- 1School of Medicine, Kabale University, Kabale, Uganda
- 2Department of Anatomy, Kampala International University, Kampala, Uganda
- 3Facultad de Medicina, Instituto de Fisiología y Biofísica (IFIBIO)-Houssay, Universidad de Buenos Aires - Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina
- 4School of Psychology, University of Sussex, Brighton, United Kingdom
- 5Department of Zoology, University of Cambridge, Cambridge, United Kingdom
- 6Brighton and Sussex Medical School, University of Sussex, Brighton, United Kingdom
- 7Anatomy Unit, Institute of Biomedical Sciences, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
Epilepsy is among the most common serious neurological disorders and affects around 50 million people worldwide, 80% of which live in developing countries. Despite the introduction of several new Anti-Epileptic Drugs (AEDs) in the last two decades, one third of treated patients have seizures refractory to pharmacotherapy. This highlights the need to develop new treatments with drugs targeting alternative seizure-induction mechanisms. Traditional medicine (TM) is used for the treatment of epilepsy in many developing countries and could constitute an affordable and accessible alternative to AEDs, but a lack of pre-clinical and clinical testing has so far prevented its wider acceptance worldwide. In this study we used Drosophila melanogaster paralytic bangsensitive (parabss) mutants as a model for epileptic seizure screening and tested, for the first time, the anti-seizure effect of a non-commercial AED. We evaluated the effect of the African custard-apple, Annona senegalensis, which is commonly used as a TM for the treatment of epilepsy in rural Africa, and compared it with the classical AED phenytoin. Our results showed that a stem bark extract from A. senegalensis was significantly more effective than a leaf extract and similar to phenytoin in the prevention and control of seizure-like behavior. These results support that Drosophila constitutes a robust animal model for the screening of TM with potential value for the treatment of intractable epilepsy.
Introduction
Epilepsy is among the most common serious neurological disorders and affects around 50 million people worldwide, with 80% of them living in developing countries (1–4). Epilepsy can cause frequent seizures, which are brief episodes of involuntary shaking involving part of or the entire body, and are sometimes accompanied by a loss of consciousness. Seizures can vary in intensity from brief lapses of attention or muscle jerks, to severe and prolonged convulsions. They can also vary in frequency, from less than one per year to several per day.
These episodes are triggered by hyper-excitation and/or abnormal synchronization of activity across neuronal circuits (5). The causes of epilepsy are attributed to acquired vs. genetic factors. Acquired epilepsy, such as those resulting from trauma, stroke, neoplasm, infection, congenital malformations, birth anoxia and autoimmune, represent slightly more than a quarter of the cases. Epilepsy with complex inheritance, ranging from mono-gene mutations to the presence of modifiers and susceptibility alleles has emerged as the main causes of idiopathic epilepsy (6). Mutations in channels, that determine cellular excitability, have been associated with a wide range of epilepsies (5, 7). Amongst them is the sodium channel SNC1A, the human ortholog of the parabss gene of Drosophila melanogaster, with over 600 different mutations found in patients (8). Links to other ionic channels (e.g., voltage-gated sodium channel a2 gene subunit SCN2A), receptors (e.g., the N-methyl-D-aspartate-type glutamate receptor NR2A subunit GRIN2A), synaptic proteins (e.g., PRRT2 synaptic release) and brain development pathways (e.g., mTOR) have also been proposed. Anti-epileptic drugs (AEDs) mechanism of action aims to control neuronal hyper-excitation by modifying ion channels functioning (8). Recent studies in both developed and developing countries have shown that up to 70% of epilepsy patients can be successfully treated (i.e., their seizures completely controlled) with AEDs (9, 10). But these drugs produce undesired secondary effects and in 30% of patients they are ineffective, highlighting the need to develop new alternative treatments (9, 10) aimed at alternative cellular mechanisms acting to stabilize neuronal activity.
In many developing countries, particularly in Africa and Asia, phenobarbital is the most commonly first-line prescribed AED as recommended by the World Health Organization (WHO) (7, 11–13), and this is likely because the other proven AEDs phenytoin, carbamazepine, and valproate are up to 5, 15, and 20 times more expensive, respectively (14–16). Despite the fact that access to medicines can cost as little as US$5 per year, three quarters of patients with epilepsy have no access to treatment (1). The availability and accessibility of Traditional Medicine (TM) means that it plays an important role in meeting the demands of primary health care. This was recognized by the 2008 WHO Congress on Traditional Medicine in Beijing (17), which resolved to promote the wider role of TM in worldwide health care and declared that TM should be “further developed based on research and innovation” (17).
The wild African custard-apple, Annona senegalensis (Magnoliales: Annonaceae) is commonly found in savannas throughout tropical Africa. Also known as soursop, dorgot (Wolof) and sunkungo (Mandinka), a decoction of its leaves and roots is used by rural African communities as a TM for the treatment of seizures, suggesting that the preparation has anticonvulsant and/or sedative properties (18). Studies have shown that extractions from root or stem have mild anticonvulsant properties on chemically induced seizures in rodents, supporting a possible role of this TM in the treatment of epilepsy (19–24). These experiments are very promising, but the screening of drugs in rodents is too costly to test the majority of ethnobiologically important candidates. Furthermore, follow-up studies investigating the pharmacological properties of novel drug candidates are intricate and even more expensive. There is therefore a need to develop a reliable, cost-effective and high throughput method for the screening of TMs with apparent anti-seizure properties that could be implemented prior to testing in animal models and clinical trials.
Adult Drosophila flies represent a genetically accessible and behaviorally tractable model for the study of seizures (25–27). Its strength resides in the high evolutionary conservation of most molecules controlling neural function (28). In particular, ion channels and synaptic transmission machinery proteins are largely comparable (29). Drosophila and humans also share several similarities in seizure phenotype thereof: (i) all individuals have a seizure threshold; (ii) seizure susceptibility can be modulated by genetic mutations; (iii) seizure activity threshold is increased by a previous electroconvulsive shock treatment; (iv) seizure activity spreads through the central nervous system (CNS) along particular pathways; (v) there is a spatial segregation of seizure activity into particular regions of the CNS; and (vi) seizure phenotypes in flies can be ameliorated by several AEDs used in humans including sodium valproate, phenytoin, gabapentin, and potassium bromide (25).
Furthermore, because Drosophila presents little gene redundancy, it offers a unique opportunity to study human mutated genes in an animal model (30, 31). Using CRISPR/Cas9 (32) it is easy to generate knock-in mutants where the endogenous copy of the gene is replaced by a mutated version of the human homolog, and these flies can then be used for drug testing to reveal behavioral, physiological and molecular effects (30, 31).
In this study we evaluated, for the first time, the effect of a non-commercially available drug for the treatment of seizure in Drosophila adult flies. We tested the hypothesis that A. senegalensis leaf and stem bark extracts affect the seizure patterns present in parabss mutant adult flies (33, 34). We showed that A. senegalensis stem bark extract was significantly more effective than a leaf extract and similar to phenytoin in the prevention and control of seizure-like behavior. These results support that Drosophila is a robust and sensitive animal model that can be used to screen pharmacologically untested compounds which, combined with centuries of transmitted knowledge about anti-convulsant TMs, could expedite the development of the most effective TMs into novel AEDs for patients irresponsive to classical treatments.
Materials and Methods
Identification and Extract Preparation of Annona senegalensis
Annona senegalensis leaves and root barks were collected from “Boroboro,” 5 Km from Lira Municipality along Soroti road, Northern Uganda. Geographic coordinates are 2.190341, 32.929115 (2011'25.2”N 32055'44.8”E). Plant was identified/deposited at Department of Biology Mbarara University of Science and Technology, Uganda and given a voucher No. Moses Odur 002.
The leaves and bark removed from stem were dried and subjected to an aqueous extraction method at the Department of Pharmacology laboratory (School of Health Sciences, Kampala International University Western Campus, Ishaka-Bushenyi, Uganda). Specifically, dry leaves and stem were grounded using a blender to obtain 200 g of powder which was mixed with 1 l of distilled water in a sterile conical flask. The mixture was placed on a shaker for 72 h and then sieved to remove debris. The remaining liquid was filtered by gravity using Whatman no. 1 filter paper. The filtrate was incubated at 35°C for 1 week to evaporate the water and obtain a dry powder (35). The powder extract, approximately 10 g, was stored at 4°C until the behavioral experiments were performed.
A phytochemical analysis was performed on the aqueous extracts of the leaf and stem bark at the Kampala International University Biochemistry department lab (Supplementary Tables 1, 2).
