- 1Department of Neurology, University of Utah, Salt Lake City, UT, United States
- 2Department of Neurology, Mayo Clinic, Rochester, MN, United States
- 3Department of Radiology, University of Utah, Salt Lake City, UT, United States
Objective: To determine whether Parkinson disease (PD) patients with (VH) have different clinical characteristics and gray-matter volume than those with visual misperceptions (VM) or other visual symptoms (OvS).
Background: The spectrum of visual complaints in PD is broad and complex.
Methods: We conducted a retrospective chart review of 525 PD patients to identify the frequency of visual symptoms and the association with clinical and radiological features. Brain volumetric MRI data was analyzed using multivariate logistic regression to differentiate cases with and without visual symptoms.
Results: Among 525 PD cases, visual complaints were documented in 177 (33.7%). Among these, 83 (46.9%) had VH, 31 (17.5%) had VM, and 63 (35.6%) had OvS (diplopia, blurry vision, photophobia, dry eyes, and eye pain or soreness). When compared to OvS, patients with VH had significantly higher age, duration of disease, rate of REM sleep behavior disorder, and cognitive impairment. Visual hallucinations patients had decreased age-adjusted volumetric averages in 28/30 gray-matter regions when compared to PD without visual symptoms and 30/30 gray-matter regions when compared to VM patients.
Conclusions: Visual symptoms in PD may represent a spectrum from OvS to VM to VH, with progression of the latter associated with older age, duration of disease, presence of REM sleep behavior disorder, cognitive impairment, and decreased gray-matter volume.
Introduction
Parkinson disease (PD) is a degenerative disorder that recognizes the abnormal aggregation and deposition of alpha-synuclein in the nervous system as the pathological hallmark. Classically, the primary target of degeneration was presumed to be the basal ganglia, causing predominantly motor disturbances. However, it is now evident that non-motor symptoms (NMS) such as autonomic dysfunction, sleep disorders, pain and sensory disorders, mood disturbances, and cognitive complaints are critical manifestations of the neurodegenerative process causing PD (1, 2). NMS of PD have been shown to have a larger negative impact over the health-related quality of life compared to PD-specific motor symptoms (3). Among NMS, visual complaints are a group of symptoms that are common and still poorly understood. Patients with PD may report or further examinations may reveal the presence of blurry vision, double vision, dry eyes, difficulty with contrast recognition, diplopia, visual misperceptions (VM), and visual hallucinations (VH) among others (4, 5). In particular, visual symptoms such as VM and VH are commonly present in alpha-synucleinopathies such as Dementia with Lewy Bodies (DLB) or Parkinson's disease Dementia (PDD) along with idiopathic PD patients. Interestingly, alpha-synuclein has been also reported to be deposited in the retina (6). Deterioration of visual function could possibly be caused by deficiencies of dopamine in the retina, unusual eye movements, decreased blinking rate or increased frequency of nuclear, and posterior sub-capsular cataract in the PD patients (5). This further highlights the role of visual symptoms as a possible clinical biomarker (7–9).
The clinical definition and diagnosis of VM and VH is still challenging. Although VM are considered benign phenomena (9), it is not clear whether they are harbingers for the future development of VH or represent a separate disease phenotype.
Although a number of studies have explored VM and VH, there are not clear data comparing the clinical, radiographic, and phenotypic characteristics of VH with VM. Indeed, a number of studies have explored the volumetric differences of cortical and subcortical structures in PD (10, 11). Nevertheless, the differences in the cortical and subcortical atrophy in PD patients with VM and VH have not been clearly defined. The aim of our study is to fill in this gap of knowledge by studying the clinical and phenotypic characteristics of PD cases affected by VM and VH and determining whether the presence of VM and VH alone or in conjunction with MRI volumetric measures can predict the progression of disease.
