- 1Key Laboratory for NeuroInformation of Ministry of Education, University of Electronic Science and Technology of China, Chengdu, China
- 2School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu, China
Background and Aim: Hormone therapy (HT) has long been thought beneficial for controlling menopausal symptoms and human cognition. Studies have suggested that HT has a positive association with working memory, but no consistent relationship between HT and neural activity has been shown in any cognitive domain. The purpose of this meta-analysis was to assess the convergence of findings from published randomized control trials studies that examined brain activation changes in postmenopausal women.
Methods: A systematic search for fMRI studies of neural responses during working memory tasks in postmenopausal women was performed. Studies were excluded if they were not treatment studies and did not contain placebo or blank controls. For the purpose of the meta-analysis, 8 studies were identified, with 103 postmenopausal women taking HT and 109 controls.
Results: Compared with controls, postmenopausal women who took HT increased activation in the left frontal lobe, including superior frontal gyrus (BA 8), right middle frontal gyrus (BA 9), anterior lobe, paracentral lobule (BA 7), limbic lobe, and anterior cingulate (BA 32). Additionally, decreased activation is noted in the right limbic lobe, including parahippocampal gyrus (BA 28), left parietal lobe, and superior parietal lobule (BA 7). All regions were significant at p ≤ 0.05 with correction for multiple comparisons.
Conclusion: Hormone treatment is associated with BOLD signal activation in key anatomical areas during fMRI working memory tasks in healthy hormone-treated postmenopausal women. A positive correlation between activation and task performance suggests that hormone use may benefit working memory.
Introduction
The influence of hormone treatment (HT) on brain and cognition in postmenopausal women has been a controversial topic. Cross-sectional studies and meta-analyses have promoted the idea that HT might enhance verbal memory performance and decrease the risk for developing dementia in postmenopausal women (Barrett-Connor and Stuenkel, 2001; LeBlanc et al., 2001; Maki, 2006). Studies indicated that HT users were better protected against cognitive decline and dementia and may have better performance during working memory tasks than non-users (Hogervorst et al., 2009; Davis et al., 2013). Similarly, a follow-up study of women who began hormone use around menopause detected lower cognitive impairment (Bagger et al., 2005). In a memory study, early initiators of HT performed better than late initiators in working memory tasks, including attention, concentration, and mental status (MacLennan et al., 2006). These studies thus suggest that HT may have beneficial effects on the central nervous system. However, other studies pointed out that HT has no detrimental effect on cognitive performance in early postmenopausal women (Davison et al., 2013). Later HTs have no preventive effect on AD and MCI in postmenopausal women (Maki, 2005; Sherwin, 2005). While most women receiving estrogen plus progestin did not experience clinically relevant adverse effects on cognition compared with placebo, a small increased risk of clinically meaningful cognitive decline occurred in the estrogen plus progestin group (Espeland et al., 2010). There are no adverse or beneficial effects of HT on cognitive function in younger postmenopausal women (Brown, 2013). Similarly, large controlled studies have suggested that HT has no beneficial effects on maintaining cognition (Henderson et al., 2000; Mulnard et al., 2000; Wang et al., 2000; Resnick et al., 2006). Moreover, data from the only large randomized controlled trial published to date, the Women’s Health Initiative Memory Study, did not confirm these observations and have even suggested an increase in dementia risk for women using hormonal replacement therapy compared to controls (Ryan et al., 2008). Based on currently available data, routine therapeutic use of estrogens in women with AD is not justified (Markou et al., 2005) and should not be used for dementia prevention (NIA, 2004). Thus the effect of HT on working memory remains unclear based on the results of studies involving direct manipulations of estradiol level.
Inconsistencies in estradiol effects are also observed in structural and functional brain imaging studies. Increases or decreases in activation are often not accompanied by changes in task performance, leading Maki and Resnick (Maki et al., 2011) suggest that brain imaging may be more sensitive to effects of estradiol or other hormones than task performance. Evidence from animal models has found estrogen receptors in numerous sites throughout the brain including the hippocampus, amygdala, hypothalamus, brainstem, and cerebral cortex suggesting that estrogen therapy may impact cognitive functioning through potential affects on these brain areas (Brake et al., 2001). It was also shown that estrogens enhance basal forebrain, hippocampus, and cortex cholinergic activity by influencing the synthetic enzyme for acetylcholine as well as by increasing the number of cholinergic neurons (Smith et al., 2001). Further, estrogen has been suggested to modulate various neurotransmitters (Moses et al., 2000; Smith et al., 2001), increase cerebral blood flow (Greene, 2000; Slopien et al., 2003), regulate the formation of synapses, affect neuronal survival (Nilsen et al., 2000; Gleason et al., 2005), influence the expression of APOE, and provide neuroprotective effects, all of which may either directly or indirectly impact cognitive functioning (Slopien et al., 2003). The fMRI results demonstrate that HT, relative to placebo, increased the response of the striatum and ventromedial prefrontal cortex, two areas that have been shown to be, respectively, involved during reward anticipation and at the time of reward delivery. Using both visual and verbal working memory tasks, one fMRI study found increased activation bilaterally in the superior frontal gyrus and in the inferior parietal lobule in subjects who were receiving high-dose conjugated equine estrogen compared with controls, while decreases in inferior parietal, which activation for storage of non-verbal material (Shaywitz et al., 1999), have also been reported. Areas of activation were deemed significantly different in the prior findings.
Although prior studies have examined the effects of HT on working memory task performance in healthy postmenopause women, to our knowledge, no brain imaging meta-analysis examined the brain activation differences in postmenopausal women. Our study assessed the convergence of findings from published randomized control trials studies that examined brain activation changes in postmenopausal women. A meta-analysis employing the activation likelihood estimate (ALE) method was performed to locate anatomical regions with significant activation differences. The ALE method permits the creation of three-dimensional (3D) probability maps that show the brain regions that are most likely to demonstrate morphometric differences between HT and controls. The frontal lobes are of paramount significance in determining emotions and judgments related to sympathy, which is defined as the ability to perform daily activities, personality manifestations, and decisions (Badre et al., 2009; Tiemeier et al., 2010). Base on the exiting studies (Shaywitz et al., 1999; Greene, 2000; Moses et al., 2000; Nilsen et al., 2000; Brake et al., 2001; Smith et al., 2001; Slopien et al., 2003; Gleason et al., 2005), we hypothesize that postmenopausal women who took HT would show increased activation in the frontal lobe, including temporal lobe and the anterior cingulate, brain regions known to be involved in cognition and working memory.
