- 1Department of Rheumatology, The First Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang, China
Purpose: To investigate the differences of retinal thickness (RT) and superficial vascular density (SVD) between patients with Sjogren's syndrome (SS) and healthy controls (HCs) using optical coherence tomography angiography (OCTA).
Methods: Individuals with SS and healthy controls were enrolled (n = 12 per group). An en-face OCTA scan was performed on each eye. Images were segmented into 9 subregions and macular RT and SVD were measured and compared between the 2 groups.
Results: Visual acuity (VA) differed significantly between patients with SS (24 eyes) and controls (24 eyes) (p < 0.001). In patients with SS, inner RT was reduced in the inner superior region, outer RT was reduced in the outer nasal (ON) region, and full RT was reduced in the ON region compared with the control group (p < 0.05). RT was negatively correlated with serum IgG level in the outer and full retina at ON regions (p < 0.05). SVD in the inner nasal, ON, and inner temporal regions was significantly lower in patients with SS than in control subjects (p < 0.05). SVD was positively correlated with full RT in the ON region in patients with SS (p < 0.05). The areas under the receiver operating characteristic (ROC) curves for the diagnostic sensitivity of outer RT and full RT in the ON region for SS were 0.828 (95% CI: 0.709–0.947) and 0.839 (95% CI: 0.715–0.963), respectively.
Conclusions: In patients with SS, retinal thinning in the macular area—which affects vision—can also reflect the severity of dry eyes in SS and has clinical value for assisted imaging diagnosis.
Introduction
Sjogren's syndrome (SS) is a long-term diffuse connective tissue disease. The global prevalence rate is 61 people per 100,000 (1). The male-to-female ratio is between 1:9 and 1:10, and most patients are 30–60 years of age (2). The clinical manifestations of SS range from exocrine to systemic with multiple extraglandular involvement (3). Although SS is associated with a lower incidence of visceral damage than systemic lupus erythematosus (SLE), the degree of disability is similar (4). Patients with SS often have physical and psychological disorders that reduce the social functioning and quality of life (5, 6). The symptomatology of SS overlaps with that of other systemic diseases, drug reactions, and viral infections. Thus, SS diagnosis and treatment represent a clinical challenge. In fact, the two-thirds of patients with SS are not correctly diagnosed initially (3, 7) and a diagnosis can take up to 10 years from initial symptom onset (8).
Since its discovery in the 1930s, the classification of SS has been continually updated. SS is suspected when the following symptoms are present: (1) constant and annoying dry eyes persisting for at least 3 months; (2) continual foreign body sensation in the eyes; (3) requiring the use of artificial tears ≥3 times a day; (4) feeling thirst every day for more than 3 months; and (5) a need to drink water to swallow solid food (9). The European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) issued classification standards for SS that included clinical and immune evaluations, with less emphasis placed on the eyes (10).
Among patients with SS, the incidences of xerophthalmia and thirst—the most common disease symptoms—were 44% and 39%, respectively (11). However, a study with a large sample size found that dry eye was much more common than dry mouth (12). Patients with SS often have ocular surface inflammation, such as eyelid swelling and corneal ulcer (8, 13–15); and ocular lesions in other parts of the eye such as optic neuritis (8), uveitis (16), and scleritis (17) have also been reported. Over one-third of patients with SS have extraglandular ocular manifestations and when these interfere with vision, patients are more likely to have fatal complications than those without such lesions (8). In patients with systemic autoimmune disorders, the choroid and retina may not only reflect ocular diseases but also indirect ocular injury (18). In SLE, retinopathy is an accurate reflection of disease activity in both recessive and dominant cases, and SLE patients with related retinopathy have a significantly lower survival rate than those without retinopathy (19). In SS, the significance of retinal changes is worth exploring.
There is currently no single clinical index or gold standard assay for the diagnosis and/or differentiation of SS, although a lip biopsy is useful (20). However, the invasiveness of the procedure precludes regular follow-up and monitoring. The main measure of disease activity in SS is the EULAR Patient Report Index (21), which includes just one eye-related assessment item. Optical coherence tomography angiography (OCTA) is a novel in vivo imaging technique that provides detailed morphologic and quantitative information on microvascular changes in the eye (22). OCTA can be used to show the microvascular structure in the macula and optic disk (23, 24), which can facilitate the diagnosis of diseases, such as Alzheimer's disease (25), diabetes (26), multiple sclerosis (27), and thyroid-related ophthalmopathy (28).
To date there have been no studies that have applied OCTA to the diagnosis of SS. In this study, we investigated the ocular status of patients with SS, and used OCTA to assess retinal thickness (RT) and vascular density (VD) in patients with SS compared with healthy controls.
Materials and Methods
Subjects
This cross-sectional study was conducted at the Department of Ophthalmology and Rheumatology of The First Affiliated Hospital of Nanchang University (Nanchang, China) in 2020. Patients diagnosed with SS were recruited from the Outpatient Department of Rheumatism Immunology; and sex- and age-matched healthy normal subjects were recruited from the Ocular Disease Clinical Research Center. An ophthalmologist from the Medical Center evaluated the absence of abnormalities in the eyes of these subjects through clinical examination and OCTA imaging. All subjects were examined by the same retina specialist.
Inclusion and Exclusion Criteria
All patients were women and met the 2002 American European Consensus Group classification criteria for SS (9). The patients ranged in age from 18 to 66 years. None of the patients showed symptoms or the signs of retinal vasculitis, choroiditis, or optic neuritis. Patients with chorioretinopathy induced by hydroxychloroquine (HCQ) were excluded.
Other exclusion criteria were as follows: (1) autoimmune diseases other than SS; (2) systemic diseases, such as endocrine, cardiovascular, or nervous system disease affecting the eye or optic nerve; (3) retinopathy or choroidal disease (e.g., arteriovenous disease, glaucoma, or high intraocular pressure (IOP); (4) any history of eye surgery or trauma or eye tumor; (5) contraindications, allergies, or intolerance to local anesthetics or mydriatic drugs; (6) diseases that could affect fundus imaging; and (7) pregnant or lactating women.
Ethical Considerations
The study was conducted in accordance with the Helsinki Declaration and was approved by the hospital's ethics council (cdyfy2018026). Before signing the informed consent statement, all participants fully understood the research methods and objectives.
Clinical Examinations
All subjects underwent the following clinical and ophthalmic examinations: (1) Immunological information, such as antinuclear antibody, anti-SS-A antibody, anti-SS-B antibody, IgG, and complement and salivary gland biopsy findings (29); (2) analysis of C-reactive protein (CRP) level and erythrocyte sedimentation rate to assess the patient's inflammatory status; (3) EULAR SS disease activity index for disease activity (ESSDAI) (21); (4) examination of mental state using the Hospital Anxiety and Depression Scale (HADS) (30); (5) ocular measurements, such as visual acuity (VA) (Snellen chart), IOP (Goldmann tonometry), spherical equivalent, and keratoconjunctivitis sicca; and (6) OCTA.
Ocular Measurements
Tear breakup time (BUT): apply fluorescein sodium evenly on the ocular surface, observe the first tear film rupture point under cobalt blue light, and calculate the tear film rupture time. Less than 10 s is positive (31).
