- 1Department of Neurology, Center of Cerebrovascular Disease, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Neurology, People's Hospital of Deyang City, Deyang, China
Background and purpose: Cerebral microbleeds (CMBs) could contribute to an increased risk of intracerebral hemorrhage in patients with antithrombotic therapy (antiplatelets or anticoagulants). Antithrombotic agents are commonly prescribed to the patients with atrial fibrillation (AF) and/or rheumatic heart disease (RHD) for preventing ischemic stroke. However, the impact of antithrombotic therapy on CMBs remained controversial. We aimed to explore the association between the prevalence of CMBs and prior antithrombotic therapy in ischemic stroke patients with AF and/or RHD.
Materials and Methods: Ischemic stroke patients with AF and/or RHD within 7 days of onset from two hospitals were enrolled. Clinical information, prior use of antiplatelets or anticoagulation, presence and location of CMBs on susceptibility weighted imaging were recorded. We investigated the association of antithrombotic use with the presence or location of CMBs using multivariable logistic regression.
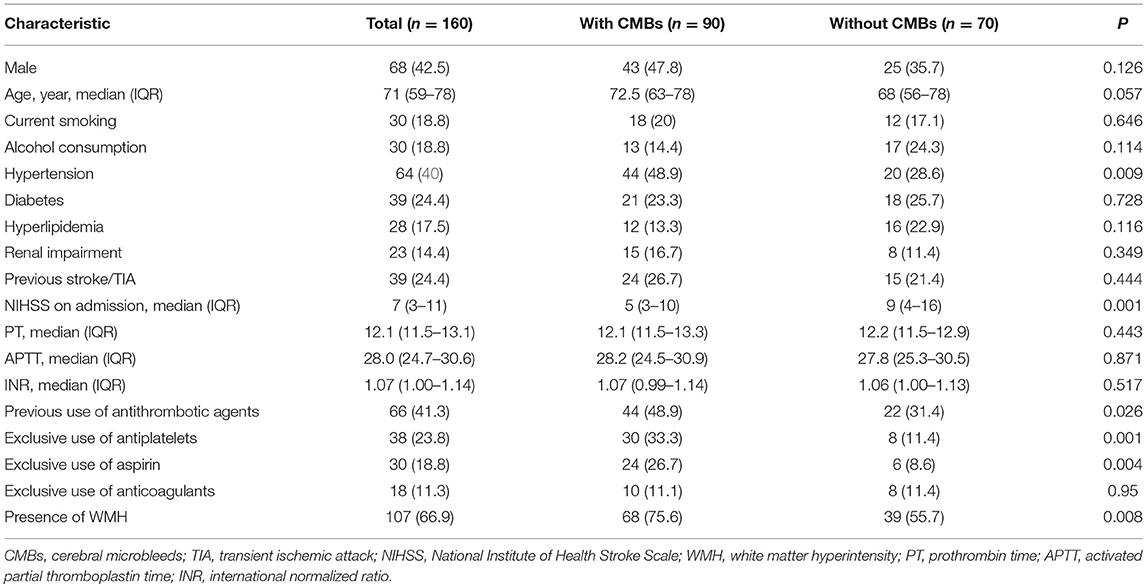
Results: A total of 160 patients (68 males; median age, 71 years) were included. CMBs were observed in 90 (56.3%) patients, of whom 37 were with strictly lobar CMBs and 53 were with deep or infratentorial CMBs. There was a significant difference in antiplatelet use between patients with and without CMBs (33.3 vs. 11.4%, P = 0.001), but not found in anticoagulants. Prior use of antiplatelets was independently associated with the presence of CMBs (OR 3.075, 95% CI 1.175–8.045, P = 0.022) and especially strictly lobar CMBs (OR 2.635, 95% CI 1.050–6.612, P = 0.039) in multivariate analysis.
Conclusions: The present study suggests that CMBs are common in ischemic stroke patients with AF and/or RHD and prior antiplatelet use may relate to the presence of CMBs predominantly in the strictly lobar region. Whether anticoagulants could cause CMBs need to be determined in future longitudinal studies.
Introduction
Ischemic stroke patients attributable to atrial fibrillation (AF) and rheumatic heart disease (RHD) are increasing in China, with a high rate of death and recurrence (1, 2). Although antithrombotic therapy (antiplatelets or oral anticoagulants) has been the mainstay for primary and secondary prevention of ischemic stroke in patients with AF and/or RHD, its use in clinical practice is suboptimal due to the feared risk of catastrophic intracerebral hemorrhage (ICH) (3). Therefore, risk stratification strategy is needed to balance the risk of ICH vs. ischemic stroke and guide the safe use of antithrombotic agents in Chinese stroke patients with AF/RHD, who have a heightened risk of ICH (4).
