- 1West China Clinical Medical School, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Neurology, West China Hospital, Sichuan University, Chengdu, China
Background: Magnetic resonance-guided laser interstitial thermal therapy (MRgLiTT) and stereoelectroencephalography-guided radiofrequency thermocoagulation (SEEG-RFTC) are two effective, minimally invasive treatments for epilepsy with focal cortical dysplasia (FCD). The purpose of this study is to conduct a meta-analysis to evaluate and compare the efficacy and safety of these two therapies in epilepsy patients with FCD.
Methods: We searched PubMed, Embase, Cochrane, and other databases for articles published before March 2023. The primary objective was to compare the effectiveness and complications of MRgLiTT and SEEG-RFTC in epilepsy patients with FCD. The second objective was to determine which method provides a better prognosis for specific subgroup patients.
Results: According to the inclusion and exclusion criteria, 18 studies were included, comprising 270 FCD patients including 37 patients from 6 MRgLiTT studies and 233 from 12 SEEG-RFTC studies. There were no significant differences between MRgLiTT and SEEG-RFTC groups in the seizure-freedom rate (59%, 95% CI 44–74%; 52%, 95% CI 47–57%, P = 0.86) and the rate of ≥50% seizure-reduction of FCD (90%, 95% CI 80–100%; 90%, 95% CI 86–94%, P = 0.42). Both methods had low complication rates (17.1%, 28/159) and long-term complication (2.5%, 4/159) rate, with no significant difference between them (P = 0.17).
Conclusion: Both MRgLiTT and SEEG-RFTC are safe and minimally invasive treatments for patients with FCD. They have comparable performance in terms of postoperative seizure-freedom rates in patients with FCD, and both can be used as treatment options for patients with FCD. Our study found that SEEG-RFTC had a better therapeutic effect in the FCD2b subgroup.
1. Introduction
Focal cortical dysplasia (FCD) is one of the most common causes of refractory epilepsy (1). Surgical excision of epileptogenic foci is an effective treatment for drug-resistant epilepsy, but only approximately one-third of patients are suitable for it due to the lack of accurate mapping of the epileptogenic zone, the location of foci in functional areas, and the presence of multiple epileptogenic zones. In recent years, stereoelectroencephalography-guided radiofrequency thermocoagulation (SEEG-RFTC) and magnetic resonance-guided laser interstitial thermal therapy (MRgLiTT) have attracted much attention for their minimally invasive nature, safety, and effectiveness (2–4). In 2004, RFTC guided by stereotactic electroencephalogram (SEEG) recording was reported to be safe and led to a significant reduction in seizure frequency (5, 6). MRgLiTT is guided by real-time MRI to selectively ablate lesions or structures using heat released by laser to treat a variety of intracranial lesions (7). MRgLiTT, regarded as a less invasive alternative to open surgery, is more precise and predictable in terms of tissue ablation volume than other techniques for local tissue ablation, resulting in less collateral damage (8, 9).
The two therapies have been well established in the treatment of various etiologies (6, 10), but it remains unknown which method is superior for FCD patients. In this meta-analysis, we address this question by comparing the seizure-freedom rate and postoperative complications between MRgLiTT and SEEG-RFTC in patients with FCD.
2. Methods
2.1. Search strategy and inclusion criteria
This study was conducted according to the preferred reporting item of the Systematic Evaluation and Meta-Analysis (PRISMA) guidelines (see the PRISMA checklist) (11). A comprehensive search was made in databases including PubMed, Embase, and Cochrane using the following terms (adding an appendix “[Title/Abstract]” to each term): stereo-electroencephalography, radiofrequency-thermocoagulation, thermo-SEEG, laser ablation, laser interstitial thermotherapy, and focal cortical dysplasia. The search ended in March 2023.
Although pathologic confirmation is the gold standard for the diagnosis of FCD, the results of the pathology report were not available because all patients received non-excision treatment. We usually make a diagnosis based on seizure characteristics (drug-refractory epilepsy with a previous diagnosis of focal seizures and recent seizures), FCD imaging features (focal cortical thickening, poorly demarcated gray and white matter, cortical and/or subcortical white matter, and T2WI/FLAIR high signal, etc.), and electroencephalographic features of FCD (rhythmic epileptiform discharges, RED). The determination of FCD in this article was based on clinical experience (drug-resistance epilepsy; MRI characteristics of the lesions; EEG shows focal rhythmic interictal epileptiform discharges that may correlate spatially with the anatomic extent of the lesion) by the authors of the cited article, and the subtypes of the FCD were also classified by the original authors according to the pathological criteria published by the International League Against Epilepsy (ILAE) in 2011 (12), in the absence of any histopathologic data. The inclusion criteria were as follows: (1) prospective or retrospective study reporting the efficacy of SEEG-RFTC or MRgLiTT in patients with FCD; (2) providing detailed information of seizure-free patients and complications; (3) more than 1 month follow-up time.
The exclusion criteria were as follows: (1) a summary or abstract without full text; (2) MRgLiTT and/or SEEG-RFTC performed as a secondary choice after the failure of a prior operation; and (3) population overlap (when there was a duplication in the inclusion populations of two articles, the earlier published study was removed); (4) case reports.
2.2. Data extraction and assessment
Two investigators worked independently on study selection, data extraction, and quality assessment (YML and JYG). To assess the quality of the search strategy, both investigators screened the titles and abstracts of articles. Disagreement was settled through consultation with the senior author (JM). Full-text versions of all eligible studies were used for quality assessment (risk of bias) and data collection. The following information was extracted from each study: first author, year of publication, country and center, surgical period, study design, sample size, gender, age range, duration of follow-up, Engel classification, and complication. Data were calculated separately by the two investigators using standard extraction rules. In addition, the risk of bias in the included studies was assessed using the MINOR (methodological index for non-randomized studies) tool (10).
The outcomes of MRgLiTT and SEEG-RFTC procedures were rated in three classes: Class 1. seizure-free, i.e., no seizure attack after the coagulation, equal to the Engle class I; Class 2. responding, i.e., patients did not develop into seizure-free but had ≥50% improvement of epilepsy, equal to the Engle classes II and III; and Class 3. non-responding, i.e., <50% improvement of epilepsy or no improvement or even becoming worse, equal to the Engle class IV. In articles using the ILAE classification, ILAE1 was considered equal to Engel 1, indicative of seizure freedom. In the analysis of complications, all postoperative complications were divided into transient and permanent complications (defined as a complication that did not resolve at the time of discharge) for separate analyses as different types of complications do not affect patients to the same degree.