Ocular staining score (OSS): corneal fluorescein staining and conjunctival lysamine green staining were used for comprehensive evaluation. The ocular surface of each eye was divided into three parts: nasal conjunctiva, cornea, and temporal conjunctiva. The nasal and temporal conjunctiva were scored according to the number of conjunctival spots in the palpebral fissure area. An OSS score ≥3 was positive (9).
Schirmer's test (SIT): fold one end of 5 mm × 35 mm filter paper into right angle, put it into conjunctival sac after disinfection. The soaking length of filter paper is normally 15 mm/5 min and <5 mm/5min is positive (9).
Tear meniscus height (TMH): infrared light was used to focus and guide patients to blink. After white light shooting, the software (Keratograph 5M) was used to measure and record (32).
Optical Coherence Tomography Angiography
We used an RTVue Avanti XR system (Optovue, Fremont, CA) to perform OCTA imaging to simultaneously display retinal cross section and microvessels. The scanning speed is set as 70,000 a-scans/s, the axial resolution is 5 mm, the horizontal resolution is 22 μm, the central wavelength is 840 nm, and the bandwidth is 45 nm. The imaging time was 3.9 s. Five angiography was performed in 3 mm × 3 mm scanning mode, focusing on the fovea along the X-axis (216 A scans in total) and along the Y-axis (216 grating positions for each scan). At 216 y positions ×5 positions, we captured 1,080 B scans at 270 frames/s (33). By 4 volume scans, OCTA images of 3 mm × 3 mm were obtained: a total of 933,120 A scans (2 horizontal scans and 2 vertical scans). A 3 mm × 3 mm en-face OCTA angiographic image was calculated for each eye.
After scanning, each retina was segmented into nine early treatment diabetic retinopathy study (ETDRS) subregions, composed of three concentric round (0.5, 1.5, and 3 mm in radius), and their thickness was analyzed. Each story of the retina covers: (1) inner retina: from internal limiting membrane (ILM) to inner plexiform layer (IPL); (2) full retina: from ILM to retinal segment epithelium (RPE). We defined outer RT as the difference between full RT and inner RT. The vascular perfusion area as a percentage of the measured area was vascular density. Using the threshold method, vascular density was reckoned by creating two-dimensional en-face images of superficial retina (the layer between the vitreous retinal interface and the front boundary of the ganglion cell layer). Determine the value of the image block and assign it to each pixel perfusion (1) or background (0). The average value of the skeleton plate in the region of interest was scaled based on the pixel size (512 pixels/3 mm) to calculate vascular density from the center of the macula to the edge of the 3 mm × 3 mm brightness gradient image. Macular RT and superficial (S) VD were measured. All subjects used the right eye first. Data from the left eye were flipped to obtain a mirror image of the right eye. Left and right eye data were averaged and analyzed together (Figure 1A).
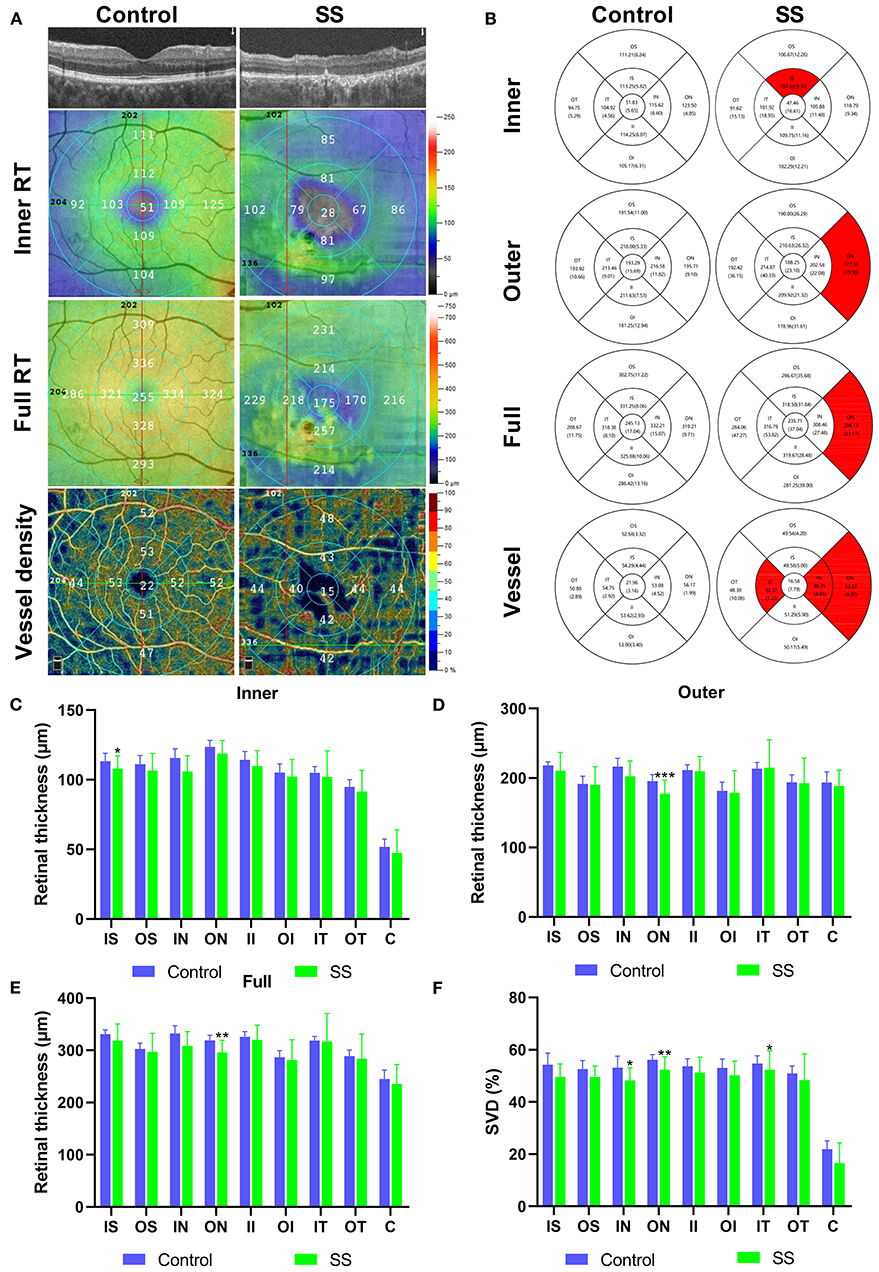
Figure 1. The OCTA images and RT and SVD analysis of control and SS groups. (A) Cross-sectional view of RT in SS and health group used OCTA. The inner RT, full RT and SVD were measured by ETDRS. (B) The results of inner RT, outer RT, full RT and SVD in the SS and healthy group were compared. (C–E) Analysis of RT results in the SS and healthy group. The vertical coordinate is the value of RT, and the horizontal coordinate is the retinal subregions. (F) Analysis of SVD results in the SS and healthy group. The vertical coordinate is the value of SVD, and the horizontal coordinate is the retinal subregions. OCTA, optical coherence tomography angiography; SS, Sjogren's syndrome; RT, retinal thickness; SVD, superficial vessel density; ETDRS, early treatment of diabetic retinopathy study; IS, inner superior; OS, outer superior; IN, inner nasal; ON, outer nasal; II, inner inferior; OI, outer inferior; IT, inner temporal; OT, outer temporal; C center. * p < 0.05; ** p < 0.01; and *** P < 0.001.
