- 1Jeonbuk National University College of Medicine, Jeonju, South Korea
- 2Department of Neurology, Jeonbuk National University Hospital and School of Medicine, Jeonju, South Korea
- 3Department of Pharmacology, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam
- 4Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, South Korea
Objectives: In this study, the specific threshold intensities and response characteristics of galvanic vestibular stimulation (GVS) on vestibular (conscious) and cutaneous (detrimental) perception as well as oculomotor nystagmus (reflex) were determined.
Methods: The threshold intensities for vestibular and cutaneous perception and oculomotor response induced by GVS were determined in 25 right-handed healthy subjects (32.6 ± 7.2 years of age; 56% female). The subjects were seated upright, and eye movements were recorded while a direct GVS current was applied with paradigms of cathode on the right and anode on the left (CRAL) and also cathode on the left and anode on the right (CLAR).
Results: Subjects experienced dizziness, sense of spinning, or fall tendency, which was more frequently directed to the cathode (76%) than the anode (24%, p < 0.001, chi-square one-variable test) at mean current greater than 0.98 ± 0.29 mA (mean vestibular threshold). The current also triggered a more frequent mild tingling sensation at the cathode (56%) than the anode (30%) or on both sides (14%; p = 0.001, chi-square one-variable test) when above the mean cutaneous threshold of 0.9 ± 0.29 mA. Above the mean oculomotor threshold of 1.61 ± 0.35 mA, combined horizontal and torsional nystagmus was more frequent toward the cathode (86%) than toward the anode (p < 0.001, chi-square one-variable test). The mean oculomotor threshold was significantly higher than both the vestibular (p < 0.001, Mann–Whitney U-test) and cutaneous (p < 0.001, Mann–Whitney U-test) thresholds, which were comparable (p = 0.317, Mann–Whitney U-test). There was no significant disparity in these specific thresholds between the two GVS paradigms. The vestibular threshold was significantly higher in males than in females [1 (0.5–1.25) mA vs. 0.75 (0.625–1.125) mA, Z = −2.241, p = 0.025, Mann–Whitney U-test]. However, the thresholds of cutaneous perception and oculomotor response did not differ by sex.
Conclusion: The findings indicate that thresholds for vestibular and somatosensory perception are lower than the oculomotor threshold. Therefore, a strategy to reduce GVS current intensity to the level of vestibular or somatosensory perception threshold could elicit beneficial vestibular effects while avoiding undesirable effects such as oculomotor consequences.
Introduction
Vestibular afferents are sensitive to motion acceleration when the head translates and rotates in space, creating rapid and accurate reflexive responses to unpredictable and high acceleration. This function is critical in maintaining balance and visual stability (1, 2). The afferents provide continuous data for exploring and comprehending a vast range of physical motions encountered in daily life, acting as an inertial sensor and significantly contributing to spatial navigation (1). Notably, the vestibular system is multimodal and integrative (1, 3, 4), involving the fusion of otolith- and canal-derived signals at the level of vestibular nuclei and multisensory integration and convergence (such as vestibular-visual, vestibular-somatosensory, and vestibular-visual-somatosensory interactions) at the vestibular nuclei, thalamus, and cerebral cortices (1, 3, 4). The coherence integration of three systems (i.e., vestibular, visual, and somatosensory) imparts the ability to determine an internal representation of space and spatial perception and navigation in three-dimensional coordinates, using both egocentric and exocentric strategies (5, 6). The interaction between body and environmental space via navigation continuously occurs based on data from the navigable space (optic flow and visual cues) and the perception of self-motion (vestibular and somatosensory) (5, 6).
Over the past century, transcutaneous delivery of electric currents to the vestibular afferents, commonly referred to as galvanic vestibular stimulation (GVS), has been used to study and understand the function of the vestibular system (7). GVS is a technique that can stimulate the spike trigger zone of primary vestibular afferents associated with both semicircular canals (SCCs) and otolith organs (8, 9), allowing perception of vestibular sensations with excellent temporal control (10, 11). Firing rates of peripheral vestibular afferents are increased by galvanic currents at the cathode and decreased at the anode (9, 12, 13). The adaptive capacity of two types of vestibular afferent fibers to each GVS waveform is strikingly different that irregular fibers are sensitive to small-amplitude, highly dynamic currents (alternating current, AC), but fail to sustain tonic firing rates with constant currents (direct current, DC), whereas regular fibers are the opposite (14). GVS has also been used in functional neuroimaging studies to investigate the vestibular system (15) and in a variety of behavioral experiments on the effects of vestibular stimulation on locomotion and cognitive processes (16). In the framework of clinical therapeutic studies, favorable advantages of GVS have been shown in the improvement of postural stability (17–19), gait performance (20), functional mobility (21, 22), sensory perception (23–25), and cognition (26, 27).
Due to an increasing interest in GVS applications in clinical neuropsychiatry and rehabilitation, it is important to determine the stimulus parameters of waveforms (DC, sinusoidal or noisy), intensity, frequency, polarity, duration, and timing of stimulation, as well as electrode size and positioning (14). When altering these parameters, different amounts of electric current can be elicited, and diverse physiologic and adverse effects induced (14). In addition to the assessment of efficacy, electrical safety is crucial for the clinical application of GVS and must be considered in the intervention. Frequently reported detrimental effects of GVS include itching, tingling, and burning at the stimulation site. In addition, high GVS amplitude leads to significantly decreased performance in short-term spatial memory or egocentric mental rotation, supported by a significant decrease in cell proliferation in the hippocampus (28), induced postural instability (29), and decreased dynamic visual acuity (30). To achieve the optimal effect and simultaneously reduce detrimental effects, it is necessary to determine the GVS threshold defined by the lowest level of stimulus required to evoke vestibular responses. GVS currents also activate other sensory inputs including those of the cutaneous, visual, and auditory systems as well as the vestibular system, which encompasses somatosensory, oculomotor, and vestibular responses during an intervention (14). Therefore, investigating the thresholds for specific points of each sensory perspective, cutaneous and vestibular perception as well as oculomotor response, is necessary. Because precise sensory feedback from the visual, vestibular, and cutaneous systems is critical for maintaining balance and motor control, determining the appropriate individualized GVS threshold will maximize the therapeutic effects of GVS intervention while minimizing the associated unpleasant sensations. In particular, due to increased clinical application of GVS, the threshold should be determined prior to the experimental session (31–33). Previous research has determined GVS threshold based on mostly vestibular perception, occasionally cutaneous feeling, and rarely oculomotor activity. To the best of our knowledge, no study has previously assessed these specific GVS thresholds concurrently. Thus, the most appropriate method to determine GVS threshold remains unclear. In this study, specific threshold intensities and response characteristics of GVS on vestibular and cutaneous perception as well as oculomotor response were determined.
Materials and methods
Participants
This study included 25 healthy subjects (32.6 ± 7.2 years; range 23–53 years; 11 males, 14 females, Table 1) recruited at Jeonbuk National University Hospital from July to September 2021. According to the Edinburgh Handedness Inventory (34), all participants were right-handed. Participants did not have any history of neurologic or neuro-otologic diseases and were not taking any medication. The subjects underwent neurotological evaluations including video oculography and head-impulse test and vestibular-evoked myogenic potential tests to screen for any vestibular impairment. They showed no abnormalities on examinations. The subjects provided written informed consent to participate in the study following an explanation of the study protocol. All experiments were reviewed and approved by the Institutional Review Board at Jeonbuk National University Hospital (No. 2021-07-013-007).
