- 1Department of Ophthalmology, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain
- 2Instituto de Investigación Sanitaria de Aragón (IIS Aragon), Zaragoza, Spain
- 3Department of Ophthalmology, Poznan City Hospital, Poznan, Poland
- 4University of Warmia and Mazury, Olsztyn, Poland
Migraine is a chronic disease characterized by unilateral, pulsating, and often moderate-to-severe recurrent episodes of headache with nausea and vomiting. It affects approximately 15% of the general population, yet the underlying pathophysiological mechanisms are not fully understood. Optical coherence tomography (OCT) is a safe and reproducible diagnostic technique that utilizes infrared wavelengths and has a sensitivity of 8–10 μm. It can be used to measure thinning of the retinal nerve fiber layer (RNFL) in some neurological disorders. Although ophthalmologists are often the first specialists to examine patients with migraine, few studies have addressed the involvement of the optic nerve and retino-choroidal structures in this group. We reviewed the literature on the etiological and pathological mechanisms of migraine and the relationship between recurrent constriction of cerebral and retrobulbar vessels and ischemic damage to the optic nerve, retina, and choroid. We also assessed the role of OCT for measuring peripapillary RNFL thickness and macular and choroidal changes in migraine patients. There is considerable evidence of cerebral and retrobulbar vascular involvement in the etiology of migraine. Transitory and recurrent constriction of the retinal and ciliary arteries may cause ischemic damage to the optic nerve, retina, and choroid in patients with migraine. OCT to assess the thickness of the peripapillary RNFL, macula, and choroid might increase our understanding of the pathophysiology of migraine and facilitate diagnosis of retino-choroidal compromise and follow-up of therapy in migraine patients. Future studies should determine the usefulness of OCT findings as a biomarker of migraine.
Introduction
Migraine is a chronic disease characterized by unilateral, pulsating, and often moderate-to-severe repetitive episodes of headache and vegetative symptoms such as nausea, vomiting, and extreme sensitivity to light and sound (1). This condition affects approximately 15% of the general population and is the most prevalent neurological disorder and the third most frequent global disorder in both genders (2). It mainly affects women aged 20–45 years (3, 4), and symptoms typically last 4–72 h (5–7).
Episodic migraine is defined as 0–14 headache days per month, whereas chronic migraine (CM) is defined as 15 or more headache days per month. Episodic migraine is classified into two major groups: migraine with aura (MwA, or classic migraine) and migraine without aura (MwoA, or common migraine) (8). Aura, which manifests as transient ocular and neurological disturbances, including flashes of light, blind spots, periorbital pain, and photophobia, affects about 25% of patients, usually immediately before but sometimes following the headache (9, 10).
Clinical data support ocular involvement in migraine. Thus, visual field compromise resembling that described in glaucoma has been reported (11). Perimetric damage progressed more quickly in patients with normal-tension glaucoma (NTG) and headache than that in individuals with NTG and no migraine (12). Several authors have explored visual acuity and visual field testing in migraineurs, although they did not observe any differences between migraineurs and controls (13, 14). Koban et al. (15) studied intraocular pressure (IOP) and biometric parameters and found no relevant differences between migraineurs during the attack and the healthy controls.
