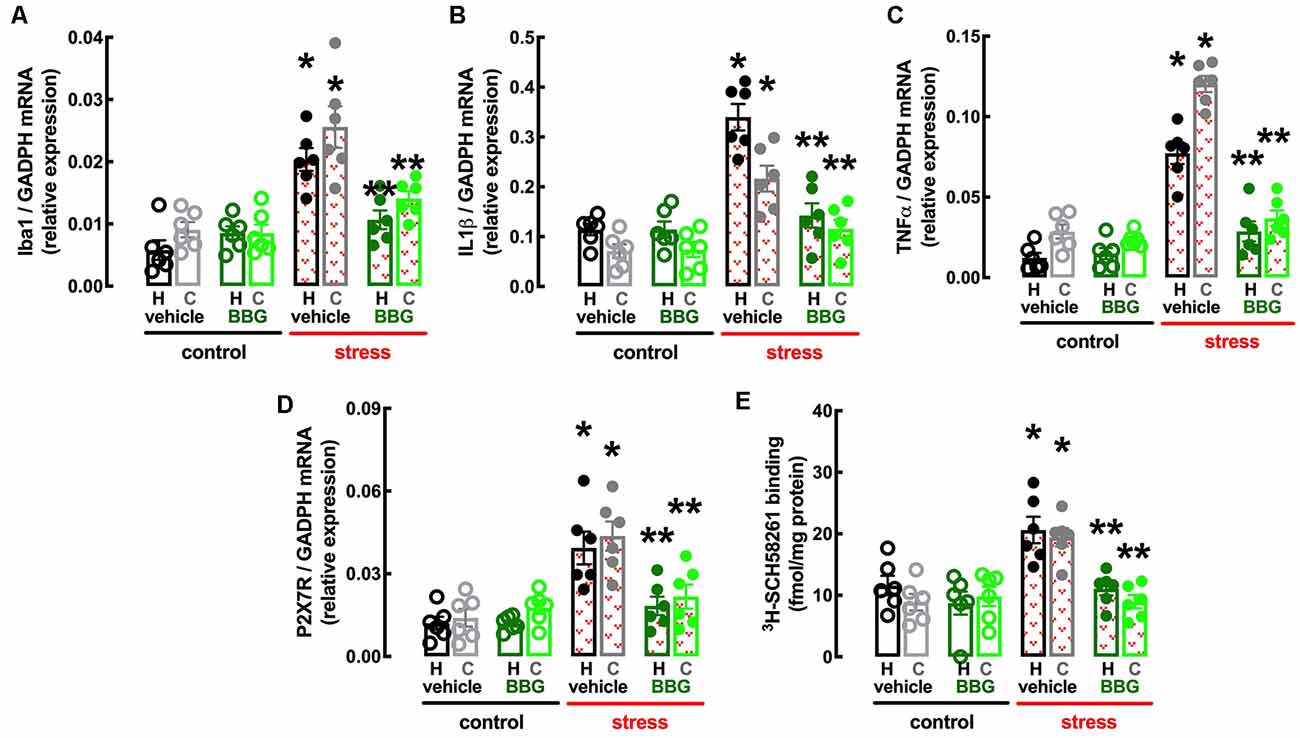
Figure 5. Male adult Wistar rats (8–10 weeks old) subject to a protocol of restraint stress (4 h/day) during 14 days display an increased expression of inflammatory markers and an up-regulation of P2X7 and A2A receptors in the hippocampus (black, dark green) and prefrontal cortex (gray, light green) which were prevented by the P2X7 receptor antagonist Brillant Blue G (BBG). Whereas BBG treatment (45 mg/kg, ip, daily, beginning 3 days before the stress protocol and until the sacrifice of the animals; green) was devoid of effects in non-stressed control rats (open bars), BBG prevented all alterations of stressed rats (red checkered bars), namely the increased expression of the microglia marker Iba1 (A), of interleukin 1β (IL1β; B), of tumor necrosis factor α (TNFα; C), and P2X7 receptors (P2X7R; D) as well as an increased density of A2A receptors (A2AR; E) as assessed by the binding density of a supramaximal concentration of the selective A2AR antagonist 3H-SCH58261 (2 nM). Data are shown as mean ± SEM; n = 5–7 rats per group. *P < 0.05 vs. control-water, **P < 0.05 vs. stress-water using a Tukey’s multiple comparisons post hoc test after a two-way ANOVA.
BBG also attenuated the stress-induced increase in the expression of the marker of “activated” microglia Iba1 in the hippocampus (effect of stress F(1,20) = 30.51, p < 0.001; effect of BBG F(1,20) = 5.295, p = 0.032; interaction F(1,20) = 16.96, p = 0.001; two-way ANOVA; Figure 5A) and prefrontal cortex (effect of stress F(1,20) = 30.52, p < 0.001; effect of BBG F(1,20) = 9.150, p = 0.007; interaction F(1,20) = 7.524, p = 0.012; two-way ANOVA; Figure 5A), as well as in the levels of mRNA of both IL1β in the hippocampus (effect of stress F(1,20) = 38.13, p < 0.001; effect of BBG F(1,20) = 23.05, p < 0.001; interaction F(1,20) = 23.15, p < 0.001; two-way ANOVA; Figure 5B) and prefrontal cortex (effect of stress F(1,20) = 24.39, p < 0.001; effect of BBG F(1,20) = 6.641, p = 0.018; interaction F(1,20) = 7.505, p = 0.013; two-way ANOVA; Figure 5B) and of TNFα in the hippocampus (effect of stress F(1,20) = 59.05, p < 0.001; effect of BBG F(1,20) = 19.99, p < 0.001; interaction F(1,20) = 24.96, p < 0.001; two-way ANOVA; Figure 5C) and prefrontal cortex (effect of stress F(1,20) = 152.8, p < 0.001; effect of BBG F(1,20) = 108.7, p < 0.001; interaction F(1,20) = 85.38, p < 0.001; two-way ANOVA; Figure 5C).
The treatment with BBG also attenuated the stress-induced up-regulation of P2X7R in the hippocampus (effect of stress F(1,20) = 21.31, p < 0.001; effect of BBG F(1,20) = 8.316, p = 0.009; interaction F(1,20) = 8.222, p = 0.009; two-way ANOVA; Figure 5D) and prefrontal cortex (effect of stress F(1,20) = 18.12, p < 0.001; effect of BBG F(1,20) = 5.305, p = 0.032; interaction F(1,20) = 10.52, p < 0.001; two-way ANOVA; Figure 5D). Remarkably, BBG also attenuated the stress-induced up-regulation of A2AR in the hippocampus (effect of stress F(1,20) = 10.85, p = 0.004; effect of BBG F(1,20) = 13.01, p = 0.002; interaction F(1,20) = 3.766, p = 0.067; two-way ANOVA; Figure 5E) and prefrontal cortex (effect of stress F(1,20) = 12.21, p = 0.002; effect of BBG F(1,20) = 11.84, p = 0.003; interaction F(1,20) = 16.96, p = 0.001; two-way ANOVA; Figure 5E).
Impact of the Adenosine Receptor Antagonist Caffeine
The non-selective adenosine receptor antagonist, caffeine (0.3 g/L, p.o.), which affords neuroprotection through the antagonism of A2AR (e.g., Dall’Igna et al., 2007; Cognato et al., 2010; Kaster et al., 2015), was devoid of effects in control rats but attenuated or prevented the behavioral and neurochemical alterations caused by repeated stress (Figures 6, 7). Thus, caffeine prevented the stress-induced decrease of the number of crossing in the central area of the open field (effect of stress F(1,32) = 8.160, p = 0.007; effect of caffeine F(1,32) = 8.459, p = 0.007; interaction F(1,32) = 8.160, p = 0.007; two-way ANOVA; Figure 6B), the stress-induced decrease of the number of entries in the open arms of the elevated plus maze (effect of stress F(1,32) = 23.91, p < 0.001; effect of caffeine F(1,32) = 1.463, p = 0.235; interaction F(1,32) = 16.27, p < 0.001; two-way ANOVA; Figure 6C), the stress-induced decrease of sucrose preference (effect of stress F1,64 = 15.96, p < 0.001; effect of caffeine F3, 64 = 6.544, p = 0.001; interaction F3, 64 = 3.828, p = 0.014; two-way ANOVA; Figure 6D), the stress-induced increase of immobility in the forced swimming test (effect of stress F(1,32) = 29.31, p < 0.001; effect of caffeine F(1,32) = 10.13, p = 0.003; interaction F(1,32) = 13.58, p = 0.001; two-way ANOVA; Figure 6E), the stress-induced decrease of the time climbing the wall in the forced swimming test (effect of stress F(1,32) = 16.45, p < 0.001; effect of caffeine F(1,32) = 8.564, p = 0.006; interaction F(1,32) = 7.247, p = 0.001; two-way ANOVA; Figure 6F), the stress-induced decrease of the time spent in the novel arm of the Y-maze (effect of stress F(1,32) = 5.879, p = 0.021; effect of caffeine F(1,32) = 9.671, p = 0.004; interaction F(1,32) = 6.851, p = 0.013; two-way ANOVA; Figure 6G), and the stress-induced decrease of the relative time exploring the displaced object (t = 1.492, p = 0.161 between displaced and non-displaced object in stress rats treated with vehicle and t = 8.637, p < 0.001 between displaced and non-displaced object in stress rats treated with caffeine, unpaired Student’s t-test; Figure 6H).