Methods
Ascertainment
We performed a retrospective chart review to identify all the patients affected with visual symptoms in PD cases. Patients were identified from a movement disorder clinic at the University of Utah Hospital and Clinics in Salt Lake City, Utah; the sole movement disorder center in a 5-state area. Our study was approved by the University of Utah's Institutional Review Board. We only included patients that were diagnosed with PD by a movement disorder specialist (DS) based on the Queen Square Brain Bank criteria (12). We reviewed the medical records of 572 PD patients seen by three providers (DS, MZ, and RS) from January 1st 2011 to December 31st 2014 searching for the presence of NMS (including hallucinations) in the clinical history. The clinical history template was a consistent collection of clinical questions been developed to ensure the completeness of clinical information. These included all motor and NMS. Exclusion criteria included charts with insufficient information, patients lost to follow-up, or patients with an unclear diagnosis. Forty-seven charts met the exclusion criteria, leaving 525 patients in our final sample. The inclusion criteria involved any PD patient who had experienced a visual symptom in one of their last three clinic visits. A neurologist (KJS) reviewed the 525 medical records to determine which patients met the inclusion criteria. One hundred and seventy-seven patients reported visual complaints, which included blurred vision, dry eyes, double vision, VH, or VM. We collected demographic information and clinical characteristics of each patient with PD and visual complaints [age, sex, age-of-onset, type of visual complaints, RBD, cognitive complaints, and use of dopamine agonists (Das)]. We took advantage of the data collected in the medical records of the movement disorders clinic that specifically addressed cognitive complaints, visual complaints, psychiatric complaints, and RBD (defined using the Mayo Clinic Questionnaire) (13). When available, the Montreal Cognitive Assessment (MoCA) was used to define and characterize the presence of cognitive complaints (MoCa score < 26); if unavailable, we used the clinical diagnosis determined by the clinician.
We differentiated between hallucinations and VM (or illusions) by using a structured set of questions that were adopted in the review of the medical records. Hallucinations were defined as a perception of an object (in this case visual) in the absence of an external stimulus (14). In comparison, VM involved identifiable external stimuli (in this case visual) that were integrated incorrectly resulting in a transient phenomenon including shadows, movement, presence, flashing figures, distortions, or corner of eye phenomena. If visual symptoms were only reported in the setting of acute delirium due to a medical illness, they were excluded from the study. We excluded all the cases that reported cataracts diagnosis, cataract surgery, macular degeneration, or had a sudden decline of visual acuity secondary to trauma, presbyopia, or other ophthalmologic reasons.
We also reviewed the available brain MRIs in order to study the volumetric structural changes among the patients with different visual symptoms. Eighty two of our 177 patients (46.3%) had a brain MRI in our hospital and clinics or at an outside institution and were scanned into PACS (picture archiving and communication system). The MRI was performed according to the clinician judgment for clinical or diagnostic reasons. In a minority of cases, MRI scan included a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence with spatial resolution of 2 mm isotropic voxels or better. Among patients in our sample, we obtained MRI scans of sufficient quality for volumetric analysis from 11 patients with VH, 4 patients with VM, and 20 patients without VH or misperceptions.
Cortical reconstruction and volumetric segmentation was performed with the FreeSurfer Image Analysis Suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (15, 16). Briefly, this processing includes motion correction and averaging of multiple volumetric T1-weighted images (when more than one is available), removal of non-brain tissue using a hybrid watershed/surface deformation procedure, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) (15, 16), intensity normalization (17), tessellation of the gray matter-white matter boundary, automated topology correction (18), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (19). This was followed by parcellation of the cerebral cortex into units with respect to gyral and sulcal structure using the Destrieux atlas (20).
Statistical Analysis
Consistent with our study design, we calculated median, 25th percentile, and 75th percentile—as well as mean and standard deviation—for continuous variables, and frequency and percent for categorical variables. To compare groups, we report p-values from the Mann–Whitney U-test (continuous variables) and Fisher's exact test (categorical variables). Multivariate logistic regression was performed to explore the correlation of age, age at disease onset, duration of disease, RBD, cognitive impairment, and DAs between clinical groups while adjusting for covariate gender. To avoid collinearity, we did not include covariates age, age at disease onset, or duration of disease simultaneously in a logistic regression model. All statistical analyses were performed at the conventional two-tailed alpha level of 0.05 using Stata-12.1 statistical software (StataCorp LP). Brain gray matter volumetric measurements were compared between groups using a two-tailed t-test in Matlab software package (Mathworks).
Ethics Approval
The Institutional Review Board of the University of Utah approved this study. Patients (or their representatives) gave written consent for the use of their medical information.
Results
Demographics
From the 525 charts reviewed 177 (33.7%) met inclusion criteria for having visual symptoms. Table 1 shows the prevalence of visual complaints in our PD sample. 83 (15.8%) patients had VH, 31 (5.9%) patients had VM and 63 (12%) patients had other visual symptoms (OvS) that included diplopia, blurry vision, photophobia, dry eyes, and eye pain or soreness.