Cerebral microbleeds (CMBs) are key neuroimaging markers of hemorrhagic small vessel disease in the brain, which can be detected on bleeding sensitive magnetic resonance imaging (MRI) sequences such as gradient-echo T2*-weighted imaging (T2*-GRE) and susceptibility-weighted imaging (SWI) (5). Histopathologically, CMBs are perivascular hemosiderin deposits after small hemorrhages, indicative of bleeding-prone microangiopathy (6). They are commonly detected in healthy elderly and more prevalent in patients with ischemic or hemorrhagic strokes (7–9). The presence of CMBs has been reported to increase the risk of ICH up to 8-fold in a pooled cohort of ischemic stroke or transient ischemic attack (TIA) (10). This is more clinically relevant in AF/RHD patients with anticoagulation needs (11). Prior studies showed that CMBs appear to be more prevalent in patients with AF (12) and ≥5 CMBs powerfully predict future ICH risk in ischemic stroke patients with AF taking oral anticoagulants (13). Consequently, a challenging clinical dilemma emerged as whether antithrombotic decisions would be influenced by the presence of CMBs in patients with AF and/or RHD.
Since CMBs may represent hemorrhage-prone pathological states, the impact of different antithrombotic therapy on the presence of CMBs has been of great concern. Some previous studies showed that antiplatelet or anticoagulant use was significantly associated with the presence of CMBs in ischemic stroke patients (12, 14). However, the results regarding this question remained controversial and uncertain (15, 16). In addition, few studies (12, 17) on this question focused on stroke patients with AF and/or RHD. Besides, most studies observed CMBs on T2*-GRE, not on SWI, though SWI has been proven to be more sensitive at detecting CMBs (18).
Hence, we aimed to investigate the relationship between prior antithrombotic therapy and prevalence of CMBs observed on SWI in ischemic stroke patients with AF and/or RHD. Furthermore, we analyzed this association in different locations of CMBs with the presumption that strictly lobar or deep/infratentorial CMBs may have different underlying pathogenesis and different bleeding risk (5).
Materials and Methods
Study Population
This research is part of the project, “Study on small vessel pathological mechanism of cerebral hemorrhage after cardioembolic stroke using SWI markers,” approved by the National Natural Science Foundation of China. Ischemic stroke patients with AF and/or RHD were consecutively registered after being admitted to the department of neurology in West China Hospital, Sichuan University (10/2013-09/2016) and People's Hospital of Deyang City (09/2014-07/2015). The study protocol was approved by the medical ethics committee in each hospital. Written informed consent was obtained from participants or their guardians.
Ischemic stroke was diagnosed according to World Health Organization criteria and confirmed by computed tomography scanning or magnetic resonance imaging. AF was diagnosed based on a history of persistent AF or paroxysmal AF, supported by previous electrocardiograms or by electrocardiography (24-h or not) on admission (1). RHD was defined according to criteria in the International Classification of Diseases (10th edition) and confirmed by echocardiography.
Data Collection
Ischemic stroke patients with AF and/or RHD within 7 days of symptom onset who had completed MRI including fluid-attenuated inversion recovery and SWI were enrolled in our study. We used a standardized form to extract patient's information including age, gender, National Institutes of Health Stroke Scale (NIHSS) score on admission, medical history of hypertension, diabetes mellitus, hyperlipidemia, and stroke/TIA, renal impairment (based on medical history or on prospective measurement of estimated glomerular filtration <60 ml/min/1.73 m) (19), current smoking and alcohol consumption, coagulation function on admission including prothrombin time (PT), activated partial thromboplastin time (APTT), and international normalized ratio (INR). We also collected information about patient-reported use and type of antithrombotic drugs before admission.
Imaging Protocol
The parameters of MRI remained the same during the study period: (1) West China Hospital, Sichuan University (3.0 Tesla Siemens Trio MR scanner); (2) People's Hospital of Deyang City (1.5 Tesla Philips Achieva MR scanner). Details of the parameters of fluid-attenuated inversion recovery and SWI sequence have been described in our previous study (20).
