- Centre for Cognitive Neuroscience, University of Salzburg, Salzburg, Austria
The present study investigated oscillatory brain dynamics during self-paced sentence-level processing. Participants read fully correct sentences, sentences containing a semantic violation and “sentences” in which the order of the words was randomized. At the target word level, fixations on semantically unrelated words elicited a lower-beta band (13–18 Hz) desynchronization. At the sentence level, gamma power (31–55 Hz) increased linearly for syntactically correct sentences, but not when the order of the words was randomized. In the 300–900 ms time window after sentence onsets, theta power (4–7 Hz) was greater for syntactically correct sentences as compared to sentences where no syntactic structure was preserved (random words condition). We interpret our results as conforming with a recently formulated predictive-coding framework for oscillatory neural dynamics during sentence-level language comprehension. Additionally, we discuss how our results relate to previous findings with serial visual presentation vs. self-paced reading.
Introduction
Recent accounts of visual word recognition describe the reading process as guided by an interplay between bottom-up incoming information and forward inferences (or predictions; Hagoort, 2005, 2013, 2014; Price and Devlin, 2011). While we read, incoming linguistic inputs are sequentially integrated into the language context (e.g., a sentence, a text or a discourse) at a rate which, in silent reading, is on the verge of 350 words per minute (e.g., Rayner, 1998). Recent findings indicate that fast readers employ highly efficient reading strategies by complementing bottom-up incoming information with predictions concerning probable upcoming words (DeLong et al., 2005; Hawelka et al., 2015). Sentence level predictions could be inferred based on previous knowledge or on context-based semantic information (Altmann and Kamide, 1999; Kamide et al., 2003).
The fact that predictions play a role during sentence-level processing is undisputed. Evidence stems, for example, from eye movement (EM) studies which assessed anticipatory looking, skipping probabilities and fixation durations—variables which are consistently affected by predictability measures (e.g., cloze probability—Balota et al., 1985; Kliegl et al., 2006; Rayner, 2009; Risse et al., 2014; Hawelka et al., 2015). Further support for context based predictions stems from electroencephalographical (EEG) measures of the reading process. Event related brain potentials (ERPs), such as the well documented N400 ERP component (for reviews see Kutas and Federmeier, 2011), were shown to reflect the degree of anomaly (“integration view”) as well as the predictability (“prediction view”) of an incoming word (for a review see Federmeier, 2007). DeLong et al. (2005) investigated the modulation of the N400 as related to expected vs. unexpected article (“a” and “an”)/noun pairs. Results showed that the size of the N400 was larger for articles and nouns that mismatched the sentence-context prediction (e.g., “an airplane” when “a kite” was the most probable continuation). Furthermore, the N400’s mean amplitudes were inversely correlated with the cloze probability of both, the articles and the nouns. These findings suggest that context-based predictions are rapidly and continuously adjusted in correspondence to incoming inputs. In line with this hypothesis, numerous magnetoencephalography (MEG) studies (Pammer et al., 2004; Cornelissen et al., 2009; Wheat et al., 2010; Dikker and Pylkkanen, 2011; Woodhead et al., 2014) as well as EEG studies (Dambacher et al., 2009) reported early effects (within 220 ms) of top-down predictions on visual word recognition. Accordingly, several neurobiological models of language comprehension embraced this predictive coding perspective (Hagoort, 2005, 2013; Jung-Beeman, 2005; Lau et al., 2008). Although partially distinct from each other, these models share two main assumptions: (1) the reading network encompasses brain areas mainly distributed across the left hemisphere; and (2) these areas interact with each other through top-down and bottom-up connections. For the purpose of the present study we will not focus on the functional neuroanatomy of the reading network (for a review see Price, 2012; Taylor et al., 2013; Martin et al., 2015), but rather on neuronal communication between and within areas as related to sentence-level predictions.
A growing body of evidence suggests that oscillatory dynamics represent a feasible mean of inter- and intra-areal communication (Gray et al., 1989; Bressler, 1995). More specifically, synchronous oscillatory activity was evidenced as a possible mechanism for the propagation of top-down and bottom-up information across cortical levels (von Stein et al., 2000; Engel et al., 2001; Varela et al., 2001; Bar et al., 2006; Hipp et al., 2011; Singer, 2011; Bressler and Richter, 2015). Accordingly, language related functions were repeatedly associated to changes in power and coherence measures in the theta (4–7 Hz), alpha (8–12 Hz), beta (13–30 Hz) and gamma (above 30 Hz) band (for reviews see Bastiaansen and Hagoort, 2006; Lewis et al., 2015). In a recent proposal Lewis and Bastiaansen (2015) suggested a strong link between oscillatory brain activity and forward inferences generated during sentence-level language comprehension. In this proposal, the authors attempted to consolidate previous language-related literature (for a review see Lewis et al., 2015) into a unified predictive coding framework for fast oscillatory neural dynamics. In this framework, two frequency bands are particularly important: the beta band (13–30 Hz) and the gamma band (35–75 Hz). According to the authors, sentence processing entails the formation of a functional network or NeuroCognitive Network (NCN—self-organized, large scale distributed cortical network) encompassing most language related areas. The maintenance or change of state in this network is supposed to be reflected in beta band oscillatory activity. More specifically, when the NCN is under revision/change (e.g., a semantically unrelated word is encountered), one would expect a beta desynchronization as compared to a synchronization for fully correct sentences. Functionally distinct, but strongly related, is the role proposed for gamma band activity. Gamma activity is expected to reflect the congruity between incoming linguistic inputs and pre-activated predictions. Gamma synchronization would reflect the successful matching of top-down predictions with bottom-up incoming information whereas no increase in gamma power (or even a desynchronization) is to be expected in case of a mismatch. This interpretation was based on Herrmann et al. (2004) “match-and-utilization” proposal as well as on recent proposals for language processing (Peña and Melloni, 2012; Wang et al., 2012b).
Electrophysiological correlates of sentence reading were, up to now, mainly recorded using “serial visual presentation” (SVP) paradigms. Thus, the wealth of evidence considered by Lewis and Bastiaansen (2015) for their framework stemmed from SVP. In the SVP approach a sentence is broken down into a series of individual words presented on the screen in isolation and separated by relatively long interstimulus intervals (ITI—usually about 500 ms). Single word presentation renders unnecessary sequential saccades to bring new stimuli to the foveal point of vision (thus avoiding horizontal EMs resulting in ocular artifacts, Henderson et al., 2013). Furthermore, long ITIs prevent the temporal overlap of the processing of successive stimuli, and thus the overlap of (and the necessity to disentangle) the corresponding brain potentials (Sereno and Rayner, 2003; Kliegl et al., 2012). SVPs were proven to be a reliable tool for the study of visual word recognition (Kutas and Federmeier, 2007). However, serial presentation might be inadequate when it comes to the study of sentence level reading. Serial presentation may distort, for instance, the normal time-course of syntactic and semantic integration and the processes of disambiguation (in case of erroneous or imprecise syntactic or semantic parsing). Whereas the former is likely to be altered by a superimposed timeline of word recognition (duration of stimulus presentation plus inter trial interval), the latter is fully inhibited as no regressive saccades can be performed to previously encountered words (Rayner, 1998; Pernet et al., 2007; Kliegl et al., 2012; Schotter et al., 2014).
For studying the reading process, fixation-related potentials (FRPs) are an alternative to the SVP paradigms (Hutzler et al., 2007; Dimigen et al., 2011, 2012). FRPs are based on the coregistration of EM and EEG data. In the FRP approach (contrary to classical ERPs), the onset of a cognitive process is not defined by an externally triggered event (i.e., stimulus onset), but it is indicated by EMs (i.e., the first fixation on a word). This allows participants to read at their own pace (self-paced reading), thus providing a higher ecological validity of the reading process and the corresponding brain signal.
In the context of neuroimaging, inconsistent findings among studies have been attributed to methodological aspects such as task demands and “unnatural” stimulus presentation (too long presentation durations; see Dehaene and Cohen, 2011; Schuster et al., 2015). Such methodological characteristics—which could induce an elevated level of processing and, ultimately, alter brain responses—can largely be circumvented by means of administering more ecologically valid approaches such those made possible by the FRP paradigm. In the context of electrophysiology, previous FRPs experiments showed how this approach permits a deeper understanding of the relationship between EM and brain signals (Dimigen et al., 2012). To illustrate, in self-paced reading the timing of word recognition can be more directly inferred (Kliegl et al., 2012). Accordingly, results from SVP and FRPs paradigms differ in temporal aspects (e.g., Hutzler et al., 2013) as well as concerning the specific cognitive processes involved. Whereas temporal aspects were proven to affect the timing of signal change (see Dimigen et al., 2011; Hutzler et al., 2013), differences in cognitive processing are most likely to affect inter- and intra-areal communication and, as a consequence, the observed oscillatory activity (for a review see Bressler, 1995).
Using the FRP paradigm, Metzner et al. (2015) re-investigated Hagoort et al.’s (2004) SVP-based results. Although being able to replicate classical ERP findings (i.e., N400), Metzner et al. (2015) reported qualitative differences in oscillatory brain dynamics for FRPs compared to serial presentation. More specifically, the authors reported an increase in power in the delta range and a decrease in power in the upper alpha range following the fixation on a word incongruent with common world knowledge (e.g., Rome is the capital of France). This result is at odds not only with SVP findings as reported by Hagoort et al. (2004), but also with predictions concerning oscillatory neural dynamics as described by Lewis and Bastiaansen’s (2015) framework. Metzner et al.’s (2015) findings, although only evidence from a single study, cast general doubts concerning the ecological validity of oscillatory dynamics stemming from SVP. As aforementioned, the rather artificial settings imposed by SVP paradigms might not be generalizable to natural reading. As an alternative explanation, the methodological approach adopted by Metzner et al. (2015) was not optimally suited to test predictions made by Lewis and Bastiaansen’s (2015) framework. To illustrate, in Metzner et al. (2015) the target word was the sentence final word in 90 out of 120 sentences, whereas this was never the case in Hagoort et al.’s (2004) stimulus material. Furthermore, differences in the experimental design as well as in the analysis (FFT vs. Wavelets) could be plausible explanations for Metzner et al.’s (2015) divergent findings.