Methods
Data Sources and Inclusion Criteria
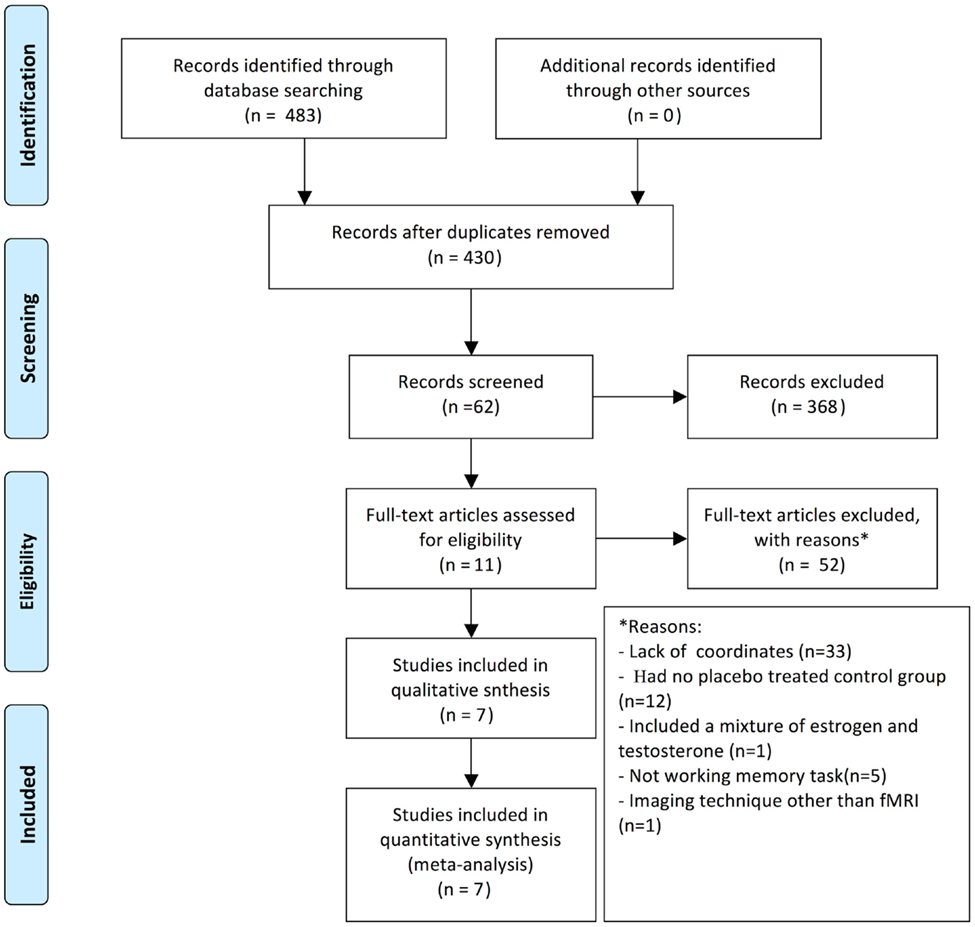
A systematic search strategy was performed to identify the relevant studies. We searched PubMed, MEDLINE, and EMBASE from 1993 to 2014 for all relevant published observational studies and clinical trials. The following key terms were employed: “HT,” “HRT,” “HT,” “ERT,” “functional MRI or fMRI,” “cognitive dysfunction,” “postmenopausal,” “brain activation,” and “working memory.” The reference lists of these articles were searched to obtain additional relevant studies. All identified articles were reviewed by at least two authors, and studies were included in the meta-analysis based on a consensus decision (see Figure 1).
To meet the inclusion criteria, the studies had to (a) report group comparisons between postmenopausal women who received HT and matched controls, (b) employ fMRI, (c) report their results in a standard stereotactic space (either Talairach or Montreal Neurological Institute space), and (d) investigate working memory or working memory-related tasks. Studies that did not fulfill these requirements were excluded. Treatment, gender, and age were not restricted and the HT brain activation studies did not include a control.
The data extracted from each study included: a description of (a) each memory task and (b) foci of task-related activation changes in which the active and control states were different between the HT group and the control as well as (c) clinical data concerning hormone dose, years of estrogen use, and the age-matching of the control group. This study examined all foci that were reported as significant based on the above criteria designated in the included studies.
ALE Meta-Analysis Procedure
Two separate ALE meta-analyses were performed: one on fMRI studies reporting that brain activation was increased in postmenopausal women receiving HT compared to controls and a second on studies reporting that brain activation was decreased.
The meta-analyses were performed using GingerALE software, version 2.1.1 (Laird et al., 2005)1. The main idea behind ALE is to treat the reported foci as centers for 3D Gaussian probability distributions to capture the spatial uncertainty associated with each focus. Foci reported for any given study were then combined to a modeled activation (MA) map. The reported foci in the original studies in MNI space were transformed into Talairach space using the tal2icbm algorithm. All ALE data processing was performed using Brain Map Search and View software2. Statistical significance was assessed using an analytical equation. The assumption is reasonable for data imaging, based on an assumption of positive dependence (Genovese et al., 2002), and the false discovery rate (FDR) was set at 0.05 to correct the p-threshold for multiple comparisons (Laird et al., 2005). Each ALE map was overlaid onto an anatomical template generated by spatially normalizing the International Consortium for Brain Mapping (ICBM) template to the Talairach space (Kochunov et al., 2002). The resulting regions were anatomically labeled by reference to probabilistic cytoarchitectonic maps of the human brain.
Results
Number of Studies Found
The literature contained eight studies in which the effect of HT was investigated in working memory. In total, the meta-analysis was conducted on 103 postmenopausal women taking HT and 109 controls (see Table 1). These studies include all relevant treatment studies through 2014. Studies were excluded if they did not perform working memory tasks, or included a combination of estrogen and testosterone, or did not include a control group, or included women suffering from cognitive impairment.