The demographic characteristics of our PD sample are summarized in Table 2; 123 (69.5%) were male. Median age of PD onset was 60 (IR: 53–68) years old without any significant differences across the three groups. The median age of patients with VH was 73 [p < 0.0039; Interquartile Range (IR): 68–78], VM was 71 (IR: 62–77) and with OvS was 65 (p < 0.003; IR: 58–74). Median PD duration was 9 (IR: 5–14) years in the VH, 7 (IR: 4–11) in VM, and 7 (IR: 4–10) in the OvS group. Those with VH compared to VM had an older median age at onset (64 vs. 63 years), longer median duration of disease (9 vs. 7 years). No significant difference was observed between the three groups regarding Dopamine agonists.
RBD and Cognitive Decline
OvS patients had a lower frequency of RBD (38.1%) as compared with the two other groups (p = 0.008). Those with VH and VM had a higher frequency of RBD as compared with OvS (60.2 vs. 58.1%) (p = 0.050).
Cognitive decline was different across the three groups (p = 0.003); in particular, OvS patients had a lower frequency of cognitive decline compared with the two other groups (p = 0.008). On the other hand, the patients with VH had a higher frequency of cognitive decline compared with the other two groups (p = 0.039).
In addition, we observed that the presence of RBD is associated with higher odds of developing VH rather than OvS (p = 0.012). In addition, cognitive complaints were more present in patients with VM and VH when compared with OvS, respectively (p = 0.047 and p = 0.005), but there was not a significant difference between VM and VH (p = 1.00).
Comparison Between Each Group of Patients With Visual Symptoms (VH vs. VM vs. OvS)
We further compared the groups individually to each other. Patients with VH had a significantly older age than patient with OvS (p = 0.001), but there was not a significant difference between VH and VM (p = 0.2926).
The age of onset of PD was not different across the different groups. On the contrary, the patients with VH had a longer duration of PD compared with OvS (p = 0.0073) but not with VM patients (0.3951).
Volumetric Comparison of Brain MRI Between Groups
Eighty two of our sample patients (14.9%) had clinically indicated brain MRI. Those with illusions were more likely than those with hallucinations to have had MRI (60.9 vs. 25.9%). For volumetric analysis, we included only MRI scans performed using a standard MPRAGE sequence at our institution for internal consistency of methodology. Only 15 brain MRI studies from our sample were obtained: 8 from patients with VH, 4 from patients with VM and 3 from patients with both VH and VM. These were compared to a control group of 20 MRIs from patients with PD who had no visual symptoms.
Given the small sample size, volumetric differences were not significant with false discovery rate multiple comparison corrections across brain regions. Nevertheless, the spatial distribution of volumetric changes was informative, particularly for the patient cohort with VH. Cortical volumes were decreased relative to control patients with Parkinson's disease but without VH or misperceptions for most cortical regions (Figure 1). Cortical volume loss was greatest along the intraparietal sulcus and precuneus bilaterally, as well as in the bilateral cerebellar hemisphere. Smaller differences in cortical volumes were noted in patients with VM (Figure 2). No significant differences were noted in the presence of visual symptoms using different antiparkinsonian agents; however, the vast majority of our patients used levodopa at maximum effective doses rather than other dopaminergic drugs.
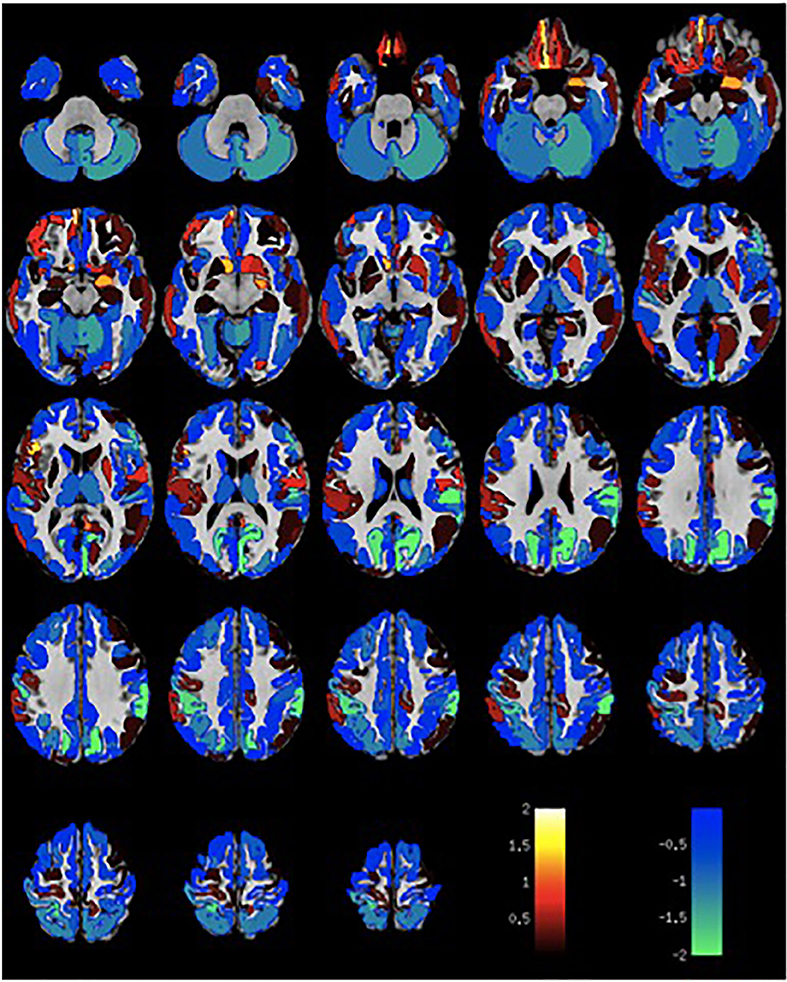
Figure 1. Differences in gray matter volume in patients with visual hallucinations and Parkinson Disease compared to patients with Parkinson Disease without hallucinations or misperceptions. Color scale shows t-statistic for differences in gray matter volume in cortical regions of the Destrieux atlas and 14 subcortical regions with automated FreeSurfer segmentation.