Statistical Analysis
The data were analyzed using SPSS version 22.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism version 8 (La Jolla, California, USA) are reported as mean ± SD. Independent-samples t-test, chi-square test, and Fisher's exact test were used to compare data between groups. The generalized estimation equation was employed to compare RT and SVD between SS eyes and control eyes and data were adjusted for known confounding variables to explain interocular correlation within subjects. The relationship between RT and systemic and ocular variables was analyzed using univariate and multivariate regression analyses. A linear correlation analysis was conducted between RT (full thickness, inner layer, and outer layer) and SVD in each group. To analyze the distinction between healthy and SS subjects, receiver operating characteristic (ROC) curves for RT (full, inner and outer) and SVD were plotted. The value of p < 0.05 was deemed statistically significant.
Results
Subjects
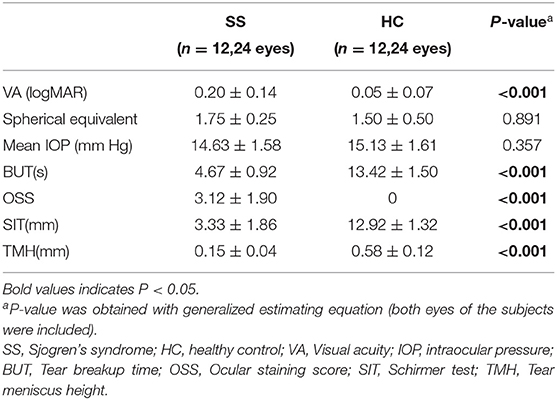
There were 12 cases (24 eyes) in each group and all were women. The patients with SS and control subjects were comparable in the terms of age (the mean age of SS was 55.17 ± 9.68 years and the mean age of the control was 54.50 ± 9.25 years, p = 0. 808). The mean time since diagnosis in the SS group was 3.92 ± 1.93 years, mean ESSDAI was 5.75 (range: 1–18), IgG was 16.11 ± 6.74 g/L, complement C3 was 0.85 ± 0.14 g/L, complement C4 was 0.21 ± 0.08 g/L. Patients with SS had a significantly higher HADS score than controls (8.08 ± 3.65 vs. 2.58 ± 0.97; p < 0.001) (Table 1).
The SS group had lower VA than the control group (p < 0.001) and had shorter BUT (4.67 ± 0.92 vs. 13.42 ± 1.50 s; p < 0.001), OSS (3.12 ± 1.90 vs. 0; p < 0.0001), lower SIT score (3.33 ± 1.86 vs. 12.92 ± 1.32 mm; p < 0.001), and lower TMH (0.15 ± 0.04 vs. 0.58 ± 0.12 mm; p < 0.001) (Table 2).
Macular RT
The subregional RT in the SS and control groups is shown in Table 3, Figure 1B. After adjusting for age, IOP, VA, and blood pressure (BP), inner RT was significantly lower in the SS group than in controls in the superior quadrant of the inner ring (p = 0.022) (Figure 1C). The other 3 regions of the inner ring (nasal, p = 0.739; inferior, p = 0.115; and temporal, p = 0.841), 4 regions of the outer ring (superior, p = 0.348; nasal, p = 0.161; inferior, p = 0.513; and temporal, p = 0.472), and foveal center (p = 0.244) did not differ significantly between groups.
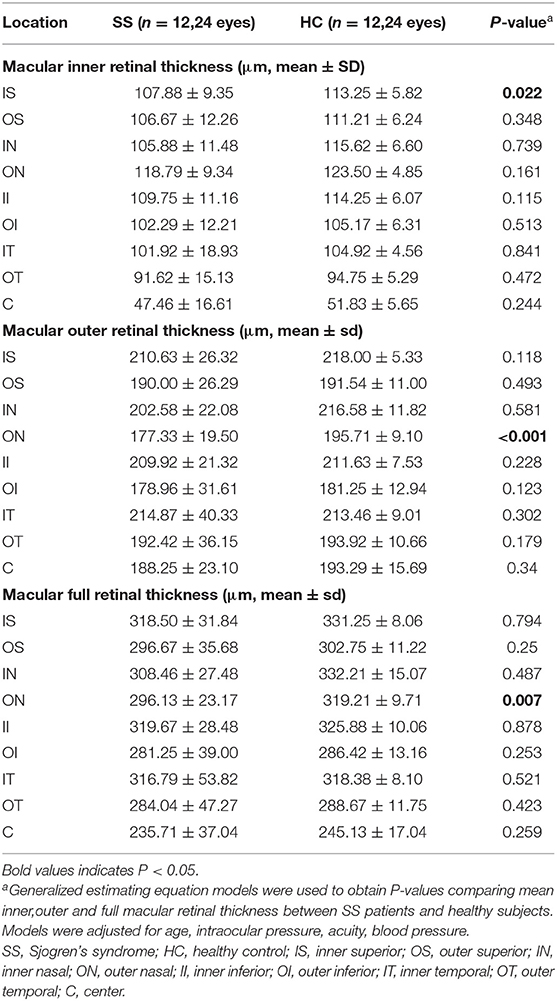
Table 3. Comparison of macular retinal thickness at different locations between patients with SS and healthy subjects.
Compared with healthy subjects, the outer RT of patients with SS was significantly lower in the outer nasal (ON) (p < 0.001) quadrants (Figure 1D). The other 3 regions of the outer ring (superior, p = 0.493; inferior, p = 0.123; and temporal, p = 0.179), the 4 regions of the inner ring (superior, p = 0.118; nasal, p = 0.581; inferior, p = 0.228; and temporal, p = 0.302), and foveal center (p = 0.340) showed no significant difference between groups.
The full RT in the nasal region of the outer ring was significantly thinner in patients with SS than in control subjects (p = 0.007) (Figure 1E). No significant differences between groups were observed in any of the other regions (p > 0.05).
In the univariate regression analysis, macular RT was negatively correlated with VA (b = −14.237, p < 0.001) but not with age, IOP, or BP. The multivariate regression analysis showed that advancing age (b = −0.149, p = 0.030) and poor VA (b = −17.758, p = 0.002) were significantly associated with thinner macular RT (Table 4).

Table 4. Univariate and multivariate regression analyses of association between macular retinal thickness with demographic and ocular parameters in patients with SS.
Superficial Macular Retinal VD
Superficial VD at different retinal subregions in the SS and control groups is shown in Table 5, Figure 1B. After adjusting for age, IOP, VA, and BP, SVD was significantly lower in patients with SS than in controls on the nasal side (inner ring, p = 0.010 and outer ring, p = 0.007) and temporal region (inner ring, p = 0.048) (Figure 1F). In the SS group, SVD was negatively correlated with disease duration (−0.464) (Figure 2A).
