- 1Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, China
- 2The First Affiliated Hospital of Shandong First Medical University, Jinan, China
Background: Behcet's disease (BD) is multi-systemic vasculitis, which generally is repeated oral and genital ulcerations as well as ocular and skin lesions. Today, the pathogenesis of BD remains mostly unknown. It is also suggested that the disease is probably related to autoinflammatory and autoimmune disorders, and innate immunity damages were perceived as key in its pathologic process. Only 5% of BD patients have neurological involvement, and it usually occurs in 4–6 years after the initial symptoms. Early onset of neurological impairment makes it difficult to diagnose and treat definitely.
Case Presentation: A 38-year-old man was admitted to our hospital with numbness and weakness of the left extremities. Diffusion magnetic resonance imaging (MRI) revealed focal infarction in the posterior limb of the internal capsule. Skin pathology suggested small vessel vasculitis, and high-resolution MRI revealed intracranial arteritis. The patient had a negative skin pathery test and then developed a scar at the venous puncture site at the early stage of disease. Laboratory examination showed that interleukin 8 (IL-8) increased. The patient was treated with an immunosuppressive agent including mycophenolate mofetil, hydroxychloroquine, and colchicine. All symptoms were alleviated after half a year's treatment. There was neither stroke nor recurrence of oral ulcer thereafter.
Conclusion: This case demonstrates that neurological involvement might be an early symptom of BD. IL-8 could act as a novel target for the treatment of BD theoretically and probably play a key role in disease recovery.
Introduction
Behcet's disease (BD) is recognized as a multi-systemic disease characterized by possible inflammatory lesions of any-size vessels (1). Although the etiopathogenesis has not yet been clarified, it is generally believed that immunological factors play an important role (2–4). It is also suggested that the disease is probably related to autoinflammatory and autoimmune disorders, and innate immunity damages were perceived as having a key role in its pathologic process (5–7). The diagnosis is established by clinical criteria only (8). The international criteria for BD (ICBD) allow for earlier recognition, thus leading to earlier and accurate diagnosis and treatment with salutary results (9).
Involvement of central nervous system symptoms is reported in 3.2–49%, mostly about 5%, in BD patients (10, 11), which is known as neuro-Behcet's disease (NBD).Nervous system impairment mostly occurs in 4–6 years after the onset of BD (12). However, some of the patients have neurological symptoms at the same time as or even before the classical BD symptoms, which may lead to diagnostic confusion and may result in there being more BD patients than actually reported (13).
In this kind of patient, the pathological feature is various types of arteriovenous vasculitis, which mainly affects small vessels in multiple systems. It appears as focal or multiple brain parenchymal damage in the central nervous system (14, 15). Artery involvement is rare compared with venous involvement, manifesting as arterial stenosis and aneurysm formation, which often lead to arterial infarction or intracranial and/or subarachnoid hemorrhage (16–18).
Case Presentation
A 38-year-old man was admitted to our hospital with numbness and weakness of the left extremities. Diffusion-weighted imaging (DWI) revealed focal infarction in the posterior limb of the internal capsule, and cranial and cervical magnetic resonance angiography was normal (Figure 1).