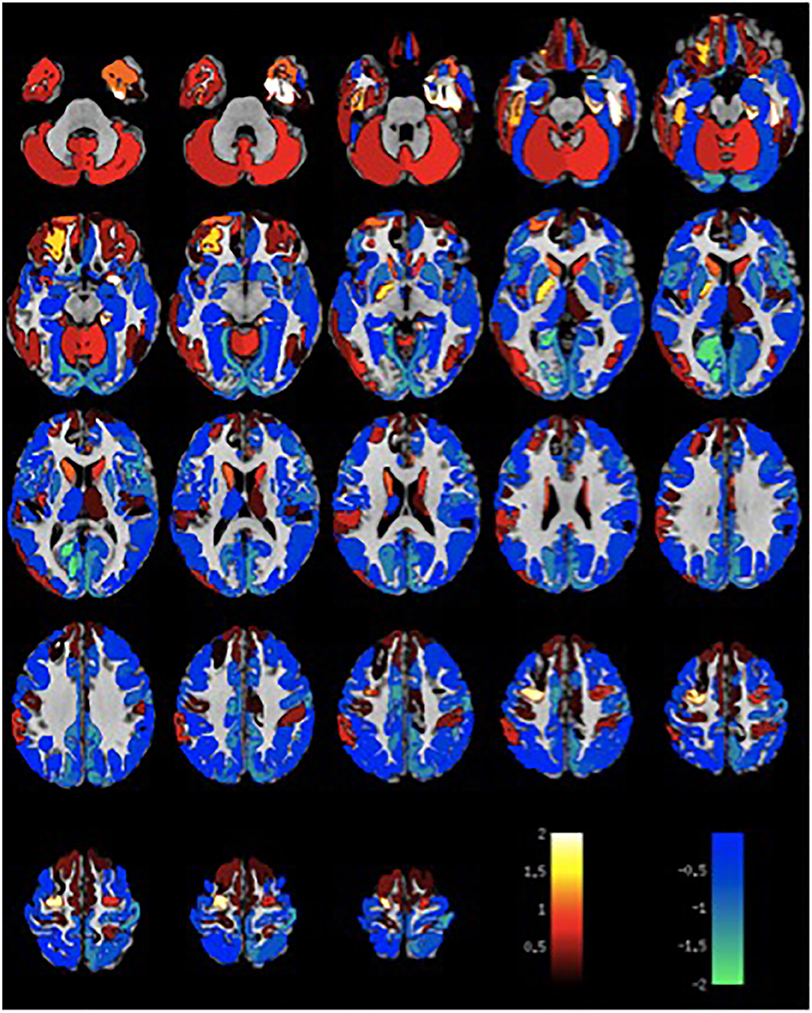
Figure 2. Differences in gray matter volume in patients with visual misperceptions and Parkinson Disease compared to patients with Parkinson Disease without hallucinations or misperceptions. Color scale shows t-statistic for differences in gray matter volume in cortical regions of the Destrieux atlas and 14 subcortical regions with automated FreeSurfer segmentation.