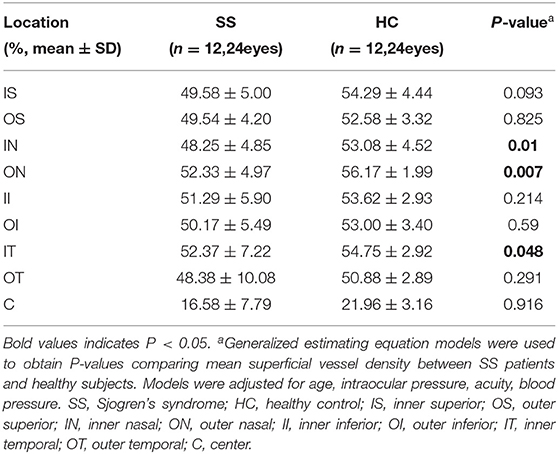
Table 5. Comparison of superficial vessel density at different locations between patients with SS and healthy subjects.
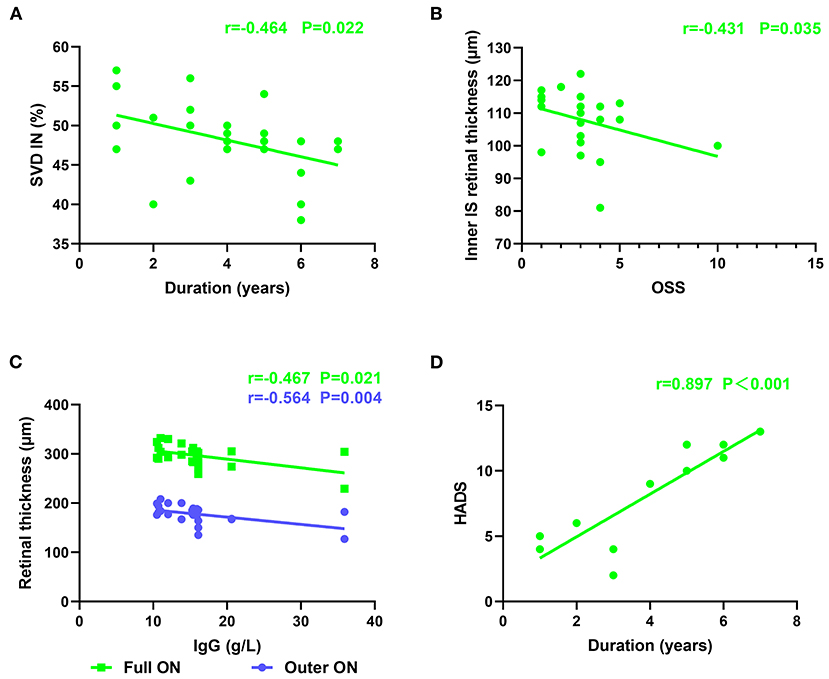
Figure 2. Retinal thickness was correlated with serum IgG level and OSS. The correlation between the duration of disease and HADS, SVD. (A) Negative correlation was found between duration and SVD at IN region (r = −0.464, p = 0.022). The vertical coordinate is the value of SVD, and the horizontal coordinate is the duration. (B) Negative correlation was found between RT and OSS in the inner retina at IS region (r = −0.431, p = 0.035). The vertical coordinate is the value of RT, and the horizontal coordinate is the value of OSS. (C) Negative correlation was found between RT and serum IgG level in the outer retina at ON region (r = −0.564, p = 0.004). Negative correlation was found between RT and serum IgG level in the full retina at ON region (r = −0.467, p = 0.021). The vertical coordinate is the value of RT, and the horizontal coordinate is the value of IgG. (D) Positive correlation was found between duration and HADS (r = 0.897, p < 0.001). The vertical coordinate is the value of HADS, and the horizontal coordinate is the duration. SS, Sjogren's syndrome; OSS, ocular staining score; HADS, hospital anxiety and Depression Scale; RT, retinal thickness; SVD, superficial vessel density; IS, inner superior.
ROC Curve Analysis of RT and SVD
Optical coherence tomography angiography data were analyzed to evaluate the specificity and sensitivity of RT and SVD as the diagnostic indicators of SS-related changes (Figure 3). Significant differences between groups were found in the inner superior (IS), outer ON, and full ON regions in the SS group. In the ON region, the area under the ROC curve for outer RT was 0.828 (95% CI: 0.709–0.947) and full RT was 0.839 (95% CI: 0.715–0.963), suggesting moderate to high diagnostic sensitivity for SS (Figure 3A).
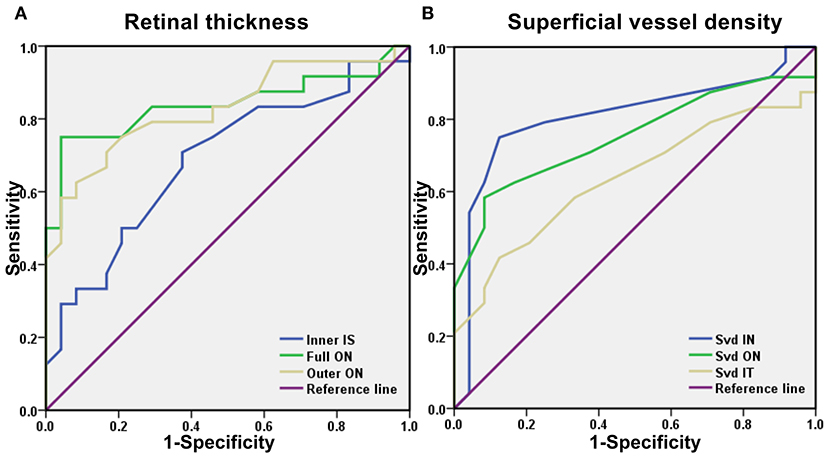
Figure 3. A receiver operating characteristic (ROC) curve analysis of RT and SVD. (A) The area under the ROC curve were 0.688 (95% CI = 0.536–0.839) for inner IS, full ON 0.839 (95% CI = 0.715–0.963), outer ON 0.828 (95% CI = 0.709–0.947). (B) The area under the ROC curve were 0.806 (95% CI = 0.671–0.942) for SVD IN, SVD ON 0.752 (95% CI = 0.607–0.896), SVD IT 0.635 (95% CI = 0.473–0.798). ROC, receiver operating characteristic; CI, confidence interval; RT, retinal thickness; SVD, superficial vessel density; ON, outer nasal; IT, inner temporal; IS, inner superior; IN, inner nasal.
Significant between-groups differences in SVD were found in the inner nasal (IN) and ON and inner temporal (IT) regions. The area under the SVD IN ROC curve was 0.806 (95% CI: 0.671–0.942), and the area under the SVD ON ROC curve was 0.752 (95% CI: 0.607–0.896), suggesting that SVD has moderate diagnostic sensitivity for SS (Figure 3B).
Relationship Between RT, SVD, IgG, and OSS
In patients with SS, full RT was correlated with SVD in the ON region (r = 0.459) (Figure 4), suggesting that retinal thinning is related to decreased VD in SS. The inner RT in the IS region of the SS group was negatively correlated with OSS (−0.431), implying that a thinner RT is related to the greater severity of xerophthalmia (Figure 2B). In patients with SS, negative correlations were found between RT and serum IgG level in the outer and full retina at ON regions (−0.564 and −0.467, respectively) (Figure 2C).