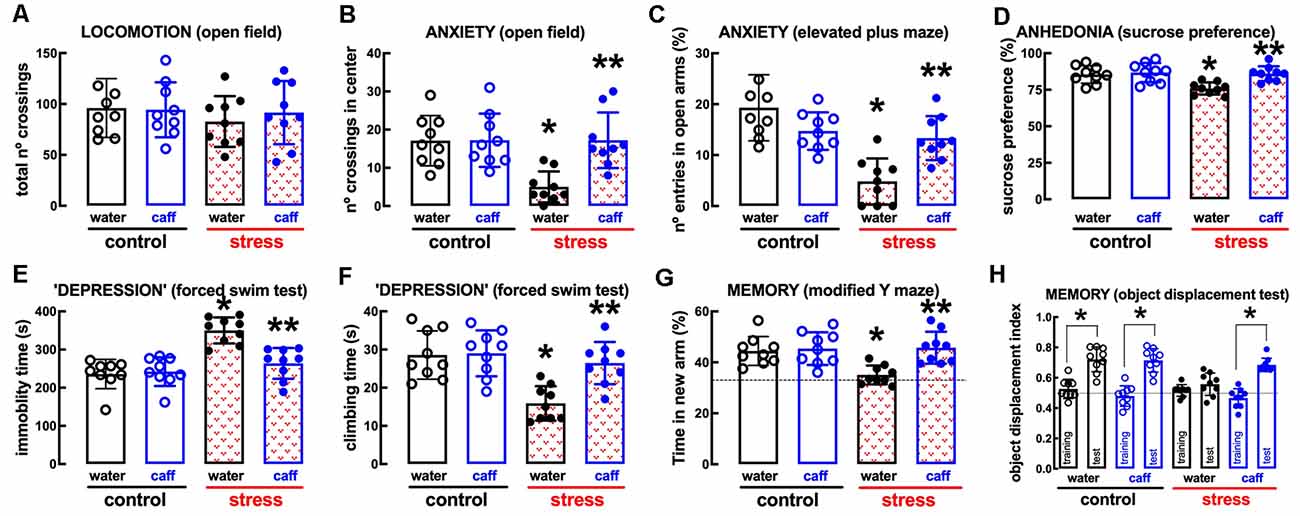
Figure 6. Male adult Wistar rats (8–10 weeks old) subject to a protocol of restraint stress (4 h/day) during 14 days display the expected features of depressed rats, which were prevented by the adenosine receptor antagonist caffeine (caff). Whereas caffeine consumption (0.3 g/L, po, beginning 3 days before the stress protocol and until the sacrifice of the animals; blue) was devoid of effects in non-stressed control rats (open bars), caffeine prevented all behavioral modifications of stressed rats (red checkered bars): without modification of locomotor activity as evaluated in the open field (A), caffeine prevented anxiety-like behavior as evaluated in the open field (B) and in the elevated plus-maze (C) tests, anhedonia as evaluated in the sucrose preference test (D), helpless-like behavior as evaluated by the forced-swimming test (E,F) and impaired memory performance as evaluated by a modified Y maze test (G) and an object-displacement test (H). Data are shown as mean ± SEM; n = 8–9 rats per group. *P < 0.05 vs. control-water, **P < 0.05 vs. stress-water using a Tukey’s multiple comparisons post hoc test after a two-way ANOVA.
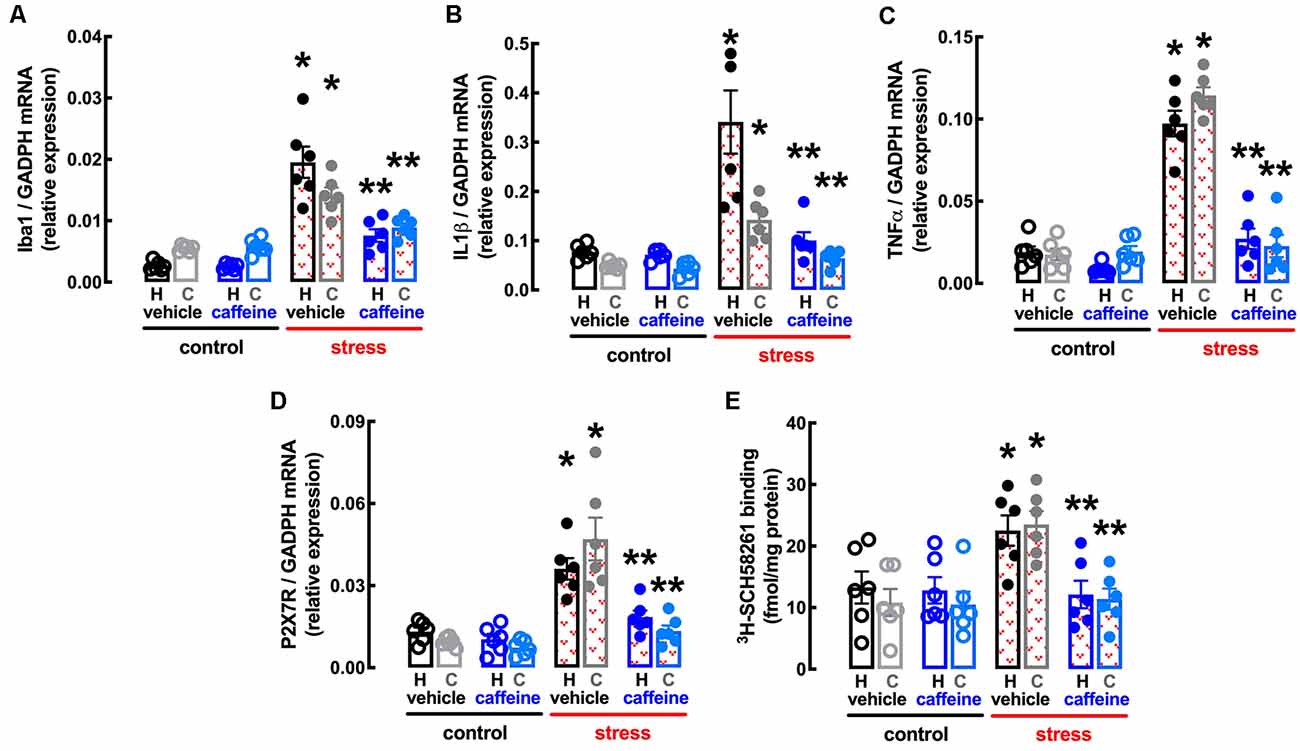
Figure 7. Male adult Wistar rats (8–10 weeks old) subject to a protocol of restraint stress (4 h/day) during 14 days display an increased expression of inflammatory markers and an up-regulation of P2X7 and of A2A receptors in the hippocampus (black, dark blue) and prefrontal cortex (gray, light blue) which were prevented by the adenosine antagonist caffeine (caff). Whereas caffeine consumption (0.3 g/L, po, beginning 3 days before the stress protocol and until the sacrifice of the animals; blue) was devoid of effects in non-stressed control rats (open bars), caffeine prevented all alterations of stressed rats (red checkered bars), namely the increased expression of the microglia marker Iba1 (A), of interleukin 1β (IL1β; B), of tumor necrosis factor α (TNFα; C) and P2X7 receptors (P2X7R; D) as well as an increased density of A2A receptors (A2AR; E) as assessed by the binding density of a supramaximal concentration of the selective A2AR antagonist 3H-SCH58261 (2 nM). Data are shown as mean ± SEM; n = 5–7 rats per group. *P < 0.05 vs. control-water, **P < 0.05 vs. stress-water using a Tukey’s multiple comparisons post hoc test after a two-way ANOVA.
Caffeine also attenuated the stress-induced increase in the expression of the marker of “activated” microglia Iba1 in the hippocampus (effect of stress F(1,20) = 63.06, p < 0.001; effect of caffeine F(1,20) = 19.07, p < 0.001; interaction F(1,20) = 18.35, p < 0.001; two-way ANOVA; Figure 7A) and prefrontal cortex (effect of stress F(1,20) = 57.53, p < 0.001; effect of caffeine F(1,20) = 10.02, p = 0.005; interaction F(1,20) = 12.92, p = 0.002; two-way ANOVA; Figure 7A), as well as in the levels of mRNA of both IL1β in the hippocampus (effect of stress F(1,20) = 19.2, p < 0.001; effect of caffeine F(1,20) = 13.58, p = 0.001; interaction F(1,20) = 12.34, p = 0.002; two-way ANOVA; Figure 7B) and prefrontal cortex (effect of stress F(1,20) = 22.15, p < 0.001; effect of caffeine F(1,20) = 9.351, p = 0.006; interaction F(1,20) = 30.24, p < 0.001; two-way ANOVA; Figure 7B) and of TNFα in the hippocampus (effect of stress F(1,20) = 82.54, p < 0.001; effect of caffeine F(1,20) = 57.24, p < 0.001; interaction F(1,20) = 31.66, p < 0.001; two-way ANOVA; Figure 7C) and prefrontal cortex (effect of stress F(1,20) = 106.0, p < 0.001; effect of caffeine F(1,20) = 85.53, p < 0.001; interaction F(1,20) = 92.56, p < 0.001; two-way ANOVA; Figure 7C).