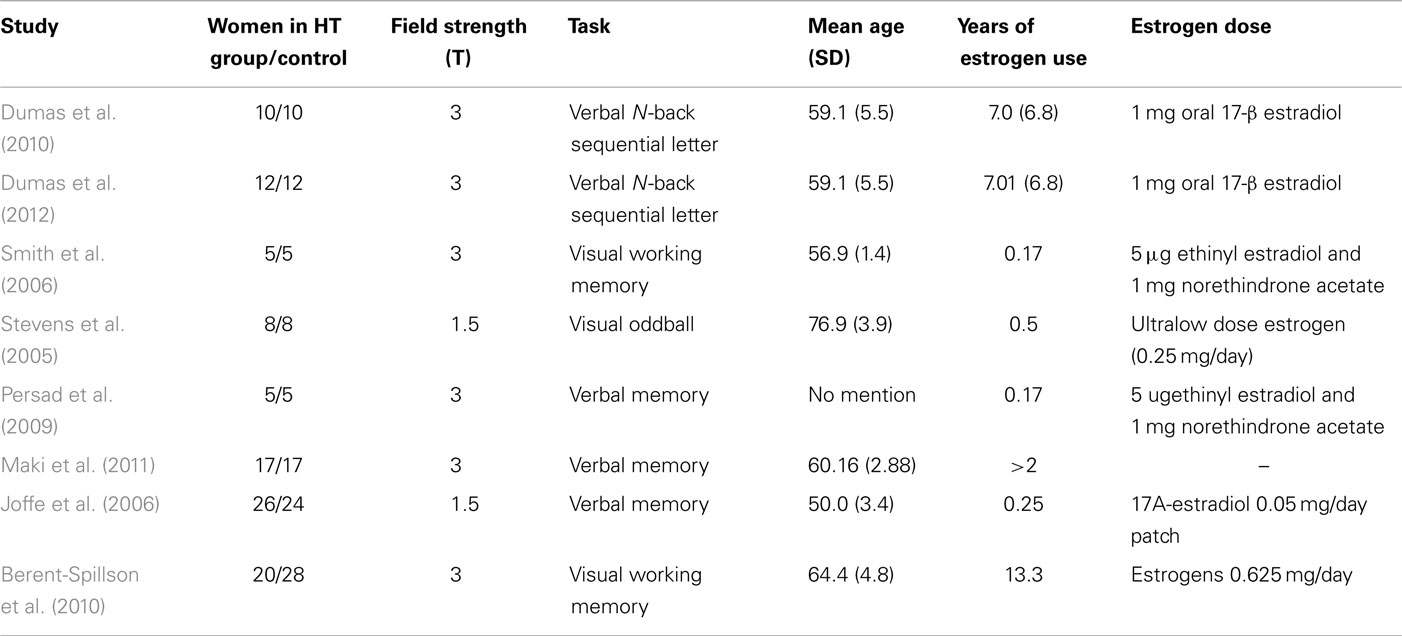
Table 1. Publications included in the meta-analysis, the tasks they employed, the number of subjects who were investigated, and the mean age of the women and their estrogen dose for the ALE meta-analysis.
Changes in Neural Activation to Working Memory Tasks
We performed two ALE analyses to investigate and aggregate the known data that exist regarding the effects of HT on neural activity in postmenopausal women during working memory tasks. The first analysis pooled the results of an increase in neural activation (8 experiments, 73 foci), and the second analysis pooled the results of a decrease in brain activation (3 experiments, 18 foci) (p ≤ 0.05 FDR-corrected; see Table 2; Figure 2).
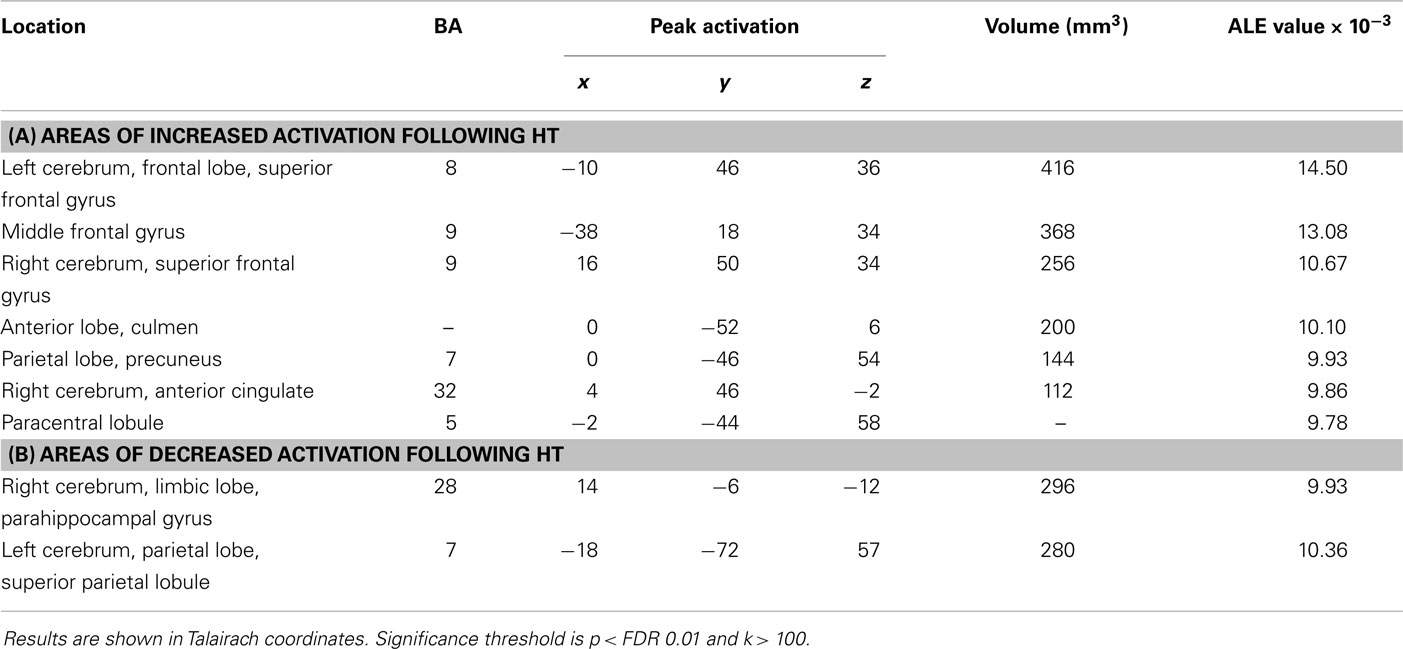
Table 2. Results from the ALE meta-analysis of treatment effects on studies in postmenopausal women (p < 0.05, FDR-corrected).