Assessment of Cerebral Microbleeds and White Matter Hyperintensity
A CMB was defined as a homogeneous, round focal area, with a diameter <10 mm and very low signal intensity on SWI (21). The location of the CMBs was classified as strictly lobar CMBs (1 or more microbleeds distributed in lobar location strictly) and deep or infratentorial CMBs (at least 1 microbleed in a deep or infratentorial brain location with or without concomitant lobar CMBs). The white matter hyperintensity (WMH) was assessed based on Fazekas scale (22). Two neurologists blinded to clinical data assessed CMBs and WMH independently. In case of disagreement, a third neurologist was consulted, and a consensus decision was reached. Our inter-rater reliability, for evaluating CMBs and WMH was 0.73 and 0.85, respectively.
Statistical Analysis
Statistical analyses were performed with SPSS version 20.0 (IBM, Chicago, IL). Continuous variables were reported as median (interquartile range [IQR]) and were analyzed with Mann–Whitney U-test or Kruskal–Wallis test. Categorical variables were presented as counts (proportions) and were analyzed with χ2-test or Fisher's exact test. Multiple comparisons were corrected by Bonferroni method. Binary logistic regression was applied to evaluate the independent relationship between antithrombotic therapy and presence of CMBs. Variables with a P < 0.1 in univariate analyses and potential confounders were included in multivariate analysis. When appropriate, results were reported as an odds ratio (OR) and 95% confidence interval (CI). Inter-rater variability was calculated using Cohen's kappa. Two-sided values of P < 0.05 were considered statistically significant.
Results
In the study period, we enrolled 160 consecutive ischemic stroke patients with AF and/or RHD within 7 days of stroke onset at admission (West China Hospital, Sichuan University, n = 125; People's Hospital of Deyang City, n = 35). Of the included patients, there were 68 (42.5%) men and the median age at stroke onset was 71 years (age range 59–78 years). Thirty-eight patients (23.8%) had been treated with antiplatelets exclusively before admission (30 for aspirin, 4 for clopidogrel, 4 for both), 18 (11.3%) patients had been treated with warfarin exclusively, and another 10 patients switching between antiplatelets and warfarin were analyzed separately. CMBs were detected in 90 patients (56.3%). Of these, 36 had single CMBs, and 54 had multiple CMBs (≥2). CMBs were most commonly present as deep or infratentorial bleeding (53/90, 58.9%), followed by strictly lobar bleeding (37/90, 41.1%).
Table 1 shows the demographic and clinical characteristics of included patients with and without CMBs. Those patients with CMBs were more likely to have a history of hypertension (48.9 vs. 28.6%; P = 0.009), lower NIHSS score on admission (median 5 vs. 9; P = 0.001), more burden of WMH (75.6 vs. 55.7%; P = 0.008) and were on exclusive antiplatelet treatment three times (33.3 vs. 11.4%; P = 0.001) as commonly compared with those without CMBs. However, patients were comparable in terms of prior use of anticoagulants and coagulation function test.
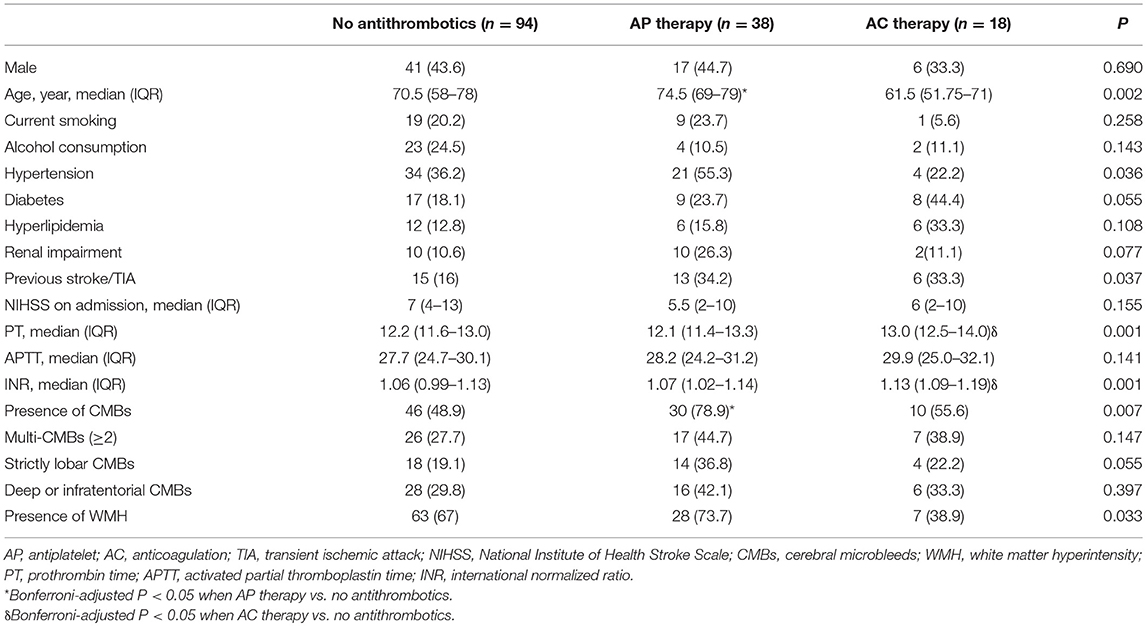
Table 2 describes the baseline demographics and CMB distribution stratified by antiplatelet or anticoagulant use. Compared with non-antithrombotic users, those with prior antiplatelet use were older, more frequently to have CMBs, and had marginally higher prevalence of strictly lobar CMBs. Patients with warfarin had higher INR values but no significant difference was found in the presence or location of CMBs.