The treatment with caffeine also attenuated the stress-induced up-regulation of P2X7R in the hippocampus (effect of stress F(1,20) = 35.32, p < 0.001; effect of caffeine F(1,20) = 15.30, p = 0.001; interaction F(1,20) = 8.046, p = 0.010; two-way ANOVA; Figure 7D) and prefrontal cortex (effect of stress F(1,20) = 5.011, p = 0.048; effect of caffeine F(1,20) = 0.569, p = 0.094; interaction F(1,20) = 51.4, p < 0.001; two-way ANOVA; Figure 7D), as well as the stress-induced up-regulation of A2AR in the hippocampus (effect of stress F(1,20) = 4.282, p = 0.045; effect of caffeine F(1,20) = 5.256, p = 0.033; interaction F(1,20) = 4.369, p = 0.050; two-way ANOVA; Figure 7E) and prefrontal cortex (effect of stress F(1,20) = 10.98, p = 0.004; effect of caffeine F(1,20) = 9.302, p = 0.006; interaction F(1,20) = 8.317, p = 0.009; two-way ANOVA; Figure 7E).
P2X7R –A2AR Interaction in Microglial N9 Cells
Since we observed crosstalk between BBG and caffeine upon restraint stress, whereby BBG controlled the up-regulation of A2AR and caffeine controlled the upregulation of P2X7R expression, and the stress-induced behavioral modifications were accompanied by a parallel control of markers of microglia “activation” and neuroinflammation, we next used a microglial N9 cell line to directly investigate a putative crosstalk between P2X7R and A2AR, since both receptors are present and functional in this microglia cell model (e.g., Ferrari et al., 1996; Gomes et al., 2013).
The P2X7R-preferring agonist BzATP (100 μM) evoked an elevation of intracellular free Ca2+ levels (Δ[Ca2+]i) of 94.8 ± 14.5 nM (n = 16), which was inhibited (−76.51 ± 20.02%, n = 10–16, F(3, 44) = 13.21, p = 0.029) in the presence of the selective P2X7R antagonist, JNJ4796556 (1 μM), added 15 min before BzATP (Figures 8A,B). The selective A2AR agonist CGS21680 (100 nM) also evoked a Δ[Ca2+]i of 79.3 ± 11.9 nM (n = 7), which was inhibited (−63.0 ± 14.0%, n = 6, F(2, 18) = 8.67, p = 0.003) in the presence of the selective A2AR antagonist, SCH58261 (50 nM), added 15 min before CGS21680 (Figures 8C,D). Notably, the Δ[Ca2+]i evoked by BzATP (100 μM) was inhibited (−69.5 ± 15.7%, n = 10–16, F(3, 44) = 12.13, p = 0.048) by SCH58261 (50 nM) and potentiated (+80.3 ± 19.5%, n = 12–16, F(3, 44) = 12.13, p = 0.012) by CGS21680 (100 nM; Figure 8B), whereas the Δ[Ca2+]i triggered by CGS21680 (100 nM) was inhibited (−54.3 ± 14.7%, n = 8, F(2, 18) = 8.67, p = 0.005) by JNJ47965567 (1 μM; Figure 8D), indicating a crosstalk between P2X7R and A2AR in the control of Δ[Ca2+]i responses in microglial N9 cells. This P2X7R-A2AR crosstalk is further reinforced by the observation that neither JNJ47965567 (1 μM) nor SCH58216 (50 nM) affected basal [Ca2+]i levels (control, no drugs: 284.2 ± 30.2 nM, n = 10; 1 μM JNJ47965567: 224.2 ± 32.0 nM, n = 9; F3, 34 = 1.92, p = 0.488 vs. control; 50 nM SCH58261: 219.1 ± 17.6 nM, n = 8, F3, 34 = 1.92, p = 0.449 vs. control), indicating a lack of tonic P2X7R- or A2AR-mediated control of Δ[Ca2+]i that could hinder the interpretation of the cross-inhibition between both purinergic receptor systems.
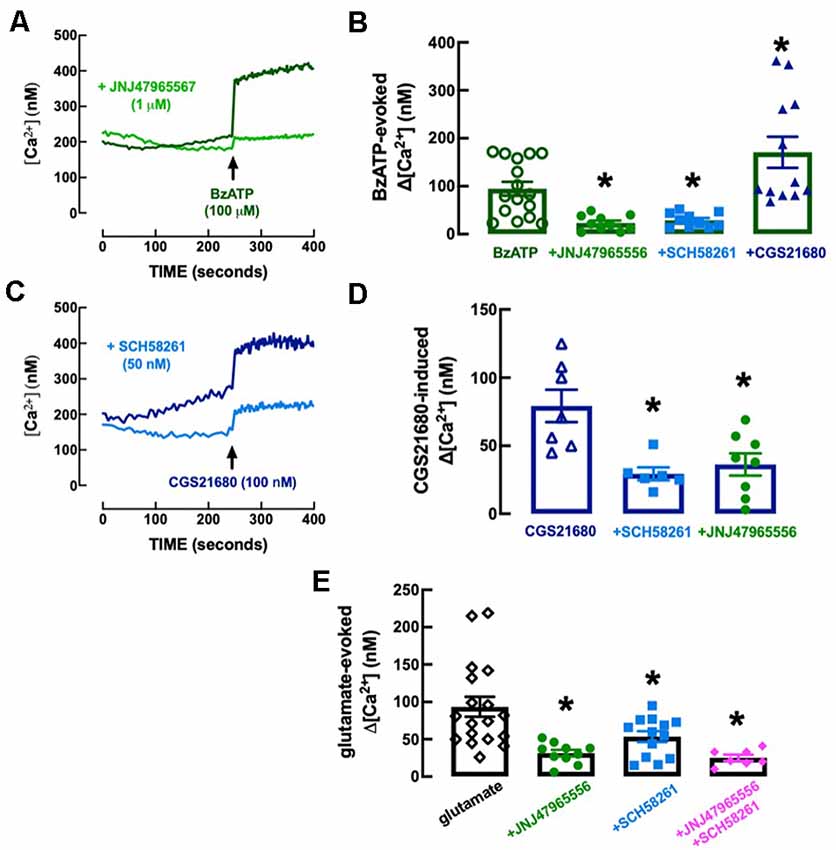
Figure 8. Functional interaction between P2X7 and A2A receptors in the control of calcium responses in N9 microglial cell lines. (A) The Fluo-4 fluorescence signal reporting alteration of intracellular free calcium levels ([Ca2+]i) was increased by the P2X7 receptor agonist BzATP (100 μM), an effect abolished by the selective P2X7 receptor antagonist JNJ47965567 (1 μM), added 15 min before BzATP. (B) Furthermore, the addition 15 min before BZATP of the A2A receptor antagonist SCH58261 (50 nM) decreased and the A2A receptor agonist CGS21680 (100 nM) increased BzATP-induced increase of [Ca2+]i (Δ[Ca2+]i). (C) CGS21680 also increased [Ca2+]i in a manner attenuated by SCH58261, as well as by JNJ47965567 (D), each added 15 min before CGS21680. (E) Glutamate (100 μM) also increased [Ca2+]i, an effect attenuated by both JNJ47965567 and by SCH58261, and their simultaneous presence caused an inhibition similar to each antagonist alone (antagonists being added 15 min before glutamate). The time course recordings are from representative experiments, whereas the bar graphs correspond to n = 6–18 independent cultures of N9 microglial cells. *p < 0.05 one-way ANOVA followed by a Dunnett’s post hoc test compared to the first bar from the left (stimulus only, without modifiers, which were added 5 min before the stimulus).
We next explored if there was a control by P2X7R and by A2AR and a crosstalk between both receptors in the control of Δ[Ca2+]i evoked by glutamate to mimic a condition of excitotoxicity-induced “activation” of microglia (reviewed in Zhang et al., 2020), irrespective of the receptors involved. Glutamate (100 mM) triggered a Δ[Ca2+]i of 93.3 ± 13.4 nM (n = 18), which was inhibited either by 1 μM JNJ47965567 (−66.4 ± 13.3%, n = 10–18, F4,53 = 13.56, p = 0.002) or by 50 nM SCH58261 (−42.67 ± 8.41%, n = 13–18, F4,53 = 13.56, p = 0.050), each added 15 min before BzATP (Figure 8E). Notably, glutamate-induced Δ[Ca2+]i was 25.1 ± 4.1 nM (n = 7) in the simultaneous presence of JNJ47965567 (1 μM) and SCH58261 (50 nM) indicating an inhibition of −73.1 ± 15.8% (Figure 8E), which was similar to that caused by JNJ47965567 alone (t = 0.997, p = 0.334).
P2X7R –A2AR Interaction in the Control of Hippocampal Synaptic Plasticity
Since we and others have collected evidence for a role of synaptic dysfunction underlying stress-associated behavioral alterations (Duman and Aghajanian, 2012; Kaster et al., 2015) and suggestions of P2X7R-mediated synaptic dysfunction add-up to the well-established ability of A2AR to control synaptic function (reviewed in Cunha, 2016), we next investigated if P2X7R and A2AR might interact in the control of synaptic plasticity in excitatory synapses of the dorsal hippocampus.
