Anesthetized animal experiments for neuroscience research
- 1Department of Neurobiology and Anatomy, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States
- 2Pathology Research Team, Faculty of Health Sciences, Kyorin University, Mitaka, Japan
- 3Department of Otorhinolaryngology, Medical School of Nihon University, Tokyo, Japan
Brain research has progressed with anesthetized animal experiments for a long time. Recent progress in research techniques allows us to measure neuronal activity in awake animals combined with behavioral tasks. The trends became more prominent in the last decade. This new research style triggers the paradigm shift in the research of brain science, and new insights into brain function have been revealed. It is reasonable to consider that awake animal experiments are more ideal for understanding naturalistic brain function than anesthetized ones. However, the anesthetized animal experiment still has advantages in some experiments. To take advantage of the anesthetized animal experiments, it is important to understand the mechanism of anesthesia and carefully handle the obtained data. In this minireview, we will shortly summarize the molecular mechanism of anesthesia in animal experiments, a recent understanding of the neuronal activities in a sensory system in the anesthetized animal brain, and consider the advantages and disadvantages of the anesthetized and awake animal experiments. This discussion will help us to use both research conditions in the proper manner.
Introduction
Anesthesia could be reversibly induced by the anesthetic agents and makes the brain and body condition into the following specific behavioral and physiological traits (Brown et al., 2010). 1. Analgesia: Animals do not perceive pain. 2. Unconsciousness: Animals are not aware of what’s happening. 3. Amnesia: Animals do not form memories. 4. Akinesia: Animals cannot move. Anesthesia decreases the painful stress and is helpful for the operation of the surgery on humans and animals. In addition, anesthesia helps monitor body and brain activity to understand the function at molecular, cellular, and circuit/tissue levels in vivo.
Brain science took advantage of anesthesia and revealed brain functions in the past centuries. In the recent decade, multiple experimental tools for monitoring the body and brain conditions have succeeded in becoming more compact and attachable to the body, directly allowing us to decrease animal stress and monitor the brain or body activity while behaving animals in awake conditions. In addition, the experimental procedures also improved to decrease the animal stress. Then, it decreased the hurdles to understanding the animal in awake conditions. The current trend is to study brain function in awake animal conditions. However, anesthesia is an important step for animal surgery, which is also necessary for many awake animal experiments, and it still has advantages in conducting experiments to understand brain function. In this review, we will shortly summarize the molecular mechanisms of popular anesthesia and clarify the difference between awake and anesthetized animal experiments to have the appropriate experimental conditions for the anesthetized and awake animal experiments.