The aim of the present study was to use an ecologically valid fixation-related brain potentials paradigm to test predictions of Lewis and Bastiaansen’s (2015) framework. To do so, we kept the experimental paradigm and methodological approach as similar as possible to previous SVP studies. More specifically, we used an adaptation of Bastiaansen et al.’s (2010) paradigm including fully correct sentences, sentences embedding a semantic violation, and two conditions where the order of the words was randomized (no syntactic structure left). According to the framework of Lewis and Bastiaansen (2015), we expected semantically unrelated words to cause a beta desynchronization and a decrease in gamma power. Similar to Bastiaansen et al. (2010) we also investigated power changes related to sentence-level processing. We traced the development of oscillatory dynamics related to the unfolding of the sentence. Furthermore, in accordance with Lewis and Bastiaansen’s (2015) framework, we expected a larger gamma and beta band power when subjects read syntactically correct sentences as compared to the condition where subjects read randomly arranged words.
Materials and Methods
Participants
Thirty-two native German speaking students (10 males, M = 23.2 years, SD = 2.3 years) participated in this study. All participants were right–handed; had normal or corrected-to-normal vision and no history of neurological or psychiatric disorders. All participants undertook a short reading test to ensure reading proficiency (reading rate above 150 words per minute). Before testing participants gave their written informed consent. The experiment was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the University of Salzburg.
Materials and Stimuli
The stimulus material consisted of 120 quadruples of sentences. A sentence quadruple comprised two syntactically correct sentences (ORD) and two random word order sentences (RDM). The ORD conditions consisted of: (i) a fully correct sentence (ORD_COR); and (ii) a version of the same sentence which contained a semantic violation (i.e., a semantically unrelated word; ORD_SEM—see Table 1). The two RDM conditions were created by pseudo-randomly arranging the words contained in the two ORD conditions (i.e., RDM_COR and RDM_SEM—see Table 1). In the randomization process we ensured that no syntactic structure was left, but we kept the target word at the original position. The average sentence length was 9.56 words (SD = 1.61) and the average position of the target word in the sentence was 7.1 (SD = 1.37). Target words were never the initial or the final word of a sentence. Moreover, the target words were matched across conditions (i.e., for the factor COR and SEM) for the following criteria: lemma word log-frequency (COR: M = 1.38, SD = 0.83; SEM: M = 1.23, SD = 0.77), length (COR: M = 6.63, SD = 2.09; SEM: M = 6.21, SD = 2.00) and number of syllables (COR: M = 2.09, SD = 0.85; SEM: M = 2.01, SD = 0.94). We splitted and pseudorandomized the items in two versions of the experiment. This procedure was applied in order to ensure that each participant read only one ORD version and the opposite RDM version of each sentence quadruplet. To illustrate, a participant received the ORD-COR/RDM-SEM pairing of a particular sentence; whereas another received the ORD-SEM/RDM-COR pairing. Participants were randomly assigned to one of the two versions of the experiment (16 participants per group).
Experimental Procedure
Participants were seated in a dimly lit room, at a distance of 50 cm from a 21-in (53.34 cm) CRT monitor (1024 × 768 pixel resolution, 120 Hz refresh rate). After the instruction screen, a three dots calibration routine was performed. Each trial sequence started with a fixation cross presented at the leftmost side of the screen for a maximum of 3 s. To trigger the stimulus appearance, participants had to maintain their gaze on the fixation cross for a minimum of 200 ms. If no fixation was detected, a new calibration routine was initiated. Stimuli were presented as whole sentences, in a mono-spaced bold font (Courier New, 14 pt). Participants were instructed to silently read the stimuli. The trials’ end was prompted by fixating a cross presented at the bottom right corner of the screen. A blank screen presented for a period ranging from 750 to 1220 ms served as intertrial interval. The experiment was divided in three blocks (80 trials each). At the end of each block participants took a short break (3–5 min). Each session lasted approximately 2 h.
Recording and Analysis
Apparatus
Electrophysiological measures were acquired with 64 active electrodes EEG system (actiCAP, Brain Products GmbH, Germany) positioned according to the standard 10–10 system. Signals were amplified using an ActiCHamp Amplifier (Brain Products GmbH, Germany). All impedances were kept below 10 kΩ. Bipolar horizontal EOG were recorded between electrodes at the outer left and right canthus. EMs were recorded monocularly from the right eye with a SR Research Eyelink 1000 desktop mount system (SR Research, ON, Canada). The head was stabilized via a chin rest. Both the eye tracker’s and the EEG’s sampling rates were set to 500 Hz. At the beginning of each block the eye tracker was calibrated with a horizontal 3-points calibration routine. The calibration was considered as successful if the average tracking error was below 0.5° of visual angle. The calibration routine was repeated every time the fixation control at the beginning of a trial (see “Experimental procedure” Section) failed. Stimulus presentation was controlled by the Experiment Builder software (SR Research Ltd., Canada).
Eye Movements
For the EMs analysis we excluded all trials where the target word was skipped. We further aggregated the remaining epochs into standard EMs measures: first fixation duration (FFD), gaze duration (GD), and total viewing time (TVT). FFD is the duration of the first fixation on a word during first-pass reading. GD is the sum of the duration of all fixations on a word during first-pass reading. TVT is the sum of the duration of all (progressive and regressive) fixations on a word (i.e., first plus second-pass reading). We excluded fixations shorter than 80 ms and longer than 3 SD from the individual mean (total data loss: 2.06% of all fixations). EM measures were log-transformed and analyzed with the package ez (Lawrence, 2011) in R.
Time-Frequency Analysis
EEG data were preprocessed and analyzed with EEGLAB (Delorme and Makeig, 2004) and ERPLAB (Lopez-Calderon and Luck, 2014) toolboxes in MATLAB (Mathworks, Natick, MA, USA). All electrodes (except EOG channels) were re-referenced against an average reference. Data was band-pass filtered between 0.1–70 Hz and a 50 Hz notch filter was applied. Ocular and muscular artifacts were corrected with Independent Component Analysis (ICA, Makeig et al., 1996, 1997) carried out separately for each subject. ICA components loading primarily in frontal sites and showing inverted polarity between the two periocular sites were defined as putative components for horizontal EMs. This procedure was conducted in accordance with standardized procedures for identification and removal of eye activity artifacts (Jung et al., 1998, 2000). For 32 participants, an average of 7.7 components were identified as putative EM components (range = 2–17, SD = 4.4). Time-frequency (TF) analysis was based on Event-Related Spectral Perturbation (ERSP) as implemented in EEGLAB (Delorme and Makeig, 2004). However, for the fixation-related analysis we will not use the acronym ERSP, instead we will adopt the nomenclature Fixation-Related Spectral Perturbation (FRSP). FRSPs represent the spectral power change of a post-fixation interval as compared to a pre-fixation baseline. In line with classical ERSPs, this measure is calculated as the log ratio of the two intervals and reported in decibel (db) units. We analyzed frequencies ranging from 3 to 70 Hz with a sliding Hanning-tapered 3-cycle sinusoidal wavelet. Our analysis focused on five frequency bands: theta (4–7 Hz), alpha (8–12 Hz), lower beta (13–18 Hz), upper beta (19–30 Hz) and gamma (31–55 Hz). Power change values were averaged into six clusters of five electrodes each, uniformly distributed across the two hemispheres: frontal left (F1, F3, FC1, FC3, FC5), frontal right (F2, F4, FC2, FC4, FC6), central left (C1, C3, C5, CP1, CP3), central right (C2, C4, C6, CP2, CP4), parieto-occipital left (P5, PO3, P7, PO7, O1), parieto-occipital right (P6, PO4, P8, PO8, O2). Clusters of electrodes were selected in order to roughly correspond to previous publications by our research group (Hutzler et al., 2004, 2007) as well as from other research groups (Barber et al., 2004, 2011). These clusters were proven to be well suited to investigate electrophysiological correlates of the reading process by means of both FRPs (Hutzler et al., 2007) and traditional ERP measures (Barber et al., 2004, 2011; Hutzler et al., 2004).
Event-Related Spectral Perturbation Time-locked to the Target Word
In order to investigate effects of semantically unrelated words on the TF data, EEG recordings were segmented from −1000 to +1500 ms time-locked to the onset of the fixation on the critical word. As baseline we used the 1000 ms period before fixation onset. Because of temporal smoothing the resulting epochs ranged from −440 to 940 ms. In order to investigate effects of the target word on TF representations (TFRs) of single data trial we will mainly focus on the comparison of the two syntactically correct conditions (i.e., ORD_COR and ORD_SEM). Mean measures of power change were included into a repeated measure analysis of variance (ANOVA). Factors in the analysis included temporal window (0–300 ms, 300–600 ms), hemisphere (left and right hemisphere), electrode cluster (frontal, central and parieto-occipital clusters) and condition (ORD_CORR vs. ORD_SEM). Post hoc contrasts between conditions were carried out for those time windows showing a significant Cluster * Condition interaction.