We first tested the effect of P2X7R agonist BzATP on basal synaptic transmission. BzATP (30 μM) decreased hippocampal synaptic transmission by 54.75 ± 3.96% (n = 4); this effect recovered fully upon washout of BzATP and repeated administrations of 30 μM BzATP caused a similar depression of synaptic transmission (p > 0.05). This allowed exploring the pharmacology of BzATP (30 μM)-induced decreased hippocampal synaptic transmission: this effect was unaffected in the presence of 1 μM BBG (−48.98 ± 4.96%, n = 4, t = 1.245, p = 0.260 vs. the effect of BzATP alone) and was fully prevented in the presence of the adenosine A1 receptor antagonist, DPCPX (50 nM; 2.97 ± 17.91% alteration of fEPSP slope, n = 4; t = 3.319, p = 0.016 vs. the effect of BzATP alone; Figure 9A). This shows the inexistence of a P2X7R-mediated effect (lack of effect of BBG) and indicates that BzATP is rapidly converted by ectonucleotidases (Cunha et al., 1998) into an adenosine analog to indirectly alter hippocampal synaptic transmission through inhibitory A1 adenosine receptors (prevention by DPCPX), as previously proposed (Kukley et al., 2004). This precludes the use of BzATP to search for P2X7R-mediated effects in hippocampal slices. Instead, we tested the impact of P2X7R antagonists on high-frequency induced LTP in Schaffer collaterals-CA1 pyramidal cell synapses. LTP magnitude was not significantly altered by either 1 μM BBG (n = 8, t = 0.493, p = 0.630 vs. LTP magnitude in control conditions, i.e., in the absence of tested drugs) or 1 μM JNJ47965567 (n = 6; t = 0.754, p = 0.468 vs. control LTP magnitude; Figures 9B,C). This does not support a role of P2X7R in the control of synaptic plasticity.
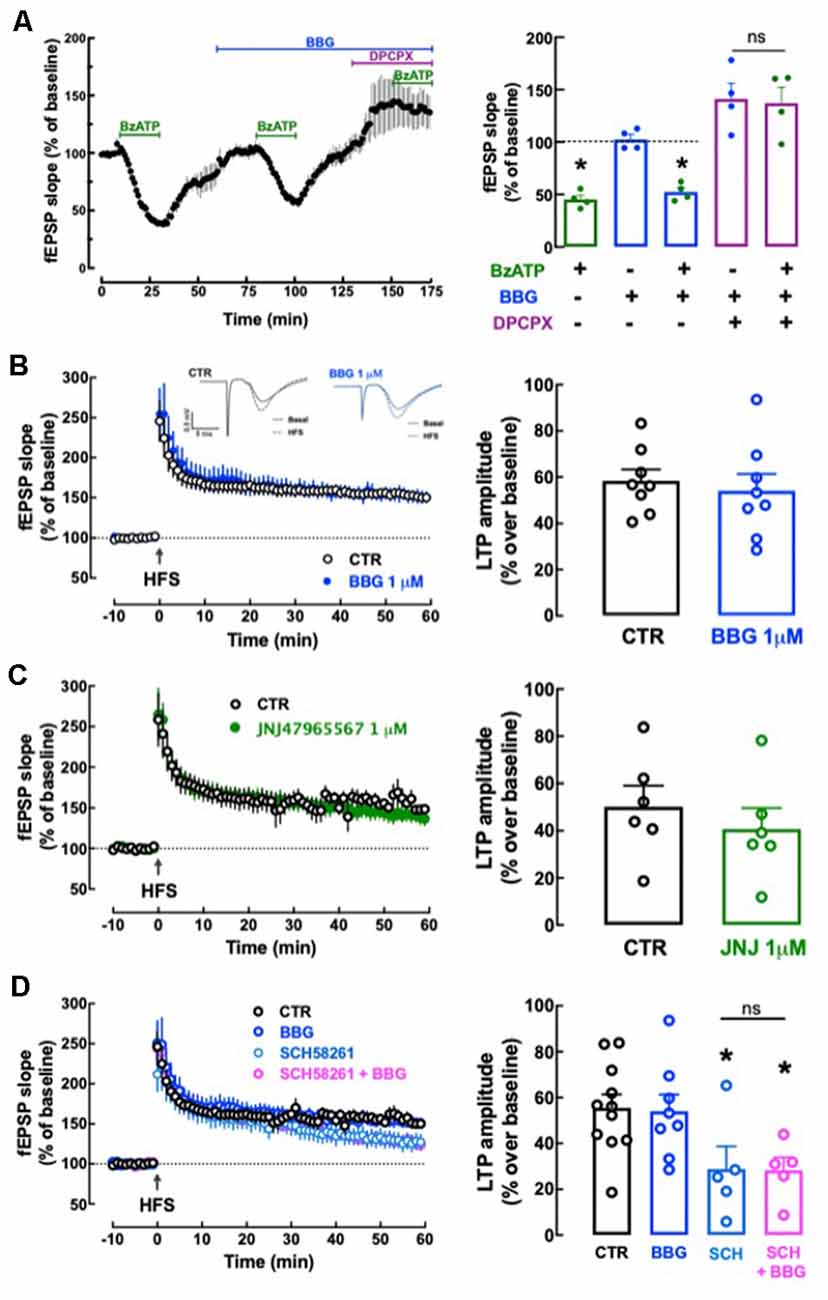
Figure 9. Lack of direct effects of P2X7 receptors on hippocampal synaptic plasticity or its modulation by A2A receptors. (A) The P2X7R agonist BzATP (30 μM) decreased synaptic transmission in Schaffer collaterals-CA1 pyramid synapses of hippocampal slices from adult rats (10–12 weeks old), but this effect was likely mediated through A1R since it was prevented by the A1R antagonist DPCPX (50 nM) but not by the P2X7R antagonist BBG (1 μM). Data are shown as mean ± SEM of n = 4; *p < 0.05 vs. control (100%, dashed line). (B–D) The P2X7R antagonists BBG (1 μM) or JNJ47965567 (JNJ, 1 μM) did not significantly modify the magnitude of Long-term potentiation (LTP; change in field excitatory post-synaptic potential (fEPSP) slope at 50–60 min) induced by a high-frequency stimulation (HFS) train concerning pre-HFS values (B,C) and also failed to alter the inhibition of LTP magnitude caused by the A2AR antagonist SCH58261 (SCH, 50 nM; D). The inserts show recordings obtained in representative experiments of fEPSP responses obtained before (filled line) and 50–60 min after (dotted line) LTP induction in the presence or in the absence (control) of BBG; each trace comprises the stimulus artifact, followed by the presynaptic volley and the fEPSP. All values are shown as mean ± SEM of 5–8 experiments; *p < 0.05 vs. LTP magnitude in the absence of drugs (control). ns: non-significant.
We next investigated if P2X7R might instead control the known ability of A2AR to control hippocampal synaptic plasticity (e.g., Costenla et al., 2011; Lopes et al., 2019). SCH58261 (50 nM) decreased LTP magnitude by −48.10 ± 10.77% (n = 5; t = 2.440, p = 0.029 vs. control LTP magnitude; Figure 9D) and a non-significantly different inhibition of −49.11 ± 7.21% (n = 5; t = 0.049, p = 0.962 vs. LTP magnitude in the SCH58261 alone) was observed in the simultaneous presence of BBG (1 μM) and SCH58261 (50 nM; Figure 9D).
Discussion
The present study provides compelling novel evidence for a hitherto unrecognized interaction between P2X7R and A2AR in the control of brain dysfunction. This conclusion is based on the parallel effects of BBG, a P2X7R preferring antagonist, and of caffeine, which antagonizes A2AR, to prevent neuroinflammation and behavioral alterations upon repeated restraint stress and on the ability of caffeine to prevent P2X7R upregulation and of BBG to prevent A2AR up-regulation; although these in vivo evidence are only suggestive of a P2X7R-A2AR interaction, this contention is further supported by the independent in vitro experiments showing that P2X7R and A2AR closely interact in the control of calcium responses in N9 microglial cells. This indicates that these two, so far considered independent, arms of the purinergic system (Agostinho et al., 2020), operated by ATP-P2R and by adenosine-P1R might actually cooperate to control adaptative brain function. Importantly, this proof-of-concept, so far only confirmed to occur in male rats (selected to cope with the “3R” guidelines), still needs to be extended to female rats, an issue of particular importance since there are gender differences in the A2AR modulation of microglia and neuroinflammatory-like responses in rodents (Caetano et al., 2017; Simões-Henriques et al., 2020).
The present study extends to a model of repeated restraint stress the ability of P2X7R blockade to attenuate behavioral modifications upon chronic stress (Iwata et al., 2016; Yue et al., 2017; Farooq et al., 2018; Aricioglu et al., 2019; reviewed in Illes et al., 2020). This is in agreement with the association of P2X7R polymorphisms with depressive symptoms (see meta-analysis in Czamara et al., 2018) and reinforces the concept of ATP as a danger signal in brain dysfunction (reviewed in Rodrigues et al., 2015). As observed by others in different animal models of brain dysfunction (Jimenez-Pacheco et al., 2013; Wang et al., 2017; Martínez-Frailes et al., 2019; Song et al., 2019), namely upon chronic stress (Yue et al., 2017; Dang et al., 2018; but see Kongsui et al., 2014), we identified an up-regulation of P2X7R and an ability of P2X7R to control different markers of neuroinflammation, as also reported in other animal models of depression (Yue et al., 2017; Bhattacharya and Jones, 2018), to mediate stress-induced behavioral modifications (Rial et al., 2016; Deng et al., 2020; Troubat et al., 2021).