2.3. Statistical analyses
Raw proportions were used to calculate prevalence and 95% confidence interval (CI). The I2 index was used to assess heterogeneity. If the I2 index is >50% or the P-value is <0.05, heterogeneity is considered to be significant, and the random effect model will be used. Otherwise, the fixed effects model would be used. For each study, forest plots were created to show a 95% CI of prevalence. Subgroup analyses were performed to investigate clinical heterogeneity. The funnel plot was used to assess potential publication bias, and Egger's linear regression test was used to determine whether the publication bias was statistically significant (13). A P-value of <0.05 was considered to be statistically significant (two-sided). R statistical (version 4.1.1) software was used for all analyses.
3. Results
3.1. Study selection and quality assessment
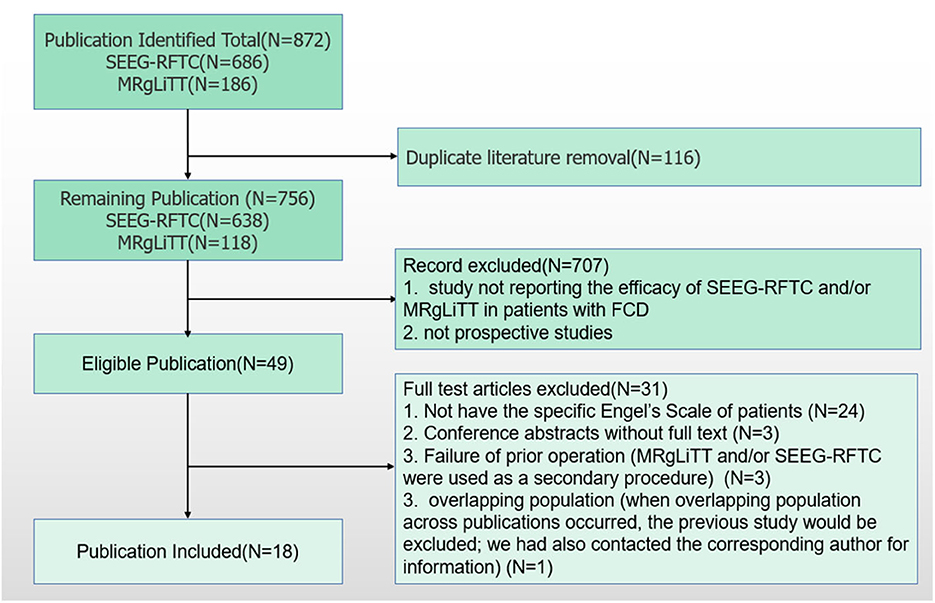
According to the search strategy, 872 articles were obtained from the online databases. After abstract screening and duplicate removal, 49 articles were remaining. By full-text reviewing, 24 articles were further excluded as they did not provide specific Engel classifications of patients, and three articles were conference abstracts without full-text. Three studies employed patients receiving MRgLiTT and/or SEEG-RFTC as a secondary procedure after failure to respond to a prior operation (14–16). Three articles used the same basic data, of which the study with the largest time span of patient enrollment was used in this study (17–19). At last, 14 articles were included for meta-analysis, including 6 for MRgLiTT (20–25) and 12 for SEEG-RFTC (15, 17, 19, 26–34). The flow chart of this study is shown in Figure 1.
3.2. Population characteristics
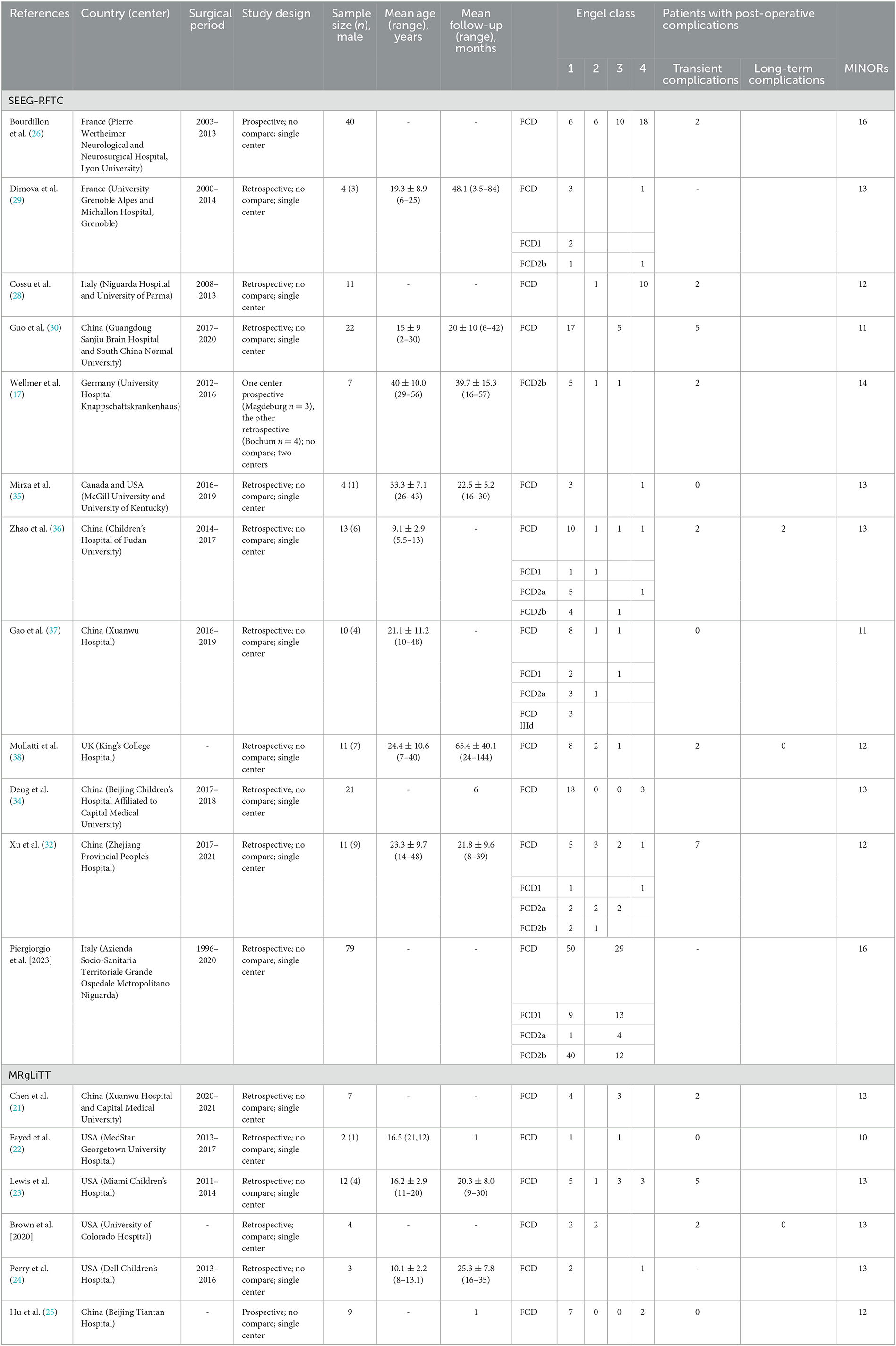
The total number of FCD patients was 270, with 37 (13.7%) in the MRgLiTT group and 233 (86.3%) in the SEEG-RFTC group. Table 1 shows the essential characteristics of the 14 studies.