Event-Related Spectral Perturbation Time-locked to Sentence Onset
For the sentence level analysis we time-locked each epoch to the onset of the fixation on the first word of the sentence. Each epoch included −1000 ms pre-stimulus baseline and +2500 ms post fixation onsets. Temporal smoothing generated epochs ranging from −440 to 1940 ms. In order to investigate the evolution of power changes across the sentence we merged FRSP values of the two ORD conditions and we compared them to the merged values of the RDM conditions. Contrary to the target word level analysis, we had no prior assumptions concerning the lateralization of the effects. We therefore did not include the factor Hemisphere in the sentence-level analysis. Mean measures of power change were included into an overall ANOVA with factors: time windows (0–300 ms, 300–600 ms, 600–900 ms, 900–1200 ms, 1200–1500 ms, 1500–1800 ms), electrode clusters (frontal left, frontal right, central left, central right, parieto-occipital left, parieto-occipital right) and conditions (ORD vs. RDM). Post hoc contrasts between conditions were carried out for those time windows showing a significant Cluster * Condition interaction. We limited our power analysis to the first 1800 ms after the fixation on the first word of the sentence. This value was chosen, because it is—on the one hand—sufficiently long so that the participants encountered the target words (in the vast majority of the cases), but it is—on the other hand—sufficiently short to avoid perturbations (as far as possible) by different reading times (due to differences in the participants’ reading rate and differences in sentence lengths).
Results
Eye Movement Results
EMs’ measures (i.e., FFDs, GDs and TVT) were analyzed with a repeated measure ANOVA with Condition (the four conditions) as within subject factor.
Separate ANOVAs for each dependent measure revealed highly significant effects of Condition in FFDs (F(3,93) = 34.27, p < 0.001), GDs (F(3,93) = 25.31, p < 0.001) and TVT (F(3,93) = 34.02, p < 0.001). Post hoc t-tests revealed significantly longer FFDs (203 vs. 198, t(31) = 2.95, p < 0.001), GDs (237 vs. 227, t(31) = 2.47, p = 0.02) and TVT (270 vs. 239, t(31) = 5.39, p < 0.001) for semantically unrelated words (see Figure 1). In the comparison between the two conditions where the order of the words was randomized (no syntactic structure left) words strings containing semantically unrelated words showed longer FFDs (217 vs. 211, t(31) = 3.30, p < 0.001), GDs (260 vs. 248, t(31) = 3.27, p < 0.01) and TVT (290 vs. 273, t(31) = 3.56, p < 0.01). The overall pattern of results is in line with previous EM findings (e.g., Rayner et al., 2004).
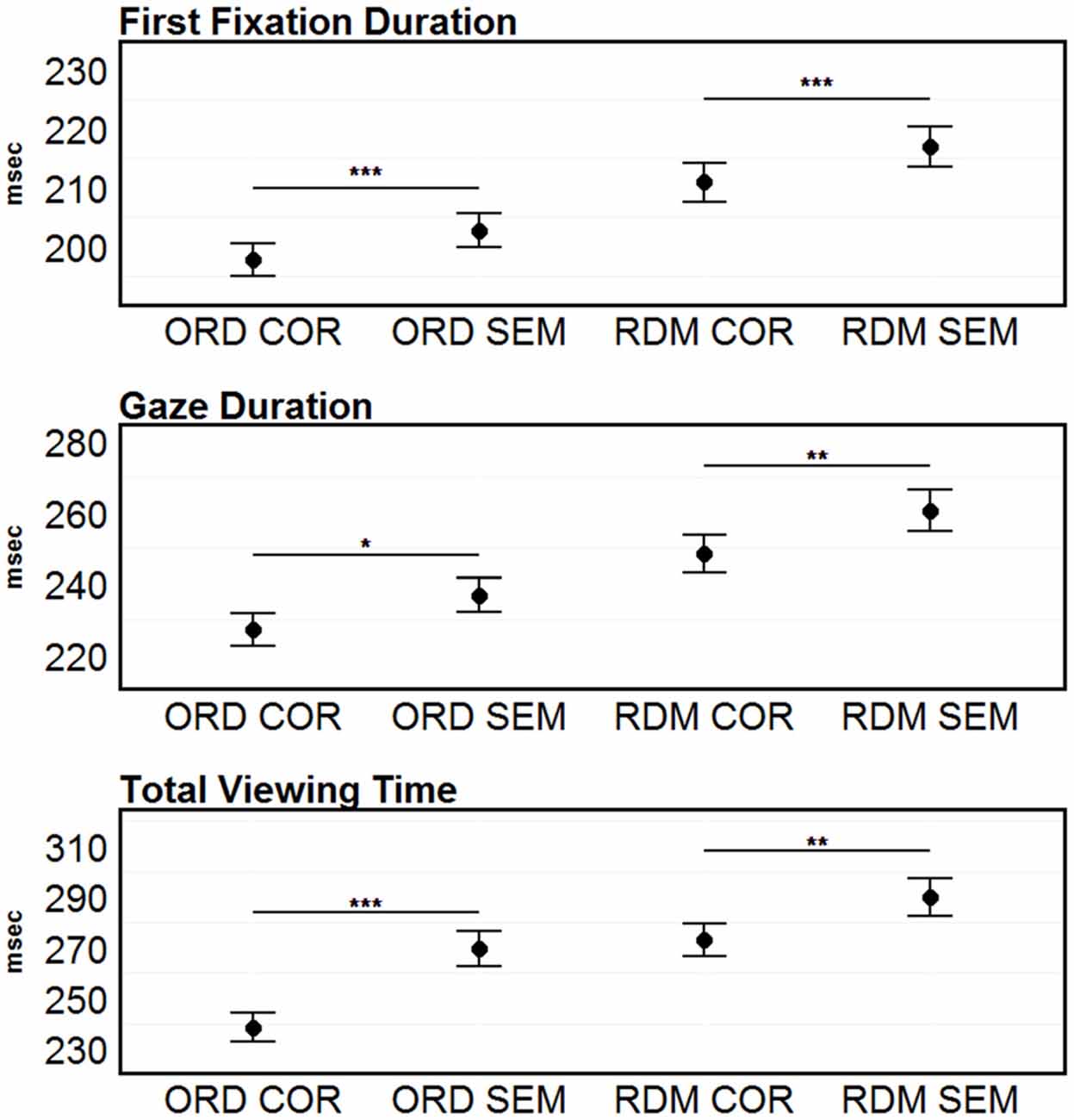
Figure 1. Eye movement (EM) results. First-fixation durations (FFDs), gaze durations (GDs) and total viewing time (TVT) on the target words. Error bars represent 95% confidence intervals. Significant differences between the correct and the semantically incorrect condition (within the ORD and COR conditions) are marked with asterisks: ***p < 0.001; **p < 0.01; *p < 0.05.
Fixation-Related Spectral Perturbation Time-locked to the Target Word
The analysis of power changes at the target word level revealed significant differences in the lower beta (13–18 Hz) band when we compared ORD_COR vs. ORD_SEM conditions in the initial 0–300 ms time window. Using the same analysis, no power differences were found in the theta (4–7 Hz), alpha (8–12 Hz), upper beta (19–30 Hz) and gamma (31–55 Hz) bands.
Lower Beta Band
The findings for the lower beta band are illustrated in Figure 2. The overall ANOVA revealed main effects of Time (F(1,31) = 13.76, p = 0.001), Hemisphere (F(1,31) = 4.46, p = 0.043) and Cluster (F(2,62) = 4.29, p = 0.018) as well as three-way interactions Time * Hemisphere * Cluster (F(2,62) = 6.40, p = 0.003) and Time * Cluster * Condition (F(2,62) = 3.77, p = 0.028). A separate ANOVA for each hemisphere with factors Time (0–300 ms and 300–600 ms time windows), Cluster (three clusters) and Condition (ORD_COR vs. ORD_SEM) revealed a significant interaction Time * Cluster * Condition in the left hemisphere (F(2,62) = 3.5, p = 0.036) but not in the right hemisphere (F(2,62) = 0.55, p = 0.578). We followed up the left lateralized effect by performing an ANOVA for the two time windows with factors Cluster and Condition (ORD_COR vs. ORD_SEM). The 0–300 ms time window revealed a significant Cluster * Condition interaction (F(2,62) = 4.98, p = 0.01). This was not the case in the 300–600 ms time window (F(2,62) = 1.11, p = 0.334; see Figure 2A). Planned t-tests for each cluster in the 0–300 ms time window revealed a significant difference for conditions at the parieto-occipital cluster (t(31) = 2.23, p = 0.033; see Figure 2C).