The present study also provides the first demonstration that a prolonged (days) intake of caffeine prevents behavioral modifications caused by repeated restraint stress, as has been observed in other animal models of stress (Pechlivanova et al., 2012; Kaster et al., 2015; Yin et al., 2015; Kasimay Cakir et al., 2017) and in individuals with mood dysfunction, namely depression (reviewed in Grosso et al., 2016; Wang et al., 2016) and suicide ideation (e.g., Lucas et al., 2014; Park et al., 2019). The protective effects of caffeine in animal stress models are mimicked by selective A2AR blockade (Kaster et al., 2015) and A2AR polymorphisms are associated with the incidence of major depression (Oliveira et al., 2019). We also observed an up-regulation of A2AR, as occurs in different conditions of brain dysfunction (reviewed in Cunha, 2016), namely upon repeated stress (Cunha et al., 2006; Kaster et al., 2015). A2AR, as well as caffeine, can control abnormal synaptic plasticity and synaptic dysfunction (e.g., Kaster et al., 2015; Temido-Ferreira et al., 2020) and also control microglia reactivity and neuro-inflammation (e.g., Brothers et al., 2010; Rebola et al., 2011; Mao et al., 2020), but the exact mechanism underlying the ability of A2AR to control mood dysfunction upon chronic stress remains to be defined.
Apart from establishing the ability of BBG and caffeine to attenuate behavioral alterations in this particular model of repeated restraint stress, the major finding of the present study is the existence of putative crosstalk between the two purinergic signaling systems operated by each of these antagonists. The inhibition of the stress-induced up-regulation of A2AR by BBG and, conversely, the inhibition of the stress-induced up-regulation of P2X7R by caffeine is suggestive of crosstalk between the two types of purinergic receptors in vivo. This was reinforced by parallel experiments studying calcium transients in microglial N9 cells. In fact, in microglial N9 cells, A2AR activation increased and A2AR blockade decreased BzATP-induced calcium transients, which was mediated by P2X7R, and conversely, a selective P2X7R antagonist attenuated CGS26180-induced calcium transients, which was largely mediated by A2AR. Since synaptic alterations have also been proposed to underlie stress-induced alterations of brain function (Duman and Aghajanian, 2012; Vose and Stanton, 2017), we also investigated if there was crosstalk between P2X7R and A2AR in synaptic alterations, namely in the process of LTP in the hippocampus. While we have previously established a selective role of A2AR controlling synaptic plasticity without an effect on basal synaptic transmission (Costenla et al., 2011; Gonçalves et al., 2019; Temido-Ferreira et al., 2020), a putative role of P2X7R on the control of hippocampal synaptic transmission has been controversial (Armstrong et al., 2002; Kukley et al., 2004; Klaft et al., 2012; Khan et al., 2019) and an eventual role of P2X7R on the control of synaptic plasticity had not yet been tested. We now show that BzATP decreases synaptic transmission, but this effect is blocked by the selective A1R antagonist DPCPX (see Kukley et al., 2004), following the remarkable efficiency of ectonucleotidases to metabolize ATP derivates into their adenosine derivative counterparts (Cunha et al., 1998) to activate the abundant and efficient presynaptic A1R that decrease excitatory transmission in the hippocampus (reviewed in Dunwiddie and Masino, 2001). Thus, we resorted to testing the impact of P2X7R antagonists (BBG and JNJ47965567) on hippocampal LTP and concluded that P2X7R does not seem to control hippocampal LTP under physiological conditions. Furthermore, we did not observe the ability of P2X7R antagonists to modify the decrease of LTP caused by the blockade of A2AR.
In contrast to the inconclusive effects on a putative P2X7R-A2AR interaction in the control of synaptic plasticity, the crosstalk between P2X7R and A2AR in the control of microglial responses suggests that the interplay between P2X7R and A2AR to control brain maladaptive function upon repeated stress might mostly be due to crosstalk in the control of neuroinflammation rather than of synaptic plasticity. Interestingly, crosstalk between P2 and P1 receptors in the control of microglia was first documented by Kettenmann’s group (Färber et al., 2008) and further developed by Koizumi’s group (reviewed in Koizumi et al., 2013); however, these P2R-P1R interactions in microglia were not characterized to involve P2X7R and A2AR, although parallel effects of P2X7R and A2AR have previously been described to control inflammatory processes (Savio et al., 2017) and brain injury (Ye et al., 2018). We now demonstrate direct crosstalk between both receptors in the control of microglial N9 cell responses, which is paralleled by the ability of antagonists of each receptor to control the other’s up-regulation upon repeated stress. This is highly suggestive of direct cooperation between the two arms of the purinergic modulation system to control neuro-inflammation and the adaptive central responses to repeated stress. However, future studies still need to detail if the P2X7R-A2AR interaction only occurs in microglia or might also take place in astrocytes. In fact, P2X7R (reviewed in Franke et al., 2012) and A2AR (reviewed in Cunha, 2016) also have profound effects on the pathophysiological roles of astrocytes and the involvement of astrocytes in the control neuroinflammation and neuronal function as well as adaptation to repeated stress (reviewed in Rial et al., 2016) cannot exclude them as a possible major locus of P2X7R-A2AR interactions to control the observed behavioral modifications upon repeated restraint stress.
The detailed mechanisms of this P2X7R-A2AR interactions also remain to be unraveled and they can involve different possibilities: one possibility is the formation of heteromers, which has been documented for P2X7R (Antonio et al., 2011) and for A2AR (reviewed in Ferré and Ciruela, 2019) and between different P2R and P1R (Namba et al., 2010); another possibility is the use of transducing systems of each receptor to control the other receptor function, as has been shown for P2X7R controlling metabotropic receptors (reviewed in Miras-Portugal et al., 2019), A2AR controlling ionotropic receptors (e.g., Garção et al., 2013; Temido-Ferreira et al., 2020) and between different P2R and P1R (George et al., 2016); a third possibility is a key role of ecto-nucleotidases metabolizing ATP into adenosine in a rapid (Dunwiddie et al., 1997; Cunha et al., 1998) and highly controlled manner (James and Richardson, 1993; Cunha, 2001) to format the balanced activation of both receptors (Kukley et al., 2004; Liston et al., 2020). After this first step establishing an interaction between A2AR and P2X7R, future work will be required to detail the mechanistic basis of this A2AR-P2X7R interaction.
In conclusion, the present study provides evidence for crosstalk between P2X7R and A2AR in the control of neuroinflammation and adaptive responses to restraint stress. The importance of these findings is best heralded by the new prospects to simultaneously target P2X7R and A2AR to maximize the neuroprotective potential of the purinergic system. The present findings place at the center-stage the need to study the purinergic system as a whole and understand the relative contribution of its different constituents to provide the required integrative views (see Agostinho et al., 2020) to justify robust protective strategies to control maladaptation of brain function characteristic of neuropsychiatric disorders.
Data Availability Statement
Data will be made available upon reasonable and justified request. Requests to access the datasets should be directed to cunharod@gmail.com.
Ethics Statement
The animal study was reviewed and approved by the Portuguese Ethical Committee (DGAV) and by the Institution’s Ethics Committee (ORBEA 238-2019/14102019).
Author Contributions
LD and AT carried out the Ca transient experiments in N9 cells. CL and FG carried out the electrophysiological recordings. AN, DP, and NM carried out the behavioral experiments. PA and RC coordinated the project and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Centro 2020 (CENTRO-01-0145-FEDER-000008:BrainHealth 2020 and CENTRO-01-0246-FEDER-000010) “La Caixa” Banking Foundation (LCF/PR/HP17/52190001) and Fundação para a Ciência e a Tecnologia (POCI-01-0145-FEDER-031274 and UIDB/04539/2020).