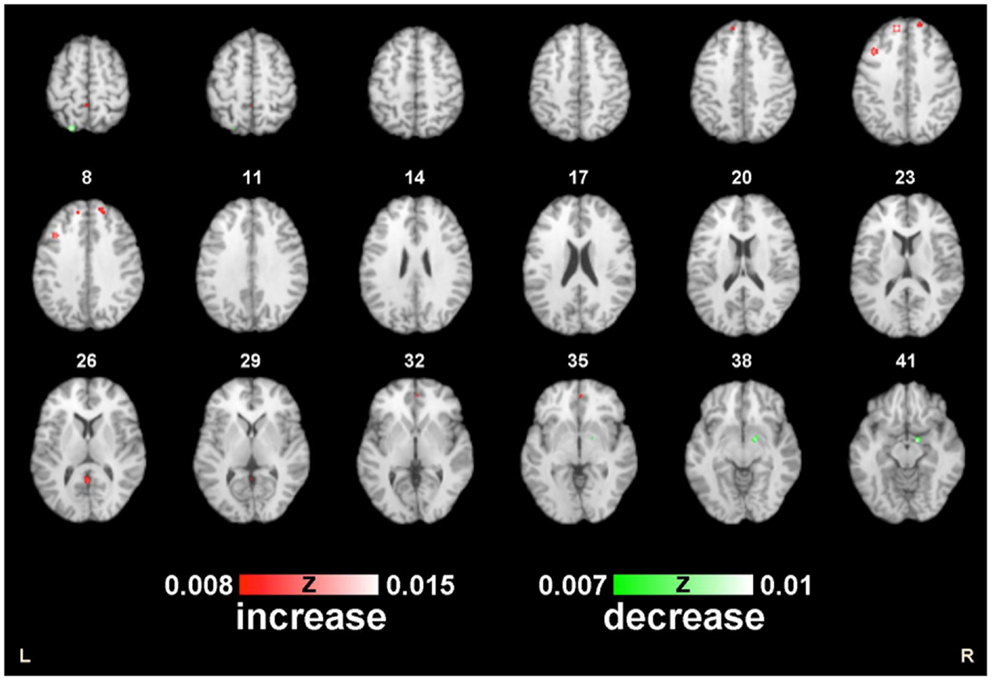
Figure 2. ALE map of the postmenopausal women during working memory. Decreased (green) and increased (red) activity following HT compared with the controls (p < 0.01; FDR-corrected; k > 100).
In the first meta-analysis, five clusters of increased task-related activity after HT were found in the left frontal lobe, superior frontal gyrus (BA 8), middle frontal gyrus (BA 9), parietal lobe, precuneus (BA 7), paracentral lobule (BA 5), limbic lobe, and anterior cingulate (BA 32) (p < 0.01 FDR-corrected, k > 100; see Figure 3).
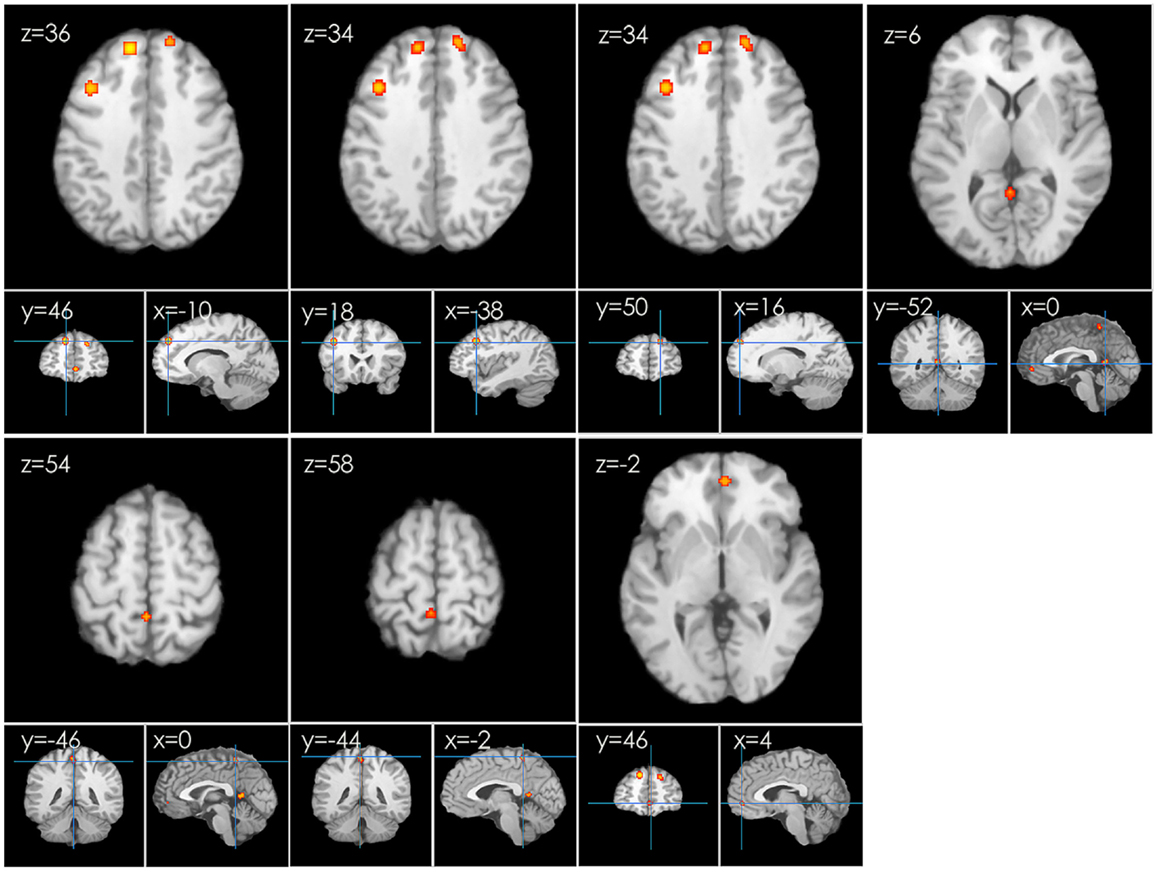
Figure 3. An ALE map presenting the increased activity in postmenopausal women undergoing HT during working memory tasks compared with the controls (p < 0.01; FDR-corrected; k > 100).