Optical Coherence Tomography (OCT), a Promising Technology in Neurology
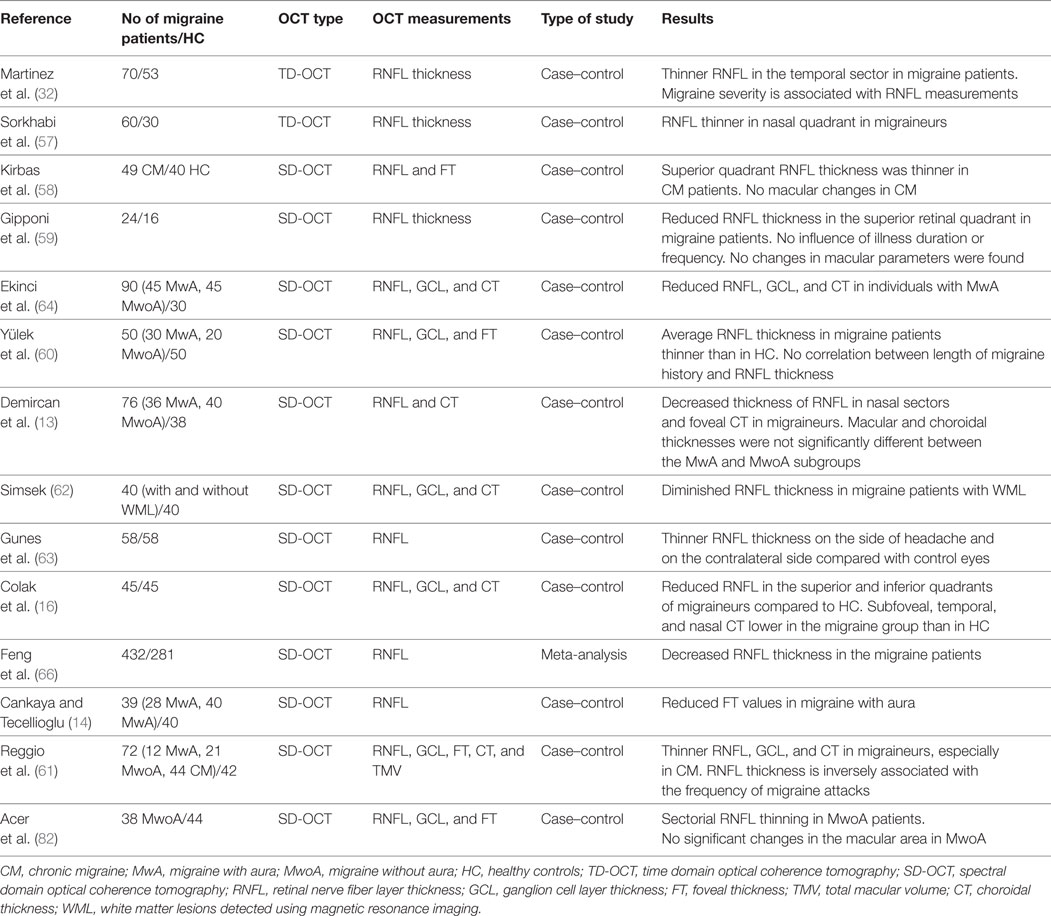
Decreased thickness of the retinal nerve fiber layer (RNFL) has been reported in several neurological disorders, such as multiple sclerosis, Alzheimer disease, and Parkinson disease (16–18). Ganglion cell layer (GCL) thickness could become a more appropriate morphological biomarker of axonal damage than RNFL in specific optic neuropathies (19, 20). OCT is a reliable, reproducible, objective, non-invasive, transpupillary diagnostic technique that enables quantitative in vivo high-resolution measurement of peripapillary RNFL, GCL, and choroid layer thickness (21–23). It generates a retinal cross-sectional map accurate to 5 µm. RNFL thickness is currently measured using OCT to assess glaucoma and maculopathies (24, 25). Decreased RNFL thickness has also been demonstrated by OCT in certain neurological disorders (26–30). In the last few years, several authors have used OCT to study whether the retina and choroid are involved in patients with migraine through the analysis of RNFL and macular and choroidal changes (Table 1).
Vascular Involvement in Migraine
Previous studies suggested that brain hypoperfusion is common in MwA (31, 32), probably because of hypercoagulability and altered endothelial and vascular smooth muscle (33). Although the underlying pathophysiological mechanisms of migraine are not fully understood and many hypotheses have been proposed (31, 34–37), there is increasing evidence that the neurovascular system is involved (10, 31–40). Migraine attacks affect the trigeminal vascular system (TGVS), including the network of intra- and extracranial meningeal blood vessels and ocular structures (41, 42), and regulate vascular tone and the transmission of pain signals (43). Following activation of the TGVS, vasoactive neurotransmitters are released from peripheral terminations of the trigeminal nerve, causing vascular and inflammatory changes that result in pain (44). Migraine typically occurs when cerebral blood vessels undergo dramatic changes in caliber (45). The reduction in blood flow—due to the transient cerebral vasospasm that emerges prior to or during pain—is often limited to the posterior area of one hemisphere, leading to cerebral hypoperfusion (46). Alternating episodes of hyperperfusion and hypoperfusion during migraine may be confined to or start in other brain regions or even outside the brain, for example, in the retina and choroid. Even though constriction of cerebral and retrobulbar arteries is a transitory event, the chronic course with repetitive attacks could lead to permanent cerebral and retinal damage (47). In fact, retinal ischemia secondary to retinal artery occlusions has been described in patients with migraine (35, 48–54). Likewise, migraine is a recognized risk factor for ischemic optic neuropathy and NTG (55).
Visual symptoms resulting from occipital cortex involvement are more common than compromise of retinal or choroidal circulation. Therefore, in patients with these symptoms, OCT changes indicate retrograde trans-synaptic neuronal degeneration (RTSD) of the retinal ganglion cells (RGCs).
Although ophthalmologists are often the first specialists to explore patients with migraine, few studies have addressed the involvement of the optic nerve, retina, and choroid in this population.