How do anesthetic agents work?
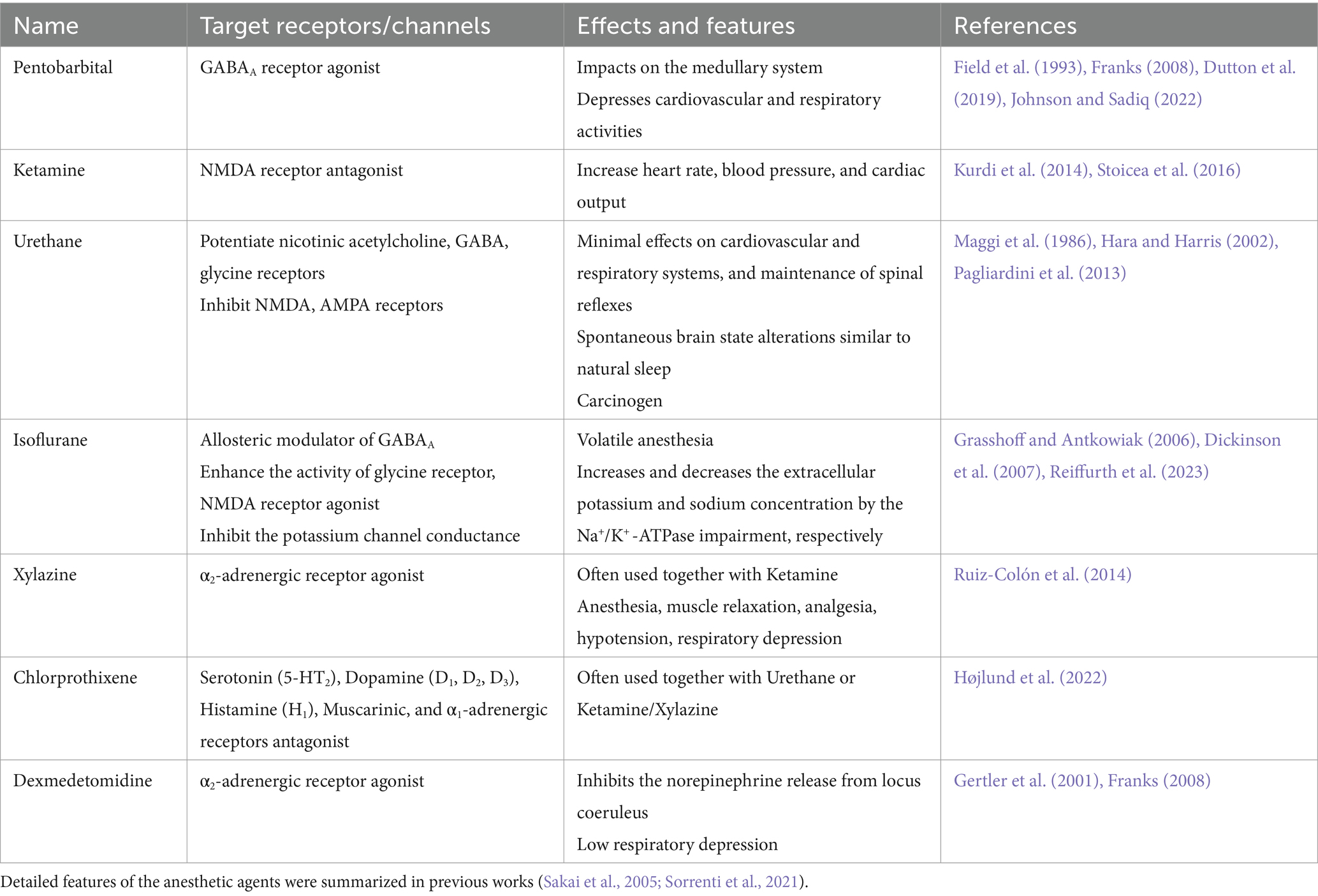
Anesthetic agents affect the animal brains and modify regular neuronal activity (Franks and Lieb, 1994). Although we frequently use anesthesia for animal experiments, we still do not understand the molecular, cellular, and circuitry mechanisms of how anesthetic agents induce Analgesic, Unconsciousness, Amnesic, and Akinetic conditions (Århem et al., 2003). Here, we quickly summarize the current knowledge of the molecular mechanism of the following major anesthesia (Pentobarbital, Ketamine, Urethane, and Isoflurane), in addition to the major sedatives (Xylazine, Chlorprothixene, and Dexmedetomidine) (Table 1).
Pentobarbital: Pentobarbital have been one of the major anesthetic agents. It binds to the gamma-aminobutyric acid (GABA) type A (GABAA) receptors (Franks, 2008; Johnson and Sadiq, 2022). It induces chloride channels to open longer, potentiate GABA effects, and induce longer hyperpolarization. The advantage of Pentobarbital anesthesia would be its reliability to induce rapid unconsciousness. It impacts the sensory systems and the medullary and depresses cardiovascular and respiratory activities (Field et al., 1993; Dutton et al., 2019). It also has the function of sedation.
Ketamine: Ketamine targets N-methyl-d-aspartate (NMDA) receptors to reduce the excitatory action of glutamate (Stoicea et al., 2016). Its prominent features as anesthesia are increased heart rate, blood pressure, and cardiac output, mediated principally through the sympathetic nervous system (Kurdi et al., 2014). It has small effects on the central respiratory drive. It increases salivation and muscle tone. Ketamine also has the function of an antidepressant.
Urethane: Urethane is one of the most popular anesthetics used for animal experiments for a long time. Although it is a carcinogen and is not proper for survival surgery, it is still well used because of its minimal effects on cardiovascular and respiratory systems, and it could maintain spinal reflexes and spontaneous brain state alterations similar to natural sleep (Maggi et al., 1986; Pagliardini et al., 2013). In addition, it can produce a long-lasting steady level of anesthesia during surgery and experiments. It is assumed that animals anesthetized with urethane represent similar physiologic and pharmacologic behaviors to those observed in unanesthetized animals. However, little is known about its mechanism. Recent research revealed the urethane effect of multiple ion channels (Hara and Harris, 2002). Urethane potentiates the functions of nicotinic acetylcholine, GABA, and glycine receptors, and it inhibits NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors in a concentration-dependent manner. Because urethane had modest effects on all channels tested in the experiment, urethane would not have a single predominant target molecule for anesthesia.