Several clusters of decreased activation after HT were detected in the second ALE analysis. Clusters of decreased activation were located in the right limbic lobe, parahippocampal gyrus (BA 28), left parietal lobe, and superior parietal lobule (BA 7) (p ≤ 0.05 FDR-corrected; see Table 2; Figure 4).
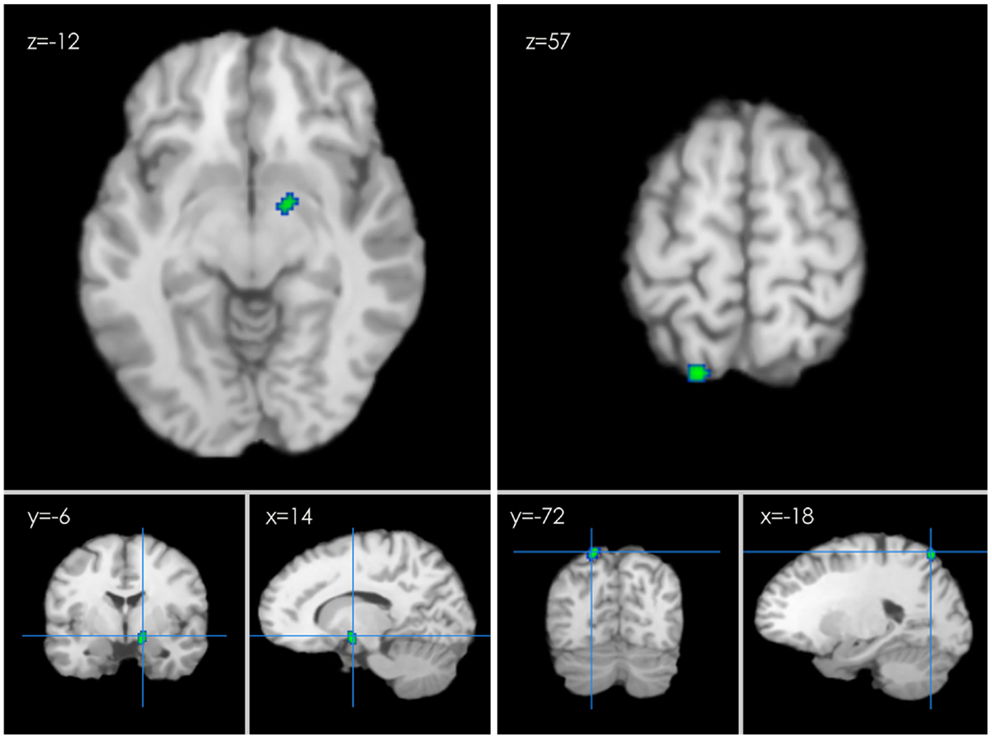
Figure 4. An ALE map presenting the decreased activity in postmenopausal women undergoing HT during working memory tasks compared with the controls (p < 0.01; FDR-corrected; k > 100).
HT and Performance of Working Memory
Four papers measured the relationship between HT and performance on verbal memory task. Two studies indicated that HT use increases frontal lobe activity during verbal memory tasks, but the degree of this increase is related to cognitive load (Dumas et al., 2010). One study demonstrated that HT results in changes in neural activation in verbal memory circuits in postmenopausal women and suggests that estrogen may enhance the overall efficiency of verbal memory processes in postmenopausal women (Persad et al., 2009). Another study suggested that HT selectively enhances verbal perseveration and is an important component of executive function by increasing activation in the inferior frontal, dorsolateral prefrontal, and posterior parietal regions (Joffe et al., 2006). One study indicated that no significant differences in visual task performance were found in response to HT. However, there was evidence that the HT group had both reductions and increases in the amplitude of hemodynamic response in some regions, including the occipital and parietal lobes, motor cortex, anterior cingulate, and PFC (Stevens et al., 2005).
Sub-Analyses for Estrogen Long-Term Use and Short-Term Use
In further sub-analysis, we investigated the effects of estrogen experience. The brain network supporting long experience [usually more than 2 years (Barrett-Connor and Stuenkel, 2001)] estrogen use (27 Foci, 4 Experiments) included cluster of activity in the left limbic lobe, right frontal lobe, and left anterior lobe. Short experience [usually <2 years (Barrett-Connor and Stuenkel, 2001)] included clusters of activity in the right front lobe, left front lobe, limbic lobe, and parietal lobe. We get similar behavioral outcomes after excluding short-term estrogen use. This suggests that long-term hormone use have a persisting chronic effect in postmenopausal women.
Discussion
Historically, cognitive benefits of postmenopausal hormone therapy (HT) have not been consistently demonstrated and the brain activation patterns are inconsistent. In this study, we addressed the cognitive effects of hormones in women and performed an ALE meta-analysis on functional brain imaging studies to investigate and aggregate the known data that exist regarding the effects of HT on neural activity in postmenopausal women during working memory tasks. Our results indicate that hormone use is associated with increased regional brain activation during working memory task, with women in hormone-treatment groups exhibiting a more robust neural response than placebo-treated women. Women receiving HT exhibited increases in brain activation in the frontal lobe and the anterior cingulate cortex (ACC) (see Table 2; Figure 3), a region where decreased activity can be predictive of Alzheimer’s disease (Rasgon et al., 2005). This finding suggests that HT may enhance brain function. The frontal lobe and the ACC may be important for processing lexical information and for the associated cognition and decision-making (Bush et al., 2000). This supports our prior hypothesis that HT maintains a positive effect on working memory processes.
Activation in the frontal lobes has been identified in postmenopausal women after estradiol treatment (Dumas et al., 2010). The frontal lobes are of paramount significance in determining emotions and judgments related to sympathy, which is defined as the ability to perform daily activities, personality manifestations, and decisions (Badre et al., 2009; Tiemeier et al., 2010). The hormone effects on working memory tasks may occur through an effect on the frontal lobe. Activation of frontal regions during memory tasks has been associated with the active maintenance of information (Cohen et al., 1997). Activation in frontal regions during a working memory task has been related to improved performance in older subjects (Davis et al., 2008). Hormones exert a greater modulatory effect on the function of the frontal lobe when working memory load is increased (Dumas et al., 2010), and episodic memory tasks are associated with the frontal lobe (Tulving et al., 1994). fMRI–BOLD signal in the frontal lobes, a region central to memory processing, positively correlated to task accuracy, implying a cognitive benefit to the increased activity measured during the memory task (Joffe et al., 2006; Dumas et al., 2010).