This update reviews the literature on the etiological and pathological mechanisms of migraine and the relationship between recurrent constriction of cerebral and retrobulbar vessels and ischemic damage to the optic nerve, retina, and choroid in affected patients. It also provides evidence in favor of OCT for measurement of RNFL thickness and macular and choroidal changes in the different variants of migraine, which could be a biomarker of RTSD of the RGCs triggered by a lesion located on the posterior visual pathway.
Methods
We searched PubMed for articles on the usefulness of OCT in patients with CM over a period of 25 years. We identified prospective and retrospective studies and case reports based on the following key words: migraine, optical coherence tomography, OCT, retinal nerve fiber layer, RNFL, retina, choroid, etiology, and physiopathology. We selected only articles written in English.
Optic Nerve Structural Changes in Migraine
Compromised choroidal blood flow can produce focal ischemic damage in the optic disk (55). By using scanning laser polarimetry in patients with migraine (both with and without aura), Tan et al. (37) reported that migraine had no effect on RNFL.
The latest technological developments have made it possible to assess whether the retina is compromised in patients with migraine by using OCT to measure RNFL (Table 1). Decreased RNFL thickness reflects a reduced number of axons in these patients (32). However, results have not been very consistent. Thus, whereas some authors observed that mean peripapillary RNFL thickness was thinner in migraine patients than in healthy controls, others reported only a thinner RNFL in a specific quadrant (16, 18, 32, 56–62). This selective RNFL involvement might be associated with differences in the vulnerability of retinal axons to ischemia and with focal perimetric changes (63). The suspected axonal damage necessitates monitoring of RNFL thickness and visual field testing in migraine patients (62).
Ekinci et al. (64) studied subgroups of MwA and MwoA patients and demonstrated thinner RNFL in all sectors in MwA, but no significant differences in MwoA. The posterior area of a single brain hemisphere usually shows cerebral hypoperfusion during the attack (32). Thus, RNFL parameters may be more altered in MwA than in MwoA (63).
As migraineurs experience headaches almost always on the same side, several authors have studied unilateral involvement. In their investigation of the association between laterality of migraine and RNFL thickness in one-sided headache, Gunes et al. (63) reported a thin RNFL in patients with migraine compared with healthy controls and found that even though thinning was more relevant on the same side of the headache, the asymmetry was not statistically significant. The possibility of more relevant thinning of RNFL on the headache side could be secondary to lateralized permanent cortical changes, indicating that the hemisphere on the side of pain is more altered. Reduced blood flow has also been demonstrated in the brain on the affected side during attacks (62). Moreover, Hougaard et al. (65) explored unilateral headaches and investigated the connection between MwA and permanent gray matter anomalies. Using magnetic resonance imaging, Simsek (62) observed that in migraine patients with white matter lesions (WML), RNFL was thinner than in controls and migraine patients with no WML. Further research should analyze the relationship between brain WML and retinal damage to clarify whether there is a common pathogenic mechanism.
As for the frequency of migraine, some authors (18, 59) did not find any correlation with the RNFL thickness, whereas others reported an inverse correlation between RNFL thickness and the total number of monthly migraine attacks (61).
Peripapillary RNFL thickness measurements could also be associated with the length of migraine history. However, whereas some authors (18, 59) found no correlation between RNFL thickness and length of migraine history, Feng et al. (66) reported significant changes in RNFL based on the length of the history of migraine. Thus, mean RNFL thickness was decreased when migraine history was longer than 15 years. While controversial, this finding could be associated with glaucoma. In fact, Phelps and Corbett (67) observed a higher incidence of low-tension glaucoma in migraineurs. The origin of migraine is associated with the constriction of cerebral and retrobulbar arteries (18). During a migraine attack, vasospasm and reduced blood perfusion are typically demonstrated in a single hemisphere, although other cerebral regions and even retinal layers may also be altered by hypoperfusion (50). Migraine episodes may be linked to diminished blood flow in the retina and optic nerve, leading to irregular ocular perfusion and therefore to ischemia, which are implicated in the progression of glaucoma (32). The chronic nature of migraine, which is characterized by recurrent vasospasms and focal ischemia during attacks, could explain structural optic nerve damage, with the subsequent reduction in peripapillary RNFL thickness (61).
Furthermore, RNFL thickness values may correlate with the severity of headache. An important relationship between mean RNFL thickness and the migraine disability assessment score has been reported (57, 68). Nevertheless, Yülek et al. (60) evaluated the severity of migraine using the visual analog scale (69) and observed that the score was not correlated with RNFL thickness. The discrepancy might be explained by the use of different scoring systems and the diversity and severity of the headache in the study groups (66).