Drug Preparation
Given the concentration of the active compound in the aqueous A. senegalensis extract or the effective dose was unknown, the initial assessments were done using a high concentration (26.66 mg.ml−1). Animals treated with this extract solution appeared healthy and showed less seizure-like behavior than controls. We decided to expand the analysis to lower extract concentrations. A. senegalensis leaf and bark stem extracts and phenytoin were administered by mixing them with standard cornmeal food, which consists of 420 g of cornmeal; 450 g of dextrose; 90 g of yeast; 42 g of agar; 140 ml of 10% Nipagin in 95% EtOH; 22 ml of propionic acid and 6.4 l of water. Drug and extract solutions were prepared fresh before each experiment. Experimental doses are expressed as mg of compound per ml of food. For 13.33 and 26.66 mg.ml−1, the powder leaf or bark stem extract was dissolved in distilled water in a beaker at room temperature. Warm food (<60°C) was then added and mixed before aliquoting approximately 1.5 ml per testing vial. For the low concentration doses of 0.26, 1.33, and 2.67 mg.ml−1 a stock solution of 2.67 mg.ml−1 was used.
For phenytoin, one 100 mg tablet (Tophen; Agog Pharma Ltd, India) was suspended in 1 ml of distilled water, and 50, 250 μl or 500 μl of drug suspension were then mixed with 5 ml of food resulting in the low and high concentrations, respectively: phenytoin 0.909, 4.76, and 9.09 mg.ml−1.
Flies Treatment and Behavioral Analysis
Flies of the bang sensitive family, specifically bang senseless (bss1) mutants of the paralytic gene parabss1 and easily shocked (eas2F) were obtained from Prof Richard Baines laboratory (University of Manchester, UK), were used (26). Oregon R (OrR) is a wildtype strain that served as a control.
Young adult flies (6–9 days) were anesthetized by cold exposure in a freezer (−20°C), separated on a cold plate into males and females and assigned to vials with 10 animals each. The flies were then randomly assigned to the different treatments vials, (standard cornmeal food with or without drug/extract). Each treatment was tested in at least 3 different days with flies from different cultures.
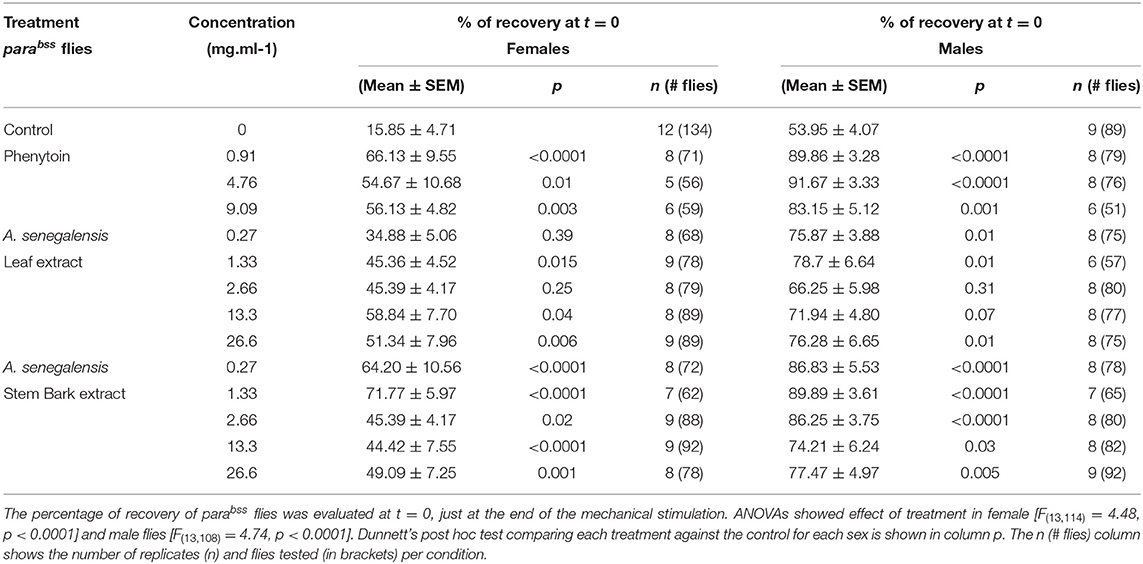
Flies were allowed to feed for 24 h prior to behavioral manipulations. On the day of behavioral assessment, flies were gently transferred into empty vials and immediately mechanically stimulated by placement on a bench-top vortex at maximum speed for 10 s (36, 37). Number of flies per vial on their backs, paralyzed, shaking or standing were recorded at 30 s intervals for 20 min. Paralyzed and shaking behaviors were recorded as seizures, whereas fully recovered flies were defined as showing standing behavior. Number of replicates and number of flies tested is detailed in Figure 1 legend and in Table 1. A few flies died before testing (sticking to the food during transfer or the 24 h drug treatment) and we had counted 1 or 2 extra flies in a few tubes. The average number of female flies tested for each treatment compared with the control was not different apart for female leaf 0.27 mg.ml−1 and 1.33 mg.ml−1 [ANOVA F(13, 101) = 4.20, p < 0.0001, η2 = 0.35; Dunnett's multiple comparisons test p = 0.008 and p= 0.01, respectively]. No differences were registered in males [F(13,95) = 1.65, p = 0.09, η2 = 0.18]. This supports that survival of flies was not affected by drug or extract treatment condition. We aimed to have 8 tube replicas for each extract or drug concentration but labeling errors between male and female flies produced variability on the final sample size.
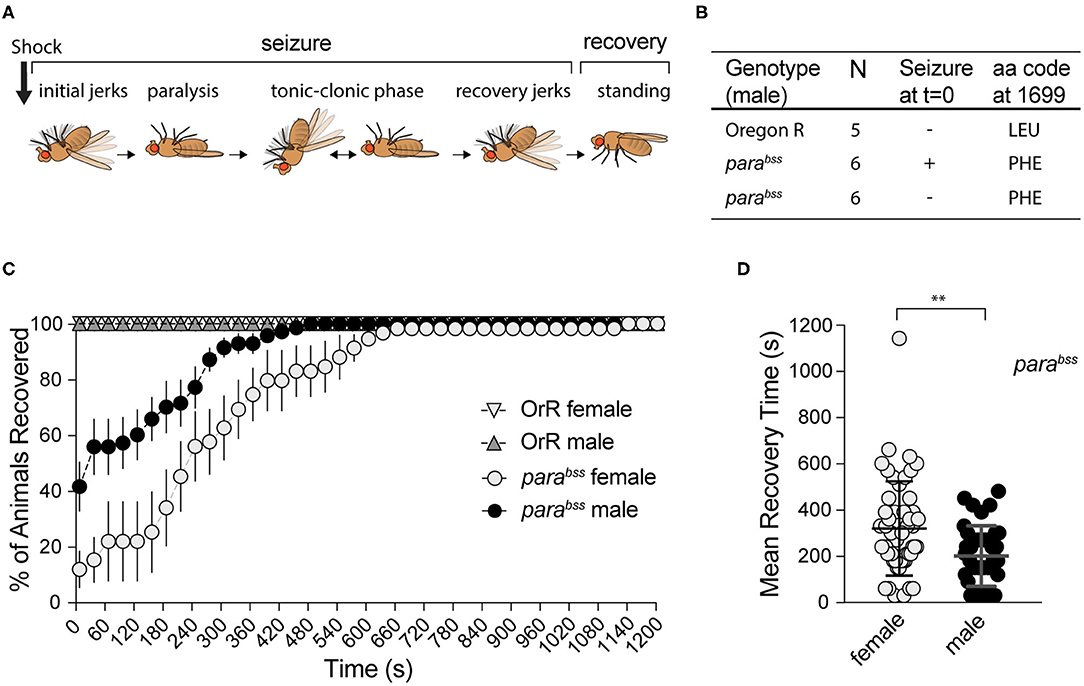
Figure 1. Effect of mechanical shock on seizuring behavior for female and male adult flies of parabss or OrR genetic background. (A) Schematic of the different phases of seizure after a mechanical shock [modified from Parker et al. (38)]. (B) Result of parabss gene sequencing in seizuring and non-seizuring males to test for the presence of the PHE mutation in position 1699 of the protein. All flies have the mutation. (C) Mechanical stimulation did not induce seizuring in OrR flies. parabss flies showed high proportion of seizuring at t = 0, the percentage of flies recovered increased as a function of time. The graph shows the mean % of flies recovered ± SEM [parabss females n = 6 (60 flies); parabss males n = 7(70 flies); OrR females n = 3 (30 flies); OrR males n = 3(30 flies)]. (D) Mean recovery time comparing males and females parabss (Mann−WhitneyU = 667, nfemales = 51, nmales = 41, P = 0.0034 two-tailed).
Mean recovery time was calculated as the mean of the time it took for each individual that showed a bang sensitive phenotype to complete recovery. Flies that never seized were not included and animals that had not completely recovered at the end of the experiments were included as 1,200 s.
Sequencing of paralytic Gene
Male flies were given a vortex shock to induce seizures as describe above. Seizing males were separated from non-seizing males. The DNA of individual parabss and OrR flies was extracted using microLysis plus (Clent-Life Science), part of gene amplified using F1: TGTCAAGTGTTTATGTCTCGAGC and R1: CAGATGTTGAACAGGGCCG and sequenced using F2: TCCAGGAGCTTTAGTCGCC and R1 primers that allow to amplify the region that encodes the amino acid in 1699 which is mutated in parabss mutants (38).