3.3. Efficacy
3.3.1. FCD control by MRgLiTT and SEEG-RFTC
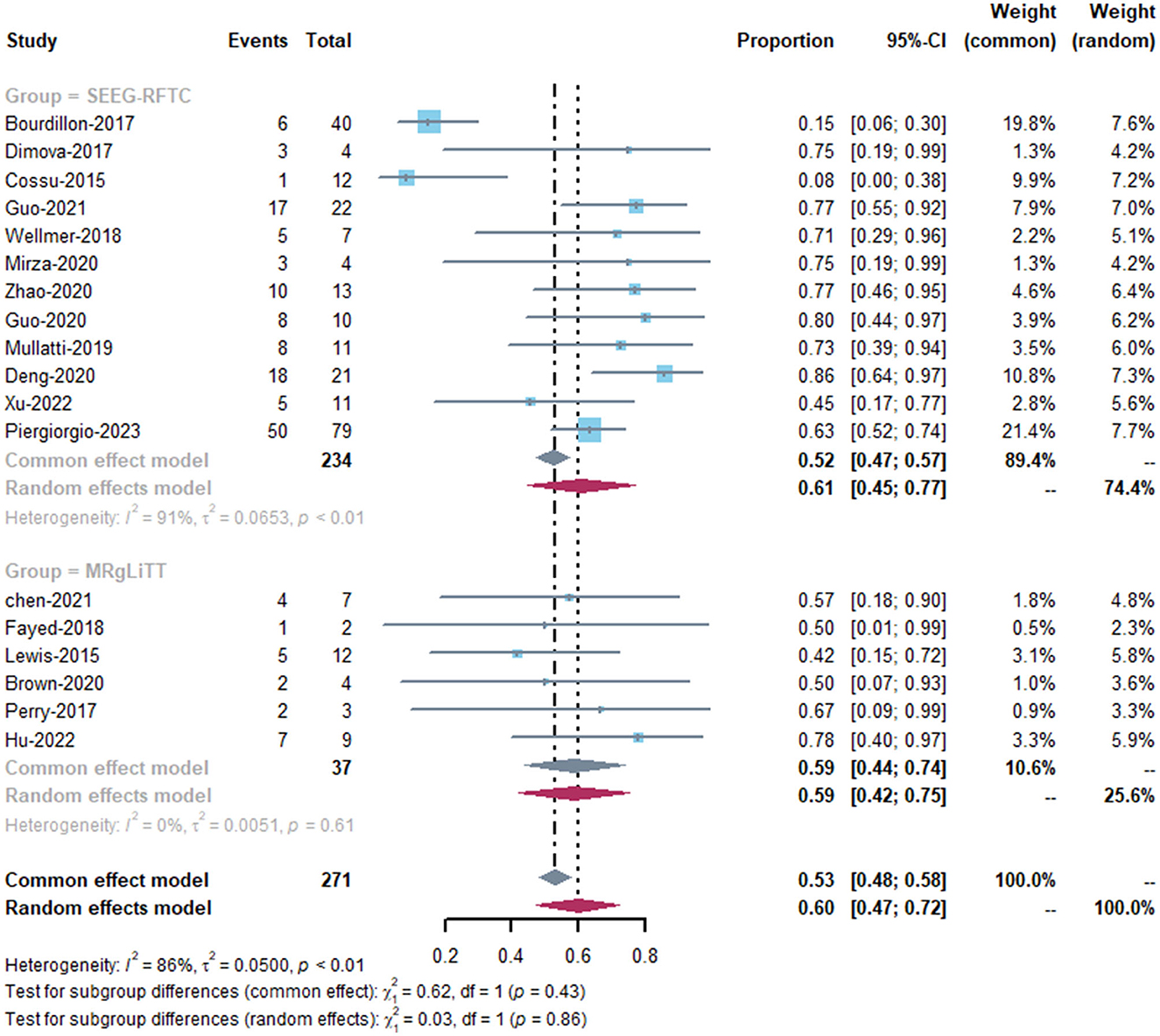
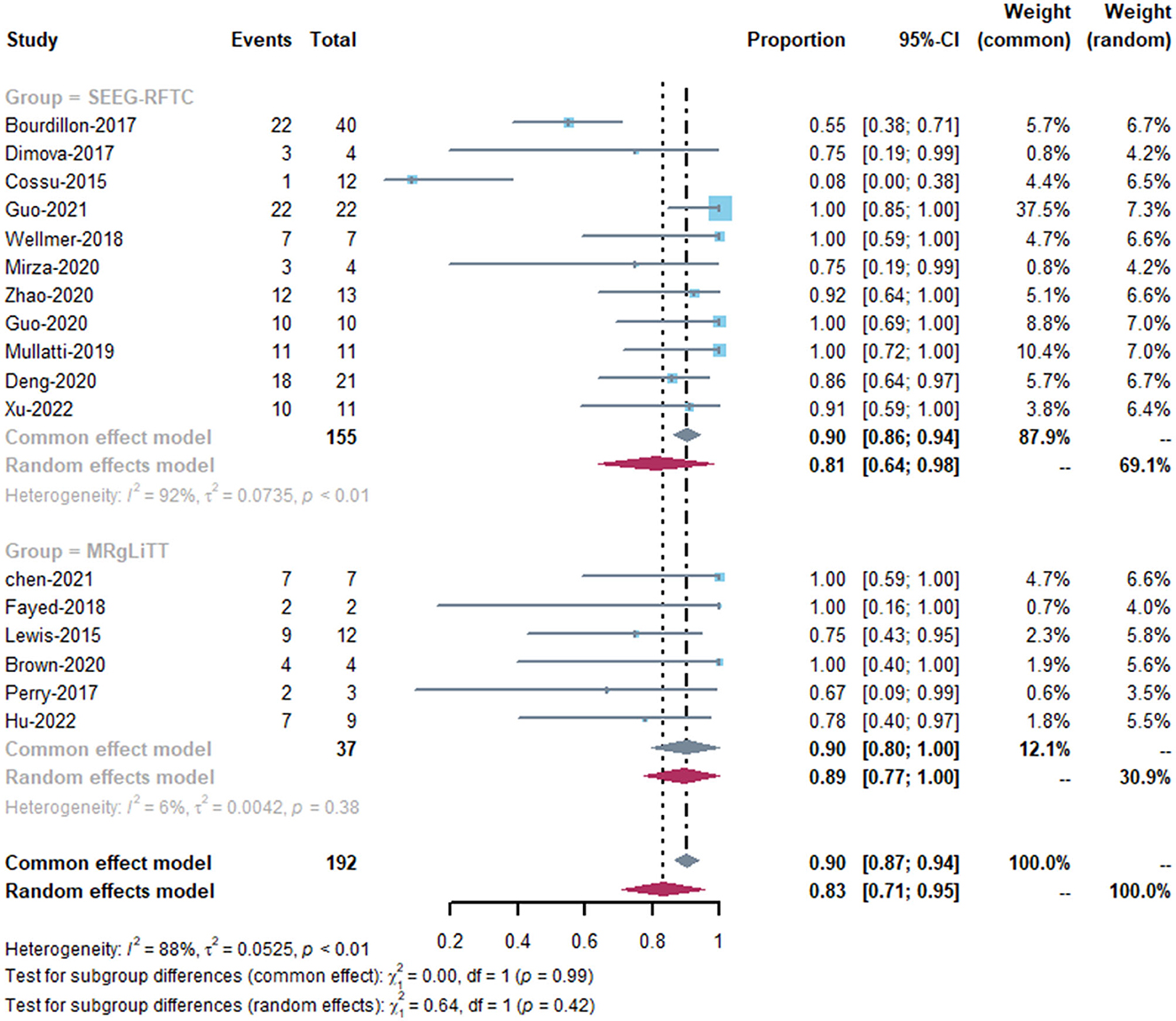
The MRgLiTT group had a seizure-freedom rate of 42 to 78%, whereas the SEEG-RFTC group had a rate of 8 to 86%. The SEEG-RFTC group had a high heterogeneity (91%), while the MRgLiTT group had a low heterogeneity (0%). The average seizure-freedom rate in the MRgLiTT group was 59% (95% CI 44–74%), while that in the SEEG-RFTC group was 52% (95% CI 47–57%), with no significant difference between the two groups (P = 0.86) (Figure 2). The average rate of ≥50% seizure reduction in the MRgLiTT group was 90% (95% CI 80–100%) and that in the SEEG-RFTC group was 90% (95% CI 86–94%), with no significant difference between the two groups (P = 0.42) (Figure 3).