Such discrepant results may be attributed to differences in methodology and sample size, ethnic variations, and the absence of standardized migraine characteristics, including severity, length of migraine history, and frequency of attacks (61). Feng et al. (66) recently performed a meta-analysis of published case–control studies to explore changes in RNFL thickness in migraine patients assessed using OCT and reported lower peripapillary RNFL in patients with migraine than in healthy subjects. Therefore, although unspecific, OCT-based measurements of RNFL in migraine patients could be a useful technique for the study of ocular compromise and for a better understanding of pathogenesis.
Macular Changes in Migraine
Retrograde trans-synaptic neuronal degeneration of the RGCs secondary to damage to structures in the posterior visual pathway has been demonstrated in various settings. Segmentation of retinal layers by spectral-domain OCT has enabled a detailed study of functional areas. This is most useful at the macula, where the density of RGCs is maximal and where other anatomical structures are absent (70, 71). Several authors have used OCT to study possible changes in macular thickness in patients with migraine. Their results are contradictory because, although most found no change in either foveal or macular parameters in migraine patients (18, 59, 62), some reported that foveal thickness (FT) values (19) or GCL (61, 64) were thinner in MwA than in MwoA. The more diminished blood flow in MwA would explain this finding, given that in MwoA, at least between attacks, pulsatile choroidal blood flow is not compromised.
Therefore, altered choroidal blood flow can produce ischemic damage in retinal tissue, leading to photoreceptor dysfunction and eventually GC death (32, 72, 73). In fact, MwA patients seem to have a higher risk of ischemic events than MwoA (31, 68).
Finally, no correlation was observed between FT and disease duration/length or number of episodes (72).
Choroidal Changes in Migraine
Choroidal vascular deficiency causes decreased choroidal thickness (CT), which results in dysfunction of retinal pigment epithelium and photoreceptors (74, 75). Choroid thickness may decrease as a consequence of decreased blood flow in retinal and ciliary vessels (31).
Today, it is possible to estimate CT using advanced OCT devices. However, major discrepancies have been observed. Thus, whereas some authors report an increase in CT during attacks, reflecting altered ocular circulation (76, 77), others report reduced CT during attacks (78, 79). Reggio et al. (61) recently reported that CT was thinner in migraineurs than in healthy individuals. Colak et al. (16) found that RNFL and CT were thinner in MwA than in healthy controls, which is secondary to an increasing loss of GC and axons. The disparity between authors could be explained by the fact that both types of migraine involve changes in blood perfusion during their course, albeit with different severity.
Ocular pulse amplitude (OPA) refers to the gap between systolic and diastolic IOP. Measured using dynamic contour tonometry, it is considered an indirect indicator of choroidal blood flow and reflects fluctuations in IOP related to the volume of blood pumped in the interior of the eye during the cardiac cycle (80, 81). Acer et al. (82) estimated choroidal flow by evaluating OPA and were unable to demonstrate relevant dissimilarities in OPA between patients with MwoA and controls, thus revealing that pulsatile choroidal blood flow is not always altered in these subjects. In fact, other studies had previously demonstrated choroidal and retinal involvement in patients with migraine (32, 59, 64).
Amaurosis fugax in migraineurs has been proposed as a sign of affected choroidal blood flow in patients with migraine (83), as have alterations in retinal pigment epithelium on fundoscopy after attacks (84).
Conclusion
The aim of this update was to review the literature on the etiological and pathogenic mechanisms of migraine. We analyze evidence on the role of OCT for measurement of peripapillary RNFL thickness and macular and choroidal changes in migraineurs. We conclude that there is abundant evidence on cerebral and retrobulbar vascular involvement in the etiology of migraine. The transitory and recurrent constriction of the retinal and ciliary arteries may cause ischemic damage to the optic nerve, retina, and choroid in migraineurs. Although OCT-based studies show conflicting results, our data suggest that the retina and choroid are altered in patients with migraine, mainly CM. Moreover, RNFL parameters and FT seem to be more altered in MwA than in MwoA. RTSD of the RGCs and RNFL following injury to the occipital lobe have been reported in experimental studies. Magnetic resonance imaging and OCT could enable us to visualize and quantify RTSD and analyze its time course (85). Therefore, OCT-based measurement of peripapillary RNFL, GCL, and macular and CT might improve our understanding of the pathophysiology of migraine and facilitate diagnosis of retino-choroidal compromise and follow-up of the effectiveness of therapies in migraine patients. Future studies should determine the usefulness of OCT findings as a biomarker of migraine. Finally, migraine is such a heterogeneous disorder that it will be difficult to cluster patients in categories. Treatment (e.g., triptans, ergot derivatives) may also lead to vasoconstriction, which could—theoretically—bring about changes in OCT values.