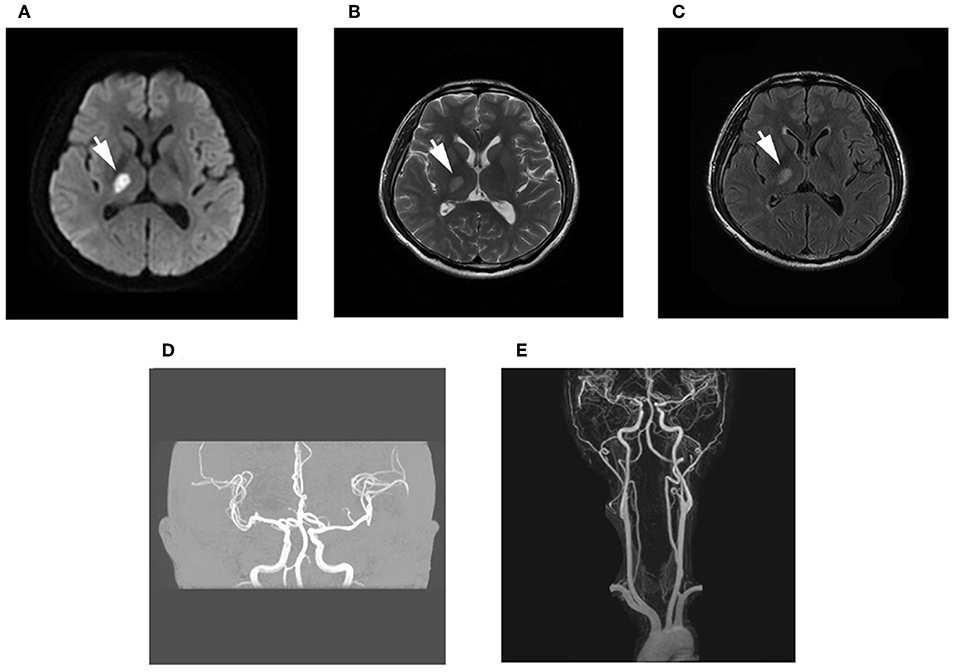
Figure 1. Brain magnetic resonance imaging (MRI). (A–C) Diffusion-weighted imaging (DWI) and T2 fluid-attenuated inversion recovery (FLAIR) imaging showed high signal, while long signal was shown in T1-weighted imaging and T2-weighted imaging of the posterior limb of the internal capsule. (D,E) Magnetic resonance angiography examination of the intracranial and cervical vascular was normal.
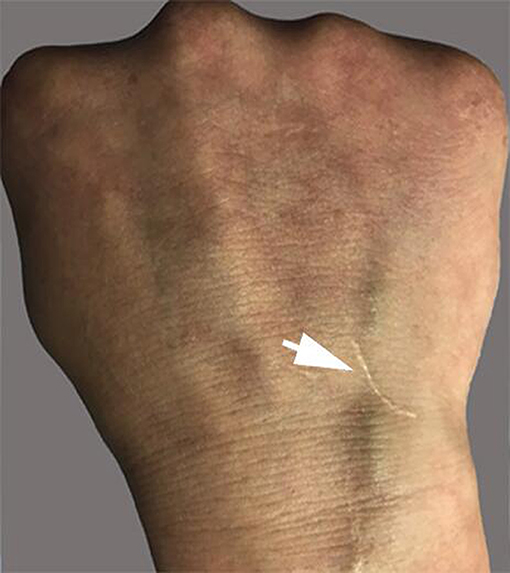
The patient had recurrent multiple and painful oral ulcers after 2 months; meanwhile, he suffered pain in both knees and the left shoulder joint. The physical examination revealed that the old and new acneiform folliculitis were alternated on the face and back and behind the ears (Figure 2); also, a scar was shown at the venous puncture site (Figure 3). The patient had no other risk factors for cerebral atherosclerotic disease and is also not a smoker. Blood routine, serologic etiology test (including syphilis, AIDS, and other pathogens), lipid profile, plasma glucose, plasma and urine homocysteine levels (indicating the possibility of a genetic predisposition to thrombosis), proteins C and S deficiency, antithrombin III deficiency, activated protein C resistance, antiphospholipid antibody (hypercoagulability markers), anticardiolipin immunoglobulin G (IgG) and immunoglobulin M (IgM), antineutrophilic cytoplasmic antibody, C-reactive protein (CRP), erythrocyte sedimentation rate, and rheumatoid factor were performed, and all of them were within normal range. Transesophageal echocardiography, contrasted transthoracic echocardiography, and 24-h dynamic electrocardiogram were normal. Interleukin 8 (IL-8) was increased to 252 pg/ml (normal value: <62 pg/ml), but IL-6, tumor necrosis factors (TNF), and other cytokines were maintained at normal levels. No cardiogenic embolism, cardiac structure and rhythm abnormalities were found.