Isoflurane: Isoflurane is volatile anesthesia. Therefore, one of the prominent features of isoflurane anesthesia is the easier control and faster anesthesia induction and recovery. The molecular mechanism of isoflurane anesthesia has not been well known (Jones et al., 1992; Jenkins et al., 1999; Krasowski and Harrison, 2000). Isoflurane acts as an allosteric modulator of the GABAA receptor, enhances the activity of glycine receptors, and decreases motor function (Grasshoff and Antkowiak, 2006). Isoflurane inhibits the activity of NMDA receptors at the same site as glycine (Dickinson et al., 2007). Isoflurane inhibits the conduction of the potassium channel (Buljubasic et al., 1992). In addition, Isoflurane also works as a burst suppression anesthesia, and increases the extracellular potassium concentration and decreases extracellular sodium concentration by the Na+/K+-ATPase impairment (Reiffurth et al., 2023).
These anesthetic agents are often used with Sedatives. The following three sedatives are popular in animal experiments.
Xylazine: Xylazine is often used together with Ketamine as a sedative, and the mixture of them is referred to as Ketamine/Xylazine (K/X). Xylazine also has effects on anesthesia, muscle relaxation, and analgesia. Xylazine is known as an α2-adrenergic receptor agonist (Ruiz-Colón et al., 2014). It also has side effects of hypotension and respiratory depression.
Chlorprothixene: Chlorprothixene is often used together with Urethane or Ketamine/Xylazine. It is an antipsychotic drug (Højlund et al., 2022). Chlorprothixene has strong impacts by blocking the Serotonin (5-HT2), Dopamine receptors (D1, D2, D3), Histamine (H1), Muscarinic, and α1-adrenergic receptors.
Dexmedetomidine: Dexmedetomidine induces sedation by agonistically binding to α2-adrenergic receptors and inhibits the norepinephrine release from locus coeruleus in the brain stem (Gertler et al., 2001; Franks, 2008). Unlike opioids and other sedatives such as propofol, dexmedetomidine can achieve its effects without causing respiratory depression.
This summary shows us that different anesthetic agents and sedatives target different ion channels or transmitter receptors. However, they induce a similar trait of anesthesia.
Neuronal mechanism of sleep and anesthesia
General anesthesia and natural sleep induce similar behaviors. Therefore, their similarities have been discussed for a long time (Shafer, 1995; Date et al., 2020). The two conditions have both similar and different functional features. Recent research revealed that sleep is a more active process than previously considered. Many of the reports especially focus on the hypothalamus area (Szymusiak et al., 2007; Sternson, 2013; Wu et al., 2014; Scott et al., 2015; Tan et al., 2016; Allen et al., 2017). It is reported that some given groups of neurons in the hypothalamus show activity under sleep or anesthesia conditions (Moore et al., 2012; Zhang et al., 2015; Gelegen et al., 2018). These neurons are called anesthetic-activated cells. More recent research revealed that anesthetic-activated cells contribute to inducing and maintaining the anesthetized and sleep conditions, in addition to the awake condition.
Jiang-Xie’s group found the anesthesia-activated cells in the supraoptic nucleus in the hypothalamus. An interesting finding was that if they activate the neuron’s chemogenetic or optogenetic method, the animal stops moving and falls into a slow wave sleep condition. If the neurons are conditionally ablated or inhibited, the mice continuously move around and cannot fall asleep. In addition, if they silence the activity of the neurons, the mice work up from anesthesia easily (Jiang-Xie et al., 2019).
Opposite functional types of neurons, whose activity is associated with induction and maintenance of awake condition, were also found. Reitz’s group showed that chemogenetic activation of tachykinin 1 expressing neurons in the preoptic area obliterates both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. Moreover, chemogenetic activation of these neurons stabilizes the waking state against both Isoflurane- and Sevoflurane-induced unconsciousness (Reitz et al., 2021).
These researches shed light on that the specific neurons and neuronal circuits in the hypothalamus would contribute to induce and maintain the anesthetized and sleep conditions, in addition to awake conditions. The anesthetic agents described in the previous section may have a strong effect on these specific neurons in the hypothalamus. However, the anesthetic agents also impact other neurons, which may not be directly associated with the induction and maintenance of sleep, anesthetized, and awake animal conditions. Further study of the molecular cellular and circuitry contributions to the induction and maintenance of sleep, anesthetized, as well as awake animal conditions, are demanded. The knowledge will help to interpret the functional research data collected in anesthetized animal conditions correctly and help to improve the method of anesthesia beyond the current anesthetic agents and procedures.
Sensory response in anesthetized and awake condition
Anesthesia induces Analgesia, Unconsciousness, Amnesia, Akinesia in animals. Therefore, the downstream (or outputs) of the neuronal circuits associated with these behavioral and psychological traits would be strongly disturbed during anesthesia. Indeed, a large number of the research reported the differences in activity of neurons, neural population, and functional connectivity of brain areas between anesthetized and awake conditions (Nallasamy and Tsao, 2011; Sellers et al., 2015; Hu et al., 2024). However, the upstream and peripheral of each circuit, such as sensory representation in the primary or secondary sensory areas, may be less disturbed by anesthesia.