In a multivariable logistic regression model after adjustment for age, gender, history of hypertension, history of stroke or TIA, NIHSS score, INR and presence of WMH, the exclusive use of antiplatelet agents was independently associated with the presence of CMBs (OR 3.075, 95% CI 1.175–8.045, P = 0.022; Table 3). In terms of CMBs' location, prior antiplatelet use was associated with strictly lobar CMBs (OR 2.635, 95% CI 1.050–6.612, P = 0.039; Table 3) but not with deep or infratentorial CMBs. Specifically, in a subgroup of exclusive aspirin users, the significant results remained. Prior use of aspirin was still independently related with the presence of CMBs (OR 3.095, 95% CI 1.085–8.832, P = 0.035) and strictly lobar CMBs (OR 3.975, 95% CI 1.506–10.495, P = 0.005) after controlling the above confounders. However, no significant association was found between the exclusive warfarin use and the presence or location of CMBs either in the univariate or multivariate analysis.
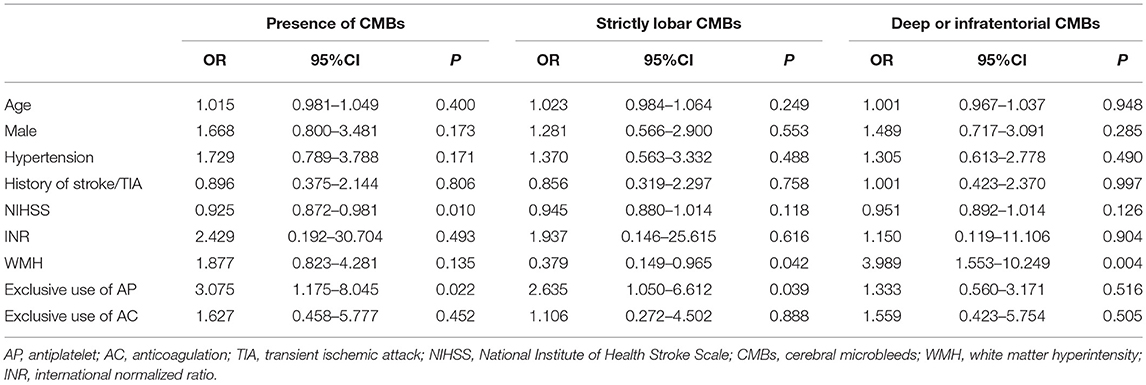
Table 3. Multivariate logistic regression analysis for the association between antithrombotic therapy and cerebral microbleeds.
Discussion
In this study, we found that CMBs were highly prevalent in Chinese ischemic stroke patients with AF and/or RHD. Prior use of the antiplatelet agents (especially aspirin) was significantly associated with presence of CMBs and especially the strictly lobar CMBs, whereas the prior use of warfarin seemed to be not related to CMBs in any location.
The frequency of CMBs in stroke patients with AF/RHD was 56.3% in the present study, which fell broadly within the range of 18% to 68% in patients with ischemic stroke (23). When focusing on stroke patients with AF, the prevalence of CMBs was reported to be around 30% (11, 12, 24, 25). The higher prevalence of CMBs in our study might be related to ethnic differences or different imaging protocol. We carefully used the SWI sequence which has better spatial resolution and post-processing ability in detecting CMBs than conventional T2*-GRE sequence (18).