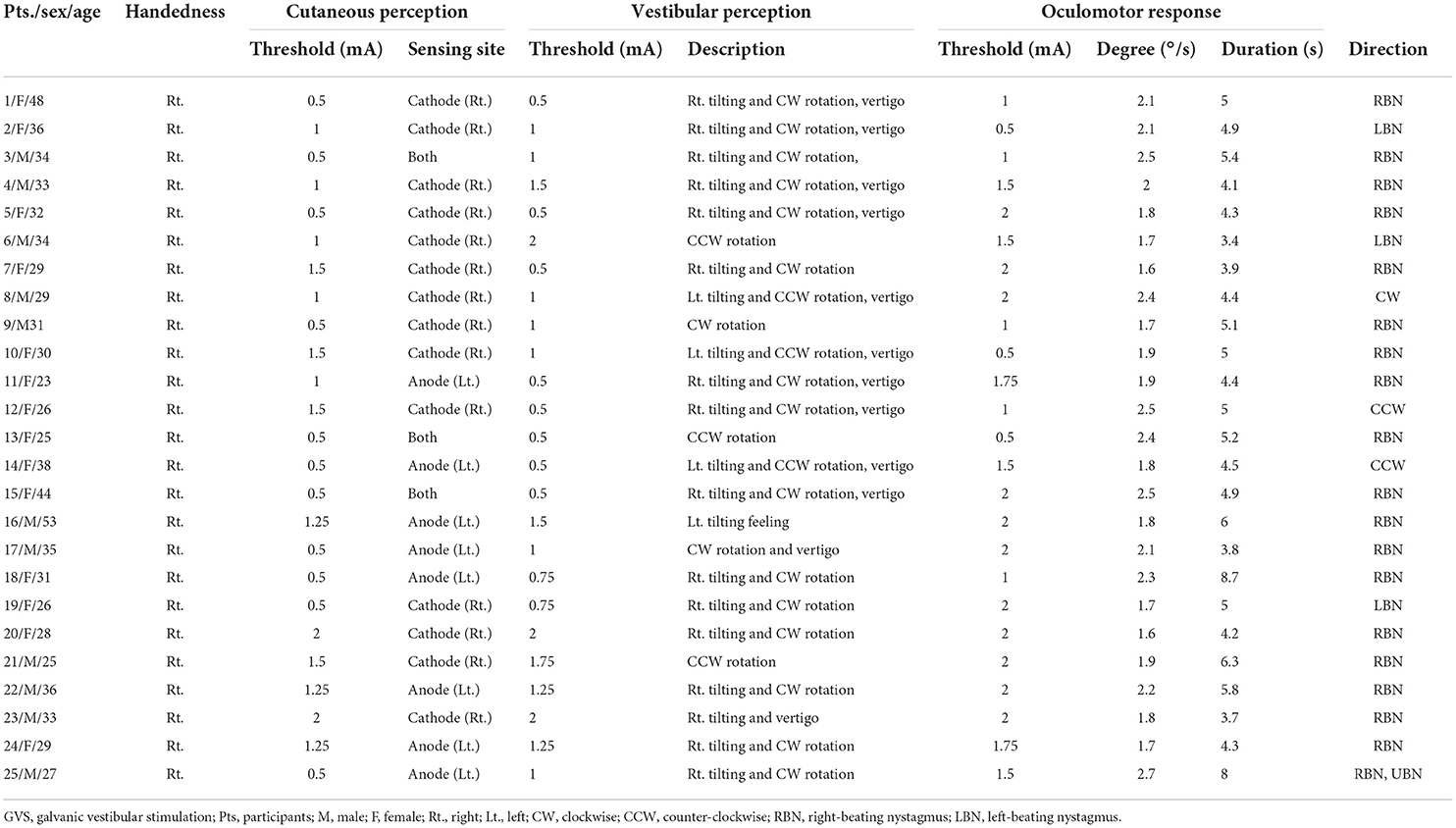
Table 1. Thresholds for somatosensory and vestibular perception and oculomotor response and their characteristics in response to GVS with the cathode on the right and the anode on the left (CRAL).
Experimental design and GVS procedure
Data from each participant were collected in two randomly ordered sessions of DC-GVS paradigms of cathode on the right and anode on the left (CRAL) or cathode on the left and anode on the right (CLAR; Tables 1, 2). The experimental design using “CLAR and CRAL” in right-handed participants is required in the context of handedness-dependent vestibular lateralization, in which the vestibular input and signaling pathways from the dominant side are more predominant than those from the non-dominant side (35, 36). This allowed the detection of any difference in the GVS threshold between CLAR and CRAL models. At the beginning of the sessions, participants received verbal and written instructions regarding the tasks. A CE-certified battery-driven constant current stimulator (neuroConn DC-Stimulator Plus; neuroConn, Ilmenau, Germany) was used to deliver the DC to subjects sitting upright in a chair via a pair of 35 cm2 (5 × 7 cm) (37–39) rectangular conductive rubber electrodes (neuroConn) coated with electrode gel and placed binaurally over both mastoids. The maximum current density in this study was estimated to be 57.14 μA/cm2 (corresponding to a charge density of 1.71 Coloumb/cm2) at the skin surface, similar to previous studies (37, 38), and well below the safety limit for tissue damage (40–42). The electrodes were positioned on the mastoid, supplemented with a conductive gel prior to testing to reduce skin impedance, and secured in place by a rubber head strap. Participants were seated in a comfortable chair in front of a target bar of the three-dimensional video oculography instrument (3D-VOG, SMI, the Netherlands, sampling rate of 60 Hz) and wore goggles that tracked their ocular movements under the supervision of a senior neurotologist (S.Y. Oh). Threshold testing started with a low current (0.5 mA), gradually increasing by 0.25 mA for a 3-s period over a 5-min inter-step interval. In the inter-step interval, the subjects were asked to describe whether they experienced any tingling/pain (cutaneous perception threshold) or dizziness/unsteadiness (vestibular perception threshold) during GVS application. The senior neurotologist also monitored for the appearance of nystagmus when recording with the VOG (oculomotor threshold). These iterations were continued with a steady increase in current until the participant concurrently expressed tingling/pain, dizziness/unsteadiness, and nystagmus. To confirm the specific thresholds, the stimulus intensity was gradually decreased by 0.25 mA from 2 mA to the level at which the tingling/pain (cutaneous threshold), dizziness/unsteadiness (vestibular threshold), and ocular responses (oculomotor threshold) disappeared (Figure 1). The procedure was repeated two times to confirm the cutaneous, vestibular, and oculomotor thresholds (43–45). When thresholds differed between sessions, the mean value was used for analysis.