In the primary visual cortex, anesthetics reduce spontaneous neuronal spike activity but promote synchronized activity (Greenberg et al., 2008; Aasebø et al., 2017; Lee et al., 2021). The change is larger in Isoflurane compared to the Ketamine/Xylazine anesthesia (Aasebø et al., 2017). Interestingly, the narrow spiking neurons (presumably inhibitory neurons) have a larger decrease in spontaneous spike activity compared to the broad spiking neurons (presumably excitatory neurons) in anesthesia (Aasebø et al., 2017). To the visual stimulation, anesthetized animals showed prolonged neuronal activity by the stimulation in a wide area in visual space. In contrast, neuronal responses to visual stimulation are more spatially selective and much briefer during wakefulness. The difference is owed to the strong inhibition of extremely broad spatial selectivity during wakefulness (Haider et al., 2012).
In the olfactory bulb, wakefulness greatly enhances the activity of inhibitory interneurons of granule cells. As a result, the odor responses of the principal neurons of mitral cells are not prominent compared to those under anesthetized animals. However, awake animals show more sparse and temporally dynamic responses (Kato et al., 2012; Blauvelt et al., 2013; Wachowiak et al., 2013). In addition, repetitive odor experiences in awake animal condition weaken the odor response of mitral cells gradually for a long time. This mitral cell plasticity is odor-specific, recovers gradually over months, and can be repeated with different odors. Furthermore, the expression of this experience-dependent plasticity is prevented by anesthesia (Kato et al., 2012).
In the barrel cortex, electrophysiological responses evoked by whisker deflection are reduced in amplitude under anesthetized condition (Simons et al., 1992; Devonshire et al., 2010). However, delayed activity, probably due to the inputs from the neighboring whisker stimulation, is more prominent and prolonged. The reduction in response amplitude was considered by the global down-scaling of the population response. Interestingly, the variation of the spike frequency is larger during wakefulness. The difference is more prominent in layer 5A, especially during whisking episodes (De Kock and Sakmann, 2009).
These researches indicate that sensory representation in the primary sensory areas could be observed in both anesthetized and awake animals. However, the amplitude, tuning specificity of sensory inputs, and temporal pattern of neuronal activity are not completely the same in anesthetized and awake animal conditions. Importantly, the anesthesia delivers different impacts on the different layers, neuronal types, and sensory systems. The majority of the reasons for the difference have not yet been clearly determined. However, it probably owes to the combination change of the direct or indirect pharmacological impact of anesthetic agents on the recording neurons and the top-down signals to each recording neuron between the anesthetized and awake animal conditions.
Advantages and disadvantages of anesthetized animal research
Advantages: Anesthetized condition is a pharmacologically induced artificial condition, which is useful for operating surgery and physiological experiments. The akinetic effect minimizes animal movement and helps us obtain high-quality experimental data such as in vivo electrophysiology or functional optical imaging experiments. In addition, the analgesic effect decreases the pain sensation and helps to decrease the animal stress of pain or uncomfortable procedures of surgery or experiments. Therefore, the biggest advantage of the anesthetized animal experiment would be that it allows us to conduct functional experiments easily and reliably.
Disadvantage 1: One of the problems of the anesthetized animal experiment is the broad and unidentified impacts of anesthetic agents on the brains. The normal neuronal activity and response to the neuronal transmitters are disturbed by the anesthetic agents. Probably, some synaptic transmission and neuronal activity would be enhanced, and others would be inhibited to some degree. In other words, the brain activity is modified in an anesthetic agent specific manner in anesthetized animals. These impacts are more remarkable when we monitor the neuronal activity in higher brain centers, where the sensory inputs reach after many synaptic transmissions. Therefore, functional research on the higher brain center may not be adequate under anesthesia in general. However, some of the peripheral sensory brain areas, which reached the sensory inputs after the small number of synaptic transmissions, would be less impacted by the anesthesia, although the centrifugal or top-down signal would be abnormal or less active in this case.
Disadvantage 2: Sensory information is actively acquired in awake-behaving rodents, such as active movement of whiskers and an increase in the sniffing cycle in olfaction (Petersen, 2007; Wachowiak, 2011). Anesthetized animals do not have such behavioral outputs. Therefore, the research using anesthetized animals allows us only to study the passive sensory processing without animal behavior attempting to intensely collect and recognize the sensory inputs. It would make neuronal response simpler and easier to interpret. However, we need to carefully interpret the data concerning the lack of active sensing top-down signals, which contribute to sensory recognition in the process of perception.