Similarly, across our ALE meta-analyses, we found that, after HT, postmenopausal women exhibited greater activity in the ACC than controls. The ACC plays an important role in integrating cognitive and emotional processes to support goal-directed behavior (Krause-Utz et al., 2014). On the other hand, ACC is involved in rational cognitive functions, including reward anticipation, impulse control decision-making, empathy (Bush et al., 2000), and emotion (Decety and Jackson, 2004; Jackson et al., 2006). This finding in the present study is consistent with the effect of HT on the emotion-processing circuitry in postmenopausal women (Frey et al., 2010; Shafir et al., 2012). However, another study revealed contradictory results in which the BOLD responses were reduced in the dorsolateral PFC and the dorsal anterior cingulate of the HT group compared with the control group during a negative emotion task. These differences between the HT and control groups may reflect a recovery of emotional responsiveness in these older women following HT (Love et al., 2010).
Additionally, decreased activation in the parahippocampal gyrus (BA 28), including left parietal lobe and superior parietal lobule (BA 7) (see Table 2; Figure 4), was observed in the HT group. Importantly, these regions may also support cognitive functions, including verbal memory (Zec and Trivedi, 2002; Sherwin, 2012). The parahippocampal gyrus is a gray matter cortical region of the brain that surrounds the hippocampus. The key role of this region is memory encoding and retrieval. Abnormalities may indicate underlying conditions, such as schizophrenia, Alzheimer’s disease, and hippocampal sclerosis (Ferreira et al., 2003). The observation of hyper-activation in the parahippocampal gyrus is consistent with the finding of Dr Maki leading investigators that women receiving HT exhibit decreased regional cerebral blood flow in the right precuneus, the dorsal frontal gyrus, and the parahippocampal gyrus during a verbal memory task (Resnick et al., 1998). Their later study suggested that deactivation in the left parahippocampal gyrus relates to better verbal memory performance, and this effect was specific to the verbal recognition condition (Maki et al., 2011). Another study found sustained decreases in activation in bilateral regions of parahippocampal cortex, suggesting that the parahippocampal gyrus might become deactivated when participants entered into a sustained state of retrieval during recognition tasks (Donaldson et al., 2001). Together, the findings of decreased parahippocampal activation in perimenopausal HT users suggest that perimenopausal HT enhances both state-dependent and recollective processes contributing to verbal recognition performance.
Sub-analyses for estrogen long-term and short-term use revealed almost the same results to the main results of HT group. The goal of this study was to evaluate differences in working memory after hormone use compared to placebo use, so women who recently quit long-term hormones were included along with current hormone users. Apart from a statistically significant difference in mean years of education, these two groups were demographically similar (although the smaller sample size used to compare current and past users may increase the likelihood of a type II error). After accounting for variations in age, education, and age at hormone initiation, long-term users did not have more activation than short-tem users in any part of the brain. Long-term users showed slightly more activation than short-term users in only one small region within the prefrontal cortex. While it is difficult to make conclusions based on one differing region, these results may be consistent with others that have indicated a persisting cognitive benefit to past hormone users (Hogervorst et al., 2009). Other than this region, the activation patterns between these two HT groups were sufficiently similar to justify including them in the same groups when comparing hormone ever-users to placebo-users.
This study has some limitations. First, methodological limitations led to a small number of available studies, and the sample sizes are small. Second, trials recruit women who may not be representative of the general population. Third, differences among the studies in the education, general health, motivation level, and age. These differences raise the possibility that the controls and HT groups may differ with respect to characteristics that could influence brain function. We consider this limitation unavoidable. Third, many trials are too short to evaluate long-term effect of HT. However, such trials provide more highly reliable evidence of the effectiveness of a treatment than observational studies (Miller and Kearney, 2004). Fourth, the functional imaging studies included employed different working memory tasks and the years of estrogen use are various, which could result in a source of heterogeneity. Fifth, the estrogen dose of HT was not identical across the included studies, which may influence the effect of estrogen on postmenopausal women.
Conclusion
In summary, the present study demonstrated that women who received HT exhibited increases in brain activation in the frontal lobe and ACC. In addition, decreased activation in the parahippocampal gyrus (BA 28), left parietal lobe, and superior parietal lobule (BA 7) were detected. The results are consistent with our hypothesis. Together, the findings of enhanced frontal lobe activation and decreased parahippocampal activation in perimenopausal HT users suggest that HT enhances both state-dependent and recollective processes contributing to cognitive performance.
Author Contributions
KL designed the study and wrote the protocol. XH and YH managed the literature searches and undertook the statistical analysis, and KL, XH, LY, JL, and DZ wrote the manuscript. JZ and YL took part in the modification and further analysis of the final manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Badre, D., Hoffman, J., Cooney, J. W., and D’Esposito, M. (2009). Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat. Neurosci. 12, 515–522. doi:10.1038/nn.2277
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bagger, Y. Z., Tanko, L. B., Alexandersen, P., Qin, G., Christiansen, C., and Group, P. S. (2005). Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause 12, 12–17. doi:10.1097/00042192-200512010-00005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barrett-Connor, E., and Stuenkel, C. A. (2001). Hormone replacement therapy (HRT) – risks and benefits. Int. J. Epidemiol. 30, 423–426. doi:10.1093/ije/30.3.423
Berent-Spillson, A. P., Persad, C. C. P., Love, T. P., Tkaczyk, A. M. S., Wang, H. M. S., and Reame, N. K. M. S. N. P. (2010). Early menopausal hormone use influences brain regions used for visual working memory. Menopause 17, 692–699. doi:10.1097/gme.0b013e3181cc49e9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brake, W. G., Alves, S. E., Dunlop, J. C., Lee, S. J., Bulloch, K., and Allen, P. B. (2001). Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology 142, 1284–1289. doi:10.1210/en.142.3.1284
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brown, S. (2013). No adverse – or beneficial – effect of HRT on cognitive function in younger postmenopausal women. Menopause Int. 19, 104–105.