Discussion
Our study assessed the clinical and radiographic characteristics of visual symptoms (VH, VM, and OvS) in a clinical series of PD patients from a tertiary movement disorder center. Our findings highlight the diversity of visual symptoms in PD and underscore the complex interaction between visual symptoms and several demographic and clinical NMS of PD. In particular, the patients with PD affected by hallucinations and misperceptions were more likely to have RBD. This supports the strong association of RBD with alpha-synuclein deposition and predates the onset of PD for several decades; therefore it would be expected to be found to a similar extent in all alpha-synucleinopathies (21, 22). In addition, our findings may indicate that patients presenting both RBD with VM and VH represent a more aggressive phenotype of alpha-synucleinopathy. Clinical differences were less evident between VH and VM; both groups had a significantly longer duration of PD as well as increased presence of RBD and cognitive complaints compared to those with OvS. The small number of patients in the VM group somewhat limited the ability to draw statistical or clinically significant differences. No significant difference in medications was observed in the three groups as well there was no difference regarding the on/off status of the cases affected by PD.
A number of studies have explored the role of visual symptoms in PD (23–25). The degeneration of dopaminergic neurons occurring in the substantia nigra seems also to occur in dopaminergic neurons of the retina, where it functions as a modulator guiding visual signal transmission (26). Visual symptoms stem from two different processes. There are basic visual processes including visual acuity, spatial contrast sensitivity, and color discrimination, which are more likely affected by retinal processes, as well as higher-order visuoperceptual systems: visuospatial processing, visuospatial problem solving, and spatial working memory, which are involved with the degeneration of central dopaminergic pathways (nigrostriatal, mesolimbic, and mesocortical) (27).
The mechanism of VH in PD is still unclear, but traditionally VH have been thought to result as a side effect of dopaminergic or even cholinergic or serotonergic medications (28). However, it is also hypothesized that VH closely correlate with impaired visual acuity. It is hypothesized that poor visual acuity is a risk factor for VH, and if this is the mechanism, proper corrections can be made to stop Parkinson's patients' VH (28).
From a pathophysiology standpoint, many different theoretical models have been postulated to explain the development of VH in PD (29); on the other hand, many of the different theories are sharing attentional and perceptual impairment as common affected functions of VH in PD (30). The role of attentional-control networks has been explored with functional MRI studies, showing the importance of the interplay between the different subdivisions of the network in patients that develop VH in PD (31).
While the later involvement of central dopaminergic pathways correlates with progression to Braak stage 3 (or 4), the involvement of the retinal dopaminergic pathway is not explained by Braak hypothesis (32). This divergent neuro-anatomic involvement is manifest in the wide variety of visual complaints reported in PD. For example, VH are most consistent with involvement of central dopaminergic pathways, due to Lewy-body pathology and nerve cell loss in the ventral-temporal regions of the brain (33). Interestingly, evidence suggests that structural changes in the fovea of patients with PD with retinal optical imaging (OCT) showed morphological changes of volume loss in the retina such as retinal thinning (8, 34, 35). To our knowledge, VM have never been systematically compared to VH in Parkinson's disease, although VM have been studied using different nosology nomenclature (minor hallucinations) that are considered a form of hallucination rather than a misidentification of a stimulus (36, 37).
The frequency of visual symptoms that we report in our study is much lower than previously described. The presence of VH in PD has been reported with an extremely wide range of occurrence from 6 to 87% (38); however, some recent studies observed that up to 50% of patients had VH (33, 38). This variability in the prevalence of VH reflects the uncertainty in the clinical definition and the diagnostic accuracy of VH. As evidence, Williams et al. found that the prevalence of hallucinations nearly doubled with the use of a structured review (39). Therefore, the low prevalence of VH in our study (15.8%) may be due to the lack of a structured interview.
In our study, we observed a number of differences between VH, VM, and OvS. Based on the different clinical characteristics found in the VH and OvS groups, we support OvS as a distinct entity from VH. Though there were some significant differences between VM and OvS, VM and VH had fewer recognizable differences. Unfortunately the small sample size of patients with VM prevents us from drawing firm conclusions; however, VM seem to represent an intermediate stage between the two other samples. A number of studies suggest that the presence of VM may be a more specific symptom of PD as compared to the presence of VH. It has been reported that visual illusions (synonymous with VM) were statistically more likely to occur in PD vs. control (17 vs. 0%), respectively, whereas simple VH were not (10.2 vs. 8.9%) (38). The benefit of studying VM as opposed to VH is the lower likelihood of VM being a side effect secondary to medication, acute medical illness, or a primary psychiatric diagnosis.
The identification of alpha-synuclein deposition as a hallmark of PD, PDD, and DLB has obscured the differentiation between these three diseases, raising the question of whether they represent different points along the same continuum.