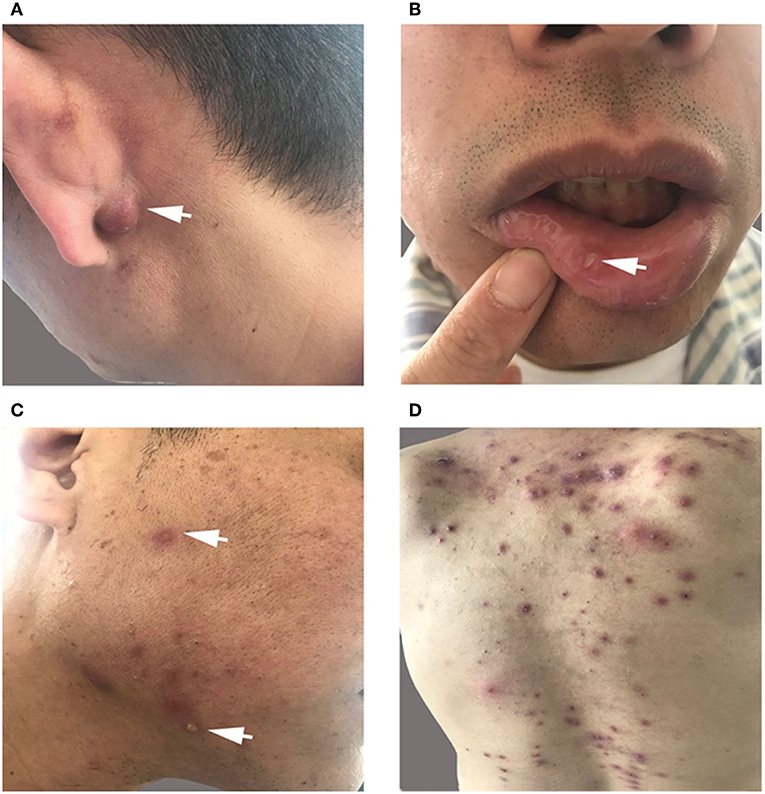
Figure 2. (A,C,D) Old and new acneiform folliculitis behind the ears (A) and on the face (C) and back (D). (B) Recurrent multiple and painful oral ulcers.
High-resolution magnetic resonance imaging (HRMRI) of the brain suggested that some blood vessel walls were slightly thickened, including the terminal portion of the left internal carotid artery, V4 segment of the bilateral vertebral artery, P2 segment of the right posterior cerebral artery (Figure 4), the terminal portion of M1 segment of the right middle cerebral artery (MCA), M2 and M3 segments of bilateral MCA, and A2 segment of the bilateral anterior cerebral artery. The enhancement scanning showed a double-track-like change and a narrowed vascular lumen, which suggested multiple intracranial arteritis. Hip magnetic resonance imaging (MRI) showed a long and flaky T1-weighted and T2 high fat-suppression signal on the lateral of the left femoral head, which suggested degenerative changes. Pathological biopsy of the skin lesion showed that some regions were infiltrated by extensively perivascular inflammatory cells, suggesting small vessel vasculitis (Figure 5). Genetic testing also was performed to analyze hereditary cerebrovascular disease elements (detected by Golden Field Medical, Inc., using analysis of missense mutations and alternative splicing; this lab has gotten the accreditation of the College of American Pathologists), and the result did not show pathogenic variation. Based on the above testing results and related to a history of genital ulcers of the patient, this case was diagnosed as BD under the direction of diagnostic criteria. The patient could not use azathioprine (AZP) to thiopurine S-methyltransferase (TPMT) mutation, and this could be checked by gene detection, which indicated that there was TPMT*3C (A719G) in this patient. Meanwhile, the patient refused to use cyclophosphamide after acquainting with its side effects (nephrotoxicity etc.) and its cumulative toxicity with long-term use (19).
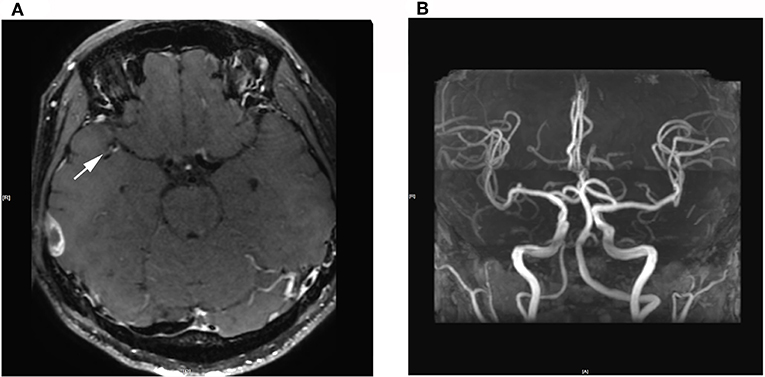
Figure 4. (A) 3D Cube T1 enhancement shows slightly thickened walls of the terminal portion of the M1 segment of the right middle cerebral artery. (B) 3D-TOF-MRA shows no abnormalities.