Advantages and disadvantages of awake animal research
Advantage: There is no doubt that animal experiments in awake conditions are one of the ideal functional experimental conditions because they allow us the naturalistic neuronal activity in the experiment. In addition, some of the disadvantages of the anesthetized animal experiments discussed above are fully or partially overcome by the awake animal experiments.
Disadvantage 1: One of the weak points of the awake animal experiments is that we could easily capture the large size of noise associated with the animal movement. Recent progress in experimental skills, such as solid attachment of the recording systems on the skull, remarkably improved the weakness. In addition, researchers were intensely challenged to compensate for the movement artifacts during the offline data analysis. As a result, we can now collect more reliable and high-quality neuronal activity in awake animals than we did previously. However, monitoring neuronal activity using recording methods sensitive to animal movement, such as in vivo intracellular or patch-clamp recording methods, is still challenging.
Disadvantage 2: Some of the experimental systems are too large and heavy to attach to the animal brain, such as optical imaging systems with high numerical aperture lenses. Some probes, such as functional ultrasound imaging systems, functional magnetic resonance imaging systems, etc., require to be moved while monitoring the brain functions. The head-restrained animal experiment would be the best choice for using such equipment. However, the head-restrained animal researches require a relatively short time experimental duration (~1 h) to decrease the head-restrained stress of animals. If it requires a longer (more than 6 h) duration, the anesthetized animal experiments have benefits. For example, the functional mapping of relatively peripheral areas, such as the olfactory bulb, barrel cortex, visual cortex, etc., has the advantage of using the anesthetized experiment.
Disadvantage 3: Massive volume of information is processed in awake animal brains simultaneously. It is one of the biggest benefits of the awake animal experiment, which allows us to understand the naturalistic brain function in vivo. However, it also has a tradeoff. Too much activity makes it difficult to capture or extract the critical signals among the flood of massive neuronal activities. Turning off some of the neuronal activity or neuronal pathways in awake animal experiments may help to dissect the brain function and focus on discussing the specific neuronal activity and pathways in some cases.
Disadvantage 4: Usually, the recording system is attached over the skull, or the recording probes are implanted in the target brain area under anesthesia before the awake animal experiment. In this case, we need to consider postoperative delirium to have the proper recovery time after the anesthetized surgery (Peng et al., 2016). Even if the animal shows normal activity after the surgery, animal performance for some experimental tasks may not be the same as that before the surgery. Therefore, the careful evaluation of the animal recovery from the anesthetized surgery is required for the awake animal experiments associated with anesthetized animal surgery.
Discussion
Considering the advantages and disadvantages discussed above session, the current method of anesthesia would be advantageous in some of the experiments, such as (1) Mapping the passive sensory inputs, (2) Evaluating the synaptic interaction or pharmacological impact in the local circuit, and (3) Measuring the neuronal activity under stressful in awake animal conditions.
In addition, some of the disadvantages of awake animal experiments will be overcome by the progress of technologies in the future. For example, the large and heavy recording systems will become more compact and lighter. They will be less stressed when attached to the animal skull. Therefore, these disadvantages could be partially solved in the future.
Recent studies showed the potential that anesthesia and sleep conditions may be inducible by the control of the specific neuronal types and circuits. These discoveries will shed light on the potential of new types of anesthesia. Suppose we will be able to control the activity of the proper number and group of anesthetic/awake associate neurons by chemogenetic or optogenetic tools and control the anesthetized/awake states of animals in the future. In that case, this procedure will become a new type of anesthesia and minimize the impact of anesthesia on other brain functions such as respiration and cardiovascular systems. It will increase the controllability of the anesthesia, may decrease the animal death associated with the failure control of the respiratory and cardiovascular systems, and increase the success rate of the animal surgery. In addition, if we know the specific neurons and neuronal circuits affected by the anesthesia, we could avoid studying these neurons and circuits, and focus on studying the other neuronal circuits, which have normal activity of received minimum impact of the new anesthesia, as like awake animal research more easily and efficiently than current awake animal experiments. Understanding the essential neuronal and circuitry mechanisms that induce sleep/anesthesia and awake animal conditions would extend the anesthetized animal research beyond the current limitation and help to understand the more naturalistic brain function in anesthetized animal conditions.
Summary
In this review, we attempt to summarize the molecular mechanism of popular anesthetic agents, the relationship between hypothalamic neurons and anesthesia/sleep/awake conditions, sensory activities under anesthesia, the advantages and disadvantages of anesthetized and awake animal experiments, and discuss the potential of future anesthesia. This would help in conducting anesthetized experiments and future animal research.