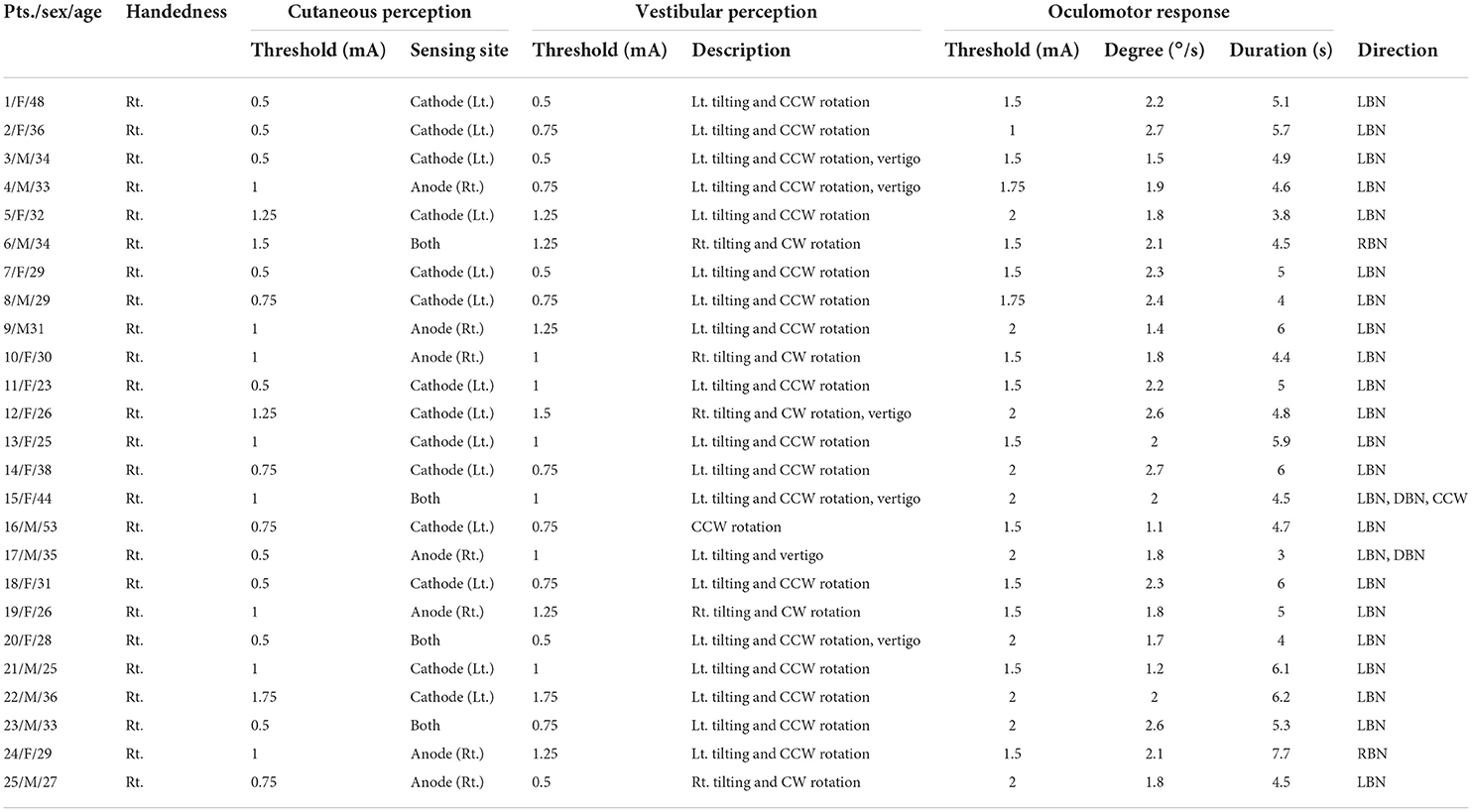
Table 2. Thresholds for somatosensory and vestibular perception and oculomotor response and their characteristics in response to GVS with the cathode on the left and the anode on the right (CLAR).
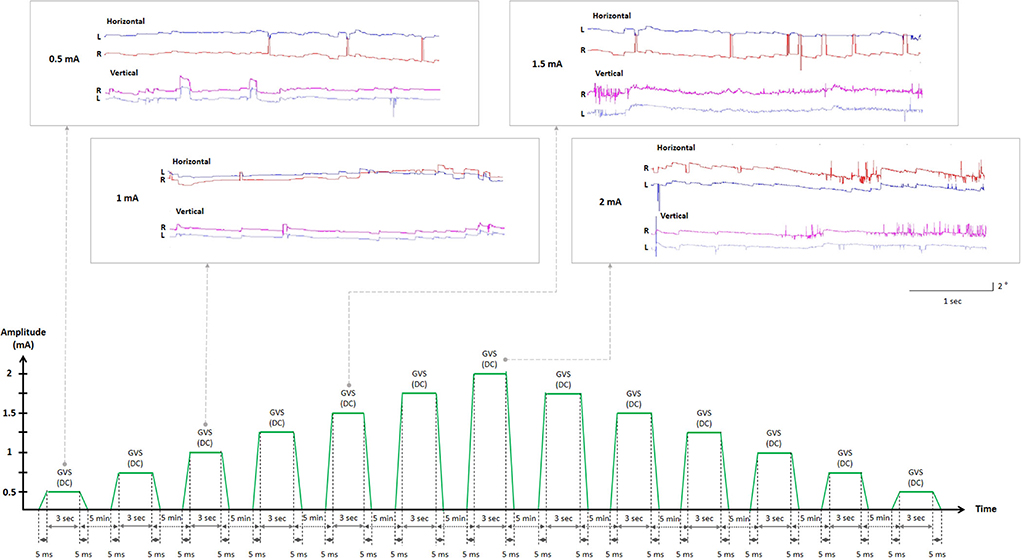
Figure 1. Experimental design flowchart. Representations of ocular movements during GVS application (in the boxes). The two-step approach to determining GVS thresholds. GVS, galvanic vestibular stimulation; DC, direct current; L, left eye (blue); R, right eye (red).
Thresholds for vestibular and cutaneous perception and oculomotor response
A verbal warning preceded each stimulus, and subjects were instructed to say “yes” when perceiving tingling, pain, or any other sensation at the electrodes. Subjects were asked to describe the sensory feelings and indicate the location of occurrence. Subjects were also instructed to focus on vestibular perception and asked to report any sense of dizziness with spinning or non-spinning and disequilibrium or any sense of motion. Each report was followed by feedback confirming the perceptions such as motion direction, and the current intensity was increased in the next step. Eye position and movement were binocularly recorded using the 3D-VOG during the entire experiment. Digitized data were analyzed using the MATLAB software (MathWorks, Natick, MA, USA). All data pertaining to the results reported in this article (text and figures) will be shared.
Statistical analysis
All data were analyzed using SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA). For each parameter, the normality of the distribution was assessed using the Shapiro-Wilk test. The variables were presented as mean ± standard deviation. The non-parametric variables were indicated as median (95% confidence interval), and the significant difference was determined using the Kruskal-Wallis test (between group comparison) and the Mann-Whitney U-test (pairwise comparisons). The chi-square one-variable test was used to compare the difference between variables in multiple categories. The Spearman's correlation coefficient was used to assess the correlations between age and the vestibular, cutaneous, and oculomotor thresholds. Statistical significance was set at a 0.05 level.
Results
Although GVS is mildly unpleasant, no participant reported particular discomfort or withdrew from the study. All participants perceived cutaneous and vestibular sensations and showed oculomotor responses confirmed by VOG recordings (Figure 1).