With the high prevalence of CMBs and a possible link to bleeding tendency, there is growing interest in demonstrating the role of CMBs in AF patients who are frequently using antithrombotic agents. Our study showed that antiplatelet use was associated with presence of CMBs in ischemic stroke patients with AF/RHD. Although controversies existed in the literature (14, 26–28), our finding is consistent with three recent meta-analyses, which all showed similar associations in patients with ischemic stroke or TIA (15, 29, 30). Some of the previous studies suggested that different type of antiplatelets may have different effects on development of CMBs. The Rotterdam Scan Study showed that aspirin was associated with lobar CMBs while clopidogrel associated with deep CMBs in general population (31, 32). A Japanese study among ICH patients reported that only aspirin, but not clopidogrel, cilostazol, or ticlopidine, was associated with the presence of CMBs (27). We also found that prior use of aspirin was associated with the presence of CMB; however, we did not evaluate the impact of clopidogrel on CMBs due to the limited number of clopidogrel users. These discrepancies among studies may be due to the difference of study population and prevalence of CMBs. Furthermore, in terms of the duration of antiplatelet therapy, a Chinese study found that CMBs are more prevalent among aspirin users with longer duration (>5 years) than those receiving aspirin <5 years (14), whereas another study from Japan failed to show this association between long duration (≥10 years) of aspirin use and CMB presence in multivariable analysis after adjusting for hypertension and other confounders (16). It is important to note that most of these studies including ours are major cross-sectional design, a conclusion on the causal relationship between antiplatelet use and development of CMBs cannot be drawn.
Different topographical associations of CMBs with the prior antiplatelet therapy were detected in our study. Prior use of antiplatelets and especially aspirin were only significantly associated with occurrence of strictly lobar CMBs rather than deep or infratentorial CMBs. Our findings are in line with the Rotterdam Scan Study (31) and the newly published meta-analysis (30). This result may be explained by the different underlying pathophysiologic mechanisms of CMBs in different locations. CMBs located in strictly lobar brain regions are associated with cerebral amyloid angiopathy, while those located in deep or infratentorial brain regions are related to hypertensive vasculopathy (5). Intriguingly, some previous study reported that the aspirin-associated hemorrhages predominantly occurred in the lobar areas (33), suggesting that aspirin favors rupture of small vessels with cerebral amyloid angiopathy. In this aspect, it is speculated that antiplatelet use would be more related to risk of lobar CMBs other than deep or infratentorial CMBs. However, due to the small sample size in subgroup analysis, there might be somewhat overfitting and possibility of chance. Moreover, whether lobar CMBs are truly attributable to CAA in patients with AF is yet to be ascertained. Further pathological studies are needed to clarify this point and explore other conditions that modulate the link between antiplatelet use and lobar CMBs.
In our study, we could not confirm an association between anticoagulation and CMBs. This result should be interpreted cautiously due to the small sample size of warfarin users. Nevertheless, previous studies have provided conflicting results regarding this question. In the Rotterdam Scan Study (31) and a Korean study of asymptomatic elderly (34), no association was found between warfarin and presence of CMBs. Another Turkish case-control study also demonstrated that warfarin treatment had no effect on CMBs in ICH patients (35). Moreover, in a longitudinal study with ischemic stroke patients on warfarin therapy for 2 years, the mean duration of warfarin treatment was not significantly related to new development of CMBs (36). Conversely, there is also evidence linking warfarin use to CMB presence. A German retrospective study of 785 patients with ischemic stroke and TIA (16.3% with AF) showed that patients with prior use of anticoagulant agents were more likely to have CMBs as compared to those without; these CMBs tended to be in lobar location (12). Unfortunately, this association was no longer present after full adjustment for demographic and clinical variables in the multivariable analysis, with the age remained the only independent factor for CMBs (12). In a large population-based study in Netherlands, higher INR values were associated with CMBs; however, we did not find the association in our study (37), which might be partly due to few patients with targeted INR. Considering INR values representing the intensity of anticoagulation that may influence the occurrence of CMBs, further prospective studies are required to clarify this association.
Notably, we also found that hypertension is an important risk factor of CMBs, which was in line with previous studies (16, 38). The chronic hypertension and associated arterial stiffness have been reported to be linked to the presence of CMBs (39) and further contribute to higher risk of hemorrhagic stroke especially after possible acute treatment such as thrombolysis (40, 41). Therefore, it is reasonable to speculate that hypertension may explained at least in part the occurrence of CMBs in addition to the effects of antithrombotic therapy. Under impaired hemostasis by antithrombotic agents, the red blood cells are prone to extravasate through the vulnerable microvasculature and this process might be aggravated by the presence of hypertension. These results suggest that vascular risk factors should be carefully monitored when prescribing antithrombotic drug treatment to patients with multiple CMBs.