Regarding the publication bias, the funnel plots showed obvious heterogeneity among studies (Supplementary Figures 1, 2), and the Egger's test showed no statistical significance for bias (SEEG-RFTC group: P = 0.20). The sample size of the MRgLiTT group is <10.
3.3.2. Subgroup analysis
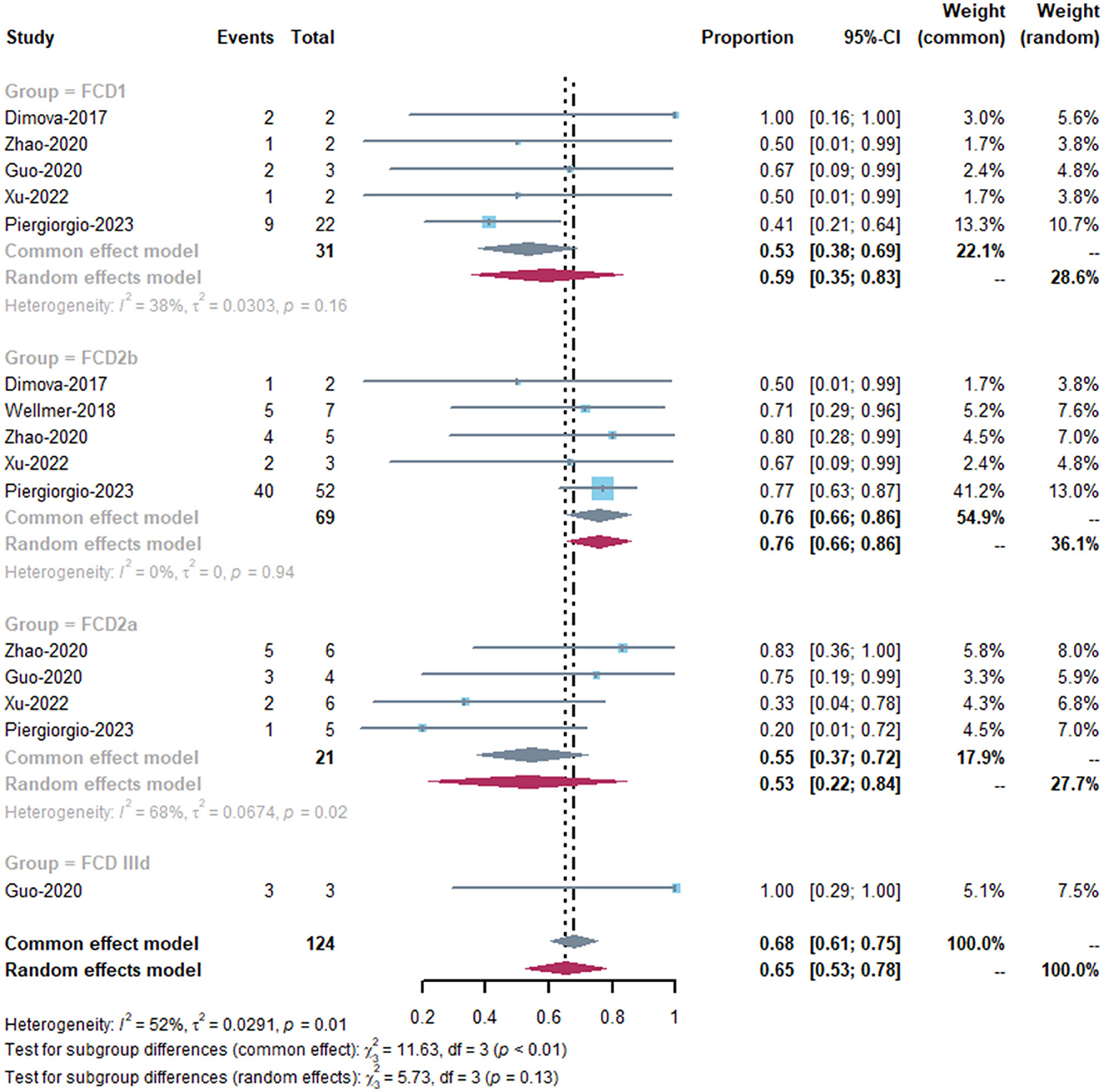
As there was no specific classification of FCD patients in the MRgLiTT group and the heterogeneity of the MRgLiTT group was low (0%), we performed subgroup analysis mainly in the SEEG-RFTC group. There were significant differences in the seizure-freedom rate among the three subtypes FCD1, FCD2a, and FCD2b (P < 0.01) (Figure 4). Patients with FCD2b had better response to SEEG-RFTC than FCD1 and FCD2a patients (P < 0.01).
In addition, we evaluated age and gender as a factor for heterogeneity. The results showed that age (>18 vs. ≤ 18; MRgLiTT: P = 0.73; SEEG-RFTC: P = 0.83) and sex (male vs. female; MRgLiTT: P = 0.73; SEEG-RFTC: P = 0.83) were not influential factors for epilepsy freedom in FCD patients in either the MRgLiTT or the SEEG-RFTC groups.