Author contributions
SN: Writing – original draft, Writing – review & editing. SH-I: Writing – review & editing. SK: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS KAKENHI Grant Numbers JP23K08930 (SK), JSPS KAKENHI Grant Numbers JP21K07280 and 24 K10495 (SH-I), Japan-US Brain Research Cooperation program (SK), and the Takeda Science Foundation (SK).
Acknowledgments
The authors apologize to those whose work was not included here due to space limitations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aasebø, I. E. J., Lepperød, M. E., Stavrinou, M., Nøkkevangen, S., Einevoll, G., Hafting, T., et al. (2017). Temporal processing in the visual cortex of the awake and anesthetized rat. eNeuro 4:59. doi: 10.1523/ENEURO.0059-17.2017
Allen, W. E., DeNardo, L. A., Chen, M. Z., Liu, C. D., Loh, K. M., Fenno, L. E., et al. (2017). Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155. doi: 10.1126/science.aan6747
Århem, P., Klement, G., and Nilsson, J. (2003). Mechanisms of anesthesia: towards integrating network, cellular, and molecular level modeling. Neuropsychopharmacology 28, S40–S47. doi: 10.1038/sj.npp.1300142
Blauvelt, D. G., Sato, T. F., Wienisch, M., and Murthy, V. N. (2013). Distinct spatiotemporal activity in principal neurons of the mouse olfactory bulb in anesthetized and awake states. Front. Neural Circuits 7:46. doi: 10.3389/fncir.2013.00046
Brown, E. N., Lydic, R., and Schiff, N. D. (2010). General anesthesia, sleep, and coma. N. Engl. J. Med. 363, 2638–2650. doi: 10.1056/NEJMra0808281
Buljubasic, N., Rusch, N. J., Marijic, J., Kampine, J. P., and Bosnjak, Z. J. (1992). Effects of halothane and isoflurane on calcium and Potassium Channel currents in canine coronary arterial cells. Anesthesiology 76, 990–998. doi: 10.1097/00000542-199206000-00020
Date, A., Bashir, K., Uddin, A., and Nigam, C. (2020). Differences between natural sleep and the anesthetic state. Future Sci. 6. doi: 10.2144/fsoa-2020-0149
De Kock, C. P. J., and Sakmann, B. (2009). Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc. Natl. Acad. Sci. USA 106, 16446–16450. doi: 10.1073/pnas.0904143106
Devonshire, I. M., Grandy, T. H., Dommett, E. J., and Greenfield, S. A. (2010). Effects of urethane anaesthesia on sensory processing in the rat barrel cortex revealed by combined optical imaging and electrophysiology. Eur. J. Neurosci. 32, 786–797. doi: 10.1111/j.1460-9568.2010.07322.x
Dickinson, R., Peterson, B. K., Banks, P., Simillis, C., Martin, J. C. S., Valenzuela, C. A., et al. (2007). Competitive inhibition at the Glycine site of the N -methyl-d-aspartate receptor by the anesthetics xenon and IsofluraneEvidence from molecular modeling and electrophysiology. Anesthesiology 107, 756–767. doi: 10.1097/01.anes.0000287061.77674.71
Dutton, J. W., Artwohl, J. E., Huang, X., and Fortman, J. D. (2019). Assessment of pain associated with the injection of sodium pentobarbital in laboratory mice (Mus musculus). J. Am. Assoc. Lab. Anim. Sci. 58, 373–379. doi: 10.30802/AALAS-JAALAS-18-000094
Field, K. J., White, W. J., and Lang, C. M. (1993). Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab. Anim. 27, 258–269. doi: 10.1258/002367793780745471
Franks, N. P. (2008). General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386. doi: 10.1038/nrn2372
Franks, N. P., and Lieb, W. R. (1994). Molecular and cellular mechanisms of general anaesthesia. Nat. Cell Biol. 367, 607–614. doi: 10.1038/367607a0
Gelegen, C., Miracca, G., Ran, M. Z., Harding, E. C., Ye, Z., Yu, X., et al. (2018). Excitatory pathways from the lateral Habenula enable Propofol-induced sedation. Curr. Biol. 28, 580–587.e5. doi: 10.1016/j.cub.2017.12.050
Gertler, R., Brown, H. C., Mitchell, D. H., and Silvius, E. N. (2001). Dexmedetomidine: a novel sedative-analgesic agent. Proc. (Bayl. Univ. Med. Cent.) 14, 13–21. doi: 10.1080/08998280.2001.11927725