Bush, G., Luu, P., and Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222. doi:10.1016/S1364-6613(00)01483-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cohen, J. D., Perlstein, W. M., Braver, T. S., Nystrom, L. E., Noll, D. C., and Jonides, J. (1997). Temporal dynamics of brain activation during a working memory task. Nature 386, 604–608. doi:10.1038/386604a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Davis, S. R., Davison, S. L., Gavrilescu, M., Searle, K., Gogos, A., and Rossell, S. L. (2013). Effects of testosterone on visuospatial function and verbal fluency in postmenopausal women: results from a functional magnetic resonance imaging pilot study. Menopause doi:10.1097/GME.0b013e3182a065ed
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Davis, S. W., Dennis, N. A., Daselaar, S. M., Fleck, M. S., and Cabeza, R. (2008). Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 18, 1201–1209. doi:10.1093/cercor/bhm155
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Davison, S. L., Bell, R. J., Robinson, P. J., Jane, F., Leech, J., and Maruff, P. (2013). Continuous-combined oral estradiol/drospirenone has no detrimental effect on cognitive performance and improves estrogen deficiency symptoms in early postmenopausal women: a randomized placebo-controlled trial. Menopause 20, 1020–1026. doi:10.1097/GME.0b013e318287474f
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Decety, J., and Jackson, P. L. (2004). The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 3, 71–100. doi:10.1177/1534582304267187
Donaldson, D. I., Petersen, S. E., Ollinger, J. M., and Buckner, R. L. (2001). Dissociating state and item components of recognition memory using fMRI. Neuroimage 13, 129–142. doi:10.1006/nimg.2000.0664
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dumas, J. A., Kutz, A. M., Naylor, M. R., Johnson, J. V., and Newhouse, P. A. (2010). Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm. Behav. 58, 929–935. doi:10.1016/j.yhbeh.2010.09.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dumas, J. A., Kutz, A. M., Naylor, M. R., Johnson, J. V., and Newhouse, P. A. (2012). Estradiol treatment altered anticholinergic-related brain activation during working memory in postmenopausal women. Neuroimage 60, 1394–1403. doi:10.1016/j.neuroimage.2012.01.043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Espeland, M. A., Brunner, R. L., Hogan, P. E., Rapp, S. R., Coker, L. H., and Legault, C. (2010). Long-term effects of conjugated equine estrogen therapies on domain-specific cognitive function: results from the women’s health initiative study of cognitive aging extension. J. Am. Geriatr. Soc. 58, 1263–1271. doi:10.1111/j.1532-5415.2010.02953.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ferreira, N. F., de Oliveira, V., Amaral, L., Mendonca, R., and Lima, S. S. (2003). Analysis of parahippocampal gyrus in 115 patients with hippocampal sclerosis. Arq. Neuropsiquiatr. 61, 707–711. doi:10.1590/S0004-282X2003000500001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frey, B. N., Hall, G. B., Attard, S., Yucel, K., Skelin, I., and Steiner, M. (2010). Shift in the brain network of emotional regulation in midlife women: is the menopausal transition the turning point? Menopause 17, 840–845. doi:10.1097/gme.0b013e3181df840f
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Genovese, C. R., Lazar, N. A., and Nichols, T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878. doi:10.1006/nimg.2001.1037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gleason, C. E., Cholerton, B., Carlsson, C. M., Johnson, S. C., and Asthana, S. (2005). Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell. Mol. Life Sci. 62, 299–312. doi:10.1007/s00018-004-4385-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Greene, R. A. (2000). Estrogen and cerebral blood flow: a mechanism to explain the impact of estrogen on the incidence and treatment of Alzheimer’s disease. Int. J. Fertil. Womens Med. 45, 253–257.
Henderson, V. W., Paganini-Hill, A., Miller, B. L., Elble, R. J., Reyes, P. F., and Shoupe, D. (2000). Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology 54, 295–301.
Hogervorst, E., Yaffe, K., Richards, M., and Huppert, F. A. (2009). Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database Syst. Rev. 4, CD003799. doi:10.1002/14651858.CD003799.pub2
Jackson, P. L., Brunet, E., Meltzoff, A. N., and Decety, J. (2006). Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 44, 752–761. doi:10.1016/j.neuropsychologia.2005.07.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Joffe, H., Hall, J. E., Gruber, S., Sarmiento, I. A., Cohen, L. S., and Yurgelun-Todd, D. (2006). Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13, 411–422. doi:10.1097/01.gme.0000189618.48774.7b
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kochunov, P., Lancaster, J., Thompson, P., Toga, A. W., Brewer, P., and Hardies, J. (2002). An optimized individual target brain in the Talairach coordinate system. Neuroimage 17, 922–927. doi:10.1006/nimg.2002.1084
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Krause-Utz, A., Elzinga, B. M., Oei, N. Y., Paret, C., Niedtfeld, I., and Spinhoven, P. (2014). Amygdala and dorsal anterior cingulate connectivity during an emotional working memory task in borderline personality disorder patients with interpersonal trauma history”. Front. Hum. Neurosci. 8:848. doi:10.3389/fnhum.2014.00848
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Laird, A. R., Fox, P. M., Price, C. J., Glahn, D. C., Uecker, A. M., and Lancaster, J. L. (2005). ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 25, 155–164. doi:10.1002/hbm.20136
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
LeBlanc, E. S., Janowsky, J., Chan, B. K., and Nelson, H. D. (2001). Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA 285, 1489–1499. doi:10.1001/jama.285.11.1489
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Love, T., Smith, Y. R., Persad, C. C., Tkaczyk, A., and Zubieta, J. K. (2010). Short-term hormone treatment modulates emotion response circuitry in postmenopausal women. Fertil. Steril. 93, 1929–1937. doi:10.1016/j.fertnstert.2008.12.056
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
MacLennan, A. H., Henderson, V. W., Paine, B. J., Mathias, J., Ramsay, E. N., and Ryan, P. (2006). Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the remember pilot study. Menopause 13, 28–36. doi:10.1097/01.gme.0000191204.38664.61
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maki, P. M. (2005). A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann. N. Y. Acad. Sci. 1052, 182–197. doi:10.1196/annals.1347.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maki, P. M. (2006). Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience 138, 1027–1030. doi:10.1016/j.neuroscience.2006.01.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maki, P. M., Dennerstein, L., Clark, M., Guthrie, J., LaMontagne, P., and Fornelli, D. (2011). Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 1379, 232–243. doi:10.1016/j.brainres.2010.11.030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Markou, A., Duka, T., and Prelevic, G. M. (2005). Estrogens and brain function. Hormones (Athens) 4, 9–17. doi:10.14310/horm.2002.11138
Miller, M., and Kearney, N. (2004). Guidelines for clinical practice: development, dissemination and implementation. Int. J. Nurs. Stud. 41, 813–821. doi:10.1016/j.ijnurstu.2003.09.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moses, E. L., Drevets, W. C., Smith, G., Mathis, C. A., Kalro, B. N., and Butters, M. A. (2000). Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry 48, 854–860.