3.4. Complications
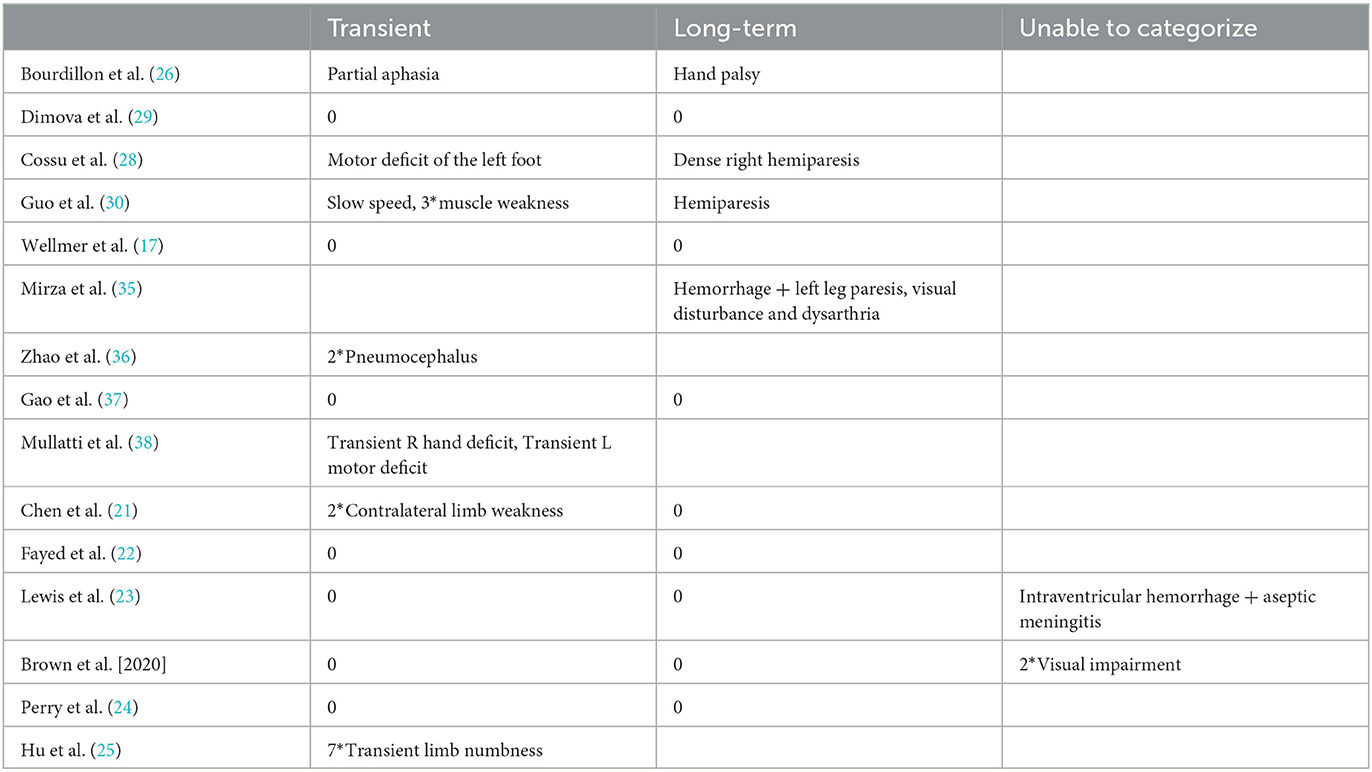
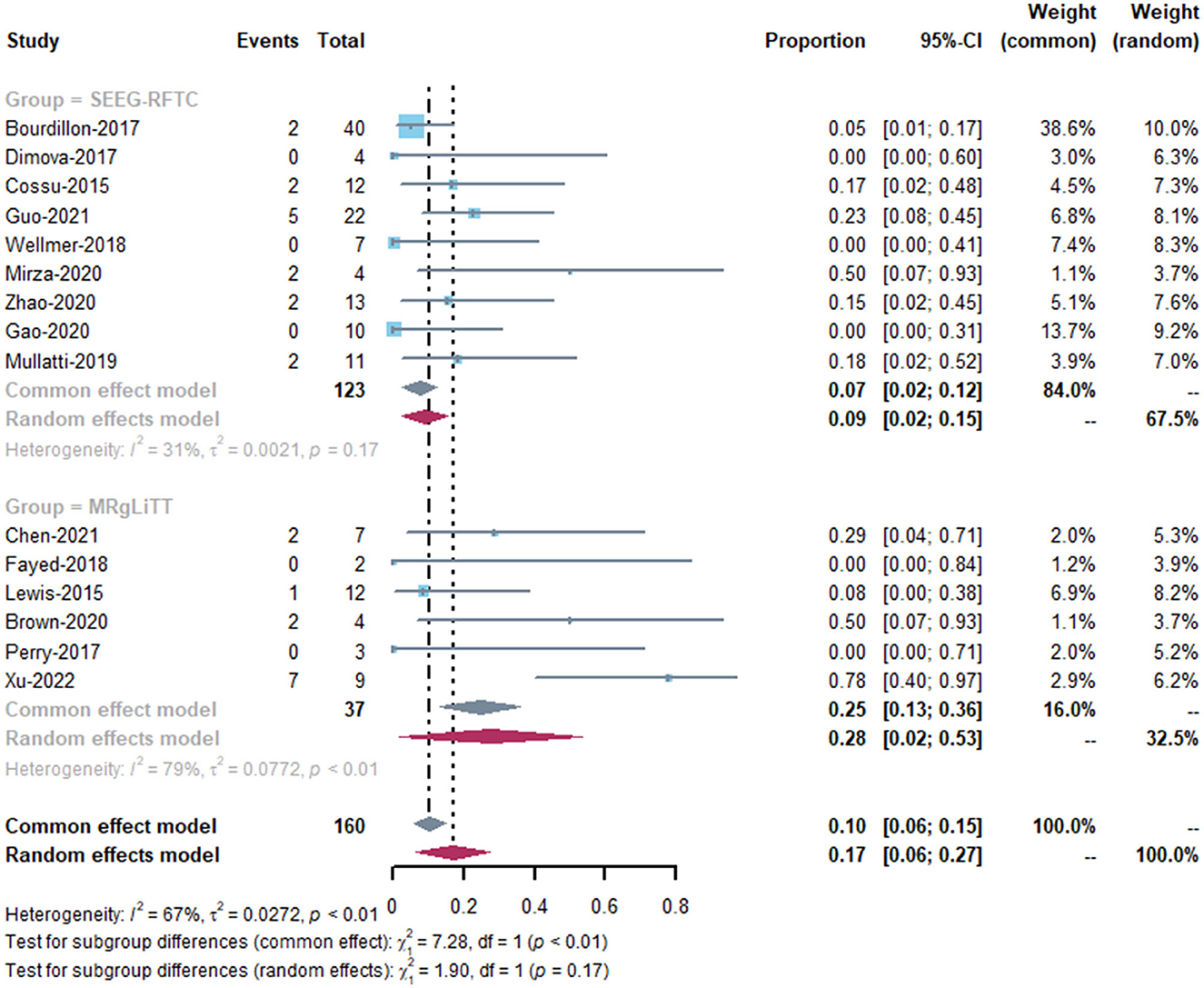
Fourteen studies reported postoperative complication rates after MRgLiTT or SEEG-RFTC (15, 17, 19–24, 26–31) (Table 2). The overall complication rate was 17.1% (27/150), with a rate of 11.9% (19/159) for transient complications, 3.1% (5/159) for long-term complications, and 2% (3/159) for complications that could not be classified due to lack of information (two cases of visual impairment and one case of intraventricular hemorrhage + aseptic meningitis). In the MRgLiTT group, postoperative complications occurred in five patients (32.4%; 12/37), whereas in the SEEG-RFTC group, 15 patients (12.3%; 15/122) had postoperative complications. However, there was no significant difference in the complication rate in FCD patients between the two surgical methods (P = 0.62) (Figure 5). There were also no significant differences between the two groups in either transient (P = 0.63) or permanent complications (P = 0.53). There were four serious complications reported (2.7%, 4/150), including hemiparesis in two patients, focal hemorrhage with left leg paralysis in one patient, and ventricular hemorrhage with aseptic meningitis in one patient.