Grasshoff, C., and Antkowiak, B. (2006). Effects of isoflurane and enflurane on GABAA and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats. Br. J. Anaesth. 97, 687–694. doi: 10.1093/bja/ael239
Greenberg, D. S., Houweling, A. R., and Kerr, J. N. D. (2008). Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat. Neurosci. 11, 749–751. doi: 10.1038/nn.2140
Haider, B., Häusser, M., and Carandini, M. (2012). Inhibition dominates sensory responses in the awake cortex. Nat. Cell Biol. 493, 97–100. doi: 10.1038/nature11665
Hara, K., and Harris, R. A. (2002). The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth. Analg. 94, 313–318. doi: 10.1213/00000539-200202000-00015
Højlund, M, Blanner Wagner, C, Wesselhoeft, R, Andersen, K, Fink-Jensen, A, and Hallas, J (2022) Use of chlorprothixene and the risk of diabetes and major adverse cardiovascular events: a nationwide cohort study. Basic Clin Pharmacol Toxicol. 130, 501–512. doi: 10.1111/bcpt.13711
Hu, Y., Du, W., Qi, J., Luo, H., Zhang, Z., Luo, M., et al. (2024). Comparative brain-wide mapping of ketamine- and isoflurane-activated nuclei and functional networks in the mouse brain. eLife 12:88420. doi: 10.7554/eLife.88420
Jenkins, A., Franks, N. P., and Lieb, W. R. (1999). Effects of temperature and volatile anesthetics on GABAAReceptors. Anesthesiology 90, 484–491. doi: 10.1097/00000542-199902000-00024
Jiang-Xie, L. F., Yin, L., Zhao, S., Prevosto, V., Han, B. X., Dzirasa, K., et al. (2019). A common neuroendocrine substrate for diverse general anesthetics and sleep. Neuron 102, 1053–1065.e4. doi: 10.1016/j.neuron.2019.03.033
Johnson, AB, and Sadiq, NM (2022) Pentobarbital - StatPearls - NCBI bookshelf. StatPearls [Internet] Available at: https://www.ncbi.nlm.nih.gov/books/NBK545288/ (Accessed April 28, 2024).
Jones, M. V., Brooks, P. A., and Harrison, N. L. (1992). Enhancement of gamma-aminobutyric acid-activated cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics. J. Physiol. 449, 279–293. doi: 10.1113/jphysiol.1992.sp019086
Kato, H. K., Chu, M. W., Isaacson, J. S., and Komiyama, T. (2012). Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron 76, 962–975. doi: 10.1016/j.neuron.2012.09.037
Krasowski, M. D., and Harrison, N. L. (2000). The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br. J. Pharmacol. 129, 731–743. doi: 10.1038/sj.bjp.0703087
Kurdi, M. S., Theerth, K. A., and Deva, R. S. (2014). Ketamine: current applications in anesthesia, pain, and critical care. Anesth. Essays Res. 8, 283–290. doi: 10.4103/0259-1162.143110
Lee, H., Tanabe, S., Wang, S., and Hudetz, A. G. (2021). Differential effect of anesthesia on visual cortex neurons with diverse population coupling. Neuroscience 458, 108–119. doi: 10.1016/j.neuroscience.2020.11.043
Maggi, C. A., Santicioli, P., and Meli, A. (1986). Somatovesical and vesicovesical excitatory reflexes in urethane-anaesthetized rats. Brain Res. 380, 83–93. doi: 10.1016/0006-8993(86)91432-0
Moore, J. T., Chen, J., Han, B., Meng, Q. C., Veasey, S. C., Beck, S. G., et al. (2012). Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr. Biol. 22, 2008–2016. doi: 10.1016/j.cub.2012.08.042
Nallasamy, N., and Tsao, D. Y. (2011). Functional connectivity in the brain: effects of anesthesia. Neuroscientist 17, 94–106. doi: 10.1177/1073858410374126
Pagliardini, S., Funk, G. D., and Dickson, C. T. (2013). Breathing and brain state: urethane anesthesia as a model for natural sleep. Respir. Physiol. Neurobiol. 188, 324–332. doi: 10.1016/j.resp.2013.05.035
Peng, M., Zhang, C., Dong, Y., Zhang, Y., Nakazawa, H., Kaneki, M., et al. (2016). Battery of behavioral tests in mice to study postoperative delirium. Sci. Rep. 6, 1–13. doi: 10.1038/srep29874
Petersen, C. C. H. (2007). The functional organization of the barrel cortex. Neuron 56, 339–355. doi: 10.1016/j.neuron.2007.09.017