Conflict of Interest
RC is a scientific consultant of the Institute for Scientific Information on Coffee (ISIC).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agostinho, P., Madeira, D., Dias, L., Simões, A. P., Cunha, R. A., and Canas, P. M. (2020). Purinergic signaling orchestrating neuron-glia communication. Pharmacol. Res. 162:105253. doi: 10.1016/j.phrs.2020.105253
Anderson, W. W., and Collingridge, G. L. (2007). Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neurosci. Methods 162, 346–356. doi: 10.1016/j.jneumeth.2006.12.018
Andlin-Sobocki, P., Jönsson, B., Wittchen, H. U., and Olesen, J. (2005). Cost of disorders of the brain in Europe. Eur. J. Neurol. 12, 1–27. doi: 10.1111/j.1468-1331.2005.01202.x
Antonio, L. S., Stewart, A. P., Xu, X. J., Varanda, W. A., Murrell-Lagnado, R. D., and Edwardson, J. M. (2011). P2X4 receptors interact with both P2X2 and P2X7 receptors in the form of homotrimers. Br. J. Pharmacol. 163, 1069–1077. doi: 10.1111/j.1476-5381.2011.01303.x
Arbeloa, J., Pérez-Samartín, A., Gottlieb, M., and Matute, C. (2012). P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol. Dis. 45, 954–961. doi: 10.1016/j.nbd.2011.12.014
Aricioglu, F., Ozkartal, C. S., Bastaskin, T., Tüzün, E., Kandemir, C., Sirvanci, S., et al. (2019). Antidepressant-like effects induced by chronic blockade of the purinergic 2X7 receptor through inhibition of non-like receptor protein 1 inflammasome in chronic unpredictable mild stress model of depression in rats. Clin. Psychopharmacol. Neurosci. 17, 261–272. doi: 10.9758/cpn.2019.17.2.261
Armstrong, J. N., Brust, T. B., Lewis, R. G., and MacVicar, B. A. (2002). Activation of presynaptic P2X7-like receptors depresses mossy fiber-CA3 synaptic transmission through p38 mitogen-activated protein kinase. J. Neurosci. 22, 5938–5945. doi: 10.1523/JNEUROSCI.22-14-05938.2002
Augusto, E., Matos, M., Sévigny, J., El-Tayeb, A., Bynoe, M. S., Müller, C. E., et al. (2013). Ecto-5’-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 33, 11390–11399. doi: 10.1523/JNEUROSCI.5817-12.2013
Berton, O., Hahn, C.-G., and Thase, M. E. (2012). Are we getting closer to valid translational models for major depression? Science 338, 75–79. doi: 10.1126/science.1222940
Bhattacharya, A., and Jones, D. N. C. (2018). Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology 98, 95–100. doi: 10.1016/j.psyneuen.2018.08.015
Brothers, H. M., Marchalant, Y., and Wenk, G. L. (2010). Caffeine attenuates lipopolysaccharide-induced neuroinflammation. Neurosci. Lett. 480, 97–100. doi: 10.1016/j.neulet.2010.06.013
Caetano, L., Pinheiro, H., Patrício, P., Mateus-Pinheiro, A., Alves, N. D., Coimbra, B., et al. (2017). Adenosine A2A receptor regulation of microglia morphological remodeling-gender bias in physiology and in a model of chronic anxiety. Mol. Psychiatry 22, 1035–1043. doi: 10.1038/mp.2016.173
Carmo, M., Gonçalves, F. Q., Canas, P. M., Oses, J.-P., Fernandes, F. D., Duarte, F. V., et al. (2019). Enhanced ATP release and CD73-mediated adenosine formation sustain adenosine A2A receptor over-activation in a rat model of Parkinson’s disease. Br. J. Pharmacol. 176, 3666–3680. doi: 10.1111/bph.14771
Carmo, M. R., Menezes, A. P., Nunes, A. C., Pliássova, A., Rolo, A. P., Palmeira, C. M., et al. (2014). The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 81, 142–152. doi: 10.1016/j.neuropharm.2014.01.045
Coelho, J. E., Alves, P., Canas, P. M., Valadas, J. S., Shmidt, T., Batalha, V. L., et al. (2014). Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Front. Psychiatry 5:67. doi: 10.3389/fpsyt.2014.00067
Cognato, G. P., Agostinho, P. M., Hockemeyer, J., Müller, C. E., Souza, D. O., and Cunha, R. A. (2010). Caffeine and an adenosine A2A receptor antagonist prevent memory impairment and synaptotoxicity in adult rats triggered by a convulsive episode in early life. J. Neurochem. 112, 453–462. doi: 10.1111/j.1471-4159.2009.06465.x
Costenla, A. R., Cunha, R. A., and de Mendonça, A. (2010). Caffeine, adenosine receptors, and synaptic plasticity. J. Alzheimers Dis. 20, S25–S34. doi: 10.3233/JAD-2010-091384
Costenla, A. R., Diógenes, M. J., Canas, P. M., Rodrigues, R. J., Nogueira, C., Maroco, J., et al. (2011). Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci. 34, 12–21. doi: 10.1111/j.1460-9568.2011.07719.x
Cunha, R. A. (2001). Regulation of the ecto-nucleotidase pathway in rat hippocampal nerve terminals. Neurochem. Res. 26, 979–991. doi: 10.1023/a:1012392719601
Cunha, R. A. (2016). How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 139, 1019–1055. doi: 10.1111/jnc.13724
Cunha, G. M., Canas, P. M., Oliveira, C. R., and Cunha, R. A. (2006). Increased density and synapto-protective effect of adenosine A2A receptors upon sub-chronic restraint stress. Neuroscience 141, 1775–1781. doi: 10.1016/j.neuroscience.2006.05.024
Cunha, R. A., Sebastião, A. M., and Ribeiro, J. A. (1998). Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J. Neurosci. 18, 1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998
Czamara, D., Müller-Myhsok, B., and Lucae, S. (2018). The P2RX7 polymorphism rs2230912 is associated with depression: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 82, 272–277. doi: 10.1016/j.pnpbp.2017.11.003
Dall’Igna, O. P., Fett, P., Gomes, M. W., Souza, D. O., Cunha, R. A., and Lara, D. R. (2007). Caffeine and adenosine A2a receptor antagonists prevent β-amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 203, 241–245. doi: 10.1016/j.expneurol.2006.08.008
Dang, R., Zhou, X., Tang, M., Xu, P., Gong, X., Liu, Y., et al. (2018). Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur. J. Nutr. 57, 893–906. doi: 10.1007/s00394-016-1373-z
de Kloet, E. R., Joëls, M., and Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475. doi: 10.1038/nrn1683
Deaglio, S., Dwyer, K. M., Gao, W., Friedman, D., Usheva, A., Erat, A., et al. (2007). Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265. doi: 10.1084/jem.20062512
Dellu, F., Fauchey, V., Le Moal, M., and Simon, H. (1997). Extension of a new two-trial memory task in the rat: influence of environmental context on recognition processes. Neurobiol. Learn. Mem. 67, 112–120. doi: 10.1006/nlme.1997.3746
Deng, S.-L., Chen, J.-G., and Wang, F. (2020). Microglia: a central player in depression. Curr. Med. Sci. 40, 391–400. doi: 10.1007/s11596-020-2193-1
Díaz-Hernández, M., Díez-Zaera, M., Sánchez-Nogueiro, J., Gómez-Villafuertes, R., Canals, J. M., Alberch, J., et al. (2009). Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 23, 1893–1906. doi: 10.1096/fj.08-122275
Díaz-Hernández, J. I., Gomez-Villafuertes, R., León-Otegui, M., Hontecillas-Prieto, L., Del Puerto, A., Trejo, J. L., et al. (2012). in vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases. Neurobiol. Aging 33, 1816–1828. doi: 10.1016/j.neurobiolaging.2011.09.040
Donnelly-Roberts, D. L., and Jarvis, M. F. (2007). Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br. J. Pharmacol. 151, 571–579. doi: 10.1038/sj.bjp.0707265
Duarte, J. M., Agostinho, P. M., Carvalho, R. A., and Cunha, R. A. (2012). Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of NONcZNO10/LTJ mice. PLoS One 7:e21899. doi: 10.1371/journal.pone.0021899
Duarte, J. M., Carvalho, R. A., Cunha, R. A., and Gruetter, R. (2009). Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J. Neurochem. 111, 368–379. doi: 10.1111/j.1471-4159.2009.06349.x
Duarte, J. M., Gaspar, R., Caetano, L., Patrício, P., Soares-Cunha, C., Mateus-Pinheiro, A., et al. (2019). Region-specific control of microglia by adenosine A2A receptors: uncoupling anxiety and associated cognitive deficits in female rats. Glia 67, 182–192. doi: 10.1002/glia.23476
Duman, R. S., and Aghajanian, G. K. (2012). Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72. doi: 10.1126/science.1222939
Dunwiddie, T. V., and Masino, S. A. (2001). The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 24, 31–55. doi: 10.1146/annurev.neuro.24.1.31
Dunwiddie, T. V., Diao, L., and Proctor, W. R. (1997). Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 17, 7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997
Färber, K., Markworth, S., Pannasch, U., Nolte, C., Prinz, V., Kronenberg, G., et al. (2008). The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia 56, 331–341. doi: 10.1002/glia.20606
Farooq, R. K., Tanti, A., Ainouche, S., Roger, S., Belzung, C., and Camus, V. (2018). A P2X7 receptor antagonist reverses behavioural alterations, microglial activation and neuroendocrine dysregulation in an unpredictable chronic mild stress (UCMS) model of depression in mice. Psychoneuroendocrinology 97, 120–130. doi: 10.1016/j.psyneuen.2018.07.016
Ferrari, D., Villalba, M., Chiozzi, P., Falzoni, S., Ricciardi-Castagnoli, P., and di Virgilio, F. (1996). Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J. Immunol. 156, 1531–1539.
Ferré, S., and Ciruela, F. (2019). Functional and neuroprotective role of striatal adenosine A2A receptor heterotetramers. J. Caffeine Adenosine Res. 9, 89–97. doi: 10.1089/caff.2019.0008
Flögel, U., Burghoff, S., van Lent, P. L., Temme, S., Galbarz, L., Ding, Z., et al. (2012). Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 4:146ra108. doi: 10.1126/scitranslmed.3003717
Flores-Santibáñez, F., Fernández, D., Meza, D., Tejón, G., Vargas, L., Varela-Nallar, L., et al. (2015). CD73-mediated adenosine production promotes stem cell-like properties in mouse Tc17 cells. Immunology 146, 582–594. doi: 10.1111/imm.12529
Franke, H., Verkhratsky, A., Burnstock, G., and Illes, P. (2012). Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 8, 629–657. doi: 10.1007/s11302-012-9300-0
Fredholm, B. B., Bättig, K., Holmén, J., Nehlig, A., and Zvartau, E. E. (1999). Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51, 83–133.