4. Discussion
This study was the first meta-analysis comparing the efficacy and safety of MRgLiTT vs. SEEG-RFTC in patients with epilepsy caused by FCD. Patients with other diseases and those who failed to respond in prior surgeries were excluded. To reduce the risk of bias, we excluded case reports and literature employing fewer than five patients to reduce the bias due to patient selection. In some articles, there were patients with multiple treatments for multiple lesions, and we counted the number of patients according to the number of procedures (23, 29).
According to this systematic review, there was no significant difference in the efficacy between MRgLiTT and SEEG-RFTC in patients with FCD. In 2021, a meta-analysis comparing the two procedures in all drug-resistant epilepsy showed a significant difference in the postoperative rate of seizure freedom between patients receiving MRgLiTT (65%; 95% CI 56–74%) and those receiving SEEG-RFTC (23%; 95% CI 10–39%) (P = 0.00). The discrepancy may be due to the different sizes of ablation lesions. MRgLiTT-induced lesions were often greater than those caused by SEEG-RFTC. The MRgLiTT treatment technique can be repeated as needed to achieve overlapping thermal ablation. In this meta-analysis, the included FCD patients had a more limited range of lesions; thus, the difference was not detected. These findings suggest that the surgical choices for patients with FCD are more varied.
The heterogeneity in the SEEG-RFTC group was very high (I2 = 91%). However, the different FCD subtypes may be responsible for the increased heterogeneity. In our subgroup analysis, SEEG-RFTC was shown to be significantly more effective in patients with the FCD2b subtype than in patients with other types of FCD. In addition, the time of surgery may be another factor contributing to the heterogeneity. We found that the results of efficacy reported in two articles (26, 28) that included FCD patients receiving SEEG-RFTC at the earliest times (2008–2013, 2003–2013) (0/11, 6/40) differed significantly from those reported by the other articles. This may be attributed to the lack of accurate patient assessment and the unskilled use of the treatment. After excluding these two articles, SEEG-RFTC achieved a cure rate of 69.8% (127/182) for epilepsy in patients with FCD that is significantly higher than MRgLITT (P < 0.01). It means that SEEG-RFTC is currently a better treatment option for patients with FCD compared to MRgLITT.
Both MRgLiTT and SEEG-RFTC are considered safe treatments. Transient somatosensory and motor dysfunction is the most common postoperative complication for both MRgLiTT and SEEG-RFTC (40, 40%), which may be due to the local edema caused by the adjacent brain areas. The dysfunction also recovered as the edema subsided. It is important to note that both surgical approaches may cause severe bleeding and require extensive attention in clinical management. In addition, other meta-analyses have also shown a lower incidence of postoperative complications with MRgLiTT and SEEG-RFTC (2, 39, 40).
MRgLiTT and SEEG-RFTC do not show better treatment outcomes or have lower complication rates compared to surgical resection. In a study that included 2,014 patients who underwent FCD resection, the overall incidence of seizure freedom (Engel Class I) after surgical treatment was 55.8 ± 16.2%, showing comparable performance to the two approaches we studied (41). In a retrospective study in 2019, the rate of complications after surgical treatment of FCD was only 9% (17/188), which is also consistent with our data (1). However, compared to surgical resection, both MRgLiTT and SEEG-RFTC are minimally invasive and still allow for secondary surgery if the postoperative outcome is unsatisfactory (42, 43). In addition, a meta-analysis in 2019 showed that histological FCD type I, incomplete resection, and extratemporal location are factors for recurrence after patients receiving epilepsy surgery for FCD (44). Additional patient data are needed to explore whether these FCD patients with poor surgical outcomes can benefit from MRgLiTT and SEEG-RFTC.
Our results suggest that both MRgLiTT and SEEG-RFTC are effective and safe treatments for patients with FCD. This is the first study to compare the efficacy and prognosis between MRgLiTT and SEEG-RFTC in patients with FCD. Our results show that there is no significant difference in the efficacy and prognosis between MRgLiTT and SEEG-RFTC. This result suggests multiple options for the treatment of patients with FCD. In this study, postoperative complications were analyzed uniformly in all patients, but in fact, different complications may have different prognoses in patients. Therefore, we recalculated permanent complications (defined as complications that require long-term treatment or have a long-term impact on the patient's quality of life). There were no serious complications in the MRgLiTT group, and five serious complications were reported in the SEEG-RFTC group. However, this lacked statistical significance due to the small number of patients.
Our study has several limitations. (1) The different inclusion criteria among the included studies led to differential variation in patient selection, resulting in heterogeneity. (2) The length of follow-up varied among the studies, and the seizure-suppression effect of surgery may deteriorate with the extension of follow-up. (3) The small number of patients undergoing MRgLiTT surgery and the lack of categorical information prevented subgroup analysis. (4) Almost all patients with FCD received MRgLiTT and SEEG-RFTC operation without pathological confirmation of the proposed diagnosis of FCD. However, although the exact type of pathology of the patient could not be confirmed, the results of this article may provide guidance on the surgical approach for patients with a proposed diagnosis of FCD. (5) Our study simply compared the effectiveness and safety of the two approaches, without considering the cost-benefit ratio and time of proficiency of new technologies. A comparison of these aspects could be considered in future studies.