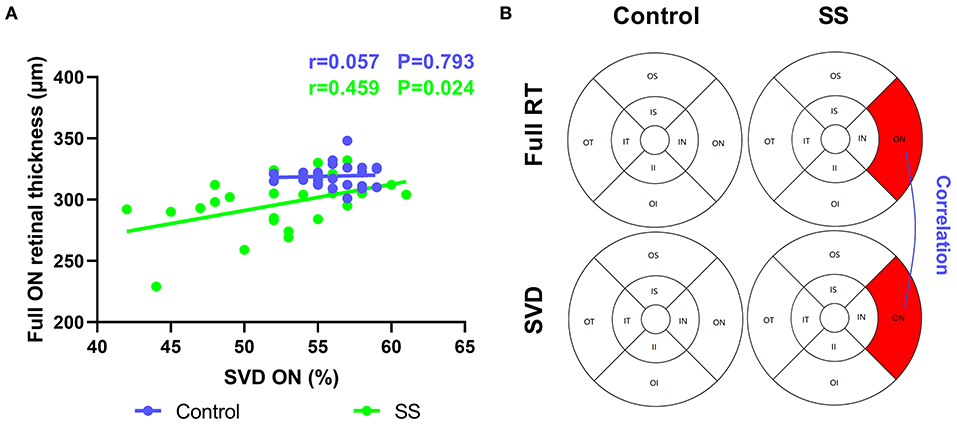
Figure 4. Correlation between RT and SVD in normal and patients with SS. The vertical coordinate is the value of RT, and the horizontal coordinate is the value of SVD. (A,B) In the SS group, SVD was positively correlated with full RT at ON region (r = 0.459, p = 0.024). SS, Sjogren's syndrome; RT, retinal thickness; SVD, superficial vessel density; ON, outer nasal.
Relationship Between Disease Duration and HADS
A longer disease course in patients with SS was associated with a higher HADS index (0.897) (Figure 2D).
Discussion
Sjogren's syndrome is characterized by exocrine gland involvement, abnormal proliferation of B lymphocytes, tissue infiltration of lymphocytes, and autoantibody production. The main clinical features dry eye, dry mouth, low fever, fatigue, and joint pain; about one-quarter of patients have visceral involvement. The risk of developing non-Hodgkin lymphoma is 40 times higher in patients with SS than in the general population (2). The EULAR and ACR issued a new classification standard for SS in 2016 (10) that emphasizes the pathologic features of the disease and the presence of autoantibodies; various indices corresponding to histology (labial gland biopsy), clinical indicators (SIT, OSS, and salivary flow rate), and immunology (anti–SS-A antibody) are stratified and weighted, and SS is diagnosed if the total score is ≥4. However, the lack of emphasis on the eyes in the assessment means that the significance of extraglandular changes in ophthalmic parameters are overlooked, although they are an important indicator of disease activity. We found that in the absence of clear ocular symptoms, patients in the SS group had significantly lower VA than healthy subjects, which has not been adequately addressed by other studies.
Sjogren's syndrome is referred as “benign lupus erythematosus” (34), which is another diffuse connective tissue disease associated with multiorgan damage; when the disease is active, the manifestations are similar to those of SLE and involve the vascular and nervous systems. The retina allows direct visualization of vascular changes (35) and provides a basis for early differentiation of patients with nervous system diseases (36). In our study, RT was calculated according to the ETDRS partition method and was found to be decreased in all areas of the retina in patients with SS, such as in the IS area of the inner retina, the ON area of the outer retina, and the ON area of the full-thickness retina. The results of the multiple regression analysis showed that visual impairment was associated with retinal thinning. This is in agreement with previous studies demonstrating that the ganglion cell inner plexiform and retinal nerve fiber layers were thinner in patients with SS compared with healthy subjects (37, 38). In healthy subjects, the inner RT is negatively correlated with age (39), which may be due to the loss of neurons, while the outer RT (especially RPE) may thicken with age (40). Our study found that full RT was negatively correlated with advancing age in patients with SS, this may need further longitudinal follow-up to confirm. The measurement of the full thickness of the retina in SS by OCTA has not yet been reported.
The choroidal blood supply is the most abundant in the body per unit weight and is therefore susceptible to inflammation in systemic diseases. Immune activation, autoantibody production, and impaired cellular immunity can damage the choroid. The activation of platelets and clotting pathways following vascular injury can lead to intravascular microthrombosis. The resultant vascular lesions and intimal hyperplasia of small arterioles followed by lumen stenosis lead to tissue hypoxia and chronic ischemia (41). The choroid is particularly sensitive to subclinical disease activity. In SLE, changes in the choroid may reflect reversible nephropathy and neuropathy (42). Similar changes in the retina may have clinical value for SS.
The retina and brain originate from the same embryonic tissue and both have comparable metabolic activity. A decrease in retinal blood supply can lead to the death of retinal cells (43). The internal blood flow of the retina is mainly supplied by the central retinal artery with the contribution from choroid vessels. The choroid can increase the partial pressure of O2 in the retina through the Root effect (44). When this occurs in the blood vessels supplying the retina, the resultant pathologic changes can affect the entire chorioretinal vascular network. Our study found that in patients with SS, the SVD showed a downward trend in all subregions with statistically significant decreases in the IN, ON, and IT regions. An increased neovascularization and the upregulation of vascular endothelial growth factor (VEGF)-A and its receptor VEGFR-2, which promote neovascularization, have been previously observed in the salivary glands of patients with SS (45), and angiogenesis is closely linked to the progression of SS (46). In contrast, we observed a decrease vascular density in the retina with increasing disease severity. Moreover, full RT was positively associated with SVD. Similar changes and associations have been reported in patients with diabetes (47) and Behcet's syndrome (18); alterations in microcirculation in these patients may precede clinically distinguishable retinopathy. We speculate that such subclinical changes exist in patients with SS. Retinal thinning was more obvious on the nasal side, while capillary loss was detected in the nasal and temporal regions. Choroidal capillary network lesions directly affect the blood supply to the outer retina; this can lead to chronic ischemia, especially of the photoreceptor layer and the death of rod cells and cone cells due to a decreased energy supply and loss of vision (43), which is supported by our findings (Figure 5).
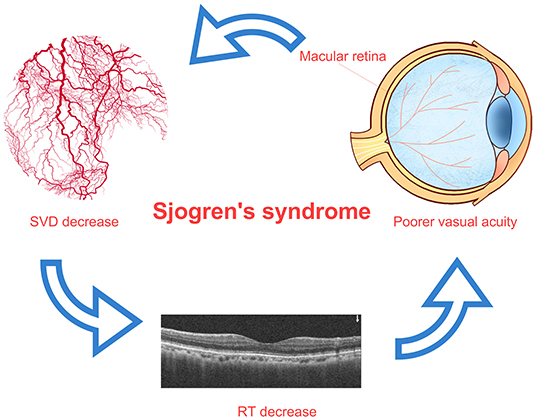
Figure 5. Relationship among SVD decrease, RT thinning, and visual impairment in patients with SS. In patients with SS, the decrease of superficial retinal vascular density in macular area may lead to the decrease of retinal thickness in related areas, and the decrease of retinal thickness in macular area may lead to the decrease of vision. The macular area is marked on the image of eyeball, which indicates the decrease of superficial vascular density in this area. SS, Sjogren's syndrome; RT, retinal thickness; SVD, superficial vessel density.