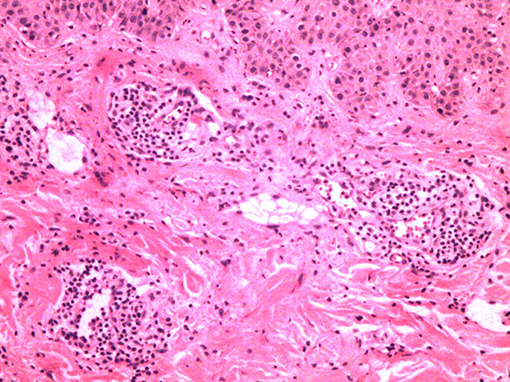
Figure 5. Extensively perivascular inflammatory cell infiltration with pathological biopsy of the skin lesion.
Therefore, the patient was treated with mycophenolate mofetil dispersible tablets (Seccopin), hydroxychloroquine tablets (Flush), and colchicine tablets. After half a year's treatment, the patient's skin rash gradually was alleviated, and the symptoms of oral ulcer and nervous system damage did not recur.
Discussion and Conclusions
Our patient fulfilled the diagnostic criteria of ICBD; he had recurrent oral ulcers (2 points), skin lesions (1 point), central nervous system lesions (1 point), vascular symptoms (1 point), and a positive pathergy test (1 point).
The patient initially consulted for acute cerebral infarction. This patient did not show risk factors for cerebral atherosclerotic disease other than smoking and also had no abnormalities in the gene test of hereditary cerebrovascular disease and other common causes, for example, hypercoagulability. According to his medical history, laboratory tests, and examination results, vasculitis caused by syphilitic vasculitis, rheumatoid arthritis, systemic lupus erythematosus, and Sjogren's syndrome can be excluded. The high-resolution MRI (HRMRI) of the brain showed intimal thickening in the wall of the intracranial and cervical arteries, which suggested arteritis. However, carotid dissection or Moyamoya symptoms were not shown. The new internal capsule lesion seen on MRI was consistent with the occlusion regions resulting from arteritis of the MCA deep perforator branches. In conclusion, the patient was diagnosed with BD.
Vasculitis is the central pathological process of BD. In this case, skin pathology was a representation of vasculitis, and it was verified by HRMRI as cerebral arteritis. Venipuncture is an inflammatory lesion of the skin, and it is similar to spontaneous skin lesions seen in the disease. Our patient had a negative skin pathery test, which developed a scar at the venous puncture site for the intravenous fluid infusion at the early stage of disease, but we have not observed the whole process of scar formation. The investigation results revealed that the presence of a positive reaction was irrelevant to the clinical manifestations of the disease (20, 21). However, the pathery reaction (PR) was reported to be significantly associated with vascular involvement (22). Chang et al. described that the intravenous Pathergy test seemed to be superior to the skin Pathergy test on specificity, sensitivity, and positive rate. Pathergy test reactions usually occur during active periods of BD as well (23). Although no peripheral vascular pathological examination was performed, the case was tested by negative classic PR, and the results suggested that the scar formation at the venipuncture site was caused by peripheral venous vasculitis.
Different immunologic abnormalities in the etiopathogenesis of BD have been reported. Increased Th1-associated cytokines such as IL-12, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α), as well as IL-2, IL-8, and IL-6, have been documented in BD patients (24). Many studies have reported that increased serum IL-8 plays an important role in BD (25–31). One recent report even implied that serum IL-8 could be a more credible marker of disease activity than CRP or erythrocyte sedimentation rate (ESR) (32). Durmazlar et al. found that IL-8 is positively correlated with the activity of BD and also closely related with the BD activity index and numbers of disease clinical manifestations. IL-8, known as neutrophil activating factor, is substantially produced by granulocytes, T cells, macrophages, and endothelial cells. A small amount of IL-8 can also be induced in keratinocytes, fibroblasts, chondrocytes, and hepatocytes (31, 33, 34). It strongly attracts and activates leukocytes. It has been supposed to establish a prominent connection between immune system activation and endothelial alterations involved in the conversion of mononuclear to granulocytic infiltration and causes raised adhesion of peripheral blood leukocytes to endothelial cells in BD. It has been found that the significant increase of IL-8 is associated with different clinical manifestations in BD, such as skin lesions, oral lesions, and ocular manifestations in one study (31). Moreover, compared with the active patient group without vascular involvement, the levels of IL-8 in patients with vascular involvement were markedly increased. On the basis of the patients' pathology and imaging, active BD patients with vascular involvement were found to possess pretty enhanced IL-8 in serum levels, which supported that endothelial cells may be partly responsible for secretion of IL-8 in the active phase of BD (35), since an IL-8 increase of fourfold in the active phase may represent endothelial injury in any part of the body. HRMRI results suggested central nervous system vasculitis. It is considered to be largely associated with a high IL-8 level, resulting in vascular endothelial destruction.