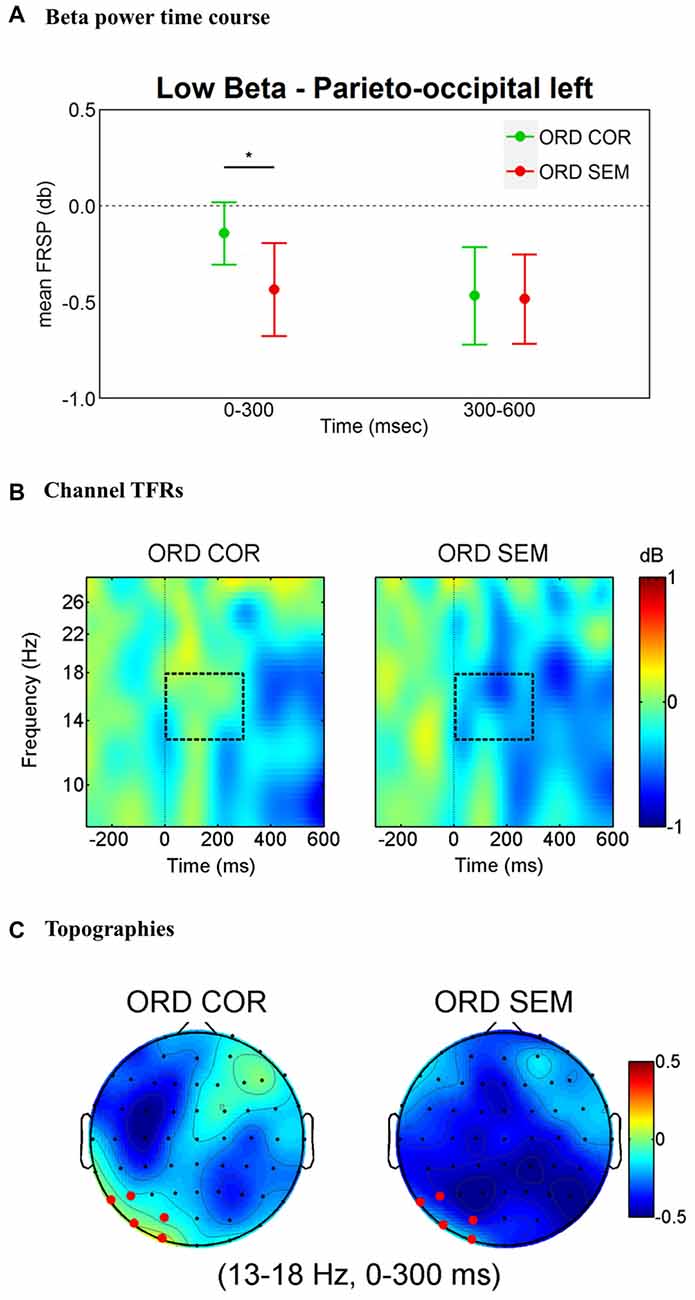
Figure 2. Lower-beta band (13–18 Hz) effects time-locked to the onset of the first fixation on the target word. (A) Left parieto-occipital cluster average lower-beta band power measures in the time windows of interest (0–300 ms and 300–600 ms). Error bars represent 95% confidence intervals. Significant differences between conditions are marked with asterisks: *p < 0.05. (B) Time-frequency (TF) representations of power changes in the target word level analysis. The black rectangle indicates the time and frequency range of interest at one representative channel (PO3). (C) Topographic maps of power change in the 0–300 ms time window. In red, the cluster of electrodes that showed a significant difference in lower-beta band activity when participants read semantically unrelated words as compared to the semantically correct target words.
Gamma Band
The overall ANOVA revealed a significant interaction for Time * Hemisphere (F(1,31) = 8.99, p = 0.005), Hemisphere * Cluster (F(2,62) = 3.24, p = 0.046) and Time * Cluster * Condition (F(2,62) = 5.68, p = 0.005). Separate ANOVAs for each hemisphere revealed a significant interaction Time * Cluster * Condition in the left hemisphere (F(2,62) = 5.03, p = 0.009) but not in the right hemisphere (F(2,62) = 2.11, p = 0.13). An ANOVA for each time window with factors Cluster (three left-lateralized clusters) and Condition (ORD_COR vs. ORD_SEM) revealed no significant Cluster * Condition interactions in both the 0–300 ms and 300–600 ms time windows (respectively: F(2,62) = 1.62, p = 0.208; F(2,62) = 0.92, p = 0.405).
Fixation-Related Spectral Perturbation Time-locked to Sentence Onset
The analysis of power changes across the sentence revealed significant differences in the theta (4–7 Hz) band when we compared ORD and RDM conditions in the 300–600 and 600–900 time windows. Additionally, analysis of individual beta weights in the gamma (31–55 Hz) band showed a linear trend in the ORD but not in the RDM condition. Using the same analysis, no power differences were found for alpha (8–12 Hz), lower beta (13–18 Hz) and upper beta (19–30 Hz) bands.
Theta Band
The findings for the theta band are illustrated in Figure 3. An overall ANOVA with factors Time * Cluster * Condition revealed a main effect for Time (F(5,155) = 15.14, p < 0.001), Cluster (F(5,155) = 3.18, p = 0.009), as well as a significant three-way interaction Time * Cluster * Condition (F(25,775) = 2.27, p < 0.001). We performed separate ANOVAs for each time window, with factors Cluster (all clusters) and Condition (ORD vs. RDM; see Figure 3A). In the 300–600 ms time window we observed main effects for Cluster (F(5,155) = 3.79, p = 0.003), Condition (F(1,31) = 5.55, p = 0.025) and a significant interaction Cluster * Condition (F(5,155) = 5.25, p < 0.001). A similar result was obtained in the 600–900 ms time window, with a main effect for Cluster (F(5,155) = 2.98, p = 0.014) and a significant interaction Cluster * Condition (F(5,155) = 4.61, p = 0.001). No other time windows revealed significant interactions Cluster * Condition (ps > 0.1). Pairwise t-tests in 300–600 ms and 600–900 ms time windows revealed significant differences across conditions in the parieto-occipital left (t(31) = 2.6, p = 0.014), parieto-occipital right (t(31) = 3.26, p = 0.003) for the 300–600 ms time window, and in the parieto-occipital right cluster (t(31) = 2.32, p = 0.027) for the 600–900 ms time window (see Figures 3B,C).
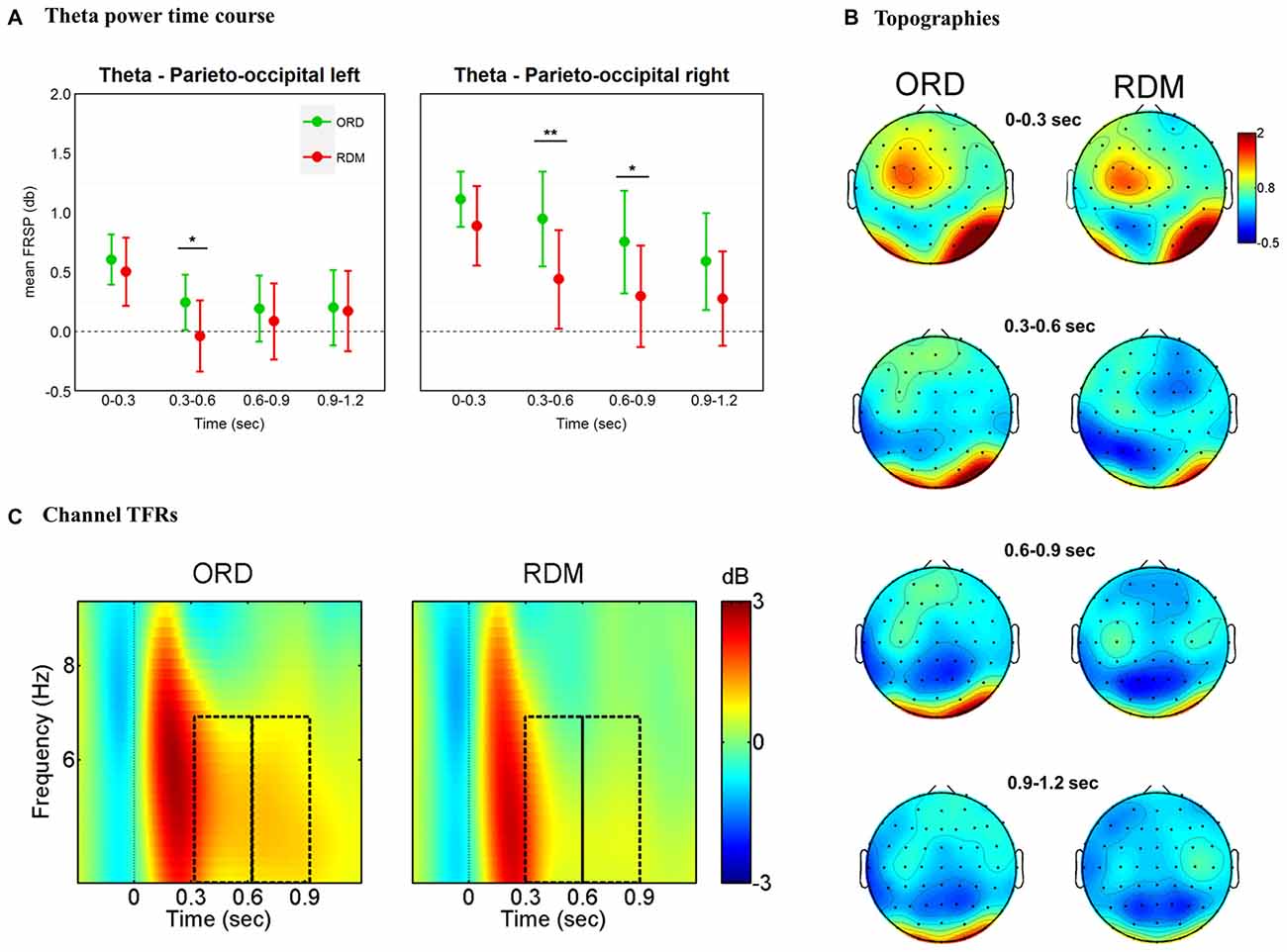
Figure 3. Theta band (4–7 Hz) effects time-locked to sentence onsets. (A) Time course of theta power for left and right parieto-occipital clusters. Mean power values are plotted in successive 300 ms time windows, up to 1200 ms. Error bars represent 95% confidence intervals. Significant differences between conditions are marked with asterisks: **p < 0.01; *p < 0.05. (B) Topographic maps of theta (4–7 Hz) power change in successive 300 ms time windows, up to 1200 ms. (C) TF representations of power changes at the sentence-level analysis. The black rectangles indicate the time windows (300–600 ms and 600–900ms) where theta power was larger for syntactically correct sentences compared to the condition where the order of the words was pseudo-randomized. Results are plotted for one representative channel (PO4).