Studying the relationship between CMBs, antithrombotic agents and AF/RHD is of great importance. Firstly, patients with AF have a significantly higher prevalence of CMBs and they tend to receive anticoagulation and antiplatelets more frequently than patients without AF (24). Secondly, CMBs are small blood extravasations and these asymptomatic leaks could evolve into a symptomatic ICH when hemostasis is damaged under antithrombotic therapy (42). There is now clear evidence that ICH incidence is higher in patients with CMBs than those without CMBs in antithrombotic users with AF (43), and the risk of ICH associated with CMBs is higher in Asians (10). With the aging population and growing prevalence of AF/RHD patients in China, it is essential to investigate the correlation of CMBs with antithrombotic therapy and determine how this small vessel disease marker can help analyze the benefit-risk ratio for stroke prevention strategies.
Our study has several limitations. First, this is a hospital-based study with a relatively small sample size and low number of the patients treated with oral anticoagulants. Second, subgroup analysis of different type of antiplatelets was not performed because most of our patients used aspirin. Nevertheless, the significant association of antiplatelets as whole remained in the subgroup of aspirin users. Third, the durations of drug use were not recorded due to the incomplete data in the medical records. It is possible that the durations of antithrombotic agents influence the occurrence of CMBs. Fourth, the selection bias cannot be avoided because only part of patients underwent SWI sequence due to the disease severity and cost of multiple MRI sequences. Finally, given the cross-sectional and retrospective nature of the study, it is possible that some CMBs may have occurred before use of antithrombotic agents. Therefore, further prospective investigation is required to confirm these findings.
Conclusion
In conclusion, prior antiplatelet use is independently associated with the presence of CMBs in ischemic patients with AF and/or RHD, especially among those with lobar CMBs. Whether anticoagulants by itself could cause CMBs remains unclear. These findings warrant future prospective longitudinal studies to clarify the relationship among antithrombotic therapy, cerebral microbleed and macrohemorrhage in stroke patients with AF and/or RHD.
Author Contributions
ML designed the study. YC, JfL, JieL, CW, and JinL collected the data. YC and JfL performed imaging analysis. YC and JfL performed statistics analysis. YC and JfL drafted the main part of the manuscript. BW and SZ provided support for imaging analysis. SZ, DW, and YW helped design the study and revised the manuscript. All authors approved the final version submitted for publication.
Funding
This research was supported by Major International (Regional) Joint Research Project, National Natural Science Foundation of China (81620108009), National Key Research and Development Program, Ministry of Science and Technology of China (2016YFC1300500-505), Key Research and Development Program, Science & Technology Department of Sichuan Province (2017SZ0007) and National Natural Science Foundation of China (81601022, 81500923).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lin S, Wu B, Hao ZL, Kong FY, Tao WD, Wang DR, et al. Characteristics, treatment and outcome of ischemic stroke with atrial fibrillation in a Chinese hospital-based stroke study. Cerebrovasc Dis. (2011) 31:419–26. doi: 10.1159/000323221
2. Wang D, Liu M, Hao Z, Tao W, Lin S, Zhang S, et al. Features of acute ischemic stroke with rheumatic heart disease in a hospitalized Chinese population. Stroke (2012) 43:2853–7. doi: 10.1161/STROKEAHA.112.670893
3. Guo J, Guan T, Fan S, Chao B, Wang L, Liu Y. Underuse of oral anticoagulants in patients with ischemic stroke and atrial fibrillation in China. Am J Cardiol. (2018). 122:2055–61. doi: 10.1016/j.amjcard.2018.08.057
4. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
5. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. (2009) 8:165–74. doi: 10.1016/S1474-4422(09)70013-4
6. Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. (2011) 32:528–34. doi: 10.1159/000331466
7. Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology (1999) 52:991–4. doi: 10.1212/WNL.52.5.991
8. Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke (2002) 33:1536–40. doi: 10.1161/01.STR.0000018012.65108.86
9. Roob G, Lechner A, Schmidt R, Flooh E, Hartung HP, Fazekas F. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke (2000) 31:2665–9. doi: 10.1161/01.STR.31.11.2665
10. Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke (2013) 44:995–1001. doi: 10.1161/STROKEAHA.111.000038
11. Haji S, Planchard R, Zubair A, Graff-Radford J, Rydberg C, Brown RD, et al. The clinical relevance of cerebral microbleeds in patients with cerebral ischemia and atrial fibrillation. J Neurol. (2016) 263:238–44. doi: 10.1007/s00415-015-7966-2
12. Horstmann S, Mohlenbruch M, Wegele C, Rizos T, Laible M, Rauch G, et al. Prevalence of atrial fibrillation and association of previous antithrombotic treatment in patients with cerebral microbleeds. Eur J Neurol. (2015) 22:1355–62. doi: 10.1111/ene.12608
13. Charidimou A, Karayiannis C, Song TJ, Orken DN, Thijs V, Lemmens R, et al. Brain microbleeds, anticoagulation, and hemorrhage risk: meta-analysis in stroke patients with AF. Neurology (2017) 89:2317–26. doi: 10.1212/wnl.0000000000004704
14. Ge L, Niu G, Han X, Gao Y, Wu Q, Wu H, et al. Aspirin treatment increases the risk of cerebral microbleeds. Can J Neurol Sci. (2011) 38:863–8. doi: 10.1017/S0317167100012440
15. Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CL, Edinburgh Stroke Study G, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke (2010) 41:1222–8. doi: 10.1161/STROKEAHA.109.572594
16. Yamashiro K, Tanaka R, Okuma Y, Ueno Y, Tanaka Y, Hattori N, et al. Associations of durations of antiplatelet use and vascular risk factors with the presence of cerebral microbleeds. J Stroke Cerebrovasc Dis. (2014) 23:433–40. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.027
17. Karayiannis C, Soufan C, Chandra RV, Phan TG, Wong K, Singhal S, et al. Prevalence of brain MRI markers of hemorrhagic risk in patients with stroke and atrial fibrillation. Front Neurol. (2016) 7:151. doi: 10.3389/fneur.2016.00151
18. Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, et al. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke (2013) 44:2782–6. doi: 10.1161/STROKEAHA.113.002267
19. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. (2006) 145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004
20. Liu J, Wang D, Li J, Xiong Y, Liu B, Wei C, et al. High serum alkaline phosphatase levels in relation to multi-cerebral microbleeds in acute ischemic stroke patients with atrial fibrillation and/or rheumatic heart disease. Curr Neurovasc Res. (2016) 13:303–8. doi: 10.2174/1567202613666160817095623
21. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
22. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, and Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
23. Wang Z, Soo YOY, Mok VCT. Cerebral microbleeds: is antithrombotic therapy safe to administer? Stroke (2014) 45:2811–7. doi: 10.1161/STROKEAHA.114.004286
24. Saito T, Kawamura Y, Tanabe Y, Asanome A, Takahashi K, Sawada J, et al. Cerebral microbleeds and asymptomatic cerebral infarctions in patients with atrial fibrillation. J Stroke Cerebrovasc Dis. (2014) 23:1616–22. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.005
25. Song TJ, Kim J, Song D, Nam HS, Kim YD, Lee HS, et al. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology (2014) 83:1308–15. doi: 10.1212/WNL.0000000000000862
26. Nighoghossian N, Hermier M, Adeleine P, Blanc-Lasserre K, Derex L, Honnorat J, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke (2002) 33:735–42. doi: 10.1161/hs0302.104615
27. Naka H, Nomura E, Kitamura J, Imamura E, Wakabayashi S, Matsumoto M. Antiplatelet therapy as a risk factor for microbleeds in intracerebral hemorrhage patients: analysis using specific antiplatelet agents. J Stroke Cerebrovasc Dis. (2013) 22:834–40. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.001