The proliferation of peripheral B cells plays an important role in the development of SS. The overactivation of B cells is the main factor of hyperglobulinemia and autoantibody production (48, 49). At the same time, the disorder of peripheral B cell subsets exists in SS, such as the significant decrease of CD27 + memory B cells, the increase of CD27 initial B cells and CD19 + B cells (50), and the positive correlation between the number of CD19 + B cells and IgG serum level and hypergammaglobulinemia (51). In SLE, the deposition of IgG immune complex in retinal vascular wall is associated with retinal nerve fiber layer infarction and ganglion cell atrophy (52). In patients with glaucoma, the loss of retinal ganglion cells is accompanied by the accumulation of IgG autoantibodies on ganglion cell layer cells (53), and the deposition of IgG autoantibodies is accompanied by CD27 +/IgG + plasma cells, which occurs under pro-inflammatory conditions, the level of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-8 were also increased (53). Immunity leads to antigen-specific and complex systemic immune responses, including the production of auto reactive antibodies against retinal and optic nerve epitopes with an increasing and time-dependent severity (53). Autoantibodies against a variety of retinal antigens have been reported, such as recoverin, α-Enolase, heat shock protein, arrestin, transducing protein, neurofilament protein, carbonic anhydrase II, and Tubby-related protein 1 (TULP1) (54). In our study, RT was found to be negatively correlated with IgG, indicating that RT may be affected by the abnormal immune state of SS disease. The exact mechanism of vascular occlusion in autoimmune diseases is still unclear, however, some possible pathogenic mechanisms include immune complex deposition, complement activation with microvascular thrombosis, and the fibrinoid degeneration of vascular wall (55). This may also be the possible reason for the decrease of SVD in patients with SS, and indirectly lead to the thinning of RT.
In patients with SS, an imbalance in the ratio of type 1 to type 2 helper T cells (56) leads to excessive interferon-γ (57), IL-17 (58), IL-21 (59), and IL-22 (60) production, which creates an inflammatory microenvironment that can cause tissue damage. Goblet cells are the main cells responsible for eye lubrication and their secretory ability is an important indicator of eye surface health. The secretory function and proliferative capacity of goblet cells are inhibited by increased inflammatory cytokine levels, resulting in changes in the ocular surface and irregular tear secretion (61). The OSS, similar to SIT test and BUT, is a reliable and objective index of the degree of dryness of the eyes. Our results showed that with the worsening of dry eye, the SIT score and BUT decreased while OSS increased. Additionally, SIT, BUT, and TMH were reduced whereas OSS was significantly higher in patients with SS compared with control subjects while inner RT was negatively correlated with OSS, which indirectly reflected the degree of dryness of the eyes.
Mental health disorders are more common in patients with SS than in healthy individuals (62, 63). The prevalence of anxiety and depression in patients with SS in China is 33.8 and 36.9%, respectively (64). Anxiety and/or depression are associated with lower physical activity and treatment compliance, which can have adverse effects on the well-being of patients with SS and may increase the incidence rate of cardiovascular disease and exacerbate SS (65). In our study, patients with SS had a higher HADS score than control subjects, which was positively correlated with disease duration.
The results of the ROC curve analysis of outer and full RT in the ON region indicated that these parameters have diagnostic utility for SS. Early diagnosis and accurate evaluation are critical for the successful treatment and good prognosis. SS has variable clinical manifestations, disease course, and prognosis, and clinical symptomatology is often unrelated to the degree of gland destruction. Classification criteria are standardized tools for the selection of appropriately defined homogeneous patient groups for research; however, they often fail to identify atypical individuals (20). OCTA is a noninvasive and convenient imaging method that can provide information on intraocular vascular network perfusion; RT measured by OCTA is a potential biomarker that can aid the diagnosis of SS. However, there is no previous report on the use of OCTA in patients with SS. So we did a preliminary study prudently. We set up a control group of healthy subjects in this study, so that we can distinguish SS from healthy people first through OCTA and then we will set up other control groups to test whether OCTA is helpful to distinguish SS from other diseases. At present, we are only exploring a possibility. We will conduct additional studies with larger samples to validate its diagnostic utility. At this stage, OCTA may be used in addition to the existing classification criteria.
This study had some limitations. First, given that patients with HCQ-induced retinochoroidosis were excluded, we did not consider the role of HCQ in SS in detail. Second, as the sample size was small, our findings require validation in a larger cohort before they can be translated into clinical practice. Due to the obvious gender bias of SS, the current study recruits female patients. With the deepening of the study, we will include more cases, both male and female will be taken into account. Third, RT is affected by many factors (such as, age and spherical equivalent). We will consider the influence of related factors as much as possible in the future study.
Conclusions
We used OCTA to evaluate the significance of RT and SVD in SS, and found that the inner, outer, and full RT were thinner while SVD was decreased in the ON, IN, and IT regions of patients with SS compared with controls; additionally, RT is positively correlated with SVD and negatively related to IgG. Thus, retinal thinning in the macular area—which affects vision—can also reflect the severity of dry eyes in SS and has clinical value for assisted imaging diagnosis.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: Figshare, https://figshare.com/s/793657ff2e10765b6616.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics of the First Affiliated Hospital of Nanchang University (cdyfy2018026). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
RL: writing the original draft and data analysis. YS: data analysis and editing. YW, QL, QX, TX, TH, SC, and SL: data collection. YS and RW: supervision and editing. All authors contributed to the article and approved the submitted version.
Funding
This research is supported by The Central Government Guides Local Science and Technology Development Foundation (20211ZDG02003); Key Research Foundation of Jiangxi Province (No: 20181BBG70004); Excellent Talents Development Project of Jiangxi Province (20192BCBL23020); Natural Science Foundation of Jiangxi Province (20181BAB205034); Grassroots Health Appropriate Technology Spark Promotion Plan Project of Jiangxi Province (No: 20188003); the Health Development Planning Commission Science Foundation of Jiangxi Province (No: 20201032); and the Health Development Planning Commission Science TCM Foundation of Jiangxi Province (No: 2018A060).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Department of Ophthalmology and Rheumatology of The First Affiliated Hospital of Nanchang University for the help during this study.
References
1. Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:1983–9. doi: 10.1136/annrheumdis-2014-205375
2. Vivino FB. Sjogren's syndrome: Clinical aspects. Clin Immunol (Orlando, Fla). (2017) 182:48–54. doi: 10.1016/j.clim.2017.04.005
3. Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T. The Diagnosis and Treatment of Sjögren's Syndrome. Dtsch Arztebl Int. (2017) 114:354–61. doi: 10.3238/arztebl.2017.0354
4. Sutcliffe N, Stoll T, Pyke S, Isenberg DA. Functional disability and end organ damage in patients with systemic lupus erythematosus (SLE), SLE and Sjögren's syndrome (SS), and primary SS. J Rheumatol. (1998) 25:63–8.