Thresholds for cutaneous and vestibular perceptions and oculomotor response
Subjects experienced mild cutaneous tingling or stinging sensations above the mean stimulation intensity of 0.9 ± 0.29 mA (cutaneous threshold, 0.85 ± 0.35 mA on CLAR, and 0.97 ± 0.50 mA on CRAL; Figure 2). These somatosensory perceptions were more frequent on the cathode (56%) than the anode (30%) or both sides (14%; p = 0.001, chi-square one-variable test; Figure 3A). Subjects also experienced various vestibular sensations evoked by GVS, as described in the subject's self-report. Some participants experienced dizziness and vertigo, likely the manifestation of whole-body angular rotation or a sense of rotation of the environment as if the objects around them were spinning. Other participants described the feeling of change in gravity. These sensations persisted for several seconds even after cessation of the stimulation above the mean stimulation intensity of 0.98 ± 0.29 mA (vestibular threshold, 0.93 ± 0.33 mA on CLAR, and 1.03 ± 0.52 mA on CRAL; Figure 2). Rotating and falling sensations were more frequently experienced toward the cathode (76%) than the anode (24%, p < 0.001, chi-square one-variable test; Figure 3B). The vestibular threshold in males was significantly higher than in females [1 (0.5–1.25) mA vs. 0.75 (0.625–1.125) mA, Z = −2.241, p = 0.025, Mann–Whitney U-test]. However, significant differences were not observed in cutaneous perception and oculomotor response between the sexes. Correlations were not found between age and vestibular, cutaneous, and oculomotor thresholds.
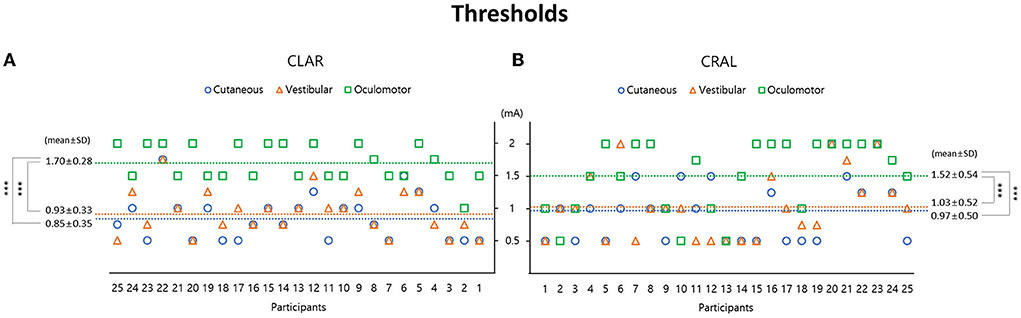
Figure 2. Galvanic vestibular stimulation (GVS) thresholds are presented separately: cutaneous and vestibular perceptions and oculomotor response using two stimulating paradigms of the cathode on the left and anode on the right (CLAR) (A) and the cathode on the right and anode on the left (CRAL) (B). In the CLAR paradigm, the oculomotor threshold (1.7 ± 0.28 mA) was significantly higher than the vestibular perception threshold (0.93 ± 0.33 mA, p < 0.001, Bonferroni test) and cutaneous perception threshold (0.85 ± 0.35 mA, p < 0.001, Bonferroni test) (A). In the CRAL paradigm, the oculomotor threshold (1.52 ± 0.54 mA) was also significantly higher than the vestibular perception threshold (1.03 ± 0.52 mA, p < 0.001, Bonferroni test) and cutaneous perception threshold (0.97 ± 0.5 mA, p < 0.001, Bonferroni test) (B). GVS threshold values are presented as mean ± standard deviation (mA).

Figure 3. Characteristics of galvanic vestibular stimulation (GVS)-induced cutaneous and vestibular perception and oculomotor responses depending on the stimulating paradigms; the cathode on the left and the anode on the right (CLAR, inside annulus) and the cathode on the right and the anode on the left (CRAL, outside annulus). Cutaneous perceptions were more frequent on the cathode side (56%) than the anode (30%) or both sides (14%; p = 0.001, chi-square one-variable test) (A). Rotating and falling sensations were more frequently experienced toward the cathode (76%) than the anode (24%, p < 0.001, chi-square one-variable test) direction (B). Nystagmoid ocular movements were observed more frequently with horizontal and torsional nystagmus toward the cathode (86%) than toward the anode (14%, p < 0.001, chi-square one-variable test) (C). Values are presented as percentages.
Short-latency eye movements were also observed above the mean stimulation intensity of 1.61 ± 0.35 mA (oculomotor threshold, 1.70 ± 0.28 mA on CLAR, and 1.52 ± 0.54 mA on CRAL; Figure 2). Nystagmoid ocular movements were observed more frequently with horizontal and torsional nystagmus toward the cathode (86%) than toward the anode (14%, p < 0.001, chi-square one-variable test; Figure 3C). The mean amplitude and the mean duration of nystagmus were, respectively, 2.03 ± 0.33°/s and 5.01 ± 1.23 s for CRAL and 2 ± 0.43°/s and 5.07 ± 0.98 s for CLAR (Table 3). Notably, the oculomotor threshold [1.75 (1.5–1.75) mA] was significantly higher than the vestibular perception threshold [1 (0.75–1.125) mA, Z = −4.866, p < 0.001, Mann–Whitney U-test] and the cutaneous perception threshold [0.875 (0.75–1.125) mA, Z = −5.184, p < 0.001, Mann–Whitney U-test; χ2 = 34.488, p < 0.001, Kruskal-Wallis test, Figure 2]. A comparison of the properties of cutaneous and vestibular perception and oculomotor responses between the CRAL and CLAR paradigms revealed no significant differences between them (Table 3).
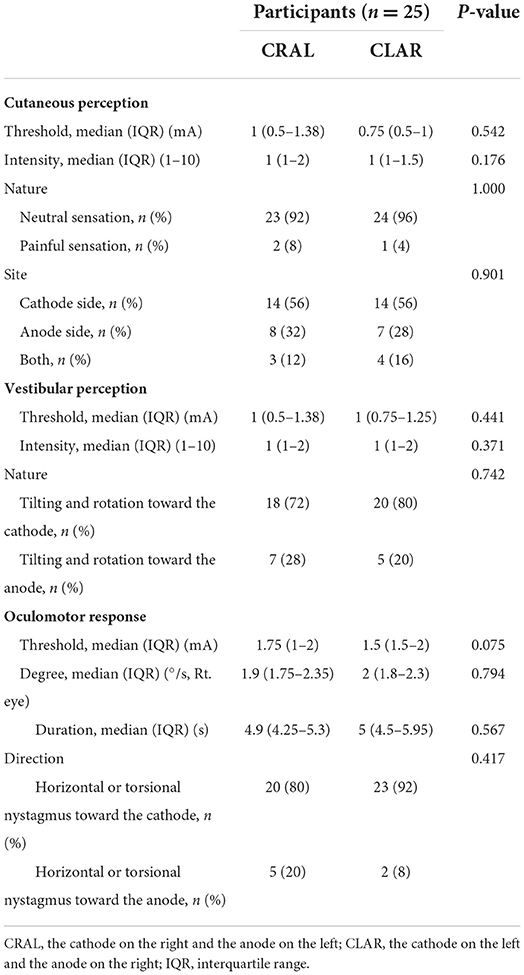
Table 3. Comparison of the properties of cutaneous and vestibular perception and oculomotor response depending on the CRAL and CLAR GVS paradigms.
Discussion
Vestibular and cutaneous perception and oculomotor response thresholds were measured in 25 healthy young subjects during DC GVS with both CRAL and CLAR paradigms, using a two-step approach with gradual increase and decrease in intensities. Vestibular and somatosensory perception thresholds were lower than the oculomotor threshold, indicating the use of the vestibular or somatosensory perception threshold to determine the GVS thresholds in the majority of human trials due to its sensitivity with minimal oculomotor responses. Theoretically, the vestibular threshold might reflect the vestibular function more directly and precisely than other motor response thresholds such as the vestibulo-ocular reflex (VOR) and the vestibulo-spinal reflex (VSR), which may be involved in central adaptation (1, 2, 14, 46, 47). Furthermore, the vestibular threshold could provide a comprehensive assay of peripheral vestibular function including the SSCs or otoliths (48, 49).