BD is not generally received as a cerebral vasculitis introducer compared with other systemic diseases known to lead to vasculitis. Only 1–5% arterial involvement is seen throughout the course of BD. The pattern of cerebral infarction and cerebral vasculitis represents a specific and uncommon form of neuro-Behcet's manifestation, and only two cases have been reported so far (10, 36). In one case, neurological deficits were considered to be infarction in the deep perforating branch of MCA, but it did not show specific change in brain imaging. The other case was an autopsy study, and it showed clearly that the infarction area was consistent with the occlusion regions resulting from arteritis of the MCA branches (37). Vascular involvement is a fatal complication for BD patients, so early diagnosis is very important.
Fortunately, for this case, the above conditions could be excluded according to the result that there was extensive cerebral artery intimal thickening from HRMRI. So we could clearly diagnose this case as BD with cerebral arterial infarction caused by cerebral arteritis, which led to early nervous system symptoms. In addition, we found that plasma IL-8 level was significantly elevated at the early stage of this case, while ESR, CRP, and other indicators have no obvious abnormalities. IL-6 is a key cytokine involved in NBD, especially since this inflammatory marker has been found to be increased also in the cerebrospinal fluid of patients with BD. However, IL-6 was not changed in the patient's plasma, which suggested that IL-8 is a dominant factor in the BD process.
AZP is the preferred treatment for BD. It commonly causes myelosuppression, presenting as megaloblastic anemia, leukopenia, severe hair loss, macrocytosis, pancytopenia, and thrombocytopenia (38–40). Leukopenia and severe hair loss are strongly associated with mutations in TPMT and NUDT15, which are widespread in the Asian population (41). Gene detection indicated that there was TPMT*3C (A719G) in this patient, so we did not choose AZP to treat patients. Mycophenolate mofetil is used in organ transplantation, multiple sclerosis, Crohn's disease and other immune-related diseases. Borhani Haghighi and Safari divide the anti-NBD armamentarium into first-line, second-line, and experimental drugs. Mycophenolate mofetil is classified as a second-line drug (42). The International Neuro-Behcet's Advisory Group has developed a consensus on NBD, which refers to mycophenolate mofetil as an alternative drug for NBD (43). Colchicine is used for the treatment of gout (44), as well as specific inflammatory conditions such as familial Mediterranean fever (FMF) (45), recurrent pericarditis (46) and BD (47). It seems to inhibit neutrophil chemotaxis and reduce plasma levels of cytokine, thereby reducing the occurrence and the duration of mucocutaneous (oral ulcers, erythema nodosum, genital) and articular manifestations of the disease (47). Hydroxychloroquine is proven to have a relatively benign toxicity profile and a low incidence of infections as an immunosuppressive agent (48, 49). A large cohort study has reported the successful empiric use of hydroxychloroquine to treat BD (50).
Considering the choice of patients and the drugs, the inflammatory activity was controlled followed by treatment with mycophenolate mofetil dispersible tablets (Seccopin), hydroxychloroquine tablets (Flush), and colchicine tablets. The clinical symptoms were ameliorated, such as suppressing oral immunity, preventing relapses in skin lesions and progressive neurological disease, as well as settling the systemic disease. However, it is unclear whether long-term immunosuppression might also work well in central nervous system (CNS) vasculitis. That is to say, the risk of recurrent cerebral infarcts remains to be elucidated in the future. Therefore, seeking new medicine targets becomes an urgent issue. IL-8, a major chemoattractant and activator of neutrophils, could act as a novel target for the treatment of BD theoretically and perhaps prove to be the key to recovery.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: hospital ID: 000444000, Shandong Povincial Qianfoshan Hospital.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Qianfoshan Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HY, YS, and MZ made substantial contributions to conception and design. HY and MZ collected the data. HY, YS, MZ, and JH contributed to analysis and interpretation of the data. HY, JH, and JT have been involved in drafting the manuscript or revising it critically for important intellectual content. All authors have given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
IL-8, interleukin 8; NBD, neuro-Behcet's disease; BD, Behcet's disease; ICBD, international criteria for Behcet's disease; AZP, azathioprine; MMF, mycophenolate mofetil; IL-1, interleukin 1; IgG, immunoglobulin G; IgM, immunoglobulin M; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; HRMRI, high-resolution MRI; MCA, middle cerebral artery; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; TPMT, thiopurine S-methyltransferase; FMF, familial Mediterranean fever.