Fredholm, B. B., Chen, J.-F., Cunha, R. A., Svenningsson, P., and Vaugeois, J.-M. (2005). Adenosine and brain function. Int. Rev. Neurobiol. 63, 191–270. doi: 10.1016/S0074-7742(05)63007-3
Garção, P., Szabó, E. C., Wopereis, S., Castro, A. A., Tomé, Â. R., Prediger, R. D., et al. (2013). Functional interaction between pre-synaptic α6β2-containing nicotinic and adenosine A2A receptors in the control of dopamine release in the rat striatum. Br. J. Pharmacol. 169, 1600–1611. doi: 10.1111/bph.12234
George, J., Cunha, R. A., Mulle, C., and Amédée, T. (2016). Microglia-derived purines modulate mossy fibre synaptic transmission and plasticity through P2X4 and A1 receptors. Eur. J. Neurosci. 43, 1366–1378. doi: 10.1111/ejn.13191
Gomes, C., Ferreira, R., George, J., Sanches, R., Rodrigues, D. I., Gonçalves, N., et al. (2013). Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflammation 10:16. doi: 10.1186/1742-2094-10-16
Gonçalves, F. Q., Lopes, J. P., Silva, H. B., Lemos, C., Silva, A. C., Gonçalves, N., et al. (2019). Synaptic and memory dysfunction in a β-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiol. Dis. 132:104570. doi: 10.1016/j.nbd.2019.104570
Grosso, G., Micek, A., Castellano, S., Pajak, A., and Galvano, F. (2016). Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol. Nutr. Food Res. 60, 223–234. doi: 10.1002/mnfr.201500620
Hamilton, S. P., Slager, S. L., De Leon, A. B., Heiman, G. A., Klein, D. F., Hodge, S. E., et al. (2004). Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology 29, 558–565. doi: 10.1038/sj.npp.1300311
Hohoff, C., Mullings, E. L., Heatherley, S. V., Freitag, C. M., Neumann, L. C., Domschke, K., et al. (2010). Adenosine A2A receptor gene: evidence for association of risk variants with panic disorder and anxious personality. J. Psychiatr. Res. 44, 930–937. doi: 10.1016/j.jpsychires.2010.02.006
Illes, P., Verkhratsky, A., and Tang, Y. (2020). Pathological ATPergic signaling in major depression and bipolar disorder. Front. Mol. Neurosci. 12:331. doi: 10.3389/fnmol.2019.00331
Iwata, M., Ota, K. T., Li, X. Y., Sakaue, F., Li, N., Dutheil, S., et al. (2016). Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol. Psychiatry 80, 12–22. doi: 10.1016/j.biopsych.2015.11.026
James, S., and Richardson, P. J. (1993). Production of adenosine from extracellular ATP at the striatal cholinergic synapse. J. Neurochem. 60, 219–227. doi: 10.1111/j.1471-4159.1993.tb05841.x
Janks, L., Sharma, C. V. R., and Egan, T. M. (2018). A central role for P2X7 receptors in human microglia. J. Neuroinflammation 15:325. doi: 10.1186/s12974-018-1353-8
Jimenez-Pacheco, A., Mesuret, G., Sanz-Rodriguez, A., Tanaka, K., Mooney, C., Conroy, R., et al. (2013). Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia 54, 1551–1561. doi: 10.1111/epi.12257
Kasimay Cakir, O., Ellek, N., Salehin, N., Hamamcı, R., Kele’, H., Kayalı, D. G., et al. (2017). Protective effect of low dose caffeine on psychological stress and cognitive function. Physiol. Behav. 168, 1–10. doi: 10.1016/j.physbeh.2016.10.010
Kaster, M. P., Machado, N. J., Silva, H. B., Nunes, A., Ardais, A. P., Santana, M., et al. (2015). Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc. Natl. Acad. Sci. U S A 112, 7833–7838. doi: 10.1073/pnas.1423088112
Khan, M. T., Deussing, J., Tang, Y., and Illes, P. (2019). Astrocytic rather than neuronal P2X7 receptors modulate the function of the tri-synaptic network in the rodent hippocampus. Brain Res. Bull. 151, 164–173. doi: 10.1016/j.brainresbull.2018.07.016
Klaft, Z.-J., Schulz, S. B., Maslarova, A., Gabriel, S., Heinemann, U., and Gerevich, Z. (2012). Extracellular ATP differentially affects epileptiform activity via purinergic P2X7 and adenosine A1 receptors in naive and chronic epileptic rats. Epilepsia 53, 1978–1986. doi: 10.1111/j.1528-1167.2012.03724.x
Koizumi, S., Ohsawa, K., Inoue, K., and Kohsaka, S. (2013). Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 61, 47–54. doi: 10.1002/glia.22358
Kongsui, R., Beynon, S. B., Johnson, S. J., Mayhew, J., Kuter, P., Nilsson, M., et al. (2014). Chronic stress induces prolonged suppression of the P2X7 receptor within multiple regions of the hippocampus: a cumulative threshold spectra analysis. Brain Behav. Immun. 42, 69–80. doi: 10.1016/j.bbi.2014.05.017
Kukley, M., Stausberg, P., Adelmann, G., Chessell, I. P., and Dietrich, D. (2004). Ecto-nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2’-3’-O-(4-benzoylbenzoyl)-ATP. J. Neurosci. 24, 7128–7139. doi: 10.1523/JNEUROSCI.2093-04.2004
Liston, T. E., Hinz, S., Müller, C. E., Holstein, D. M., Wendling, J., Melton, R. J., et al. (2020). Nucleotide P2Y1 receptor agonists are in vitro and in vivo prodrugs of A1/A3 adenosine receptor agonists: implications for roles of P2Y1 and A1/A3 receptors in physiology and pathology. Purinergic Signal. 16, 543–559. doi: 10.1007/s11302-020-09732-z
Lopes, L. V., Halldner, L., Rebola, N., Johansson, B., Ledent, C., Chen, J. F., et al. (2004). Binding of the prototypical adenosine A2A receptor agonist CGS 21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. Br. J. Pharmacol. 141, 1006–1014. doi: 10.1038/sj.bjp.0705692
Lopes, J. P., Pliássova, A., and Cunha, R. A. (2019). The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem. Pharmacol. 166, 313–321. doi: 10.1016/j.bcp.2019.06.008
Lucas, M., O’Reilly, E. J., Pan, A., Mirzaei, F., Willett, W. C., Okereke, O. I., et al. (2014). Coffee, caffeine and risk of completed suicide: Results from three prospective cohorts of American adults. World J. Biol. Psychiatr. 15, 377–386. doi: 10.3109/15622975.2013.795243
Machado, N. J., Simões, A. P., Silva, H. B., Ardais, A. P., Kaster, M. P., Garção, P., et al. (2017). Caffeine reverts memory but not mood impairment in a depression-prone mouse strain with up-regulated adenosine A2A receptor in hippocampal glutamate synapses. Mol. Neurobiol. 54, 1552–1563. doi: 10.1007/s12035-016-9774-9
Madeira, M. H., Boia, R., Elvas, F., Martins, T., Cunha, R. A., Ambrósio, A. F., et al. (2016). Selective A2A receptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Transl. Res. 169, 112–128. doi: 10.1016/j.trsl.2015.11.005
Mahmut, A., Boulanger, M.-C., Bouchareb, R., Hadji, F., and Mathieu, P. (2015). Adenosine derived from ecto-nucleotidases in calcific aortic valve disease promotes mineralization through A2a adenosine receptor. Cardiovasc. Res. 106, 109–120. doi: 10.1093/cvr/cvv027
Mao, Z.-F., Ouyang, S.-H., Zhang, Q.-Y., Wu, Y.-P., Wang, G.-E., Tu, L. F., et al. (2020). New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: inhibition of CORT-induced microglia activation. FASEB J. 34, 10998–11014. doi: 10.1096/fj.202000146RR
Martínez-Frailes, C., Di Lauro, C., Bianchi, C., de Diego-García, L., Sebastián-Serrano, Á., Boscá, L., et al. (2019). Amyloid peptide induced neuroinflammation increases the P2X7 receptor expression in microglial cells, impacting on its functionality. Front. Cell. Neurosci. 13:143. doi: 10.3389/fncel.2019.00143
Matheus, F. C., Rial, D., Real, J. I., Lemos, C., Takahashi, R. N., Bertoglio, L. J., et al. (2016). Temporal dissociation of striatum and prefrontal cortex uncouples anhedonia and defense behaviors relevant to depression in 6-OHDA-lesioned rats. Mol. Neurobiol. 53, 3891–3899. doi: 10.1007/s12035-015-9330-z
Ménard, C., Hodes, G. E., and Russo, S. J. (2016). Pathogenesis of depression: insights from human and rodent studies. Neuroscience 321, 138–162. doi: 10.1016/j.neuroscience.2015.05.053
Meng, F., Guo, Z., Hu, Y., Mai, W., Zhang, Z., Zhang, B., et al. (2019). CD73-derived adenosine controls inflammation and neurodegeneration by modulating dopamine signalling. Brain 142, 700–718. doi: 10.1093/brain/awy351