Structural imaging found that patients with parkinsonism (PD, PDD, and DLB) who experienced delusional misidentification and those with VH had volume loss within attentional regions of the parietal lobe (40) including intraparietal sulcus and precuneus (41). This may suggest that visual symptoms involve disrupted integration of dorsal visual attentional processing or control of attention to visual percepts. A recent study focusing on the neural correlates of minor hallucinations (passage and presence hallucinations) utilizing voxel-based morphometry found that patients with minor hallucinations had more gray-matter volume loss in multiple regions, most dramatically in the precuneus, compared with controls (11). These findings are in line with our results that support a role for the precuneus and the default network in the development of VH. Interestingly, in light of the evidence of structural changes in the fovea and retina of patients with PD, we may speculate that the brain volumetric changes that we observed correspond or correlate with retinal or foveal changes. Typically, VM and VH are characteristic of PD and DLB but can also be seen in other types of parkinsonism, such as Multiple System Atrophy and progressive supranuclear palsy (42). The presence of VH and their relationship with volumetric brain changes seen at the MRI in specific areas of the brain, can help make a differential clinical diagnosis amongst the Lewy body disorders; for example, a possible to fiber tracts connecting the nucleus basalis of Meynert to the cerebral cortex may contribute to VH in PD, possibly due to a loss of cholinergic innervation. Thus, additional mechanisms and hypotheses can be evoked to explain the generation of high-order visual symptoms in PD (43). Further studies are needed to correlate the eye-findings of OCT with the brain structural changes across the different groups of patients with alpha synucleinopathies.
We propose a possible spectrum of progression of disease and severity of visual symptoms that can be observed in patients affected by synucleinopathies. There can be a possible continuum of progression and severity from a specific visual symptoms to formed hallucinations: the visual symptoms may indeed correlated with a more severe and more diffuse deposit of Lewy Bodies in the patients affected by synucleinopathies.
Limitations
Our study has a number of limitations. First, the retrospective nature of the study is prone to recall bias. Thus, it is possible that some information was not reported or elicited by the clinician. The presence of a recall bias and under- reporting of visual information is, unfortunately, an inherent part of the study design and warrants caution in the interpretation of the data. However, over the past 3 years, the clinical notes have become standardized, including key NMS (visual complaints, RBD, cognitive complaints, and DA agonists), improving the data collection. Second, the small number of MRI studies limits the volumetric analysis. Additionally, MRIs were obtained at multiple locations with slightly variable techniques, which frequently were not compatible with post-procurement processing. Prior to 2010, very few MRIs included high spatial resolution sequences needed for volumetric processing. To achieve a homogenously acquired imaging sample, only a relatively small minority of cases could be included; thus, the findings of the volumetric analyses should be considered preliminary, hypothesis-generating, and warranting caution in the interpretation. Further studies are definitively needed, involving multimodal imaging, to differentiate between such different visual symptoms in PD.
Conclusion
Although there is a large spectrum of visual complaints in PD patients, there were some clear clinical distinctions between PD patients with VH and OvS supported by different prevalence of RBD and cognitive complaints as well as a different age of onset and duration. Differences between VH and VM were not statistically significant; rather, VM seemed to be intermediate to VH and OvS in regard to all demographic and clinical features except DA agonists and cognitive complaints. This observation may reflect distinct clinical phenotypes at different stages along the clinical spectrum of alpha synucleinopathies. Future studies are needed to support the difference between VM and VH and the possible role of VM as a predictor of phenotypic characteristics and prognosis of PD.
Ethics Statement
This study was carried out in accordance with the recommendations of the Institutional Review Board of the University of Utah Medical Center with written informed consent from all subjects or their authorized representatives. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of the University of Utah.
Author Contributions
KB data collection, editing, first draft. BB and PT editing and data management. GP data analyses. JA and AM data analyses and cases collection. DS, MZ, and EZ data collection, editing. RS study conception, data analyses, data collection, editing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Department of Neurology University of Utah supported the research reported in this manuscript. The authors retained full independence in the conduct of this research and have no relevant conflicts of interest to report.
Abbreviations
DA, Dopamine agonists; DLB, Dementia with Lewy bodies; IR, Interquartile range; MoCA, Montreal cognitive assessment; MPRAGE, Magnetization prepared rapidly acquired gradient echo; NMS, Non-motor systems; OCT, Retinal optical imaging; OvS, Other visual symptoms; PD, Parkinson disease; PDD, Parkinson disease dementia; RBD, REM sleep behavior disorder; VH, Visual hallucinations; VM, Visual misperceptions.