5. Conclusion
In conclusion, both MRgLiTT and SEEG-RFTC are currently safe and effective minimally invasive procedures for patients with FCD. Subgroup analysis further revealed that SEEG-RFTC is significantly more effective for FCD Type 2b than other FCD types, suggesting that SEEG-RFTC is the optimal choice for this type of patients. Our conclusions are based on limited retrospective analysis, and randomized controlled trials are still needed to validate the conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YL proposed the study purpose. Searching strategy was developed and revised by JG and ZY. The revision and guidance of the article are carried out by MJ. All authors have contributed to the revision of the manuscript.
Funding
This study was funded by the 1.3.5 Talent Excellence Development Project - West China Hospital of Sichuan University (ZYGD20011) and Chengdu Science and Technology Bureau key demonstration project (2019-YF09-00215-SN).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1241763/full#supplementary-material
Abbreviations
FCD, focal cortical dysplasia; RF-TC, radiofrequency thermal coagulation; MRgLiTT, magnetic resonance-guided laser interstitial thermal therapy; FDA, food and drug administration; SEEG, stereoelectroencephalography.
References
1. Jayalakshmi S, Nanda SK, Vooturi S, Vadapalli R, Sudhakar P, Madigubba S, et al. Focal cortical dysplasia and refractory epilepsy: role of multimodality imaging and outcome of surgery. AJNR Am J Neuroradiol. (2019) 40:892–8. doi: 10.3174/ajnr.A6041
2. Wang Y, Xu J, Liu T, Chen F, Chen S, Xie Z, et al. Magnetic resonance-guided laser interstitial thermal therapy versus stereoelectroencephalography-guided radiofrequency thermocoagulation for drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsy Res. (2020) 166:106397. doi: 10.1016/j.eplepsyres.2020.106397
3. Lagman C, Chung LK, Pelargos PE, Ung N, Bui TT, Lee SJ, et al. Laser neurosurgery: a systematic analysis of magnetic resonance-guided laser interstitial thermal therapies. J Clin Neurosci. (2017) 36:20–6. doi: 10.1016/j.jocn.2016.10.019
4. Kerezoudis P, Parisi V, Marsh WR, Kaufman TJ, Lehman VT, Worrell GA, et al. Surgical outcomes of laser interstitial thermal therapy for temporal lobe epilepsy: systematic review and meta-analysis. World Neurosurg. (2020) 143:527–36.e3. doi: 10.1016/j.wneu.2020.07.194
5. Guénot M, Isnard J, Ryvlin P, Fischer C, Mauguière F, Sindou M. SEEG-guided RF thermocoagulation of epileptic foci: feasibility, safety, and preliminary results. Epilepsia. (2004) 45:1368–74. doi: 10.1111/j.0013-9580.2004.17704.x
6. Horsley V. The structures and functions of the cerebellum examined by a new method. Brain. (1908) 31:45–124. doi: 10.1093/brain/31.1.45
7. Norred SE, Johnson JA. Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: a review. Biomed Res Int. (2014) 2014:761312. doi: 10.1155/2014/761312
8. Banerjee C, Snelling B, Berger MH, Shah A, Ivan ME, Komotar RJ. The role of magnetic resonance-guided laser ablation in neurooncology. Br J Neurosurg. (2015) 29:192–6. doi: 10.3109/02688697.2014.996527
9. Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. (2012) 71:133–44, 144–5. doi: 10.1227/NEU.0b013e31826101d4
10. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
12. Blümcke I, Spreafico R. An international consensus classification for focal cortical dysplasias. Lancet Neurol. (2011) 10:26–7. doi: 10.1016/S1474-4422(10)70225-8
13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
14. Hyslop A, Wang S, Bryant JP, Bhatia S, Sandoval-Garcia C, Karkare K, et al. Stereo-electroencephalography (SEEG) in pediatric epilepsy: utility in children with and without prior epilepsy surgery failure. Epilepsy Res. (2021) 177:106765. doi: 10.1016/j.eplepsyres.2021.106765
15. Mirza FA, Hall JA. Radiofrequency thermocoagulation in refractory focal epilepsy: the montreal neurological institute experience. Can J Neurol Sci. (2021) 48:626–39. doi: 10.1017/cjn.2020.263
16. Zhang C, Zhao BT, McGonigal A, Hu WH, Wang X, Shao XQ, et al. Superior frontal sulcus focal cortical dysplasia type II: an MRI, PET, and quantified SEEG study. Front Neurol. (2019) 10:1253. doi: 10.3389/fneur.2019.01253
17. Wellmer J. Lesion focused radiofrequency thermocoagulation of bottom-of-sulcus focal cortical dysplasia type IIb: conceptional considerations with regard to the epileptogenic zone. Epilepsy Res. (2018) 142:143–8. doi: 10.1016/j.eplepsyres.2018.02.009
18. Wellmer J, Kopitzki K, Voges J. Lesion focused stereotactic thermo-coagulation of focal cortical dysplasia IIB: a new approach to epilepsy surgery? Seizure. (2014) 23:475–8. doi: 10.1016/j.seizure.2014.01.024
19. Wellmer J, Parpaley Y, Rampp S, Popkirov S, Kugel H, Aydin Ü, et al. Lesion guided stereotactic radiofrequency thermocoagulation for palliative, in selected cases curative epilepsy surgery. Epilepsy Res. (2016) 121:39–46. doi: 10.1016/j.eplepsyres.2016.01.005
20. Brown MG, Drees C, Nagae LM, Thompson JA, Ojemann S, Abosch A. Curative and palliative MRI-guided laser ablation for drug-resistant epilepsy. J Neurol Neurosurg Psychiatry. (2018) 89:425–33. doi: 10.1136/jnnp-2017-316003
21. Chen SC, Wang YH, Fan XT, Wei PH, Ren LK, Shan YZ, et al. Safety and short-term efficacy of domestic magnetic resonance-guided laser interstitial thermotherapy in the treatment of drug-resistant epilepsy. Zhonghua Yi Xue Za Zhi. (2021) 101:3399–403. doi: 10.3760/cma.j.cn112137-20210501-01046
22. Fayed I, Sacino MF, Gaillard WD, Keating RF, Oluigbo CO. MR-guided laser interstitial thermal therapy for medically refractory lesional epilepsy in pediatric patients: experience and outcomes. Pediatr Neurosurg. (2018) 53:322–9. doi: 10.1159/000491823
23. Lewis EC, Weil AG, Duchowny M, Bhatia S, Ragheb J, Miller I. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia. (2015) 56:1590–8. doi: 10.1111/epi.13106