Miras-Portugal, M. T., Queipo, M. J., Gil-Redondo, J. C., Ortega, F., Gómez-Villafuertes, R., Gualix, J., et al. (2019). P2 receptor interaction and signalling cascades in neuroprotection. Brain Res. Bull. 151, 74–83. doi: 10.1016/j.brainresbull.2018.12.012
Namba, K., Suzuki, T., and Nakata, H. (2010). Immunogold electron microscopic evidence of in situ formation of homo- and heteromeric purinergic adenosine A1 and P2Y2 receptors in rat brain. BMC Res. Notes 3:323. doi: 10.1186/1756-0500-3-323
Oliveira, S., Ardais, A. P., Bastos, C. R., Gazal, M., Jansen, K., de Mattos Souza, L., et al. (2019). Impact of genetic variations in ADORA2A gene on depression and symptoms: a cross-sectional population-based study. Purinergic Signal. 15, 37–44. doi: 10.1007/s11302-018-9635-2
Orr, A. G., Orr, A. L., Li, X.-J., Gross, R. E., and Traynelis, S. F. (2009). Adenosine A2A receptor mediates microglial process retraction. Nat. Neurosci. 12, 872–878. doi: 10.1038/nn.2341
Park, H., Suh, B. S., and Lee, K. (2019). Relationship between daily coffee intake and suicidal ideation. J. Affect. Disord. 256, 468–472. doi: 10.1016/j.jad.2019.06.023
Pechlivanova, D. M., Tchekalarova, J. D., Alova, L. H., Petkov, V. V., Nikolov, R. P., and Yakimova, K. S. (2012). Effect of long-term caffeine administration on depressive-like behavior in rats exposed to chronic unpredictable stress. Behav. Pharmacol. 23, 339–347. doi: 10.1097/FBP.0b013e3283564dd9
Rebola, N., Canas, P. M., Oliveira, C. R., and Cunha, R. A. (2005). Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience 132, 893–903. doi: 10.1016/j.neuroscience.2005.01.014
Rebola, N., Lujan, R., Cunha, R. A., and Mulle, C. (2008). Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57, 121–134. doi: 10.1016/j.neuron.2007.11.023
Rebola, N., Simões, A. P., Canas, P. M., Tomé, A. R., Andrade, G. M., Barry, C. E., et al. (2011). Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J. Neurochem. 117, 100–111. doi: 10.1111/j.1471-4159.2011.07178.x
Rial, D., Lemos, C., Pinheiro, H., Duarte, J. M., Gonçalves, F. Q., Real, J. I., et al. (2016). Depression as a glial-based synaptic dysfunction. Front. Cell. Neurosci. 9:521. doi: 10.3389/fncel.2015.00521
Ribeiro, D. E., Roncalho, A. L., Glaser, T., Ulrich, H., Wegener, G., and Joca, S. (2019). P2X7 receptor signaling in stress and depression. Int. J. Mol. Sci. 20:E2778. doi: 10.3390/ijms20112778
Rodrigues, R. J., Tomé, A. R., and Cunha, R. A. (2015). ATP as a multi-target danger signal in the brain. Front. Neurosci. 9:148. doi: 10.3389/fnins.2015.00148
Savio, L. E. B., de Andrade Mello, P., Figliuolo, V. R., de Avelar Almeida, T. F., Santana, P. T., Oliveira, S. D. S., et al. (2017). CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J. Hepatol. 67, 716–726. doi: 10.1016/j.jhep.2017.05.021
Silva, C. G., Métin, C., Fazeli, W., Machado, N. J., Darmopil, S., Launay, P. S., et al. (2013). Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci. Transl. Med. 5:197ra104. doi: 10.1126/scitranslmed.3006258
Simões, A. P., Duarte, J. A., Agasse, F., Canas, P. M., Tomé, A. R., Agostinho, P., et al. (2012). Blockade of adenosine A2A receptors prevents interleukin-1β-induced exacerbation of neuronal toxicity through a p38 mitogen-activated protein kinase pathway. J. Neuroinflammation 9:204. doi: 10.1186/1742-2094-9-204
Simões-Henriques, C., Mateus-Pinheiro, M., Gaspar, R., Pinheiro, H., Mendes Duarte, J., Baptista, F. I., et al. (2020). Microglia cytoarchitecture in the brain of adenosine A2A receptor knockout mice: brain region and sex specificities. Eur. J. Neurosci. 51, 1377–1387. doi: 10.1111/ejn.14561
Song, P., Hu, J., Liu, X., and Deng, X. (2019). Increased expression of the P2X7 receptor in temporal lobe epilepsy: animal models and clinical evidence. Mol. Med. Rep. 19, 5433–5439. doi: 10.3892/mmr.2019.10202
Temido-Ferreira, M., Ferreira, D. G., Batalha, V. L., Marques-Morgado, I., Coelho, J. E., Pereira, P., et al. (2020). Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatry 25, 1876–1900. doi: 10.1038/s41380-018-0110-9
Troubat, R., Barone, P., Leman, S., Desmidt, T., Cressant, A., Atanasova, B., et al. (2021). Neuroinflammation and depression: a review. Eur. J. Neurosci. 53, 151–171. doi: 10.1111/ejn.14720
Vose, L. R., and Stanton, P. K. (2017). Synaptic plasticity, metaplasticity and depression. Curr. Neuropharmacol. 15, 71–86. doi: 10.2174/1570159x14666160202121111
Wang, Y.-C., Cui, Y., Cui, J.-Z., Sun, L.-Q., Cui, C.-M., Zhang, H.-A., et al. (2015). Neuroprotective effects of brilliant blue G on the brain following traumatic brain injury in rats. Mol. Med. Rep. 12, 2149–2154. doi: 10.3892/mmr.2015.3607
Wang, L., Shen, X., Wu, Y., and Zhang, D. (2016). Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust. N Z J. Psychiatry 50, 228–242. doi: 10.1177/0004867415603131
Wang, X.-H., Xie, X., Luo, X.-G., Shang, H., and He, Z.-Y. (2017). Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson’s disease. Mol. Med. Rep. 15, 768–776. doi: 10.3892/mmr.2016.6070
Yamada, K., Kobayashi, M., Mori, A., Jenner, P., and Kanda, T. (2013). Antidepressant-like activity of the adenosine A2A receptor antagonist, istradefylline (KW-6002), in the forced swim test and the tail suspension test in rodents. Pharmacol. Biochem. Behav. 114–115, 23–30. doi: 10.1016/j.pbb.2013.10.022
Yang, J.-N., Chen, J.-F., and Fredholm, B. B. (2009). Physiological roles of A1 and A2A adenosine receptors in regulating heart rate, body temperature, and locomotion as revealed using knockout mice and caffeine. Am. J. Physiol. 296, H1141–H1149. doi: 10.1152/ajpheart.00754.2008
Ye, X.-C., Hu, J.-X., Li, L., Li, Q., Tang, F.-L., Lin, S., et al. (2018). Astrocytic Lrp4 (Low-Density Lipoprotein Receptor-Related Protein 4) contributes to ischemia-induced brain injury by regulating ATP release and adenosine-A2AR (adenosine A2A receptor) signaling. Stroke 49, 165–174. doi: 10.1161/STROKEAHA.117.018115
Yin, Y.-Q., Zhang, C., Wang, J.-X., Hou, J., Yang, X., and Qin, J. (2015). Chronic caffeine treatment enhances the resilience to social defeat stress in mice. Food Funct. 6, 479–491. doi: 10.1039/c4fo00702f
Yue, N., Huang, H., Zhu, X., Han, Q., Wang, Y., Li, B., et al. (2017). Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J. Neuroinflammation 14:102. doi: 10.1186/s12974-017-0865-y
Zhang, X., Wang, D., Zhang, B., Zhu, J., Zhou, Z., and Cui, L. (2020). Regulation of microglia by glutamate and its signal pathway in neurodegenerative diseases. Drug Discov. Today 25, 1074–1085. doi: 10.1016/j.drudis.2020.04.001
Keywords: ATP P2X7 receptor, adenosine A2A receptor, stress, behavior, microglia, neuroinflammation, synaptic plasticity
Citation: Dias L, Lopes CR, Gonçalves FQ, Nunes A, Pochmann D, Machado NJ, Tomé AR, Agostinho P and Cunha RA (2021) Crosstalk Between ATP-P2X7 and Adenosine A2A Receptors Controlling Neuroinflammation in Rats Subject to Repeated Restraint Stress. Front. Cell. Neurosci. 15:639322. doi: 10.3389/fncel.2021.639322
Received: 08 December 2020; Accepted: 08 February 2021;
Published: 01 March 2021.
Edited by:
Robson Xavier Faria, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Hércules Rezende Freitas, Federal University of Rio de Janeiro, BrazilAmeneh Rezayof, University of Tehran, Iran
Enric I. Canela, University of Barcelona, Spain
Joana Esteves Coelho, University of Lisbon, Portugal
Copyright © 2021 Dias, Lopes, Gonçalves, Nunes, Pochmann, Machado, Tomé, Agostinho and Cunha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodrigo A. Cunha, cunharod@gmail.com
 Liliana Dias1
Liliana Dias1  Cátia R. Lopes
Cátia R. Lopes Francisco Q. Gonçalves
Francisco Q. Gonçalves Ana Nunes
Ana Nunes Nuno J. Machado
Nuno J. Machado Angelo R. Tomé
Angelo R. Tomé Paula Agostinho
Paula Agostinho Rodrigo A. Cunha
Rodrigo A. Cunha