A previous study with a similar model reported a vestibular perception threshold of DC of 1.0 ± 0.2 mA, which is very similar to the results of our study (45). Recently, the vestibular threshold was shown to significantly increase after the age of 40 years (49), which has been considered a central gain enhancement for compensating the reduced peripheral vestibular input due to age-related degeneration of vestibular hair cells and afferents (50–52). In particular, prior studies have demonstrated that the aging-related degeneration of vestibular irregular afferent fibers, which are six times more sensitive to GVS than regular fibers, was faster and more potent than those in regular fibers (51, 53). Significant changes, however, have not been observed in the GVS threshold dependent on temporal effects, polarity effects, or body positions (10). Additionally, this study confirmed that there were no significant differences in the specific thresholds between the CRAL and CLAR configurations. Notably, a substantial difference associated with sex was found in vestibular perception but did not occur in cutaneous and oculomotor response thresholds, consistent with a recent trial (54). These sex-related threshold differences were most likely because they have a substantially different size of vestibular apparatus and numbers of vestibular afferent fibers (55, 56). In particular, the total number of myelinated axons in vestibular nuclei is significantly lower in females than in males (55). Regarding the otolith organs, the surface areas of the utricular and saccular maculae, the width of the utricular macula, and the length of the saccular macula are significantly greater in males than in females (56). In addition, diameters of the three SCCs are larger in males than in females, and the difference was statistically significant for the superior SCC (56). These anatomical distinctions might cause vestibular-related behavioral differences. For example, the GVS-induced ocular vestibular-evoked myogenic potential (oVEMP) amplitude was reported higher in males than in females (57). Males were shown to normally perform better in vestibular function-related tasks, in particular, visuospatial cognition such as 3D figures, spatial orientation, and maze navigation (58, 59).
Cutaneous sensations underneath and around the stimulus electrodes, such as burning, tingling, itching, and pain, mostly depend on the stimulus amplitude (14, 33) and have commonly been described as minor adverse effects of GVS intervention. The use of large surface electrodes on the mastoid, covering with electrode gel or topical anesthesia, or controlling the current amplitude helps to minimize the risk of skin irritations, burns, and patient discomfort (14, 60). The cutaneous threshold, also known as the nociceptive threshold, was determined to help manage these disruptions and enhance treatment compliance (17, 26, 61, 62). The cutaneous threshold was determined to be 0.9 mA in this study, with tingling sensation occurring mostly at the cathode side (Figure 3A). The findings were in agreement with a previous study conducted on healthy volunteers utilizing the staircase approach to assess the cutaneous threshold in which a mean value of 0.8 mA was reported (63). Although several previous studies have revealed that a threshold of ~1 mA produced no significant difference in somatosensory sensations between the anode and cathode sides (64–68), the sensory ratings were consistently higher at the cathode than at the anode for pain, tingling, piercing, electrifying, tugging, and pinching senses in other recent studies (Figure 3A) (68, 69). The tingling and itching sensations under the electrodes were usually short lasting and disappeared after a few seconds, similar to our findings. However, in some studies, the sensations were reportedly prolonged (64, 65, 70).
Regarding the characteristics of the vestibular responses to bipolar GVS, rotation and tilt were more frequently experienced toward the cathode (76%) than toward the anode (Figure 3B). Our present findings were in agreement with results from previous studies showing that DC-form GVS induces a tilt of subjective vertical toward the anode and simultaneously induces some illusion of self-tilt or spin toward the cathodal side (8, 71, 72). This dissociation between subjective vertical and body orientation elicited by GVS is mainly determined by central multisensory integration processes involved in the estimation of sensory cue reliability (72). Interestingly, the direction of tilt illusion was associated with posture only when the duration of the GVS was longer than 5 s, and it was toward the anode in freestanding posture, and toward the cathode in an immobilized posture (71). GVS-induced perception of motion is as context-dependent as oculomotor and postural responses, which are specified by the anatomic orientation of the SCC (SCC-driven signals) and the macular surfaces in the otolith organs (otolith-driven signals) (8). Cathodal GVS increases the firing rate of all responsive afferents, and anodal GVS decreases the firing rate of all responsive afferents; consequently, their effects at a canal are represented by a sum of geometric rotational stimuli (9, 40, 73). After calculating both the position of the canals in the plane of the head during upright standing and the incomplete orthogonal of SCCs, the summing vector representing the synergistic effects of SCC afferent activation evoked by GVS from both sides will be a signal roll with a yaw component, both directed toward the cathode, and a perceived visual tilt toward the anode (8, 11, 73). Furthermore, bilateral bipolar GVS produces a utricular firing pattern consistent with a natural stimulus of linear acceleration toward the cathodal side or visual tilt toward the anodal side (8, 11). However, in some psychophysical studies, the otolith contributions to GVS-induced postural responses were negligible compared with the canal input (74, 75).
As GVS can elicit ocular movement in the absence of head movement, it is frequently used to characterize potential pathologies in peripheral and central vestibular signal transmission (14). GVS activates both afferent fibers, including regular and irregular afferents, from all three SCCs to generate a horizontal-torsional nystagmus as a summative effect (14, 76). Regarding the time-related eye movements evoked by the application of a constant current application (DC-GVS) in this study, horizontal nystagmus frequently appeared at the onset of stimuli and immediately subsided, which was consistent with rapid activation but prompt adaptation characterized by thick, irregular fibers. However, non-adapting ocular torsion prevailed for the duration of the stimulus, which was the result of regular SCC fiber activation (14). Due to the oculomotor response threshold as the only method for estimating the GVS threshold in animal trials, it was often used as a control GVS (16, 77). In humans, however, due to discomforts such as oscillopsia and blurred vision caused by ocular movements, measurement of oculomotor threshold is less widely used to trigger vestibular stimulation with GVS. In previous studies with vestibular dysfunction patients, the GVS-induced eye movements were used to evaluate the reduction or absence of oculomotor contribution from the vestibular end organs (60, 78–80). Patients with bilateral vestibular nerve dissection or other pathological disorders affecting both vestibular nerves did not reveal GVS-induced oculomotor responses; therefore, these GVS-induced eye movements are considered a strong predictor of the residual function of the injured vestibular nerve (81–83). In this study, the oculomotor threshold was significantly higher than the vestibular and cutaneous perception thresholds (1.61 mA vs. 0.98 mA and 0.9 mA, Figure 2). Some previous studies have investigated GVS-induced nystagmus in normal subjects (80, 84), in which the oculomotor response appeared with intensities above 1.5 mA, similar to this study (80). Because the entire populations of both SCCs and otolith afferents are susceptible to GVS (76), GVS-induced oculomotor responses are likely to be exceedingly diversified, including the roll, yaw, and pitch components (76). In this study, the most prevalent components were horizontal and torsional nystagmus, primarily beating toward the cathode, compatible with vectored activation of afferent fibers from all three SCCs on one side (51, 76). Other studies have reported obvious GVS-induced oculomotor response of mainly conjugate ocular torsion with the upper pole of both eyes rotating toward the side of the anode, slight skew deviation with hypertropia of the eye ipsilateral to the cathode and hypotropia with the eye of the anode, and small conjugate horizontal eye movements toward the anode (80). Amplitudes of GVS-induced eye movements apparently depend on the stimulating current level or the group of vestibular afferents engaged with regular or irregular firing rates (76, 80, 85). Furthermore, GVS-induced nystagmus was suppressed by visual fixation in light (60, 79, 85), and horizontal nystagmus was mainly observed at the onset and offset of the current step, with ocular torsion prevailing for the entire duration of the stimulus up to several minutes (79).