References
1. Yazici H. Behcet's syndrome: an update. Curr Rheumatol Rep. (2003) 5:195–9. doi: 10.1007/s11926–003-0066–9
2. Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, et al. The long-term mortality and morbidity of Behcet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine. (2003) 82:60–76. doi: 10.1097/00005792–200301000-00006
3. Tsuruta D, Dainichi T, Hamada T, Ishii N, Hashimoto T. Molecular diagnosis of autoimmune blistering diseases. Methods Mol Biol. (2013) 961:17–32. doi: 10.1007/978–1-62703–227-8_2
4. Direskeneli H. Behcet's disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis. (2001) 60:996–1002. doi: 10.1136/ard.60.11.996
5. McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. (2006) 3:e297. doi: 10.1371/journal.pmed.0030297
6. Cantarini L, Lopalco G, Vitale A, Coladonato L, Rigante D, Lucherini OM, et al. Paradoxical mucocutaneous flare in a case of Behcet's disease treated with tocilizumab. Clin Rheumatol. (2015) 34:1141–3. doi: 10.1007/s10067–014-2589-z
7. Vitale A, Rigante D, Lopalco G, Selmi C, Galeazzi M, Iannone F, et al. Interleukin-1 Inhibition in Behcet's disease. Isr Med Assoc J. (2016) 18:171–6.
8. Criteria for diagnosis of Behcet's disease. International Study Group for Behcet's Disease. Lancet. (1990) 335:1078–80. doi: 10.1016/0140–6736(90)92643-V
9. The International Criteria for Behcet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. (2014) 28:338–47. doi: 10.1111/jdv.12107
10. Akman-Demir G, Serdaroglu P, Tasci B. Clinical patterns of neurological involvement in Behcet's disease: evaluation of 200 patients. The Neuro-Behcet Study Group. Brain. (1999) 122 (Pt 11):2171–82. doi: 10.1093/brain/122.11.2171
11. Siva A, Kantarci OH, Saip S, Altintas A, Hamuryudan V, Islak C, et al. Behcet's disease: diagnostic and prognostic aspects of neurological involvement. J Neurol. (2001) 248:95–103. doi: 10.1007/s004150170242
12. Serdaroglu P. Behcet's disease and the nervous system. J Neurol. (1998) 245:197–205. doi: 10.1007/s004150050205
13. Kidd D, Steuer A, Denman AM, Rudge P. Neurological complications in Behcet's syndrome. Brain. (1999) 122 (Pt 11):2183–94. doi: 10.1093/brain/122.11.2183
14. Reynolds N. Vasculitis in Behcet's syndrome: evidence-based review. Curr Opin Rheumatol. (2008) 20:347–52. doi: 10.1097/BOR.0b013e3282ff0d51
15. Mendes D, Correia M, Barbedo M, Vaio T, Mota M, Goncalves O, et al. Behcet's disease–a contemporary review. J Autoimmun. (2009) 32:178–88. doi: 10.1016/j.jaut.2009.02.011
17. Zelenski JD, Capraro JA, Holden D, Calabrese LH. Central nervous system vasculitis in Behcet's syndrome: angiographic improvement after therapy with cytotoxic agents. Arth Rheum. (1989) 32:217–20. doi: 10.1002/anr.1780320217
18. Pannone A, Lucchetti G, Stazi G, Corvi F, Ferguson TL, Massucci M, et al. Internal carotid artery dissection in a patient with Behcet's syndrome. Ann Vasc Surg. (1998) 12:463–7. doi: 10.1007/s100169900185
19. Shugaiv E, Tuzun E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behcet's disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol. (2011) 29 (Suppl. 67):S64–7.