Reiffurth, C., Berndt, N., Gonzalez Lopez, A., Schoknecht, K., Kovács, R., Maechler, M., et al. (2023). Deep isoflurane anesthesia is associated with alterations in ion homeostasis and specific Na+/K+-ATPase impairment in the rat brain. Anesthesiology 138, 611–623. doi: 10.1097/ALN.0000000000004553
Reitz, S. L., Wasilczuk, A. Z., Beh, G. H., Proekt, A., and Kelz, M. B. (2021). Activation of preoptic tachykinin 1 neurons promotes wakefulness over sleep and volatile anesthetic-induced unconsciousness. Curr. Biol. 31, 394–405.e4. doi: 10.1016/j.cub.2020.10.050
Ruiz-Colón, K., Chavez-Arias, C., Díaz-Alcalá, J. E., and Martínez, M. A. (2014). Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: a comprehensive review of the literature. Forensic Sci. Int. 240, 1–8. doi: 10.1016/j.forsciint.2014.03.015
Sakai, E. M., Connolly, L. A., and Klauck, J. A. (2005). Inhalation anesthesiology and volatile liquid anesthetics: focus on isoflurane, desflurane, and sevoflurane. Pharmacotherapy 25, 1773–1788. doi: 10.1592/phco.2005.25.12.1773
Scott, N., Prigge, M., Yizhar, O., and Kimchi, T. (2015). A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nat. Cell Biol. 525, 519–522. doi: 10.1038/nature15378
Sellers, K. K., Bennett, D. V., Hutt, A., Williams, J. H., and Fröhlich, F. (2015). Awake vs. anesthetized: layer-specific sensory processing in visual cortex and functional connectivity between cortical areas. J. Neurophysiol. 113, 3798–3815. doi: 10.1152/jn.00923.2014
Shafer, A. (1995). Metaphor and anesthesia. Anesthesiology 83, 1331–1342. doi: 10.1097/00000542-199512000-00024
Simons, D. J., Carvell, G. E., Hershey, A. E., and Bryant, D. P. (1992). Responses of barrel cortex neurons in awake rats and effects of urethane anesthesia. Exp. Brain Res. 91, 259–272. doi: 10.1007/BF00231659
Sorrenti, V., Cecchetto, C., Maschietto, M., Fortinguerra, S., Buriani, A., and Vassanelli, S. (2021). Understanding the effects of anesthesia on cortical electrophysiological recordings: a scoping review. Int. J. Mol. Sci. 22:1286. doi: 10.3390/ijms22031286
Sternson, S. M. (2013). Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77, 810–824. doi: 10.1016/j.neuron.2013.02.018
Stoicea, N., Versteeg, G., Florescu, D., Joseph, N., Fiorda-Diaz, J., Navarrete, V., et al. (2016). Ketamine-based anesthetic protocols and evoked potential monitoring: a risk/benefit overview. Front. Neurosci. 10:180420. doi: 10.3389/fnins.2016.00037
Szymusiak, R., Gvilia, I., and McGinty, D. (2007). Hypothalamic control of sleep. Sleep Med. 8, 291–301. doi: 10.1016/j.sleep.2007.03.013
Tan, C. L., Cooke, E. K., Leib, D. E., Lin, Y. C., Daly, G. E., Zimmerman, C. A., et al. (2016). Warm-sensitive neurons that control body temperature. Cell 167, 47–59.e15. doi: 10.1016/j.cell.2016.08.028
Wachowiak, M. (2011). All in a sniff: olfaction as a model for active sensing. Neuron 71, 962–973. doi: 10.1016/j.neuron.2011.08.030
Wachowiak, M., Economo, M. N., Díaz-Quesada, M., Brunert, D., Wesson, D. W., White, J. A., et al. (2013). Optical dissection of odor information processing in vivo using GCaMPs expressed in specified cell types of the olfactory bulb. J. Neurosci. 33, 5285–5300. doi: 10.1523/JNEUROSCI.4824-12.2013
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M., and Dulac, C. G. (2014). Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. doi: 10.1038/nature13307
Keywords: anesthesia, brain, experimental animal, anesthetic-activated cells, sensory representation
Citation: Nagayama S, Hasegawa-Ishii S and Kikuta S (2024) Anesthetized animal experiments for neuroscience research. Front. Neural Circuits. 18:1426689. doi: 10.3389/fncir.2024.1426689
Edited by:
Kensaku Mori, RIKEN, JapanReviewed by:
Haruki Takeuchi, The University of Tokyo, JapanCopyright © 2024 Nagayama, Hasegawa-Ishii and Kikuta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin Nagayama, shin.nagayama@uth.tmc.edu
 Shin Nagayama
Shin Nagayama Sanae Hasegawa-Ishii2
Sanae Hasegawa-Ishii2  Shu Kikuta
Shu Kikuta