Gut Content Quantification
Groups of 10 flies were fed with the extract solutions plus 0.5% (w/v) bromophenol blue salt (B5525, Sigma) for 24 h, as in the behavioral experiments. Sub-groups of 3 flies where homogenized in 30 μl of PBS (1x) with a plastic pestle in a 1.5 ml Eppendorf tube, centrifuged twice at 13,000 rpm for 2 min (39). Dye content of the second supernatant was quantified at 594 nm using a NanodropOne spectrophotometer (Thermo Scientific). The background absorbance was obtained processing groups of flies reared in the same manner but on dye-free food. The gut food content was calculated based on a standard curve done with serial dilutions of 0.5% (w/v) bromophenol blue in PBS.
Seizuring Data Normalization
Seizure data for each sex was normalized independently. Mean recovery of control flies (without extract/drug) at t = 0 (Rct0) was considered as 0% recovery, and used to normalize recovery for each experimental condition at t = 0 (Rtit0). Therefore, recovery normalized was calculated as Rnorm = (Rtit0–Rct0)/(100–Rct0) × 100. Normalized values were used to calculate mean and SEM.
Statistical Analysis
All behavioral data was expressed as the mean % of flies per experimental vial ± SEM. Mixed ANOVAs, with group (sex or treatment) as the between-subject factor and time as the within-subject factor, were used to analyse recovering behavior. If the ANOVA showed a significant effect of time, drug treatment or the interaction between time and drug treatment, we performed a Dunnett's post hoc test to compare between sexes and/or drug concentrations. An ANOVA and Dunnett's post hoc test comparing treatments was applied to evaluate differences at t = 0.
Because the distribution of MRT is not normal (as tested with D'Agostino & Pearson's normality test), a Kruskal-Wallis test followed by Dunn's multiple comparisons test to compare the MRT for each drug treatment to the control was done. A two-way ANOVA with post hoc Dunn's multiple comparisons test was performed to analyse the food intake experiment. The α level was set at 0.05 for all analyses.
Biosecurity Statement
The animal facility, flyroom, is a secure facility with access only granted to researchers who have had an induction to the animal facility covering health and safety and biosecurity arrangements. Access is through a swipe card system with approval only given on completion of the induction. To prevent animals escaping, the flyroom windows are sealed and the doors remain closed except when people are coming in and out and when moving equipment. In all cases there are at least three doors between the flyroom and the outside to further minimize the risk of animals escaping from the facility.
Results
Behavioral Characterization of parabss Mutants
In order to evaluate the effect of A. senegalensis extracts on seizures, we first characterized the seizure-like phenotype (from now on referred to as seizuring) of our parabss mutant strain. parabss is a bang-sensitive mutant, extremely sensitive to seizures that are characterized by 6 phases (Figure 1A). In response to a mechanical shock, many flies become immediately paralyzed while others have a short period of shaking accompanied by leg twitching, abdominal muscle contractions, rapid wing flapping and proboscis extensions before paralysis. In a fraction of animals' paralysis is interrupted by muscle jerks, in what has been described as a tonic-clonic phase. Most flies recover within 10 min following the mechanical shock, some of them after a short period of recovery shaking. After 20 min all animals are capable of maintaining their standing posture and can walk or fly (38) (Figure 1A).
The seizure phenotype observed in parabss is due to a gain of function mutation, L1699F, in the sole fly voltage gated sodium channel gene paralytic (38) (Figure 1B). This mutation alters the voltage dependency of channel inactivation, making neurons more excitable and increasing the risk of aberrant electrical activity and seizures (38). Mutations in the human ortholog, SCN1A, are associated with a wide spectrum of epilepsies with over 600 mutations registered in this sole locus (8).
To induce seizures, we performed a mechanical stimulation (10 s vortex) to parabss and wildtype OrR adult flies. We evaluated behavioral patterns of female and male flies separately because parabss mutation is located in the X chromosome.
Immediately after induction (t = 0), none of the 60 (10 per vial) wildtype OrR flies evaluated showed a seizuring phenotype, while 88 ± 5% of parabss females and 59 ± 8% of males seizuring (Two-way ANOVA, p = 0.023; Figure 1C). As expected, after a few seconds paralyzed, flies started to recover. Some of them recovered their standing posture directly while others went through a tonic-clonic phase, showing strong wing flapping and leg shaking, before standing. Mean recovery time (MRT) for flies showing a seizure-like phenotype at the beginning of the experiment was longer for females (319 ± 28 s) than for males (201 ± 20 s; Mann-Whitney test: U = 677, p = 0.0034; Figure 1D).
A mixed ANOVA on percentage of animals recovered (Figure 1C) showed an effect of time [time: F(40,440) = 34.03, p < 0.001, η2 = 0.76] and sex [F(1,11) = 11.16, p = 0.007, η2 = 0.50], and an interaction [time X sex: F(40,440) = 3.17, p < 0.001, η2 = 0.22]. These results showed an unexpected sexual dimorphism in seizuring phenotype, with males being more resistant to the initiation of seizures and recovering faster than females if and when they showed the seizure phenotype. We confirmed that our stock still carries the mutation described in the literature, sequencing the paralytic gene in OrR wildtype flies and parabss mutants. All 12 mutants, 6 of which did not seize at t = 0, had LEU mutated to PHE in position 1699. Therefore, despite these differences, the high penetrance of seizure-like behavior and the reproducibility of the phenotype confirm that parabss flies are well suited to evaluate anti-seizure properties of candidate compounds as well as the dynamics of recovery from seizuring and indicate that male and female behavior need to be assessed separately.
Effect of Annona senegalensis Extracts: Female Flies
To evaluate the effect of A. senegalensis as an anticonvulsant, we compared the seizure-like behavior of parabss flies kept in control food to animals treated with food mixed with aqueous extract from leaf or stem bark of A. senegalensis. As a positive control, a group of flies was treated with phenytoin, a commonly prescribed AED which has already been tested in flies (36) (Figure 2). Flies received an acute drug treatment consisting of feeding on control or treated food for 24 h before behavioral testing. Seizure-like behavior measured as the % of recovered animals across time was analyzed by mixed ANOVA comparing the effect of drug and time for female flies. An effect of time [time: F(40,4040) = 238.83, p < 0.0001, η2 = 0.70], drug [drug: F(13,101) = 4.53, p < 0.0001, η2 = 0.37] and an interaction [time X treatment: F(520,4040) = 3.29, p < 0.0001, η2 = 0.30] was found in females confirming that the treatments modified the time of recovery from seizures. We therefore analyzed the individual effect of each drug treatment on the seizure-like phenotype.
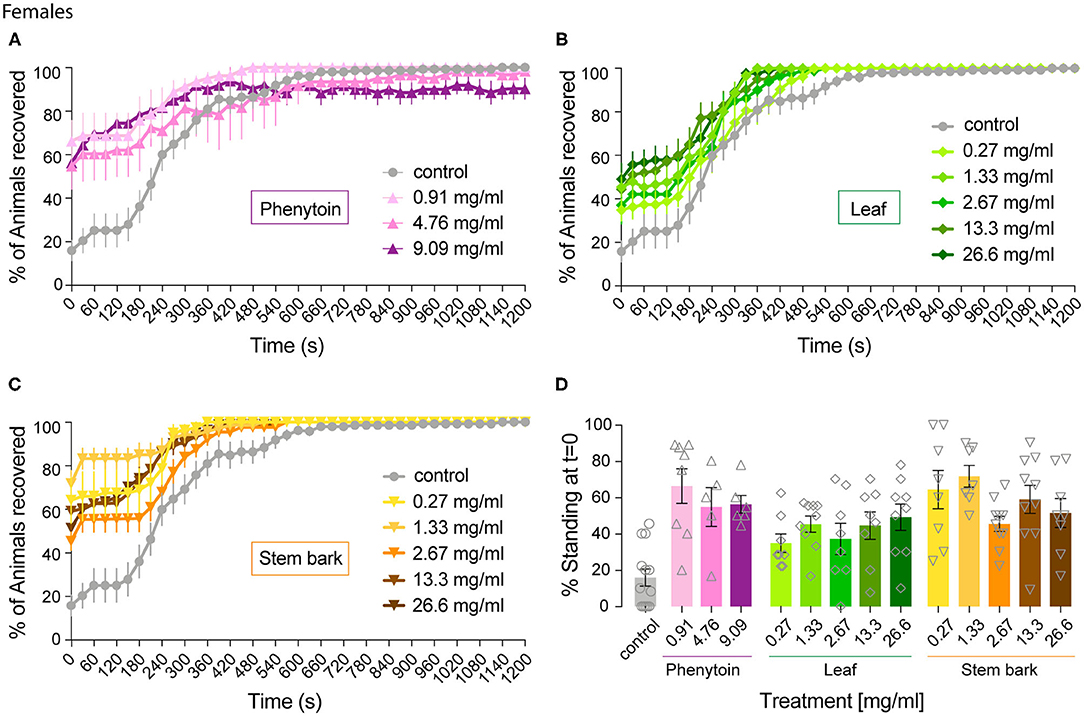
Figure 2. Effect of phenytoin and A. senegalensis leaf and stem extracts on recovery from seizures of parabss female flies. parabss flies treated with control food showed a very low percentage of flies recovered from seizures after mechanical perturbation, which improved over time. (A) Treatment with phenytoin increased the percentage of recovered flies at three different doses. (B) Treatment with A. senegalensis leaf extracts increased the percentage of flies recovered. (C) A. senegalensis stem extract doses showed a greater effect than the leaf extract. (D) Dose response analysis at t = 0 for all treatments. Graphs show the mean % of flies seizuring ± SEM (for number of replicates, flies and significance values see Table 1).