References
1. Sung VW, Nicholas AP. Nonmotor symptoms in Parkinson's disease: expanding the view of Parkinson's disease beyond a pure motor, pure dopaminergic problem. Neurol Clin. (2013) 31:S1–16. doi: 10.1016/j.ncl.2013.04.013
2. Bayulkem K, Lopez G. Nonmotor fluctuations in Parkinson's disease: clinical spectrum and classification. J Neurol Sci. (2010) 289:89–92. doi: 10.1016/j.jns.2009.08.022
3. Santos-Garcia D, de la Fuente-Fernandez R. Impact of non-motor symptoms on health-related and perceived quality of life in Parkinson's disease. J Neurol Sci. (2013) 332:136–40. doi: 10.1016/j.jns.2013.07.005
4. Hori N, Takamori M, Hirayama M, Watanabe H, Nakamura T, Yamashita F, et al. Pupillary supersensitivity and visual disturbance in Parkinson's disease. Clin Auton Res. (2008) 18:20–7. doi: 10.1007/s10286-008-0453-4
5. Nowacka B, Lubinski W, Honczarenko K, Potemkowski A, Safranow K. Ophthalmological features of Parkinson disease. Med Sci Monit. (2014) 20:2243–9. doi: 10.12659/MSM.890861
6. Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S. alpha-synuclein in the inner retina in parkinson disease. Ann Neurol. (2014) 75:964–6. doi: 10.1002/ana.24182
7. Bodis-Wollner I. Foveal vision is impaired in Parkinson's disease. Parkinsonism Relat Disord. (2013) 19:1–14. doi: 10.1016/j.parkreldis.2012.07.012
8. Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. (2009) 127:737–41. doi: 10.1001/archophthalmol.2009.106
9. Pagonabarraga J, Martinez-Horta S, Fernandez de Bobadilla R, Perez J, Ribosa-Nogue R, Marin J, et al. Minor hallucinations occur in drug-naive Parkinson's disease patients, even from the premotor phase. Mov Disord. (2016) 31:45–52. doi: 10.1002/mds.26432
10. Häussermann P, Boecker H, Förstl H, Granert O, Ceballos-Baumann A, Feurer R, et al. 184. Neuroanatomy of cognition, delusions and visual hallucinations in Lewy body and Parkinson‘s disease dementia. Clin Neurophysiol. (2009) 120:e76. doi: 10.1016/j.clinph.2008.07.182
11. Pagonabarraga J, Soriano-Mas C, Llebaria G, Lopez-Sola M, Pujol J, Kulisevsky J. Neural correlates of minor hallucinations in non-demented patients with Parkinson's disease. Parkinsonism Relat Disord. (2014) 20:290–6. doi: 10.1016/j.parkreldis.2013.11.017
12. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry (1992) 55:181–4. doi: 10.1136/jnnp.55.3.181
13. Boeve BF, Molano JR, Ferman TJ, Lin SC, Bieniek K, Tippmann-Peikert M, et al. Validation of the mayo sleep questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med. (2013) 9:475–80. doi: 10.5664/jcsm.2670
14. Teeple RC, Caplan JP, Stern TA. Visual hallucinations: differential diagnosis and treatment. Prim Care Companion J Clin Psychiatry (2009) 11:26–32. doi: 10.4088/PCC.08r00673
15. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron (2002) 33:341–55. doi: 10.1016/S0896-6273(02)00569-X
16. Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage (2004) 23 (Suppl. 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016
17. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging (1998) 17:87–97. doi: 10.1109/42.668698
18. Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging (2007) 26:518–29. doi: 10.1109/TMI.2006.887364
19. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. (2000) 97:11050–5. doi: 10.1073/pnas.200033797
20. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage (2010) 53:1–15. doi: 10.1016/j.neuroimage.2010.06.010
21. Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. (2013) 14:744–8. doi: 10.1016/j.sleep.2012.10.009
22. McCarter SJ, St Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. (2012) 12:182–92. doi: 10.1007/s11910-012-0253-z
23. Regan BC, Freudenthaler N, Kolle R, Mollon JD, Paulus W. Colour discrimination thresholds in Parkinson's disease: results obtained with a rapid computer-controlled colour vision test. Vision Res. (1998) 38:3427–31. doi: 10.1016/S0042-6989(97)00402-1
24. Sampaio J, Bobrowicz-Campos E, Andre R, Almeida I, Faria P, Januario C, et al. Specific impairment of visual spatial covert attention mechanisms in Parkinson's disease. Neuropsychologia (2011) 49:34–42. doi: 10.1016/j.neuropsychologia.2010.11.002