Gamma Band
The findings for the gamma band are illustrated in Figure 4. The overall ANOVA revealed main effects for Cluster (F(5,155) = 13.6, p = 0.000), Condition (F(5,155) = 11.2, p = 0.002) and significant interaction Time * Cluster * Condition (F(25,775) = 1.76, p = 0.013). A separate ANOVA for each time window with factors Cluster (all clusters) and Condition (ORD vs. RDM) revealed a significant main effect for Condition in all time windows except the initial one (0–300 ms). In order to further investigate the time course of power in the gamma band we followed a procedure similar to the one applied by Bastiaansen et al. (2010). We extracted average power change values in each cluster and we fitted a linear regression line for each individual subject (see Figure 4A). Hence, for each condition, we performed a one-sample t-test on individual beta weights—testing whether the regression slopes were significantly greater than zero (i.e., one-sided test). Results showed a linear trend in the ORD condition at parieto-occipital and central right clusters (t(31) = 1.74, p = 0.045 and t(31) = 1.7, p = 0.049, respectively) but not in the RDM condition for the same clusters (ts < 1; see Figures 4B,C). Pairwise t-tests comparing beta weights between conditions, revealed significantly larger regression coefficients for the ORD than the RDM condition in the right parieto-occipital cluster (t(31) = 2.73, p = 0.01) but not in the right central cluster (t(31) = 1.19, p = 0.24).
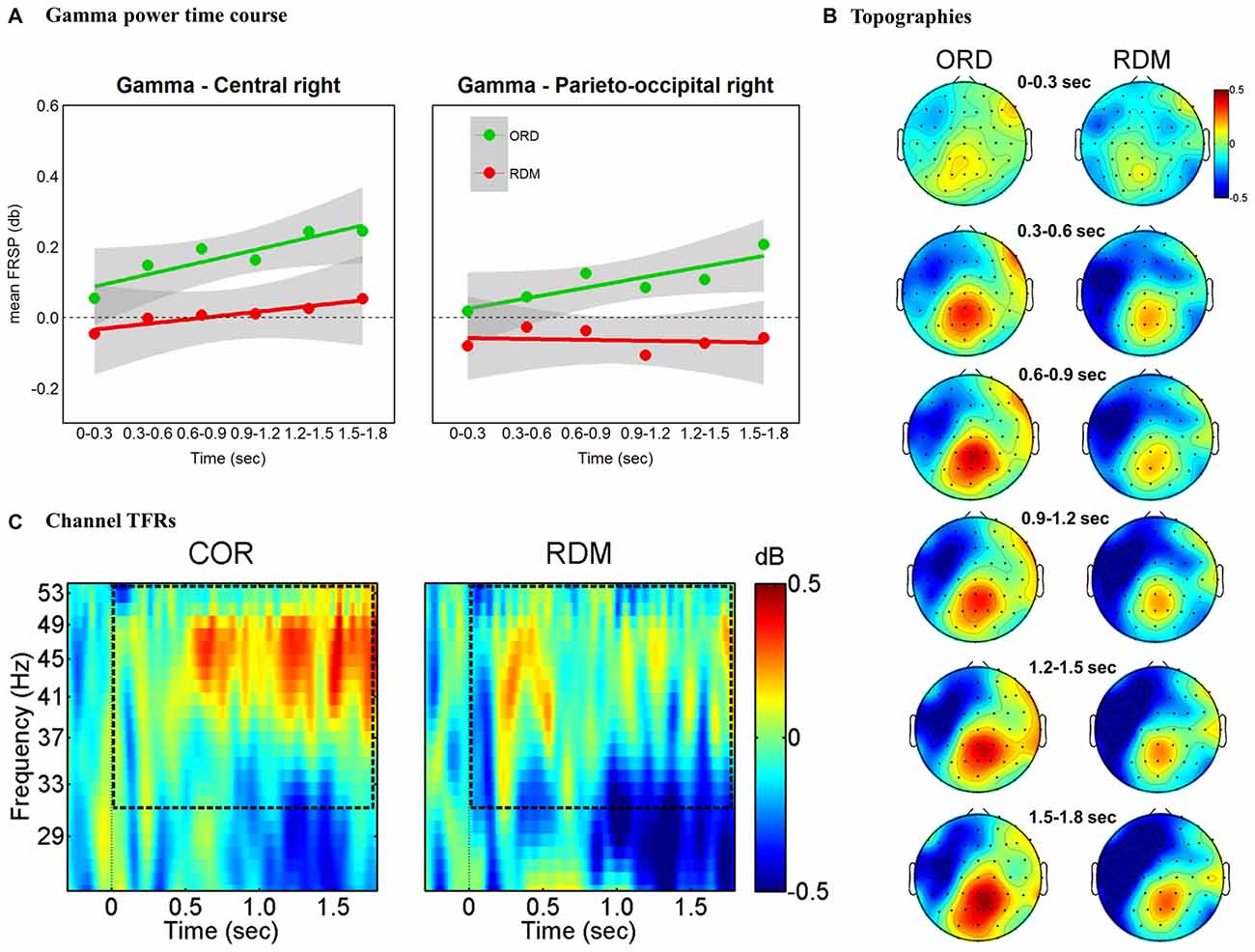
Figure 4. Gamma band (31–55 Hz) effects time-locked to sentence onsets. (A) Time course of gamma power for right parieto-occipital and central clusters. Mean power values are plotted in successive 300 ms time windows, up to 1800 ms. We fitted a linear regression line for each condition (green and red lines). The shaded gray regions represent a pointwise 95% confidence interval. (B) Topographic maps of gamma (31–55 Hz) power change in successive 300 ms time windows, up to 1800 ms. (C) TF representations of power changes at the sentence-level analysis. The black rectangles indicate the frequency range of interest. Results are plotted at one representative channel (PO4).
Discussion
The main objectives of the present study were to investigate oscillatory brain dynamics during self-paced sentence-level reading and compare our fixation-related results with previous SVP findings. Although several different frequency bands were previously associated to language related functions (for reviews see Bastiaansen and Hagoort, 2006; Lewis et al., 2015), we focused on beta and gamma band modulation as predicted by Lewis and Bastiaansen’s (2015) framework for neural dynamics during sentence-level language comprehension. We used an adaptation of Bastiaansen et al.’s (2010) paradigm and asked participants to silently read semantically correct sentences, sentences containing a semantically unrelated word (henceforth: target word) and “sentences” in which the order of the words was randomized (no syntactic structure left). At the target word level, words that were both syntactically and semantically congruous with the sentential context showed typical eye-movement measures for self-paced reading (i.e., FFDs of M ~ 200 ms, GDs of M ~ 230 ms and TVTs of M ~ 240 ms—c.f., Rayner, 1998; Sereno and Rayner, 2003). Expectedly, semantically unrelated words embedded in otherwise correct sentences led to longer TVT (M = 270 ms) due to more regressive refixations after the first encounter of the word (i.e., during second-pass reading).
For the analysis of oscillatory brain dynamics we investigated power changes time-locked to the onset of the first fixation on the target word (target word analysis) and traced the evolution of power in the theta and gamma bands during the syntactic parsing of the sentence (sentence-level analysis). The target word analysis focused on the comparison between the two conditions with preserved syntactic structure (i.e., fully correct sentences vs. sentences containing a semantic violation). Larger beta band (13–18 Hz) desynchronization was found when participants read semantically incongruent words as compared to congruent words. Sentence-level analysis focused on comparing the conditions with a correct syntactic structure vs. the “sentences” with a random word order. On the sentence level, gamma power (31–55 Hz) increased linearly during parsing of syntactically correct sentences, whereas this effect was not observed when the order of the words was randomized. Furthermore, in the 300–900 ms time window after sentence onset, theta power (4–7 Hz) was greater for syntactically correct sentences than for the random word sequences.
Semantic Violations Induced a Lower-Beta band Desynchronization
At the target word level our results confirmed the expected modulation of beta band activity. The expectation—derived from previous studies—was a beta band desynchronization time-locked to syntactically and semantically unrelated words—previously reported by Weiss et al. (2005), Davidson and Indefrey (2007), Luo et al. (2010) and Meyer et al. (2013). In these studies, semantic violations elicited a beta suppression as early as 200 ms after stimulus onsets (Luo et al., 2010; Wang et al., 2012a) and the localization of the effect was over areas typically associated with the reading network (for a review see Price, 2012; Taylor et al., 2013; Martin et al., 2015). These locations included bilateral parietal and occipital sites as well as left temporal and inferior frontal regions (Wang et al., 2012a; Kielar et al., 2014, 2015). Accordingly, we found that semantically unrelated words induced an early (0–300 ms after fixation onsets) lower-beta band desynchronization—mainly distributed over left parietal and occipital sites.