As expected, parabss flies kept in control food were significantly affected by the mechanical stimulation, with 15.85 ± 9.55% of flies standing at t = 0 and a MRT of 284 ± 21 s (Figure 3A).
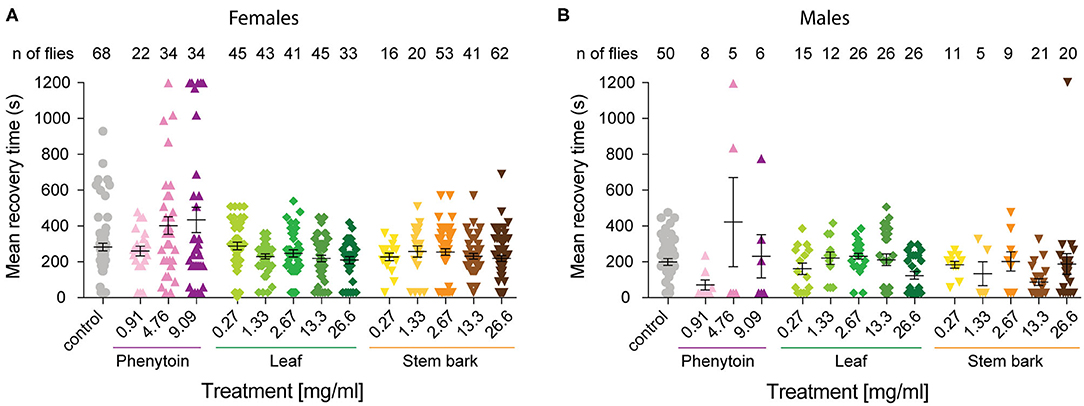
Figure 3. Mean recovery time for parabss flies treated with phenytoin and, leaf and stem bark A. senegalensis extracts. The time it took for each individual that showed a bang sensitive phenotype to complete recovery was calculated. Flies not seizuring were not included and if a fly was still seizuring at the end of the experiment its time was recorded as 1,200 s. Mean ± SEM is shown. (A) The MRT was significantly affected by the treatment in females (Kruskal-Wallis test H(14) = 30.21, p = 0.004). There were no differences between the control and other treatments as tested with a Dunn's post hoc test. (B) The MRT was significantly affected by the treatment in males (Kruskal-Wallis test H(14) = 31.04, p = 0.003). Dunn's post hoc test showed that the control was only different with the stem bark 13.3 mg.ml−1 treatment (p = 0.02). Number of males is lower because MRT measures the time to recovery of paralyzed flies, and fewer males than females where paralyzed at t = 0.
As a positive control we evaluated phenytoin, a drug known to modulate the gating of voltage gated sodium channels (40). Phenytoin has already been shown to diminish seizures in parabss flies, but those tests used different concentrations to the range used in our experiments and males and females were analyzed together. In the present study, phenytoin significantly improved the seizure-like phenotype of parabss females over time. A mixed ANOVA on % of animals recovered in control vs. the three phenytoin concentrations showed significant interaction effects of treatment across time [time X treatment: F(120,1120) = 5.07, p < 0.0001, η2 = 0.35] and time alone [time: F(40,120) = 39.94, p < 0.0001, η2 = 0.59], and an effect of treatment [F(3,28) = 3.69, p = 0.02, η2 = 0.28; Figure 2A]. A follow-on post hoc analysis showed a significant effect of phenytoin at 0.91 mg.ml−1 (Dunnett test: vs. control p = 0.007) but no effect at the higher doses, 4.76 and 9.09 mg.ml−1 (p > 0.3). At 4.76 and 9.09 mg.ml−1 we observed that the curves of % of recovery were below the curve of control after 480 s indicating that parabss flies were recovering faster than the flies treated with the higher concentrations (Figure 2A). Indeed, phenytoin failed to shorten the MRT at 4.76 and 9.09 mg.ml−1 and the tendency was to increase it (Figure 3A), suggesting that the higher concentrations might have a small toxic effect on the flies. However, phenytoin had a clear prophylactic effect at t = 0. The three concentrations increased the percentage of recovered flies being the lower concentration the most effective (Dunnett test: control vs. 0.91 mg.ml−1 p < 0.0001; vs. 4.76 mg.ml−1 p = 0.009 and vs. 9.09 mg.ml−1 p = 0.003; Figure 2D and Table 1). At these concentrations, phenytoin protected against the initiation of seizure (Figures 2A,D, 3).
We then analyzed the effect of A. senegalensis leaf extract (Figures 2B,D). The five curves of animals treated with the leaf extract showed an improvement in the percentage of flies recovered from seizure over time. This observation was confirmed by a mixed ANOVA on the percentage of flies recovered, which showed significant effects of time [time: F(40,1960) = 189.37, p < 0.0001, η2 = 0.79], treatment [drug: F(5,49) = 6.56, p < 0.0001, η2 = 0.40] and their interaction [time X drug: F(200,1960) = 3.07, p < 0.0001, η2 = 0.24]. A follow-on Dunnett's post hoc analysis comparing each drug with the control showed no effect at 0.27 mg.ml−1 (p = 0.31) and a greater time-dependent recovery in animals treated with the leaf extract above 1.33 mg.ml−1 (control vs.: 1.33 mg.ml−1 p < 0.0001; 2.67 mg.ml−1 p = 0.05; 13.3 mg.ml−1 p = 0.002 and 26.6 mg.ml−1 p = 0.001). The quantification at t = 0 showed an improvement in the percentage of flies insensitive to seizure depending on the concentration with a significant prophylactic effect at 0.27, 1.33, 13.33, and 26.6 mg.ml−1 (Figure 2D and Table 1). To test if the leaf extract alleviates the seizure by reducing their duration, we quantified the MRT. There was no shortening of the MRT comparing the parabss control to the five concentrations of leaf extract (Kruskal- Wallis with Dunn's post hoc test; Figure 3A). All together, these results indicate an anti-seizure effect of A. senegalensis leaf extract on parabss female flies that is mainly attributed to a prophylactic effect of the drug.
Finally, we evaluated the anti-convulsant properties of A. senegalensis stem bark extract (Figures 2C,D). A mixed ANOVA on percentage of flies recovered showed significant effects of time [time: F(40,1920) = 105.02, p < 0.0001, η2 = 0.69], treatment [F(5,48) = 9.67, p < 0.0001, η2 = 0.50] and of their interaction [time X treatment: F(200,1920) = 5.05, p < 0.0001, η2 = 0.35]. A follow-on post hoc analysis comparing the different concentrations to the control showed that the stem bark extract treatment produced a very significant improvement of the % of recovery (Dunnett test: control vs. 0.27, 1.33, 13.33, and 26.6 mg.ml−1 p < 0.0001 and 2.6 mg.ml−1 p = 0.006). Furthermore, the five concentrations of the drug showed a significant increment of the % of flies recovered compared to control at t = 0 (Figure 2D and Table 1). However, this differences over time were not reflected in shorter MRTs (Figure 3A). Meaning that if a fly has started seizing it will recover on average at the same pace as a parabss mutant which was not treated with the stem extract. As for the other drugs tested, the improvement in seizure-like behavior quantified in flies treated with the stem bark was mainly due to its prophylactic effect.
Effect of Annona senegalensis Extracts on Male Flies
We then evaluated the seizure phenotypes of males exposed to the same treatments as females. In males under control conditions, the percentage of flies recovered after mechanical stimulation is significantly higher than in females (200 ± 21 s; Figure 1C) and the MRT is significantly shorter (204 ± 19 s; Figure 3B). Significant drug effects were therefore expected mainly at the early time points of behavioral analysis. A mixed ANOVA on the percentage of male flies recovered after mechanical stimulation showed an effect of time [time: F(40,3800) = 96.55, p < 0.0001, η2 = 0.50], an interaction between time and treatment [time X treatment: F(520,3800) = 108.97, p < 0.0001, η2 = 0.32], and an effect of treatment alone [treatment: F(13,95) = 4.41, p < 0.0001, η2 = 0.38].