25. van Laar T, Mosimann UP, Burn DJ. C.1 Visual hallucinations in Parkinson's Disease - cause and treatment. Parkinsonism Relat Disord. (2006) 12:17. doi: 10.1016/S1353-8020(07)70054-8
26. Huang YM, Yin ZQ. Minor retinal degeneration in Parkinson's disease. Med Hypotheses (2011) 76:194–6. doi: 10.1016/j.mehy.2010.09.016
27. Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res. (2005) 45:1285–96. doi: 10.1016/j.visres.2004.11.006
28. Matsui H, Udaka F, Tamura A, Oda M, Kubori T, Nishinaka K, et al. Impaired visual acuity as a risk factor for visual hallucinations in Parkinson's disease. J Geriatr Psychiatry Neurol. (2006) 19:36–40. doi: 10.1177/0891988705284739
29. Muller AJ, Shine JM, Halliday GM, Lewis SJ. Visual hallucinations in Parkinson's disease: theoretical models. Mov Disord. (2014) 29:1591–8. doi: 10.1002/mds.26004
30. Lewis SJ, Shine JM, Duffy S, Halliday G, Naismith SL. Anterior cingulate integrity: executive and neuropsychiatric features in Parkinson's disease. Mov Disord. (2012) 27:1262–7. doi: 10.1002/mds.25104
31. Shine JM, Halliday GM, Gilat M, Matar E, Bolitho SJ, Carlos M, et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson's disease. Hum Brain Mapp. (2014) 35:2206–19. doi: 10.1002/hbm.22321
32. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging (2003) 24:197–211. doi: 10.1016/S0197-4580(02)00065-9
33. Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson's disease: a retrospective autopsy study. Lancet Neurol. (2005) 4:605–10. doi: 10.1016/S1474-4422(05)70146-0
34. Spund B, Ding Y, Liu T, Selesnick I, Glazman S, Shrier EM, et al. Remodeling of the fovea in Parkinson disease. J Neural Trans. (2013) 120:745–53. doi: 10.1007/s00702-012-0909-5
35. Bodis-Wollner I, Miri S, Glazman S. Venturing into the no-man's land of the retina in Parkinson's disease. Mov Disord. (2014) 29:15–22. doi: 10.1002/mds.25741
36. Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain (2000) 123:733–45. doi: 10.1093/brain/123.4.733
37. Shine JM, Halliday GM, Naismith SL, Lewis SJ. Visual misperceptions and hallucinations in Parkinson's disease: dysfunction of attentional control networks? Mov Disord. (2011) 26:2154–9. doi: 10.1002/mds.23896
38. Urwyler P, Nef T, Killen A, Collerton D, Thomas A, Burn D, et al. Visual complaints and visual hallucinations in Parkinson's disease. Parkinsonism Relat Disord. (2014) 20:318–22. doi: 10.1016/j.parkreldis.2013.12.009
39. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson's disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry (2008) 79:652–5. doi: 10.1136/jnnp.2007.124677
40. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. (2002) 3:201–15. doi: 10.1038/nrn755
41. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain (2006) 129:564–83. doi: 10.1093/brain/awl004
42. Bertram K, Williams DR. Visual hallucinations in the differential diagnosis of parkinsonism. J Neurol Neurosurg Psychiatry (2012) 83:448–52. doi: 10.1136/jnnp-2011-300980
Keywords: Parkinson's disease, visual symptoms, visual hallucinations, visual misperceptions, gray-matter volume, REM sleep behavior disorder, cognitive impairment
Citation: Barrell K, Bureau B, Turcano P, Phillips GD, Anderson JS, Malik A, Shprecher D, Zorn M, Zamrini E and Savica R (2018) High-Order Visual Processing, Visual Symptoms, and Visual Hallucinations: A Possible Symptomatic Progression of Parkinson's Disease. Front. Neurol. 9:999. doi: 10.3389/fneur.2018.00999
Received: 20 August 2018; Accepted: 05 November 2018;
Published: 27 November 2018.
Edited by:
Tim Anderson, University of Otago, Christchurch, New ZealandReviewed by:
Gennaro Pagano, King's College London, United KingdomPedro Chana, Universidad de Santiago de Chile, Chile
Copyright © 2018 Barrell, Bureau, Turcano, Phillips, Anderson, Malik, Shprecher, Zorn, Zamrini and Savica. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodolfo Savica, savica.rodolfo@mayo.edu
 Kelsey Barrell1
Kelsey Barrell1 Jeffrey S. Anderson
Jeffrey S. Anderson David Shprecher
David Shprecher Rodolfo Savica
Rodolfo Savica