The DC-GVS is useful for rescuing vestibular function in unilateral vestibular disorders by rebalancing the vestibular deafferentation effect proved in previous studies (77, 86). Therefore, in this study, we investigated only the GVS thresholds using DC. Based on the components of tonic or phasic vestibular fibers that are likely to be recruited for the GVS response, we surmise threshold differences between DC and AC stimulation. Thus, further research is needed to determine the GVS thresholds for the AC (sinusoidal, noisy). Regarding the statistically non-significant results in our study such as age-related GVS threshold, it could be due to a type 2 error related to the small sample size.
In conclusion, GVS is a safe stimulation method when certain standard procedures are followed. Due to the variables influencing GVS-induced cortical excitability, the same quantity of current is likely to have non-uniform effects in each subject with different conditions. Individual factors are a source of heterogeneity in clinical research, which is a significant obstacle for routine use in clinical settings. Therefore, the patient-specific customized threshold should be determined rather than population thresholds. The results of this study show more sensitive vestibular and somatosensory perception thresholds than the oculomotor threshold, which allows the selection of the appropriate current that induces the vestibular effect while simultaneously preventing disturbing ocular movements during the intervention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
All experiments were reviewed and approved by the Institutional Review Board at Jeonbuk National University Hospital (No. 2021-07-013-007). The patients/participants provided their written informed consent to participate in this study.
Author contributions
TTN collected data, conducted the experiment, and wrote the manuscript. J-JK collected data and drew the figures. S-YO designed and supervised the study and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (Ministry of Science and ICT; No. 2022R1A2B5B01001933) and by the Fund of the Biomedical Research Institute, Jeonbuk National University Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. (2008) 31:125–50. doi: 10.1146/annurev.neuro.31.060407.125555
2. Vidal P-P, Cullen K, Curthoys I, Du Lac S, Holstein G, Idoux E, et al. The Vestibular System. The Rat Nervous System. Amsterdam: Elsevier (2015). p. 805–64. doi: 10.1016/B978-0-12-374245-2.00028-0
3. Ferrè ER, Walther LE, Haggard P. Multisensory interactions between vestibular, visual and somatosensory signals. PLoS ONE. (2015) 10:e0124573. doi: 10.1371/journal.pone.0124573
4. Zaleski-King AC, Lai W, Sweeney AD. Anatomy and Physiology of the Vestibular System. Diagnosis and Treatment of Vestibular Disorders. Berlin: Springer (2019). p. 3–16. doi: 10.1007/978-3-319-97858-1_1
5. Brandt T. Vestibular Cortex: Its Locations, Functions, and Disorders. New York, NY: Vertigo (2003). p. 219–31. doi: 10.1007/978-1-4757-3801-8_13
6. Pissaloux E, Velázquez R. On spatial cognition and mobility strategies. Mobility oVisually Impaired People. (2018) 2018:137–66. doi: 10.1007/978-3-319-54446-5_5
7. Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabat L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. (2012) 5:175–95. doi: 10.1016/j.brs.2011.03.002
8. Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. (2004) 96:2301–16. doi: 10.1152/japplphysiol.00008.2004
9. Goldberg J, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. (1984) 51:1236–56. doi: 10.1152/jn.1984.51.6.1236
10. Ertl M, Klimek M, Boegle R, Stephan T, Dieterich M. Vestibular perception thresholds tested by galvanic vestibular stimulation. J Neurol. (2018) 265:54–6. doi: 10.1007/s00415-018-8808-9
11. Bent LR, McFadyen BJ, Merkley VF, Kennedy PM, Inglis JT. Magnitude effects of galvanic vestibular stimulation on the trajectory of human gait. Neurosci Lett. (2000) 279:157–60. doi: 10.1016/S0304-3940(99)00989-1
12. Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci. (1991) 11:1636–48. doi: 10.1523/JNEUROSCI.11-06-01636.1991
13. Courjon J, Precht W, Sirkin D. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Exp Brain Res. (1987) 66:41–8. doi: 10.1007/BF00236200
14. Dlugaiczyk J, Gensberger KD, Straka H. Galvanic vestibular stimulation: from basic concepts to clinical applications. J Neurophysiol. (2019) 121:2237–55. doi: 10.1152/jn.00035.2019
15. Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol. (2001) 85:886–99. doi: 10.1152/jn.2001.85.2.886
16. Nguyen TT, Nam G-S, Kang J-J, Han GC, Kim JS, Dieterich M, et al. Galvanic vestibular stimulation improves spatial cognition after unilateral labyrinthectomy in mice. Front Neurol. (2021) 2021:1261. doi: 10.3389/fneur.2021.716795
17. Iwasaki S, Yamamoto Y, Togo F, Kinoshita M, Yoshifuji Y, Fujimoto C, et al. Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology. (2014) 82:969–75. doi: 10.1212/WNL.0000000000000215
18. Goel R, Kofman I, Jeevarajan J, De Dios Y, Cohen HS, Bloomberg JJ, et al. Using low levels of stochastic vestibular stimulation to improve balance function. PLoS ONE. (2015) 10:e0136335. doi: 10.1371/journal.pone.0136335
19. Stefani SP, Serrador JM, Breen PP, Camp AJ. Impact of galvanic vestibular stimulation-induced stochastic resonance on the output of the vestibular system: a systematic review. Brain Stimul. (2020) 13:533–5. doi: 10.1016/j.brs.2020.01.006
20. Iwasaki S, Fujimoto C, Egami N, Kinoshita M, Togo F, Yamamoto Y, et al. Noisy vestibular stimulation increases gait speed in normals and in bilateral vestibulopathy. Brain Stimul. (2018) 11:709–15. doi: 10.1016/j.brs.2018.03.005
21. Mulavara AP, Kofman IS, De Dios YE, Miller C, Peters BT, Goel R, et al. Using low levels of stochastic vestibular stimulation to improve locomotor stability. Front Syst Neurosci. (2015) 9:117. doi: 10.3389/fnsys.2015.00117
22. Putman EJ, Galvan-Garza RC, Clark TK. The effect of noisy galvanic vestibular stimulation on learning of functional mobility and manual control nulling sensorimotor tasks. Front Hum Neurosci. (2021) 15:756674. doi: 10.3389/fnhum.2021.756674
23. Voros JL, Sherman SO, Rise R, Kryuchkov A, Stine P, Anderson AP, et al. Galvanic vestibular stimulation produces cross-modal improvements in visual thresholds. Front Neurosci. (2021) 15:311. doi: 10.3389/fnins.2021.640984
24. Keywan A, Wuehr M, Pradhan C, Jahn K. Noisy galvanic stimulation improves roll-tilt vestibular perception in healthy subjects. Front Neurol. (2018) 9:83. doi: 10.3389/fneur.2018.00083