Author Contributions
FA, JM, SM, MM, OE, and AG: substantial contributions to the conception or design of the work; the acquisition, analysis, and interpretation of data for the work; FA, JM, SM, MM, OE, and AG: drafting the work or revising it critically for important intellectual content; FA, JM, SM, MM, OE, and AG: final approval of the version to be published; FA, JM, SM, MM, OE, and AG: agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Dinkin M. Trans-synaptic retrograde degeneration in the human visual system: slow, silent, and real. Curr Neurol Neurosci Rep (2017) 17:16. doi:10.1007/s11910-017-0725-2
2. Ripa P, Ornello R, Degan D, Tiseo C, Stewart J, Pistoia F, et al. Migraine in menopausal women: a systematic review. Int J Womens Health (2015) 20:773–82. doi:10.2147/IJWH.S70073
3. Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci (2003) 4:386–98. doi:10.1038/nrn1102
4. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry (1975) 38:613–6. doi:10.1136/jnnp.38.6.613
5. Friberg L, Olesen J, Lassen NA, Olsen TS, Karle A. Cerebral oxygen extraction, oxygen consumption, and regional cerebral blood flow during the aura phase of migraine. Stroke (1994) 25:974–9. doi:10.1161/01.STR.25.5.974
6. Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Headache (2013) 53:227–9. doi:10.1111/head.12034
7. Malone CD, Bhowmick A, Wachholtz AB. Migraine: treatments, comorbidities, and quality of life, in the USA. J Pain Res (2015) 12:537–47. doi:10.2147/JPR.S88207
8. The International Classification of Headache Disorders. 2nd edition. Headache Classification Subcommittee of the International Headache Society. Cephalalgia (2004) 24:9–160.
9. Sharma P, Sridhar J, Mehta S. Flashes and floaters. Prim Care (2015) 42:425–35. doi:10.1016/j.pop.2015.05.011
10. Charles A, Hansen JM. Migraine-aura: new ideas about cause, classification and clinical significance. Curr Opin Neurol (2015) 28:255–60. doi:10.1097/WCO.0000000000000193
11. McKendrick AM, Vingrys AJ, Badcock DR, Heywood JT. Visual field losses in subjects with migraine headaches. Invest Ophthalmol Vis Sci (2000) 41:1239–47.
12. Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol (2001) 131:699–708. doi:10.1016/S0002-9394(01)00964-3
13. Demircan S, Ataş M, Arık Yüksel S, Ulusoy MD, Yuvacı İ, Arifoğlu HB, et al. The impact of migraine on posterior ocular structures. J Ophthalmol (2015) 2015:868967. doi:10.1155/2015/868967
14. Cankaya C, Tecellioglu M. Foveal thickness alterations in patients with migraine. Med Arch (2016) 70:123–6. doi:10.5455/medarh.2016.70.123-126
15. Koban Y, Ozlece HK, Bilgin G, Koc M, Cagatay HH, Durgunlu EI, et al. Intraocular pressure and ocular biometric parameters changes in migraine. BMC Ophthalmol (2016) 16:70. doi:10.1186/s12886-016-0258-5
16. Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics (2011) 8:117–32. doi:10.1007/s13311-010-0005-1
17. Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in Alzheimer disease. J Neuroophthalmol (2013) 33:58–61. doi:10.1097/WNO.0b013e318267fd5f
18. Monterio ML, Fermandes DB, Apóstolos-Pereira SL, Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci (2012) 53:3959–66. doi:10.1167/iovs.11-9324
19. Colak HN, Kantarcı FA, Tatar MG, Eryilmaz M, Uslu H, Goker H, et al. Retinal nerve fiber layer, ganglion cell complex, and choroidal thicknesses in migraine. Arq Bras Oftalmol (2016) 79:78–81. doi:10.5935/0004-2749.20160024
20. Kardon RH. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J Neuroophthalmol (2011) 31:353–61. doi:10.1097/WNO.0b013e318238b9cb
21. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science (1991) 254:1178–81. doi:10.1126/science.1957169
22. Drexler W, Sattmann H, Hermann B, Ko TH, Stur M, Unterhuber A, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol (2003) 121:695–706. doi:10.1001/archopht.121.5.695
23. Fercher AF. Optical coherence tomography – development, principles, applications. Z Med Phys (2010) 20:251–76. doi:10.1016/j.zemedi.2009.11.002
24. Yang Z, Tatham AJ, Zangwill LM, Weinreb RN, Zhang C, Medeiros FA. Diagnostic ability of retinal nerve fiber layer imaging by swept-source optical coherence tomography in glaucoma. Am J Ophthalmol (2015) 159:193–201. doi:10.1016/j.ajo.2014.10.019
25. Sung KR, Wollstein G, Kim NR, Na JH, Nevins JE, Kim CY, et al. Macular assessment using optical coherence tomography for glaucoma diagnosis. Br J Ophthalmol (2012) 96:1452–5. doi:10.1136/bjophthalmol-2012-301845
26. Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci (1999) 40:2520–7.