20. Davies PG, Fordham JN, Kirwan JR, Barnes CG, Dinning WJ. The pathergy test and Behcet's syndrome in Britain. Ann Rheum Dis. (1984) 43:70–3. doi: 10.1136/ard.43.1.70
21. Krause I, Molad Y, Mitrani M, Weinberger A. Pathergy reaction in Behcet's disease: lack of correlation with mucocutaneous manifestations and systemic disease expression. Clin Exp Rheumatol. (2000) 18:71–4.
22. Koc Y, Gullu I, Akpek G, Akpolat T, Kansu E, Kiraz S, et al. Vascular involvement in Behcet's disease. J Rheumatol. (1992) 19:402–10.
23. Chang HK, Cheon KS. The clinical significance of a pathergy reaction in patients with Behcet's disease. J Korean Med Sci. (2002) 17:371–4. doi: 10.3346/jkms.2002.17.3.371
24. Ben Ahmed M, Houman H, Miled M, Dellagi K, Louzir H. Involvement of chemokines and Th1 cytokines in the pathogenesis of mucocutaneous lesions of Behcet's disease. Arth Rheum. (2004) 50:2291–5; doi: 10.1002/art.20334
25. Itoh R, Takenaka T, Okitsu-Negishi S, Matsushima K, Mizoguchi M. Interleukin 8 in Behcet's disease. J Dermatol. (1994) 21:397–404. doi: 10.1111/j.1346–8138.1994.tb01762.x
26. Ozoran K, Aydintug O, Tokgoz G, Duzgun N, Tutkak H, Gurler A. Serum levels of interleukin-8 in patients with Behcet's disease. Ann Rheum Dis. (1995) 54:610. doi: 10.1136/ard.54.7.610
27. Bardak Y, Aridogan BC. The demonstration of serum interleukin 6–8, tumor necrosis factor-alpha, complement, and immunoglobulin levels in Behcet's disease with ocular involvement. Ocul Immunol Inflamm. (2004) 12:53–8. doi: 10.1076/ocii.12.1.53.28062
28. Kaburaki T, Fujino Y, Kawashima H, Merino G, Numaga J, Chen J, et al. Plasma and whole-blood chemokine levels in patients with Behcet's disease. Graefes Arch Clin Exp Ophthalmol. (2003) 241:353–8. doi: 10.1007/s00417–003-0668-y
29. Freire Ade L, Bertolo MB, de Pinho AJ Jr, Samara AM, Fernandes SR. Increased serum levels of interleukin-8 in polyarteritis nodosa and Behcet's disease. Clin Rheumatol. (2004) 23:203–5. doi: 10.1007/s10067–003-0851-x
30. Evereklioglu C, Er H, Turkoz Y, Cekmen M. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behcet's disease. Mediators Inflamm. (2002) 11:87–93. doi: 10.1080/09629350220131935
31. Gur-Toy G, Lenk N, Yalcin B, Aksaray S, Alli N. Serum interleukin-8 as a serologic marker of activity in Behcet's disease. Int J Dermatol. (2005) 44:657–60. doi: 10.1111/j.1365–4632.2004.02184.x
32. Katsantonis J, Adler Y, Orfanos CE, Zouboulis CC. Adamantiades-Behcet's disease: serum IL-8 is a more reliable marker for disease activity than C-reactive protein and erythrocyte sedimentation rate. Dermatology. (2000) 201:37–9. doi: 10.1159/000018426
33. Durmazlar SP, Ulkar GB, Eskioglu F, Tatlican S, Mert A, Akgul A. Significance of serum interleukin-8 levels in patients with Behcet's disease: high levels may indicate vascular involvement. Int J Dermatol. (2009) 48:259–64. doi: 10.1111/j.1365–4632.2009.03905.x
34. Pineton de Chambrun M, Wechsler B, Geri G, Cacoub P, Saadoun D. New insights into the pathogenesis of Behcet's disease. Autoimmun Rev. (2012) 11:687–98. doi: 10.1016/j.autrev.2011.11.026
35. Zouboulis CC, Katsantonis J, Ketteler R, Treudler R, Kaklamani E, Hornemann S, et al. Adamantiades-Behcet's disease: interleukin-8 is increased in serum of patients with active oral and neurological manifestations and is secreted by small vessel endothelial cells. Arch Dermatol Res. (2000) 292:279–84. doi: 10.1007/s004030000128