We then analyzed the behavioral effect for each drug treatment independently. A mixed ANOVA on percentage of flies recovered comparing phenytoin treated animals with controls showed an effect of time [time: F(40,1080) = 30.95, p < 0.0001, η2 = 0.53], an interaction between time and treatment [time X treatment: F(120,1080) = 12.40, p < 0.0001, η2 = 0.58] and of treatment [F(3,27) = 21.94, p < 0.0001, η2 = 0.71]. Further post hoc analysis showed that all three concentrations were significantly better than the control (Dunnett: control vs. 0.91, 4.76 and 9.09 mg.ml−1 of phenytoin p < 0.001; Figure 4A). Significant behavioral differences (p < 0.001) were found at t = 0 for the three concentrations tested (Figures 4A,D and Table 1). We did not observe differences in MRT across groups, probably due to a low number of flies seizuring at early time points. However, as observed in females, at 4.76 and 9.09 mg.ml−1 of phenytoin, a few flies had longer MRT then in controls, suggesting that the highest concentrations tested have a slight toxic effect on male flies as well (Figure 3B).
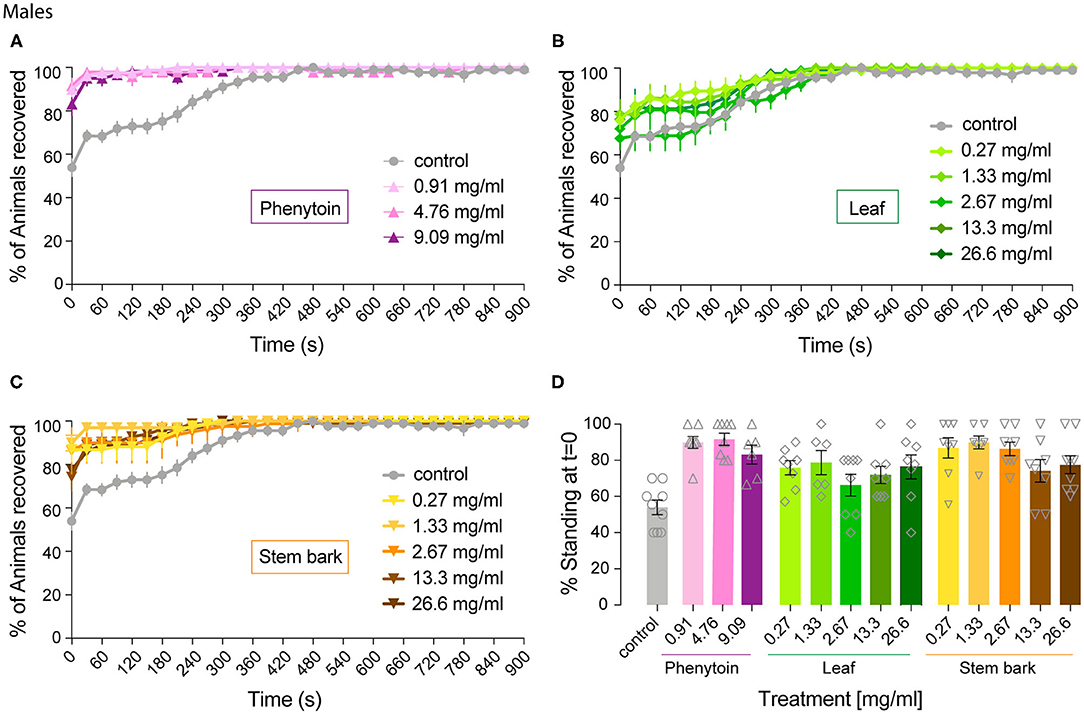
Figure 4. Effect of phenytoin and A. senegalensis extracts on recovery from seizures of parabss males. Mechanical stimulation of parabss control male flies induced a seizure-like phenotype that was less penetrant than in females. The effects of phenytoin (A), A. senegalensis leaf (B) or stem extract (C) are shown. (D) Dose response analysis at t = 0 for all treatments. Graphs show the mean % of flies seizuring ± SEM (for number of replicates, flies and significance see Table 1).
We then evaluated the effect of A. senegalensis leaf extract treatment. A mixed ANOVA on the percentage of flies recovered showed a significant effect of time [time: F(40,1640) = 68.50, p < 0.0001, η2 = 0.63], and a significant interaction between time and drug treatment [time X treatment: F(200,1640) = 1.46, p < 0.0001, η2 = 0.15], but no effect of drug alone [treatment: F(3,29) = 1.77, p = 0.14, η2 = 0.18; Figure 4B]. The lower effect of the leaf extract on males was further supported by a generalized more modest improvement at t = 0 (Figure 4D and Table 1). The MRT was not significantly improved for any concentration of the leaf extract (Figure 3B).
An effect of time, treatment and their interaction were present after treatment with stem bark extract of A. senegalensis in males [time: F(40,1720) = 48.57, p < 0.0001, η2 = 0.53; treatment: F(5,43) = 6.84, p < 0.0001, η2 = 0.44; time X treatment: F(200,1720) = 4.10, p < 0.0001, η2 = 0.32]. A Dunnett's post hoc analysis confirmed the improvement in the % of recovery of the flies treated with the stem bark extract compare to the untreated control, all concentrations being highly significant (control vs. 0.27, 1.33, and 26.6 mg.ml−1 p < 0.0001 and vs. 2.67 mg.ml−1 p = 0.001 and 13.3 mg.ml−1 p = 0.002; Figure 4C). As in phenytoin treated animals, stem bark extracts very significantly decreased the percentage of animals seizuring at t = 0 pointing to a prophylactic effect against the initiation of seizure-like behavior (Figure 4D and Table 1). Furthermore, the treatment with stem bark extract at 13.3 mg.ml−1 was the only treatment to significantly decrease the MRT (p = 0.02; Figure 3B) supporting the effectiveness of the drug.
Altogether, these results show that recovery in both female and male flies was improved by treatments with A. senegalensis stem and leaf extract as well as with the commonly used AED phenytoin. To gain a better insight into the dose-response curve, we normalized the percentage of recovery. This allowed us to only evaluate the effect of the drug and extracts on flies seizuring at t = 0 (now at t = 0 there is 0 ± SEM % recovery for all treatments; Figure 5A). In this condition the sexual dimorphism is no longer present, and we therefore pooled the data of both sexes. The three curves are in the plateau phase and no significant effect of concentration was found, apart for when comparing with the control. At saturated doses, the prophylactic effect of phenytoin is comparable to A. senegalensis stem bark administration (p = 0.66; Figure 5A). There is a slight but non-significant decrease of phenytoin and stem bark effects, suggesting that higher concentrations of these compounds may have toxic or sedative effect. The leaf extract of A. senegalensis showed a lower protective effect than phenytoin (p < 0.0001) and stem bark (p = 0.0002), present at all concentrations (Figure 5A). Differences of prophylactic effects are not explained by differences in amount of food ingested since there were no significant differences in gut content between treatments (Figure 5B). The strong effect of A. senegalensis stem extract suggests the existence of an AED compound that could offer an alternative for the treatment of intractable epilepsy.
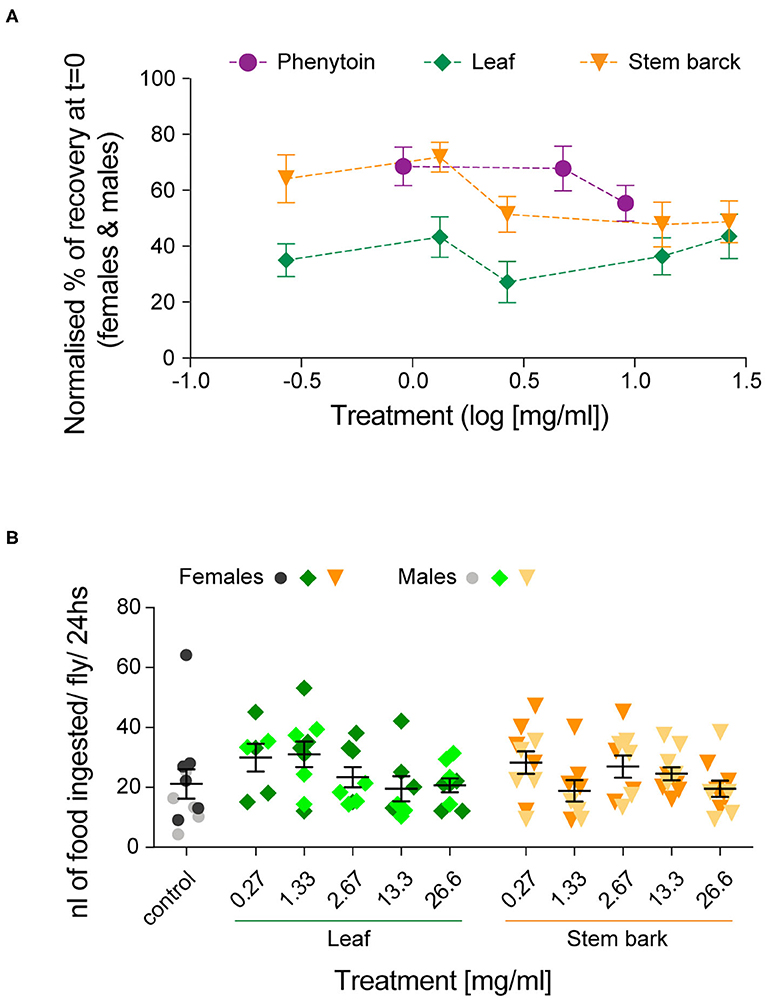
Figure 5. Dose-dependent prophylactic effect of phenytoin and, leaf and stem bark A. senegalensis extracts. (A) Normalized % of recovery at t = 0 for females and males. The concentration is in log10 scale. An ANOVA comparing the effect of the three drugs treatment was significant [F(2,199) = 15.06, p < 0.0001, η2 = 0.13]. A post hoc Bonferroni multiple comparisons test was used to test for differences between the drugs (phenytoin vs. leaf p < 0.0001; vs. stem p = 0.35 and leaf vs. stem p = 0.0001). No significant differences between concentrations of each drug were found, apart when comparing with the control (Tukey's multiple comparisons test). (B) Amount of food ingested per fly and per 24 h. A two way ANOVA showed no effect of drug [F(1,103) = 0.03, p = 0.86], no effect of concentration [F(5,103) = 1.63, p = 0.16] and no interaction [F(5,103) = 1.26, p = 0.28]. A post hoc Dunnett's multiple comparisons test comparing the control with each treatment did not yield any significant differences.