Based on the work by Herrmann et al. (2004), we expected—concomitant with the beta band desynchronization—that congruent words would generate a larger gamma band synchronization than semantically incongruent words. Our results did not fulfill this expectation. However, it is important to note that findings reported in the literature are inconsistent. On the one hand, several studies did not report gamma band effects for semantic violations (Hagoort et al., 2004; Davidson and Indefrey, 2007; Wang et al., 2012a; Kielar et al., 2014, 2015). On the other hand, numerous studies reported significant gamma band modulation (Hald et al., 2006; Penolazzi et al., 2009; Wang et al., 2012b; Rommers et al., 2013). Lewis and Bastiaansen (2015) interpreted these discrepancies in the literature to reflect differences due to different presentation modalities (i.e., single word presentations, sentence-level language comprehension, speech processing). In our FRSP analysis we used a 1000 ms baseline previous to the onset of the fixation on the target word. Thus, during the baseline, the reading process was already ongoing. This is not the case in SVP paradigms, where the inter-stimulus interval (i.e., a blank screen) is usually used as baseline period. The different cognitive affordances of the chosen baseline periods are a potential explanation for the divergent results when we compare findings stemming from different presentation modalities. However, of main interest for our target word analysis were changes in the ongoing sentence-level oscillatory dynamics during natural reading of continuous sentences (realized by the fixation-related setup). Using a blank screen as baseline period would create different cognitive affordances in the pre- and post-fixation onsets intervals—probably influencing the results.
Importantly, at the target word level, our beta and gamma effects are inconsistent with those from a previous fixation-related study (Metzner et al., 2015), whereas we share crucial commonalities with previous SVP findings (Kielar et al., 2014, 2015). Metzner et al.’s (2015) divergent findings (with regard to the original findings of Hagoort et al., 2004) led to the conclusion that oscillatory brain dynamics are qualitatively different when one compares self-paced reading with serial-visual presentation. In the present study, at the target word level, the observed pattern of results is in agreement with predictions of Lewis and Bastiaansen’s (2015) framework (which is, to a great extent—based on the work of Hagoort et al., 2004). This is noteworthy since this framework is primarily based on findings from SVP. Accordingly, we interpret the beta band desynchronization to reflect the revision/change of the ongoing NCN prompted by semantically unrelated words (see also Engel and Fries, 2010; Bressler and Richter, 2015). Furthermore, we expected semantic violations to generate gamma band desynchronization resulting from the mismatch between top-down sentence-level predictions and bottom-up incoming semantic violations (Herrmann et al., 2004). The gamma band effect did not reach significance at the target word level, however support for this hypothesis can be found in our sentence-level analysis.
Theta and Gamma Power During Syntactic Parsing of the Whole Sentences
For the sentence-level analysis Bastiaansen et al. (2010) reported a linear increase in power of lower beta (13–18 Hz) and theta (4–8 Hz) bands for syntactically correct sentences. The authors did not observe such an effect when the order of the words was randomized. In the present study, sentence-level analysis did not reveal significant differences in beta band power when we compared fully correct sentences to “sentences” with a random word order. According to Lewis and Bastiaansen’s (2015) framework, an increase in beta activity is to be expected under the condition in which supplementary load is added to the current NCN. NCN’s load has been associated to beta band activity during experiments investigating syntactic unification processes (Weiss et al., 2005; Bastiaansen and Hagoort, 2006) and in experiments investigating subject-verb agreement dependencies (Meyer et al., 2013). As aforementioned, in SVP paradigms the pace at which words are presented is externally controlled. Presentation rates in the range of 500–1000 ms per word may lead to processing differences when compared to the average 200–250 ms fixation duration in natural reading (Rayner, 1998; Pernet et al., 2007; Kliegl et al., 2012). An externally controlled (i.e., superimposed) timeline of visual word recognition is likely to call for additional processing load on the NCN which may be reflected in the beta increase observed by Bastiaansen et al. (2010). On the contrary, in self-paced reading paradigms, subjects are free to read at their own pace without any further restrictions above and beyond visual word recognition. Thus, differences in the NCNs’ processing load, imposed by manners of presentation, might have led to the pattern of results observed in the present study.
Additional support for the interpretation that the divergent findings are due to differences in the processing load of the NCN can be found in speech processing experiments. Peña and Melloni (2012) had participants listen to utterances played in their native language and utterances played in foreign languages. Both native and foreign languages were played either forward or backwards. As in our self-paced reading experiment, the authors did not find a linear increase in beta band power for utterances played forward and in the native language. Results in the beta band are inconsistent when we consider similar experimental conditions, but different manners of presentation (i.e., SVP, self-paced reading, spoken sentence processing). Task-related effects on beta band modulation will require further empirical investigation. Besides no beta effects, Peña and Melloni (2012) reported a significant increase in theta and gamma band power when participants listened to utterances played forward and in their native language. In the present experiment, we observed a linear increase in gamma power at the posterior right site when participants read syntactically correct sentences. For the same site, this trend was not observed when the order of the words was randomized. We interpret the gamma modulation as reflecting the successful sequential matching of top-down sentence-level predictions and bottom-up information (Herrmann et al., 2004; Lewis and Bastiaansen, 2015).
It could be argued that the observed gamma modulation reflects the sequential encoding of words into a multi-item working memory system. This hypothesis would be in line with studies reporting a linear increase in gamma power as a function of increasing working memory load (Howard et al., 2003). However, we consider this to be an unlikely explanation, as no such a trend was observed in previous SVP experiments (where the memory load is greater compared to natural reading paradigms; Kliegl et al., 2012) or when the order of the words was randomized. In previous sentence-level SVP studies (see Bastiaansen et al., 2010) not gamma, but theta power linearly increased across the sentences. Theta band modulation was associated with memory and unification processes (Hald et al., 2006; Meyer et al., 2015) as well as retrieval of specific items from a working memory set (Bastiaansen and Hagoort, 2006). We observed larger theta power for syntactically correct sentences compared to the random words condition—restricted to the 300–900 ms time window after sentence onset. Again, these findings could stem from the different working memory loads imposed by manners of presentation. In Bastiaansen et al. (2010), sentence presentation lasted up to 6 s, whereas in our case the average time needed by participants to complete sentences was just above 2 s. Unification and retrieval of specific items from a working memory set might be more challenging when words constituting a sentence are presented one by one and separated by long ITIs (Raghavachari et al., 2001).
Conclusions
The pattern of results evidenced by the present study is in line with the predictive coding framework for rapid oscillatory neuronal dynamics described by Lewis and Bastiaansen (2015). Unlike Metzner et al.’s (2015) results, our fixation-related analysis revealed qualitatively comparable findings as the SVP literature. Despite several shared commonalities, presentation modality was identified as the most likely explanation for the observed differences in the temporal distribution of the effects.
For the present study, the analysis of power measures was performed on the basis of pre-defined clusters of electrodes (see “Time-frequency analysis” Section). However, it should be noted that more sophisticated data-driven clustering methods, such as Threshold Free Cluster Enhancement (TFCE, Smith and Nichols, 2009; Ehinger et al., 2015; Pernet et al., 2015) and cluster-based permutation test (Maris and Oostenveld, 2007) would provide a valuable alternative to literature-based clusters. One additional challenge associated to the fixation-related approach, was the correction of EM related artifacts. For the present study we adopted a classical approach (Jung et al., 1998, 2000) which proved to be sufficiently accurate in detecting and removing EM related activity. However, more advanced methods for the identification, characterization and correction of EM artifacts, could further improve the quality of the signal, hence the stability of the results in future studies (see Dimigen et al., 2011; Plöchl et al., 2012). Last, methodological choices (e.g., the baseline period, the experimental design and the preferred power analysis method) are well established sources of heterogeneity in the field and issues for future research.
To date, SVP paradigms were the most extensively used tool to investigate word recognition in sentential context (for a review see Bastiaansen and Hagoort, 2006). SVP paradigms were, however, criticized for the restrictions imposed on reading-related EM behavior (e.g., word skippings, regressive saccades) and reading-related attentional processes (e.g., parafoveal preprocessing; Sereno and Rayner, 2003; Schotter et al., 2012). FRPs and FRSPs were proven to be reliable tools in the study of neural underpinnings of the reading process. We hope that future research will benefit from these newly pioneered techniques by studying reading and reading related processes from a more ecologically valid perspective.
Author Contributions
Conceptualized and designed the experiment (LV, FH, SH); acquired the data (LV, NAH); analyzed the data (LV, FR); wrote the article (LV, NAH, FH, SH, FR). All authors have approved the final version of the article and agree to be accountable for all aspects of this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF P 25799-B23).