25. Kerkhoff G, Hildebrandt H, Reinhart S, Kardinal M, Dimova V, Utz KS, et al. long-lasting improvement of tactile extinction after galvanic vestibular stimulation: two Sham-stimulation controlled case studies. Neuropsychologia. (2011) 49:186–95. doi: 10.1016/j.neuropsychologia.2010.11.014
26. Hilliard D, Passow S, Thurm F, Schuck NW, Garthe A, Kempermann G, et al. Noisy galvanic vestibular stimulation modulates spatial memory in young healthy adults. Sci Rep. (2019) 9:1–11. doi: 10.1038/s41598-019-45757-0
27. Wilkinson D, Zubko O, DeGutis J, Milberg W, Potter J. Improvement of a figure copying deficit during subsensory galvanic vestibular stimulation. J Neuropsychol. (2010) 4:107–18. doi: 10.1348/174866409X468205
28. Dilda V, MacDougall HG, Curthoys IS, Moore ST. Effects of Galvanic vestibular stimulation on cognitive function. Exp Brain Res. (2012) 216:275–85. doi: 10.1007/s00221-011-2929-z
29. MacDougall HG, Moore ST, Curthoys IS, Black FO. Modeling postural instability with Galvanic vestibular stimulation. Exp Brain Res. (2006) 172:208–20. doi: 10.1007/s00221-005-0329-y
30. Moore ST, MacDougall HG, Peters BT, Bloomberg JJ, Curthoys IS, Cohen HS. Modeling locomotor dysfunction following spaceflight with galvanic vestibular stimulation. Exp Brain Res. (2006) 174:647–59. doi: 10.1007/s00221-006-0528-1
31. Kennedy PM, Cressman EK, Carlsen AN, Chua R. Assessing vestibular contributions during changes in gait trajectory. Neuroreport. (2005) 16:1097–100. doi: 10.1097/00001756-200507130-00013
32. Bent LR, Bolton PS, Macefield VG. Modulation of muscle sympathetic bursts by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. (2006) 174:701–11. doi: 10.1007/s00221-006-0515-6
33. Utz KS, Korluss K, Schmidt L, Rosenthal A, Oppenländer K, Keller I, et al. Minor adverse effects of galvanic vestibular stimulation in persons with stroke and healthy individuals. Brain Injury. (2011) 25:1058–69. doi: 10.3109/02699052.2011.607789
34. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. (1971) 9:97–113. doi: 10.1016/0028-3932(71)90067-4
35. Dieterich M, Kirsch V, Brandt T. Right-sided dominance of the bilateral vestibular system in the upper brainstem and thalamus. J Neurol. (2017) 264:55–62. doi: 10.1007/s00415-017-8453-8
36. Kirsch V, Boegle R, Keeser D, Kierig E, Ertl-Wagner B, Brandt T, et al. Handedness-dependent functional organizational patterns within the bilateral vestibular cortical network revealed by fMRI connectivity based parcellation. Neuroimage. (2018) 178:224–37. doi: 10.1016/j.neuroimage.2018.05.018
37. Sandrini M, Fertonani A, Cohen LG, Miniussi C. Double dissociation of working memory load effects induced by bilateral parietal modulation. Neuropsychologia. (2012) 50:396–402. doi: 10.1016/j.neuropsychologia.2011.12.011
38. Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. (2007) 72:208–14. doi: 10.1016/j.brainresbull.2007.01.004
39. Nooristani M, Maheu M, Houde M-S, Bacon B-A, Champoux F. Questioning the lasting effect of galvanic vestibular stimulation on postural control. PLoS ONE. (2019) 14:e0224619. doi: 10.1371/journal.pone.0224619
40. Utz KS, Dimova V, Oppenländer K, Kerkhoff G. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—a review of current data and future implications. Neuropsychologia. (2010) 48:2789–810. doi: 10.1016/j.neuropsychologia.2010.06.002
41. Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. (2003) 114:2220–3. doi: 10.1016/S1388-2457(03)00235-9
42. Yuen TG, Agnew WF, Bullara LA, Jacques S, McCreery DB. Histological evaluation of neural damage from electrical stimulation: considerations for the selection of parameters for clinical application. Neurosurgery. (1981) 9:292–9. doi: 10.1227/00006123-198109000-00013
43. Helmchen C, Machner B, Rother M, Spliethoff P, Göttlich M, Sprenger A. Effects of galvanic vestibular stimulation on resting state brain activity in patients with bilateral vestibulopathy. Hum Brain Mapp. (2020) 41:2527–47. doi: 10.1002/hbm.24963
44. Helmchen C, Rother M, Spliethoff P, Sprenger A. Increased brain responsivity to galvanic vestibular stimulation in bilateral vestibular failure. NeuroImage. (2019) 24:101942. doi: 10.1016/j.nicl.2019.101942
45. Preuss N, Kalla R, Müri R, Mast FW. Framing susceptibility in a risky choice game is altered by galvanic vestibular stimulation. Sci Rep. (2017) 7:1–7. doi: 10.1038/s41598-017-02909-4
46. Hartmann M, Furrer S, Herzog MH, Merfeld DM, Mast FW. Self-motion perception training: thresholds improve in the light but not in the dark. Exp Brain Res. (2013) 226:231–40. doi: 10.1007/s00221-013-3428-1
47. Cullen K. Chapter 2—Physiology of central pathways. In: Furman JM, editors, Handbook of Clinical Neurology. Amsterdam: T. Lempert, Elsevier B.V, (2016). p. 17–40. doi: 10.1016/B978-0-444-63437-5.00002-9
48. Priesol AJ, Valko Y, Merfeld DM, Lewis RF. Motion perception in patients with idiopathic bilateral vestibular hypofunction. Otolaryngology. (2014) 150:1040–2. doi: 10.1177/0194599814526557
49. Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM. Vestibular perceptual thresholds increase above the age of 40. Front Neurol. (2016) 7:162. doi: 10.3389/fneur.2016.00162
50. Peters RM, Blouin J-S, Dalton BH, Inglis JT. Older adults demonstrate superior vestibular perception for virtual rotations. Exp Gerontol. (2016) 82:50–7. doi: 10.1016/j.exger.2016.05.014
51. Jahn K, Naeßl A, Schneider E, Strupp M, Brandt T, Dieterich M. Inverse U-shaped curve for age dependency of torsional eye movement responses to galvanic vestibular stimulation. Brain. (2003) 126:1579–89. doi: 10.1093/brain/awg163
52. Dalton BH, Blouin J-S, Allen MD, Rice CL, Inglis JT. The altered vestibular-evoked myogenic and whole-body postural responses in old men during standing. Exp Gerontol. (2014) 60:120–8. doi: 10.1016/j.exger.2014.09.020
53. Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. (2000) 130:277–97. doi: 10.1007/s002210050033
54. Geng B, Yoshida K, Jensen W. Impacts of selected stimulation patterns on the perception threshold in electrocutaneous stimulation. J Neuroeng Rehabil. (2011) 8:1–10. doi: 10.1186/1743-0003-8-9
55. Moriyama H, Itoh M, Shimada K, Otsuka N. Morphometric analysis of fibers of the human vestibular nerve: sex differences. Eur Archiv Oto-rhino-laryngol. (2007) 264:471–5. doi: 10.1007/s00405-006-0197-5
56. Sato H, Sando I, Takahashi H. Computer-aided three-dimensional measurement of the human vestibular apparatus. Otolaryngology. (1992) 107:405–9. doi: 10.1177/019459989210700311