Effect of Annona senegalensis Extracts on easily shocked Mutants
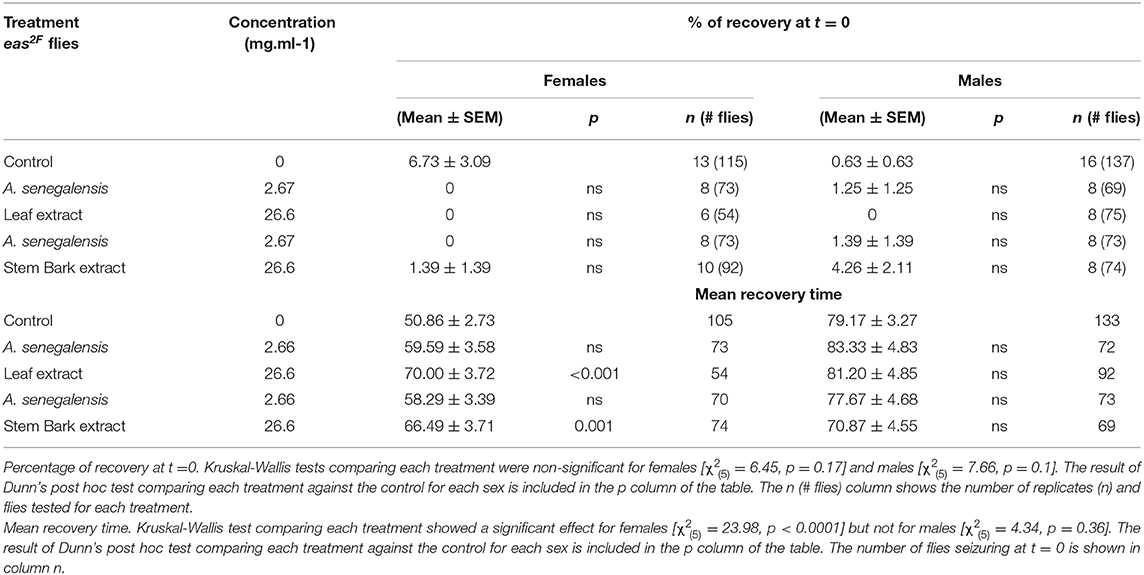
To test the efficacity of A. senegalensis when the molecular cause of the hyperexcitability is other than mutations of an ionic channel, we decided to evaluate its effect on easily shocked mutants (eas2F), which carry a mutation in an ethanolamine kinase which affects the phospholipid composition of neuronal membranes (41). This bang sensitive mutant is known to improve upon treatment with phenytoin but is partially or completely insensitive to other drugs like carbamazeprine, ethosuximide, and vigabactrin (36). When we tested the leaf and stem bark extract at 2.67 and 26.6 mg.ml−1 on eas2F flies, we did not observe any improvement in their percentage of recovery (Table 2). Rather the mutants were more paralyzed and the only significant changes in the MRT were due to a delay in recovery (Table 2).
This experiment shows that despite the similarity in the prophylactic effect between phenytoin and A. senegalensis extract on parabss, it is likely that their mode of action differs.
Discussion
Approximately 30 AEDs are currently approved for treatment of patients affected by epilepsy, an aetiologically complex and variable disease. A non-negligible percentage of epileptic experience undesired secondary effects of these AEDs, and 30% of patients are insensitive to them altogether (9, 10), highlighting the necessity of discovering alternative compounds to incorporate in the design of novel therapies.
Almost all commonly prescribed AEDs were discovered by animal screenings (40) and their mechanisms of action were also studied in non-human species, emphasizing the importance of animal testing for drug discovery in epilepsy. Medicinal plant extracts have been historically used for the treatment of various diseases, including epilepsy. The knowledge gathered and transmitted for generations about the anti-seizure properties of herbal TM preparations constitute a rich source of information for the discovery of new drug candidates. However, an empirical testing of their efficacy as AED and the pharmacological characterization of the active compound is required. A few hundred drugs used in TM have been tested in epilepsy animal models but the lack of follow up clinical and pharmacological studies places constraints on the clinical recommendation of herbal TMs (42).
In the present study, we used a Drosophila seizure model to test the effect of A. senegalensis extracts as an anti-seizure treatment (25–27). We used a fly with a gain of function mutation in the unique voltage gated sodium channel of Drosophila genome, parabss, which is extremely prone to seizures. parabss mutant flies are generally accepted as a model for intractable epilepsy due to the complexity of their seizures, including a tonic-clonic-like phase, and their significant resistance to AED treatments (38). A. senegalensis was used because of its strong reputation as a TM potentially effective for the treatment of seizures and because several studied performed in rodents have tested its anticonvulsant properties (19–24). In these studies, extracts of A. senegalensis were prepared with different methods (in general with polar solvents) and from different parts of the plant (leaf, root or stem). In all cases, an acute treatment with the extract, via intraperitoneal injection or via oral administration, produced a significant prophylactic effect against seizures induced by pentylenetetrazole, picrotoxin, pilocarpin or maximal electroshock (19–24). However, drug testing in mammals is costly and slow, reducing the number of compounds that can be subject to thorough analysis. Using Drosophila as a model system offers an opportunity to overcome such limitations and isolate active compounds in a high throughput and cost-effective manner.
parabss flies showed a strong seizure-like phenotype with significant differences between males and females. Approximately 85% of females were paralyzed after the mechanical stimulation, while only 50% of the males were. Despite this level of seizuring been below the one described in previous reports (36–38), the aminoacidic residue leucine in position 1699 was mutated to Phenylalanine in our parabss stock. This suggests that a repressor mutation in the X chromosome might be compensating for the L1699P mutation or, less likely, that our stock has lost an enhancer mutation that was generating the original fully penetrant phenotype.
Alternatively, this could be a consequence of the lack of controlled circadian synchronization in our experimental animals. In humans, it is known that the onset of seizures and the interictal epileptiform discharges (IED) have a tendency to occur in specific times of the day. For example, tonic-clonic seizures occur more frequently during sleep. This rhythmicity is controlled by both the circadian clock and sleep-wake state (43). In our experimental conditions, flies were inadvertently tested at different endogenous circadian time. This factor might have influenced the seizure threshold across animals, generating heterogeneity in the penetrance of the seizure phenotype. In this scenario the known behavioral differences in the rest-activity cycles between females (more active) and males might have resulted in the sexual dimorphism observed (44). It would be interesting to take advantage of the knowledge of circadian rhythms in Drosophila to further investigate the molecular and cellular mechanisms underlying the circadian and vigilance regulation of seizures.
Although presenting lower levels of seizuring than in the literature and sexual differences in phenotype penetrance, both sexes showed a measurable level of paralysis and recovered gradually, so they could be used to test the anti-seizure effect of A. senegalensis. Our experimental design was therefore suited to analyse the overall effect of drug treatments, from seizure induction to complete recovery. More precisely, we evaluated (1) the prophylactic effect against seizure induction and (2) the effect on recovery dynamics. We compared the effect of A. senegalensis extracts, to the commonly used AED phenytoin. Phenytoin was chosen as our positive control since its anti-convulsion properties have been documented in flies before. When administered chronically and at low doses (0.03–0.3 mg.ml−1) it induced a decrease in MRT in a mixed population of males and females parabss (36).