Mulnard, R. A., Cotman, C. W., Kawas, C., van Dyck, C. H., Sano, M., and Doody, R. (2000). Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA 283, 1007–1015. doi:10.1001/jama.283.8.1007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nilsen, J., Mor, G., and Naftolin, F. (2000). Estrogen-regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. J. Neurobiol. 43, 64–78. doi:10.1002/(SICI)1097-4695(200004)43:1<64::AID-NEU6>3.0.CO;2-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Persad, C. C., Zubieta, J. K., Love, T., Wang, H., Tkaczyk, A., and Smith, Y. R. (2009). Enhanced neuroactivation during verbal memory processing in postmenopausal women receiving short-term hormone therapy. Fertil. Steril. 92, 197–204. doi:10.1016/j.fertnstert.2008.04.040
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rasgon, N. L., Silverman, D., Siddarth, P., Miller, K., Ercoli, L. M., and Elman, S. (2005). Estrogen use and brain metabolic change in postmenopausal women. Neurobiol. Aging 26, 229–235. doi:10.1016/j.neurobiolaging.2004.03.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Resnick, S. M., Maki, P. M., Golski, S., Kraut, M. A., and Zonderman, A. B. (1998). Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm. Behav. 34, 171–182. doi:10.1006/hbeh.1998.1476
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Resnick, S. M., Maki, P. M., Rapp, S. R., Espeland, M. A., Brunner, R., and Coker, L. H. (2006). Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J. Clin. Endocrinol. Metab. 91, 1802–1810. doi:10.1210/jc.2005-2097
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ryan, J., Scali, J., Carriere, I., Ritchie, K., and Ancelin, M. L. (2008). Hormonal treatment, mild cognitive impairment and Alzheimer’s disease. Int. Psychogeriatr. 20, 47–56.
Shafir, T., Love, T., Berent-Spillson, A., Persad, C. C., Wang, H., and Reame, N. K. (2012). Postmenopausal hormone use impact on emotion processing circuitry. Behav. Brain Res. 226, 147–153. doi:10.1016/j.bbr.2011.09.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shaywitz, S. E., Shaywitz, B. A., Pugh, K. R., Fulbright, R. K., Skudlarski, P., and Mencl, W. E. (1999). Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA 281, 1197–1202. doi:10.1001/jama.281.13.1197
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sherwin, B. B. (2005). Estrogen and memory in women: how can we reconcile the findings? Horm. Behav. 47, 371–375. doi:10.1016/j.yhbeh.2004.12.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sherwin, B. B. (2012). Estrogen and cognitive functioning in women: lessons we have learned. Behav. Neurosci. 126, 123–127. doi:10.1037/a0025539
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Slopien, R., Junik, R., Meczekalski, B., Halerz-Nowakowska, B., Maciejewska, M., and Warenik-Szymankiewicz, A. (2003). Influence of hormonal replacement therapy on the regional cerebral blood flow in postmenopausal women. Maturitas 46, 255–262. doi:10.1016/S0378-5122(03)00144-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smith, Y. R., Love, T., Persad, C. C., Tkaczyk, A., Nichols, T. E., and Zubieta, J.-K. (2006). Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J. Clin. Endocrinol. Metabol. 91, 4476–4481. doi:10.1210/jc.2006-0907
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smith, Y. R., Minoshima, S., Kuhl, D. E., and Zubieta, J. K. (2001). Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J. Clin. Endocrinol. Metab. 86, 679–684. doi:10.1210/jcem.86.2.7222
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stevens, M. C., Clark, V. P., and Prestwood, K. M. (2005). Low-dose estradiol alters brain activity. Psychiatry Res. 139, 199–217. doi:10.1016/j.pscychresns.2005.04.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tiemeier, H., Lenroot, R. K., Greenstein, D. K., Tran, L., Pierson, R., and Giedd, J. N. (2010). Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage 49, 63–70. doi:10.1016/j.neuroimage.2009.08.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tulving, E., Kapur, S., Craik, F. I., Moscovitch, M., and Houle, S. (1994). Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A 91, 2016–2020. doi:10.1073/pnas.91.6.2016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wang, P. N., Liao, S. Q., Liu, R. S., Liu, C. Y., Chao, H. T., and Lu, S. R. (2000). Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology 54, 2061–2066. doi:10.1212/WNL.54.11.2061
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
National Institute on Aging (NIA). (2004). Estrogen-alone hormone therapy could increase risk of dementia in older women. J. Investig. Med. 52, 356–357.
Zec, R. F., and Trivedi, M. A. (2002). The effects of estrogen replacement therapy on neuropsychological functioning in postmenopausal women with and without dementia: a critical and theoretical review. Neuropsychol. Rev. 12, 65–109. doi:10.1023/A:1016880127635
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: hormone therapy, ALE meta-analysis, functional magnetic resonance imaging, postmenopause, working memory, neural activation
Citation: Li K, Huang X, Han Y, Zhang J, Lai Y, Yuan L, Lu J and Zeng D (2015) Enhanced neuroactivation during working memory task in postmenopausal women receiving hormone therapy: a coordinate-based meta-analysis. Front. Hum. Neurosci. 9:35. doi: 10.3389/fnhum.2015.00035
Received: 15 June 2014; Accepted: 13 January 2015;
Published online: 11 February 2015.
Edited by:
John J. Foxe, Albert Einstein College of Medicine, USAReviewed by:
Roberta Brinton, University of Southern California, USAAnnie Duchesne, Bishop’s University, Canada
Copyright: © 2015 Li, Huang, Han, Zhang, Lai, Yuan, Lu and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Li, Key Laboratory for NeuroInformation of Ministry of Education, School of Life Science and Technology, University of Electronic Science and Technology of China, No.4, Section 2, North Jianshe Road, Chengdu 610054, China e-mail:bGlrZUB1ZXN0Yy5lZHUuY24=
 Ke Li
Ke Li Xiaoyan Huang1
Xiaoyan Huang1 Yingping Han
Yingping Han