57. Sung P-H, Cheng P-W, Young Y-H. Effect of gender on ocular vestibular-evoked myogenic potentials via various stimulation modes. Clin Neurophysiol. (2011) 122:183–7. doi: 10.1016/j.clinph.2010.06.004
58. Rilea SL. A lateralization of function approach to sex differences in spatial ability: a reexamination. Brain Cogn. (2008) 67:168–82. doi: 10.1016/j.bandc.2008.01.001
59. Mueller SC, Jackson CP, Skelton RW. Sex differences in a virtual water maze: an eye tracking and pupillometry study. Behav Brain Res. (2008) 193:209–15. doi: 10.1016/j.bbr.2008.05.017
60. MacDougall HG, Brizuela AE, Curthoys IS. Linearity, symmetry and additivity of the human eye-movement response to maintained unilateral and bilateral surface galvanic (DC) vestibular stimulation. Exp Brain Res. (2003) 148:166–75. doi: 10.1007/s00221-002-1289-0
61. Yamamoto Y, Struzik ZR, Soma R, Ohashi K, Kwak S. Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Ann Neurol. (2005) 58:175–81. doi: 10.1002/ana.20574
62. Wuehr M, Nusser E, Krafczyk S, Starube A, Brandt T, Jahn K, et al. Noise-enhanced vestibular input improves dynamic walking stability in healthy subjects. Brain Stimul. (2016) 9:109–16. doi: 10.1016/j.brs.2015.08.017
63. Wilkinson D, Nicholls S, Pattenden C, Kilduff P, Milberg W. Galvanic vestibular stimulation speeds visual memory recall. Exp Brain Res. (2008) 189:243–8. doi: 10.1007/s00221-008-1463-0
64. Ambrus GG, Paulus W, Antal A. Cutaneous perception thresholds of electrical stimulation methods: comparison of tDCS and tRNS. Clin Neurophysiol. (2010) 121:1908–14. doi: 10.1016/j.clinph.2010.04.020
65. Dundas J, Thickbroom G, Mastaglia F. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol. (2007) 118:1166–70. doi: 10.1016/j.clinph.2007.01.010
66. Ferrè ER, Day BL, Bottini G, Haggard P. How the vestibular system interacts with somatosensory perception: a sham-controlled study with galvanic vestibular stimulation. Neurosci Lett. (2013) 550:35–40. doi: 10.1016/j.neulet.2013.06.046
67. Lenggenhager B, Lopez C, Blanke O. Influence of galvanic vestibular stimulation on egocentric and object-based mental transformations. Exp Brain Res. (2008) 184:211–21. doi: 10.1007/s00221-007-1095-9
68. McFadden JL, Borckardt JJ, George MS, Beam W. Reducing procedural pain and discomfort associated with transcranial direct current stimulation. Brain Stimul. (2011) 4:38–42. doi: 10.1016/j.brs.2010.05.002
69. Borckardt JJ, Linder K, Ricci R, Li X, Anderson B, Arana A, et al. Focal electrically administered therapy (FEAT): device parameter effects on stimulus perception in humans. J ECT. (2009) 25:91. doi: 10.1097/YCT.0b013e318183c6a4
70. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. (2006) 117:845–50. doi: 10.1016/j.clinph.2005.12.003
71. Wardman DL, Taylor JL, Fitzpatrick RC. Effects of galvanic vestibular stimulation on human posture and perception while standing. J Physiol. (2003) 551:1033–42. doi: 10.1113/jphysiol.2003.045971
72. Mars F, Vercher J-L, Popov K. Dissociation between subjective vertical and subjective body orientation elicited by galvanic vestibular stimulation. Brain Res Bull. (2005) 65:77–86. doi: 10.1016/j.brainresbull.2004.11.012
73. Day B, Severac Cauquil A, Bartolomei L, Pastor M, Lyon I. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. (1997) 500:661–72. doi: 10.1113/jphysiol.1997.sp022051
74. Mian OS, Dakin CJ, Blouin JS, Fitzpatrick RC, Day BL. Lack of otolith involvement in balance responses evoked by mastoid electrical stimulation. J Physiol. (2010) 588:4441–51. doi: 10.1113/jphysiol.2010.195222
75. Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. (2005) 563:229–34. doi: 10.1113/jphysiol.2004.079525
76. Zink R, Bucher S, Weiss A, Brandt T, Dieterich M. Effects of galvanic vestibular stimulation on otolithic and semicircular canal eye movements and perceived vertical. Electroencephalogr Clin Neurophysiol. (1998) 107:200–5. doi: 10.1016/S0013-4694(98)00056-X
77. Nam G-S, Nguyen TT, Kang J-J, Han GC, Oh S-Y. Effects of galvanic vestibular stimulation on vestibular compensation in unilaterally labyrinthectomized mice. Front Neurol. (2021) 12:736849. doi: 10.3389/fneur.2021.736849
78. MacDougall H, Brizuela A, Burgess A, Curthoys I, Halmagyi G. Patient and normal three-dimensional eye-movement responses to maintained (DC) surface galvanic vestibular stimulation. Otol Neurotol. (2005) 26:500–11. doi: 10.1097/01.mao.0000169766.08421.ef
79. MacDougall HG, Brizuela AE, Burgess AM, Curthoys IS. Between-subject variability and within-subject reliability of the human eye-movement response to bilateral galvanic (DC) vestibular stimulation. Exp Brain Res. (2002) 144:69–78. doi: 10.1007/s00221-002-1038-4
80. Cauquil AS, Faldon M, Popov K, Day BL, Bronstein AM. Short-latency eye movements evoked by near-threshold galvanic vestibular stimulation. Exp Brain Res. (2003) 148:414–8. doi: 10.1007/s00221-002-1326-z
81. Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. (2010) 121:132–44. doi: 10.1016/j.clinph.2009.09.027
82. Dieterich M, Zink R, Weiss A, Brandt T. Galvanic stimulation in bilateral vestibular failure: 3-D ocular motor effects. Neuroreport. (1999) 10:3283–7. doi: 10.1097/00001756-199911080-00007
83. Aw ST, Todd MJ, Lehnen N, Aw GE, Weber KP, Eggert T, et al. Electrical vestibular stimulation after vestibular deafferentation and in vestibular schwannoma. PLoS ONE. (2013) 8:e82078. doi: 10.1371/journal.pone.0082078
84. Kim HJ, Choi JY, Son EJ, Lee WS. Response to galvanic vestibular stimulation in patients with unilateral vestibular loss. Laryngoscope. (2006) 116:62–6. doi: 10.1097/01.mlg.0000184525.14825.f4
85. Curthoys IS, MacDougall HG. What galvanic vestibular stimulation actually activates. Front Neurol. (2012) 3:117. doi: 10.3389/fneur.2012.00117
Keywords: galvanic vestibular stimulation (GVS), vestibular perception threshold, cutaneous threshold, nystagmus, vertigo
Citation: Nguyen TT, Kang J-J and Oh S-Y (2022) Thresholds for vestibular and cutaneous perception and oculomotor response induced by galvanic vestibular stimulation. Front. Neurol. 13:955088. doi: 10.3389/fneur.2022.955088
Received: 28 May 2022; Accepted: 18 July 2022;
Published: 12 August 2022.
Edited by:
Denise Utsch Gonçalves, Federal University of Minas Gerais, BrazilReviewed by:
Theodore Raphan, Brooklyn College (CUNY), United StatesStefan Kammermeier, Ludwig Maximilian University of Munich, Germany
Copyright © 2022 Nguyen, Kang and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sun-Young Oh, ohsun@jbnu.ac.kr
†These authors have contributed equally to this work
 Thanh Tin Nguyen
Thanh Tin Nguyen Jin-Ju Kang
Jin-Ju Kang Sun-Young Oh
Sun-Young Oh