24. Perry MS, Donahue DJ, Malik SI, Keator CG, Hernandez A, Reddy RK, et al. Magnetic resonance imaging-guided laser interstitial thermal therapy as treatment for intractable insular epilepsy in children. J Neurosurg Pediatr. (2017) 20:575–82. doi: 10.3171/2017.6.PEDS17158
25. Hu WH, Mo JJ, Yang BW, Liu HG, Zhang C, Wang X, et al. Voxel-based morphometric mri postprocessing-assisted laser interstitial thermal therapy for focal cortical dysplasia-suspected lesions: technique and outcomes. Oper Neurosurg. (2022) 23:334–41. doi: 10.1227/ons.0000000000000328
26. Bourdillon P, Isnard J, Catenoix H, Montavont A, Rheims S, Ryvlin P, et al. Stereo electroencephalography-guided radiofrequency thermocoagulation (SEEG-guided RF-TC) in drug-resistant focal epilepsy: results from a 10-year experience. Epilepsia. (2017) 58:85–93. doi: 10.1111/epi.13616
27. Chipaux M, Taussig D, Dorfmuller G, Dorison N, Tisdall MM, Boyd SG, et al. SEEG-guided radiofrequency thermocoagulation of epileptic foci in the paediatric population: feasibility, safety and efficacy. Seizure. (2019) 70:63–70. doi: 10.1016/j.seizure.2019.07.004
28. Cossu M, Fuschillo D, Casaceli G, Pelliccia V, Castana L, Mai R, et al. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: a retrospective study on 89 cases. J Neurosurg. (2015) 123:1358–67. doi: 10.3171/2014.12.JNS141968
29. Dimova P, de Palma L, Job-Chapron AS, Minotti L, Hoffmann D, Kahane P. Radiofrequency thermocoagulation of the seizure-onset zone during stereoelectroencephalography. Epilepsia. (2017) 58:381–92. doi: 10.1111/epi.13663
30. Guo Q, Tan HP, Chen J, Chen MB, Zhang LM, Zhang W, et al. Efficacy and safety of conformal thermocoagulation guided by stereotactic electroencephalogram in the treatment of epilepsy caused by focal cortical dysplasia in eloquent cortex. Zhonghua Yi Xue Za Zhi. (2021) 101:3393–8. doi: 10.3760/cma.j.cn112137-20210418-00927
31. Yanming H, Wenzhen Y, Yunjuan S, Zhe S, Zhengbo L, Yali L, et al. Radiofrequency thermocoagulation of the sulcus bottom in type II focal cortical dysplasia-related epilepsy with tapered implantation of electrodes: a case report. Acta Neurochir. (2021) 163:3045–50. doi: 10.1007/s00701-021-04998-7
32. Xu Y, Wang H, Zhao Y, Feng X, Wu L, Lou L. Stereoelectroencephalography-guided radiofrequency thermocoagulation of epileptic foci in the eloquent motor cortex: feasibility, safety, and efficacy. World Neurosurg. (2022) 164:e492–500. doi: 10.1016/j.wneu.2022.04.133
33. d'Orio P, Revay M, Bevacqua G, Battista F, Castana L, Squarza S, et al. Stereo-electroencephalography (SEEG)-guided surgery in epilepsy with cingulate gyrus involvement: electrode implantation strategies and postoperative seizure outcome. J Clin Neurophysiol. (2023) 40:516–28. doi: 10.1097/WNP.0000000000001000
34. Deng JFT, Xie Z, Fang F, Chen C, Wang X, Li H, et al. Efficacy of stereoelectroencephalography-guided radiofrequency thermocoagulation in the treatment of drug-resistant epilepsy in children: a report of 71 cases. Chin J Neurosurg. (2020) 36:342–7. doi: 10.3760/cma.j.cn112050-20191012-00438
35. Mirza FA, Hall JA. Radiofrequency thermocoagulation in refractory focal epilepsy: the montreal neurological institute experience. Can J Neurol Sci. (2021) 48:626–39.
36. Zhao R, Xue P, Zhou Y, Yang H, Zhou S, Wang Y, et al. Application of robot-assisted frameless stereoelectroencephalography based on multimodal image guidance in pediatric refractory epilepsy: experience of a pediatric center in a developing country. World Neurosurg. (2020) 140:e161–8. doi: 10.1016/j.wneu.2020.04.218
37. Gao R, Yu T, Xu C, Zhang X, Yan X, Ni D, et al. The value of magnetoencephalography for stereo-EEG-guided radiofrequency thermocoagulation in MRI-negative epilepsy. Epilepsy Res. (2020) 163: 106322.
38. Mullatti N, Landre E, Mellerio C, Oliveira AJ, Laurent A, Turak B. Stereotactic thermocoagulation for insular epilepsy: Lessons from successes and failures. Epilepsia. (2019) 60: 1565–79.
39. Bourdillon P, Cucherat M, Isnard J, Ostrowsky-Coste K, Catenoix H, Guénot M, et al. Stereo-electroencephalography-guided radiofrequency thermocoagulation in patients with focal epilepsy: a systematic review and meta-analysis. Epilepsia. (2018) 59:2296–304. doi: 10.1111/epi.14584
40. Xue F, Chen T, Sun H. Postoperative outcomes of magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT) in the treatment of drug-resistant epilepsy: a meta-analysis. Med Sci Monit Int Med J Exp Clin Res. (2018) 24:9292–9. doi: 10.12659/MSM.911848
41. Rowland NC, Englot DJ, Cage TA, Sughrue ME, Barbaro NM, Chang EF, et al. meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg. (2012) 116:1035–41. doi: 10.3171/2012.1.JNS111105
42. Leiphart JW, Young RM, Shields DC. A historical perspective: stereotactic lesions for the treatment of epilepsy. Seizure. (2014) 23:1–5. doi: 10.1016/j.seizure.2013.10.006
43. Quigg M, Harden C. Minimally invasive techniques for epilepsy surgery: stereotactic radiosurgery and other technologies. J Neurosurgery. (2014) 121:232-40. doi: 10.3171/2014.8.GKS141608
Keywords: magnetic resonance-guided laser interstitial thermal therapy, stereoelectroencephalography-guided radiofrequency thermocoagulation, focal cortical dysplasia, seizure, seizure-free
Citation: Li Y, Gao J, Ye Z and Mu J (2023) Magnetic resonance-guided laser interstitial thermal therapy vs. stereoelectroencephalography-guided radiofrequency thermocoagulation in epilepsy patients with focal cortical dysplasia: a systematic review and meta-analysis. Front. Neurol. 14:1241763. doi: 10.3389/fneur.2023.1241763
Received: 17 June 2023; Accepted: 05 September 2023;
Published: 20 October 2023.
Edited by:
Jesse Skoch, Cincinnati Children's Hospital Medical Center, United StatesReviewed by:
Xiaoqiu Shao, Capital Medical University, ChinaAndrea Landi, University of Padua, Italy
Copyright © 2023 Li, Gao, Ye and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Mu, bXVqaWUyMDEwJiN4MDAwNDA7Zm94bWFpbC5jb20=
 Yiming Li
Yiming Li Jiayi Gao1
Jiayi Gao1