27. Iseri PK, Altinas O, Tokay T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol (2006) 26:18–24. doi:10.1097/01.wno.0000204645.56873.26
28. Yu JG, Feng YF, Xiang Y, Huang JH, Savini G, Parisi V, et al. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One (2014) 9:e85718. doi:10.1371/journal.pone.0085718
29. Ascaso FJ, Rodriguez-Jimenez R, Cabezón L, López-Antón R, Santabárbara J, De la Cámara C, et al. Retinal nerve fiber layer and macular thickness in patients with schizophrenia: influence of recent illness episodes. Psychiatry Res (2015) 229:230–6. doi:10.1016/j.psychres.2015.07.028
30. Casas P, Ascaso FJ, Vicente E, Tejero-Garcés G, Adiego MI, Cristóbal JA. Retinal and optic nerve evaluation by optical coherence tomography in adults with obstructive sleep apnea-hypopnea syndrome (OSAHS). Graefes Arch Clin Exp Ophthalmol (2013) 251:1625–34. doi:10.1007/s00417-013-2268-9
31. Sacco S, Ricci S, Carolei A. Migraine and vascular diseases: a review of the evidence and potential implications for management. Cephalalgia (2012) 32:785–95. doi:10.1177/0333102412451361
32. Martinez A, Proupim N, Sanchez M. Retinal nerve fibre layer thickness measurements using optical coherence tomography in migraine patients. Br J Ophthalmol (2008) 92:1069–75. doi:10.1136/bjo.2008.137471
33. Larrosa-Campo D, Ramón-Carbajo C, Para-Prieto M, Calleja-Puerta S, Cernuda-Morollón E, Pascual J. Migraine as a vascular risk factor. Rev Neurol (2012) 55:349–58.
34. Gonçalves FM, Luizon MR, Speciali JG. Haplotypes in candidate genes related to nitric oxide pathway and vascular permeability associated with migraine and aura. J Headache Pain (2012) 13:335–6. doi:10.1007/s10194-012-0438-5
35. Tzourio C, Iglesias S, Hubert JB, Visy JM, Alpérovitch A, Tehindrazanarivelo A, et al. Migraine and risk of ischaemic stroke: a case-control study. BMJ (1993) 307:289–92. doi:10.1136/bmj.307.6899.289
36. Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia (2004) 24:2–7. doi:10.1111/j.1468-2982.2004.00892.x
37. Tan FU, Akarsu C, Gullu R. Retinal nerve fiber layer thickness is unaffected in migraine patients. Acta Neurol Scand (2005) 112:19–23. doi:10.1111/j.1600-0404.2005.00423.x
38. Goadsby PJ. Pathophysiology of migraine. Neurol Clin (2009) 27:335–60. doi:10.1016/j.ncl.2008.11.012
39. Perko D, Pretnar-Oblak J, Šabovič M, Zaletel M, Žvan B. Associations between cerebral and systemic endothelial function in migraine patients: a post-hoc study. BMC Neurol (2011) 11:146. doi:10.1186/1471-2377-11-146
40. Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology (2007) 68:1563–70. doi:10.1212/01.wnl.0000260964.28393.ed
41. Aguggia M, Saracco MG, Cavallini M, Bussone G, Cortelli P. Sensitization and pain. Neurol Sci (2013) 34:S37–40. doi:10.1007/s10072-013-1382-0
42. Friedman DI. The eye and headache. Continuum (2015) 21(4 Headache):1109–17. doi:10.1212/CON.0000000000000204
43. Russo A, Tessitore A, Tedeschi G. Migraine and trigeminal system-I can feel it coming. Curr Pain Headache Rep (2013) 17:367. doi:10.1007/s11916-013-0367-2
44. Ayata C. Cortical spreading depression triggers migraine attack pro. Headache (2010) 50:725–30. doi:10.1111/j.1526-4610.2010.01647.x
45. Kara SA, Erdemoglu AK, Karadeniz MY, Altinok D. Colour Doppler sonography of orbital and vertebral arteries in migraineurs without aura. J Clin Ultrasound (2003) 31:308–14. doi:10.1002/jcu.10181
46. Wang SJ. Epidemiology of migraine and other types of headache in Asia. Curr Neurol Neurosci Rep (2003) 3:104–8. doi:10.1007/s11910-003-0060-7