In our experimental conditions (24 h drug exposure in the food, 0.91, 4.76 and 9.09 mg.ml−1), phenytoin produced the expected improvement on seizuring phenotype. This was characterized by a clear prophylactic effect of all three doses, with fewer male or female flies showing seizures compare to controls at the beginning of the experiment (t = 0). The MRT was not improved at any concentration but this might be due to a slight toxic effect of phenytoin at 4.76 and 9.09 mg.ml−1. At this concentration the MRT slightly increased and a few flies remained paralyzed for the entire duration of the experiment (1,200 s), a phenotype not observed in parabss mutants.
The treatment with A. senegalensis stem bark extract effectively improved the seizure-like phenotype. At the lower doses (below 2.67 mg.ml−1) approximately 60% of the flies did not seizure at t = 0, showing a similar effect to the one quantified with phenytoin. We could not quantify a decrease in the MRT indicating that once the seizure has started the drug barely ameliorates the symptoms. In comparison, treatments with A. senegalensis leaf extracts were less effective than stem bark extracts, suggesting that the active compound of the plant is most concentrated in the stem bark.
These findings are significant because they lay the bases for future studies aiming to discover the active chemical compound present in A. senegalensis stem bark extract. In that direction, it would be possible to use this experimental approach to test the anti-seizure effect of samples coming from an extensive sub-fractioning of stem bark extracts. This would allow further purification and identification of the active compound.
One such simple sub-fractioning experiment was performed from A. senegalensis roots. It identified a putative candidate belonging to the diterpenoid family, Kar-16a-19oic acid, that showed promising sedative and anti-seizure properties in rodents (21). The candidate compound was enriched in an ethyl-acetate fraction and showed an acute toxicity in mice of LD50 = 2154 mg/kg compared to LD50 = 150 mg/kg for phenytoin (pubchem). Toxicity of an aqueous extract, like the one used in our experiments, in mice is lower, LD50 > 5,000 mg/kg (45), which would make it ideal for its clinical recommendation as a TM (9). This and other candidates could be further tested using the present animal model.
The difference in prophylactic effect observed on parabss and eas2F mutant flies gives an indication regarding the possible mechanism of action of A. senegalensis extract. It has already been shown that the percentage of eas2F mutants seizuring improves when treated with phenytoin (36) but that the mutants are resistant to treatment with several AEDs (36): carbamazepine a sodium channel blocker; ethosuximide that blocks T-type calcium channels, and may include effects on other classes of ion channel; vigabatrin that increases GABA in the brain blocking the activity of the gamma-aminobutyric acid aminotransferase and gabapentin which has recently been shown to selectively inhibit calcium channels containing the a2d-1 subunit (46). Because A. senegalensis improves the phenotype of parabss but not eas2F, it is likely that the active compound acts via a mechanism different than phenytoin, not acting on voltage gated sodium channels (40). On the other hand, an anticonvulsant effect of A. senegalensis leaf extract was shown on pentylenetetrazol (PTZ) induces convulsions in mice (19). PTZ antagonizes GABAA receptor Cl– channel complex (19) attenuating GABA-dependent inhibition and inducing seizures. It is possible that A. senegalensis effect is partially due to enhancement of GABAergic pathways. If this were the case, a high concentration of the plant extract could produce a sedative effect and induce more paralysis as observed when testing eas2F. Ultimately, experiments to test the mechanism of action of A. senegalensis extract should be performed.
The simplicity, reproducibility, potential high throughput and low cost of the testing method reported here constitutes an ideal setup to isolate the active anti-seizure compound present in A. senegalensis and potentially other TMs. Molecules controlling the development and function of the nervous system (28, 29) in Drosophila and humans present high structural and functional conservation. Drosophila could provide an early neurophysiological characterization of the mechanisms of action of new candidate compounds with potential translational anti-seizure effects (25–27, 47, 48). Specific neurons whose neurotransmitter identity and connectivity are known could be patch-clamped in parabss and the effect of the drug tested (49). The contribution of inherited or de novo mutations in the etiology of epilepsy, including focal epilepsy (6, 50), is growing. Sodium channels, that play a crucial function for cellular excitability, have been associated with a wide range of epilepsies (5). Amongst them is SNC1A, the human ortholog of parabss, with over 600 different mutations found in patients (8). Links to other ionic channels, receptors synaptic proteins and brain development pathways have also been proposed. The genetic malleability of Drosophila, where specific gene mutations can easily be generated, strengthens its validity for screening and early characterization of neural mechanism underlying hyperexcitability.
Screening TMs in several distinct Drosophila seizuring mutants will most likely help isolating new compounds for intractable epilepsy. This is exemplified by the lack of prophylactic effect induced by A. senegalensis extract on eas2F mutants, which are insensitive to many EADs but might benefit from new drugs (36).
However, fruit flies and humans also show fundamental differences that need to be taken into consideration when interpreting screening results. From a circuit point to view, the fly brain is much simpler with no cortical and subcortical regions where most focal source of seizure are located in humans. From the aetiological perspective, the causes of epilepsy in humans are varied ranging from inherited (genetic) to acquire through brain injury (e.g., prenatal or perinatal causes, severe head or brain injury, stroke, infections) (11). Treatment with AEDs aims to re-balance neuronal activity but fly models of epilepsy might fail to capture the full spectrum of neural and brain dysfunctions. Limited behavioral phenotypes in flies suggest that only seizure resembling the “grand mal” with strong spams and muscular manifestations could be studied. More complex or subtle cognitive and emotional aspects of epilepsies are not accessible in flies. Finally, absence of a proper brain blood barrier, an open hemolymph circulation and the distinctive renal system of Drosophila compromises translation of pharmacokinetics and toxicity data to humans.
Ultimately, pre-clinical research in different animal models complemented with clinical trials in humans will be essential in establishing effectivity and safety profiles for TM compounds for the treatment of epilepsy.
In conclusion, our work is important because it confirms how powerful Drosophila is as a model system for screening of putative new AEDs. Regardless of the fact that there was little information about how to prepare the plant extract, we did not know the identity of the active compound, its effective dose, pharmacokinetic, or toxicity in flies, we were able to find a strong anti-seizure effect of A. senegalensis stem bark extracts on parabss flies. This method could be used for high throughput screening of many TM with suspected anti-convulsion properties, hence offering a robust platform for the identification and isolation of novel AEDs that could be the basis of new treatments for intractable epilepsy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the animal study because the use of Drosophila is not included the UK Home Office regulation.
Author Contributions
SD and JB conceived and designed the experiments. SD, JB, JRC, and NH performed the experiments. EM and JB analyzed the data and EM did the statistics. PE identified the plants and prepared the extracts. EM and JB wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The project was funded by Cambridge in Africa programme the Alborada Trust Fund. JB is funded by a Sir Henry Dale Fellowship (Wellcome Trust and the Royal Society) Grant 105568/Z/14/Z.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Nara Muraro for constructive comments on the manuscript, Richard Baines for sharing the parabss and eas2F flies and Flybase for data curation, Oksana Elliot for fly maintenance and Darron Cullen for doing the PCRs. We thank the Institute of Biomedical Research Laboratory, Kampala International University Western Campus and Mbarara University of Science and Technology, for providing laboratory space and support, and the Teaching and Research in Natural Sciences for Development in Africa (TReND in Africa) organization for setting up the laboratory and for training SD during their Drosophila neurogenetics course funded by the IBRO, that we also thanks.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.606919/full#supplementary-material
References
1. World Health Organization. Epilepsy, a Public Health Imperative. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization (2019). Available online at: https://www.ilae.org/files/dmfile/19053_Epilepsy_A-public-health-imperative-For-Web.pdf
2. Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. (2017) 88:296–303. doi: 10.1212/WNL.0000000000003509
3. Collaborators GBDE. Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:357–75. doi: 10.1016/S1474-4422(18)30454-X
4. Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. (2010) 51:883–90. doi: 10.1111/j.1528-1167.2009.02481.x
5. Catterall WA. Forty years of sodium channels: structure, function, pharmacology, and epilepsy. Neurochem Res. (2017) 42:2495–504. doi: 10.1007/s11064-017-2314-9
6. Thomas RH, Berkovic SF. The hidden genetics of epilepsy-a clinically important new paradigm. Nat Rev Neurol. (2014) 10:283–92. doi: 10.1038/nrneurol.2014.62
7. Relationship between epilepsy and tropical diseases. Commission on Tropical Diseases of the International League Against Epilepsy. Epilepsia. (1994) 35:89–93. doi: 10.1111/j.1528-1157.1994.tb02916.x
8. Claes LR, Deprez L, Suls A, Baets J, Smets K, Van Dyck T, et al. The SCN1A variant database: a novel research and diagnostic tool. Hum Mutat. (2009) 30:E904–20. doi: 10.1002/humu.21083
9. Tang F, Hartz AMS, Bauer B. Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol. (2017) 8:301. doi: 10.3389/fneur.2017.00301
10. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
11. World Health Organization. Division of Mental Health, Initiative of Support to People With Epilepsy. (1990). Available online at: https://apps.who.int/iris/handle/10665/61822
12. De Silva M, MacArdle B, McGowan M, Hughes E, Stewart J, Reynolds EH, et al. Randomised comparative monotherapy trial of phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed childhood epilepsy. Lancet. (1996) 347:709–13. doi: 10.1016/S0140-6736(96)90074-4