5. Koh JH, Kwok SK, Lee J, Son CN, Kim JM, Kim HO, et al. Pain, xerostomia, and younger age are major determinants of fatigue in Korean patients with primary Sjögren's syndrome: a cohort study. Scand J Rheumatol. (2017) 46:49–55. doi: 10.3109/03009742.2016.1153142
6. Kotsis K, Voulgari PV, Tsifetaki N, Drosos AA, Carvalho AF, Hyphantis T. Illness perceptions and psychological distress associated with physical health-related quality of life in primary Sjögren's syndrome compared to systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. (2014) 34:1671–81. doi: 10.1007/s00296-014-3008-0
7. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. (2004) 164:1275–84. doi: 10.1001/archinte.164.12.1275
8. Akpek EK, Mathews P, Hahn S, Hessen M, Kim J, Grader-Beck T, et al. Ocular and systemic morbidity in a longitudinal cohort of Sjögren's syndrome. Ophthalmology. (2015) 122:56–61. doi: 10.1016/j.ophtha.2014.07.026
9. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. (2002) 61:554–8. doi: 10.1136/ard.61.6.554
10. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. (2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren's Syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol (Hoboken, NJ). (2017) 69:35–45. doi: 10.1002/art.39859
11. Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, et al. Primary Sjögren's Syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. (2009) 7:46. doi: 10.1186/1477-7525-7-46
12. Saldanha IJ, Petris R, Han G, Dickersin K, Akpek EK. Research Questions and Outcomes Prioritized by Patients With Dry Eye. JAMA Ophthalmol. (2018) 136:1170–9. doi: 10.1001/jamaophthalmol.2018.3352
13. Cohen KL. Sterile corneal perforation after cataract surgery in Sjögren's syndrome. Br J Ophthalmol. (1982) 66:179–82. doi: 10.1136/bjo.66.3.179
14. Gottsch JD, Akpek EK. Topical cyclosporin stimulates neovascularization in resolving sterile rheumatoid central corneal ulcers. Trans Am Ophthalmol Soc. (2000) 98:81–7; discussion 7–90.
15. Ou JI, Manche EE. Corneal perforation after conductive keratoplasty in a patient with previously undiagnosed Sjögren syndrome. Arch Ophthalmol (Chicago, Ill: 1960). (2007) 125:1131–2. doi: 10.1001/archopht.125.8.1131
16. Rosenbaum JT, Bennett RM. Chronic anterior and posterior uveitis and primary Sjögren's syndrome. Am J Ophthalmol. (1987) 104:346–52. doi: 10.1016/0002-9394(87)90223-6
17. Bamrolia NR, Arora R, Yadava U. Unusual presentation of a case of Sjogren's syndrome with neurological and ocular manifestation. Cont Lens Anterior Eye. (2012) 35:85–8. doi: 10.1016/j.clae.2011.10.002
18. Steiner M, Esteban-Ortega MDM, Muñoz-Fernández S. Choroidal and retinal thickness in systemic autoimmune and inflammatory diseases: a review. Surv Ophthalmol. (2019) 64:757–69. doi: 10.1016/j.survophthal.2019.04.007
19. Mukherjee B, Nair AG. Principles and practice of external digital photography in ophthalmology. Indian J Ophthalmol. (2012) 60:119–25. doi: 10.4103/0301-4738.94053
20. Jonsson R, Brokstad KA, Jonsson MV, Delaleu N, Skarstein K. Current concepts on Sjögren's syndrome - classification criteria and biomarkers. Eur J Oral Sci. (2018) 126 Suppl 1:37–48. doi: 10.1111/eos.12536
21. Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis. (2010) 69:1103–9. doi: 10.1136/ard.2009.110619
22. Chen M, Dai J, Gong L. Changes in retinal vasculature and thickness after small incision lenticule extraction with optical coherence tomography angiography. J Ophthalmol. (2019) 2019:3693140. doi: 10.1155/2019/3693140
23. Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. J AMA Ophthalmol. (2015) 133:45–50. doi: 10.1001/jamaophthalmol.2014.3616
24. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. (2018) 64:1–55. doi: 10.1016/j.preteyeres.2017.11.003
25. Veverka KK, AbouChehade JE, Iezzi R, Pulido JS. NONINVASIVE GRADING OF RADIATION RETINOPATHY: the use of optical coherence tomography angiography. Retina (Philadelphia, Pa). (2015) 35:2400–10. doi: 10.1097/IAE.0000000000000844
26. Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Invest Ophthalmol Vis Sci. (2016) 57:Oct348–55. doi: 10.1167/iovs.15-18782
27. Wang X, Jia Y, Spain R, Potsaid B, Liu JJ, Baumann B, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol. (2014) 98:1368–73. doi: 10.1136/bjophthalmol-2013-304547
28. Ye L, Zhou SS, Yang WL, Bao J, Jiang N, Min YL, et al. RETINAL MICROVASCULATURE ALTERATION IN ACTIVE THYROID-ASSOCIATED OPHTHALMOPATHY. Endocr Pract. (2018) 24:658–67. doi: 10.4158/EP-2017-0229
29. Kroese FGM, Haacke EA, Bombardieri M. The role of salivary gland histopathology in primary Sjögren's syndrome: promises and pitfalls. Clin Exp Rheumatol. (2018) 36 Suppl 112:222–33.
30. Mackenzie LJ, Carey ML, Sanson-Fisher RW, D'Este CA, Paul CL, Yoong SL. Agreement between HADS classifications and single-item screening questions for anxiety and depression: a cross-sectional survey of cancer patients. Ann Oncol. (2014) 25:889–95. doi: 10.1093/annonc/mdu023
31. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocular Surface. (2007) 5:108–52. doi: 10.1016/S1542-0124(12)70083-6
32. Arita R, Yabusaki K, Hirono T, Yamauchi T, Ichihashi T, Fukuoka S, et al. Automated measurement of tear meniscus height with the kowa dr-1α tear interferometer in both healthy subjects and dry eye patients. Invest Ophthalmol Vis Sci. (2019) 60:2092–101. doi: 10.1167/iovs.18-24850
33. Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. (2017) 60:66–100. doi: 10.1016/j.preteyeres.2017.07.002
34. Diamond B. Systemic lupus erythematosus and Sjögren's syndrome. Curr Opin Rheumatol. (2007) 19:405. doi: 10.1097/BOR.0b013e3282b971f5
35. Md Noh UK, Zahidin AZ, Yong TK. Retinal vasculitis in systemic lupus erythematosus: an indication of active disease. Clin Pract. (2012) 2:e54. doi: 10.4081/cp.2012.e54
36. Karadag AS, Bilgin B, Soylu MB. Comparison of optical coherence tomographic findings between Behcet disease patients with and without ocular involvement and healthy subjects. Arq Bras Oftalmol. (2017) 80:69–73. doi: 10.5935/0004-2749.20170018
37. Arikan S, Gokmen F, Comez AT, Gencer B, Kara S, Akbal A. Evaluation of possible factors affecting contrast sensitivity function in patients with primary Sjögren's syndrome. Arq Bras Oftalmol. (2015) 78:150–3. doi: 10.5935/0004-2749.20150039
38. Yang JM, Sung MS, Ji YS, Heo H, Park SW. Analysis of clinical factors associated with retinal morphological changes in patients with primary Sjögren's syndrome. PLoS ONE. (2016) 11:e0157995. doi: 10.1371/journal.pone.0157995
39. Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. (2000) 41:741–8.
40. Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN∙OCT consensus. Ophthalmology. (2014) 121:1572–8. doi: 10.1016/j.ophtha.2014.02.023
41. Ferreira CS, Beato J, Falcão MS, Brandão E, Falcão-Reis F, Carneiro  M, et al. MANIFESTATIONS. Retina (Philadelphia, Pa). (2017) 37:529–35. doi: 10.1097/IAE.0000000000001193
42. Nguyen QD, Uy HS, Akpek EK, Harper SL, Zacks DN, Foster CS. Choroidopathy of systemic lupus erythematosus. Lupus. (2000) 9:288–98. doi: 10.1191/096120300680199024
43. Country MW. Retinal metabolism: a comparative look at energetics in the retina. Brain Res. (2017) 1672:50–7. doi: 10.1016/j.brainres.2017.07.025
44. Waser W, Heisler N. Oxygen delivery to the fish eye: root effect as crucial factor for elevated retinal PO2. J Exp Biol. (2005) 208:4035–47. doi: 10.1242/jeb.01874
45. Sisto M, Lisi S, Ingravallo G, Lofrumento DD, D'Amore M, Ribatti D. Neovascularization is prominent in the chronic inflammatory lesions of Sjögren's syndrome. Int J Exp Pathol. (2014) 95:131–7. doi: 10.1111/iep.12061
46. Rodrigues AR, Soares R. Inflammation in Sjögren's syndrome: cause or consequence? Autoimmunity. (2017) 50:141–50. doi: 10.1080/08916934.2017.1280027
47. Kim K, Kim ES, Kim DG, Yu SY. Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: longitudinal study using OCT angiography. Acta Diabetol. (2019) 56:1275–82. doi: 10.1007/s00592-019-01395-6
48. Hershberg U, Meng W, Zhang B, Haff N, St Clair EW, Cohen PL, et al. Persistence and selection of an expanded B-cell clone in the setting of rituximab therapy for Sjögren's syndrome. Arthritis Res Ther. (2014) 16:R51. doi: 10.1186/ar4481
49. Zheng J, Huang Q, Huang R, Deng F, Yue X, Yin J, et al. B Cells Are Indispensable for a Novel Mouse Model of Primary Sjögren's Syndrome. Front Immunol. (2017) 8:1384. doi: 10.3389/fimmu.2017.01384
50. Ibrahem HM B. cell dysregulation in primary Sjögren's syndrome: a review. Jpn Dent Sci Rev. (2019) 55:139–44. doi: 10.1016/j.jdsr.2019.09.006
51. Ishioka-Takei E, Yoshimoto K, Suzuki K, Nishikawa A, Yasuoka H, Yamaoka K, et al. Increased proportion of a CD38(high)IgD(+) B cell subset in peripheral blood is associated with clinical and immunological features in patients with primary Sjögren's syndrome. Clin Immunol (Orlando, Fla). (2018) 187:85–91. doi: 10.1016/j.clim.2017.10.008
52. Liu GY, Utset TO, Bernard JT. Retinal nerve fiber layer and macular thinning in systemic lupus erythematosus: an optical coherence tomography study comparing SLE and neuropsychiatric SLE. Lupus. (2015) 24:1169–76. doi: 10.1177/0961203315582285
53. Gramlich OW, Beck S, von Thun Und Hohenstein-Blaul N, Boehm N, Ziegler A, Vetter JM, et al. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS ONE. (2013) 8:e57557. doi: 10.1371/journal.pone.0057557
54. Abazari A, Allam SS, Adamus G, Ghazi NG. Optical coherence tomography findings in autoimmune retinopathy. Am J Ophthalmol. (2012) 153:750–6, 6.e1. doi: 10.1016/j.ajo.2011.09.012
55. Yen YC, Weng SF, Chen HA, Lin YS. Risk of retinal vein occlusion in patients with systemic lupus erythematosus: a population-based cohort study. Br J Ophthalmol. (2013) 97:1192–6. doi: 10.1136/bjophthalmol-2013-303265
56. Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, et al. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. (2002) 128:562–8. doi: 10.1046/j.1365-2249.2002.01869.x
57. Gliozzi M, Greenwell-Wild T, Jin W, Moutsopoulos NM, Kapsogeorgou E, Moutsopoulos HM, et al. A link between interferon and augmented plasmin generation in exocrine gland damage in Sjögren's syndrome. J Autoimmun. (2013) 40:122–33. doi: 10.1016/j.jaut.2012.09.003
58. Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am J Pathol. (2009) 175:1167–77. doi: 10.2353/ajpath.2009.090319
59. Scofield RH. IL-21 and Sjögren's syndrome. Arthritis Res Ther. (2011) 13:137. doi: 10.1186/ar3518
60. Ciccia F, Guggino G, Rizzo A, Ferrante A, Raimondo S, Giardina A, et al. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren's syndrome. Ann Rheum Dis. (2012) 71:295–301. doi: 10.1136/ard.2011.154013
61. Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: role of programmed cell death and mesenchymal stem cells. Ocul Surf. (2010) 8:60–9. doi: 10.1016/S1542-0124(12)70070-8
62. Anyfanti P, Pyrpasopoulou A, Triantafyllou A, Triantafyllou G, Gavriilaki E, Chatzimichailidou S, et al. Association between mental health disorders and sexual dysfunction in patients suffering from rheumatic diseases. J Sex Med. (2014) 11:2653–60. doi: 10.1111/jsm.12672
63. Milin M, Cornec D, Chastaing M, Griner V, Berrouiguet S, Nowak E, et al. Sicca symptoms are associated with similar fatigue, anxiety, depression, and quality-of-life impairments in patients with and without primary Sjögren's syndrome. Joint bone spine. (2016) 83:681–5. doi: 10.1016/j.jbspin.2015.10.005
64. Koçer B, Tezcan ME, Batur HZ, Haznedaroglu S, Göker B, Irkeç C, et al. Cognition, depression, fatigue, and quality of life in primary Sjögren's syndrome: correlations. Brain Behav. (2016) 6:e00586. doi: 10.1002/brb3.586
Keywords: retinal thickness, Sjogren's syndrome, optical coherence tomography angiography, biomarker, vascular density
Citation: Liu R, Wang Y, Li Q, Xia Q, Xu T, Han T, Cai S, Luo S, Wu R and Shao Y (2022) Optical Coherence Tomography Angiography Biomarkers of Retinal Thickness and Microvascular Alterations in Sjogren's Syndrome. Front. Neurol. 13:853930. doi: 10.3389/fneur.2022.853930
Received: 13 January 2022; Accepted: 09 February 2022;
Published: 08 March 2022.
Edited by:
Yuzhen Xu, Tongji University, ChinaReviewed by:
Guanghui Liu, Fujian Provincial People's Hospital, ChinaGuigang Li, Huazhong University of Science and Technology, China
Copyright © 2022 Liu, Wang, Li, Xia, Xu, Han, Cai, Luo, Wu and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Wu, tcmclinic@163.com; Yi Shao, freebee99@163.com
 Ren Liu
Ren Liu Yan Wang1
Yan Wang1 Tian Xu
Tian Xu Rui Wu
Rui Wu Yi Shao
Yi Shao