28. Marti-Fabregas J, Medrano-Martorell S, Merino E, Prats-Sanchez L, Marin R, Delgado-Mederos R, et al. Statins do not increase markers of cerebral angiopathies in patients with cardioembolic stroke. Sci Rep. (2018) 8:1492. doi: 10.1038/s41598-018-20055-3
29. Liu S, Li C. Antiplatelet drug use and cerebral microbleeds: a meta-analysis of published studies. J Stroke Cerebrovasc Dis. (2015) 24:2236–44. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.022
30. Qiu J, Ye H, Wang J, Yan J, Wang J, Wang Y. Antiplatelet therapy, cerebral microbleeds, and intracerebral hemorrhage: a meta-analysis. Stroke (2018) 49:1751–4. doi: 10.1161/strokeaha.118.021789
31. Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. (2009) 66:714–20. doi: 10.1001/archneurol.2009.42
32. Darweesh SK, Leening MJ, Akoudad S, Loth DW, Hofman A, Ikram MA, et al. Clopidogrel use is associated with an increased prevalence of cerebral microbleeds in a stroke-free population: the Rotterdam study. J Am Heart Assoc. (2013) 2:e000359. doi: 10.1161/jaha.113.000359
33. Wong KS, Mok V, Lam WW, Kay R, Tang A, Chan YL, et al. Aspirin-associated intracerebral hemorrhage: clinical and radiologic features. Neurology (2000) 54:2298–2301. doi: 10.1212/WNL.54.12.2298
34. Kim CK, Kwon HT, Kwon HM. No significant association of aspirin use with cerebral microbleeds in the asymptomatic elderly. J Neurol Sci. (2012) 319:56–8. doi: 10.1016/j.jns.2012.05.017
35. Orken DN, Kenangil G, Uysal E, Forta H. Cerebral microbleeds in ischemic stroke patients on warfarin treatment. Stroke (2009) 40:3638–40. doi: 10.1161/strokeaha.109.559450
36. Orken DN, Uysal E, Timer E, Kuloglu-Pazarci N, Mumcu S, Forta H. New cerebral microbleeds in ischemic stroke patients on warfarin treatment: two-year follow-up. Clin Neurol Neurosurg. (2013) 115:1682–5. doi: 10.1016/j.clineuro.2013.03.004
37. Akoudad S, Darweesh SK, Leening MJ, Koudstaal PJ, Hofman A, van der Lugt A, et al. Use of coumarin anticoagulants and cerebral microbleeds in the general population. Stroke (2014) 45:3436–9. doi: 10.1161/strokeaha.114.007112
38. Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain (2007) 130:1988–2003. doi: 10.1093/brain/awl387
39. Song TJ, Kim J, Kim YD, Nam HS, Lee HS, Nam CM, et al. The distribution of cerebral microbleeds determines their association with arterial stiffness in non-cardioembolic acute stroke patients. Eur J Neurol. (2014) 21:463–9. doi: 10.1111/ene.12332
40. Acampa M, Guideri F, Di Donato I, Tassi R, Marotta G, Lo Giudice G, et al. Arterial stiffness in patients with deep and lobar intracerebral hemorrhage. J Stroke (2014) 16:184–8. doi: 10.5853/jos.2014.16.3.184
41. Acampa M, Camarri S, Lazzerini PE, Guideri F, Tassi R, Valenti R, et al. Increased arterial stiffness is an independent risk factor for hemorrhagic transformation in ischemic stroke undergoing thrombolysis. Int J Cardiol. (2017) 243:466–70. doi: 10.1016/j.ijcard.2017.03.129
42. Wilson D, Jager HR, Werring DJ. Anticoagulation for atrial fibrillation in patients with cerebral microbleeds. Curr Atheroscler Rep. (2015) 17:47. doi: 10.1007/s11883-015-0524-7
43. Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. (2018) 17:539–47. doi: 10.1016/S1474-4422(18)30145-5
Keywords: antithrombotic therapy, cerebral microbleeds, ischemic stroke, atrial fibrillation, rheumatic heart disease
Citation: Cheng Y, Liu J, Zhang S, Li J, Wei C, Wang D, Lin J, Wang Y, Wu B, Zhang S and Liu M (2019) Prior Antithrombotic Therapy Is Associated With Cerebral Microbleeds in Ischemic Stroke Patients With Atrial Fibrillation and/or Rheumatic Heart Disease. Front. Neurol. 9:1184. doi: 10.3389/fneur.2018.01184
Received: 28 July 2018; Accepted: 21 December 2018;
Published: 11 January 2019.
Edited by:
Phyo Kyaw Myint, University of Aberdeen, United KingdomReviewed by:
Maurizio Acampa, Azienda Ospedaliera Universitaria Senese, ItalyAlexander Tsiskaridze, Tbilisi State University, Georgia
Copyright © 2019 Cheng, Liu, Zhang, Li, Wei, Wang, Lin, Wang, Wu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Liu, wyplmh@hotmail.com
†These authors have contributed equally to this work
 Yajun Cheng
Yajun Cheng Junfeng Liu1†
Junfeng Liu1† Shuting Zhang
Shuting Zhang Deren Wang
Deren Wang Yanan Wang
Yanan Wang