13. Dekker PA. Epilepsy a Manual for Medical and Clinical Officers in Africa. Geneva: World Health Organization (2002).
14. Shorvon SD, Farmer PJ. Epilepsy in developing countries: a review of epidemiological, sociocultural, and treatment aspects. Epilepsia. (1988) 29 (Suppl. 1):S36–54. doi: 10.1111/j.1528-1157.1988.tb05790.x
15. Jost J, Millogo A, Preux PM. Antiepileptic treatments in developing countries. Curr Pharm Des. (2017) 23:5740–8. doi: 10.2174/1381612823666170809103202
16. Kvalsund MP, Birbeck GL. Epilepsy care challenges in developing countries. Curr Opin Neurol. (2012) 25:179–86. doi: 10.1097/WCO.0b013e328350baf8
17. World Health Organization. Beijin Declaration. (2008). Available online at: https://www.who.int/medicines/areas/traditional/congress/beijing_declaration/en/
18. Adjakpa JB, Ahoton LE, Obossou FK, Ogougbe C. Ethnobotanical study of Senegal custard apple (Annona senegalensis Pers.) in Dassa-Zoumétownship, Republic of Benin. Int J Bio Chem Sci. (2017) 10:2123–25. doi: 10.4314/ijbcs.v10i5.15
19. Okoli CO, Onyeto CA, Akpa BP, Ezike AC, Akah PA, Okoye TC. Neuropharmacological evaluation of Annona senegalensis leaves. Afr J Biotechnol. (2010) 9:8435–44. doi: 10.4314/AJB.V9I49
20. Okoye TC, Akah PA, Ezike AC, Okoye MO, Onyeto CA, Ndukwu F, et al. Evaluation of the acute and sub acute toxicity of Annona senegalensis root bark extracts. Asian Pac J Trop Med. (2012) 5:277–82. doi: 10.1016/S1995-7645(12)60039-X
21. Okoye TC, Akah PA, Omeje EO, Okoye FB, Nworu CS. Anticonvulsant effect of kaurenoic acid isolated from the root bark of Annona senegalensis. Pharmacol Biochem Behav. (2013) 109:38–43. doi: 10.1016/j.pbb.2013.05.001
22. Okoye TC, Akah PA, Omeke CP. Evaluation of the anticonvulsant and muscle relaxant effects of the methanol root bark extracts of Annona senegalensis. Asian Pac J Trop Med. (2010) 3:25–8. doi: 10.1016/S1995-7645(10)60025-9
23. Konate A, Sawadogo WR, Dubruc F, Caillard O, Ouedraogo M, Guissou IP. Phytochemical and anticonvulsant properties of Annona senegalensis Pers. (Annonaceae), Plant used in Burkina folk medicine to treat epilepsy and convulsions. Br J Pharmacol Toxicol. (2012) 3:245–50.
24. Konate A, Sawadogo WR, Dubruc F, Caillard O, Guissou IP. Anticonvulsant effects of the stem bark extract of Annona senegalensis Pers. Mol Clin Pharmacol. (2012) 3:62–72.
25. Song J, Tanouye MA. From bench to drug: human seizure modeling using Drosophila. Prog Neurobiol. (2008) 84:182–91. doi: 10.1016/j.pneurobio.2007.10.006
26. Muraro NI, Baines RA. Drosophila melanogaster in the study of epilepsy. SEB Exp Biol Ser. (2008) 60:141–60. doi: 10.1201/9781003060796-9
27. Stone B, Burke B, Pathakamuri J, Coleman J, Kuebler D. A low-cost method for analyzing seizure-like activity and movement in Drosophila. J Vis Exp. (2014) 84:e51460. doi: 10.3791/51460
28. Ganetzky B. Genetic analysis of ion channel dysfunction in Drosophila. Kidney Int. (2000) 57:766–71. doi: 10.1046/j.1523-1755.2000.00913.x
29. Yoshihara M, Ensminger AW, Littleton JT. Neurobiology and the Drosophila genome. Funct Integr Genom. (2001) 1:235–40. doi: 10.1007/s101420000029
30. Sun L, Gilligan J, Staber C, Schutte RJ, Nguyen V, O'Dowd DK, et al. A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J Neurosci. (2012) 32:14145–55. doi: 10.1523/JNEUROSCI.2932-12.2012
31. Schutte RJ, Schutte SS, Algara J, Barragan EV, Gilligan J, Staber C, et al. Knock-in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J Neurophysiol. (2014) 112:903–12. doi: 10.1152/jn.00135.2014
32. Port F, Chen HM, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci USA. (2014) 111:E2967–76. doi: 10.1073/pnas.1405500111
33. Benzer S. From the gene to behavior. JAMA. (1971) 218:1015–22. doi: 10.1001/jama.1971.03190200047010
34. Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics. (1982) 100:597–614.
35. Ijaiya IS, Arzika S, Abdulkadir M. Extraction and phytochemical screening of the root and leave of Annona Senegalesis (Wild Custad Apple). Acad J Interdiscip Stud. (2014) 3:9. doi: 10.5901/ajis.2014.v3n7p9
36. Reynolds ER, Stauffer EA, Feeney L, Rojahn E, Jacobs B, McKeever C. Treatment with the antiepileptic drugs phenytoin and gabapentin ameliorates seizure and paralysis of Drosophila bang-sensitive mutants. J Neurobiol. (2004) 58:503–13. doi: 10.1002/neu.10297
37. Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. (2000) 83:998–1009. doi: 10.1152/jn.2000.83.2.998
38. Parker L, Padilla M, Du Y, Dong K, Tanouye MA. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. (2011) 187:523–34. doi: 10.1534/genetics.110.123299
39. Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. (2011) 13:92–104. doi: 10.1016/j.cmet.2010.12.010
40. Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. (2004) 5:553–64. doi: 10.1038/nrn1430
41. Pavlidis P, Ramaswami M, Tanouye MA. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. (1994) 79:23–33. doi: 10.1016/0092-8674(94)90397-2
42. Liu W, Ge T, Pan Z, Leng Y, Lv J, Li B. The effects of herbal medicine on epilepsy. Oncotarget. (2017) 8:48385–97. doi: 10.18632/oncotarget.16801
43. Jin B, Aung T, Geng Y, Wang S. Epilepsy and its interaction with sleep and circadian rhythm. Front Neurol. (2020) 11:327. doi: 10.3389/fneur.2020.00327
44. Helfrich-Forster C. Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster–sex-specific differences suggest a different quality of activity. J Biol Rhythms. (2000) 15:135–54. doi: 10.1177/074873040001500208
45. Ilboudo S, Ouedraogo GG, Ouedraogo S, Guissou IP. Phytochemical, acute and subacute toxicity studies of Annona senegalensis Pers. (Annonaceae) root wood extracts. Afr J Biochem Res. (2019) 3:44–55. doi: 10.5897/AJBR2019.1030
46. Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. (2006) 6:108–13. doi: 10.1016/j.coph.2005.11.003
47. Stilwell GE, Saraswati S, Littleton JT, Chouinard SW. Development of a Drosophila seizure model for in vivo high-throughput drug screening. Eur J Neurosci. (2006) 24:2211–22. doi: 10.1111/j.1460-9568.2006.05075.x
48. Baines RA, Giachello CNG, Lin WH. Models of Seizures and Epilepsy. New York, NY: Academic Press (2017). p. 345–58.
49. Giachello CN, Zarin AA, Kohsaka H, Fan YN, Nose A, Landgraf M, et al. Electrophysiological validation of premotor interneurons monosynaptically connected to the aCC motoneuron in the Drosophila larval CNS. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.06.17.156430
Keywords: Annona senegalensis, bang sensitive, Drosophila melanogaster, epilepsy, parabss, phenytoin, seizure, eas2F
Citation: Dare SS, Merlo E, Rodriguez Curt J, Ekanem PE, Hu N and Berni J (2021) Drosophila parabss Flies as a Screening Model for Traditional Medicine: Anticonvulsant Effects of Annona senegalensis. Front. Neurol. 11:606919. doi: 10.3389/fneur.2020.606919
Received: 17 September 2020; Accepted: 09 December 2020;
Published: 13 January 2021.
Edited by:
Sherifa Ahmed Hamed, Assiut University Hospitals, EgyptReviewed by:
Yatinesh Kumari, Monash University Malaysia, MalaysiaYam Nath Paudel, Monash University Malaysia, Malaysia
Richard A. Baines, The University of Manchester, United Kingdom
Copyright © 2021 Dare, Merlo, Rodriguez Curt, Ekanem, Hu and Berni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jimena Berni, J.berni@sussex.ac.uk
 Samuel S. Dare
Samuel S. Dare Emiliano Merlo
Emiliano Merlo Jesus Rodriguez Curt
Jesus Rodriguez Curt Peter E. Ekanem7
Peter E. Ekanem7 Jimena Berni
Jimena Berni