47. Schwedt TJ, Chiang CC, Chong CD, Dodick DW. Functional MRI of migraine. Lancet Neurol (2015) 14:81–91. doi:10.1016/S1474-4422(14)70193-0
49. Abdul-Rahman AM, Gilhotra JS, Selva D. Dynamic focal retinal arteriolar vasospasm in migraine. Indian J Ophthalmol (2011) 59:51–3. doi:10.4103/0301-4738.73717
50. Killer HE, Forrer A, Flammer J. Retinal vasospasm during an attack of migraine. Retina (2003) 23:253–4. doi:10.1097/00006982-200304000-00023
51. Banik S, Bhutto HU, Bagga P. Recurrent branch retinal vein occlusion with factor V Leiden mutation. Eye (Lond) (2006) 20:948–9. doi:10.1038/sj.eye.6702060
52. Agostoni E, Rigamonti A. Migraine and small vessel diseases. Neurol Sci (2012) 33:51–4. doi:10.1007/s10072-012-1041-x
53. Hykin PG, Gartry D, Brazier DJ, Graham E. Bilateral cilio-retinal artery occlusion in classic migraine. Postgrad Med J (1991) 67:282–4. doi:10.1136/pgmj.67.785.282
54. Beversdorf D, Stommel E, Allen C, Stevens R, Lessell S. Recurrent branch retinal infarcts in association with migraine. Headache (1997) 37:396–9. doi:10.1046/j.1526-4610.1997.3706396.x
55. Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res (2001) 20:319–49. doi:10.1016/S1350-9462(00)00028-8
56. Martínez A, Proupim N, Sanchez M. Scanning laser polarimetry with variable corneal compensation in migraine patients. Acta Ophthalmol (2009) 87:746–53. doi:10.1111/j.1755-3768.2008.01356.x
57. Sorkhabi R, Mostafaei S, Ahoor M, Talebi M. Evaluation of retinal nerve fiber layer thickness in migraine. Iran J Neurol (2013) 12:51–5.
58. Kirbas S, Tufekci A, Turkyilmaz K, Kirbas A, Oner V, Durmus M. Evaluation of the retinal changes in patients with chronic migraine. Acta Neurol Belg (2013) 113:167–72. doi:10.1007/s13760-012-0150-x
59. Gipponi S, Scaroni N, Venturelli E, Forbice E, Rao R, Liberini P, et al. Reduction in retinal nerve fiber layer thickness in migraine patients. Neurol Sci (2013) 34:841–5. doi:10.1007/s10072-012-1103-0
60. Yülek F, Dirik EB, Eren Y, Simavlı H, Ugurlu N, Cagil N, et al. Macula and retinal nerve fiber layer in migraine patients: analysis by spectral domain optic coherence tomography. Semin Ophthalmol (2015) 30:124–8. doi:10.3109/08820538.2013.833270
61. Reggio E, Chisari CG, Ferrigno G, Patti F, Donzuso G, Sciacca G, et al. Migraine causes retinal and choroidal structural changes: evaluation with ocular coherence tomography. J Neurol (2017) 264:494–502. doi:10.1007/s00415-016-8364-0
62. Simsek IB. Retinal nerve fibre layer thickness of migraine patients with or without white matter lesions. Neuroophthalmology (2016) 41:7–11. doi:10.1080/01658107.2016.1243131
63. Gunes A, Demirci S, Tok L, Tok O, Demirci S, Kutluhan S. Is retinal nerve fiber layer thickness change related to headache lateralization in migraine? Korean J Ophthalmol (2016) 30:134–9. doi:10.3341/kjo.2016.30.2.134
64. Ekinci M, Ceylan E, Cagatay HH, Keles S, Hüseyinoglu N, Tanyildiz B, et al. Retinal nerve fibre layer, ganglion cell layer and choroid thinning in migraine with aura. BMC Ophthalmol (2014) 14:75. doi:10.1186/1471-2415-14-75
65. Hougaard A, Amin FM, Hoffmann MB, Larsson HB, Magon S, Sprenger T, et al. Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia (2015) 35:3–9. doi:10.1177/0333102414532378
66. Feng YF, Guo H, Huang JH, Yu JG, Yuan F. Retinal nerve fiber layer thickness changes in migraine: a meta-analysis of case-control studies. Curr Eye Res (2016) 41:814–22. doi:10.3109/02713683.2015.1056373
67. Phelps CD, Corbett JJ. Migraine and low-tension glaucoma. A case-control study. Invest Ophthalmol Vis Sci (1985) 26:1105–8.
68. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache related disability. Neurology (2001) 56:S20–8. doi:10.1212/WNL.56.suppl_1.S20
69. Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health (1990) 13:227–36. doi:10.1002/nur.4770130405
70. Keller J, Sánchez-Dalmau BF, Villoslada P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion cells. PLoS One (2014) 9:e97444. doi:10.1371/journal.pone.0097444
71. Gupta S, Zivadinov R, Ramanathan M, Weinstock-Guttman B. Optical coherence tomography and neurodegeneration: are eyes the windows to the brain? Expert Rev Neurother (2016) 16:765–75. doi:10.1080/14737175.2016.1180978
72. Shiragami C, Shiraga F, Matsuo T, Tsuchida Y, Ohtsuki H. Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol (2002) 240:436–42. doi:10.1007/s00417-002-0451-5
73. Cohen AS, Goadsby PJ. Functional neuroimaging of primary headache disorders. Curr Pain Headache Rep (2005) 9:141–6. doi:10.1007/s11916-005-0053-0
74. Osmanbasoglu OA, Alkin Z, Ozkaya A, Ozpınar Y, Yazici AT, Demirok A. Diurnal choroidal thickness changes in normal eyes of Turkish people measured by spectral domain optical coherence tomography. J Ophthalmol (2013) 2013:687165. doi:10.1155/2013/687165
75. Lee SW, Yu SY, Seo KH, Kim ES, Kwak HW. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy Korean subjects. Retina (2014) 34:385–93. doi:10.1097/IAE.0b013e3182993f29
76. Dadaci Z, Doganay F, Acir NO, Aydin HD, Borazan M. Enhanced depth imaging optical coherence tomography of the choroid in migraine patients: implications for the association of migraine and glaucoma. Br J Ophthalmol (2014) 98:972–5. doi:10.1136/bjophthalmol-2013-304711
77. Karalezli A, Simsek C, Celik G, Eroglu FC. Evaluation of choroidal thickness using spectral-domain optical coherence tomography in migraine patients during acute migraine attacks: a comparative study. Eye (Lond) (2014) 28:1477–81. doi:10.1038/eye.2014.218
78. Zengin MO, Elmas Z, Cinar E, Kucukerdonmez C. Choroidal thickness changes in patients with migraine. Acta Neurol Belg (2015) 115:33–7. doi:10.1007/s13760-014-0301-3
79. Dervisogullari MS, Totan Y, Gençler OS. Choroid thickness and ocular pulse amplitude in migraine during attack. Eye (2015) 29:371–5. doi:10.1038/eye.2015.4
80. Schwenn O, Troost R, Vogel A, Grus F, Beck S, Pfeiffer N. Ocular pulse amplitude in patients with open angle glaucoma, normal tension glaucoma, and ocular hypertension. Br J Ophthalmol (2002) 86:981–4. doi:10.1136/bjo.86.9.981
81. Knecht PB, Menghini M, Bachmann LM, Baumgartner RW, Landau K. The ocular pulse amplitude as a noninvasive parameter for carotid artery stenosis screening: a test accuracy study. Ophthalmology (2012) 119:1244–9. doi:10.1016/j.ophtha.2011.12.040
82. Acer S, Oğuzhanoğlu A, Çetin EN, Ongun N, Pekel G, Kaşıkçı A, et al. Ocular pulse amplitude and retina nerve fiber layer thickness in migraine patients without aura. BMC Ophthalmol (2016) 16:1. doi:10.1186/s12886-015-0180-2
83. O’Sullivan F, Rossor M, Elston JS. Amaurosis fugax in young people. Br J Ophthalmol (1992) 76:660–2. doi:10.1136/bjo.76.11.660
84. Connor RC. Complicated migraine. A study of permanent neurological and visual defects caused by migraine. Lancet (1962) 2:1072–5. doi:10.1016/S0140-6736(62)90782-1
Keywords: migraine, optical coherence tomography, retinal nerve fiber layer, retina, choroid
Citation: Ascaso FJ, Marco S, Mateo J, Martínez M, Esteban O and Grzybowski A (2017) Optical Coherence Tomography in Patients with Chronic Migraine: Literature Review and Update. Front. Neurol. 8:684. doi: 10.3389/fneur.2017.00684
Received: 19 August 2017; Accepted: 30 November 2017;
Published: 13 December 2017
Edited by:
Heather Moss, Stanford University, United StatesReviewed by:
Jorge Kattah, University of Illinois College of Medicine Peoria, United StatesLisanne J. Balk, VU University Medical Center, Netherlands
Copyright: © 2017 Ascaso, Marco, Mateo, Martínez, Esteban and Grzybowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrzej Grzybowski, ae.grzybowski@gmail.com
 Francisco J. Ascaso
Francisco J. Ascaso Sara Marco
Sara Marco Javier Mateo
Javier Mateo Mireya Martínez1
Mireya Martínez1 Olivia Esteban
Olivia Esteban Andrzej Grzybowski
Andrzej Grzybowski