36. Krespi Y, Akman-Demir G, Poyraz M, Tugcu B, Coban O, Tuncay R, et al. Cerebral vasculitis and ischaemic stroke in Behcet's disease: report of one case and review of the literature. Eur J Neurol. (2001) 8:719–22. doi: 10.1046/j.1468–1331.2001.00300.x
37. Nishimura M, Satoh K, Suga M, Oda M. Cerebral angio- and neuro-Behcet's syndrome: neuroradiological and pathological study of one case. J Neurol Sci. (1991) 106:19–24. doi: 10.1016/0022–510X(91)90188-D
38. Gisbert JP, Gomollon F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. (2008) 103:1783–800. doi: 10.1111/j.1572–0241.2008.01848.x
39. Gisbert JP, Gonzalez-Lama Y, Mate J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. (2007) 102:1518–27. doi: 10.1111/j.1572–0241.2007.01187.x
40. Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Theoret Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. (2000) 118:705–13. doi: 10.1016/S0016–5085(00)70140–5
41. Sato T, Takagawa T, Kakuta Y, Nishio A, Kawai M, Kamikozuru K, et al. NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases. Intest Res. (2017) 15:328–37. doi: 10.5217/ir.2017.15.3.328
42. Borhani Haghighi A, Safari A. Proposing an algorithm for treatment of different manifestations of neuro-Behcet's disease. Clin Rheumatol. (2010) 29:683–6. doi: 10.1007/s10067–010-1414–6
43. Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, et al. Diagnosis and management of Neuro-Behcet's disease: international consensus recommendations. J Neurol. (2014) 261:1662–76. doi: 10.1007/s00415–013-7209–3
44. Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med. (2015) 128:461–70. doi: 10.1016/j.amjmed.2014.12.010
45. Akar S, Yuksel F, Tunca M, Soysal O, Solmaz D, Gerdan V, et al. Familial Mediterranean fever: risk factors, causes of death, and prognosis in the colchicine era. Medicine. (2012) 91:131–6. doi: 10.1097/MD.0b013e3182561a45
46. Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2015) 36:2921–64. doi: 10.1093/eurheartj/ehv318
47. Yurdakul S, Mat C, Tuzun Y, Ozyazgan Y, Hamuryudan V, Uysal O, et al. A double-blind trial of colchicine in Behcet's syndrome. Arth Rheum. (2001) 44:2686–92. doi: 10.1002/1529-0131(200111)44:11<2686::aid-art448>3.0.co;2-h
48. Louyot P, Gaucher A, Mathieu J. [Intermittent hydarthrosis of the knee as a component in the picture of Behcet's disease. Apparent cure of the recurrent articular effusion using chloroquine]. Ann Med Nancy. (1962) 1:241–9.
49. Gonzalez-Echavarri C, Capdevila O, Espinosa G, Suarez S, Marin-Ballve A, Gonzalez-Leon R, et al. Infections in newly diagnosed Spanish patients with systemic lupus erythematosus: data from the RELES cohort. Lupus. (2018) 27:2253–61. doi: 10.1177/0961203318811598
Keywords: Behcet's disease, neuro-Behcet's disease, cerebral infarction, cerebral arteritis, IL-8
Citation: Yin H, Song Y, Zheng M, Han J and Tang J (2019) Behcet's Disease With Cerebral Artery Infarction Caused by Cerebral Arteritis as an Early Symptom Only With Elevated Interleukin-8. Front. Neurol. 10:1102. doi: 10.3389/fneur.2019.01102
Received: 04 June 2019; Accepted: 01 October 2019;
Published: 22 October 2019.
Edited by:
Johannes Boltze, University of Warwick, United KingdomReviewed by:
Giuseppe Lopalco, Interdisciplinary Department of Medicine, ItalyAlexander Tsiskaridze, Tbilisi State University, Georgia
Copyright © 2019 Yin, Song, Zheng, Han and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiyou Tang, jytang001@sohu.com
 Hao Yin
Hao Yin Yun Song1,2
Yun Song1,2 Meimei Zheng
Meimei Zheng Ju Han
Ju Han Jiyou Tang
Jiyou Tang