References
Altmann, G. T., and Kamide, Y. (1999). Incremental interpretation at verbs: restricting the domain of subsequent reference. Cognition 73, 247–264. doi: 10.1016/s0010-0277(99)00059-1
Balota, D. A., Pollatsek, A., and Rayner, K. (1985). The interaction of contextual constraints and parafoveal visual information in reading. Cogn. Psychol. 17, 364–390. doi: 10.1016/0010-0285(85)90013-1
Bar, M., Kassam, K. S., Ghuman, A. S., Boshyan, J., Schmid, A. M., Dale, A. M., et al. (2006). Top-down facilitation of visual recognition. Proc. Natl. Acad. Sci. U S A 103, 449–454. doi: 10.1073/pnas.0507062103
Barber, H. A., Ben–Zvi, S., Bentin, S., and Kutas, M. (2011). Parafoveal perception during sentence reading? An ERP paradigm using rapid serial visual presentation (RSVP) with flankers. Psychophysiology 48, 523–531. doi: 10.1111/j.1469-8986.2010.01082.x
Barber, H., Vergara, M., and Carreiras, M. (2004). Syllable-frequency effects in visual word recognition: evidence from ERPs. Neuroreport 15, 545–548. doi: 10.1097/00001756-200403010-00032
Bastiaansen, M., and Hagoort, P. (2006). Oscillatory neuronal dynamics during language comprehension. Prog. Brain Res. 159, 179–196. doi: 10.1016/s0079-6123(06)59012-0
Bastiaansen, M., Magyari, L., and Hagoort, P. (2010). Syntactic unification operations are reflected in oscillatory dynamics during on-line sentence comprehension. J. Cogn. Neurosci. 22, 1333–1347. doi: 10.1162/jocn.2009.21283
Bressler, S. L. (1995). Large-scale cortical networks and cognition. Brain Res. Brain Res. Rev. 20, 288–304. doi: 10.1016/0165-0173(94)00016-i
Bressler, S. L., and Richter, C. G. (2015). Interareal oscillatory synchronization in top-down neocortical processing. Curr. Opin. Neurobiol. 31, 62–66. doi: 10.1016/j.conb.2014.08.010
Cornelissen, P. L., Kringelbach, M. L., Ellis, A. W., Whitney, C., Holliday, I. E., and Hansen, P. C. (2009). Activation of the left inferior frontal gyrus in the first 200 ms of reading: evidence from magnetoencephalography (MEG). PLoS One 4:e5359. doi: 10.1371/journal.pone.0005359
Dambacher, M., Rolfs, M., Göllner, K., Kliegl, R., and Jacobs, A. J. M. (2009). Event-Related Potentials Reveal Rapid Verification of Predicted Visual Input. Konstanz: Bibliothek der Universität.
Davidson, D. J., and Indefrey, P. (2007). An inverse relation between event-related and time-frequency violation responses in sentence processing. Brain Res. 1158, 81–92. doi: 10.1016/j.brainres.2007.04.082
Dehaene, S., and Cohen, L. (2011). The unique role of the visual word form area in reading. Trends Cogn. Sci. 15, 254–262. doi: 10.1016/j.tics.2011.04.003
DeLong, K. A., Urbach, T. P., and Kutas, M. (2005). Probabilistic word pre-activation during language comprehension inferred from electrical brain activity. Nat. Neurosci. 8, 1117–1121. doi: 10.1038/nn1504
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Dikker, S., and Pylkkanen, L. (2011). Before the N400: effects of lexical-semantic violations in visual cortex. Brain Lang. 118, 23–28. doi: 10.1016/j.bandl.2011.02.006
Dimigen, O., Kliegl, R., and Sommer, W. (2012). Trans-saccadic parafoveal preview benefits in fluent reading: a study with fixation-related brain potentials. Neuroimage 62, 381–393. doi: 10.1016/j.neuroimage.2012.04.006
Dimigen, O., Sommer, W., Hohlfeld, A., Jacobs, A. M., and Kliegl, R. (2011). Coregistration of eye movements and EEG in natural reading: analyses and review. J. Exp. Psychol. Gen. 140, 552–572. doi: 10.1037/a0023885
Ehinger, B. V., König, P., and Ossandón, J. P. (2015). Predictions of visual content across eye movements and their modulation by inferred information. J. Neurosci. 35, 7403–7413. doi: 10.1523/JNEUROSCI.5114-14.2015
Engel, A. K., and Fries, P. (2010). Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165. doi: 10.1016/j.conb.2010.02.015
Engel, A. K., Fries, P., and Singer, W. (2001). Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716. doi: 10.1038/35094565
Federmeier, K. D. (2007). Thinking ahead: the role and roots of prediction in language comprehension. Psychophysiology 44, 491–505. doi: 10.1111/j.1469-8986.2007.00531.x
Gray, C. M., König, P., Engel, A. K., and Singer, W. (1989). Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338, 334–337. doi: 10.1038/338334a0
Hagoort, P. (2005). On broca, brain and binding: a new framework. Trends Cogn. Sci. 9, 416–423. doi: 10.1016/j.tics.2005.07.004
Hagoort, P. (2013). MUC (memory, unification, control) and beyond. Front. Psychol. 4:416. doi: 10.3389/fpsyg.2013.00416
Hagoort, P. (2014). Nodes and networks in the neural architecture for language: broca’s region and beyond. Curr. Opin. Neurobiol. 28, 136–141. doi: 10.1016/j.conb.2014.07.013
Hagoort, P., Hald, L., Bastiaansen, M., and Petersson, K. M. (2004). Integration of word meaning and world knowledge in language comprehension. Science 304, 438–441. doi: 10.1126/science.1095455
Hald, L. A., Bastiaansen, M. C., and Hagoort, P. (2006). EEG theta and gamma responses to semantic violations in online sentence processing. Brain Lang. 96, 90–105. doi: 10.1016/j.bandl.2005.06.007
Hawelka, S., Schuster, S., Gagl, B., and Hutzler, F. (2015). On forward inferences of fast and slow readers. an eye movement study. Sci. Rep. 5:8432. doi: 10.1038/srep08432
Henderson, J. M., Luke, S. G., Schmidt, J., and Richards, J. E. (2013). Co-registration of eye movements and event-related potentials in connected-text paragraph reading. Front. Syst. Neurosci. 7:28. doi: 10.3389/fnsys.2013.00028
Herrmann, C. S., Munk, M. H., and Engel, A. K. (2004). Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn. Sci. 8, 347–355. doi: 10.1016/j.tics.2004.06.006
Hipp, J. F., Engel, A. K., and Siegel, M. (2011). Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 69, 387–396. doi: 10.1016/j.neuron.2010.12.027
Howard, M. W., Rizzuto, D. S., Caplan, J. B., Madsen, J. R., Lisman, J., Aschenbrenner-Scheibe, R., et al. (2003). Gamma oscillations correlate with working memory load in humans. Cereb. Cortex 13, 1369–1374. doi: 10.1093/cercor/bhg084
Hutzler, F., Bergmann, J., Conrad, M., Kronbichler, M., Stenneken, P., and Jacobs, A. M. (2004). Inhibitory effects of first syllable-frequency in lexical decision: an event-related potential study. Neurosci. Lett. 372, 179–184. doi: 10.1016/j.neulet.2004.07.050
Hutzler, F., Braun, M., Võ, M. L., Engl, V., Hofmann, M., Dambacher, M., et al. (2007). Welcome to the real world: validating fixation-related brain potentials for ecologically valid settings. Brain Res. 1172, 124–129. doi: 10.1016/j.brainres.2007.07.025
Hutzler, F., Fuchs, I., Gagl, B., Schuster, S., Richlan, F., Braun, M., et al. (2013). Parafoveal X-masks interfere with foveal word recognition: evidence from fixation-related brain potentials. Front. Syst. Neurosci. 7:33. doi: 10.3389/fnsys.2013.00033
Jung, T. P., Humphries, C., Lee, T. W., Makeig, S., McKeown, M. J., Iragui, V., et al. (1998). Extended ICA removes artifacts from electroencephalographic recordings. Adv. Neural Inf. Process. Syst. 10, 894–900.
Jung, T. P., Makeig, S., Westerfield, M., Townsend, J., Courchesne, E., and Sejnowski, T. J. (2000). Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 111, 1745–1758. doi: 10.1016/s1388-2457(00)00386-2
Jung-Beeman, M. (2005). Bilateral brain processes for comprehending natural language. Trends Cogn. Sci. 9, 512–518. doi: 10.1016/j.tics.2005.09.009
Kamide, Y., Altmann, G. T., and Haywood, S. L. (2003). The time-course of prediction in incremental sentence processing: evidence from anticipatory eye movements. J. Mem. Lang. 49, 133–156. doi: 10.1016/s0749-596x(03)00023-8
Kielar, A., Meltzer, J. A., Moreno, S., Alain, C., and Bialystok, E. (2014). Oscillatory responses to semantic and syntactic violations. J. Cogn. Neurosci. 26, 2840–2862. doi: 10.1162/jocn_a_00670
Kielar, A., Panamsky, L., Links, K. A., and Meltzer, J. A. (2015). Localization of electrophysiological responses to semantic and syntactic anomalies in language comprehension with MEG. Neuroimage 105, 507–524. doi: 10.1016/j.neuroimage.2014.11.016
Kliegl, R., Dambacher, M., Dimigen, O., Jacobs, A. M., and Sommer, W. (2012). Eye movements and brain electric potentials during reading. Psychol. Res. 76, 145–158. doi: 10.1007/s00426-011-0376-x
Kliegl, R., Nuthmann, A., and Engbert, R. (2006). Tracking the mind during reading: the influence of past, present and future words on fixation durations. J. Exp. Psychol. Gen. 135, 12–35. doi: 10.1037/0096-3445.135.1.12
Kutas, M., and Federmeier, K. D. (2007). “Event-related brain potential (ERP) studies of sentence processing,” in The Oxford Handbook of Psycholinguistics, ed. G. Gaskell (Oxford, UK: Oxford University Press), 385–406.
Kutas, M., and Federmeier, K. D. (2011). Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Annu. Rev. Psychol. 62, 621–647. doi: 10.1146/annurev.psych.093008.131123
Lau, E. F., Phillips, C., and Poeppel, D. (2008). A cortical network for semantics: (de)constructing the N400. Nat. Rev. Neurosci. 9, 920–933. doi: 10.1038/nrn2532
Lawrence, M. A. (2011). Ez: easy analysis and visualization of factorial experiments. Computer Software Manual](R Package Version 3.0–0) Available online at: http://cran.r-project.org/package=ez. Accessed on December 22, 2011.
Lewis, A. G., and Bastiaansen, M. (2015). A predictive coding framework for rapid neural dynamics during sentence-level language comprehension. Cortex 68, 155–168. doi: 10.1016/j.cortex.2015.02.014
Lewis, A. G., Wang, L., and Bastiaansen, M. (2015). Fast oscillatory dynamics during language comprehension: unification versus maintenance and prediction? Brain Lang. 148, 51–63. doi: 10.1016/j.bandl.2015.01.003
Lopez-Calderon, J., and Luck, S. J. (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 8:213. doi: 10.3389/fnhum.2014.00213
Luo, Y., Zhang, Y., Feng, X., and Zhou, X. (2010). Electroencephalogram oscillations differentiate semantic and prosodic processes during sentence reading. Neuroscience 169, 654–664. doi: 10.1016/j.neuroscience.2010.05.032
Makeig, S., Bell, A. J., Jung, T., and Sejnowski, T. J. (1996). Independent component analysis of electroencephalographic data. Adv. Neural Inf. Process. Syst. 8,145–151.
Makeig, S., Jung, T. P., Bell, A. J., Ghahremani, D., and Sejnowski, T. J. (1997). Blind separation of auditory event-related brain responses into independent components. Proc. Natl. Acad. Sci. U S A 94, 10979–10984. doi: 10.1073/pnas.94.20.10979
Maris, E., and Oostenveld, R. (2007). Nonparametric statistical testing of EEG-and MEG-data. J. Neurosci. Methods 164, 177–190. doi: 10.1016/j.jneumeth.2007.03.024
Martin, A., Schurz, M., Kronbichler, M., and Richlan, F. (2015). Reading in the brain of children and adults: a meta–analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 36, 1963–1981. doi: 10.1002/hbm.22749
Metzner, P., von der Malsburg, T., Vasishth, S., and Rösler, F. (2015). Brain responses to world knowledge violations: a comparison of stimulus-and fixation-triggered event-related potentials and neural oscillations. J. Cogn. Neurosci. 27, 1017–1028. doi: 10.1162/jocn_a_00731
Meyer, L., Grigutsch, M., Schmuck, N., Gaston, P., and Friederici, A. D. (2015). Frontal-posterior theta oscillations reflect memory retrieval during sentence comprehension. Cortex 71, 205–218. doi: 10.1016/j.cortex.2015.06.027
Meyer, L., Obleser, J., and Friederici, A. D. (2013). Left parietal alpha enhancement during working memory-intensive sentence processing. Cortex 49, 711–721. doi: 10.1016/j.cortex.2012.03.006
Pammer, K., Hansen, P. C., Kringelbach, M. L., Holliday, I., Barnes, G., Hillebrand, A., et al. (2004). Visual word recognition: the first half second. Neuroimage 22, 1819–1825. doi: 10.1016/j.neuroimage.2004.05.004
Peña, M., and Melloni, L. (2012). Brain oscillations during spoken sentence processing. J. Cogn. Neurosci. 24, 1149–1164. doi: 10.1162/jocn_a_00144
Penolazzi, B., Angrilli, A., and Job, R. (2009). Gamma EEG activity induced by semantic violation during sentence reading. Neurosci. Lett. 465, 74–78. doi: 10.1016/j.neulet.2009.08.065
Pernet, C. R., Latinus, M., Nichols, T. E., and Rousselet, G. A. (2015). Cluster-based computational methods for mass univariate analyses of event-related brain potentials/fields: a simulation study. J. Neurosci. Methods 250, 85–93. doi: 10.1016/j.jneumeth.2014.08.003
Pernet, C., Uusvuori, J., and Salmelin, R. (2007). Parafoveal-on-foveal and foveal word priming are different processes: behavioral and neurophysiological evidence. Neuroimage 38, 321–330. doi: 10.1016/j.neuroimage.2007.07.035
Plöchl, M., Ossandón, J. P., and König, P. (2012). Combining EEG and eye tracking: identification, characterization and correction of eye movement artifacts in electroencephalographic data. Front. Hum. Neurosci. 6:278. doi: 10.3389/fnhum.2012.00278
Price, C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62, 816–847. doi: 10.1016/j.neuroimage.2012.04.062
Price, C. J., and Devlin, J. T. (2011). The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 15, 246–253. doi: 10.1016/j.tics.2011.04.001
Raghavachari, S., Kahana, M. J., Rizzuto, D. S., Caplan, J. B., Kirschen, M. P., Bourgeois, B., et al. (2001). Gating of human theta oscillations by a working memory task. J. Neurosci. 21, 3175–3183.
Rayner, K. (1998). Eye movements in reading and information processing: 20 years of research. Psychol. Bull. 124, 372–422. doi: 10.1037/0033-2909.124.3.372
Rayner, K. (2009). Eye movements and attention in reading, scene perception and visual search. Q. J. Exp. Psychol. (Hove) 62, 1457–1506. doi: 10.1080/17470210902816461
Rayner, K., Warren, T., Juhasz, B. J., and Liversedge, S. P. (2004). The effect of plausibility on eye movements in reading. J. Exp. Psychol. Learn. Mem. Cogn. 30, 1290–1301. doi: 10.1037/0278-7393.30.6.1290
Risse, S., Hohenstein, S., Kliegl, R., and Engbert, R. (2014). A theoretical analysis of the perceptual span based on SWIFT simulations of the n 2 boundary paradigm. Vis. Cogn. 22, 283–308. doi: 10.1080/13506285.2014.881444
Rommers, J., Dijkstra, T., and Bastiaansen, M. (2013). Context-dependent semantic processing in the human brain: evidence from idiom comprehension. J. Cogn. Neurosci. 25, 762–776. doi: 10.1162/jocn_a_00337
Schotter, E. R., Angele, B., and Rayner, K. (2012). Parafoveal processing in reading. Atten. Percept. Psychophys. 74, 5–35. doi: 10.3758/s13414-011-0219-2
Schotter, E. R., Reichle, E. D., and Rayner, K. (2014). Rethinking parafoveal processing in reading: serial-attention models can explain semantic preview benefit and N 2 preview effects. Vis. Cogn. 22, 309–333. doi: 10.1080/13506285.2013.873508
Schuster, S., Hawelka, S., Richlan, F., Ludersdorfer, P., and Hutzler, F. (2015). Eyes on words: a fixation-related fMRI study of the left occipito-temporal cortex during self-paced silent reading of words and pseudowords. Sci. Rep. 5:12686. doi: 10.1038/srep12686
Sereno, S. C., and Rayner, K. (2003). Measuring word recognition in reading: eye movements and event-related potentials. Trends Cogn. Sci. 7, 489–493. doi: 10.1016/j.tics.2003.09.010
Singer, W. (2011). Dynamic formation of functional networks by synchronization. Neuron 69, 191–193. doi: 10.1016/j.neuron.2011.01.008
Smith, S. M., and Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98. doi: 10.1016/j.neuroimage.2008.03.061
Taylor, J., Rastle, K., and Davis, M. H. (2013). Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol. Bull. 139, 766–791. doi: 10.1037/a0030266
Varela, F., Lachaux, J., Rodriguez, E., and Martinerie, J. (2001). The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239. doi: 10.1038/35067550
von Stein, A., Chiang, C., and König, P. (2000). Top-down processing mediated by interareal synchronization. Proc. Natl. Acad. Sci. U S A 97, 14748–14753. doi: 10.1073/pnas.97.26.14748
Wang, L., Jensen, O., van den Brink, D., Weder, N., Schoffelen, J., Magyari, L., et al. (2012a). Beta oscillations relate to the N400m during language comprehension. Hum. Brain Mapp. 33, 2898–2912. doi: 10.1002/hbm.21410
Wang, L., Zhu, Z., and Bastiaansen, M. (2012b). Integration or predictability? A further specification of the functional role of gamma oscillations in language comprehension. Front. Psychol. 3:187. doi: 10.3389/fpsyg.2012.00187
Weiss, S., Mueller, H. M., Schack, B., King, J. W., Kutas, M., and Rappelsberger, P. (2005). Increased neuronal communication accompanying sentence comprehension. Int. J. Psychophysiol. 57, 129–141. doi: 10.1016/j.ijpsycho.2005.03.013
Wheat, K. L., Cornelissen, P. L., Frost, S. J., and Hansen, P. C. (2010). During visual word recognition, phonology is accessed within 100 ms and may be mediated by a speech production code: evidence from magnetoencephalography. J. Neurosci. 30, 5229–5233. doi: 10.1523/JNEUROSCI.4448-09.2010
Keywords: brain oscillations, electroencephalography, sentence processing, eye movements, semantic violation
Citation: Vignali L, Himmelstoss NA, Hawelka S, Richlan F and Hutzler F (2016) Oscillatory Brain Dynamics during Sentence Reading: A Fixation-Related Spectral Perturbation Analysis. Front. Hum. Neurosci. 10:191. doi: 10.3389/fnhum.2016.00191
Received: 22 January 2016; Accepted: 15 April 2016;
Published: 29 April 2016.
Edited by:
Lutz Jäncke, University of Zurich, SwitzerlandReviewed by:
Peter König, University of Osnabrück, GermanyRoel M. Willems, Donders Institute for Brain, Cognition and Behavior, Netherlands
Copyright © 2016 Vignali, Himmelstoss, Hawelka, Richlan and Hutzler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florian Hutzler, Zmxvcmlhbi5odXR6bGVyQHNiZy5hYy5hdA==
 Lorenzo Vignali
Lorenzo Vignali Nicole A. Himmelstoss
Nicole A. Himmelstoss Stefan Hawelka
Stefan Hawelka Fabio Richlan
Fabio Richlan Florian Hutzler
Florian Hutzler